Summary
Patients with both heart failure and obstructive sleep apnea often have poor, repeatedly disrupted sleep, and yet they frequently do not complain of excessive daytime sleepiness. Understanding this lack of perceived sleepiness is crucial for the case identification and treatment of obstructive sleep apnea in the heart failure population at high risk for this disease, especially given the association between untreated obstructive sleep apnea and mortality among patients with heart failure. In this review, we present epidemiologic evidence concerning the lack of sleepiness symptoms in heart failure and obstructive sleep apnea, explore possible mechanistic explanations for this relationship, assess the benefits of treatment in this population, discuss implications for clinical practice, and explore directions for future research.
Keywords: Heart failure, Obstructive sleep apnea, Cardiovascular disease, Excessive daytime sleepiness
Introduction and Overview
Obstructive sleep apnea (OSA) is characterized by the interruption of ventilation due to a repetitive collapse of the upper airway during sleep (Somers et al., 2008) and is a major public health problem affecting five to 15% of adults in the United States (Young et al., 1993). Among patients with heart failure (HF), which is a clinical syndrome that occurs when there is a decline in the pumping ability of the heart (Lymperopoulos et al., 2013) sleep apnea, or sleep disordered breathing (SDB) is a common issue. We focus our review on reduced ejection fraction (HFrEF aka systolic HF) which occurs when the heart fails to contract properly and ejects less blood as a result (Borlaug and Paulus, 2011). SDB includes obstructive sleep apnea, as well as central sleep apnea (CSA), which occurs when ventilation is interrupted as a result of an abnormality in the brainstem’s regulation of respiration. In both disorders, the apnea hypopnea index (AHI), or total number of apneas and hypopneas per hour, is elevated from normal. OSA and CSA have different pathophysiologies, and, for this review, we explore the relationship between HF and OSA specifically. Using an apnea hypopnea index cut-off of 15 with 50% or more obstructive events, OSA is diagnosed in varying proportions of HF patients, ranging from approximately 16% (Oldenburg et al., 2016) to 37% (Sin et al., 1999) to 46% (Arzt et al., 2016) of HF patients. To further compound the problem, OSA remains under-diagnosed (Kapur et al., 2002).
The Adult OSA Task Force of the American Academy of Sleep Medicine categorizes patients with heart failure as a group at high risk of OSA (Epstein et al., 2009). According to the Task Force, these patients should be evaluated for symptoms such as witnessed apneas, snoring, gasping episodes, sleep fragmentation, morning headaches, decreased concentration, and excessive daytime sleepiness not explained by other factors” (Epstein et al., 2009). However, including these symptoms, such as excessive daytime sleepiness, as important indicators of OSA in patients with HF may be problematic, since patients with HF appear to have fewer sleepiness complaints than the general OSA population. Sleepiness is the physiological state in which the body needs sleep and may also be an individuals’ perception of physiological and psychological symptoms pertaining to sleepiness; thus, different individuals may perceive sleepiness symptoms differently (Hublin et al., 1996, Johns, 2000).
Excessive daytime sleepiness (EDS) is commonly measured by the Epworth Sleepiness Scale (ESS), which is a validated questionnaire that asks subjects to rate their likelihood of falling asleep in several common situations (Johns, 1991). As the scale is subjective, patients with HF and OSA who do not experience this symptom will not report it. This decreases the likelihood of testing and eventual diagnosis and treatment of OSA. This is especially concerning because those patients with heart failure treated for OSA have decreased two-year mortality rates compared to HF patients not treated for OSA (HR: 0.33 [95% confidence interval, 0.21–0.51], P < 0.0001) (Javaheri et al., 2011) Despite these potential consequences, there currently are no comprehensive reviews exploring excessive sleepiness in this population. This review seeks to address this gap by detailing the epidemiology and mechanisms for the lack of association between EDS and OSA among patient with CHF, explaining the treatment options, screening recommendations, and implications for future research.
Epidemiological studies on Sleepiness Symptoms in Heart Failure patients with Obstructive Sleep Apnea
Findings in several reviewed studies generally provide evidence that patients with both OSA and HF do not commonly complain of excessive daytime sleepiness (Javaheri et al., 1998, Kaneko et al., 2003, Arzt et al., 2006, Rao et al., 2006, Mansfield et al., 2004, Wang et al., 2009). Arzt et al compared HF patients to community controls and found that HF patients at any AHI slept less and yet still had less subjective daytime sleepiness than community controls (p<.01) (Arzt et al., 2006). Kaneko et al and Mansfield et al explored baseline sleepiness levels in patients with HF and OSA (Kaneko et al., 2003, Mansfield et al., 2004) and several studies have previously compared EDS in HF patients with and without sleep apnea or sleep-disordered breathing (Dolliner et al., 2013, Herrscher et al., 2014, Javaheri et al., 2011, Redeker et al., 2010, Wang et al., 2009, Yumino et al., 2009). These studies found that most HF patients, regardless of whether or not they had OSA, endorsed similar levels of daytime sleepiness. See Table 1 for detail.
