Abstract
OBJECTIVE
To better understand how to educate patients and providers about study findings relevant to treatment guidelines, we assessed pre- versus post-Women’s Health Initiative (WHI) differences in menopausal hormone therapy (MHT) initiation and continuation and their correlates, and in women’s reasons for initiation and discontinuation.
METHODS
We analyzed survey data from up to 14 approximately annual visits over 17 years (1996–2013) from 3018 participants in the Study of Women’s Health Across the Nation, a prospective cohort study. We used logistic regression to compare pre- versus post-WHI associations of covariates with MHT initiation and continuation, and to compare pre- versus post-WHI reasons for initiation and continuation.
RESULTS
MHT initiation dropped from 8.6% pre-WHI to 2.8% post-WHI (p<.0001), and the corresponding decrease in MHT continuation was 84.0% to 62.0% (p<.0001). Decreases in MHT initiation and continuation occurred across a range of participant subgroups, consistent with wide dissemination of post-WHI recommendations. However, contrary to current guidelines, we found large declines in MHT use in subgroups for whom MHT is often recommended, i.e., younger women and those with more vasomotor symptoms. Post-WHI, women’s reasons for MHT initiation and discontinuation reflected concerns highlighted by WHI results. The largest declines in initiation reasons were for reducing risks of osteoporosis and heart disease, while the largest increases in discontinuation reasons were for media reports and provider advice.
CONCLUSIONS
Immediate post-WHI recommendations for MHT use were widely adopted. MHT risks documented in older women, however, may have led younger symptomatic women to forgo MHT for symptom relief.
Keywords: hormone therapy, vasomotor symptoms, Women’s Health Initiative
After the July 2002 Women’s Health Initiative (WHI) trial announcement that estrogen-progestin therapy was being halted prematurely due to safety concerns, prevalence of prescription menopausal hormone therapy (MHT) declined dramatically.1–3 Less clear is whether trends were similar for MHT initiation and continuation, and whether declines differed by factors such as frequency of vasomotor symptoms (VMS), for which MHT is recognized as the most effective treatment.4–6 Also not well studied are changes in women’s reasons for initiating or discontinuing MHT. MHT use recommendations have been modified since 2002 in response to additional information on risks and benefits,7 moving from a uniform guideline for the shortest duration at the lowest possible dose primarily in women younger than 60 years8–9 to guidelines allowing for greater flexibility and individualized treatment.4–6,10–12 Moreover, recent WHI findings indicate no MHT-related difference in long-term all-cause mortality.13 In light of these updates, it is important to assess women’s and providers’ responsiveness to shifts in clinical evidence and recommendations, to understand how to improve education regarding new findings with implications for treatment guidelines.
A number of studies of MHT trends utilized pharmacy or insurance databases, with large samples and information on preparation and prescription dates. However, many cannot distinguish initiation or continuation from prevalence, and have limited or no data on correlates or reasons for initiation and discontinuation. Conversely, many studies with woman-level data cannot make explicit pre- versus post-WHI comparisons. The Study of Women’s Health Across the Nation (SWAN), an ongoing longitudinal study of menopause, assessed MHT initiation and continuation, woman-level predictors, and reasons for initiation and discontinuation, approximately annually during 1996–2013.
The present study analyzed data from 14 visits to assess pre- versus post-WHI differences in MHT initiation and continuation, their correlates, and women’s reasons for initiating and discontinuing MHT. We hypothesized that compared with pre-WHI, both initiation and continuation would be lower post-WHI, across a variety of subgroups. We also hypothesized that reasons for initiating MHT would shift from health maintenance and prevention of chronic conditions such as cardiovascular disease and osteoporosis to VMS management, and that discontinuation reasons would shift to concerns highlighted by WHI results.
METHODS
Study Population
SWAN is a seven-site, community-based longitudinal study of the menopausal transition.14 In 1995–1997, each site recruited non-Hispanic white women and one other racial/ethnic group – Black, Chinese, Japanese, and Hispanic (total 3,302 participants). Eligibility criteria included age 42–52 years, intact uterus and 1–2 ovaries, a menstrual cycle without exogenous reproductive hormones use in the prior 3 months, and not pregnant/lactating. Each site’s institutional review board approved protocols. All participants provided written informed consent. At an in-person baseline and up to 13 annual follow-up visits, participants completed surveys on lifestyle behaviors, symptoms, and health care utilization. Analyses excluded women with no follow-up data (N=284). Analyses of the remaining 3,018 participants omitted visits during current or prior-visit pregnancy/lactation (N=9 visits from 5 women) or with missing covariate data (N=699 visits from 83 women, due primarily to suspended data collection at the Newark site during visits 6–8, 10, 11); MHT continuation analyses of race/ethnicity and site omitted post-WHI observations from Hispanic and Newark participants, respectively, due to small sample sizes.
Measures
At each visit, trained interviewers asked participants about prescription medication use since the prior visit and whether use was recent (2+ times per week for the last month for visits 1–10, use in the previous 3 months for visits 11–13) and transcribed preparation names from the participant’s medication containers. Beginning in 2014, SWAN coded all preparations based on the Iowa Drug Information Service system. For these analyses, we classified prescription medications as MHT if they contained estrogen with or without progestogens, including oral contraceptives used for non-contraception purposes. We distinguished prescription medications containing estrogen without progestogens as ET, and prescriptions containing both estrogen and progestogens as EPT. Eligible medications included systemic preparations likely to affect menstrual bleeding, e.g., oral, injection, and patch formulations, and high-dose gels or vaginal rings, as well as non-systemic preparations such as low-dose gels, vaginal rings, and vaginal suppositories. Exceptions were oral contraceptives reported as used for contraception, and treatment of conditions unrelated to menopause, e.g., ovarian cysts. Analyses used coded MHT except at the eleventh annual follow-up, for which self-reported MHT was obtained via telephone. We defined MHT initiation as no recent use at the prior visit followed by any use since the prior visit; this included first use between the prior and current visits, with or without discontinuation before the current visit, as well as re-initiation after first initiation and discontinuation before the prior visit. We defined MHT continuation as recent use at the current visit for women with initiation since the prior visit or earlier; this included recent use at both the prior and current visit, as well as initiation/re-initiation since the prior visit with recent use at the current visit.
Participants with self-reported MHT initiation since the prior visit were asked a list of possible reasons for starting MHT, including a choice to specify other reasons; verbatim responses were recorded and categorized. Participants with self-reported MHT discontinuation since the prior visit were asked one open-ended question regarding reasons for stopping MHT; verbatim responses were recorded and categorized.
The primary predictor was whether the visit occurred before or after the July 2002 WHI announcement. Time-invariant covariates included race/ethnicity, education, and site; because each site recruited white participants and participants from one other racial/ethnic group, so that race/ethnicity and site were confounded to some extent, analyses of site included only white participants to distinguish geographic variation from racial/ethnic differences.
