Abstract
In the United States each year, more than 300,000 infants are admitted to neonatal intensive care units (NICU) where they are exposed to a chemical-intensive hospital environment during a developmentally vulnerable period. Although multiple studies have demonstrated elevated phthalate biomarkers in NICU patients, specific sources of NICU-based phthalate exposure have not been identified.
In this study, premature newborns with birth weight <1500g were recruited to participate in a prospective environmental health cohort during the NICU hospitalization. Exposure to specific NICU equipment was recorded daily during the NICU hospitalization. One hundred forty-nine urine specimens from 71 infants were analyzed for phthalate metabolites using high-performance liquid chromatography/tandem mass spectrometry.
In initial analyses, exposure to medical equipment was directly related to phthalate levels, with DEHP biomarkers 95% - 132% higher for infants exposed to specific medical equipment types compared to those without that equipment exposure (p<0.001–0.023). This association was mirrored for clinically relevant phthalate mixtures whether composed of DEHP metabolites or not (p=0.002–0.007). In models accounting for concurrent equipment use, exposure to respiratory support was associated with DEHP biomarkers 50%−136% higher in exposed compared to unexposed infants (p=0.007–0.036). Phthalate mixtures clinically relevant to neurobehavioral development were significantly associated with noninvasive respiratory support (p=0.008– 0.026). Feeding supplies and intravenous lines were not significantly associated with clinically important phthalate mixtures.
Respiratory support equipment may be a significant and clinically relevant NICU source of phthalate exposure. Although manufacturers have altered feeding and intravenous supplies to reduce DEHP exposure, other sources of exposure to common and clinically impactful phthalates persist in the NICU.
Keywords: Phthalate, Prematurity, Neurodevelopment, Neonatal Intensive Care Unit, Children’s Environmental Health, Exposure Assessment
INTRODUCTION
Premature infants, those born before 37 weeks gestational age, spend a developmentally vulnerable time period equivalent to the third trimester of gestation in a chemical-intensive hospital environment for which they are physiologically unprepared.(1–4) Prematurity-related neurobehavioral impairments commonly include developmental delay, cognitive dysfunction, attention deficit hyperactivity disorder, and autism (7–10) and are only partially explained by degree of prematurity or severity of illness in infancy.(11, 12) NICU inpatients are known to be exposed to phthalates, chemical plasticizers found in medical equipment.(13–17) Specific sources of NICU-based phthalate exposure have not been identified previously, despite the need for source identification to mitigate exposure.
Phthalates are a family of industrial chemicals non-covalently bonded to plastic materials to enhance flexibility and durability.(18) Because they are not chemically integral to the plastic scaffold, phthalates leach from the solid plastic matrix -- particularly in conditions of elevated heat or humidity that are present in neonatal incubators, intravenous catheter bundles, and respiratory support circuits.(15, 16, 18) In non-NICU populations, phthalate exposure is known to occur through ingestion, inhalation, dermal absorption, and parenteral routes.(18) In the body, parent phthalate diesters undergo hydrolysis and glucuronidation, followed by excretion of monoester metabolite(s) in urine and stool.(19) Both parent diesters and monoester metabolites are biologically active.(20) Phthalate exposure can be studied by single diester exposure, or by commonly occurring coincident exposure to multiple diesters via single or multiple sources. As phthalate bioavailability varies under differing conditions of heat and humidity, studies typically rely on testing biomarkers of exposure rather than the exposure source itself. Readily available biomarkers of phthalate exposure are the urinary monoester metabolites.
The chemical structure of phthalates is similar to that of naturally occurring steroid hormones, and can interact with endogenous receptors in multiple organ systems. As such, phthalates belong to a class of synthetic chemicals referred to as “endocrine disruptors”. The ability of phthalates to mimic, block, or alter normal signaling in hormone responsive tissues in vitro is well accepted.
Phthalates are present in adult urine for at least 48 hours after exposure via ingestion, with a half-life of 4–24 hours depending on the specific phthalate and metabolite evaluated.(28–32) The half-life in preterm infants is likely longer due to immature enzymatic, intestinal, hepatic, and renal function.(33, 34) Phthalate toxicity during critical periods of developmental programming results in adverse outcomes that do not manifest until later in life.(21, 23, 35)
Prenatal or early childhood exposure to both low- and high-molecular weight phthalates (LMWP; HMWP) are associated with alterations in multiple domains of neurodevelopment(25, 36). Elevated in utero exposures to mixtures of HMWP, those likely prevalent in NICU equipment, are associated with worse performance in studies of term-born infant executive function, attention, and motor reflexes(26, 33). Exposure to DEHP, a well-studied ubiquitous HMWP, during the third trimester is associated independently with impairments in cognitive, motor, and executive function, as well as with hyperactivity, poor attention,(24, 35) and autism spectrum behaviors(37) through middle childhood.(21, 23, 35, 38)
Multiple groups, including ours, hypothesize that NICU-based exposure to phthalates contributes to altered development in preterm infants.(4, 39–41) We previously demonstrated the association of NICU-based phthalate exposure with early neurobehavioral outcome in a cohort of premature infants.(27) In the present analysis, we extend that work to use urinary biomarkers of exposure to identify potential NICU-based sources of exposure to clinically relevant phthalate mixtures.
MATERIALS/SUBJECTS AND METHODS
Participant identification and enrollment
Between September 2011 and June 2013, very low birth weight infants (those with birth weight < 1500g) were recruited upon admission to the Mount Sinai Hospital NICU to participate in phase I of the NICU-HEALTH (Hospital Exposures and Long-Term Health) study, part of the DINE (Developmental Impact of NICU Exposures) cohort included in the ECHO (Environmental influences on Child Health Outcomes) program (ClinicalTrials.gov NCT01420029, NCT01963065, NCT03061890; http://www.nih.gov/echo). Very low birth weight infants were targeted as those with a NICU stay of duration long enough (generally 4 weeks or more) to convey exposure to the hospital environment with the potential to impact long-term outcome during the sensitive third trimester developmental stage. Length of stay in the NICU tends to be bimodal, with a subset of infants born close to or at term and with birth weight > 2000g admitted to the NICU for 72 hours or less. We wanted to focus our efforts on infants with chronic exposure, so defined our population to target longer-stay patients. Birth weight was chosen as a more objective enrolment criterion than gestational age: as it is not always possible to accurately estimate gestational age and as the population of term infants with birth weight less than 1500g is exceedingly small, we felt that weight was the preferred objective entry criteria. Informed consent to participate in the study was obtained from participants’ parents prior to enrollment. The institutional review board of the Icahn School of Medicine at Mount Sinai approved the study.
Specimen collection
Urine was collected by trained study staff between birth and NICU discharge using noninvasive methods described previously.(27) Briefly, cotton balls were placed in the infant diaper and retrieved three hours later. Urine was squeezed from cotton not contaminated with stool, specimens were immediately refrigerated on the unit, then frozen within 6 hours at −80°C pending batch analysis. Urine specific gravity was measured by refractometry (Atago PAL-10S) prior to freezing. Initially, the study protocol called for collection of 3 urine specimens spanning the NICU hospitalization, with the first collection during the first week of life. After an initial start-up period, urine specimens were collected on a weekly basis throughout the NICU stay. Contamination from collection materials was not anticipated as we measured phthalate metabolites in urine rather than the parent phthalate diester prior to in vivo conversion. Nonetheless, collection materials were screened for phthalate metabolite contamination. Field blanks were also analyzed for phthalate metabolites.
