Abstract
This study aimed to assess the effects of dehydration on echocardiographic indices in healthy cats: specifically, it aimed to assess the effects of volume depletion on diastolic function. Nine experimental cats were subjected to both a dehydration and placebo protocol separated by a 21-day washout period. Echocardiography was performed at baseline and on completion of each protocol. Results were compared between the two protocols. Volume depletion was induced by intravenous administration of furosemide. Volume depletion showed a significant association with increased interventricular septal and left ventricular free wall thickness at end-diastole, decreased left ventricular internal diameter at end-diastole, and left atrial diameter at end-systole. The peak early (E) and late (A) diastolic filling velocities, and the peak early diastolic velocities (E′) were significantly decreased by dehydration. Volume depletion did not affect peak longitudinal strain rate during early diastole, E/A, or E/E′. Volume depletion significantly affected the echocardiographic diastolic indices and conventional echocardiographic parameters in healthy cats.
Keywords: Diastolic, feline, furosemide, hypertrophy
INTRODUCTION
Echocardiography is essential in the diagnosis of hypertrophic cardiomyopathy (HCM) in cats. Two-dimensional (2D) echocardiography is used to diagnose HCM based primarily on the presence of morphological abnormalities of the left ventricle (LV), such as LV hypertrophy and narrowing of the LV lumen. These morphological changes can also be induced by dehydration. This condition is known as pseudohypertrophy [1], which is a mimic of LV hypertrophy. Pseudohypertrophy (i.e., increased thickness of the LV walls without change in the LV mass) has been observed in cats that were volume depleted by administration of furosemide [1]. It can be difficult to distinguish between pseudohypertrophy and HCM based on echocardiographic assessment of morphology alone.
Dehydration is a commonly encountered clinical situation in cats [2,3,4], which can be corrected using fluid therapy. However, fluid therapy can influence the occurrence of congestive heart failure in cats with concurrent HCM. Making an appropriate diagnosis in a cat with a thickened heart is therefore important in deciding on the appropriate fluid regimen.
Diastolic dysfunction is regarded as the main abnormality in HCM [5,6]. Transmitral flow (TMF) assessment, tissue Doppler imaging (TDI), and speckle-tracking echocardiography (STE) have revealed diastolic dysfunction in cats with HCM [7,8,9,10]. Furthermore, impairment of diastolic function has been observed during the asymptomatic stage in which the left atrium is not yet enlarged [7,10,11].
We hypothesized that assessment of the diastolic function using echocardiography would aid in the diagnosis of LV pseudohypertrophy induced by volume depletion. This study aimed to assess the echocardiographic indices, particularly with respect to diastolic function, in healthy cats with hypovolemia.
MATERIALS AND METHODS
Animals
This experimental study protocol was approved by the Azabu University Animal Care and Use Committee (No. 170203-2).
Nine experimental cats were used in this study (4 females, 5 males; age 60 month; weight 2.58–4.49 kg). No cats were undergoing any treatment. In all cats, a complete clinical assessment was performed to exclude systemic diseases; this comprised a physical examination, complete blood count, performance of biochemical blood tests, electrocardiography, echocardiography, and thoracic radiography. There were no abnormal findings in any cats in this study.
The cats were housed in separate cages (1 cat per cage); they were provided commercially available cat food, and had access to water ad libitum.
Experimental protocol
Cats were subjected to both a placebo and a dehydration protocol. Echocardiographic parameters were measured, as well as body weight (BW) and systolic blood pressure (SBP); a blood sample was collected for measurement of the serum total protein (TP), packed cell volume (PCV), and serum angiotensin-converting enzyme (ACE) activity at the start of each intervention. An intravenous (IV) catheter was placed in the cephalic vein through which saline or furosemide could subsequently be administered. All cats were assigned to the dehydration intervention first. Subsequently, a complete clinical assessment was re-performed after a 21-day wash-out period, at which point it was confirmed that baseline conditions had returned to normal and were the same as the baseline conditions prior to the dehydration protocol.
