Abstract
Enterococcus spp. are opportunistic pathogens that cause lameness in broiler chickens, resulting in serious economic losses worldwide. Virulence of Enterococcus spp. is associated with several putative virulence genes including fsr, efm, esp, cylA, cad1, ace, gelE, and asa1. In this study, multiplex polymerase chain reaction (PCR) for the simultaneous detection of these virulence genes in Enterococcus spp. was developed, and detection limits for E. faecium, E. faecalis, and E. hirae were 64.0 pg/µL, 320.0 pg/µL, and 1.6 ng/µL DNA, respectively. Among 80 Enterococcus isolates tested, efm and cad1 were detected in all 26 E. faecium samples, and only cad1 was observed in E. hirae. Additionally, the presence of virulence genes in 25 E. faecalis isolates were 100% for cad1, 88.0% for gelE, 64.0% for fsr, 44.0% for asa1, 16.0% for cylA, and 4.0% for esp. No virulence genes were found in E. gallinarum isolates. A total of 49 isolates were resistant to tigecycline and to at least 2 different classes of antibiotics. The most prevalent resistance was to ciprofloxacin (73.5%), quinupristin/dalfopristin (55.1%), and tetracycline (49.0%). No strains were resistant to vancomycin or linezolid. This is the first multiplex PCR assay to simultaneously detect eight virulence genes in Enterococcus spp., and the method provides diagnostic value for accurate, rapid, and convenient detection of virulence genes. Additionally, we report the prevalence of virulence genes and antimicrobial resistance in Enterococcus isolates from commercial broiler chickens suffering lameness.
Keywords: Multiplex polymerease chain reaction, virulence genes, antimicrobial drug resistance, Enterococcus spp., broiler chickens
INTRODUCTION
Enterococcus spp. are opportunistic pathogens that inhabit the intestines of many warm-blooded animals, including humans, and have been isolated from several sources such as food, plants, water, and soil [1]. Enterococcus spp. can infect birds through various routes including the respiratory system, gastrointestinal tract, and integument, particularly in poultry environments where birds are hatched, reared, or processed. After infection, bacteria circulate in the bloodstream and form abscesses that cause arthritis, osteomyelitis, spondylitis, and femoral head necrosis in broiler and broiler breeder flocks [2-4]. Virulence of Enterococcus spp. is associated with several genes including fsr (regulator of gelE expression), efm (E. faecium-specific cell wall adhesin), esp (enterococcal surface protein), cylA (hemolysin), cad1 (pheromone cAD1 precursor lipoprotein), ace (collagen-binding cell wall protein), gelE (gelatinase), asa1 (aggregation substance), acm (surface-exposed antigen), cpd1 (pheromone cPD1 lipoprotein), efaAEfs (endocarditis-specific antigen), and sagA (secreted antigen) [5,6]. These virulence factors are involved in attachment to target organs, colonization, invasion of host tissues, diminished host immune function, and the production of enzymes that increase the severity of infection and regulate extracellular production of toxins [7-9].
The use of antimicrobials to enhance the production of animals for human consumption of meat and eggs is leading to increased antimicrobial resistance worldwide. Enterococcus spp. acquire antibiotic resistance via transfer of plasmids and transposons, chromosomal exchange, and spontaneous mutations [6]. Consequently, the use of antibiotics for animal growth promotion exposes humans to antibiotic-resistant pathogens through food and contaminates the environment, which facilitates spreading.
Herein, we developed a multiplex polymerase chain reaction (PCR) method for simultaneous detection of 8 virulence genes in Enterococcus spp. and investigated potential virulence factors and antimicrobial resistance using E. faecium, E. faecalis, E. hirae, and E. gallinarum isolates from broiler chickens suffering lameness in 2 commercial poultry farms.
MATERIALS AND METHODS
Isolation of bacteria
Enterococcus spp. were isolated from various samples of 13 different flocks suffering from lameness in 2 commercial poultry farms over a period of 7 weeks in 2016. Broilers with lameness varied in age from 2 to 15 days. Swabs from liver, femur, and joint tissue were inoculated onto Enterococcosel agar and blood agar plates (BD Diagnostics, Germany) and cultivated at 37°C for 18–24 h. After the initial growth period, dark brown colonies on selective Enterococcosel agar medium were presumptively characterized as Enterococcus spp. and subcultured in brain heart infusion (BHI) agar for Enterococcus species-specific identification using 16s rRNA sequencing analysis and conventional biochemical testing, including Gram staining and Vitek2.
DNA extraction
Total genomic DNA was isolated from 80 different bacterial species using a QIAamp DNA mini kit (Qiagen, Germany) according to the manufacturer's instructions.
Oligonucleotide primer design
Specific primers were designed based on published fsr (GenBank accession No. JN246675), efm (GenBank accession No. AF097414), esp (GenBank accession No. AH013271 and JF826520), cylA (GenBank accession No. JQ794947 and KY613925), cad1 (GenBank accession No. AF421355, NC017960, CP003504, and AOSM01000007), ace (GenBank accession No. AF159247 and KY613927), gelE (GenBank accession No D85393, KT598464, EU423275, and KY613931), and asa1 (GenBank accession No. KT598461, KY613929, and U91527) gene sequences using OligoAnalyzer (Integrated DNA Technologies, USA). Primer specificity was confirmed by BLAST searches against the National Center for Biotechnology Information (NCBI) database (https://www.ncbi.nlm.nih.gov/). The eight pairs of primers and amplicon sizes are listed in Table 1.
