Abstract
Purpose of review:
This review investigates the potential bi-directional relation between sleep and diet in considering their contribution to cardiovascular health. We further explore the involvement of the gut microbiome in the relationships between poor sleep and dietary intakes and increased cardiovascular disease (CVD) risk.
Recent findings:
There is strong evidence that sleep restriction leads to unhealthy food choices and increased energy intake. The diet may impact sleep, as well. Epidemiological studies show that higher adherence to a Mediterranean dietary pattern predicts healthier sleep. One factor that could underlie these relationships is the gut microbiome. Although data are mixed, there is some evidence that sleep restriction can influence the composition of the gut microbiome in humans. Similarly, Mediterranean diets and other plant-rich diets are related to increased diversity of the microbiota. At present, few studies have investigated the influence of the microbiome on sleep; however, limited evidence from epidemiological and intervention studies suggest that the composition of the microbiome may relate to sleep quality. More research is needed to better understand the role of the microbiome in the multi-directional relationship between sleep, diet and CVD.
Summary:
There is growing evidence of a bi-directional relationship between sleep and the diet, which could act in concert to influence CVD risk. Diets such as the Mediterranean diet, comprised of high intakes of fruits, vegetables, and other plant-based foods, may promote healthy sleep and beneficial gut microflora. The gut microbiome may then underlie the relation between diet, sleep, and CVD risk.
Keywords: Sleep, Diet, CVD, Mediterranean diet, Microbiome
Introduction
Cardiovascular disease (CVD) is a primary public health concern. Despite efforts to promote awareness and develop effective strategies to treat and prevent CVD (1), it remains the leading cause of mortality in both men and women in the United States (2). Given the relation between CVD and obesity (3), with the prevalence of the latter continuing to escalate (4), it is likely that CVD will remain a principal barrier to health and longevity for years to come. Lifestyle behaviors to reduce both obesity and CVD risk are therefore critical. Diet and physical activity have traditionally been the primary targets of lifestyle interventions for obesity and CVD risk reduction, but sleep has recently garnered significant interest. Importantly, sleep is related to cardiovascular health (5), partially attributable to its influence on obesity risk via alterations in energy intake and food choice (6). It is also notable that recent research strongly suggests multi-directional relations among diet, sleep, and CVD risk. Thus, the purpose of this review is to elaborate on the roles of sleep and diet on CVD risk, and to examine the potential mechanisms underlying the complex relation between these two lifestyle factors as they relate to cardiovascular health. We use this information to propose a model by which sleep and the diet interact to affect CVD risk with the microbiome moderating these relationships.
Diet, Sleep and CVD Risk
The effect of diet on the risk of developing CVD is well-established (7). Overall dietary patterns that are high in fruits and vegetables (FV) (7–10 servings/day) and favor intakes of unsaturated over saturated fats, such as the Mediterranean diet, have been dubbed as “heart-healthy” diets (8). Indeed, the Mediterranean diet and others with overlapping concepts (e.g. increased FV intake) promote weight loss (9-11). Beyond this, higher adherence to a Mediterranean diet has been shown to directly reduce CVD risk and events compared to a lower fat diet (12).
Just as diet can influence the risk of CVD, short sleep duration and poor sleep quality are also associated with increased CVD risk (5). Briefly, epidemiological and experimental evidence demonstrate relationships between inadequate sleep and other modifiable risk factors including: blood pressure (13,14), lipid levels (15,16), type 2 diabetes (17), and inflammation (18). Given that these CVD-related outcomes can also be influenced by the diet (7), we argue that a pathway by which sleep increases CVD risk may be via alterations in food choice and dietary intakes. This will be discussed in the following sections.
Sleep and the diet
Cross-sectional studies support a relation between sleep and diet. Short sleepers (those with sleep <7 h/night) report lower intakes of FV (19) and fiber (20) and higher saturated fat intake (20) than adequate sleepers (those sleeping at least 7 h/night). For example, individuals who slept 7–8 h/night consumed approximately 12% more FV per day than those sleeping less than 7 h/night (19). Associations are also found between food intake and sleep quality, which includes higher self-reported consumption of FV (e.g. 132.8 vs 121.7 g/day) (21) as well as oily fish (188 g/day vs 164 g/day) (22) by those who describe their sleep as being of good quality compared to those with poor sleep quality. These observations helped to establish a sleep-diet relation, but questions are raised regarding the direction and causality of the relationship, given the epidemiological nature of the studies.
To support a direct impact of sleep on the diet, sleep restriction studies have clearly shown increases in food intake relative to adequate sleep (5,23). In fact, a meta-analysis of 11 randomized controlled sleep restriction studies concluded that the average increase in daily energy intake following partial sleep deprivation (sleep ranging from 3.75 h to 5.5 h/night for several days) relative to adequate sleep (7–9 h/night) was 385 kcal/d (24). Greater energy intake during a period of sleep restriction can be attributed to increased eating frequency (25) as well as greater intakes of total and saturated fats (6) and snacks (26,27). Given that these eating behaviors can contribute to weight gain (28-30), it is likely that changes to dietary patterns as a result of sleep restriction are one mechanism underlying the relation between sleep duration and CVD risk. At present, the impact of short sleep duration on CVD risk is not fully understood, since no study has tested sleep restriction beyond 3 wk (31). We are currently working to fill this gap by assessing the influence over a 6-wk period.
While sleep clearly affects food choice and energy intake, whether the diet can influence sleep duration and quality is less certain. Intervention studies, which can establish causality, have been conducted for some specific foods: milk fortified with Horlicks powder (32) and melatonin-rich milk (33); cherry juice (34); B vitamins (35,36); and kiwifruit (37). The results of these studies have been summarized elsewhere (38,39) with the conclusion that these foods likely improve sleep quality. However, further studies are needed to elucidate the magnitude and long-term impact of specific dietary patterns on sleep health, particularly in adults with poor sleep quality.
