Abstract
The receptor for activated c-kinase (RACK1, Asc1 in yeast) is a eukaryotic ribosomal protein located in the head region of the 40S subunit near the mRNA exit channel. This WD-repeat β-propeller protein acts as a signaling molecule and is involved in metabolic regulation, cell cycle progression, and translational control. However, the exact details of the RACK1 recruitment and stable association with the 40S ribosomal subunit remain only partially known. X-ray analyses of the yeast, Saccharomyces cerevisiae, ribosome revealed that the RACK1 propeller blade (4-5) interacts with the eukaryote-specific C-terminal domain (CTD) of ribosomal protein S3 (uS3 family). To check the functional significance of this interaction, we generated mutant yeast strains harboring C-terminal deletions of uS3. We found that deletion of the 20 C-terminal residues (interacting with blade 4-5) from the uS3-CTD abrogates RACK1 binding to the ribosome. Strains with truncated uS3-CTD exhibited compromised cellular growth and protein synthesis similar to that of RACK1Δ strain, thus suggesting that the uS3-CTD is crucial not only for the recruitment and association of RACK1 with the ribosome, but also for its intracellular function. We suggest that eukaryote-specific RACK1-uS3 interaction has evolved to act as a link between the ribosome and the cellular signaling pathways.
Keywords: Eukaryotic ribosome, RACK1/Asc1, rpS3/uS3, translation, yeast
1. Introduction
Ribosomes are large macromolecular ribonucleoprotein complexes which decode mRNAs to make proteins. The small ribosomal subunit decodes the message, while the large subunit catalyzes peptide bond formation in its peptidyl transferase center. Eukaryotic ribosomes are larger in size than their bacterial counterparts due to the presence of additional ribosomal proteins and ribosomal RNA (rRNA)/rRNA expansion segments. Almost two-thirds of the yeast, S. cerevisiae, ribosomal proteins belong to the universally conserved protein families that are also present in bacteria and archaea (Melnikov et al., 2012). The remaining ribosomal proteins are unique to eukaryotes (Melnikov et al., 2012). Many of the universally conserved ribosomal proteins have also evolved eukaryote-specific extension segments (Melnikov et al., 2012; Ghosh and Komar, 2015). As a rule, these extension segments are present on the N- or C- terminal (or both) ends of the conserved proteins and vary widely in length, structure, and function (Ghosh and Komar, 2015). The extension segments are known to affect ribosome function/protein synthesis as a result of their interaction(s) with other ribosomal proteins, mRNA and/or the translation factors (Ghosh and Komar, 2015). It has been speculated that such extra proteins/protein extension segments have evolved to allow eukaryote-specific translational control of gene expression (Hashem et al., 2013; Ghosh et al., 2014; Ghosh and Komar, 2015; Simms et al., 2018; Jindal et al., 2019). It ought to be also noted that in addition to their canonical role in ribosome function(s), many ribosomal proteins are involved in extra-ribosomal activities (Ardini et al., 1998; Nelson et al., 2008; Melamed et al., 2010; Poddar et al., 2013; Ban et al., 2014; Han et al., 2017; Kershner and Welshhans 2017; Ono et al., 2017; Derylo et al., 2018; Dionne et al., 2019). Such activities in eukaryotes may also rely on eukaryote-specific extension segments of these proteins. Detailed mechanistic understanding of how the extension segments modulate ribosome-associated function and extra-ribosomal activity of these proteins is slowly emerging (Ghosh and Komar, 2015).
The receptor for activated c-kinase 1 (RACK1, known as Asc1 in yeast and mentioned hereafter as RACK1, according to the new nomenclature of ribosomal proteins) is a eukaryote-specific ribosomal protein known to be located in the head region of the small ribosomal subunit (40S) (Ben-Shem et al., 2010; Ban et al., 2014). RACK1 is the only ribosomal protein belonging to the tryptophan-aspartate repeat (WD-repeat) family of proteins (Ben-Shem et al., 2010; Ban et al., 2014). It is made up of seven repeats of the WD40 motifs (each ~40 residues long) forming a β-propeller structure (Ben-Shem et al., 2010; Ban et al., 2014). RACK1 has several independent protein binding sites and individual WD40 repeats can interact with different molecules simultaneously, thus allowing RACK1 to integrate inputs from distinct signaling pathways (Schmitt et al., 2017). Although RACK1 is a non-essential protein, its depletion is known to compromise cellular stress response and translation efficiency (Schmitt et al., 2017). Presence of RACK1 is also important for ensuring correct ribosome-associated quality control pathways (Gerbasi et al., 2004; Majzoub et al., 2014; Wolf and Grayhack, 2015; Ikeuchi and Inada, 2016; Thompson et al., 2016; Nielsen et al., 2017). Apart from the ribosome-associated functions, cytosolic RACK1 is also known to play role in different cellular signaling pathways (Melamed et al., 2010; Kershner and Welshhans, 2017; Schmitt et al., 2017). As such, RACK1 is believed to act as a link between the cellular signaling pathways and ribosome function. Original/early Cryo-EM studies that identified RACK1 as an integral part of the eukaryotic ribosome, have shown that the majority of the protein moiety remains solvent exposed with only one side of the protein interacting with the ribosome (Sengupta et al., 2004). Such structural organization allows the protein to bind many different regulatory factors while being attached to the 40S ribosomal subunit (Sengupta et al., 2004; Coyle et al., 2009).
It should be noted that the interface between RACK1 and the 40S ribosomal subunit is highly conserved within eukaryotes (Sengupta et al., 2004; Coyle et al., 2009). It is predominantly formed by a set of positively charged RACK1 amino acid (aa) residues on the protein side and the 18S rRNA (helices 39 and 40) on the other (RNA) side (Sengupta et al., 2004; Coyle et al., 2009). Altering the nature/charge of these positively charged residues in yeast (manifested most notably in the RACK1-R38DK40E double mutant) was shown to reduce the ribosomal association of RACK1 (Coyle et al., 2009). Such RACK1 mutant strains with severely compromised 40S association ability exhibited decreased cellular growth, altered cellular signaling and compromised cell wall integrity (Coyle et al., 2009). In addition to the 18S rRNA helices 39 and 40, RACK1 was also shown to form contacts with several ribosomal proteins (namely, S3, S5, S16 and S17) (Sengupta et al., 2004; Ghosh and Komar, 2015). However, despite of this knowledge, many details of the mechanism(s) ensuring efficient RACK1 recruitment to and stable association with the 40S ribosomal subunit remain unknown. Recent high-resolution structures of the yeast 40S ribosomal subunit revealed that RACK1 interaction with 40S subunit is mediated through the eukaryote-specific extension segments of ribosomal protein S3 (mentioned as uS3 hereafter) located in its C-terminal domain (CTD) (Fig. 1A) (Ben-Shem et al., 2010; Melnikov et al., 2012; Ghosh and Komar, 2015). We therefore hypothesized that this eukaryote-specific uS3-CTD may have evolved to facilitate stable association between RACK1 and the ribosome.
