Abstract
One of the mechanisms of rapid adaptation or acclimatization to environmental changes in corals is through the dynamics of the composition of their associated endosymbiotic Symbiodiniaceae community. The various species of these dinoflagellates are characterized by different biological properties, some of which can confer stress tolerance to the coral host. Compelling evidence indicates that the corals’ Symbiodiniaceae community can change via shuffling and/or switching but the ecological relevance and the governance of these processes remain elusive. Using a qPCR approach to follow the dynamics of Symbiodiniaceae genera in tagged colonies of three coral species over a 10–18 month period, we detected putative genus-level switching of algal symbionts, with coral species-specific rates of occurrence. However, the dynamics of the corals’ Symbiodiniaceae community composition was not driven by environmental parameters. On the contrary, putative shuffling event were observed in two coral species during anomalous seawater temperatures and nutrient concentrations. Most notably, our results reveal that a suit of permanent Symbiodiniaceae genera is maintained in each colony in a specific range of quantities, giving a unique ‘Symbiodiniaceae signature’ to the host. This individual signature, together with sporadic symbiont switching may account for the intra-specific differences in resistance and resilience observed during environmental anomalies.
Subject terms: Molecular biology, Ecology
Introduction
Dinoflagellate algae from the family Symbiodiniaceae are one of the keystone taxa for coral reef ecosystems. Their importance lies in that in tandem to living free in the environment1,2, they also form photo-symbiotic associations with corals and several other invertebrates (e.g.3,4). Their extraordinary diversity encompasses at least nine major lineages5, labelled A to I in the literature some of which were recently erected to genus level5: Symbiodinium (clade A), Breviolum (clade B), Cladocopium (clade C) and Durusdinium (clade D). Moreover, each lineage encompasses multiple distinct genetic types6,7. The acquisition of Symbiodiniaceae by corals is initiated during early life stages via vertical/maternal transfer or through horizontal pathways (reviewed in8). This fine-tuned partnership between the metazoan coral and the dinoflagellates enables the coral-guild (i.e. the holobiont) to thrive in oligotrophic waters (e.g.9–14). The collapse of this symbiosis is becoming a common global phenomenon as environmental anomalies are becoming more and more frequent and severe, leading to mass coral bleaching and mortality events globally15–18.
The coral holobiont’s resilience to environmental stressors is in large part based on various genotypes and biological traits19. This includes the complex interaction and differential physiological responses of symbiotic Symbiodiniaceae populations that may be composed of one single genus or multiple genera and species20,21 and the host22–25. A plethora of in situ and ex situ studies have reported highly dynamic coral-Symbiodiniaceae associations in response to environmental changes, contrasts or extremes26–31. This is a particularly complex mechanism to study, as the free-living Symbiodiniaceae communities may be altered depending on habitat quality32,33. Shifts that occur over the coral’s ontogeny have been related to physiological states (e.g. diseased vs. healthy, temperature resilience)34–37. These ecological observations support the idea that flexibility in the coral-Symbiodiniaceae partnership enables the coral holobiont to adapt rapidly to environmental stressors38, a paradigm that is encapsulated in the Adaptive Bleaching Hypothesis39. In contrast, the analysis of ancient DNA from octocoral species revealed a stable coral host-Symbiodiniaceae association over the last century40. Moreover, comparative genomic and transcriptomic analyses showed evidence of a metabolic continuum between the genomes of corals and associated symbionts, supporting the hypothesis that co-evolutionary mechanisms in corals play important roles in the maintenance and adaptation of the symbiosis41–43. In line with these observations, coral-Symbiodiniaceae associations can exhibit a high degree of specificity44–47 at the level of both genera and species, and there is a lack of evidence for adult coral colonies to form stable partnerships with newly acquired exogenous Symbiodiniaceae48,49. Therefore, corals have to balance two apparently incompatible traits: Symbiodiniaceae genus fidelity versus Symbiodiniaceae genus flexibility50.
Deciphering the extent of symbiont change potentially occurring among different coral species through shuffling (intrinsic changes) and/or switching (extrinsic changes) of Symbiodiniaceae communities39, requires the consideration of cryptic taxonomic units present at trace levels21,51–53. While an increasing attention has been directed towards these symbiotic cryptic populations since the development of high-sensitivity molecular techniques such as real-time quantitative Polymerase Chain Reaction (qPCR;21,51) and Next-Generation Sequencing54–59, their ecological role in the holobiont’s performance is still poorly understood. To date, studies investigating cryptic Symbiodiniaceae populations have mostly focused on the presumably stress tolerant genus Durusdinium, and the potential for change in the relative abundance of pre-existing Symbiodiniaceae genotypes in response to heat stress23,28,31,53,56,60,61. Recent studies also using random sampling surveys revealed that possible de novo acquisition of exogenous Symbiodiniaceae species can occur associated with environmental changes47,56. In contrast, a long-term survey of diseased A. cytherea corals found that the acquisition of unusual Symbiodiniaceae genera by corals facing environmental stressors is sporadic rather than specific36.
In the present study we evaluate the potential of Symbiodiniaceae genus dynamics as a rapid adaptive mechanism for the coral holobiont. We study the composition of Symbiodiniaceae communities and their quantitative regulation in tagged colonies of three coral species over a period of 10–18 months, among environmentally contrasting locations of Moorea (French Polynesia).
Material and Methods
Monitoring design: reefs and corals
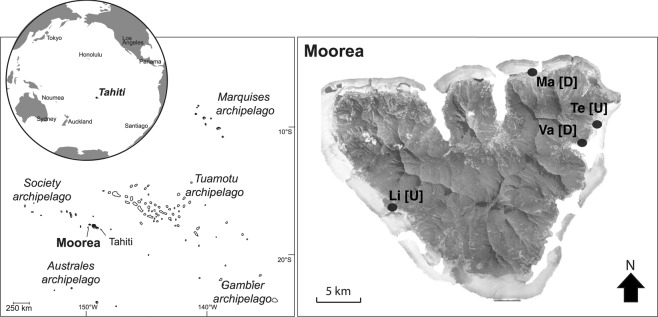
Sampling was conducted in the Moorea lagoon, in the Society Archipelago, French Polynesia (Fig. 1) from February 2011 to August 2012, i.e. our study period included two wet (November to April) and two dry (May to October) seasons (Table S2). The spatio-temporal dynamics of Symbiodiniaceae genera were investigated on three dominant Pacific coral species (Pocillopora acuta, Acropora cytherea, and Porites rus) with contrasting biological traits (Table 1). Sample collection took place every two months in four inshore fringing reef locations (1–2 m depth; Fig. 1) over a period of 10 months for P. rus, 14 months for A. cytherea, and 18 months for P. acuta (P. damicornis type β sensu62 genetically identified in50). At every timepoint, small fragments (0.5–1 cm3) were collected from each of 5 to 6 tagged adult colonies per coral species per study site (Table 1). For Acropora cytherea 11 colonies correspond to those used by Rouzé et al.36 and 5 new colonies were added for this study. The four study sites (Fig. 1) were selected for their contrasting environmental conditions: exposed to human activities (Disturbed: D) at Vaiare (in the direct vicinity of a highly frequented ferry wharf) and Maharepa (anthropogenic impacts from hotel facilities, aquatic activities and close to a river mouth subject to intense terrestrial runoffs) versus limited to human activities (Undisturbed: U) at Linareva and Teavaro63.
Figure 1.
Map of Moorea island (Archipelago of society, French Polynesia) and the locations of the fringing reefs studied: disturbed [D] sites Vaiare (Va) and Maharepa (Ma) versus undisturbed [U] sites Linareva (Li) and Teavaro (Te) modified from50.
Table 1.
Biological traits and conditions of the surveys of the tagged coral colonies Acropora cytherea (ACY), Pocillopora acuta (PAC) and Porites rus (PRU).
| Species | Biological Traits | Survey | |||
|---|---|---|---|---|---|
| Morphology | Resistance | Symb. acquisition | Period | Site (N) | |
| PAC | Branching | Moderate | Vertical | Feb11-Aug12 | Li (5), Te (5), Va (5), Ma (5) |
| PRU | Massive-branching | High | Vertical | Oct11-Aug12 | Li (5), Te (5), Va (5), Ma (5) |
| ACY | Tabular | Low | Horizontal | Jun11-Aug12 | Va (5), Li (5)*, LI2 (6)* |
Reef sites are coded as follows: Linareva (Li), Teavaro (Te), Vaiare (Va) and Maharepa (Ma) with the number of colonies tagged in each of them (N). Asterisk indicates samples used in Rouzé et al. 201636.