The scope of studies has been expanded slightly to focus on the effects of carbohydrate intake on sleep health (40-43). It is difficult, however, to interpret the clinical impact of the macronutrient composition of the diet on sleep architecture from the studies available. This is due in part to the finding that increased carbohydrate had a positive influence on rapid-eye movement sleep, but reduced slow-wave sleep (40,41). In addition, whether effects on sleep resulted from the increase in carbohydrate or the concomitant reduction in fat is rather difficult to isolate. These studies are also limited by their short duration, substantial heterogeneity in the extent to which macronutrients were varied, and study of populations with generally healthy sleep. Although these experiments were a progression from studying single foods, they did not address the complete diet, which would be more informative and likely have a more positive impact on sleep (38).
Importantly, the field of nutrition has moved away from specific macronutrient recommendations to offer greater focus on whole foods and overall dietary patterns. As an example, the Dietary Guidelines for Americans 2015–2020 recommend following a healthy eating pattern that includes FV, whole grains, dairy, lean unprocessed protein sources, and vegetable oils and limits saturated and trans fats, added sugars, and sodium (44). Data on the relation between complete dietary patterns and sleep are rather limited, but there is a growing body of literature on the Mediterranean diet and sleep, particularly insomnia symptoms, sleep duration, and sleep quality (Table 1).
Table 1:
Studies assessing the relationship between adherence to the Mediterranean Diet and Sleep
| Study (ref) | Population | Dietary measures | Sleep measures | Pertinent outcomes |
|---|---|---|---|---|
| Jaussent et al., 2011 (45) | Sample size: 5,886 French men and women Age range: 65 y and older (Three-City Study) |
Self-reported frequency of consuming food components of the Med. Diet. Frequent consumption of 7 or more components was classified as Med. Diet |
Insomnia symptoms (IS): self-reported sleep complaint questionnaire, history of medication for sleep disorders, self-reported daytime sleepiness | Women had lower adherence to the Med. Diet In women only, Med. Diet predicted decreased risk for IS. |
| Castro-Diehl et al., 2018 (46) | Sample size: 2,068 men and women from six sites in the US (observed at 2 time points; sample size differed by available data) Age range: 45-84 y (at baseline) (MESA Study) |
Modified FFQ; Adherence to the Med. Diet was determined through the Alternate Med. Diet (aMed) score aMed score range: 0-10; scores of 6-10 are moderate-high and 0-5 are low |
Insomnia symptoms (IS): self-reported through Women’s health Insomnia Rating Scale (WHIIRS) Sleep duration: objectively measured through 7-day wear of wrist actigraphy Subjects further categorized into categories of sleep duration + IS |
Higher aMed score: more likely to sleep 6-7 h/night, less likely to have IS, less likely to have IS + <6 h sleep Increase in aMed score: less likely to have IS Increase and no change in aMed Score: less likely to have IS + < 6 h sleep (*) |
| Campanini et al., 2016 (47) | Sample size: 1,596 Spanish men and women Age range: 60 y and older (Seniors-ENRICA Study) |
Modified, computer-assisted FFQ; Score (0-14) on Med. Diet Adherence Screener (MEDAS) MEDAS score ≥9 is high adherence, 8 is moderate |
Sleep duration: Self-reported duration of sleep/night (short: ≤6, normal: 7–8, long: ≥ 9 h); Change in sleep duration also calculated Sleep Quality: 5-point scale: very poor to very good; self-reported frequency of indicators of poor nighttime sleep; Epworth Sleepiness Scale |
Highest vs lowest tertile of MEDAS: lower risk of large increases or decreases in sleep duration Highest tertile of MEDAS: lower risk of reporting multiple indicators of poor sleep quality; trend towards inverse association with general sleep quality |
| Mamalaki et al., 2018 (49) | Sample size: 1639 men and women Age range: 65 y and older (Hellenic Longitudinal Study) |
Validated FFQ; frequency of intake of food consistent with Med. Diet scored from 1 to 5 on 11-question MedDietScore Scores ranged from 0-55; higher scores mean greater adherence |
Sleep duration: Self-reported duration of sleep/night Sleep quality: Self-report on two measures: Sleep Scale and Sleep Index II; scores for sleep quality on Sleep Index II ranged from 1 to 54 with higher scores meaning more dysfunction |
Higher adherence to the Med. Diet was associated with better sleep quality. *Further analysis indicated this is only true in those under 75 y No significant relationship observed between Med. Diet adherence and sleep duration |
| Ferranti et al., 2016 (48) | Sample size: 1586 Italian boys and girls Age range: 11-14 y |
Revised FFQ. Data used to determine adherence to Med. Diet through an index for children and adolescents (KIDMED) |
Sleep duration: Calculated from self-reported bed and wake times Type of sleeper (e.g. early bed, early wake) also determined Daytime sleepiness assessed by Pediatric Daytime Sleepiness Scale (PDSS) |
Med. Diet adherence was positively associated with total sleep time and inversely associated with daytime sleepiness FV intake inversely related to total sleep time *Only 84 (6%) of sample met criteria for good adherence to Med. Diet |
(*): Some of these relationships failed to maintain statistical significance after full adjustment in the statistical model
The first study to investigate whether adherence to the Mediterranean diet was related to sleep outcomes was conducted in French men and women taking part in the Three-City Study (45). Results showed that greater conformity to a Mediterranean diet predicted reduced risk (by approximately 20%) for self-reported insomnia symptoms in women, but not men. These findings were extended by the Sleep ancillary to the Multi-Ethnic Study of Atherosclerosis (MESA): individuals with higher adherence to a Mediterranean-style diet had a lower likelihood of experiencing insomnia symptoms (OR: 0.81) or insomnia symptoms with short sleep duration (OR: 0.65) than those with low adherence (46).
Associations between the Mediterranean diet and sleep duration have also been observed, although results are somewhat less straightforward. Data from a study in Spain indicated that those most adherent to the Mediterranean diet had more stability in sleep duration over a 3-y period than did those that were least adherent, exemplified by a 42% lower risk of losing more than 2 hours of sleep per night (47). Moreover, the majority of those individuals conforming closely to the Mediterranean diet also tended to achieve adequate sleep. Further support for a role of the Mediterranean diet on sleep duration was provided in a study of 11–14 y-olds (48). In contrast, a study of older adults in Greece showed no relation between Mediterranean diet score and sleep duration (49). Discordance in results can likely be explained by differences in study populations and measures of diet adherence and sleep across studies.