Figure 1.
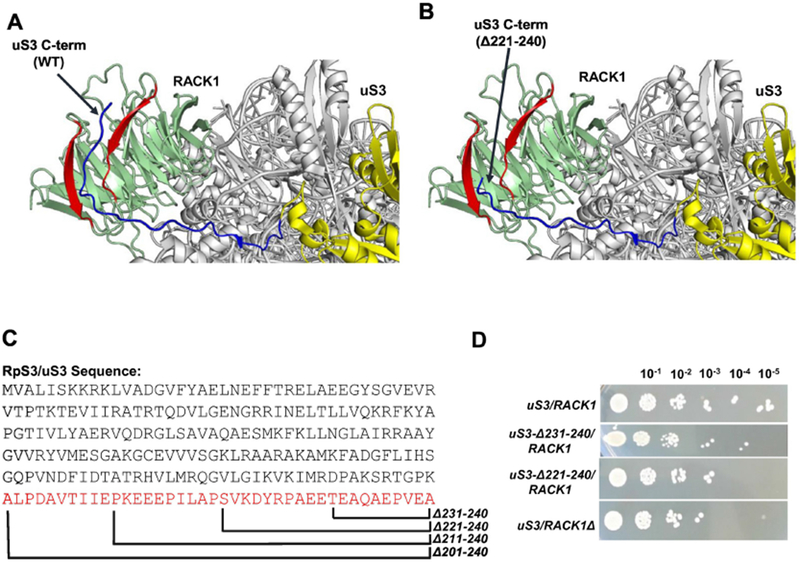
Interaction between uS3 eukaryote-specific C-terminal domain (CTD) and RACK1: (A) Ribosomal location of uS3 and RACK1 in the yeast 40S head region is shown. RACK1 is in green with the two blades interacting with the uS3-CTD shown in red. uS3 is in yellow with its CTD shown in blue. PDB:4V88 was used for the analysis (Ben-Shem et al., 2010). (B) Deletion of the last 20 residues of the uS3-CTD abolishes the uS3-RACK1 interaction. PDB:4V88 was used for the analysis (Ben-Shem et al., 2010). (C) The sequence of uS3 (Saccharomyces cerevisiae) is shown with the C-terminal deletion mutants. (D) Cellular growth of the wild-type, uS3-CTD mutants (lacking 10 and 20 C-terminal amino acid residues), and the RACK1 deletion strain.
To test our hypothesis, we generated mutant yeast strains expressing C-terminally truncated uS3 proteins (lacking 10, 20, 30 and 40 amino acid residues). We found that while deletion of 30 and 40 uS3 C-terminal amino acid residues leads to cellular inviability, the absence of just 20 residues from the uS3-CTD results in only moderately reduced yeast cell growth rates (comparable to that for yeast strains lacking RACK1) and complete abrogation of RACK1 binding to the ribosome. Importantly, the phenotype of a uS3 strain lacking 20 residues from the uS3-CTD was very similar to that of RACK1 deletion strain showing comparable defects in protein synthesis fidelity. Evidence presented here suggest that the uS3-CTD plays an important role in ribosome-associated functioning of RACK1 and may have evolved to act as a link between the ribosome and the cellular signaling pathways.
2. Materials and Methods
2.1. Yeast strain and growth methods
Yeast strains used in the study are listed in Table 1. RACK1 deletion strain was constructed by replacing RACK1/ASC1 gene in W303 yeast strain with KANMX cassette lifted from BY4741-RACK1 deletion strain (a kind gift of Dr. Yoav Arava, Department of Biology, Technion, Israel Institute of Technology, Haifa, Israel) (Melamed et al., 2010). To construct uS3 mutants, a genomic copy of the uS3 ORF with 400bp upstream and 300bp downstream sequences was amplified and cloned into a pRS314-trp plasmid with primer set 746/747. Different uS3 mutants were constructed by PCR based method using the primer set 757/769, 757/770, 757/771 and 757/772 for uS3Δ231-240/RACK1, uS3Δ221-240/RACK1, uS3Δ211-240 RACK1, and uS3Δ201-240 RACK1 respectively and confirmed by sequencing using primer 754 (Table 2). To construct yeast strains with mutant uS3 gene, the above mentioned plasmids containing wild-type and mutant copies of the uS3 gene were transformed into yeast W303 Matα strain (uS3::natNT2, ade3::kanMX4 <pRS316-uS3-GFP, URA3>) (a kind gift of Dr. Brigitte Pertschy, University of Fribourg, Fribourg, Switzerland) (Koch et al., 2012) and selected on SD-trp plates. Positive strains were counter-selected in 5-FOA containing plates to retrieve strains expressing either wild-type or mutant uS3 from the pRS314-trp plasmid. Only uS3/RACK1, uS3Δ231-240/RACK1, and uS3Δ221-240/RACK1 mutants were viable and yielded colonies after 5-FOA selection. Yeast cultures were grown as indicated using either synthetic media containing 0.67% Difco yeast nitrogen base, 1% ammonium sulfate, 2% glucose and supplemented with the appropriate amino acids or YEPD medium. Yeast cells were transformed using the lithium acetate method (Ito et al., 1983).
Table 1.
Yeast strains used in the study.
| Strain | Genotype | Source |
|---|---|---|
| uS3/RACK1Δ | asc1::KanMX4 | This work |
| uS3/RACK1 | nS3::natNT2 <pRS314-uS3> | This work |
| uS3Δ231-240/RACK1 | uS3::natNT2 <pRS314-uS3Δ231-240> | This work |
| uS3Δ221-240/RACK1 | uS3::natNT2 <pRS314-uS3Δ221-240> | This work |
All strains are in W303 (ade2-1 his3-11,15 leu2-3,112 trp1-1 ura3-1 can1-100) background
Table 2.
List of primers used in the study.
| Primer | Sequence (5’ to 3’) |
|---|---|
| 746 | AAG AGC TCC GTA TAC AAG TGG TGA |
| 747 | TTG GAT CCA CGT GAA CGG GAT TA |
| 754 | GAC ACT GCT ACT AGA CAC G |
| 757 | TAG ATT TAA TTA TTA AAT ACA TAA ATA AAC |
| 769 | TTG GAT CCC TAT TCT TCA GCT GGT CTG TAG TC |
| 770 | TTG GAT CCC TAT GGA GCA AGA ATT GGT TCT TC |
| 771 | TTG GAT CCC TAT TCA ATG ATG GTG ACA GCA TC |
| 772 | TTG GAT CCC TAC TTT GGA CCA GTT CTG CTC TTA G |
2.2. Reporter plasmids
Reporter constructs used for testing PRF and stop codon recognition efficiencies were a kind gift of Dr. Jonathan D. Dinman (University of Maryland) (Harger and Dinman, 2003). Reporters were transformed into respective strains and expression of Renilla and Firefly luciferase was measured using with dual luciferase assay kit (Promega) as described before (Lumsden et al., 2010).
