Abstract
Pancreatic neuroendocrine tumours (PNETs) may occur as a non-familial isolated endocrinopathy or as part of a complex hereditary syndrome, such as multiple endocrine neoplasia type 1 (MEN1), which is an autosomal dominant disorder characterised by the combined occurrence of PNETs with tumours of the parathyroids and anterior pituitary. Treatments for primary PNETs, include surgery, and for nonresectable PNETs and metastases, treatments include biotherapy (e.g. somatostatin analogues, inhibitors of receptors, and monoclonal antibodies), chemotherapy, and radionuclide therapy. However, treatment of PNETs in patients with MEN1 is challenging due to concomitant development of tumours, which may have metastasised, and there is a scarcity of clinical trials reporting the effects of these anti-tumour therapies in PNETs of MEN1 patients. For example, clinical trials have shown that inhibitors of receptor tyrosine kinases (RTKs) and the mechanistic target of rapamycin receptor (mTOR) pathway, and antibodies to vascular endothelial growth factor A (VEGFA) are effective treatments for PNETs in non-MEN1 patients, but data from MEN1 patients is lacking. Recent preclinical studies have identified potentially new therapeutic targets for treating MEN1-associated NETs, and these include epigenetic modification, the β-catenin/Wnt-pathway, hedgehog signalling, and somatostatin receptors, as well as MEN1 gene replacement therapy. This review discusses these advances.
Introduction
Pancreatic neuroendocrine tumours (PNETs) have a reported incidence of 0.48 per 100,000 of the population, although they are found more frequently in 0.8% to 1.0% of patients undergoing post-mortem examinations1–3. PNETs usually occur as a non-familial (i.e. sporadic) isolated endocrinopathy, but they may also occur as part of a complex hereditary syndrome, such as multiple endocrine neoplasia type 1 (MEN1), von-Hipple Lindau disease, von Recklinghausen’s syndrome (Neurofibromatosis type 1, NF1), and tuberous sclerosis4,5. PNETs have been reported to occur in 30%-80% of MEN1 patients, >15% of VHL patients, <10% of NFI patients, and <1% patients with tuberose sclerosis. Thus, MEN1 is the most common hereditary syndrome associated with PNETs, and ~10% of all PNETs are associated with MEN16. Moreover, somatic mutations of the MEN1 gene, which are found in virtually all PNETs of MEN1 patients7 are also found to occur in >40% of sporadic PNETs, indicating that MEN1 mutations are “major drivers” in the development of all PNETs8,9. Current treatment of PNETs, which comprise drugs (e.g. chemotherapy and biotherapies), surgery, and radiotherapy (Figure 1 and Table 1) are often not successful, such that the median survival time for patients with PNETs is ~3.6 years1. Thus, there is a clinically unmet need for better treatments, which may arise from a greater understanding of PNET biology and the role of the MEN1 gene and its encoded protein menin. This review will focus on providing an overview of the clinical features (Figure 2) and genetics of MEN1, the functions of menin (Figure 3), the current therapies for PNETs in non-MEN1 patients and their use in treating PNETs in MEN1 patients (Table 1 and Supplementary Table 1), and emerging therapies of which some are based on the function of menin (Figure 3).
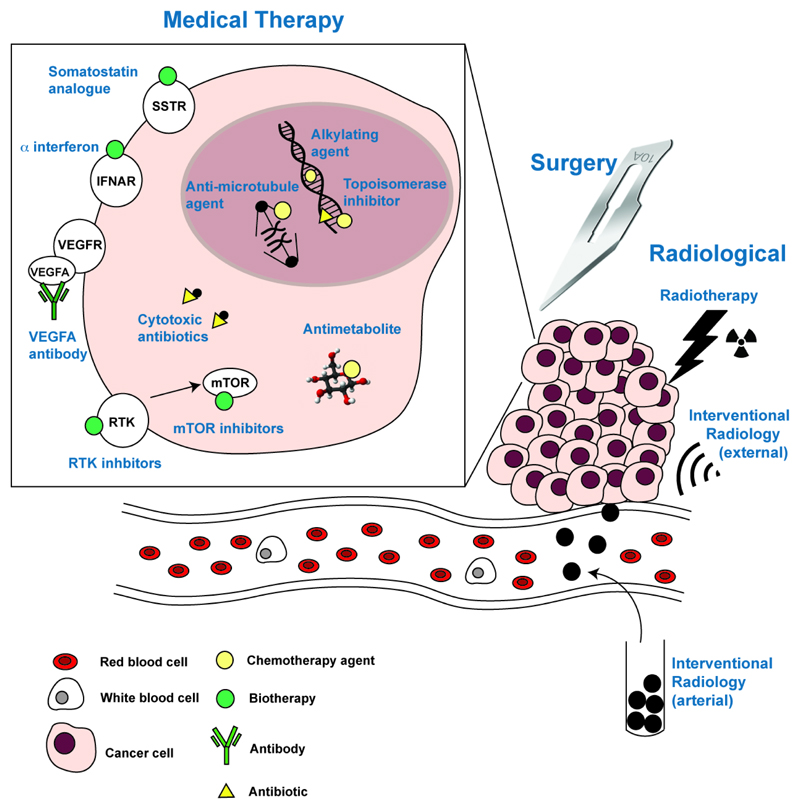
Figure 1.
Current treatments for pancreatic neuroendocrine tumours (PNETs). Treatments are: medical, which includes drugs and antibodies that that target different pathways of cancer cells; surgical, i.e. removal or resection of the NET; and radiological, in which particles or high frequency waves are delivered externally or internally (e.g. intra-arterially) to the tumour. SSTR – somatostatin receptor; IFNAR – interferon alpha/beta reception; VEGFR – vascular endothelial growth factor receptor; VEGFA – vascular endothelial growth factor A; RTK – receptor tyrosine kinase; mTOR – mechanistic target of rapamycin.
Table 1. Current treatment options for pancreatic NETs (PNETs).
| Medical | |
| Biotherapy | |
Chemotherapy
|
|
| Surgery | |
| Curative | |
| Cytoreduction | |
| Radiological | |
Radiotherapy
|
|
Interventional Radiology
|
|
PDGFR – platelet-derived growth factor receptor; and VEGFR – vascular endothelial growth factor receptor are both TKI inhibitors. Inhibitors may have multiple targets, for example sunitinib and sorafenib inhibit PDGFR and VEGFRs; imatinib inhibits PDGFRs, Abelson murine leukemia viral oncogene homolog 1 (vABl) and proto-oncogene c-Kit (c-kit); and vandetanib inhibits VEGFRs and epidermal growth factor receptors (EGFRs)
VEGFA – vascular endothelial growth factor A
Nuclear targets
cytoplasmic targets; capecitabine is the orally administered pro-drug of 5’fluorouracil (5FU).
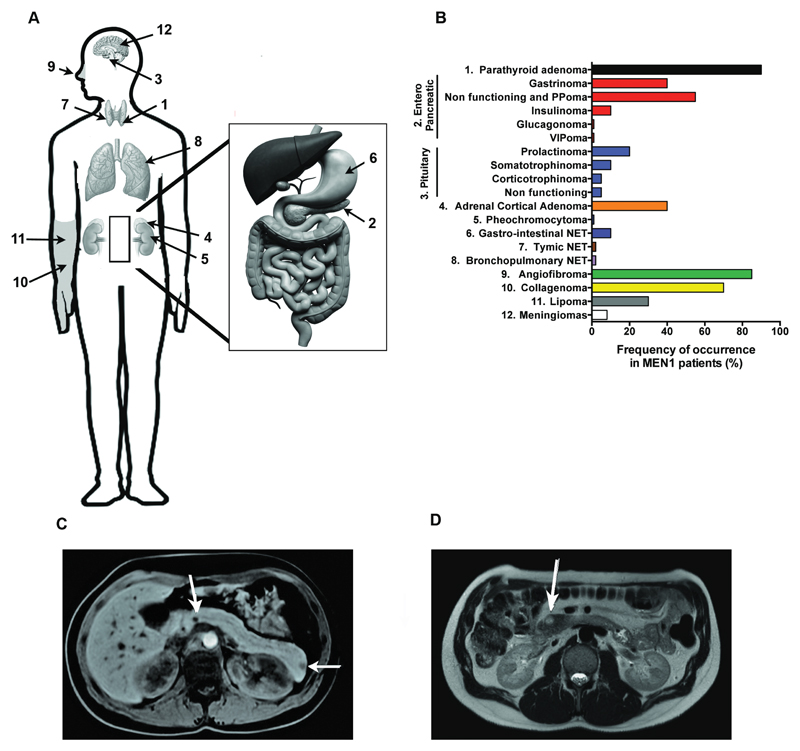
Figure 2.
Distribution of endocrine and non-endocrine tumours in MEN1 patients. (A) MEN1 patients may develop: endocrine tumours involving the parathyroids (labelled number 1), pancreas (2), pituitary (3), adrenal cortex (4) and medulla (5), gastro-intestinal tract (6), thymus (7) and bronchial tree (8); and non-endocrine tumours such as facial angiofibromas (9), collagenomas (10), lipomas (11) and meningiomas (12). (B) Frequencies of MEN1-associated tumours. The most frequently occurring endocrine tumours in MEN1 patients are: parathyroid adenomas, which occur in >95% of patients; pancreatic neuroendocrine tumours (PNETs), which occur in 50-70% of patients, with ~40% of patients having gastrinomas, ~10% having insulinomas, <1% having glucagonomas, <1% having VIPomas, and ~20-50% having PPomas or non-functioning tumours; anterior pituitary tumours, which occur in 20-40% of patients, with ~20% having prolactinomas, ~10% having somatotrophinomas, <5% having corticotrophinomas, and ~5% having non-functioning tumours; and adrenal tumours, which occur in 20-40% of patients, with ~40% having cortical adenomas that are usually non-secreting, but may occasional secrete glucocorticoids, or aldosterone causing Cushing’s or Conn’s syndrome, respectively, and <1% having pheochromocytoma tumours arising from the medulla. The most frequently occurring non-endocrine tumours in MEN1 patients are angiofibromas, collagenomas, and lipomas, which are reported to occur in 0-85%, 0-70%, and ~30% of patients, respectively. (C) Magnetic Resonance Imaging (MRI) sagittal section of multiple PNETs (indicated by white arrows) in an MEN1 patient. (D) MRI sagittal section of a non-MEN1 PNET (the tumour is indicated by a white arrow).
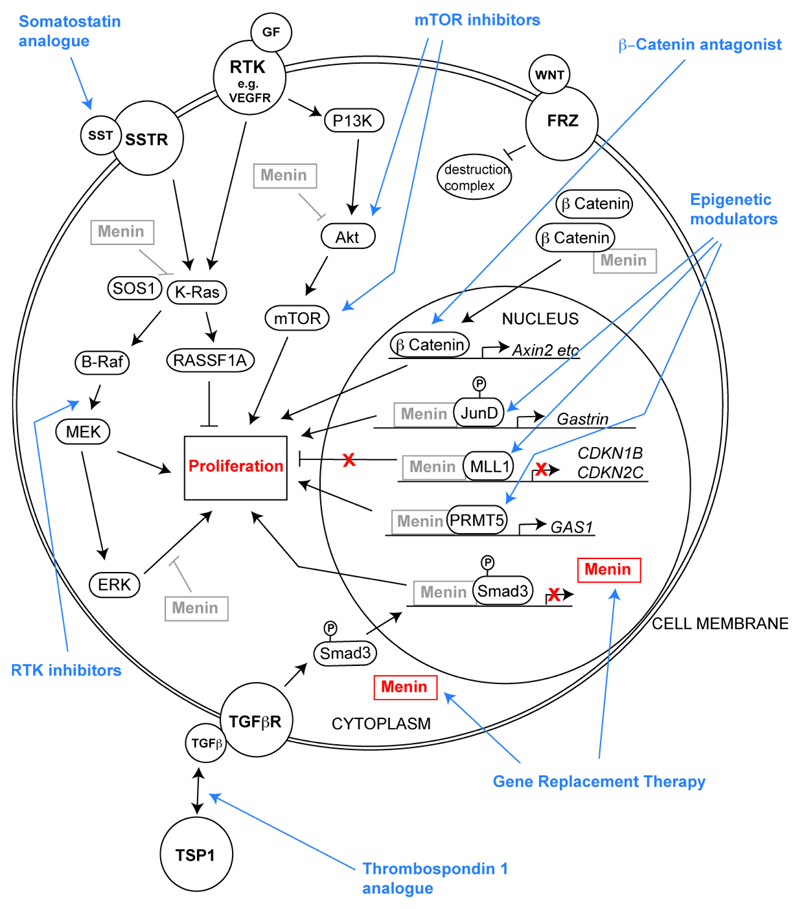
Figure 3.