Urinary phthalate biomarker analysis
As described previously(27), concentrations of fifteen metabolites of eleven phthalate diesters were measured. These were monomethyl phthalate (MMP), monoethyl phthalate (MEP), mono-iso-propyl phthalate (MiPP), mono-n-pentyl phthalate (MnPP), monobenzyl phthalate (MBzP), mono-n-butyl phthalate (MnBP), mono-iso-butyl phthalate (MiBP), mono(3-carboxypropyl) phthalate (MCPP), mono-n-octyl phthalate (MOP), mono(2-ethylhexyl) phthalate (MEHP), mono(2-ethyl-5-hydroxyhexyl) phthalate (MEHHP), mono(2-ethyl-5-oxohexyl) phthalate (MEOHP), mono(2-ethyl-5-carboxypentyl) phthalate (MECPP), mono(2-carbox-hexyl) phthalate (MCHP), mono-iso-nonyl phthalate (MiNP). All analyses were completed at the Senator Frank R. Lautenberg Environmental Health Sciences Laboratory at the Icahn School of Medicine at Mount Sinai via enzymatic deconjugation of glucuronidated phthalate metabolites, solid phase extraction, and triple quadrupole mass spectrometry. Samples, reagent blanks, and quality control (QC) materials were analyzed in random order and processed identically. QC material consisted of a pooled urine matrix spiked with low, medium, and high levels of target analytes. The National Institute of Standards and Technology reference material 3673 Organic Contaminants in Non-Smokers’ Urine was analyzed along with the study samples to ensure the accuracy and reliability of the data. Supplementary detail on laboratory analysis techniques can be found in Appendix 1.
Equipment Exposure Assessment
From enrollment until NICU discharge, trained study staff kept a prospective daily inventory of medical equipment exposure for each infant. This inventory included equipment and supplies directly in contact with the infant by name and brand, as well as all elements of circuits directly communicating with the infant that could contribute to inhalational or intravenous exposure (Appendix 2). For example, a participant infant receiving intravenous (IV) crystalloid solution would have every element of the IV set up recorded: the IV base bag, IV tubing, filters, IV hub or stop-cock, IV catheter, etc. These exposures were subsequently collapsed into 7 categories representing bundles used for a dedicated purpose: peripheral IV access, central venous or arterial access, IV access for blood product transfusion, invasive respiratory support, non-invasive respiratory support, gavage feeding, and the bed type (closed incubator vs open crib). For each day of tracking, categories were assigned a value of 0 or 1, corresponding to the absence or presence of that care bundle in contact with the infant on that day.
Neurobehavioral Assessment
The NICU Network Neurobehavioral Scale (NNNS), a standardized exam of infant neurobehavior, motor function, and stress response,(42–47) was administered by a certified examiner before NICU discharge at 33 – 37 weeks postmenstrual age (PMA). NNNS performance is reported as 13 summary scores (Supporting Information 2) of the newborn’s motor and behavioral performance and maturity and has been associated with motor, cognitive, and behavioral function in later childhood.(48) It is an established method for early detection of attention and motor deficits in preterm and toxin-exposed populations.(49–51)
Statistical Analysis
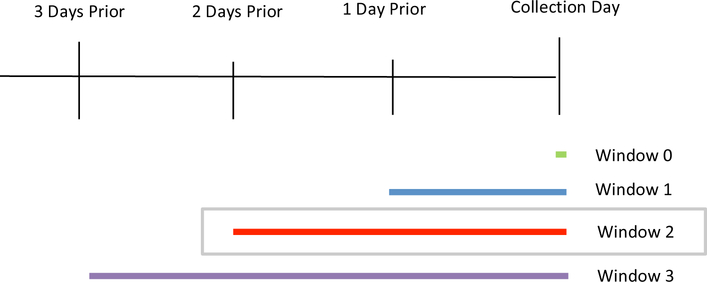
Binary exposure assignment (equipment category present or absent) was determined based on various time windows prior to urine specimen collection. As the half-life for phthalate metabolites has not been well established in the premature infant population, multiple time windows of medical equipment exposure were assessed for each biospecimen collected (Figure 1). Four consecutive windows of exposure were examined in separate models to account for the short metabolism of phthalates and to examine differences in exposure date and collection date over a 3-day period. Window 0 represented the exposure of each source only on the day of urine collection. If a participant was not exposed to any sources on the collection day, all exposure source variables were assigned 0 for that window only. Window 1 examined the day of collection and one day prior to collection. Window 2 ranged from two days prior to the day of collection and window 3 ranged from three days prior to the day of collection, assigning a value of 1 if exposed at any time within the total window. If a participant was not exposed to any source equipment category within 3 days of urine collection, all exposures source variables were assigned a value of 0. If exposure to a source occurred on multiple days within the window, the infant still received a value of 1 for exposure assignment to the specified source. Window 2 was determined to be the most relevant both due to the biological likelihood that exposure within the 48 hours prior to specimen collection would be measurable, and by empiric sensitivity analysis. Blood product transfusions are known to confer high phthalate exposure.(52) Beyond confirming that this association was seen in our data, we did not include biomarkers reflecting a transfusion in further analyses.
Figure 1:
Time windows evaluated in separate mixed models.
After ascertaining the appropriate time window to consider, we chose two distinct estimates of NICU- based phthalate exposure: (A) the log-transformed molar sum of di(2-ethylhexyl) phthalate (∑DEHP) metabolites -- (MEHP, MEHHP, MEOHP, MECPP, and MCHP) levels -- and (B) levels of clinically relevant mixtures of phthalate metabolites as identified previously using a weighted quantile sum (WQS) regression approach(53). As described previously,(27) we used a WQS approach to quantify the association between specific mixtures of phthalate metabolites and performance on individual subscales of the NICU Network Neurobehavioral Scale (NNNS).(43–47, 54) WQS regression analyses included 10 of 15 phthalate monoesters measured, all those measured above the limit of detection in greater than 85% of specimens collected from the cohort. For infants who had more than one urine collection, the geometric mean of biomarker levels was calculated across the several time points, such that one concentration per metabolite per infant was used for weight estimation.
Each of these modeling strategies included relevant covariates identified through directed acyclic graph (DAG) analysis (Appendix 3)(27, 55). As described previously,(56) we initially tested factors in our model that have been associated with neurodevelopmental outcomes of preterm infants including gestational age and size at birth, and specific medical comorbidities before and after birth. We found the following factors relevant to reduce confounding in the WQS model of the association between phthalate biomarkers and NNNS performance: infant gender, gestational age at birth, status as a small for gestational age, severity of illness at birth, and NICU-based medical morbidity. We initially planned to use the updated Clinical Risk Index for Babies (CRIB II) score(57) as a proxy for severity of illness at birth. The CRIB II includes birth weight, gestational age, infant gender, and arterial blood gas measurement of base deficit, a measure of metabolic acidosis. After reviewing our DAG, we chose to include base deficit rather than the CRIB II score to account for illness severity at birth. This reduced the risk of over-specification in the model if gestational age, birth weight, and appropriateness of birth weight were included both as components of the CRIB II and as independently variables. We created a composite dichotomous variable of NICU-based morbidity that indicated whether the infant experienced necrotizing enterocolitis, culture-proven sepsis, stage II-IV retinopathy of prematurity, grade II-IV intraventricular hemorrhage, or bronchopulmonary dysplasia. A participant was considered to have experienced a NICU-based morbidity if he/she carried one or more than one of the specified diagnoses. In models based on the sum of DEHP metabolites without consideration of neurodevelopmental outcome, the following the following factors relevant to reduce confounding: gestational age at birth, status as a small for gestational age at birth, and infant gender.