Biological and physical environmental parameters (i.e. seawater temperature, nutrients, phytoplankton and sedimentation) were also collected at each timepoint at each location as described in Rouzé et al.63 (Fig. S1 and Table S2).
DNA extraction and Symbiodiniaceae/host ratio quantification
The fragments collected from each coral colony were stored individually in 80% ethanol (EtOH) at −20 °C until genomic DNA extraction. All CTAB-based DNA extractions, qPCR assays (i.e. tests for specificity and efficiency of each genus-specific primer) and Symbiodiniaceae/host ratio measurements, including the conditions of qPCR assays, were conducted as described in50. Briefly, Symbiodiniaceae from the genus Symbiodinium, Breviolum, Cladocopium and Durusdinium and clades E and F64 were targeted and quantified using the nuclear ribosomal large subunit (28S rRNA) gene as a template. More specifically, the sensitivity degree of this qPCR method was estimated at the order of 1 algal cell for Symbiodinium, Cladocopium and Durusdinium from assays performed on series of counted symbiotic cells isolated from corals (see50), which allows for the detection of background populations. Each sample was analyzed twice on the same plate, as one technical replicate, and averaged when the variation between both Ct values was not exceeding 1. An inter-plate calibrator (i.e. positive control with known concentrations and Ct values: mixture of DNA from different Symbiodiniaceae genera50, tested in triplicates (one technical replicate), was added to each plate to calibrate Ct values (performed manually on the MxPro software to set the fluorescent threshold to a fixed Ct value) among different plates of coral DNA samples. The proportion of Symbiodiniacea genera was then estimated by measuring the symbiont/host ratio (S/H ratio) between gene copy numbers of the Symbiodiniaceae 28S and the coral 18S rRNA gene copy numbers in each colony (transformed by log +1). This enabled the simultaneous comparison of Symbiodiniaceae genera within and amongst coral DNA extracts.
Defining the stability of Symbiodiniaceae communities
Based on the presence over time of each Symbiodiniaceae genus in each surveyed coral colony, we defined an arbitrary ‘genus stability status’, as follows: 1) ‘Permanent’ (detected in more than 80% of sampling timepoints) and 2) ‘non-permanent’ (detected in less than or equal to 80% of sampling timepoints) subdivided into ‘temporal’ (detected in more than two consecutive sampling timepoints) or ‘sporadic’ (detected in less than three consecutive sampling timepoints) based on the global genera distribution compared to random distribution (SI 2).
Statistical analysis
For each Symbiodiniaceae genus that was quantified in any given sample, the S/H ratios were estimated and log +1 transformed for further analyses. Differences in S/H ratio of stable Symbiodiniaceae genera were tested among coral species and study sites using either analyses of variance (ANOVA) in case of homogeneity of variance; or non-parametric Kruskal-Wallis tests on ranks.
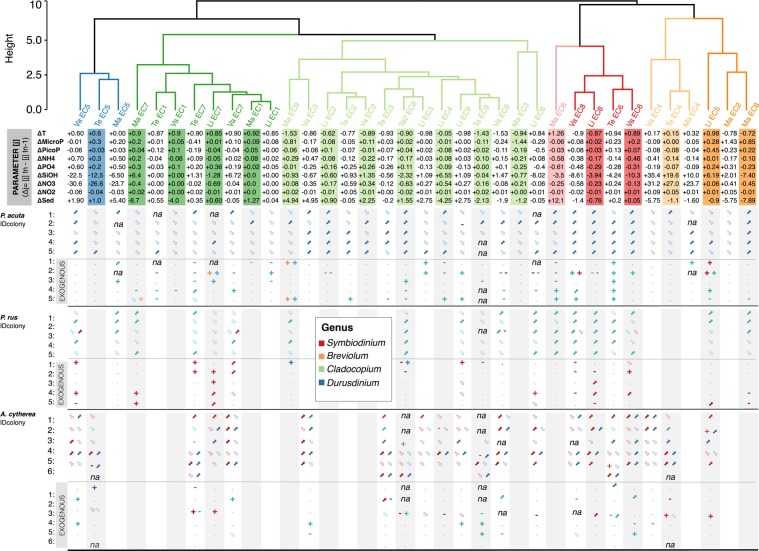
Symbiodiniaceae genus dynamics were estimated for each coral host by the calculation of the difference of S/H ratio of two successive sampling times tn and tn+1 (genus i, sampling time n: ∆genus i,n = [Si/H]tn+1 − [Si/H]tn). Similarly, the delta values of each environmental parameter (from Table 1 in63) were calculated for each period (parameter j: ∆j = [j]tn+1 − [j]tn), and then combined to consider the overall Environmental Context (EC) at each period. A hierarchical clustering of ECs was done using Euclidian metrics and the Ward method, yielding seven clusters, each characterized by a unique set of environmental variations (Fig. 3).
Figure 3.
Results of the cluster analysis of environmental contexts from reef sites Linareva (Li), Teavaro (Te), Maharepa (Ma) and Vaiare Va) and the corresponding dynamics of Symbiodiniaceae genera associated to Pocillopora acuta (PAC), Porites rus (PRU) and Acropora cytherea (ACY) from February 2011 to August 2012, between two successive sampling times tn+1 and tn. The Hierarchical Clustering Analysis was applied on the matrix with delta values of each environmental parameters using the Euclidean distance coefficient to compare ECs and Ward’s method of minimum variance to assemble clusters (i.e. package ‘pvclust’ in R 101). ECs are coded according to the chronological sampling periods as follows: EC1 (Feb11-Apr11), EC2 (Apr11-Jun11), EC3 (Jun11-Aug11), EC4 (Aug11-Oct11), EC5 (Oct11-Dec11), EC6 (Dec11-Feb12), EC7 (Feb12-Apr12), EC8 (Apr12-Jun12) and EC9 (Jun12-Aug12). The analysis is based on the quantitative variation of the different environmental parameters of sea surface temperature (T), Phytoplankton >2 μm (Micro) and <2 μm (PicoP), ammonium (NH4+), phosphate (PO43-), silicate (SIOH), nitrite (NO3-), nitrate (NO2-) and sedimentation (Sed), described in63. The Symbiodiniaceae genus dynamics recorded into different tagged coral hosts dissociated with their label number (IDcol) from different species are described through their quantitative variation (increase: ‘↑’, decrease: ‘↓’, loss: ‘−’ or re-acquisition: ‘+’) of permanent clades and/or non-permanent clades (exogenous exchanges with temporary and sporadic clades).
All statistical analyses were performed using R software (R Foundation for Statistical Computing, version 2.15) using the package ‘stats’ for the ANOVAs (normality and homoscedasticity of variances screened) and corresponding pairwise posthoc Tukey’s tests or chi-squared tests, and simple or multiple linear regressions. For all analyses, the confidence interval was set to 0.95.
Results
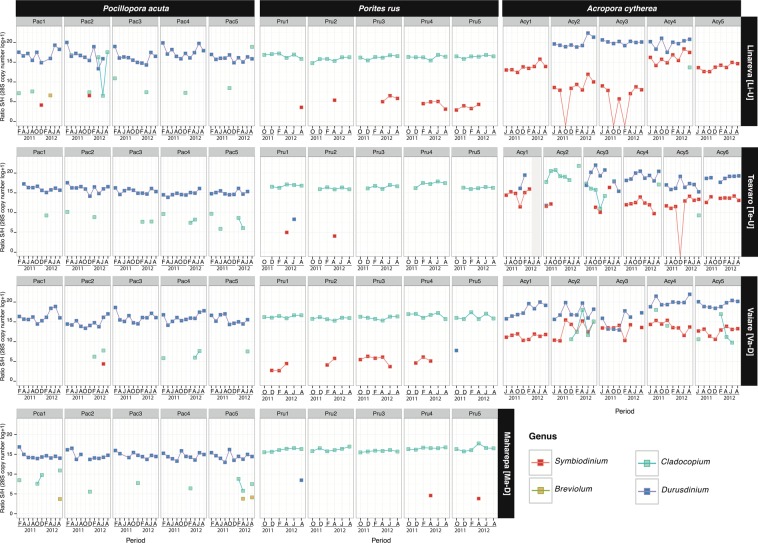
In total, the composition of Symbiodiniaceae genera from 20 P. acuta, 20 P. rus, and 16 A. cytherea colonies were screened over a 10 to 18-month survey at four reef locations (Table 1). Symbiodiniaceae genera Symbiodinium, Cladocopium and Durusdinium were recorded at least once in every coral species, while clades E and F were never recorded. Genus Breviolum was exclusively detected in P. acuta, and only at three occasions, always as background population representing less than 5% of the relative proportion of total Symbiodiniaceae47,50,51 (S/H ratio: <5 28S copy number in log +1, Fig. 2).
