Abstract
This paper compares and describes the tidal volume (Vt) used in mechanically ventilated dogs under a range of clinical conditions. Twenty-eight dogs requiring mechanical ventilation (MV) were classified into 3 groups: healthy dogs mechanically ventilated during surgery (group I, n = 10), dogs requiring MV due to extra-pulmonary reasons (group II, n = 7), and dogs that required MV due to pulmonary pathologies (group III, n = 11). The median Vt used in each group was 16 mL/kg (interquartile range [IQR], 15.14–21) for group I, 12.59 mL/kg (IQR, 9–14.25) for group II, and 12.59 mL/kg (IQR, 10.15–14.96) for group III. The Vt used was significantly lower in group III than in group I (p = 0.016). The thoraco-pulmonary compliance was significantly higher in group I than in groups II and III (p = 0.011 and p = 0.006, respectively). The median driving pressure was similar among the groups with a median of 9, 11, and 10 cmH2O in groups I, II, and III, respectively (p = 0.260). Critically-ill dogs requiring MV due to the primary pulmonary pathology received a significantly lower Vt than healthy dogs but with a range of values that were markedly higher than those recommended by human guidelines.
Keywords: Ventilator-induced lung injury, mechanical ventilation, Compliance, tidal volume
INTRODUCTION
The use of a protective ventilatory strategy with a tidal volume (Vt) of 6 mL/kg that limits the end-inspiratory pressure in human patients with acute respiratory distress syndrome (ARDS) [5] can be considered a gold standard. Indeed, this recommendation might be considered a standard of practice for people receiving mechanical ventilation (MV) due to reasons other than ARDS, as with people under general anesthesia for abdominal surgery and no lung pathology [10]. The recommended Vt for the MV of healthy dogs ranges from 10 to 20 mL/kg [2,14], even though the use of a high Vt level of 20 mL/kg in patients could lead to overdistention and lung injury, making these recommendations questionable. On the other hand, the use of Vt levels of 10 mL/kg could generate cyclic opening and closing, which is another potential mechanism for parenchymal damage. Bumbacher et al. [4] reported that setting a Vt of 15 mL/kg resulted in better alveolar ventilation with less dead space compared to Vt values of 10 to 12 mL/kg in dogs. They showed in a previous study on volumetric capnography that the average anatomic dead space in dogs is 7 mL/kg [20]. In a pilot study conducted in healthy Beagle dogs, however, the use of 6 to 8 mL/kg of Vt (maintaining constant the minute volume at 200 mL/kg/min) allowed for adequate alveolar ventilation [24]. The differences observed in previous studies probably reflect the variability that may exist between different dog breeds and respective thoracic morphologies. On the other hand, the functional lung size varies greatly in patients with ARDS, both in humans and dogs [17,19]. These results put into question the use of extrapolating human medicine guidelines when using Vt of 6 mL/kg in critically-ill dogs.
The driving pressure (DP), which is defined as the difference between the plateau pressure and the total positive end expiratory pressure (PEEP), depends on Vt and thoraco-pulmonary elastance in such a way that for the same DP, the Vt decreases with increasing thoraco-pulmonary elastance and vice versa [27]. Amato et al. [1] reported an association between a low DP (< 15 cmH2O) and reduced mortality in people requiring MV due to ARDS. Because the specific pulmonary elastance and the airway pressure at the total lung capacity are similar in dogs and people [9,18], it is likely that the information on the airway pressures can be extrapolated from people to dogs, which is in contrast to the guidelines about the use of low Vt. This paper describes and compares the Vt used in healthy and critically-ill dogs requiring MV with an adequate arterial partial pressure of carbon dioxide (PaCO2) and DP.
MATERIALS AND METHODS
Patient population
The medical records of dogs in the intensive care unit of Unidad de Cuidados Intensivos Cooperativa Veterinaria (UCICOOP) requiring MV between April 2017 and February 2018, and the records of healthy dogs that had been mechanically ventilated under anesthesia for elective procedures were reviewed. For data analysis, the dogs were stratified into 3 groups according to their clinical condition: group I, healthy dogs under anesthesia with MV for elective procedures; group II, critically ill dogs that required MV due to extra-pulmonary reasons; and group III, critically-ill dogs that required MV due to pulmonary reasons. All dogs were ventilated using a micro processed mechanical ventilator (Leistung PR4G or NeumoventGraph, Argentina).