Table 1. Sequences of oligonucleotide primers used to detect putative virulence genes in Enterococcus isolates.
| Virulence gene | Gene description | Primer | Sequence (5′ to 3′) | Amplicon size (bp) |
|---|---|---|---|---|
| fsr | Regulator of gelE and sprE expression | Forward primer | CAA GGC ACT ATT TCT TAC TTA GG | 1,016 |
| Reverse primer | AGC GCA TAA ATC AAC CAA G | |||
| efm | E. faecium-specific cell wall adhesin | Forward primer | GAA AAG TTG TCA GTC GTG G | 818 |
| Reverse primer | TGT TTG TGA CAA ACC TTC ATG | |||
| esp | Enterococcal surface protein | Forward primer | CAT CTT TGA TTC TTG GTT GTC G | 695 |
| Reverse primer | GTT ATA GGT ACG TAT GTT GCA TCA | |||
| cylA | Hemolysin | Forward primer | GAG TTA GAT GAA TAT GGT CAT GGT | 521 |
| Reverse primer | AGA AAC TAG CGA TGT AGG GTA ATA | |||
| cad1 | Pheromone cAD1 precursor lipoprotein | Forward primer | TTC CAA AAC TAC GCA CAA CA | 423 |
| Reverse primer | CTT TTT CAG CAG CAT TCA CTA ATT | |||
| ace | Collagen-binding cell wall protein | Forward primer | ATA GAA ACG GAT TTC GGA ACA G | 298 |
| Reverse primer | TCA AAC TCG GCA AGT GAA ATA T | |||
| gelE | Gelatinase | Forward primer | TAT GAC AAT GCT TTT TGG GAT G | 208 |
| Reverse primer | GCA CCC GAA ATA ATA TAA CCC | |||
| asa1 | Aggregation substance | Forward primer | AAC AAG CTT GGT CTG TGT ATC | 168 |
| Reverse primer | TCT TCC CCT TTC TTG TTA TGA AC |
Multiplex PCR
PCR assays (20 µL) were conducted using PCR premix (iNtRON Biotechnology, Seongnam, Korea). Each reaction mixture contained 2 µL bacterial genomic DNA, 0.1 µM of each primer for fsr, efm, esp, cylA, gelE, and asa1, 0.16 µM of each primer for cad1 and ace, 2.5 mM of each dNTP, 1.5 mM MgCl2, 10× reaction buffer, and 1 U Taq polymerase. The thermal cycler (Eppendorf, Hamburg, Germany) was programmed for initial denaturation at 94°C for 5 min, followed by 35 cycles of 94°C for 1 min, 55°C for 1 min, and 72°C for 1 min, and then final elongation at 72°C for 7 min. PCR products were analyzed under UV light after separation by 1.5% agarose gel electrophoresis.
Determining the multiplex PCR detection limit
The detection limit of multiplex PCR assays was determined by testing 5-fold serial dilutions of bacterial DNA of E. faecalis (field isolate) containing virulence genes fsr, esp, cylA, cad1, ace, gelE, and asa1. Similarly, the sensitivity for detecting the pheromone cAD1 precursor lipoprotein gene and E. faecium-specific cell wall adhesin gene was tested using E. hirae (field isolate) possessing the cAD1 gene and E. faecium (field isolate) harboring efm and cAD1 genes, respectively.
Sequencing method
To confirm the exact virulence genes, all PCR products amplified by multiplex and simplex PCR were purified using a QIAquick Gel Extraction Kit (Qiagen) and sequencing was performed by Cosmogenetech Co. (Korea). Nucleotide sequences were analyzed by BLAST searching against the NCBI database.
Screening of virulence genes in Enterococcus spp.
Multiplex PCR was used to analyze eight virulence genes in 26 E. faecalis, 25 E. faecium, 20 E. hirae, and 9 E. gallinarum isolates from liver, femur and joint tissues of broiler chickens suffering lameness.
Antibiotic susceptibility testing
The 16 antimicrobial minimal inhibitory concentrations (MICs) of Enterococcus isolates were determined using the Sensititre automated system (Trek Diagnostic Systems, USA) according to the guidelines of the Clinical and Laboratory Standards Institute (CLSI, 2013). Ampicillin, chloramphenicol, ciprofloxacin, daptomycin, erythromycin, florfenicol, gentamicin, kanamycin, linezolid, quinupristin-dalfopristin, salinomycin, streptomycin, tetracycline, tigecycline, tylosin, and vancomycin antibiotics were tested with Sensititre plates. E. faecalis ATCC 29212 and S. aureus ATCC 25923 strains were used as quality controls.
RESULTS
Development of multiplex PCR
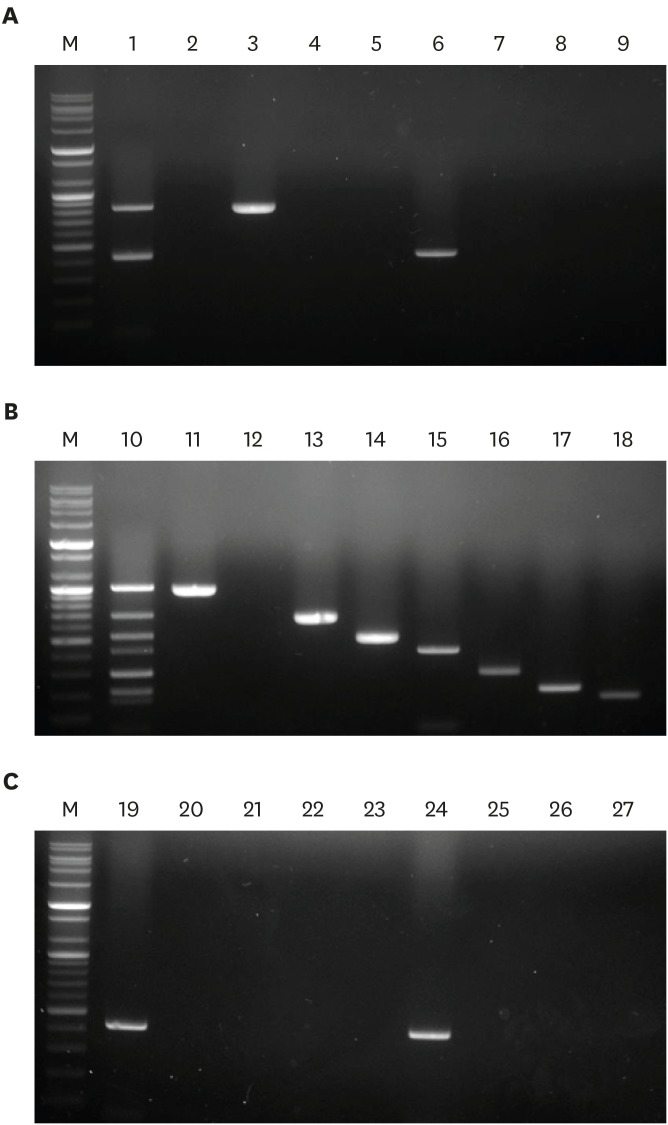
Multiplex PCR assays were conducted using various concentrations of each primer pair to optimize the PCR conditions. Increasing the primer concentrations for cad1 and ace genes facilitated the production of the expected PCR products at low DNA template concentrations. Multiplex PCR mixtures containing efm and cAD1 genes in E. faecium genomic DNA generated 2 bands on 1.5% agarose gels: an 818 bp band for efm and a 423 bp band for cad1 (Fig. 1A, lane 1). Samples containing fsr, esp, cylA, cad1, ace, gelE, and asa1 genes in E. faecalis genomic DNA yielded 7 bands: 1,016 bp for fsr, 695 bp for esp, 521 bp for cylA, 423 bp for cad1, 298 bp for ace, 208 bp for gelE, and 168 bp for asa1 (Fig. 1B, lane 10). Similarly, a multiplex PCR mixture containing only E. hirae genomic DNA amplified the cad1 gene (Fig. 1A, lane 19).