Figure 2.
Spatio-temporal survey of Symbiodiniaceae of genus Symbiodinium, Breviolum, Cladocopium and Durusdinium associated in 56 tagged coral colonies of 3 coral species (Pocillopora acuta [Pac], Porites rus [Pru] and Acropora cytherea [Acy]) at four reef sites (Linareva [Li], Teavaro [Te], Vaiare [Va] and Maharepa [Ma]) from February 2011 to August 2012. Between 5 and 6 tagged colonies were sampled for each coral species from each study reef site, each represented by a panel (e.g. Pac1 at Li is colony 1 of P. acuta at Linareva).
Individual coral colonies exhibit a stable S/H ratio of permanent Symbiodiniaceae genera over time
Symbiodiniaceae genera Cladocopium and Durusdinium are the sole permanent genera in P. rus (Cladocopium permanence, hereafter Cladocopium-pattern) and P. acuta (Durusdinium permanence, hereafter Durusdinium-pattern), respectively (Fig. 2). Porites rus also harbored non-permanent background populations of Symbiodinium and Durusdinium, while P. acuta harbored populations of Symbiodinium, Breviolum and Cladocopium.
In addition to the specificity of Symbiodiniaceae genus-patterns, each host colony was quantified with a specific sustainable S/H ratio range of its associated permanent genera over time (Fig. 2). Pocillopora acuta exclusively associated with Durusdinium-pattern communities with significant variation among colonies (Table 2) and sites (ANOVA: df = 3, F = 38.391, P < 0.001), leading to a total of nine distinct groups (Table 2). The recorded range of S/H ratio of Durusdinium were highest in Linareva (mean: 16.6 ± 1.5S/H ratio), lowest in Maharepa (mean: 14.6 ± 0.8S/H ratio) and intermediate at Vaiare and Teavaro (mean: 15.7 ± 1.3 and 15.3 ± 0.8S/H ratio, respectively). In P. rus intra-specific variation of Cladocopium-pattern community composition was also significant, albeit in lower magnitude (Table 2), with a total of 19 colonies partitioned into five groups varying between 17.2 ± 0.6 to 15.7 ± 0.6S/H ratio without significant site effect (ANOVA: df = 3, F = 2.56, P = 0.06). In addition, for the single P. rus colony that also harbored Symbiodinium as permanent member of its dinoflagellate community (Cladocopium and Symbiodinium-pattern), the S/H ratio of Cladocopium fell within the same range as for four other colonies with Cladocopium-pattern (Table 2; Sign.4 at Maharepa, Teavaro and Vaiare). Conversely, intraspecific variation in Symbiodiniaceae community composition was most pronounced in A. cytherea (Fig. 2): the majority of colonies (n = 10) simultaneously hosted Durusdinium and Symbiodinium (Durusdinium and Symbiodinium-pattern); three colonies hosted only genus Symbiodinium (Symbiodinium-pattern); two colonies hosted only genus Durusdinium, and one colony hosted only genus Cladocopium. Among the various symbiotic patterns observed in A. cytherea, significantly distinct groups were identified based on the S/H ratios (ANOVA; Symbiodinium: df = 12, F = 12.82 and clade D: df = 11, F = 9.3, p < 0.001). Table 2 shows 9 distinct groups among the 10 Symbiodinium and Durusdinium-pattern colonies, 2 distinct groups among the 3 Symbiodinium-pattern colonies and 2 distinct groups between 2 Durusdinium-pattern colonies. The S/H ratio range of permanent genera was similar for Symbiodinium regardless of sites (df = 2, F = 2.004, P = 0.141), and even following its punctual loss (e.g. Acy2 at Li October 2011). In contrast, the S/H ratio range for Durusdinium was significantly different among study sites (ANOVA: clade D: df = 2, F = 14.775, P < 0.001). Overall, the colonies from Linareva characterized by a genus pattern involving Durusdinium displayed the highest ranges of S/H ratio (Table 2; mean ‘genus 1 v’: 19.9 ± 1.0 S/H ratio), when compared to those detected at sites Teavaro and Vaiare (from 15.2 ± 2.1 to 20.1 ± 1.0 S/H ratio; Table 2). Acropora cytherea colonies associated with a stable Cladocopium-pattern community had a mean S/H ratio of 19.6 ± 1.5.
Table 2.
Symbiodiniaceae signatures (GROUP) of each surveyed coral colony (in rows) from Pocillopora acuta, Porites rus, and Acropora cytherea, based on significant differences in the quantities of their permanent Symbiodiniaceae genera (ANOVA analyses on genus quantity among coral hosts and their corresponding post-hoc Tukey tests p < 0.05). The significant groups discriminated with the Post-hoc Tukey tests are indicated with the following lowercase letters: v, w, x, y, z, i.
| Coral-algae | Group | Post-hoc TUKEY | Site (ID colony) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genus 1 | med ± SD | Genus 2 | med ± SD | ||||||||||||
| Pocillopora acuta | v | w | x | y | z | * | v | w | x | y | z | i | * | ||
| Durusdinium | Sign. 1 | • | 17.6 ± 1.4 | Li (#4) | |||||||||||
| Sign. 2 | • | • | 17.0 ± 1.4 | Li (#1) | |||||||||||
| Sign. 3 | • | • | • | 16.7 ± 1.9 | Li (#2) | ||||||||||
| Sign. 4 | • | • | • | • | 16.3 ± 1.4 | Va (#1) | |||||||||
| Sign. 5 | • | • | • | • | • | 16.0 ± 1.0 | Li (#3, #5) | ||||||||
| Va (#3, #4) | |||||||||||||||
| Te (#1, #2) | |||||||||||||||
| Sign. 6 | • | • | • | • | 15.4 ± 0.8 | Va (#5) | |||||||||
| Te (#3) | |||||||||||||||
| Sign. 7 | • | • | • | 14.9 ± 0.5 | Te (#5) | ||||||||||
| Sign. 8 | • | • | 14.7 ± 0.9 | Ma (#2, #3) | |||||||||||
| Va (#2) | |||||||||||||||
| Te (#4) | |||||||||||||||
| Sign. 9 | • | 14.5 ± 0.8 | Ma (#1, #4, #5) | ||||||||||||
| Porites rus | |||||||||||||||
| Cladocopium | Sign. 1 | • | 17.2 ± 0.6 | Te (#4) | |||||||||||
| Sign. 2 | • | • | 16.7 ± 0.3 | Te (11) | |||||||||||
| Sign. 3 | • | • | • | 16.4 ± 0.5 | Li (#1, #3, #4, #5) | ||||||||||
| Va (#1, #4, #5) | |||||||||||||||
| Ma (#2, #4, #5) | |||||||||||||||
| Te (#3, #5) | |||||||||||||||
| Sign. 4 | • | • | 16.0 ± 0.4 | Ma (#1, #3) | |||||||||||
| Te (#2) | |||||||||||||||
| Va (#2) | |||||||||||||||
| Sign. 5 | • | 15.7 ± 0.6 | Li (#2) | ||||||||||||
| Cladocopium > Symbiodinium | Sign. 6 | • | • | 15.9 ± 0.4 | • | 5.8 ± 1.0 | Va (#3) | ||||||||
| Acropora cytherea | |||||||||||||||
| Durusdinium > Symbiodinium | Sign. 1 | • | 19.7 ± 1.2 | • | 16.1 ± 1.4 | Li (#4) | |||||||||
| Sign. 2 | • | 20.1 ± 1.0 | • | 14.0 ± 1.2 | Va (#4) | ||||||||||
| Sign. 3 | • | 19.9 ± 1.3 | • | 9.2 ± 1.4 | Li (#2) | ||||||||||
| Sign. 4 | • | • | 19.3 ± 0.9 | • | • | 12.4 ± 1.1 | Va (#5), Te (#4) | ||||||||
| Sign. 5 | • | • | • | 18.8 ± 0.6 | • | 13.6 ± 0.6 | Te (#6) | ||||||||
| Sign. 6 | • | • | • | 17.9 ± 1.6 | • | • | 11.3 ± 0.6 | Va (#1) | |||||||
| Sign. 7 | • | • | • | 17.4 ± 1.6 | • | • | 13.2 ± 2.1 | Va (#2) | |||||||
| Sign. 8 | • | • | 16.8 ± 1.2 | • | • | 12.6 ± 1.1 | Te (#5) | ||||||||
| Sign. 9 | • | 15.2 ± 2.1 | • | • | 13.2 ± 1.3 | Va (#3) | |||||||||
| Symbiodinium | Sign. 10 | • | • | 14.5 ± 1.6 | Te (#1) | ||||||||||
| Sign. 11 | • | 13.7 ± 0.9 | Li (#1, #5) | ||||||||||||
| Durusdinium | Sign. 12 | • | 20.0 ± 0.4 | Li (#3) | |||||||||||
| Sign. 13 | • | • | • | 18.9 ± 2.3 | Te (#3) | ||||||||||
| Cladocopium | Sign. 14 | • | 19.6 ± 1.5 | Te (#2) | |||||||||||
Reef sites are coded as follows: Linareva (Li), Teavaro (Te), Vaiare (Va) and Maharepa (Ma).