Initially, linear models were created to compare the adjusted difference in the log-transformed molar sum of DEHP between exposed and unexposed participants, per source. The same models were created using the WQS index as the endpoint. These models did not account for concurrent exposure to multiple medical equipment source categories.
To further identify specific NICU equipment contributing to higher levels of clinically relevant phthalate metabolites, we constructed mixed models using each estimate of clinically relevant phthalate exposure. Two DEHP outliers, with values greater than 2 standard deviations from the mean, were removed from this analysis to meet normality assumptions (W-Sq >0.25). In models based on estimate (B), the WQS index for each significant NNNS summary score, metabolites of DEHP contributed 20–92% of the weighted mixture with the remaining weight made up of metabolites of other phthalate diesters.(27) Within subject correlation, due to repeated measures, was accounted for in both models by including a random effect for intra-subject correlations assuming a compound symmetry correlation structure. Similarly, intra-family between subject correlation, due to the high percentage of twins and triplets in the cohort, was accounted for by including a random effect for family and a compound symmetry intra-family correlation structure. Results were considered significant if p<0.05. Analyses were conducted using SAS (version 9.4; SAS Institute Inc., Cary, NC).
RESULTS
All 148 infants meeting entry criteria during the study period were targeted for study participation. Five died prior to the enrollment discussion and two were inadvertently missed by the enrollment coordinator. Of the remaining 141 eligible children, 81 or 57% were enrolled in phase I of NICU-HEALTH. The seventy-three participants with medical equipment exposure and phthalate biomarker data available contributed 149 urine specimens to this analysis.
NNNS assessment results for the WQS-driven analyses were available for 64 of these participants. Table 1 shows demographic and clinical characteristics of the analytic cohorts. Participants ranged in gestational age at birth from 22 to 33 weeks (mean 28.3 ± 2), with birth weights ranging from 510 to 1500g (mean 1088 ± 277). 48% of infants were male, and 48% were racial/ethnic minorities. Figure 2 shows participants’ chronological age at the time of specimen collection and the number of specimens collected per participant.
Table 1:
Demographic characteristics of cohort participants
| Mean ± STD, Number (%), or Median (IQR) | |||
|---|---|---|---|
| WQS Analysis Cohort (n=64) | ∑DEHP Analysis Cohort (n=71) | ||
| Demographic factors | Private insurance (#, %, vs Medicaid) | 49 (76.6%) | 62 (80.1%) |
| Racial/ethnic minority | 31 (48.4%) | 37 (48.1%) | |
| White | 37 (57.8%) | 45 (58.4%) | |
| Black | 18 (28.1%) | 22 (28.6%) | |
| Asian | 6 (9.4%) | 7 (9.1%) | |
| Hispanic | 5 (7.8%) | 5 (6.5%) | |
| Male infant gender | 31 (48.4%) | 37 (48.1%) | |
| Maternal/antenatal factors | Maternal parity at index pregnancy | 2 (1, 3) | 2 (1, 3) |
| Maternal Age at delivery, years | 33.1 ± 7.3 | 33.2 ± 6.6 | |
| Multiple gestation | 38 (59.4%) | 43 (55.8%) | |
| Antenatal steroids | 63 (98.4%) | 76 (98.7%) | |
| Antenatal magnesium sulfate | 62 (96.9%) | 73 (94.8%) | |
| PPROM | 24 (37.5%) | 28 (36.4%) | |
| IUGR | 10 (15.6%) | 15 (19.5%) | |
| Pre-eclampsia | 12 (18.8%) | 17 (22.1%) | |
| Assisted reproductive technology | 28 (43.8%) | 31 (40.3%) | |
| Cesarean delivery | 57 (89.0%) | 68 (88.3%) | |
| Birth Characteristics | Gestational age at birth (weeks) | 28.3 ± 2.2 | 28.4 ± 2.3 |
| Birth weight (g) | 1080 ±270 | 1088±277 | |
| Small for gestational age | 4 (6.3%) | 6 (7.8%) | |
| 1 min Apgar | 8 (5, 9) | 8 (6, 9) | |
| 5 min Apgar | 9 (8, 9) | 9 (8, 9) | |
| Clinical Risk Index for Babies II Score | 8 (6, 11) | 8 (6, 11) | |
| Morbidities of prematurity | Hypotension requiring pressors | 21 (32.8%) | 23 (29.9%) |
| Bronchopulmonary dysplasia | 25 (39.1%) | 27 (35.1%) | |
| Necrotizing enterocolitis | 1 (1.6%) | 2 (2.6%) | |
| Culture proven sepsis | 11 (17.2%) | 14 (18.2%) | |
| Retinopathy of prematurity stage 2–4 | 12 (18.8%) | 12 (15.6%) | |
| Intraventricular hemorrhage grade II-IV | 7 (10.9%) | 7 (9.1%) | |
| PMA at NNNS assessment (weeks) | 34.6 ± 0.6 | N/A | |
| NICU | Duration of NICU hospitalization (days) | 69 (50, 97) | 62 (48, 93) |
| Number of urine specimens per participant | 2 (1, 3) | 2 (1, 3) | |
Figure 2:
The number and timing of collected urine specimens per infant. Each colored shape represents a urine specimen with identical shape/color combinations representing specimens from the same participant. Day of life is days since birth with the birthday being day of life 1
Ten of the 15 phthalate monoesters measured – MMP, MCPP, MEP, MECPP, MnBP, MiBP, MEHHP, MBzP, MEHP, and MEOHP – were above the limit of detection in greater than 85% of specimens collected from the cohort. Table 2 shows summary information about biomarker levels for each metabolite. Further detail can be found in previous work with this cohort.(56)
Table 2:
Distribution of specific gravity-adjusted phthalate concentrations (ng/mL)
| LOD(ng/mL) | N (%) > LOD | median | range | |
|---|---|---|---|---|
| MMP | 0.10 | 100 | 6.64 | 0.5, 67 |
| MCPP | 0.10 | 89 | 2.16 | 0.05, 19 |
| MEP | 0.10 | 100 | 21.13 | 0.8, 171 |
| MECPP | 0.10 | 100 | 49.72 | 0.2, 305 |
| MNBP | 0.10 | 100 | 36.82 | 0.3, 191 |
| MiBP | 0.10 | 100 | 15.26 | 0.3, 294 |
| MEHHP | 0.10 | 100 | 11.84 | 0.6, 165 |
| MBzP | 0.10 | 99 | 27.37 | 0.2, 289 |
| MEHP | 0.10 | 100 | 7.12 | 0.5, 72 |
| MEOHP | 0.10 | 100 | 11.95 | 0.6, 149 |
We first assessed whether estimates of phthalate exposure were higher in participants exposed to each category of medical equipment compared to those who were not exposed to that category of medical equipment, not accounting for concurrent exposure to multiple medical equipment categories. For the WQS-focused analysis, we used the most robust NNNS outcome identified previously, Attention. (27) Table 3 demonstrates the association between exposure to each of our medical equipment categories, not accounting for other concurrent equipment use, and our two estimates of phthalate exposure, ∑DEHP and NNNS Attention subscale-associated mixture. Participants with greater exposure to PIV, non-invasive respiratory support, and a closed incubator had significantly greater estimated phthalate exposure than those participants without exposure to the equipment category of interest within the 2 days prior to specimen collection. For example, a beta of 0.84 for noninvasive respiratory support and DEHP indicates that the molar sum of DEHP is E^0.84–1 or 132% higher for those exposed to noninvasive respiratory support compared to those who were not exposed to noninvasive respiratory support, not accounting for other concurrent medical equipment exposures. This analysis does not identify a specific source of exposure, as infants receiving noninvasive respiratory support often had other medical equipment exposures sources, but does directly link phthalate exposure to medical equipment use in the NICU-HEALTH cohort. This finding held for the WQS-based analysis as well, as participants receiving noninvasive respiratory support had, on average, an outcome-specific association that was 2.06 units greater than those without noninvasive respiratory support.
