Extracellular vesicles (EVs) are major vehicles for transporting viruses en bloc among hosts. While RNA viruses make up the great majority of transmission by EVs, in a recent article in mBio (mBio 10:e00379-19, 2019, https://mbio.asm.org/content/10/2/e00379-19.long), Morris-Love and colleagues revealed that a double-stranded DNA (dsDNA) virus, JC polyomavirus (JCPyV), a major cause of progressive multifocal leukoencephalopathy (PML), can be released from and transmitted to other glia in EVs.
KEYWORDS: demyelinating disease, exosome, extracellular vesicle, glia, polyomavirus
ABSTRACT
Extracellular vesicles (EVs) are major vehicles for transporting viruses en bloc among hosts. While RNA viruses make up the great majority of transmission by EVs, in a recent article in mBio (mBio 10:e00379-19, 2019, https://mbio.asm.org/content/10/2/e00379-19.long), Morris-Love and colleagues revealed that a double-stranded DNA (dsDNA) virus, JC polyomavirus (JCPyV), a major cause of progressive multifocal leukoencephalopathy (PML), can be released from and transmitted to other glia in EVs. This mode of transmission appears to be highly infectious, independent of the free virus attachment and entry receptors LSTc and 5-HT2, and protected from neutralizing antibodies. This novel form of JCPyV transmission may potentially explain its dissemination into the central nervous system (CNS) and its increased virulence.
COMMENTARY
Extracellular vesicles (EVs) are emerging as major vehicles for transporting viruses among hosts. Both nonenveloped and enveloped viruses, including poliovirus, coxsackievirus, rhinovirus, hepatitis A virus, hepatitis C virus, Dengue virus, rotavirus, and norovirus, exploit EVs to transmit viral particles and/or naked infectious genomes (1). Viral transmission by EVs can increase virulence and has distinct advantages over transmission by free virus particles (2, 3). These advantages include the following: increasing the multiplicity of infection by allowing multiple viral particles and/or infectious naked genomes to be transported together to infect hosts en bloc; shielding by EV membranes of viral cargo against neutralizing antibodies as well as containment by EV membranes of lipids such as phosphatidylserine (PS) that suppress immune responses; and protection of cargo by EV membranes from environmental assaults, including those represented by proteases and nucleases, detergents, and temperature fluctuations, during transmission (1). While RNA viruses make up the great majority of viruses transmitted by EVs that have been detected thus far, in a recent article in mBio, Morris-Love et al. (4) proposed that a DNA virus, JC polyomavirus (JCPyV), a major cause of progressive multifocal leukoencephalopathy (PML), also uses EVs to traffic itself among cells. They further conjectured that this mode of transmission may explain the dissemination of JCPyV into the central nervous system (CNS) and its increased virulence.
JCPyV is a nonenveloped double-stranded DNA (dsDNA) virus that belongs to the Polyomaviridae family. Other members of this family include simian virus 40 (SV40), Bk polyomavirus, and Merkel cell polyomavirus. JCPyV is widespread in the human population and largely presents as an asymptomatic kidney and B lymphocyte infection. However, within immunocompromised individuals, such as those previously infected with HIV or undergoing chemotherapy, JCPyV can spread to the CNS and cause PML, a severe demyelinating disease that frequently leads to death (5, 6). In the CNS, JCPyV primarily infects glia, including oligodendrocytes and astrocytes, as well as the cells of the choroid plexus, the latter a critical component of the blood-brain barrier (7). In immunocompetent individuals, JCPyV infection is mediated through a two-step process that starts with the attachment of the virus capsid protein VP1 to the cell surface through the sialic acid moiety of host lactoseries tetrasaccharide c (LSTc) protein, followed by binding to one of the host serotonin 5-hydroxytryptamine (5-HT) receptors for internalization (8, 9). Virus and receptor are cointernalized through clathrin-mediated endocytosis, followed by virus particle trafficking to the endoplasmic reticulum for partial uncoating and retrotranslocation to the cytosol. Once in the cytosol, the virus shuttles to the nucleus for replication (6). While this model suffices for infectivity of renal cells, oligodendrocytes and astrocytes lack LSTc receptors (10) and viruses isolated from PML patients contain mutations in the LSTc binding site of their VP1 capsid protein.
