Abstract
This study examined the effects of a caffeine treatment to improve nuclear reprogramming in porcine cloned embryos. Embryonic development and the expression of genes related to pluripotency (POU5F1, SOX2, NANOG, and CDX2) were compared after caffeine supplementation during manipulation at different concentrations (0, 1.25, 2.5, and 5.0 mM) and after varying the delayed activation time (control, 1, 2, and 4 h) after fusion. Caffeine added to media during manipulation produced a higher rate of development to blastocysts in the 1.25 mM group than in the other concentration groups (22.8% vs. 16.1%, 16.2%, and 19.2%; p < 0.05). When caffeine was added during the 4 h delayed activation, the 1.25 mM caffeine concentration produced a significantly higher rate of development than those in the other 4 h-activation-delayed caffeine concentration groups (22.4% vs. 9.4%, 14.0%, and 11.1%; p < 0.05). On the other hand, no significant improvement over that in the control group was observed when caffeine was supplemented during both the manipulation period and delayed activation period (16.0% vs. 15.2%), respectively. The levels of POU5F1, SOX2, and NANOG expression in blastocysts were significantly higher in the delayed activation caffeine group (4 h, 1.25 mM) than in the control group (1 h, 0 mM; p < 0.05). In conclusion, a caffeine treatment at 1.25 mM during delayed activation for 4 h can improve the preimplantation development of porcine somatic cell nuclear transfer embryos by activating nuclear reprogramming.
Keywords: Caffeine, Development, Embryo, Porcine, Somatic cell nuclear transfer
INTRODUCTION
The first cloned animal was produced 20 years ago using an adult somatic cell [1]. Since then, mice [2], cattle [3], goat [4], horse [5], and others have been cloned by researchers using the somatic cell nuclear transfer (SCNT) technique. Currently, transgenic animals are used only for special purposes. For example, a transgenic cow can provide benefits, such as changes in the milk composition or it can produce novel proteins in milk [6,7], while transgenic mice can be used as models of specific human diseases [8].
Porcine SCNT has been performed to produce transgenic pigs for use in xenotransplantation, and many transgenic pigs have been produced over the past decade [9,10,11]. According to previous studies, pigs have specific physiological similarities to humans [12]. Among the other transgenic animals that have been produced, the transgenic pig is particularly useful in xenotransplantation [13], treatment of human diseases [14], and therapeutic protein production. Moreover, transgenic pigs have been used in bioreactors [15] and agricultural industries, as well as in studies aimed at enhancing the meat quality and quantity [16]. On the other hand, there is low efficiency in transgenic animal production. Many studies have focused on identifying the causes of the low efficiency as well as the reasons for the many failures of various elements in transgenic animal production [17,18]. The key important factors that have been identified for increasing the efficiency of transgenic animal production by SCNT are the quality of the recipient oocyte and the efficiency of nuclear reprogramming of the donor cell in the recipient oocyte [19,20,21,22].
Therefore, efficient nuclear reprogramming of the donor cell in the oocyte determines the ultimate production efficiency of producing transgenic animals. Of the many factors related to nuclear reprogramming of donor nuclei, it was reported that the maturation promoting factor (MPF) activity is important [23]. A high level of MPF activity affects nuclear envelope breakdown (NEBD) and premature chromosome condensation (PCC) of the donor cell nucleus in the cytoplasm of the recipient oocyte [24,25]. In SCNT experiments, the MPF levels can be reduced by adjusting the manipulation time during the enucleation and donor cell injection period. The MPF activity of the recipient oocyte cytoplasm can be enhanced by preventing the shift from an active MPF form to an inactive pre-MPF form [26].
The addition of caffeine to the recipient oocyte cytoplasm and/or SCNT embryos can promote PCC in pigs. Previous research has shown that the addition of caffeine, a phosphatase inhibitor [27], can increase the MPF activity in pig oocytes [28]. Caffeine elevation of MPF activity has been reported to induce the dephosphorylation of the catalytic subunit of MPF [29]. Moreover, caffeine is effective in treating aged oocytes [30,31], preventing aging and increasing the number of cells in bovine blastocysts [27]. In particular, caffeine has been used to establish an embryonic stem cell line from human SCNT embryos [32].
Therefore, it was hypothesized that caffeine supplementation could increase the development efficiency of porcine SCNT embryos and induce nuclear reprogramming of donor cells more efficiently. This study examined the effects of caffeine supplementation on the preimplantation development of porcine SCNT embryos. For that purpose, caffeine was supplemented to the manipulation media at different concentrations (0, 1.25, 2.5, and 5.0 mM) and/or after different delayed activation time after fusion (control, 1, 2, and 4 h) to determine the optimal caffeine concentration and caffeine treatment timing/duration. In addition, the relative mRNA transcript levels of POU5F1, SOX2, NANOG, and CDX2 genes related to pluripotency, were compared in blastocysts from the caffeine-treated and control groups.
MATERIALS AND METHODS
Chemicals and culture media
All chemical reagents used in this study were obtained from Sigma-Aldrich Chemical Company (USA). Tissue culture medium-199 (TCM-199, Gibco, USA) was used as the base medium for in vitro maturation (IVM). TCM-199 was supplemented with 0.6 mM cysteine, 0.91 mM pyruvate, 10 ng/mL epidermal growth factor, 75 mg/mL kanamycin, 1 μg/mL insulin, and 10% (v/v) porcine follicular fluid [33]. The modified porcine zygote medium (PZM5) was used for the in vitro development of SCNT embryos.
