Abstract
Objectives
This manuscript describes the development, validation and clinical application of a novel method for the quantification of the antiretroviral drug efavirenz in dried breast milk spots using liquid chromatography-mass spectrometry.
Methods
Dried breast milk spots were prepared by spotting 30μL of human breast milk on each circle of Whatman 903 Protein Saver cards. Chromatographic separation was achieved on a reverse phase C18 column with 1mM ammonium acetate in water/acetonitrile using a solvent gradient program at a flow rate of 400μL/min and detection was by TSQ Quantum Access triple quadrupole mass spectrometer equipped with a heated electrospray ionisation source. The method was applied to characterise the breast milk pharmacokinetic profile of efavirenz in HIV positive nursing mothers receiving regimens containing 600 mg efavirenz once daily.
Results
The assay was validated over the concentration range of 50-7500 ng/mL. Accuracy ranged between 95.2 and 102.5% and precision ranged between 1.05 and 9.53%. The average recovery of efavirenz from dried breast milk spots was 106.4% and matrix effect was 8.14%. Stability of efavirenz in dried breast milk spots and processed samples at room temperature, -40°C, and -80°C was demonstrated. In the pharmacokinetic study, the mean (SD) AUC0-24, Cmax and Cmin of efavirenz in breast milk were 59620 ng.h/mL (17440), 4527 ng/mL (1767) and 1261 ng/mL (755.9), respectively. Mean (range) milk-to-plasma concentration ratio over the dosing interval was 0.78 (0.57-1.26).
Conclusions
The dried breast milk spot method is simple, robust, accurate and precise, and can be used in settings with limited resources.
Keywords: efavirenz, breast milk, dried breast milk spots, mass spectrometry
Introduction
The WHO recommends exclusive, “on demand” breastfeeding starting within one hour of birth, up to 6 months of age, and continued with gradual introduction of appropriate complementary foods up to 2 years of age or beyond. In addition to its nutritional benefits, ready availability and affordability, the health benefits of breastfeeding for both infant and mother have long been recognised.1–4 However, breastfeeding in the presence of maternal drug use is widespread despite lack of safety data for either proscriptions or permissive statements. In fact, over 90% of nursing mothers take at least one drug during the first week after delivery, 17% take at least one drug until 4 months after delivery, and 5% receive drugs for chronic conditions, giving rise to concerns over the presence of drugs in breast milk and their potential effects on the nursing infant.5
Making an informed decision requires an accurate evaluation of the potential risks versus benefits based on knowledge of the extent of the drug’s excretion in human breast milk.6 For instance, HIV positive mothers breastfeed their babies while taking antiretroviral drugs (ARVs) started during pregnancy for their own health and for prevention of mother-to-child transmission (PMTCT) of HIV. Understanding the dynamics of HIV replication in breast milk and the safety of maternal drugs for the breastfed infant requires understanding of the pharmacokinetics of ARVs in breast milk.7 Therapeutic drug concentrations in breast milk can prevent ongoing localised replication of viral reservoir 8 and development of drug resistant virus, which may otherwise be passed to infants who become infected if PMTCT fails.9 On the other hand, high concentrations may lead to toxicity in exposed infants.
Very few bioanalytical methods have been described in the literature for the quantification of ARV drugs in breast milk. The complexity of breast milk makes available methods either difficult to validate because of inadequate sample clean-up or complicated due to multiple sample clean-up steps involving a combination of liquid-liquid and solid phase extraction in a single method.10, 11 In addition, lack of standardisation makes cross-study comparisons difficult, particularly given differential drug accumulation within specific fractions of milk. For instance, studies reporting the excretion of abacavir, efavirenz, etravirine, lamivudine, lopinavir, nevirapine,zidovudine, tenofovir and emtricitabine in human breast milk have used methods validated only in plasma.12–17 A fully validated method was described for the quantification of lamivudine, lopinavir, nelfinavir, nevirapine, ritonavir, stavudine, and zidovudine (7 out of 25 ARVs in use) in breast milk.18 However, the multiple extraction steps make the method time-consuming and expensive, which is a barrier to implementation in resource-limited settings. No fully validated method has been described for the quantification of other ARV drugs in breast milk, including efavirenz, a HIV non-nucleoside reverse transcriptase inhibitor which is recommended by the WHO as a regular component of first-line regimens across different populations.19
The use of dried matrix spot in the bioanalysis of drugs is increasingly becoming acceptable. This approach is characterised by several advantages compared to the traditional methods: low sample volume, ease of collection, biosafety, room temperature storage, low-cost shipping, enhanced stability of some analytes,20 and the potential application in intensive pharmacokinetic studies in special populations. This technique has been described for whole blood21 and transparent matrices like plasma,22 cerebrospinal fluid,20 urine23 and synovial fluid.24 However, drug quantification in dried breast milk spots has not been reported. Here we describe a novel and simple method for the quantification of efavirenz in total (unfractionated) breast milk extracted from dried filter paper using liquid chromatography-mass spectrometry (LC-MS). Using this method, we describe for the first time the pharmacokinetics of efavirenz in human breast milk over a full dosing interval.
