Abstract
Objective:
To examine the associations between vaginal estrogen use and multiple health outcomes including cardiovascular disease (total myocardial infarction, stroke, and pulmonary embolism/deep vein thrombosis), cancer (total invasive, breast, endometrial, ovarian, and colorectal cancer), and hip fracture.
Methods:
We included postmenopausal women from the Nurses’ Health Study (1982–2012) who were not current users of systemic hormone therapy at the start of the study or during follow-up. Vaginal estrogen use was self-reported on the biennial questionnaires. Information on incident health outcomes were self-reported and confirmed by medical records. We used Cox proportional hazards regression to model the multivariable adjusted hazard ratios and the 95% confidence intervals for vaginal estrogen use and multiple health outcomes.
Results:
Over 18 years of follow-up, after adjusting for covariates, risks for cardiovascular disease, cancer, and hip fracture were not different between users and non-users of vaginal estrogen. No statistically significant increase in risk of any health outcome was observed with vaginal estrogen use. In sensitivity analyses, when we examined associations by hysterectomy status, the stratified results were generally similar to those for the total cohort.
Conclusions:
Vaginal estrogen use was not associated with a higher risk of cardiovascular disease or cancer. Our findings lend support to the safety of vaginal estrogen use, a highly effective treatment for genitourinary syndrome of menopause.
Keywords: vaginal estrogen, vaginal estradiol, chronic disease, cardiovascular disease, cancer, hormone therapy
INTRODUCTION
Genitourinary syndrome of menopause (GSM) is a chronic condition that is associated with a decrease in exposure of the urogenital tissues to estrogen.1 Vulvovaginal atrophy and atrophic vaginitis are components of GSM, which affects a substantial proportion of postmenopausal women with prevalence estimates ranging from as low as 25% to as high as 70%.2–4 GSM encompasses a constellation of signs and symptoms including genital symptoms of dryness, burning, and irritation; sexual symptoms of lack of lubrication, discomfort or dyspareunia; and urinary symptoms of urgency, dysuria, and recurrent urinary tract infections.5 Unlike vasomotor symptoms, symptoms of GSM do not resolve over time, are chronic, and can become progressively worse without treatment.6 They can significantly impair quality of life7, 8 and despite their high prevalence, they remain largely under diagnosed.6
Low-dose vaginal estrogen therapy is the preferred and most effective treatment for GSM and is recommended by the North American Menopause Society, the American College of Obstetricians and Gynecologists, the Endocrine Society, and other professional societies.6,9,10 11Although non-hormonal options are available, a systematic review of evidence from randomized controlled trials and prospective comparative studies has shown that vaginal estrogen therapy was superior to non-hormonal therapies in improving symptoms in patients with two or more complaints.12 A meta-analysis of 58 comparative studies of postmenopausal women with GSM found that vaginal estrogen therapy correlated with better patient reports of symptom-relief compared to oral estrogen therapy.13 A more recent Cochrane Database Systematic Review found that all commercially available vaginal estrogens effectively relieve symptoms with no difference in efficacy between the various regimens (creams, tablets, rings).14 Although a recent 12-week multicenter randomized clinical trial showed that neither a vaginal tablet nor moisturizer provided additional benefit over a placebo gel in the relief of postmenopausal vulvovaginal symptoms,15 this was a short-duration trial with several limitations.16 Despite the availability of strong and generally consistent data to show its effectiveness, low-dose vaginal estrogen therapy remains underutilized owing to perceived risks associated with menopausal hormone therapy.17 In addition, the FDA-issued black-box warning on the low-dose vaginal estrogen package label discourages clinicians from prescribing the product and women from using prescribed therapy.18 However, these warnings stem from evidence generated by randomized clinical trials of systemic hormone therapy19 which utilized much higher doses of estrogen. Unlike oral estrogen therapy, vaginal estrogen is not subject to gastrointestinal conversion of estradiol (E2) to estrone and avoids the first-pass liver metabolism associated with increased hepatic synthesis of thrombotic and other factors. 18,20 Further, the substantial increases in blood hormone levels seen with systemic estrogen treatment are not observed in treatment with the recommended low-doses of vaginal estrogen where serum hormone concentrations remain within the postmenopausal range.21–26
Randomized clinical trial data on the effect of low-dose vaginal estrogen therapy on major chronic disease outcomes such as cardiovascular disease and cancer are lacking. Evidence from population-based cohort studies has been limited with one study reporting no association between vaginal estrogen use and risk of breast cancer and endometrial cancer,27 another reporting a higher risk of endometrial cancer,28 and a few others demonstrating a lower risk of coronary heart disease and stroke.27,29,30 Given the overall limited data on risks and benefits associated with long-term use of low-dose vaginal estrogen use among women not using systemic hormone therapy, we aimed to examine the prospective associations between vaginal estrogen use and chronic disease outcomes among postmenopausal women in the Nurses’ Health Study.
METHODS
Study Population
The Nurses’ Health Study (NHS) began in 1976 as a long- term prospective investigation of the health effects of various contraceptive methods in female registered nurses residing in 11 U.S. states. Every 2 years, participants returned a mailed validated questionnaire that obtained detailed and updated information on their lifestyle, behavioral, personal, and reproductive factors, medical history, health status, and a range of other exposures and covariates.31–34 A response rate of at least 90% has been achieved in most follow-up cycles.
Assessment of vaginal estrogen use
Beginning in 1982, use of vaginal estrogen was ascertained through self-report on the main questionnaire. We did not collect information on dose or specific type of vaginal estrogen regimen (cream, ring, tablet, or suppository). When information regarding vaginal estrogen use was missing, we carried forward information from the previous cycle.
Outcome ascertainment
In order to understand whether the risks and benefits associated with systemic hormone therapy also apply to vaginal estrogen use, we considered the following outcomes in our analyses: cancer outcomes (all cancer types and site-specific cancers including invasive breast, ovarian, endometrial, and colorectal cancer), cardiovascular outcomes (total myocardial infarction [MI], stroke, and pulmonary embolism/deep vein thrombosis), and hip fracture.