IVM
The porcine ovaries were collected from a slaughterhouse immediately after slaughter, stored in prepared thermo flasks, and transported to the laboratory within 4 h. The temperature of the saline was checked immediately upon arrival at the laboratory. The ovaries were then washed with 38°C saline (osmolality = 280). The fluid in the follicles was aspirated using a 10 mL syringe with the aid of an 18-gauge needle. Follicles more than 3 mm in diameter were selected for aspiration. The aspirate was placed in a 15 mL conical tube and kept for 5 min to allow the sediments to settle. The supernatant was discarded by pipetting without disturbing the sediments on the bottom. HEPES-buffered Tyrode's medium (TLH) containing 0.05% (w/v) polyvinyl alcohol (TLH-PVA) was added to the sediment, and the mixed solution was poured into a 60 mm petri dish for observation under a stereomicroscope. The cumulus-oocyte complexes (COCs) having more than three layers of compact cumulus cells were obtained using a mouse pipette, washed three times in TLH-PVA, and finally washed in P-IVM medium. Fifty COCs were transferred to each well of a four-well multi-dish (SPL, Pocheon, South Korea) containing 500 μL of IVM medium to which 10 IU/mL human chorionic gonadotrophin (hCG, Intervet International BV, Boxmeer, Netherlands) was added. The COCs were incubated at 39°C in an incubator with a humidified atmosphere containing 5% CO2. The COCs were allowed to mature for 22 h in the incubator. After maturation, the COCs were washed three times in fresh hormone-free IVM medium. Finally, the COCs were cultured in hormone-free IVM medium for an additional 18–21 h.
Production of in vitro fertilized (IVF) embryos
Porcine IVF embryos were produced using the method described elsewhere [34]. The basic medium used for IVF was referred to as modified Tris-buffered medium (mTBM). Oocytes cultured for 44 h in maturation medium were stripped of cumulus mechanically by gentle aspiration with a pipette. The denuded oocytes were washed three times in mTBM medium and groups of 30–35 oocytes were transferred to each well of four-well dishes containing TALP medium and equilibrated overnight at 38.5°C under 5% CO2. Sperm suspensions (250 µL) for each treatment group were added to each fertilization well to obtain a final concentration of 5 × 104 cells/mL. After incubation for 6 h, the IVF embryos were cultured in PZM5 medium with 25 µL drops, covered with mineral oil, and incubated at 39°C in 5% CO2 and 5% O2 at a saturating humidity.
Production of cloned embryos
Kidney cells were obtained from a miniature pig, added to four-well plates, and grown in Dulbecco's modified Eagle's medium with 1% penicillin/streptomycin (Gibco), 1% nonessential amino acids (Gibco), and β-mercaptoethanol that had been supplemented with 10% (v/v) fetal bovine serum. The cells were incubated until a complete monolayer of cells had formed. The donor cells were maintained at the G0/G1 stage of the cell cycle for 48–72 h by contact inhibition. Each replicate used cells of the same passage (4–8 passages). The cultured cells were separated to form a single cell suspension using EDTA-trypsin. Before performing nuclear transfer, TLH containing 0.4% (w/v) bovine serum albumin (BSA) (TLH-BSA) was added to the single cell suspension.
After 40 h of IVM, the cumulus cells were removed by pipetting in 0.1% (w/v) hyaluronidase. Subsequently, manipulation medium (calcium-free TLH-BSA) containing 5 mg/mL Hoechst 33342 was used to incubate the denuded oocytes for 10 min. The oocytes were then washed twice with fresh manipulation medium. The washed oocytes were introduced to a drop of the manipulation medium containing 5 mg/mL cytochalasin B and were covered with mineral oil. The polar body and MII chromosomes were aspirated using a 17 mm beveled glass pipette (Humagen, USA). Enucleation success was determined by viewing the oocytes under an epifluorescence microscope (Axiovert 200; ZEISS, Germany). Finally, a single donor cell was inserted into the perivitelline space of the enucleated recipient oocyte. According to the experimental design, the above experiment was repeated but with the addition of different concentrations of caffeine into the manipulation medium.
After injecting donor cells, a 1 mm fusion chamber was overlaid with a 280 mM mannitol solution containing 0.001 mM CaCl2, and 0.05 mM MgCl2 was used to place the cell-injected oocytes [35]. Premature oocyte activation was prevented using a low calcium concentration. Membrane fusion was induced by applying an alternating current of 2 V cycling at 1 MHz for 2 sec, followed by two pulses of a 160 V/mm direct current for 40 μsec using a cell fusion generator. After fusion, the oocytes were incubated for 1 h in a M199 solution containing 0.1% BSA. The reconstructed oocytes were activated with 2 pulses of 120 V/mm of direct electrical activation current for 60 μsec in a 280 mM mannitol solution containing 0.01 mM CaCl2 and 0.05 mM MgCl2. In the post-activation process, the activated embryos were incubated 4 h in M199 solution containing 0.4 μg/mL demecolcine and 2 mM 6-dimethylaminopurine. The SCNT embryos were washed three times in fresh IVC medium and transferred to 25 μL IVC droplets covered with mineral oil. The embryos were then cultured in PZM5 at 39°C in a humidified atmosphere containing 5% CO2 and 5% O2.
Differential staining
Porcine cloned blastocysts from each treatment group were removed from the culture with a small amount of medium. They were then inoculated in the Hoechst 33342 solution (5 µg/mL) and kept for 40 min. Subsequently, the embryos were washed in phosphate-buffered saline and inoculated in 500 μL of the Triton X-100 solution (0.1%). The embryos were then inoculated in 500 μL of a propidium iodide (PI; 10 mg/mL) solution for 35–40 sec. Finally, the embryos were transferred to microscope slides and a small volume of glycerol solution was added and a coverslip mounted. The embryos were observed immediately under UV light (200–400 nm) and the number of inner cell mass (ICM) and trophectoderm (TE) were counted.