Furosemide (Lasix [20 mg/2 mL], Nichi-Iko Pharmaceutical, Toyama, Japan) was administered via IV at a dose of 2–4 mg/kg/h until a body weight reduction of 7%–10% was reached; if weight reduction was < 7%, furosemide was administered to a cumulative total of 14 mg/kg over a period of 5 h, based on the dose previously reported [1]. If the target BW was achieved, SBP and echocardiographic parameters were measured (Dehydration protocol). BW was determined without emptying the urinary bladder. The cats had free access to water during the experiment. Immediately after the dehydration protocol was completed, isotonic saline (0.9% NaCl, Otsuka Pharmaceutical, Japan) was administered over several hours, in accordance with standard fluid therapy guidelines, until cats had achieved rehydration and BW reached baseline [1].
For each cat, saline was administered using the same volume and at the same intervals as those for furosemide during the dehydration protocol (Placebo protocol), thus ensuring an identical administration regimen for both protocols.
Echocardiographic examination
In this study, echocardiographic images were taken using an ultrasound unit equipped with a 7-MHz transducer (Vivid 7 dimension, GE Medical System, Japan). All echocardiographic examinations were performed by one sonographer (KS). Cats were gently restrained in a lateral recumbent position without sedation or anesthesia. All echocardiographic images were analyzed with commercial software (EchoPAC PC, GE Medical System). Three consecutive measurements were performed and an average of the 3 values was obtained for each parameter.
Aortic diameter (AoD) and left atrial diameter (LAD) at end-systole were assessed by using the right-sided parasternal short-axis view at the level of the aortic valve with an existing 2D method [12,13]. Left ventricular internal diameter (LVIDd), LV free wall thickness (LVFWd), and interventricular septum thickness (IVSd) at end-diastole were assessed by using the right parasternal short-axis view at the level of the chordae tendineae with M-mode images. The heart rate (HR) was also determined on the basis of M-mode images.
Diastolic function was evaluated with a pulse wave, color TDI, and STE. The left apical four-chamber view was used to characterize TMF patterns. Color flow Doppler imaging was applied to facilitate positioning of a cursor in line with mitral inflow. In addition, a sampling gate was positioned in line with this flow at the level of the open mitral valve tips [13]. The peak early (E) and late (A) diastolic filling velocities were measured and used to calculate the E/A ratio.
All color TDI examinations were performed using an existing method [14]. Lateral and septal aspects of the mitral annulus were evaluated with the left 4-chamber apical view. A 2×2-mm sample volume was used without angle correction. Lateral and septal peak early diastolic velocities (Lat E′ and Sept E′, respectively) were measured and used to calculate the E/E′ ratio.
Longitudinal strain rate was measured using STE as previously described [10]. Peak longitudinal strain rate during early diastole (SrLe) was measured with the left apical 4-chamber view in 6 segments: basal, middle, and apical regions of both lateral and septal walls. Average measurements from these 6 ventricular segments were used to calculate the SrLe used for statistical analysis.
Serum ACE activity
Blood samples (approximately 1 mL) were collected in tubes without anticoagulant before and after furosemide or saline administration. All samples were centrifuged to obtain serum (3000 rpm, 10 min). ACE activity was measured with an ACE assay kit (ACE Color, Fujirebio, Japan).
Systolic blood pressure
Non-invasive measurement of SBP was performed with Doppler sphygmomanometry (Hadeco, Japan), using an inflatable cuff on the tail as previously described [15]. Hair was clipped before positioning of the probe, and the cuff was inflated manually such that the pulse signal was not discernible; it was then gradually deflated. In this study, SBP was the pressure at which the Doppler signal became discernible during cuff deflation. Multiple measurements were performed at each time point in a consecutive manner. The mean value of 5 stable measurements was used for statistical analyses.
Statistical analysis
All measurements are expressed as mean ± standard deviation. Statistical analyses were performed using SPSS Statistics version 21.0 (IBM, USA). Cats that showed separation of peak early diastolic velocity from peak atrial systolic velocity on echocardiography in both protocols were used for all statistical analysis.
BW, PCV, SBP, serum ACE activity, conventional echocardiographic indices, E, E′, and SrLe recorded before each protocol were compared with the Wilcoxon signed-rank test to evaluate carry-over effects of furosemide.
To fulfill the primary aim of this study (i.e., evaluating the effects of the interventions), the difference between the baseline value (pre-) and the value recorded following the protocol (post-) was calculated for all measurement variables, and a comparison between the protocols was performed using the Wilcoxon signed-rank test.
To evaluate the effects of intervention, affected indices were detected by comparing values recorded pre- and post-each protocol using the Wilcoxon signed-rank test. Differences were regarded as significant when p < 0.05.
