Abstract
Background:
Studies associate sleeping and eating late in the day with poor dietary quality and higher obesity risk but differences in sleep duration confound this association. We aimed to determine whether sleep and meal timing, independent of sleep duration, influenced food intake in healthy adults.
Methods:
This was a controlled, 2×2 inpatient crossover study with normal (0000–0800h) or late (0330–1130h) sleep and normal (1, 5, 11, and 12.5h after awakening) or late (4.5, 8.5, 14.5, and 16h after awakening) meals. Food intake was controlled while blood samples were obtained for determination of appetite-regulating hormones on days 3–4. Self-selected food intake was assessed on day 5. Data were analyzed using linear mixed model analysis with sleep, meal, and sleep × meal interaction as dependent variables.
Results:
Five participants completed all phases (mean age 25.1 ± [SD] 3.9y, body mass index 29.2 ± 2.7kg/m2). There was a significant sleep × meal interaction on energy intake (P=0.035) and trends on fat and sodium intakes (P<0.10). Overnight ghrelin concentrations were higher under normal sleep and meal conditions relative to late (P<0.005) but lower when both were combined (P<0.001). Overnight leptin concentrations were higher under normal meal conditions (P=0.012). There was a significant sleep × meal interaction on ghrelin (P=0.032) and glucagon-like peptide 1 (P=0.041) concentrations, but not leptin (P=0.83), in response to a test meal.
Conclusions:
Our results suggest that alignment of sleep and meals may influence food choice and energy balance. Additional research is necessary to expand and confirm our findings.
Keywords: Sleep time, meal time, energy intake, ghrelin, leptin, glucagon-like peptide 1, food choice
Introduction
There is observational evidence that eating and sleeping at unconventional hours is associated with greater risk of obesity and an adverse metabolic profile. Indeed, studies have shown greater prevalence of obesity in those with shift work1, 2 and social jetlag3 (shifts in the timing of sleep between work days and free days). Sleeping later in the day is also related to greater body mass index (BMI) and poor dietary habits: lower intakes of fruits and vegetables and greater intakes of sugar-sweetened beverages and fast foods4. Those who sleep late also eat more after 2000h, a behavior associated with greater BMI, independent of sleep timing and duration. However, human studies in this field have largely been observational. This is problematic because individuals who sleep late also have short sleep duration and the timing of their food intake is shifted relative to those who sleep earlier4, making inferences related to sleep timing and meal timing difficult to isolate. We5 and others6–8 have previously shown that sleep restriction increases food intake. Furthermore, in sleep restriction studies when the timing of sleep is delayed to the second half of the night, or early morning hours, there is increased eating at night7.
Although animal studies cannot model for changes in sleep timing, rodent studies support the notion that eating during the biological night has negative effects on weight status. Mice fed during their light cycle (inactive period) gain more weight and tend to have greater percent body fat despite similar energy intakes and physical activity as mice fed during the active period9. Others have found greater weight gain as a result of increased food intake and reduced fat oxidation in mice fed only during the light cycle10 or maintained in constant light or on a light/dim light cycle11. Corresponding data in humans can be provided from shift work interventions: working at night work leads to increased intake of high-fat foods compared to a control day condition12.
This intervention study was conducted to isolate the impact of sleep and meal times on appetite-regulating hormones and food intake, under conditions of identical sleep duration. We hypothesized that delaying sleep and meal times by 3.5 h would lead to a hormonal profile indicative of greater appetite and lower satiety and that this would result in greater overall food intake relative to sleeping and eating at earlier times. We further expected greater fat and carbohydrate intakes, particularly saturated fat and sugar, in late sleep and meal conditions.