Gene expression analysis by real-time reverse transcription (RT) polymerase chain reaction (PCR)
For the gene expression analysis of reprogramming genes, the POU5F1, SOX2, NANOG, and CDX2 pluripotency genes were analyzed. To that end, the total RNA was isolated from porcine blastocysts after 6 d of culture in the control (0 mM caffeine, activation at 1 h post-fusion) and caffeine (1.25 mM caffeine during all procedures, activation at 4 h post-fusion) groups. The total RNA was isolated from the blastocysts using Trizol reagent; subsequently, cDNA was synthesized from 300 ng of the total RNA using reverse transcription 2 × RT Pre-Mix (BioFACT, Korea) and oligo dT primers (18-mers; Neoprove). Quantitative real-time PCR was performed using 1 μL of the cDNA template and a 2 × real-time PCR Pre-Mix (BioFACT) containing the specific primers. The reactions were performed for 39 cycles using the following cycle parameters: denaturation at 95°C for 15 min 20 sec, annealing and extension at 60°C for 40 sec. Table 1 lists the primer sequences. The expression levels of the target genes were quantified as the expression relative to that of an internal control gene (β-actin). The PCR specificity was checked by performing melting curve data analysis. The relative expression quantification was based on a comparison of the threshold cycles (Ct) and constant fluorescence intensity. The relative mRNA is expressed and calculated as the mRNA expression = 2 −(∆Ct sample − ∆Ct control). To determine the normalized arbitrary value of each gene, each value was normalized to β-actin.
Table 1. Sequence of the primers for gene expression analysis by real-time RT PCR.
| Gene | NCBI reference sequence | Primer sequences (5′–3′) | Product size (base pair, bp) |
|---|---|---|---|
| β-actin | NC_010454 | F: CCCTGGAGAAGAGCTACGAG | 172 |
| R: TCCTTCCTGATGTCCACGTC | |||
| POU5F1 | NM_001113060 | F: AGTGAGAGGCAACCTGGAGA | 166 |
| R: TCGTTGCGAATAGTCACTGC | |||
| SOX2 | NM_001123197 | F; GCCCTGCAGTACAACTCCAT | 216 |
| R: GCTGATCATGTCCCGTAGGT | |||
| NANOG | XM_013993362 | F: AAGTACCTCAGCCTCCAGCA | 202 |
| R: GGCATCCTTGGTGATAGGAA | |||
| CDX2 | NM_001278769 | F: AGCCAAGTGAAAACCAGGAC | 178 |
| R: TGCGGTTCTGAAACCAGATT |
RT, reverse transcription; PCR, polymerase chain reaction; NCBI, National Center for Biotechnology Information, F, forward; R, Reverse.
Experimental design
Experiment 1 shows the in vitro development of porcine embryos treated with different caffeine concentrations (0 [control], 1.25, 2.5, and 5 mM) during enucleation and/or during cell injection. In experiment 2, electrical activation was delayed for different times (0 [control], 1, 2, and 4 h) to determine the effective time for introducing the donor cell into the oocyte cytoplasm with high MPF and caffeine supplementation. In experiment 3, the results of experiments 1 and 2 were combined to assess the effects of the caffeine concentration and delayed activation on embryo development (0 mM caffeine and activation at 1 h post-fusion; 0 mM caffeine and activation at 4 h post-fusion; 0 mM caffeine and activation at 4 h post-fusion). In experiment 4, the combined conditions during manipulation and activation considering experiments 1, 2, and 3 that were best for the embryonic development and ICM/TE cells of porcine blastocysts were identified. Experiment 5 used real-time RT PCR to determine and analyze the mRNA expression of the pluripotent genes, POU5F1, SOX2, NANOG, and CDX2 in porcine blastocysts.
Statistical analysis
Each experiment included at least three replicates. The differences among the groups were analyzed using the one-way analysis of variance (ANOVA) procedure in the SPSS program (Release 21.0, SPSS Inc., USA). The data presented in Tables 2, 3, and 4 and Fig. 1 were analyzed using one-way of the variance followed by a least significant difference test. The data in Table 5 and Fig. 2 were analyzed using a Student's t-test. The data are presented as the mean or mean ± standard error of the mean values and a p value < 0.05 was considered significant.
Table 2. Development of porcine SCNT embryos by caffeine supplementation in manipulation media with four different concentrations.
| Caffeine concentration | No. of reconstructed oocytes | No. of embryos (%; mean ± SEM) developed to | ||||
|---|---|---|---|---|---|---|
| 2-cell | 4-cell | 8-cell | Morula | Blastocyst | ||
| 0 (control) | 95 | 57 (60.1 ± 1.3)* | 48 (50.9 ± 2.0) | 26 (27.6 ± 2.6) | 20 (21.3 ± 3.0) | 15 (16.1 ± 2.8) |
| 1.25 mM | 95 | 68 (71.8 ± 6.7)* | 58 (61.2 ± 7.5) | 41 (42.7 ± 4.1) | 27 (27.9 ± 4.5) | 21 (22.8 ± 4.3) |
| 2.5 mM | 91 | 73 (80.6 ± 6.4)† | 65 (71.4 ± 5.8) | 34 (37.8 ± 8.0) | 19 (20.5 ± 2.1) | 15 (16.2 ± 2.9) |
| 5.0 mM | 96 | 73 (76.4 ± 6.7)* | 63 (65.6 ± 6.0) | 33 (34.8 ± 7.0) | 24 (25.2 ± 7.0) | 18 (19.1 ± 4.0) |
SCNT, somatic cell nuclear transfer; SEM, standard error of the mean; conc., concentration.
*,†Values in the same column with different superscript letters are significantly different (p < 0.05).