To confirm cases of MI, we used the World Health Organization criteria which include typical symptoms and either elevated enzymes or diagnostic electrocardiographic findings.35 Further, as specified by the European Society of Cardiology and the American College of Cardiology, elevated cardiac specific troponin are diagnostic of MI when accompanied by pain or EKG changes.36 We confirmed MI deaths if the autopsy report showed evidence of fresh infarction or thrombus or if there were EKG and enzyme changes characteristic of MI prior to death by medical records. We did not include sudden cardiac deaths without evidence of MI in our analyses. We classified stroke according to the National Survey of Stroke criteria37 which require evidence of a neurological deficit with sudden or rapid onset that persisted for more than 24 hours or until death. We excluded cerebrovascular pathology due to infection, trauma, or malignancy, and “silent” strokes discovered only by radiologic imaging. Pulmonary embolism cases were confirmed if a ventilation/perfusion lung scan was read by a radiologist as high probability for pulmonary embolism, or if there was a filling defect on contrast-enhanced computed tomography of the pulmonary vasculature or on catheter-based pulmonary angiography. We included both “idiopathic” (defined by the absence of recent surgery, major trauma, or active malignancy) and “non-idiopathic” cases (associated with recent surgery, trauma, or malignancy) in our analyses.38,39 Physician diagnosed deep vein thrombosis was identified from participants writing in this diagnosis on the biennial questionnaires on a blank line reserved for “other conditions”. For the current analysis, we included any report of pulmonary embolism or deep vein thrombosis that were confirmed by medical records or by additional evidence from the participant.
For all cancer types, we considered all pathologically confirmed and probable cases of invasive cancer (except non-melanoma skin cancer). For breast cancer, we included only confirmed cases with evidence of invasion (including microinvasion) on the pathology report. Cases of carcinoma in situ were not included in our analyses. Cases of ovarian and endometrial cancer were reported on the biennial questionnaires. For all reported cases, we requested medical records pertaining to the diagnosis. For cases where records were unavailable, we confirmed diagnoses through state cancer registries. For ovarian and endometrial cancer, incident cases were confirmed after review of pathology reports by a gynecologic pathologist.40 Incident cases of colorectal cancer were confirmed by a review of medical records by study physicians. Colorectal cancer and sub-sites were defined according to the International Classification of Diseases, Ninth Revision.41
Deaths were identified by reports from next-of-kin or from the U.S. postal service when a questionnaire or newsletter mailed to a participant is returned. Deaths were also identified through a search of the National Death Index. To identify the primary cause of death, attempts were made to contact the next-of-kin to request permission to obtain medical records. Information was also obtained from the National Death Index, from tumor registries, and from death certificates obtained from state vital statistics departments. Deaths were classified according to the International Classification of Diseases, Eighth Revision (ICD-8) as cardiovascular deaths (ICD-8 codes 390–458) or cancer deaths (ICD-8 codes 140–207). Follow-up for deaths was >98% complete.42,43
Assessment of covariates
In the biennial follow-up questionnaires, we updated information on age, weight, smoking status, physical activity, aspirin use, history of bilateral oophorectomy, personal history of chronic disease, and mammogram screening in the previous cycle. Height and age at first birth were determined in 1976. Information on race was obtained in 1992 and 2004. Parental history of MI was ascertained in 1976 and in 1984. Family history of cancer was first ascertained in 1976 and again in the years1982, 1992, 1996, 2004, and 2008. Parity was assessed from 1976 to1984 and again in 1996. Cumulative duration of systemic hormone therapy use and vaginal estrogen use was calculated for the years that women reported their use. Alcohol intake was measured every 4 years using the food frequency questionnaire.
Statistical analysis
Beginning in 1982 we included women on a rolling basis as they became postmenopausal. For the current analysis, we excluded current (but not past) users of systemic hormone therapy at the time of study enrollment. We also excluded women with previously diagnosed cancer (except non-melanoma skin cancer). For cardiovascular outcomes, we also excluded participants with self-reported cardiovascular disease. Person-time was calculated from the time a participant entered the analysis to the first diagnosis of an outcome, start of systemic hormone therapy use, loss to follow-up, death, or the cut-off date (June 2012) whichever came first.
For all outcomes, we used time-varying updates of vaginal estrogen use. We used Cox proportional hazards regression model to estimate the age- and multivariable-adjusted hazard ratios (HRs) for the association between vaginal estrogen use (current users versus non-users) and various clinical end points. The Cox proportional hazards regression models included age in years as the time scale, stratified by calendar time in 2-year intervals, and allowed for the possible interaction between calendar time and age in the baseline hazards to be accounted for non-parametrically. Covariates included in the multivariable adjusted models were selected based on apparent differences in study characteristics between users and non-users of vaginal estrogen and a prior knowledge of variables that could confound the association between hormone therapy use and chronic disease risk. In multivariable adjusted model 1, we adjusted for race, smoking status, alcohol intake, physical activity, BMI, aspirin use, age at menopause, bilateral oophorectomy status (except for ovarian cancer models), and past systemic hormone therapy use. Models with cardiovascular end-points and mortality end-points additionally adjusted for aspirin use. In multivariable adjusted model 2, we further adjusted for parental history of cancer. Models with cardiovascular events and mortality as the endpoint additionally adjusted for history of chronic disease including a history of type 2 diabetes, hypertension, hypercholesterolemia, and parental history of MI before age 60. Models with breast cancer as an outcome additionally adjusted model 2 for height, parity, age at first birth, BMI at age 18, and history of benign breast disease. Because women who had mammogram screening in the previous cycle were more likely to be diagnosed with invasive breast cancer,44 we also adjusted for this surveillance-related variable. To fully and completely account for any effect of systemic hormone therapy use on risk of invasive breast cancer, in sensitivity analyses for breast cancer models, we further excluded past systemic hormone therapy users at study enrollment and follow-up. For endometrial cancer models, we conducted two additional sensitivity analyses. First, because NHS women during early follow-up were more likely to use vaginal estrogen with much higher doses, we conducted a sensitivity analysis based on starting follow-up in 1992 (instead of 1982). Second, because systemic progestin is prescribed to protect the endometrium, in sensitivity analyses, for endometrial cancer models, we only censored women upon starting systemic estrogen therapy and we adjusted for past use of systemic estrogen plus progestin therapy and systemic progestin therapy. Because the risks associated with hormone therapy (estrogen alone or with progestin) differ by hysterectomy status, results for all outcomes are also presented by hysterectomy status (time-varying) in sensitivity analyses. All statistical tests were 2-sided and were conducted using SAS for UNIX (version 9.3, SAS Institute Inc., Cary, NC, USA).