The inclusion criteria were complete medical records that contained signalment, relevant history, and appropriate documentation of MV settings (Vt, PEEP, DP), in addition to blood gas analysis values, where PaCO2 could be analyzed in relation to the MV settings. The blood gas analysis and electrolyte panel were performed in all patients using the same analyzer (EPOC Analizer; Siemens Healthineers, Germany). Dogs were excluded from the study if the DP was above 15 cmH2O, PaCO2 values during MV were outside the normal range (30–45 mmHg) for dogs in groups I and II or outside the range of 30–50 mmHg for dogs in group III (to allow for permissive hypercapnia due to the pulmonary pathology).
Statistical analysis
The qualitative variables are expressed as the absolute and relative frequencies. The quantitative variables are expressed as the mean (± standard deviation) or median (interquartile range [IQR]) by distribution. The distribution was evaluated by observing the histograms and the Shapiro-Wilk test. Comparisons between more than 2 groups were performed using the Kruskal-Wallis test. Pair-wise comparisons were made using the Mann-Whitney U test with a Bonferroni correction. Multivariate linear regression analysis was performed to evaluate the effects of weight and age on the Vt of the different groups. Post hoc analysis for thoraco-pulmonary compliance was performed using the body weight and clinical condition as independent variables. A logarithmic transformation of the variables was performed to comply with the homoscedasticity and normality assumptions of the residuals. All statistical analyses were performed using commercial software (STATA 13.0; STATA Corporation, USA).
RESULTS
A total of 10 healthy dogs that underwent general anesthesia for elective procedures and were mechanically ventilated during the study period were included in the study. The medical records of 75 patients admitted to the intensive care unit and required MV were evaluated. Twenty-three patients were excluded due to a lack of complete medical records, and 34 were excluded because the PaCO2 values were outside the range described in the methods section, resulting in a final population of 18 critically ill dogs. From a total of 28 dogs in the 3 groups, 15 were females and 13 males: 19 mixed breeds, 2 Labrador Retrievers, 1 Golden Retrievers, 1 Doberman, 2 Poodles, 1 Pitt bull, and 2 French Bulldogs. The working diagnosis in the 18 critically ill hospitalized were shock (n = 3), laryngeal paralysis (n = 2), hypoxic encephalopathy (n = 1), status epilepticus (n = 1), and gastric dilatation volvulus (n = 1) for group II; and bacterial bronchopneumonia (n = 1), neurogenic pulmonary edema (n = 1), aspiration pneumonia (n = 2), and unknown diagnosis (n = 6) for group III. The healthy dogs were significantly younger and had a significantly lower body weight than the critically ill dogs (Table 1).
Table 1. Description of the study groups.
| Variable | Group I (n = 10) | Group II (n = 7) | Group III (n = 11) | p |
|---|---|---|---|---|
| Age (yr) | 4 (3–5) | 10 (8–12) | 10 (4–13) | 0.029 |
| Weight (kg) | 10 (7–20) | 40 (6–40) | 20 (9–40) | 0.013 |
| Vt (mL/kg) | 16 (15.14–21) | 12.59 (9–14.25) | 12.59 (10.15–14.96) | 0.015 |
| DP (cmH2O) | 9 (8–10) | 11 (9–12) | 10 (9–11) | 0.264 |
| PEEP (cmH2O) | 0 (0–0) | 2 (0–3) | 2 (0–3) | 0.071 |
| RR (breaths/min) | 15 (15–15) | 20 (17–25) | 19 (15–22.5) | 0.026 |
| Minute volume (mL/kg/minute) | 240 (227.14–315) | 214.07 (205–292.5) | 224.4 (213.75–333.3) | 0.538 |
| Compliance (mL/cmH2O/Kg) | 2.12 (1.51–2.35) | 1.21 (0.86–1.58) | 1.27 (1.04–1.58) | 0.031 |
| PaCO2 (mmHg) | 34.1 (31.3–39.1) | 35 (33–43) | 33.9 (33.6–43) | 0.675 |
Values expressed in median and interquartile range. The dogs were classified into 3 groups: group I, healthy canines mechanically ventilated during general anesthesia undergoing a surgical procedure; group II, canines that require mechanical ventilation due to extrapulmonary pathologies; group III, canines that require mechanical ventilation due to pulmonary pathology.