2.3. Sucrose density gradient fractionation, western blotting and rRNA analysis
Sucrose density gradient centrifugation and polysome analysis were done as described previously (Lumsden et al., 2010). Fractions from respective gradients were collected, proteins were precipitated using Trichloroacetic acid (TCA), solubilized in SDS-polyacrylamide gel electrophoresis loading buffer and analyzed by western blotting as described (Lumsden et al., 2010). The rpS5 antibody has been described previously (Galkin et al., 2007). The polyclonal anti Rabbit anti-RACK1 (1:1000; cat. no. PA5-17484) and anti-Rps3 (1:1000; cat. no. D50G7) antibodies were from Thermo Scientific and Cell Signaling Technology respectively. Secondary anti-rabbit horseradish peroxidase-conjugated antibody (1:5000, Cell Signaling Technology) and enhanced chemiluminescence detection kit (ECL™, GE Healthcare) was used for detection. Blots were scanned with Odessey system from Li-cor. For RNA analysis, Yeast strains were grown at 30°C in complete medium till mid-logarithmic phase. Total RNA was extracted, resolved by denaturing gel electrophoresis, stained with ethidium bromide and scanned using a Typhoon imaging scanner as described previously (Lumsden et al., 2010).
2.4. Miscellaneous
Molecular cloning was done using common procedures as outlined in Sambrook et. al., (Sambrook et al., 1989). DNA sequencing was accomplished by the DNA Sequencing Core facility at Cleveland Clinic, Cleveland Ohio, USA. Sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis was done according to the procedure of Laemmli (Laemmli, 1970).
3. Results
3.1. RACK1 interacts with uS3 C-terminal eukaryote-specific extension
Apart from 18S rRNA, several ribosomal proteins are known to interact with RACK1 and may modulate its ribosomal recruitment (Sengupta et al., 2004; Coyle et al., 2009; Ben-Shem et al., 2010). Recent high resolution 40S ribosomal structures have however revealed that the most extensive set of contacts is formed between RACK1 and the ribosomal protein uS3 (Ben-Shem et al., 2010; Ghosh and Komar, 2015). The unstructured uS3-CTD runs diagonally across the edge of blade 4 and towards the blade 5 C-D loop of RACK1 (Fig. 1A). In this model, removal of the last 20 residues of uS3-CTD was seen to completely upset the uS3-RACK1 interaction (Fig. 1B). To experimentally check the importance of this uS3-RACK1 interaction, we generated yeast strains expressing either wild-type or mutant uS3 proteins lacking 10, 20, 30 or 40 aa residues from its C-terminal end (Fig. 1C). As such, we have used a yeast strain in which the chromosomal uS3 copy was disrupted and the normal cellular growth was supported by a plasmid (pRS316, URA3) borne uS3 copy (Melamed et al., 2010). This strain was further transformed with a plasmid (pRS314, TRP1) expressing either the wild-type or truncated (deletion) uS3 gene copies under the control of the uS3 endogenous promoter and terminator sequences. Transformants were counter-selected in 5-FOA (5-Fluoroorotic acid) to generate yeast strains expressing either wild-type or mutant uS3 exclusively from the pRS314 plasmid. While colonies were seen after 5-FOA counter-selection for strains expressing uS3-CTD variants truncated by 10 and 20 aa, deletion of 30 and 40 aa residues yielded no colonies at all (not shown). These experiments therefore revealed that cells harboring uS3 mutants with deletions of 30 or 40 aa residues from uS3-CTD are not viable.
Next, we compared the cellular growth of uS3-CTD 10 and 20 aa deletion mutants to that of the wild-type strain and the uS3/RACK1Δ strain. Wild-type and mutant yeast strains were grown to the same OD600 levels, serial diluted and spotted on YPDA plates. While the uS3Δ231-240/RACK1 strain (with 10 residues deleted from uS3-CTD) showed only moderate growth deficiency as compared to the wild-type strain, the uS3Δ221-240/RACK1 strain (with 20 residues deleted from uS3-CTD) showed a stronger slow growth phenotype (Fig. 1D). Interestingly, the decreased growth rates observed for the uS3Δ221-240/RACK1 strain was similar to that observed for the uS3/RACK1Δ strain (Fig. 1D) suggesting that the defects seen in the uS3Δ221-240/RACK1 strain could potentially stem from the lack of RACK1 binding with the ribosome.
3.2. Interaction between RACK1 and uS3 is necessary for ribosomal recruitment of RACK1
To further assess whether the lack of the 20 aa residues from uS3-CTD in the uS3Δ221-240/RACK1 strain indeed affects the ribosomal association of RACK1, we performed co-immunoprecipitation analysis. Lysates from the wild-type and uS3Δ221-240/RACK1 strains were precipitated with anti-uS3 antibody and further probed with anti-RACK1 antibody to check for the presence of RACK1 within the precipitated ribosomal fractions. As expected, RACK1 was readily detected in the precipitated fraction for the wild-type uS3/RACK1 strain, however it was not detected in case of the precipitate from the uS3Δ221-240/RACK1 strain (Fig. 2A). This suggests that uS3-CTD is indeed necessary for the stable binding of RACK1 to the ribosome.
Figure 2.