Table 3:
The difference in estimated phthalate exposure between specific medical equipment-exposed and non-exposed participants.
| ln (molar sum DEHP (ng/ml)) | Average adjusted WQS based on NNNS Attention subscale performance | ||||||
|---|---|---|---|---|---|---|---|
| N(exposed) | Beta | 95% CI | P-value | Beta | 95% CI | P-value | |
| PIV | 35 | 0.67 | 0.33,1.01 | <0.001* | 1.37 | 0.37, 2.36 | 0.007* |
| Invasive respiratory support | 81 | 0.35 | 0.05, 0.65 | 0.023* | 0.74 | −0.14, 1.61 | 0.101 |
| Noninvasive respiratory support | 19 | 0.84 | 0.38,1.30 | <0.001* | 2.06 | 0.72, 3.40 | 0.003* |
| Feeding tube | 126 | 0.28 | −0.14, 0.70 | 0.190 | 0.85 | −0.35, 2.06 | 0.163 |
| Central line | 25 | 0.56 | 0.13, 0.94 | 0.014* | 0.74 | −0.42, 1.90 | 0.213 |
| Incubator | 101 | 0.56 | 0.25, 0.87 | <0.001* | 1.46 | 0.56, 2.37 | 0.002* |
p<0.05
Models adjusted for the following covariates: gestational age at birth, status as small for gestational age at birth, and infant gender.
Next, we calculated the strength of association between exposure to specific medical equipment bundles and ∑DEHP in multivariable models accounting for concurrent exposures. Exposure to invasive and non-invasive respiratory support were associated with elevated biomarkers of DEHP metabolites (Table 4). Neither the presence of intravascular access (peripheral or central) nor an indwelling feeding tube were significantly associated with DEHP biomarkers in multivariable analysis.
Table 4:
Association of sources in the NICU with DEHP biomarkers
| ln molar sum DEHP (ng/ml) | |||
|---|---|---|---|
| Source of Exposure | beta | 95% CI | p-value |
| PIV | 0.19 | −0.20, 0.58 | 0.334 |
| Invasive respiratory support | 0.41 | 0.03, 0.80 | 0.036* |
| Noninvasive respiratory support | 0.86 | 0.25, 1.57 | 0.007* |
| Feeding tube | −0.16 | −0.62, 0.31 | 0.507 |
| Central line | −0.02 | −0.48, 0.43 | 0.922 |
| Incubator | 0.31 | −0.08, 0.70 | 0.120 |
p<0.05
Models adjusted for the following covariates: gestational age at birth, status as small for gestational age at birth, and infant gender.
Finally, we quantified the strength of association between exposure to specific medical equipment bundles and clinically relevant phthalate mixtures as estimated by WQS indices(27) in multivariable models. Exposure to non-invasive respiratory support was again significantly associated with clinically-relevant phthalate metabolite levels as represented by unique mixtures significantly associated with NNNS subscale performance in our previous WQS analysis (Table 5).(27)
Table 5:
Association of sources in the NICU to a WQS index estimated using weights from significant NNNS scales.
| Attention | Habituation | Handling | Excitability | Regulation | Non-Optimal Reflexes | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Relevant phthalate mixture (27) | MEOHP MEHHP | MECPP MEP MMP | MECPP MEP MMP | MEOHP MCPP MEP | MEOHP MEP | MEOHP MCPP MEP | ||||||||||||
| Source of Exposure | beta | 95% CI | p-value | beta | 95% CI | p-value | beta | 95% CI | p-value | beta | 95% CI | p-value | beta | 95% CI | p-value | beta | 95% CI | p-value |
| PIV | 0.45 | −0.66, 1.56 | 0.419 | 0.64 | −0.59, 1.87 | 0.299 | 0.44 | −0.64, 1.52 | 0.419 | 0.51 | −0.57, 1.59 | 0.352 | 0.49 | −0.62, 1.60 | 0.383 | 0.45 | −0.63, 1.53 | 0.406 |
| Invasive respiratory support | −0.53 | −0.53, 1.59 | 0.324 | 0.41 | −0.83, 1.65 | 0.512 | 0.89 | −0.12, 1.90 | 0.083 | 0.88 | −0.13, 1.88 | 0.086 | 0.64 | −0.40, 1.69 | 0.222 | 0.90 | −0.10, 1.90 | 0.077 |
| Noninvasive respiratory support | 1.87 | 0.23, 3.52 | 0.026* | 2.30 | 0.53, 4.06 | 0.011* | 2.15 | 0.59, 3.71 | 0.008* | 2.05 | 0.49, 3.61 | 0.011* | 1.89 | 0.27, 3.52 | 0.023* | 2.01 | 0.45, 3.56 | 0.012* |
| Feeding tube | −0.57 | −1.94, 0.81 | 0.412 | −0.42 | −1.89, 1.05 | 0.573 | −0.59 | −1.91, 0.72 | 0.370 | −0.53 | −1.84, 0.79 | 0.427 | −0.65 | −2.02, 0.71 | 0.341 | −0.55 | −1.86, 0.76 | 0.406 |
| Central line | −0.71 | −2.00, 0.59 | 0.279 | −1.36 | −2.87, 0.14 | 0.076 | −0.48 | −1.71, 0.76 | 0.445 | −0.56 | −1.80, 0.67 | 0.365 | −0.48 | −1.77, 0.80 | 0.457 | −0.56 | −1.80. 0.68 | 0.368 |
| Incubator | 1.16 | −0.04, 2.27 | 0.042* | 0.95 | −0.21, 2.12 | 0.107 | 0.85 | −0.24, 1.94 | 0.123 | 0.76 | −0.33, 1.85 | 0.169 | 1.04 | −0.08, 2.15 | 0.068 | 0.78 | −0.30, 1.86 | 0.153 |
p <0.05
- WQS regression analyses included 10 of 15 phthalate monoesters measured, all those measured above the limit of detection in greater than 85% of specimens collected from the cohort. Models adjusted for the following covariates: gestational age at birth, status as small for gestational age at birth, and infant gender.
