Abstract
Porcine proliferative enteropathy (PPE) caused by Lawsonia intracellularis (LI) is a global cause for substantial economic losses in the swine industry. Here, we constructed live attenuated Salmonella typhimurium (ST) mutant strains expressing and secreting 4 selected immunogenic LI antigens, namely, optA, optB, Lawsonia flagellin (LfliC), and Lawsonia hemolysin (Lhly); the resultant recombinant strains were designated Sal-optA, Sal-optB, Sal-LfliC, or Sal-Lhly, respectively. Using the BALB/c mouse model, we demonstrate that mice vaccinated once orally, either with a mixture of all 4 recombinant strains or with an individual recombinant strain, show significant (p < 0.05) production of LI-specific systemic immunoglobulin (Ig) G and mucosal IgA responses compared to the Salmonella alone group. Upon restimulation of vaccinated splenocytes with the LI-specific antigens, significant (p < 0.05) and comparable production of interferon-γ responses are found in all vaccinated groups, except the Sal-Lhly group, which shows non-significant levels. Challenge studies were performed in C57BL/6 vaccinated mice. On challenge with the LI (106.9 50% tissue culture infectious dose) 14 days post-vaccination, 20% (1/5) of mice in all vaccinated groups, except Sal-Lhly group, show the presence of the LI-specific genomic DNA (gDNA) in stool samples. In contrast, 40% (2/5) and 60% (3/5) of mice vaccinated with the Sal-Lhly strain and the attenuated Salmonella alone, respectively, were found positive for the LI-specific gDNA. Furthermore, 0% mortality was observed in mice vaccinated against the ST challenge compared to the 30% mortality observed in the unvaccinated control group. In conclusion, we demonstrate that the Salmonella-based LI-vaccines induce LI-specific humoral and cell-mediated immunities, and encompass the potential to offer dual protection against PPE and salmonellosis.
Keywords: Lawsonia intracellularis, Salmonellosis, attenuated vaccine, mice
INTRODUCTION
Lawsonia intracellularis (LI) is a gram-negative obligate intracellular bacterium causing proliferative enteropathy (PE) in various animals, most notably pigs [1,2]. The infection is characterized by proliferation of immature crypt cells and thickening of the intestinal mucosa, which is manifested by diarrhea and decreased growth rate in weaned piglets [3]. Porcine PE (PPE) is prevalent in various pig rearing countries, including Korea, Sweden, Denmark, and other European countries, triggering substantial economic losses in the global swine industry [3,4,5]. Antibiotics, particularly chlortetracycline, tiamulin, tylosin, and lincomycin, have been used in susceptible populations to control the disease [1]. However, due to the emergence of antibiotic resistance and increased stringency in regulations of antibiotic application in the meat industry, the use of antibiotics is restricted and impractical [6]. Considering the above constraints, vaccination of pigs with an effective LI vaccine is a viable strategy to prevent PPE. Although live attenuated commercially available LI vaccines mitigate the clinical symptoms, the vaccination fails to completely prevent the infection [7]. In contrast, natural infection elicits sterile immunity and provides complete protection against re-infection [7]. It has been reported that pigs infected with LI bacteria have a higher risk factor for Salmonella shedding in feces [8]. Like PPE, salmonellosis, caused by Salmonella typhimurium (ST), has a similar infectious niche and clinical outcomes. Both pathogens likely have indirect interactions that might be mediated by other members of the gastrointestinal microbes, resulting in altering the composition of the intestinal microbiome [9]. Moreover, profound disease symptoms have been noticed in pigs upon concurrent infections with LI and Salmonella. In accordance with this notion, novel vaccination strategies need to be devised that are not only safe, but can provide dual protection against PPE and salmonellosis in pigs.
Till date, virulence factors related to LI pathogenesis have rarely been identified. Recently, the intensive immunoreactive band of ~72 kDa, which was identified as Lawsonia autotransporter A (LatA) protein, has been detected in sera collected from the LI-infected pigs [10]. Based on their potential antigenicity and functionality, the LatA protein located on the LI outer membrane is cleaved into 2 highly immunogenic domains, namely optA and optB [11]. Furthermore, a single flagellar motor on LI is associated with the intracellular motility and initial invasive characteristics [12]. A previous study indicated that a putative LI flagellin and related hook-associated protein show immunogenic traits comprising toll-like receptor 5-stimulating activity and interleukin (IL)-8 cytokine expression [13]. The antigenic features of putative Lawsonia hemolysin (Lhly) A protein is also demonstrated in a recent study [14], which is highly similar to RNA methyltransferase of hemolysin-like molecule (i.e. TlyA protein), a virulence factor found in Mycobacterium tuberculosis pathogenesis [15]. In this study, vaccine candidates against PE were developed using the live attenuated Salmonella vector system employing the optA, optB, Lawsonia flagellin (LfliC) and Lhly proteins [16,17]. This strategy is expected to allow differentiation of infected from vaccinated animals. Here, we report the construction of attenuated ST mutant strains expressing and secreting optA, optB, LfliC, and Lhly, and evaluate the immunogenicity of these vaccine constructs in mice model. We show that vaccination of mice, either with a cocktail of all 4 recombinant strains or with an individual recombinant strain, induces LI-specific humoral and cell mediated immune responses and partial protection against the LI challenge. We further demonstrate that Salmonella-based LI vaccines offer complete protection against salmonellosis.
MATERIALS AND METHODS
Bacterial strains and plasmids
The bacterial strains, plasmids and primer pairs used in this study are listed in Table 1. The JOL912 ST mutant strain was used to deliver the LI-specific vaccine antigens. An asd+ plasmid (pJHL65) was used for cloning and expression of LI antigens in the JOL912 strain. All bacterial strains were incubated at 37°C in Luria-Bertani broth. The live attenuated LI strain (Enterisol Ileitis; Boehringer Ingelheim Vetmedica, Inc., USA) was purchased, and dissolved in sterile phosphate buffered saline (PBS; pH = 7.2). Final concentration of the strain was adjusted to 106.9 50% tissue culture infectious dose (TCID50) per 100 µL volume for the challenge experiment.
