Abstract
Technologies enabling new enzyme discovery and efficient protein engineering have spurred intense interest in the development of biocatalytic reactions. In recent years, whole-cell biocatalysis has received attention as a simple, efficient, and scalable biocatalytic reaction platform. Inspired by these developments, we have established a whole-cell protocol for oxidative dearomatization of phenols using the flavin-dependent monooxygenase, TropB. This approach provides a scalable biocatalytic platform for accessing gram-scale quantities of chiral synthetic building blocks.
Keywords: biocatalysis, whole-cell reactions, oxidative dearomatization, preparative-scale, flavin-dependent monooxygenase
Graphical Abstract
The development of scalable and economical biocatalytic reaction platforms is critical for the application of biocatalysis in synthetic chemistry. Therefore, we have established a whole cell method for the oxidative dearomatization of phenols using the flavin-dependent monooxygenase, TropB. In comparison to reactions using isolated enzyme, our whole-cell method allowed us to perform tenfold more reactions per liter of cell culture, without loss of site- or stereoselectivity.

Introduction
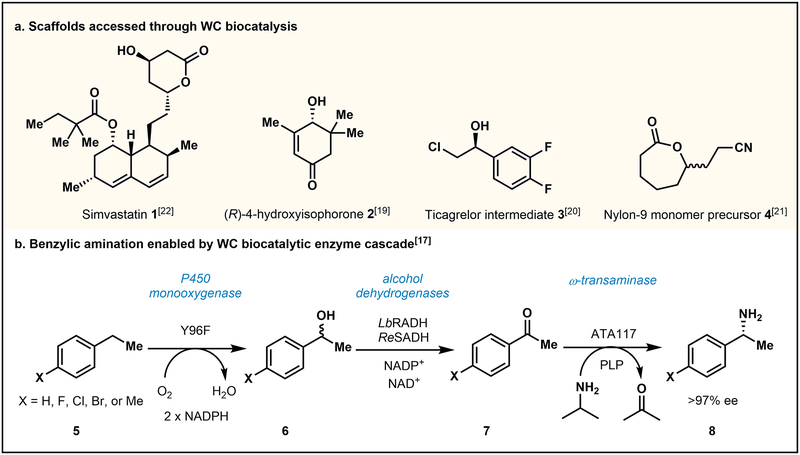
Growth in the areas of new enzyme discovery and development of novel biocatalytic reactions, combined with advances in protein engineering strategies, have positioned biocatalysis to have a greater footprint in synthetic chemistry.[1,2] Biocatalytic methods also provide green alternatives to traditional chemical reactions by avoiding the use of organic solvents, minimizing hazardous waste streams, and using abundant materials.[2] However, the adoption of biocatalytic methods relies on the development of scalable, economical, and operationally accessible reaction platforms. Recently, significant effort has been dedicated to establishing efficient biocatalytic reaction platforms for a wide variety of transformations. A number of approaches have been developed for conducting preparative-scale biocatalytic reactions, including reactions with purified enzymes, crude cell lysates,[1,2] immobilized enzymes,[3–5] lyophilized lysates, and wet whole cells.[6–8] Enzymes in various forms can be employed in batch or flow reactors.[9–10] Whole-cell (WC) transformations present several advantages over using isolated enzymes for in vitro reactions. For example, supply costs associated with the generation of WC preparations are much lower than for isolated proteins. Specifically, the cost of affinity chromatography resins, protein concentration devices, and cell lysis equipment is avoided for WC methods. WC preparations are also less time intensive, as laborious protein purification steps are not required. Similarly, crude cell lysate preparations also avoid a full protein purification, capturing some of these advantages. These attributes make WC biocatalysts more accessible to chemists and help promote the broader use of biocatalysts in organic synthesis. Capitalizing on these advantages, the use of WC biocatalysis for chemical synthesis has become increasingly prevalent in both academic and industrial settings.[6,11–16] Recently, Flitsch and Turner reported a WC biocatalytic process for the stereoselective amination of benzylic C–H bonds.[17] This feat was accomplished by co-transforming two plasmids containing genes corresponding to four enzymes necessary for a biocatalytic cascade (a monooxygenase, two alcohol dehydrogenases, and a ω-transaminase, Fig. 1B). By incubating the reaction components with the requisite biocatalysts in WC form in a sealed reaction vessel, they were able to produce chiral amines (8) with moderate yields and high enantioselectivity. Without a WC biocatalytic platform, this process would have been logistically cumbersome, requiring the expression and potential isolation of each enzyme individually.