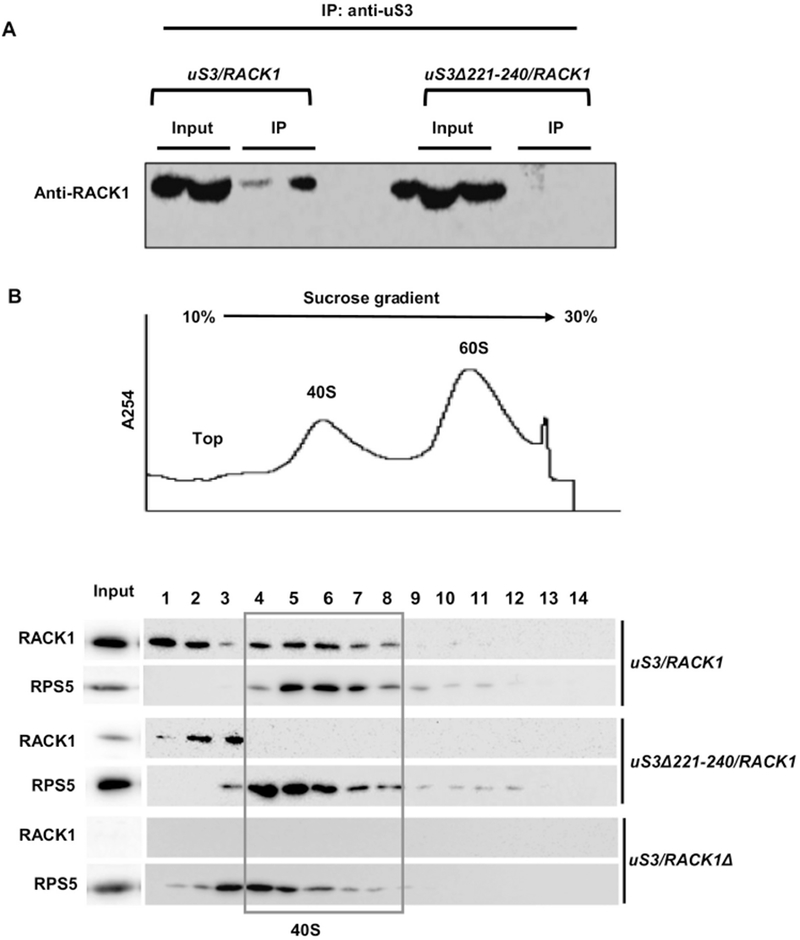
uS3-RACK1 interaction is essential for ribosomal recruitment of RACK1: (A) Co-immunoprecipitation analysis showing that deletion of the last 20 aa residues from uS3-CTD abrogates RACK1 interaction with the ribosome; (B) Polysome profiling analysis showing that RACK1 is unable to associate with the mutant 40S ribosomal subunits harboring uS3Δ221-240. Whole cell extracts from wild-type and mutant strains were resolved in 10-30% sucrose gradients and the fractions were probed to detect the presence of RACK1 with the anti-RACK1 antibody. A representative UV trace at 254 nm is shown above. RACK1 does not bind to the 40S in the uS3Δ221-240/RACK1 strain. Input - represents a 7% portion of each gradient fraction. Western blotting with anti-rpS5 antibody is also shown to detect the 40S subunit fractions.
We have further resolved the whole cell lysates from the wild-type, uS3Δ221-240/RACK1 and uS3/RACK1Δ strains using 10-30% sucrose gradient ultracentrifugation and checked for the presence of the RACK1 protein in the 40S ribosomal fractions. Total protein was TCA precipitated and probed for the presence of RACK1 by western blotting. In case of the wild-type strain, RACK1 was detected both in the top (free) fractions and the 40S ribosomal fractions (Fig. 2B, bottom panel). However, in case of strain uS3Δ221-240/RACK1, RACK1 was detected only in the top fractions, but not in the 40S ribosomal fractions (Fig. 2B, bottom panel). This clearly suggests that RACK1 is not able to associate with the mutant 40S ribosomal subunits harboring uS3 with 20 residues deleted from uS3-CTD. In the uS3/RACK1Δ strain, RACK1 was absent across all the fractions, as expected (Fig. 2B, bottom panel). Although previous studies have shown that RACK1 interacts with other ribosomal proteins like rpS16 and rpS17 (in addition to uS3), the fact that uS3-CTD deletion resulted in complete loss of RACK1 from the 40S fractions suggests that uS3-CTD is critical for the proper ribosomal recruitment of RACK1 (Sengupta et al., 2004; Ben-Shem et al., 2010).
3.3. Disruption of RACK1 binding to the ribosome perturbs global translation
Recent studies have shown that ribosomal binding of RACK1 is important for both cap-dependent and cap-independent modes of translation (Adams et al., 2011; Majzoub et al., 2014; Thompson et al., 2016; Gallo et al., 2018). A distinct part of uS3 (within its N-terminal globular domain) has also been implicated in the stabilization of the pre-initiation complexes on the start codons in yeast (Dong et al., 2017). In view of these findings, we wanted to check, if our mutant uS3Δ221-240/RACK1 strain may also exhibit any translational defects and if these defects could be similar to those observed for RACK1 deletion strains. To investigate the global translational status of the mutant yeast strains we performed polysome profiling analysis. As such, whole cell extracts of the uS3/RACK1, uS3Δ221-240/RACK1 and uS3/RACK1Δ strains were resolved by sedimentation through sucrose density gradients. (7-50%) (Fig. 3A–C). This analysis revealed a reduced polyribosome (P) to monosome (80S) ratio (P/M) in uS3/RACK1 (P/M=1.43±0.48) and uS3Δ221-240/RACK1 (P/M=1.52±0.56) strains as compared to the wild-type strain (P/M=1.97±0.3) (Fig. 3A–C). Since a reduced P/M ratio is a characteristic phenotype of mutations that impair translation initiation, these data suggest uS3Δ221-240/RACK1 strains harbors a translation initiation defect.
Figure 3.
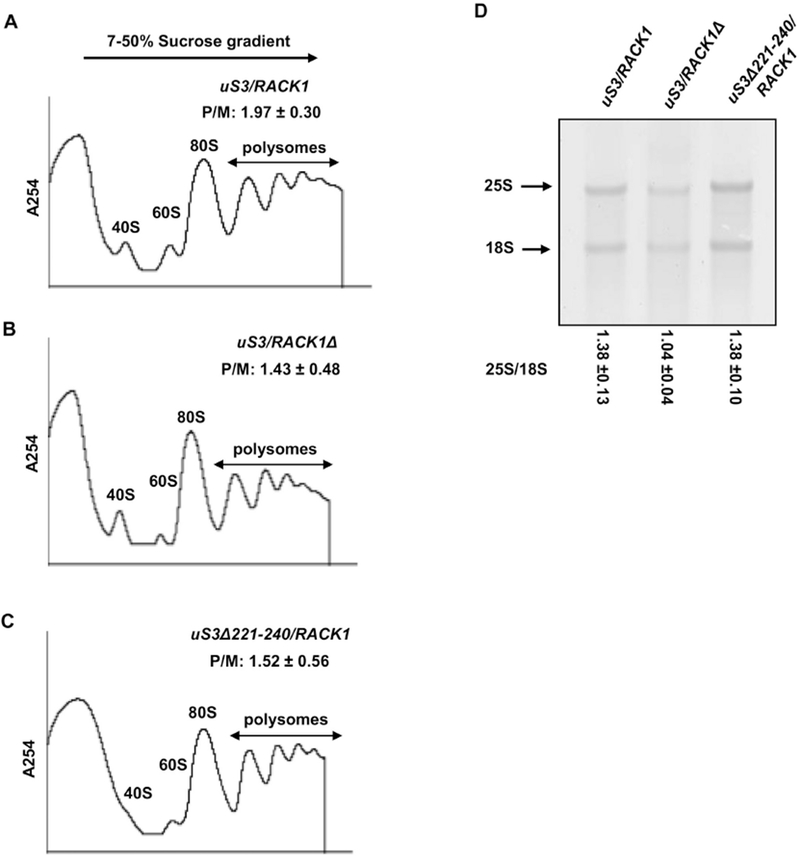
Disruption of uS3-RACK1 interaction results in translation initiation defects: Polysome profiles of (A) uS3/RACK1 (B) uS3/RACK1Δ and (C) uS3Δ221-240/RACK1 strain are shown with their P/M ratios; (D) Ethidium bromide staining of the 25S and 18S rRNA of the wild-type and the mutant strains (denaturing agarose gel electrophoresis). The 25S/18S ratio is mentioned for each strain.