Table 1. Bacterial strains and plasmids used in this study.
| Strain/plasmid | Description | Reference | |
|---|---|---|---|
| ST | |||
| JOL401 | ST wild type, challenge strain | Lab stock | |
| JOL912 | Δlon, ΔcpxR, Δasd mutant ofST | [27] | |
| JOL1592 | JOL912 containing pJHL65-optA and expressing optB | This study | |
| JOL1607 | JOL912 containing pJHL65-optB and expressing optA | This study | |
| JOL1659 | JOL912 containing pJHL65-LfliC and expressing LfliC | This study | |
| JOL1744 | JOL912 containing pJHL65-Lhly and expressing Lhly | This study | |
| E. coli | |||
| BL21 (DE3) pLysS | F−, ompT, hsdSB (rB−, mB−), dcm, gal, λ (DE3), pLysS, Cmr | Progma, USA | |
| JOL1593 | BL21 with pET-optA | This study | |
| JOL1586 | BL21 with pET-OptB | This study | |
| JOL1682 | BL21 with pET-LfliC | This study | |
| JOL1742 | BL21 with pET-Lhly | This study | |
| JOL232 | F− λ− ϕ80 Δ (lacZYA-argF) endA1 recA1 hadR17 deoR thi-1 glnV44 gyrA96 relA1 ΔasdA4 | Lab stock | |
| JOL1601 | JOL232 containing pJHL65-optA | This study | |
| JOL1902 | JOL232 containing pJHL65-OptB | This study | |
| JOL1658 | JOL232 containing pJHL65-LfliC | This study | |
| JOL1743 | JOL232 containing pJHL65-Lhly | This study | |
| Plasmids | |||
| pET28a (+) | IPTG-inducible expression vector; Kanamycin resistant | Novagen, USA | |
| pET32a | IPTG-inducible expression vector; Kanamycin resistant | Lab stock | |
| pET-optA | pET32a derivative containing optA | This study | |
| pET-optB | pET28a (+) derivative containing optB | This study | |
| pET-LfliC | pET28a (+) derivative containing LfliC | This study | |
| pET-Lhly | pET28a (+) derivative containing Lhly | This study | |
| pJHL65 | asd+ vector, pBR ori, bla SS-based periplasmic secretion plasmid, 6xHis, high copy number | [16] | |
| pJHL65-optA | pJHL65 harboring optA of LI | This study | |
| pJHL65-optB | pJHL65 harboring optB of LI | This study | |
| pJHL65-LfliC | pJHL65 harboring LfliC of LI | This study | |
| Gene | |||
| optA | F: GTC GAC ATT TTT AAT CCT CAA GAT | ||
| R: CTC GAG TTA GAA TCT ATA AGT AGC A | |||
| optB | F: GTC GAC ATT TTT AAT CCT CAA GAT | ||
| R: CTG CAG TTA GAA TCT ATA AGT AGC A | |||
| LfliC | F: CCGAATTCTCTTTGGTCATTAACAACAACATGA | ||
| R: CCG TCG ACG CCA CTA ATG AGT TGG CTTG | |||
| Lhly | F: GAA TTC GCC AAA CAT AAA GTA CGT GCT GAT | ||
| R: AAGCTTACGTTTTTTCAAGTAAATAAGATATCTTG | |||
ST, Salmonella typhimurium; LI, Lawsonia intracellularis; IPTG, isopropyl β-D-1-thiogalactopyranoside; bla SS, beta-lactamase signal sequence.
Construction of attenuated Salmonella mutants expressing and secreting LI antigens
The optA, optB, LfliC, and Lhly gene sequences of LI strain acquired from the National Center for Biotechnology Information databases were chemically synthesized (Bioneer, Korea) and built into the pJHL65 plasmid, an asd+ vector for protein expression, as previously described [18]. The selected antigenic genes were cloned in the frame downstream to the beta-lactamase signal sequence (bla SS), thereby facilitating secretion of the target proteins from the Salmonella strain (Supplementary Fig. 1). The recombinant plasmids, pJHL65-optA, pJHL65-optB, pJHL65-LfliC, and pJHL65-Lhly, were subsequently transformed into an asd gene-deleted (Δasd) mutant of attenuated ST, JOL912, and the resultant strains were designated as JOL1607 (Sal-optA), JOL1592 (Sal-optB), JOL1659 (Sal-LfliC), or JOL1744 (Sal-Lhly), respectively. Presence of the respective gene in JOL912 was confirmed by colony polymerase chain reaction (PCR) and restriction enzyme analysis. The JOL912 strain was attenuated by the deletion of lon, cpxR, and asd genes from the wild type ST (JOL401 isolate) by applying an allelic exchange method, as described in a previous study [18]. The LI-specific proteins expressed by the recombinant strains were confirmed in the culture supernatants by performing Western blot analysis using polyclonal LI-specific rabbit hyperimmune serum.
To determine the LI-specific antibody responses in vivo, optA, optB, LfliC, and Lhly genes were inserted into a pET28a (+) expression vector (Novagen, USA), and subsequently transformed into Escherichia coli BL21 plys strain (Novagen) for acquiring the purified protein [16]. The proteins expressed in the E. coli strain were confirmed by sodium dodecyl sulfate polyacrylamide gel electrophoresis and Western blot analysis. The expressed proteins were purified by Ni-NTA chromatographic column. Purified protein concentration was determined by the Bradford assay [19]. For determination of immunogenic domains, BepiPred 1.0 Server (http://www.cbs.dtu.dk/services/BepiPred/) was used to predict the location of linear B-cell epitopes in each LI-specific protein.
Vaccination and challenge studies
All animal experiments involving the use of specific pathogen-free BALB/c and C57BL/6 mice were approved by the Chonbuk National University Animal Ethics Committee (CBNU2015-00085) in accordance with guidelines of the Korean Council on Animal Care and Korean Animal Protection Law, 2007; article 13 (experiments with animals).
