Abstract
It is well-documented in the literature that individuals repeatedly exposed to cocaine exhibit cognitive impairment and that cognitive dysfunction is a risk factor for poor treatment outcomes in those with cocaine use disorder (CUD). Specific deficits related to attention, episodic memory, working memory, and executive functioning are the most common deficits noted in this population. Given that cognitive impairment is a risk factor for poor treatment outcomes in those with CUD, identifying possible moderating factors contributing to and/or exacerbating cocaine-related cognitive deficits is of great importance. Some of these factors may include premorbid intellectual functioning, cocaine use patterns, polysubstance use, comorbid emotional symptoms, and sleep dysfunction. It is plausible that by identifying moderating factors impacting cognition, behavioral interventions can then be modified accordingly and/or treatment regimens can be augmented with pharmacological interventions (e.g., cognitive enhancing agents), leading to a reduction in treatment attrition and improved treatment outcomes. The currently available treatments for CUD are mainly behavioral with variable efficacy, and even though there have been great preclinical and clinical research efforts focused on medication development for CUD, there are currently no Food and Drug Administration-approved medications for CUD. A description of some of the several potential moderating factors, along with some pharmacological treatments which have been shown to ameliorate, at least to some extent, cognitive dysfunction in those with CUD are discussed.
Keywords: cocaine, cocaine use disorder, cognition, intellectual functioning, cognitive deficits
According to the 2017 National Survey on Drug Use and Health (NSDUH), an estimated 2.2 million of Americans reported using cocaine within the last month (473,000 reported using crack cocaine), approximately half of which met criteria for cocaine use disorder (CUD; Center for Behavioral Health Statistics and Quality, 2018). The current conceptualization regarding the neural effects of cocaine use (and substance use in general) focuses on dopamine (DA) and the nucleus accumbens (NAc; Di Chiara, 2002; Koob & Volkow, 2016; Volkow, Koob, & McLellan, 2016; Volkow & Morales, 2015; Volkow, Wang, Fowler, & Tomasi, 2012). The NAc maintains direct and indirect involvement with several brain regions which are associated with emotions, self-regulation, disinhibition, insight, craving, and habit forming, including the dorsal striatum, amygdala, hippocampus, and prefrontal cortex (Volkow et al., 2016). The acute effects of cocaine use result in a surge of DA throughout the reward circuitry, subse quently increasing the probability of continued and future cocaine use, given the reinforcing effects. Chronic cocaine use, however, leads to a suppression of DA availability over time. The prefrontal cortex also becomes hypoactive, secondary to this reduction of DA, and an individual is therefore less likely to inhibit impulsive behaviors, including cocaine use. The insula and anterior cingulate cortex, both of which are associated with insight and self-monitoring, are also critical as they too become hypoactive secondary to this chronic suppression of DA. As a result, the individual is less aware of these cyclical, impulsive cocaine seeking/taking behaviors.
It is well-documented in the literature that individuals repeatedly exposed to cocaine exhibit cognitive impairment (Bolla & Cadet, 2007; Jovanovski, Erb, & Zakzanis, 2005; Sofuoglu, De-Vito, Waters, & Carroll, 2013; Spronk, van Wel, Ramaekers, & Verkes, 2013). In a recent meta-analytic review of 46 studies comprised of 1,452 chronic cocaine users and 1,411 controls, findings demonstrated that cocaine users evidenced deficits related to attention, episodic memory, and working memory with effect sizes of moderate or greater magnitude (Potvin, Stavro, Rizkallah, & Pelletier, 2014). These reported deficits were also consistent with previous meta-analyses exploring cognitive functioning in cocaine users (Jovanovski et al., 2005). Although the preceding deficits appear to be the most prominent, individuals with CUD also demonstrate deficits in executive functioning (e.g., reduced insight, judgment, decision making, impulsivity, disinhibition), visuoperception, psychomotor speed, and manual dexterity (Cadet & Bisagno, 2016; Fernandez-Serrano, Perales, Moreno-Lopez, Perez-Garcia, & Verdejo-Garcia, 2012; Fillmore & Rush, 2002; Li, Milivojevic, Kemp, Hong, & Sinha, 2006; Winhusen et al., 2013).
Impact of Cognitive Dysfunction on Treatment Outcomes
It is also well-documented in the literature that cognitive impairment is a risk factor for poor treatment outcomes in those with CUD (Aharonovich, Hasin, et al., 2006; Aharonovich, Nunes, & Hasin, 2003; Brewer, Worhunsky, Carroll, Rounsaville, & Potenza, 2008; Sofuoglu, 2010; Streeter et al., 2008; Turner, La-Rowe, Horner, Herron, & Malcolm, 2009). The rate of treatment attrition for individuals receiving behavioral treatment for CUD (e.g., cognitive-behavioral therapy [CBT]), ranges from 29% to 41% (Kang et al., 1991; Simpson, Joe, Fletcher, Hubbard, & Anglin, 1999) and rates for trials combining pharmacological and behavioral treatments range are similar, ranging from 30% to 40% (Carroll et al., 1994; Elkashef et al., 2005). Although there are many possible contributory factors accounting for treatment attrition, one prominent risk factor for premature discontinuation is cognitive impairment. For example, in a sample of cocaine users receiving CBT, patients who discontinued treatment performed significantly worse across several cognitive domains when compared to those who successfully completed treatment, including reduced performances on tasks related to attention, memory, executive functioning, and processing speed (Aharonovich, Hasin, et al., 2006). Further supporting this, baseline executive function in individuals with CUD significantly predicted treatment retention (e.g., greater executive dysfunction was associated with higher attrition; Verdejo-Garcia et al., 2012). Similarly, individuals with CUD who demonstrated executive deficits evidenced a shorter duration of commitment to treatment (Brewer et al., 2008), were less likely to complete treatment (Streeter et al., 2008; Turner et al., 2009), and provided a greater number of cocaine-positive urine samples after treatment initiation (Moeller et al., 2010). In addition, high working memory demand, reduced working memory function, and/or reduced response inhibition has been associated with drug craving and/or relapse (Chambers, Garavan, & Bell-grove, 2009; Hester & Garavan, 2004).
Although there is consensus in the literature that individuals who use cocaine repeatedly exhibit cognitive impairment, an important factor to consider is whether those deficits can improve following sustained abstinence. A meta-analytic review indicated that cognitive impairments remain stable during the first few months of abstinence with improvements noted following five months of sustained abstinence (Potvin et al., 2014). In support of this, stable and persistent cognitive deficits have been noted following shorter periods of abstinence (~1 month; Bauer, 1996; Bolla, Rothman, & Cadet, 1999; Rosselli & Ardila, 1996; Rosselli, Ardila, Lubomski, Murray, & King, 2001); however, improvements in cognitive performance (e.g., working memory and verbal declarative memory) have been reported following longer durations of abstinence (~6 months or longer; De Oliveira et al., 2009; Di Sclafani, Tolou-Shams, Price, & Fein, 2002).
Given that cognitive impairment is a risk factor for poor treatment outcomes in those with CUD, identifying possible moderating factors which contribute to or exacerbate cocaine-related cognitive deficits is of importance. It is plausible that by identifying moderating factors impacting cognition, behavioral interventions can then be modified accordingly and/or treatment regimens can be augmented with pharmacological interventions (e.g., cognitive enhancing agents), leading to a reduction in treatment attrition and improved outcomes. The currently available treatments for CUD are mainly behavioral with variable efficacy (Dutra et al., 2008) and, even though there have been great preclinical and clinical research efforts focused on medication development, there are currently no Food and Drug Administration (FDA)-approved medications for CUD (Forray & Sofuoglu, 2014). A description of some of the several potential moderating factors, along with some pharmacological treatments which have been shown to ameliorate, at least to some extent, cognitive dysfunction in those with CUD are discussed. Given that this is a review of the literature with no new data collected or procedures performed, institutional review board approval was not necessary.
Potential Moderating Factors Impacting Cognition in Individuals with CUD
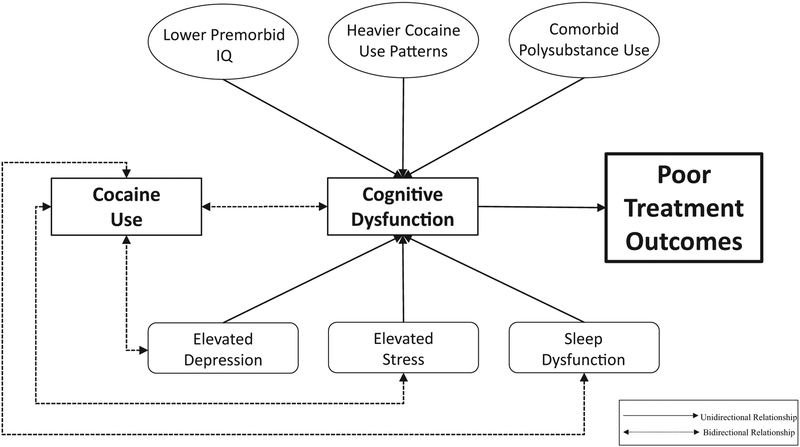
As displayed in Figure 1, there are several potential moderating factors which may contribute to cognitive dysfunction, subsequently leading to negative treatment outcomes in individuals with CUD. Some examples include lower premorbid IQ, cocaine use patterns (greater years, frequency, and daily cocaine use), and polysubstance use. In addition, there are several factors that not only detrimentally impact cognition, but also lead to continued, and possibly increased, cocaine use. For example, cocaine users with elevated symptoms of depression and elevated stress may have greater difficulty achieving abstinence because their continued cocaine use may serve as a maladaptive coping mechanism for dealing with their emotional symptoms. In addition, if an individual experiences sleep dysfunction causing subsequent fatigue, continued cocaine use may be serve as a way to combat this fatigue given the stimulant properties of the drug. Furthermore, the relationship between these factors are very likely cyclical (e.g., using cocaine in the context of elevated depression and/or stress may lead to an exacerbation of these symptoms subsequently leading to continued and increased cocaine use).
Figure 1.