Time-varying covariates included menopausal stage: premenopausal (bleeding in past 3 months, no change in regularity since last year), early perimenopausal (bleeding in past 3 months, change in regularity since last year), late perimenopausal (3–11 months amenorrhea), postmenopausal (12+ consecutive months of amenorrhea), bilateral salpingo-oophorectomy (BSO) with or without hysterectomy, hysterectomy with 1+ ovary retained, and undetermined due to recent MHT or other reproductive hormone use. We subdivided hysterectomy and BSO categories into prevalent at the prior visit versus incident since the prior visit. We further subdivided BSO categories by whether current age was younger than 51 years, the median age at menopause.15
Other time-varying covariates included age quartile and number of days in the past 2 weeks with VMS, categorized as in prior analyses.16 We dichotomized specialty of the participant’s provider (asked at visits 4–10) as obstetrics and gynecology (ob/gyn) versus other. For initiation analyses, we categorized prior MHT as: “no” (no prior use); and “yes” (prior initiation and discontinuation before the prior visit). For continuation analyses, we categorized prior MHT as: “new use” (initiation since the prior visit); “persistent use” (recent use at prior and current visit); and “interrupted use” (past use with discontinuation, re-initiation since the prior visit). Analyses for non-surgical menopausal stage and VMS frequency used values from prior visits, as MHT could affect these measures.
Statistical Analyses
To compare pre- versus post-WHI associations of covariates with MHT initiation and continuation, we estimated binomial logistic regressions, using generalized estimating equations (GEE) to handle within-woman correlation.17 Each model included the covariate of interest, an indicator for pre- versus post-WHI, and their interaction, indicating whether the association of initiation or continuation with the covariate differed pre- versus post-WHI, and conversely, whether the WHI-related difference varied by the covariate. To compare pre- versus post-WHI reasons for initiation and discontinuation, we estimated a GEE binomial logistic regression for each reason as a function of pre- versus post-WHI. Because the addition of progestogen to systemic estrogen is recommended for women with a uterus but not post-hysterectomy, we conducted supplemental analyses of initiation and continuation for 3-category MHT – none, ET, and EPT – comparing women with and without a hysterectomy pre- and post-WHI; these analyses used multinomial logistic regression with bootstrapped standard errors (1000 bootstrapping samples) to account for within-woman correlation.18 In additional supplemental analyses, the post-WHI interval was divided into 1, 2, and 2+ years post-WHI based on nonparametric locally-weighted scatterplot smoothing (LOESS) regression.19 Results are presented as percentages.
Due to SWAN’s longitudinal cohort design, pre- versus post-WHI differences in MHT outcomes reflected WHI-related impacts as well as within-woman changes in factors such as age. To account for the latter, we employed a propensity-score approach,20 by estimating a logistic regression for the “exposure,” post- versus pre-WHI, as a function of covariates jointly related to MHT,21 including race/ethnicity, site, financial strain, past oral contraceptive or MHT use at screening, prior-visit menopausal stage, and concurrent age. The estimated propensity score (probability of being a post-WHI visit) was a time-varying covariate in all analyses. Inclusion of site in the propensity score also addressed possible nonresponse bias from suspended data collection at the Newark site. Because age distributions differed for pre- and post-WHI in the top and bottom propensity-score quartiles,22 we conducted sensitivity analyses including only observations with ages between the minimum post-WHI age (46.9) and the maximum pre-WHI age (58.9); all results were consistent (data not shown). Analyses used SAS version 9.4 (SAS Institute, Cary, NC).
RESULTS
Relative to the analytic sample for initiation (3018 women), those who initiated MHT during follow-up and thus were included in MHT continuation analyses (990 women) were more likely to be white, from Los Angeles (among whites), have higher education, be early peri- rather than premenopausal at baseline, and have more frequent VMS at baseline (Table 1). Overall, the per-woman median number of follow-up visits was 5 pre-WHI and 7 post-WHI.
Table 1:
Baseline characteristics of participants in Study of Women’s Health Across the Nation (SWAN), for hormone initiation and continuation analytic samples
| Mean (SD) or No. (%) of Participants | ||
|---|---|---|
| Characteristic | Participants in initiation analyses (n=3,018 women contributing 28,715 observations) | Participants in continuation analyses (n=990 women contributing 4,251 observations) |
| Age, y | 46.34 (2.69) | 46.8 (2.7) |
| Race/ethnicity: | ||
| White | 1432 (47.45) | 569 (57.5) |
| Black | 865 (28.66) | 223 (22.5) |
| Japanese | 271 (8.98) | 87 (8.8) |
| Chinese | 243 (8.05) | 76 (7.7) |
| Hispanic | 207 (6.86) | 35 (3.5) |
| Educational level: | ||
| ≤ high school | 705 (23.48) | 174 (17.6) |
| Some college | 976 (32.51) | 318 (32.2) |
| College or beyond | 1321 (44.00) | 495 (50.2) |
| Missing | 16 | 3 |
| Site, white participants only: | ||
| Detroit, MI | 203 (14.18) | 70 (12.3) |
| Boston, MA | 226 (15.78) | 79 (13.9) |
| Chicago, IL | 202 (14.11) | 75 (13.2) |
| Oakland, CA | 201 (14.04) | 83 (14.6) |
| Los Angeles, CA | 210 (14.66) | 114 (20.0) |
| Newark, NJ | 104 (7.26) | 26 (4.6) |
| Pittsburgh, PA | 286 (19.97) | 122 (21.4) |
| Menopause status: | ||
| Premenopausal | 1614 (53.71) | 463 (46.9) |
| Early perimenopausal | 1391 (46.29) | 524 (53.1) |
| Missing | 13 | 3 |
| Days in past 2 weeks with VMS: | ||
| 0 | 1825 (60.81) | 543 (55.0) |
| 1 – 5 | 848 (28.26) | 300 (30.4) |
| 6+ | 328 (10.93) | 144 (14.6) |
| Missing | 17 | 3 |
MHT initiation
Overall, initiation dropped from 8.6% pre-WHI to 2.8% post-WHI (p<0.001). In supplemental analyses, initiation declined from 6.3% in the first year post-WHI to 1.9% after 2 years beyond the WHI announcement. The pre- versus post-WHI decline was largest in white and smallest in Hispanic women (Figure 1a; interaction p=.002 for racial/ethnic differences in pre- versus post-WHI comparison). All education levels had lower initiation post-WHI than pre-WHI (Figure 1al; interaction p=.012). Age-related patterns differed pre- and post-WHI, with the highest pre-WHI initiation among the two middle age quartiles (49.6–57.1 years), but a decrease in initiation with increasing age post-WHI (Figure 1a; interaction p=.002). Pre- versus post-WHI decline in initiation varied by site (range 3.1%−10.2%, interaction p=.0002). Both pre-and post-WHI, initiation was more common in women with an ob/gyn provider – 12.3% versus 5.0% pre-WHI, 5.6% versus 2.2% post-WHI – but the decline post-WHI was similar (interaction p=.849).