Table 3. Development of porcine SCNT embryos by the delayed activation of three different times with 1.25 mM caffeine supplementation.
| Duration of caffeine treatment (h) | No. of reconstructed oocytes | No. of embryos (%; mean ± SEM) developed to | ||||
|---|---|---|---|---|---|---|
| 2-cell | 4-cell | 8-cell | Morula | Blastocyst | ||
| Control (1 h) | 95 | 61 (64.0 ± 3.8) | 50 (52.2 ± 6.0) | 24 (25.3 ± 2.2) | 18 (18.5 ± 3.2) | 9 (9.4 ± 1.4)* |
| 1 h | 98 | 63 (64.2 ± 5.9) | 57 (58.1 ± 5.9) | 30 (31.0 ± 3.2) | 21 (21.6 ± 2.4) | 13 (14.0 ± 3.5)* |
| 2 h | 93 | 63 (67.9 ± 5.9) | 51 (54.3 ± 6.7) | 30 (31.8 ± 7.4) | 21 (22.3 ± 6.5) | 10 (11.1 ± 2.0)* |
| 4 h | 88 | 58 (65.8 ± 3.6) | 52 (59.1 ± 4.1) | 31 (36.3 ± 4.9) | 25 (27.9 ± 5.3) | 20 (22.4 ± 6.1)† |
SCNT, somatic cell nuclear transfer; SEM, standard error of the mean.
*,†Values in the same column with different superscript letters are significantly different (p < 0.05).
Table 4. Effect of 1.25 mM caffeine treatment delayed activation according to the time duration on preimplantation development of porcine SCNT embryos.
| Caffeine treatment time (conc.) | No. of reconstructed oocytes | No. of embryos (%; mean ± SEM) developed to | ||||
|---|---|---|---|---|---|---|
| 2-cell | 4-cell | 8-cell | Morula | Blastocyst | ||
| 1 h (0 mM) | 159 | 116 (72.9 ± 4.8) | 105 (66.0 ± 5.2) | 70 (44.1 ± 6.2) | 36 (22.9 ± 2.6) | 24 (15.2 ± 1.9)* |
| 4 h (0 mM) | 178 | 139 (78.0 ± 4.9) | 125 (70.3 ± 4.4) | 72 (40.6 ± 3.5) | 45 (25.2 ± 2.7) | 23 (12.9 ± 3.0)* |
| 4 h (1.25 mM) | 178 | 145 (81.2 ± 5.5) | 128 (71.9 ± 6.2) | 76 (42.8 ± 3.8) | 51 (28.8 ± 1.7) | 40 (22.5 ± 2.1)† |
SCNT, somatic cell nuclear transfer; SEM, standard error of the mean; conc., concentration.
*,†Values in the same column with different superscript letters are significantly different (p < 0.05).
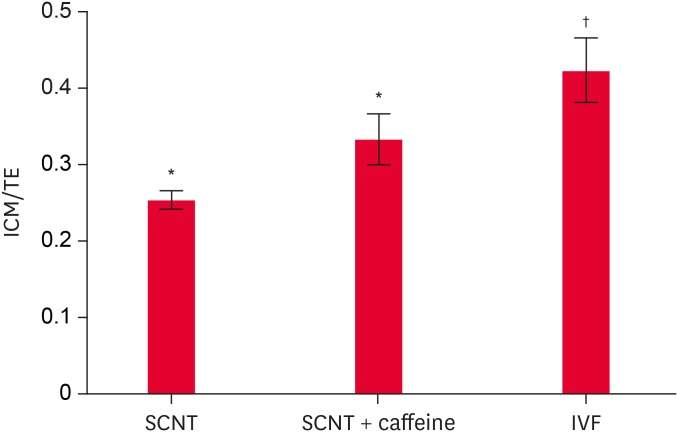
Fig. 1. Comparison of ICM/TE cells in blastocysts on day 6: control, caffeine treatment and IVF group. Caffeine was supplemented with 1.25 mM for 4 h after fusion in the caffeine treatment group.
SCNT, somatic cell nuclear transfer; IVF, in vitro fertilized; ICM, inner cell mass; TE, trophectoderm.
*,†The values are significantly different (p < 0.05).
Table 5. Preimplantation development of porcine SCNT embryos with caffeine supplement both of oocyte manipulation and 4 h post-fusion based on the results of previous experiments.
| Caffeine concentration (mM) | No. of reconstructed oocytes | No. of embryos (%; mean ± SEM) developed to | ||||
|---|---|---|---|---|---|---|
| 2-cell | 4-cell | 8-cell | Morula | Blastocyst | ||
| 0 | 131 | 117 (89.6 ± 2.9) | 101 (77.1 ± 3.6) | 39 (29.5 ± 1.9) | 24 (18.3 ± 1.3) | 20 (15.2 ± 1.2) |
| 1.25 | 119 | 102 (85.7 ± 3.0) | 85 (71.7 ± 4.5) | 35 (29.6 ± 2.6) | 23 (19.3 ± 1.7) | 19 (16.0 ± 1.6) |
SCNT, somatic cell nuclear transfer; SEM, standard error of the mean.
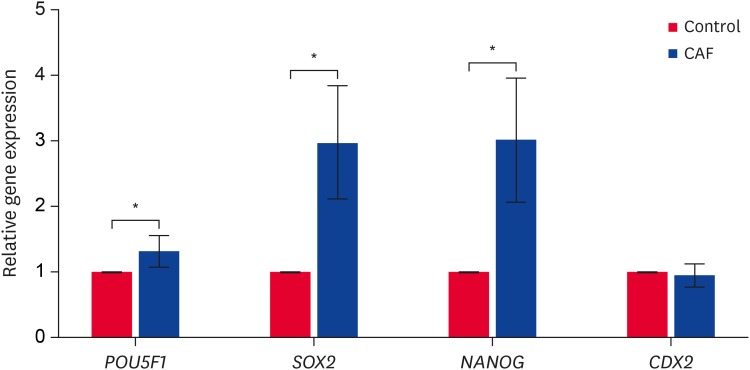
Fig. 2. Relative mRNA expression of POU5F1, SOX2, NANOG, and CDX2 in the blastocysts of the control and caffeine treatment group.