Interestingly, very similar P/M ratios were seen for the uS3Δ221-240/RACK1 and the uS3/RACK1Δ (Fig. 3B,C). Our findings are in line with the previous results suggesting that the deletion of RACK1 and/or abrogation of its ribosomal association impairs translation (Coyle et al., 2009; Adams et al., 2011; Gallo et al., 2018). However, it can’t be completely excluded that these defects may also originate from the mutation in uS3 itself (Dong et al., 2017; Ochkasova et al., 2018).
It is known that many ribosomal proteins play important roles in ribosome biogenesis and mutations introduced in these proteins often result in accumulation of unprocessed immature ribosomes (Biedka et al., 2018; Espinar-Marchena et al., 2018; Tomioka et al., 2018; Cheng et al., 2019). Previous studies have also shown that specific mutations/deletions of RACK1 or uS3 impact ribosome biogenesis (Larburu et al., 2016; Mitterer et al., 2016; Limoncelli et al., 2017). It thus can’t be completely excluded that the observed decrease in the P/M ratio for the mutant strains can stem from the impaired ribosome biogenesis. To test this possibility, total RNA was extracted from the wild-type and mutant yeast strains and resolved by agarose gel electrophoresis under denaturing conditions. Substantial ribosome biogenesis defects in these mutants would be expected to alter the ratios of the 25S rRNA to the 18S rRNA species (25S/18S). Analysis of rRNA under denaturing conditions however indicated that ribosome biogenesis is not severely affected in the uS3Δ221-240/RACK1 strain, since its 25 S/18S ratio was comparable to that in the wild-type strain (Fig. 3D). However, the 25S/18S ratio for the uS3/RACK1Δ mutant was slightly altered as compared to the wild-type (Fig. 3D). This change was expected due to the previously defined role of RACK1 in the ribosome biogenesis and the maturation of the small subunit (Larburu et al., 2016). However, neither we detected any accumulation of 20S pre-rRNA species in our assay, nor the 25S/18S ratio was drastically different to that in the wild-type strain, suggesting that this defect was a modest one (as has been reported previously (Larburu et al., 2016)). Based on these observations, we concluded that the observed defects in global translation initiation do not originate from impaired ribosome biogenesis, but rather stem from the absence and/or impaired interaction between RACK1 and the ribosome, specifically in strain uS3Δ221-240/RACK1.
3.4. Communication between uS3 and RACK1 is essential for maintenance of translation fidelity and correct stop codon recognition
It has been previously shown that RACK1 controls read-through of inhibitory sequences like CGA codon repeats and affects ribosome-associated quality control pathways (Wolf and Grayhack, 2015; Ikeuchi and Inada, 2016; Wang et al., 2018). We therefore wanted to check whether disruption of the uS3-RACK1 interaction in the uS3Δ221-240/RACK1 mutant may have also affected translation fidelity and/or stop codon recognition in this strain. To monitor translation fidelity, we took advantage of the previously described dual-luciferase reporter system optimized for studies in yeast (Harger and Dinman, 2003). In this system, frameshifting signals derived from the L-A virus and Ty1 or Ty3 retrotransposons are inserted between the Renilla and Firefly luciferase genes. While L-A virus derived signal induces −1 programmed ribosomal frameshifting (PRF), sequences derived from Ty1 and Ty3 induce +1 PRF (Harger and Dinman, 2003). The Firefly luciferase is translated only by those ribosomes which undergo PRF at the indicated sequences. A comparison of the Firefly luciferase activity with respect to the Renilla luciferase activity demonstrates the relative efficiency of PRF for each strain. Relative −1 PRF efficiencies from the L-A virus-derived sequence for the uS3Δ221-240/RACK1 and the uS3/RACK1Δ mutants were comparable to those seen for the wild-type strain, suggesting that neither uS3-CTD deletion nor lack of RACK1 affects −1 PRF efficiency under these conditions (Fig. 4A). However, for the Ty1 derived sequence, the +1 PRF efficiency increased ~6-fold for the uS3/RACK1Δ mutant compared to that of the wild-type (Fig. 4A). Similar enhanced +1 PRF efficiency was also seen for the uS3Δ221-240/RACK1 strain (~8-fold more than the wild-type) (Fig. 4A). For both the L-A derived −1 PRF and Ty1 derived +1 PRF, the frameshift efficiencies for the uS3/RACK1Δ and the uS3Δ221-240/RACK1 strains were comparable (Fig. 4A). However, in case of the Ty3 sequence, the +1 PRF efficiency in the uS3Δ221-240/RACK1 strain was found to be similar to that in the wild-type, while the same for the uS3/RACK1Δ mutant was ~2-fold more than that for the wild-type (Fig. 4A). It is known that different sequences induce −1 or +1 PRF using different mechanisms. The differences observed for the Ty3 +1 PRF between the uS3Δ221-240/RACK1 and the uS3/RACK1Δ mutant points towards different mechanisms being utilized in these mutants. Thus, at least for the L-A and Ty1 PRF signals, deletion of uS3-CTD abrogates RACK1 function in the preservation of translation fidelity through common mechanisms.
Figure 4.