Potential moderating factors impacting cognition and cocaine use in individuals with cocaine use disorder.
Premorbid IQ
Whether there is a linear relationship between IQ and neuro-psychological test performance has been questioned on several occasions (e.g., Bell & Roper, 1998; Dodrill, 1997, 1999; Horton, 1999; Larrabee, 2000; Tremont, Hoffman, Scott, & Adams, 1998). Previous finding have demonstrated a strong relationship between cognitive impairment, as assessed with the Halstead Reitan Battery (Halstead Impairment Index; Allen, 2011), and intelligence at lower IQ levels, but little relationship once IQ exceeded 90 or 95 (Dodrill, 1997). Regardless, the relationship between reduced intellectual functioning and cognitive deficits have been well-documented in the literature (Diaz-Asper, Schretlen, & Pearlson, 2004; Tremont et al., 1998). For example, Diaz-Asper et al. reported that individuals with above average IQ (>109) performed significantly better than those with average IQ (range: 99–109) across several cognitive domains including attention, memory, language abilities, and executive functions (Diaz-Asper et al., 2004). Also, and with an even greater discrepancy between performances, those with average IQ performed significantly better than those with below average IQ (<90) across those domains. The primary theory accounting for the impact of IQ on cognition is the concept of cognitive reserve (Roldan-Tapia, Garcia, Canovas, & Leon, 2012; Satz, Cole, Hardy, & Rassovsky, 2011; Satz et al., 1993; Stern, 2011). One conceptualization of cognitive reserve involves the brain compensating for compromise by recruiting alternate, more efficient, neural networks and those individuals with higher IQ may demonstrate more effective cognitive compensation in the context of brain pathology (Stern, 2002).
Similar to the findings in healthy controls mentioned previously, lower premorbid IQ was associated with reduced performances on measures of verbal learning, verbal recall, and working memory in individuals with CUD (Mahoney, Kalechstein, De Marco, Newton, & De La Garza, 2017). Similarly, the discrepancy of scores on these cognitive measures was more robust when comparing individuals with average and below average IQ versus those with above average and average IQ. There were also no differences between the three classification groups in this sample with regard to cocaine use patterns (i.e., years of use, recent use in the last 30 days, and daily use), suppressing the impact of this possible confound. One possible explanation for these findings is that individuals with higher intellectual functioning may be less susceptible to the detrimental cognitive effects produced by cocaine. Another possible explanation is that higher IQ may serve as a protective factor, in other words, slowing cognitive decline related to cocaine use, supporting the theory of cognitive reserve. As such, given that cognitive impairment is a risk factor for poor treatment outcomes, along with the high rate of relapse soon after treatment initiation, assessing premorbid IQ and addressing cognitive deficits early in the treatment process may be advantageous to promoting successful outcomes. By doing so, treatment plans can be modified accordingly and tailored specifically to the patient and, by accounting for these deficits, treatment outcomes will hopefully be improved for those with CUD.
Cocaine Use Patterns
Another potential moderating factor to consider is the association between patterns of cocaine use (e.g., years of use, recent use over the last month, and daily use) and cognition. As previously mentioned, cognitive impairments persisted during short-term abstinence (a few months) with improvement noted following approximately 5 months (Potvin et al., 2014). When compared with individuals who used <2 g of cocaine per week, those who used >2 g of cocaine per week evidenced greater impairments in attention, working memory, and processing speed (Bolla et al., 1999). Also, quantity (grams/month) and duration of cocaine use (years) have been negatively correlated with performance on measures of abstract reasoning and working memory (Fernandez-Serrano, Perez-Garcia, Perales, & Verdejo-Garcia, 2010; Fernandez-Serrano, Perez-Garcia, Schmidt Rio-Valle, & Verdejo-Garcia, 2010). Negative correlations have been reported between cognitive performance and cumulative cocaine dose, duration of cocaine use, and cocaine metabolites in hair (indicative of recent use; Vonmoos et al., 2013). Also, individuals who began using cocaine prior to 18 years of age demonstrated greater cognitive deficits than did those with a later onset of use, even when controlling for duration of use and age (Vonmoos et al., 2013). Early onset of use has been associated with reduced recovery of working memory when the cocaine use was decreased (Vonmoos et al., 2014).
When compared with healthy controls, cocaine users who were abstinent for ~3 days performed worse on tests of episodic memory, visuospatial skills, and attention/concentration, and these deficits persisted after 2 weeks of abstinence (Berry et al., 1993). Individuals with CUD who provided a urine toxicology screen positive for cocaine at the time of the assessment (suggestive of use within the last 3 days), evidenced better performance on measures of attention, executive functioning, psychomotor speed, and verbal memory as compared to those who were cocaine negative at evaluation (Berry et al., 1993; Spronk et al., 2013; Woicik et al., 2009). Although the negative impact of higher duration and frequency of use on cognitive functioning is expected, the findings that recent use is associated with improved cognitive functioning is not entirely unexpected either. For example, recent cocaine use may possibly mitigate impairments observed during the early phases of abstinence given the psycho-stimulant effects of the drug.
Neuroimaging findings have demonstrated that lifetime amount of cocaine was negatively correlated with activity in the left inferior parietal lobe extending to the left postcentral gyrus (Moreno-Lopez et al., 2012). Other neuroimaging findings noted lower activation in the right frontoparietal regions during cognitive measures of attention, working memory, executive control, and vigilance (Barros-Loscertales et al., 2011; Bustamante et al., 2011; Tomasi et al., 2007). In addition, grams of cocaine used per week was negatively correlated with activation in the left orbitofrontal cortex (Bolla et al., 2003). MRI has also shown reduction in gray matter volume in orbitofrontal, cingulate, insular, temporoparietal and cerebellar cortex, and these changes in gray matter volume were correlated with the duration of cocaine use (Ersche et al., 2011). Cocaine use is associated with changes in both gray and white matter structure, some which may be partially reversible, as prefrontal gray matter has been shown to improve with abstinence (Parvaz et al., 2017).
Although prior literature suggests that greater cognitive decrement may result from more years, recent, and daily cocaine use, results from a recent study did not support these findings. Specifically, these usage characteristics did not modulate performance on tasks of attention, working memory, and episodic memory (Mahoney, Kalechstein, Newton, & De La Garza, 2017). These findings were unexpected given the significantly discrepant usage patterns between the higher and lower comparison groups (years of cocaine use: ~25 vs. ~10 years; recent use over the last month: ~26 vs. ~6 days; daily use: ~1.8 vs. ~0.7 g). One potential, yet speculative, explanation for these findings is that after using cocaine for a certain number of years, the deleterious effects on cognition stabilize and increased use does not exacerbate those impairments. This possibility may be supported to some extent by the findings that recreational cocaine users exhibited significant impairments similar to individuals with CUD in the domains of attention, working memory, memory and executive functions (Vonmoos et al., 2013).
Some methodological differences may have also accounted for the discrepancy in these abovementioned findings and other studies which noted differences, at least to some degree. For example, the participants in the study referenced above had abstained for a much shorter duration of time in comparison to those in prior reports. In addition, different cognitive measures were used across studies, possibly also contributing to the discrepancy between these findings. Differences in sample characteristics (e.g., severity of use, polydrug use, psychiatric comorbidities, main route of administration) and potentially relevant modulating factors (e.g., purity of the drug, lifestyle differences, genetic predispositions, environmental factors), which are difficult to scientifically address and control, also add complexity to measuring the impact of cocaine use patterns (Vonmoos & Quednow, 2017). Regardless of the mixed findings from a cognitive standpoint, drug use characteristics still warrant consideration during treatment planning given the known association between factors such as longer duration of cocaine use and increased addiction severity.
Polysubstance Use
Individuals diagnosed with CUD are also more likely to use other substances, including nicotine, alcohol, and cannabis (Aharonovich, Garawi, et al., 2006; Brewer, Mahoney, Nerumalla, Newton, & De La Garza, 2013; Budney, Higgins, Hughes, & Bickel, 1993; Carroll, Rounsaville, & Bryant, 1993; Roll, Higgins, Budney, Bickel, & Badger, 1996). As such, it is important to consider whether the use of these other substances moderates cognition or exacerbates deficits that commonly occur among individuals with CUD. In a recent review of the literature, cigarette smokers who were not cocaine users exhibited deficits in executive functioning, learning, episodic memory, processing speed, and working memory (Durazzo et al., 2010). Individuals often report smoking cigarettes as a means to improve cognition, specifically increasing attention and reducing distractibility (West, 1993). Because nicotine use is associated with a number of cognitive deficits (Durazzo et al., 2010), it is plausible that the synergistic effects of cocaine and nicotine could adversely affect cognition over and above the independent effects of either substance alone.
Studies have shown that 60% to 80% of individuals with CUD also meet criteria for alcohol use disorder (Carroll et al., 1993; Regier et al., 1990) and the percentage of those with CUD who engage in moderate alcohol use (but not to the extent of use disorder criteria), likely exceeds that number. In a meta-analysis, which included 143 published articles related to alcohol use and cognitive functioning, “heavy” drinking (3 to 4 drinks/day) was associated with an increased risk of cognitive impairment; however, “light to moderate” drinking (1 to 2 drinks/day) was not associated with any cognitive deficits (Neafsey & Collins, 2011). Because heavy alcohol use is associated with cognitive decline, it is plausible that the additive effects of cocaine in combination with alcohol use may adversely affect cognition at a greater level than either substance alone. In support of this, comorbid CUD and alcohol use disorder was associated with greater impairment on measures of executive functioning when compared with those who met criteria for one of the two SUDs, but not both (Bolla, Funderburk, & Cadet, 2000).
