Abstract
Quiescent stem cells in adult tissues can be activated for homeostasis or repair. Neural stem cells (NSCs) in Drosophila are reactivated from quiescence in response to nutrition by the insulin signalling pathway. It is widely accepted that quiescent stem cells are arrested in G0. In this study, however, we demonstrate that quiescent NSCs (qNSCs) are arrested in either G2 or G0. G2/G0 heterogeneity directs NSC behavior: G2 qNSCs reactivate before G0 qNSCs. In addition, we show that the evolutionarily conserved pseudokinase Tribbles (Trbl) induces G2 NSCs to enter quiescence by promoting degradation of Cdc25String and subsequently maintains quiescence by inhibiting Akt activation. Insulin signalling overrides repression of Akt and silences trbl transcription, allowing NSCs to exit quiescence. Our results have implications for identifying and manipulating quiescent stem cells for regenerative purposes.
As in mammals, NSCs in Drosophila proliferate during embryogenesis, become quiescent in the late embryo and then proliferate again (reactivate) post-embryonically to produce neurons and glia (Fig. 1A) (1, 2). A nutritional stimulus induces reactivation (3); specifically, dietary amino acids induce glial cells in the blood brain barrier to secrete insulin/IGF-like peptides (dILPs) (4, 5). dILPs activate the insulin signalling pathway in neighboring qNSCs, prompting them to exit quiescence (4, 6).
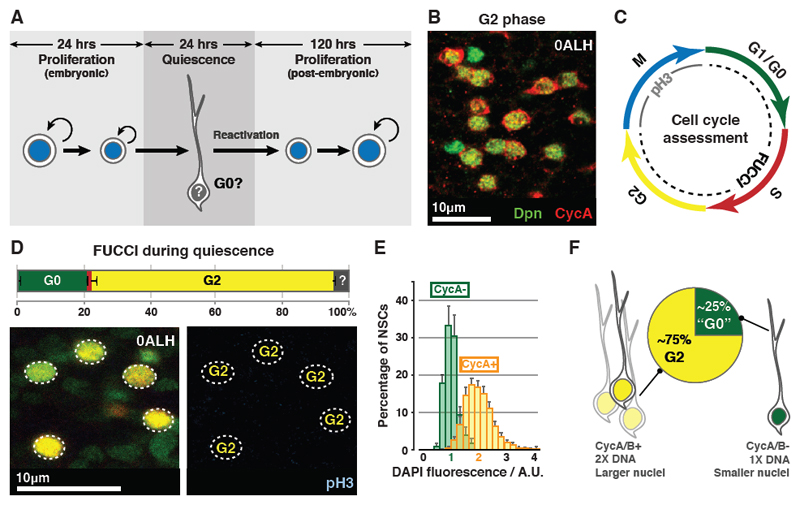
Fig. 1. qNSCs arrest in G0 or G2.
(A) qNSCs are smaller than proliferating NSCs and extend a primary process, which is retracted upon activation from quiescence. Proliferating NSCs in the embryo do not exhibit a primary process prior to entering quiescence.
(B) 73±0.79% of qNSCs (green) are CycA+ (red). n=10 tVNCs, ~150 NSCs each.
(C) Cell cycle phase assessment using FUCCI and pH3.
(D) Percentages of NSCs (outlined) in each cell cycle phase during quiescence. Colours as in (C). n=5 tVNCs, ~150 NSCs each. ?: undetermined.
(E) DAPI intensities of CycA+ and CycA- qNSC nuclei are significantly different (p=2.20x10-16, Kolgomorov-Smirnov test). n=10 tVNCs, ~75 NSCs each. A.U.: arbitrary units. 1 A.U.: mean DAPI intensity of the CycA- population. Error bars indicate S.E.M.
(F) Features of G2 and G0 qNSCs.
Images are single section confocal images, unless indicated otherwise, and anterior is up in this and all subsequent figures.