The MESA Sleep ancillary study was the first to show a relationship between the Mediterranean diet and objectively-measured sleep duration; higher adherence to a Mediterranean-type diet was associated with a 43% greater likelihood of achieving 6–7 h of sleep/night compared to 6 h/night (46). Although not an experimental study, there is some indication of directionality given that increases in adherence over a period of time were also associated with measures of healthier sleep; this included an increased likelihood of achieving more adequate sleep duration (OR: 1.36) and reduced probability of experiencing insomnia symptoms with short sleep duration (OR: 0.70). Based on these findings, it would be warranted to experimentally test whether adopting a dietary pattern closely resembling the Mediterranean diet can improve sleep health and, in particular, promote adequate sleep duration.
When examining sleep quality as an outcome, we again find evidence of a relation with the Mediterranean diet. In addition to decreased risk for insomnia symptoms (45,46), data from the Seniors-ENRICA and Hellenic Longitudinal studies both indicate a lower likelihood of poor sleep quality for those who more closely follow a Mediterranean diet (47,49). Results from a study in our lab may provide insight into a potential mechanism. A secondary analysis of sleep quality (using polysomnography) following a day of ad libitum food intake revealed that time spent in slow-wave sleep was positively associated with fiber and negatively associated with saturated fat intakes (50). For every 10 g increase in fiber intake, there was a 2.6% increase in percent of total sleep time spent in slow-wave sleep; in contrast, time in slow-wave sleep was reduced by 7.1% for every 10% increase in energy intake from saturated fat. Given that the Mediterranean diet is comprised of foods high in fiber and low in saturated fat, such as FV, whole grains, olive oil and nuts (51), these data would support a potential causal influence of this particular dietary pattern for sleep health.
The studies described above are some of the first to examine the relation between a heart-healthy diet and sleep but are not without limitations. Well-designed clinical trials are needed to determine a causal relation between consumption of a Mediterranean diet and objective measures of sleep duration and quality. Studies would also benefit from consistent use of a standard assessment of adherence to the Mediterranean diet, which would improve homogeneity of classification across studies. Nevertheless, data from the studies available provide further support of a bi-directional relationship between the diet and sleep and extend previous work by investigating the complete diet. Maybe more importantly, the biochemical and physiological consequences of diets rich in FV, whole grains, and fiber and low in saturated fat could be used to explain the mechanisms underlying an effect of diet on sleep as described in a review by St-Onge et al. (2018) (52).
Potential mechanisms underlying the relations between sleep, diet and CVD
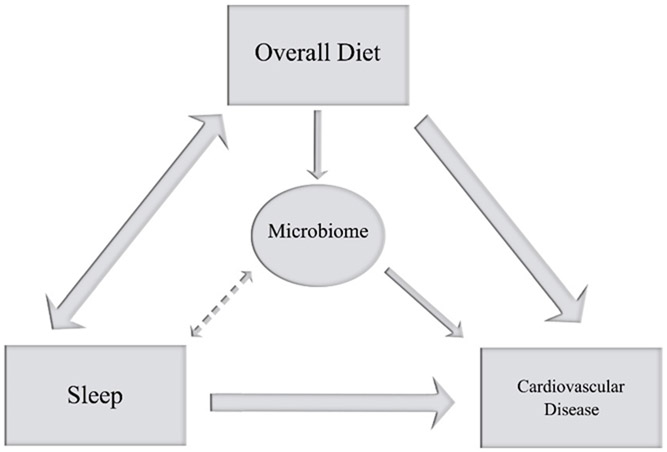
Few mechanisms have been put forth to explain the relation between sleep and CVD risk. In a recent review, we posited that sleep may influence CVD risk via changes in diet, which could also help to explain the potential bi-directional relationship between sleep and the diet (52). As described in sections above, sleep restriction increases overall food intake with a preference towards high-carbohydrate, high-fat foods, which are associated with an adverse cardiometabolic health profile (53-55). We suggested that plant-rich diets, such as the Mediterranean diet, may reduce CVD risk by improving sleep via changes in tryptophan intakes (52). Diets with a preponderance of plant foods, as recommended by the Dietary Guidelines and exemplified by the Mediterranean diet, are rich in tryptophan, which is a precursor to melatonin and serotonin, both of which influence sleep duration and quality. The sleep improvements may act to reduce CVD. Herein, we propose another mechanism by which such diets may improve sleep and reduce CVD risk: via the gut microbiome (Figure 1).
Figure 1:
Recent evidence suggests a bi-directional relationship between sleep and the diet, both of which impact CVD risk. We propose that the microbiome contributes to the relationship between sleep and the diet and, consequently, their influence on CVD risk.
Relation between gut microbiome and cardiovascular disease risk
Increased microbial richness has been associated with reduced lifetime CVD risk (56). In particular, genera from Prevotellaceae (including Bacteroidetes), taxa from Firmicutes, and genus from Proteobacteria have been associated with CVD risk, often with opposing relations of different genera within the same phylum. Gut microbiota metabolize nutrients, with the main end products being trimethylamine N-oxide (TMAO) and short-chain fatty acids (SCFA) (57), which have been associated with increased and reduced CVD risk, respectively (58). Dietary intakes of choline, phosphatidylchoine, and L-carnitine lead to increased trimethylamine production by gut bacteria which is then oxidized in the liver to TMAO and excreted in the urine (57). Trimethylamine N-oxide reduces reverse cholesterol transport and enhances platelet responsiveness, contributing to the development of atherosclerosis. Vegans and vegetarians have reduced capacity to form trimethylamine from carnitine, which may contribute to their lower risk of CVD (59).
Short-chain fatty acids, on the other hand, have been shown to promote cardiovascular health via maintenance of the intestinal barrier, which prevents translocation of bacterial pro-inflammatory molecules, such as lipopolysaccharides, and promotion of regulatory T cells expansion and immunity (60). The SCFA act as natural ligands for G-protein coupled receptors that may play a role in inflammation and blood pressure regulation (57,59,60).
