Abstract
Gastric cancer (GC) is one of the most common malignancies worldwide. Due to the low rate of early detection, most GC patients were diagnosed as advance stages and had poor response to chemotherapy. Some studies found that Fumarate hydratase (FH) participated in the DNA damage response and its deficiency was associated with tumorigenesis in some cancers. In this study, we investigated the relationship between FH and cisplatin (CDDP) sensitivity in GC cell lines. We found that FH was the most significant gene which induced by CDDP treatment and the suppression of FH could enhance the cytotoxicity of CDDP. Miconazole Nitrate (MN) could inhibit FH activity and enhance the effect of CDDP in vitro and in vivo. We also investigated the significance of expression of FH in GC tissues. The FH expression, which was higher in GC tissues than in noncancerous tissues, was negatively associated with the prognosis of patients. Together, these results revealed that FH is a reliable indicator for response to CDDP treatment in GC and the inhibition of FH may be a potential strategy to improve the effects of CDDP-based chemotherapy.
Subject terms: Gastric cancer, Translational research
Introduction
Gastric cancer (GC) is one of the most common human malignancies. GC is currently the fifth most frequently diagnosed cancer and it carries the third highest mortality rate worldwide and is the most common malignancy in East Asia. China is greatly affected by the high morbidity rate of GC, which accounts for 13.08% of overall malignancies1,2. Because of the latent biological behavior of GC, almost 60% of patients are diagnosed in late stages and exhibit a poor treatment response3,4. Although researchers have proposed hypotheses about GC treatments’ models5–7, tumor invasions and nodal metastasis lead to the poor survival rate. Therefore, discovering new molecular targets for studies to determine proper GC treatment is quite urgent.
Fumarate hydratase (FH), an enzyme participating in the tricarboxylic acid (TCA) cycle, catalyzes the reversible hydration of fumarate to generate malate8–10. Researchers first described FH mutation while studying a tumor susceptibility syndrome, hereditary leiomyomatosis and renal cell cancer (HLRCC),whose characteristics are benign cutaneous and uterine leiomyomas, renal cell carcinomas, and uterine leiomyosarcomas11–13. However, the roles of FH in nonmetabolic functions and the possible mechanisms underlying these roles attracted our interest, and some researchers have focused on these topics14,15. Ohad Yogev et al. and Zhimin Lu et al. investigated the relationship between FH and DNA damage repair. The process is activated by FH and lead to enhance DNA damage repair16,17. Rui Liu et al. hypothesized that enhancement of FH inactivation or fumarate accumulation could lead to HIF-1α over-expression of observed during their exploration of the relationship between GC and triiodothyronine(T3)18. However, the role of FH in human GC has not yet been investigated, and the expression of FH in GC and its effect on GC treatment are unknown.
Miconazole nitrate (MN) is an imidazole antifungal agent approved for the treatment of yeast infections of the skin or vagina and some other applications. Previous investigation showed that miconazole induces apoptosis via the death receptor 5-dependent and mitochondrial-mediated pathways in human bladder cancer cells as well as colon cancer cells19,20. However, the probable mechanism should be studied.
In this study, we investigated the relationship between FH and cisplatin (CDDP) sensitivity in GC cell lines. We found that FH was the most significant gene which induced by CDDP treatment and the suppression of FH could enhance the cytotoxicity of CDDP. Miconazole Nitrate (MN) could inhibit FH activity and enhance the effect of CDDP in vitro and in vivo. We also investigated the significance of expression of FH in GC tissues. The FH expression, which was higher in GC tissues than in noncancerous tissues, was negatively associated with the prognosis of patients. Together, these results revealed that FH is a reliable indicator for response to CDDP treatment in GC and the inhibition of FH may be a potential strategy to improve the effects of CDDP-based chemotherapy.
Materials and methods
Cell culture
RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) containing penicillin and streptomycin was used to grow the GC cell lines SGC7901, HGC27 and BGC823 in a humidified atmosphere with 5% CO2 at 37 °C. The GC cell lines were obtained from the Cell Bank of Chinese Academy of Sciences (Shanghai, China), with validation of identity based on American Type Culture Collection standards.
Reagents and antibodies
CDDP, 5-fluorouracil (5-Fu) and MN were purchased from Selleck Chemicals (Houston, USA). Antibodies specific for the following proteins were used in this study were as follow: p-ATM, p-Chk1, p-Chk2, cleaved-PARP, cleaved-caspase 3, Vinculin (all from Cell Signaling Technology, Beverly, MA, USA), Lamin B1, FH, ATM, Chk1, Chk2, γ-H2AX (all from Abcam, Cambridge, UK), GAPDH (Sigma-Aldrich, St Louis, USA), DNA-PK (Affinity Biosciences, Cincinnati, OH, USA) and Ki-67 (Zhongshan Golden Bridge Biotech, Beijing, China).
Real-time PCR and metabolism array
We used TRIzol (Invitrogen, Carlsbad, California, USA) to extract total RNA. Then, cDNA was generated using a PrimeScript One-step RT-PCR kit (TaKaRa, Dalian, China). The cDNA template was amplified by RT-PCR using GoTaq qPCR Master Mix (Promega, Madison, Wisconsin, USA). The primer sequences used are listed in Table S1. The reactions were performed in a LightCycler480 system (Roche, Mannheim, Germany). The relative expression of mRNA was calculated by the 2−ΔCt or 2−ΔΔCt method depending on the purpose.
cDNA from the indicated samples was added to a NuRNA™ Human Central Metabolism PCR Array (Arraystar Inc., Rockville, USA). The following procedure was as the description previously.
RNA interference and over-expression plasmid
For RNA interference mediated depletion (knockdown) of FH, human FH specific and control siRNAs were synthesized by RiboBio (Guangzhou, Guangdong, China). GC cells were transfected with siRNAs using Lipofectamine 3000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The sequences targeting FH were GATCTACGATGAACTTTAA (#1) and GGATGCTGTTCCACTTACT (#2).
RNA interference in vivo was also synthesized by RiboBio (Guangzhou, Guangdong, China). The tumors in nude mice were injected cholesterol-conjugated siRNA every 3 days21,22 and the following treatment was adopted as the procedure of ‘Pharmacologic study in vivo’.
FH over-expression (OE) plasmid and empty vectors (EV) were purchased from Kidan bio. co. Ltd (Guangzhou, Guangdong, China). After transfection, the cells were selected by G418 (MedChemExpress, USA). The over-expression level was confirmed by Western blot.
Rescue test
After the cells were transfected by siRNA of FH, CDDP and/or Fumarate were treated in the full medium with the indicated cells for 3 days. MTS assay was used to detect the cell viability as the procedure described in ‘Cell proliferation and apoptosis assays’.
Immunoblotting and immunofluorescence analysis
Tissue samples and GC cell lines were lysed in RIPA lysis buffer (Beyotime, Jiangsu, China) at 4 °C for 15 min. Lysates were cleared by centrifugation (12,000 rpm) at 4 °C for 15 min to collect total protein. Protein concentrations were estimated using a BCA protein assay (Thermo Fisher, MA, USA). Equal amounts of protein in the lysates were resolved on an 8–15% gel by electrophoresis and electrotransferred to a polyvinylidene fluoride (PVDF) membranes. The PVDF membrane was blocked with 5% skim milk for 1 h and probed with primary antibody (1:2000 dilution of anti-FH; 1:10,000 dilution of anti-GAPDH) at 4 °C overnight. Antibody binding was detected with horseradish peroxidase-conjugated IgG secondary antibody for 1 h at room temperature. Finally, the membrane was treated with an enhanced ECL kit for visualization (Thermo Fisher, MA, USA). Immunofluorescence of c-PARP and γ-H2AX was detected using a standard protocol.