Vt, tidal volume; DP, driving pressure; PEEP, positive end expiratory pressure; RR, respiratory rate; Minute volume, respiratory minute volume; Compliance, thoraco-pulmonary compliance indexed to weight; PaCO2: partial pressure of carbon dioxide.
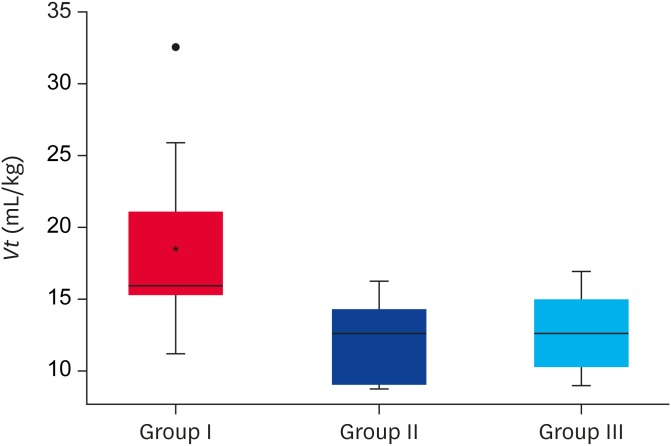
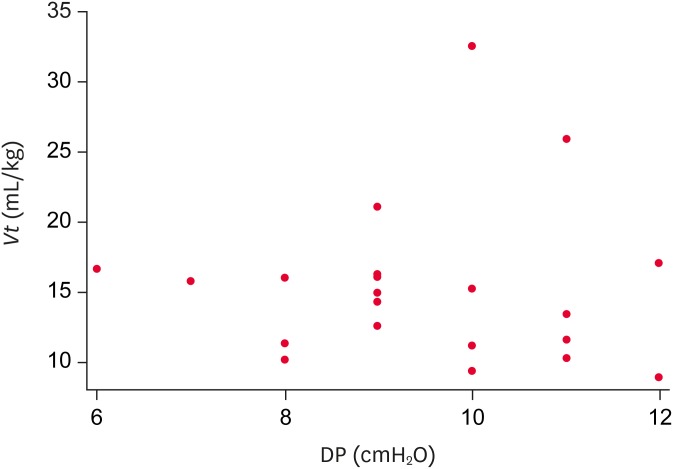
The dogs in (group III) were ventilated with a significantly lower Vt (p = 0.010) than the healthy dogs (group I) (Fig. 1). Paired analysis with an adjustment using a Bonferroni correction for alpha type errors identified statistically significant differences in Vt only between groups I and III. The difference remained statistically significant after Vt was adjusted for weight and age by linear regression analysis (dependent variable logVt, independent variable group III compared to group I adjusting for weight and age: b = −0.167; 95% confidence interval [CI], −0.293 to −0.0428; p = 0.001). The median DP with IQR was 9 cmH2O (8–11) for group I, 11 cmH2O (9–12) for group II, and 10 cmH2O (9–11) for group III (p = 0.260) (Table 1), resulting in a wide range of Vt for each of these values (Fig. 2). No significant differences in the PaCO2 values were observed between the 3 groups (p = 0.670).
Fig. 1. Vt used during MV in healthy dogs (group I), critically ill dogs that required MV due to extra-pulmonary reasons (group II), and pulmonary reasons (group III). The values are expressed in the median and interquartile range.
Vt, tidal volume; MV, mechanical ventilation.
*Indicates statistically significant difference (p < 0.05).
Fig. 2. Vt values in relation to the driving pressure in healthy and critically ill dogs receiving mechanical ventilation. For each DP value there is a wide range of Vt.
Vt, tidal volume; DP, driving pressure.