Materials and Methods
Reference standard of efavirenz was obtained from Toronto Research Chemicals Inc. (North York, ON, Canada) and hexobarbital, used as an internal standard (IS), was obtained from Sigma-Aldrich (Gillingham, Dorset, UK). LC-MS grade acetonitrile was obtained from Fisher scientific (Loughborough, Leicestershire, UK), methanol from VWR International (Lutterworth, Leicestershire, UK) and water was produced from an Elga Option 4 water purifier (Elga Labwater, High Wycombe, Buckinghamshire, UK) and was further purified to 18.2 MΩ with a Purelab Classic UVF (Elga LabWater, High Wycombe, Buckinghamshire, UK). Whatman 903 Protein Saver cards were obtained from Scientific Laboratory Supplies (Hessle, East Yorkshire, UK). Blank breast milk samples were obtained (with ethics approval) from Wirral Mothers’ Milk Bank, Clatterbridge Hospital, Wirral, UK and whole blood was obtained from drug-free healthy volunteers.
LC-MS/MS systems
The LC system consisted of a variable loop Accela autosampler and an Accela LC-Pump (Thermo Electron Corporation, Hemel Hempstead, Hertfordshire, UK). A reverse phase Fortis™ C18 column: 3μm, 100mm x 2.1mm (Fortis Technologies Ltd, Neston, Cheshire, UK) was used to resolve analytes, using a 2μm C18 Quest column-saver (Thermo Electron Corporation, Hemel Hempstead, Hertfordshire, UK) as a guard column. The HPLC was connected to a TSQ Quantum Access triple quadrupole mass spectrometer (Thermo Electron Corporation, Hemel Hempstead, Hertfordshire, UK) equipped with a heated electrospray ionisation source. TSQ tune software (Thermo Electron Corporation, Hemel Hempstead, Hertfordshire, UK) enabled the optimisation of tuning parameters while LC Quan software (Thermo Electron Corporation, Hemel Hempstead, Hertfordshire, UK) was used for data acquisition and processing.
LC-MS/MS conditions
Chromatographic separation was achieved using a solvent gradient program at a flow rate of 400μL/min. The two mobile phases consisted of 1mM ammonium acetate in water (mobile phase A) and 1mM ammonium acetate in acetonitrile (mobile phase B). The gradient programme started with 70% mobile phase A, decreasing to 10% over 3 minutes. This was maintained for 1 minute, followed by column equilibration to the initial conditions over 2 minutes. The total run time was 5 minutes. Injection volume was 10 μL and the needle was washed twice with 3 mL MeOH:water 1:1 (v/v) between injections. The mass spectrometer was operated in negative ionization mode to produce characteristic fragmentation patterns and precursor ions ([M-H]-) for efavirenz and IS which were then monitored by selective reaction monitoring (SRM).
The electrospray voltage was set at 3.0 kV, the capillary temperature at 270 °C and vaporiser temperature at 350 °C. The sheath and auxiliary gas pressures were set to 60 and 15 arbitrary units, respectively. Argon was used as the collision gas at a pressure of 1.5 mTorr. Product ion characterisation was done by directly infusing 1 μg/mL solutions of efavirenz and hexobarbital separately into the MS using a syringe at a flow rate of 5μL/min. The transitions were m/z 313.979 → 241.991 and 244.025 for efavirenz and 235.071 → 42.449 for hexobarbital with optimal collision energies of 20 and 19 and tube lenses of 76 and 84 respectively. The scan width was set at 0.01 m/z and the scan time at 0.05 s. The peak width settings for Q1 and Q3 were set at 0.7 μm.
Stock solutions, calibration standards (STD) and quality controls (QC)
Stock solutions of efavirenz and the IS were prepared from the pure compounds in 100% methanol to obtain a final concentration of 1 mg/mL and refrigerated at 4 °C until use. A 10 μg/mL working stock of efavirenz in breast milk was prepared by adding a predetermined volume of efavirenz stock solution to drug-free breast milk, tumbled for 60 minutes and used the same day. Nine calibration standards in the range of 50-7500 ng/mL, together with low quality control (LQC, 100 ng/mL), medium quality control (MQC, 1500 ng/mL) and high quality control (HQC, 6000 ng/mL) were prepared from separate working stocks by serial dilution with drug-free breast milk. Working solution of efavirenz at concentrations equivalent to the QCs and 100 μg/mL of the IS were prepared in methanol:water (50:50, v/v).