Menin-associated pathways with potential targeted therapies. Menin, which is encoded by the MEN1 gene, has roles in the nucleus and cytoplasm, and loss of menin expression (represented by grey boxes in the diagram) results in increased cell proliferation by multiple different pathways. Thus, in the nucleus, menin: interacts with the transcription factor JunD and the protein arginine methyltransferase (PRMT) 5 to repress transcription of target genes, for example Gastrin and Gas1, respectively115,116; binds to mixed linage leukaemia proteins MLL1 and/or MLL2, and Smad3, which is a TGF-β signalling component, to promote transcription of target genes117–121; and regulates the Wnt pathway by preventing β-catenin from entering the nucleus and therefore preventing transcription of Wnt pathway target genes110,113. In the cytoplasm, menin inhibits: the mTOR pathway, by binding to Akt, which is downstream of PI3K that is part of the RTK signalling pathway, and preventing its translocation to the plasma membrane127; and K-Ras induced proliferation, by possible inhibition of ERK dependent phosphorylation and prevention of the interaction between SOS and K-Ras123,124. These advances in understanding the function of menin, have helped to identify potential new and targeted therapies (indicated in blue), which include: mTOR inhibitors; Wnt pathway inhibitors e.g. β-catenin antagonists; epigenetic modulators; MEN1 gene replacement; analogues of thrombospondin-1 (TSP1), which interact and alter TGF-β signalling, that includes the menin-interacting protein Smad3; RTK inhibitors; and somatostatin analogues which act on a broader spectrum of SSTRs. All pathways affect proliferation (shown in the cytoplasm only), which involves both nuclear and cytoplasmic process and mechanisms; RTK – receptor tyrosine kinase; GF – growth factor; P13K – phosphoinositide 3-kinase; Akt – protein kinase B; mTOR – mechanistic target of rapamycin; FRZ – fizzled; MLL – mixed lineage leukemia; CDKN – cyclin dependent kinase inhibitor; PRMT5 – protein arginine N-methytransferase 5; GAS1 – growth arrest specific 1; SMAD3 – mothers against decapentaplegic hormone 3; TGFβ(R) – transforming growth factor beta (receptor); TSP1 – thrombospondin 1; SST(R) – somatostatin (receptor); SOS1 – sons of sevenless 1; RASSF1A – ras associated domain family member 1 isoform A; MEK – mitogen activated protein kinase kinase; ERK – extra signal-related kinase.
Multiple Endocrine Neoplasia Type 1 (MEN1)
Multiple endocrine neoplasia type 1 (MEN1) is characterised by the combined occurrence of two or more tumours that usually involve the parathyroids, pancreatic islets, anterior pituitary and adrenals (Figure 2A-C)10. In addition, <5% of MEN1 patients may develop carcinoid tumours of the thymus, bronchus, and gut, and women may have an increased occurrence of breast cancer10,11. The majority of MEN1-patients will have developed manifestations of an MEN1-tumour by the age of 45 years12, with primary hyperparathyroidism (PHPT), due to parathyroid tumours, being the most common, and occurring in >80% of MEN1 patients by the age of 50 years13. Among the pancreatic islet cell tumours, also referred to as PNETs, ~60% will secrete polypeptide hormones such as gastrin, insulin, glucagon, or vasoactive intestinal peptide (VIP), and cause multiple ulcers of the stomach and duodenum, hypoglycaemia with seizures, glucose intolerance, and watery diarrhoea with hypokalaemic alkalosis (WDHA) syndrome, respectively14,15. However, 40% of these pancreatic NETs may not secrete hormones, and these are referred to as non-functioning (i.e. non-secreting) PNETs10,14,15. Among the anterior pituitary tumours in MEN1 patients, ~90% will secrete prolactin, growth hormone (GH) or adrenalcorticotropic hormone (ACTH), and the remaining ~10% are non-functioning (or glycoprotein subunit secreting) adenomas10,15. Among the adrenal cortical tumours in MEN1 patients ~10% will hypersecrete glucocorticoids or mineralocorticoids, whilst ~90% will be non-functioning. Hormonal hypersecretion by these MEN1-associated endocrine tumours, the majority of which are benign adenomas, will result in hormone related disorders with specific symptoms and morbidities that are not always similar to those in non-MEN1 patients10,15. This is because non-MEN1 patients will only have a tumour of one endocrine gland, whereas MEN1 patients will have tumours occurring in two or more glands (Figure 2A-D). These endocrine tumours in MEN1 patients are associated with a decreased 20-year survival of <65%, when compared to that of >80% in an age and sex-matched US population16.
Genetics of MEN1
MEN1 is an autosomal dominant disorder caused by mutations of the MEN1 tumour suppressor gene, which encodes a 610 amino acid protein called menin. MEN1 germline mutations are found in >90% of patients7,10, and comprise whole or partial gene deletions, frameshift deletions or insertions, in-frame deletions or insertions, and splice site, missense, and nonsense mutations, and result in a functional deficiency of menin10. There appear to be no genotype-phenotype correlations7,17,18. MEN1 tumours will have somatic mutations as well as the germline mutations, consistent with the Knudson two-hit hypothesis for the role of tumour suppressor genes in oncogenesis, and in the majority (>90%) of MEN1 tumours the somatic abnormality is loss of heterozygosity (LOH), with the remaining 10% having intrageneic deletions or point mutations10,16,19,20. Moreover, the MEN1 gene is involved in the aetiology of non-MEN1 parathyroid tumours, PNETs, pituitary adenomas, and adrenal cortical adenomas, as ~20%, ~40%, ~4% and ~2% of these have somatic MEN1 mutations, respectively10,21.
Current treatment of endocrine tumours in MEN1
The choice of optimal anti-tumour therapies, which comprise medical, surgical, and radiological approaches (Table 1 and Figure 1), for MEN1 patients is frequently challenging, as such therapies have not been formally evaluated with clinical trials in MEN1 patients but instead have often been extrapolated from outcomes of clinical trials reported from non-MEN1 patients who are affected with a single endocrine tumour (Supplementary Table 1)21. This increases reliance on consensus expert opinions, which acknowledge the uncertainties in the provisions of optimal treatments. However, all recognise that in the absence of treatment, endocrine tumours in MEN1 patients are associated with an earlier mortality21. Thus, untreated patients with MEN1 tumours have a decreased life expectancy with a 50% probability of death by the age of 50 years, and the cause of death in 50-70% of patients with MEN1 is usually a malignant tumour process or sequelae of the disease19,20. This increased mortality in MEN1 patients can be attributed to PNETs, which may metastasise19,20. However, the implementation of genetic diagnosis and regular screening for MEN1-associated tumours for their earlier detection and treatment21, has been reported to result in a shift towards less advanced clinical presentations, in those MEN1 patients undergoing screening when compared to those not undergoing screening, with lower rates of malignant PNETs (0% versus 14%), metastases (0% versus 7%) and death (0% versus 7%)22. PNETs in MEN1 patients, which are usually diagnosed between <10 and 50 years of age, are frequently multiple, although small (i.e. <1cm), and occur on a background of diffuse microdenomatosis23,24. In addition, PNETs in MEN1 patients occur concomitantly with other tumours and the associated comorbidities may also decrease survival rates. Thus, the mean age of death for MEN1 patients with PNETs is 55 years, which is lower than that expected for the general population, and approximately 40% of these deaths are due to malignant PNETs, which are usually non-functioning PNETs, and are not associated with a clinical syndrome19,20. These non-functioning PNETs in MEN1 patients are invariably located within the pancreas and ~33% and 15% will be associated with lymph node and hepatic metastases, respectively24,25, and are the commonest cause of death in MEN1 patients, with 5 and 10 year survival being about 75% and 50%, respectively26–28. In contrast, non-MEN1 PNETS are usually solitary pancreatic lesions that are diagnosed between 50-80 years of age, and ~50-75% of these will be associated with regional lymph node or distant hepatic metastasis at diagnosis29,30. These differences between MEN1 and non-MEN1 PNETs make it difficult to extrapolate results of treatment outcomes from studies of non-MEN1 PNETs to MEN1 PNETs14,21.
Current Therapies for PNETs in Non-MEN1 Patients and Their Repurposing for PNETs in MEN1 Patients
Current therapies for PNETs in non-MEN1 patients include medical drugs, surgery, and radiology interventions (Figure 1), which have been repurposed for treatment of PNETs in MEN1 patients, despite a lack of their formal evaluation in MEN1 patients. Thus, the current treatments for PNETs in MEN1 are similar to those for PNETs in non-MEN1 patients, and evidence for the effectiveness of these treatments comprise anecdotal case reports or small case series. These treatments and their limitations will be briefly reviewed.
Medical Therapies
Medical therapies for PNETs can be broadly divided into biotherapies, which target tumour-specific receptors and intracellular pathways, or chemotherapies, which generally target cell division (Table 1 and Figure 1).
Biotherapies
Biotherapies for PNETs can be hormonal, which are based on somatostatin (a peptide hormone that inhibits release of other hormones, cell proliferation and angiogenesis31), or targeted to tumour-specific molecular changes (e.g. in receptors and signalling pathways) that help the tumours to grow and spread, and these include: mechanistic target of rapamycin (mTOR) signalling inhibitors, receptor tyrosine kinase (RTK) inhibitors, and antibodies targeting the vascular endothelial growth factor (VEGF) or its receptor (VEGFR) (Table 1 and Figure 1).
Somatostatin analogues (e.g. octreotide and lanreotide) have been used to control excessive hormone secretion and for their potential anti-proliferative effects in patients with low-grade (Ki67<5%) PNETs that express somatostatin receptors (SSTRs), which are G-protein-coupled receptors (GPCRs)32–35. There are 5 SSTRs (SSTR1-5) and PNETs may express all 5 subtypes, although ~80% of PNETs will predominantly express SSTR2, for which octreotide and lanreotide have high affinities32. Treatment with lanreotide has been reported to result in a ~50% reduction in the risk of disease progression and a prolonged progression-free survival (PFS) (median in lanreotide treated group not reached versus median in placebo treated group of 18 months, p<0.001) in non-MEN1 patients with treatment naive well-differentiated advanced gastroenteropancreatic NETs (Supplementary Table 1)34,36,37. However, this trial, which studied 204 patients did not contain any MEN1 patients, who were excluded36. However, a retrospective evaluation of 40 MEN1 patients with dudeno-pancreatic NETs who were treated with long-acting octreotide, reported tumour response in 10%, stable disease in 80%, and progression of disease in 10% of patients over 12-15 months of treatment38, thereby suggesting that somatostatin analogue treatment may have some anti-oncogenic benefits for MEN1 patients.