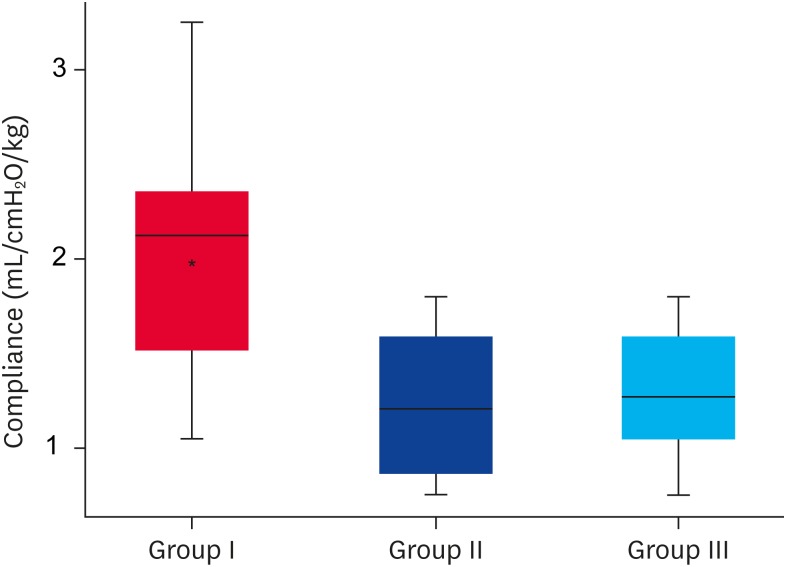
The group of healthy dogs had a significantly higher compliance during MV (p = 0.030) than the critically ill dogs (Fig. 3). Paired analysis with an adjustment using a Bonferroni correction for alpha type errors revealed a significant difference between groups I and II (p = 0.011) and between groups I and III (p = 0.006). In addition, when evaluating Vt used by different compliance ranges (Table 2), Vt was higher as the thoraco-pulmonary compliance increased. (p < 0.001). Post hoc analysis showed that after controlling for the clinical status of the dogs (healthy vs. critically ill), thoraco-pulmonary compliance was associated significantly with the body weight (dependent variable logCompliance, independent variables weight, and clinical status of the dog: b = −0.0043; 95% CI, −0.0085 to −0.0033; p = 0.003). Dogs with a larger body weight showed significantly lower thoraco-pulmonary compliance.
Fig. 3. Thoraco-pulmonary compliance indexed to the body weight during MV in healthy dogs (group I), critically ill dogs that required MV due to extra-pulmonary reasons (group II), and pulmonary reasons (group III). The values are expressed in the median and interquartile range.
MV, mechanical ventilation.
*Indicates statistically significant difference (p < 0.05).
Table 2. Relationship between the thoraco-pulmonary compliance and the amount of Vt received.
| Thoraco-pulmonary compliance (mL/cmH2O/kg) | Vt (mL/kg) |
|---|---|
| Less than 1 (< 1) | 9 (9–10.25) |
| 1–1.5 | 12.06 (11.11–13.33) |
| 1.5–2 | 15.57 (14.60–16.12) |
| Greater than 2 (> 2) | 21 (16.6–25.9) |
Values expressed in median and interquartile range.
Vt, tidal volume.
DISCUSSION
The selection of an adequate Vt value for MV is essential to avoid hypoventilation and hypercapnia, as well as to prevent excessive pulmonary stress and strain development. These results show that the critically ill dogs received a significantly lower Vt than the healthy patient group, which is consistent with previous publications in human studies [5,10]. On the other hand, according to these results, the range of Vt needed to achieve acceptable blood gas values could be broader than what is usually proposed [2,11,13,14]. In the majority of the veterinary literature describing MV in dogs, the Vt used was greater than 10 mL/kg of weight [12,13]. Some authors proposed the use of a Vt of 6 mL/kg in dogs with lung injury, but this recommendation was based on human literature [21]. A recent report showed two dogs suffering from ARDS that had been ventilated successfully using a Vt of 12 mL/kg [15], which is a volume well above the recommendation in human guidelines. The “low” Vt values in most studies using dogs suffering from ARDS as an experimental model, correspond to the values currently considered harmful in human medicine [16,22,25].