Table 1:
Epidemiologic studies exploring sleepiness in heart failure (HFreF) and obstructive sleep apnea
| Authors | Subjects | Definition of Heart Failure (HF) | Method of Sleepiness Assessment | Control for medications in main analyses | Results |
|---|---|---|---|---|---|
| Arzt et al 2006 1 | n=48 patients with HF and obstructive sleep apnea (OSA) (Apnea hypopnea index (AHI) > 5) n=1139 community controls (328 subjects had OSA with AHI > 5) |
Ischemic, non-ischemic, or hypertensive cardiomyopathy with systolic dysfunction (left ventricular ejection fraction (LVEF) ≤ 45%) | Epworth sleepiness scale (ESS) | No control for medications 81% of HF patients were on B-blockers |
HF patients at any severity of OSA had significantly lower mean ESS (AHI < 5: 7.1±0.4 vs 8.3±0.2 [p=.005]; AHI 5–14: 6.7±0.7 vs 9.2±0.3 [p<.001]; and AHI >=15:7.8±0.7 vs 9.8±0.4 [p=.01]), despite sleeping less than community controls (p<.001). |
| Dolliner et al 20132 | n = 26 HF patients with OSA (AHI ≥ 15 and >50% obstructive events) n = 88 HF patients without sleep-disordered breathing (SDB) |
Recently hospitalized for heart failure or for heart transplantation evaluation with LVEF ≤ 35% and brain natriuretic peptide > 200 pg/mL | ESS | No control for medications 90.3% of patients in this study were on Beta-blockers |
No statistically significant difference in ESS between patients with HF and OSA (ESS = 8) and patients with HF and no SDB (ESS = 6.5). |
| Herrscher et al (2014)3 | n = 62 HF patients with OSA (AHI > 5 and >50% obstructive events) n = 22 HF patients with no SDB (AHI ≤ 5) |
Clinically stable heart failure patients with fully titrated medications in NYHA class II-IV with preserved or unpreserved ejection fractions | ESS | No control for medications 96% of no SDB (AHI<5) patients were on Beta-blockers 94% of mild SDB (5≤AHI <15) were on Beta-blockers 85% of moderate/severe SDB (AHI≥15) were on Beta -blockers |
No statistically significant difference in ESS between HF patients with OSA (ESS = 5.9+/− 4.3) and HF patients without SDB (ESS = 5.0+/− 3.2). |
| Javaheri et al 1998 4 | n= 9 male HF patients with sleep apnea (AHI ≥ 15) n= 72 male HF patients without sleep apnea (AHI <15) |
Stable HF due to systolic dysfunction (LVEF fraction < 45%) | Non-ESS ^ symptom scoring system | No control for medications | No statistically significant difference in the percent of HF patients with and without sleep disordered breathing who reported excessive daytime sleepiness symptoms (24% vs.15%; p >.05). |
| Kaneko et al 2003 5 | N=24 patients with HF and severe OSA (AHI >20 with > 50% obstructive events) | HF due to ischemic or non-ischemic dilated cardiomyopathy for at least 6 months; LVEF<45% at rest | ESS | No control for medications | Patients with HF and severe OSA did not generally complain of excessive sleepiness (mean ESS between 5.7 ±.9 and 6.8 ±.7). |
| Rao et al 2006 7 | N=84 ambulatory HF patients; n = 64 with AHI <15 and n = 20 with AHI >15 | HF defined using the European Society of Cardiology criteria, i.e. symptoms of HF, objective evidence of LVEF and/or response to treatment directed towards HF. | ESS | No control for medications | No statistically significant difference in ESS scores between HF patients with and without sleep disordered breathing (mean ESS of 7.8 ± 4.7 versus 7.5 ±3.6; p = .87). |
| Redeker et al (2010)8 | n = 37 HF patients with OSA (apnea index ≥ 5, < 50% central apneas) n = 27 HF patients without SDB (apnea index < 5) |
Stable chronic HF patients from structured HF disease management programs. | ESS | No control for medications | No statistically significant difference in ESS score between HF patients with OSA (ESS = 7.9 +/− 4.5) and HF patients without SDB (ESS = 8.4+/− 5.4). |
| Wang et al 20099 | N = 195 HF patients: n = 103 with OSA (AHI >15 with >50% obstructive), n = 39 without SDB | HF defined as LVEF ≤45% | ESS | No control for medications | No statistically significant difference in ESS between HF patients with OSA and HF patients without sleep disordered breathing (N-SDB vs OSA: 6.7 +/− 0.6 vs 7.6 +/− 0.4, p = 0.105). |
| Yumino et al 2009 10 | n = 56 patients with HF and OSA (AHI >=15 with >=50% obstructive events) n = 117 patients with HF and mild or no sleep apnea (AHI < 25) |
Ischemic or no-ischemic dilated cardiomyopathy for >= 6 months, LVEF <=45% and NYHA Class II-IV after optimized medical therapy | ESS | β-blockers were controlled for using a stepwise variable selection in a generalized logistic model. β-blockers had no statistically significant association with sleepiness. | No statistically significant difference in ESS between HF patients with OSA (ESS = 7.5+/−3.5) and those with mild or no sleep apnea (ESS = 7.3+/− 4.0) |
Arzt M, Young T, Finn L, et al. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Archives of internal medicine. 2006;166(16):1716–1722.
Dolliner P, Brammen L, Graf S, et al. Portable recording for detecting sleep disorder breathing in patients under the care of a heart failure clinic. Clinical research in cardiology : official journal of the German Cardiac Society. 2013;102(7):535–542.
Herrscher TE, Akre H, Overland B, Sandvik L, Westheim AS. Clinical predictors of sleep apnoea in heart failure outpatients. International journal of clinical practice. 2014;68(6):725–730.
Javaheri S, Parker TJ, Liming JD, et al. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation. 1998;97(21):2154–2159.
Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. The New England journal of medicine. 2003;348(13):1233–1241.
Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P, Naughton MT. Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. American journal of respiratory and critical care medicine. 2004;169(3):361–366.
Rao A, Georgiadou P, Francis DP, et al. Sleep-disordered breathing in a general heart failure population: relationships to neurohumoral activation and subjective symptoms. J Sleep Res. 2006;15(1):81–88.
Redeker NS, Muench U, Zucker MJ, et al. Sleep disordered breathing, daytime symptoms, and functional performance in stable heart failure. Sleep. 2010;33(4):551–560.
Wang HQ, Chen G, Li J, et al. Subjective sleepiness in heart failure patients with sleep-related breathing disorder. Chinese medical journal. 2009;122(12):1375–1379.
Yumino D, Wang H, Floras JS, et al. Prevalence and physiological predictors of sleep apnea in patients with heart failure and systolic dysfunction. Journal of cardiac failure. 2009;15(4):279–285.
Luyster FS, Buysse DJ, Strollo PJ. Comorbid insomnia and obstructive sleep apnea: challenges for clinical practice and research. Journal of Clinical Sleep Medicine. 2010;6(02):196–204.
Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30(2):213–218.