Five weeks old female mice (BALB/c for immunogenicity analysis and C57BL/6 for challenge study against LI) were maintained in a clean room equipped with exhaust fan and air conditioner. Groups of 5 mice were housed together in a cage, and all animals were provided antibiotics free deionized water and feed ad libitum. One week later, BALB/c mice were assigned into 6 groups (n = 10) and immunized once orally with either JOL1563, Sal-optA, Sal-optB, Sal-LfliC, Sal-Lhly, or a co-mixture of Sal-optA plus Sal-optB plus Sal-LfliC plus Sal-Lhly. The JOL1563 strain carries an empty pJHL65 vector. In this study, 107 colony-forming unit (CFU) of each bacterial strain or the co-mixture (i.e. 2.5 × 106 CFU each) diluted to a final volume of 100 µL in PBS, were used for vaccination. Serum (n = 5) and vaginal wash samples (n = 5) were obtained on the day of vaccination (pre-vaccination) and weekly, to evaluate the LI-specific and Salmonella ompA-specific serum immunoglobulin (Ig) G and vaginal secretory IgA (sIgA) humoral responses until week 6 post-vaccination. For the challenge studies, C57BL/6 mice were randomly assigned into 6 groups (n = 30) and immunized once with either JOL1563, Sal-optA, Sal-optB, Sal-LfliC, Sal-Lhly, or a co-mixture of Sal-optA plus Sal-optB plus Sal-LfliC plus Sal-Lhly, via the oral route. Immunization was achieved by administering 107 CFU of each bacterial strain and the co-mixture (i.e. 2.5 × 106 CFU each). At 2 weeks after the vaccination, all immunized mice were challenged with oral administration of 106.9 TCID50 live attenuated LI bacteria. Stool samples (n = 5) were obtained to determine the presence of LI-specific genomic DNA (gDNA) by PCR assay at days 0, 3, 6, 7, 8, and 9 post-challenge [20]. The LI-specific protection is presented as the number of animals positive for gDNA to the total number of animals in that group. Furthermore, we separately maintained 2 BALB/c mice groups (n = 10) vaccinated once orally with either the co-mixture of 4 vaccine candidates (total 107 CFU) or sterile PBS (100 µL); 4 weeks later, these animals were inoculated with 100 µL of a suspension containing 2 × 109 CFU of a wild type ST strain JOL401 via the oral route, as described previously [16]. The Salmonella-specific protection efficacy was evaluated in the context of bacterial recovery from the spleen, and mortality of animals post-challenge.
Antigen-specific enzyme-linked immunosorbent assay (ELISA)
An indirect ELISA was used to measure the LI antigen-specific IgG and sIgA responses in sera (n = 5) and in vaginal wash samples (n = 5), respectively, as described previously [21]. Salmonella-specific IgG and sIgA responses was also evaluated in vaccinated mice. Recombinant LI proteins (500 ng/well) and ompA (250 ng/well) were applied as the coating antigens to determine the titers of LI-specific and Salmonella-specific antibody, respectively.
Analysis of cytokine responses in vaccinated mice
Splenocytes (1 × 106 cells/mL, n = 5) were isolated from the BALB/c vaccinated mice at day 14 post-vaccination, and subsequently pulsed with 200 ng LI-specific antigens for 24 h. Total RNA was isolated by the RNeasy Mini kit (Qiagen, Germany), as per the manufacturer's instructions. Complementary DNA was prepared by using the SuperScript™ III Reverse Transcriptase kit (Invitrogen, USA), as previously described [16]. Quantitative real time PCR (qRT-PCR) assay was performed to assess the cytokine gene expression using Power SYBR Green PCR Master Mix (Applied Biosystems, USA) [16]. The primer pairs were obtained from previous literature [22]. The relative amounts of cytokine messenger RNA (mRNA) present (normalized with glyceraldehyde 3-phosphate dehydrogenase) was determined by the 2-∆∆CT method [23].
Statistical analysis
All data are analyzed using the GraphPad prism 6.00 program (GraphPad, USA). Statistical significance in antibody responses and LI-specific protection post-challenge are examined using the Kruskal-Wallis test with Dunn's multiple comparison test. The significance in cytokine responses are examined using 1-way analysis of variance with Tukey's multiple comparison tests. A non-parametric χ2 test analyzes significant differences in mortality post-Salmonella challenge. The results are expressed as mean ± standard deviation. The p values less than 0.05 are considered significant.
RESULTS
JOL912 efficiently expressed and secreted LI-specific proteins
To construct the attenuated Salmonella mutant strains expressing and secreting LI antigens, we predicted highly antigenic epitopes of LatA, LfliC, and Lhly using the BepiPred 1.0 Server program (http://www.cbs.dtu.dk/services/BepiPred/) based on protein characteristics such as structural domains, hydrophilicity residues, antigenicity and surface possibility (Supplementary Fig. 2). Two immunogenic regions consisting of amino acid residues from 101–200 and 534–851 were chosen in LatA and designated as optA and optB, respectively, while the full length LfliC and Lhly were selected for expression in the Salmonella system. The insertion of optA, optB, LfliC, or Lhly into the pJHL65 vector was confirmed by digestion of the positive clones with EcoR1 and HindIII to release fragments of 300, 957, 885, and 756 bp, respectively. Subsequently, the pJHL65-optA, pJHL65-optB, pJHL65-LfliC, or pJHL65-Lhly recombinant plasmid was electroporated into the JOL912 strain for protein expression. Immunoblotting analysis demonstrated that the JOL912 strain efficiently expresses and secretes optA, optB, LfliC, and Lhly proteins in the culture supernatants of the respective recombinant strain (Supplementary Fig. 3). In this study, we observed that the LI proteins were biologically active, as evidenced by the elicitation of the antigen-specific immune responses in vaccinated mice.