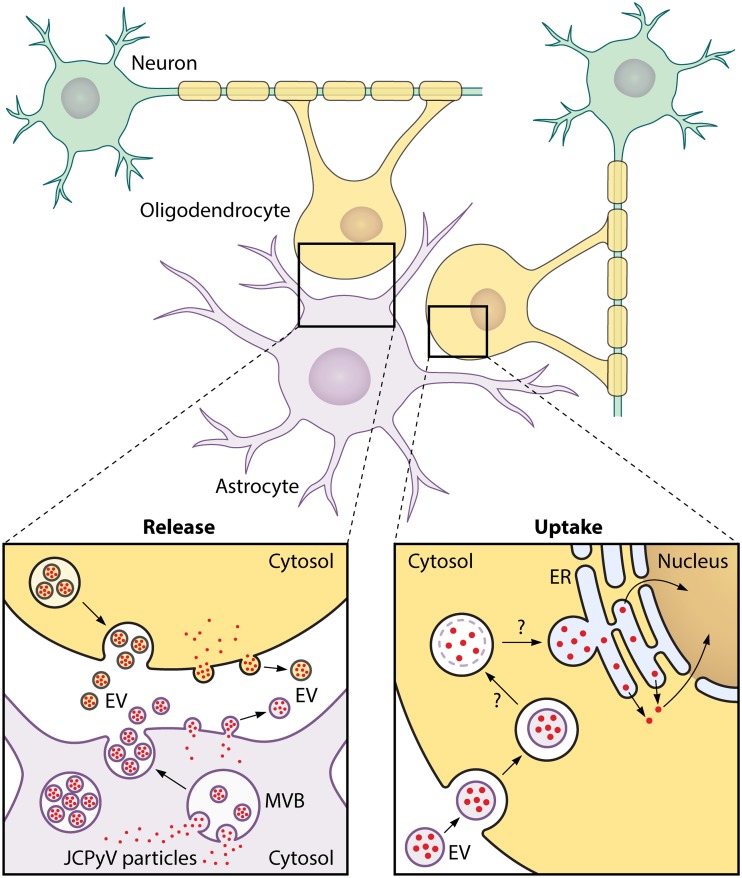
These findings have highlighted the need to determine what, if any, additional mode(s) of transmission, other than free particles, exists for JCPyV. The findings reported here by Morris-Love et al. reveal a novel mode of infection whereby JCPyV is transmitted among glial cells by EVs. Using JCPyV-infected primary human fetal glia-derived SVG-A cells, Morris-Love et al. found substantial amounts of virus-loaded EVs released from these cells into the extracellular medium (Fig. 1, “Release”). Each virus-transporting EV is 100 to 200 nm in diameter and contains many JCPyV particles. It remains to be determined which cellular organelles produce the virus-loaded EVs, but the presence of CD9, CD81, flotillin-1, annexin-V, and TSG-101 proteins in the EVs suggests either a multivesicular body or plasma membrane origin. Those authors showed that the EV membrane protects JCPyV cargo from neutralizing antibodies, a finding consistent with previous studies of EV-shuttled viruses such hepatitis A virus (11). Moreover, they provide evidence that JCPyV infection mediated by EVs is not dependent on the sialic acid moiety of LSTc as neuraminidase treatment of the cells does not block infection and EVs carrying pseudoviruses with VP1 capsid protein mutations similar to those found in PML patients are still infectious and also are not dependent on the JCPyV entry receptor 5-HT2.
FIG 1.
JC Polyomavirus transmission by extracellular vesicles. (Release) JCPyV-infected glial cells (oligodendrocytes and astrocytes) produce extracellular vesicles (EVs) containing multiple JCPyV particles. These JCPyV-loaded EVs are 100 to 200 nm in diameter and have cell host markers that suggest either multivesicular body (MVB) or plasma membrane origin. (Uptake) JCPyV-loaded EVs are endocytosed by oligodendrocytes independently of LSTc and 5HT2 receptors. We conjecture, on the basis of previous studies performed with other EV-transported nonenveloped viruses, that the EV membrane becomes disrupted by endosomal lipases (2, 15). Subsequently, the endosomes transfer their JCPyV particles to the endoplasmic reticulum (ER) (by fusion?). In the ER lumen, JCPyV particles undergo partial uncoating prior to retrotranslocation into the cytosol and eventual traffic of viral DNA into the nucleus for replication, similarly to what has been reported with SV40 particles (14).
These findings are of potential significance for JCPyV virulence and tropism. First, by traveling in an EV as a cluster of viral particles, these viruses can infect en bloc and increase their multiplicity of infection and this can lead to enhanced and efficient viral replication (1). Second, lack of dependence of EV-mediated transmission on LSTc and 5-HT2 receptors may explain how these viruses can infect the CNS.
The findings from Morris-Love et al. also trigger many new lines of inquiry. First and foremost is the issue of whether JCPyV-loaded EVs are present in PML patients. Sera and cerebrospinal fluid biopsy samples from PML patients are available and should be readily testable with extracellular vesicle isolation techniques. The second line of inquiry concerns how widespread transmission by EVs is among the members of the Polyomaviridae family. Do other members such as SV40 also exploit these organelles to transmit themselves en bloc?