Methods
Four men and two women, 20–49y of age, with a body mass index between 25–34.9kg/m2, were recruited to participate in this study. All participants underwent 2 weeks of sleep monitoring with actigraphy as part of the screening process, as previously reported5. Participants were required to sleep an average of 7–9h/night, have an intermediate chronotype based on the Ostberg Morningness-Eveningness questionnaire, and a midpoint of sleep 0400h or earlier, to be eligible. We excluded individuals who did not habitually eat breakfast, defined as food intake within 1h of awakening at least 4 times/week, and those with sleep, eating, or other psychological disorders. The study was approved by the institutional review boards of Columbia University Medical Center and New York University Langone Medical Center (New York, NY) and was registered on Clinicaltrials.gov (#NCT02347020). All participants were given the opportunity to review and ask questions about the protocol prior to providing informed consent.
Experimental design
This study employed a 2×2 factorial design with two sleep (normal or late) and meal (normal or late) times (Table 1), resulting in 24 possible phase order combinations. Once enrolled, participants were randomly assigned one of those combinations generated from a randomization schedule. Bedtimes, wake times, and meal times in the normal conditions were based on data from Baron et al.4 We modeled bedtimes for the late sleep (Ls) phases based on self-reported bedtimes for late sleepers from that same study4 but delayed wake time to achieve the same duration of time in bed as the normal sleep (Ns) condition. Differences in bed times between Ns and Ls in the study by Baron et al. was 3.5 h.4 Meal times for the late meal (Lm) phases were delayed by this same amount relative to normal meal (Nm) times to achieve equal inter-meal intervals between study phases.
Table 1.
Timing of sleep and meals in each study phase.
| Normal sleep/ Normal meals | Normal sleep/ Late meals | Late sleep/ Normal meals | Late sleep/ Late meals | |
|---|---|---|---|---|
| Sleep, h | 0000–0800 | 0000–0800 | 0330–1130 | 0330–1130 |
| Breakfast, h | 0900 | 1230 | 1230 | 1600 |
| Lunch, h | 1300 | 1630 | 1630 | 2000 |
| Dinner, h | 1900 | 2230 | 2230 | 0200 |
| Snack, h | 2030 | 0000 | 0000 | 0330 |
Participants were inpatients in the Columbia University Medical Center Irving Institute for Clinical and Translational Research (CTSA) for all 4 study phases. The first 3 d of each phase were conducted under controlled feeding conditions, wherein participants consumed a weight-maintenance diet, as estimated using the Mifflin-St. Jeor equation13. Breakfast provided 25% of estimated energy requirements, lunch 30%, dinner 35%, and the remaining 10% was provided as a post-dinner snack. All foods and beverages were consumed in their entirety during this period. All meals were prepared at the CTSA Bionutrition Unit by study personnel.
On day 3, visual analog scales to assess appetite were filled out immediately before and at 15, 30, 45, 60, 90, and 120min after meals. Questions included: (1) how hungry do you feel? (2) how satisfied do you feel? (3) how full do you feel? (4) how much food would you like to eat? (5) how energetic do you feel? (6) how sluggish do you feel? (7) how much would like to eat something sweet? (8) something salty? (9) something savory? (10) fruits and vegetables? Questions were anchored with “not at all” at 0 and “very much so” at 100mm.14
Blood samples were obtained starting on the night of day 3 and into day 4 for hormone and metabolite assessments. An insulin-modified frequently-sampled i.v. glucose tolerance test was performed at scheduled breakfast time and a meal tolerance test (MTT) was done at the scheduled lunch time. The meal consisted of a liquid meal replacement (Boost Plus, Nestle Nutritionals, Highland Park, MI). Blood samples during the overnight period and in response to the MTT were analyzed for leptin, ghrelin, and glucagon-like peptide 1 (GLP-1). Serum leptin was measured using a double-antibody RIA (Linco Research Products Inc., St. Charles, MO). Blood samples were collected in EDTA-coated chilled tubes for the measurement of peptide hormones. The tubes were pre-treated with addition of aprotinin (0.6 TIU/mL of blood) and DPP-IV inhibitor (10 μL/mL of blood) to prevent hormone degradation. Total ghrelin was assessed using RIA (Linco Research). Total GLP-1 was measured by RIA (Phoenix Pharmaceutical, Belmont, CA) after plasma extraction with 95% ethanol.