RESULTS
Animals
All protocols were performed within 5 h to 5 h 30 min. Out of the nine cats, six had E and A waves that were suitably separated in both protocols for them to be included in all subsequent analyses. Out of these six cats, there were four females and two males. All cats received a total of 14 mg/kg of furosemide in the dehydration protocol.
BW, PCV, TP, SBP, and serum ACE activity
The results of measurements of BW, PCV, TP and SBP are shown in Table 1. The post-intervention TP measurements were significantly increased with the dehydration protocol compared to placebo. There were no significant differences in other indices pre- compared to post-intervention for either protocol (p > 0.05). SBP was statistically unchanged when compared before and after the Dehydration protocol, although the one cat showed decreased SBP from 167 mmHg to 90 mmHg. During the placebo protocol, pre and post-intervention SBP measurements increased from 140 mmHg to 190 mmHg in one cat, but were not statistically different in the rest of the sample.
Table 1. The results of measurements of BW, SBP, and PCV in both protocols (mean ± SD).
| Index | Dehydration protocol | Placebo protocol | ||
|---|---|---|---|---|
| Baseline | After | Baseline | After | |
| BW (kg) | 3.70 ± 0.57 | 3.49 ± 0.56 | 3.54 ± 0.60 | 3.54 ± 0.60 |
| SBP (mmHg) | 151.3 ± 11.0 | 140.3 ± 26.7 | 144.8 ± 14.4 | 165.2 ± 17.8 |
| PCV (%) | 39.7 ± 9.1 | 46.5 ± 6.2 | 44.3 ± 9.1 | 44.8 ± 9.0 |
| TP (g/dL) | 6.8 ± 0.2 | 9.4 ± 0.6* | 7.0 ± 0.3 | 7.1 ± 0.4 |
BW, body weight; SBP, systolic blood pressure; TP, serum total protein; SD, standard deviation.
*p < 0.01 vs. Placebo protocol.
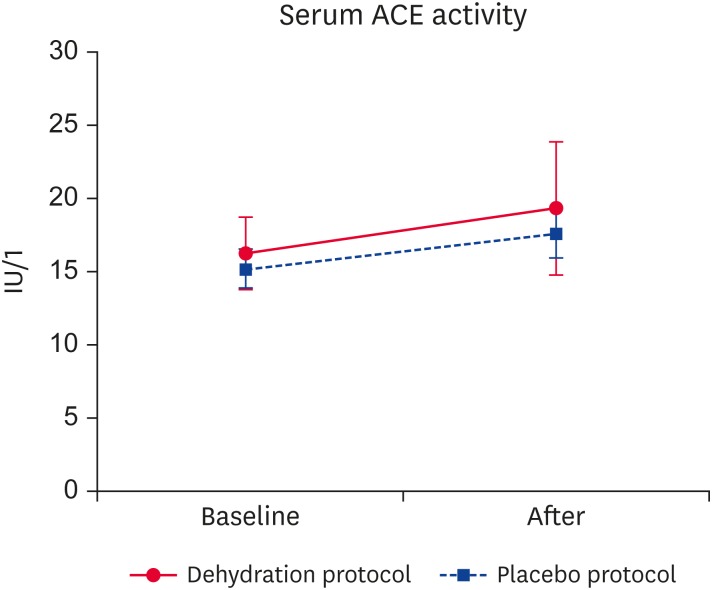
Serum ACE activity measurements are depicted in Fig. 1; there was no significant difference pre- compared to post-intervention for either protocol (p > 0.05).
Fig. 1. Results of serum ACE activity measurements. No significant differences were present between the Dehydration and Placebo protocols.
ACE, angiotensin-converting enzyme.