Figure 1a:
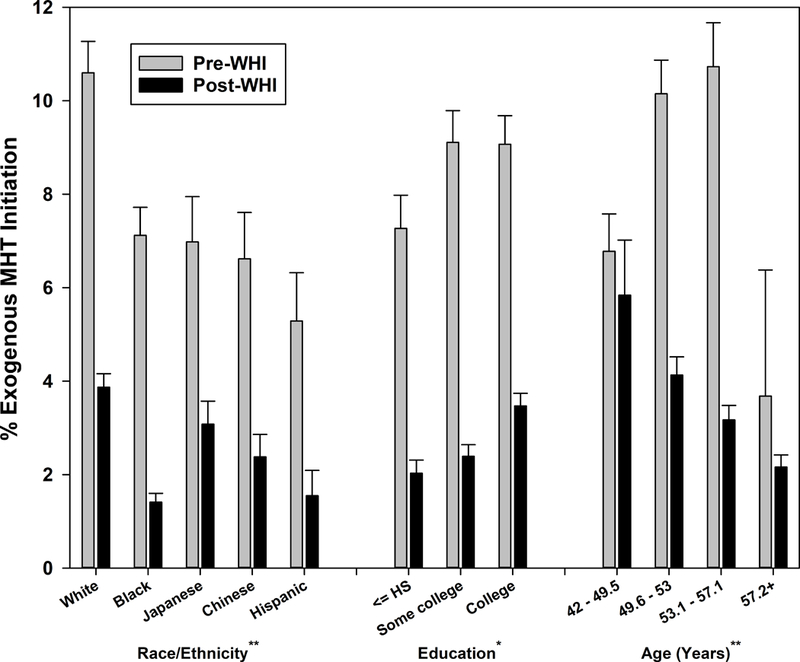
Menopausal hormone therapy initiation before and after Women’s Health Initiative July 2002 announcement by sociodemographic characteristics
p-values for interaction between pre-WHI versus post-WHI and characteristic indicated by *p<0.05, **p<0.01, ***p<0.001
Higher VMS frequency at the preceding visit was associated with greater MHT initiation both pre- and post-WHI (p<.001 both pre- and post-WHI). The largest pre- versus post-WHI decline occurred for the most frequent VMS category – 9.4% decline versus 6.0% decline in infrequent and 3.9% decline in asymptomatic (Figure 1b; interaction p=.089). The decline in initiation did not vary by prior MHT use patterns (Figure 1b; interaction p=.979).
Figure 1b:
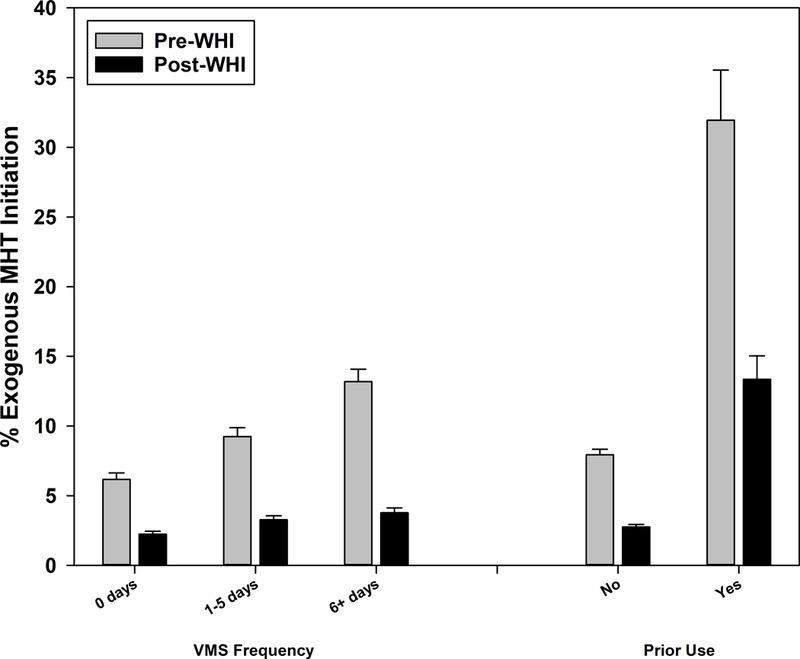
Menopausal hormone therapy initiation before and after Women’s Health Initiative July 2002 announcement by prior vasomotor symptom frequency and MHT use
p-value for interaction between pre-WHI versus post-WHI and characteristic indicated by **p<0.01
Both pre- and post-WHI, ET was the predominant form of MHT initiated by women with a hysterectomy (Supplemental Table 1), accounting for 90.5% of MHT initiation pre-WHI and 85.6% post-WHI in this subgroup. Any MHT initiation was much lower post-WHI than pre-WHI (39.3% versus 4.9%). In contrast, in women with a uterus, EPT was the primary form of MHT initiated pre-WHI, accounting for 87.2% of MHT initiation pre-WHI in this subgroup. Post-WHI, ET and EPT accounted for more similar percentages of MHT initiation, at 55.2% and 44.8%, respectively; the percentage of ET preparations that were non-systemic shifted from 13.3% pre-WHI to 65.3% post-WHI. Initiation of any MHT declined from 7.3% pre-WHI to 2.8% post-WHI.
Comparisons of MHT (ET or EPT) initiation by menopausal stage are presented in Figure 1c. Pre- versus post-WHI declines in MHT initiation differed to some extent across categories (for all categories, interaction p=.091). Among women with an incident BSO, those younger than 51 had a smaller decline in MHT initiation than those aged 51 and older (25.7% versus 44.4%), although this difference was not statistically significant (interaction p=.395) due to the small number of women in the latter category pre-WHI (N=11). Post-WHI declines also did not differ by age in those with a prevalent BSO (interaction p=.321). Conversely, post-WHI declines did not differ by incident versus prevalent BSO within current age category (interaction p-values .916 for younger than 51, .837 for 51 and older), again likely due to small sample sizes for several categories. In contrast, post-WHI decline in MHT initiation – primarily ET – was significantly greater for prevalent versus incident hysterectomy (8.5% versus 1.7%, interaction p=.020). For non-surgical menopausal categories, the post-WHI decline ranged from 10.6% for undetermined stage to 1.3% for premenopause (interaction p=.051 including non-surgical categories only).
Figure 1c:
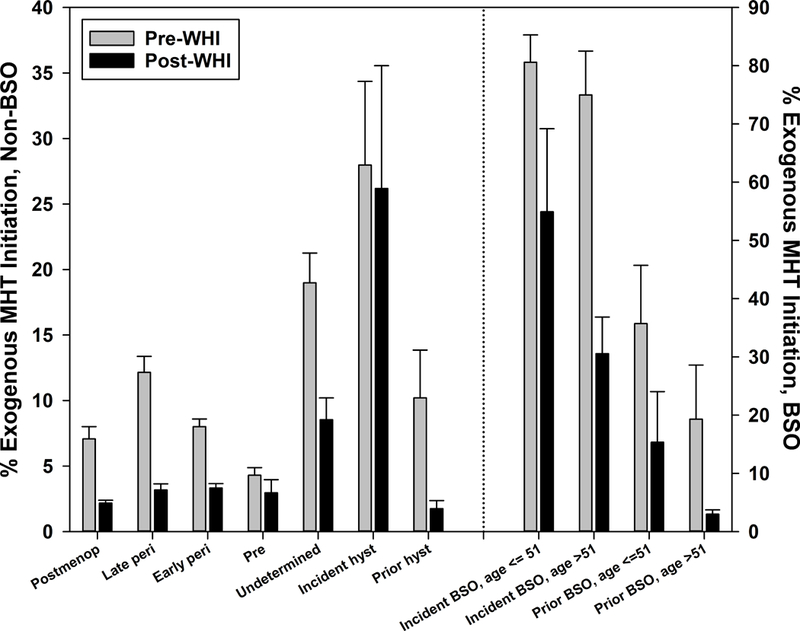
Menopausal hormone therapy initiation before and after Women’s Health Initiative July 2002 announcement by menopausal stage
MHT continuation
Overall, MHT continuation dropped from 84.0% pre-WHI to 62.0% post-WHI (p<.001). In supplemental analyses, continuation was lowest in the second year post-WHI (54.4%), rebounding subsequently to 65.0% but still significantly lower than pre-WHI. Associations of sociodemographic characteristics with continuation were weaker than corresponding associations with initiation both pre- and post-WHI, and effect modification of these associations by pre- versus post-WHI was not statistically significant (Figure 2a; all interaction p>.05). Pre- versus post-WHI declines also did not vary significantly by site (range 15.5% – 24.8%, interaction p=.842) or by provider type (range 17.8% – 22.6%, interaction p=.340).