Systemic IgG and mucosal IgA antibody responses post-vaccination
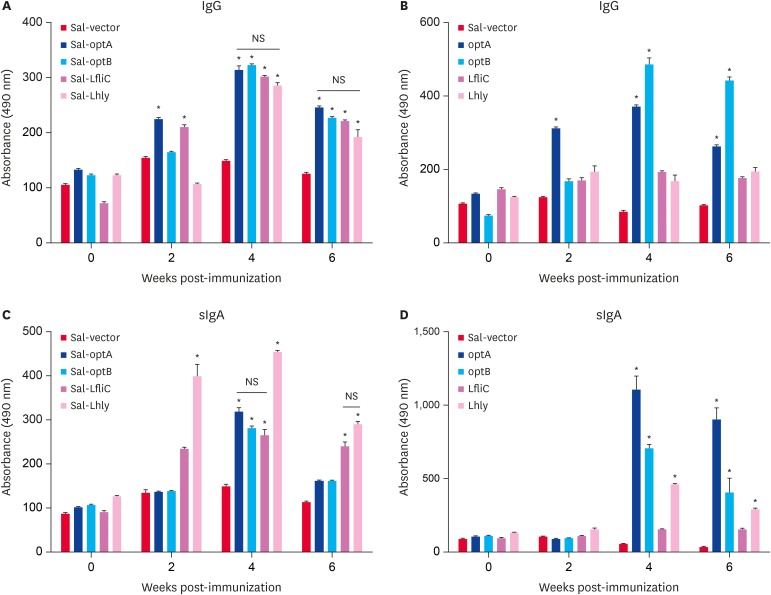
To investigate the effect of Salmonella-based LI vaccines on the systemic and mucosal antibody responses, serum and vaginal wash samples collected from BALB/c vaccinated mice were analyzed for IgG and sIgA responses, respectively. All the vaccinated groups showed significant (p < 0.05) increase in L1-specific IgG and sIgA responses compared to the Salmonella alone group. Among the groups vaccinated by individual Salmonella-based vaccine candidates, significantly (p < 0.05) higher IgG responses were observed in Sal-optA and Sal-LfliC groups at 2 weeks post-vaccination as compared to the Sal-optB and Sal-Lhly vaccinated groups; however, comparable IgG responses were observed in all groups at 4 and 6 weeks post-vaccination (Fig. 1A). Peak IgG titers were observed at 4 weeks post-vaccination in all groups, which remained constant till 6 weeks post-vaccination (Fig. 1A). Considering the vaccination with mixture of all 4 strains, significantly (p < 0.05) higher IgG responses were observed against optB followed by optA, while the titers were comparable for LfliC and Lhly (Fig. 1B). Our results further demonstrate that peak IgG titers were found at 4 weeks post-vaccination, and the titers were slightly higher than that observed with individual vaccine candidates (Fig. 1A and B). Among the groups vaccinated with individual vaccine candidates, the sIgA titers were significantly (p < 0.05) higher in animals receiving Sal-Lhly at 2 and 4 weeks post-vaccination as compared to other groups, where comparable sIgA responses are observed at 4 weeks post-vaccination (Fig. 1C). Vaccination with a mixture of all the 4 strains highest sIgA responses were observed against optA, followed by optB and Lhly, and the least response against LfliC (Fig. 1D). Peak sIgA responses were observed at 4 weeks post-vaccination against all the LI-specific antigens, which were maintained till 6 weeks (Fig. 1D). Our data further demonstrates that animals vaccinated with a mixture of all the 4 strains show significantly (p < 0.05) higher LI-specific sIgA responses compared to vaccination with the individual vaccine strains.
Fig. 1. Systemic IgG and mucosal IgA antibody responses in BALB/c mice (n = 5) vaccinated orally with Sal-vector, Sal-optA, optB, Sal-LfliC, Sal-Lhly, or a mixture of Sal-optA, Sal-optB, Sal-LfliC, and Sal-Lhly. (A) Serum IgG obtained after vaccination with the individual vaccine strain. (B) Serum IgG obtained after vaccination with a co-mixture of all the 4 strains. (C) Intestinal sIgA obtained after vaccination with the individual vaccine strain. (D) Vaginal sIgA obtained after vaccination with a co-mixture of all the 4 strains. IgG and IgA responses measured against each LI protein by an indirect enzyme-linked immunosorbent assay using individual recombinant proteins as coating antigens.
Each data point represents mean ± standard deviation of 5 mice per group. The significance of the data was examined using the Kruskal-Wallis test with Dunn's multiple comparison test.
Ig, immunoglobulin; NS, not significant; LI, Lawsonia intracellularis; LfliC, Lawsonia flagellin; Lhly, Lawsonia hemolysin; sIgA, secretory immunoglobulin A.
*p < 0.05.
Cytokine responses post-vaccination
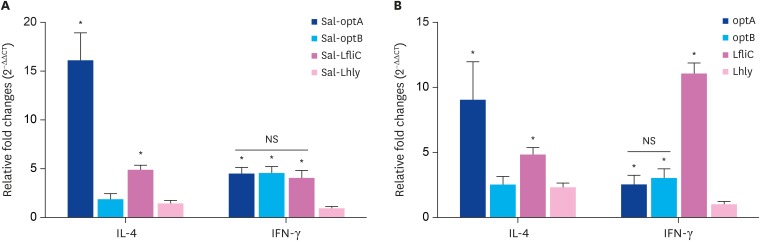
To investigate the effect of Salmonella-based LI vaccines on the cytokine responses post-vaccination, we sacrificed mice (n = 5) at 14 days post-vaccination. Splenocytes were subsequently collected for analysis of T helper cell type (Th) 1 (interferon [IFN]-γ) and Th2 (IL-4) cytokines by qRT-PCR assay. Considering the groups vaccinated by individual vaccine candidates, IL-4 mRNA induction levels were found significantly (p < 0.05) higher in the Sal-optA group followed by the Sal-LfliC group, whereas least responses were observed in the Sal-optB and Sal-hly groups (Fig. 2A). Lowest levels of IFN-γ were observed in the Sal-Lhly group, whereas Sal-optA, Sal-optB, and Sal-LfliC groups showed significantly (p < 0.05) higher levels (Fig. 2A). Similar kinetics and induction levels were observed in the group vaccinated with a mixture of all 4 strains (Fig. 2B). However, the IFN-γ levels observed against LfliC were significantly (p < 0.05) higher compared to the levels observed against optA and optB. Our results further indicate that IL-4 and IFN-γ levels are almost comparable in all except the Sal-optA vaccinated group, which shows significantly (p < 0.05) higher IL-4 levels compared to the IFN-γ levels. These results therefore suggest that vaccination with the Salmonella-based L1 vaccines stimulates mixed type of Th1 and Th2 immunities.