CAF, caffeine.
*The values are significantly different (p < 0.05).
RESULTS
Effect of a caffeine treatment on embryonic development
The assessment of the treatments with various concentrations of caffeine during manipulation revealed the 2-cell cleavage rate to be significantly higher in the 2.5 mM group in the control group and the other two caffeine-treated groups (80.6% vs. 60.1%, 71.8%, and 76.4%; p < 0.05), respectively. The blastocyst formation rate of the reconstructed oocytes treated during manipulation was higher in the 1.25 mM caffeine-treated group (22.8%) than in the control group and other two caffeine-treated groups (16.1%, 16.2%, and 19.1%), but the increase was not significant (p > 0.05; Table 2).
The assessment of different caffeine treatment durations (control, 1, 2, and 4 h) following fusion revealed the 2-cell cleavage rates for all caffeine treatment durations to be similar (64.0%, 64.2%, 67.9%, and 65.8%, respectively) with no significant differences (p > 0.05). On the other hand, the analysis of the different caffeine treatment durations (control, 1, 2, and 4 h) following fusion revealed the 4 h post-fusion caffeine treatment to produce a significantly different blastocyst formation rate (22.4%) in reconstructed oocytes compared to the rates in the other groups (p < 0.05; Table 3).
The 4 h delayed activation with 1.25 mM caffeine group had a higher 2-cell cleavage rate (81.2%) than the other groups (72.9% and 78.0%, respectively) but the differences were not significant (p < 0.05). On the other hand, the caffeine-treated 4 h delayed activation group had a significantly different blastocyst formation rate (22.5%) compared to those in the other two delayed groups (p < 0.05; Table 4).
Among the caffeine-treated groups, the addition of caffeine during manipulation (enucleation) and during the delayed activation procedure were investigated to determine if there were synergic effects. No significant differences in preimplantation development, including 2-cell cleavage rates (89.6% vs. 85.7%) and blastocyst rates (15.2% vs. 16.0%), respectively, were observed with the caffeine treatment during both the manipulation and delayed activation periods (Table 5).
Effect of a caffeine treatment on the ICM/TE ratios
A comparison of ICM/TE ratios, which indicated embryo quality after differential staining, revealed the caffeine-treated group to have a higher ICM/TE ratio but there were no significant differences among the groups. On the other hand, the ICM/TE ratio was significantly lower in both groups of cloned embryos than that of the IVF embryos (Fig. 1).
Effect of a caffeine treatment on gene expression
In the caffeine-treated, post-fusion blastocysts, the mRNA expression of the four different pluripotency genes (POU5F1, SOX2, NANOG, and CDX2) were compared with those in the untreated control group. POU5F1, SOX2, and NANOG were expressed more strongly (p < 0.05) in the caffeine-treated group than in the control group, but CDX2 expression was not significantly different among the groups. Moreover, in the caffeine-treated groups, SOX2 and NANOG were expressed more strongly than POU5F1 and CDX2 (Fig. 2).
DISCUSSION
This study examined effects of the addition of a caffeine supplement during oocyte manipulation and post-fusion culture on in vitro development and on the expression of genes related to nuclear reprogramming in porcine cloned embryos. The caffeine treatment during oocyte manipulation resulted in a significantly high rate of development to the blastocyst stage in the 1.25 mM caffeine groups (Table 3). The development rates of porcine cloned embryos were also increased significantly by delaying oocyte activation for 4 h with 1.25 mM caffeine (Table 3). These increased preimplantation development rates were observed when supplemented with caffeine, whereas no significant improvement was noted without caffeine even after 4 h delayed activation (Table 4). On the other hand, the development rates were not increased when supplemented with caffeine during both the oocyte manipulation and delayed activation periods when the synergic effects of the caffeine treatment in manipulation and post-activation periods were investigated (Table 5). The results suggest that caffeine is important in nuclear reprogramming in porcine SCNT embryos during the post-activation periods.
In this study, caffeine was supplemented during the manipulation procedure, such as the previous study in human SCNT for establishment of cloned embryonic stem cell lines [32]. The blastocyst formation rates was increased in the caffeine-treated group compared to the control group. Therefore, the beneficial effects of a caffeine treatment were observed, but there was no significant difference, which was also reported in a previous study in human cloned embryo stem cells [32]. In addition, porcine oocytes were activated after 4 h instead of at the conventional time (1 h) to improve the reprogramming of donor cells by exposing them to oocyte cytoplasm with a high MPF, as was reported for bovine SCNT [36]. The blastocyst development rates were significantly higher after 4 h delayed activation, indicating that the caffeine treatment has a positive effect on damage to the oocyte cytoplasm related to reconstruction during manipulation and fusion. In addition, caffeine induced the reprogramming and condensation of donor nuclei of somatic cells. In primates, the activation of oocytes after 2 h fusion with caffeine supplementation (2 mM) was effective in increasing the MPF level and the blastocyst development rate [37]. Compared to the control groups, the caffeine treatment at a concentration of 5 mM after electrical fusion was reported to increase the cleavage (77.8% vs. 82.4%) and blastocyst (15.2% vs. 21.4%) rates compared to a control group, respectively [38]. In addition, the expression of the heat shock protein family genes (HSPA1B, HSPA5, HS90A, and HSP90B1) involved in stress was reduced to 1/3 the level of the control level after the caffeine treatment in the porcine oocytes [31]. In Tables 2 and 3, the blastocyst rates of the control groups in the 2 different experiments were shown with high differences (16.1% vs. 9.4%), even though the SCNT conditions were the same. The lower development rates of the control group in Table 3 was caused by seasonal effects in that SCNT was performed in summer. Porcine oocytes are one species with weaker oocytes compared to other mammalian oocytes. Porcine oocyte die most quickly after ovulation, and survive only 8 h, even though most mammalian oocytes can survive for more than 1 day. In addition, they are prone to heat shock, so they are fragile in summer.