On day 5, participants were permitted to self-select their food intake, both food type and amount, at their scheduled meal and snack times. All foods were dispensed by research personnel and weighed prior to and post consumption. Participants could choose foods from our standard diet menu or elsewhere. They were given a $25 allowance to purchase foods of their liking from neighborhood food establishments (fast food restaurants and grocery stores). The only restriction placed on purchases was the availability of nutrition information for the foods and beverages chosen. Dietary intakes were analyzed using Nutrition Data System for Research (NDSR, Nutrition Coordinating Center, University of Minnesota, Minneapolis, MN).
Statistical methods
Food intake data were analyzed using linear mixed model analysis with energy and macronutrient variables as outcomes, and sex, weight, sleep timing, meal timing, sleep × meal timing interaction as independent variables. When significant, phase was used as an independent variable. Subject ID was used as a grouping variable.
Clock time was categorized as morning (0600–1200h), afternoon (1200–1800h), evening (1800–2400h), and overnight (0000–0600h) for use as an independent variable. For MTT outcomes, only evening and afternoon clock times are present in the data. Linear mixed model analyses were used to assess ghrelin and leptin as outcomes. In each case, subject ID was used the grouping variable. Sleep timing, meal timing and their interaction were used as independent variables. The analyses were done separately for overnight and MTT. GLP-1 data were tested for normality using Shapiro-Wilk test of normality, and were determined to be non-normal; subsequently data were transformed using log2. The log2 transformation was chosen so that one unit change in the transformed outcome variable corresponds to doubling of the raw value of the outcome variable. Repeated measures ANOVA and mixed-model regression analysis were performed on each outcome (leptin, ghrelin, GLP-1). The independent variables were sleep timing, meal timing and their interaction. Raw data are presented in tables and figures as means ± SD. Statistical significance was set at P < 0.05. Data are available, upon request, from the authors.