Figure 1.
Examples of WC biocatalytic processes. (a) Select scaffolds accessed through WC biocatalytic reactions.[18–24] (b) WC biocatalysis utilizing a multi-enzyme cascade to generate chiral benzylic amines.[17]
On an industrial scale, several processes have been designed to employ WC biocatalysts. Notably, WC biocatalysis has been utilized for the generation of valuable chemical feedstocks such as enantio-enriched alcohols,[18] performance material monomers,[19] and pharmaceutically-relevant intermediates,[20] as well as the late-stage functionalization of active pharmaceutical ingredients during route development (Fig. 1A).[20–22] Recently, researchers at GlaxoSmithKline demonstrated that a WC biocatalysis platform is also useful for discovery of new synthetic routes on the gram-scale, developing several biocatalytic routes to chiral 1,3-substituted cyclohexanones, such as 2.[23] After obtaining initial hits from enzyme libraries, the use of WC biocatalysts allowed the GlaxoSmithKline team to rapidly scale reactions and ultimately provide the desired chiral cyclohexanone products in gram quantities.
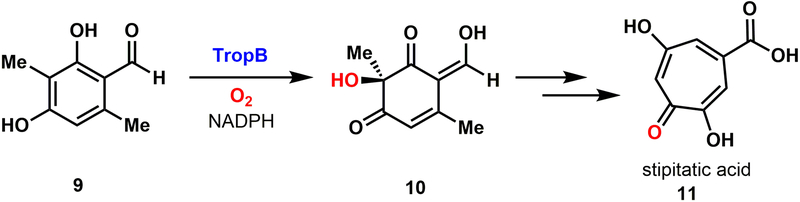
We previously established a platform for WC biocatalytic oxidative dearomatization of phenols using the flavin-dependent monooxygenase TropB.[24] The native biosynthetic function of TropB was first characterized by Cox and coworkers in 2012.[25,26] TropB is an oxygenase, involved in the biosynthesis of stipitatic acid, that naturally converts 3-methyl-orcinaldehyde (9) to dienone 10 with perfect site- and stereoselectivity (Fig. 2).[24–26] We have previously demonstrated that TropB possesses a broad substrate scope and is able to generate a diverse array of dienone products.[24] The products generated through this powerful reaction can serve as building blocks to valuable bioactive natural products, including tropolones and azaphilones.[24–27] Over the last two decades, several chemical reagents have been developed to affect this difficult transformation.[28–32] Despite advances in synthetic oxidative dearomatization methodology, these reactions often exhibit substrate-controlled site-selectivity, low levels of stereoselectivity, and can require stoichiometric chiral reagents. In contrast, Nature has conquered this reaction by using common biochemical cofactors and molecular oxygen, along with an evolved active site to provide impeccable site- and stereoselectivity. The advantages of biocatalytic oxidative dearomatization inspired our interest in developing TropB as a synthetically useful catalyst.
Figure 2.
Native reactivity of TropB within the stipitatic acid (11) biosynthetic pathway.