- MEOHP = mono(2-ethyl-5-oxohexyl) phthalate; MEHHP = mono(2-ethyl-5-hydrohexyl) phthalate; MECPP = mono(2-ethyl-5-carboxypentyl) phthalate; MEP = monoethyl phthalate; MMP = monomethyl phthalate; MCPP = mono(3-carboxypropyl) phthalate
DISCUSSION
In this report, we document an association between exposure to non-invasive respiratory support in the NICU and phthalate mixtures with known association to neurobehavioral development in a cohort of premature infants. Our findings are novel: noninvasive respiratory support – the equipment bundles used to deliver pressure and supplemental oxygen via nasal cannula – may be a significant source of exposure to potentially neuroactive phthalates in the NICU. As previous studies and hypotheses of hospital-based phthalate exposure have focused on intravenous and enteral exposure to phthalates, this unexpected finding offers a new avenue for exposure reduction.
Phthalates are common in medical materials used in the NICU(15, 16, 52, 58), and multiple groups have documented elevated biomarkers of phthalate metabolites in NICU patients.(13, 14, 17, 59) In order to estimate phthalate exposures, research groups have used single metabolites, the sum of the multiple metabolite breakdown products of di(2-ethylhexyl) phthalate (DEHP) (21, 22), phthalate mixtures based on molecular weight (low versus high) (23–26), or empirically derived mixtures of bioactive phthalate metabolites.(27) In a pilot study of phthalate exposure in the NICU conducted in 2003, Green et a/.(14) demonstrated an association between intensity of medical care and phthalate biomarker levels, but did not identify specific exposure source materials. Numerous materials used in NICU care, such as respiratory circuits, intravenous equipment, enteral feeding supplies, and incubators are made from plastics that could act as vehicles of phthalate exposure.(58) Beyond that limited, single center study, the relationship between phthalate exposure in the NICU and specific care practices has not been comprehensively explored. A more recent secondary analysis of published data calculated the dose-response toxicity thresholds for DEHP, and estimated the magnitude of DEHP exposures in NICU infants from polyvinyl chloride devices.(40) This report estimated that daily DEHP intake reaches 16 mg/kg/day for critically ill preterm infants; this is on the order of 4,000 and 160,000 times higher than desired to avoid reproductive and hepatic toxicities, respectively.(40) Our study confirmed significant exposure to phthalates in the NICU. The 10 monoester metabolites detected in >85% of specimens collected represent exposure to two high molecular weight phthalates (di-n-octyl phthalate (DOP) and di(2-ethylhexyl) phthalate (DEHP)) and five low molecular weight phthalates (dimethyl phthalate (DMP), diethyl phthalate (DEP), butylbenzyl phthalate (BBzP), di-n-butyl phthalate (DnBP), di-iso-butyl phthalate (DiBP)). A small number of previous human studies have documented exposure to DEHP metabolites and select other phthalate metabolites in the NICU.(60–62) This is the first study to document NICU-based exposure to DOP. Specific gravity-adjusted urinary phthalate concentrations were lower than reported in older studies of NICU patients,(13, 14, 17) but comparable to or higher than exposure in a termborn cohort born at approximately the same time as our participants.(61)
In the present study, we provide evidence that a major clinically relevant source of phthalate exposure in the NICU may be non-invasive respiratory support circuits. From a neonatal care perspective, this finding is impactful due to the wide use of non-invasive respiratory support (i.e. via nasal cannula) preferentially over invasive respiratory support (i.e. via endotracheal tube) to protect infants from the barotrauma and tracheal injury associated with intubation and mechanical ventilation. The majority of NICU inpatients require noninvasive respiratory support during their hospitalization, sometimes for a prolonged period of weeks to months, making the population of exposed infants likely 200,000 or more every year in the United States alone. The finding that noninvasive respiratory support circuits may convey exposure to potentially neuroactive phthalates provides a possible explanation for the lack of strong association between classical markers of severe NICU illness and the neurobehavioral impairments common among NICU graduates.(63, 64) Many infants, particularly those born in the moderate or late preterm period, require prolonged non-invasive respiratory support for pulmonary immaturity but have relatively low incidence of physiological derangements, hypoxia, intracranial hemorrhage, sepsis, and other major morbidities of prematurity. If phthalate exposure from non-invasive respiratory support contributes to adverse long-term behavioral outcome in children who have an otherwise benign perinatal course, the result could contribute to the disconnect between benign medical history and poor behavioral outcome that is well-documented in the premature population.(11)
Additionally, we found an association between mixtures of phthalates associated with performance on the NNNS Attention subscale and care in an incubator rather than an open crib. This finding was only for this specific mixture, and could indicate an association between the incubator plastic directly, or from the existence of a closed environment potentially entrapping volatile organics.
Our study has a number of strengths. It was designed to address limitations of the few other biomarker-based studies of NICU-based phthalate exposure in the literature,(13, 14, 17) which were limited by cross-sectional design, heterogeneous enrollment without attention to factors likely to affect phthalate metabolism (e.g., gestational age, PMA), and absence of prospectively-designed clinical outcome assessments. All study participants were cared for in the same unit using standardized care approaches. Although individual care practices may have varied somewhat, the level of medical intervention for a given clinical presentation was fairly uniform in this single ICU population, reducing the risk of confounding by provision of medical care. Our statistical approach focused on mixtures is highly relevant to studies of NICU-based phthalate exposure. NICU inpatients are exposed to phthalate mixtures through the complex materials used concurrently in NICU care; respiratory circuits, intravenous equipment, enteral feeding supplies, and incubators are likely vehicles of exposure to different phthalate mixtures and are rarely used in isolation.(58)
Our study does have some limitations. Our patient population, very low birth weight infants cared for at a level IV academic regional perinatal center, may not be representative of the entire NICU population. As data on “typical” community-based exposure to phthalates in early infancy in non-NICU and non-preterm populations is not available (the youngest patients with phthalate biomarker measures in the National Health and Nutrition Examination Survey,(65) for example, are age 6), and comparisons to a relevant non-NICU group is not possible. The half-life of phthalate diester metabolism in preterm infants is not well characterized, but is known to vary between 4 and 24 hours in adults.(28, 29, 31, 66) This short half-life indicates that phthalate levels likely reflect source exposure in the preceding day or two. As is common in environmental health research, we used the geometric mean of serial participant biomarker levels to estimate changing exposure over the course of the NICU stay. For participants with only a single biomarker level available, that biomarker was used in place of the geometric mean in all analyses. If a different estimate (e.g. peak, arithmetic mean, median) is actually the best estimate of clinically-relevant phthalate exposure during the NICU stay, we may have under- or over-estimated exposure for patients with wider ranges of biomarker levels. Although used in other NICU(49, 54, 67) and environmental health(26, 68, 69) studies, the NNNS is a very early outcome measure and may not be fully representative of later development. Covariates were selected using rigorous and accepted methodology; nonetheless it is possible that confounders not considered or not measured could impact results. Given the limited sample size in this study, we were unable to consider sex-specific associations that have been reported in some of the previous studies of phthalates and neurobehavioral outcomes. Possible episodic NICU-based sources of phthalate including contact with fragranced personal care products worn by family members or NICU staff in contact with the infant, in cleaning supplies used in the NICU, in medications administered to the infant, or contamination of home-pumped breast milk that could vary with maternal diet or mode of collection could not be rigorously addressed. As the wearing of fragrance is discouraged in the NICU, as cleaning with volatile chemicals is not performed inside the closed incubator when occupied, and as the phthalates are primarily added to medications in pill form that are not used by neonates, these potential exposure sources are likely minimal. Additionally, as the focus of this cohort study is modifiable NICU-based phthalate exposure, it was not designed to assess potential prenatal exposure. These deficiencies will be addressed in ongoing larger studies.