Coral hosts harbour temporary and/or sporadic Symbiodiniaceae genera
The abundance and diversity of non-permanent Symbiodiniaceae genera (temporary and/or sporadic) was highly variable among coral species in the study period. The majority of P. acuta colonies harboured sporadic clades (18/20), in contrast with less than half of the colonies in P. rus (9/20) and A. cytherea (7/16) (chi-squared test: χ2 = 11.19 df = 2, P = 0.004). Despite the stability of the Durusdinium-pattern observed in P. acuta, this coral species was simultaneously flexible in its ability to associate with up to three additional sporadic genera (Symbiodinium, Breviolum and Cladocopium simultaneously in Pac1 at Li; or individually with Symbiodinium in Pac2 from sites Li and Va; Breviolum in Pac1 and Pca5 at site Ma; and Cladocopium in 17 colonies; Fig. 2). Similarly, P. rus displayed a stable Cladocopium-pattern with occasional sporadic Symbiodinium (n = 7; e.g. Pru2 at Li and Te, Pru4 at Ma) and/or Durusdinium (n = 3; i.e. Pru1 at Te, Pru5 at Va, Pru1 at Ma). Acropora cytherea had complex Symbiodiniaceae community patterns with occasional sporadic Symbiodinium (n = 2; Acy2 and Acy3 at Te), Cladocopium (n = 4; e.g. Acy4 at Li, Te and Va, Acy5 at Te) and/or Durusdinium (n = 1; Acy1 at Te). Nearly all of the sporadic Symbiodiniaceae genus detections appeared with S/H ratio quantities much lower than the ones detected in permanent genera, especially for P. acuta and P. rus.
In contrast to sporadic genera, temporary genera were only harbored by low numbers of colonies, and their frequency did not significantly differ among coral species: P. acuta (1/20; Pac2 at Li), P. rus (5/20; Pru3, Pru4 and Pru5 at Li and Pru1 and Pru4 at Va) and A. cytherea (3/16; Acy3 at Te, Acy2 and Acy5 at Va) (chi-squared test: χ2 = 3.08, df = 2, P = 0.214). Temporary clades had a low diversity in all coral species: only Cladocopium in P. acuta (Pac2 at Li), only Symbiodinium in P. rus (n = 5 colonies), and Cladocopium or Symbiodinium in A. cytherea, in three colonies (e.g. Acy3 at Te and Acy5 at Va) and one colony (Acy3 at Li), respectively.
Dynamics of Symbiodiniaceae genera in response to environmental variation
The permanent Symbiodiniaceae genus-patterns of each coral species were similar among study sites, despite the environmental contrasts (Fig. 3). In particular, no significant correlation was detected between the S/H ratio of Symbiodiniaceae genera and any environmental parameter or sites.
There were no common patterns in Symbiodiniaceae genus dynamics (S/H ratio variation during a period = Δgenus i, n) among and within species when correlated to clusters of environmental contexts, except for EC8 (Figs 2 and 3). This environmental context cluster was characterized by a decrease in sediment rates, a decrease in sea surface temperature (T) and nutrient concentrations (i.e. ammonium [NH4+], phosphate [PO43−], silicates [SiOH], nitrates [NO3−] and nitrites [NO2−]) and an increase in phytoplankton concentrations (micro- and pico- fractions). Indeed, between April and June 2012, there was a significant interaction between site and sampling time for the permanent genus Durusdinium in P. acuta and a significant effect of sampling time for Cladocopium in P. rus (Table S1). At that period, we observed a significant increase in Durusdinium in P. acuta at all four reef sites (Fig. 3). A pairwise analysis revealed that the variations of sea surface temperature, PO43−, SiOH and NO2− were significantly and negatively correlated with changes in the abundance of Durusdinium (Fig. S2a). EC8 was also significantly associated with changes in the abundance of Cladocopium in P. rus, which increased in nearly all tagged colonies (17/20) from undisturbed or disturbed sites, and the pairwise analysis showed that PO43− drove this negative correlation (Fig. S2b).
In contrast, the permanent genus Symbiodinium and/or Durusdinium S/H ratio in A. cytherea did not show any significant variations with respect to EC (Fig. S2c).
The overall distribution of non-permanent genera did not significantly differ from random patterns in the study period in any of the three coral species (p > 0.05 compared to Binomial and Poisson distributions, Supplementary Information SI 2). Variations of Symbiodinium abundance in P. rus were correlated to changes in SST, PO43− and NH4+ (Fig. S2b), however the dynamics of temporary or sporadic genera (appearance or disappearance; Fig. 2) did not show clear patterns in relation to any particular environmental change (within or between ECs, Fig. 3) for any of the species (Fig. S1a–c).
Discussion
The choice of the qPCR assay employed in the present study was based on its ability to detect Symbiodiniaceae genera in hospite at the level of ≤200 28S copies, which approximates the number of 28S copies in a single algal cell50. The combination of this highly sensitive method with a sampling every two months of 56 tagged colonies of three coral host species enabled us to identify putative inferences of both newly acquired Symbiodiniaceae genera (switching) and shifts in the abundance of genera (shuffling) over a period that spans multiple seasons. The overall dataset provides a comprehensive picture of the diversity and quantitative variations of Symbiodiniaceae in various coral hosts over time and in different environments.
Random acquisition and short-term maintenance of Symbiodiniaceae
The detection of de novo Symbiodiniaceae genera in all three coral species suggests that symbiont switching may be a common natural phenomenon in healthy adult scleractinians. Interestingly, while clade F was presents in the surrounding environment50, it was never detected in coral samples, indicative of an active control of symbiont uptake by the host. Previous studies suggested that Symbiodiniaceae switching occurs readily in juvenile stages23, while in adults it only happens when the health of the colony is compromised during infected experimental conditions49,65 or in situ36. While exogenous symbiont acquisition may be more common in healthy adult corals than previously thought, the rate at which it occurs varies substantially among species. Of the three coral species studied here, P. acuta showed a significantly higher affinity to integrate sporadic clades compared to P. rus and A. cytherea. This interspecific difference may reflect divergent strategies for coping with fluctuating environmental conditions. A higher flexibility in symbiont switching in P. acuta may be a biological mechanism adapted to compensate for the dominance of thermally tolerant but low efficiency genus Durusdinium66–70 in this coral’s microbiome. Supportive of the symbiont switching hypothesis our study also found that six out of twenty (i.e. 30%) Cladocopium-dominated P. rus colonies acquired a background population of Symbiodinium over several consecutive months at some study reef sites but not for all surveyed colonies and without any temporal patterns. These results contradict previous reports71–73 that describe an exclusive association of P. rus with a single ITS2 genotype (C15 of genus Cladocopium) and could suggest a potential ecological role of the cryptic Symbiodinium in this coral species. Thus, while it is impossible to rule out that some of the de novo genera are merely consumed Symbiodiniaceae that are not in symbiosis with the coral (see47), these can also be the first steps of ‘symbiont switching’ (Fig. 2). Although apparently not driven by external factors (within the study period), such random sporadic and/or temporary symbionts switching might strongly impact the fitness and behavior of coral colonies. This has been previously shown for A. cytherea in which the external acquisition of Durusdinium was correlated by the loss of a Vibrio spp.36. Furthermore, our results suggest that the mechanism of acquisition and the maintenance of non-permanent Symbiodiniaceae genera is largely labile (e.g. change in Pattern Recognition Receptors, or the lack of their maintenance74,75), colony dependent and extends the mechanism of symbiont acquisition/selection already observed in symbiont-free offspring76.