Quiescent stem cells are widely accepted to be arrested in G0, a poorly-understood state characterised by a 2n DNA content and a lack of expression of cell cycle progression factors (7). We assessed whether Drosophila qNSCs are G0-arrested (Fig. S1A). As expected, we did not detect the M phase marker phospho-Histone H3 (pH3) in qNSCs (Fig. S1B). Previous studies demonstrated that qNSCs do not express the G1 marker Cyclin E, or incorporate the S phase markers BrdU/EdU (1, 3, 6, 8). However, we found that 73% of quiescent NSCs express the G2 markers Cyclin A (CycA) and Cyclin B (CycB) (Figs. 1B, S1C). This suggests that (i) most qNSCs arrest in G2 and that (ii) qNSCs arrest heterogeneously in the cell cycle.
We verified that ~75% of qNSCs are G2-arrested by comparing the FUCCI/pH3 profiles of qNSCs and proliferating NSCs (Figs. 1C-D, S1D) (9, 10). CycA+ qNSCs had twice the DNA content (Fig. 1E) and larger nuclei (30.5±0.66µm3 vs 18.1±0.32µm3, n=10 tVNCs, ~75 NSCs each) when compared to CycA- qNSCs. Thus qNSCs exhibit two types of stem cell quiescence: the majority arrests in G2 and a minority in G0 (Fig. 1F). G2 quiescence has not been reported previously in stem cells in mammals or Drosophila.
The choice of G2 or G0 arrest could be stochastic or pre-programmed. We found 7 G0 qNSCs in the T1 hemi-segment and 8 G0 qNSCs each in T2 and T3. A consistent subset of qNSCs always arrested in G0, namely NB2-2, NB2-4, NB2-5, NB3-4, NB5-3 and NB7-4 (Figs. 2A-B, S2A-F, Table S1). Of these, NB2-4 disappears from T1 during embryogenesis (11, 12), explaining why fewer qNSCs are G0-arrested in T1 than T2/T3. NB5-4/NB5-7 were G2-arrested in 50% of hemi-segments but did not always arrest in the same cell cycle phase either side of the midline (Fig. S2F). We conclude that, with the exception of NB5-4 and NB5-7, the choice of G2 or G0 quiescence is entirely invariant.
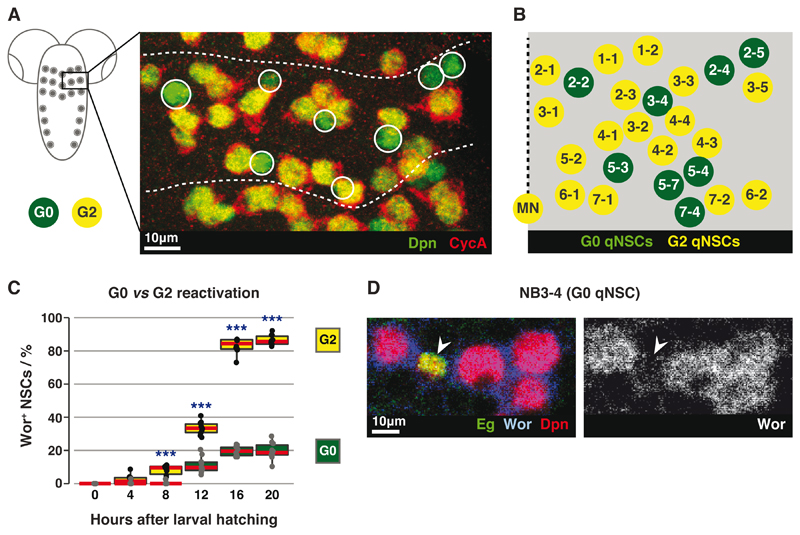
Fig. 2. G2 qNSCs reactivate before G0 qNSCs.
(A) Maximum intensity projection of a tVNC hemi-segment, stained for G2 (yellow) and G0 (green; circled) qNSCs. Dotted lines indicate hemi-segment boundaries.
(B) Positions and identities of G0 qNSCs (green) within a hemi-segment. Dotted line represents the midline. Schematic modified from (12).
(C) Quantification of Wor+ G0 or G2 qNSCs. n=10 tVNCs/time point, ~150 NSCs each. ***: p<1.39x10-5, two-tailed paired t-tests. Red lines indicate medians.
(D) NB3-4 (Eg+; arrowed), remains small and Wor-negative at 20ALH, while neighbouring G2 qNSCs have reactivated.