Oldenburg O, Lamp B, Faber L, Teschler H, Horstkotte D, Töpfer V. Sleep‐disordered breathing in patients with symptomatic heart failure A contemporary study of prevalence in and characteristics of 700 patients. European journal of heart failure. 2007;9(3):251–257.
In the majority of the reviewed studies that used the Epworth sleepiness scale, scores for patients with HF and OSA were generally within the normal range of scores for healthy adults (zero to ten) (Dolliner et al., 2013, Herrscher et al., 2014, Javaheri et al., 2011, Redeker et al., 2010, Wang et al., 2009, Yumino et al., 2009, Kaneko et al., 2003, Mansfield et al., 2004). Additionally, HF patients with and without OSA report normal levels of sleepiness symptoms. These findings suggest that there are differences between both diseases that decreases HF/OSA patients’ ability to experience the symptoms of excessive daytime sleepiness that are often experienced by OSA patients without HF.
Weaknesses of the existing epidemiologic studies include concerns about different measures of exposure and outcome, control of confounding variables, inclusion criteria, and power (Arzt et al., 2006). In the majority of these studies (Arzt et al., 2006, Kaneko et al., 2003, Rao et al., 2006, Mansfield et al., 2004, Wang et al., 2009), sleepiness was measured via the Epworth sleepiness scale (ESS). However, in the study by Javaheri et al, sleepiness was assessed by asking several questions regarding subjects’ tendencies to fall asleep in various situations (Javaheri et al., 1998); as this work preceded the other manuscripts cited, it was published prior to widespread use of the ESS (See Table 1). Thus varying methods are used for assessing subjective sleepiness, from the methods used in Javaheri et al. to the ESS (Riegel et al., 2013). Beyond concerns about the different measures of subjective sleepiness used, there is also the possibility that objective measures of sleepiness, such as the Psychomotor Vigilance Task (PVT), (Dinges and Powell, 1985) may be a more accurate reflection of sleepiness. PVT measures sustained attention as an indicator of sleepiness by counting the subject’s lapses in attention. Creber et al explored sleepiness in a chronic heart failure population in which, 64% of subjects had mild to severe sleep apnea and 72% reported poor sleep. Subjective measures of sleepiness, such as the ESS, Pittsburgh Sleep Quality Index, and Stanford Sleepiness Scale, did not demonstrate sleepiness, but importantly the objective PVT measure showed evidence of the effects of poor sleep by demonstrating poor behavioral alertness (Creber et al., 2015). Additionally, the ESS may not be an accurate predictor of sleep-disordered breathing even in patients without heart failure, as some studies have shown it does not have a statistically significant correlation with measures of sleep apnea severity or mean sleep latency (MSL), which is considered the gold standard for excessive daytime sleepiness (Chervin and Aldrich, 1999, Abrishami et al., 2010, Benbadis et al., 1999). Other studies suggest that the ESS is the most sensitive and specific test when it comes to distinguishing between narcolepsy and normal EDS (Johns, 2000). A review of several studies found that the multiple sleep latency test (MSLT), previously considered the gold standard for measuring EDS, was the least discriminating test of daytime sleepiness, compared to the maintenance of wakefulness test (MWT) and the ESS, which was considered the most discriminating (Johns, 2000).
Furthermore, current studies use inconsistent inclusion criteria, with some including central sleep apnea and others including only obstructive sleep apnea, and inconsistently controlling for confounding variables. Even in studies that separated patients with OSA and CSA, these conditions can coexist in the same patient, making it challenging to correctly classify patients’ disease. All studies had small sample sizes, which limited statistical power to confirm hypotheses. In spite of these limitations, the studies consistently found that HF patients generally did not complain of excessive daytime sleepiness. This suggests that there are mechanisms specific to heart failure that reduce disrupted sleepiness perception. As the HF patients’ poor PVT results demonstrated, even when these patients did not perceive their sleepiness, their behavioral alertness was negatively impacted.
Sympathetic Activation as a Mechanism for the Lack of Self-Reported Sleepiness in Heart Failure and OSA
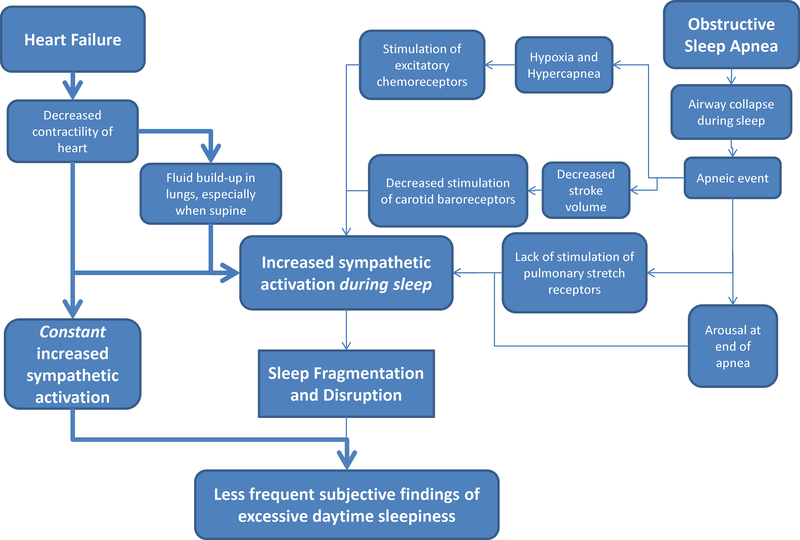
Mechanistically, it is hypothesized that the lack of symptomatic sleepiness in patients with heart failure and OSA may be due to an increase in sympathetic activity, which increases arousal and wakefulness and decreases daytime sleepiness symptoms (See Figure 1). The proposed mechanism is similar to patients with atrial fibrillation, with patients not reporting excessive daytime sleepiness (Albuquerque et al., 2012). The plausibility of this explanation will be discussed in the following sections.
Figure 1: Proposed mechanism of excessive daytime sleepiness in patients with heart failure and obstructive sleep apnea.