Dynamics of permanent Symbiodiniaceae genera
Variations of the abundance of permanent Symbiodiniaceae genera in correlation with environmental changes suggests that the holobiont can rapidly modulate its autotrophic activity by increasing its Symbiodiniaceae densities to counterbalance conditions for limited heterotrophy. For example, one event of putative Symbiodiniaceae shuffling was observed between April and June 2012 in all tagged P. acuta (Durusdinium) colonies and at all four reef sites, as well as in P. rus (Cladocopium) colonies at the Linareva (undisturbed) and Vaiare (disturbed) sites. This period was characterized by a decrease in sea surface temperature and in nutrient concentrations (i.e. inorganic nitrogen, phosphate, silicates) and an increase in phytoplankton concentration (micro- and pico- fractions). Previous reports (reviewed in77) stated that increased inorganic nitrogen levels boost Symbiodiniaceae densities under high irradiance conditions, as they are normally nitrogen-limited, while increased inorganic phosphate does not have a major effect. Therefore, the negative correlation detected between inorganic phosphate and Symbiodiniaceae densities is unexpected and could be explained by the fact that our study was conducted in situ, or that other parameters not measured here drive symbiont dynamics. Alternatively, this result suggests novel mechanisms currently not fully understood.
Only one potential shuffling event was detected in this study that supports the ABH39,61. Indeed, there were no clear patterns in symbiont dynamics in response to environmental changes, i.e. neither for changes in one particular environmental parameter or the combination of multiple parameters. This is consistent with previous ecological observations showing that even if coral species are able to host a large diversity of symbionts, they will not necessarily activate rapid partnership changes as a response to acute environmental change36,47,49,65,78. Although changes in the abundance of non-permanent Symbiodiniacea genera were observed in all studied coral species, our results indicate that these acquisitions are random and their maintenance limited, supporting the idea that even under environmental changes, established symbioses are highly stable over time with only transient modifications47. This finding has also been highlighted in a recent reciprocal transplant experiment along a depth gradient78 in which corals that vertically transmit their symbionts reversed after several months to the original symbiotic communities even if these were well-suited to the transplantation depth.
Long term stability of permanent clades: coral colonies with unique Symbiodiniaceae signatures
The stability and host specificity for particular Symbiodiniaceae genera has been well-established for most coral taxa in both tropical36,44–46,78,79 and high-latitudinal47,80,81 regions. In the present study, both coral species that vertically transmit their symbionts (i.e. from parent to offspring) displayed strict host specificity for a single permanent clade (Durusdinium in P. acuta and Cladocopium in P. rus), whereas the horizontally transmitting coral species A. cytherea exhibited multiple host clade-patterns (Durusdinium/Symbiodinium, Symbiodinium, Durusdinium or Cladocopium). These findings corroborate the hypothesis that the reproductive strategy of corals plays a key role in the establishment of host-symbiont associations, with more specific and stable patterns characteristic for vertically transmitting ‘symbiont specialist’ corals45,82–85, compared with the enhanced flexibility often encountered in horizontally transmitting or ‘symbiont generalist’ corals80,83,85.
Most notably, this study also reveals that the coral-Symbiodiniaceae specificity is a trait that is shaped at the colony scale. Permanent Symbiodiniaceae genera are regulated by the host in a non-random manner, within a specific density range, which we coined ‘Symbiodiniaceae signature’. This signature reflects high intraspecific variability in symbiont communities in all three-coral species, which enables distinguishing individual coral colonies by their microbiome. In non-stressful conditions of symbiosis the Symbiodiniaceae densities are regulated in the host at an equilibrium state to sustain both partners in a position of mutual benefit86. Therefore, based on the diversity of Symbiodiniaceae signatures observed in the present study, we suggest that the symbiosis equilibrium state might be host dependent. This host regulation can be synergistically affected by environmental filtering. For example, P. acuta and A. cytherea from the undisturbed reef of Linareva had higher Durusdinium quantities in their Symbiodiniaceae signatures. It is potentially one way for the hosts to compensate for the poor contribution of Durusdinium symbionts to the holobiont’s metabolism, as previously described in non-stressful conditions53,68,69. The control of Symbiodiniaceae community composition can be reached through intrinsic and extrinsic factors (reviewed in87,88), including direct expulsion64,89 or in hospite degradation of Symbiodiniaceae cells (e.g. necrosis, apoptosis;87). Regardless of the control mechanism involved, each individual coral colony harbors a specific suite and abundance of Symbiodiniaceae genera that likely reflect on their unique plasticity in endosymbiont regulation.
Our findings indicate that each holobiont is characterized by a host with a specific Symbiodiniaceae signature of permanent genera and a constitutive flexibility to associate with additional temporary or sporadic genera. In the context of the current model of Symbiodiniaceae-coral winnowing14,90–94, this new theory of Symbiodiniaceae signatures suggests the existence of regulatory mechanisms within the host that enable them to maintain and regulate their permanent Symbiodiniaceae genera homeostasis within a particular range. When identical genera are present but in different quantity ranges, this signature can be considered as distinct amounts of Symbiodiniaceae ‘genomes’ modulating the hologenome (sensu95), which could account for differences in the host’s behavior92,96. Additionally, the appearance of sporadic and/or temporary non-permanent genera in the host may contribute to the diversity of the coral hologenome (see95). This diversity may contribute to explaining intraspecific differences in the ecological success of distinct holobionts (Hologenome Dependent Susceptibility) and may constitute an important factor underpinning their resistance or resilience to environmental stressors.
As ocean warming progresses, more research on the ecological relevance of particular coral hologenomes, including the Symbiodiniacea signature within and among coral species (e.g. physiological or gene expression responses to multiple stressors), will be critically important to better predict the fate of coral reefs and to assist in the elaboration of effective conservation plans.
Supplementary information
Acknowledgements
We thank members of the IFREMER from Taravao (Saulnier D., Gueguen Y., Fievet J., Santini A., C. Belliard) for fruitful discussions and technical help with qPCR, technicians from CRIOBE (Espiau B., Ung P. and Lerouvreur F.) and volunteers from Planète Urgence for their technical assistance. We are grateful to L. Hédouin for providing material and field assistance. We also thank M.A. Coffroth and her laboratory (BURR; http://www.nsm.buffalo.edu/Bio/burr/) for providing the Symbiodiniaceae strains. This work was funded and supported by Polynesian associations Proscience and TeMana o teMoana and the French association Planète Urgence. Additional funding was provided by the Délégation à la Recherche of French Polynesia, the Ministère de l’Outre-Mer and the Contrat de Projet Etat-Polynésie Française (041-12).
Author Contributions
H.R., V.B.L. and G.L. designed the project. H.R. performed the fieldwork and the molecular analyses. H.R., V.B.L. and G.L. analyzed the results. H.R. wrote the first draft of the paper. H.R., V.B.L., G.L., X.P. and G.T. discussed the results and reviewed the paper.
Competing Interests
The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary information accompanies this paper at 10.1038/s41598-019-44017-5.