Is G2/G0 heterogeneity in qNSCs significant? We assessed reactivation of G2 and G0 qNSCs by tracking expression of the reactivation marker worniu (wor) (Figs. S3A-C). Over 86% of G2 qNSCs reactivated by 20 hours after larval hatching (ALH), as compared to 20% of G0 qNSCs (Fig. 2C, n=10 tVNCs, ~150 NSCs each). For example NB3-4, a G0 qNSC, reactivated in fewer than 7% of hemi-segments (n=10 tVNCs, 6 hemi-segments each) (Fig. 2D). All NSCs reactivated by 48 hours ALH (Fig. S3C). Thus G2 qNSCs are faster-reactivating stem cells than G0 qNSCs.
We next profiled gene expression in qNSCs using Targeted DamID (TaDa) (13), identifying 1656 genes with GO terms including ‘nervous system development’ (35 genes, corrected p value: 2.70x10-6) and ‘neuroblast (NSC) development’ (10 genes, corrected p value: 8.40x10-4) (Tables S2 and S3). To identify quiescence-specific genes, we eliminated genes common to quiescent and proliferating NSCs (13) such as deadpan (dpn) (Figs. S4A-B). tribbles (trbl) is one of the most significantly expressed protein-coding genes specific to qNSCs (Fig. S4C). trbl encodes an evolutionarily conserved pseudokinase with three human homologues that have been implicated in insulin and MAPK signalling (reviewed by (14)). We confirmed that trbl labels quiescent but not proliferating NSCs in vivo (Figs. 3A, S4D-F). To date, no other gene has been identified that labels qNSCs specifically.
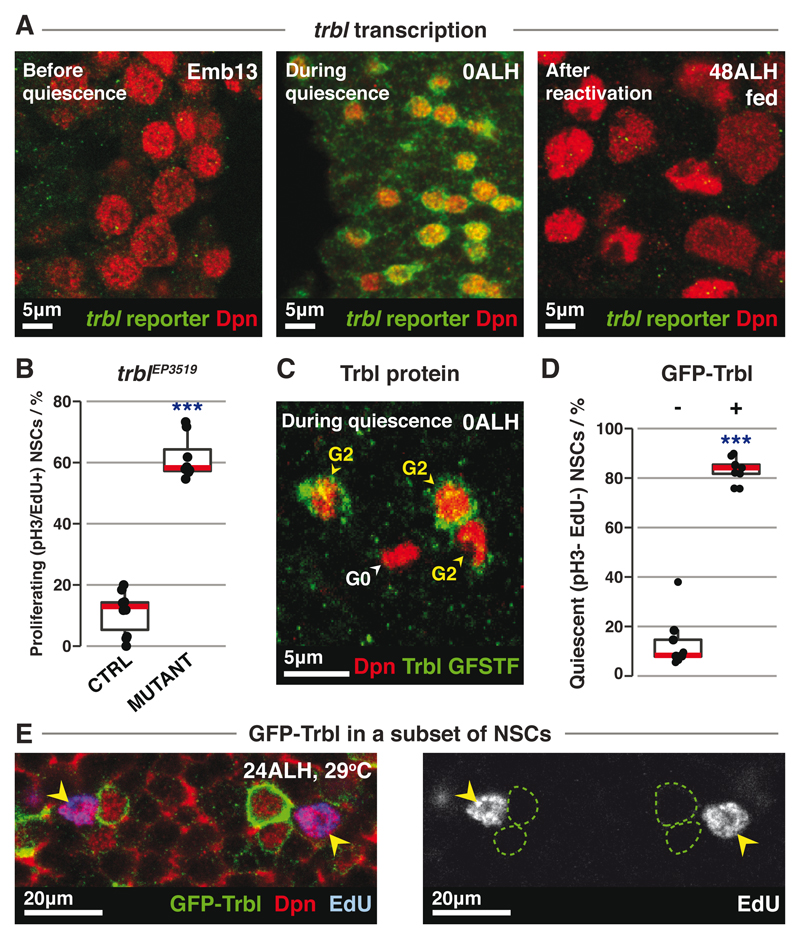
Fig. 3. trbl regulates G2 qNSCs.