Results
A total of 6 participants were recruited and enrolled into the study (Table 2). Of those, 5 completed all 4 phases; one failed to complete the last phase (Ls/Lm). Information on the phase of the menstrual cycle for the 2 female participants is unknown; phases were scheduled 4 wk apart to approximate a regular cycle length of 28 d.
Table 2.
Participant characteristics.
| Mean ± SD | |
|---|---|
| Age, y | 25.1 ± 3.9 |
| Sex, M/F | 4/2 |
| Race, White/Black | 3/3 |
| Body mass index, kg/m2 | 29.2 ± 2.7 |
| Sleep duration, h:min | 7:38 ± 0:28 |
| Midpoint of sleep, h:min | 4:00 ± 0:50 |
Hormone data
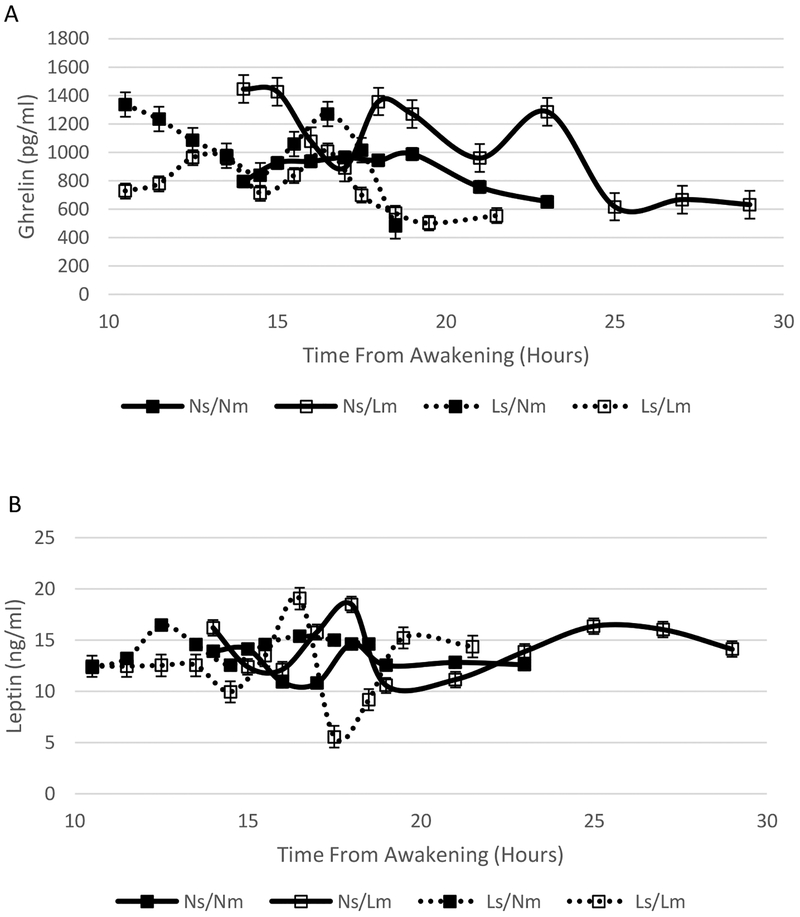
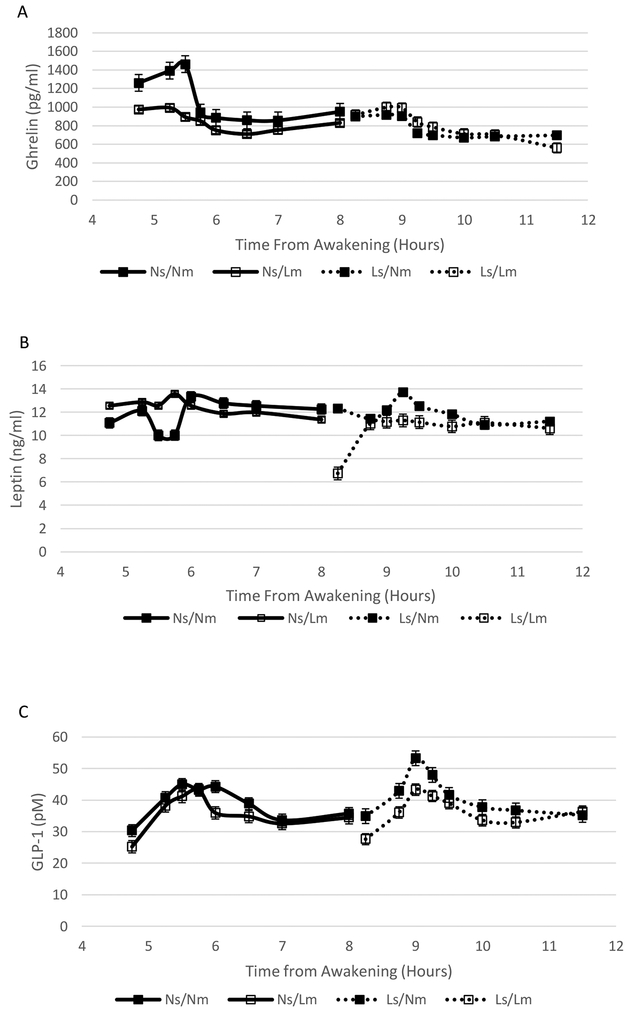
Ghrelin concentrations during the overnight hours were significantly affected by sleep timing (P < 0.0001), meal timing (P = 0.0044), and sleep × meal timing interaction (P < 0.0001) (Figure 1A). There was also a significant sleep × meal timing interaction on ghrelin concentrations during the MTT (P = 0.032) (Figure 2A). The timing of the test was also a significant variable affecting ghrelin concentrations during the MTT (P = 0.041) with evening times resulting in lower ghrelin levels and the combination of Ns and Nm associated with higher ghrelin concentrations.
Figure 1.
Ghrelin (A) and Leptin (B) overnight concentrations under late and normal sleep and meal timing conditions: Ns/Nm solid line/sold markers; Ns/Lm, solid line/open markers; Ls/Nm, dotted line/solid markers; Ls/Lm, dotted line/open markers. Ghrelin concentrations were significantly affected by sleep timing (P < 0.0001), meal timing (P = 0.0044), and sleep × meal timing interaction (P < 0.0001). Leptin concentrations were significantly affected by meal timing (P=0.012).