Fig. 2. Cytokine responses following vaccination with the individual vaccine strain or a cocktail of all 4 vaccine strains. Splenocytes (1 × 106/mL, n = 5) isolated from BALB/c mice at day 14 post-vaccination were stimulated with their respective vaccine antigen for 24 h, and subsequently analyzed for induction of IL-4 and IFN-γ mRNA transcription levels by quantitative real time polymerase chain reaction assay. (A) IL-4 and IFN-γ induction levels in splenocytes of mice vaccinated by the individual vaccine strains. (B) IL-4 and IFN-γ induction levels in splenocytes of mice vaccinated with a cocktail of all 4 strains. Results are expressed as relative fold change in mRNA transcription levels of stimulated splenocytes from vaccinated mice compared to the Salmonella vector control group. Gene expressions are normalized to glyceraldehyde 3-phosphate dehydrogenase, and mRNA levels of naive stimulated cells are used as the calibrator.
Data presented are mean ± standard deviation of 5 mice per group. The significance of the data was examined using 1-way analysis of variance with Tukey's multiple comparison test.
mRNA, messenger RNA; NS, not significant; IL, interleukin; IFN, interferon; LfliC, Lawsonia flagellin; Lhly, Lawsonia hemolysin.
*p < 0.05.
Vaccination with Salmonella-based LI vaccines provide partial protection against the LI challenge
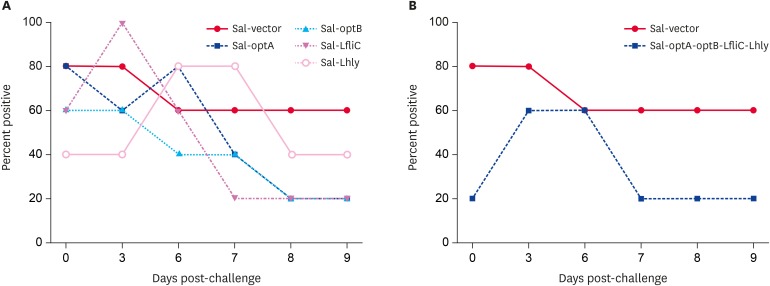
To assess the protection offered by the Salmonella-based LI vaccines, C57BL6 vaccinated mice were orally challenged with 106.9 TCID50 live attenuated LI bacteria. Stool samples were subsequently analyzed for the presence of gDNA to determine the protective efficacy (Supplementary Fig. 4). Our results demonstrate that vaccination with either individual vaccine candidates or cocktail of all 4 strains reduces the gDNA in vaccinated mice by days 7, 8, and 9 post-challenge. In all the vaccinated groups, only 20% (1/5) of mice show gDNA in stool samples by day 8 and 9 post-challenge; however, 40% (2/5) of mice are seen to be positive in the Sal-Lhly group (Fig. 3A and B). In contrast, 60% (3/5) of mice in the Salmonella alone group show the presence of gDNA by day 8 and 9 post-challenge. These results thus indicate that vaccination with Salmonella-based L1 vaccines provide partial protection against LI infection, and an individual vaccine candidate may be sufficient to mediate this partial protection.
Fig. 3. Protection efficacies of Salmonella-based LI vaccines. All the vaccinated C57BL/6 mice groups were orally challenged with 106.9 50% tissue culture infectious dose LI strain at 2 weeks post-vaccination. The protection against LI was assessed based on the presence of gDNA in stool samples. (A) Protection shown by an individual vaccine strain. (B) Protection shown by a cocktail of all 4 strains. Protection is presented as the number of animals showing gDNA in stool samples to the total number of animals in that group. Five animals were sacrificed at each time-point indicated. The significance of the data was examined using the Kruskal-Wallis test with Dunn's multiple comparison test.
gDNA, genomic DNA; LI, Lawsonia intracellularis; LfliC, Lawsonia flagellin; Lhly, Lawsonia hemolysin.
Evaluation of immune responses and protective efficacy against salmonellosis
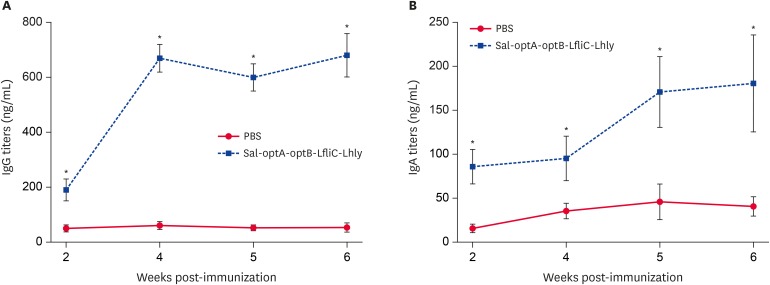
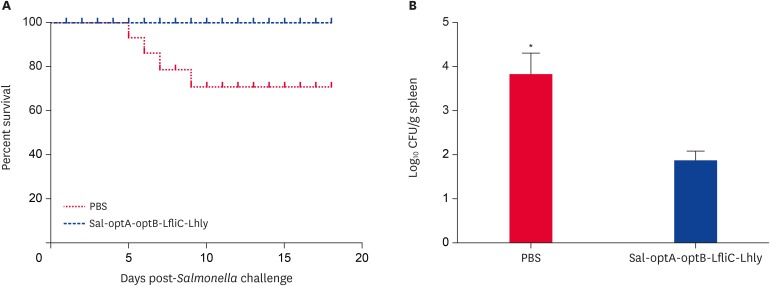
We further evaluated the Salmonella-specific systemic IgG and mucosal IgA responses post-vaccination, and subsequent protection against Salmonella challenge. Salmonella ompA-specific IgG and sIgA titers induced by vaccination with the respective candidates were significantly (p < 0.05) higher from the 2nd week till the end of the experiment. Peak IgG titers were observed at 4 weeks post-vaccination that remain constant till 6 weeks (Fig. 4A), whereas IgA titers peak at 6 weeks post-vaccination (Fig. 4B). Furthermore, compared to the sIgA titers, IgG titers were significantly higher at all time points post-vaccination. The survival rates of vaccinated and PBS control mice following lethal challenge with wild-type ST were evaluated to assess the protection rate against salmonellosis. Our results demonstrate that mice vaccinated with Salmonella-based LI vaccine candidates show 100% survivability, whereas 30% mortality is observed in the PBS control group (Fig. 5A). All the surviving mice from PBS control and vaccination groups were euthanized 18 days post-challenge, and their spleens were collected aseptically for determination of bacterial count. Our data shows that the PBS control mice have a significantly (p < 0.05) higher bacterial load than the vaccinated mice (Fig. 5B). The challenge strain recovered from spleen of unimmunized mice (3.82 ± 0.45 log10 CFU/g) was 76 times higher than the recovery from immunized mice (1.92 ± 0.22 log10 CFU/g), thereby indicating that the Salmonella-based LI vaccines offer significant protection against Salmonella infection.