(A) trbl reporter expression (green) in NSCs (red) before, during and after quiescence.
(B) Quantification of proliferating NSCs in control (n=10) vs trblEP3519 mutant (n=8) tVNCs, with ~120 NSCs each. ***: p=7.06x10-14, Student’s t-test.
(C) Trbl protein expression (green) in G2 (CycA+) or G0 (CycA-) qNSCs (red).
(D) Quantification of qNSCs in grh-GAL4>GFP-Trbl tVNCs. “-” and “+” denote GFP-Trbl- and GFP-Trbl+ NSCs respectively. n=9 tVNCs, ~150 NSCs each. ***: p=3.90x10-4, Wilcoxon signed-rank test.
(E) In grh-GAL4>GFP-Trbl brains, GFP-Trbl+ NSCs (green outlines) do not incorporate EdU, while control NSCs (yellow arrowheads) do.
Red lines indicate medians.
trbl is necessary for quiescence entry, as NSCs continued to divide during late embryogenesis in trbl hypomorphic mutants or when trbl was knocked down specifically in NSCs (Figs. 3B, S5A-D). trbl regulates quiescence entry specifically, without affecting division mode or cell viability (Fig. S5E-F). The ectopically dividing NSCs in the trblEP3519 mutant were G2, not G0, qNSCs (Fig. S5G). G2, but not G0, qNSCs also became significantly smaller in trblEP3519 mutants (Figs. S5H-J). As embryonic NSCs do not re-grow between cell divisions (15), the size reduction is consistent with excessive divisions. Consistent with a function in G2 quiescence, Trbl was expressed primarily in G2 qNSCs (Figs. 3C, S5K).
Trbl is also required to maintain quiescence. RNAi-mediated knockdown of trbl in qNSCs caused NSCs to leave quiescence and divide (Fig. S5L-M). We generated transgenic flies carrying UAS-GFP-Trbl and drove expression with grainyhead (grh)-GAL4 (4) to assess whether Trbl is sufficient to maintain G2 quiescence. grh-GAL4 expression initiates at quiescence entry and is expressed in ~67% of NSCs, allowing comparison between neighboring GFP-Trbl-expressing and non-expressing NSCs. Almost all GFP-Trbl-expressing NSCs remained in G2 quiescence and expressed CycA (91.8±0.88%, n=10 tVNCs, ~120 NSCs each) (Figs. 3D-E, S6A-C). GFP-Trbl-expressing NSCs retained the primary process that is extended specifically by quiescent NSCs (see Fig. 1A), unlike control NSCs, which had begun to divide (Figs. S6B-C) (1, 4, 16). Thus Trbl is sufficient to maintain G2 quiescence.
In the embryonic mesoderm, trbl induces G2 arrest by promoting Cdc25String protein degradation (17–19). We found that Cdc25String protein is reduced in NSCs at quiescence entry whereas cdc25string mRNA is maintained (Fig. 4A). Therefore, Cdc25String is regulated post-transcriptionally at quiescence entry. Significantly more NSCs were Cdc25String protein-positive in trblEP3519 mutants (Figs. 4B, S7A-B). This increase in Cdc25String is sufficient to explain the excessive NSC proliferation in trbl mutants (Figs. S7C-D). Thus Trbl initiates quiescence entry by promoting Cdc25String protein degradation during late embryogenesis.