Echocardiography
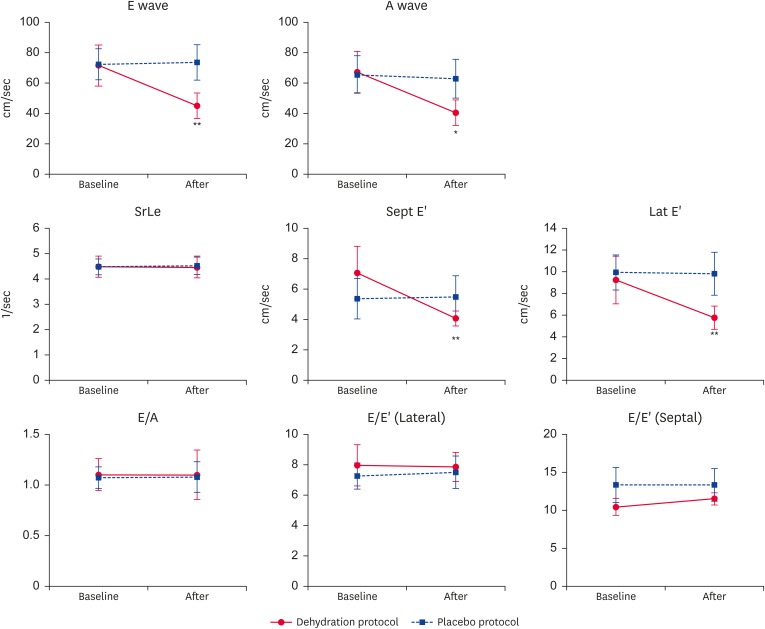
Tables 2 and 3 and Fig. 2 show echocardiographic examination results. No significant differences were observed in echocardiographic indices between the baseline of the two protocols. Administration of furosemide induced significant increases in the LV wall thickness (IVSd and LVFWd) and diminished chamber diameters (LVIDd and LAD). There were 2 cats whose IVSd dimensions exceeded 5.5 mm after furosemide administration. Neither protocol altered the HR or AoD. Volume depletion did not affect SrLe, E/E′ ratio, or E/A ratio; however; the A wave, E wave, Lat E′, and Sept E′ were significantly decreased during the dehydration protocol compared to placebo protocol. During the dehydration protocol, the A wave, E wave, Lat E′, and Sept E′ were significantly decreased post- compared to pre-furosemide administration. There were no significant differences in these indices pre- compared to post-saline administration during the placebo protocol.
Table 2. The results of conventional echocardiographic examination in both protocols (mean ± SD).
| Index | Dehydration protocol | Placebo protocol | ||
|---|---|---|---|---|
| Baseline | After | Baseline | After | |
| IVSd (mm) | 4.12 ± 0.52 | 5.05 ± 0.56* | 3.9 ± 0.74 | 3.5 ± 0.65 |
| LVIDd (mm) | 14.72 ± 1.16 | 12.12 ± 2.26** | 15.73 ± 1.79 | 15.37 ± 2.51 |
| LVFWd (mm) | 4.05 ± 0.79 | 4.52 ± 0.66* | 4.53 ± 1.56 | 3.85 ± 0.67 |
| AoD (mm) | 8.53 ± 0.90 | 8.21 ± 0.65 | 8.42 ± 1.10 | 8.90 ± 0.86 |
| LAD (mm) | 11.72 ± 1.58 | 9.82 ± 0.95* | 10.32 ± 1.04 | 10.57 ± 1.56 |
| HR (bpm) | 186.9 ± 13.9 | 176.6 ± 24.5 | 184.0 ± 20.1 | 182.8 ± 15.2 |
IVSd, interventricular septal thickness at end-diastole; LVIDd, left ventricular internal diameters at end-diastole; LVFWd, left ventricular free wall thickness at end-diastole; AoD, aortic diameter at end-systole; LAD, left atrial diameter at end-systole; HR, heart rate; SD, standard deviation.
*p < 0.05 compared to Placebo protocol; **p < 0.01 compared to Placebo protocol.
Table 3. Echocardiographic diastolic indices in both protocols (mean ± SD).
| Index | Dehydration protocol | Placebo protocol | ||
|---|---|---|---|---|
| Baseline | After | Baseline | After | |
| E wave (cm/sec) | 72.3 ± 10.9 | 44.9 ± 5.1* | 72.4 ± 10.1 | 73.7 ± 11.5 |
| A wave (cm/sec) | 67.2 ± 13.6 | 40.4 ± 8.4** | 65.7 ± 12.3 | 62.8 ± 12.7 |
| E/A | 1.10 ± 0.16 | 1.10 ± 0.24 | 1.08 ± 0.11 | 1.08 ± 0.15 |
| Lat E′ (cm/sec) | 9.28 ± 2.20 | 5.78 ± 0.73* | 9.96 ± 1.62 | 9.83 ± 1.98 |
| Sept E′ (cm/sec) | 7.07 ± 1.75 | 4.07 ± 0.49* | 5.39 ± 1.33 | 5.50 ± 1.39 |
| E/E′ (Lateral) | 7.98 ± 1.36 | 7.88 ± 0.96 | 7.26 ± 0.87 | 7.52 ± 1.07 |
| E/E′ (Septal) | 10.49 ± 1.13 | 11.43 ± 0.79 | 13.37 ± 2.31 | 13.39 ± 2.14 |
| SrLe (1/sec) | 4.49 ± 0.42 | 4.48 ± 0.42 | 4.49 ± 0.31 | 4.53 ± 0.34 |
SD, standard deviation; E wave, peak early diastolic filling velocity; A wave, peak late diastolic filling velocity; Lat E′, lateral peak early diastolic velocity; Sept E′, septal peak early diastolic velocity; SrLe, peak longitudinal strain rate during early diastole.