Fig. 4. Salmonella ompA-specific serum IgG and vaginal sIgA responses in BALB/c vaccinated mice. BALB/c mice were vaccinated with either the co-mixture of 4 vaccine candidates or sterile PBS, and serum and vaginal wash samples were collected at different time points for IgG and IgA analysis, respectively, by an indirect enzyme-linked immunosorbent assay. (A) ompA-specific IgG measured in serum. (B) ompA-specific IgA measured in vaginal wash samples.
Each data point represents mean ± standard deviation of 5 mice per group. The significance of the data was examined using the Kruskal-Wallis test with Dunn's multiple comparison test.
PBS, phosphate buffered saline; Ig, immunoglobulin; sIgA, secretory immunoglobulin A; LfliC, Lawsonia flagellin; Lhly, Lawsonia hemolysin.
*p < 0.05.
Fig. 5. Protection efficacies of Salmonella-based LI vaccines against S. typhimurium. BALB/c mice (n = 10) vaccinated with either Salmonella-based LI vaccine constructs (the co-mixture of 4 vaccine candidates) or PBS were challenged at 6 weeks post-vaccination with a virulent Salmonella strain. Mortality and bacterial recovery from spleen were recorded. (A) Mortality in vaccinated and PBS control groups. (B) Bacterial load in survived mice post-challenge.
Each data point represents mean ± standard deviation of 5 mice per group. A non-parametric χ2 test was used to analyze significant differences in mortality, and Student's t-test for bacterial load.
LI, Lawsonia intracellularis; PBS, phosphate buffered saline; LfliC, Lawsonia flagellin; Lhly, Lawsonia hemolysin.
*p < 0.05.
DISCUSSION
This study aimed to investigate whether Salmonella-based LI vaccines induce LI-specific immune responses and subsequent protection against PPE and salmonellosis. Causative pathogens for PPE and salmonellosis are both intracellular enteric bacteria and have similarity in both the infection route and resident niche [1,24]. It has been reported that concurrent infections of LI and Salmonella in pigs result in more profound symptoms rather than those associated with either type of infection, suggesting a synergistic type of interaction between LI and Salmonella [9]. Considering the pathogenic mechanisms of 2 enteric pathogens, the development of efficient vaccines that provide concurrent protections against salmonellosis and PPE may have enormous advantages for the swine industries. In this context, we genetically engineered a live attenuated Salmonella based recombinant system delivering LI-specific antigens to elicit efficient protection against both PPE and salmonellosis. For the development of an efficient vaccine against a pathogenic organism, target antigens are generally determined based on the virulence factors such as toxins, fimbriae, flagellin, and membrane proteins. In this study, based on their antigenic and virulence characteristic features, the LI proteins including Lat2, LfliC, and Lhly were selected for vaccine development. Our study demonstrates that mice vaccinated with either the individual vaccine candidates or a cocktail of recombinant Salmonella strains induce LI-specific and Salmonella-specific systemic and mucosal immunities, providing partial protection against PPE and complete protection against salmonellosis.
Using bla SS, we further demonstrate that LI-specific antigens are efficiently expressed and actively secreted by the Salmonella system. The expressed proteins are immunologically active, as evidenced by the immunoreactivity with the polyclonal LI-specific sera and elicitation of antigen-specific immune response in vaccinated mice. The in vitro expression of optA and LFliC in Western blot analysis is relatively weak compared to expression levels of optB and LHly. Considering the live Salmonella vector system was employed in this study, the amount of protein expressed in vivo in the live vector may not be exactly reflected by in vitro expression of the antigenic protein. The present study indicates that vaccination with Salmonella-based LI vaccines generate efficient systemic and mucosal antibody responses, although the LI-specific antibody response does not correlate with the clinical protection observed against PPE. The LI-specific sIgA responses were highest in mice administered the Sal-Lhly vaccine, but this group shows least protection against the LI challenge. The LI-specific IgG responses are comparable in all vaccinated groups, suggesting that humoral immunity has a minimal role in preventing the clinical infection. Given that cellular immune response plays a pivotal role in controlling the PE infection [25], we evaluated the production of IFN-γ responses in the stimulated splenocytes following inoculation with the Salmonella-based LI vaccines. We observed significantly higher IFN-γ responses in groups vaccinated with Sal-optA, Sal-optB, and Sal-LfliC, compared to the mice administered the Sal-Lhly vaccine. Earlier studies have validated the primers and reaction method used in this study for quantification of copy number in mouse samples [22]. Although ELISA results are considerably more accurate for cytokine quantifications, the mRNA levels obtained in this study also represent an indirect indication in increase of cytokine levels. Consequently, the percentage of mice showing LI infection was low (1/5) in these groups. The mice vaccinated with a mixture of all 4 strains show significantly less IFN-γ responses against Lhly, which explains why the protection rate is lower in the group receiving the Sal-Lhly vaccine. In a previous study, Smith et al. [26] also indicated that IFN-γ is responsible for limiting intracellular dissemination of LI in a mouse model. Our study further demonstrates that the Salmonella-based LI-vaccine candidates simultaneously offer protection against both LI infection and salmonellosis in a mouse model, thereby demonstrating their potential to act as cost-effective bivalent vaccines.