Approximately 15% of PNETs have somatic mutations of genes associated with the mTOR pathway8, which regulates cell proliferation and growth (Figures 1 and 3), and the mTOR inhibitor everolimus has been reported to increase PFS from ~6 to 11 months in patients with advanced NETs, including PNETs39–42. Details of the occurrence of MEN1, MEN1 mutations, or mutations of the components of the mTOR pathway in the 401 patients in this trial were not provided39–41, and thus it remains to be established whether such mutations may be associated with any differential responses to mTOR inhibitor therapy. PNETs are highly vascular and frequently express VEGFRs43,44, and RTK inhibitors, e.g. sunitinib, which targets VEGFRs, and platelet-derived growth factor receptors (PDGFRs) have also been reported to increase PFS from ~5.5 to 11.4 months, in patients with PNETs45. However, the efficacy of sunitinib in treating PNETs in MEN1 patients remains to be evaluated, because this trial, which comprised a total of 171 patients, included only 2 MEN1 patients and both of these were not in the treatment arm of the study45. Pazopanib, another RTK inhibitor, has also been reported, in a phase 2 trial, to result in response and disease control rates of ~20% and >75%, respectively of non-MEN1 patients with metastatic gastroenteropancreatic NETs46.
Combination therapy using biotherapies that act on the different receptors and signalling pathways that regulate PNET proliferation and growth have been reported to result in beneficial effects and improved outcomes, when compared to monotherapy for gastro-intestinal NETs and PNETs that occurred in non-MEN1 patients, or in patients whose MEN1 status was unknown (Supplementary Table 1). For example, combined use of: 1) octreotide with everolimus (in a phase 3 study) or pazopanib (in a phase 3 study), increased PFS or PNET responses, respectively47,48; 2) temsirolimus, a mTOR inhibitor, with bevacizumab, a monoclonal antibody directed at VEGF, in a phase 2 study, reduced tumour size and increased PFS in >40% and ~80% of patients, respectively, with PNETs49; 3) bevacizumab, everolimus and octreotide, in a phase 2 study, increased PFS more than everolimus and octreotide in patients with PNETs50,51; and 4) pasireotide, a somatostatin analogue which targets SSTR2 and SSTR5, which in a phase 2 monotherapy study seemed to inhibit growth of metastatic NETS, including PNETs52, but was associated, in phase 1 and 2 trials, with hyperglycaemia and bradycardia in ~80% and 30% of patients, respectively52,53 when used with everolimus decreased tumour size in >80% of the patients with unresectable or metastatic PNETs, in a phase 1 trial54. However, some targeted therapies and their combinations are not always successful, and examples include: 1) lanreotide with interferon alpha (IFNα), which induces cell cycle arrest and also has anti-angiogenic effects in a randomized trial treating metastatic gastroentero pancreatic NETs, resulted only in antiproliferative results that were similar to treatment with lanreotide or IFNα55; 2) everolimus and octreotide combined with a monoclonal antibody to the insulin-like growth factor-1 receptor (IGF1R), which is a RTK that is expressed in PNETs and that promotes cell proliferation by activation of PI3K/AKT signalling and subsequently mTOR activity and whose blockade impairs NET cell growth, in combined therapy, during phase 1 and 2 trials, were found to be unsafe and to not result in a PNET response56–60; 3) octreotide and bevacizumab, with pertuzumab, a dimerization inhibitor of the epidermal growth factor receptor (HER1), which is overexpressed in NETs, in a phase 2 study, failed to result in adequate response rates in patients with advanced PNETs61; and 4) dactosilib, an inhibitor of PI3K and the mTOR complex 2, which is not inhibited by everolimus, in a phase 2 study, failed to improve outcome in patients with PNETs that were inadequately treated with everolimus62.
The use of these biotherapies varies in different international centres, and generally the somatostatin analogues (octreotide and lanreotide), the mTOR inhibitor (everolimus), and the RTK inhibitor (sunitinib) are accepted for treatment of PNET.
Chemotherapy
Chemotherapy is reserved to treat patients who have PNETs associated with: metastases; a high tumour burden; a high proliferative index (i.e. Ki67 >5% or mitosis >5/10 per high powered field); rapid tumour progression; and/or symptoms not controlled by biotherapy14,63,64. Chemotherapy drugs can be classified in 6 categories (Tables 1 and Supplementary Table 2, and Figure 1), and drugs from each of these, except the non-classical compounds, have been used to treat PNETs14,63,64. These chemotherapy drugs have actions at different stages of mitosis and include: alkylating agents (e.g. streptozocin, temozolomide and cisplatin), which are cell cycle-independent drugs that covalently bind to DNA via their alkylating groups, disrupt DNA replication and cause apoptosis; anti-microtubule agents (e.g. etoposide and docetaxel) that disrupt the function of microtubules, which are required for cell division; topoisomerase inhibitors (e.g. doxorubicin and irinotecan) that prevent the normal unwinding of DNA that is required during replication or transcription, by blocking the activity of topoisomerase enzymes, which produce single or double-strand breaks in DNA, thereby reducing the tension in DNA strands adjacent to the unwound double-stranded DNA helix; antimetabolites (e.g. 5fluorouracil (5FU) and its pro-drug capecitabine, and gemcitabine), which are cell cycle dependent, block enzymes required for DNA synthesis or are incorporated into DNA, thereby damaging it and inducing apoptosis; and cytotoxic antibiotics (e.g. actinomycin D, mitomycin C, doxorubicin and mixoxantrone), which either alkylate DNA, become intercalated into DNA, or generate highly reactive free radicals that damage intracellular molecules or inhibit topoisomerases.
Combination therapy using cytotoxic drugs that act on different cell division components result in better tumour responses, when compared to monotherapy, in patients with PNETs, and streptozocin- and temozolomide-based regimes have been reported to yield substantially higher response rates. For example, studies in the 1990’s reported that monotherapy with streptozocin (an alkylating agent) and 5FU (an antimetabolite) resulted in PNET response rates of ~40% and 35%, respectively65; and that combined therapies with streptozocin and 5FU or doxorubicin (a topoisomerase inhibitor) resulted in response rates of >60% and ~70%, respectively66. However, these early studies used non-standard response rates, and more recent studies using World Health Organisation (WHO) criteria and response evaluation criteria in solid tumours (RECIST) have reported lower PNET response rates of ~35% for streptozocin-based combined regimes67. Temozolomide (an alkylating agent) monotherapy resulted in PNET response rates of <10%68, whereas temozolomide-based combined therapies with: capecitabine (an antimetabolite) was reported from a retrospective review to result in PNET response rates of ~60%69; everolimus, in a phase1/2 study, was reported to result in tumour regression in 40% of patients with metastatic or locally unresectable PNETs70; and with bevacizumab in combination with streptozocin or capecitabine, in phase 2 studies, improved PFS in patients with metastatic NETs, including PNETs71–73. None of these studies reported on the occurrence of MEN1 in the patients (Supplementary Table 1).
Surgery
The ideal treatment for a non-metastatic single PNET is surgical excision, as this offers the only potentially curative treatment. However, surgery is often not successful in MEN1 patients compared to that in non-MEN1 patients, for the following reasons: first, MEN1-PNETs are usually multiple (Figure 2C) with sizes varying from micro-adenomas to larger than 4cm4,24,74,75 thereby making it difficult to achieve a successful surgical cure; and second, occult metastatic disease may be present76. In addition, the clinical behaviour of these PNETs also varies and it is generally considered that all macroscopic PNETs are potentially malignant, although the aggressiveness of an individual PNET cannot be accurately predicted by tumour size, radiological features, or hormone production77–79. However, studies have shown that most microadenomas are stable and infrequently increase in size; less than 20% of macroadenomas smaller than 2cm will increase in size over 10 years; approximately 50-70% of PNETs between 2-3cm will be associated with lymph node metastasis; and that 25-40% of PNETs greater than 4cm will be associated with hepatic metastasis19,24,29,80,81. Survival in patients with MEN1 correlates with non-metastatic disease; for example, survival at 15 years in MEN1 patients with gastrinomas smaller than 2.5cm in size that are associated with non-metastatic disease or metastatic disease, is reported to be 100% and 50%, respectively80–83. Thus, the occurrence of multiple PNETs and their varied and unpredictable malignant potential in MEN1 patients pose difficulties for the timing and extent of curative surgery. As a result of this MEN1 patients with PNETs frequently require additional non-surgical treatments, such as biotherapies, chemotherapy (see above) or radiological-based therapies (see below).
Radiological Therapies
Radiological therapies for PNETs include external beam radiotherapy, peptide receptor radionuclide therapy (PRRT), and interventional radiology (Table 1 and Figure 1). PNETs are not sensitive to external beam radiotherapy and PRRT is the preferred treatment14,63,64.
Peptide receptor radionuclide therapy (PRRT)
PRRT is usually based on a somatostatin analogue (e.g. octreotide, octreotate, dototate, and dotatoc) that has been labelled with a β-emitting nuclide in the form of either 177Lutetium or 90Yttrium. After binding to SSTRs (Figure 1) and internalisation of the receptor complex, the ionising radiation is released, causing damage to tumour DNA and cell death. To date, the only results assessing the efficacy of PRRT in PNETs are from cohort studies of patients, which have also included patients with other gastro-intestinal NETs and metastatic NETS84–89. These studies, which do not comment on the occurrence of MEN1 in the patients, have assessed the effects of 177Lutetium- or 90Yttrium-octreotide, and have reported objective response rates of ~20-60%84,86–88, PFS of 20-34 months86,88, and an overall survival of 53 months86. Moreover, combining 177Lutetium and 90Yttrium nuclides increased survival in a nonrandomized trial in patients with NETs including PNETs89, and the combination of 177Lu-octreotate, capecitabine and temozolomide, in a phase1/2 study, resulted in complete or partial response in >50% of the patients with in advanced NETs (including PNETs)85.
Interventional Radiology
Interventional radiology (Table 1 and Figure 1) using radiofrequency ablation (RA), transarterial embolization (TAE), transarterial chemoembolisation (TACE), and selective internal radiation therapy (SIRT) have been shown to be effective treatments in selected cases of PNETs occurring in non-MEN1 patients, or in whom the MEN1 status of the patient was not reported (Supplementary Table 1). RFA has been reported to be effective in treating primary PNETs in patients, who are unable or unwilling to undergo surgical intervention90, or who have solitary PNET hepatic metastases91. TAE, which involves systemic infusion of microparticles that cause ischemia and tissue necrosis, is reported to be effective for inoperable primary PNETs92,93, and hepatic PNET metastases94. Combining TAE of the hepatic artery with sunitinib in patients with PNETs, in a phase 2 study, has been reported to result in >65% PFS, and ~60% overall survival rates95. TACE combines TAE with locoregional chemotherapy, and TACE, using doxorubicin-eluting beads, of hepatic metastases from gastrointestinal NETs, of which ~40% were from PNETs, has been shown, in a phase 2 study, to be effective96,97, although this was associated with severe adverse events97. TACE and TAE have similar antitumour effects, but post-embolisation syndrome is commoner with TACE treatment, and the superiority of TACE over TAE in treating NETs remains unproven98. SIRT, which is used to deliver 90Yttrium-labelled spheres in the hepatic artery to provide local radiotherapy for hepatic metastases, was associated with objective tumour response in ~35% and stabile disease in ~55% of patients with NETs (10% of whom had PNETs) after 20 months99.