In obstructive sleep apnea (OSA) and heart failure (HF), multiple events during sleep, including hypoxia, decreased stroke volume, and fluid build-up in the lungs, lead to increased sympathetic activation, which results in sleep disruption and poor sleep quality. However, the effects of poor sleep, which usually lead to symptoms of excessive daytime sleepiness, are counteracted by the constant increase in sympathetic activation that occurs in heart failure. This may explain the lack of reports of excessive daytime sleepiness in patients with both HF and OSA.
Sympathetic Activation in Heart Failure
The most prominent neurohumoral mechanism at play in individuals with HF is the adrenergic, or sympathetic nervous system (SNS), whose activity and outflow are significantly elevated in HF. When the SNS is activated, two catecholamines, norepinephrine and epinephrine, increase the rate and force of contraction of the heart and increase blood pressure. Under normal conditions when the heart is functioning properly, these effects work as a compensatory mechanism to maintain cardiac function (Lymperopoulos et al., 2013). However, in patients with HF, sympathetic outflow to the heart, kidney, and skeletal muscle is chronically increased as a reflex response to the hemodynamic disturbances of HF and myocardial ischemia (Floras, 2009, Azevedo et al., 2001). With continued cardiac insult, the SNS’s ability to compensate for the decreased contractile function of the failing heart eventually is overwhelmed, and the heart progresses into chronic decompensated HF. When this occurs, the hyperactive SNS continues to fire and stimulate the heart to work at a higher level than it can handle. This response confers significant toxicity to the failing heart and increases morbidity and mortality (Lymperopoulos et al., 2013).
In addition to increased sympathetic activation, sleep disruption is another common consequence of HF. The causes of poor sleep in this population include gender, age, pathophysiology of HF, medications, comorbid health problems, such as pain and depression, and primary sleep disorders, such as obstructive sleep apnea (Redeker NS). The pathophysiology of HF causes poor sleep due to fluid build-up in the lungs, which leads to patients waking with difficulty breathing after a period in the supine position. This sleep disruption leads to sympathoexcitation. Because the arousal system has sympathetic inputs (22), this excitation may carry over into wakefulness and establish a state of heightened arousal (Spaak et al., 2005, Somers et al., 1995). In rats, this sympathetic input has been shown to occur through the norepinephrine nuclei in the locus coeruleus, which are associated with arousal and vigilance (16).
As a result of this increased sympathetic activation both from the failing heart and the resulting fluid accumulation on the lungs, patients with HF sleep fewer hours than those without HF and suffer from interrupted sleep more frequently than individuals without HF (Kaneko et al., 2003). This type of interrupted sleep, which is characteristic of systolic dysfunction, (Arzt et al., 2006) increases the integrated 24-hour sympathetic burden upon the failing heart and circulation (Floras, 2009), which can impact patients’ morbidity and mortality.
Sympathetic Activation in OSA
As in patients with HF, increased SNS activation also occurs in patients with OSA, thus exacerbating the already highly activated sympathetic nervous system. During non-rapid eye movement (NREM) sleep, daytime muscle sympathetic nervous activation (MSNA), as measured by microneurography, and heart rate usually fall (Somers et al., 1993), while vagal activity and arterial baroreflex control of heart rate rise (Van de Borne et al., 1994, Smyth et al., 1969). However, during episodes of apnea in OSA, the tonic inhibition during NREM is interrupted by an increase in sympathetic nerve discharge to skeletal muscle and other vascular beds. This sympathetic activation is triggered by a combination of the following events: the lack of reflex inhibition of MSNA by pulmonary stretch receptors during apneic events (Narkiewicz et al., 1998b), stimulation of excitatory chemoreceptors by hypoxia and hypercapnia (Narkiewicz et al., 1998b, Kasai and Bradley, 2011)), unloading of carotid baroreceptors caused by acute reductions in stroke volume (Bradley et al., 2003), and arousal from sleep at the end of apnea (Somers et al., 1995, Horner et al., 1995).
Sympathetic activation in both HF and OSA
In exploring sympathetic activation in both diseases, micrographic recordings have documented significantly elevated MSNA firing rates in individuals with HF or OSA, when studied while awake and at rest, compared to healthy control subjects, (Floras, 2009, Kasai and Bradley, 2011, Narkiewicz et al., 1998b). This is important because subjective daytime sleepiness assessed by the ESS has been found to decrease with increased sympathetic activity, as quantified by daytime MSNA burst incidence and frequency (Taranto Montemurro et al., 2012). Furthermore, when HF and OSA exist simultaneously, MSNA has been found to be significantly greater during wakefulness than in patients who have HF without OSA (Spaak et al., 2005). This shows the combined effect that both conditions have on increasing sympathetic activation. Although the findings in the epidemiologic studies discussed above did not support this result by finding that patients with both conditions had even lower rates of excessive daytime sleepiness (Javaheri et al., 1998, Rao et al., 2006), this may be due to the increase in MSNA in patients with both diseases being too small to translate into a detectable symptomatic decrease in subjective sleepiness. It is also possible that HF patients’ baseline levels of sleepiness are low enough that measures of sleepiness are not able to detect a decrease in sleepiness symptoms between HF patients with and without OSA. The role of sympathetic hyperactivation in sleep apnea and sleepiness has been assessed by Donadio and colleagues (Donadio et al., 2007). As OSAS is associated with higher resting MSNA (Carlson et al., 1993, Narkiewicz et al., 1998a, Narkiewicz et al., 1998b), it is possible that the sleepiest patients have the highest MSNA levels as suggested by Donadio and colleagues (Donadio et al., 2007). In summary, increased SNS activation in patients with both HF and OSA may counteract the effects of the poor sleep commonly found in patients with HF or OSA, leading to an insensitivity to sleepiness.
Treatment of OSA in HF: Impact on Cardiovascular Function
Treatment with continuous positive airway pressure (CPAP) is the most studied treatment in OSA, with or without comorbid HF, and has the most evidence of benefit (Redeker NS). In patients with normal cardiac function, randomized trials looking at OSA and excessive daytime sleepiness have shown that CPAP decreases apneic events, improves sleep quality, reduces daytime sleepiness, improves left ventricular function, and lowers nocturnal and daytime blood pressure (Engleman et al., 1994, Pepperell et al., 2002).