References
- 1.Coffroth MA, Lewis CF, Santos SR, Weaver JL. Environmental populations of symbiotic dinoflagellates in the genus Symbiodinium can initiate symbioses with reef cnidarians. Current Biology. 2006;16:985–987. doi: 10.1016/j.cub.2006.10.049. [DOI] [PubMed] [Google Scholar]
- 2.Pochon X, et al. Comparison of endosymbiotic and free-living Symbiodinium (Dinophyceae) diversity in a Hawaiian reef environment. J. Phycol. 2010;46:53–65. doi: 10.1111/j.1529-8817.2009.00797.x. [DOI] [Google Scholar]
- 3.LaJeunesse TC, et al. High diversity and host specificity observed among symbiotic dinoflagellates in reef coral communities from Hawaii. Coral Reefs. 2004;23:596–603. [Google Scholar]
- 4.Stat M, Carter D, Hoegh-Guldberg O. The evolutionary history of Symbiodinium and scleractinian hosts-symbiosis, diversity, and the effect of climate change. Perspect. Plant Ecol. Evol. Syst. 2006;8:23–43. doi: 10.1016/j.ppees.2006.04.001. [DOI] [Google Scholar]
- 5.LaJeunesse TC, et al. Systematic revision of Symbiodiniaceae highlights the antiquity and diversity of coral endosymbionts. Curr. Biol. 2018;28:2570–2580e6. doi: 10.1016/j.cub.2018.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Pochon X, Putnam HM, Gates RD. Multi-gene analysis of Symbiodinium dinoflagellates: a perspective on rarity, symbiosis, and evolution. PeerJ. 2014;2:e394. doi: 10.7717/peerj.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.LaJeunesse TC. Validation and description of Symbiodinium microadriaticum, the type species of Symbiodinium (Dinophyta) Journal of Phycology. 2017;53:1109–1114. doi: 10.1111/jpy.12570. [DOI] [PubMed] [Google Scholar]
- 8.Baird AH, Guest JR, Willis BL. Systematic and biogeographical patterns in the reproductive biology of scleractinian corals. Annu. Rev. Ecol. Evol. Syst. 2009;40:551–571. doi: 10.1146/annurev.ecolsys.110308.120220. [DOI] [Google Scholar]
- 9.Muscatine L, Porter JW. Reef corals: mutualistic symbioses adapted to nutrient-poor environments. Bioscience. 1977;27:454–460. doi: 10.2307/1297526. [DOI] [Google Scholar]
- 10.Furla P, Galgani I, Durand I, Allemand D. Sources and mechanisms of inorganic carbon transport for coral calcification and photosynthesis. J. Exp. Biol. 2000;203:3445–3457. doi: 10.1242/jeb.203.22.3445. [DOI] [PubMed] [Google Scholar]
- 11.Whitehead LF, Douglas AE. Metabolite comparisons and the identity of nutrients translocated from symbiotic algae to an animal host. J. Exp. Biol. 2003;206:3149–3157. doi: 10.1242/jeb.00539. [DOI] [PubMed] [Google Scholar]
- 12.Grover R, Maguer J-F, Allemand D, Ferrier-Pagès C. Uptake of dissolved free amino acids by the scleractinian coral Stylophora pistillata. J. Exp. Biol. 2008;211:860–865. doi: 10.1242/jeb.012807. [DOI] [PubMed] [Google Scholar]
- 13.Burriesci MS, Raab TK, Pringle JR. Evidence that glucose is the major transferred metabolite in dinoflagellate-cnidarian symbiosis. J. Exp. Biol. 2012;215:3467–3477. doi: 10.1242/jeb.070946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davy SK, Allemand D, Weis VM. Cell biology of cnidarian-dinoflagellate symbiosis. Microbiol. Mol. Biol. Rev. 2012;76:229–261. doi: 10.1128/MMBR.05014-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heron SF, Maynard JA, Van Hooidonk R, Eakin CM. Warming trends and bleaching stress of the world’s coral reefs 1985–2012. Sci. Rep. 2016;6:38402. doi: 10.1038/srep38402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hughes TP, et al. Coral reefs in the Anthropocene. Nature. 2017;546:82–90. doi: 10.1038/nature22901. [DOI] [PubMed] [Google Scholar]
- 17.Camp EF, et al. The future of coral reefs subject to rapid climate change: lessons from natural extreme environments. Front. Mar. Sci. 2018;5:1–21. doi: 10.3389/fmars.2018.00001. [DOI] [Google Scholar]
- 18.Hughes TP, et al. Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science. 2018;359:80–83. doi: 10.1126/science.aan8048. [DOI] [PubMed] [Google Scholar]
- 19.Yost DM, et al. Diversity in skeletal architecture influences biological heterogeneity and Symbiodinium habitat in corals. Zoology. 2013;116:262–269. doi: 10.1016/j.zool.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Baker AC. Flexibility and specifictity in coral-algal symbiosis: diversity, ecology, and biogeography of Symbiodinium. Annu. Rev. Ecol. Evol. Syst. 2003;34:661–689. doi: 10.1146/annurev.ecolsys.34.011802.132417. [DOI] [Google Scholar]
- 21.Silverstein RN, Correa AMS, Baker AC. Specificity is rarely absolute in coral-algal symbiosis: implications for coral response to climate change. Proc. R. Soc. B Biol. Sci. 2012;279:2609–2618. doi: 10.1098/rspb.2012.0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baird AH, Bhagooli R, Ralph PJ, Takahashi S. Coral bleaching: the role of the host. Trends Ecol. Evol. 2009;24:16–20. doi: 10.1016/j.tree.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Mieog JC, et al. The roles and interactions of symbiont, host and environment in defining coral fitness. PLoS One. 2009;4:e6364. doi: 10.1371/journal.pone.0006364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barshis DJ, et al. Protein expression and genetic structure of the coral Porites lobata in an environmentally extreme Samoan back reef: does host genotype limit phenotypic plasticity? Mol. Ecol. 2010;19:1705–20. doi: 10.1111/j.1365-294X.2010.04574.x. [DOI] [PubMed] [Google Scholar]
- 25.Wall CB, et al. The effects of environmental history and thermal stress on coral physiology and immunity. Mar. Biol. 2018;165:1–15. doi: 10.1007/s00227-018-3317-z. [DOI] [Google Scholar]
- 26.Berkelmans R, Van Oppen MJH. The role of zooxanthellae in the thermal tolerance of corals: a ‘nugget of hope’ for coral reefs in an era of climate change. Proc. R. Soc. B Biol. Sci. 2006;273:2305–2312. doi: 10.1098/rspb.2006.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Howells EJ, et al. Coral thermal tolerance shaped by local adaptation of photosymbionts. Nat. Clim. Chang. 2012;2:116–120. doi: 10.1038/nclimate1330. [DOI] [Google Scholar]
- 28.LaJeunesse TC, Smith RT, Finney J, Oxenford H. Outbreak and persistence of opportunistic symbiotic dinoflagellates during the 2005 Caribbean mass coral ‘bleaching’ event. Proc. R. Soc. London B Biol. Sci. 2009;276:4139–4148. doi: 10.1098/rspb.2009.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cooper TF, et al. Environmental factors controlling the distribution of Symbiodinium harboured by the coral Acropora millepora on the Great Barrier Reef. PLoS One. 2011;6:e25536. doi: 10.1371/journal.pone.0025536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cunning R, Muller EB, Gates RD, Nisbet RM. A dynamic bioenergetic model for coral- Symbiodinium symbioses and coral bleaching as an alternate stable state. J. Theor. Biol. 2017;431:49–62. doi: 10.1016/j.jtbi.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Silverstein RN, Cunning R, Baker AC. Change in algal symbiont communities after bleaching, not prior heat exposure, increases heat tolerance of reef corals. Glob. Chang. Biol. 2015;21:236–249. doi: 10.1111/gcb.12706. [DOI] [PubMed] [Google Scholar]
- 32.Nitschke MR, Davy SK, Ward S. Horizontal transmission of Symbiodinium cells between adult and juvenile corals is aided by benthic sediment. Coral Reefs. 2016;35:335–344. doi: 10.1007/s00338-015-1349-0. [DOI] [Google Scholar]
- 33.Decelle J, et al. Worldwide occurrence and activity of the reef- building coral symbiont Symbiodinium in the open Ocean. Curr. Biol. 2018;28:1–9. doi: 10.1016/j.cub.2018.09.024. [DOI] [PubMed] [Google Scholar]