Rates of concurrent cannabis use in those with CUD ranges from 59% to 89% (Aharonovich, Garawi, et al., 2006; Aharonovich et al., 2005; Lindsay, Stotts, Green, Herin, & Schmitz, 2009; Miller, Klahr, Gold, Sweeney, & Cocores, 1990). Cannabis use is also a risk factor for cognitive deficits related to attention, information processing speeding, learning and memory, and executive/frontal functions (Bolla, Brown, Eldreth, Tate, & Cadet, 2002; Meier et al., 2012; Solowij, 1998; Solowij & Battisti, 2008; Solowij, Stephens, Roffman, & Babor, 2002; Solowij, Stephens, Roffman, Babor, et al., 2002). Individuals with CUD who were moderate users of alcohol and marijuana (but did not meet use disorder criteria for the latter two substances) exhibited decrements in declarative recall and attention when compared to moderate users of marijuana and alcohol who do not use cocaine (Vadhan et al., 2014). As such, these findings support the possible deleterious cognitive effects of polysubstance use. As clinicians providing SUD treatment routinely do, continued assessment of comorbid substance use is critical as the use of multiple substances may serve as barrier to successful treatment outcomes, given the greater negative impact of polysubstance use on cognitive functioning.
Depression
Another factor possibly modulating cognition are emotional symptoms such as depression, a common comorbid diagnosis occurring in ~45% of individuals with CUD (Conway, Compton, Stinson, & Grant, 2006). The Diagnostic and Statistical Manual of Mental Disorders – Fifth ed. (DSM-5) also includes specific criteria related to cognition for major depressive disorder: “the diminished ability to think or concentrate, or indecisiveness” (American Psychiatric Association, 2013). Depression has been associated with cognitive dysfunction across several domains including attention, executive functions, memory, and psychomotor speed (Clark, DiBenedetti, & Perez, 2016). Also, comorbid de pression is a known risk factor for relapse in patients receiving treatment for cocaine use (Poling, Kosten, & Sofuoglu, 2007).
When compared with healthy controls, although none of the individuals with CUD met DSM-5 diagnostic criteria for any depressive disorder, cocaine users reported significantly more symptoms of depression evidenced by higher scores on the Beck Depression Inventory – 2nd Ed. (BDI-II; Mahoney et al., 2015). These findings may have treatment implications given that individuals with lower rates of depression were more likely to remain cocaine abstinent after treatment (McKay et al., 2013). Given the relationship between elevated depression and cognitive deficits, those individuals may have had greater difficulty practicing the techniques provided during treatment, such as relapse prevention strategies, making these individuals more susceptible to relapse. Identifying depression early in the treatment process and modifying treatment plans to address and treat these symptoms will therefore be of benefit to maximize the potential for successful treatment outcomes.
Stress and Trauma
Elevated stress and past trauma have also been found to detrimentally impact learning and memory, working memory, and visuospatial abilities (Bremner, Krystal, Southwick, & Charney, 1995; Morgan, Doran, Steffian, Hazlett, & Southwick, 2006). In addition, stress and trauma are known risk factors for relapse following treatment for CUD (Back et al., 2000; Brady & Sinha, 2005; McMahon, 2001; Sinha, 2001), and those with CUD have reported greater use of cocaine following stressful events (Wal-drop, Back, Verduin, & Brady, 2007). One possibility for this involves cocaine use serving as a maladaptive coping mechanism for dealing with distress. In other words, given that chronic stress is aversive, an individual may “self-medicate” using cocaine, supported by the association between increased cocaine craving and increased psychological stress (Sinha, Fuse, Aubin, & O’Malley, 2000). Also, elevated lifetime stress has been associated with higher addiction severity in individuals with CUD (Mahoney, Newton, Omar, Ross, & De la Garza, 2013). Given that elevated stress is independently known to contribute to cognitive dysfunction, it is plausible that cognitive deficits produced by elevated levels of stress in combination with cocaine use, may exceed deficits produced by either stress or cocaine alone. As such, during substance use treatment, concurrently addressing and treating past trauma, along with providing strategies to adaptively cope with ongoing distress, is of importance especially since craving and relapse is more prominent in the context of increased stress.
Sleep Dysfunction
Cognitive deficits associated with sleep dysfunction include impairments related to attention, processing, executive functioning, and memory (Durmer & Dinges, 2005; Goel, Rao, Durmer, & Dinges, 2009; Jones & Harrison, 2001; Lowe, Safati, & Hall, 2017; Walker, 2008). In addition, it is well known that individuals with CUD experience disrupted sleep as acute cocaine use and withdrawal adversely affect objective measures of sleep quality (Gawin & Kleber, 1986; Morgan & Malison, 2007; Weddington et al., 1990). Specifically, sleep onset latency is prolonged, total sleep time is reduced, and sleep efficiency is decreased (Johanson, Roehrs, Schuh, & Warbasse, 1999; Post, Gillin, Wyatt, & Goodwin, 1974; Schierenbeck, Riemann, Berger, & Hornyak, 2008; Watson, Bakos, Compton, & Gawin, 1992).
There are differential effects of acute cocaine use and withdrawal, as acute use reduces REM sleep (Johanson et al., 1999; Post et al., 1974; Schierenbeck et al., 2008; Watson et al., 1992), whereas withdrawal increases REM sleep percentage and decreases REM latency (Gillin, Pulvirenti, Withers, Golshan, & Koob, 1994; Johanson et al., 1999; Kowatch, Schnoll, Knisely, Green, & Elswick, 1992; Post et al., 1974; Schierenbeck et al., 2008; Watson et al., 1992). This can, however, be remediated following sustained abstinence, as improvements have been noted in subjective measures of sleep quality following abstinence (Matuskey, Pittman, Forselius, Malison, & Morgan, 2011). Excessive daytime sleepiness in cocaine users, which could result from poor sleep quality and/or reduced sleep time, may lead to partial sleep deprivation and deleteriously affect cognitive functioning. Individuals with CUD report poorer sleep quality and elevated daytime sleepiness, and self-reported sleep dysfunction was more common in those reporting greater recent cocaine use over the last month (Mahoney, De La Garza, et al., 2014).
Thus, given that sleep dysfunction is known to impair cognitive function, possibly exacerbating deficits related to an individual’s cocaine use, addressing and treating sleep abnormalities concurrently may be of benefit, at least to some extent, in improving treatment adherence. In addition, by treating sleep abnormalities and improving sleep hygiene (through the implementation of a structured routine), the individual will hopefully be able to carry this behavior to other aspects of his or her life, given that structure and routine is critical for successfully for SUD treatment.
Medication Development for CUD
There are currently no FDA-approved medications for CUD; however, research has provided evidence that medications with cognitive enhancing properties have successfully improved cognition in individuals with CUD. Given the association between cognitive impairment and poor treatment outcomes in those with CUD (Aharonovich, Brooks, Nunes, & Hasin, 2008; Aharonovich, Hasin, et al., 2006; Aharonovich et al., 2003; McKellar, Harris, & Moos, 2006; McKellar, Kelly, Harris, & Moos, 2006; Sofuoglu, 2010; Sofuoglu, Sugarman, & Carroll, 2010), remediating these deficits pharmacologically warrants investigation and many medications with cognitive-enhancing properties have been examined as potential treatments for neuropsychiatric disorders and SUDs (Sofuoglu, 2010; Wallace, Ballard, Pouzet, Riedel, & Wettstein, 2011). Cognition is modulated by several neurotransmitters, including dopamine (DA), acetylcholine (ACh), serotonin, gluta-mate, GABA, and norepinephrine (NE) and the role of the catecholamine neurotransmitters in cognition, motivation, and reward is well known (Berridge & Waterhouse, 2003; Wise, 1978, 2004a, 2004b).
Modafinil for CUD
One cognitive enhancing medication, modafinil, currently approved by the FDA for narcolepsy, sleep apnea, and shift work sleep disorder, has mixed neurotransmitter actions in multiple brain regions (including GABA, glutamate, and dopaminergic transmitters). Modafinil has been shown to improve cognitive function in healthy controls (Baranski, Pigeau, Dinich, & Jacobs, 2004; Turner et al., 2003) as well as in individuals with attention-deficit/hyperactivity disorder (ADHD; Turner, Clark, Dowson, Robbins, & Sahakian, 2004) and methamphetamine use disorder (Ghahremani et al., 2011; Kalechstein, De La Garza, & Newton, 2010). Modafanil exposure is associated with increased daytime sleep latency and decreased daytime sleepiness in abstinent cocaine and methamphetamine dependent individuals (Mahoney et al., 2012; Morgan, Pace-Schott, Pittman, Stickgold, & Malison, 2010).
Modafinil has also shown promise in some clinical trials for CUD. For example, in nontreatment seeking individuals with CUD, short-term administration of modafinil improved performance on two measures of working memory and demonstrated a trend toward significant improvement on a measure of visual working memory, two measures of sustained attention, and reduced impulsivity (Kalechstein, Mahoney, Yoon, Bennett, & De la Garza, 2013). In comparison to placebo, treatment-seeking individuals with CUD randomized to modafinil were significantly more likely to be abstinent overall over an 8-week treatment period (Kampman et al., 2015). In addition, as mentioned above, there is a high comorbidity of depression and CUD, and results from a meta-analysis in individuals with major depressive disorder found significant effects of modafinil and improvements in overall depression scores and fatigue symptoms (Goss, Kaser, Costafreda, Sahakian, & Fu, 2013). That being said, in a recent meta-analytic review of 11 studies, findings revealed that there was no evidence to conclude superiority of modafinil in increasing cocaine abstinence and treatment retention rate; however, subgroup analysis of six studies conducted in the United States demonstrated that modafinil was superior to placebo in improving cocaine abstinence (Sangroula et al., 2017). Although the results were mixed, the authors concluded that due to the promising results in this subgroup analysis, along with the good safety profile, larger studies to derive more conclusive results are warranted.
Rivastigmine and Galantamine for CUD
The neurotransmitter acetylcholine is heavily involved in the cognitive and behavioral processes of SUDs through interactions with the dopaminergic reward system in the NAc, prefrontal cortex, as well as other areas (Sofuoglu & Mooney, 2009). Acetylcholine is known to play a role in several cognitive functions including motor processing, attention, arousal, declarative memory, and working memory (Pepeu, Spignoli, Giovannini, & Magnani, 1989; Woolf, 2006).