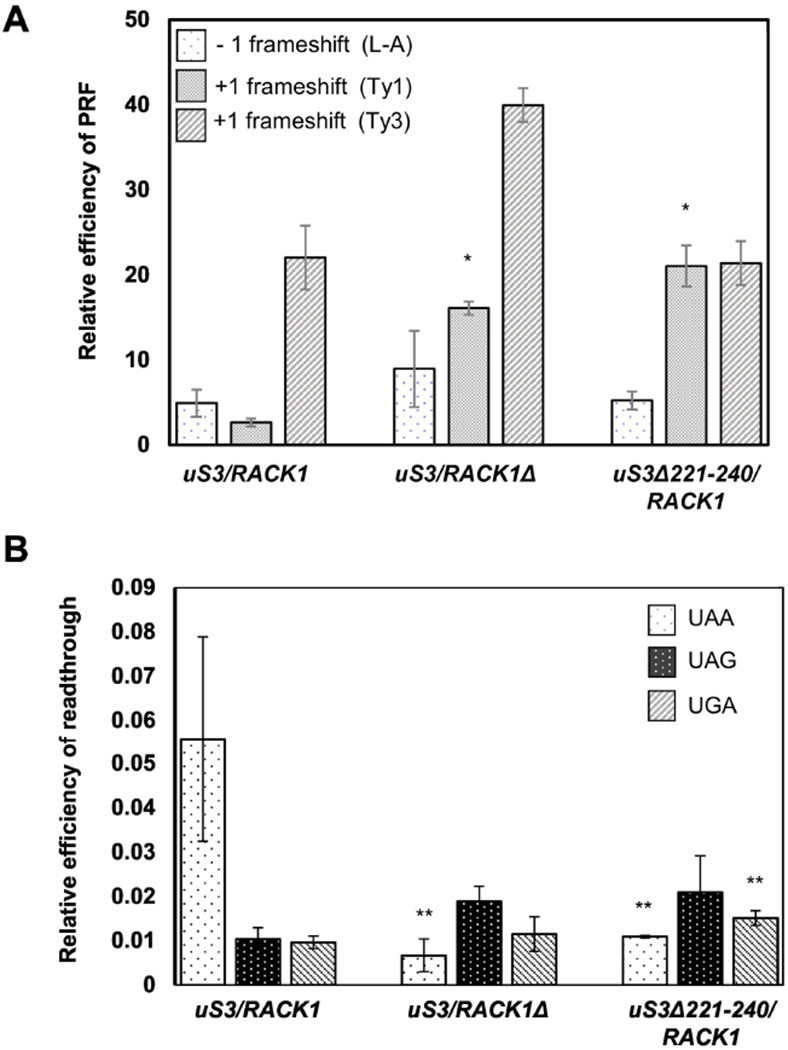
Abrogation of uS3-RACK1 interaction with the ribosome impairs translation fidelity and stop codon recognition efficiency: (A) Relative frameshifting efficiency of L-A, Ty1 and Ty3 reporters from the wild-type and mutants strains are shown; (B) Efficiency of stop codon recognition in the wild-type and mutants strains are shown. *P <0.001, **P <0.05.
We were further interested to check if deletion of uS3-CTD also affects the efficiency of stop codon recognition. We used the same dual luciferase reporter system described above for this study with the only exception that the PRF signals were replaced with the three stop codons (UAA, UAG, and UGA) (Harger and Dinman, 2003). Only those ribosomes which are unable to efficiently recognize the stop codons readthrough them, thus resulting in the expression of Firefly luciferase in these constructs. The uS3/RACK1Δ and uS3Δ221-240/RACK1 mutants exhibited hyper-accurate recognition of the UAA stop codon as shown by ~5-fold less readthrough efficiency in the uS3/RACK1Δ and uS3Δ221-240/RACK1 mutants with respect to the wild-type (Fig. 4B). Recognition of the UAG stop codon occurred with similar efficiencies in all the strains (Fig. 4B). We also observed a modest increase in the readthrough of UGA codon in the uS3Δ221-240/RACK1 mutant, but not in the uS3/RACK1Δ strain as compared to the wild-type (Fig. 4B).
Taken together, our data suggest that deletion of the last 20 aa residues of uS3-CTD modulates fidelity of both translation elongation and stop codon recognition. These defects were similar to those attributed to the absence of RACK1, suggesting that defects observed in both uS3/RACK1Δ and uS3Δ221-240/RACK1 mutant may stem from the same mechanism.
4. Discussion
Presence of additional ribosomal proteins/ribosomal protein extension segments in eukaryotic ribosomes has resulted in complex networks of ribosomal protein interactions on the outer shell of the eukaryotic ribosome (Klinge et al., 2012; Melnikov et al., 2012; Ghosh and Komar, 2015). It has been hypothesized that these protein-protein interactions evolved to accommodate specific features of the eukaryotic translational apparatus and fulfill important functions in the regulation of gene expression in eukaryotes (Klinge et al., 2012; Melnikov et al., 2012; Ghosh and Komar, 2015). However, direct evidence in support of this hypothesis is limited. Previous work from our laboratory has, for example, shown that the eukaryote-specific N-terminal extension of ribosomal protein S5 (uS7) has evolved to accommodate interaction with ribosomal protein S16 (uS9) and that this cross-talk is important for efficient translation initiation in yeast (Ghosh et al., 2014; Jindal et al., 2019). Disruption of the interaction between uS7 and uS9 affected recruitment of Met-tRNAiMet in the P site of 48S complex, leading to a reduced rate of bulk translation initiation (decreased polysome content), impaired derepression of GCN4 mRNA translation, and accumulation of eIF2 on 40S ribosomal subunits (Ghosh et al., 2014; Jindal et al., 2019). This study provided one of the first experimental evidences supporting the functional significance of protein-protein interactions within the ribosome that are absent in prokaryotes but represent a defining feature of eukaryotic ribosomes.
To gain insights into the mechanism which ensures stable association of the eukaryote-specific RACK1 protein with the eukaryotic ribosome, we performed here deletion analysis of uS3 protein. It has been previously shown that RACK1 interacts with 18S rRNA (helices 39 and 40) and forms contacts with several ribosomal proteins, including S3, S5, S16 and S17 (Sengupta et al., 2004; Ghosh and Komar, 2015). However, the exact details of the RACK1 recruitment and stable association with the 40S ribosomal subunit remain poorly understood. Recent high resolution X-ray crystal structures of the yeast 80S ribosome (Ben-Shem et al., 2010; Melnikov et al., 2012) permitted detailed analysis of the structural organization of the eukaryotic ribosome of the yeast, S. cerevisiae and in particular revealed that the RACK1 propeller blade (4-5) interacts with the eukaryote-specific C-terminal extension segment of uS3 (Ben-Shem et al., 2010; Ghosh and Komar, 2015). We have therefore suggested that this uS3 region may have evolved to ensure stable recruitment and association of RACK1 with the eukaryotic ribosome. Indeed, we found that deletion of just 20 C-terminal uS3 amino acid residues forming contacts with RACK1 blade (4-5) (Fig 1) was sufficient to completely abrogate uS3-RACK1 interaction and RACK1-ribosomal association (Fig. 2). Interestingly, we found that the phenotype of this uS3Δ221-240/RACK1 mutant strain appeared to be very similar to that of the uS3/RACK1Δ mutant strain, revealing similar defects in cell growth, polysome distribution and translation fidelity (Fig. 3,4). Altered fidelity of translation elongation and stop codon recognition observed both in the uS3Δ221-240/RACK1 and the uS3/RACK1Δ mutants suggests a cooperative role played by these two proteins in ribosome function further suggesting that communication between RACK1 and the ribosome and, more specifically, between RACK1 and uS3 is essential for RACK1 intracellular function. It has been previously shown that cells harboring RACK1R36D/K38E mutant variant (incapable of efficient ribosome binding), exhibit defects similar to that of RACK1 deletion mutant, suggesting that ribosomal association of RACK1 is a critical determinant of its regulatory functions (Coyle et al., 2009; Kim et al., 2017; Gallo et al., 2018). Many of the observed defects in this mutant are believed to stem from the inability of RACK1 deficient ribosomes to efficiently translate messages (Gallo et al., 2018). Our data further corroborate these observations and suggest that eukaryote-specific uS3-CTD is essential for ribosome-associated functioning of RACK1 and that eukaryote-specific uS3-RACK1 interaction has evolved to act as a critical link between cellular signaling pathways and ribosome-mediated translational control of gene expression. Further in-depth analysis of the signaling pathways and specific messages affected by the absence of uS3-RACK1 communication may shed additional light on RACK1 intracellular function as it relates to its ribosomal location.