Figure 2.
Ghrelin (A), Leptin (B), and GLP-1 (C) concentrations during the MTT under late and normal sleep and meal timing conditions: Ns/Nm solid line/sold markers; Ns/Lm, solid line/open markers; Ls/Nm, dotted line/solid markers; Ls/Lm, dotted line/open markers. There was a significant sleep × meal timing interaction on ghrelin concentrations (P = 0.032). There was no effect of sleep timing, meal timing, and their interaction on leptin concentrations. There was a significant sleep × meal timing interaction on GLP-1 concentrations (P = 0.041).
Overnight leptin concentrations were significantly affected by meal timing (P = 0.012), with higher concentrations associated with Nm (Figure 1B). There was no effect of sleep timing or sleep × meal interaction on overnight leptin concentrations. Sleep timing, meal timing, and their interaction did not influence leptin concentrations during the MTT although tests conducted in the evening hours tended to be associated with lower concentrations (P = 0.070) (Figure 2B).
GLP-1 concentrations were only assessed during the MTT. There were no main effects of meal and sleep timing on GLP-1 but there was a significant sleep × meal timing interaction (P = 0.041) (Figure 2C). Having Ns and Nm together yielded higher GLP-1 concentrations relative to either one separately or having both Ls and Lm.
Food intake data
There was a significant effect of sleep timing (P = 0.019) and sleep × meal timing interaction (P = 0.035) on energy intake during the ad libitum food consumption day (Table 3). There was also a trend for an effect of meal timing (P = 0.095). In general, independently, Ns and Nm timing were associated with increased intake relative to late timing but, when combined, resulted in a negative coefficient. Similarly, there were significant effects of sleep timing on total fat (P = 0.010), saturated fat (P = 0.039), monounsaturated fat (P = 0.0025), and polyunsaturated fat (P = 0.032) intakes. There was a significant effect of meal timing on monounsaturated fat intakes (P = 0.016) with a trend for polyunsaturated fat intakes (P = 0.074). The sleep timing × meal timing interaction was significant for monounsaturated fat intakes only (P = 0.0086) but trends were observed for total fat (P = 0.091) and polyunsaturated fat (P = 0.089) intakes. There were no effects of sleep timing, meal timing, or their interaction on carbohydrate, protein, fiber, sugar, or sodium intakes although there was a trend for a sleep × meal timing interaction on sodium intakes (P = 0.092), favoring lower sodium intakes when sleep and meal timing were normal relative to late.
Table 3.
24-h energy and macronutrient intakes during ad libitum food consumption.
| Normal sleep/ Normal meals | Normal sleep/ Late meals | Late sleep/ Normal meals | Late sleep/ Late meals | |
|---|---|---|---|---|
| Energy, kcal | 3309 ± 611 | 3762 ± 812 | 3490 ± 900 | 2811 ± 649 |
| Fat, g | 150.4 ± 42.2 | 169.8 ± 57.8 | 139.7 ± 55.5 | 96.1 ± 32.9 |
| Saturated fat, g | 51.6 ± 19.8 | 59.4 ± 26.0 | 43.4 ± 18.8 | 33.1 ± 15.2 |
| Monounsaturated fat, g | 50.2 ± 11.8 | 58.4 ± 20.1 | 49.7 ± 22.5 | 27.0 ± 14.6 |
| Polyunsaturated fat, g | 32.8 ± 5.4 | 35.5 ± 5.9 | 33.4 ± 13.2 | 24.0 ± 8.2 |
| Carbohydrates, g | 375. 7 ± 71.1 | 421.6 ± 112.4 | 462.9 ± 27.0 | 412.0 ± 111.9 |
| Protein, g | 122.8 ± 25.4 | 131.1 ± 23.0 | 134.4 ± 47.0 | 101.5 ± 26.5 |
| Fiber, g | 27.6 ± 7.2 | 30.6 ± 8.9 | 31.5 ± 8.5 | 34.8 ± 17.3 |
| Sugar, g | 120.1 ± 35.1 | 150.9 ± 71.6 | 162.7 ±75.1 | 153.6 ± 59.3 |
| Sodium, mg | 4577 ± 884 | 5485 ± 1821 | 5185 ± 2157 | 3706 ± 1180 |
Data are unadjusted means ± SD, n = 6 except for Ls/Lm (n = 5).