*p < 0.01 compared to Placebo protocol; **p < 0.05 compared to Placebo protocol.
Fig. 2. Echocardiographic diastolic indices. No significant differences were present in SrLe, E/A ratio, and E/E′ ratio between the Dehydration and Placebo protocols.
SrLe, peak longitudinal strain rate during early diastole; E wave, peak early filling velocity; A wave, late filling velocity; E′, peak early diastolic myocardial velocity at the mitral annulus, Lat E′, lateral peak early diastolic velocity; Sept E′, septal peak early diastolic velocity; SrLe, peak longitudinal strain rate during early diastole.
*p < 0.05 compared to Placebo protocol; **p < 0.01 compared to Placebo protocol.
DISCUSSION
In our model, volume depletion was induced by administration of furosemide, a potent short-acting diuretic. Although BW and PCV were not changed, LVIDd and LAD were significantly decreased in all cats during dehydration compared to the placebo protocol. Thus, the reduction in the dimensions of the LV and LA showed a decreased preload. In addition, the LV wall thickness was significantly increased in all cats during volume depletion, and there were 2 cats whose LV thickness exceeded 5.5 mm (pseudohypertrophy). Since PCV was measured several hours after collecting the blood sample in some cats, this may have led to some technical errors in this measurement. This aside, these results show that volume depletion was successfully induced in this study.
It has previously been reported that E wave, E′, E/A, and SrLe were decreased in cats with HCM and E/E′ was increased in human patients with HCM [7,8,9,10]. We hypothesized that these indices were useful to distinguish pathological hypertrophy due to HCM from pseudohypertrophy. The current study revealed that E/E′, SrLe, and E/A showed no changes after dehydration. However, the E wave and E′ were significantly affected by volume depletion. Therefore E/A, SrLe and E/E′ could be indices that distinguish HCM and pseudohypertrophy.
This study revealed that Lat E′ and Sept E′ were significantly decreased in cats with normal diastolic function who were volume depleted. E′ is generally known as a preload-independent index of diastolic function [16], and several studies have demonstrated that E′ is decreased in cats with HCM [9,14,17,18]. In humans, relatively abrupt changes in preload have been observed after hemodialysis is performed. Tamano et al. [19] demonstrated that E′ did not change after hemodialysis in human patients; however, Ie et al. [20] have reported contrary results that showed E′ was decreased after hemodialysis. Tamano et al. [19] reported that discrepancies in the results were due to differences in the amount of fluid removed from the patients, the basal conditions of the patients, and differences in cardiac function. Diastolic function in our cats was normal at baseline, and volume depletion was acutely induced. Therefore, E′ was possibly affected by the rapid reduction of the preload.
TDI is angle dependent [21], therefore, technical error can be another possible cause of the decreased E′. Reduction of the preload significantly reduced the LV lumen, resulting in changes in the position of the mitral annulus. A previous report stated that the movement of the septal side of the annulus was more parallel to the ultrasonic beam; thus, it showed less effects due to translational movement of the heart, relative to the movements of other regions [22].
The results of TMF assessment in the current study showed that the E and A waves were both significantly decreased during the dehydration protocol, resulting in an unchanged E/A ratio. Several studies have revealed that the E wave is strongly influenced by preload, although the extent of preload reduction influence on the A wave is reportedly inconsistent [23,24,25].
Our group previously reported that STE showed greater sensitivity than TDI for evaluation of left ventricular diastolic function in cats with HCM [10]. One of the diastolic STE parameters, SrLe, did not change during dehydration in the current study. Strain and strain rate during systole are reportedly affected by the preload and afterload [26,27]; however, to the best of our knowledge, the influence of the preload reduction on SrLe is not well known. Fredholm et al. [28] reported that an increased preload or an elevated HR increases the strain rate during diastole. The HR in the current study was not significantly changed; therefore, a further study is warranted to determine the factors that influence the diastolic STE parameters when the preload is changed.