Figure 2a:
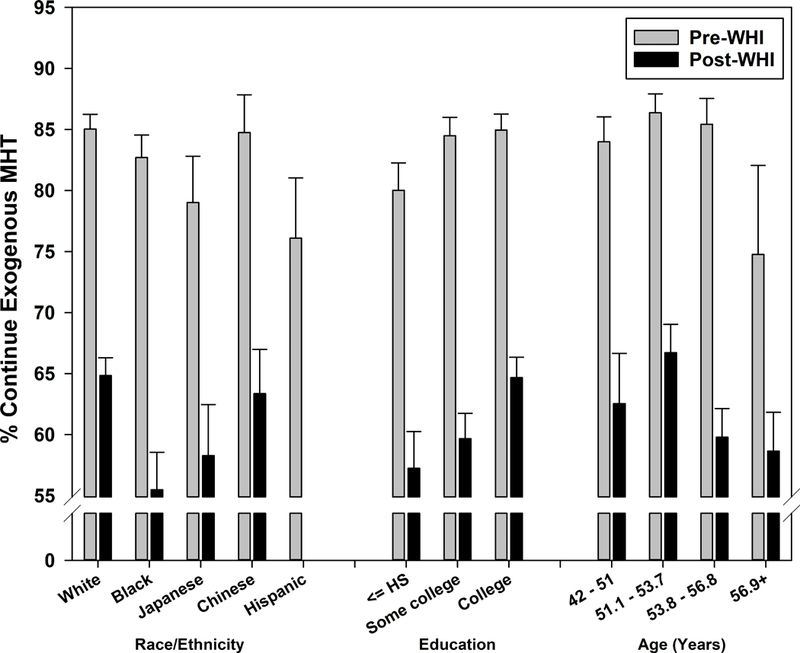
Menopausal hormone therapy continuation before and after Women’s Health Initiative July 2002 announcement by sociodemographic characteristics
VMS frequency at the visit prior to MHT initiation was positively related to continuation pre-WHI (p=.056). Conversely, the association post-WHI was weakly negative (p=.308), and the post-WHI decline in continuation was significantly larger for more frequent VMS categories: 18.6 for asymptomatic, 20.8% for infrequent, and 27.6% for frequent (Figure 2b; interaction p=.012). In supplemental analyses, continuation was lowest in the second year post-WHI for all three VMS categories (51.6–58.3%), increasing subsequently but 15.1–23.8% lower than pre-WHI. Prior MHT use also significantly modified pre-post WHI differences, with a decline of 26.4% for persistent use versus 11.2–13.8% for new and interrupted use (Figure 2b; interaction p=.002).
Figure 2b:
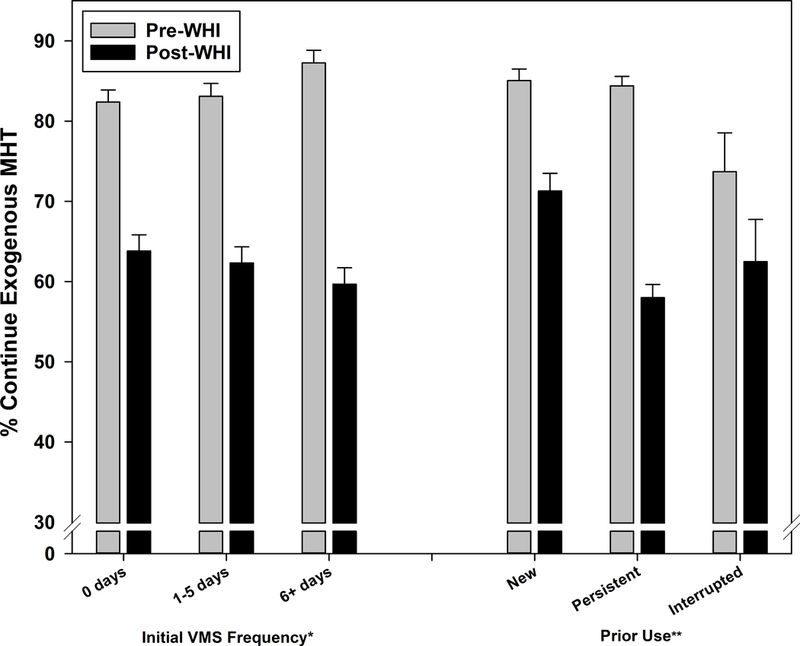
Menopausal hormone therapy continuation before and after Women’s Health Initiative July 2002 announcement by prior vasomotor symptom frequency and MHT use
p-values for interaction between pre-WHI versus post-WHI and characteristic indicated by *p<0.05, **p<0.01
Consistent with results for MHT initiation, prior-visit MHT was predominantly ET for women who had undergone a hysterectomy, at 91.6% pre-WHI and 94.6% post-WHI (Supplemental Table 2). In this hysterectomized subgroup on ET, fewer continued any MHT post-WHI than pre-WHI – 65.2% versus 90.4%, respectively – but ET accounted for virtually all of the continued MHT use both pre- and post-WHI. Among the small group of women with a hysterectomy and EPT use at the prior visit, continuation of any MHT also was lower post-WHI than pre-WHI – 30.8% versus 87.9%, respectively. Of continuers, switching to ET occurred for 70.5% pre-WHI and 86.5% post-WHI. Among women with a uterus, prior-visit MHT was largely EPT, at 89.5% pre-WHI and 69.2% post-WHI; the lower percent post-WHI is consistent with higher post-WHI ET initiation presented in Supplemental Table 1 (largely non-systemic preparations). In this subgroup, fewer continued any MHT post-WHI than pre-WHI – 62.2% versus 84.1%, respectively – but EPT accounted for 97.1% of continued MHT use pre-WHI and 95.0% post-WHI. Among women with a uterus and ET at the prior visit, continuation of any MHT also was lower post-WHI than pre-WHI – 59.9% versus 70.4%, respectively; ET accounted for 72.1% of continued MHT pre-WHI and 90.8% post-WHI.
Comparisons of MHT (ET or EPT) continuation by menopausal stage are presented in Figure 2c. These analyses combined younger and older incident BSO, younger and older prevalent BSO, pre- and early perimenopausal, and incident and prevalent hysterectomy, due to small cell counts, similar percentages of MHT continuation both pre- and post-WHI, and lack of statistically significant pairwise differences. Post-WHI declines in MHT continuation varied significantly by menopausal stage (interaction p=0.016), ranging from a decline in continuation of 9.0% for late perimenopause to 37.3% for incident BSO.