In terms of dietary patterns, the Mediterranean diet may reduce CVD risk via the mechanisms highlighted above. Vegetarian protein sources increase SCFA while red meat intakes are associated with increased TMAO (61). Polyphenols in red wine, a key component of the Mediterranean diet, increase abundance of Bacteroides and reduce pathogenic Clostridium species (61), further contributing to its health benefits.
Role of sleep on gut microbiome
The gut microbiome may be susceptible to changes in sleep patterns. It has been proposed that sleep loss itself acts as a stressor that shifts the gut microbial composition to a profile that is indicative of metabolic dysfunction (62). However, in a small study of sleep restriction with a controlled diet, Benedict et al. showed that 2 nights of 4.25 h bedtimes tended to increase the Firmicutes to Bacteroidetes ratio compared to 2 nights of adequate sleep (63). Moreover, inadequate sleep resulted in lower counts from Tenericutes phylum and greater counts from the Coriobacteriaceae and Erysipeltrichacaea families. Species from these bacterial families are positively correlated with liver fat content and obesity (64,65). On the other hand, another experiment performed in rats and humans showed no overt effects of 2 cycles of 5 nights of sleep restriction, separated by 5 nights of recovery sleep, on microbial beta diversity with no population shifts in neither humans nor rats (66). These studies had small sample sizes (n=9–11) and may have been too short in duration for an influence on gut microbiome composition to be observed; nevertheless, the available data do not point to a strong effect of short sleep on the gut microbial content. Rather, given the known impact of severe sleep restriction on food intake and diet quality, it may be that any influence of inadequate sleep on the gut microbiome is the result of shifts in dietary intakes. Alternatively, it may be that inappropriate changes in the timing of sleep or reductions in sleep quality (including insomnia and obstructive sleep apnea), may be of importance in influencing shifts in gut microbiome. Additional research is needed, however, to clearly determine the influence of inadequate sleep on the gut microbiome.
Influence of diet on gut microbiome
It is well known that diet has a profound influence on the composition and diversity of the gut microbiome (61). Plant-based diets, and, in particular, the Mediterranean diet, have attracted much attention for their potential effects on the microbiome. A recent review summarized studies comparing the gut microbiome of individuals based on their level of adherence to the Mediterranean diet (58); results of these studies indicate that adults who follow the Mediterranean dietary pattern more closely, as well as those with a largely vegetarian diet, have a lower ratio of Firmicutes to Bacteroidetes and a shift in more beneficial bacterial flora than adults who are less adherent to theses dietary patterns.
Several observational studies support the notion that the gut microbiome of adults following vegetarian and Mediterranean diets differs from that of adults consuming more omnivorous diets. For example, De Filippis et al. (67) compared the gut microbiome of vegetarians, vegans, and omnivores living in Italy. In this population, high adherence to the Mediterranean diet was noted in 88% of vegans, 65% of vegetarians, and 30% of omnivores. Microbiota alpha diversity, which is positively correlated with cardiometabolic health (68), did not differ by diet type or Mediterranean adherence. However, Bacteroidetes were more abundant in vegetarians and vegans than omnivores and omnivores had higher Firmicutes to Bacteroidetes ratio. Fecal content of Roseburia, Lachnospira, and Prevotella correlated with plant-based diet composition while L. Ruminococcus and Streptococcus were associated with animal-based nutrients (67). Others have also found that higher Mediterranean diet adherence scores were associated with abundance of Bacteroidetes and Prevotellaceae and inversely associated with Firmicutes and Ruminococcaceae families (69) and were related to lower Firmicutes to Bacteroidetes ratio (70). On the other hand, Mitsou et al. (2017) failed to observe any relation between Mediterranean diet scores and Prevotella, Bacteroides, and Roseburia in 116 adults from Greece (71). These findings have important health implications given that Bacteroides, Prevotella, Roseburia, Faecalibacterium, and Ruminococcus and higher Streptococcus and Clostridium genera have been observed in patients with obesity and metabolic syndrome compared to individuals without metabolic syndrome or obesity (72). Differences between studies may be due to method of collection of dietary information, participant characteristics, or study location.
There are only a few intervention studies that have tested the effects of plant-based foods and diets on the gut microbiome. For example, provision of 3-d diet consisting only of fruit juice decreased fecal content of Firmicutes while increasing Bacteriodetes and Cyanobacteria compared to baseline (73). These changes were related to changes in body weight, which was decreased following the diet. As a result, and because there was no control group, it is unclear if changes to the microbiome were due to the diet or the weight loss.
Another study compared the effects of a Mediterranean diet versus a low-fat diet on the gut microbiome of men at varying levels of cardiometabolic risk: metabolic syndrome with obesity; obesity alone; or no obesity nor metabolic syndrome (72). Participants received intensive dietary counseling to either consume a Mediterranean diet (35% of energy from fat, <10% of energy from saturated fat) or a low-fat diet (American Heart Association Step I diet: <30% of energy from fat and <10% from saturated fat) and were followed for 2 y. Groups did not differ with respect to bacterial diversity at baseline or follow-up. However, there were differences at the phylum level between groups at baseline: participants with metabolic syndrome and obesity had a higher Firmicutes to Bacteroidetes ratio and lower Actinobacteria and Bacteroidetes. At the genus level, this group had lower P. distanosis and F. prausnitzii. Interestingly, in participants with the metabolic syndrome, both dietary interventions increased Bacteroides, Prevotella, Faecalibacteria, and the low-fat diet increased Bacteroidetes and decreased the Firmicutes to Bacteroidetes ratio, Streptococcus, and Clostridium relative to baseline whereas the Mediterranean diet increased Roseburia and Ruminococcus genera and P. distanosis and F. prausnitzii species (72). The authors proposed that chronic consumption of a healthy dietary pattern restores gut dysbiosis depending on the degree of metabolic dysfunction.