Dried milk spot STD & QC preparation
STDs and QCs were prepared by carefully spotting 30 μL of spiked and well mixed breast milk on each circle of Whatman 903 Protein Saver cards. Spotted cards were left to dry at room temperature overnight and stored with desiccant sachets in ziplock bags. QC samples for stability testing were stored at room temperature, -40 °C and -80 °C.
Sample pre-treatment
In each case, the entire spot was removed using a 13 mm hole punch, folded into a 7 mL screw cap tube, and extracted with 1mL of methanol by tumbling for 30 minutes in the presence of 20 μL of IS. The extract was centrifuged at 4000 rpm for 5 minutes, supernatant was transferred into a 5mL tube and evaporated to dryness in a centrifugal rotary vacuum evaporator (Thermo Electron Industries, Chateau Gontier, France) operated at 40 °C. The residue was reconstituted in 500 μL mobile phase A and B (50:50, v/v) and aliquots were transferred into autosampler vials. Each STD level was prepared in duplicate (n = 2) while the QCs were prepared in sextuplicate (n = 6).
Standard curve, accuracy and precision
Validation of the method was carried out as per FDA guidelines.25 Ten separate assays, each consisting of a zero blank (n = 2), nine STDs between 50 and 7500 ng/mL (n = 2), and QCs (n = 6) were run. Calibration curves were constructed using a linear regression equation of analyte/IS peak area ratios versus nominal concentrations with a 1/concentration weighting. Accuracy was defined as percentage deviation of measured concentration from the nominal value and precision was defined as the percentage coefficient of variation (%CV). Inter-individual precision was assessed using a validation assay run by a different operator. At least 75% of STDs were required to have percentage deviation within ±15%, except for the lower limit of quantification (LLOQ) which is allowed to be ±20% of the nominal value. A percentage deviation and a %CV within ±20% for LQC and within ±15% for MQC and HQC were set as acceptance criteria for ≥ 67% of all QC samples and ≥ 50% of QCs at each level.25, 26
Recovery, matrix effect and dilution integrity
Recovery was assessed by comparing peak area obtained from replicates of each of the extracted QCs with the peak area obtained from the corresponding solutions of efavirenz in mobile phase, as recommended by Matuszewski et al.27 To evaluate matrix effect, six drug-free breast milk samples from different donors were spotted on Whatman 903 Protein Saver cards and extracted as previously described. Each of the blank extracts was spiked with the appropriate efavirenz working solution (100 ng/mL, 1500 ng/mL or 6000 ng/mL) to obtain final concentrations equivalent to extracted dried breast milk spots at LQC, MQC or HQC. Identical concentrations of unextracted samples were prepared by directly spiking working solutions into mobile phase. Each concentration was evaluated in sextuplicate for each of the six breast milk samples. The overall recovery was calculated as the ratio (expressed as a %) of the absolute peak-area response of plasma samples spiked with drug prior to extraction to the peak area response of spiked mobile phase samples. The % matrix effect was calculated as the ratio (expressed as a percentage) of the peak area response of blank plasma extracts spiked post extraction to the peak areas of spiked mobile phase samples. A relative standard deviation (RSD) of ≤ 15% was set as the level of acceptance for both recovery and matrix effect in line with the FDA and EMA guidelines.25, 26 In addition, we stipulated that mean recovery should be < 115% at any concentration. Dilution integrity was evaluated to investigate the applicability of the method to patient samples with efavirenz concentrations above 7500 ng/mL. For this, 10μg/mL efavirenz in breast milk was prepared and 30 μL was spotted on each circle of Whatman 903 card, dried and extracted as previously described. The extract was diluted 2x and 4x using blank dried breast milk spots similarly extracted.
Stability and re-injection reproducibility
The stability of efavirenz in dried breast milk spots at different storage and processing conditions was investigated. Short-term stability was evaluated by storing extracted QC samples at room temperature and in the autosampler (4 °C) for 24 hours and over the weekend (72 hours). Short-term stability of processed patient samples (n = 10) from an accepted validation assay run was also evaluated. For long-term stability at room temperature, -40 °C and -80 °C stability, QC samples were stored at these temperatures for 6 months. The concentrations of the stored samples were determined using STDs and QCs prepared with a freshly made efavirenz stock solution. To assess re-injection reproducibility in the event of instrument interruption, an accepted validation assay run was re-injected after 24 hours in the autosampler.