Discussion
Our study showed that, in the absence of sleep restriction and under identical sleep duration and food intake, conditions that combine earlier sleep and meal times lead to lower overnight ghrelin but higher concentrations in response to a test meal. On the other hand, GLP-1 concentrations were higher in conditions combining earlier sleep and meal times in response to a test meal. Additionally, earlier meal times resulted in higher leptin concentrations overnight with no clear impact of sleep or meal times on its responses to a meal. We also showed that sleep timing tended to exert a greater influence on food intake parameters than meal timing. However, the effects of sleep timing were influenced by meal timing, as reflected by the significant sleep × meal interactions.
We expected the Ls/Lm conditions to result in higher ghrelin and lower leptin and GLP-1 concentrations overall compared to Ns/Nm and that Ns/Lm and Ls/Nm conditions would have intermediate effects. However, literature shows that sleep stimulates ghrelin secretion, with levels peaking at night15 in healthy participants, as observed in our Ns/Nm phase. Natalucci et al. also observed that ghrelin was secreted in a pulsatile fashion over a 24-h fasting period in healthy individuals16. In that small study, higher ghrelin levels were observed in participants with low BMI and lower levels were noted in those with higher BMI. In the present study, there was no effect of meal or sleep time, or their interaction, on the timing of the ghrelin peak and trough, peak and trough values, and average ghrelin daily concentrations (data not presented). Therefore, our results related to Ns and Nm seem to correspond to habitual ghrelin rhythms, whereas delaying sleep or meal times may disrupt this balance. For the other hormones under consideration in this study, Scheer and colleagues concluded that there was no evidence of an endogenous circadian pattern for GLP-1 secretion or leptin, independent of sleep/wake, feeding/fasting cycles under forced desynchrony conditions17. Indeed, we also found that sleep and meal times influenced these hormones throughout the measurement period. Higher GLP-1 concentrations in response to a meal consumed closer to wake time relative to later may lead to improved satiation and this may be more important in those with late sleep patterns. There is evidence that skipping breakfast, equivalent to our Lm conditions, increases hunger and reduces satiety in habitual breakfast eaters and reduces GLP-1 concentrations at the lunch meal in both habitual breakfast eaters and skippers18. Moreover, we have previously shown that sleep restriction reduces GLP-1 levels in women19. Combined, these results suggest that sleep and meal timing, along with the duration of sleep, could influence hormonal levels related to satiety and food intake.
We expected Ls/Lm to result in greatest energy intakes, followed by Ns/Lm and Ls/Nm. Reid et al, in a convenient sample of young adults, found that time of last meal and time lapse between last meal and sleep onset were associated with energy intakes after controlling for age, sex, sleep duration and sleep timing20. Eating frequency, however, explained the relation between eating close to bedtime and energy intake. In the present study, we found Ls/Lm to produce lowest energy intake and Ns/Lm, the highest energy intake. Those 2 conditions both provided participants with the opportunity to consume a snack immediately before bedtime. Interestingly, under Ls/Lm condition, only one participant chose to consume a snack at the scheduled snack time, 0330h, (62 kcal) whereas 3/6 participants consumed a snack in the Ns/Lm condition, at midnight (average intake 363.7 ± 118.1 kcal). In contrast, in the Nm conditions, all participants consumed a snack in Ns, at 2030h (351.5 ± 177.4 kcal) and 5/6 participants consumed one in Ls, at midnight (277.7 ± 257.7 kcal). Interestingly, and in line with data from Reid et al.20, intakes were highest when a large snack was consumed close to bedtime (Ns/Lm) and lowest when food intake was further from bedtime (3.5 h in Ns/Nm and Ls/Nm).