These medical and radiological based therapies potentially should be effective in MEN1 patients, but they require formal evaluation, as the effects of comorbidities from other endocrine and non-endocrine tumours in MEN1 patients may affect the outcomes. To date, the majority of trials have either excluded MEN1 patients, or only included occasional MEN1 patients in the non-treatment arm (Supplementary Table 1). Thus, to facilitate re-purposing of these therapies it would be important to undertake their evaluations in MEN1 patients. In addition, new therapeutic modalities based on the function of menin may help in improving the outcome and prognosis for MEN1 patients.
Emerging Therapies for MEN1-Associated PNETs from Preclinical Studies
Therapies based on increased understanding of menin and of receptors and signalling pathways in PNETs (Figure 3) are emerging and assessment of their efficacy have been facilitated by cellular and in vivo models which include conventional and conditional Men1 knockout mouse models that develop MEN1-assocaited tumours, including PNETs100–109. Menin is a ubiquitously expressed protein that functions as a nuclear scaffold protein with roles in transcriptional regulation, genome stability, cell division, cell cycle control and epigenetic regulation110–112, that enable it to influence pathways of cellular proliferation (Figure 3). For example, menin inhibits: Wnt signalling by transferring β-catenin from the nucleus, which reduces cell proliferation110,113; the activity of JunD by blocking its phosphorylation and therefore potentially subsequent interaction with co-activators111,114, causing JunD to prevent rather than promote cell growth115; and Hedgehog pathway signalling, which influences several functions including tumorigenesis, by recruitment of a protein arginine methyltransferase (PRMT5), which inactivates the Hedgehog pathway promotor Gas1116. Moreover, menin interacts with the mixed lineage leukaemia protein 1 (MLL1) histone methyltransferase complex to methylate histone H3 (Lys4), causing chromatin modification and increased transcriptional activity of genes including cyclin dependent kinase inhibitors p27 and p18, which are involved in cell cycle regulation117,118. Menin also promotes the cytostatic effects of transforming growth factor-beta (TGF-B) by interaction with the Smad pathway119–121, as well as interacting with NF-kB proteins to modulate NF-kB transactivation122. In addition, menin has roles that include interaction with the GTPase K-Ras and sons of sevenless (SOS), which prevents K-Ras-SOS interaction that is essential for K-Ras activation123. Moreover, in murine pancreatic β-cells menin activates opposing K-Ras pathways that comprise a proliferative pathway, likely via regulation of MAPK and ERK phosphorylation, and an anti-proliferative pathway via RASSF1A124. Thus, menin is considered to interact with K-Ras to block the MAPK/ERK pathway, thereby inhibiting proliferation, and menin loss removes this inhibition and leads to increased cell proliferation124. In addition, SSTR modulation of proliferation may also occur through K-Ras signalling, thereby highlighting the importance of K-Ras signalling in PNETs, and indicating that menin may play a role in SSTR downstream signalling125. Furthermore, menin acts as a suppressor of ERK-dependent phosphorylation of target proteins126, and an inhibitor of the mTOR signalling pathway, by binding to Akt and preventing its translocation to the plasma membrane127. Some recent therapies emerging from pre-clinical studies that target these menin-specific pathways (Figure 3) will be discussed, and include MEN1 gene therapy, epigenetic modulators, Wnt signalling modulators, anti-angiogeneic agents, and use of a somatostatin analogue as a chemopreventative agent.
MEN1 gene therapy
The role of menin as a tumour suppressor was supported by in vitro studies which demonstrated that menin overexpression, by use of recombinant plasmid adenoviral or retroviral vectors, in menin-null mouse embryonic fibroblasts (MEFs), RAS-transformed NIH3T3 MEFs, and rat insulinoma cell lines resulted in decreased cell proliferation and increased apoptosis127–131. In addition, injection of RAS-transformed NIH3T3 MEFs that over expressed menin, into athymic nude mice was associated with reduced tumour growth, further supporting a likely tumour suppressor role for menin in vivo128. These observations, which showed that over-expression of menin could reduce proliferation, suggested that MEN1 gene replacement therapy may be efficacious, and this was evaluated in a mouse model for MEN1, using a recombinant non-replicating adenoviral serotype 5 vector (rAd5), containing Men1 cDNA under the control of a cytomeglavirus promoter (rAd5-MEN1). To establish proof-of-principle for the efficacy of MEN1 gene therapy, the rAd5-MEN1 vector was injected into pituitary NETs that developed in conventional knockout mice lacking one allele of Men1 (Men1+/-). This Men1 gene therapy resulted in increased menin expression with decreased proliferation of the pituitary NETs, without inducing an immune response or increased apoptosis132. Moreover, the adenoviral gene therapy was not associated with a higher mortality, and these results indicate the potential utility of MEN1 gene replacement therapy for in vivo treatment of MEN1-associated NETs132. Use of a hybrid adeno-associated virus and phage (AAVP) vector displaying biologically active octreotide on the viral surface for ligand-directed delivery of the pro-apoptotic tumour necrosis factor (TNF) transgene to PNETs developing in a pancreatic specific (Pdx1-Cre) Men1 knockout mouse model, has also been reported to reduce tumour size, and improve survival of the mutant mice133. These results suggest that systemic, ligand-directed transgene treatment of MEN1-related tumours could evolve as a novel and effective treatment of MEN1-related tumours.
Epigenetic modulators
Cancer is associated with alterations in epigenetic mechanisms such as histone modifications and methylation of DNA, and epigenetic modulators represent a novel class of anti-cancer drugs134. Menin interacts with a number of histone modifying proteins including histone methyltransferase (MLL1 and PRMT5, Figure 3) and deacetylase complexes (mSin3A-histone deacetylase)10, and the use of epigenetic modulators to treat PNETs was therefore assessed utilising JQ1, an inhibitor of the bromo and extra terminal domain (BET) family of proteins that bind to acetylated histone residues to promote gene transcription. In vitro studies revealed that JQ1 decreased proliferation and increased apoptosis of PNET and bronchial NET cell lines135. These anti-proliferative effects of JQ1 were associated with: increased numbers of NET cells in G1, and decreased numbers in S and G2 phases of the cell cycle; and with increased expression of histone 2B, which was likely mediated through altered activity of BET proteins135. Assessment of JQ1 in vivo, using a pancreatic β-cell specific conditional (RIP2-Cre) Men1 knockout mouse model that develops PNETs, revealed that JQ1 decreased proliferation and increased apoptosis of PNETs. CP103, another BET inhibitor, has also been reported to reduce PNET proliferation in a human PNET cell line (BON-1) xenograft mouse model136. Thus, epigenetic modulators, e.g. via BET inhibition, may offer potential therapies for MEN1-associated PNETs.
Wnt signalling modulators
Menin inhibits Wnt signalling, as it promotes phosphorylation of β-catenin and its transfer from the nucleus, which reduces cell proliferation (Figure 3)110,113. Moreover, the conditional knockout of β-catenin in MEN1-deficient PNETs of mice with pancreatic β-cell (RIP-Cre) conditional knockout of menin, decreased the number and size of PNETs, as well as increasing survival137. These findings suggest that modulation of Wnt signalling may represent another approach for treatment of MEN1 PNETs, and use of a β-catenin antagonist (PKF115-584) decreased PNET cell proliferation in β-cell menin knockout mice137. Thus, Wnt-signalling modulators may provide a novel approach for treatment of PNETs in MEN1 patients.
Anti-angiogenic compounds
The majority of anti-angiogenic compounds block the actions of VEGF, a cytokine that promotes the growth and survival of blood vessels, and treatment with bevacizumab, an anti-VEGF monoclonal antibody, in combination with chemotherapy delayed progression of moderately well-differentiated and advanced gastro-intestinal NETs, which included metastatic well-differentiated, PNETs71–73,138. However, such inhibition of VEGF signalling has been reported to be accompanied by increased invasiveness and metastasis of cancers and PNETs developing in a transgenic mouse model that expressed the large T antigen (Tag) encoded by the simian virus 40 (SV40) under the control of the rat insulin promoter (RIP) and had been rendered null for the recombinase activator gene Rag1 (RIP-Tag2,Rag1 knockout mice)139. This progression of the cancers and PNETs was reported to be associated with increased tumour hypoxia and expression of pro-angiogeneic factors including VEGFA, members of the fibroblast growth factor (FGF) family, Ephrin A1, and c-Met activation139. In addition, treatment with RTK inhibitors (e.g. sunitinib), which act on VEGF and PDGF receptors, decrease growth of PNETs in mice, but increase tumour invasiveness and liver metastases, possibly by upregulation of proangiogenic factors including FGFs140, and thus targeting FGFs in addition to VEGF and PDGF may improve efficacy141. Furthermore, combining sunitinib, or an anti-VEGF antibody, with inhibitors (e.g. PF-04217903 or PF-0241066 (crizotinib)) of c-Met, which promotes cell proliferation, invasion and metastasis, prevented these increases in tumour aggressiveness without any impairment to restriction of tumour growth142. These findings indicate that the invasion and metastasis that are promoted by selective inhibition of VEGF signalling, may be reduced by combined treatment with a c-met inhibitor. In addition, inhibition of nitric oxide (NO) synthase may have a role, as demonstrated by use of L-arginine methyl ester (L-NAME), a NO synthase inhibitor, which in ex vivo treatment of PNETs from conventional Men1+/- knockout mice, caused impaired blood perfusion and increased constriction of the tumour supplying arterioles143. Trombospondin-1 (Figure 3) analogues may also have a role, as administration of thrombospondin-1 suppressed angiogenesis and tumour growth of PNETs in a transgenic mouse model (RIP-Tag2)144, and it is of interest to note that menin interacts with Smad3 which is downstream of the thrombospondin-1-transforming growth factor beta (TGFβ) signalling pathway145.
Use of somatostatin analogue for tumor chemoprevention
Cancer chemoprevention involves the chronic administration of a synthetic, natural, or biological agent to reduce or delay the occurrence of tumours, and its value has been demonstrated in breast, prostate, and colon cancer trials146–149. Currently, individuals with a MEN1 mutation are entered into a screening programme for MEN1-associated tumours, including PNETs, commencing from the first or second decade of life21. This approach will detect tumours early, thereby facilitating earlier treatment (e.g. surgery), but will not prevent or delay the occurrence of tumours, i.e. chemoprevention. Somatostatin analogues may have a potential role in chemoprevention of MEN1-associated NETs as they have been shown to have antiproliferative, and antisecretary effects on NETs36,37,150,151. Thus, treatment with pasireotide, which is a multiple-receptor-targeted somatostatin analogue that acts via SSTR1,2,3 and SSTR532, decreased proliferation and increased apoptosis of PNETs in Men1+/- and Pdx-Cre Men1 knockout mutant mice152,153. Pasireotide also decreased proliferation and increased apoptosis of pituitary NETs, in Men1+/- mice, as well as increasing survival of the Men1+/- mutant mice152,153. In addition to these anti-proliferative and pro-apoptotic effects, pasireotide was also found to inhibit the development of PNETs in the Men1+/- mutant mice152. Thus, PNET occurrence was significantly lower in Men1+/- mice treated with pasireotide when compared to Men1+/- mice treated with control phosphate buffered saline (PBS) (87% of pasireotide-treated versus ~97% of PBS-treated mice, p<0.05)152. Moreover, the mean number of PNETs per pancreas was also significantly lower in the pasireotide-treated Men1+/- mice when compared to PBS-treated Men1+/- mice (2.36± 0.25 in pasireotide-treated versus 3.72± 0.32, p<0.001). These findings, which indicate that pasireotide-treatment resulted in fewer Men1+/- mice with PNETs who also had fewer PNETs per pancreas, when compared to PBS-treated Men1+/- mice, are consistent with a lower development of new NETs, in pasireotide treated Men1+/- mice152. Moreover, the pasireotide treated Men1+/- mice appeared healthier and had increased survival than the placebo treated Men1+/- mice, and adverse effects from the pasireotide treatment were not detected152. These findings, suggest that somatostatin analogues may have a chemopreventative role in the treatment of MEN1-associated PNETs in humans, and two studies have reported that somatostatin analogues have anti-proliferative actions in PNETs of MEN1 patients38,154. In one study octreotide was given to MEN1 patients to treat duodeno-pancreatic NETs, and retrospective analysis revealed that 10% of PNETs had tumour response, and that 80% had stable disease38; and in another study, octreotide was given prospectively to 8 MEN1 patients with GEP-NETs, and found to be safe and decrease gastro-intestinal hormone secretion, and to be associated with stable PNET disease154.