Figure 2c:
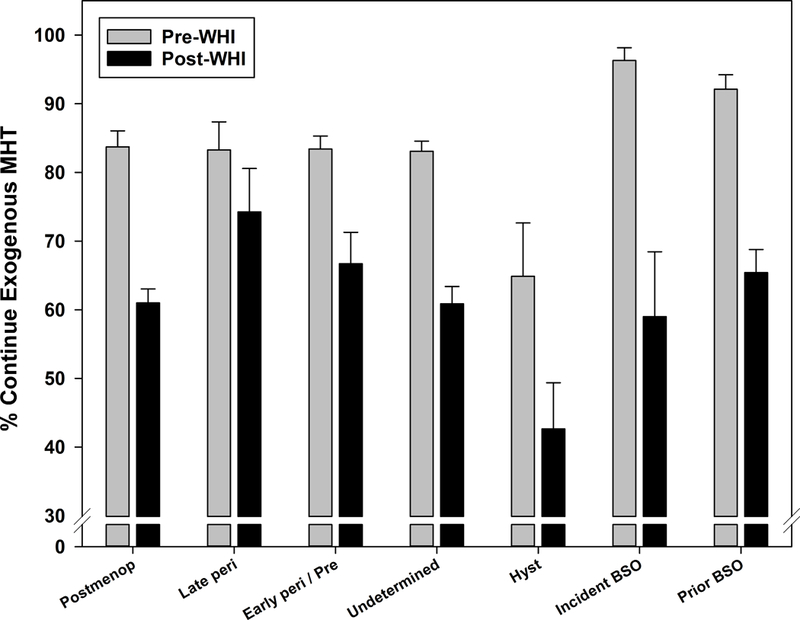
Menopausal hormone therapy continuation before and after Women’s Health Initiative July 2002 announcement by menopausal stage
Reasons for MHT initiation
Menopausal symptom relief and provider advice were common MHT initiation reasons both pre- and post-WHI, with the former mentioned only slightly less often post-WHI (Figure 3a). In contrast, reducing risks of heart disease and osteoporosis were cited by 29.2–40.9% of initiators pre-WHI, but declined dramatically post-WHI to 2.0–10.5%. Several reasons – friend/relative advice, improving memory, and staying young-looking – were mentioned infrequently pre-WHI and even less often post-WHI. The percentage mentioning regulation of menstrual bleeding was similar pre- and post-WHI, at 20.2% and 21.4%, respectively.
Figure 3a:
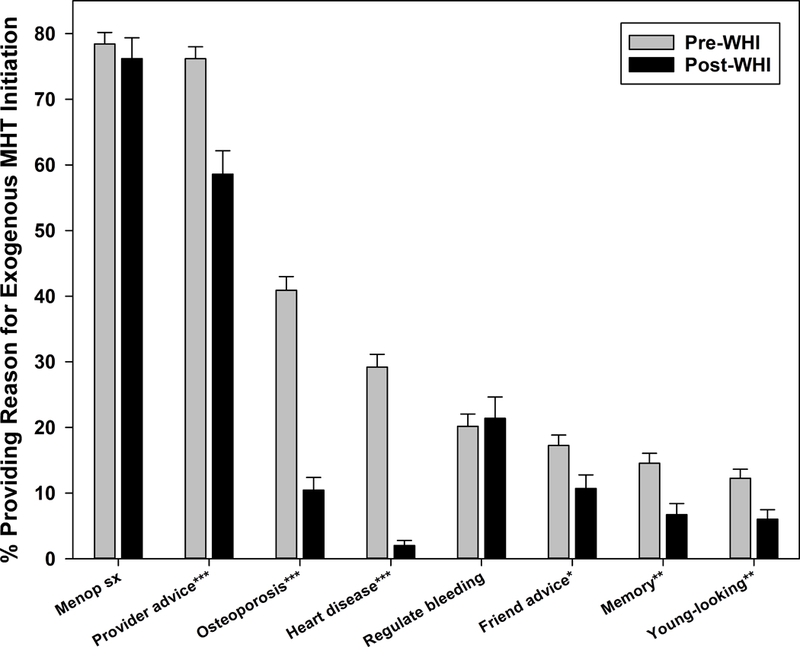
Reasons for menopausal hormone therapy initiation before and after Women’s Health Initiative July 2002 announcement
p-values for pre-WHI versus post-WHI difference indicated by *p<0.05, **p<0.01, ***p<0.001
Reasons for discontinuing MHT
Media reports had the largest increase pre- to post-WHI, followed by provider advice, as reasons for MHT discontinuation (Figure 3b). In supplemental analyses, the increase in citing media reports occurred primarily in the first 2 years post-WHI (33.5–46.6% versus 10.7% 2+ years post-WHI). Dislike of MHT was reported by approximately one in four participants discontinuing MHT both pre- and post-WHI (pre- versus post-WHI p=0.819). Prescription finished / no longer needed was mentioned less often than dislike, but increased post-WHI compared with pre-WHI (p=.057). Menstrual bleeding and adverse effects, e.g. weight gain and headaches, were reported significantly less often post-WHI than pre-WHI, decreasing from 19.8–21.4% to 7.6–10.2%. In contrast, worry regarding possible side effects and cancer risk demonstrated a temporary increase in the first year post-WHI (46.2% and 37.6% respectively) versus subsequent years (13.5–18.9%), although the overall pre- versus post-WHI differences were not statistically significant.
Figure 3b:
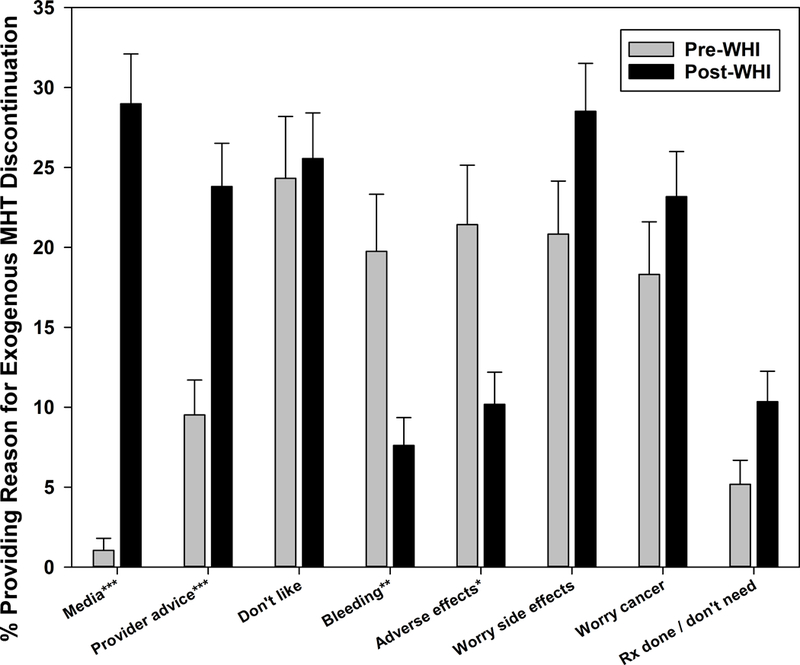
Reasons for menopausal hormone therapy discontinuation before and after Women’s Health Initiative July 2002 announcement
p-values for pre-WHI versus post-WHI difference indicated by *p<0.05, **p<0.01, ***p<0.001
DISCUSSION
In this multi-racial/ethnic cohort of 3,018 midlife women, we found decreases in both MHT initiation and continuation following the 2002 WHI announcement highlighting the risks and benefits of MHT, based on a median within-woman follow-up of 5.0 years before and 9.8 years after the announcement. Virtually all subgroups exhibited post-WHI declines in both MHT initiation and continuation. However, we observed larger decreases and more effect modification by participant characteristics for initiation than for continuation, emphasizing the importance of analyzing initiation and continuation separately. Pervasive declines across a variety of subgroups based on sociodemographic characteristics, provider specialty, menopausal stage, and patterns of prior MHT use suggests wide dissemination of WHI findings. An important exception is women with VMS, for whom MHT use declined despite lack of applicability of the WHI announcement to VMS.