One class of medications that has demonstrated an indication for the attenuation of cognitive impairment are acetylcholinesterase inhibitors which increase levels of synaptic acetylcholine (Sofuoglu & Mooney, 2009). Given that individuals with substance use disorders display altered cholinergic responses in brain areas relevant to craving, learning, and memory, the cholinergic system may be a promising pharmacological treatment target (Adinoff et al., 2010). These medications have been studied extensively and are FDA-approved for the treatment of Alzheimer’s disease, due to their effects on dementia-associated cognitive and functional impairments (M. Farlow, 2002; M. R. Farlow, 2002). Administration of rivastigmine improved performance on tests of attention and memory in individuals diagnosed with Alzheimer’s disease (Feldman, Lane, & the Study 304 Group, 2007; Frankfort et al., 2007) and improved information processing, episodic memory (Silver et al., 2009), and vigilance (Tenovuo, Alin, & Helenius, 2009) in individuals who sustained a traumatic brain injury. Rivastigmine has also shown benefit in improving cognition in those with CUD as acute, low-dose treatment with rivastigmine improved span of working memory (Mahoney, Kalechstein, et al., 2014). In addition, a double-blind, randomized trial of galantamine treatment in recently abstinent chronic cocaine abusers demonstrated selective improvement in measures of sustained attention and working memory functions (Sofuoglu, Waters, Poling, & Carroll, 2011). In a separate study, relative to placebo, participants with CUD and opioid use disorder randomized to galantamine had fewer self-reported or urine-confirmed cocaine use days over an 8-week treatment course (Sofuoglu & Carroll, 2011). A recent study revealed that while galantamine did not improve measures of cognitive functioning, it was associated with a significant reduction of cocaine use (Carroll, Nich, DeVito, Shi, & Sofuoglu, 2018), findings that were also consistent with prior literature (Sofuoglu & Carroll, 2011). Given these positive findings related to a reduction in cocaine use, further investigations of acetylcholinesterase inhibitors for CUD are warranted.
Conclusions
Although it is well-established that cocaine users exhibit cognitive impairment across several domains, there are potential moderating factors possibly exacerbating these cocaine-related deficits. Given that cognitive impairment is a risk factor for poor treatment outcomes in those with CUD, accounting for and addressing these potential moderating factors at the onset of treatment initiation, may hopefully improve treatment outcomes including retention and sustained abstinence. For example, one such factor to consider is premorbid intellectual functioning given the known relationship between lower premorbid IQ and reduced cognitive functioning. Specifically, by evaluating premorbid IQ and subsequent cognitive dysfunction early in the treatment process, behavioral therapies (i.e., cognitive-behavioral therapy) can be modified appropriately and tailored to the patient’s level of functioning (e.g., simplifying strategies and techniques), hopefully leading to improved treatment outcomes.
The identified behavioral interventions can also be augmented with additional components to address other factors possibly exacerbating cognitive deficits. For example, sleep dysfunction is known to contribute to reduced cognitive performance, and individuals with CUD experience reduced sleep quality and quantity. If an individual with CUD endorses sleep dysfunction, behavioral providers can then provide evidenced-based recommendations for improving sleep (e.g., sleep hygiene strategies and education) possibly remediating, at least to some extent, cognitive deficits related to their sleep abnormalities. In addition, emotional symptoms (including depression, stress, and trauma), all of which are prevalent in individuals with CUD, are also known to independently interfere with cognition. By assessing for and aggressively treating these symptoms early in treatment, behavioral interventions can then be tailored specifically to addressing the emotional symptoms the patient is endorsing. For example, treatment plans can be modified to include the instruction, practice, and imple mentation of adaptive coping mechanisms in combination with the processing of past distress. By doing so, cognitive deficits related to these emotional symptoms can hopefully be remediated to some degree.
It is important to note that while individuals with CUD demonstrate multiple cognitive deficits, it cannot be stated with certainty that these deficits are a direct result of cocaine use, especially in the absence of baseline (pre-cocaine use) data. This has been previously reported in the literature, specifically that cocaine users may experience cognitive deficits predating the onset of cocaine use (Spronk et al., 2013) and that preexisting abnormalities may be linked to frontal deficits in cocaine users (Winhusen et al., 2013). This is important to consider given that individuals with preexisting cognitive deficits may also be more vulnerable to initiating drug use and/or more susceptible to relapse following abstinence (Sofuoglu, DeVito, Waters, & Carroll, 2016). In addition, genetic predispositions must also be considered given the distinct neuro-biological phenotypes associated with a familial vulnerability for developing CUD (Ersche et al., 2013). Regardless of the etiology of the cognitive dysfunction (secondary to chronic cocaine use, premorbid cognitive deficits predating the onset of cocaine use, and/or preexisting vulnerability factors including comorbid psychiatric symptoms), treating these cognitive deficits remains an important target given that they are known to have an adverse impact on successful treatment outcomes (Sofuoglu et al., 2016).
It is also important to note that by addressing and treating these potential moderating factors, these individuals may not fully return to their baseline level of cognitive functioning. That being said, it can be expected that some of these deficits should improve to the extent that these individuals will be able to better engage, learn, and practice the techniques they are instructed through behavioral intervention and treatment. For example, as previously mentioned, the most prominent deficits cocaine users endorse are with regard to attention, episodic memory, working memory, and executive functioning. Deficits related to attention will serve as a barrier to patients fully engaging during treatment, preventing them from successfully learning the techniques (e.g., relapse prevention strategies) presented to them during therapy. Also, deficits related to episodic memory will also serve as a barrier as patients will have difficulty recalling the information they were provided during therapy sessions and translating the techniques into their “real life” environments. Finally, deficits related to working memory and executive functioning will also serve as a barrier as patients will have difficulty modifying previously established maladaptive coping mechanisms through cognitive restructuring, reorganization, and problem solving.
In addition to addressing the possible moderating factors, augmenting behavioral treatment with psychotropic medication with cognitive enhancing properties may provide added benefit to improving cognition. Although there are no FDA-approved medications for CUD currently, several medications have shown an indication for improving cognitive functioning. Two such medications, modafinil and rivastigmine, have shown some promise in improving cognition in those with CUD. Specifically, modafinil, which targets the neurotransmitter dopamine, improved performance on measures of working memory and sustained attention (Kalechstein et al., 2013), whereas rivastigmine, which targets the neurotransmitter acetylcholine, improved span of working memory in individuals with CUD (Mahoney, Kalechstein, et al., 2014). In conclusion, by taking a multidimensional approach, accounting for and treating these other possible moderating factors that contribute to and/or exacerbate cognitive deficits, along with augmenting behavioral therapies with pharmacological treatments, cognitive deficits in individuals with CUD can be, at least, partially remediated, hopefully leading to improved engagement in therapy, and more successful treatment outcomes.
Public Health Significance.
Individuals repeatedly exposed to cocaine exhibit cognitive impairment and cognitive dysfunction is a risk factor for poor treatment outcomes. Identifying potential moderating factors contributing to and/or exacerbating cognitive deficits in cocaine users is of importance given the relationship between cognition and treatment outcomes. By accounting for possible moderating factors early in treatment and modifying and/or augmenting treatment plans accordingly, treatment outcomes may subsequently be improved.
Acknowledgments
The author receives support from the National Institute of General Medical Sciences of the National Institutes of Health (NIH) under Award U54GM104942–03. The funding source had no other role other than financial support. The content is solely the responsibility of the author and does not necessarily represent the official views of the NIH.
The findings reported in this article were presented at the following national conferences: American College of Neuropsychopharmacology (12/4–12/8/2011, Waikoloa, Hawaii; 12/6–12/7/2012, Hollywood, Florida; 12/8–12/12/2013, Hollywood, Florida), American Psychological Association (8/2–8/5/2012, Orlando, Florida; 8/3–8/6/2017, Washington, DC), College on Problems of Drug Dependence (6/18–6/23/2011, Hollywood, Florida; 6/9–6/14/2012, Palm Spring, California), International Neuropsychological Society (2/4–2/7/2015, Denver, Colorado; 2/3–2/6/2016, Boston, Massachusetts). The author declares no conflicts of interest.