Conclusions
MEN1 is an autosomal dominant disorder characterised by the combined occurrence of tumours in different endocrine glands. The associated hypersecretion of hormones and malignant potential of these tumours severely reduces life expectancy for MEN1 patients. Current medical, surgical and radiological treatments for MEN1 patients, which are based on their effectiveness in treating endocrine tumours in non-MEN1 patients, are associated with inferior outcomes. MEN1 is caused by mutations of the MEN1 gene, encoding the tumour suppressor protein, menin, and increased understanding of the role of menin in tumourigenesis and cell proliferation, has enabled targeted therapies to be identified. These new treatments, which have been evaluated in pre-clinical studies include: adenoviral MEN1 gene replacement therapy; epigenetic modulators; Wnt pathway-targeting β-catenin antagonists; thrombospondin-1 analogues; and a multiple-receptor-targeted somatostatin analogue. Clinical evaluation of such emerging treatments targeting menin-associated pathways may provide new therapies for improving outcomes, and life expectancy in MEN1 patients.
Supplementary Material
Box 1. Multiple Endocrine Neoplasia type 1 (MEN1) clinical features and emerging therapies.
Clinical features and genetics
Multiple Endocrine Neoplasia type 1 (MEN1) is an autosomal dominant disorder characterised by the combined occurrence of tumours of the parathyroids or neuroendocrine tumours (NETs) of the pancreas, pituitary and adrenal. Patients may also develop other endocrine or non-endocrine tumours. The 20-year survival of MEN1 patients is lower than the general population, and >65% of MEN1 patients die from MEN1-related disease. MEN1 is caused by mutations of the MEN1 gene, encoding the tumour suppressor protein menin, a nuclear scaffold protein with roles in gene transcription, genome stabilisation, cell cycle and epigenetic regulation.
Current treatments
Current medical, surgical and radiological treatments, have not been evaluated in MEN1 patients, but instead successful treatments for endocrine tumours in non-MEN1 patients have been extrapolated as treatments for MEN1 patients. However, the outcome of such treatments in MEN1 patients are greatly inferior to those in non-MEN1 patients. This may be due to the: concomitant occurrence of multiple tumours in different glands; presence of occult metastatic disease; associated co-morbidities that may decrease survival rates; and the younger onset that may result in larger and more aggressive tumours that are resistant to treatment. Improved treatments for MEN1 tumours are required.
Emerging therapies
Increased understanding of the role of menin in cell proliferation has enabled targeted therapy of endocrine tumours in mouse models for NETs with MEN1 mutations. These include: adenoviral MEN1 gene replacement therapy; a hybrid adeno-associated virus and phage vector containing a ligand motif and tumour necrosis factor transgene for tumour directed therapy, and inducing apoptosis; epigenetic modulators; Wnt pathway targeting β-catenin antagonists; Thrombospondin-1 analogues; receptor tyrosine kinase inhibitors; mammalian target of rapamycin (mTOR) inhibitors; and somatostatin analogues, which target a broader spectrum of receptors and may have a potential role in tumour chemoprevention.
Key points.
Patients with MEN1 may develop hormone secreting and non-secreting tumours in endocrine organs including the pancreas, which decreases their life expectancy substantially.
MEN1-related tumours are difficult to treat due to differences in growth potential, concomitant development of other tumours, and relative insensitivity to treatment.
Current medical, surgical and radiological treatments have not been formally assessed in MEN1 patients, but instead been used on the basis of their effects on endocrine tumours in non-MEN1 patients.
Therapies targeting MEN1 tumours are required, and preclinical studies indicate that gene therapy, epigenetic modifiers such as bromo- and extra terminal domain (BET) inhibitors, which are acetyl code-reader inhibitors, and Wnt pathway and VEGF-signalling antagonists may be promising treatments.
Chemoprevention aimed at reducing or delaying the occurrence of MEN1-pancreatic neuroendocrine tumours may be possible by chronic administration of somatostatin analogues, which have anti-proliferative and anti-secretary actions.
Acknowledgements
This work was funded by the United Kingdom Medical Research Council (MRC) program Grants G9825289 and G1000467 (KEL, and RVT), Danish Council for Independent Research (MF) and National Institute for Health Research (NIHR)-Oxford Biomedical Research Centre Programme. RVT is a Wellcome Trust Investigator and NIHR Senior Investigator.
Author Biographies
Morten Frost has been a clinician at the Endocrine Research Unit, University of Southern Denmark, since 2003, and received his Ph.D. from the University of Southern Denmark in 2011. In 2016, he visited Professor Rajesh Thakker’s laboratory at the University of Oxford to research the genetics of neuroendocrine tumour patients, as well as calcium sensing receptor signalling. His research currently involves investigating monogenic bone diseases and calcium metabolic disorders.
Kate Lines received her PhD in Molecular Oncology from the Barts Cancer Institute, Queen Mary University of London, UK in 2011, and is currently a postdoctoral research assistant in the laboratory of Professor Raj Thakker at the University of Oxford. Her research currently focuses on elucidating the epigenetic mechanisms occurring in neuroendocrine tumours, and targeting these mechanisms to develop novel therapeutic approaches.
Rajesh Thakker is the May Professor of Medicine at the University of Oxford, and a Fellow of the Royal Society. He has pursued molecular, cellular, and physiological analyses of >15 disorders, with identification of their defective genes and functional studies that explain the disease phenotypes. This has resulted in the elucidation of: molecular mechanisms of endocrine tumour formation and potential new therapeutic targets; signalling and regulatory pathways downstream of the calcium-sensing receptor, and molecular aspects of renal tubular physiology. He was the lead author for the recently published clinical guidelines for multiple endocrine neoplasia type 1 (MEN1).
Footnotes
Disclosures: The authors declare that they have no competing interests as defined by Springer Nature, or other interests that might be perceived to influence the interpretation of the article.
References
- 1.Dasari A, et al. Trends in the Incidence, Prevalence, and Survival Outcomes in Patients With Neuroendocrine Tumors in the United States. JAMA oncology. 2017 doi: 10.1001/jamaoncol.2017.0589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Halfdanarson TR, Rabe KG, Rubin J, Petersen GM. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol. 2008;19:1727–1733. doi: 10.1093/annonc/mdn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lepage C, et al. Incidence and management of malignant digestive endocrine tumours in a well defined French population. Gut. 2004;53:549–553. doi: 10.1136/gut.2003.026401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anlauf M, et al. Hereditary neuroendocrine tumors of the gastroenteropancreatic system. Virchows Arch. 2007;451(Suppl 1):S29–38. doi: 10.1007/s00428-007-0450-3. [DOI] [PubMed] [Google Scholar]
- 5.Falconi M, et al. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology. 2016;103:153–171. doi: 10.1159/000443171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marx SJ, Simonds WF. Hereditary hormone excess: genes, molecular pathways, and syndromes. Endocrine reviews. 2005;26:615–661. doi: 10.1210/er.2003-0037. [DOI] [PubMed] [Google Scholar]
- 7.Lemos MC, Thakker RV. Multiple endocrine neoplasia type 1 (MEN1): analysis of 1336 mutations reported in the first decade following identification of the gene. Hum Mutat. 2008;29:22–32. doi: 10.1002/humu.20605. [DOI] [PubMed] [Google Scholar]
- 8.Jiao Y, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199–1203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scarpa A, et al. Corrigendum: Whole-genome landscape of pancreatic neuroendocrine tumours. Nature. 2017 doi: 10.1038/nature24026. [DOI] [PubMed] [Google Scholar]
- 10.Thakker RV. Multiple endocrine neoplasia type 1 (MEN1) and type 4 (MEN4) Molecular and cellular endocrinology. 2014;386:2–15. doi: 10.1016/j.mce.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dreijerink KM, Goudet P, Burgess JR, Valk GD. Breast-cancer predisposition in multiple endocrine neoplasia type 1. N Engl J Med. 2014;371:583–584. doi: 10.1056/NEJMc1406028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marx S, et al. Multiple endocrine neoplasia type 1: clinical and genetic topics. Ann Intern Med. 1998;129:484–494. doi: 10.7326/0003-4819-129-6-199809150-00011. [DOI] [PubMed] [Google Scholar]
- 13.Schaaf L, et al. Developing effective screening strategies in multiple endocrine neoplasia type 1 (MEN 1) on the basis of clinical and sequencing data of German patients with MEN 1. Exp Clin Endocrinol Diabetes. 2007;115:509–517. doi: 10.1055/s-2007-970160. [DOI] [PubMed] [Google Scholar]
- 14.Yates CJ, Newey PJ, Thakker RV. Challenges and controversies in management of pancreatic neuroendocrine tumours in patients with MEN1. Lancet Diabetes Endocrinol. 2015;3:895–905. doi: 10.1016/s2213-8587(15)00043-1. [DOI] [PubMed] [Google Scholar]
- 15.Frilling A, et al. Neuroendocrine tumor disease: an evolving landscape. Endocr Relat Cancer. 2012;19:R163–185. doi: 10.1530/erc-12-0024. [DOI] [PubMed] [Google Scholar]
- 16.Dean PG, et al. Are patients with multiple endocrine neoplasia type I prone to premature death? World J Surg. 2000;24:1437–1441. doi: 10.1007/s002680010237. [DOI] [PubMed] [Google Scholar]
- 17.Bassett JH, et al. Characterization of mutations in patients with multiple endocrine neoplasia type 1. Am J Hum Genet. 1998;62:232–244. doi: 10.1086/301729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Concolino P, Costella A, Capoluongo E. Multiple endocrine neoplasia type 1 (MEN1): An update of 208 new germline variants reported in the last nine years. Cancer genetics. 2016;209:36–41. doi: 10.1016/j.cancergen.2015.12.002. [DOI] [PubMed] [Google Scholar]
- 19.Goudet P, et al. Risk factors and causes of death in MEN1 disease. A GTE (Groupe d'Etude des Tumeurs Endocrines) cohort study among 758 patients. World J Surg. 2010;34:249–255. doi: 10.1007/s00268-009-0290-1. [DOI] [PubMed] [Google Scholar]