Fig. 1. Validation of eight primer pairs by simplex and multiplex PCR assays. To confirm that primer pairs correctly amplified their respective regions they were evaluated in simplex and multiplex PCR assays using (A) E. faecium, (B) E. faecalis, and (C) E. hirae genomic DNA isolated from broiler chickens suffering lameness. Lane M, 100 bp DNA markers; lane 1, multiplex PCR with E. faecium genomic DNA; lane 10, multiplex PCR with E. faecalis genomic DNA; lane 19, multiplex PCR with E. hirae genomic DNA; lanes 2, 11, and 20, simplex PCR with only fsr primers; lanes 3, 12, and 21, simplex PCR with only efm primers; lanes 4, 13, and 22, simplex PCR with only esp primers; lanes 5, 14, and 23, simplex PCR with only cylA primers; lanes 6, 15, and 24, simplex PCR with only cad1 primers; lanes 7, 16, and 25, simplex PCR with only ace primers; lanes 8, 17, and 26, simplex PCR with only gelE primers; lanes 9, 18, and 27, simplex PCR with only asa1 primers.
PCR, polymerase chain reaction.
Sequencing of PCR products
The eight amplicons were successfully amplified in eight single PCRs containing each gene-specific primer pair, as indicated by the expected PCR products on 1.5% agarose gels (Fig. 1A, lanes 2–8; 1B, lanes 11–18; and 1C, lanes 20–27). All PCR products amplified in multiplex and single PCRs were confirmed by DNA sequencing, and sequence analysis showed that both multiplex and single PCR sequences were identical in the amplified regions (data not shown).
Detection limit of multiplex PCR
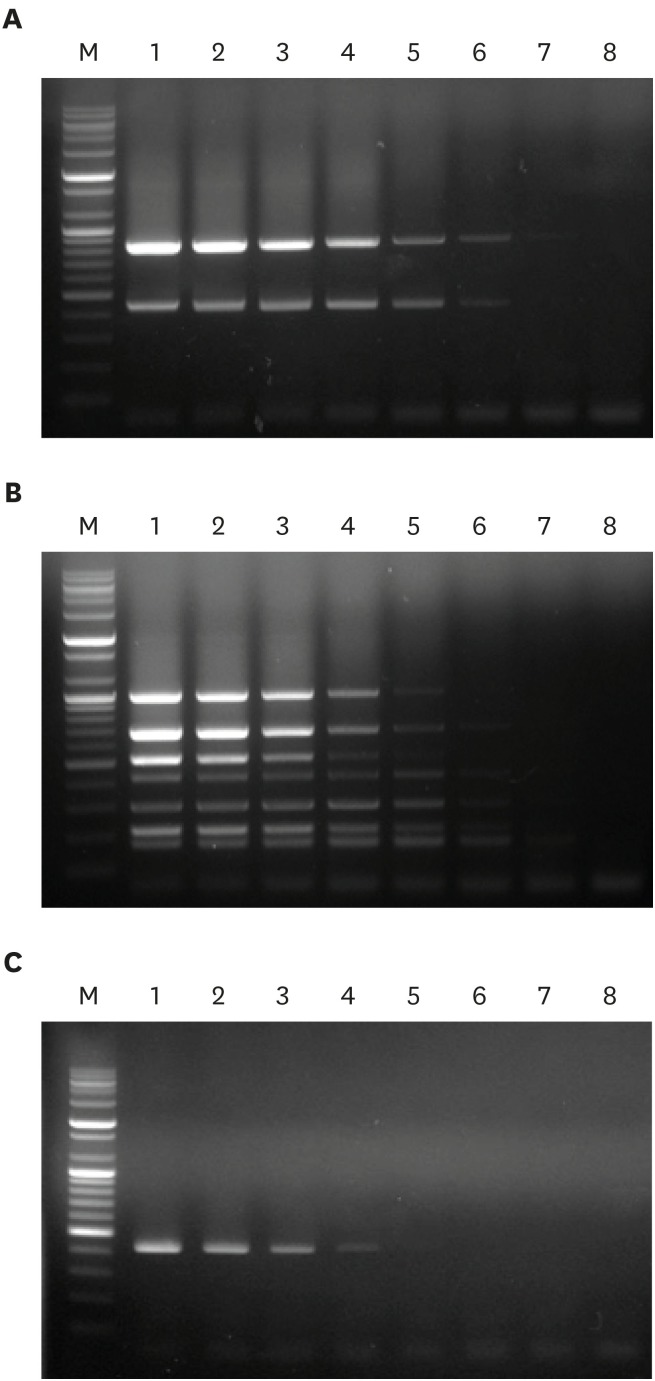
To examine the detection limit of optimized multiplex PCR, genomic DNA from E. faecium (containing efm and cAD1 genes), E. faecalis (containing fsr, esp, cylA, cad1, ace, gelE, and asa1 genes), and E. hirae (containing the cad1 gene) was prepared at DNA concentrations ranging from 12.8 pg to 200 ng. Multiplex PCR using the eight gene-specific primer pairs achieved detection limits of 64.0 pg/µL, 320.0 pg/µL, and 1.6 ng/µL DNA for E. faecium, E. faecalis, and E. hirae, respectively (Fig. 2).