In summary, we found that Salmonella-based LI vaccine candidates have a potential preventive role in the affected host, considering that the injection effectively elicits pathogen-specific humoral and cellular immunity and confers robust protection against both PE and salmonellosis. This work is a preliminary study to determine the potential immunogenicity and protective efficacy of vaccine candidates against LI. Although all the immunological parameters tested in mice may not exactly represent the true immune response of pigs, a certain level of humoral immunity or CMI may be expected in the pig model via this preliminary data. Further studies are required to assess the immunogenicity and efficacy of these vaccine candidates, and elucidate their defense mechanism in natural hosts.
Footnotes
Funding: This work was supported by the Technological Innovation R&D Program (S2448723) funded by the Small and Medium Business Administration (SMBA, Korea).
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Lee JH.
- Data curation: Park S.
- Formal analysis: Won G.
- Funding acquisition: Lee JH.
- Investigation: Park S, Won G.
- Methodology: Won G, Park S.
- Project administration: Lee JH.
- Resources: Lee JH.
- Software: Park S, Won G.
- Supervision: Lee JH.
- Validation: Lee JH, Won G.
- Visualization: Won G, Park S.
- Writing - original draft: Park S.
- Writing - review & editing: Won G, Lee JH.
SUPPLEMENTARY MATERIALS
Components of the pJHL65 plasmid carrying LI-specific genes. The asd+ plasmid with pBR ori has a constitutive expression promoter system and bla SS that directs the expressed protein out of the cell.
In silico prediction of the linear B-cell epitopes of each LI antigen. Linear B-cell epitopes were predicted for antigenicity of LI antigens. The BepiPred program (http://www.cbs.dtu.dk/services/BepiPred/) assigns a score to each individual amino acid in a specific sequence.
Immunoblot analysis of optA, optB, LfliC, and Lhly antigens expressed in JOL1607, JOL1592, JOL1659, and JOL1744 strains, respectively. The respective vaccine strains were rown in Luria-Bertani broth to mid-log phase; culture supernatants were subsequently collected and subjected to Western blot analysis using protein-specific hyperimmune sera raised in rabbits. (A) Western blot of Sal-optA, (B) Western blot of Sal-optB, (C) Western blot of Sal-LfliC, and (D) Western blot of Sal-Lhly.
Quantitative PCR for LI-specific gDNA recovered in stool samples collected from vaccinated C57BL/6 mice post-challenge. C57BL/6 mice (n = 30) vaccinated with either Salmonella-based LI vaccines or Salmonella alone were orally challenged with 106.9 50% tissue culture infectious dose LI bacteria. Stool samples were collected on days 0, 3, 5, 7, 8, and 9 post-challenge for analysis of LI-specific gDNA by quantitative PCR. Here, we provide the data for days 6 and 9 post-challenge only. (A) Gel electrophoresis of quantitative PCR done on day 6. (B) Gel electrophoresis of quantitative PCR done on day 9 post-challenge.
References
- 1.Kroll JJ, Roof MB, Hoffman LJ, Dickson JS, Harris DL. Proliferative enteropathy: a global enteric disease of pigs caused by Lawsonia intracellularis . Anim Health Res Rev. 2005;6:173–197. doi: 10.1079/ahr2005109. [DOI] [PubMed] [Google Scholar]
- 2.Lee SW, Kim TJ, Park SY, Song CS, Chang HK, Yeh JK, Park HI, Lee JB. Prevalence of porcine proliferative enteropathy and its control with tylosin in Korea. J Vet Sci. 2001;2:209–212. [PubMed] [Google Scholar]
- 3.McOrist S, Smith SH, Green LE. Estimate of direct financial losses due to porcine proliferative enteropathy. Vet Rec. 1997;140:579–581. doi: 10.1136/vr.140.22.579. [DOI] [PubMed] [Google Scholar]
- 4.Just SD, Thoen CO, Thacker BJ, Thompson JU. Monitoring of Lawsonia intracellularis by indirect serum immunofluorescence assay in a commerical swine production system. J Swine Health Prod. 2001;9:57–61. [Google Scholar]
- 5.Park S, Lee JB, Kim KJ, Oh YS, Kim MO, Oh YR, Hwang MA, Lee JA, Lee SW. Efficacy of a commercial live attenuated Lawsonia intracellularis vaccine in a large scale field trial in Korea. Clin Exp Vaccine Res. 2013;2:135–139. doi: 10.7774/cevr.2013.2.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koluman A, Dikici A. Antimicrobial resistance of emerging foodborne pathogens: status quo and global trends. Crit Rev Microbiol. 2013;39:57–69. doi: 10.3109/1040841X.2012.691458. [DOI] [PubMed] [Google Scholar]
- 7.Riber U, Heegaard PM, Cordes H, Ståhl M, Jensen TK, Jungersen G. Vaccination of pigs with attenuated Lawsonia intracellularis induced acute phase protein responses and primed cell-mediated immunity without reduction in bacterial shedding after challenge. Vaccine. 2015;33:156–162. doi: 10.1016/j.vaccine.2014.10.084. [DOI] [PubMed] [Google Scholar]
- 8.Beloeil PA, Fravalo P, Fablet C, Jolly JP, Eveno E, Hascoet Y, Chauvin C, Salvat G, Madec F. Risk factors for Salmonella enterica subsp. enterica shedding by market-age pigs in French farrow-to-finish herds. Prev Vet Med. 2004;63:103–120. doi: 10.1016/j.prevetmed.2004.01.010. [DOI] [PubMed] [Google Scholar]
- 9.Borewicz KA, Kim HB, Singer RS, Gebhart CJ, Sreevatsan S, Johnson T, Isaacson RE. Changes in the porcine intestinal microbiome in response to infection with Salmonella enterica and Lawsonia intracellularis. PLoS One. 2015;10:e0139106. doi: 10.1371/journal.pone.0139106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watson E, Clark EM, Alberdi MP, Inglis NF, Porter M, Imrie L, McLean K, Manson E, Lainson A, Smith DG. A novel Lawsonia intracellularis autotransporter protein is a prominent antigen. Clin Vaccine Immunol. 2011;18:1282–1287. doi: 10.1128/CVI.05073-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wells TJ, Tree JJ, Ulett GC, Schembri MA. Autotransporter proteins: novel targets at the bacterial cell surface. FEMS Microbiol Lett. 2007;274:163–172. doi: 10.1111/j.1574-6968.2007.00833.x. [DOI] [PubMed] [Google Scholar]