- 20.Ito T, Igarashi H, Uehara H, Berna MJ, Jensen RT. Causes of death and prognostic factors in multiple endocrine neoplasia type 1: a prospective study: comparison of 106 MEN1/Zollinger-Ellison syndrome patients with 1613 literature MEN1 patients with or without pancreatic endocrine tumors. Medicine (Baltimore) 2013;92:135–181. doi: 10.1097/MD.0b013e3182954af1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thakker RV, et al. Clinical practice guidelines for multiple endocrine neoplasia type 1 (MEN1) J Clin Endocrinol Metab. 2012;97:2990–3011. doi: 10.1210/jc.2012-1230. [DOI] [PubMed] [Google Scholar]
- 22.Pieterman CR, et al. Multiple endocrine neoplasia type 1 (MEN1): its manifestations and effect of genetic screening on clinical outcome. Clin Endocrinol (Oxf) 2009;70:575–581. doi: 10.1111/j.1365-2265.2008.03324.x. [DOI] [PubMed] [Google Scholar]
- 23.Newey PJ, et al. Asymptomatic children with multiple endocrine neoplasia type 1 mutations may harbor nonfunctioning pancreatic neuroendocrine tumors. J Clin Endocrinol Metab. 2009;94:3640–3646. doi: 10.1210/jc.2009-0564. [DOI] [PubMed] [Google Scholar]
- 24.Jensen RT, Berna MJ, Bingham DB, Norton JA. Inherited pancreatic endocrine tumor syndromes: advances in molecular pathogenesis, diagnosis, management, and controversies. Cancer. 2008;113:1807–1843. doi: 10.1002/cncr.23648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Conemans EB, et al. Prognostic Factors For Survival Of MEN1 Patients With Duodenopancreatic Tumours Metastatic To The Liver: Results From The DMSG. Endocrine practice : official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2017;23:641–648. doi: 10.4158/ep161639.or. [DOI] [PubMed] [Google Scholar]
- 26.Akerstrom G, Hellman P. Surgery on neuroendocrine tumours. Best Pract Res Clin Endocrinol Metab. 2007;21:87–109. doi: 10.1016/j.beem.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 27.Jensen RT. Management of the Zollinger-Ellison syndrome in patients with multiple endocrine neoplasia type 1. J Intern Med. 1998;243:477–488. doi: 10.1046/j.1365-2796.1998.00281.x. [DOI] [PubMed] [Google Scholar]
- 28.Trouillas J, et al. Pituitary tumors and hyperplasia in multiple endocrine neoplasia type 1 syndrome (MEN1): a case-control study in a series of 77 patients versus 2509 non-MEN1 patients. Am J Surg Pathol. 2008;32:534–543. doi: 10.1097/PAS.0b013e31815ade45. [DOI] [PubMed] [Google Scholar]
- 29.Fraenkel M, Kim MK, Faggiano A, Valk GD. Epidemiology of gastroenteropancreatic neuroendocrine tumours. Best practice & research. Clinical gastroenterology. 2012;26:691–703. doi: 10.1016/j.bpg.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Rindi G, et al. TNM staging of neoplasms of the endocrine pancreas: results from a large international cohort study. Journal of the National Cancer Institute. 2012;104:764–777. doi: 10.1093/jnci/djs208. [DOI] [PubMed] [Google Scholar]
- 31.Cakir M, Dworakowska D, Grossman A. Somatostatin receptor biology in neuroendocrine and pituitary tumours: part 1--molecular pathways. J Cell Mol Med. 2010;14:2570–2584. doi: 10.1111/j.1582-4934.2010.01125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmid HA, Silva AP. Short- and long-term effects of octreotide and SOM230 on GH, IGF-I, ACTH, corticosterone and ghrelin in rats. J Endocrinol Invest. 2005;28:28–35. [PubMed] [Google Scholar]
- 33.Walter T, Brixi-Benmansour H, Lombard-Bohas C, Cadiot G. New treatment strategies in advanced neuroendocrine tumours. Digestive and liver disease : official journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver. 2012;44:95–105. doi: 10.1016/j.dld.2011.08.022. [DOI] [PubMed] [Google Scholar]
- 34.Martin-Richard M, et al. Antiproliferative effects of lanreotide autogel in patients with progressive, well-differentiated neuroendocrine tumours: a Spanish, multicentre, open-label, single arm phase II study. BMC Cancer. 2013;13:427. doi: 10.1186/1471-2407-13-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Palazzo M, et al. Ki67 proliferation index, hepatic tumor load, and pretreatment tumor growth predict the antitumoral efficacy of lanreotide in patients with malignant digestive neuroendocrine tumors. European journal of gastroenterology & hepatology. 2013;25:232–238. doi: 10.1097/MEG.0b013e328359d1a6. [DOI] [PubMed] [Google Scholar]
- 36.Caplin ME, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224–233. doi: 10.1056/NEJMoa1316158. [DOI] [PubMed] [Google Scholar]
- 37.Caplin ME, et al. Anti-tumour effects of lanreotide for pancreatic and intestinal neuroendocrine tumours: the CLARINET open-label extension study. Endocr Relat Cancer. 2016;23:191–199. doi: 10.1530/ERC-15-0490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ramundo V, et al. Impact of long-acting octreotide in patients with early-stage MEN1-related duodeno-pancreatic neuroendocrine tumours. Clin Endocrinol (Oxf) 2014;80:850–855. doi: 10.1111/cen.12411. [DOI] [PubMed] [Google Scholar]
- 39.Yao JC, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–523. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lombard-Bohas C, et al. Impact of prior chemotherapy use on the efficacy of everolimus in patients with advanced pancreatic neuroendocrine tumors: a subgroup analysis of the phase III RADIANT-3 trial. Pancreas. 2015;44:181–189. doi: 10.1097/MPA.0000000000000262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yao JC, et al. Everolimus for the Treatment of Advanced Pancreatic Neuroendocrine Tumors: Overall Survival and Circulating Biomarkers From the Randomized, Phase III RADIANT-3 Study. J Clin Oncol. 2016 doi: 10.1200/JCO.2016.68.0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oh DY, et al. Phase 2 study of everolimus monotherapy in patients with nonfunctioning neuroendocrine tumors or pheochromocytomas/paragangliomas. Cancer. 2012;118:6162–6170. doi: 10.1002/cncr.27675. [DOI] [PubMed] [Google Scholar]
- 43.Hanahan D, Christofori G, Naik P, Arbeit J. Transgenic mouse models of tumour angiogenesis: the angiogenic switch, its molecular controls, and prospects for preclinical therapeutic models. Eur J Cancer. 1996;32A:2386–2393. doi: 10.1016/s0959-8049(96)00401-7. [DOI] [PubMed] [Google Scholar]
- 44.Scoazec JY. Angiogenesis in neuroendocrine tumors: therapeutic applications. Neuroendocrinology. 2013;97:45–56. doi: 10.1159/000338371. [DOI] [PubMed] [Google Scholar]
- 45.Raymond E, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–513. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 46.Ahn HK, et al. Phase II study of pazopanib monotherapy in metastatic gastroenteropancreatic neuroendocrine tumours. Br J Cancer. 2013;109:1414–1419. doi: 10.1038/bjc.2013.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pavel ME, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005–2012. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]
- 48.Phan AT, et al. Pazopanib and depot octreotide in advanced, well-differentiated neuroendocrine tumours: a multicentre, single-group, phase 2 study. Lancet Oncol. 2015;16:695–703. doi: 10.1016/S1470-2045(15)70136-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hobday TJ, et al. Multicenter Phase II Trial of Temsirolimus and Bevacizumab in Pancreatic Neuroendocrine Tumors. J Clin Oncol. 2015;33:1551–1556. doi: 10.1200/JCO.2014.56.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kulke M. Randomized phase II study of everolimus (E) versus everolimus plus bevacizumab (E+B) in patients (Pts) with locally advanced or metastatic pancreatic neuroendocrine tumors (pNET), CALGB 80701 (Alliance) J Clin Oncol. 2015;33(Suppl) abstr 4005. [Google Scholar]
- 51.Yao JC, et al. Perfusion computed tomography as functional biomarker in randomized run-in study of bevacizumab and everolimus in well-differentiated neuroendocrine tumors. Pancreas. 2015;44:190–197. doi: 10.1097/MPA.0000000000000255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cives M, et al. Phase II clinical trial of pasireotide long-acting repeatable in patients with metastatic neuroendocrine tumors. Endocr Relat Cancer. 2015;22:1–9. doi: 10.1530/ERC-14-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yao JC, et al. Phase I dose-escalation study of long-acting pasireotide in patients with neuroendocrine tumors. OncoTargets and therapy. 2017;10:3177–3186. doi: 10.2147/ott.s128547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chan JA, et al. Phase I study of pasireotide (SOM 230) and everolimus (RAD001) in advanced neuroendocrine tumors. Endocr Relat Cancer. 2012;19:615–623. doi: 10.1530/ERC-11-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Faiss S, et al. Prospective, randomized, multicenter trial on the antiproliferative effect of lanreotide, interferon alfa, and their combination for therapy of metastatic neuroendocrine gastroenteropancreatic tumors--the International Lanreotide and Interferon Alfa Study Group. J Clin Oncol. 2003;21:2689–2696. doi: 10.1200/JCO.2003.12.142. [DOI] [PubMed] [Google Scholar]
- 56.Hopfner M, Baradari V, Huether A, Schofl C, Scherubl H. The insulin-like growth factor receptor 1 is a promising target for novel treatment approaches in neuroendocrine gastrointestinal tumours. Endocr Relat Cancer. 2006;13:135–149. doi: 10.1677/erc.1.01090. [DOI] [PubMed] [Google Scholar]
- 57.von Wichert G, et al. Insulin-like growth factor-I is an autocrine regulator of chromogranin A secretion and growth in human neuroendocrine tumor cells. Cancer Res. 2000;60:4573–4581. [PubMed] [Google Scholar]
- 58.Reidy-Lagunes DL, et al. A phase 2 study of the insulin-like growth factor-1 receptor inhibitor MK-0646 in patients with metastatic, well-differentiated neuroendocrine tumors. Cancer. 2012;118:4795–4800. doi: 10.1002/cncr.27459. [DOI] [PubMed] [Google Scholar]
- 59.Strosberg JR, et al. A multi-institutional, phase II open-label study of ganitumab (AMG 479) in advanced carcinoid and pancreatic neuroendocrine tumors. Endocr Relat Cancer. 2013;20:383–390. doi: 10.1530/ERC-12-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dasari A, et al. Phase I study of the anti-IGF1R antibody cixutumumab with everolimus and octreotide in advanced well-differentiated neuroendocrine tumors. Endocr Relat Cancer. 2015;22:431–441. doi: 10.1530/ERC-15-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Bendell JC, et al. A Phase II Study of the Combination of Bevacizumab, Pertuzumab, and Octreotide LAR for Patients with Advanced Neuroendocrine Cancers. Cancer Invest. 2016;34:213–219. doi: 10.3109/07357907.2016.1174257. [DOI] [PubMed] [Google Scholar]
- 62.Fazio N, et al. A Phase II Study of BEZ235 in Patients with Everolimus-resistant, Advanced Pancreatic Neuroendocrine Tumours. Anticancer Res. 2016;36:713–719. [PMC free article] [PubMed] [Google Scholar]
- 63.Strosberg J. Advances in the Treatment of Pancreatic Neuroendocrine Tumors (pNETs) Gastrointestinal cancer research : GCR. 2013;6:S10–12. [PMC free article] [PubMed] [Google Scholar]
- 64.Hammel P, et al. New treatment options with cytotoxic agents in neuroendocrine tumours. Target Oncol. 2012;7:169–172. doi: 10.1007/s11523-012-0228-7. [DOI] [PubMed] [Google Scholar]