Earlier studies found little long-term MHT use even prior to the July 2002 WHI announcement, with 20% discontinuing after the first prescription,23 discontinuation within a year in one-third to one-half of users,24–27 and discontinuation by the end of the study in 42% of WHI participants randomized to EPT.28 Pre- versus post-WHI comparisons of MHT patterns in health maintenance organizations found lower MHT initiation and higher discontinuation post-WHI compared with pre-WHI,29–30 as in SWAN.
Consistent with post-WHI clinical guidelines highlighting the most favorable MHT risk-benefit ratio for women under age 60,4–6,10, 12 age was negatively associated with MHT initiation post-WHI, and the youngest age category had the smallest post-WHI decline. Post-WHI declines in MHT continuation were slightly larger in the two oldest age groups, consistent with previous findings of lower HT prevalence and higher discontinuation among older women post-WHI.29, 31–33 For incident BSO, the decrease in initiation was larger in those aged 51+ versus those who were younger – although the difference was not statistically significant due to the small sample size for the older category – perhaps because women in the younger category were more likely to use MHT for VMS or to sustain higher estrogen levels until the mean menopause age for cardiovascular and bone protection.4–6,34–37
WHI-related changes in MHT use varied by prior use and demographic characteristics, but not by provider specialty. Both pre- and post-WHI, re-initiation of MHT was higher than first-time initiation, but the decline post-WHI was similar for both categories. Lower post-WHI continuation in persistent users and higher post-WHI continuation in new users may indicate adherence to recommendations that MHT be used for the shortest time needed.8–9 Results regarding WHI-related changes by race and education are similar to findings in two prior studies,30,38 although other studies found less discontinuation post-WHI among higher income women39 and noted lower familiarity with WHI results and possible MHT side effects among black women and those with lower socioeconomic status.40–42 As in previous studies,1,32,43 women with ob/gyn providers were more likely to initiate MHT both pre- and post-WHI. Post-WHI declines in both initiation and discontinuation, however, were consistent across provider specialties.
VMS frequency was more strongly associated with MHT initiation pre-WHI than post-WHI. Moreover, declines in MHT continuation post-WHI were significantly larger in women with more frequent VMS prior to MHT initiation. Two smaller studies reported limited MHT use in women with VMS post-WHI. A longitudinal Norwegian study found a drop in MHT initiation within 2 years post-WHI and subsequent stable prevalence, despite a concomitant rise in daily VMS prevalence;44 in a cross-sectional Australian study, only 14% of peri- and postmenopausal women with moderate or severe VMS were using MHT post-WHI.45 Results of our study extend prior work by including explicit pre- versus post-WHI comparisons of VMS associations with MHT initiation and continuation and indicate that women with VMS are less likely to receive MHT post-WHI than pre-WHI. These observations suggest that the translation of WHI findings regarding MHT’s disease prevention-related risks and benefits affected approaches to VMS treatment, as others have noted,12,46,47 despite the explicit message that recommendations regarding primary prevention in asymptomatic women are not applicable to those seeking relief from menopausal symptoms.48
Changes in MHT initiation and discontinuation reasons in SWAN were largely consistent with post-WHI clinical guidelines, reflecting wide dissemination. For participants initiating MHT, the sharp post-WHI decline in reported initiation to prevent heart disease is consistent with recommendations against use for cardiovascular prevention.4–6,48,49 The post-WHI decline in reported initiation for reducing osteoporosis risk is consistent with the US Preventive Services Task Force recommendation against MHT use for primary prevention of chronic diseases,48 although the North American Menopause Society recognizes MHT’s beneficial effect on fracture risk and recommends individualized treatment that balances a woman’s risks and benefits.4 Although MHT use declined post-WHI in women with VMS, among those initiating MHT the proportion reporting initiation for symptom relief was similar pre- and post-WHI.
Both pre- and post-WHI, one in four participants reported discontinuing MHT due to dislike, and almost one in five mentioned bleeding as a reason for discontinuation; previous studies also point to the latter as a common discontinuation reason.25,50–53 Reporting of discontinuation due to actual adverse effects declined post-WHI, while there was a temporary increase in the first year post-WHI in discontinuation due to concerns about possible side effects and cancer; the latter also has been reported pre-WHI as a reason for discontinuation. Prior studies have reported that discontinuation was more strongly associated with concern about possible side effects than with side effects that actually occurred.44,54 Fear of cancer also has been noted in previous studies pre-WHI as a discontinuation reason.51,53 Notably, media reports were mentioned more often than provider advice post-WHI. Our longitudinal findings extend prior studies31,33,53,55–62 by providing formal pre- versus post-WHI comparisons.
A limitation of our study is lack of detail regarding dates of MHT initiation and discontinuation due to the annual nature of data collection, which did not facilitate analyses of MHT duration. Instead, we focused on visit-to-visit continuation. Also, our sample size is smaller than those in studies utilizing insurance or pharmacy data, particularly for several menopausal stage categories (Supplemental Table 3), although total sample size is consistent with or larger than those in other studies with more detailed individual-level data.
Strengths of the study include a large sample of women with prospectively assessed characteristics over 17 years across the 2002 WHI announcement (1996 – 2013). Distinguishing MHT initiation from continuation provides critical insights that previous studies have not demonstrated. The cohort was multi-racial/ethnic and drawn from diverse geographic areas, and prospective data collection enabled direct assessment of within-woman changes in MHT use based on centralized coding. Finally, MHT use and reasons for initiation and discontinuation were assessed concurrently.
CONCLUSIONS
The WHI demonstrated that the risks of MHT use may outweigh benefits for disease prevention, particularly in women initiating MHT after age 60 or 10+ years beyond menopause.4–6,10,12,48 The pre- to post-WHI changes in MHT use patterns and in the reasons for initiation and discontinuation seen in SWAN provide evidence of rapid and widespread dissemination of the main WHI findings. Post-WHI recommendations against MHT use for prevention appear to have been adopted across the U.S. regardless of natural or surgical menopausal stage, age, race/ethnicity, geographic area, education, or provider type. However, our findings also suggest, as others have noted,12,46,47 that these recommendations have not been appropriately interpreted by women with VMS or their providers. Many symptomatic women may forgo MHT for symptom relief because of concerns about study findings that were not truly applicable to this population of women in their 50s when VMS are most prevalent but risks of MHT are lower.4–6,10,12 The results of this analysis support the need for more education about personalized risk/benefit profiles for women and providers as MHT research translates to public health recommendations, to ensure evidence-based care of women through menopause and beyond.