RESULTS
Characteristics of participants at entry into analysis
In the overall analytic sample, users of vaginal estrogen had an overall favorable risk profile (Table 1). Compared to non-users, vaginal estrogen users were more likely to be never smokers, had a lower BMI, a marginally higher level of physical activity, and a lower prevalence of hypertension. However, these women were more likely to have had a bilateral oophorectomy, a hysterectomy, and a history of benign breast disease. They were also more likely to have a family history of cancer. Women using vaginal estrogen were more likely to be younger at first birth, more likely to have 2 or more children, and more likely to be past users of systemic hormone therapy. There were no appreciable differences in age at study entry, race, height, history of diabetes, parental history of MI, or aspirin use between users and non-users of vaginal estrogen. The average duration of vaginal estrogen use, over follow-up, was 35.7 months.
TABLE 1.
Characteristics of participants at entry into analysis by vaginal estrogen (VE) use in postmenopausal women
| Characteristica | Overall |
|
|---|---|---|
| No VE use | VE use | |
| n | 52901 | 896 |
| Ageb, y | 54.4 (3.9) | 54.8 (4.0) |
| White, % | 97 | 98 |
| Age at menopause, y | 49.6 (4.4) | 49.0 (4.8) |
| Age at first birth, y | 25.5 (12.4) | 24.1 (12.6) |
| Body mass index, kg/m2 | 25.9 (5.2) | 24.2 (4.1) |
| Height, inches | 64.3 (3.3) | 64.3 (2.3) |
| Smokingc | ||
| Never, % | 43 | 49 |
| Past, % | 31 | 36 |
| Current,% | 27 | 15 |
| Physical activity, MET-h/week | 14.9 (21.3) | 15.2 (17.4) |
| Alcohol intake, g/d | 6.3 (10.8) | 6.5 (9.7) |
| Bilateral oophorectomy, % | 11 | 16 |
| Hysterectomy, % | 22 | 32 |
| Parityc | ||
| Nulliparous, % | 6 | 10 |
| 1 child, % | 7 | 6 |
| 2–3 children, % | 52 | 57 |
| >3 children, % | 36 | 27 |
| History of past systemic hormone therapy use, % | 19 | 47 |
| Hypertension, % | 33 | 28 |
| Hypercholesterolemia, % | 23 | 24 |
| History of diabetes, % | 12 | 11 |
| History of benign breast disease, % | 34 | 45 |
| Parental history of early MI, % | 14 | 14 |
| Family history of cancer, % | 19 | 21 |
| Aspirin used, % | 15 | 16 |
MET, metabolic equivalent task; VE, vaginal estrogen
Values are means(SD) or percentages and are standardized to the age distribution of the study population.
Not age adjusted
Values for polytomous variables do not add up to a 100 due to rounding
At least 1 tablet per day
Risk for major health outcomes
In the overall sample, in the age adjusted model, compared to non-users, users of vaginal estrogen had a lower risk of total MI (HR 0.56, 95% confidence interval [CI] 0.36–0.87) and a marginally but non-significant lower risk of total stroke (HR 0.71, 95% CI 0.47–1.09). (Table 2) There were no significant differences in the age-adjusted risk of pulmonary embolism/deep vein thrombosis, total invasive cancer, invasive breast cancer, ovarian cancer, endometrial cancer, colorectal cancer, or hip fracture between users and non-users of vaginal estrogen. In the fully adjusted model, after accounting for differences in race, smoking status, alcohol intake, physical activity, BMI, aspirin use, age at menopause, bilateral oophorectomy, past systemic hormone therapy use, history of hypertension, hypercholesterolemia, type 2 diabetes, parental history of early MI, and family history of cancer, we found no statistically significant difference between users and non-users of vaginal estrogen in the risk of all major cardiovascular outcomes (including total MI, stroke, pulmonary embolism/deep vein thrombosis), cancer outcomes (including total invasive cancer, ovarian cancer, endometrial cancer, and colorectal cancer), or hip fracture. When we further adjusted breast cancer models for additional covariates such as height, parity, age at first birth, BMI at age 18, history of benign breast disease, and mammogram screening in the previous cycle, results remained null and non-significant (HR 1.07, 95% CI 0.78–1.47). When we further excluded past systemic hormone therapy users at entry and follow-up, the HR for invasive breast cancer among vaginal estrogen users remained unchanged (HR 1.07, 95% CI 0.77–1.47).
TABLE 2.