In an experimental canine model of ARDS induced by saline solution lavage, the dogs showed significant improvement in oxygenation when the Vt values were increased from 15 to 20 mL/kg [25]. The use of a “higher” Vt was justified by the experimental observations, showing that dogs required a relatively higher Vt to avoid the excessive accumulation of PaCO2. Another experimental model of canine ARDS induced by acid inhalation reported that unlike what is observed in people with ARDS, 15 mL/kg represents a low Vt value [7]. In this study, the authors evaluated the use 10 mL/kg of Vt but normocapnia could not be achieved. Therefore, the Vt was reduced to the lowest possible value that would maintain PaCO2 values between 35 and 40 mmHg. Furthermore, a study carried out on healthy Beagles showed that the use of a Vt of 6 mL/kg was well tolerated when used together with respiratory rates that could reach a minute volume at 200 mL/kg/min, resulting in PaCO2 values of 40.4 ± 2 mmHg [24].
Taking into consideration previous reports together with the results of this study, it has been suggested that it is very difficult to find a unique Vt value for adequate MV in dogs, and it becomes even more difficult to extrapolate the recommendations from human medicine. In dogs, anatomical variations due to breed differences are probably the reason for the broad variability in Vt values used to keep alveolar ventilation within the physiological ranges. A previous study reported that the lung volume/body weight unit ratio is greater in dogs than in other species [26]. This emphasizes that a single value of Vt, and its extrapolation from human medicine, is unsuitable for dogs. A previous study performed by the authors showed that successful MV could be achieved in both healthy and critically-ill patients by measuring the inspiratory capacity and by selecting the Vt as a percentage of this capacity. This is because measurement of this inspiratory capacity provides a better determination of the volume of an aerated lung [9].
The DP of the respiratory system has been evaluated as a monitoring parameter to limit the stress and strain in people suffering from ARDS [1]. Because the DP reflects the relationship between Vt and compliance, control of this parameter allows the Vt to be adjusted to the functional lung size, meaning that the Vt value employed must be low to reach a certain DP at a lower compliance [27]. This rationale for lung protection was shown in the results of this study, where the lower the thoraco-pulmonary compliance indexed to weight, the lower Vt the dogs received. Post hoc analysis also showed that dogs with larger body weights had a significantly lower thoraco-pulmonary compliance that was independent of their clinicopathological status (healthy vs. critically ill), emphasizing that Vt should be targeted to the needs and physiological variables of each patient during MV. The inclusion criteria for critically ill dogs in groups I and II was selected from previously reported normal values [8]. The decision to allow PaCO2 values up to 50 mmHg in ventilated dogs with a pulmonary pathology is a known strategy called “permissive hypercapnia” [3]. This condition is a protective ventilatory strategy used to reduce the risk of lung injury induced by MV. In this strategy, the use of a low Vt to reduce the transpulmonary pressure (stress) and strain decreases the alveolar ventilation, and hypercapnia is usually generated as a consequence. The strategy of permissive hypercapnia allows high PaCO2 values provided that no contraindications are present (e.g. traumatic brain injury) and the blood pH is over 7.2 [6]. Recently, a cohort study conducted on people suffering from ARDS showed that the presence of severe hypercapnia (PaCO2 over 50 mmHg) is an independent marker of intra-hospital mortality, calling into question the permissive hypercapnia strategy when the PaCO2 values exceed 50 mmHg [23]. As shown in the present study, the PaCO2 values remained relatively stable in all dogs despite the wide variability of Vt values used.
The limitations of this study include the low number of patients, particularly in the group of critically ill dogs receiving MV for non-pulmonary reasons, where neuromuscular pathologies leading to hypoventilation are not represented. Owing to this limitation, further clinical studies will be needed to understand the application of the results presented here in the general population of dogs admitted to critical care units and receiving MV. On the other hand, the low number of dogs included in this retrospective study limits the statistical power of some of these results by increasing the risk of a type II statistical error.
In conclusion, critically-ill dogs with adequate DP values and lower pulmonary compliance were mechanically ventilated with lower Vt values than those used in healthy dogs. Moreover, the Vt was higher as the thoraco-pulmonary compliance increased. The range of Vt required to achieve acceptable blood gas values and normal lung mechanics could be broader than what is usually recommended and appears to be different from the 6 ml/kg used in human medicine for lung-protective strategies.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Donati PA, Plotnikow G, Londoño L.
- Data curation: Donati PA, Benavides G, Belerenian G, Jensen M.
- Formal analysis: Donati PA.
- Investigation: Donati PA, Plotnikow G, Benavides G, Londoño L, Belerenian G, Jensen M.
- Methodology: Donati PA, Plotnikow G.