References
- Adinoff B, Devous MD Sr., Williams MJ, Best SE, Harris TS, Minhajuddin A, … Cullum M (2010). Altered neural cholinergic receptor systems in cocaine-addicted subjects. Neuropsychopharmacology, 35, 1485–1499. 10.1038/npp.2010.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharonovich E, Brooks AC, Nunes EV, & Hasin DS (2008). Cognitive deficits in marijuana users: Effects on motivational enhancement therapy plus cognitive behavioral therapy treatment outcome. Drug and Alcohol Dependence, 95, 279–283. 10.1016/j.drugalcdep.2008.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharonovich E, Garawi F, Bisaga A, Brooks D, Raby WN, Rubin E, … Levin FR (2006). Concurrent cannabis use during treatment for comorbid ADHD and cocaine dependence: Effects on outcome. American Journal of Drug and Alcohol Abuse, 32, 629–635. 10.1080/00952990600919005 [DOI] [PubMed] [Google Scholar]
- Aharonovich E, Hasin DS, Brooks AC, Liu X, Bisaga A, & Nunes EV (2006). Cognitive deficits predict low treatment retention in cocaine dependent patients. Drug and Alcohol Dependence, 81, 313–322. 10.1016/j.drugalcdep.2005.08.003 [DOI] [PubMed] [Google Scholar]
- Aharonovich E, Liu X, Samet S, Nunes E, Waxman R, & Hasin D (2005). Postdischarge cannabis use and its relationship to cocaine, alcohol, and heroin use: A prospective study. The American Journal of Psychiatry, 162, 1507–1514. 10.1176/appi.ajp.162.8.1507 [DOI] [PubMed] [Google Scholar]
- Aharonovich E, Nunes E, & Hasin D (2003). Cognitive impairment, retention and abstinence among cocaine abusers in cognitive-behavioral treatment. Drug and Alcohol Dependence, 71, 207–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen DN (2011). Halstead Impairment Index In Kreutzer JS, DeLuca J, & Caplan B (Eds.), Encyclopedia of clinical neuropsychology (pp. 1197–1199). New York, NY: Springer. [Google Scholar]
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, DC: Author. [Google Scholar]
- Back S, Dansky BS, Coffey SF, Saladin ME, Sonne S, & Brady KT (2000). Cocaine dependence with and without post-traumatic stress disorder: A comparison of substance use, trauma history and psychiatric comorbidity. The American Journal on Addictions, 9, 51–62. 10.1080/10550490050172227 [DOI] [PubMed] [Google Scholar]
- Baranski JV, Pigeau R, Dinich P, & Jacobs I (2004). Effects of modafinil on cognitive and meta-cognitive performance. Human Psychopharmacology, 19, 323–332. 10.1002/hup.596 [DOI] [PubMed] [Google Scholar]
- Barrós-Loscertales A, Bustamante JC, Ventura-Campos N, Llopis JJ, Parcet MA, & Avila C (2011). Lower activation in the right frontoparietal network during a counting Stroop task in a cocaine-dependent group. Psychiatry Research, 194, 111–118. 10.1016/j.pscychresns.2011.05.001 [DOI] [PubMed] [Google Scholar]
- Bauer LO (1996). Psychomotor and electroencephalographic sequelae of cocaine dependence. NIDA Research Monograph, 163, 66–93. [PubMed] [Google Scholar]
- Bell BD, & Roper BL (1998). “Myths of neuropsychology”: Another view. The Clinical Neuropsychologist, 12, 237–244. 10.1076/clin.12.2.237.1995 [DOI] [Google Scholar]
- Berridge CW, & Waterhouse BD (2003). The locus coeruleusnoradrenergic system: Modulation of behavioral state and state-dependent cognitive processes. Brain Research Reviews, 42, 33–84. 10.1016/S0165-0173(03)00143-7 [DOI] [PubMed] [Google Scholar]
- Berry J, van Gorp WG, Herzberg DS, Hinkin C, Boone K, Steinman L, & Wilkins JN (1993). Neuropsychological deficits in abstinent cocaine abusers: Preliminary findings after two weeks of abstinence. Drug and Alcohol Dependence, 32, 231–237. 10.1016/0376-8716(93)90087-7 [DOI] [PubMed] [Google Scholar]
- Bolla KI, Brown K, Eldreth D, Tate K, & Cadet JL (2002). Dose-related neurocognitive effects of marijuana use. Neurology, 59, 1337–1343. 10.1212/01.WNL.0000031422.66442.49 [DOI] [PubMed] [Google Scholar]
- Bolla KI, & Cadet JL (2007). Cocaine In A. K. W. v. Gorp (Ed.), Neuropsychology and substance use (pp. 111–138). New York, NY: Taylor & Francis. [Google Scholar]
- Bolla KI, Eldreth DA, London ED, Kiehl KA, Mouratidis M, Contoreggi C, … Ernst M (2003). Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. NeuroImage, 19, 1085–1094. 10.1016/S1053-8119(03)00113-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Funderburk FR, & Cadet JL (2000). Differential effects of cocaine and cocaine alcohol on neurocognitive performance. Neurology, 54, 2285–2292. 10.1212/WNL.54.12.2285 [DOI] [PubMed] [Google Scholar]
- Bolla KI, Rothman R, & Cadet JL (1999). Dose-related neurobehavioral effects of chronic cocaine use. The Journal of Neuropsychiatry and Clinical Neurosciences, 11, 361–369. 10.1176/jnp.11.3.361 [DOI] [PubMed] [Google Scholar]
- Brady KT, & Sinha R (2005). Co-occurring mental and substance use disorders: The neurobiological effects of chronic stress. The American Journal of Psychiatry, 162, 1483–1493. 10.1176/appi.ajp.162.8.1483 [DOI] [PubMed] [Google Scholar]
- Bremner JD, Krystal JH, Southwick SM, & Charney DS (1995). Functional neuroanatomical correlates of the effects of stress on memory. Journal of Traumatic Stress, 8, 527–553. 10.1002/jts.2490080403 [DOI] [PubMed] [Google Scholar]
- Brewer AJ III, Mahoney JJ III, Nerumalla CS, Newton TF, & De La Garza R II. (2013). The influence of smoking cigarettes on the high and desire for cocaine among active cocaine users. Pharmacology, Biochemistry, and Behavior, 106, 132–136. 10.1016/j.pbb.2013.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, & Potenza MN (2008). Pretreatment brain activation during stroop task is associated with outcomes in cocaine-dependent patients. Biological Psychiatry, 64, 998–1004. 10.1016/j.biopsych.2008.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST, Hughes JR, & Bickel WK (1993). Nicotine and caffeine use in cocaine-dependent individuals. Journal of Substance Abuse, 5, 117–130. 10.1016/0899-3289(93)90056-H [DOI] [PubMed] [Google Scholar]
- Bustamante JC, Barrós-Loscertales A, Ventura-Campos N, Sanjuán A, Llopis JJ, Parcet MA, & Avila C (2011). Right parietal hypoactivation in a cocaine-dependent group during a verbal working memory task. Brain Research, 1375, 111–119. 10.1016/j.brainres.2010.12.042 [DOI] [PubMed] [Google Scholar]
- Cadet JL, & Bisagno V (2016). Neuropsychological consequences of chronic drug use: Relevance to treatment approaches. Frontiers in Psychiatry, 6, 189 10.3389/fpsyt.2015.00189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Nich C, DeVito EE, Shi JM, & Sofuoglu M (2018). Galantamine and computerized cognitive behavioral therapy for cocaine dependence: A randomized clinical trial. The Journal of Clinical Psychiatry, 79, 17m11669 10.4088/JCP.17m11669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, & Bryant KJ (1993). Alcoholism in treatment-seeking cocaine abusers: Clinical and prognostic significance. Journal of Studies on Alcohol, 54, 199–208. 10.15288/jsa.1993.54.199 [DOI] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Gordon LT, Nich C, Jatlow P, Bisighini RM, & Gawin FH (1994). Psychotherapy and pharmacotherapy for ambulatory cocaine abusers. Archives of General Psychiatry, 51, 177–187. 10.1001/archpsyc.1994.03950030013002 [DOI] [PubMed] [Google Scholar]
- Center for Behavioral Health Statistics and Quality. (2018). 2017 National Survey on Drug Use and Health: Detailed tables. Rockville, MD: Substance Abuse and Mental Health Services Administration. [Google Scholar]
- Chambers CD, Garavan H, & Bellgrove MA (2009). Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neuroscience and Biobehavioral Reviews, 33, 631–646. 10.1016/j.neubiorev.2008.08.016 [DOI] [PubMed] [Google Scholar]
- Clark M, DiBenedetti D, & Perez V (2016). Cognitive dysfunction and work productivity in major depressive disorder. Expert Review of Pharmacoeconomics & Outcomes Research, 16, 455–463. 10.1080/14737167.2016.1195688 [DOI] [PubMed] [Google Scholar]
- Conway KP, Compton W, Stinson FS, & Grant BF (2006). Lifetime comorbidity of DSM–IV mood and anxiety disorders and specific drug use disorders: Results from the National Epidemiologic Survey on Alcohol and Related Conditions. The Journal of Clinical Psychiatry, 67, 247–257. 10.4088/JCP.v67n0211 [DOI] [PubMed] [Google Scholar]
- De Oliveira LG, Barroso LP, Silveira CM, Sanchez ZV, De Carvalho Ponce J, Vaz LJ, & Nappo SA (2009). Neuropsycho-logical assessment of current and past crack cocaine users. Substance Use & Misuse, 44, 1941–1957. 10.3109/10826080902848897 [DOI] [PubMed] [Google Scholar]
- Diaz-Asper CM, Schretlen DJ, & Pearlson GD (2004). How well does IQ predict neuropsychological test performance in normal adults? Journal of the International Neuropsychological Society, 10, 82–90. 10.1017/S1355617704101100 [DOI] [PubMed] [Google Scholar]
- Di Chiara G (2002). Nucleus accumbens shell and core dopamine: Differential role in behavior and addiction. Behavioural Brain Research, 137(1–2), 75–114. 10.1016/S0166-4328(02)00286-3 [DOI] [PubMed] [Google Scholar]
- Di Sclafani V, Tolou-Shams M, Price LJ, & Fein G (2002). Neuro-psychological performance of individuals dependent on crack-cocaine, or crack-cocaine and alcohol, at 6 weeks and 6 months of abstinence. Drug and Alcohol Dependence, 66, 161–171. 10.1016/S0376-8716(01)00197-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodrill CB (1997). Myths of neuropsychology. The Clinical Neuropsychologist, 11, 1–17. 10.1080/13854049708407025 [DOI] [PubMed] [Google Scholar]
- Dodrill CB (1999). Myths of neuropsychology: Further considerations. The Clinical Neuropsychologist, 13, 562–572. 10.1076/1385-4046(199911)13:04;1-Y;FT562 [DOI] [PubMed] [Google Scholar]