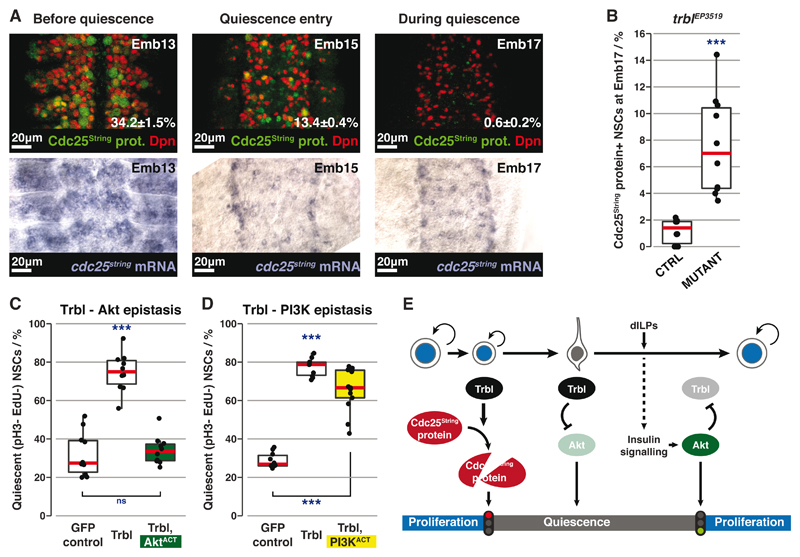
Fig. 4. Trbl induces and maintains quiescence through different mechanisms.
(A) Top row: Cdc25String protein (green) expression in NSCs (red) before and during quiescence (percentages are mean±SEM). n=10 tVNCs/time point, ~130 NSCs each. Maximum intensity projections. Bottom row: In situ hybridisation against cdc25string mRNA at the same stages.
(B) Percentages of Cdc25String protein+ NSCs in control (trblEP3519 heterozygote) vs mutant tVNCs. n=10 tVNCs/genotype, ~110 NSCs each. ***: p=9.08x10-5, Kolgomorov-Smirnov test.
(C and D) Quantification of qNSCs in epistasis experiments between GFP-Trbl and AktACT (C) or PI3KACT (D). n>10 tVNCs/condition, ~80 NSCs each. ***: p<3.39x10-9. ns: p>0.05, one way ANOVA followed by post-hoc Tukey’s HSD test. In (D), there is no significant difference between GFP-Trbl alone and GFP-Trbl+PI3KACT.
(E) Three-step model for Trbl activity.
Red lines indicate medians.
Trbl also maintains NSC quiescence post-embryonically, however, it must act through another mechanism as Cdc25String is no longer expressed in post-embryonic qNSCs (Fig. S8A). Trbl is known to inhibit insulin signalling through binding Akt and preventing its phosphorylation (Fig. S8B) (20). Consistent with this, Trbl-expressing NSCs had less p4E-BP than control NSCs (Fig. S8C). If Trbl inhibits Akt to maintain quiescence, constitutively active Akt (myr-Akt (21); AktACT) should rescue Trbl-induced quiescence: AktACT fully rescued NSC reactivation (Figs. 4C, S8D). In contrast, as Trbl is thought to act downstream of PI3K (20), constitutively active PI3K (dp110CAAX, (22); PI3KACT) should not rescue reactivation, which it did not (Figs. 4D, S8D). Thus Trbl maintains quiescence by blocking activation of Akt. This role is specific to post-embryonic NSCs since embryonic NSCs do not depend on insulin signalling to proliferate (Fig. S8E).
trbl expression must be repressed to allow NSC reactivation. We found that insulin signalling is necessary and sufficient to repress trbl transcription. NSCs misexpressing PTEN, an insulin pathway inhibitor, failed to down-regulate trbl transcription (Fig. S8F). In contrast, activating the insulin pathway by expressing AktACT in NSCs was sufficient to switch off trbl transcription (Fig. S8G).
Here we have discovered the mechanisms by which Drosophila NSCs enter, remain in, and exit quiescence in response to nutrition (Fig. 4E). Trbl pseudokinase (1) promotes degradation of Cdc25String protein to induce quiescence, (2) blocks insulin signalling by inhibiting Akt in the same NSCs to maintain quiescence, and (3) is overridden by nutrition-dependent secretion of dILPs from blood-brain barrier glia, which activate insulin signalling in qNSCs, repress trbl expression and enable reactivation.
Contrary to the prevailing dogma, we found that qNSCs are pre-programmed to arrest in G2 or G0. G2 qNSCs reactivate earlier and generate neurons before G0 qNSCs; this may ensure that neurons form the correct circuits in the appropriate order during brain development. G2 arrest also enables high fidelity homologous recombination-mediated repair in response to DNA damage, preserving genomic integrity during quiescence. Quiescent stem cells in mammals may also arrest in G2, with implications for isolating and manipulating quiescent stem cells for therapeutic purposes.