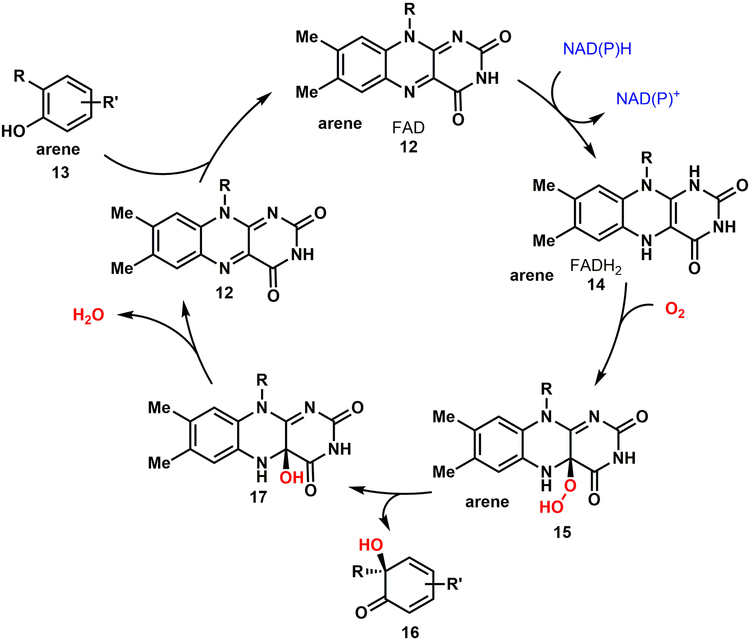
Flavin-dependent monooxygenases employ the non-covalent cofactor flavin adenine dinucleotide (FAD, 12), which following reduction to FADH2 (14) can react with molecular oxygen to form C4-α-hydroperoxyflavin 15 (Fig. 3).[33] This highly reactive species serves as an electrophilic source of oxygen for the oxidative dearomatization of electron-rich phenolic substrates. In the final step, loss of water from 17 regenerates FAD to close the catalytic cycle. We initially recognized that the requirement for stoichiometric NADPH is a potentially limiting factor in the scalability of this reaction. To address this limitation, we employed a well-established NADPH recycling system. Using this system, cofactor recycling could be successfully integrated into our WC reactions with native and non-native substrates on gram-scale to obtain complete conversion of substrates to dearomatized products.[24]
Figure 3.
Proposed catalytic cycle of type A flavin-dependent monooxygenases in oxidative dearomatization of phenolic substrates.[33]
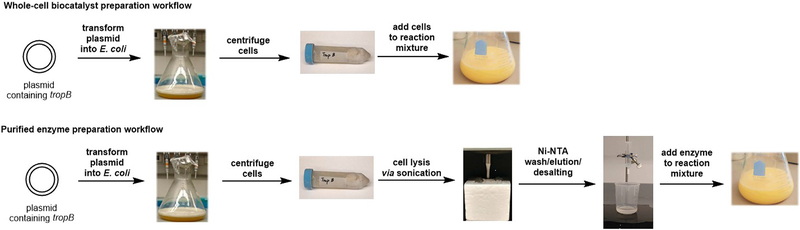
Our platform for WC reactions utilizes well-established biochemical tools to rapidly generate biocatalyst in high quantities (Fig. 4). The general workflow begins with transformation of an expression plasmid containing the target gene, tropB, into E. coli BL21(DE3) cells, followed by overexpression of TropB in nutrient-rich media. After harvesting and flash-freezing the cells containing TropB, these cells can be immediately used for oxidative dearomatization reactions. In contrast, obtaining protein for in vitro reactions requires several additional steps beyond protein expression, including cell lysis, purification by immobilized metal affinity chromatography, and desalting procedures. Thus, WC methods significantly decrease the time and resources required to prepare the catalyst for use in reactions.
Figure 4.
Workflow of whole-cell reaction versus purified enzyme for TropB biocatalysis. Plasmid containing tropB is transformed into E. coli and then expressed. E. coli cells harboring TropB are harvested by centrifugation and frozen. The pellet can be added directly to the reaction flask. Additional steps are required to prepare purified protein.
The workflow employed for WC biocatalysis included the following steps. A gene, codon-optimized for heterologous expression in E. coli, was synthesized and cloned into pET-151 by GeneArt (ThermoFisher). The plasmid DNA was transformed into chemically competent E. coli BL21(DE3) by standard heat-shock technique. An individual colony was selected and grown overnight in a 5 mL culture of LB media supplemented with ampicillin for plasmid maintenance. A 500 mL culture of sterilized Terrific Broth was supplemented with ampicillin and inoculated with 5 mL of overnight culture. This culture was incubated at 37 °C until the cell density (OD600) reached 0.6–0.8, at which point the culture was cooled to 20 °C and protein overexpression was induced by addition of IPTG (0.1 mM final concentration). The culture was incubated at 20 °C for 14–16 h before harvesting the cells via centrifugation (4000 x g for 20 min).