- Durazzo TC, Fryer SL, Rothlind JC, Vertinski M, Gazdzinski S, Mon A, & Meyerhoff DJ (2010). Measures of learning, memory and processing speed accurately predict smoking status in short-term abstinent treatment-seeking alcohol-dependent individuals. Alcohol and Alcoholism, 45, 507–513. 10.1093/alcalc/agq057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durmer JS, & Dinges DF (2005). Neurocognitive consequences of sleep deprivation. Seminars in Neurology, 25, 117–129. 10.1055/s-2005-867080 [DOI] [PubMed] [Google Scholar]
- Dutra L, Stathopoulou G, Basden SL, Leyro TM, Powers MB, & Otto MW (2008). A meta-analytic review of psychosocial interventions for substance use disorders. The American Journal of Psychiatry, 165, 179–187. 10.1176/appi.ajp.2007.06111851 [DOI] [PubMed] [Google Scholar]
- Elkashef A, Holmes TH, Bloch DA, Shoptaw S, Kampman K, Reid MS, … Vocci F (2005). Retrospective analyses of pooled data from CREST I and CREST II trials for treatment of cocaine dependence. Addiction, 100(Suppl. 1), 91–101. 10.1111/j.1360-0443.2005.00986.x [DOI] [PubMed] [Google Scholar]
- Ersche KD, Barnes A, Jones PS, Morein-Zamir S, Robbins TW, & Bullmore ET (2011). Abnormal structure of frontostriatal brain systems is associated with aspects of impulsivity and compulsivity in cocaine dependence. Brain: A Journal of Neurology, 134(Part 7), 2013–2024. 10.1093/brain/awr138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Jones PS, Williams GB, Smith DG, Bullmore ET, & Robbins TW (2013). Distinctive personality traits and neural correlates associated with stimulant drug use versus familial risk of stimulant dependence. Biological Psychiatry, 74, 137–144. 10.1016/j.biopsych.2012.11.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farlow MR (2002). Cholinesterase inhibitors: Relating pharmacological properties to clinical profiles: Introduction. International Journal of Clinical Practice Supplement, 127, 1–5. [PubMed] [Google Scholar]
- Farlow M (2002). A clinical overview of cholinesterase inhibitors in Alzheimer’s disease. International Psychogeriatrics, 14(Suppl. 1), 93–126. 10.1017/S1041610203008688 [DOI] [PubMed] [Google Scholar]
- Feldman HH, Lane R, & the Study 304 Group. (2007). Rivastigmine: A placebo controlled trial of twice daily and three times daily regimens in patients with Alzheimer’s disease. Journal of Neurology, Neurosurgery, and Psychiatry, 78, 1056–1063. 10.1136/jnnp.2006.099424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Serrano MJ, Perales JC, Moreno-López L, Pérez-García M, & Verdejo-García A (2012). Neuropsychological profiling of impulsivity and compulsivity in cocaine dependent individuals. Psychopharmacology, 219, 673–683. 10.1007/s00213-011-2485-z [DOI] [PubMed] [Google Scholar]
- Fernández-Serrano MJ, Pérez-García M, Perales JC, & Verdejo-García A (2010). Prevalence of executive dysfunction in cocaine, heroin and alcohol users enrolled in therapeutic communities. European Journal of Pharmacology, 626, 104–112. 10.1016/j.ejphar.2009.10.019 [DOI] [PubMed] [Google Scholar]
- Fernández-Serrano MJ, Pérez-García M, Schmidt Río-Valle J, & Verdejo-García A (2010). Neuropsychological consequences of alcohol and drug abuse on different components of executive functions. Journal of Psychopharmacology, 24, 1317–1332. 10.1177/0269881109349841 [DOI] [PubMed] [Google Scholar]
- Fillmore MT, & Rush CR (2002). Impaired inhibitory control of behavior in chronic cocaine users. Drug and Alcohol Dependence, 66, 265–273. 10.1016/S0376-8716(01)00206-X [DOI] [PubMed] [Google Scholar]
- Forray A, & Sofuoglu M (2014). Future pharmacological treatments for substance use disorders. British Journal of Clinical Pharmacology, 77, 382–400. 10.1111/j.1365-2125.2012.04474.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankfort SV, Appels BA, de Boer A, Tulner LR, van Campen JP, Koks CH, … Schmand BA (2007). Identification of responders and reactive domains to rivastigmine in Alzheimer’s disease. Pharmacoepidemiology and Drug Safety, 16, 545–551. 10.1002/pds.1345 [DOI] [PubMed] [Google Scholar]
- Gawin FH, & Kleber HD (1986). Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Archives of General Psychiatry, 43, 107–113. 10.1001/archpsyc.1986.01800020013003 [DOI] [PubMed] [Google Scholar]
- Ghahremani DG, Tabibnia G, Monterosso J, Hellemann G, Poldrack RA, & London ED (2011). Effect of modafinil on learning and task-related brain activity in methamphetamine-dependent and healthy individuals. Neuropsychopharmacology, 36, 950–959. 10.1038/npp.2010.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillin JC, Pulvirenti L, Withers N, Golshan S, & Koob G (1994). The effects of lisuride on mood and sleep during acute withdrawal in stimulant abusers: A preliminary report. Biological Psychiatry, 35, 843–849. 10.1016/0006-3223(94)90019-1 [DOI] [PubMed] [Google Scholar]
- Goel N, Rao H, Durmer JS, & Dinges DF (2009). Neurocognitive consequences of sleep deprivation. Seminars in Neurology, 29, 320–339. 10.1055/s-0029-1237117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goss AJ, Kaser M, Costafreda SG, Sahakian BJ, & Fu CH (2013). Modafinil augmentation therapy in unipolar and bipolar depression: A systematic review and meta-analysis of randomized controlled trials. The Journal of Clinical Psychiatry, 74, 1101–1107. 10.4088/JCP.13r08560 [DOI] [PubMed] [Google Scholar]
- Hester R, & Garavan H (2004). Executive dysfunction in cocaine addiction: Evidence for discordant frontal, cingulate, and cerebellar activity. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 24, 11017–11022. 10.1523/JNEUROSCI.3321-04.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton AM Jr. (1999). Above-average intelligence and neuropsycho-logical test score performance. The International Journal of Neuroscience, 99, 221–231. 10.3109/00207459908994326 [DOI] [PubMed] [Google Scholar]
- Johanson CE, Roehrs T, Schuh K, & Warbasse L (1999). The effects of cocaine on mood and sleep in cocaine-dependent males. Experimental and Clinical Psychopharmacology, 7, 338–346. 10.1037/1064-1297.7.4.338 [DOI] [PubMed] [Google Scholar]
- Jones K, & Harrison Y (2001). Frontal lobe function, sleep loss and fragmented sleep. Sleep Medicine Reviews, 5, 463–475. 10.1053/smrv.2001.0203 [DOI] [PubMed] [Google Scholar]
- Jovanovski D, Erb S, & Zakzanis KK (2005). Neurocognitive deficits in cocaine users: A quantitative review of the evidence. Journal of Clinical and Experimental Neuropsychology, 27, 189–204. 10.1080/13803390490515694 [DOI] [PubMed] [Google Scholar]
- Kalechstein AD, De La Garza R II, & Newton TF (2010). Modafinil administration improves working memory in methamphetamine-dependent individuals who demonstrate baseline impairment. The American Journal on Addictions, 19, 340–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalechstein AD, Mahoney JJ III, Yoon JH, Bennett R, & De la Garza R II. (2013). Modafinil, but not escitalopram, improves working memory and sustained attention in long-term, high-dose cocaine users. Neuropharmacology, 64, 472–478. 10.1016/j.neuropharm.2012.06.064 [DOI] [PubMed] [Google Scholar]
- Kampman KM, Lynch KG, Pettinati HM, Spratt K, Wierzbicki MR, Dackis C, & O’Brien CP (2015). A double blind, placebo controlled trial of modafinil for the treatment of cocaine dependence without co-morbid alcohol dependence. Drug and Alcohol Dependence, 155, 105–110. 10.1016/j.drugalcdep.2015.08.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang SY, Kleinman PH, Woody GE, Millman RB, Todd TC, Kemp J, & Lipton DS (1991). Outcomes for cocaine abusers after once-a-week psychosocial therapy. The American Journal of Psychiatry, 148, 630–635. 10.1176/ajp.148.5.630 [DOI] [PubMed] [Google Scholar]
- Koob GF, & Volkow ND (2016). Neurobiology of addiction: A neurocircuitry analysis. The Lancet Psychiatry, 3, 760–773. 10.1016/S2215-0366(16)00104-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowatch RA, Schnoll SS, Knisely JS, Green D, & Elswick RK (1992). Electroencephalographic sleep and mood during cocaine withdrawal. Journal of Addictive Diseases, 11, 21–45. 10.1300/J069v11n04_03 [DOI] [PubMed] [Google Scholar]
- Larrabee GJ (2000). Association between IQ and neuropsychological test performance: Commentary on Tremont, Hoffman, Scott, and Adams (1998). The Clinical Neuropsychologist, 14, 139–145. 10.1076/1385-4046(200002)14:1;1-8;FT139 [DOI] [PubMed] [Google Scholar]
- Li CS, Milivojevic V, Kemp K, Hong K, & Sinha R (2006). Performance monitoring and stop signal inhibition in abstinent patients with cocaine dependence. Drug and Alcohol Dependence, 85, 205–212. 10.1016/j.drugalcdep.2006.04.008 [DOI] [PubMed] [Google Scholar]
- Lindsay JA, Stotts AL, Green CE, Herin DV, & Schmitz JM (2009). Cocaine dependence and concurrent marijuana use: A comparison of clinical characteristics. The American Journal of Drug and Alcohol Abuse, 35, 193–198. 10.1080/00952990902933860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe CJ, Safati A, & Hall PA (2017). The neurocognitive consequences of sleep restriction: A meta-analytic review. Neuroscience and Biobehavioral Reviews, 80, 586–604. 10.1016/j.neubiorev.2017.07.010 [DOI] [PubMed] [Google Scholar]
- Mahoney JJ III, De La Garza R II, Jackson BJ, Verrico CD, Ho A, Iqbal T, & Newton TF (2014). The relationship between sleep and drug use characteristics in participants with cocaine or methamphetamine use disorders. Psychiatry Research, 219, 367–371. 10.1016/j.psychres.2014.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney JJ III, Kalechstein AD, Newton TF, & De La Garza R II. (2017). The limited impact that cocaine use patterns have on neuro-cognitive functioning in individuals with cocaine use disorder. Journal of Psychopharmacology, 31, 989–995. 10.1177/0269881117715606 [DOI] [PubMed] [Google Scholar]