A caffeine treatment during both the micromanipulation and delayed activation periods was performed based on the results obtained during the individual periods. Nevertheless, with the dual-period caffeine treatment, no significant differences were detected in either the blastocyst development rate or the ICM/TE rate. In a previous study, the total cell number of blastocysts were significantly higher in the caffeine-treated group (142.7 vs. 116.4) in ovine embryos [39]. In another study of the total cell numbers in ovine embryos, there were no significant differences in the parthenogenetic (107.4 vs. 102.5) and IVF (128.2 vs. 127.6) blastocysts, but a significant difference was detected in the cloned embryos (98.5 vs. 76.5) [40]. A beneficial effect of the caffeine treatment was also reported in a study of human embryonic cloning [27]. In that study, caffeine supplementation (1.25 mM) during the enucleation and fusion processes improved the blastocyst development rate compared to that in the control group (23.5% vs. 11.5%) and embryonic stem cell lines were derived from cloned embryos that had been established with a caffeine treatment [27]. In this study, however, the caffeine treatment had no beneficial effects on embryonic development when it was used during both the manipulation and delayed activation periods, whereas significant benefits of higher development rates were noted when caffeine was used in a single period. These results suggest that the lack of a beneficial effect from a caffeine treatment during both procedures is due to the relatively short survival time after the maturation of porcine oocytes. Moreover, there were no synergistic effects observed with the continuous supplementation of caffeine for more than 6 h in both the manipulation and delayed activation periods. In addition, the inhibitor had an antagonistic effect on the decrease in MPF and proteasome in porcine oocytes.
The porcine POU5F1 gene is well-expressed in IVF embryos but not in cloned embryos that are produced by adult cells, and POU5F1 is well-expressed in the blastocyst and hatching stages [41]. Therefore, the gene expression of POU5F1 in cloned blastocysts from mini pig kidney donor cells was analyzed to determine the involvement of caffeine in nuclear reprogramming. POU5F1, SOX2, and NANOG had significantly different levels of expression than that of β-actin, whereas CDX2 showed no significant difference. The expression of reprogramming-related genes (POU5F1, DPPA2, DPPA3, and DPPA5) and the division-related genes NDP52I1 in porcine cloned embryos were reported to be significantly higher when a proteasome inhibitor was used after fusion [38]. In ovine oocytes, the caffeine treatment can increase the accessibility of DNase I to the donor chromatin and reduce apoptosis in cloned embryos. Moreover, POU5F1, NANOG, and FGF4 were expressed strongly, but HSP70.1 and HSP27 were not expressed in the blastocyst stages [40]. Considering the similarity of these results with the results obtained in other species, the results suggest that the caffeine treatment has a beneficial effect on the nuclear reprogramming of cloned embryos and can increase the embryonic development rate.
In conclusion, caffeine can be used effectively to increase the efficiency of in vitro development and nuclear reprogramming of porcine cloned embryos. The results showed that a 1.25 mM concentration of caffeine applied during manipulation can effectively increase the cleavage formation rate. Although there were no significant differences detected after the treatment with 1.25 mM caffeine on blastocyst formation, the results revealed an increased blastocyst formation rate. Moreover, a 4 h post-fusion caffeine treatment time can enhance the blastocyst formation rate.
Footnotes
Funding: This study was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI13C0954).
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Kim G, Cho J.
- Data curation: Cho J.
- Formal analysis: Kim G, Cho J.
- Funding acquisition: Cho J.
- Methodology: Kim G, Roy PK, Fang X, Hassan BM.
- Project administration: Cho J.
- Resources: Cho J.
- Supervision: Cho J.
- Writing - original draft: Kim G.
- Writing - review & editing: Cho J.
References
- 1.Campbell KH, McWhir J, Ritchie WA, Wilmut I. Sheep cloned by nuclear transfer from a cultured cell line. Nature. 1996;380:64–66. doi: 10.1038/380064a0. [DOI] [PubMed] [Google Scholar]
- 2.Wakayama T, Perry AC, Zuccotti M, Johnson KR, Yanagimachi R. Full-term development of mice from enucleated oocytes injected with cumulus cell nuclei. Nature. 1998;394:369–374. doi: 10.1038/28615. [DOI] [PubMed] [Google Scholar]
- 3.Kato Y, Tani T, Sotomaru Y, Kurokawa K, Kato J, Doguchi H, Yasue H, Tsunoda Y. Eight calves cloned from somatic cells of a single adult. Science. 1998;282:2095–2098. doi: 10.1126/science.282.5396.2095. [DOI] [PubMed] [Google Scholar]