Although patients with both HF and OSA often do not experience sleepiness symptoms, they have been found to benefit from CPAP treatment in other ways. In patients with HF and OSA, CPAP was found to decrease the excess MSNA experienced by these patients to the level experienced by patients with only one condition (Usui et al., 2005), demonstrating that treatment successfully decreases sympathetic activation. CPAP was also found to increase heart rate variability during morning wakefulness, indicating improved vagal modulation of heart rate, which may improve prognosis (Gilman et al., 2008). This evidence of physiologic benefit is also supported by studies looking at more clinical outcome measures. For example, CPAP in patients with HF and OSA was found to result in beneficial decreases in blood pressure and heart rate (Kaneko et al., 2003), as well as improvements in cardiovascular function, such as increased left ventricular ejection fraction (LVEF) (Kaneko et al., 2003, Mansfield et al., 2004). These findings contradict the conclusions of the Barbe et al randomized trial, which included patients with severe OSA (AHI>30) who did not experience excessive daytime sleepiness. This trial found that patients randomized to CPAP did not derive any symptomatic, neurocognitive, or cardiovascular benefits from the treatment and concluded that in patients with OSA and no sleepiness symptoms, CPAP should not be recommended (Barbe et al., 2001, Damy et al., 2012). Finally, CPAP has been shown to decrease morbidity and mortality in patients with HF and OSA. A study by Kasai et al demonstrated significantly decreased rates of hospitalization or death in patients with HF and OSA treated with CPAP compared to patients with these conditions not treated with CPAP (HR 2.03, 95% CI 1.07–3.68, P =0.03) (Kasai et al., 2008). Two other studies by Damy et al and Javaheri et al also found similar results with sleep apnea treatment, consisting mostly of CPAP, in patients with HF and SDB. In the study by Javaheri et al, treatment decreased mortality (p = 0.009) (Javaheri et al., 2011), while Damy et al discovered treatment decreased rates of mortality, heart transplant, and assist device implantation (p = 0.017) (Damy et al., 2012). However, in spite of clear health benefits to HF patients, with fewer symptomatic benefits, the issue of whether patients comply with treatment becomes a major concern. Although anecdotal reports indicate that 80% of HF patients comply with long-term mask therapy if they “are aware of the rationale for treatment,” further research needs to be done that directly compares CPAP adherence in HF and non-HF populations (Pearse and Cowie, 2016).
There is also the assumption that treating OSA in a person with heart failure is mandatory, or at least advisable. It is important to note that an RCT assessing the treatment of OSA with adaptive servo ventilation (ASV) in patients with heart failure and reduced ejection fraction in reducing mortality is currently underway (Lyons et al., 2017) to explore treatment options further. ADVENT-HF is a randomized, open-label trial with blinded assessment designed to determine the effects of treating SDB with ASV on morbidity and mortality in patients with HFrEF (LVEF ≤45%) and SDB (apnoea-hypopnoea index ≥15) during a maximum follow-up time of 5 years (Lyons et al., 2017). SERVE- HF studied the effects of ASV in patients who had HFrEF and predominantly central sleep apnea (Cowie et al., 2015) and showed there is an equally strong association with poor outcome, an apparent lack of daytime sleepiness, and a strong suggestion of harm from treating the sleep apnea with pressure therapy (Cowie et al., 2015). The study randomly assigned n=1325 patients with a LVEF of <45%, an apnea–hypopnea index (AHI) of 15 ≥ per hour, and a predominance of central events to receive guideline-based medical treatment with ASV or solely guideline-based medical treatment (control) (Cowie et al., 2015). The primary end point in the time-to-event analysis was the first event of death, lifesaving cardiovascular intervention, or unplanned hospitalization for worsening heart failure (Cowie et al., 2015). ASV had no significant effect, although all-cause and cardiovascular mortality were both increased with this therapy (Cowie et al., 2015).
Conclusion and Future Implications
Clinicians should be aware that there is excess sympathetic activation in patients with heart failure, and as a result, subjective sleepiness is not a good indicator of whether or not patients with HF have OSA. If a patient with HF denies feeling sleepy, they should still be evaluated for other symptoms of OSA, such as insomnia, snoring, witnessed apneas, or morning headaches, especially if they have other risk factors, such as obesity. If the patient screens positive for these symptoms or risk factors, clinicians should consider ordering an overnight sleep study to screen for OSA in this high-risk group of patients. This maximizes the likelihood that their OSA is detected and treated. Thus, if there is clinical suspicion of obstructive sleep apnea, a sleep study should be initiated in heart failure patients even without sleepiness symptoms.
It will be important to research further the effect of CPAP treatment in reducing blood pressure and sympathetic activation to improve cardiovascular function. A prior RCT found that treating OSA patients with CPAP who had CVD was not associated with a reduction in cardiovascular events (McEvoy et al., 2016). As there has been suggestion of harm with treatment of ASV seen in SERVE- HF which studied the effects of ASV in patients who had HFrEF and predominantly central sleep apnea (Cowie et al., 2015). SERVE-HF found, although not significantly, that all-cause mortality and cardiovascular mortality were higher among the group receiving ASV (Cowie et al., 2015).
Although the reviewed studies provide evidence of a relationship between decreased sleepiness symptoms and the combination of HF and OSA, further investigation is needed to understand the mechanism. The current epidemiologic studies have methodologic flaws and inconsistencies: they have small samples, do not consistently control for fatigue, use various measures of sleepiness and heart failure definitions, do not compare sleepy (ESS>10) versus non-sleepy groups in the main analyses, do not consider insomnia which may worsen symptoms of heart failure such as fatigue and low energy (Skotzko, 2009), and do not adequately control for potential confounders. Studies also do not consistently explore beta blockers, which are commonly used in heart failure and contribute to fatigue (Fotino et al., 2013, Kishi et al., 1977) (See Table 1). Gender differences are also not consistently controlled for, which may contribute to varying degrees of fatigue in OSA (Quintana-Gallego et al., 2004, Chotinaiwattarakul et al., 2009, Lee et al., 2014) and HF patients (Ekman and Ehrenberg, 2002).