- Mahoney JJ III,Kalechstein AD, Verrico CD, Arnoudse NM, Shapiro BA, & De La Garza R II. (2014). Preliminary findings of the effects of rivastigmine, an acetylcholinesterase inhibitor, on working memory in cocaine-dependent volunteers. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 50, 137–142. 10.1016/j.pnpbp.2013.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney JJ III, Newton TF, Omar Y, Ross EL, & De La Garza R II. (2013). The relationship between lifetime stress and addiction severity in cocaine-dependent participants. European Neuropsychopharmacology, 23, 351–357. 10.1016/j.euroneuro.2012.05.016 [DOI] [PubMed] [Google Scholar]
- Mahoney JJ III, Thompson-Lake DG, Cooper K, Verrico CD, Newton TF, & De La Garza R II. (2015). A comparison of impulsivity, depressive symptoms, lifetime stress and sensation seeking in healthy controls versus participants with cocaine or methamphetamine use disorders. Journal of Psychopharmacology, 29, 50–56. 10.1177/0269881114560182 [DOI] [PubMed] [Google Scholar]
- Mahoney JJ, Jackson BJ, Kalechstein AD, De La Garza R II, Chang LC, & Newton TF (2012). Acute modafinil exposure reduces daytime sleepiness in abstinent methamphetamine-dependent volunteers. The International Journal of Neuropsychopharmacology, 15, 1241–1249. 10.1017/S1461145711001805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahoney JJ, Kalechstein AD, De Marco AP, Newton TF, & De La Garza R (2017). The relationship between premorbid IQ and neurocognitive functioning in individuals with cocaine use disorders. Neuropsychology, 31, 311–318. 10.1037/neu0000344 [DOI] [PubMed] [Google Scholar]
- Matuskey D, Pittman B, Forselius E, Malison RT, & Morgan PT (2011). A multistudy analysis of the effects of early cocaine abstinence on sleep. Drug and Alcohol Dependence, 115(1–2), 62–66. 10.1016/j.drugalcdep.2010.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JR, Van Horn D, Rennert L, Drapkin M, Ivey M, & Koppenhaver J (2013). Factors in sustained recovery from cocaine dependence. Journal of Substance Abuse Treatment, 45, 163–172. 10.1016/j.jsat.2013.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKellar JD, Harris AH, & Moos RH (2006). Predictors of outcome for patients with substance-use disorders five years after treatment dropout. Journal of Studies on Alcohol, 67, 685–693. 10.15288/jsa.2006.67.685 [DOI] [PubMed] [Google Scholar]
- McKellar J, Kelly J, Harris A, & Moos R (2006). Pretreatment and during treatment risk factors for dropout among patients with substance use disorders. Addictive Behaviors, 31, 450–460. 10.1016/j.addbeh.2005.05.024 [DOI] [PubMed] [Google Scholar]
- McMahon RC (2001). Personality, stress, and social support in cocaine relapse prediction. Journal of Substance Abuse Treatment, 21, 77–87. 10.1016/S0740-5472(01)00187-8 [DOI] [PubMed] [Google Scholar]
- Meier MH, Caspi A, Ambler A, Harrington H, Houts R, Keefe RS, … Moffitt TE (2012). Persistent cannabis users show neuro-psychological decline from childhood to midlife. Proceedings of the National Academy of Sciences of the United States of America, 109(40), E2657–E2664. 10.1073/pnas.1206820109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller NS, Klahr AL, Gold MS, Sweeney K, & Cocores JA (1990). The prevalence of marijuana (cannabis) use and dependence in cocaine dependence. New York State Journal of Medicine, 90, 491–492. [PubMed] [Google Scholar]
- Moeller FG, Steinberg JL, Schmitz JM, Ma L, Liu S, Kjome KL, … Narayana PA (2010). Working memory fMRI activation in cocaine-dependent subjects: Association with treatment response. Psychiatry Research, 181, 174–182. 10.1016/j.pscychresns.2009.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-López L, Stamatakis EA, Fernández-Serrano MJ, Gómez-Río M, Rodríguez-Fernández A, Pérez-García M, & Verdejo-García A (2012). Neural correlates of the severity of cocaine, heroin, alcohol, MDMA and cannabis use in polysubstance abusers: A resting-PET brain metabolism study. PLoS ONE, 7(6), e39830 10.1371/journal.pone.0039830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan CA III, Doran A, Steffian G, Hazlett G, & Southwick SM (2006). Stress-induced deficits in working memory and visuo-constructive abilities in Special Operations soldiers. Biological Psychiatry, 60, 722–729. 10.1016/j.biopsych.2006.04.021 [DOI] [PubMed] [Google Scholar]
- Morgan PT, & Malison RT (2007). Cocaine and sleep: Early abstinence. The Scientific World Journal, 7, 223–230. 10.1100/tsw.2007.209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PT, Pace-Schott E, Pittman B, Stickgold R, & Malison RT (2010). Normalizing effects of modafinil on sleep in chronic cocaine users. The American Journal of Psychiatry, 167, 331–340. 10.1176/appi.ajp.2009.09050613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neafsey EJ, & Collins MA (2011). Moderate alcohol consumption and cognitive risk. Neuropsychiatric Disease and Treatment, 7, 465–484. 10.2147/NDT.S23159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvaz MA, Moeller SJ, d’Oleire Uquillas F, Pflumm A, Maloney T, Alia-Klein N, & Goldstein RZ (2017). Prefrontal gray matter volume recovery in treatment-seeking cocaine-addicted individuals: A longitudinal study. Addiction Biology, 22, 1391–1401. 10.1111/adb.12403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepeu G, Spignoli G, Giovannini MG, & Magnani M (1989). The relationship between the behavioral effects of cognition-enhancing drugs and brain acetylcholine. Nootropic drugs and brain acetylcholine. Pharmacopsychiatry, 22(Suppl. 2), 116–119. 10.1055/s-2007-1014630 [DOI] [PubMed] [Google Scholar]
- Poling J, Kosten TR, & Sofuoglu M (2007). Treatment outcome predictors for cocaine dependence. The American Journal of Drug and Alcohol Abuse, 33, 191–206. 10.1080/00952990701199416 [DOI] [PubMed] [Google Scholar]
- Post RM, Gillin JC, Wyatt RJ, & Goodwin FK (1974). The effect of orally administered cocaine on sleep of depressed patients. Psycho-pharmacology, 37, 59–66. 10.1007/BF00426683 [DOI] [PubMed] [Google Scholar]
- Potvin S, Stavro K, Rizkallah E, & Pelletier J (2014). Cocaine and cognition: A systematic quantitative review. Journal of Addiction Medicine, 8, 368–376. 10.1097/ADM.0000000000000066 [DOI] [PubMed] [Google Scholar]
- Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, & Goodwin FK (1990). Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study. Journal of the American Medical Association, 264, 2511–2518. 10.1001/jama.1990.03450190043026 [DOI] [PubMed] [Google Scholar]
- Roldán-Tapia L, García J, Cánovas R, & León I (2012). Cognitive reserve, age, and their relation to attentional and executive functions. Applied Neuropsychology Adult, 19, 2–8. 10.1080/09084282.2011.595458 [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Budney AJ, Bickel WK, & Badger GJ (1996). A comparison of cocaine-dependent cigarette smokers and non-smokers on demographic, drug use and other characteristics. Drug and Alcohol Dependence, 40, 195–201. 10.1016/0376-8716(96)01219-7 [DOI] [PubMed] [Google Scholar]
- Rosselli M, & Ardila A (1996). Cognitive effects of cocaine and poly-drug abuse. Journal of Clinical and Experimental Neuropsychology, 18, 122–135. 10.1080/01688639608408268 [DOI] [PubMed] [Google Scholar]
- Rosselli M, Ardila A, Lubomski M, Murray S, & King K (2001). Personality profile and neuropsychological test performance in chronic cocaine-abusers. The International Journal of Neuroscience, 110(1–2), 55–72. 10.3109/00207450108994221 [DOI] [PubMed] [Google Scholar]
- Sangroula D, Motiwala F, Wagle B, Shah VC, Hagi K, & Lippmann S (2017). Modafinil treatment of cocaine dependence: A systematic review and meta-analysis. Substance Use & Misuse, 52, 1292–1306. 10.1080/10826084.2016.1276597 [DOI] [PubMed] [Google Scholar]
- Satz P, Cole MA, Hardy DJ, & Rassovsky Y (2011). Brain and cognitive reserve: Mediator(s) and construct validity, a critique. Journal of Clinical and Experimental Neuropsychology, 33, 121–130. 10.1080/13803395.2010.493151 [DOI] [PubMed] [Google Scholar]
- Satz P, Morgenstern H, Miller EN, Selnes OA, McArthur JC, Cohen BA, et al. (1993). Low education as a possible risk factor for cognitive abnormalities in HIV-1: Findings from the multicenter AIDS Cohort Study (MACS). Journal of Acquired Immune Deficiency Syndromes, 6, 503–511. [PubMed] [Google Scholar]
- Schierenbeck T, Riemann D, Berger M, & Hornyak M (2008). Effect of illicit recreational drugs upon sleep: Cocaine, ecstasy and marijuana. Sleep Medicine Reviews, 12, 381–389. 10.1016/j.smrv.2007.12.004 [DOI] [PubMed] [Google Scholar]
- Silver JM, Koumaras B, Meng X, Potkin SG, Reyes PF, Harvey PD, … Arciniegas DB (2009). Long-term effects of rivastigmine capsules in patients with traumatic brain injury. Brain Injury, 23, 123–132. 10.1080/02699050802649696 [DOI] [PubMed] [Google Scholar]
- Simpson DD, Joe GW, Fletcher BW, Hubbard RL, & Anglin MD (1999). A national evaluation of treatment outcomes for cocaine dependence. Archives of General Psychiatry, 56, 507–514. 10.1001/archpsyc.56.6.507 [DOI] [PubMed] [Google Scholar]
- Sinha R (2001). How does stress increase risk of drug abuse and relapse? Psychopharmacology, 158, 343–359. 10.1007/s002130100917 [DOI] [PubMed] [Google Scholar]
- Sinha R, Fuse T, Aubin LR, & O’Malley SS (2000). Psychological stress, drug-related cues and cocaine craving. Psychopharmacology, 152, 140–148. 10.1007/s002130000499 [DOI] [PubMed] [Google Scholar]