Fig. 2. Evaluation of multiplex PCR detection limits using genomic DNA from (A) E. faecalis (harboring fsr, esp, cylA, cad1, ace, gelE, and asa1), (B) E. faecium (harboring efm and cad1), and (C) E. hirae (harboring cad1). Lane M, 100 bp DNA markers; lane 1, 200.0 ng/µL DNA; lane 2, 40.0 ng/µL DNA; lane 3, 8.0 ng/µL DNA; lane 4, 1.6 ng/µL DNA; lane 5, 320.0 pg/µL DNA; lane 6, 64.0 pg/µL DNA; lane 7, 12.8 pg/µL DNA; lane 8, negative control.
PCR, polymerase chain reaction.
Screening of virulence genes in Enterococcus spp.
Optimized multiplex PCR was conducted to confirm the presence of the 8 virulence genes (fsr, efm, esp, cylA, cad1, ace, gelE, and asa1) in 80 Enterococcus strains isolated from clinical samples. Virulence gene profiles for E. faecium, E. faecalis, E. hirae, and E. gallinarum isolates are shown in Table 2. All 26 E. faecium isolates were positive for the E. faecium-specific cell wall adhesin gene efm and the pheromone cAD1 precursor lipoprotein gene cAD1. In contrast, the fsr, esp, cylA, ace, gelE, and asa1 genes were not detected. Among the 25 E. faecalis isolates, positive results were obtained for 16 (64.0%) isolates for fsr, 1 (4.0%) for esp, 4 (16.0%) for cylA, 22 (88.0%) for gelE, and 11 (44.0%) for asa1. Additionally, cAD1 and ace genes were positive in all E. faecalis isolates. Although all E. hirae isolates were positive for cAD1, no other virulence genes were found in E. hirae isolates. All 8 virulence factors were negative in 9 E. gallinarum isolates (Table 2). No significant difference in the prevalence of fsr, efm, esp, cylA, cad1, ace, gelE, or asa1 was observed between isolates from farms A or B.
Table 2. Prevalence of virulence genes in 80 enterococcal isolates from liver, joint, and femur samples from commercial broiler chickens suffering lameness.
| Species | Farm | Origin | Number of virulence genes (%) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| fsr | efm | esp | cylA | cad1 | ace | gelE | asa1 | |||
| E. faecium (n = 26) | A | Liver (n = 3) | 0 (0.0) | 3 (100.0) | 0 (0.0) | 0 (0.0) | 3 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Joint (n = 7) | 0 (0.0) | 7 (100.0) | 0 (0.0) | 0 (0.0) | 7 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Femur (n = 5) | 0 (0.0) | 5 (100.0) | 0 (0.0) | 0 (0.0) | 5 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| B | Liver (n = 3) | 0 (0.0) | 3 (100.0) | 0 (0.0) | 0 (0.0) | 3 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Joint (n = 4) | 0 (0.0) | 4 (100.0) | 0 (0.0) | 0 (0.0) | 4 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Femur (n = 4) | 0 (0.0) | 4 (100.0) | 0 (0.0) | 0 (0.0) | 4 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Total | 0 (0.0) | 26 (100.0) | 0 (0.0) | 0 (0.0) | 26 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| E. faecalis (n = 25) | A | Liver (n = 1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (100.0) | 1 (100.0) | 1 (100.0) | 0 (0.0) |
| Joint (n = 6) | 4 (66.7) | 0 (0.0) | 1 (16.7) | 2 (33.3) | 6 (100.0) | 6 (100.0) | 6 (100.0) | 3 (50.0) | ||
| Femur (n = 5) | 4 (80.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (100.0) | 5 (100.0) | 5 (100.0) | 4 (80.0) | ||
| B | Liver (n = 5) | 2 (40.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 5 (100.0) | 5 (100.0) | 2 (40.0) | 1 (20.0) | |
| Joint (n = 6) | 4 (66.7) | 0 (0.0) | 0 (0.0) | 2 (33.3) | 6 (100.0) | 6 (100.0) | 6 (100.0) | 3 (50.0) | ||
| Femur (n = 2) | 2 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (100.0) | 2 (100.0) | 2 (100.0) | 0 (0.0) | ||
| Total | 16 (64.0) | 0 (0.0) | 1 (4.0) | 4 (16.0) | 25 (100.0) | 25 (100.0) | 22 (88.0) | 11 (44.0) | ||
| E. hirae (n = 20) | A | Liver (n = 7) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 7 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Joint (n = 3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Femur (n = 8) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 8 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| B | Liver (n = 0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Joint (n = 2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Femur (n = 0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Total | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 20 (100.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| E. gallinarum (n = 9) | A | Liver (n = 0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Joint (n = 3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Femur (n = 0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| B | Liver (n = 3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Joint (n = 2) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Femur (n = 1) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
| Total | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | ||
Antibiotic susceptibility
The MICs for 22 E. faecium, 22 E. faecalis, 3 E. hirae, and 2 E. gallinarum isolates were tested, and resistance breakpoints are presented in Table 3. All 49 isolates were resistant to tigecycline, while none were resistant to linezolid or vancomycin. Among the 16 antimicrobial agents tested, resistance to daptomycin (77.3% in E. faecium and 100% in E. gallinarum), erythromycin (50% in E. faecalis and 66.7% in E. hirae), ciprofloxacin (100% in E. faecium, 54.6% in E. faecalis, and 66.7% in E. gallinarum), quinupristin-dalfopristin (95.5% in E. faecalis), tetracycline (63.8% in E. faecalis and 100% in E. gallinarum), and tylosin (40.9% in E. faecalis and 66.7% in E. hirae) was most frequent among Enterococcus isolates. In addition, low levels of resistance to chloramphenicol, kanamycin, and streptomycin were observed. Multi-resistance patterns of isolates are shown in Table 4. All Enterococcus isolates were resistant to at least 2 different classes of antimicrobials, with 43 (87.8%) of 49 strains being resistant to 3 or more antimicrobials.