- Resources: Belerenian G, Donati PA, Benavides G, Jensen M.
- Writing - original draft: Donati PA.
- Writing - review & editing: Plotnikow G, Londoño L.
References
- 1.Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, Stewart TE, Briel M, Talmor D, Mercat A, Richard JC, Carvalho CR, Brower RG. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]
- 2.Balakrishnan A, King LG. Updates on pulmonary function testing in small animals. Vet Clin North Am Small Anim Pract. 2014;44:1–18. doi: 10.1016/j.cvsm.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 3.Barnes T, Zochios V, Parhar K. Re-examining permissive hypercapnia in ARDS: a narrative review. Chest. 2018;154:185–195. doi: 10.1016/j.chest.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 4.Bumbacher S, Schramel JP, Mosing M. Evaluation of three tidal volumes (10, 12 and 15 mL kg−1) in dogs for controlled mechanical ventilation assessed by volumetric capnography: a randomized clinical trial. Vet Anaesth Analg. 2017;44:775–784. doi: 10.1016/j.vaa.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 5.Acute Respiratory Distress Syndrome Network. Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 6.Cortes GA, Marini JJ. Update: adjuncts to mechanical ventilation. Curr Opin Anaesthesiol. 2012;25:156–163. doi: 10.1097/ACO.0b013e32834f8c65. [DOI] [PubMed] [Google Scholar]
- 7.Corbridge TC, Wood LD, Crawford GP, Chudoba MJ, Yanos J, Sznajder JI. Adverse effects of large tidal volume and low PEEP in canine acid aspiration. Am Rev Respir Dis. 1990;142:311–315. doi: 10.1164/ajrccm/142.2.311. [DOI] [PubMed] [Google Scholar]
- 8.Daly ML. Hypoventilation. In: Silverstein DC, Hopper K, editors. Small Animal Critical Care Medicine. 2nd ed. St. Louis: Saunders; 2015. pp. 86–92. [Google Scholar]
- 9.Donati PA, Gogniat E, Madorno M, Guevara JM, Guillemi EC, Lavalle MD, Scorza FP, Mayer GF, Rodriguez PO. Sizing the lung in dogs: the inspiratory capacity defines the tidal volume. Rev Bras Ter Intensiva. 2018;30:144–152. doi: 10.5935/0103-507X.20180028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Futier E, Constantin JM, Paugam-Burtz C, Pascal J, Eurin M, Neuschwander A, Marret E, Beaussier M, Gutton C, Lefrant JY, Allaouchiche B, Verzilli D, Leone M, De Jong A, Bazin JE, Pereira B, Jaber S IMPROVE Study Group. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med. 2013;369:428–437. doi: 10.1056/NEJMoa1301082. [DOI] [PubMed] [Google Scholar]
- 11.Haskins S, King LG. Positive pressure ventilation. In: King LG, editor. Textbook of Respiratory Disease in Dogs and Cats. 1st ed. St. Louis: Saunders; 2004. pp. 217–229. [Google Scholar]
- 12.Hoareau GL, Mellema MS, Silverstein DC. Indication, management, and outcome of brachycephalic dogs requiring mechanical ventilation. J Vet Emerg Crit Care (San Antonio) 2011;21:226–235. doi: 10.1111/j.1476-4431.2011.00635.x. [DOI] [PubMed] [Google Scholar]
- 13.Hopper K, Haskins SC, Kass PH, Rezende ML, Aldrich J. Indications, management, and outcome of long-term positive-pressure ventilation in dogs and cats: 148 cases (1990–2001) J Am Vet Med Assoc. 2007;230:64–75. doi: 10.2460/javma.230.1.64. [DOI] [PubMed] [Google Scholar]
- 14.Hopper K, Powell LL. Basics of mechanical ventilation for dogs and cats. Vet Clin North Am Small Anim Pract. 2013;43:955–969. doi: 10.1016/j.cvsm.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 15.Kelmer E, Love LC, Declue AE, Cohn LA, Bruchim Y, Klainbart S, Sura PA, Merbl Y. Successful treatment of acute respiratory distress syndrome in 2 dogs. Can Vet J. 2012;53:167–173. [PMC free article] [PubMed] [Google Scholar]