It is important to contrast our study and prior research. In previous studies, participants were examined in free-living situations where sleep and meals were not controlled but were measured. In our study, participants were required to eat at fixed times, although food intake was self-selected and measured. It is possible that the rigid meal schedule prevented participants from eating at their preferred time and led to reductions in intake. This seems most evident in the Ls/Lm phase when bedtimes and meals were delayed relative to the participants’ preferred behavior (all participants were regular breakfast eaters with midpoint of sleep ~0400h). We observed that skipping breakfast, as in Lm conditions, seems to induce increased food intake when sleep is normal (0000–0800h) but not when sleep is delayed (0330–1130h). However, in pilot testing a prior version of this protocol, allowing participants to consume meals at self-selected times during the ad libitum feeding portion of the study resulted in participants eating a meal immediately upon awakening in all 4 conditions, essentially obliterating the Lm conditions. This observation guided our decision to implement the fixed meal schedule in this iteration of the protocol. Another limitation of this study is the small and heterogenous sample size. However, the within subject, cross over design of the study and our excellent participant retention rate provide robustness to our results. Finally, all participants had a BMI in the overweight and obese categories and results may not be generalizable to those in the normal weight category.
Future studies should be done in a larger group of men and women to uncover the causality of the relation between sleep timing, meal timing, and food choice. It is possible that shifting the alignment of sleep and meals in relation to each other is a critical factor in the association between shift work and obesity and cardiometabolic risk. With this information, additional examinations of personalized sleep/meal time recommendations should be done to determine whether risk can be minimized for those who need to work or maintain wakefulness at unconventional times.
Acknowledgment:
This article is published as part of a supplement sponsored by the Mediterranean Diet Foundation and the Diputació de Barcelona.
Funding Information: This study was funded by the National Institutes of Health grants R56HL0119945 (MPSO) and DK26687, and also in part by Columbia University’s CTSA grant UL1 TR000040 from NCATS/NIH.
Conflicts of interest: M-PS-O received grant support from the National Institutes of Health. ARC owns stock in Alphabet Inc and received grant support National Institutes of Health and National Science Foundation.
References
- 1.Di Lorenzo L, De Pergola G, Zocchetti C, L’Abbate N, Basso A, Pannacciulli N et al. Effect of shift work on body mass index: results of a study performed in 319 glucose-tolerant men working in a Southern Italian industry. Int J Obes Relat Metab Disord 2003; 27(11): 1353–1358. doi: 10.1038/sj.ijo.0802419 [DOI] [PubMed] [Google Scholar]
- 2.van Drongelen A, Boot CR, Merkus SL, Smid T, van der Beek AJ. The effects of shift work on body weight change - a systematic review of longitudinal studies. Scandinavian journal of work, environment & health 2011; 37(4): 263–275. doi: 10.5271/sjweh.3143 [DOI] [PubMed] [Google Scholar]
- 3.Roenneberg T, Allebrandt KV, Merrow M, Vetter C. Social jetlag and obesity. Curr Biol 2012; 22(10): 939–943. doi: 10.1016/j.cub.2012.03.038 [DOI] [PubMed] [Google Scholar]
- 4.Baron KG, Reid KJ, Kern AS, Zee PC. Role of sleep timing in caloric intake and BMI. Obesity (Silver Spring) 2011; 19(7): 1374–1381. e-pub ahead of print 2011/04/30; doi: oby2011100 [pii] 10.1038/oby.2011.100 [DOI] [PubMed] [Google Scholar]