Although many NICUs - including ours - are increasingly labeled “phthalate-free” to signify the use of DEHP-free equipment whenever available, replacements for DEHP are often other phthalates(70) or non-phthalate organic chemicals that may be neuro-active but not included in our current biomarker testing panel. Nonetheless, modern NICUs convey significant DEHP and measureable non-DEHP phthalate exposure through the many plastic medical materials that are not available in phthalate-free form.(58)
CONCLUSION
Non-invasive respiratory support circuits may be an important source of phthalate exposure in the NICU. Further studies evaluating long-term multi-organ system outcomes in a larger population are needed to confirm this association. Exposure reduction efforts in the hospital setting may need to be broadened to include inhalational sources of phthalate exposure.
Supplementary Material
Appendix 3: Directed acyclic graphs depicting covariate selection. Models based on WQS regressions involving NNNS subscales included covariates representing severity of illness at birth (base deficit on central blood sampling in the first 12 hours of life), gestational age at birth, a variable representing composite medical illness in the NICU, and gender. For models based on DEHP exposure that did not take into accounte neurodevelopmental outcome (i.e., NNNS performance), gestational age at birth, status as a small for gestational age at birth, and infant gender were included as covariates in the model.
ACKNOWLEDGEMENTS
Dr. Stroustrup is supported by a career development award, K23ES022268, from the National Institutes of Environmental Health Sciences (NIEHS) and a cooperative agreement, UG3OD023320, from the National Institutes of Health for the Environmental Influences on Child Health Outcomes (ECHO) program. Additional funding for the investigators and this project came through pilot grants from the Passport Foundation, the Mount Sinai Children’s Environmental Health Center, and an NIEHS center grant P30ES023515.
Funding Sources: Dr. Stroustrup is supported by a career development award, K23ES022268, from the National Institutes of Environmental Health Sciences (NIEHS) and a cooperative agreement, UG3OD023320, from the National Institutes of Health for the Environmental Influences on Child Health Outcomes (ECHO) program. Additional funding for the investigators and this project came through pilot grants from the Passport Foundation, the Mount Sinai Children’s Environmental Health Center, and an NIEHS center grant P30ES023515.
Abbreviations
- DEHP
di(2-ethylhexyl) phthalate
- LOD
level of detection
- NICU
neonatal intensive care unit
- NICU-HEALTH
NICU Hospital Exposures and Long-Term Health study
- NNNS
NICU Network Neurobehavioral Scale
- PMA
postmenstrual age
- PIV
peripheral intravenous catheter
- QC
quality control
- WQS
weighted quantile sum
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest or financial relationships relevant to this article to disclose.
REFERENCES
- 1.Barker DJ. The origins of the developmental origins theory. J Intern Med. 2007;261(5):412–7. [DOI] [PubMed] [Google Scholar]
- 2.Meredith RM. Sensitive and critical periods during neurotypical and aberrant neurodevelopment: a framework for neurodevelopmental disorders. Neurosci Biobehav Rev. 2015;50:180–8. [DOI] [PubMed] [Google Scholar]
- 3.Mueller CA, Eme J, Burggren WW, Roghair RD, Rundle SD. Challenges and opportunities in developmental integrative physiology. Comp Biochem Physiol A Mol Integr Physiol. 2015;184:113–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stroustrup A, Teitelbaum SL, Aschner JL. The Value of Preterm Infant Environmental Health Cohorts: The Canary in the Coal Mine. JAMA Pediatr. 2017;171(12):1139–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kraft AD, Aschner M, Cory-Slechta DA, Bilbo SD, Caudle WM, Makris SL. Unmasking silent neurotoxicity following developmental exposure to environmental toxicants. Neurotoxicol Teratol. 2016;55:38–44. [DOI] [PubMed] [Google Scholar]
- 6.Mundy WR, Padilla S, Breier JM, Crofton KM, Gilbert ME, Herr DW, et al. Expanding the test set: Chemicals with potential to disrupt mammalian brain development. Neurotoxicol Teratol. 2015;52(Pt A):25–35. [DOI] [PubMed] [Google Scholar]
- 7.Aarnoudse-Moens CS, Weisglas-Kuperus N, van Goudoever JB, Oosterlaan J. Meta-analysis of neurobehavioral outcomes in very preterm and/or very low birth weight children. Pediatrics. 2009;124(2):717–28. [DOI] [PubMed] [Google Scholar]
- 8.Scharf RJ, Stroustrup A, Conaway MR, DeBoer MD. Growth and development in children born very low birthweight. Arch Dis Child Fetal Neonatal Ed. 2016;101(5):F433–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Delobel-Ayoub M, Arnaud C, White-Koning M, Casper C, Pierrat V, Garel M, et al. Behavioral problems and cognitive performance at 5 years of age after very preterm birth: the EPIPAGE Study. Pediatrics. 2009;123(6):1485–92. [DOI] [PubMed] [Google Scholar]
- 10.Treyvaud K, Ure A, Doyle LW, Lee KJ, Rogers CE, Kidokoro H, et al. Psychiatric outcomes at age seven for very preterm children: rates and predictors. J Child Psychol Psychiatry. 2013;54(7):772–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Andrews B, Lagatta J, Chu A, Plesha-Troyke S, Schreiber M, Lantos J, et al. The Non-impact of Gestational Age on Neuro-developmental Outcome for Ventilated Survivors Born at 23 – 28 Weeks Gestation. Acta Paediatr. 2012;101(6):574–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Conrad AL, Richman L, Lindgren S, Nopoulos P. Biological and environmental predictors of behavioral sequelae in children born preterm. Pediatrics. 2010;125(1):e83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Calafat AM, Needham LL, Silva MJ, Lambert G. Exposure to di-(2-ethylhexyl) phthalate among premature neonates in a neonatal intensive care unit. Pediatrics. 2004;113(5):e429–34. [DOI] [PubMed] [Google Scholar]
- 14.Green R, Hauser R, Calafat AM, Weuve J, Schettler T, Ringer S, et al. Use of di(2-ethylhexyl) phthalate-containing medical products and urinary levels of mono(2-ethylhexyl) phthalate in neonatal intensive care unit infants. Environmental health perspectives. 2005;113(9):1222–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Latini G, De Felice C, Del Vecchio A, Barducci A, Ferri M, Chiellini F. Di-(2-ethylhexyl)phthalate leakage and color changes in endotracheal tubes after application in high-risk newborns. Neonatology. 2009;95(4):317–23. [DOI] [PubMed] [Google Scholar]
- 16.Latini G, Ferri M, Chiellini F. Materials degradation in PVC medical devices, DEHP leaching and neonatal outcomes. Curr Med Chem. 2010;17(26):2979–89. [DOI] [PubMed] [Google Scholar]