Supplementary Material
ACKNOWLEDGMENTS
The Study of Women’s Health Across the Nation (SWAN) has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Funding for this work was received from the National Institutes of Health
Dr. Joffe receives research funding from Merck and Pfizer, and is a consultant/advisor to Merck, KaNDY, and Sojournix.
We thank the study staff at each site and all the women who participated in SWAN.
Footnotes
Potential conflicts of interest: No other potential conflicts of interest.
References
- 1.Ettinger B, Wang SM, Leslie RS, et al. Evolution of postmenopausal hormone therapy between 2002 and 2009. Menopause 2012;19:610–615. [DOI] [PubMed] [Google Scholar]
- 2.Steinkellner AR, Denison SE, Eldridge SL, Lenzi LL, Chen W, Bowlin SJ. A decade of postmenopausal hormone therapy prescribing in the United States: long-term effects of the Women’s Health Initiative. Menopause 2012;19:616–621. [DOI] [PubMed] [Google Scholar]
- 3.Weissfeld JL, Liu W, Woods C, et al. Trends in oral and vaginally administered estrogen use among US women 50 years of age and older with commercial health insurance. Menopause 2018;25:611–614. [DOI] [PubMed] [Google Scholar]
- 4.North American Menopause Society. The 2017 position statement of the North American Menopause Society. Menopause 2017;24;728–753. [DOI] [PubMed] [Google Scholar]
- 5.Stuenkel CA, Davis SR, Gompel A, et al. Treatment of symptoms of the menopause: An Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab 2015;100:3975–4011. [DOI] [PubMed] [Google Scholar]
- 6.de Villers TJ, Hall JE, Pinkerton JV, et al. Revised global consensus statement on menopausal hormone therapy. Maturitas 2016;91:153–155. [DOI] [PubMed] [Google Scholar]
- 7.Kling JM, Manson JE. The Women’s Health Initiative: evolving insights over 15 years. Menopause 2017;24:355–357. [DOI] [PubMed] [Google Scholar]
- 8.American College of Obstetricians and Gynecologists Committee on Practice Bulletins. ACOG practice bulletin: clinical management guidelines for obstetrician-gynecologists. Number 50, January 2004. Obstet Gynecol 2004;103:203–216. [PubMed] [Google Scholar]
- 9.North American Menopause Society. Recommendations for estrogen and progestogen use in peri- and postmenopausal women: October 2004 position statement of the North American Menopause Society. Menopause 2004;11(6, pt 1):589–600. [DOI] [PubMed] [Google Scholar]
- 10.North American Menopause Society. The North American Menopause Society statement on continuing use of systemic hormone therapy after age 65. Menopause 2015;22:693. [DOI] [PubMed] [Google Scholar]
- 11.ACOG Practice Bulletin No. 141: management of menopausal symptoms. Obstet Gynecol 2014;123:202–216. [DOI] [PubMed] [Google Scholar]
- 12.Sturdee DW, Pines A; International Menopause Society Writing Group. Updated IMS recommendations on postmenopausal hormone therapy and preventive strategies for midlife health. Climacteric 2011;14;302–20. [DOI] [PubMed] [Google Scholar]
- 13.Manson JE, Aragaki AK, Rossouw JE, et al. Menopausal hormone therapy and long-term all-cause and cause-specific mortality. JAMA 2017;318:927–938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sowers M, Crawford S, Sternfeld B, et al. Design, survey, sampling and recruitment methods of SWAN: a multi-center, multi-ethnic, community-based cohort study of women and the menopausal transition. In: Lobo RA, Kelsey J, Marcus R, editors. Menopause: Biology and Pathobiology San Diego: Academic Press, 2000:175–188. [Google Scholar]
- 15.Gold EB, Bromberger J, Crawford S, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol 2001;153:865–874. [DOI] [PubMed] [Google Scholar]
- 16.Gold EB, Crawford SL, Shelton JF, et al. Longitudinal analysis of changes in weight and waist circumference in relation to incident vasomotor symptoms: the Study of Women’s Health Across the Nation (SWAN). Menopause 2017;24:9–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Molenberghs G, Verbeke G. Models for discrete longitudinal data New York: Springer Science+Business Media, Inc., 2005. [Google Scholar]
- 18.Chernick MR. Bootstrap methods: a guide for practitioners and researchers, 2nd ed. Wiley: New York, 2008. [Google Scholar]
- 19.Cleveland WS, Devlin SJ. Locally weighted regression: an approach to regression analysis by local fitting. J Am Stat Assoc 1998;83:596–610. [Google Scholar]
- 20.Austin PC. An introduction to propensity score methods for reducing the effect of confounding in observational studies. Multiv Behav Res 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity models. Am J Epidemiol 2006;163:1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Austin PC, Mamdani MM. A comparison of propensity score methods: A case-study estimating the effectiveness of post-AMI statin use. Stat Med 2006;25:2084–2106. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds RF, Walker AM, Obermeyer CM, Rahman O, Guilbert D. Discontinuation of postmenopausal hormone therapy in a Massachusetts HMO. J Clin Epidemiol 2001;54:1056–1064. [DOI] [PubMed] [Google Scholar]
- 24.Ettinger B, Pressman A, Bradley C. Comparison of continuation of postmenopausal hormone replacement therapy: transdermal versus oral estrogen. Menop 1998;5:152–156. [PubMed] [Google Scholar]
- 25.Prevalence Barrett-Connor E., initiation, and continuation of hormone replacement therapy. J Womens Health 1995;4:143–148. [Google Scholar]
- 26.Faulkner DL, Young C, Hutchins D, McCollam JS. Patient noncompliance with hormone replacement therapy: a nationwide estimate using a large prescription claims database. Menop 1998;5:226–229. [PubMed] [Google Scholar]
- 27.Connelly T, Richardson M, Platt R. Prevalence and duration of postmenopausal hormone replacement therapy use in a managed care organization, 1990–1995. J Gen Intern Med 2000;15:542–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rossouw JE, Anderson GL, Prentice GL, LaCroix AX, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results from the Women’s Health Initiative randomized controlled trial. JAMA 2002;288:321–333. [DOI] [PubMed] [Google Scholar]
- 29.Buist DS, Newton KM, Miglioretti DL, et al. Hormone therapy prescribing patterns in the United States. Obstet Gynecol 2004;104(5 Pt 1):1042–1050. [DOI] [PubMed] [Google Scholar]
- 30.Wei F, Miglioretti DL, Connelly MT, et al. Changes in women’s use of hormones after the Women’s Health Initiative Estrogen and Progestin Trial by race, education, and income. J Natl Cancer Inst Monogr 2005;35:106–112. [DOI] [PubMed] [Google Scholar]