Cell proliferation and apoptosis assays
We used MTS (Promega, Madison, Wisconsin, USA) and colony formation assays to detect cell viability and proliferation. In addition, IC50 values were determined by an MTS assay. Annexin-V/propidium iodide (PI) (KeyGEN,Nanjing, China) were used to detect cell apoptosis induced by MN or CDDP via flow cytometric analysis (Beckman Coulter, California, USA) according to the manufacturer’s instructions.
Determination of FH enzymatic activity and fumarate concentration
GC cell samples were treated with Fumarase Assay Buffer from a Fumarase Activity Colorimetric Assay Kit (Biovision, CA, USA). The samples, Fumarase Positive Control and the NADH Standard were then added to a the 96-well clear-bottom plate according to the protocols. Absorbance at 450 nm was measured immediately in kinetic mode for 10–60 min at 37 °C. The relative FH activity was calculated by dividing the values for the agent-treated group by those for the negative control group.
Another portion of the samples was analyzed by a Fumarate Assay Kit (Sigma-Aldrich, St Louis, USA) following the manufacturer’s instructions. The results are shown as fumarate concentrations (ng/μl).
DNA fragment detection and comet assay
For electrophoretic detection of DNA from GC samples, 1% agarose gels were prepared. For the comet assay, GC cells were centrifuged, collected and resuspended at a density of 1 × 106/ml. The first layer of agarose gel, with a normal melting point, was flattened on a microscope slide. After this agarose gel solidified, the second layer of agarose gel, with a low melting point, was mixed with cells and placed atop the first layer. After the second layer solidified, the third layer of agarose gel, with a normal melting point, was overlaid. The slides were placed in cold lysis buffer to lyse the cells and were then placed in the alkaline buffer for 60 min to allow DNA unwinding. We set a power supply at 25 V and adjusted the current to 300 mA by slowly raising or lowering the buffer level for 20 min. After dealing with neutralization buffer 3 times, 30 μl of PI staining solution was added to the slides, and the slides were covered with a coverslip. The slides were then observed under a fluorescence microscope at 515–560 nm. The result was evaluated by the tail Moment (pixels), which was calculated by OpenComet software.
Pharmacologic study in vivo
We constructed SGC7901 GC cell-derived xenograft (CDX) and patient-derived xenograft (PDX) models to assess the pharmacological function of the combination of MN and CDDP. Four- to five-weeks-old female BALB/c nude mice were purchased from Beijing Vital River Laboratory Animal Technology Co., Ltd. We used CDXs for the experiments, which were designed as follows: A total of 2 × 106 SGC7901 cells were injected subcutaneously into the flanks of each mouse. Beginning on the 7th day, the mice were randomly divided into 4 groups: (1) The control group, which received 200 μl of PBS every 3 days; (2) The MN group, which received 5 mg/kg MN every 3 days by intraperitoneal (i.p.) injection23; (3) The CDDP group, which received 4 mg/kg CDDP every 3 days by i.p. injection24; and (4) The combination group, which received both MN and CDDP. We measured the tumor sizes every 3 days during the next 25 days of treatment. The tumor volumes were calculated using the formula V = 0.5 × L × W2. In addition, PDX models were established in NOD/SCID mice. After the tumors from GC patients successfully grew to approximately 500 mm2 (P1), the xenografts were transplanted to the other mice (P2). The same procedure was followed to raise the P3 graft-bearing mice for therapeutic study. The subsequent steps were performed as described above. All animal studies were performed in biological triplicacte and approved by the Institutional Animal Care and Use Committee of Sun Yat-sen University.
Patients and samples
We received approval from the Ethics Committee of the Cancer Center of Sun Yat-sen University (SYSUCC, Guangdong, China). All GC specimens were collected from GC patients who received radical gastrectomy without preoperative treatment between 2007 and 2011. Final diagnosis was confirmed by pathological examination. TNM staging was assessed based on the 7th edition of the International Union Against Cancer (UICC) staging system.
Immunohistochemical (IHC) analysis
Paraffin-embedded tumor samples from patients with GC were prepared following a standard protocol. The slides were incubated with rabbit polyclonal antibody against FH (1:200 dilution), cleaved-caspase 3 (1:400 dilution), γ-H2AX (1:400 dilution) and Ki-67 (according to the manufacturer’s instructions).
The results were evaluated by two pathologists blinded to the clinical outcomes of each patient. FH expression was scored by the combination of staining intensity and extent. Staining intensity was quantified as follows: negative (0), weak (1), moderate (2) or strong (3). Staining extent was scored according to the percentage of positive cells as follows: none (0), <10% (1), 10–50% (2), 50–80% (3) or >80% (4). The product of the intensity score and the extent score was taken as the final score.
Statistical analysis
We evaluated the differences between each group by the unpaired Student’s t-test. The χ2 test was used to analyze the relationships between FH expression and various clinicopathological parameters. For survival analysis, we used the Kaplan-Meier method and compared the data by the log-rank test to calculate the survival curve. The overall survival (OS) time was defined as the time from the first treatment to the date of death or last follow-up. Univariate and multivariate analyses were performed to explore association between FH expression and clinicopathological variables by using the Cox proportional hazards regression model. All statistical analyses were performed with GraphPad Prism 7(GraphPad Software Inc., La Jolla, CA) as well as SPSS 19.0 for Windows (SPSS Inc., Chicago, IL), and a two-sided P value of less than 0.05 was considered statistically significant.
Results
FH was related to CDDP sensitivity in GC
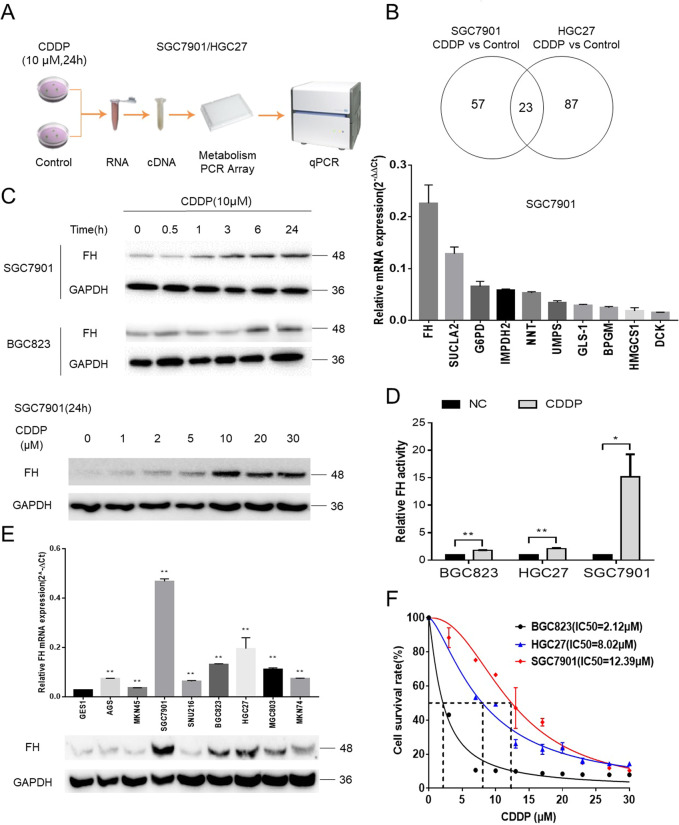
To compare the differences in gene expression between CDDP-treated GC cells and untreated GC cells, we utilized a metabolic PCR assay to examine alterations in mRNA levels in two GC cell lines, SGC7901 and HGC27 (Fig. 1a). As shown in Fig. 1b and Fig. S1A, 23 genes were significantly different in both groups, indicating that these genes were associated with CDDP sensitivity. As expected, FH was among these genes. To confirm these results, these 23 genes were analyzed by qPCR; FH was the most significantly differentially expressed gene of the top 10 most highly expressed transcripts among the 23 genes.