Application in a pharmacokinetic study
The method was applied in a preliminary study to evaluate the breast milk pharmacokinetic profile of efavirenz in HIV positive nursing mothers (n = 5) receiving regimens containing 600 mg efavirenz once daily. Patients were recruited from Bishop Murray Medical Centre, Makurdi, Nigeria and written informed consent was obtained prior to enrolment. Mothers taking antimalarial, anti-tuberculosis or other drugs known to interact with efavirenz were excluded. Breast milk was manually expressed by the mothers mid-feed into 5 mL tubes, 0.5, 1, 2, 4, 8, 12, 24 hours after an observed evening dose of 600 mg efavirenz. To reflect real-life situations, patients took a standard local meal about 30 minutes before drug administration. A 30 μL aliquot was immediately spotted on each circle of Whatman 903 cards, dried and stored as previously described. Within 2 minutes of breast milk collection, whole blood samples were collected as dried blood spots (DBS) after sterile skin cleaning and finger prick using a 2mm safety lancet (BD, Oxford, Oxfordshire, UK). The first drop of blood was discarded and subsequent blood drops were collected on Whatman 903 cards, dried and stored as described above. Samples were shipped at room temperature to the Department of Molecular and Clinical Pharmacology, University of Liverpool, United Kingdom for analysis. Efavirenz in dried breast milk spots was quantified using the method described above and efavirenz in DBS was quantified using a method described elsewhere.28 Plasma efavirenz concentrations were estimated using [DBS[EFV]/(1-HCT)]*fbpp, where DBS[EFV] is efavirenz concentration in DBS, HCT is the patient specific haematocrit and fbpp (0.995) is the fraction of efavirenz bound to plasma protein.29 The study protocol and the material transfer agreement (MTA) were approved by the National Health Research and Ethics Committee (NHREC), Abuja and Obafemi Awolowo University Teaching Hospitals (OAUTHC) Ethics and Research Committee, Ile-Ife, Nigeria.
Results
LC-MS/MS conditions
The total run time was 5 minutes and retention times of efavirenz and IS were 2.27 and 1.67 minutes, respectively. Representative chromatograms are presented in Figure S1.
Linearity, accuracy and precision
The method was linear, accurate and precise in the range of 50-7500 ng/mL. Mean regression coefficient (r2) was 0.9994 and the average relative standard deviation of the IS response was 3.7%. Accuracy (% bias) was between 95.2 and 102.5% and precision (% CV) was between 1.05 and 9.53% (Table 1). These values are within bioanalytical method validation acceptance criteria as per FDA and EMA guidelines.25, 26
Table 1. Accuracy (%) and precision (%) for the quantification of efavirenz in dried breast milk spot.
| Concentration | Inter-day | Intra-day | ||||||
|---|---|---|---|---|---|---|---|---|
| Calibration standards (ng/mL) | Mean | SD | Precision (%CV) | Accuracy (%) | Mean | SD | Precision (%CV) | Accuracy (%) |
| 50 | 50.4 | 2.78 | 5.51 | 100.8 | 51.0 | 3.48 | 6.83 | 101.9 |
| 100 | 98.2 | 3.04 | 3.09 | 98.2 | 98.6 | 1.54 | 1.56 | 98.7 |
| 200 | 198.9 | 10.05 | 5.05 | 99.5 | 193.5 | 2.92 | 1.51 | 96.8 |
| 500 | 500.8 | 11.59 | 2.31 | 100.2 | 495.7 | 5.19 | 1.05 | 99.1 |
| 1000 | 1017.7 | 41.40 | 4.07 | 101.8 | 1045.4 | 35.31 | 3.38 | 104.5 |
| 2000 | 2012.4 | 36.51 | 1.81 | 100.6 | 2013.8 | 27.55 | 1.37 | 100.7 |
| 3500 | 3473.9 | 23.90 | 0.69 | 99.3 | 3464.4 | 27.00 | 0.78 | 99.0 |
| 5000 | 4961.2 | 75.80 | 1.53 | 99.2 | 4918.8 | 23.01 | 0.47 | 98.4 |
| 7500 | 7539.5 | 87.62 | 1.16 | 100.5 | 7574.4 | 83.04 | 1.10 | 100.0 |
| Quality control (QC)* | ||||||||
| LQC (100) | 99.5 | 9.48 | 9.53 | 99.5 | 101.7 | 6.29 | 6.19 | 101.7 |
| MQC (1500) | 1427.7 | 72.73 | 5.09 | 95.2 | 1492.2 | 40.98 | 2.75 | 99.5 |
| HQC (6000) | 6125.1 | 313.10 | 5.11 | 102.1 | 6087.1 | 330.04 | 5.37 | 102.5 |
LQC, low quality control; MQC, medium quality control; HQC, high quality control.