Hazard ratio (HR) and 95% confidence interval (CI) for health outcomes among postmenopausal users by vaginal estrogen (VE) use, 1982–2012
| Outcome | No VE | VE | |||||
|---|---|---|---|---|---|---|---|
| Cases | Person years |
Cases | Person years |
Age-adjusted model |
Multivariable adjusted model 1a |
Multivariable adjusted model 2b |
|
| Total MI | 1339 | 696454 | 20 | 17884 | 0.56 (0.36–0.87) | 0.71 (0.45–1.10) | 0.73 (0.47–1.13) |
| Stroke | 1188 | 699023 | 22 | 17972 | 0.71 (0.47–1.09) | 0.82 (0.54–1.25) | 0.85 (0.56–1.29) |
| PE/DVT | 524 | 648536 | 11 | 16502 | 0.88 (0.48–1.60) | 1.05 (0.58–1.92) | 1.06 (0.58–1.93) |
| All cancer types | 5444 | 670766 | 139 | 17534 | 0.97 (0.82–1.15) | 1.05 (0.89–1.25) | 1.05 (0.89–1.25) |
| Invasive breast cancer | 1570 | 510880 | 40 | 11826 | 1.06 (0.77–1.44) | 1.13 (0.82–1.55) | 1.07 (0.78–1.47) |
| Ovarian cancer c | 202 | 621565 | 6 | 15798 | 1.12 (0.50–2.54) | 1.17 (0.52–2.66) | 1.17 (0.52–2.65) |
| Endometrial cancer d | 344 | 540085 | 11 | 13166 | 1.30 (0.71–2.38) | 1.62 (0.88–2.97) | 1.62 (0.88–2.97) |
| Colorectal cancer | 649 | 711936 | 13 | 18199 | 0.73 (0.42–1.27) | 0.78 (0.45–1.35) | 0.77 (0.45–1.34) |
| Hip fracture | 1055 | 708827 | 23 | 18148 | 0.88 (0.58–1.33) | 0.88 (0.58–1.34) | 0.91 (0.60–1.38) |
MI, myocardial infarction; PE/DVT, pulmonary embolism/deep vein thrombosis; VE, vaginal estrogen
Model 1 was adjusted for age (y), calendar time, race, smoking status, alcohol intake, physical activity (MET-h/wk), BMI (kg/m2), age at menopause, hysterectomy (except for endometrial cancer), bilateral oophorectomy (except for ovarian cancer), and history of past systemic hormone therapy use (y/n). Cardiovascular outcomes were also adjusted for aspirin use (at least 1/day).
Model 2 was adjusted for variables in model 1 and parental history of cancer. Cardiovascular models were also adjusted for history of high blood pressure, hypercholesterolemia, history of diabetes, and parental history of early MI. Breast cancer models were also adjusted for height, parity, age at first birth, BMI at age 18, history of benign breast disease, and mammogram screening in the previous cycle.
Excluded women with bilateral oophorectomy.
Endometrial cancer results are only shown for women with an intact uterus
In sensitivity analyses, for endometrial cancer, when we started follow-up in 1992 (instead of 1982), results remained null and non-significant (HR=1.52, 95% CI 0.78–2.98). In additional analyses, when we only censored women for past systemic estrogen use and adjusted for past systemic estrogen plus progestin or progestin alone use, the HR for endometrial cancer among VE users was attenuated and remained non-significant (HR=1.24, 95% CI 0.64–2.41). When we examined the association between vaginal estrogen use and health outcomes by hysterectomy status, results remained similar to the overall sample with no differences in risk for cardiovascular and cancer outcomes (Figure 1).
Figure 1.
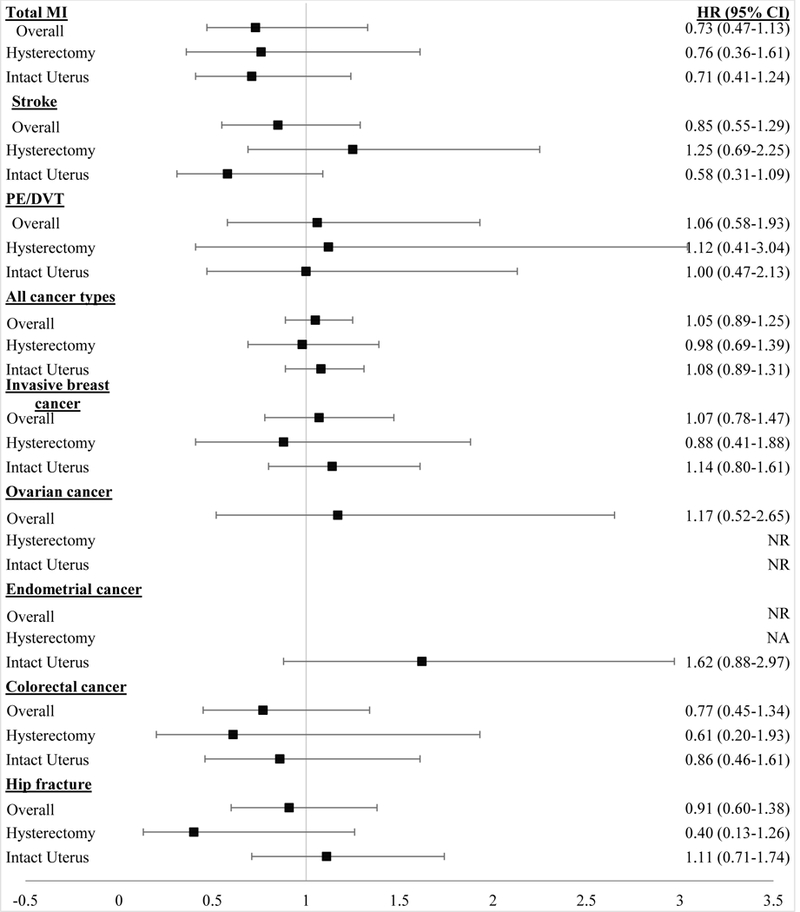
Hazard Ratio (HR) and 95% Confidence Interval (CI) for health outcomes among Vaginal Estrogen (VE) users Overall and by Hysterectomy Status. VE and hysterectomy status were included in the model as time varying covariates. Analysis for ovarian cancer was not conducted on women with a bilateral oophorectomy. Ovarian cancer results are not presented by hysterectomy status due to model convergence issues. Endometrial cancer analysis was only conducted on women with an intact uterus. All models were adjusted for age, calendar time, race, smoking status, alcohol intake, physical activity, BMI, age at menopause, hysterectomy status (for all overall models except endometrial cancer), bilateral oophorectomy (except for ovarian cancer), past systemic hormone therapy use, and parental history of cancer. In addition to these covariates, breast cancer models were also adjusted for height, parity, age at first birth, BMI at age 18, history of benign breast disease, and mammogram screening in the previous cycle. Cardiovascular endpoints were additionally adjusted for aspirin use, history of high blood pressure, hypercholesterolemia, history of type 2 diabetes, and parental history of early MI. CI, confidence interval; HR, hazard ratio; MI, myocardial infarction; NA, not applicable; NR, not reported
DISCUSSION
In this large prospective study of postmenopausal nurses’, over a period of 18 years of follow-up, after accounting for differences in major confounders, we found that users and non-users of vaginal estrogen did not have different risks for major cardiovascular outcomes (including total MI, stroke, pulmonary embolism/deep vein thrombosis), cancer outcomes (total invasive cancer, invasive breast, ovarian, endometrial, or colorectal cancer), or hip fracture. When we examined associations by hysterectomy status, results remained similar to the overall cohort.