- 12.Lawson GH, Gebhart CJ. Proliferative enteropathy. J Comp Pathol. 2000;122:77–100. doi: 10.1053/jcpa.1999.0347. [DOI] [PubMed] [Google Scholar]
- 13.Won G, Lee JH. Antigenic and functional profiles of a Lawsonia intracellularis protein that shows a flagellin-like trait and its immuno-stimulatory assessment. Vet Res. 2018;49:17. doi: 10.1186/s13567-018-0515-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim J, Won G, Park S, Lee JH. Identification of Lawsonia intracellularis putative hemolysin protein A and characterization of its immunoreactivity. Vet Microbiol. 2017;205:57–61. doi: 10.1016/j.vetmic.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Rahman A, Srivastava SS, Sneh A, Ahmed N, Krishnasastry MV. Molecular characterization of tlyA gene product, Rv1694 of Mycobacterium tuberculosis: a non-conventional hemolysin and a ribosomal RNA methyl transferase. BMC Biochem. 2010;11:35. doi: 10.1186/1471-2091-11-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hajam IA, Lee JH. An influenza HA and M2e based vaccine delivered by a novel attenuated Salmonella mutant protects mice against homologous H1N1 infection. Front Microbiol. 2017;8:872. doi: 10.3389/fmicb.2017.00872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pei Z, Jiang X, Yang Z, Ren X, Gong H, Reeves M, Sheng J, Wang Y, Pan Z, Liu F, Wu J, Lu S. Oral delivery of a novel attenuated Salmonella vaccine expressing influenza a virus proteins protects mice against H5N1 and H1N1 viral infection. PLoS One. 2015;10:e0129276. doi: 10.1371/journal.pone.0129276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hur J, Lee JH. Enhancement of immune responses by an attenuated Salmonella enterica serovar Typhimurium strain secreting an Escherichia coli heat-labile enterotoxin B subunit protein as an adjuvant for a live Salmonella vaccine candidate. Clin Vaccine Immunol. 2011;18:203–209. doi: 10.1128/CVI.00407-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 20.Suh DK, Lym SK, Bae YC, Lee KW, Choi WP, Song JC. Detection of Lawsonia intracellularis in diagnostic specimens by one-step PCR. J Vet Sci. 2000;1:33–37. [PubMed] [Google Scholar]
- 21.Won G, Hajam IA, Lee JH. Improved lysis efficiency and immunogenicity of Salmonella ghosts mediated by co-expression of λ phage holin-endolysin and ɸX174 gene E. Sci Rep. 2017;7:45139. doi: 10.1038/srep45139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Overbergh L, Valckx D, Waer M, Mathieu C. Quantification of murine cytokine mRNAs using real time quantitative reverse transcriptase PCR. Cytokine. 1999;11:305–312. doi: 10.1006/cyto.1998.0426. [DOI] [PubMed] [Google Scholar]
- 23.Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Altier C. Genetic and environmental control of Salmonella invasion. J Microbiol. 2005;43:85–92. [PubMed] [Google Scholar]
- 25.Cordes H, Riber U, Jensen TK, Jungersen G. Cell-mediated and humoral immune responses in pigs following primary and challenge-exposure to Lawsonia intracellularis . Vet Res (Faisalabad) 2012;43:9. doi: 10.1186/1297-9716-43-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith DG, Mitchell SC, Nash T, Rhind S. Gamma interferon influences intestinal epithelial hyperplasia caused by Lawsonia intracellularis infection in mice. Infect Immun. 2000;68:6737–6743. doi: 10.1128/iai.68.12.6737-6743.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hajam IA, Lee JH. Preexisting Salmonella-specific immunity interferes with the subsequent development of immune responses against the Salmonella strains delivering H9N2 hemagglutinin. Vet Microbiol. 2017;205:117–123. doi: 10.1016/j.vetmic.2017.05.021. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Components of the pJHL65 plasmid carrying LI-specific genes. The asd+ plasmid with pBR ori has a constitutive expression promoter system and bla SS that directs the expressed protein out of the cell.
In silico prediction of the linear B-cell epitopes of each LI antigen. Linear B-cell epitopes were predicted for antigenicity of LI antigens. The BepiPred program (http://www.cbs.dtu.dk/services/BepiPred/) assigns a score to each individual amino acid in a specific sequence.
Immunoblot analysis of optA, optB, LfliC, and Lhly antigens expressed in JOL1607, JOL1592, JOL1659, and JOL1744 strains, respectively. The respective vaccine strains were rown in Luria-Bertani broth to mid-log phase; culture supernatants were subsequently collected and subjected to Western blot analysis using protein-specific hyperimmune sera raised in rabbits. (A) Western blot of Sal-optA, (B) Western blot of Sal-optB, (C) Western blot of Sal-LfliC, and (D) Western blot of Sal-Lhly.
Quantitative PCR for LI-specific gDNA recovered in stool samples collected from vaccinated C57BL/6 mice post-challenge. C57BL/6 mice (n = 30) vaccinated with either Salmonella-based LI vaccines or Salmonella alone were orally challenged with 106.9 50% tissue culture infectious dose LI bacteria. Stool samples were collected on days 0, 3, 5, 7, 8, and 9 post-challenge for analysis of LI-specific gDNA by quantitative PCR. Here, we provide the data for days 6 and 9 post-challenge only. (A) Gel electrophoresis of quantitative PCR done on day 6. (B) Gel electrophoresis of quantitative PCR done on day 9 post-challenge.