- 4.Baguisi A, Behboodi E, Melican DT, Pollock JS, Destrempes MM, Cammuso C, Williams JL, Nims SD, Porter CA, Midura P, Palacios MJ, Ayres SL, Denniston RS, Hayes ML, Ziomek CA, Meade HM, Godke RA, Gavin WG, Overström EW, Echelard Y. Production of goats by somatic cell nuclear transfer. Nat Biotechnol. 1999;17:456–461. doi: 10.1038/8632. [DOI] [PubMed] [Google Scholar]
- 5.Galli C, Lagutina I, Crotti G, Colleoni S, Turini P, Ponderato N, Duchi R, Lazzari G. Pregnancy: a cloned horse born to its dam twin. Nature. 2003;424:635. doi: 10.1038/424635a. [DOI] [PubMed] [Google Scholar]
- 6.Brophy B, Smolenski G, Wheeler T, Wells D, L'Huillier P, Laible G. Cloned transgenic cattle produce milk with higher levels of beta-casein and kappa-casein. Nat Biotechnol. 2003;21:157–162. doi: 10.1038/nbt783. [DOI] [PubMed] [Google Scholar]
- 7.Richt JA, Kasinathan P, Hamir AN, Castilla J, Sathiyaseelan T, Vargas F, Sathiyaseelan J, Wu H, Matsushita H, Koster J, Kato S, Ishida I, Soto C, Robl JM, Kuroiwa Y. Production of cattle lacking prion protein. Nat Biotechnol. 2007;25:132–138. doi: 10.1038/nbt1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Justice MJ, Siracusa LD, Stewart AF. Technical approaches for mouse models of human disease. Dis Model Mech. 2011;4:305–310. doi: 10.1242/dmm.000901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cheng W, Zhao H, Yu H, Xin J, Wang J, Zeng L, Yuan Z, Qing Y, Li H, Jia B, Yang C, Shen Y, Zhao L, Pan W, Zhao HY, Wang W, Wei HJ. Efficient generation of GGTA1-null Diannan miniature pigs using TALENs combined with somatic cell nuclear transfer. Reprod Biol Endocrinol. 2016;14:77. doi: 10.1186/s12958-016-0212-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kang JT, Kwon DK, Park AR, Lee EJ, Yun YJ, Ji DY, Lee K, Park KW. Production of α1,3-galactosyltransferase targeted pigs using transcription activator-like effector nuclease-mediated genome editing technology. J Vet Sci. 2016;17:89–96. doi: 10.4142/jvs.2016.17.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Naeimi Kararoudi M, Hejazi SS, Elmas E, Hellström M, Naeimi Kararoudi M, Padma AM, Lee D, Dolatshad H. Clustered regularly interspaced short palindromic repeats/Cas9 gene editing technique in xenotransplantation. Front Immunol. 2018;9:1711. doi: 10.3389/fimmu.2018.01711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lunney JK. Advances in swine biomedical model genomics. Int J Biol Sci. 2007;3:179–184. doi: 10.7150/ijbs.3.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SJ, Kim JS, Chee HK, Yun IJ, Park KS, Yang HS, Park JH. Seven years of experiences of preclinical experiments of xeno-heart transplantation of pig to non-human primate (cynomolgus monkey) Transplant Proc. 2018;50:1167–1171. doi: 10.1016/j.transproceed.2018.01.041. [DOI] [PubMed] [Google Scholar]
- 14.Zhao YD, Liu BT, Guo LL, Shan H, Fang BH. A novel experimental intraperitoneal infection model for Haemophilus parasuis in neutropenic guinea pigs. J Pharmacol Toxicol Methods. 2019;95:27–35. doi: 10.1016/j.vascn.2018.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Noble MS, Rodriguez-Zas S, Cook JB, Bleck GT, Hurley WL, Wheeler MB. Lactational performance of first-parity transgenic gilts expressing bovine alpha-lactalbumin in their milk. J Anim Sci. 2002;80:1090–1096. doi: 10.2527/2002.8041090x. [DOI] [PubMed] [Google Scholar]
- 16.Lai L, Kang JX, Li R, Wang J, Witt WT, Yong HY, Hao Y, Wax DM, Murphy CN, Rieke A, Samuel M, Linville ML, Korte SW, Evans RW, Starzl TE, Prather RS, Dai Y. Generation of cloned transgenic pigs rich in omega-3 fatty acids. Nat Biotechnol. 2006;24:435–436. doi: 10.1038/nbt1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Callesen H, Liu Y, Pedersen HS, Li R, Schmidt M. Increasing efficiency in production of cloned piglets. Cell Reprogram. 2014;16:407–410. doi: 10.1089/cell.2014.0053. [DOI] [PubMed] [Google Scholar]
- 18.Cho J, Kang S, Lee BC. Identification of abnormal gene expression in bovine transgenic somatic cell nuclear transfer embryos. J Vet Sci. 2014;15:225–231. doi: 10.4142/jvs.2014.15.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee GS, Hyun SH, Kim HS, Kim DY, Lee SH, Lim JM, Lee ES, Kang SK, Lee BC, Hwang WS. Improvement of a porcine somatic cell nuclear transfer technique by optimizing donor cell and recipient oocyte preparations. Theriogenology. 2003;59:1949–1957. doi: 10.1016/s0093-691x(02)01294-3. [DOI] [PubMed] [Google Scholar]
- 20.Mizutani E, Ohta H, Kishigami S, Van Thuan N, Hikichi T, Wakayama S, Kosaka M, Sato E, Wakayama T. Developmental ability of cloned embryos from neural stem cells. Reproduction. 2006;132:849–857. doi: 10.1530/rep.1.01010. [DOI] [PubMed] [Google Scholar]
- 21.Sirard MA, Richard F, Blondin P, Robert C. Contribution of the oocyte to embryo quality. Theriogenology. 2006;65:126–136. doi: 10.1016/j.theriogenology.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 22.Whitworth KM, Li R, Spate LD, Wax DM, Rieke A, Whyte JJ, Manandhar G, Sutovsky M, Green JA, Sutovsky P, Prather RS. Method of oocyte activation affects cloning efficiency in pigs. Mol Reprod Dev. 2009;76:490–500. doi: 10.1002/mrd.20987. [DOI] [PubMed] [Google Scholar]