Only a few previous studies have examined the association between vaginal estrogen use and various health outcomes, and only one previous study comprehensively examined the balance of risks and benefits associated with vaginal estrogen use.27 Although use of oral conjugated equine estrogens (0.625 mg/d) was associated with an increased risk of stroke and deep vein thrombosis in the intervention phase of the Women’s Health Initiative (WHI) Hormone Therapy (HT) trials,45 we found no evidence for a higher risk for either of these outcomes with use of vaginal estrogen. Our findings for stroke are consistent with WHI Observational Study (WHI-OS) findings27 that found no association with vaginal estrogen use. However, two studies from Scandinavian nations found a lower risk of stroke with vaginal estrogen use. A Danish national cohort of 980,003 postmenopausal women, aged 51–70 years,29 documented a 35% (RR=0.65, 95% CI 0.59–0.70) lower risk of stroke among vaginal estrogen users while a pooled analysis of 5 Swedish cohort studies30 found that late initiation (>5 years since menopause onset) of vaginal HT was associated with a longer stroke-free period. The apparent discrepancies in these findings may be attributed to differences in study populations, more comprehensive adjustment for potential confounding variables in our study and the types of vaginal hormones used. For example, while the Swedish analysis examined associations with vaginal HT which could include progestin creams, it remains unclear if the inverse association persisted when limited to vaginal estrogen use. For total MI, similar to the WHI-OS analysis (which examined CHD as an outcome),27 we found an inverse association, albeit non-significant, with vaginal estrogen use.
For breast cancer, similar to the results of the WHI-OS analysis27 and a study of postmenopausal women from Finland,46 we found no association between vaginal estrogen use and incident breast cancer. Moreover, because use of systematic unopposed estrogen was not associated with an increased risk of breast cancer in either the intervention or the cumulative follow-up phases of the WHI HT trials,45 it is unlikely that use of a low-dose vaginal estrogen would have an effect on risk of breast cancer.
With respect to endometrial cancer, consistent with the findings of Crandall et al27 we found no evidence for a difference in risk between users and non-users of vaginal estrogen. However, contrary to our findings, a Danish national cohort study found that compared to never users, users of vaginal estrogen therapy had a nearly 2-fold risk of endometrial cancer (RR=1.96, 95% CI 1.77–2.17).28 The potential reasons underlying these differing findings, as articulated in a recent editorial,47 included potential confounding by concomitant use of systemic hormone therapy in the Danish cohort, as a substantial proportion of women were using unopposed systemic estrogen. In both the WHI-OS cohort and the current study, current users of systemic hormone therapy were excluded at baseline and women were censored at the start of systemic hormone therapy during follow-up. This analytic approach allowed us to minimize any “effects” of unopposed systemic hormone therapy use on the endometrium. We also adjusted for the past systemic hormone therapy use. In sensitivity analyses, when we only censored who were current users of systemic estrogen therapy and adjusted for systemic estrogen plus progestin or systemic progestin therapy, results were attenuated and remained non-significant. Second, data from the National Danish Prescription Registry indicate that doses of vaginal estrogen in Denmark were generally higher (25 μg E2) than those in the US (10 μg E2).47 Because systemic absorption has been shown in women using vaginal E2 at doses of 25 μg,48 the safety of estrogen on the endometrium at these higher doses remains unknown. At the same time, 10 μg vaginal E2 results in at least 50% lower mean E2 concentrations than with the 25 μg dose within 24h after dosing.25 Because NHS women used vaginal estrogen therapy with much higher doses during early follow-up, when we initiated follow-up in 1992 instead of 1982, results for endometrial cancer were null and non-significant. A 2006 Cochrane review of 4 trials found that, when compared to a placebo, vaginal estrogen therapy was not associated with a statistically significant risk of endometrial hyperplasia among postmenopausal women, although no data were available to evaluate safety beyond six months of use.49 However, our findings combined with those of the WHI-OS27 provide reassurance that use of low-dose vaginal estrogen for more than six months is not associated with a higher risk of endometrial cancer. Still, because GSM symptoms could last for a long time thereby warranting a longer treatment duration, additional studies are needed to examine the safety of longer duration of use beyond what was observed in the WHI-OS and the NHS. Nonetheless, our conclusions are in line with recommendations by the North American Menopause Society and the American College of Obstetricians and Gynecologists that state that low-dose vaginal estrogen can be used indefinitely, if needed, and do not advise use of concomitant progestion to protect the endometrium.9,10
Although systemic HT use has consistently been shown to be associated with a lower risk of hip fracture, we did not document such an association with vaginal estrogen use. In line with our results, a case-control study of 4589 postmenopausal women also found no association between low potency vaginal estrogen and hip fracture risk (OR=0.82, 95% CI 0.50–1.36).50 However, the WHI-OS analysis documented a strong and inverse association between current vaginal estrogen use and hip fracture risk (RR=0.40, 95% CI 0.19–0.85) although this estimate is based on only 10 cases of hip fracture in vaginal estrogen users.27 In a small randomized controlled trial of 30 health women, aged 60 years or more, ultralow doses of parenteral E2 (7.5 μg/24 hours) for 6 months increased forearm bone mineral density compared to non-users51, suggesting a potentially causal role for E2 in lowering fracture risk.