Table 3. Distribution of antimicrobial MICs among 22 E. faecium, 22 E. faecalis, 3 E. hirae, and 2 E. gallinarum isolates from broiler chickens suffering lameness.
| CLSI subclass | Antimicrobials | Species | MIC50 | MIC90 | Resistance (%) | Total resistance (%) | Number of strains with MIC (µg/mL)* | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | 128 | 256 | 512 | 1,024 | 2,048 | > 2,048 | |||||||
| Penicillins | AMP | E. faecium | 1 | 4 | 1 (4.6) | 1 (2.0) | 18 | 1 | 2 | 1 | |||||||||||
| E. faecalis | 1 | 1 | 0 (0.0) | 22 | |||||||||||||||||
| E. hirae | 1 | 2 | 0 (0.0) | 2 | 1 | ||||||||||||||||
| E. gallinarum | 1 | 1 | 0 (0.0) | 2 | |||||||||||||||||
| Phenicols | CHL | E. faecium | 8 | > 32 | 3 (13.6) | 7 (14.3) | 18 | 1 | 3 | ||||||||||||
| E. faecalis | 8 | > 32 | 3 (13.6) | 13 | 6 | 3 | |||||||||||||||
| E. hirae | 8 | > 32 | 1 (33.3) | 2 | 1 | ||||||||||||||||
| E. gallinarum | 8 | 8 | 0 (0.0) | 2 | |||||||||||||||||
| FFN | E. faecium | 4 | > 32 | 3 (13.6) | 4 (8.2) | 18 | 1 | 3 | |||||||||||||
| E. faecalis | 4 | 8 | 1 (4.6) | 17 | 4 | 1 | |||||||||||||||
| E. hirae | 4 | 4 | 0 (0.0) | 3 | |||||||||||||||||
| E. gallinarum | 4 | 4 | 0 (0.0) | 2 | |||||||||||||||||
| Lipopeptides | DAP† | E. faecium | 8 | 16 | 17 (77.3) | 21 (42.9) | 1 | 4 | 14 | 2 | 1 | ||||||||||
| E. faecalis | 2 | 4 | 1 (4.6) | 2 | 11 | 8 | 1 | ||||||||||||||
| E. hirae | 2 | 8 | 1 (33.3) | 1 | 1 | 1 | |||||||||||||||
| E. gallinarum | 8 | 8 | 2 (100) | 2 | |||||||||||||||||
| Macrolides | ERY | E. faecium | 2 | > 64 | 5 (22.7) | 18 (36.7) | 3 | 10 | 4 | 5 | |||||||||||
| E. faecalis | 2 | > 64 | 11 (50.0) | 8 | 3 | 1 | 10 | ||||||||||||||
| E. hirae | 32 | > 64 | 2 (66.7) | 1 | 1 | 1 | |||||||||||||||
| E. gallinarum | 1 | 1 | 0 (0.0) | 2 | |||||||||||||||||
| Aminoglycosides | GEN | E. faecium | 128 | 128 | 1 (4.6) | 3 (6.1) | 20 | 1 | 1 | ||||||||||||
| E. faecalis | 128 | 128 | 1 (4.6) | 21 | 1 | ||||||||||||||||
| E. hirae | 128 | 1,024 | 1 (33.3) | 2 | 1 | ||||||||||||||||
| E. gallinarum | 128 | 128 | 0 (0.0) | 2 | |||||||||||||||||
| KAN | E. faecium | 128 | 1,024 | 3 (13.6) | 10 (20.4) | 15 | 4 | 1 | 2 | ||||||||||||
| E. faecalis | 128 | > 2,048 | 6 (27.3) | 16 | 1 | 5 | |||||||||||||||
| E. hirae | 128 | > 2,048 | 1 (33.3) | 2 | 1 | ||||||||||||||||
| E. gallinarum | 128 | 128 | 0 (0.0) | 2 | |||||||||||||||||
| STR | E. faecium | 128 | 128 | 2 (9.1) | 11 (22.4) | 20 | 1 | 1 | |||||||||||||
| E. faecalis | 128 | > 2,048 | 8 (36.4) | 14 | 8 | ||||||||||||||||
| E. hirae | 128 | > 2,048 | 1 (33.3) | 2 | 1 | ||||||||||||||||
| E. gallinarum | 128 | 128 | 0 (0.0) | 2 | |||||||||||||||||
| Fluoroquinolone | CIP | E. faecium | 4 | > 16 | 22 (100) | 36 (73.5) | 11 | 7 | 4 | ||||||||||||
| E. faecalis | 4 | > 16 | 12 (54.6) | 10 | 2 | 10 | |||||||||||||||
| E. hirae | 16 | > 16 | 2 (66.7) | 1 | 2 | ||||||||||||||||
| E. gallinarum | 1 | 2 | 0 (0.0) | 1 | 1 | ||||||||||||||||
| Oxazolidinones | LZD | E. faecium | 4 | 4 | 0 (0.0) | 0 (0.0) | 7 | 15 | |||||||||||||
| E. faecalis | 2 | 4 | 0 (0.0) | 16 | 6 | ||||||||||||||||
| E. hirae | 2 | 4 | 0 (0.0) | 2 | 1 | ||||||||||||||||
| E. gallinarum | 4 | 4 | 0 (0.0) | 2 | |||||||||||||||||
| Streptogramins | SYN | E. faecium | 2 | 4 | 5 (22.7) | 27 (55.1) | 9 | 8 | 3 | 2 | |||||||||||
| E. faecalis | 8 | 16 | 21 (95.5) | 1 | 2 | 16 | 3 | ||||||||||||||
| E. hirae | 2 | 4 | 1 (33.3) | 2 | 1 | ||||||||||||||||
| E. gallinarum | 2 | 2 | 0 (0.0) | 2 | |||||||||||||||||
| Glycylcyclines | TGC‡ | E. faecium | 1 | 4 | 22 (100.0) | 49 (100.0) | 5 | 14 | 3 | ||||||||||||
| E. faecalis | 2 | 16 | 22 (100.0) | 1 | 7 | 14 | |||||||||||||||
| E. hirae | 0.5 | 4 | 3 (100.0) | 2 | 1 | ||||||||||||||||
| E. gallinarum | 1 | 2 | 2 (100.0) | 2 | |||||||||||||||||
| Tetracyclines | TET | E. faecium | 2 | > 128 | 7 (31.8) | 24 (49.0) | 14 | 1 | 1 | 1 | 5 | ||||||||||
| E. faecalis | 64 | 128 | 14 (63.8) | 8 | 1 | 5 | 8 | ||||||||||||||
| E. hirae | 2 | > 128 | 1 (33.3) | 2 | 1 | ||||||||||||||||