Recovery, matrix effect and dilution integrity
The average (%CV) recovery of efavirenz from dried breast milk spots at LQC, MQC and HQC was 101.6% (7.40), 109.4% (2.58) and 108.2% (2.97), respectively. Matrix effect was within ±15% for each of the six different matrices, with an average (%CV) of 9.05% (4.94), 8.15% (5.35), and 7.27% (4.17 at LQC, MQC and HQC), respectively. The average efavirenz concentration in the 10000 ng/mL dried breast milk spots diluted 2x and 4x were 10147 and 10893 ng/mL, representing 101.5 and 108.9% dilution integrity, respectively.
Stability and re-injection reproducibility
Processed QC and incurred patient samples were stable at room temperature and in the autosampler (4°C) for 24 hours and over the weekend (72 hours) with percentage deviations from nominal concentrations (for QC samples) or from the original assay results (for patient samples) within ± 15%. The stability of efavirenz in dried breast milk spots after storage for 6 months ranged from 92 to 101% at room temperature, 105 to 111% at -40 °C and 110 to 113% at -80 °C. Reinjection reproducibility was demonstrated in a repeat analysis of an accepted validation batch with all assay validation parameters within acceptable limits.
Application in a pharmacokinetic study
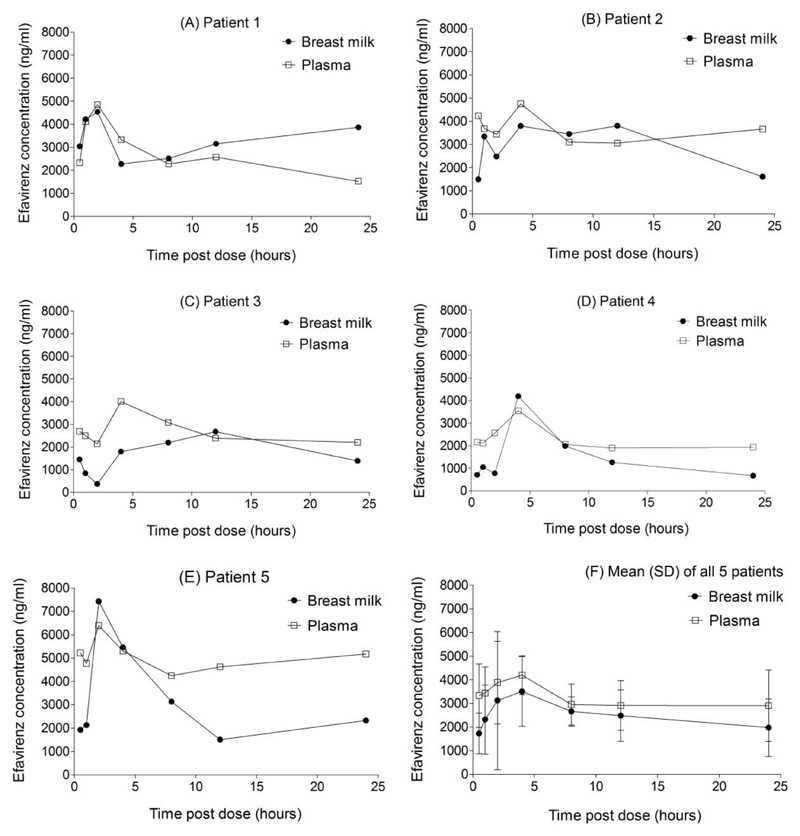
The method was successfully applied to investigate the pharmacokinetics of efavirenz in human breast milk. Median (range) age of mothers included was 29 years (25-34) and weight was 55 kg (45-71). All five mothers started efavirenz-containing regimen before delivery and samples were collected at steady state, 146 days (108-194) after delivery. As shown in Figure 1F, the breast milk profile approximately paralleled the plasma profile. As with the plasma, breast milk concentrations also exhibited considerable inter-individual variability (Figure 1A-1E). The mean (SD) AUC, Cmax and Cmin were 59620 ng.h/mL (17440), 4527 ng/mL (1767) and 1261 ng/mL (755.9), respectively in breast milk. Corresponding values in plasma were 74230 ng.h/mL (25610), 4711 ng/mL (1083) and 2571 ng/mL (1092), respectively (Figure 1). Interestingly, the breast milk efavirenz concentration exceeded the plasma concentration at some point during the dosing interval in all five patients. Also, the breast milk concentration was predominantly higher than plasma concentration in patient 1. The average (range) milk-to-plasma ratio over the dosing interval was 0.78 (0.57-1.26). The milk-to-plasma concentration (M/P) ratio was 0.83 at 4 hours post-dose.