The findings of our study together with those of the WHI-OS provide substantial and consistent evidence for the safety of vaginal estrogen use in postmenopausal women in relation to multiple health outcomes. Despite lack of any observational or clinical trial evidence for chronic disease risks related to vaginal estrogen use, the FDA has issued a boxed warning on the package label for low-dose vaginal estrogen. This black-box warning, which is the highest level of warning information in labeling, is designed to call attention to serious or life-threatening risks. The specified risks which include endometrial cancer, cardiovascular disorders, breast cancer, and probable dementia stem from the findings of the WHI HT trials19 that used systemic doses of hormone therapy and should not be extrapolated to the lower doses found in vaginal estrogen regimens. Unlike doses of estrogen therapy used to treat vasomotor symptoms, low-dose vaginal estrogen results in minimal systemic absorption and circulating E2 and estrone concentrations generally remain within the normal postmenopausal range.23,24,26
It is worth mentioning that prevalence of vaginal estrogen use in our study (<3%) was much lower than what was observed in the WHI-OS (~10%). While the reasons for the lower prevalence are not entirely clear, one possible reason is that women who reported using vaginal estrogen use in a questionnaire cycle and who also reported using a systemic form of hormone therapy were excluded from the analysis. Along with the low prevalence of vaginal estrogen use, the average duration of use (37.5 months) was also low in our population. Given the long lasting duration of GSM symptoms, additional data on the safety of longer duration of use of vaginal estrogen is warranted.
The strengths of the current study include prospective collection of hormone therapy use and health outcomes, confirmation of clinical endpoints, high rates of follow-up, and our ability to examine the risk-benefit profile by hysterectomy status. Still, several limitations are worth mentioning. First, although we comprehensively adjusted for a number of covariates that could confound the association between vaginal estrogen use and health outcomes, residual confounding remains a possibility given the observational nature of the study. Second, we did not have information on the individual types of vaginal therapy formulations such as creams, rings, tablets, and suppositories, or the doses used. During early follow-up in the NHS, it is possible that some women were using vaginal estrogen with much higher doses rather than the currently prescribed low-dose regimens. However, we did not have information to address this issue. Third, we did not have information on Femring which contains systemic doses of estrogen vaginally. Still, Femring use is unlikely to affect results, as it was not approved until 2003 and is an uncommonly prescribed form of systemic HT. A small open-label, randomized, multiple-dose, two-treatment crossover study among 24 postmenopausal women showed that when women received 0.5 g Premarin vaginal cream (equivalent to 0.3 mg conjugated estrogens, 0.625 mg/g) for 7 days, unconjugated plasma E2 and estrone levels remained within the normal postmenopausal range.24 Still the safety of these “higher” doses of vaginal estrogen need to be ascertained in future studies. Fourth, our study includes only health professionals mainly of European ancestry (~97%) with a high educational status. While the lack of racial diversity limits our ability to generalize our findings to other ethnic groups, the consistency of these results with those of the WHI-OS supports the generalizability of our study. Further, the high educational status can be perceived as an advantage because high quality and reliable data can be collected from our study participants. Finally, given the observational nature of our study, we cannot establish cause and effect relationships..
CONCLUSIONS
In conclusion, our data lend support to the safety of vaginal estrogen use, as no excess risk of cardiovascular disease or cancer was observed among women who self-reported use of various delivery systems and doses of vaginal estrogen. Our findings provide a comprehensive summary of the relationship between vaginal estrogen and multiple health outcomes and offer reassurance regarding the safety of low-dose vaginal estrogen to treat GSM.
ACKNOWLEDGEMENTS
We would like to thank the participants and staff of the Nurses’ Health Study that contributed data] for their valuable contributions as well as the following state cancer registries for their help: AL, AZ, AR, CA, CO, CT, DE, FL, GA, ID, IL, IN, IA, KY, LA, ME, MD, MA, MI, NE, NH, NJ, NY, NC, ND, OH, OK, OR, PA, RI, SC, TN, TX, VA, WA, WY. The authors assume full responsibility for analyses and interpretation of these data.
Sources of financial support: Dr. Bhupathiraju is supported by a Career Development Grant from the NIH (K01 DK107804).
Funding disclosures/conflicts of interest: The Nurses’ Health Study is supported by grants from the National Institutes of Health UM1 CA186107, P01 CA87969, R01 HL034594, and R01 HL088521.
REFERENCES
- 1.Bachmann GA, Nevadunsky NS. Diagnosis and treatment of atrophic vaginitis. American family physician. 2000;61(10):3090–6. [PubMed] [Google Scholar]
- 2.Santoro N, Komi J. Prevalence and impact of vaginal symptoms among postmenopausal women. The journal of sexual medicine. 2009;6(8):2133–42. [DOI] [PubMed] [Google Scholar]
- 3.Nappi RE, Palacios S, Panay N, Particco M, Krychman ML. Vulvar and vaginal atrophy in four European countries: evidence from the European REVIVE Survey.. [Climacteric : the journal of the International Menopause Society Research Support, Non-U.S. Gov’t]. 2016;19(2):188–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moral E, Delgado JL, Carmona F, et al. Genitourinary syndrome of menopause. Prevalence and quality of life in Spanish postmenopausal women. The GENISSE study. Climacteric : the journal of the International Menopause Society. 2018;21(2):167–73. [DOI] [PubMed] [Google Scholar]