- 31.Haskell SG, Bean-Mayberry B, Goulet JL, Skanderson M, Good CB, Justice AC. Determinants of hormone therapy discontinuation among female veterans nationally. Mil Med 2008;173:91–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hing E, Brett KM. Changes in U.S. prescribing patterns of menopausal hormone therapy, 2001–2003. Obstet Gynecol 2006;108:33–40. [DOI] [PubMed] [Google Scholar]
- 33.Jalava-Broman J, Mäkinen J, Ojanlatva A, Jokinen K, Sillanmäki L, Rautava P. Change in the frequency of HRT use from 2000 to 2005 and reasons to discontinue: follow-up of a normal cohort in Finland. Acta Obstetricia et Gynecologica Scandinavica 2011;90;351–357. [DOI] [PubMed] [Google Scholar]
- 34.Muka T, Oliver-Williams C, Kunutsor S, et al. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality. JAMA Cardiology 2016;1:767–776. [DOI] [PubMed] [Google Scholar]
- 35.American College of Obstetricians and Gynecologists. Hormone therapy in primary ovarian insufficiency. Committee Opinion No. 698. Obstet Gynecol 2017;129:e134–141. [DOI] [PubMed] [Google Scholar]
- 36.Christ JP, Gunning MN, Palla G, et al. Estrogen deprivation and cardiovascular disease risk in primary ovarian insufficiency. Fertil Steril 2018;109:594.e1.600.el. [DOI] [PubMed] [Google Scholar]
- 37.Faubion SS, Kuhle CL, Shuster LT, Rocca WA. Long-term health consequences of premature or early menopause and considerations for management. Climacteric 2015;18:483–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Haas JS, Kaplan CP, Gerstenberger EP, Kerlikowske K. Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Ann Intern Med 2004;140:184–188. [DOI] [PubMed] [Google Scholar]
- 39.Bosworth HB, Bastian LA, Grambow SC, et al. Initiation and discontinuation of hormone therapy for menopausal symptoms: results from a community sample. J Behav Med 2005;28:105–114. [DOI] [PubMed] [Google Scholar]
- 40.Blümel JE, Castelo-Branco C, Chedraui PA, et al. Patients’ and clinicians’ attitudes after the Women’s Health Initiative study. Menopause 2004;11:57–61. [DOI] [PubMed] [Google Scholar]
- 41.Breslau ES, Davis WW, Doner L, et al. The hormone therapy dilemma: women respond. J Am Med Womens Assoc 2003;58:33–43. [PubMed] [Google Scholar]
- 42.Rigby AJ, Ma J, Stafford RS. Women’s awareness and knowledge of hormone therapy post-Women’s Health Initiative. Menopause 2007;14:853–858. [DOI] [PubMed] [Google Scholar]
- 43.Spangler L, Reed SD, Nekhyludov L, Grothaus L, LaCroiz AZ, Newton KM. Provider attributes associated with hormone therapy prescribing frequency. Menopause 2009;16:810–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gjelsvik B, Straand J, Hunskaar S, Dalen I, Rosvold EO. Use and discontinued use of menopausal hormone therapy by healthy women in Norway: the Hordaland Women’s Cohort study. Menopause 2014;21:459–468. [DOI] [PubMed] [Google Scholar]
- 45.Worsley R, Bell RJ, Gartoulla P, Davis SR. Low use of effective and safe therapies for moderate to severe menopausal symptoms: a cross-sectional community study of Australian women. Menopause 2016;23:1–17. [DOI] [PubMed] [Google Scholar]
- 46.Grady D Evidence for postmenopausal hormone therapy to prevent chronic conditions: Success, failure, and lessons learned. JAMA Int Med 2018;178:185–186. [DOI] [PubMed] [Google Scholar]
- 47.Pines A Women’s Health Initiative and rate of hormone use: a study that impacted a whole generation. Menopause 2018;25:586–588. [DOI] [PubMed] [Google Scholar]
- 48.US Preventive Services Task Force. Hormone therapy for the primary prevention of chronic conditions in postmenopausal women: US Preventive Services Task Force recommendation statement 2017;318:2224–2233. [DOI] [PubMed] [Google Scholar]
- 49.American College of Obstetricians and Gynecologists. Hormone therapy and heart disease. Committee Opinion No. 565. Obstet Gynecol 2013;121:1407–1410. [DOI] [PubMed] [Google Scholar]
- 50.Ryan PJ, Harrison R, Blake GM, Fogelman I. Compliance with hormone replacement therapy (HRT) after screening for postmenopausal osteoporosis. Br J Obstet Gynaecol 1992;99:325–328. [DOI] [PubMed] [Google Scholar]
- 51.Wren BG, Brown L. Compliance with hormonal replacement therapy. Maturitas 1991;13:17–21. [DOI] [PubMed] [Google Scholar]
- 52.Hahn RG. Compliance considerations with estrogen replacement: withdrawal bleeding and other factors. Am J Obstet Gynecol 1989;161:854–858. [DOI] [PubMed] [Google Scholar]
- 53.Newton KM, LaCroix AZ, Leveille SG, Rutter C, Keenan NL, Anderson LA. Women’s beliefs and decisions about hormone replacement therapy. J Women’s Health 1997;6:459–465. [DOI] [PubMed] [Google Scholar]
- 54.Reynolds RF, Obermeyer CM, Walker AM, Guilbert D. The role of treatment intentions and concerns about side effects in women’s decision to discontinue postmenopausal hormone therapy. Maturitas 2002;43:183–194. [DOI] [PubMed] [Google Scholar]
- 55.Gass MLS, Rebar RW, Liu JH, Cedars MI. Characteristics of women who continue using hormone replacement therapy. Menopause 1997;4:19–23. [Google Scholar]
- 56.French LM, Smith MA, Holtrop JS, Holmes-Rovner M. Hormone therapy after the Women’s Health Initiative: a qualitative study. BMC Fam Prac 2006;7:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Helenius IM, Korenstein D, Halm EA. Changing use of hormone therapy among minority women since the Women’s Health Initiative. Menopause 2007;14:216–222. [DOI] [PubMed] [Google Scholar]
- 58.Keinan-Boker L, Dictiar R, Garty N, Green MS. Prevalence and correlates of hormone therapy among Israeli women in the post-WHI era. Maturitas 2005;52:364–373. [DOI] [PubMed] [Google Scholar]
- 59.Ness J, Aronow WS. Prevalence and causes of persistent use of hormone replacement therapy among postmenopausal women: a follow-up study. Amer J Therapeutics 2006;13:109–112. [DOI] [PubMed] [Google Scholar]
- 60.Ness J, Aronow WS, Newkirk E, McDanel D. Use of hormone replacement therapy by postmenopausal women after publication of the Women’s Health Initiative trial. J Geront: Med Sci 2005;60A:460–462. [DOI] [PubMed] [Google Scholar]
- 61.Kang BM, Kim M-R, Park HM, et al. Attitudes of Korean clinicians to postmenopausal hormone therapy after the Women’s Health Initiative study. Menopause 2006;13:125–129. [DOI] [PubMed] [Google Scholar]
- 62.McIntosh J, Blalock SJ. Effects of media coverage of Women’s Health Initiative study on attitudes and behavior of women receiving hormone replacement therapy. Am J Health-Syst Pharm 2005;62:69–74. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