- 23.Kikuchi K, Izaike Y, Noguchi J, Furukawa T, Daen FP, Naito K, Toyoda Y. Decrease of histone H1 kinase activity in relation to parthenogenetic activation of pig follicular oocytes matured and aged in vitro. J Reprod Fertil. 1995;105:325–330. doi: 10.1530/jrf.0.1050325. [DOI] [PubMed] [Google Scholar]
- 24.Macháty Z, Prather RS. Strategies for activating nuclear transfer oocytes. Reprod Fertil Dev. 1998;10:599–613. doi: 10.1071/rd98048. [DOI] [PubMed] [Google Scholar]
- 25.Tani T, Kato Y, Tsunoda Y. Direct exposure of chromosomes to nonactivated ovum cytoplasm is effective for bovine somatic cell nucleus reprogramming. Biol Reprod. 2001;64:324–330. doi: 10.1095/biolreprod64.1.324. [DOI] [PubMed] [Google Scholar]
- 26.Kikuchi K, Naito K, Noguchi J, Kaneko H, Tojo H. Maturation/M-phase promoting factor regulates aging of porcine oocytes matured in vitro . Cloning Stem Cells. 2002;4:211–222. doi: 10.1089/15362300260339494. [DOI] [PubMed] [Google Scholar]
- 27.Lee JH, Campbell KH. Caffeine treatment prevents age-related changes in ovine oocytes and increases cell numbers in blastocysts produced by somatic cell nuclear transfer. Cloning Stem Cells. 2008;10:381–390. doi: 10.1089/clo.2007.0091. [DOI] [PubMed] [Google Scholar]
- 28.Li YH, Kang H, Xu YN, Heo YT, Cui XS, Kim NH, Oh JS. Greatwall kinase is required for meiotic maturation in porcine oocytes. Biol Reprod. 2013;89:53. doi: 10.1095/biolreprod.113.109850. [DOI] [PubMed] [Google Scholar]
- 29.Ariu F, Bogliolo L, Leoni G, Falchi L, Bebbere D, Nieddu SM, Zedda MT, Pau S, Ledda S. Effect of caffeine treatment before vitrification on MPF and MAPK activity and spontaneous parthenogenetic activation of in vitro matured ovine oocytes. Cryo Lett. 2014;35:530–536. [PubMed] [Google Scholar]
- 30.Ono T, Mizutani E, Li C, Yamagata K, Wakayama T. Offspring from intracytoplasmic sperm injection of aged mouse oocytes treated with caffeine or MG132. Genesis. 2011;49:460–471. doi: 10.1002/dvg.20756. [DOI] [PubMed] [Google Scholar]
- 31.Jiang GJ, Wang K, Miao DQ, Guo L, Hou Y, Schatten H, Sun QY. Protein profile changes during porcine oocyte aging and effects of caffeine on protein expression patterns. PLoS One. 2011;6:e28996. doi: 10.1371/journal.pone.0028996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tachibana M, Amato P, Sparman M, Gutierrez NM, Tippner-Hedges R, Ma H, Kang E, Fulati A, Lee HS, Sritanaudomchai H, Masterson K, Larson J, Eaton D, Sadler-Fredd K, Battaglia D, Lee D, Wu D, Jensen J, Patton P, Gokhale S, Stouffer RL, Wolf D, Mitalipov S. Human embryonic stem cells derived by somatic cell nuclear transfer. Cell. 2013;153:1228–1238. doi: 10.1016/j.cell.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song K, Hyun SH, Shin T, Lee E. Post-activation treatment with demecolcine improves development of somatic cell nuclear transfer embryos in pigs by modifying the remodeling of donor nuclei. Mol Reprod Dev. 2009;76:611–619. doi: 10.1002/mrd.20989. [DOI] [PubMed] [Google Scholar]
- 34.Malaweera DB, Wu JB, Oh SK, Kim SH, Kim SJ, Shin ST, Cho JK. Establishment of efficient microinjection system in the porcine embryos. J Embryo Transf. 2014;29:59–66. [Google Scholar]
- 35.Walker SC, Shin T, Zaunbrecher GM, Romano JE, Johnson GA, Bazer FW, Piedrahita JA. A highly efficient method for porcine cloning by nuclear transfer using in vitro-matured oocytes. Cloning Stem Cells. 2002;4:105–112. doi: 10.1089/153623002320253283. [DOI] [PubMed] [Google Scholar]
- 36.Wells DN, Misica PM, Tervit HR. Production of cloned calves following nuclear transfer with cultured adult mural granulosa cells. Biol Reprod. 1999;60:996–1005. doi: 10.1095/biolreprod60.4.996. [DOI] [PubMed] [Google Scholar]
- 37.Mitalipov SM, Zhou Q, Byrne JA, Ji WZ, Norgren RB, Wolf DP. Reprogramming following somatic cell nuclear transfer in primates is dependent upon nuclear remodeling. Hum Reprod. 2007;22:2232–2242. doi: 10.1093/humrep/dem136. [DOI] [PubMed] [Google Scholar]
- 38.You J, Lee J, Kim J, Park J, Lee E. Post-fusion treatment with MG132 increases transcription factor expression in somatic cell nuclear transfer embryos in pigs. Mol Reprod Dev. 2010;77:149–157. doi: 10.1002/mrd.21115. [DOI] [PubMed] [Google Scholar]
- 39.Lee JH, Campbell KH. Effects of enucleation and caffeine on maturation-promoting factor (MPF) and mitogen-activated protein kinase (MAPK) activities in ovine oocytes used as recipient cytoplasts for nuclear transfer. Biol Reprod. 2006;74:691–698. doi: 10.1095/biolreprod.105.043885. [DOI] [PubMed] [Google Scholar]
- 40.Choi I, Campbell KH. Treatment of ovine oocytes with caffeine increases the accessibility of DNase I to the donor chromatin and reduces apoptosis in somatic cell nuclear transfer embryos. Reprod Fertil Dev. 2010;22:1000–1014. doi: 10.1071/RD09144. [DOI] [PubMed] [Google Scholar]
- 41.Cervera RP, Martí-Gutiérrez N, Escorihuela E, Moreno R, Stojkovic M. Trichostatin A affects histone acetylation and gene expression in porcine somatic cell nucleus transfer embryos. Theriogenology. 2009;72:1097–1110. doi: 10.1016/j.theriogenology.2009.06.030. [DOI] [PubMed] [Google Scholar]