Fig. 1. FH was related to CDDP sensitivity in GC.
a The Metabolism Array workflow to identify differentially expressed genes between CDDP-treated SGC7901 and HGC27 cells and negative control cells. b Venn diagram of the differentially expressed genes (P < 0.05) between control cells and two CDDP-treated GC cell lines, demonstrating that FH was the most differentially expressed among the 23 genes that were differentially expressed in both groups. FH was reconfirmed as the most significantly differentially expressed gene among the 23 differentially expressed genes in SGC7901 cells. Data are presented as the mean ± s.d. (n = 3). c FH expression gradually increased along the time or concentration gradient of CDDP in GC cell lines. d FH activity was increased in the indicated cells after CDDP treatment (*P < 0.05, **P < 0.01, Student’s t-test). Data are presented as the mean ± s.d. (n = 3). e qPCR and immunoblotting analysis of FH expression in one gastric epithelial cell line and eight GC cell lines (**P < 0.01, Student’s t-test). Data are presented as the mean ± s.d. (n = 3). f The IC50 values for CDDP in three GC cell lines increased following the trend in the FH expression level
Next, we considered whether the alteration in FH expression at the protein level was consistent with that at the mRNA level. After treatment with the same concentration (10 μM) of CDDP for the indicated durations or treatment for the same duration (24 h) with different concentrations of CDDP, FH protein expression increased gradually following the concentration or time gradient of CDDP exposure (Fig. 1c). As FH is an enzyme, its activity should also be considered. FH activity was significantly higher in the selected GC cell lines treated with CDDP than in the control cell lines (Fig. 1d). Although the expression of FH varied among the common GC cell lines and the normal gastric cell line (Fig. 1e), it was positively correlated with the CDDP IC50 value (Fig. 1f). These results suggested that FH was related to CDDP sensitivity in GC cell lines.
FH expression and enzymatic activity were induced by CDDP and reduced CDDP cytotoxicity
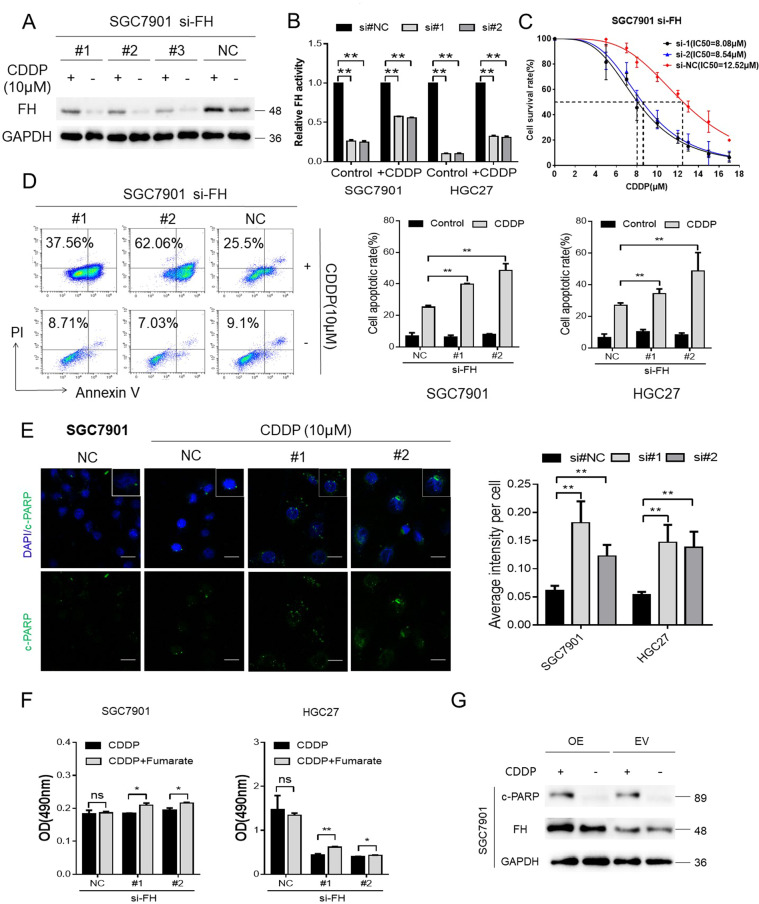
Furthermore, we knocked down FH expression to examine the relationship between FH and CDDP. Knockdown of FH expression not only reduced the expression of FH but also decreased its enzymatic activity. Interestingly, FH expression and enzymatic activity increased after CDDP treatment, although FH expression had been knocked down (Fig. 2a, b). Moreover, the IC50 of CDDP in SGC7901 cells with FH knockdown was lower than that in negative control cells (Fig. 2c).
Fig. 2. FH expression and enzymatic activity were induced by CDDP and reduced CDDP cytotoxicity.
a Western blot of FH expression in CDDP-treated SGC7901 cells with FH knockdown. b FH enzymatic activity in cells treated with or without CDDP after FH knockdown (**P < 0.01, Student’s t-test). Data are presented as the mean ± s.d. (n = 3). c IC50 of CDDP in SGC7901 cells with FH knockdown. d An annexin-V/PI assay detected the apoptosis of FH knockdown and control cells treated with CDDP (**P < 0.01, Student’s t-test). Data are presented as the mean ± s.d. (n = 3). e Representative images (left) and quantification (right) of immunofluorescence staining of c-PARP in SGC7901 and HGC27 cells treated with CDDP after FH knockdown (scale bar: 100 μm, **P < 0.01, Student’s t-test). Data are presented as the mean ± s.d. (n = 3). f CDDP and/or exogenous fumarate (5 mM) were utilized to the GC cell lines with FH knockdown or control. MTS assay determined the viability of the indicated groups (72 h, *P < 0.05, Student’s t-test). Data are presented as the mean ± s.d. (n = 3). g FH and c-PARP expression in FH over-expression cells with or without CDDP treated
Because of the pharmacological function of CDDP in tumor cells, annexin-V/PI assays were used to evaluate the apoptotic effects of FH knockdown in cells treated with this cytotoxic agent. The results, as shown in Fig. 2d, indicated that FH knockdown promoted the cytotoxicity of CDDP to induce GC cell apoptosis. Although different siRNAs were used to treat the cells, the apoptosis rates were not significantly different between the siRNA-treated groups. Immunofluorescence staining of c-PARP reconfirmed the consequence of the more effective proapoptotic function of CDDP after FH knockdown (Fig. 2e). After FH was inhibited, exogenous fumarate could rescue the inhibition and reduce the cytotoxicity of CDDP (Fig. 2f and Fig. S2). The over-expression of FH (OE) made no difference in the c-PARP expression with the cells transfected by empty vector (EV) after CDDP treated (Fig. 2g). Cell viability between the indicated groups didn’t reveal the significant difference either (Fig. S3).