- 34.Stat M, Morris E, Gates RD. Functional diversity in coral-dinoflagellate symbiosis. Proc. Natl. Acad. Sci. USA. 2008;105:9256–61. doi: 10.1073/pnas.0801328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quigley KM, Willis BL, Bay LK. Maternal effects and Symbiodinium community composition drive differential patterns in juvenile survival in the coral Acropora tenuis. R. Soc. Open Sci. 2016;3:160471. doi: 10.1098/rsos.160471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rouzé H, Lecellier G, Saulnier D, Berteaux-Lecellier V. Symbiodinium clades A and D differentially predispose Acropora cytherea to disease and Vibrio spp. colonization. Ecol. Evol. 2016;6:560–572. doi: 10.1002/ece3.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silverstein RN, Cunning R, Baker AC. Tenacious D: Symbiodinium in clade D remain in reef corals at both high and low temperature extremes despite impairment. J. Exp. Biol. 2017;220:1192–1196. doi: 10.1242/jeb.148239. [DOI] [PubMed] [Google Scholar]
- 38.Torda G, et al. Rapid adaptive responses to climate change in corals. Nat. Clim. Chang. 2017;7:627–636. doi: 10.1038/nclimate3374. [DOI] [Google Scholar]
- 39.Buddemeier RW, Fautin DG. Coral bleaching as an adaptive mechanism. Bioscience. 1993;43:320–326. doi: 10.2307/1312064. [DOI] [Google Scholar]
- 40.Baker DM, Weigt L, Fogel M, Knowlton N. Ancient DNA from coral-hosted Symbiodinium reveal a static mutualism over the last 172 years. PLoS One. 2013;8:e55057. doi: 10.1371/journal.pone.0055057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lin S, et al. The Symbiodinium kawagutii genome illuminates dinoflagellate gene expression and coral symbiosis. Science. 2015;240:13201–13202. doi: 10.1126/science.aad0408. [DOI] [PubMed] [Google Scholar]
- 42.Lin Z, et al. Transcriptome profiling of Galaxea fascicularis and its endosymbiont Symbiodinium reveals chronic eutrophication tolerance pathways and metabolic mutualism between partners. Sci. Rep. 2017;7:42100. doi: 10.1038/srep42100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aranda M, et al. Genomes of coral dinoflagellate symbionts highlight evolutionary adaptations conducive to a symbiotic lifestyle. Sci. Rep. 2016;6:39734. doi: 10.1038/srep39734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thornhill DJ, Fitt WK, Schmidt GW. Highly stable symbioses among western Atlantic brooding corals. Coral Reefs. 2006;25:515–519. doi: 10.1007/s00338-006-0157-y. [DOI] [Google Scholar]
- 45.Thornhill DJ, LaJeunesse TC, Kemp DW, Fitt WK, Schmidt GW. Multi-year, seasonal genotypic surveys of coral-algal symbioses reveal prevalent stability or post-bleaching reversion. Mar. Biol. 2006;148:711–722. doi: 10.1007/s00227-005-0114-2. [DOI] [Google Scholar]
- 46.Stat M, Loh WKW, LaJeunesse TC, Hoegh-Guldberg O, Carter DA. Stability of coral-endosymbiont associations during and after a thermal stress event in the southern Great Barrier Reef. Coral Reefs. 2009;28:709–713. doi: 10.1007/s00338-009-0509-5. [DOI] [Google Scholar]
- 47.Lee MJ, et al. Most low-abundance “background” Symbiodinium spp. are transitory and have minimal functional significance for symbiotic corals. Microb. Ecol. 2016;71:771–783. doi: 10.1007/s00248-015-0724-2. [DOI] [PubMed] [Google Scholar]
- 48.Little AF, Van Oppen MJH, Willis BL. Flexibility in algal endosymbioses shapes growth in reef corals. Science. 2004;304:1492–1494. doi: 10.1126/science.1095733. [DOI] [PubMed] [Google Scholar]
- 49.Coffroth MA, Poland DM, Petrou EL, Brazeau DA, Holmberg JC. Environmental symbiont acquisition may not be the solution to warming seas for reef-building corals. PLoS One. 2010;5:e13258. doi: 10.1371/journal.pone.0013258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rouzé H, et al. An updated assessment of Symbiodinium spp. that associate with common scleractinian corals from Moorea (French Polynesia) reveals high diversity among background symbionts and a novel finding of clade B. PeerJ. 2017;5:e285–556. doi: 10.7717/peerj.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mieog JC, van Oppen MJH, Cantin NE, Stam WT, Olsen JL. Real-time PCR reveals a high incidence of Symbiodinium clade D at low levels in four scleractinian corals across the Great Barrier Reef: implications for symbiont shuffling. Coral Reefs. 2007;26:449–457. doi: 10.1007/s00338-007-0244-8. [DOI] [Google Scholar]
- 52.Correa AMS, McDonald MD, Baker AC. Development of clade-specific Symbiodinium primers for quantitative PCR (qPCR) and their application to detecting clade D symbionts in Caribbean corals. Mar. Biol. 2009;156:2403–2411. doi: 10.1007/s00227-009-1263-5. [DOI] [Google Scholar]
- 53.Cunning R, Silverstein RN, Baker AC. Investigating the causes and consequences of symbiont shuffling in a multi-partner reef coral symbiosis under environmental change. Proc. R. Soc. B Biol. Sci. 2015;282:20141725. doi: 10.1098/rspb.2014.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Arif C, et al. Assessing Symbiodinium diversity in scleractinian corals via next-generation sequencing-based genotyping of the ITS2 rDNA region. Mol. Ecol. 2014;23:4418–4433. doi: 10.1111/mec.12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Edmunds PJ, et al. Long-term changes in Symbiodinium communities in Orbicella annularis in St. John, US Virgin Islands. Mar. Ecol. Prog. Ser. 2014;506:129–144. doi: 10.3354/meps10808. [DOI] [Google Scholar]
- 56.Boulotte NM, et al. Exploring the Symbiodinium rare biosphere provides evidence for symbiont switching in reef-building corals. Int. Soc. Microb. Ecol. J. 2016;10:2693–2701. doi: 10.1038/ismej.2016.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cunning R, Gates RD, Edmunds PJ. Using high-throughput sequencing of ITS2 to describe Symbiodinium metacommunities in St. John, US Virgin Islands. PeerJ. 2017;5:e3472. doi: 10.7717/peerj.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ziegler M, et al. Biogeography and molecular diversity of coral symbionts in the genus Symbiodinium around the Arabian Peninsula. J. Biogeogr. 2017;44:674–686. doi: 10.1111/jbi.12913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ziegler M, Eguíluz VM, Duarte CM, Voolstra CR. Rare symbionts may contribute to the resilience of coral–algal assemblages. ISME J. 2017;12:161–172. doi: 10.1038/ismej.2017.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cunning R, Glynn PW, Baker AC. Flexible associations between Pocillopora corals and Symbiodinium limit utility of symbiosis ecology in defining species. Coral Reefs. 2013;32:795–801. doi: 10.1007/s00338-013-1036-y. [DOI] [Google Scholar]
- 61.Cunning R, Silverstein RN, Baker AC. Symbiont shuffling linked to differential photochemical dynamics of Symbiodinium in three Caribbean reef corals. Coral Reefs. 2018;37:145–152. doi: 10.1007/s00338-017-1640-3. [DOI] [Google Scholar]
- 62.Schmidt-Roach S, Miller KJ, Lundgren P, Andreakis N. With eyes wide open: a revision of species within and closely related to the Pocillopora damicornis species complex (Scleractinia; Pocilloporidae) using morphology and genetics. Zool. J. Linn. Soc. 2014;170:1–33. doi: 10.1111/zoj.12092. [DOI] [Google Scholar]
- 63.Rouzé H, Lecellier G, Langlade M, Planes S, Berteaux-Lecellier V. Fringing reefs exposed to different levels of eutrophication and sedimentation can support the same benthic communities. Mar. Pollut. Bull. 2015;92:212–221. doi: 10.1016/j.marpolbul.2014.12.016. [DOI] [PubMed] [Google Scholar]