- 65.Moertel CG, Hanley JA, Johnson LA. Streptozocin alone compared with streptozocin plus fluorouracil in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1980;303:1189–1194. doi: 10.1056/nejm198011203032101. [DOI] [PubMed] [Google Scholar]
- 66.Moertel CG, Lefkopoulo M, Lipsitz S, Hahn RG, Klaassen D. Streptozocin-doxorubicin, streptozocin-fluorouracil or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1992;326:519–523. doi: 10.1056/nejm199202203260804. [DOI] [PubMed] [Google Scholar]
- 67.Kouvaraki MA, et al. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol. 2004;22:4762–4771. doi: 10.1200/jco.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 68.Ekeblad S, et al. Temozolomide as monotherapy is effective in treatment of advanced malignant neuroendocrine tumors. Clin Cancer Res. 2007;13:2986–2991. doi: 10.1158/1078-0432.ccr-06-2053. [DOI] [PubMed] [Google Scholar]
- 69.Isacoff WH, Moss RA, Pecora AL, Fine RL. Temozolomide/capecitabine therapy for metastatic neuroendocrine tumors of the pancreas. A retrospective review. Journal of Clinical Oncology. 2006;24:14023–14023. doi: 10.1200/jco.2006.24.18_suppl.14023. [DOI] [Google Scholar]
- 70.Chan JA, et al. A prospective, phase 1/2 study of everolimus and temozolomide in patients with advanced pancreatic neuroendocrine tumor. Cancer. 2013;119:3212–3218. doi: 10.1002/cncr.28142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ducreux M, et al. Bevacizumab combined with 5-FU/streptozocin in patients with progressive metastatic well-differentiated pancreatic endocrine tumours (BETTER trial)--a phase II non-randomised trial. Eur J Cancer. 2014;50:3098–3106. doi: 10.1016/j.ejca.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 72.Berruti A, et al. Bevacizumab plus octreotide and metronomic capecitabine in patients with metastatic well-to-moderately differentiated neuroendocrine tumors: the XELBEVOCT study. BMC Cancer. 2014;14:184. doi: 10.1186/1471-2407-14-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chan JA, et al. Prospective study of bevacizumab plus temozolomide in patients with advanced neuroendocrine tumors. J Clin Oncol. 2012;30:2963–2968. doi: 10.1200/JCO.2011.40.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Anlauf M, et al. Microadenomatosis of the endocrine pancreas in patients with and without the multiple endocrine neoplasia type 1 syndrome. Am J Surg Pathol. 2006;30:560–574. doi: 10.1097/01.pas.0000194044.01104.25. [DOI] [PubMed] [Google Scholar]
- 75.Trump D, et al. Clinical studies of multiple endocrine neoplasia type 1 (MEN1) QJM : monthly journal of the Association of Physicians. 1996;89:653–669. doi: 10.1093/qjmed/89.9.653. [DOI] [PubMed] [Google Scholar]
- 76.Anlauf M, et al. Primary lymph node gastrinoma or occult duodenal microgastrinoma with lymph node metastases in a MEN1 patient: the need for a systematic search for the primary tumor. Am J Surg Pathol. 2008;32:1101–1105. doi: 10.1097/PAS.0b013e3181655811. [DOI] [PubMed] [Google Scholar]
- 77.Lowney JK, Frisella MM, Lairmore TC, Doherty GM. Pancreatic islet cell tumor metastasis in multiple endocrine neoplasia type 1: correlation with primary tumor size. Surgery. 1998;124:1043–1048. doi: 10.1067/msy.1998.92561. discussion 1048-1049. [DOI] [PubMed] [Google Scholar]
- 78.Triponez F, et al. Is surgery beneficial for MEN1 patients with small (< or = 2 cm), nonfunctioning pancreaticoduodenal endocrine tumor? An analysis of 65 patients from the GTE. World J Surg. 2006;30:654–662. doi: 10.1007/s00268-005-0354-9. discussion 663-654. [DOI] [PubMed] [Google Scholar]
- 79.Sakurai A, et al. Long-term follow-up of patients with multiple endocrine neoplasia type 1. Endocrine journal. 2007;54:295–302. doi: 10.1507/endocrj.k06-147. [DOI] [PubMed] [Google Scholar]
- 80.Norton JA. Surgical treatment and prognosis of gastrinoma. Best practice & research. Clinical gastroenterology. 2005;19:799–805. doi: 10.1016/j.bpg.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 81.Norton JA, et al. Surgery to cure the Zollinger-Ellison syndrome. N Engl J Med. 1999;341:635–644. doi: 10.1056/nejm199908263410902. [DOI] [PubMed] [Google Scholar]
- 82.Cadiot G, et al. Prognostic factors in patients with Zollinger-Ellison syndrome and multiple endocrine neoplasia type 1. Groupe d'Etude des Neoplasies Endocriniennes Multiples (GENEM and groupe de Recherche et d'Etude du Syndrome de Zollinger-Ellison (GRESZE) Gastroenterology. 1999;116:286–293. doi: 10.1016/s0016-5085(99)70124-1. [DOI] [PubMed] [Google Scholar]
- 83.Wells SA, Norton JA, Thompson NW, Friesen SR. Comparison of surgical results in patients with advanced and limited disease with multiple endocrine neoplasia type 1 and Zollinger-Ellison syndrome - Discussion. Annals of Surgery. 2001;234:505–506. doi: 10.1097/00000658-200110000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bodei L, et al. Peptide receptor radionuclide therapy with (1)(7)(7)Lu-DOTATATE: the IEO phase I-II study. Eur J Nucl Med Mol Imaging. 2011;38:2125–2135. doi: 10.1007/s00259-011-1902-1. [DOI] [PubMed] [Google Scholar]
- 85.Claringbold PG, Price RA, Turner JH. Phase I-II study of radiopeptide 177Lu-octreotate in combination with capecitabine and temozolomide in advanced low-grade neuroendocrine tumors. Cancer Biother Radiopharm. 2012;27:561–569. doi: 10.1089/cbr.2012.1276. [DOI] [PubMed] [Google Scholar]
- 86.Ezziddin S, et al. Outcome of peptide receptor radionuclide therapy with 177Lu-octreotate in advanced grade 1/2 pancreatic neuroendocrine tumours. Eur J Nucl Med Mol Imaging. 2014;41:925–933. doi: 10.1007/s00259-013-2677-3. [DOI] [PubMed] [Google Scholar]
- 87.Imhof A, et al. Response, survival, and long-term toxicity after therapy with the radiolabeled somatostatin analogue [90Y-DOTA]-TOC in metastasized neuroendocrine cancers. J Clin Oncol. 2011;29:2416–2423. doi: 10.1200/JCO.2010.33.7873. [DOI] [PubMed] [Google Scholar]
- 88.Sansovini M, et al. Treatment with the radiolabelled somatostatin analog Lu-DOTATATE for advanced pancreatic neuroendocrine tumors. Neuroendocrinology. 2013;97:347–354. doi: 10.1159/000348394. [DOI] [PubMed] [Google Scholar]
- 89.Villard L, et al. Cohort study of somatostatin-based radiopeptide therapy with [(90)Y-DOTA]-TOC versus [(90)Y-DOTA]-TOC plus [(177)Lu-DOTA]-TOC in neuroendocrine cancers. J Clin Oncol. 2012;30:1100–1106. doi: 10.1200/JCO.2011.37.2151. [DOI] [PubMed] [Google Scholar]
- 90.Rossi S, et al. Radiofrequency ablation of pancreatic neuroendocrine tumors: a pilot study of feasibility, efficacy, and safety. Pancreas. 2014;43:938–945. doi: 10.1097/MPA.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 91.Gamblin TC, Christians K, Pappas SG. Radiofrequency ablation of neuroendocrine hepatic metastasis. Surg Oncol Clin N Am. 2011;20:273–279. doi: 10.1016/j.soc.2010.11.002. vii-viii. [DOI] [PubMed] [Google Scholar]
- 92.Orgera G, et al. Current status of Interventional Radiology in the management of Gastro-Entero-Pancreatic Neuroendocrine Tumours (GEP-NETs) Cardiovasc Intervent Radiol. 2015;38:13–24. doi: 10.1007/s00270-014-1005-z. [DOI] [PubMed] [Google Scholar]
- 93.Peppa M, et al. Embolization as an alternative treatment of insulinoma in a patient with multiple endocrine neoplasia type 1 syndrome. Cardiovasc Intervent Radiol. 2009;32:807–811. doi: 10.1007/s00270-008-9499-x. [DOI] [PubMed] [Google Scholar]
- 94.Pavel M, et al. ENETS Consensus Guidelines for the management of patients with liver and other distant metastases from neuroendocrine neoplasms of foregut, midgut, hindgut, and unknown primary. Neuroendocrinology. 2012;95:157–176. doi: 10.1159/000335597. [DOI] [PubMed] [Google Scholar]
- 95.Strosberg JR, et al. A phase II clinical trial of sunitinib following hepatic transarterial embolization for metastatic neuroendocrine tumors. Ann Oncol. 2012;23:2335–2341. doi: 10.1093/annonc/mdr614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gaur SK, et al. Hepatic arterial chemoembolization using drug-eluting beads in gastrointestinal neuroendocrine tumor metastatic to the liver. Cardiovasc Intervent Radiol. 2011;34:566–572. doi: 10.1007/s00270-011-0122-1. [DOI] [PubMed] [Google Scholar]
- 97.Bhagat N, et al. Phase II study of chemoembolization with drug-eluting beads in patients with hepatic neuroendocrine metastases: high incidence of biliary injury. Cardiovasc Intervent Radiol. 2013;36:449–459. doi: 10.1007/s00270-012-0424-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Fiore F, et al. Transarterial embolization (TAE) is equally effective and slightly safer than transarterial chemoembolization (TACE) to manage liver metastases in neuroendocrine tumors. Endocrine. 2014;47:177–182. doi: 10.1007/s12020-013-0130-9. [DOI] [PubMed] [Google Scholar]
- 99.Barbier CE, Garske-Roman U, Sandstrom M, Nyman R, Granberg D. Selective internal radiation therapy in patients with progressive neuroendocrine liver metastases. Eur J Nucl Med Mol Imaging. 2016;43:1425–1431. doi: 10.1007/s00259-015-3264-6. [DOI] [PubMed] [Google Scholar]
- 100.Wiedemann T, Pellegata NS. Animal models of multiple endocrine neoplasia. Molecular and cellular endocrinology. 2016;421:49–59. doi: 10.1016/j.mce.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 101.Bertolino P, Tong WM, Galendo D, Wang ZQ, Zhang CX. Heterozygous Men1 mutant mice develop a range of endocrine tumors mimicking multiple endocrine neoplasia type 1. Mol Endocrinol. 2003;17:1880–1892. doi: 10.1210/me.2003-0154. [DOI] [PubMed] [Google Scholar]
- 102.Bertolino P, et al. Pancreatic beta-cell-specific ablation of the multiple endocrine neoplasia type 1 (MEN1) gene causes full penetrance of insulinoma development in mice. Cancer Res. 2003;63:4836–4841. [PubMed] [Google Scholar]
- 103.Biondi CA, et al. Conditional inactivation of the MEN1 gene leads to pancreatic and pituitary tumorigenesis but does not affect normal development of these tissues. Mol Cell Biol. 2004;24:3125–3131. doi: 10.1128/MCB.24.8.3125-3131.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Crabtree JS, et al. A mouse model of multiple endocrine neoplasia, type 1, develops multiple endocrine tumors. Proc Natl Acad Sci U S A. 2001;98:1118–1123. doi: 10.1073/pnas.98.3.1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Crabtree JS, et al. Of mice and MEN1: Insulinomas in a conditional mouse knockout. Mol Cell Biol. 2003;23:6075–6085. doi: 10.1128/MCB.23.17.6075-6085.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Gannon M, Shiota C, Postic C, Wright CV, Magnuson M. Analysis of the Cre-mediated recombination driven by rat insulin promoter in embryonic and adult mouse pancreas. Genesis (New York, N.Y. : 2000) 2000;26:139–142. doi: 10.1002/(sici)1526-968x(200002)26:2<139::aid-gene12>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 107.Harding B, et al. Multiple endocrine neoplasia type 1 knockout mice develop parathyroid, pancreatic, pituitary and adrenal tumours with hypercalcaemia, hypophosphataemia and hypercorticosteronaemia. Endocr Relat Cancer. 2009;16:1313–1327. doi: 10.1677/erc-09-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li F, et al. Conditional deletion of Men1 in the pancreatic beta-cell leads to glucagon-expressing tumor development. Endocrinology. 2015;156:48–57. doi: 10.1210/en.2014-1433. [DOI] [PubMed] [Google Scholar]