MN influenced the activity of FH to promote the proapoptotic effect of CDDP
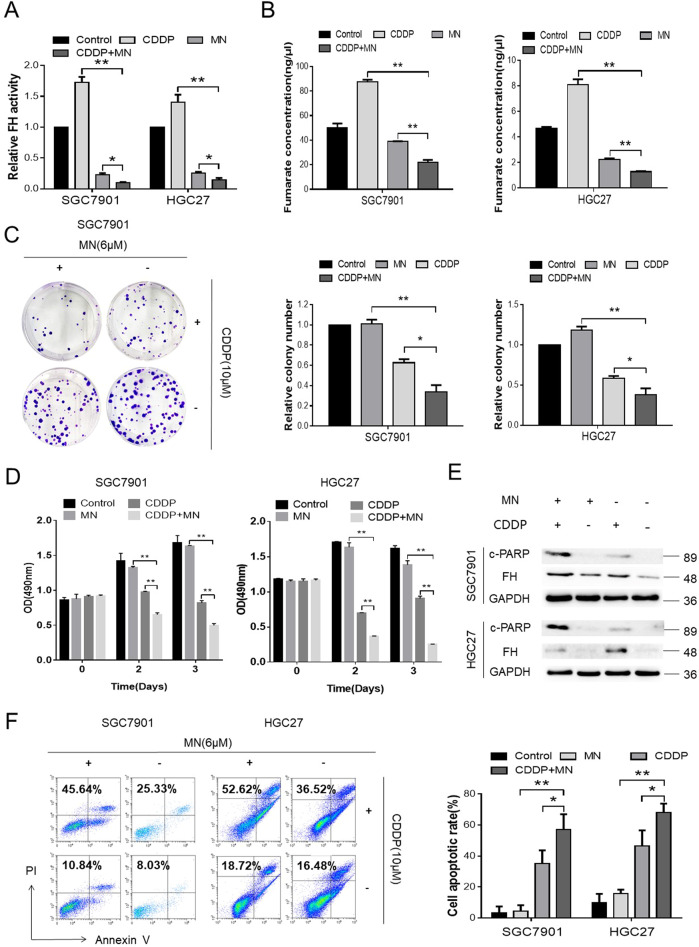
A previous study and data from a database (GeneCards®) revealed that MN targets FH in some species25. Because of the evolutionary conservation of FH, we tried to verify whether MN effectively affected the FH promoted CDDP cytotoxicity in human GC cell lines. First, we used different concentrations of MN to verify the appropriate concentration for further procedures. At an MN concentration of 6 μM, the enzymatic activity of FH decreased dramatically, to nearly 20% (Fig. S4). Therefore, we selected this concentration for further analysis.
The FH activity in SGC7901 or HGC27 cells treated with MN or with MN in combination with CDDP was much lower than that in cells treated with CDDP alone and in negative control cells (Fig. 3a). Fumarate, one of the factors participating in the enzymatic reaction, exhibited the same trend as that described in the above result (Fig. 3b).
Fig. 3. MN influenced the activity of FH to promote the proapoptotic effect of CDDP.
a Relative FH activity in SGC7901 and HGC27 cells after treatment with MN (6 μM), CDDP (10 μM) or the combination of the agents described above (*P < 0.05, **P < 0.01, Student’s t-test). Data are presented as the mean ± s.d. (n = 3). b Fumarate concentrations in the indicated groups of SGC7901 and HGC27 cells (**P < 0.01, Student’s t-test). Data are presented as the mean± s.d. (n = 3). c Images (left) and quantification (right) of colony formation in the indicated cells cultured for 14 days with MN (6 μM), CDDP (10 μM) or the combination (*P < 0.05, **P < 0.01, Student’s t-test). Data are presented as the mean ± s.d. (n = 3). d MTS assay to determine the viability of the indicated cells exposed to MN, CDDP or the combination (48 and 72 h, **P < 0.01, Student’s t-test). Data are presented as the mean ± s.d. (n = 3). e FH and c-PARP expression in the indicated groups of cells were detected by Western blotting. f Images (left) and quantification (right) of the percentage of apoptotic cells in the indicated cells treated for 48 h with MN (6 μM), CDDP (10 μM) or the combination (*P < 0.05, **P < 0.01, Student’s t-test). Data are presented as the mean ± s.d. (n = 3)
Compared with CDDP or MN alone, as well as the negative control, the combination of MN and CDDP significantly inhibited GC cell colony formation and growth (Fig. 3c, d). Moreover, the combination group expressed more c-PARP than the single-agent groups (Fig. 3e), indicating a higher level of apoptosis. In addition, the apoptosis rates in the combination group in both GC cell lines (SGC7901 and HGC27) were significantly higher than those in the corresponding single-agent groups or negative control groups (Fig. 3f).
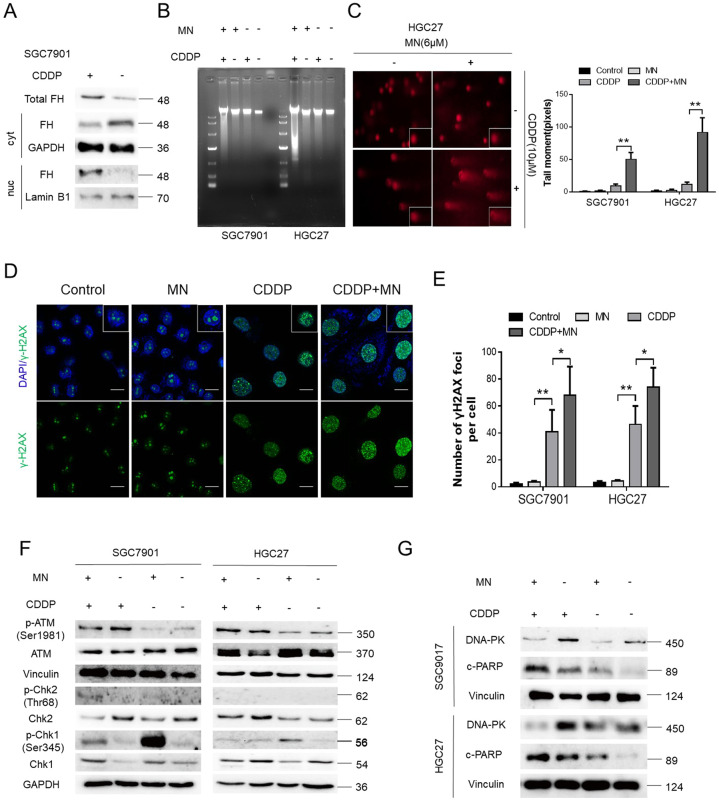
FH contributed to CDDP sensitivity via affecting DNA damage repair
DNA damage repair is a mechanism by which tumor cells become resistant to cytotoxic agents. First, we considered the localization of FH in cells. After CDDP treated, FH appeared more in cell nucleus while it was expressed less in cytoplasm (Fig. 4a). Then DNA damage was detected in GC cells by agarose gel electrophoresis and a comet assay. As Fig. 4b to Fig. 4c and Fig. S5 show, more DNA fragments appeared in the group treated with the combination of MN and CDDP, than in the group treated with the single-agent and the negative control group. The number of γ-H2AX foci was greatly increased in the combination group (Fig. 4d, e). Moreover, Western blot analysis revealed that ATM, a biomarker of homologous recombination (HR), was phosphorylated after CDDP treatment. However, downstream ATM-Chk2 pathway components26 and the Chk1 kinase in the HR pathway did not exhibit the same trend in any groups in both GC cell lines (Fig. 4f). Nonhomologous end joining (NHEJ), another type of DNA damage repair, was suppressed by the combination of MN and CDDP, as DNA-PK expression was downregulated, but c-PARP expression was upregulated. DNA-PK expression was higher in the CDDP group than in the other groups, but the expression of c-PARP was lower in the CDDP group than in the combination group (Fig. 4g). In summary, MN influenced the contribution of FH to CDDP sensitivity via affecting DNA damage repair.