Two methods were used to prepare the cell pellet for use in WC reactions: flash freezing or lyophilization. For the former, the crude cell pellet was flash frozen in liquid nitrogen in small aliquots. This method greatly alleviated difficulties in weighing out wet cell pellet for accurate quantification of our WC reactions. For the preparation of lyophilized cells, the crude cell pellet was aliquoted into 1 gram portions and resuspended in an excipient solution (0.5 L, 10% w/v excipient in Milli-Q water) and pelleted in several 50 mL Falcon tubes. The tubes were then covered with a KimWipe and lyophilized for 48–72 h. To identify the ideal preparation method for lyophilized cell pellet for use in our WC reactions, we added various excipients to cells prior to lyophilization based upon a survey of common excipients used in the literature.[34–36] We then compared the activity of each preparation in 1 g scale reactions with the non-native substrate 18 (Table 1). The results of these experiments indicated that enzyme activity was maintained in lyophilized cells, and only improved nominally by the addition of 10 wt % sucrose or PEG 4000 (entries 3 and 4, respectively).[34–36] This method simplified the handling of the biocatalytic reagent, streamlining the set-up of large-scale reactions.
Table 1.
Optimization of gram-scale reactions with lyophilized cell pellet.

| Entry | Excipient | % conversion |
|---|---|---|
| 1 | No additive | 98% |
| 2 | 10 wt% skim milk | 83% |
| 3 | 10 wt% sucrose | >99% |
| 4 | PEG 4000 | >99% |
Preparative-scale WC enzymatic reactions were conducted on 1 g of substrate under the following conditions: 20 weight equivalents of wet cell pellet, 5 mM substrate, 10% (v/v) toluene (caution: highly flammable solvent), 0.1 mM NADP+, 0.1 U/mL glucose-6-phosphate dehydrogenase (G6PDH) and 10 mM glucose-6-phosphate for NADPH generation in reaction buffer (50 mM potassium phosphate buffer, pH 8.0). The reaction components were combined in a 1 L Erlenmeyer flask and incubated at 30 °C for 2 h at 100 rpm. The reaction mixture was filtered through Celite, acidified to pH 2.0 and extracted with ethyl acetate (3 × 500 mL). The combined organic layers were dried over sodium sulfate, filtered and concentrated under reduced pressure. The resulting mixture was purified on silica gel (methanol/acetic acid/dichloromethane, 1:1:10) to afford the o-quinol product. For smaller scale reactions (5 mg), the same procedure was employed, except a 100 mL Erlenmeyer flask was used in place of a 1 L flask. To monitor reaction progress, 50 μL aliquots were removed from the reaction and quenched with 75 μL of methanol supplemented with an internal standard, 2.5 mM pentamethyl benzene. Reaction conversion was monitored by UPLC-DAD and starting material consumption was quantified by comparison with a substrate calibration curve (Figure 5).
Figure 5.
UPLC trace for oxidative dearomatization of 9 by TropB.
Results and Discussion
Whole cell reactions with TropB were initially performed in aqueous buffer alone. Under these conditions, conversions of starting material were low, which we hypothesized was due to poor substrate solubility in the aqueous reaction environment. To resolve this issue, we tested the effect of both water-miscible and immiscible solvents on the WC reaction (Table 2). Toluene improved conversion to dienone 10, with full conversion in 2 h under the described conditions. Additionally, we screened reactions with a reduced amount of wet cells and noted complete conversion with cell loading as low as 20 weight equivalents. These optimized conditions were applied to gram-scale reactions, further demonstrating the appeal of this method for synthetic applications.
Table 2.
Optimization of WC TropB reaction with co-solvents.