Supplementary Material
One Sentence Summary.
G2-arrested quiescent stem cells reactivate more readily than G0 cells and are regulated by the pseudokinase Tribbles.
Acknowledgments
We thank P. Callaerts, F. Díaz-Benjumea, J. Dods, C. Q. Doe, B. Edgar, E. Higginbotham, Y. Kimata, J. Ng, J. Urban, U. Walldorf, E. Wieschaus, Bloomington Drosophila Stock Centre and Developmental Studies Hybridoma Bank (DSHB) for generously providing reagents; T. Southall and O. J. Marshall for updating the TaDa microarray data to Release 6 of the Drosophila genome and for gene expression analysis; F. Doetsch, A. C. Delgado, F. J. Livesey, D. St. Johnston and the Brand laboratory members for discussion.
Funding: This work was funded by the Royal Society Darwin Trust Research Professorship, Wellcome Trust Senior Investigator Award 103792 and Wellcome Trust Programme grant 092545 to A.H.B., and Wellcome Trust PhD Studentship 097423 to L.O. A.H.B acknowledges core funding to the Gurdon Institute from the Wellcome Trust (092096) and CRUK (C6946/A14492).
Footnotes
This manuscript has been accepted for publication in Science. This version has not undergone final editing. Please refer to the complete version of record at http://www.sciencemag.org/. The manuscript may not be reproduced or used in any manner that does not fall within the fair use provisions of the Copyright Act without the prior, written permission of AAAS.
Author contributions: L.O. and A.H.B. designed the experiments, analysed the data and wrote the manuscript. L.O. performed the experiments.
Competing interests: The authors declare no conflict of interest.
Data and Materials accessibility: Microarray data have been deposited with GEO: accession GSE81745.
References
- 1.Truman JW, Bate M. Developmental Biology. 1988;125:145–157. doi: 10.1016/0012-1606(88)90067-x. [DOI] [PubMed] [Google Scholar]
- 2.Prokop A, Technau GM. Development. 1991 [Google Scholar]
- 3.Britton JS, Edgar BA. Development. 1998;125:2149–2158. doi: 10.1242/dev.125.11.2149. [DOI] [PubMed] [Google Scholar]
- 4.Chell JM, Brand AH. Cell. 2010;143:1161–1173. doi: 10.1016/j.cell.2010.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spéder P, Brand AH. Developmental Cell. 2014;30:309–321. doi: 10.1016/j.devcel.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sousa-Nunes R, Yee LL, Gould AP. Nature. 2011;471:508–512. doi: 10.1038/nature09867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheung TH, Rando TA. Nat Rev Mol Cell Biol. 2013;14:329–340. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lai S-L, Doe CQ. eLife. 2014;3 doi: 10.7554/eLife.03363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakaue-Sawano A, et al. Cell. 2008;132:487–498. doi: 10.1016/j.cell.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 10.Zielke N, et al. Cell Rep. 2014;7:588–598. doi: 10.1016/j.celrep.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 11.Truman JW, Schuppe H, Shepherd D, Williams DW. Development. 2004;131:5167–5184. doi: 10.1242/dev.01371. [DOI] [PubMed] [Google Scholar]
- 12.Lacin H, Truman JW, VijayRaghavan K. eLife. 2016;5:e13399. doi: 10.7554/eLife.13399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Southall TD, et al. Developmental Cell. 2013;26:101–112. doi: 10.1016/j.devcel.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eyers PA, Keeshan K, Kannan N. Trends Cell Biol. 2016 doi: 10.1016/j.tcb.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hartenstein V, Rudloff E, Ortega JAC. Roux's Arch Dev Biol. 1987;196:473–485. doi: 10.1007/BF00399871. [DOI] [PubMed] [Google Scholar]