The E/E′ ratio may be a useful tool for evaluation of LV filling pressures because it combines the effects of transmitral driving pressure and myocardial relaxation [29,30,31]. In our study, both the E wave and E′ were significantly decreased, resulting in an unchanged E/E′ ratio. Although the E/E′ ratio was applied to estimate LV filling pressure, we found that it not was affected by preload reduction.
The present study had a number of limitations. First, we used an acute dehydration model. Serum ACE activity and SBP did not significantly change during volume depletion. These results implied that experimentally-induced dehydration may be different from dehydration occurring in cats with chronic diseases. Second, the E wave and the A wave are often summated because of high HR in cats, and this summation was present in three of nine cats in the present study. Third, the sample size was small. Fourth, echocardiographic indices are influenced by many other factors associated with volume depletion. Acute changes in echocardiographic parameters after hemodialysis reportedly may be explained by several mechanisms, including the change in serum ionized calcium concentration, increased oxidative stress during hemodialysis, and sympathetic hyperactivity [30]. Similar changes may have occurred in cats that were administered furosemide. Fifth, echocardiographic examination and analysis were not performed in a blinded manner.
In conclusion, we showed that acute dehydration induced by using furosemide affected the echocardiographic parameters, especially those of the diastolic function, in healthy cats. Volume depletion significantly affected both echocardiographic diastolic indices and conventional echocardiographic parameters. The cats' hydration status should be considered when interpreting echocardiographic parameters.
Footnotes
Conflict of Interest: The authors declare no conflicts of interest.
- Conceptualization: Sugimoto K, Fujii Y.
- Data curation: Sugimoto K, Kawase N.
- Formal analysis: Sugimoto K, Kawase N, Fujii Y.
- Investigation: Sugimoto K, Kawase N.
- Methodology: Sugimoto K, Fujii Y.
- Project administration: Fujii Y.
- Resources: Aoki T, Fujii Y.
- Supervision: Aoki T, Fujii Y.
- Validation: Sugimoto K, Kawase N, Aoki T, Fujii Y.
- Visualization: Sugimoto K, Fujii Y.
- Writing - original draft: Sugimoto K, Fujii Y.
- Writing - review & editing: Sugimoto K, Kawase N, Aoki T, Fujii Y.
References
- 1.Campbell FE, Kittleson MD. The effect of hydration status on the echocardiographic measurements of normal cats. J Vet Intern Med. 2007;21:1008–1015. doi: 10.1892/0891-6640(2007)21[1008:teohso]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 2.Greene JP, Lefebvre SL, Wang M, Yang M, Lund EM, Polzin DJ. Risk factors associated with the development of chronic kidney disease in cats evaluated at primary care veterinary hospitals. J Am Vet Med Assoc. 2014;244:320–327. doi: 10.2460/javma.244.3.320. [DOI] [PubMed] [Google Scholar]
- 3.Sparkes AH, Cannon M, Church D, Fleeman L, Harvey A, Hoenig M, Peterson ME, Reusch CE, Taylor S, Rosenberg D, ISFM ISFM consensus guidelines on the practical management of diabetes mellitus in cats. J Feline Med Surg. 2015;17:235–250. doi: 10.1177/1098612X15571880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tello L, Perez-Freytes R. Fluid and electrolyte therapy during vomiting and diarrhea. Vet Clin North Am Small Anim Pract. 2017;47:505–519. doi: 10.1016/j.cvsm.2016.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fox PR, Liu SK, Maron BJ. Echocardiographic assessment of spontaneously occurring feline hypertrophic cardiomyopathy. An animal model of human disease. Circulation. 1995;92:2645–2651. doi: 10.1161/01.cir.92.9.2645. [DOI] [PubMed] [Google Scholar]