Finally, a study in non-human primates evaluated the effects of a Mediterranean diet, versus a Western diet, on gut microbiome over a 30 mo period (74). The Mediterranean diet consisted of fish oil, olive oil, fish meal, butter, eggs, black and garbanzo bean flours, wheat flour, V-8, fruit puree and sucrose (31% of energy from fat, 25% of fat as saturated fat, 13% fiber) whereas the Western diet consisted of lard, beef tallow, butter, eggs, cholesterol, casein, lactalbumin, dextrin, high fructose corn syrup and sucrose (31% of energy from fat, 39% of fat as saturated fat, 9% fiber). At end point, macaques fed the Mediterranean diet had higher microbial diversity, higher Bacteroidetes, Proteobacteria, Fibrobacteres, Spirochaetes, and lower Firmicutes and Verrucomicrobia (lower Firmicutes to Bacteroidetes ratio) than those fed the Western diet. These studies clearly lend support to an impact of the Mediterranean diet in producing a gut microbiome profile that is representative of better health status.
Diet, the microbiome and sleep quality
Given that diet may influence sleep quality, we sought to query the literature on the role of the microbiome on sleep health. We found few studies examining the relation between gut microbiome and sleep health. In a small cohort of elderly adults, Anderson et al. observed inverse relations between Lentisphaerae and Verrucomicrobia and scores on the Pittsburgh Sleep Quality Index questionnaire, indicating that these phyla are related to better sleep quality (75). In a double-blind, placebo-controlled study of young adults subjected to academic stress, Takada et al. tested the effect of an 8 wk dietary supplementation of fermented milk containing the probiotic Lactobacillus casei Shirota on sleep measured prior to and 3 weeks after an exam. Results showed that probiotic milk suppressed the increase in sleep onset latency observed in the placebo milk group (76). Additionally, in comparison to controls, participants in the intervention arm exhibited better sleep quality and intensity, assessed through EEG, as the exam approached. Although microbiota was not assessed in this study, that provision of probiotics can have beneficial effects on sleep could indicate a role of the microbiome in sleep quality. Effects could depend on the strain of probiotic or age of the host; supplementation with Lactobacillus helveticus-fermented milk for 3 wk had similar effects as a non-fermented milk placebo in elderly adults (77). It is important to note that gut microbiome composition and function were not assessed in the latter two intervention studies and, consequently, it is unknown whether colonization with the probiotic was achieved or whether any changes in the gut microbiome were obtained as a result of probiotic consumption to effect changes in sleep quality. It is also possible that milk placebo could also cause changes in gut microbiome that may have attenuated the findings. An adequate control for such studies of fermented milk is often difficult to choose.
Conclusion
Based on the studies reviewed here, we propose a cyclical pattern implicating sleep, diet, and the gut microbiome in determining the risk of CVD. On the one hand, poor sleep (low quality and/or quantity) affects dietary patterns towards ones that would predispose to a shift in gut microbiome associated with increased CVD risk: higher Firmicutes:Bacteroidetes ratio, Prevotellae, and Proteobacteria that generate higher TMAO and less SCFA. These metabolites in turn promote inflammation which contributes to the development of atherosclerosis. In addition, SCFA have been shown, in animal models, to have important functions in circadian clock rhythmicity (78,79), which may be important for cardiometabolic health. We also have accumulating evidence that poor dietary quality is associated with poor sleep. However, there is currently insufficient evidence to suggest that inadequate sleep affects the gut microbiome directly but this could propel a cycle of poor sleep, leading to an unhealthy dietary pattern/microbiome, further reinforcing inadequate sleep and increased CVD risk.
Acknowledgments
This is funded in part by AHA Go Red for Women 16SFRN27950012 and NIH R01HL128226 and R01HL142648 (St-Onge, PI). Dr. Zuraikat is supported by an AHA Go Red for Women Post-Doctoral Fellowship (16SFRN27880000).
Footnotes
The authors declare no conflicts of interest.
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and compiled with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/institutional guidelines).
References
- 1.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women−−2011 update: a guideline from the american heart association. Circulation 2011:123(11):1243–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.CDC. Know the Facts About Heart Disease. 2017. [cited 2018 November 4]. Available from: www.cdc.gov/nchs/
- 3.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, et al. Obesity and Cardiovascular Disease. Arterioscler Thromb Vasc Biol 2006:26(5):968–76. [DOI] [PubMed] [Google Scholar]
- 4.Hales CM, Fryar CD, Carroll MD, Freedman DS, Ogden CL. Trends in Obesity and Severe Obesity Prevalence in US Youth and Adults by Sex and Age, 2007–2008 to 2015–2016. JAMA 2018:319(16):1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.St-Onge M-P, Grandner MA, Brown D, Conroy MB, Jean-Louis G, Coons M, et al. Sleep Duration and Quality: Impact on Lifestyle Behaviors and Cardiometabolic Health: A Scientific Statement From the American Heart Association. Circulation 2016:134(18). e367–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.St-Onge M-P, Roberts AL, Chen J, Kelleman M, O’Keeffe M, RoyChoudhury A, et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011:94(2):410–6.This study shows that sleep restriction leads to increased energy intake, particularly from fat.