- 16.Narbonne-Reveau K, et al. eLife. 2016;5:e13463. doi: 10.7554/eLife.13463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mata J, Curado S, Ephrussi A, Rørth P. Cell. 2000;101:511–522. doi: 10.1016/s0092-8674(00)80861-2. [DOI] [PubMed] [Google Scholar]
- 18.Grosshans J, Wieschaus E. Cell. 2000;101:523–531. doi: 10.1016/s0092-8674(00)80862-4. [DOI] [PubMed] [Google Scholar]
- 19.Seher TC, Leptin M. Curr Biol. 2000;10:623–629. doi: 10.1016/s0960-9822(00)00502-9. [DOI] [PubMed] [Google Scholar]
- 20.Das R, Sebo Z, Pence L, Dobens LL. PLoS ONE. 2014;9:e109530. doi: 10.1371/journal.pone.0109530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Britton JS, Lockwood WK, Li L, Cohen SM, Edgar BA. Developmental Cell. 2002;2:239–249. doi: 10.1016/s1534-5807(02)00117-x. [DOI] [PubMed] [Google Scholar]
- 22.Leevers SJ, Weinkove D, MacDougall LK, Hafen E, Waterfield MD. EMBO J. 1996;15:6584–6594. [PMC free article] [PubMed] [Google Scholar]
- 23.Campos-Ortega JA, Hartenstein V. The Embryonic Origin of Drosophila melanogaster. 1985 [Google Scholar]
- 24.Quiñones-Coello AT, et al. Genetics. 2007;175:1089–1104. doi: 10.1534/genetics.106.065995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rørth P, et al. Development. 1998;125:1049–1057. doi: 10.1242/dev.125.6.1049. [DOI] [PubMed] [Google Scholar]
- 26.Nagarkar-Jaiswal S, et al. eLife. 2015;4:e05338. [Google Scholar]
- 27.Albertson R, Chabu C, Sheehan A, Doe CQ. J Cell Sci. 2004;117:6061–6070. doi: 10.1242/jcs.01525. [DOI] [PubMed] [Google Scholar]
- 28.Luo L, Liao YJ, Jan LY, Jan YN. Genes & Development. 1994;8:1787–1802. doi: 10.1101/gad.8.15.1787. [DOI] [PubMed] [Google Scholar]
- 29.Lee T, Luo L. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
- 30.McGuire SE, Le PT, Osborn AJ, Matsumoto K, Davis RL. Science. 2003;302:1765–1768. doi: 10.1126/science.1089035. [DOI] [PubMed] [Google Scholar]
- 31.Huang H, et al. Development. 1999;126:5365–5372. doi: 10.1242/dev.126.23.5365. [DOI] [PubMed] [Google Scholar]
- 32.Estacio-Gómez A, et al. Development. 2013;140:2139–2148. doi: 10.1242/dev.090423. [DOI] [PubMed] [Google Scholar]
- 33.Li H-H, et al. Cell Rep. 2014;8:897–908. doi: 10.1016/j.celrep.2014.06.065. [DOI] [PubMed] [Google Scholar]
- 34.Di Talia S, et al. Curr Biol. 2013;23:127–132. doi: 10.1016/j.cub.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Whitfield WG, Gonzalez C, Maldonado-Codina G, Glover DM. EMBO J. 1990;9:2563–2572. doi: 10.1002/j.1460-2075.1990.tb07437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dittrich R, Bossing T, Gould AP, Technau GM, Urban J. Development. 1997;124:2515–2525. doi: 10.1242/dev.124.13.2515. [DOI] [PubMed] [Google Scholar]
- 37.Kosman D, Small S, Reinitz J. Dev Genes Evol. 1998;208:290–294. doi: 10.1007/s004270050184. [DOI] [PubMed] [Google Scholar]
- 38.Isshiki T, Takeichi M, Nose A. Development. 1997;124:3099–3109. doi: 10.1242/dev.124.16.3099. [DOI] [PubMed] [Google Scholar]
- 39.Rozen S, Skaletsky H. Methods Mol Biol. 2000;132:365–386. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 40.Cox KH, DeLeon DV, Angerer LM, Angerer RC. Developmental Biology. 1984;101:485–502. doi: 10.1016/0012-1606(84)90162-3. [DOI] [PubMed] [Google Scholar]
- 41.Wilkie GS, Shermoen AW, O'Farrell PH, Davis I. Curr Biol. 1999;9:1263–1266. doi: 10.1016/s0960-9822(99)80509-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.