Fig. 4. FH contributed to CDDP sensitivity via affecting DNA damage repair.
a FH expression in GC cells’ cytoplasm (cyt) or nucleus (nuc) with or without CDDP treated. Total FH expression was also shown. Lamin B1 was the nucleus internal reference and GAPDH was the cytoplasmic internal reference. b Agarose gel electrophoresis to detect DNA fragments in the MN (6 μM), CDDP (10 μM), combination or negative control groups of SGC7901 and HGC27 cells. c Representative images from the comet assay for the indicated groups in GC cell lines (left). DNA damage, expressed as the tail moment (pixels) in the comet assay, for the indicated groups (right, **P < 0.01, Student’s t-test). Data are presented as the mean ± s.e.m. d Representative images of immunofluorescence staining of γ-H2AX foci in GC cells treated with the indicated agents (scale bar: 100 μm). e Quantification of γ-H2AX foci in GC cells treated with the indicated agents (*P < 0.05, **P < 0.01, Student’s t-test). Data are presented as the mean ± s.d. (n = 3). f Immunoblotting for HR pathway components in the indicated groups. g Immunoblotting for NHEJ pathway components in the indicated groups
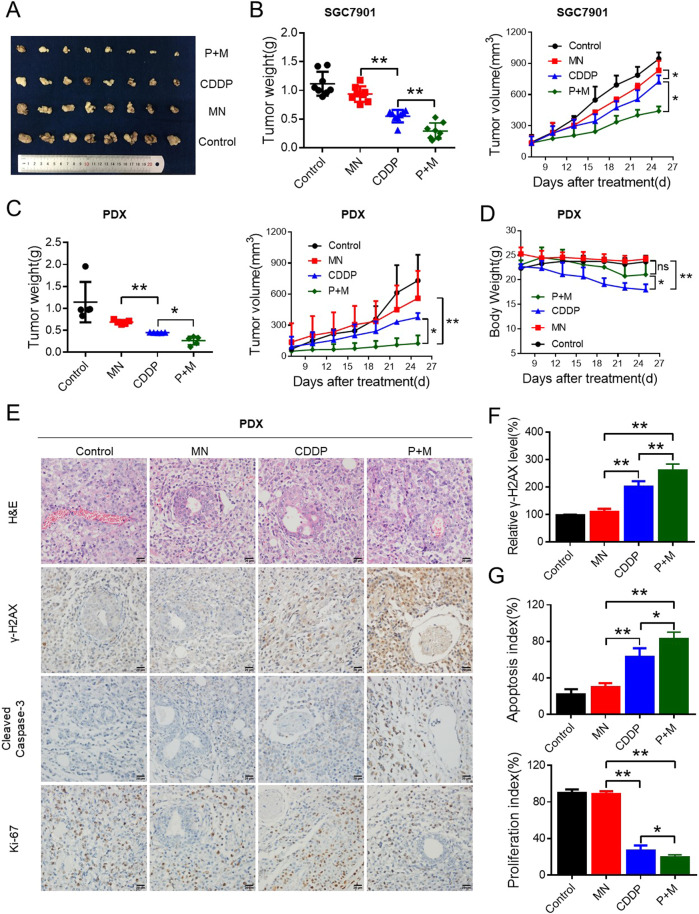
The combination of MN and CDDP inhibited GC growth in vivo
Before we determined the pharmacological effect of MN on CDDP sensitivity in vivo, the relationship between FH and CDDP sensitivity in vivo should be considered. First, we established a model for SGC7901 injected subcutaneously in nude mice. RNA interference of FH in vivo and CDDP were adopted to treated the tumors. As shown in Fig. S6, siRNA of FH with CDDP demonstrated the significant inhibition of tumor growth compared to the control cells with CDDP. Then, the pharmacological effect of MN on CDDP sensitivity in vivo would verify from the following experiments. We tested SGC7901 cells, which exhibit high FH expression. Like the negative control xenografts, xenografts treated with MN showed no growth inhibition, while those treated with CDDP exhibited decreases in weight and volume. Encouragingly, cotreatment with both agents induced marked suppression of tumor growth. The weights and volumes of the tumors in mice treated with the combination of these two agents were the lowest among the groups (Fig. 5a, b).
Fig. 5. The combination of MN and CDDP inhibited GC growth in vivo.
a SGC7901 cells (2 × 106) were injected subcutaneously into nude mice. After the tumor volumes in the control group reached 100 mm3, the mice were treated with MN, CDDP or the combination (M+P). Images of tumors derived from the groups described above are shown. b The tumor weights and tumor growth curves of the indicated groups of mice were measured and generated, respectively (*P < 0.05, **P < 0.01, Student’s t-test). Data are presented as the mean ± s.d. (n = 8). c The tumor weights and tumor growth curves of PDX model mice treated with MN, CDDP or the combination were measured and recorded for 26 days (*P < 0.05, **P < 0.01, Student’s t-test). Data are presented as the mean ± s.d. (n = 5). d The body weights of PDX model mice in the indicated groups were measured and recorded for 26 days (*P < 0.05, **P < 0.01, Student’s t-test). e Paraffin-embedded tumor sections derived from PDX tumors were stained with H&E, and antibodies specific for γ-H2AX, cleaved caspase-3 and Ki-67 (scale bar: 20 μm). f The relative γ-H2AX level was measured by quantification of γ-H2AX (**P < 0.01, Student’s t-test). Data are presented as the mean ± s.d. (n = 5). g The apoptosis index and proliferation index were measured by quantification of cleaved-caspase 3 and Ki-67 levels respectively (*P < 0.05, **P < 0.01, Student’s t-test). Data are presented as the mean ± s.d. (n = 5). The animal studies were performed in biological triplicate
To emulate realistic experimental and preclinical conditions, we adopted PDX models to evaluate the effectiveness of cotreatment with CDDP and MN. Like CDXs, PDXs in the combination group exhibited markedly reduced weights and volumes (Fig. 5c), while the body weight loss in the combination group mice was not significantly different from that in the MN group and control group mice. The adverse effect of CDDP was the appreciably reduced body weight of the mice, but combination with MN ameliorated this adverse effect; the body weight loss was reduced (Fig. 5d). Excised CDX and PDX tumor sections were further analyzed by IHC staining. The IHC score for Ki-67 was significantly lower in the combination group than in the single-agent-treated group. The IHC scores of cleaved caspase-3 and γ-H2AX, however, demonstrated the opposite trend from Ki-67 (Fig. S7A and Fig. 5e). The relative γ-H2AX level in the combination group was the highest among the four groups, indicating increased DNA damage in that group (Fig. S7B and Fig. 5f). In the combination group, the apoptosis index dramatically increased, and the proliferation index decreased, suggesting that the combination treatment effectively repressed growth (Fig. S7C and Fig. 5g).
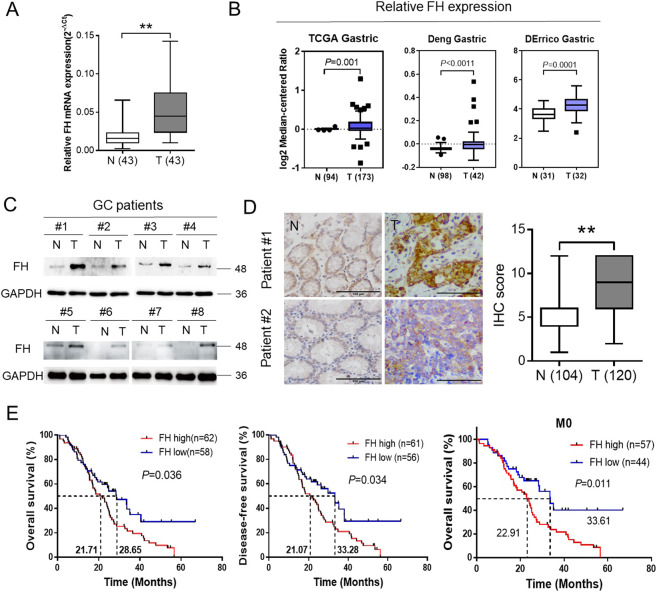
FH expression was an independent prognostic factor in GC patients
To examine the clinical significance of FH expression, we investigated the expression status of FH in human GC tissues. The mRNA level was significantly increased in human GC tissues in 43 pairs of patient samples from our hospital (SYSUCC), as evidenced by qPCR analysis, and in a microarray data set available from Oncomine (Fig. 6a, b). Moreover, protein was extracted from eight pairs of GC and adjacent nontumor tissues, and FH expression assessed by Western blotting. The protein expression levels were consistent with the mRNA levels determined in our previous experiment (Fig. 6c).