Future studies should address these issues by more consistently controlling for medications linked to sleepiness, insomnia, gender differences, fatigue, and measuring sleepiness via established subjective and objective measures. Studies should also consider comparing sleepy versus non-sleepy groups and controlling for sleep measures, such as sleep quality and total sleep time. We have highlighted a potential pathway for the lack of sleepiness in HF through the failing heart’s activation of the sympathetic nervous system, which may suppress daytime sleepiness by stimulating alertness and arousal. It will be important to consider possible unidentified risk factors that contribute to the lack of subjective sleepiness in HF patients in order to optimize the ability to diagnose and treat these patients.
Practice Points.
Patients with heart failure and obstructive sleep apnea generally do not report excessive daytime sleepiness.
A possible mechanism explaining this phenomenon is that patients with heart failure and obstructive sleep apnea do not perceive sleepiness as a result of the constant sympathetic activation that occurs with heart failure.
In heart failure patients with no symptomatic sleepiness, continuous positive airway pressure treatment of obstructive sleep apnea has beneficial effects including decreased sympathetic activation, improved cardiovascular function, and decreased risk of functional compromise and possibly even death.
If there is clinical suspicion of obstructive sleep apnea, a sleep study should be initiated in heart failure patients without sleepiness symptoms.
Research Agenda.
Future epidemiologic studies investigating sleepiness in patients with heart failure and obstructive sleep apnea should improve on the existing literature by measuring sleepiness via established subjective and objective measures, controlling for known confounders, and utilizing adequate sample sizes.
Further mechanistic studies are needed to explore the role of the sympathetic nervous system on sleepiness symptoms in obstructive sleep apnea and heart failure.
Acknowledgments
This work is supported by the National Institutes of Health K99NR014675-01/ R00NR014675-03 (Pak) “Mechanisms of Sleepiness Symptoms in Obstructive Sleep Apnea and Cardiovascular Disease.”
Abbreviations
- AHI
apnea hypopnea index
- CPAP
continuous positive airway pressure
- EDS
excessive daytime sleepiness
- ESS
Epworth sleepiness scale
- HF
heart failure
- HR
heart rate
- LVEF
left ventricular ejection fraction
- MSNA
muscle sympathetic nervous activation
- NREM
non-rapid eye movement
- OSA
obstructive sleep apnea
- SDB
sleep disordered breathing
- SNS
sympathetic nervous system
Footnotes
Conflicts of interest: Authors declare no conflicts of interest.
References
- Abrishami A, Khajehdehi A and Chung F A systematic review of screening questionnaires for obstructive sleep apnea. Canadian Journal of Anesthesia/Journal canadien d’anesthésie, 2010, 57: 423–38. [DOI] [PubMed] [Google Scholar]
- Albuquerque FN, Calvin AD, Sert Kuniyoshi FH et al. Sleep-disordered breathing and excessive daytime sleepiness in patients with atrial fibrillation. Chest, 2012, 141: 967–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arzt M, Woehrle H, Oldenburg O et al. Prevalence and Predictors of Sleep-Disordered Breathing in Patients With Stable Chronic Heart Failure: The SchlaHF Registry. JACC: Heart Failure, 2016, 4: 116–25. [DOI] [PubMed] [Google Scholar]
- Arzt M, Young T, Finn L et al. Sleepiness and sleep in patients with both systolic heart failure and obstructive sleep apnea. Archives of internal medicine, 2006, 166: 1716–22. [DOI] [PubMed] [Google Scholar]
- Azevedo ER, Kubo T, Mak S et al. Nonselective versus selective beta-adrenergic receptor blockade in congestive heart failure: differential effects on sympathetic activity. Circulation, 2001, 104: 2194–9. [DOI] [PubMed] [Google Scholar]
- Barbe F, Mayoralas LR, Duran J et al. Treatment with continuous positive airway pressure is not effective in patients with sleep apnea but no daytime sleepiness. a randomized, controlled trial. Annals of Internal Medicine, 2001, 134: 1015–23. [DOI] [PubMed] [Google Scholar]
- Benbadis SR, Mascha E, Perry MC, Wolgamuth BR, Smolley LA and Dinner DS Association between the Epworth sleepiness scale and the multiple sleep latency test in a clinical population. Annals of internal medicine, 1999, 130: 289–92. [DOI] [PubMed] [Google Scholar]
- Borlaug BA and Paulus WJ Heart failure with preserved ejection fraction: pathophysiology, diagnosis, and treatment. European heart journal, 2011, 32: 670–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley TD, Tkacova R, Hall MJ, Ando S and Floras JS Augmented sympathetic neural response to simulated obstructive apnoea in human heart failure. Clinical science, 2003, 104: 231–8. [DOI] [PubMed] [Google Scholar]
- Carlson JT, Hedner J, Elam M, Ejnell H, Sellgren J and Wallin BG Augmented resting sympathetic activity in awake patients with obstructive sleep apnea. Chest, 1993, 103: 1763–8. [DOI] [PubMed] [Google Scholar]
- Chervin RD and Aldrich MS The Epworth Sleepiness Scale may not reflect objective measures of sleepiness or sleep apnea. Neurology, 1999, 52: 125. [DOI] [PubMed] [Google Scholar]
- Chotinaiwattarakul W, O’brien LM, Fan L and Chervin RD Fatigue, Tiredness, and Lack of Energy Improve with Treatment for OSA. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine, 2009, 5: 222–27. [PMC free article] [PubMed] [Google Scholar]