| Entry | Cell Pellet (mg) | Co-solvent (10% v/v) | % conversion to 10 |
|---|---|---|---|
| 1 | 500 | No co-solvent | 23% |
| 2 | 500 | Ethanol | 54% |
| 3 | 500 | Acetonitrile | <5% |
| 4 | 500 | Methanol | 62% |
| 5 | 500 | Tetrahydrofuran | <5% |
| 6 | 500 | Toluene | >99% |
| 7 | 100 | Toluene | >99% |
| 8 | 50 | Toluene | 86% |
Using our optimized WC biocatalysis platform, the overall yield of usable biocatalyst from each protein expression was significantly higher than what was obtained following protein purification for in vitro reactions. In this case, more than 20 WC preparative scale (100 mg) reactions could be carried out for every liter of TropB expression cell culture. In comparison, from 1 L of expression culture the quantity of purified protein was sufficient for 2–3 reactions on the same scale (Table 3). We suspect this was due to a loss of protein and activity over the course of the purification procedure. Reactions carried out with crude cell lysate gave diminished conversions relative to both in vitro and WC reactions, indicating that some enzyme stabilization may have been provided by the cellular environment. The WC biocatalyst also required significantly less time to prepare, saving 5–6 h for each batch of catalyst. Therefore, our WC platform demonstrated superior efficiency over in vitro reactions in both preparation time and scalability of the reaction.
Table 3.
Scalability of WC platform compared to in vitro platform.
| Platform | Scale of reactions (mg) | Reactions per liter cell culture |
|---|---|---|
| in vitro | 100 | 2–3 |
| WC | 100 | >20 |
Conclusion
In summary, we have developed a scalable and economical WC biocatalytic method for oxidative dearomatization of phenols. This biocatalytic method improves upon previously developed chemical methods in terms of environmental sustainability, as it uses water as a solvent and a renewable biological catalyst as the oxidant. In comparison to in vitro reactions, WC biocatalytic methods can provide distinct and significant advantages. With no requirement for enzyme purification, WC procedures reduce the overall time, cost and specialized equipment associated with catalyst production. After the simple optimization of several parameters, such as cell preparation method and reaction co-solvent, our WC platform allowed us to perform tenfold more reactions per liter of cell culture when compared to purified enzyme. This improvement was achieved without loss of catalytic function or selectivity. Moreover, this highly scalable method provides a simple and accessible reaction platform, promoting use of biocatalysts in synthetic chemistry.
Acknowledgements
This work was supported by funds from the University of Michigan Life Sciences Institute and the National Institutes of Health R35 GM124880 (to A.R.H.N.). S.B.D. is supported by the Chemical-Biology Interface Training Program at the University of Michigan. T.J.D. thanks the National Science Foundation for a Graduate Research Fellowship.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- [1].Rudroff F, Mihovilovic MD, Gröger H, Snajdrova R, Iding H, Bornscheuer UT, Nat. Catal 2018, 1, 12–22. [Google Scholar]
- [2].Sheldon RA, Woodley JM, Chem. Rev 2018, 118, 801–838. [DOI] [PubMed] [Google Scholar]
- [3].Liang JF, Li YT, Yang VC, J. Pharm. Sci 2000, 89, 979–990. [DOI] [PubMed] [Google Scholar]
- [4].Sheldon RA, van Pelt S, Chem. Soc. Rev 2013, 42, 6223–6235. [DOI] [PubMed] [Google Scholar]
- [5].Soares JC, Moreira PR, Queiroga AC, Morgado J, Malcata FX, Pintado ME, Biocatal. Biotransformation 2011, 29, 223–237. [Google Scholar]