Fig. 6. FH expression was an independent prognostic factor in GC patients.
a–b FH was overexpressed in GC tissues, as determined by qPCR analysis of 43 pairs of patient samples from our hospital (SYSUCC) and a microarray data set available from Oncomine (https://www.oncomine.com/, **P < 0.01, Student’s t-test). c Immunoblotting of FH for eight pairs of GC and adjacent nontumor tissues. GAPDH was used as the internal reference. d IHC images (left) of tumor(T) and adjacent nontumor tissues (N) from two representative GC patients of. The scale bars represent 100 μm for 400× images. IHC score for 120 tumor tissues and 104 adjacent nontumor tissues from GC patients (right, **P < 0.01, Student’s t-test). e The OS (P = 0.0357) and DFS (P = 0.0339) curves for patients with low and high levels of FH expression were generated using the Kaplan-Meier method. Compared with patients with metastasis, patients without metastasis in the indicated groups showed a significant difference in OS time (P = 0.0108). P values were calculated using the log-rank test. The median survival time for each group in different situations is shown by the numbers on the curves
Next, we further analyzed the clinical relevance of FH expression. Tissue samples from 130 GC patients were examined by IHC methods. The IHC staining results suggested that FH expression was sharply increased in human paraffin-embedded GC tissues (Fig. 6d). Moreover, the immunoblotting results suggested that FH expression was significantly higher in GC tissues than in adjacent normal tissues (Fig. 6d).
We divided the patients into two groups according to the IHC score: the high FH expression group and the low FH expression group, based on a cutoff score of 6. By the criteria described above, 62 patients (51.67%) were as assigned to the high FH expression group. Additionally, based on the clinicopathological parameters, we found that FH expression was correlated with age (P = 0.01) and distant metastasis (M stage, P = 0.02), but not with sex, depth of tumor infiltration (T stage), local lymph node metastasis (N stage), TNM stage or treatment with adjuvant chemotherapy (Table S2).
Finally, we tried to evaluate the prognostic value of FH in GC patients. We compared survival status among GC patients with high and low FH expression. The median OS times of patients with high and low FH expression were 21.71 months and 28.65 months, respectively. The OS time of patients with high FH expression was shorter than that of patients with low expression (P = 0.036, log-rank test), based on assessment of the Kaplan-Meier curves. In addition, the disease-free survival (DFS, P = 0.034, log-rank test) time was also assessed. Furthermore, FH expression could predict the prognoses of GC patients without metastasis (Fig. 6e). According to the univariate and multivariate Cox regression analysis for each variable, distant metastasis (P = 0.03), TNM stage (P < 0.001) and FH expression (P = 0.02) were independent prognostic factors for OS in patients with GC. Patients with low FH expression had a significantly lower relative risk of death than those with high FH expression (hazard ratio (HR) = 0.56, Table S3).
Discussion
Platinum-mediated chemotherapy, especially CDDP, is the main therapeutic strategy for GC. Researchers primarily seek to optimize therapeutic strategy by improving the effectiveness of CDDP. The anticancer mechanism of CDDP operates by the promotion of platinum-DNA adduct-induced cell death27. Therefore, CDDP sensitivity can be increased through this mechanism.
DNA double-strand breaks (DSBs) are the main type of DNA damage by which cytotoxic agents defeat tumor cells28,29. DNA damage repair pathways respond to such damage to protect cells from this damage30. As mentioned in a literature review, DNA damage repair pathways, including HR, nucleotide excision repair (NER), and nonhomologous end joining (NHEJ), are well-known mechanisms31. In mammalian cells, NHEJ pathway activity dramatically increases to reduce harm from cytotoxic agents after DNA damage occurs32. Therefore, inhibiting the NHEJ pathway could accelerate cytotoxic cell death33.
In this study, we suggest a new approach to increase the sensitivity of GC cells to CDDP via FH inhibition. The metabolism assay verified that FH activity was significantly different after CDDP treatment in two GC cell lines. Thus, FH might play an important role in mediating CDDP sensitivity.
Then, we used CDDP or 5-Fu to treat GC cells, and the effect was quite consistent in CDDP-treated cells. However, cells treated with 5-Fu showed no change in FH expression (Fig. S1B). CDDP induced DSBs, while 5-Fu induced the inhibition of thymidine synthesis34,35. The trend in FH expression levels between the normal gastric cell line (GES1) and GC cell lines was the same as that between normal tissues compared with GC tissues. These results indicate that the upregulation of FH is related to tumorigenesis and CDDP sensitivity in GC.
Apoptosis levels in cells with FH knockdown alone were not different than those in negative control cells. In addition, FH knockdown alone did not affect cell growth (data not shown). A possible explanation for this similarity might be that FH itself was not the main factor maintaining the malignant biological properties of tumor cells. In combination with CDDP, FH knockdown enhanced CDDP cytotoxicity, but FH expression, as well as activity rose. These results provided further confirmation that FH is involved in regulating CDDP sensitivity. Exogenous fumarate could partly rescue the function of FH after FH was knocked down. But the over-expression of FH didn’t improve cell survival in response to CDDP treatment. Because of the better prognosis for patients with low expression of FH, the possible explanation for the phenomenon was that the basal expression of FH dictated CDDP sensitivity.
Targeting FH is a possible approach to sensitize GC cells to CDDP. Zhimin Lu et al. investigated the relationship between FH and histone H3 in the osteosarcoma cell line U2OS and found that in the nucleus, p-FH (Thr236) combined with the histone complex under exposure to DNA damaging agents. However, the combination of DNA-dependent protein kinase (DNA-PK) and FH abrogated the conversion of malate to fumarate catalyzed by Thr236-phosphorylated FH, and the accumulation of fumarate repressed the expression of Lysine Demethylase 2B (KDM2B), which participates in histone demethylation. This process eventually led to enhanced promotion of DNA repair. The results indicated that the enzymatic activities of FH and its product, fumarate, were responsible for the DNA damage response and that FH deficiency promoted tumorigenesis due to the impairment of DNA repair17. Valery Barillaro et al. noted that MN could combine with fumaric acid via hydrogen bonding25. These results suggested that MN could inhibit the enzymatic activity of FH in converting fumaric acid to malic acid. In fact, the following study also verified this hypothesis. At a concentration of 6 μM (Supplementary Fig. 3), MN inhibited FH activity to 20% of the baseline activity and led to a decrease amount in fumarate. Alone, MN exerted almost no influence on GC cell growth. However, when MN was combined with CDDP, apoptosis increased because the cytotoxicity was augmented. Furthermore, we preliminarily discussed the mechanism by which FH mediates CDDP sensitivity. After CDDP treated, FH expression in total protein was increased, which was consistent with the results described above. In the cell nucleus, FH expression was higher than the control group while the opposite trend was occurred in the cytoplasm. It is possible that FH plays a role in DNA damage repair which occurs in cell nucleus. The work of Zhimin Lu and colleagues indicated that FH participates in the NHEJ DNA damage repair pathway17. We examined both the HR and NHEJ pathways. ATM was phosphorylated after CDDP treatment, but neither Chk1 nor Chk2 were noticeably phosphorylated. As the initial marker of NHEJ repair, DNA-PK expression indicated that repair occurred36. The higher the DNA-PK expression, the more NHEJ repair occurred37. The combination group exhibited downregulated DNA-PK expression, while the CDDP group exhibited upregulated DNA-PK expression, indicating that the NHEJ pathway and not the HR pathway was the main pathway mediating CDDP sensitivity. MN inhibited NHEJ repair after CDDP damaged tumor cell DNA. Additional evidence from agarose gel electrophoresis and the comet assay revealed the generation of DNA fragments via the DSB DNA damage. In addition, the number of γ-H2AX foci increased dramatically in the combination group, indicating that MN could promote the cytotoxicity of CDDP38. In summary, FH contributed to CDDP sensitivity via affecting DNA damage repair.