- 7.Badimon L, Chagas P, Chiva-Blanch G. Diet and Cardiovascular Disease: Effects of Foods and Nutrients in Classical and Emerging Cardiovascular Risk Factors. Curr Med Chem. 2017:24:1–12. [DOI] [PubMed] [Google Scholar]
- 8.Mayo Clinic. Mediterranean diet for heart health. 2017. [cited 2018 November 4]. Available from: https://www.mayoclinic.org/healthy-lifestyle/nutrition-and-healthy-eating/in-depth/mediterranean-diet/art-20047801
- 9.Mancini JG, Filion KB, Atallah R, Eisenberg MJ. Systematic Review of the Mediterranean Diet for Long-Term Weight Loss. Am J Med 2016:129(4):407–15.e4. [DOI] [PubMed] [Google Scholar]
- 10.Siervo M, Lara J, Chowdhury S, Ashor A, Oggioni C, Mathers JC. Effects of the Dietary Approach to Stop Hypertension (DASH) diet on cardiovascular risk factors: a systematic review and meta-analysis. Br J Nutr 2015:113(01):1–15. [DOI] [PubMed] [Google Scholar]
- 11.Ledikwe JH, Rolls BJ, Smiciklas-Wright H, Mitchell DC, Ard JD, Champagne C, et al. Reductions in dietary energy density are associated with weight loss in overweight and obese participants in the PREMIER trial. Am J Clin Nutr 2007: 85(5):1212–21. [DOI] [PubMed] [Google Scholar]
- 12.Estruch R, Ros E, Salas-Salvadó J, Covas M-I, Corella D, Arós F, et al. Primary Prevention of Cardiovascular Disease with a Mediterranean Diet Supplemented with Extra-Virgin Olive Oil or Nuts. N Engl J Med 2018:378(25):e34.This study shows, in an at risk population, that the Mediterranean diet supplemented with olive oil or nuts leads to significantly reduced risk of cardiovascular events compared to a control diet.
- 13.Fang J, Wheaton AG, Keenan NL, Greenlund KJ, Perry GS, Croft JB. Association of Sleep Duration and Hypertension Among US Adults Varies by Age and Sex. Am J Sleep Hypertens 2012:25(3):335–41. [DOI] [PubMed] [Google Scholar]
- 14.Yang H, Haack M, Gautam S, Meier-Ewert HK, Mullington JM. Repetitive exposure to shortened sleep leads to blunted sleep-associated blood pressure dipping. J Hypertens 2017:35(6):1187–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin P, Chang K-T, Lin Y-A, Tzeng I-S, Chuang H-H, Chen J-Y, et al. Association between self-reported sleep duration and serum lipid profile in a middle-aged and elderly population in Taiwan: a community-based, cross-sectional study. BMJ Open 2017:7(10):e015964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aho V, Ollila HM, Kronholm E, Bondia-Pons I, Soininen P, Kangas AJ, et al. Prolonged sleep restriction induces changes in pathways involved in cholesterol metabolism and inflammatory responses. Sci Rep 2016:6(1):24828 DOI: 10.1038/srep24828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shan Z, Mo H, Xie M, Yan P, Guo Y, Bao W, et al. Sleep Duration and Risk of Type 2 Diabetes: A Meta-analysis of Prospective Studies. Diabetes Care 2015:38:529–37. [DOI] [PubMed] [Google Scholar]
- 18.Aggarwal B, Makarem N, Shah R, Emin M, Wei Y, St‐Onge M, et al. Effects of Inadequate Sleep on Blood Pressure and Endothelial Inflammation in Women: Findings From the American Heart Association Go Red for Women Strategically Focused Research Network. J Am Heart Assoc 2018:7(12). e0088590.This study shows that poor sleep quality is related to higher blood pressure and vascular inflammation.
- 19.Noorwali EA, Cade JE, Burley VJ, Hardie LJ. The relationship between sleep duration and fruit/vegetable intakes in UK adults: a cross-sectional study from the National Diet and Nutrition Survey. BMJ Open 2018:8:20810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grandner MA, Jackson N, Gerstner JR, Knutson KL. Dietary nutrients associated with short and long sleep duration. Data from a nationally representative sample. Appetite 2013:64:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katagiri R, Asakura K, Kobayashi S, Suga H, Sasaki S, Sasaki S. Low Intake of Vegetables, High Intake of Confectionary, and Unhealthy Eating Habits are Associated with Poor Sleep Quality among Middle-aged Female Japanese Workers. J Occup Health 2014:56(5):359–68 [DOI] [PubMed] [Google Scholar]
- 22.Del Brutto OH, Mera RM, Ha J, Gillman J, Zambrano M, Castillo PR. Dietary fish intake and sleep quality: a population-based study. Sleep Med 2016:17:126–8. [DOI] [PubMed] [Google Scholar]
- 23.St-Onge M-P. Sleep-obesity relation: underlying mechanisms and consequences for treatment. Obes Rev 2017:18:34–9. [DOI] [PubMed] [Google Scholar]
- 24.Al Khatib HK, Harding SV, Darzi J, Pot GK. The effects of partial sleep deprivation on energy balance: a systematic review and meta-analysis. Eur J Clin Nutr 2017:71(5):614–24. [DOI] [PubMed] [Google Scholar]
- 25.Spaeth AM, Dinges DF, Goel N. Effects of Experimental Sleep Restriction on Weight Gain, Caloric Intake, and Meal Timing in Healthy Adults. Sleep 2013:36(7):981–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Broussard JL, Kilkus JM, Delebecque F, Abraham V, Day A, Whitmore HR, et al. Elevated ghrelin predicts food intake during experimental sleep restriction. Obesity 2016:24(1):132–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr 2009:89(1):126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bray GA, Popkin BM. Dietary fat intake does affect obesity! Am J Clin Nutr 1998:68(6):1157–73. [DOI] [PubMed] [Google Scholar]