- Cowie MR, Woehrle H, Wegscheider K et al. Adaptive Servo-Ventilation for Central Sleep Apnea in Systolic Heart Failure. New England Journal of Medicine, 2015, 373: 1095–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creber RM, Pak VM, Varrasse M, Dinges DF, Wald J and Riegel B Determinants of Behavioral Alertness in Adults with Heart Failure. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damy T, Margarit L, Noroc A et al. Prognostic impact of sleep-disordered breathing and its treatment with nocturnal ventilation for chronic heart failure. European journal of heart failure, 2012, 14: 1009–19. [DOI] [PubMed] [Google Scholar]
- Dinges DF and Powell J Microcomputer analyses of performance on a portable, simple visual RT task during sustained operations. Beh Res Meth, Inst & Comp, 1985, 17: 652–55. [Google Scholar]
- Dolliner P, Brammen L, Graf S et al. Portable recording for detecting sleep disorder breathing in patients under the care of a heart failure clinic. Clinical research in cardiology : official journal of the German Cardiac Society, 2013, 102: 535–42. [DOI] [PubMed] [Google Scholar]
- Donadio V, Liguori R, Vetrugno R et al. Daytime sympathetic hyperactivity in OSAS is related to excessive daytime sleepiness. Journal of Sleep Research, 2007, 16: 327–32. [DOI] [PubMed] [Google Scholar]
- Ekman I and Ehrenberg A Fatigue in Chronic Heart Failure – Does Gender Make a Difference? European Journal of Cardiovascular Nursing, 2002, 1: 77–82. [DOI] [PubMed] [Google Scholar]
- Engleman HM, Martin SE, Deary IJ and Douglas NJ Effect of continuous positive airway pressure treatment on daytime function in sleep apnoea/hypopnoea syndrome. Lancet, 1994, 343: 572–5. [DOI] [PubMed] [Google Scholar]
- Epstein LJ, Kristo D, Strollo PJ Jr. et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. Journal of clinical sleep medicine : JCSM : official publication of the American Academy of Sleep Medicine, 2009, 5: 263–76. [PMC free article] [PubMed] [Google Scholar]
- Floras JS Sympathetic nervous system activation in human heart failure: clinical implications of an updated model. Journal of the American College of Cardiology, 2009, 54: 375–85. [DOI] [PubMed] [Google Scholar]
- Fotino AD, Thompson-Paul AM and Bazzano LA Effect of coenzyme Q(1)(0) supplementation on heart failure: a meta-analysis. The American journal of clinical nutrition, 2013, 97: 268–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman MP, Floras JS, Usui K, Kaneko Y, Leung RS and Bradley TD Continuous positive airway pressure increases heart rate variability in heart failure patients with obstructive sleep apnoea. Clinical science, 2008, 114: 243–9. [DOI] [PubMed] [Google Scholar]
- Herrscher TE, Akre H, Overland B, Sandvik L and Westheim AS Clinical predictors of sleep apnoea in heart failure outpatients. International journal of clinical practice, 2014, 68: 725–30. [DOI] [PubMed] [Google Scholar]
- Horner RL, Brooks D, Kozar LF, Tse S and Phillipson EA Immediate effects of arousal from sleep on cardiac autonomic outflow in the absence of breathing in dogs. Journal of applied physiology, 1995, 79: 151–62. [DOI] [PubMed] [Google Scholar]
- Hublin C, Kaprio J, Partinen M, Heikkilä K and Koskenvuo M Daytime sleepiness in an adult, Finnish population. Journal of Internal Medicine, 1996, 239: 417–23. [DOI] [PubMed] [Google Scholar]
- Javaheri S, Caref EB, Chen E, Tong KB and Abraham WT Sleep apnea testing and outcomes in a large cohort of Medicare beneficiaries with newly diagnosed heart failure. American journal of respiratory and critical care medicine, 2011, 183: 539–46. [DOI] [PubMed] [Google Scholar]
- Javaheri S, Parker TJ, Liming JD et al. Sleep apnea in 81 ambulatory male patients with stable heart failure. Types and their prevalences, consequences, and presentations. Circulation, 1998, 97: 2154–9. [DOI] [PubMed] [Google Scholar]
- Johns MW A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep, 1991, 14: 540–45. [DOI] [PubMed] [Google Scholar]
- Johns MW Sensitivity and specificity of the multiple sleep latency test (MSLT), the maintenance of wakefulness test and the Epworth sleepiness scale: Failure of the MSLT as a gold standard. Journal of Sleep Research, 2000, 9: 5–11. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Floras JS, Usui K et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. The New England journal of medicine, 2003, 348: 1233–41. [DOI] [PubMed] [Google Scholar]
- Kapur V, Strohl KP, Redline S, Iber C, O’connor G and Nieto J Underdiagnosis of sleep apnea syndrome in U.S. communities. Sleep & breathing = Schlaf & Atmung, 2002, 6: 49–54. [DOI] [PubMed] [Google Scholar]
- Kasai T and Bradley TD Obstructive sleep apnea and heart failure: pathophysiologic and therapeutic implications. Journal of the American College of Cardiology, 2011, 57: 119–27. [DOI] [PubMed] [Google Scholar]
- Kasai T, Narui K, Dohi T et al. Prognosis of patients with heart failure and obstructive sleep apnea treated with continuous positive airway pressure. Chest, 2008, 133: 690–6. [DOI] [PubMed] [Google Scholar]
- Kishi T, Watanabe T and Folkers K Bioenergetics in clinical medicine XV. Inhibition of coenzyme Q10-enzymes by clinically used adrenergic blockers of beta-receptors. Research communications in chemical pathology and pharmacology, 1977, 17: 157–64. [PubMed] [Google Scholar]
- Lee M-H, Lee S-A, Lee G-H et al. Gender differences in the effect of comorbid insomnia symptom on depression, anxiety, fatigue, and daytime sleepiness in patients with obstructive sleep apnea. Sleep and Breathing, 2014, 18: 111–17. [DOI] [PubMed] [Google Scholar]
- Lymperopoulos A, Rengo G and Koch WJ Adrenergic nervous system in heart failure: pathophysiology and therapy. Circulation research, 2013, 113: 739–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons OD, Floras JS, Logan AG et al. Design of the effect of adaptive servo-ventilation on survival and cardiovascular hospital admissions in patients with heart failure and sleep apnoea: the ADVENT-HF trial. European journal of heart failure, 2017, 19: 579–87. [DOI] [PubMed] [Google Scholar]