In order to consider the influence factor for the off-target effects of miconazole In vivo, RNA interference was adopted. The results suggested that FH was still effective in CDDP sensitivity in vivo. The combination of MN and CDDP demonstrated an inhibitory effect on CDX growth. The growth of patient-derived xenografts (PDXs), which were consistent with the patients’ actual tumor statuses39–41, was also inhibited. The combination of MN and CDDP was less toxic than CDDP alone, as evidenced by the reduction in body weight loss. Although both DNA damage and apoptosis were enhanced, the proliferation index was reduced, demonstrating that GC growth was inhibited via molecular mechanisms. Further analysis of clinical samples suggested that the FH expression level was significantly increased in GC tissues compared with that in adjacent normal tissues. As an independent prognostic factor, the FH expression level showed excellent predictive value for the prognosis of GC patients, especially for those in the early stage of disease.
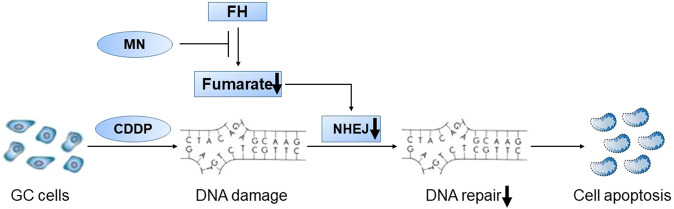
In summary, our study suggested a possible approach using MN to pharmacologically inhibit FH enzymatic activity via the DNA damage repair pathway. MN-mediated inhibition of FH enzymatic activity increased the sensitivity of cells to CDDP in vitro and in vivo (Fig. 7). Moreover, the combination of MN and CDDP showed lower toxicity and higher effectiveness than CDDP alone. Furthermore, the FH expression level could predict GC patients’ prognoses. Our data suggest that the combination of MN, an enzymatic inhibitor of FH, with CDDP may be a unique therapeutic approach in GC. The FH expression level could guide the treatment strategy, especially for GC patients without distant metastasis.
Fig. 7. Schematic illustration of the effects observed in this study.
Once DNA damage occurred in GC cells with CDDP, MN inhibited FH enzymatic activity and reduced the production of fumarate. The decrease of fumarate induced tumor cells’ apoptosis via weakening NHEJ in the DNA repair pathway
Supplementary information
Acknowledgements
This research was supported by the National Key R&D Program of China (2018YFC1313300), National Natural Science Foundation of China (81871951, 81602137, 81572392); Natural Science Foundation of Guangdong Province (2018B030306049, 2017A030313485, 2014A030312015); Science and Technology Program of Guangdong (2015B020232008), Tip-top Scientific and Technical Innovative Youth Talents of Guangdong special support program (2016TQ03R614).
Authors’ contributions
H.E.Y. conducted most of the experiments, with assistance from F.Y., Y.W., Y.X.L., Y.J., D.S.W. and M.Z.Q.; H.E.Y. analyzed the results, produced figures and wrote preliminary draft of the paper; F.W. and Z.L.Z. provided fresh gastric cancer samples and advice; H.Y.P., T.B.K. and D.X. supervised and analyzed results and edited the paper. H.Q.J., R.H.X. and H.Y.L. designed, supervised and wrote the final draft of the paper. All authors approved final version of the manuscript.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Edited by M. Agostini
This article has been retracted. Please see the retraction notice for more detail: 10.1038/s41419-023-05604-7
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Hong-En Yu, Feng Wang, Fang Yu, Zhao-Lei Zeng
Change history
1/20/2023
This article has been retracted. Please see the Retraction Notice for more detail: 10.1038/s41419-023-05604-7
Contributor Information
Huai-Qiang Ju, Email: juhq@sysucc.org.cn.
Rui-Hua Xu, Email: xurh@sysucc.org.cn.
Hui-Yan Luo, Email: luohy@sysucc.org.cn.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41419-019-1652-8).
References
- 1.Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Torre LA, Siegel RL, Ward EM, Jemal A. Global Cancer Incidence and Mortality Rates and Trends--An Update. Cancer Epidemiol Biomarkers Prev. 2016;25:16–27. doi: 10.1158/1055-9965.EPI-15-0578. [DOI] [PubMed] [Google Scholar]
- 3.Cunningham D, et al. Peri-operative chemotherapy with or without bevacizumab in operable oesophagogastric adenocarcinoma (UK Medical Research Council ST03): primary analysis results of a multicentre, open-label, randomised phase 2-3 trial. Lancet Oncol. 2017;18:357–370. doi: 10.1016/S1470-2045(17)30043-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mizoshita T, et al. Gastric phenotype signet-ring cell carcinoma of the stomach with multiple bone metastases effectively treated with sequential methotrexate and 5-fluorouracil. Int J Clin Oncol. 2008;13:373–376. doi: 10.1007/s10147-007-0750-2. [DOI] [PubMed] [Google Scholar]
- 5.Kang YK, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20:666–673. doi: 10.1093/annonc/mdn717. [DOI] [PubMed] [Google Scholar]
- 6.Kim JG, et al. Phase II study of docetaxel and capecitabine in patients with metastatic or recurrent gastric cancer. Oncology. 2005;68:190–195. doi: 10.1159/000086773. [DOI] [PubMed] [Google Scholar]
- 7.Giordano KF, et al. Docetaxel and capecitabine in patients with metastatic adenocarcinoma of the stomach and gastroesophageal junction: a phase II study from the North Central Cancer Treatment Group. Ann Oncol. 2006;17:652–656. doi: 10.1093/annonc/mdl005. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki T, Sato M, Yoshida T, Tuboi S. Rat liver mitochondrial and cytosolic fumarases with identical amino acid sequences are encoded from a single gene. J Biol Chem. 1989;264:2581–2586. doi: 10.1016/S0021-9258(19)81652-6. [DOI] [PubMed] [Google Scholar]
- 9.Wu M, Tzagoloff A. Mitochondrial and cytoplasmic fumarases in Saccharomyces cerevisiae are encoded by a single nuclear gene FUM1. J Biol Chem. 1987;262:12275–12282. doi: 10.1016/S0021-9258(18)45347-1. [DOI] [PubMed] [Google Scholar]
- 10.Woods SA, Miles JS, Roberts RE, Guest JR. Structural and functional relationships between fumarase and aspartase. Nucleotide sequences of the fumarase (fumC) and aspartase (aspA) genes of Escherichia coli K12. Biochem J. 1986;237:547–557. doi: 10.1042/bj2370547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tomlinson IP, et al. Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nature genetics. 2002;30:406–410. doi: 10.1038/ng849. [DOI] [PubMed] [Google Scholar]
- 12.Schmidt LS, Linehan WM. Hereditary leiomyomatosis and renal cell carcinoma. International journal of nephrology and renovascular disease. 2014;7:253–260. doi: 10.2147/IJNRD.S42097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Llamas-Velasco M, et al. Fumarate hydratase immunohistochemical staining may help to identify patients with multiple cutaneous and uterine leiomyomatosis (MCUL) and hereditary leiomyomatosis and renal cell cancer (HLRCC) syndrome. Journal of Cutaneous Pathology. 2014;41:859–865. doi: 10.1111/cup.12396. [DOI] [PubMed] [Google Scholar]