- [6].de Carvalho CC, Microb. Biotechnol 2017, 10, 250–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Lin B, Tao Y, Microb. Cell. Fact 2017, 16, 106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Wachtmeister J, Mennicken P, Hunold A, Rother D, ChemCatChem, 2016, 8, 607–614. [Google Scholar]
- [9].Britton J, Majumdar S, Weiss GA, Chem. Soc. Rev 2018, 47, 5891–5918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Tamborini L, Fernandes P, Paradisi F, Molinari F, Trends Biotechnol. 2018, 36, 73–88. [DOI] [PubMed] [Google Scholar]
- [11].Bommarius AS, Annu. Rev. Chem. Biomol. Eng 2015, 6, 319–345. [DOI] [PubMed] [Google Scholar]
- [12].Wachtmeister J, Rother D, Curr. Opin. Biotechnol 2016, 42, 169–177. [DOI] [PubMed] [Google Scholar]
- [13].Schrittwieser JH, Velikogne S, Hall M, Kroutil W, Chem. Rev 2018, 118, 270–348. [DOI] [PubMed] [Google Scholar]
- [14].Parmeggiani F, Weise NJ, Ahmed ST, Turner NJ, Chem. Rev 2018, 118, 73–118. [DOI] [PubMed] [Google Scholar]
- [15].Bornscheuer UT, Huisman GW, Kazlauskas RJ, Lutz S, Moore JC, Robins K, Nature 2012, 485, 185. [DOI] [PubMed] [Google Scholar]
- [16].Li A, Ilie A, Sun Z, Lonsdale R, Xu J-H, Reetz MT, Angew. Chem. Int. Ed 2016, 55, 12026–12029. [DOI] [PubMed] [Google Scholar]
- [17].Both P, Busch H, Kelly PP, Mutti FG, Turner NJ, Flitsch SL, Angew. Chem. Int. Ed 2016, 55, 1511–1513. [DOI] [PubMed] [Google Scholar]
- [18].Matsuyama A, Yamamoto H, Kobayashi Y, Org. Proc. Res. Dev 2002, 6, 558–561. [Google Scholar]
- [19].Kaluzna I, Schmitges T, Straatman H, van Tegelen D, Müller M, Schürmann M, Mink D, Org. Proc. Res. Dev 2016, 20, 814–819. [Google Scholar]
- [20].Guo X, Tang J-W, Yang J-T, Ni G-W, Zhang F-L, Chen S-X, Org. Proc. Res. Dev 2017, 21, 1595–1601. [Google Scholar]
- [21].Milker S, Fink MJ, Rudroff F, Mihovilovic MD, Biotechnol. Bioeng 2017, 114, 1670–1678. [DOI] [PubMed] [Google Scholar]
- [22].Xie X, Tang Y, Appl. Environ. Microbiol 2007, 73, 2054–2060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hadi T, Díaz-Rodríguez A, Khan D, Morrison JP, Kaplan JM, Gallagher KT, Schober M, Webb MR, Brown KK, Fuerst D, Snajdrova R, Roiban G-D, Org. Proc. Res. Dev 2018, 22, 871–879. [Google Scholar]
- [24].Baker Dockrey SA, Lukowski AL, Becker MR, Narayan ARH, Nat. Chem 2018, 10, 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Davison J, al Fahad A, Cai M, Song Z, Yehia SY, Lazarus CM, Bailey AM, Simpson TJ, Cox RJ, Proc. Natl. Acad. Sci. U S A, 2012, 109, 7642–7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Abood A, Al-Fahad A, Scott A, Hosny AE-DMS, Hashem AM, Fattah AMA, Race PR, Simpson TJ, Cox RJ, RSC Adv. 2015, 5, 49987–49995. [Google Scholar]
- [27].Gao JM, Yang SX, Qin JC, Chem. Rev 2013, 113, 4755–4811. [DOI] [PubMed] [Google Scholar]
- [28].Bosset C, Coffinier R, Peixoto PA, El Assal M, Miqueu K, Sotiropoulos JM, Pouysegu L, Quideau S, Angew. Chem. Int. Ed. Engl 2014, 53, 9860–9864. [DOI] [PubMed] [Google Scholar]
- [29].Roche SP, Porco JA Jr., Angew. Chem. Int. Ed. Engl 2011, 50, 4068–4093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Volp KA, Harned AM, Chem. Commun. (Camb), 2013, 49, 3001–3003. [DOI] [PubMed] [Google Scholar]
- [31].Wu WT, Zhang L, You SL, Chem. Soc. Rev 2016, 45, 1570–1580. [DOI] [PubMed] [Google Scholar]
- [32].Zhu J, Grigoriadis NP, Lee JP, Porco JA, J. Am. Chem. Soc 2005, 127, 9342–9343. [DOI] [PubMed] [Google Scholar]
- [33].Ullrich R, Hofrichter M, Cell. Mol. Life. Sci 2007, 64, 271–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Baheti A, Kumar L, Bansal AK, J. Excipients and Food Chem 2010, 41, 41–54. [Google Scholar]
- [35]. Randolph TW, J. Pharm. Sci 1997, 86, 1198–1203 [DOI] [PubMed] [Google Scholar]
- [36]. Bedu-Addu FK, Pharm. Technol. (Lyophilization) 2004, 10–18. [Google Scholar]