Figure 1.
Concentration-time profiles of efavirenz in breast milk and plasma of five HIV positive nursing mothers (A) to (E) taking regimens containing 600mg of efavirenz once daily. The mean (standard deviation, SD) of all five patients is shown in (F). The mean (range) M/P ratio over the dosing interval was 0.78 (0.57, 1.26).
Discussion
To our knowledge, this is the first description of a drug quantification method in dried breast milk spots. Additionally, the full pharmacokinetic profile of efavirenz in human breast milk has been described for the first time. The simplicity, accuracy and precision of the developed method will facilitate inter-laboratory transfer. The stability of efavirenz in dried breast milk spots at room temperature will facilitate studies in settings with inadequate cold storage facilities and shipping without the need for dry ice, thus extending its utility in resource-limited settings. Other advantages include patient acceptability, and reduction in perceived or actual risks to the patient resulting from multiple sampling of large volumes. The low sample volume required per assay may also extend application of the method to PK studies in animals in which adequate breast milk volumes may be difficult to obtain because of low rate of milk production.10
As a result of the complexity of human breast milk, previously described methods involve complicated extraction procedures with a combination of liquid-liquid extraction and solid phase extraction for sample clean-up.11 Some authors have used skimmed milk for validation which has the potential of underestimating breast milk concentrations and hence the M/P ratio of drugs that are highly bound to breast milk proteins and lipids. The use of dried matrix spot allows quantification of drug concentrations in whole milk because the cellulose material entraps milk proteins and lipids on drying, allowing selective extraction of analytes.
The antiretroviral drug efavirenz, which is an essential component of first-line regimens and frequently used by HIV positive nursing mothers in developing countries, is not licensed for use in children < 3 months old or < 3.5 kg30 because optimal dosing and safety have not been fully evaluated in this age group. In the first report of its breast milk excretion by Schneider et al., single-point breast milk and plasma sample were collected 3 to 4 hours post-dose and the M/P ratio was 0.54,12 compared with the M/P ratio of 0.83 at 4 hours post-dose in the present study. This difference may be due to the use of skimmed milk instead of whole milk in the former study, resulting in underestimation of the breast milk concentrations of efavirenz which in plasma is 99.5% protein bound. In addition, changes in milk production, composition, and infant feeding patterns may cause variations in the M/P ratio during the dosing interval,31 making single point estimates inaccurate and often misleading.6 Breast milk is more lipophilic than plasma; efavirenz may preferentially partition into this compartment and accumulate after maternal dose before the next infant feed. In fact, we observed intra- and inter-individual variations in the M/P ratio during the dosing interval in the present study. In addition to the factors highlighted above, genetic factors may also play some role.32 Using our method, M/P ratio ranged from 0.57 to 1.26 with an average of 0.78 over the 24-hour dosing interval.
Uncertainties surrounding the use of many drugs during lactation may result in suboptimal adherence to essential pharmacotherapy or premature recourse to formula feeding by nursing mothers. In the context of HIV/AIDS, this will not be affordable, feasible, acceptable, sustainable or safe for the majority of the world’s HIV positive women living in resource-limited countries. The availability of validated bioanalytical methods for the quantification of drugs in human breast milk is an important step towards resolving some of these uncertainties and clarifying the pharmacokinetics of drugs in this compartment. The dried milk spot method presented here is simple, accurate and precise and further studies to investigate applications for other drugs that may be used during lactation are warranted. A useful approach will be to modify methods already validated for the drug of interest in other matrices. Additional validation steps to establish a suitable extraction procedure, optimal recovery, and drug stability in dried breast milk spot will be needed as per FDA guidelines.25
In the present study, a potential limitation is that a cross validation comparing the dried breast milk spot method with traditional liquid breast milk method was not conducted. Also, unlike dried blood spots which can be collected directly from patients after finger prick, the dried breast milk method relies on accurate pipetting from expressed breast milk and spotting on sample collection cards. It is also essential that spotting be limited to the marked sample collection areas on the cards since breast milk is colourless.
Supplementary Material
Figure S1 is available as Supplementary data at JAC Online (http://jac.oxfordjournals.org/).
Acknowledgements
We thank staff at Liverpool Bioanalytical Facility for their input during the method development and validation. We would also like to thank the participating patients, staff and management of Bishop Murray Medical Centre, Makurdi, Nigeria for their support in the preliminary pharmacokinetic study.
Funding
The method development and validation was carried out as part of our routine work at Liverpool Bioanalytical Facility, Department of Molecular and Clinical Pharmacology, University of Liverpool, UK. Adeniyi Olagunju received funding from HIV Research Trust, UK for the preliminary pharmacokinetic study. Catriona Waitt was funded by an Academy of Medical Sciences Starter Grant for Clinical Lecturers subsequently by a Wellcome Clinical Postdoctoral Training Fellowship WT104422MA.