- 16.Kloot TE, Blanch L, Melynne Youngblood A, Weinert C, Adams AB, Marini JJ, Shapiro RS, Nahum A. Recruitment maneuvers in three experimental models of acute lung injury. Effect on lung volume and gas exchange. Am J Respir Crit Care Med. 2000;161:1485–1494. doi: 10.1164/ajrccm.161.5.9809014. [DOI] [PubMed] [Google Scholar]
- 17.Liu Q, Gao YH, Hua DM, Li W, Cheng Z, Zheng H, Chen RC. Functional residual capacity in beagle dogs with and without acute respiratory distress syndrome. J Thorac Dis. 2015;7:1459–1466. doi: 10.3978/j.issn.2072-1439.2015.08.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Q, Li W, Zeng QS, Zhong NS, Chen RC. Lung stress and strain during mechanical ventilation in animals with and without pulmonary acute respiratory distress syndrome. J Surg Res. 2013;181:300–307. doi: 10.1016/j.jss.2012.07.010. [DOI] [PubMed] [Google Scholar]
- 19.Mattingley JS, Holets SR, Oeckler RA, Stroetz RW, Buck CF, Hubmayr RD. Sizing the lung of mechanically ventilated patients. Crit Care. 2011;15:R60. doi: 10.1186/cc10034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mosing M, Staub L, Moens Y. Comparison of two different methods for physiologic dead space measurements in ventilated dogs in a clinical setting. Vet Anaesth Analg. 2010;37:393–400. doi: 10.1111/j.1467-2995.2010.00548.x. [DOI] [PubMed] [Google Scholar]
- 21.Mueller ER. Suggested strategies for ventilation management of veterinary patients with acute respiratory distress syndrome. J Vet Emerg Crit Care. 2001;11:191–198. [Google Scholar]
- 22.Nahum A, Hoyt J, Schmitz L, Moody J, Shapiro R, Marini JJ. Effect of mechanical ventilation strategy on dissemination of intratracheally instilled Escherichia coli in dogs. Crit Care Med. 1997;25:1733–1743. doi: 10.1097/00003246-199710000-00026. [DOI] [PubMed] [Google Scholar]
- 23.Nin N, Muriel A, Peñuelas O, Brochard L, Lorente JA, Ferguson ND, Raymondos K, Ríos F, Violi DA, Thille AW, González M, Villagomez AJ, Hurtado J, Davies AR, Du B, Maggiore SM, Soto L, D'Empaire G, Matamis D, Abroug F, Moreno RP, Soares MA, Arabi Y, Sandi F, Jibaja M, Amin P, Koh Y, Kuiper MA, Bülow HH, Zeggwagh AA, Anzueto A, Sznajder JI, Esteban A VENTILA Group. Severe hypercapnia and outcome of mechanically ventilated patients with moderate or severe acute respiratory distress syndrome. Intensive Care Med. 2017;43:200–208. doi: 10.1007/s00134-016-4611-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oura T, Rozanski EA, Buckley G, Bedenice D. Low tidal volume ventilation in healthy dogs. J Vet Emerg Crit Care (San Antonio) 2012;22:368–371. doi: 10.1111/j.1476-4431.2012.00749.x. [DOI] [PubMed] [Google Scholar]
- 25.Pelosi P, Goldner M, McKibben A, Adams A, Eccher G, Caironi P, Losappio S, Gattinoni L, Marini JJ. Recruitment and derecruitment during acute respiratory failure: an experimental study. Am J Respir Crit Care Med. 2001;164:122–130. doi: 10.1164/ajrccm.164.1.2007010. [DOI] [PubMed] [Google Scholar]
- 26.Robinson NE, Gillespie JR, Berry JD, Simpson A. Lung compliance, lung volumes, and single-breath diffusing capacity in dogs. J Appl Physiol. 1972;33:808–812. doi: 10.1152/jappl.1972.33.6.808. [DOI] [PubMed] [Google Scholar]
- 27.Tonetti T, Vasques F, Rapetti F, Maiolo G, Collino F, Romitti F, Camporota L, Cressoni M, Cadringher P, Quintel M, Gattinoni L. Driving pressure and mechanical power: new targets for VILI prevention. Ann Transl Med. 2017;5:286. doi: 10.21037/atm.2017.07.08. [DOI] [PMC free article] [PubMed] [Google Scholar]