- 5.Portman DJ, Gass ML. Genitourinary syndrome of menopause: new terminology for vulvovaginal atrophy from the International Society for the Study of Women’s Sexual Health and the North American Menopause Society. Maturitas. [Consensus Development Conference Research Support, Non-U.S. Gov’t]. 2014;79(3):349–54. [DOI] [PubMed] [Google Scholar]
- 6.Management of symptomatic vulvovaginal atrophy: 2013 position statement of The North American Menopause Society. Menopause. 2013;20(9):888–902; quiz 3–4. [DOI] [PubMed] [Google Scholar]
- 7.DiBonaventura M, Luo X, Moffatt M, Bushmakin AG, Kumar M, Bobula J. The Association Between Vulvovaginal Atrophy Symptoms and Quality of Life Among Postmenopausal Women in the United States and Western Europe. J Womens Health (Larchmt). [Comparative Study]. 2015;24(9):713–22. [DOI] [PubMed] [Google Scholar]
- 8.Nappi RE, Palacios S. Impact of vulvovaginal atrophy on sexual health and quality of life at postmenopause. Climacteric : the journal of the International Menopause Society. [Review]. 2014;17(1):3–9. [DOI] [PubMed] [Google Scholar]
- 9.ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstetrics and gynecology. [Practice Guideline]. 2014;123(1):202–16. [DOI] [PubMed] [Google Scholar]
- 10.The 2017 hormone therapy position statement of The North American Menopause Society. Menopause. 2017;24(7):728–53. [DOI] [PubMed] [Google Scholar]
- 11.Stuenkel CA, Davis SR, Gompel A, et al. Treatment of Symptoms of the Menopause: An Endocrine Society Clinical Practice Guideline. The Journal of clinical endocrinology and metabolism. [Practice Guideline Research Support, Non-U.S. Gov’t]. 2015;100(11):3975–4011. [DOI] [PubMed] [Google Scholar]
- 12.Rahn DD, Carberry C, Sanses TV, et al. Vaginal estrogen for genitourinary syndrome of menopause: a systematic review. Obstetrics and gynecology. [Review]. 2014;124(6):1147–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cardozo L, Bachmann G, McClish D, Fonda D, Birgerson L. Meta-analysis of estrogen therapy in the management of urogenital atrophy in postmenopausal women: second report of the Hormones and Urogenital Therapy Committee. Obstetrics and gynecology. [Meta-Analysis Research Support, Non-U.S. Gov’t]. 1998;92(4 Pt 2):722–7. [DOI] [PubMed] [Google Scholar]
- 14.Lethaby A, Ayeleke RO, Roberts H. Local oestrogen for vaginal atrophy in postmenopausal women. The Cochrane database of systematic reviews. [Meta-Analysis Research Support, Non-U.S. Gov’t Review]. 2016(8):CD001500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mitchell CM, Reed SD, Diem S, et al. Efficacy of Vaginal Estradiol or Vaginal Moisturizer vs Placebo for Treating Postmenopausal Vulvovaginal Symptoms: A Randomized Clinical Trial. JAMA internal medicine. 2018;178(5):681–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pinkerton JV, Kaunitz AM, Manson JE. Not time to abandon use of local vaginal hormone therapies. Menopause. 2018;25(8):855–8. [DOI] [PubMed] [Google Scholar]
- 17.Kingsberg SA, Krychman M, Graham S, Bernick B, Mirkin S. The Women’s EMPOWER Survey: Identifying Women’s Perceptions on Vulvar and Vaginal Atrophy and Its Treatment. The journal of sexual medicine. 2017;14(3):413–24. [DOI] [PubMed] [Google Scholar]
- 18.Manson JE, Goldstein SR, Kagan R, et al. Why the product labeling for low-dose vaginal estrogen should be changed. Menopause. [Editorial]. 2014;21(9):911–6. [DOI] [PubMed] [Google Scholar]
- 19.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women’s Health Initiative randomized controlled trial. Jama. [Clinical Trial Multicenter Study Randomized Controlled Trial Research Support, U.S. Gov’t, P.H.S.]. 2002;288(3):321–33. [DOI] [PubMed] [Google Scholar]
- 20.Archer DF . Efficacy and tolerability of local estrogen therapy for urogenital atrophy. Menopause. [Research Support, Non-U.S. Gov’t Review]. 2010;17(1):194–203. [DOI] [PubMed] [Google Scholar]
- 21.Vulvar Lev-Sagie A. and Vaginal Atrophy: Physiology, Clinical Presentation, and Treatment Considerations. Clinical obstetrics and gynecology. [Review]. 2015;58(3):476–91. [DOI] [PubMed] [Google Scholar]
- 22.Naessen T, Rodriguez-Macias K. Endometrial thickness and uterine diameter not affected by ultralow doses of 17beta-estradiol in elderly women. American journal of obstetrics and gynecology. [Clinical Trial Randomized Controlled Trial Research Support, Non-U.S. Gov’t]. 2002;186(5):944–7. [DOI] [PubMed] [Google Scholar]
- 23.Weisberg E, Ayton R, Darling G, et al. Endometrial and vaginal effects of low-dose estradiol delivered by vaginal ring or vaginal tablet. Climacteric : the journal of the International Menopause Society. [Clinical Trial Randomized Controlled Trial Research Support, Non-U.S. Gov’t]. 2005;8(1):83–92. [DOI] [PubMed] [Google Scholar]
- 24.Dorr MB, Nelson AL, Mayer PR, et al. Plasma estrogen concentrations after oral and vaginal estrogen administration in women with atrophic vaginitis. Fertility and sterility. [Randomized Controlled Trial Research Support, Non-U.S. Gov’t]. 2010;94(6):2365–8. [DOI] [PubMed] [Google Scholar]
- 25.Eugster-Hausmann M, Waitzinger J, Lehnick D. Minimized estradiol absorption with ultra-low-dose 10 microg 17beta-estradiol vaginal tablets. Climacteric : the journal of the International Menopause Society. [Randomized Controlled Trial]. 2010;13(3):219–27. [DOI] [PubMed] [Google Scholar]
- 26.Santen RJ, Pinkerton JV, Conaway M, et al. Treatment of urogenital atrophy with low-dose estradiol: preliminary results. Menopause. [Clinical Trial]. 2002;9(3):179–87. [DOI] [PubMed] [Google Scholar]