- 14.Yogev O, Naamati A, Pines O. Fumarase: a paradigm of dual targeting and dual localized functions. The FEBS journal. 2011;278:4230–4242. doi: 10.1111/j.1742-4658.2011.08359.x. [DOI] [PubMed] [Google Scholar]
- 15.Dik E, Naamati A, Asraf H, Lehming N, Pines O. Human Fumarate Hydratase Is Dual Localized by an Alternative Transcription Initiation Mechanism. Traffic. 2016;17:720–732. doi: 10.1111/tra.12397. [DOI] [PubMed] [Google Scholar]
- 16.Yogev O, et al. Fumarase: a mitochondrial metabolic enzyme and a cytosolic/nuclear component of the DNA damage response. PLoS biology. 2010;8:e1000328. doi: 10.1371/journal.pbio.1000328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang Y, et al. Local generation of fumarate promotes DNA repair through inhibition of histone H3 demethylation. Nature cell biology. 2015;17:1158–1168. doi: 10.1038/ncb3209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu R, et al. Mechanism of cancer cell adaptation to metabolic stress: proteomics identification of a novel thyroid hormone-mediated gastric carcinogenic signaling pathway. Mol Cell Proteomics. 2009;8:70–85. doi: 10.1074/mcp.M800195-MCP200. [DOI] [PubMed] [Google Scholar]
- 19.Yuan SY, et al. Miconazole induces apoptosis via the death receptor 5-dependent and mitochondrial-mediated pathways in human bladder cancer cells. Oncol Rep. 2017;37:3606–3616. doi: 10.3892/or.2017.5608. [DOI] [PubMed] [Google Scholar]
- 20.Wu CH, et al. Antitumor effects of miconazole on human colon carcinoma xenografts in nude mice through induction of apoptosis and G0/G1 cell cycle arrest. Toxicol Appl Pharmacol. 2002;180:22–35. doi: 10.1006/taap.2002.9352. [DOI] [PubMed] [Google Scholar]
- 21.Hou J, et al. Hepatic RIG-I predicts survival and interferon-alpha therapeutic response in hepatocellular carcinoma. Cancer Cell. 2014;25:49–63. doi: 10.1016/j.ccr.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 22.Cai WY, et al. The Wnt-beta-catenin pathway represses let-7 microRNA expression through transactivation of Lin28 to augment breast cancer stem cell expansion. J Cell Sci. 2013;126:2877–2889. doi: 10.1242/jcs.123810. [DOI] [PubMed] [Google Scholar]
- 23.Zheng T, Wang X, Yim M. Miconazole inhibits receptor activator of nuclear factor-kappaB ligand-mediated osteoclast formation and function. Eur J Pharmacol. 2014;737:185–193. doi: 10.1016/j.ejphar.2014.04.047. [DOI] [PubMed] [Google Scholar]
- 24.Wu QN, et al. Pharmacological inhibition of DUSP6 suppresses gastric cancer growth and metastasis and overcomes cisplatin resistance. Cancer Lett. 2018;412:243–255. doi: 10.1016/j.canlet.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 25.Barillaro V, et al. Theoretical and experimental vibrational study of miconazole and its dimers with organic acids: application to the IR characterization of its inclusion complexes with cyclodextrins. Int J Pharm. 2008;350:155–165. doi: 10.1016/j.ijpharm.2007.08.048. [DOI] [PubMed] [Google Scholar]
- 26.Chaturvedi P, et al. Mammalian Chk2 is a downstream effector of the ATM-dependent DNA damage checkpoint pathway. Oncogene. 1999;18:4047–4054. doi: 10.1038/sj.onc.1202925. [DOI] [PubMed] [Google Scholar]
- 27.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 28.Bhide SA, et al. Delayed DNA double-strand break repair following platin-based chemotherapy predicts treatment response in head and neck squamous cell carcinoma. Br J Cancer. 2016;115:825–830. doi: 10.1038/bjc.2016.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldstein M, Kastan MB. The DNA damage response: implications for tumor responses to radiation and chemotherapy. Annu Rev Med. 2015;66:129–143. doi: 10.1146/annurev-med-081313-121208. [DOI] [PubMed] [Google Scholar]
- 30.Willers H, Dahm-Daphi J, Powell SN. Repair of radiation damage to DNA. Br J Cancer. 2004;90:1297–1301. doi: 10.1038/sj.bjc.6601729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tian H, et al. DNA damage response--a double-edged sword in cancer prevention and cancer therapy. Cancer Lett. 2015;358:8–16. doi: 10.1016/j.canlet.2014.12.038. [DOI] [PubMed] [Google Scholar]
- 32.Shibata A. Regulation of repair pathway choice at two-ended DNA double-strand breaks. Mutat Res. 2017;803–805:51–55. doi: 10.1016/j.mrfmmm.2017.07.011. [DOI] [PubMed] [Google Scholar]
- 33.Bredemeyer AL, et al. High-throughput screening approach for identifying compounds that inhibit nonhomologous end joining. SLAS Discov. 2018;23:624–633. doi: 10.1177/2472555217746324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inoue A, et al. A small molecule inhibitor of monoubiquitinated Proliferating Cell Nuclear Antigen (PCNA) inhibits repair of interstrand DNA cross-link, enhances DNA double strand break, and sensitizes cancer cells to cisplatin. J Biol Chem. 2014;289:7109–7120. doi: 10.1074/jbc.M113.520429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Longley DB, et al. Characterization of a thymidylate synthase (TS)-inducible cell line: a model system for studying sensitivity to TS- and non-TS-targeted chemotherapies. Clin Cancer Res. 2001;7:3533–3539. [PubMed] [Google Scholar]
- 36.Roos WP, Krumm A. The multifaceted influence of histone deacetylases on DNA damage signalling and DNA repair. Nucleic Acids Res. 2016;44:10017–10030. doi: 10.1093/nar/gkw922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang W, et al. Differential phosphorylation of DNA-PKcs regulates the interplay between end-processing and end-ligation during nonhomologous end-joining. Mol Cell. 2015;58:172–185. doi: 10.1016/j.molcel.2015.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mah LJ, El-Osta A, Karagiannis TC. GammaH2AX: a sensitive molecular marker of DNA damage and repair. Leukemia. 2010;24:679–686. doi: 10.1038/leu.2010.6. [DOI] [PubMed] [Google Scholar]
- 39.Tentler JJ, et al. Patient-derived tumour xenografts as models for oncology drug development. Nat Rev Clin Oncol. 2012;9:338–350. doi: 10.1038/nrclinonc.2012.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kopetz S, Lemos R, Powis G. The promise of patient-derived xenografts: the best laid plans of mice and men. Clin Cancer Res. 2012;18:5160–5162. doi: 10.1158/1078-0432.CCR-12-2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Byrne AT, et al. Interrogating open issues in cancer precision medicine with patient-derived xenografts. Nat Rev Cancer. 2017;17:254–268. doi: 10.1038/nrc.2016.140. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.