| E. gallinarum | 64 | 64 | 2 (100.0) | 2 | |||||||||||||||||
| TYLT | E. faecium | 4 | > 64 | 5 (22.7) | 16 (32.7) | 9 | 2 | 6 | 5 | ||||||||||||
| E. faecalis | 4 | > 64 | 9 (40.9) | 9 | 4 | 9 | |||||||||||||||
| E. hirae | 64 | > 64 | 2 (66.7) | 1 | 2 | ||||||||||||||||
| E. gallinarum | 2 | 2 | 0 (0.0) | 2 | |||||||||||||||||
| Ionophore coccidiostats | SAL | E. faecium | 2 | 4 | 1 (4.6) | 1 (2.0) | 19 | 1 | 1 | 1 | |||||||||||
| E. faecalis | 2 | 4 | 0 (0.0) | 19 | 3 | ||||||||||||||||
| E. hirae | 4 | 4 | 0 (0.0) | 1 | 2 | ||||||||||||||||
| E. gallinarum | 2 | 2 | 0 (0.0) | 2 | |||||||||||||||||
| Glycopeptides | VAN | E. faecium | 2 | 2 | 0 (0.0) | 0 (0.0) | 21 | 1 | |||||||||||||
| E. faecalis | 2 | 2 | 0 (0.0) | 22 | |||||||||||||||||
| E. hirae | 2 | 2 | 0 (0.0) | 3 | |||||||||||||||||
| E. gallinarum | 8 | 8 | 0 (0.0) | 2 | |||||||||||||||||
MIC, minimal inhibitory concentration; CLSI, Clinical and Laboratory Standards Institute; AMP, ampicillin; CHL, chloramphenicol; FFN, florfenicol; DAP, daptomycin; ERY, erythromycin; GEN, gentamicin; KAN, kanamycin; STR, streptomycin; CIP, ciprofloxacin; LZD, linezolid; SYN, quinupristin/dalfopristin; TGC, tigecycline; TET, tetracycline; TYLT, tylosin; SAL, salinomycin; VAN, vancomycin.
*Solid and dashed lines indicate the breakpoints of intermediate and complete resistance established by CLSI, respectively; †The susceptibility breakpoint has only been established for daptomycin (≤ 4 µg/mL). In this study, isolates with an MIC ≥ 8 µg/mL were classified as resistant; ‡The susceptibility breakpoint has only been established for tigecycline (≤ 0.25 µg/mL). In this study, isolates with an MIC ≥ 0.5 µg/mL were classified as resistant.
Table 4. Distribution of antimicrobial resistance patterns among 49 Enterococcus isolates from liver, joint, and femur samples from broilers suffering lameness.
| Resistance patterns | Number of strains with antimicrobial resistance | Total (%) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| E. faecium | E. faecalis | E. hirae | E. gallinarum | ||||||||||
| Liver | Joint | Femur | Liver | Joint | Femur | Liver | Joint | Femur | Liver | Joint | Femur | ||
| TGC-CIP | 2 | 1 | 6 (12.2) | ||||||||||
| TGC-SYN | 1 | 1 | 6 (12.2) | ||||||||||
| TGC-DAP | 1 | 6 (12.2) | |||||||||||
| TGC-CIP-DAP | 2 | 4 | 3 | 19 (38.8) | |||||||||
| TGC-CIP-KAN | 1 | 19 (38.8) | |||||||||||
| TGC-SYN-TET | 1 | 2 | 2 | 19 (38.8) | |||||||||
| TGC-SYN-CIP | 2 | 19 (38.8) | |||||||||||
| TGC-DAP-TET | 1 | 1 | 19 (38.8) | ||||||||||
| TGC-CIP-DAP-TET | 2 | 7 (14.3) | |||||||||||
| TGC-CIP-DAP-SYN | 1 | 1 | 7 (14.3) | ||||||||||
| TGC-SYN-CIP-TET | 2 | 7 (14.3) | |||||||||||
| TGC-CIP-ERY-TYLT | 1 | 7 (14.3) | |||||||||||
| TGC-SYN-TYLT-CIP-ERY | 1 | 2 (4.1) | |||||||||||
| TGC-SYN-ERY-STR-TET | 1 | 2 (4.1) | |||||||||||
| TGC-CIP-DAP-TET-SYN-ERY | 1 | 3 (6.1) | |||||||||||
| TGC-SYN-CIP-TYLT-ERY-DAP | 1 | 3 (6.1) | |||||||||||
| TGC-SYN-CIP-TYLT-ERY-CHL | 1 | 3 (6.1) | |||||||||||
| TGC-CIP-TET-SYN-ERY-TYLT-STR | 1 | 1 | 6 (12.2) | ||||||||||
| TGC-SYN-TYLT-ERY-STR-TET-KAN | 2 | 6 (12.2) | |||||||||||
| TGC-CIP-STR-ERY-KAN-SYN-TET | 1 | 6 (12.2) | |||||||||||
| TGC-CIP-STR-ERY-KAN-TYLT-CHL | 1 | 6 (12.2) | |||||||||||
| TGC-CIP-DAP-TET-ERY-TYLT-CHL-FFN | 1 | 2 (4.1) | |||||||||||
| TGC-SYN-CIP-TET-STR-ERY-TYLT-KAN | 1 | 2 (4.1) | |||||||||||
| TGC-CIP-DAP-TET-SYN-ERY-TYLT-CHL-FFN-SAL | 1 | 2 (4.1) | |||||||||||
| TGC-CIP-ERY-TYLT-SYN-STR-TET-CHL-KAN-GEN | 1 | 2 (4.1) | |||||||||||
| TGC-SYN-CIP-TET-STR-ERY-TYLT-KAN-CHL-FFN-GEN | 1 | 1 (2.0) | |||||||||||
| TGC-CIP-DAP-TET-SYN-ERY-TYLT-STR-CHL-FFN-KAN-GEN-AMP | 1 | 1 (2.0) | |||||||||||
| Total | 5 | 9 | 8 | 5 | 10 | 7 | 0 | 1 | 2 | 0 | 1 | 1 | 49 |
TGC, tigecycline; CIP, ciprofloxacin; SYN, quinupristin/dalfopristin; DAP, daptomycin; KAN, kanamycin; TET, tetracycline; ERY, erythromycin; TYLT, tylosin; STR, streptomycin; CHL, chloramphenicol; FFN, florfenicol; SAL, salinomycin; GEN, gentamicin; AMP, ampicillin.