- 5.St-Onge M-P, Roberts A, Chen J, Kelleman M, O’Keeffe M, Jones P. Short sleep duration increases energy intakes but does not change expenditure in normal weight individuals. The American journal of clinical nutrition 2011; 94(2): 410–416. e-pub ahead of print 2011 Jun 29.; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. The American journal of clinical nutrition 2009; 89(1): 126–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spaeth AM, Dinges DF, Goel N. Effects of experimental sleep restriction on weight gain, caloric intake and meal timing in healthy adults. Sleep 2013; 36(7): 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proceedings of the National Academy of Sciences of the United States of America 2013; 110(14): 5695–5700. doi: 10.1073/pnas.1216951110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arble DM, Bass J, Laposky AD, Vitaterna MH, Turek FW. Circadian timing of food intake contributes to weight gain. Obesity (Silver Spring) 2009; 17(11): 2100–2102. e-pub ahead of print 2009/09/05; doi: oby2009264 [pii] 10.1038/oby.2009.264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bray MS, Ratcliffe WF, Grenett MH, Brewer RA, Gamble KL, Young ME. Quantitative analysis of light-phase restricted feeding reveals metabolic dyssynchrony in mice. International journal of obesity 2012. e-pub ahead of print 2012/08/22; doi: 10.1038/ijo.2012.137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fonken LK, Workman JL, Walton JC, Weil ZM, Morris JS, Haim A et al. Light at night increases body mass by shifting the time of food intake. Proceedings of the National Academy of Sciences of the United States of America 2010; 107(43): 18664–18669. e-pub ahead of print 2010/10/13; doi: 1008734107 [pii] 10.1073/pnas.1008734107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cain SW, Filtness AJ, Phillips CL, Anderson C. Enhanced preference for high-fat foods following a simulated night shift. Scandinavian journal of work, environment & health 2015; 41(3): 288–293. doi: 10.5271/sjweh.3486 [DOI] [PubMed] [Google Scholar]
- 13.Mifflin MD, St Jeor ST, Hill LA, Scott BJ, Daugherty SA, Koh YO. A new predictive equation for resting energy expenditure in healthy individuals. The American journal of clinical nutrition 1990; 51(2): 241–247. e-pub ahead of print 1990/02/01; [DOI] [PubMed] [Google Scholar]
- 14.Kissileff HR, Wentzlaff TH, Guss JL, Walsh BT, Devlin MJ, Thornton JC. A direct measure of satiety disturbance in patients with bulimia nervosa. Physiology & behavior 1996; 60(4): 1077–1085. e-pub ahead of print 1996/10/01; [DOI] [PubMed] [Google Scholar]
- 15.Dzaja A, Dalal MA, Himmerich H, Uhr M, Pollmacher T, Schuld A. Sleep enhances nocturnal plasma ghrelin levels in healthy subjects. American journal of physiology. Endocrinology and metabolism 2004; 286(6): E963–967. doi: 10.1152/ajpendo.00527.2003 [DOI] [PubMed] [Google Scholar]
- 16.Natalucci G, Riedl S, Gleiss A, Zidek T, Frisch H. Spontaneous 24-h ghrelin secretion pattern in fasting subjects: maintenance of a meal-related pattern. Eur J Endocrinol 2005; 152(6): 845–850. doi: 10.1530/eje.1.01919 [DOI] [PubMed] [Google Scholar]
- 17.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proceedings of the National Academy of Sciences of the United States of America 2009; 106(11): 4453–4458. e-pub ahead of print 2009/03/04; doi: 0808180106 [pii] 10.1073/pnas.0808180106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thomas EA, Higgins J, Bessesen DH, McNair B, Cornier MA. Usual breakfast eating habits affect response to breakfast skipping in overweight women. Obesity (Silver Spring) 2015; 23(4): 750–759. doi: 10.1002/oby.21049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.St-Onge MP, O’Keeffe M, Roberts AL, RoyChoudhury A, Laferrere B. Short sleep duration, glucose dysregulation and hormonal regulation of appetite in men and women. Sleep 2012; 35(11): 1503–1510. e-pub ahead of print 2012/11/02; doi: 10.5665/sleep.2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reid KJ, Baron KG, Zee PC. Meal timing influences daily caloric intake in healthy adults. Nutr Res 2014; 34(11): 930–935. doi: 10.1016/j.nutres.2014.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]