- Sofuoglu M (2010). Cognitive enhancement as a pharmacotherapy target for stimulant addiction. Addiction, 105, 38–48. 10.1111/j.1360-0443.2009.02791.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, & Carroll KM (2011). Effects of galantamine on cocaine use in chronic cocaine users. The American Journal on Addictions, 20, 302–303. 10.1111/j.1521-0391.2011.00130.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, DeVito EE, Waters AJ, & Carroll KM (2013). Cognitive enhancement as a treatment for drug addictions. Neuropharmacology, 64, 452–463. 10.1016/j.neuropharm.2012.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, DeVito EE, Waters AJ, & Carroll KM (2016). Cognitive function as a transdiagnostic treatment target in stimulant use disorders. Journal of Dual Diagnosis, 12, 90–106. 10.1080/15504263.2016.1146383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, & Mooney M (2009). Cholinergic functioning in stimulant addiction: Implications for medications development. CNS Drugs, 23, 939–952. 10.2165/11310920-000000000-00000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Sugarman DE, & Carroll KM (2010). Cognitive function as an emerging treatment target for marijuana addiction. Experimental and Clinical Psychopharmacology, 18, 109–119. 10.1037/a0019295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Waters AJ, Poling J, & Carroll KM (2011). Galantamine improves sustained attention in chronic cocaine users. Experimental and Clinical Psychopharmacology, 19, 11–19. 10.1037/a0022213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solowij N (1998). Cannabis and cognitive functioning. New York, NY: Cambridge University Press; 10.1017/CBO9780511526824 [DOI] [Google Scholar]
- Solowij N, & Battisti R (2008). The chronic effects of cannabis on memory in humans: A review. Current Drug Abuse Reviews, 1, 81–98. 10.2174/1874473710801010081 [DOI] [PubMed] [Google Scholar]
- Solowij N, Stephens R, Roffman RA, & Babor T (2002). Does marijuana use cause long-term cognitive deficits? Journal of the American Medical Association, 287, 2653–2654. [PubMed] [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, … the Marijuana Treatment Project Research Group. (2002). Cognitive functioning of long-term heavy cannabis users seeking treatment. Journal of the American Medical Association, 287, 1123–1131. 10.1001/jama.287.9.1123 [DOI] [PubMed] [Google Scholar]
- Spronk DB, van Wel JH, Ramaekers JG, & Verkes RJ (2013). Characterizing the cognitive effects of cocaine: A comprehensive review. Neuroscience and Biobehavioral Reviews, 37, 1838–1859. 10.1016/j.neubiorev.2013.07.003 [DOI] [PubMed] [Google Scholar]
- Stern Y (2002). What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsycho-logical Society, 8, 448–460. 10.1017/S1355617702813248 [DOI] [PubMed] [Google Scholar]
- Stern Y (2011). Elaborating a hypothetical concept: Comments on the special series on cognitive reserve. Journal of the International Neuro-psychological Society, 17, 639–642. 10.1017/S1355617711000579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streeter CC, Terhune DB, Whitfield TH, Gruber S, Sarid-Segal O, Silveri MM, … Yurgelun-Todd DA (2008). Performance on the Stroop predicts treatment compliance in cocaine-dependent individuals. Neuropsychopharmacology, 33, 827–836. 10.1038/sj.npp.1301465 [DOI] [PubMed] [Google Scholar]
- Tenovuo O, Alin J, & Helenius H (2009). A randomized controlled trial of rivastigmine for chronic sequels of traumatic brain injury-what it showed and taught? Brain Injury, 23, 548–558. 10.1080/02699050902926275 [DOI] [PubMed] [Google Scholar]
- Tomasi D, Goldstein RZ, Telang F, Maloney T, Alia-Klein N, Caparelli EC, & Volkow ND (2007). Widespread disruption in brain activation patterns to a working memory task during cocaine abstinence. Brain Research, 1171, 83–92. 10.1016/j.brainres.2007.06.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremont G, Hoffman RG, Scott JG, & Adams RL (1998). Effect of intellectual level on neuropsychological test performance: A response to Dodrill (1997). The Clinical Neuropsychologist, 12, 560–567. 10.1076/clin.12.4.560.7238 [DOI] [Google Scholar]
- Turner DC, Clark L, Dowson J, Robbins TW, & Sahakian BJ (2004). Modafinil improves cognition and response inhibition in adult attention-deficit/hyperactivity disorder. Biological Psychiatry, 55, 1031–1040. 10.1016/j.biopsych.2004.02.008 [DOI] [PubMed] [Google Scholar]
- Turner DC, Robbins TW, Clark L, Aron AR, Dowson J, & Sahakian BJ (2003). Cognitive enhancing effects of modafinil in healthy volunteers. Psychopharmacology, 165, 260–269. 10.1007/s00213-002-1250-8 [DOI] [PubMed] [Google Scholar]
- Turner TH, LaRowe S, Horner MD, Herron J, & Malcolm R (2009). Measures of cognitive functioning as predictors of treatment outcome for cocaine dependence. Journal of Substance Abuse Treatment, 37, 328–334. 10.1016/j.jsat.2009.03.009 [DOI] [PubMed] [Google Scholar]
- Vadhan NP, Myers CE, Benedict E, Rubin E, Foltin RW, & Gluck MA (2014). A decrement in probabilistic category learning in cocaine users after controlling for marijuana and alcohol use. Experimental and Clinical Psychopharmacology, 22, 65–74. 10.1037/a0034506 [DOI] [PubMed] [Google Scholar]
- Verdejo-García A, Betanzos-Espinosa P, Lozano OM, Vergara-Moragues E, González-Saiz F, Fernández-Calderón F, … Pérez-García M (2012). Self-regulation and treatment retention in cocaine dependent individuals: A longitudinal study. Drug and Alcohol Dependence, 122(1–2), 142–148. 10.1016/j.drugalcdep.2011.09.025 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Koob GF, & McLellan AT (2016). Neurobiologic advances from the brain disease model of addiction. The New England Journal of Medicine, 374, 363–371. 10.1056/NEJMra1511480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, & Morales M (2015). The brain on drugs: From reward to addiction. Cell, 162, 712–725. 10.1016/j.cell.2015.07.046 [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, & Tomasi D (2012). Addiction circuitry in the human brain. Annual Review of Pharmacology and Toxicology, 52, 321–336. 10.1146/annurev-pharmtox-010611-134625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonmoos M, Hulka LM, Preller KH, Jenni D, Baumgartner MR, Stohler R, … Quednow BB (2013). Cognitive dysfunctions in recreational and dependent cocaine users: Role of attention-deficit hyperactivity disorder, craving and early age at onset. The British Journal of Psychiatry, 203, 35–43. 10.1192/bjp.bp.112.118091 [DOI] [PubMed] [Google Scholar]
- Vonmoos M, Hulka LM, Preller KH, Minder F, Baumgartner MR, & Quednow BB (2014). Cognitive impairment in cocaine users is drug-induced but partially reversible: Evidence from a longitudinal study. Neuropsychopharmacology, 39, 2200–2210. 10.1038/npp.2014.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonmoos M, & Quednow BB (2017). Cognitive dysfunction in chronic cocaine users In Preedy VR (Ed.), The neuroscience of cocaine (pp. 395–405). New York, NY: Academic Press; 10.1016/B978-0-12-803750-8.00040-3 [DOI] [Google Scholar]
- Waldrop AE, Back SE, Verduin ML, & Brady KT (2007). Triggers for cocaine and alcohol use in the presence and absence of posttraumatic stress disorder. Addictive Behaviors, 32, 634–639. 10.1016/j.addbeh.2006.06.001 [DOI] [PubMed] [Google Scholar]
- Walker MP (2008). Cognitive consequences of sleep and sleep loss. Sleep Medicine, 9(Suppl. 1), S29–S34. 10.1016/S1389-9457(08)70014-5 [DOI] [PubMed] [Google Scholar]
- Wallace TL, Ballard TM, Pouzet B, Riedel WJ, & Wettstein JG (2011). Drug targets for cognitive enhancement in neuropsychiatric disorders. Pharmacology, Biochemistry, and Behavior, 99, 130–145. 10.1016/j.pbb.2011.03.022 [DOI] [PubMed] [Google Scholar]
- Watson R, Bakos L, Compton P, & Gawin F (1992). Cocaine use and withdrawal: The effect on sleep and mood. The American Journal of Drug and Alcohol Abuse, 18, 21–28. 10.3109/00952999209001608 [DOI] [PubMed] [Google Scholar]
- Weddington WW, Brown BS, Cone EJ, Haertzen CA, Dax EM, Herning RI, & Michaelson BS (1990). Changes in mood, craving and sleep during acute abstinence reported by male cocaine addicts. NIDA Research Monograph, 105, 453–454. [PubMed] [Google Scholar]
- West R (1993). Beneficial effects of nicotine: Fact or fiction? Addiction, 88, 589–590. 10.1111/j.1360-0443.1993.tb02067.x [DOI] [PubMed] [Google Scholar]
- Winhusen T, Lewis D, Adinoff B, Brigham G, Kropp F, Donovan DM, … Somoza E (2013). Impulsivity is associated with treatment non-completion in cocaine- and methamphetamine-dependent patients but differs in nature as a function of stimulant-dependence diagnosis. Journal of Substance Abuse Treatment, 44, 541–547. 10.1016/j.jsat.2012.12.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA (1978). Catecholamine theories of reward: A critical review. Brain Research, 152, 215–247. 10.1016/0006-8993(78)90253-6 [DOI] [PubMed] [Google Scholar]
- Wise RA (2004a). Dopamine, learning and motivation. Nature Reviews Neuroscience, 5, 483–494. 10.1038/nrn1406 [DOI] [PubMed] [Google Scholar]
- Wise RA (2004b). Rewards wanted: Molecular mechanisms of motivation. Discovery Medicine, 4, 180–186. [PubMed] [Google Scholar]
- Woicik PA, Moeller SJ, Alia-Klein N, Maloney T, Lukasik TM, Yeliosof O, … Goldstein RZ (2009). The neuropsychology of cocaine addiction: Recent cocaine use masks impairment. Neuropsycho-pharmacology, 34, 1112–1122. 10.1038/npp.2008.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf NJ (2006). Acetylcholine, cognition, and consciousness. Journal of Molecular Neuroscience, 30(1–2), 219–222. 10.1385/JMN:30:1:219 [DOI] [PubMed] [Google Scholar]