- 17.Weuve J, Sanchez BN, Calafat AM, Schettler T, Green RA, Hu H, et al. Exposure to phthalates in neonatal intensive care unit infants: urinary concentrations of monoesters and oxidative metabolites. Environmental health perspectives. 2006;114(9):1424–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Department of Health and Human Services, Centers for Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals; 2009. Atlanta, GA. [Google Scholar]
- 19.Calafat AM, Ye X, Silva MJ, Kuklenyik Z, Needham LL. Human exposure assessment to environmental chemicals using biomonitoring. Int J Androl. 2006;29(1):166–71; discussion 81–5. [DOI] [PubMed] [Google Scholar]
- 20.Ventrice P, Ventrice D, Russo E, De Sarro G. Phthalates: European regulation, chemistry, pharmacokinetic and related toxicity. Environ Toxicol Pharmacol. 2013;36(1):88–96. [DOI] [PubMed] [Google Scholar]
- 21.Tellez-Rojo MM, Cantoral A, Cantonwine DE, Schnaas L, Peterson K, Hu H, et al. Prenatal urinary phthalate metabolites levels and neurodevelopment in children at two and three years of age. Sci Total Environ. 2013;461–462:386–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Whyatt RM, Liu X, Rauh VA, Calafat AM, Just AC, Hoepner L, et al. Maternal prenatal urinary phthalate metabolite concentrations and child mental, psychomotor, and behavioral development at 3 years of age. Environ Health Perspect. 2012;120(2):290–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cho SC, Bhang SY, Hong YC, Shin MS, Kim BN, Kim JW, et al. Relationship between environmental phthalate exposure and the intelligence of school-age children. Environ Health Perspect. 2010;118(7):1027–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park S, Kim BN, Cho SC, Kim Y, Kim JW, Lee JY, et al. Association between urine phthalate levels and poor attentional performance in children with attention-deficit hyperactivity disorder with evidence of dopamine gene-phthalate interaction. Int J Environ Res Public Health. 2014;11(7):6743–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Swan SH. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ Res. 2008;108(2):177–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yolton K, Xu Y, Strauss D, Altaye M, Calafat AM, Khoury J. Prenatal exposure to bisphenol A and phthalates and infant neurobehavior. Neurotoxicol Teratol. 2011;33(5):558–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stroustrup A, Bragg JB, Andra S, Curtin P, Spear EA, Sison DB, et al. Neonatal intensive care unit phthalate exposure and preterm infant neurobehavioral performance. PLoS ONE. 2018;13(3):e0193835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson WA, Castle L, Hird S, Jeffery J, Scotter MJ. A twenty-volunteer study using deuterium labelling to determine the kinetics and fractional excretion of primary and secondary urinary metabolites of di-2- ethylhexylphthalate and di-iso-nonylphthalate. Food Chem Toxicol. 2011;49(9):2022–9. [DOI] [PubMed] [Google Scholar]
- 29.Kessler W, Numtip W, Volkel W, Seckin E, Csanady GA, Putz C, et al. Kinetics of di(2-ethylhexyl) phthalate (DEHP) and mono(2-ethylhexyl) phthalate in blood and of DEHP metabolites in urine of male volunteers after single ingestion of ring-deuterated DEHP. Toxicol Appl Pharmacol. 2012;264(2):284–91. [DOI] [PubMed] [Google Scholar]
- 30.Koch HM, Angerer J. Di-iso-nonylphthalate (DINP) metabolites in human urine after a single oral dose of deuterium-labelled DINP. Int J Hyg Environ Health. 2007;210(1):9–19. [DOI] [PubMed] [Google Scholar]
- 31.Koch HM, Bolt HM, Preuss R, Angerer J. New metabolites of di(2-ethylhexyl)phthalate (DEHP) in human urine and serum after single oral doses of deuterium-labelled DEHP. Arch Toxicol. 2005;79(7):367–76. [DOI] [PubMed] [Google Scholar]
- 32.Leng G, Koch HM, Gries W, Schutze A, Langsch A, Bruning T, et al. Urinary metabolite excretion after oral dosage of bis(2-propylheptyl) phthalate (DPHP) to five male volunteers--characterization of suitable biomarkers for human biomonitoring. Toxicol Lett. 2014;231(2):282–8. [DOI] [PubMed] [Google Scholar]
- 33.Engel SM, Zhu C, Berkowitz GS, Calafat AM, Silva MJ, Miodovnik A, et al. Prenatal phthalate exposure and performance on the Neonatal Behavioral Assessment Scale in a multiethnic birth cohort. Neurotoxicology. 2009;30(4):522–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferguson KK, Peterson KE, Lee JM, Mercado-Garcia A, Blank-Goldenberg C, Tellez-Rojo MM, et al. Prenatal and peripubertal phthalates and bisphenol A in relation to sex hormones and puberty in boys. Reprod Toxicol. 2014;47:70–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim Y, Ha EH, Kim EJ, Park H, Ha M, Kim JH, et al. Prenatal exposure to phthalates and infant development at 6 months: prospective Mothers and Children’s Environmental Health (MOCEH) study. Environ Health Perspect. 2011;119(10):1495–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ejaredar M, Nyanza eC, Ten Eycke K, Dewey D Phthalate exposure and childrens neurodevelopment: A systematic review. Environ Res. 2015;142:51–60. [DOI] [PubMed] [Google Scholar]
- 37.Testa C, Nuti F, Hayek J, De Felice C, Chelli M, Rovero P, et al. Di-(2-ethylhexyl) phthalate and autism spectrum disorders. aSn Neuro. 2012;4(4):223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Factor-Litvak P, Insel B, Calafat AM, Liu X, Perera F, Rauh VA, et al. Persistent Associations between Maternal Prenatal Exposure to Phthalates on Child IQ at Age 7 Years. PLoS One. 2014;9(12):e114003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fischer CJ, Bickle Graz M, Muehlethaler V, Palmero D, Tolsa JF. Phthalates in the NICU: is it safe? J Paediatr Child Health. 2013;49(9):E413–9. [DOI] [PubMed] [Google Scholar]
- 40.Mallow EB, Fox MA. Phthalates and critically ill neonates: device-related exposures and non-endocrine toxic risks. J Perinatol. 2014;34(12):892–7. [DOI] [PubMed] [Google Scholar]
- 41.Santos J, Pearce SE, Stroustrup A . Impact of hospital-based environmental exposures on neurodevelopmental outcomes of preterm infants. Curr Opin Pediatr. 2015;27(2):254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boukydis CF, Bigsby R, Lester BM. Clinical use of the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics. 2004;113(3 Pt 2):679–89. [PubMed] [Google Scholar]
- 43.Lester BM, Tronick EZ. History and description of the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics. 2004;113(3 Pt 2):634–40. [PubMed] [Google Scholar]
- 44.Lester BM, Tronick EZ, Brazelton TB. The Neonatal Intensive Care Unit Network Neurobehavioral Scale procedures. Pediatrics. 2004;113(3 Pt 2):641–67. [PubMed] [Google Scholar]
- 45.Lester BM, Tronick EZ, LaGasse L, Seifer R, Bauer CR, Shankaran S, et al. Summary statistics of neonatal intensive care unit network neurobehavioral scale scores from the maternal lifestyle study: a quasinormative sample. Pediatrics. 2004;113(3 Pt 2):668–75. [PubMed] [Google Scholar]