5. Conclusions
Despite the recent progress in the structural studies of ribosomes, assignment of specific functions to individual ribosomal proteins and/or eukaryote-specific protein extensions remains challenging. In this study, we found that deletion of just 20 C-terminal residues from the eukaryote-specific C-terminal extension of ribosomal protein uS3 abrogates RACK1 binding to the ribosome. We further showed that yeast strains with truncated uS3-CTD exhibited compromised cellular growth and protein synthesis similar to that of RACK1Δ strain, thus suggesting that the uS3-CTD is crucial not only for the recruitment and association of RACK1 with the ribosome, but also for its intracellular function. Our data therefore further suggest that eukaryote-specific uS3-CTD may have evolved to act as a critical link between cellular signaling pathways and ribosome-mediated translational control of gene expression.
Highlights:
Receptor for Activated c-Kinase 1 (RACK1) is a eukaryotic ribosomal protein located in the head region of the 40S subunit near the mRNA exit channel.
RACK1 interacts with uS3 on ribosome along with rRNA and other ribosomal proteins.
C-terminal deletions of uS3 abrogate binding of RACK1 to ribosome in S. cerevisiae.
C-terminal deletion mutants of uS3 show similar phenotypic and translational defects to that of RACK1Δ strain.
Acknowledgments:
The authors would like to thank Drs Yoav Arava, Jonathan D. Dinman and Brigitte Pertschy for generous gifts of plasmids and strains used in this study.
Funding: This work was in part supported by National Institute of Health (NIH) grant HL121779 (to A.A.K.), and by the Center for Gene Regulation in Health and Disease (GRHD).
Abbreviation list:
- RACK1
The receptor for activated c-kinase
- CTD
C-terminal domain
- rRNA
ribosomal RNA
- YEPD (medium)
Yeast extract peptone dextrose
- SD (medium)
Synthetic Defined
- 5-FOA
5-Fluoroorotic Acid
- TCA
Trichloroacetic acid
- SDS
Sodium dodecyl sulfate
- PRF
Programmed ribosomal frameshifting
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: The authors declare no conflict of interests.
References
- Adams DR, Ron D, Kiely PA, 2011. RACK1, A multifaceted scaffolding protein: Structure and function. Cell Commun Signal 9, 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ardini E, Pesole G, Tagliabue E, Magnifico A, Castronovo V, Sobel ME., Colnaghi MI, Menard S, 1998. The 67-kDa laminin receptor originated from a ribosomal protein that acquired a dual function during evolution. Mol Biol Evol 15, 1017–1025. [DOI] [PubMed] [Google Scholar]
- Ban N, Beckmann R, Cate JH, Dinman JD, Dragon F, Ellis SR, Lafontaine DL, Lindahl L, Liljas A, Lipton JM, et al. 2014. A new system for naming ribosomal proteins. Curr Opin Struct Biol 24, 165–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shem A, Jenner L, Yusupova G, Yusupov M, 2010. Crystal structure of the eukaryotic ribosome. Science 330, 1203–1209. [DOI] [PubMed] [Google Scholar]
- Biedka S, Micic J, Wilson D, Brown H, Diorio-Toth L, Woolford JL Jr. 2018. Hierarchical recruitment of ribosomal proteins and assembly factors remodels nucleolar pre-60S ribosomes. J Cell Biol 217, 2503–2518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Z, Mugler CF, Keskin A, Hodapp S, Chan LY, Weis K, Merlins P, Regev A, Jovanovic M, Brar GA, 2019. Small and Large Ribosomal Subunit Deficiencies Lead to Distinct Gene Expression Signatures that Reflect Cellular Growth Rate. Mol Cell 73, 36–47 e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyle SM, Gilbert WV, Doudna JA, 2009. Direct link between RACK1 function and localization at the ribosome in vivo. Mol Cell Biol 29, 1626–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derylo K, Michalec-Wawiorka B, Krokowski D, Wawiorka L, Hatzoglou M, Tchorzewski M, 2018. The uL10 protein, a component of the ribosomal P-stalk, is released from the ribosome in nucleolar stress. Biochim Biophys Acta Mol Cell Res 1865, 34–47. [DOI] [PubMed] [Google Scholar]
- Dionne KL, Bergeron D, Landry-Voyer AM, Bachand F, 2019. The 40S ribosomal protein uS5 (RPS2) assembles into an extraribosomal complex with human ZNF277 that competes with the PRMT3-uS5 interaction. J Biol Chem 294, 1944–1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong J, Aitken CE, Thakur A, Shin BS, Lorsch JR, Hinnebusch AG, 2017. Rps3/uS3 promotes mRNA binding at the 40S ribosome entry channel and stabilizes preinitiation complexes at start codons. Proc Natl Acad Sci U S A 114, E2126–E2135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinar-Marchena F, Rodriguez-Galan O, Fernandez-Fernandez J, Linnemann J, de la Cruz J. 2018. Ribosomal protein L14 contributes to the early assembly of 60S ribosomal subunits in Saccharomyces cerevisiae. Nucleic Acids Res 46: 4715–4732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galkin O, Bentley AA, Gupta S, Compton BA, Mazumder B, Kinzy TG, Merrick WC, Hatzoglou M, Pestova TV, Hellen CU, et al. 2007. Roles of the negatively charged N-terminal extension of Saccharomyces cerevisiae ribosomal protein S5 revealed by characterization of a yeast strain containing human ribosomal protein S5. RNA 13, 2116–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo S, Ricciardi S, Manfrini N, Pesce E, Oliveto S, Calamita P, Mancino M, Maffioli E, Moro M, Crosti M, et al. 2018. RACK1 Specifically Regulates Translation through its Binding to Ribosomes. Mol Cell Biol doi: 10.1128/MCB.00230-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerbasi VR, Weaver CM, Hill S, Friedman DB, Link AJ, 2004. Yeast Asc1p and mammalian RACK1 are functionally orthologous core 40S ribosomal proteins that repress gene expression. Mol Cell Biol 24, 8276–8287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Jindal S, Bentley AA, Hinnebusch AG, Komar AA, 2014. Rps5-Rps16 communication is essential for efficient translation initiation in yeast S. cerevisiae. Nucleic Acids Res 42, 8537–8555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A, Komar AA, 2015. Eukaryote-specific extensions in ribosomal proteins of the small subunit: Structure and function. Translation (Austin) 3, e999576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SH, Chung JH, Kim J, Kim KS, Han YS, 2017. New role of human ribosomal protein S3: Regulation of cell cycle via phosphorylation by cyclin-dependent kinase 2. Oncol Lett 13, 3681–3687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harger JW, Dinman JD, 2003. An