- 6.Kittleson MD, Meurs KM, Munro MJ, Kittleson JA, Liu SK, Pion PD, Towbin JA. Familial hypertrophic cardiomyopathy in maine coon cats: an animal model of human disease. Circulation. 1999;99:3172–3180. doi: 10.1161/01.cir.99.24.3172. [DOI] [PubMed] [Google Scholar]
- 7.Bright JM, Herrtage ME, Schneider JF. Pulsed Doppler assessment of left ventricular diastolic function in normal and cardiomyopathic cats. J Am Anim Hosp Assoc. 1999;35:285–291. doi: 10.5326/15473317-35-4-285. [DOI] [PubMed] [Google Scholar]
- 8.Koffas H, Dukes-McEwan J, Corcoran BM, Moran CM, French A, Sboros V, Simpson K, Anderson T, McDicken WN. Colour M-mode tissue Doppler imaging in healthy cats and cats with hypertrophic cardiomyopathy. J Small Anim Pract. 2008;49:330–338. doi: 10.1111/j.1748-5827.2008.00543.x. [DOI] [PubMed] [Google Scholar]
- 9.Koffas H, Dukes-McEwan J, Corcoran BM, Moran CM, French A, Sboros V, Simpson K, McDicken WN. Pulsed tissue Doppler imaging in normal cats and cats with hypertrophic cardiomyopathy. J Vet Intern Med. 2006;20:65–77. doi: 10.1892/0891-6640(2006)20[65:ptdiin]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 10.Sugimoto K, Fujii Y, Sunahara H, Aoki T. Assessment of left ventricular longitudinal function in cats with subclinical hypertrophic cardiomyopathy using tissue Doppler imaging and speckle tracking echocardiography. J Vet Med Sci. 2015;77:1101–1108. doi: 10.1292/jvms.14-0354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Golden AL, Bright JM. Use of relaxation half-time as an index of ventricular relaxation in clinically normal cats and cats with hypertrophic cardiomyopathy. Am J Vet Res. 1990;51:1352–1356. [PubMed] [Google Scholar]
- 12.Chetboul V, Concordet D, Pouchelon JL, Athanassiadis N, Muller C, Benigni L, Munari AC, Lefebvre HP. Effects of inter- and intra-observer variability on echocardiographic measurements in awake cats. J Vet Med A Physiol Pathol Clin Med. 2003;50:326–331. doi: 10.1046/j.1439-0442.2003.00546.x. [DOI] [PubMed] [Google Scholar]
- 13.Simpson KE, Devine BC, Gunn-Moore DA, French AT, Dukes-McEwan J, Koffas H, Moran CM, Corcoran BM. Assessment of the repeatability of feline echocardiography using conventional echocardiography and spectral pulse-wave Doppler tissue imaging techniques. Vet Radiol Ultrasound. 2007;48:58–68. doi: 10.1111/j.1740-8261.2007.00205.x. [DOI] [PubMed] [Google Scholar]
- 14.Granström S, Pipper CB, Møgelvang R, Sogaard P, Willesen JL, Koch J. Effect of sample volume size and sampling method on feline longitudinal myocardial velocity profiles from color tissue Doppler imaging. J Vet Cardiol. 2012;14:479–488. doi: 10.1016/j.jvc.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Brown S, Atkins C, Bagley R, Carr A, Cowgill L, Davidson M, Egner B, Elliott J, Henik R, Labato M, Littman M, Polzin D, Ross L, Snyder P, Stepien R American College of Veterinary Internal Medicine. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med. 2007;21:542–558. doi: 10.1892/0891-6640(2007)21[542:gftiea]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 16.Sohn DW, Chai IH, Lee DJ, Kim HC, Kim HS, Oh BH, Lee MM, Park YB, Choi YS, Seo JD, Lee YW. Assessment of mitral annulus velocity by Doppler tissue imaging in the evaluation of left ventricular diastolic function. J Am Coll Cardiol. 1997;30:474–480. doi: 10.1016/s0735-1097(97)88335-0. [DOI] [PubMed] [Google Scholar]