- 64.Yamashita H, Suzuki G, Hayashibara T, Koike K. Do corals select zooxanthellae by alternative discharge? Mar. Biol. 2011;158:87–100. doi: 10.1007/s00227-010-1544-z. [DOI] [Google Scholar]
- 65.Lewis CL, Coffroth MA. The acquisition of exogenous algal symbionts by an octocoral after bleaching. Science. 2004;304:1490–1492. doi: 10.1126/science.1097323. [DOI] [PubMed] [Google Scholar]
- 66.Stat M, Gates RD, Clade D. Symbiodinium in Scleractinian corals: a “Nugget” of hope, a selfish opportunist, an ominous sign, or all of the above? J. Mar. Biol. 2011;2011:1–9. doi: 10.1155/2011/730715. [DOI] [Google Scholar]
- 67.Lesser MP, Stat M, Gates RD. The endosymbiotic dinoflagellates (Symbiodinium sp.) of corals are parasites and mutualists. Coral Reefs. 2013;32:603–611. doi: 10.1007/s00338-013-1051-z. [DOI] [Google Scholar]
- 68.Cantin NE, Oppen MJH, Willis BL, Mieog JC, Negri AP. Juvenile corals can acquire more carbon from high-performance algal symbionts. Coral Reefs. 2009;28:405–414. doi: 10.1007/s00338-009-0478-8. [DOI] [Google Scholar]
- 69.Baker DM, Andras JP, Jordán-Garza AG, Fogel ML. Nitrate competition in a coral symbiosis varies with temperature among Symbiodinium clades. ISME J. 2013;7:1248–51. doi: 10.1038/ismej.2013.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Cunning R, Yost DM, Guarinello ML, Putnam HM, Gates RD. Variability of Symbiodinium communities in waters, sediments, and corals of thermally distinct reef pools in American Samoa. PLoS One. 2015;10:e0145099. doi: 10.1371/journal.pone.0145099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fitt WK, et al. Response of two species of Indo-Pacific corals, Porites cylindrica and Stylophora pistillata, to short-term thermal stress: the host does matter in determining the tolerance of corals to bleaching. J. Exp. Mar. Bio. Ecol. 2009;373:102–110. doi: 10.1016/j.jembe.2009.03.011. [DOI] [Google Scholar]
- 72.Fabricius KE, et al. Losers and winners in coral reefs acclimatized to elevated carbon dioxide concentrations. Nat. Clim. Chang. 2011;1:165–169. doi: 10.1038/nclimate1122. [DOI] [Google Scholar]
- 73.Putnam HM, Stat M, Pochon X, Gates RD. Endosymbiotic flexibility associates with environmental sensitivity in scleractinian corals. Proc. R. Soc. B Biol. Sci. 2012;279:4352–4361. doi: 10.1098/rspb.2012.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Weis VM, Reynolds WS, DeBoer MD, Krupp DA. Host-symbiont specificity during onset of symbiosis between the dinoflagellates Symbiodinium spp. and planula larvae of the scleractinian coral Fungia scutaria. Coral Reefs. 2001;20:301–308. doi: 10.1007/s003380100179. [DOI] [Google Scholar]
- 75.Rodriguez-Lanetty M, Wood-Charlson EM, Hollingsworth LL, Krupp DA, Weis VM. Temporal and spatial infection dynamics indicate recognition events in the early hours of a dinoflagellate-coral symbiosis. Mar. Biol. 2006;149:713–719. doi: 10.1007/s00227-006-0272-x. [DOI] [Google Scholar]
- 76.Wolfowicz I, et al. Aiptasia sp. larvae as a model to reveal mechanisms of symbiont selection in cnidarians. Sci. Rep. 2016;6:1–12. doi: 10.1038/srep32366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fabricius KE. Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Mar. Pollut. Bull. 2005;50:125–46. doi: 10.1016/j.marpolbul.2004.11.028. [DOI] [PubMed] [Google Scholar]
- 78.Sampayo EM, et al. Coral symbioses under prolonged environmental change: living near tolerance range limits. Sci. Rep. 2016;6:36271. doi: 10.1038/srep36271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Suwa R, Hirose M, Hidaka M. Seasonal fluctuation in zooxanthellar genotype composition and photophysiology in the corals Pavona divaricata and P. decussata. Mar. Ecol. Prog. Ser. 2008;361:129–137. doi: 10.3354/meps07372. [DOI] [Google Scholar]
- 80.LaJeunesse TC, et al. Specificity and stability in high latitude eastern Pacific coral-algal symbioses. Limnol. Oceanogr. 2008;53:719–727. doi: 10.4319/lo.2008.53.2.0719. [DOI] [Google Scholar]
- 81.De Palmas S, et al. Symbiodinium spp. associated with high-latitude scleractinian corals from Jeju Island, South Korea. Coral Reefs. 2015;34:919–925. doi: 10.1007/s00338-015-1286-y. [DOI] [Google Scholar]
- 82.Yamamura N. Evolution of mutualistic symbiosis: a differential equation model. Popul. Ecol. 1996;38:211–218. doi: 10.1007/BF02515729. [DOI] [Google Scholar]
- 83.Loh WKW, Loi T, Carter D, Hoegh-guldberg O. Genetic variability of the symbiotic dinoflagellates from the wide ranging coral species Seriatopora hystrix and Acropora longicyathus in the Indo-West Pacific. Mar. Ecol. Prog. Ser. 2001;222:97–107. doi: 10.3354/meps222097. [DOI] [Google Scholar]
- 84.Barneah O, Weis VM, Perez S, Benayahu Y. Diversity of dinoflagellate symbionts in Red Sea soft corals: mode of symbiont acquisition matters. Mar. Ecol. Prog. Ser. 2004;275:89–95. doi: 10.3354/meps275089. [DOI] [Google Scholar]
- 85.Stat M, Loh WKW, Hoegh-Guldberg O, Carter DA. Symbiont acquisition strategy drives host-symbiont associations in the southern Great Barrier Reef. Coral Reefs. 2008;27:763–772. doi: 10.1007/s00338-008-0412-5. [DOI] [Google Scholar]
- 86.Muscatine L, Falkowski PG, Dubinsky Z, Cook PA, McCloskey LR. The effect of external nutrient resources on the population dynamics of zooxanthellae in a reef coral. Proc. R. Soc. London. B. Biol. Sci. 1989;236:311–324. doi: 10.1098/rspb.1989.0025. [DOI] [Google Scholar]
- 87.Wooldridge SA. Is the coral-algae symbiosis really ‘mutually beneficial’ for the partners? Bioessays. 2010;32:615–25. doi: 10.1002/bies.200900182. [DOI] [PubMed] [Google Scholar]
- 88.Fransolet D, Roberty S, Plumier J. Establishment of endosymbiosis: the case of cnidarians and Symbiodinium. J. Exp. Mar. Bio. Ecol. 2012;421:1–7. doi: 10.1016/j.jembe.2012.03.015. [DOI] [Google Scholar]
- 89.Baghdasarian G, Muscatine L. Preferential expulsion of dividing algal cells as a mechanism for regulating algal-cnidarian symbiosis. Biol. Bull. 2000;199:278–86. doi: 10.2307/1543184. [DOI] [PubMed] [Google Scholar]
- 90.Nyholm SV, Mcfall-Ngai MJ. The winnowing: establishing the squid- Vibrio symbiosis. Nat. Rev. Microbiol. 2004;2:632–642. doi: 10.1038/nrmicro957. [DOI] [PubMed] [Google Scholar]
- 91.Dunn SR, Weis VM. Apoptosis as a post-phagocytic winnowing mechanism in a coral-dinoflagellate mutualism. Environ. Microbiol. 2009;11:268–276. doi: 10.1111/j.1462-2920.2008.01774.x. [DOI] [PubMed] [Google Scholar]
- 92.Ganot P, et al. Adaptations to endosymbiosis in a cnidarian-dinoflagellate association: differential gene expression and specific gene duplications. PLoS Genet. 2011;7:e1002187. doi: 10.1371/journal.pgen.1002187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Poland D, Coffroth M. Trans-generational specificity within a cnidarian-algal symbiosis. Coral Reefs. 2017;36:119–129. doi: 10.1007/s00338-016-1514-0. [DOI] [Google Scholar]
- 94.Mies M, et al. Expression of a symbiosis-specific gene in Symbiodinium type A1 associated with coral, nudibranch and giant clam larvae. R. Soc. Open Sci. 2017;4:170253. doi: 10.1098/rsos.170253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Rosenberg E, Zilber-Rosenberg I. Bacterial bleaching of corals leads to hologenome concept. Microbe. 2016;11:27–31. [Google Scholar]
- 96.Parkinson JE, et al. Gene expression variation resolves species and individual strains among coral-associated dinoflagellates within the genus Symbiodinium. Genome Biol. Evol. 2016;8:665–680. doi: 10.1093/gbe/evw019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.