in vivo dual-luciferase assay system for studying translational recoding in the yeast Saccharomyces cerevisiae. RNA 9, 1019–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashem Y, des Georges A, Dhote V, Langlois R, Liao HY, Grassucci RA, Hellen CU, Pestova TV, Frank J, 2013. Structure of the mammalian ribosomal 43S preinitiation complex bound to the scanning factor DHX29. Cell 153, 1108–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeuchi K, Inada T, 2016. Ribosome-associated Asc1/RACK1 is required for endonucleolytic cleavage induced by stalled ribosome at the 3′ end of nonstop mRNA. Sci Rep 6, 28234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Fukuda Y, Murata K, Kimura A, 1983. Transformation of intact yeast cells treated with alkali cations. J Bacteriol 153,163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal S, Ghosh A, Ismail A, Singh N, Komar AA, 2019. Role of the uS9/yS16 C-terminal tail in translation initiation and elongation in Saccharomyces cerevisiae. Nucleic Acids Res 47, 806–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershner L, Welshhans K, 2017. RACK1 regulates neural development. Neural Regen Res 12, 1036–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HD, Kong E, Kim Y, Chang JS, Kim J, 2017. RACK1 depletion in the ribosome induces selective translation for non-canonical autophagy. Cell Death Dis 8, e2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinge S, Voigts-Hoffmann F, Leibundgut M, Ban N, 2012. Atomic structures of the eukaryotic ribosome. Trends Biochem Sci 37, 189–198. [DOI] [PubMed] [Google Scholar]
- Koch B, Mitterer V, Niederhauser J, Stanborough T, Murat G, Rechberger G, Bergler H, Kressler D, Pertschy B, 2012. Yar1 protects the ribosomal protein Rps3 from aggregation. J Biol Chem 287, 21806–21815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK, 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227, 680–685. [DOI] [PubMed] [Google Scholar]
- Larburu N, Montellese C, O’Donohue MF, Kutay U, Gleizes PE, Plisson-Chastang C, 2016. Structure of a human pre-40S particle points to a role for RACK1 in the final steps of 18S rRNA processing. Nucleic Acids Res 44, 8465–8478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limoncelli KA, Merrikh CN, Moore MJ, 2017. ASC1 and RPS3: new actors in 18S nonfunctional rRNA decay. RNA 23, 1946–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden T, Bentley AA, Beutler W, Ghosh A, Galkin O, Komar AA, 2010. Yeast strains with N-terminally truncated ribosomal protein S5: implications for the evolution, structure and function of the Rps5/Rps7 proteins. Nucleic Acids Res 38, 1261–1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majzoub K, Hafirassou ML, Meignin C, Goto A, Marzi S, Fedorova A, Verdier Y, Vinh J, Hoffmann JA, Martin F, et al. 2014. RACK1 controls IRES-mediated translation of viruses. Cell 159, 1086–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melamed D, Bar-Ziv L, Truzman Y, Arava Y, 2010. Asc1 supports cell-wall integrity near bud sites by a Pkc1 independent mechanism. PLoS One 5, e11389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melnikov S, Ben-Shem A, Garreau de Loubresse N, Jenner L, Yusupova G, Yusupov M, 2012. One core, two shells: bacterial and eukaryotic ribosomes. Nat Struct Mol Biol 19, 560–567. [DOI] [PubMed] [Google Scholar]
- Mitterer V, Murat G, Rety S, Blaud M, Delbos L, Stanborough T, Bergler H, Leulliot N, Kressler D, Pertschy B, 2016. Sequential domain assembly of ribosomal protein S3 drives 40S subunit maturation. Nat Commun 7, 10336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson J, McFerran NV, Pivato G, Chambers E, Doherty C, Steele D, Timson DJ, 2008. The 67 kDa laminin receptor: structure, function and role in disease. Biosci Rep 28, 33–48. [DOI] [PubMed] [Google Scholar]
- Nielsen MH, Flygaard RK, Jenner LB, 2017. Structural analysis of ribosomal RACK1 and its role in translational control. Cell Signal 35, 272–281. [DOI] [PubMed] [Google Scholar]
- Ochkasova AS, Meschaninova MI, Venyaminova AG, Ivanov AV, Graifer DM, Karpova GG, 2018. The human ribosome can interact with the abasic site in mRNA via a specific peptide of the uS3 protein located near the mRNA entry channel. Biochimie 158, 117–125. [DOI] [PubMed] [Google Scholar]
- Ono H, Iizumi Y, Goi W, Sowa Y, Taguchi T, Sakai T, 2017. Ribosomal protein S3 regulates XIAP expression independently of the NF-kappaB pathway in breast cancer cells. Oncol Rep 38, 3205–3210. [DOI] [PubMed] [Google Scholar]
- Poddar D, Basu A, Baldwin WM 3rd, Kondratov RV, Barik S, Mazumder B, 2013. An extraribosomal function of ribosomal protein L13a in macrophages resolves inflammation. J Immunol 190, 3600–3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch FF, Maniatis T, 1989. Molecular Cloning: A Laboratory Manual, 2nd edn; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA. [Google Scholar]
- Schmitt K, Smolinski N, Neumann P, Schmaul S, Hofer-Pretz V, Braus GH, Valerius O, 2017. Asc1p/RACK1 Connects Ribosomes to Eukaryotic Phosphosignaling. Mol Cell Biol 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta J, Nilsson J, Gursky R, Spahn CM, Nissen P, Frank J, 2004. Identification of the versatile scaffold protein RACK1 on the eukaryotic ribosome by cryo-EM. Nat Struct Mol Biol 11, 957–962. [DOI] [PubMed] [Google Scholar]
- Simms CL, Kim KQ, Yan LL, Qiu J, Zaher HS, 2018. Interactions between the mRNA and Rps3/uS3 at the entry tunnel of the ribosomal small subunit are important for no-go decay. PLoS Genet 14, e1007818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson MK, Rojas-Duran MF, Gangaramani P, Gilbert WV, 2016. The ribosomal protein Asc1/RACK1 is required for efficient translation of short mRNAs. Elife 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomioka M, Shimobayashi M, Kitabatake M, Ohno M, Kozutsumi Y, Oka S, Takematsu H, 2018. Ribosomal protein uS7/Rps5 serine-223 in protein kinase-mediated phosphorylation and ribosomal small subunit maturation. Sci Rep 8, 1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Zhou J, Yang Q, Grayhack EJ, 2018. Multi-protein bridging factor 1(Mbf1), Rps3 and Asc1 prevent stalled ribosomes from frameshifting. Elife 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf AS, Grayhack EJ, 2015. Asc1, homolog of human RACK1, prevents frameshifting in yeast by ribosomes stalled at CGA codon repeats. RNA 21, 935–945. [DOI] [PMC free article] [PubMed] [Google Scholar]