DISCUSSION
Many Enterococcus spp. from various sources including humans, animals, birds, and the environment are generally considered harmless, although some can become opportunistic pathogens by acquiring antibiotic resistance and putative virulence genes from other bacteria through horizontal gene transfer [10-15]. Herein, the pathogenicity of isolates was evaluated by screening for the presence of virulence factors, and a multiplex PCR method was developed for the simultaneous detection of 8 enterococcal virulence genes (fsr, efm, esp, cylA, cad1, ace, gelE, and asa1). All primer pairs were validated using simplex PCR before being employed in multiplex PCR and only 1 amplified product of the expected size was observed in each simplex PCR as expected. In addition, we confirmed that each primer pair amplified the correct putative virulence gene by DNA sequencing.
To evaluate the sensitivity of multiplex PCR, DNA isolated from E. faecium harboring efm and cAD1 genes, E. faecalis harboring fsr, esp, cylA, cad1, ace, gelE, and asa1 genes, and E. hirae harboring the cad1 gene was tested at different concentrations. The detection limits of E. faecium, E. faecalis, and E. hirae were 64.0 pg/µL, 320.0 pg/µL, and 1.6 ng/µL DNA, respectively. Moreover, multiplex PCR was carried out using 5 µL heat-treated suspensions from individual colonies as DNA template, and the sensitivity results mirrored the detection limits obtained using genomic DNA isolated with a DNA extraction kit (data not shown). Thus, the multiplex PCR method for the 8 virulence genes was shown to be a reliable and rapid alternative for phenotypic testing and simplex PCR.
Furthermore, the multiplex PCR method was applied to screen 26 E. faecium, 25 E. faecalis, 20 E. hirae, and 9 E. gallinarum isolates from broiler chickens suffering lameness for the presence of these virulence genes, and the results showed that the assay could detect all 8 virulence genes in Enterococcus spp. isolates. Additionally, all 26 E. faecium isolates were positive for efm, whereas all E. faecalis isolates were positive for ace. Among the virulence genes analyzed, the sex pheromone cAD1 precursor lipoprotein gene cad1, which is related to the secretion of signaling molecules for intercellular communication, was detected in all 26 E. faecium, 25 E. faecalis, and 20 E. hirae isolates. Interestingly, aggregation substances encoded by sex pheromone plasmids are known to mediate aggregation between bacterial cells and facilitate transfer of plasmids [16]. Hemolysin secreted by bacteria damages cell membranes and facilitates the infection process [16,17].
The distribution of asa1 and cylA among E. faecalis isolates was 44% and 16%, respectively. The gelE gene is believed to enhance the survivability of Enterococcus spp. in extra-intestinal environments, and this gene was found in 88% of the 25 E. faecalis isolates. Gelatinase activity is known to be co-controlled by gelE and fsr genes, and lack of fsr affects the production of gelatinase [18,19]. The fsr gene product, which hydrolyzes gelatin, casein, hemoglobin, and other bioactive peptides, was detected in 64% of E. faecalis isolates. Among E. faecalis isolates harboring the esp gene, which contributes to enterococcal biofilm formation, resistance to environmental stresses, and adhesion to eukaryotic cells, only 1 of 25 isolates was positive fpr esp. Meanwhile, fsr, esp, cylA, ace, gelE, and asa1 genes were not detected in 26 E. faecium isolates, and fsr, esp, cylA, cad1, ace, gelE, and asa1 genes were detected in 2 of 25 E. faecalis isolates. No virulence factors were found in any of the 9 E. gallinarum isolates.
Enterococcus spp. are widespread, resistant to various antimicrobial agents, and have the ability to rapidly acquire resistance to various antimicrobial agents [5,20]. Herein, all 49 Enterococcus isolates were resistant to tigecycline, followed by ciprofloxacin (73.5%), quinupristin/dalfopristin (55.1%), tetracycline (49.0%), daptomycin (42.9%), erythromycin (36.7%), and tylosin (32.7%). Resistance to linezolid and vancomycin was not observed in any of the E. faecium, E. faecalis, E. hirae, or E. gallinarum isolates. Among the 49 Enterococcus isolates, 22 E. faecium isolates were resistant to ciprofloxacin (100.0%) and daptomycin (77.3%).
Moreover, E. faecalis displayed 95.5%, 63.8%, 54.6%, and 50.0% resistance to high levels of quinupristin/dalfopristin, tetracycline, ciprofloxacin, and erythromycin, respectively. Additionally, 66.7% of E. hirae isolates were resistant to tylosin, ciprofloxacin, and erythromycin. Resistance to daptomycin, tetracycline, and tigecycline was 100% in all E. gallinarum isolates.
Comparison of antimicrobial agents revealed that the frequency of resistance to daptomycin and ciprofloxacin was much higher in E. faecium (77.3% and 100.0%, respectively) than in E. faecalis (4.6% and 54.6%, respectively). Similarly, resistance to erythromycin, streptomycin, quinupristin/dalfopristin, and tetracycline was higher in E. faecalis (50.0%, 36.4%, 95.5%, and 63.8%, respectively) than E. faecium (22.7%, 9.1%, 22.7%, and 31.8%, respectively). Multidrug resistance was observed in the majority of isolates, and the prevalence of multidrug resistance (resistant to at least 3 antimicrobials) was 19/22 (86.4%) for E. faecium, 20/22 (90.9%) for E. faecalis, 2/3 (66.7%) for E. hirae, and 2/2 (100.0%) for E. gallinarum.