Footnotes
Transparency declarations
David Back, Saye Khoo, Andrew Owen and Laura Else have received research grants and/or travel bursaries from Merck, Bristol Myers and Squibb, GlaxoSmithKline, Pfizer, Abbott, ViiV, Boehringer Ingelheim and Janssen Pharmaceuticals. The remaining authors have none to declare.
References
- 1.Ebbs JH, Mulligan F. The incidence and mortality of breast- and artificially-fed infants admitted to hospital with infections. Arch Dis Child. 1942;17:217–9. doi: 10.1136/adc.17.92.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gyllensward C. Breast-fed children compared to artificially fed children in a series from a children's home. Acta Soc Med Ups. 1953;58:285–311. [PubMed] [Google Scholar]
- 3.Levin B, Mackay HM, Neill CA, et al. Weight gains, serum protein levels and health of breast fed and artificially fed infants, full term and premature. Memo Med Res Counc. 1959;296:1–154. [PubMed] [Google Scholar]
- 4.Fallot ME, Boyd JL, 3rd, Oski FA. Breast-feeding reduces incidence of hospital admissions for infection in infants. Pediatrics. 1980;65:1121–4. [PubMed] [Google Scholar]
- 5.McNamara PJ, Abbassi M. Neonatal exposure to drugs in breast milk. Pharm Res. 2004;21:555–66. doi: 10.1023/b:pham.0000022401.14710.c5. [DOI] [PubMed] [Google Scholar]
- 6.Ito S. Drug therapy for breast-feeding women. N Engl J Med. 2000;343:118–26. doi: 10.1056/NEJM200007133430208. [DOI] [PubMed] [Google Scholar]
- 7.Marzolini C, Gray GE. Maternal antiretroviral prophylaxis and breastfeeding. Antivir Ther. 2012;17:1503–6. doi: 10.3851/IMP2314. [DOI] [PubMed] [Google Scholar]
- 8.Van de Perre P, Rubbo PA, Viljoen J, et al. HIV-1 reservoirs in breast milk and challenges to elimination of breast-feeding transmission of HIV-1. Sci Transl Med. 2012;4:143sr3. doi: 10.1126/scitranslmed.3003327. [DOI] [PubMed] [Google Scholar]
- 9.Kuhn L, Hunt G, Technau KG, et al. Drug resistance among newly diagnosed HIV-infected children in the era of more efficacious antiretroviral prophylaxis. AIDS. 2014 doi: 10.1097/QAD.0000000000000261. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rossi DT, Wright DS. Analytical considerations for trace determinations of drugs in breast milk. J Pharm Biomed Anal. 1997;15:495–504. doi: 10.1016/s0731-7085(96)01880-8. [DOI] [PubMed] [Google Scholar]
- 11.Rezk NL, Abdel-Megeed MF, Kashuba AD. Development of a highly efficient extraction technique and specific multiplex assay for measuring antiretroviral drug concentrations in breast milk. Ther Drug Monit. 2007;29:429–36. doi: 10.1097/FTD.0b013e318074db39. [DOI] [PubMed] [Google Scholar]
- 12.Schneider S, Peltier A, Gras A, et al. Efavirenz in human breast milk, mothers', and newborns' plasma. J Acquir Immune Defic Syndr. 2008;48:450–4. doi: 10.1097/QAI.0b013e31817bbc21. [DOI] [PubMed] [Google Scholar]
- 13.Shapiro RL, Rossi S, Ogwu A, et al. Therapeutic levels of lopinavir in late pregnancy and abacavir passage into breast milk in the Mma Bana Study, Botswana. Antivir Ther. 2013;18:585–90. doi: 10.3851/IMP2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shapiro RL, Holland DT, Capparelli E, et al. Antiretroviral concentrations in breast-feeding infants of women in Botswana receiving antiretroviral treatment. J Infect Dis. 2005;192:720–7. doi: 10.1086/432483. [DOI] [PubMed] [Google Scholar]
- 15.Mirochnick M, Thomas T, Capparelli E, et al. Antiretroviral Concentrations in Breast-Feeding Infants of Mothers Receiving Highly Active Antiretroviral Therapy. Antimicrob Agents Chemother. 2009;53:1170–6. doi: 10.1128/AAC.01117-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spencer LY, Liu S, Wang C, et al. Intensive Etravirine PK and HIV-1 Viral Load in Breast Milk and Plasma in HIV+ Women Receiving HAART. Top Antivir Med. 2014;22:466. [Abstract 891] [Google Scholar]