- 46.Tronick E, Lester BM. Grandchild of the NBAS: the NICU network neurobehavioral scale (NNNS): a review of the research using the NNNS. J Child Adolesc Psychiatr Nurs. 2013;26(3):193–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tronick EZ, Olson K, Rosenberg R, Bohne L, Lu J, Lester BM. Normative neurobehavioral performance of healthy infants on the Neonatal Intensive Care Unit Network Neurobehavioral Scale. Pediatrics. 2004;113(3 Pt 2):676–8. [PubMed] [Google Scholar]
- 48.Liu J, Bann C, Lester B, Tronick E, Das A, Lagasse L, et al. Neonatal neurobehavior predicts medical and behavioral outcome. Pediatrics. 2010;125(1):e90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lester BM, Tronick EZ, LaGasse L, Seifer R, Bauer CR, Shankaran S, et al. The maternal lifestyle study: effects of substance exposure during pregnancy on neurodevelopmental outcome in 1-month-old infants. Pediatrics. 2002;110(6):1182–92. [DOI] [PubMed] [Google Scholar]
- 50.Montirosso R, Del Prete A, Bellu R, Tronick E, Borgatti R, Neonatal Adequate Care for Quality of Life Study G. Level of NICU quality of developmental care and neurobehavioral performance in very preterm infants. Pediatrics. 2012;129(5):e1129–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Smith JR, McGrath J, Brotto M, Inder T. A randomized-controlled trial pilot study examining the neurodevelopmental effects of a 5-week M Technique intervention on very preterm infants. Adv Neonatal Care. 2014;14(3):187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sampson J, de Korte D. DEHP-plasticised PVC: relevance to blood services. Transfus Med. 2011;21(2):73–83. [DOI] [PubMed] [Google Scholar]
- 53.Carrico C, Gennings C, Wheeler D, Factor-Litvak P. Characterization of a weighted quartile sum regression for highly correlated data in a risk analysis setting. J Agric Biol Environ Stat. 2014;20(1):100–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lester BM, Andreozzi-Fontaine L, Tronick E, Bigsby R. Assessment and evaluation of the high risk neonate: the NICU Network Neurobehavioral Scale. J Vis Exp. 2014(90). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Textor J, Hardt J, Knuppel S. DAGitty: a graphical tool for analyzing causal diagrams. Epidemiology. 2011;22(5):745. [DOI] [PubMed] [Google Scholar]
- 56.Stroustrup A, Bragg JB, Andra S, Curtin P, Spear EA, Sison DB, et al. Neonatal intensive care unit phthalate exposure and preterm infant neurobehavioral performance. PLoS ONE. 2018;13(3):e0193835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Parry G, Tucker J, Tarnow-Mordi W, Group UKNSSC. CRIB II: an update of the clinical risk index for babies score. Lancet. 2003;361(9371):1789–91. [DOI] [PubMed] [Google Scholar]
- 58.Van Vliet ED, Reitano EM, Chhabra JS, Bergen GP, Whyatt RM. A review of alternatives to di (2- ethylhexyl) phthalate-containing medical devices in the neonatal intensive care unit. Journal of perinatology : official journal of the California Perinatal Association. 2011;31(8):551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Su PH, Chang YZ, Chang HP, Wang SL, Haung HI, Huang PC, et al. Exposure to di(2-ethylhexyl) phthalate in premature neonates in a neonatal intensive care unit in Taiwan. Pediatr Crit Care Med. 2012;13(6):671–7. [DOI] [PubMed] [Google Scholar]
- 60.Demirel A, Coban A, Yildirim S, Dogan C, Sanci R, Ince Z. Hidden Toxicity in Neonatal Intensive Care Units: Phthalate Exposure in Very Low Birth Weight Infants. J Clin Res Pediatr Endocrinol. 2016;8(3):298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Frederiksen H, Kuiri-Hanninen T, Main KM, Dunkel L, Sankilampi U. A longitudinal study of urinary phthalate excretion in 58 full-term and 67 preterm infants from birth through 14 months. Environ Health Perspect. 2014;122(9):998–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Strommen K, Lyche JL, Blakstad EW, Moltu SJ, Veierod MB, Almaas AN, et al. Increased levels of phthalates in very low birth weight infants with septicemia and bronchopulmonary dysplasia. Increased levels of phthalates in very low birth weight infants with septicemia and bronchopulmonary dysplasia. 2016;89–90:228–34. [DOI] [PubMed] [Google Scholar]
- 63.Johnson S, Marlow N. Preterm birth and childhood psychiatric disorders. Pediatr Res. 2011;69(5 Pt 2):11R–8R. [DOI] [PubMed] [Google Scholar]
- 64.Montagna A, Nosarti C. Socio-Emotional Development Following Very Preterm Birth: Pathways to Psychopathology. Front Psychol. 2016;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Services DoHaH. Fourth National Report on Human Exposure to Environmental Chemicals. 2009. Atlanta, GA. [Google Scholar]
- 66.Koch HM, Muller J, Angerer J. Determination of secondary, oxidised di-iso-nonylphthalate (DINP) metabolites in human urine representative for the exposure to commercial DINP plasticizers. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;847(2):114–25. [DOI] [PubMed] [Google Scholar]
- 67.Madlinger-Lewis L, Reynolds L, Zarem C, Crapnell T, Inder T, Pineda R. The effects of alternative positioning on preterm infants in the neonatal intensive care unit: a randomized clinical trial. Res Dev Disabil. 2014;35(2):490–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Donauer S, Chen A, Xu Y, Calafat AM, Sjodin A, Yolton K. Prenatal exposure to polybrominated diphenyl ethers and polyfluoroalkyl chemicals and infant neurobehavior. J Pediatr. 2015;166(3):736–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yolton K, Xu Y, Sucharew H, Succop P, Altaye M, Popelar A, et al. Impact of low-level gestational exposure to organophosphate pesticides on neurobehavior in early infancy: a prospective study. Environmental health : a global access science source. 2013;12(1):79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bernard L, Decaudin B, Lecoeur M, Richard D, Bourdeaux D, Cueff R, et al. Analytical methods for the determination of DEHP plasticizer alternatives present in medical devices: a review. Talanta. 2014;129:39–54. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 3: Directed acyclic graphs depicting covariate selection. Models based on WQS regressions involving NNNS subscales included covariates representing severity of illness at birth (base deficit on central blood sampling in the first 12 hours of life), gestational age at birth, a variable representing composite medical illness in the NICU, and gender. For models based on DEHP exposure that did not take into accounte neurodevelopmental outcome (i.e., NNNS performance), gestational age at birth, status as a small for gestational age at birth, and infant gender were included as covariates in the model.