- 29.Hill JO, Melanson EL, Wyatt HT. Dietary Fat Intake and Regulation of Energy Balance: Implications for Obesity. J Nutr 2000:130(2):284S–8S. [PubMed] [Google Scholar]
- 30.Bellisle F Meals and snacking, diet quality and energy balance. Physiol Behav 2014:134:38–43. [DOI] [PubMed] [Google Scholar]
- 31.Robertson MD, Russell-Jones D, Umpleby AM, Dijk D-J. Effects of three weeks of mild sleep restriction implemented in the home environment on multiple metabolic and endocrine markers in healthy young men. Metabolism 2013:62(2):204–11. [DOI] [PubMed] [Google Scholar]
- 32.Brezinová V, Oswald I. Sleep after a bedtime beverage. Br Med J 1972:2(5811):431–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valtonen M, Niskanen L, Kangas A-P, Koskinen T. Effect of melatonin-rich night-time milk on sleep and activity in elderly institutionalized subjects. Nord J Psychiatry 2005:59(3):217–21. [DOI] [PubMed] [Google Scholar]
- 34.Losso JN, Finley JW, Karki N, Liu AG, Prudente A, Tipton R, et al. Pilot Study of the Tart Cherry Juice for the Treatment of Insomnia and Investigation of Mechanisms. Am J Ther 2018:25(2):e194–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mayer G, Kröger M, Meier-Ewert K. Effects of Vitamin B12 on Performance and Circadian Rhythm in Normal Subjects. Neuropsychopharmacology 1996:15(5):456–64. [DOI] [PubMed] [Google Scholar]
- 36.Aspy DJ, Madden NA, Delfabbro P. Effects of Vitamin B6 (Pyridoxine) and a B Complex Preparation on Dreaming and Sleep. Percept Mot Skills 2018:125(3): 451–462. DOI: 10.1177/00331512518770326. [DOI] [PubMed] [Google Scholar]
- 37.Lin H-H, Tsai P-S, Fang S-C, Liu J-F. Effect of kiwifruit consumption on sleep quality in adults with sleep problems. Asia Pac J Clin Nutr 2011:20(2):169–74. [PubMed] [Google Scholar]
- 38.Peuhkuri K, Sihvola N, Korpela R. Diet promotes sleep duration and quality. Nutr Res 2012:32:309–19. [DOI] [PubMed] [Google Scholar]
- 39.St-Onge M-P, Mikic A, Pietrolungo CE. Effects of Diet on Sleep Quality. Adv Nutr 2016:7(5):938–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phillips F, Crisp A., Mcguinness B, Kalucy E, Chen C, Koval J, et al. Isolcaloric diet changes and electroencephalographic sleep. Lancet 1975:306(7938):723–5. [DOI] [PubMed] [Google Scholar]
- 41.Porter J, Horne J Bed-time food supplements and sleep: Effects of different carbohydrate levels. Electroencephalogr Clin Neurophysiol 1981:51(4):426–33. [DOI] [PubMed] [Google Scholar]
- 42.Afaghi A, O’Connor H, Chow CM. Acute effects of the very low carbohydrate diet on sleep indices. Nutr Neurosci 2008:11(4):146–54. [DOI] [PubMed] [Google Scholar]
- 43.Yajima K, Seya T, Iwayama K, Hibi M, Hari S, Nakashima Y, et al. Effects of Nutrient Composition of Dinner on Sleep Architecture and Energy Metabolism during Sleep. J Nutr Sci Vitaminol (Tokyo) 2014:60(2):114–21. [DOI] [PubMed] [Google Scholar]
- 44.USDA. 2015–2020 Dietary Guidelines for Americans. 2015. [cited 2018 November 4]. Available from: http://health.gov/dietaryguidelines/2015/guidelines/.
- 45.Jaussent I, Dauvilliers Y, Ancelin M-L, Dartigues J-F, Tavernier B, Touchon J, et al. Insomnia Symptoms in Older Adults: Associated Factors and Gender Differences. Am J Geriatr Psychiatry 2011:19(1):88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castro-Diehl C, Wood AC, Redline S, Reid M, Johnson DA, Maras JE, et al. Mediterranean diet pattern and sleep duration and insomnia symptoms in the Multi-Ethnic Study of Atherosclerosis. Sleep 2018:41(11). Doi: 10.1093/sleep/zsy158/5077799.This is the first study to relate sleep quality and objectively measured sleep duration to the Mediterranean Diet. Higher adherence to the Mediterranean diet was associated with healthier sleep.
- 47.Campanini MZ, Guallar-Castillón P, Rodríguez-Artalejo Fernando, Lopez-Garcia E Mediterranean Diet and Changes in Sleep Duration. Sleep 2017:40(3). Doi: 10.1093/sleep/zsw083. [DOI] [PubMed] [Google Scholar]
- 48.Ferranti R, Marventano S, Castellano S, Giogianni G, Nolfo F, Rametta S, et al. Sleep quality and duration is related with diet and obesity in young adolescent living in Sicily, Southern Italy. Sleep Sci 2016:9:117–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mamalaki E, Anastasiou CA, Ntanasi E, Tsapanou A, Kosmidis MH, Dardiotis E, et al. Associations between the mediterranean diet and sleep in older adults: Results from the hellenic longitudinal investigation of aging and diet study. Geriatr Gerontol Int 2018:18(11):1543–8. [DOI] [PubMed] [Google Scholar]
- 50.St-Onge M-P, Roberts A, Shechter A, Choudhury AR. Fiber and Saturated Fat Are Associated with Sleep Arousals and Slow Wave Sleep. J Clin Sleep Med 2016:12(01):19–24.This is one of the first studies to demonstrate an influence of diet on sleep quality; lower fiber and higher saturated fat intake were associated with reduced sleep quality.