- 27.Crandall CJ, Hovey KM, Andrews CA, et al. Breast cancer, endometrial cancer, and cardiovascular events in participants who used vaginal estrogen in the Women’s Health Initiative Observational Study. Menopause. 2018;25(1):11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morch LS, Kjaer SK, Keiding N, Lokkegaard E, Lidegaard O. The influence of hormone therapies on type I and II endometrial cancer: A nationwide cohort study. International journal of cancer. [Research Support, Non-U.S. Gov’t]. 2016;138(6):1506–15. [DOI] [PubMed] [Google Scholar]
- 29.Lokkegaard E, Nielsen LH, Keiding N. Risk of Stroke With Various Types of Menopausal Hormone Therapies: A National Cohort Study. Stroke. 2017;48(8):2266–9. [DOI] [PubMed] [Google Scholar]
- 30.Carrasquilla GD, Frumento P, Berglund A, et al. Postmenopausal hormone therapy and risk of stroke: A pooled analysis of data from population-based cohort studies. PLoS medicine. [Observational Study]. 2017;14(11):e1002445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Belanger C, Speizer FE, Hennekens CH, Rosner B, Willett W, Bain C. The nurses’ health study: current findings. The American journal of nursing. [Research Support, U.S. Gov’t, P.H.S.]. 1980;80(7):1333. [DOI] [PubMed] [Google Scholar]
- 32.Belanger CF, Hennekens CH, Rosner B, Speizer FE. The nurses’ health study. The American journal of nursing. 1978;78(6):1039–40. [PubMed] [Google Scholar]
- 33.Colditz GA, Manson JE, Hankinson SE. The Nurses’ Health Study: 20-year contribution to the understanding of health among women. Journal of women’s health / the official publication of the Society for the Advancement of Women’s Health Research. [Research Support, U.S. Gov’t, P.H.S.]. 1997;6(1):49–62. [DOI] [PubMed] [Google Scholar]
- 34.Bao Y, Bertoia ML, Lenart EB, et al. Origin, Methods, and Evolution of the Three Nurses’ Health Studies. American journal of public health. 2016;106(9):1573–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organization; IHD Registers: Report of the Fifth Working Group. . In: Organization WH, editor. Copenhagen; 1971. [Google Scholar]
- 36.Alpert JS, Thygesen K, Antman E, Bassand JP. Myocardial infarction redefined--a consensus document of The Joint European Society of Cardiology/American College of Cardiology Committee for the redefinition of myocardial infarction. Journal of the American College of Cardiology. [Guideline Practice Guideline]. 2000;36(3):959–69. [DOI] [PubMed] [Google Scholar]
- 37.Walker AE, Robins M, Weinfeld FD. The National Survey of Stroke. Clinical findings. Stroke. [Research Support, U.S. Gov’t, P.H.S.]. 1981;12(2 Pt 2 Suppl 1):I13–44. [PubMed] [Google Scholar]
- 38.Pun VC, Hart JE, Kabrhel C, Camargo CA Jr., Baccarelli AA, Laden F Prospective Study of Ambient Particulate Matter Exposure and Risk of Pulmonary Embolism in the Nurses’ Health Study Cohort. Environmental health perspectives. [Research Support, N.I.H., Extramural Research Support, Non-U.S. Gov’t]. 2015;123(12):1265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kabrhel C, Varraso R, Goldhaber SZ, Rimm E, Camargo CA, Jr. Physical inactivity and idiopathic pulmonary embolism in women: prospective study. BMJ. [Research Support, N.I.H., Extramural]. 2011;343:d3867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Poole EM, Lin WT, Kvaskoff M, De Vivo I, Terry KL, Missmer SA. Endometriosis and risk of ovarian and endometrial cancers in a large prospective cohort of U.S. nurses. Cancer causes & control : CCC. 2017;28(5):437–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puckett CD. The Educational Annotation of ICD-9-CM; Diseases and Procedures Tabular Lists. Reno, NV; 1986. [Google Scholar]
- 42.Rich-Edwards JW, Corsano KA, Stampfer MJ. Test of the National Death Index and Equifax Nationwide Death Search. American journal of epidemiology. [Research Support, U.S. Gov’t, P.H.S.]. 1994;140(11):1016–9. [DOI] [PubMed] [Google Scholar]
- 43.Stampfer MJ, Willett WC, Speizer FE, et al. Test of the National Death Index. American journal of epidemiology. [Research Support, U.S. Gov’t, P.H.S.]. 1984;119(5):837–9. [DOI] [PubMed] [Google Scholar]
- 44.Zahl PH, Jorgensen KJ, Maehlen J, Gotzsche PC. Biases in estimates of overdetection due to mammography screening. The Lancet Oncology. [Comment Letter]. 2008;9(3):199–201; author reply −2. [DOI] [PubMed] [Google Scholar]
- 45.Manson JE, Chlebowski RT, Stefanick ML, et al. Menopausal hormone therapy and health outcomes during the intervention and extended poststopping phases of the Women’s Health Initiative randomized trials. Jama. [Multicenter Study Randomized Controlled Trial Research Support, N.I.H., Intramural]. 2013;310(13):1353–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lyytinen H, Pukkala E, Ylikorkala O. Breast cancer risk in postmenopausal women using estrogen-only therapy. Obstetrics and gynecology. [Research Support, Non-U.S. Gov’t]. 2006;108(6):1354–60. [DOI] [PubMed] [Google Scholar]
- 47.Pinkerton JV, Kaunitz AM, Manson JE. Vaginal estrogen in the treatment of genitourinary syndrome of menopause and risk of endometrial cancer: an assessment of recent studies provides reassurance. Menopause. 2017;24(12):1329–32. [DOI] [PubMed] [Google Scholar]
- 48.Labrie F, Cusan L, Gomez JL, et al. Effect of one-week treatment with vaginal estrogen preparations on serum estrogen levels in postmenopausal women. Menopause. [Randomized Controlled Trial Research Support, Non-U.S. Gov’t]. 2009;16(1):30–6. [DOI] [PubMed] [Google Scholar]
- 49.Suckling J, Lethaby A, Kennedy R. Local oestrogen for vaginal atrophy in postmenopausal women. The Cochrane database of systematic reviews. [Meta-Analysis Review] 2006(4):CD001500. [DOI] [PubMed] [Google Scholar]
- 50.Michaelsson K, Baron JA, Farahmand BY, Ljunghall S. Use of low potency estrogens does not reduce the risk of hip fracture. Bone. [Research Support, Non-U.S. Gov’t]. 2002;30(4):613–8. [DOI] [PubMed] [Google Scholar]
- 51.Naessen T, Berglund L, Ulmsten U. Bone loss in elderly women prevented by ultralow doses of parenteral 17beta-estradiol. American journal of obstetrics and gynecology. [Clinical Trial Randomized Controlled Trial Research Support, Non-U.S. Gov’t]. 1997;177(1):115–9. [DOI] [PubMed] [Google Scholar]