To the best of our knowledge, this report is the first to provide detailed antibiotic resistance patterns for Enterococcus spp. isolated from commercial broiler chickens suffering lameness. The developed multiplex PCR method may have diagnostic value for the reliable, cost- and time-effective detection of 8 putative virulence genes in Enterococcus spp. simultaneously using a single assay.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Joh S.
- Investigation: Bae Y, Jeon E.
- Resources: Bae Y.
- Supervision: Kwon Y.
- Validation: Kwon Y.
- Writing - original draft: Song H, Joh S.
References
- 1.Murray BE. The life and times of the Enterococcus. Clin Microbiol Rev. 1990;3:46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kolbjørnsen Ø, David B, Gilhuus M. Bacterial osteomyelitis in a 3-week-old broiler chicken associated with Enterococcus hirae . Vet Pathol. 2011;48:1134–1137. doi: 10.1177/0300985810396513. [DOI] [PubMed] [Google Scholar]
- 3.Stalker MJ, Brash ML, Weisz A, Ouckama RM, Slavic D. Arthritis and osteomyelitis associated with Enterococcus cecorum infection in broiler and broiler breeder chickens in Ontario, Canada. J Vet Diagn Invest. 2010;22:643–645. doi: 10.1177/104063871002200426. [DOI] [PubMed] [Google Scholar]
- 4.Wideman RF., Jr Bacterial chondronecrosis with osteomyelitis and lameness in broilers: a review. Poult Sci. 2016;95:325–344. doi: 10.3382/ps/pev320. [DOI] [PubMed] [Google Scholar]
- 5.Champagne J, Diarra MS, Rempel H, Topp E, Greer CW, Harel J, Masson L. Development of a DNA microarray for enterococcal species, virulence, and antibiotic resistance gene determinations among isolates from poultry. Appl Environ Microbiol. 2011;77:2625–2633. doi: 10.1128/AEM.00263-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Diarra MS, Rempel H, Champagne J, Masson L, Pritchard J, Topp E. Distribution of antimicrobial resistance and virulence genes in Enterococcus spp. and characterization of isolates from broiler chickens. Appl Environ Microbiol. 2010;76:8033–8043. doi: 10.1128/AEM.01545-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Borgmann S, Niklas DM, Klare I, Zabel LT, Buchenau P, Autenrieth IB, Heeg P. Two episodes of vancomycin-resistant Enterococcus faecium outbreaks caused by two genetically different clones in a newborn intensive care unit. Int J Hyg Environ Health. 2004;207:386–389. doi: 10.1078/1438-4639-00304. [DOI] [PubMed] [Google Scholar]
- 8.Jett BD, Huycke MM, Gilmore MS. Virulence of enterococci. Clin Microbiol Rev. 1994;7:462–478. doi: 10.1128/cmr.7.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin X, Singh KV, Weinstock GM, Murray BE. Effects of Enterococcus faecalis fsr genes on production of gelatinase and a serine protease and virulence. Infect Immun. 2000;68:2579–2586. doi: 10.1128/iai.68.5.2579-2586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.An FY, Clewell DB. Identification of the cAD1 sex pheromone precursor in Enterococcus faecalis . J Bacteriol. 2002;184:1880–1887. doi: 10.1128/JB.184.7.1880-1887.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Arias CA, Panesso D, Singh KV, Rice LB, Murray BE. Cotransfer of antibiotic resistance genes and a hylEfm-containing virulence plasmid in Enterococcus faecium . Antimicrob Agents Chemother. 2009;53:4240–4246. doi: 10.1128/AAC.00242-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Bandyopadhyay A, Kozlowicz BK, Haemig HA, Tai A, Hu WS, Dunny GM. Mechanisms of peptide sex pheromone regulation of conjugation in Enterococcus faecalis . MicrobiologyOpen. 2017;6:e00492. doi: 10.1002/mbo3.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coburn PS, Baghdayan AS, Dolan GT, Shankar N. Horizontal transfer of virulence genes encoded on the Enterococcus faecalis pathogenicity island. Mol Microbiol. 2007;63:530–544. doi: 10.1111/j.1365-2958.2006.05520.x. [DOI] [PubMed] [Google Scholar]
- 14.Flannagan SE, Clewell DB. Identification and characterization of genes encoding sex pheromone cAM373 activity in Enterococcus faecalis and Staphylococcus aureus. Mol Microbiol. 2002;44:803–817. doi: 10.1046/j.1365-2958.2002.02922.x. [DOI] [PubMed] [Google Scholar]
- 15.Wirth R. Sex pheromones and gene transfer in Enterococcus faecalis. Res Microbiol. 2000;151:493–496. doi: 10.1016/s0923-2508(00)00163-7. [DOI] [PubMed] [Google Scholar]
- 16.Fisher K, Phillips C. The ecology, epidemiology and virulence of Enterococcus. Microbiology. 2009;155:1749–1757. doi: 10.1099/mic.0.026385-0. [DOI] [PubMed] [Google Scholar]
- 17.Galloway-Peña JR, Bourgogne A, Qin X, Murray BE. Diversity of the fsr-gelE region of the Enterococcus faecalis genome but conservation in strains with partial deletions of the fsr operon. Appl Environ Microbiol. 2011;77:442–451. doi: 10.1128/AEM.00756-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mundy LM, Sahm DF, Gilmore M. Relationships between enterococcal virulence and antimicrobial resistance. Clin Microbiol Rev. 2000;13:513–522. doi: 10.1128/cmr.13.4.513-522.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Waters CM, Antiporta MH, Murray BE, Dunny GM. Role of the Enterococcus faecalis GelE protease in determination of cellular chain length, supernatant pheromone levels, and degradation of fibrin and misfolded surface proteins. J Bacteriol. 2003;185:3613–3623. doi: 10.1128/JB.185.12.3613-3623.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barlow RS, McMillan KE, Duffy LL, Fegan N, Jordan D, Mellor GE. Antimicrobial resistance status of Enterococcus from Australian cattle populations at slaughter. PLoS One. 2017;12:e0177728. doi: 10.1371/journal.pone.0177728. [DOI] [PMC free article] [PubMed] [Google Scholar]