- 51.Davis C, Bryan J, Hodgson J, Murphy K. Definition of the Mediterranean Diet: A Literature Review. Nutrients 2015:7(11):9139–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.St-Onge M-P, Crawford A, Aggarwal B. Plant-based diets: Reducing cardiovascular risk by improving sleep quality? Curr Sleep Med Rep 2018:4(1):74–8. [PMC free article] [PubMed] [Google Scholar]
- 53.Abdesselam I, Pepino P, Troalen T, Macia M, Ancel P, Masi B, et al. Time course of cardiometabolic alterations in a high fat high sucrose diet mice model and improvement after GLP-1 analog treatment using multimodal cardiovascular magnetic resonance. J Cardiovasc Magn Reson 2015:17(1):95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Drake I, Sonestedt E, Ericson U, Wallström P, Orho-Melander M. A Western dietary pattern is prospectively associated with cardio-metabolic traits and incidence of the metabolic syndrome. Br J Nutr 2018:119(10):1168–76. [DOI] [PubMed] [Google Scholar]
- 55.Heidemann C, Scheidt-Nave C, Richter A, Mensink GBM. Dietary patterns are associated with cardiometabolic risk factors in a representative study population of German adults. Br J Nutr 2011:106(8):1253–62. [DOI] [PubMed] [Google Scholar]
- 56.Kelly TN, Bazzano LA, Ajami NJ, He H, Zhao J, Petrosino JF, et al. Gut Microbiome Associates With Lifetime Cardiovascular Disease Risk Profile Among Bogalusa Heart Study Participants. Circ Res 2016:119(8):956–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peng J, Xiao X, Hu M, Zhang X. Interaction between gut microbiome and cardiovascular disease. Life Sci 2018:214:153–7. [DOI] [PubMed] [Google Scholar]
- 58.Tindall AM, Petersen KS, Kris-Etherton PM. Dietary Patterns Affect the Gut Microbiome - The Link to Risk of Cardiometabolic Diseases. J Nutr 2018:148(9):1402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tang WHW, Kitai T, Hazen SL. Gut Microbiota in Cardiovascular Health and Disease. Circ Res 2017:120(7):1183–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chambers ES, Preston T, Frost G, Morrison DJ. Role of Gut Microbiota-Generated Short-Chain Fatty Acids in Metabolic and Cardiovascular Health. Curr Nutr Rep 2018:7(4):198–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Singh RK, Chang H-W, Yan D, Lee KM, Ucmak D, Wong K, et al. Influence of diet on the gut microbiome and implications for human health. J Transl Med 2017:15(1):73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Reynolds AC, Paterson JL, Ferguson SA, Stanley D, Wright KP, Dawson D. The shift work and health research agenda: Considering changes in gut microbiota as a pathway linking shift work, sleep loss and circadian misalignment, and metabolic disease. Sleep Med Rev 2017:34:3–9. [DOI] [PubMed] [Google Scholar]
- 63.Benedict C, Vogel H, Jonas W, Woting A, Blaut M, Schürmann A, et al. Gut microbiota and glucometabolic alterations in response to recurrent partial sleep deprivation in normal-weight young individuals. Mol Metab 2016:5(12):1175–86.This is the first study to demonstrate that sleep restriction has deleterious effects on the composition of the microbiome in humans.
- 64.Spencer MD, Hamp TJ, Reid RW, Fischer LM, Zeisel SH, Fodor AA. Association Between Composition of the Human Gastrointestinal Microbiome and Development of Fatty Liver With Choline Deficiency. Gastroenterology 2011:140(3):976–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci 2009:106(7):2365–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang SL, Bai L, Goel N, Bailey A, Jang CJ, Bushman FD, et al. Human and rat gut microbiome composition is maintained following sleep restriction. Proc Natl Acad Sci 2017:114(8):E1564–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut 2016:65(11):1812–21.This study shows that the Mediterranean and plant-rich diets are associated with favorable microbiota composition.
- 68.Hansen TH, Gøbel RJ, Hansen T, Pedersen O. The gut microbiome in cardio-metabolic health. Genome Med 2015:7(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gutiérrez-Díaz I, Fernández-Navarro T, Sánchez B, Margolles A, González S. Mediterranean diet and faecal microbiota: a transversal study. Food Funct 2016:7(5):2347–56. [DOI] [PubMed] [Google Scholar]
- 70.Garcia-Mantrana I, Selma-Royo M, Alcantara C, Collado MC. Shifts on Gut Microbiota Associated to Mediterranean Diet Adherence and Specific Dietary Intakes on General Adult Population. Front Microbiol 2018:9:890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mitsou EK, Kakali A, Antonopoulou S, Mountzouris KC, Yannakoulia M, Panagiotakos DB, et al. Adherence to the Mediterranean diet is associated with the gut microbiota pattern and gastrointestinal characteristics in an adult population. Br J Nutr 2017:117(12):1645–55. [DOI] [PubMed] [Google Scholar]
- 72.Haro C, García-Carpintero S, Rangel-Zúñiga OA, Alcalá-Díaz JF, Landa BB, Clemente JC, et al. Consumption of Two Healthy Dietary Patterns Restored Microbiota Dysbiosis in Obese Patients with Metabolic Dysfunction. Mol Nutr Food Res 2017:61(12):1700300 Doi: 10.1002/mnfr.201700300.This study shows that consumption of a Mediterranean diet can improve gut microbiome dysbiosis in individuals with severe metabolic syndrome.
- 73.Henning SM, Yang J, Shao P, Lee R-P, Huang J, Ly A, et al. Health benefit of vegetable/fruit juice-based diet: Role of microbiome. Sci Rep 2017:7(1):2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nagpal R, Shively CA, Appt SA, Register TC, Michalson KT, Vitolins MZ, et al. Gut Microbiome Composition in Non-human Primates Consuming a Western or Mediterranean Diet. Front Nutr 2018:5:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Anderson JR, Carroll I, Azcarate-Peril MA, Rochette AD, Heinberg LJ, Peat C, et al. A preliminary examination of gut microbiota, sleep, and cognitive flexibility in healthy older adults. Sleep Med 2017:38:104–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Takada M, Nishida K, Gondo Y, Kikuchi-Hayakawa H, Ishikawa H, Suda K, et al. Beneficial effects of Lactobacillus casei strain Shirota on academic stress-induced sleep disturbance in healthy adults: a double-blind, randomised, placebo-controlled trial. Benef Microbes 2017:8(2):153–62. [DOI] [PubMed] [Google Scholar]
- 77.Yamamura S, Morishima H, Kumano-go T, Suganuma N, Matsumoto H, Adachi H, et al. The effect of Lactobacillus helveticus fermented milk on sleep and health perception in elderly subjects. Eur J Clin Nutr 2009:63(1):100–5. [DOI] [PubMed] [Google Scholar]
- 78.Leone V, Gibbons SM, Martinez K, Hutchison AL, Huang EY, Cham CM, et al. Effects of diurnal variation of gut microbes and high-fat feeding on host circadian clock function and metabolism. Cell Host Microbe 2015:17(5):681–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tahara Y, Yamazaki M, Motohashi H, Sasaki H, Miyakawa H, Haraguchi A, et al. Gut microbiota-derived short chain fatty acids induce circadian clock entrainment in mouse peripheral tissue. Sci Rep 2018:8(1):1395. [DOI] [PMC free article] [PubMed] [Google Scholar]