- 109.Loffler KA, et al. Broad tumor spectrum in a mouse model of multiple endocrine neoplasia type 1. Int J Cancer. 2007;120:259–267. doi: 10.1002/ijc.22288. [DOI] [PubMed] [Google Scholar]
- 110.Cao Y, et al. Nuclear-cytoplasmic shuttling of menin regulates nuclear translocation of {beta}-catenin. Mol Cell Biol. 2009;29:5477–5487. doi: 10.1128/MCB.00335-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huang J, et al. The same pocket in menin binds both MLL and JUND but has opposite effects on transcription. Nature. 2012;482:542–546. doi: 10.1038/nature10806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Matkar S, Thiel A, Hua X. Menin: a scaffold protein that controls gene expression and cell signaling. Trends Biochem Sci. 2013;38:394–402. doi: 10.1016/j.tibs.2013.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Klaus A, Birchmeier W. Wnt signalling and its impact on development and cancer. Nat Rev Cancer. 2008;8:387–398. doi: 10.1038/nrc2389. [DOI] [PubMed] [Google Scholar]
- 114.Agarwal SK, et al. Menin interacts with the AP1 transcription factor JunD and represses JunD-activated transcription. Cell. 1999;96:143–152. doi: 10.1016/s0092-8674(00)80967-8. [DOI] [PubMed] [Google Scholar]
- 115.Agarwal SK, et al. Transcription factor JunD, deprived of menin, switches from growth suppressor to growth promoter. Proc Natl Acad Sci U S A. 2003;100:10770–10775. doi: 10.1073/pnas.1834524100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gurung B, et al. Menin epigenetically represses Hedgehog signaling in MEN1 tumor syndrome. Cancer Res. 2013;73:2650–2658. doi: 10.1158/0008-5472.CAN-12-3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Hughes CM, et al. Menin associates with a trithorax family histone methyltransferase complex and with the hoxc8 locus. Mol Cell. 2004;13:587–597. doi: 10.1016/s1097-2765(04)00081-4. [DOI] [PubMed] [Google Scholar]
- 118.Milne TA, et al. Menin and MLL cooperatively regulate expression of cyclin-dependent kinase inhibitors. Proc Natl Acad Sci U S A. 2005;102:749–754. doi: 10.1073/pnas.0408836102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Attisano L, Wrana JL. Signal transduction by the TGF-beta superfamily. Science. 2002;296:1646–1647. doi: 10.1126/science.1071809. [DOI] [PubMed] [Google Scholar]
- 120.Hendy GN, Kaji H, Sowa H, Lebrun JJ, Canaff L. Menin and TGF-beta superfamily member signaling via the Smad pathway in pituitary, parathyroid and osteoblast. Horm Metab Res. 2005;37:375–379. doi: 10.1055/s-2005-870152. [DOI] [PubMed] [Google Scholar]
- 121.Canaff L, Vanbellinghen JF, Kaji H, Goltzman D, Hendy GN. Impaired transforming growth factor-beta (TGF-beta) transcriptional activity and cell proliferation control of a menin in-frame deletion mutant associated with multiple endocrine neoplasia type 1 (MEN1) J Biol Chem. 2012;287:8584–8597. doi: 10.1074/jbc.M112.341958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Heppner C, et al. The tumor suppressor protein menin interacts with NF-kappaB proteins and inhibits NF-kappaB-mediated transactivation. Oncogene. 2001;20:4917–4925. doi: 10.1038/sj.onc.1204529. [DOI] [PubMed] [Google Scholar]
- 123.Wu Y, et al. Interplay between menin and K-Ras in regulating lung adenocarcinoma. J Biol Chem. 2012;287:40003–40011. doi: 10.1074/jbc.M112.382416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chamberlain CE, et al. Menin determines K-RAS proliferative outputs in endocrine cells. J Clin Invest. 2014;124:4093–4101. doi: 10.1172/JCI69004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Patel YC. Somatostatin and its receptor family. Frontiers in neuroendocrinology. 1999;20:157–198. doi: 10.1006/frne.1999.0183. [DOI] [PubMed] [Google Scholar]
- 126.Gallo A, et al. Menin uncouples Elk-1, JunD and c-Jun phosphorylation from MAP kinase activation. Oncogene. 2002;21:6434–6445. doi: 10.1038/sj.onc.1205822. [DOI] [PubMed] [Google Scholar]
- 127.Wang Y, et al. The tumor suppressor protein menin inhibits AKT activation by regulating its cellular localization. Cancer Res. 2011;71:371–382. doi: 10.1158/0008-5472.can-10-3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Kim YS, et al. Stable overexpression of MEN1 suppresses tumorigenicity of RAS. Oncogene. 1999;18:5936–5942. doi: 10.1038/sj.onc.1203005. [DOI] [PubMed] [Google Scholar]
- 129.Sayo Y, et al. The multiple endocrine neoplasia type 1 gene product, menin, inhibits insulin production in rat insulinoma cells. Endocrinology. 2002;143:2437–2440. doi: 10.1210/endo.143.6.8950. [DOI] [PubMed] [Google Scholar]
- 130.Schnepp RW, et al. Menin induces apoptosis in murine embryonic fibroblasts. J Biol Chem. 2004;279:10685–10691. doi: 10.1074/jbc.M308073200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.La P, et al. Menin-mediated caspase 8 expression in suppressing multiple endocrine neoplasia type 1. J Biol Chem. 2007;282:31332–31340. doi: 10.1074/jbc.M609555200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Walls GV, et al. MEN1 gene replacement therapy reduces proliferation rates in a mouse model of pituitary adenomas. Cancer Res. 2012;72:5060–5068. doi: 10.1158/0008-5472.CAN-12-1821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Smith TL, et al. AAVP displaying octreotide for ligand-directed therapeutic transgene delivery in neuroendocrine tumors of the pancreas. Proc Natl Acad Sci U S A. 2016;113:2466–2471. doi: 10.1073/pnas.1525709113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kumar R, Li DQ, Muller S, Knapp S. Epigenomic regulation of oncogenesis by chromatin remodeling. Oncogene. 2016 doi: 10.1038/onc.2015.513. [DOI] [PubMed] [Google Scholar]
- 135.Lines KE, et al. Epigenetic pathway inhibitors represent potential drugs for treating pancreatic and bronchial neuroendocrine tumors. Oncogenesis. 2017;6:e332. doi: 10.1038/oncsis.2017.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wong C, et al. The bromodomain and extra-terminal inhibitor CPI203 enhances the antiproliferative effects of rapamycin on human neuroendocrine tumors. Cell death & disease. 2014;5:e1450. doi: 10.1038/cddis.2014.396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Jiang X, et al. Targeting beta-catenin signaling for therapeutic intervention in MEN1-deficient pancreatic neuroendocrine tumours. Nat Commun. 2014;5:5809. doi: 10.1038/ncomms6809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mitry E, et al. Bevacizumab plus capecitabine in patients with progressive advanced well-differentiated neuroendocrine tumors of the gastro-intestinal (GI-NETs) tract (BETTER trial)--a phase II non-randomised trial. Eur J Cancer. 2014;50:3107–3115. doi: 10.1016/j.ejca.2014.10.001. [DOI] [PubMed] [Google Scholar]
- 139.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 140.Paez-Ribes M, et al. Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer cell. 2009;15:220–231. doi: 10.1016/j.ccr.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bill R, et al. Nintedanib Is a Highly Effective Therapeutic for Neuroendocrine Carcinoma of the Pancreas (PNET) in the Rip1Tag2 Transgenic Mouse Model. Clin Cancer Res. 2015;21:4856–4867. doi: 10.1158/1078-0432.CCR-14-3036. [DOI] [PubMed] [Google Scholar]
- 142.Sennino B, et al. Suppression of tumor invasion and metastasis by concurrent inhibition of c-Met and VEGF signaling in pancreatic neuroendocrine tumors. Cancer Discov. 2012;2:270–287. doi: 10.1158/2159-8290.CD-11-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chu X, et al. Multiple microvascular alterations in pancreatic islets and neuroendocrine tumors of a Men1 mouse model. The American journal of pathology. 2013;182:2355–2367. doi: 10.1016/j.ajpath.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 144.Xie L, et al. Counterbalancing angiogenic regulatory factors control the rate of cancer progression and survival in a stage-specific manner. Proc Natl Acad Sci U S A. 2011;108:9939–9944. doi: 10.1073/pnas.1105041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kaji H, Canaff L, Lebrun JJ, Goltzman D, Hendy GN. Inactivation of menin, a Smad3-interacting protein, blocks transforming growth factor type beta signaling. Proc Natl Acad Sci U S A. 2001;98:3837–3842. doi: 10.1073/pnas.061358098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Padamsee TJ, Wills CE, Yee LD, Paskett ED. Decision making for breast cancer prevention among women at elevated risk. Breast cancer research : BCR. 2017;19:34. doi: 10.1186/s13058-017-0826-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Cuzick J, et al. Tamoxifen for prevention of breast cancer: extended long-term follow-up of the IBIS-I breast cancer prevention trial. Lancet Oncol. 2015;16:67–75. doi: 10.1016/s1470-2045(14)71171-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Lazzeroni M, DeCensi A. Alternate dosing schedules for cancer chemopreventive agents. Seminars in oncology. 2016;43:116–122. doi: 10.1053/j.seminoncol.2015.09.014. [DOI] [PubMed] [Google Scholar]
- 149.Ricciardiello L, Ahnen DJ, Lynch PM. Chemoprevention of hereditary colon cancers: time for new strategies. Nature reviews. Gastroenterology & hepatology. 2016;13:352–361. doi: 10.1038/nrgastro.2016.56. [DOI] [PubMed] [Google Scholar]
- 150.Rinke A, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–4663. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 151.Rinke A, et al. Placebo-Controlled, Double-Blind, Prospective, Randomized Study on the Effect of Octreotide LAR in the Control of Tumor Growth in Patients with Metastatic Neuroendocrine Midgut Tumors (PROMID): Results of Long-Term Survival. Neuroendocrinology. 2017;104:26–32. doi: 10.1159/000443612. [DOI] [PubMed] [Google Scholar]
- 152.Walls GV, et al. Pasireotide Therapy of Multiple Endocrine Neoplasia Type 1-Associated Neuroendocrine Tumors in Female Mice Deleted for an Men1 Allele Improves Survival and Reduces Tumor Progression. Endocrinology. 2016;157:1789–1798. doi: 10.1210/en.2015-1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Quinn TJ, et al. Pasireotide (SOM230) is effective for the treatment of pancreatic neuroendocrine tumors (PNETs) in a multiple endocrine neoplasia type 1 (MEN1) conditional knockout mouse model. Surgery. 2012;152:1068–1077. doi: 10.1016/j.surg.2012.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Cioppi F, Cianferotti L, Masi L, Giusti F, Brandi ML. The LARO-MEN1 study: a longitudinal clinical experience with octreotide Long-Acting Release in patients with Multiple Endocrine Neoplasia type 1 Syndrome. Clinical Cases in Mineral and Bone Metabolism. 2017;14:123–130. doi: 10.11138/ccmbm/2017.14.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.