- Mansfield DR, Gollogly NC, Kaye DM, Richardson M, Bergin P and Naughton MT Controlled trial of continuous positive airway pressure in obstructive sleep apnea and heart failure. American journal of respiratory and critical care medicine, 2004, 169: 361–6. [DOI] [PubMed] [Google Scholar]
- Mcevoy RD, Antic NA, Heeley E et al. CPAP for Prevention of Cardiovascular Events in Obstructive Sleep Apnea. The New England journal of medicine, 2016, 375: 919–31. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, Pesek CA, Kato M, Phillips BG, Davison DE and Somers VK Baroreflex control of sympathetic nerve activity and heart rate in obstructive sleep apnea. Hypertension, 1998a, 32: 1039–43. [DOI] [PubMed] [Google Scholar]
- Narkiewicz K, Van De Borne PJ, Cooley RL, Dyken ME and Somers VK Sympathetic activity in obese subjects with and without obstructive sleep apnea. Circulation, 1998b, 98: 772–6. [DOI] [PubMed] [Google Scholar]
- Oldenburg O, Wellmann B, Buchholz A et al. Nocturnal hypoxaemia is associated with increased mortality in stable heart failure patients. European heart journal, 2016, 37: 1695–703. [DOI] [PubMed] [Google Scholar]
- Pearse SG and Cowie MR Sleep-disordered breathing in heart failure. European journal of heart failure, 2016 [DOI] [PubMed] [Google Scholar]
- Pepperell JC, Ramdassingh-Dow S, Crosthwaite N et al. Ambulatory blood pressure after therapeutic and subtherapeutic nasal continuous positive airway pressure for obstructive sleep apnoea: a randomised parallel trial. Lancet, 2002, 359: 204–10. [DOI] [PubMed] [Google Scholar]
- Quintana-Gallego E, Carmona-Bernal C, Capote F et al. Gender differences in obstructive sleep apnea syndrome: a clinical study of 1166 patients. Respiratory Medicine, 2004, 98: 984–89. [DOI] [PubMed] [Google Scholar]
- Rao A, Georgiadou P, Francis DP et al. Sleep-disordered breathing in a general heart failure population: relationships to neurohumoral activation and subjective symptoms. J Sleep Res, 2006, 15: 81–8. [DOI] [PubMed] [Google Scholar]
- Redeker Ns BK Sleep Disorders in Patients with Heart Failure. In), American Association of Heart Failure Nurses. [Google Scholar]
- Redeker NS, Muench U, Zucker MJ et al. Sleep disordered breathing, daytime symptoms, and functional performance in stable heart failure. Sleep, 2010, 33: 551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riegel B, Hanlon AL, Zhang X et al. What is the best measure of daytime sleepiness in adults with heart failure? Journal of the American Association of Nurse Practitioners, 2013, 25: 272–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin DD, Fitzgerald F, Parker JD, Newton G, Floras JS and Bradley TD Risk factors for central and obstructive sleep apnea in 450 men and women with congestive heart failure. American journal of respiratory and critical care medicine, 1999, 160: 1101–6. [DOI] [PubMed] [Google Scholar]
- Skotzko CE Symptom perception in CHF: (why mind matters). Heart Failure Reviews, 2009, 14: 29–34. [DOI] [PubMed] [Google Scholar]
- Smyth HS, Sleight P and Pickering GW Reflex regulation of arterial pressure during sleep in man. A quantitative method of assessing baroreflex sensitivity. Circulation research, 1969, 24: 109–21. [DOI] [PubMed] [Google Scholar]
- Somers VK, Dyken ME, Clary MP and Abboud FM Sympathetic neural mechanisms in obstructive sleep apnea. The Journal of clinical investigation, 1995, 96: 1897–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somers VK, Dyken ME, Mark AL and Abboud FM Sympathetic-nerve activity during sleep in normal subjects. The New England journal of medicine, 1993, 328: 303–7. [DOI] [PubMed] [Google Scholar]
- Somers VK, White DP, Amin R et al. Sleep apnea and cardiovascular disease: an American Heart Association/american College Of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council On Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health). Circulation, 2008, 118: 1080–111. [DOI] [PubMed] [Google Scholar]
- Spaak J, Egri ZJ, Kubo T et al. Muscle sympathetic nerve activity during wakefulness in heart failure patients with and without sleep apnea. Hypertension, 2005, 46: 1327–32. [DOI] [PubMed] [Google Scholar]
- Taranto Montemurro L, Floras JS, Millar PJ et al. Inverse relationship of subjective daytime sleepiness to sympathetic activity in patients with heart failure and obstructive sleep apnea. Chest, 2012, 142: 1222–8. [DOI] [PubMed] [Google Scholar]
- Usui K, Bradley TD, Spaak J et al. Inhibition of awake sympathetic nerve activity of heart failure patients with obstructive sleep apnea by nocturnal continuous positive airway pressure. Journal of the American College of Cardiology, 2005, 45: 2008–11. [DOI] [PubMed] [Google Scholar]
- Van De Borne P, Nguyen H, Biston P, Linkowski P and Degaute JP Effects of wake and sleep stages on the 24-h autonomic control of blood pressure and heart rate in recumbent men. The American journal of physiology, 1994, 266: H548–54. [DOI] [PubMed] [Google Scholar]
- Wang HQ, Chen G, Li J et al. Subjective sleepiness in heart failure patients with sleep-related breathing disorder. Chinese medical journal, 2009, 122: 1375–9. [PubMed] [Google Scholar]
- Young T, Palta M, Dempsey J, Skatrud J, Weber S and Badr S The occurrence of sleep-disordered breathing among middle-aged adults. N Eng J Med, 1993, 328: 1230–35. [DOI] [PubMed] [Google Scholar]
- Yumino D, Wang H, Floras JS et al. Prevalence and physiological predictors of sleep apnea in patients with heart failure and systolic dysfunction. Journal of cardiac failure, 2009, 15: 279–85. [DOI] [PubMed] [Google Scholar]