- 17.Benaboud S, Pruvost A, Coffie PA, et al. Concentrations of Tenofovir and Emtricitabine in Breast Milk of HIV-1-Infected Women in Abidjan, Cote d'Ivoire, in the ANRS 12109 TEmAA Study, Step 2. Antimicrob Agents Chemother. 2011;55:1315–7. doi: 10.1128/AAC.00514-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rezk NL, White N, Bridges AS, et al. Studies on antiretroviral drug concentrations in breast milk: Validation of a liquid chromatography-tandem mass spectrometric method for the determination of 7 anti-human immunodeficiency virus medications. Ther Drug Monit. 2008;30:611–9. doi: 10.1097/FTD.0b013e318186e08e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organisation. Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection. [7 July 2014, date last accessed];2013 Available at: http://www.who.int/iris/bitstream/10665/85321/1/9789241505727_eng.pdf. [PubMed]
- 20.Delaby C, Gabelle A, Meynier P, et al. Development and validation of dried matrix spot sampling for the quantitative determination of amyloid beta peptides in cerebrospinal fluid. Clin Chem Lab Med. 2013:1–7. doi: 10.1515/cclm-2013-0611. [DOI] [PubMed] [Google Scholar]
- 21.Zheng JH, Guida LA, Rower C, et al. Quantitation of tenofovir and emtricitabine in dried blood spots (DBS) with LC-MS/MS. J Pharm Biomed Anal. 2013;88C:144–51. doi: 10.1016/j.jpba.2013.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baietto L, D'Avolio A, Ariaudo A, et al. Development and validation of a new UPLC-PDA method to quantify linezolid in plasma and in dried plasma spots. J Chromatogr B Analyt Technol Biomed Life Sci. 2013;936:42–7. doi: 10.1016/j.jchromb.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 23.Otero-Fernandez M, Cocho JA, Tabernero MJ, et al. Direct tandem mass spectrometry for the simultaneous assay of opioids, cocaine and metabolites in dried urine spots. Anal Chim Acta. 2013;784:25–32. doi: 10.1016/j.aca.2013.04.061. [DOI] [PubMed] [Google Scholar]
- 24.Christianson CD, Laine DF, Zimmer JS, et al. Development and validation of an HPLC-MS/MS method for the analysis of dexamethasone from pig synovial fluid using dried matrix spotting. Bioanalysis. 2010;2:1829–37. doi: 10.4155/bio.10.137. [DOI] [PubMed] [Google Scholar]
- 25.Food and Drug Administration. Guidance for Industry: Bioanalytical Method Validation. Maryland: Food and Drug Administration, US Department of Health and Human Services; [7 July 2014, date last accessed]. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm070107.pdf. [Google Scholar]
- 26.European Medicines Agency. Guideline on Validation of Bioanalytical Methods. London: Committee for Medicinal Products for Human Use; [7 July 2014, date last accessed]. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2011/08/WC500109686.pdf. [Google Scholar]
- 27.Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem. 2003;75:3019–30. doi: 10.1021/ac020361s. [DOI] [PubMed] [Google Scholar]
- 28.Amara AB, Else LJ, Tjia J, et al. A Validated Method for Quantification of Efavirenz in Dried Blood Spots (DBS) using HPLC-MS/MS. Ther Drug Monit. 2014 doi: 10.1097/FTD.0000000000000127. [in press] [DOI] [PubMed] [Google Scholar]
- 29.Kromdijk W, Mulder JW, Rosing H, et al. Use of dried blood spots for the determination of plasma concentrations of nevirapine and efavirenz. J Antimicrob Chemother. 2012;67:1211–6. doi: 10.1093/jac/dks011. [DOI] [PubMed] [Google Scholar]
- 30.Panel on Antiretroviral Therapy and Medical Management of HIV-Infected Children. Guidelines for the Use of Antiretroviral Agents in Pediatric HIV Infection. [7 July 2014, date last accessed]; http://aidsinfo.nih.gov/contentfiles/lvguidelines/pediatricguidelines.pdf.
- 31.Wilson JT, Brown RD, Cherek DR, et al. Drug excretion in human breast milk: principles, pharmacokinetics and projected consequences. Clin Pharmacokinet. 1980;5:1–66. doi: 10.2165/00003088-198005010-00001. [DOI] [PubMed] [Google Scholar]
- 32.Olagunju A, Owen A, Creesey TR. Potential effect of pharmacogenetics on maternal, fetal and infant antiretroviral drug exposure during pregnancy and breastfeeding. Pharmacogenomics. 2012;13:1501–22. doi: 10.2217/pgs.12.138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.