- 17.Carlos Sampedrano C, Chetboul V, Gouni V, Nicolle AP, Pouchelon JL, Tissier R. Systolic and diastolic myocardial dysfunction in cats with hypertrophic cardiomyopathy or systemic hypertension. J Vet Intern Med. 2006;20:1106–1115. doi: 10.1892/0891-6640(2006)20[1106:sadmdi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 18.Simpson KE, Gunn-Moore DA, Shaw DJ, French AT, Dukes-McEwan J, Moran CM, Corcoran BM. Pulsed-wave Doppler tissue imaging velocities in normal geriatric cats and geriatric cats with primary or systemic diseases linked to specific cardiomyopathies in humans, and the influence of age and heart rate upon these velocities. J Feline Med Surg. 2009;11:293–304. doi: 10.1016/j.jfms.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tamano K, Kobayashi T, Takahashi M, Honda T, Uetake S, Mitobe R, Futoo Y, Matsuoka H. Effect of hemodialysis on peak velocity of early diastolic mitral annulus motion. J Cardiol. 2004;44:147–152. [PubMed] [Google Scholar]
- 20.Ie EH, Vletter WB, ten Cate FJ, Nette RW, Weimar W, Roelandt JR, Zietse R. Preload dependence of new Doppler techniques limits their utility for left ventricular diastolic function assessment in hemodialysis patients. J Am Soc Nephrol. 2003;14:1858–1862. doi: 10.1097/01.asn.0000072745.94551.fc. [DOI] [PubMed] [Google Scholar]
- 21.van Dalen BM, Bosch JG, Kauer F, Soliman OI, Vletter WB, ten Cate FJ, Geleijnse ML. Assessment of mitral annular velocities by speckle tracking echocardiography versus tissue Doppler imaging: validation, feasibility, and reproducibility. J Am Soc Echocardiogr. 2009;22:1302–1308. doi: 10.1016/j.echo.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 22.Dincer I, Kumbasar D, Nergisoglu G, Atmaca Y, Kutlay S, Akyurek O, Sayin T, Erol C, Oral D. Assessment of left ventricular diastolic function with Doppler tissue imaging: effects of preload and place of measurements. Int J Cardiovasc Imaging. 2002;18:155–160. doi: 10.1023/a:1014697208218. [DOI] [PubMed] [Google Scholar]
- 23.Choong CY, Herrmann HC, Weyman AE, Fifer MA. Preload dependence of Doppler-derived indexes of left ventricular diastolic function in humans. J Am Coll Cardiol. 1987;10:800–808. doi: 10.1016/s0735-1097(87)80273-5. [DOI] [PubMed] [Google Scholar]
- 24.Courtois M, Vered Z, Barzilai B, Ricciotti NA, Pérez JE, Ludbrook PA. The transmitral pressure-flow velocity relation. Effect of abrupt preload reduction. Circulation. 1988;78:1459–1468. doi: 10.1161/01.cir.78.6.1459. [DOI] [PubMed] [Google Scholar]
- 25.Sztajzel J, Ruedin P, Monin C, Stoermann C, Leski M, Rutishauser W, Lerch R. Effect of altered loading conditions during haemodialysis on left ventricular filling pattern. Eur Heart J. 1993;14:655–661. doi: 10.1093/eurheartj/14.5.655. [DOI] [PubMed] [Google Scholar]
- 26.Abali G, Tokgözoğlu L, Ozcebe OI, Aytemir K, Nazli N. Which Doppler parameters are load independent? A study in normal volunteers after blood donation. J Am Soc Echocardiogr. 2005;18:1260–1265. doi: 10.1016/j.echo.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 27.Burns AT, La Gerche A, D'hooge J, MacIsaac AI, Prior DL. Left ventricular strain and strain rate: characterization of the effect of load in human subjects. Eur J Echocardiogr. 2010;11:283–289. doi: 10.1093/ejechocard/jep214. [DOI] [PubMed] [Google Scholar]
- 28.Fredholm M, Jörgensen K, Houltz E, Ricksten SE. Load-dependence of myocardial deformation variables - a clinical strain-echocardiographic study. Acta Anaesthesiol Scand. 2017;61:1155–1165. doi: 10.1111/aas.12954. [DOI] [PubMed] [Google Scholar]
- 29.Graham RJ, Gelman JS, Donelan L, Mottram PM, Peverill RE. Effect of preload reduction by haemodialysis on new indices of diastolic function. Clin Sci (Lond) 2003;105:499–506. doi: 10.1042/CS20030059. [DOI] [PubMed] [Google Scholar]
- 30.Hayashi SY, Brodin LA, Alvestrand A, Lind B, Stenvinkel P, Mazza do Nascimento M, Qureshi AR, Saha S, Lindholm B, Seeberger A. Improvement of cardiac function after haemodialysis. Quantitative evaluation by colour tissue velocity imaging. Nephrol Dial Transplant. 2004;19:1497–1506. doi: 10.1093/ndt/gfh205. [DOI] [PubMed] [Google Scholar]
- 31.Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: a comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]