Another issue that arises from that study is whether JCPyV-containing EVs exploit attachment and entry receptors other than LSTc and 5-HT2. Geoghegan el al. (12) reported that JCPyV pseudovirus particles can use nonsialylated glycosaminoglycans (GAG), which are present in oligodendrocytes and astrocytes, as alternative entry pathway attachment receptors. It remains to be determined if JCPyV-containing EVs exploit these nonsialylated GAGs for attachment. Another mode of attachment for EVs may be that of binding to specific phosphatidylserine receptors on the surface of oligodendrocytes and astrocytes. EV membrane outer leaflets are highly enriched in PS lipids (13), and, at least for norovirus-, poliovirus-, coxsackievirus-, and rhinovirus-containing EVs, PS-PS receptor interactions are required for EV attachment to the cell surface (2, 3). An alternative and unorthodox mode of EV-mediated infection may be that of completely receptor-independent fusion of EVs with cells, followed by postentry regulation of viral replication through cell-to-cell differences in innate immune responses, presence of host factors needed by JCPyV for retrotranslocation, transport to the nucleus, DNA synthesis, etc. It will be intriguing to determine if the EVs produced from SVG-A glial cells can fuse with other cell types, including neurons, renal cells, and B cells.
Studies performed with free SV40 particles have revealed that once viruses are internalized into endosomes, they are transferred to the ER lumen, where an initial uncoating of the capsid is catalyzed by lumenal thiol oxidoreductases and chaperones. From there, the partially uncoated viruses are retrotranslocated into the cytosol and viral DNA is trafficked to the nucleus for replication (14). In the case of uptake of EVs containing JCPyV, on the basis of previous studies performed with other EV-transported nonenveloped viruses (2, 15), it seems likely the EV membrane becomes disrupted by endosomal lipases to release viruses into the endosome before the latter transfers the viral cargo to the ER lumen (Fig. 1, “Uptake”).
Finally, the limited data on CNS pathology in PML patients and humanized mouse models is consistent with viral egress through EVs, as infected astrocytes and oligodendrocytes do not appear to undergo lysis and the latter become apoptotic only through viral T-antigens forcing cell cycle entry (16). Given that demyelination is a major pathological hallmark of PML, it will be important to determine if and how EV production can perturb glial myelination pathways.
The views expressed in this article do not necessarily reflect the views of the journal or of ASM.
Footnotes
Citation Santiana M, Altan-Bonnet N. 2019. Insane in the membrane: glial extracellular vesicles transmit polyomaviruses. mBio 10:e01024-19. https://doi.org/10.1128/mBio.01024-19.
REFERENCES
- 1.Altan-Bonnet N, Perales C, Domingo E. 2019. Extracellular vesicles: vehicles of en bloc viral transmission. Virus Res 265:143–149. doi: 10.1016/j.virusres.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 2.Chen YH, Du W, Hagemeijer MC, Takvorian PM, Pau C, Cali A, Brantner CA, Stempinski ES, Connelly PS, Ma HC, Jiang P, Wimmer E, Altan-Bonnet G, Altan-Bonnet N. 2015. Phosphatidylserine vesicles enable efficient en bloc transmission of enteroviruses. Cell 60:619–630. doi: 10.1016/j.cell.2015.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Santiana M, Ghosh S, Ho BA, Rajasekaran V, Du WL, Mutsafi Y, De Jésus-Diaz DA, Sosnovtsev SV, Levenson EA, Parra GI, Takvorian PM, Cali A, Bleck C, Vlasova AN, Saif LJ, Patton JT, Lopalco P, Corcelli A, Green KY, Altan-Bonnet N. 2018. Vesicle-cloaked virus clusters are optimal units for inter-organismal viral transmission. Cell Host Microbe 24:208. doi: 10.1016/j.chom.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris-Love J, Gee GV, O'Hara BA, Assetta B, Atkinson AL, Dugan AS, Haley SA, Atwood WJ. 2019. JC polyomavirus uses extracellular vesicles to infect target cells. mBio 10:e00379-19. doi: 10.1128/mBio.00379-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayberry CL, Nelson CDS, Maginnis MS. 2017. JC polyomavirus attachment and entry: potential sites for PML therapeutics. Curr Clin Microbiol Rep 4:132–141. doi: 10.1007/s40588-017-0069-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maginnis MS, Nelson CD, Atwood WJ. 2015. JC polyomavirus attachment, entry, and trafficking: unlocking the keys to a fatal infection. J Neurovirol 21:601–613. doi: 10.1007/s13365-014-0272-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Hara BA, Gee GV, Atwood WJ, Haley SA. 2018. Susceptibility of primary human choroid plexus epithelial cells and meningeal cells to infection by JC virus. J Virol 92:e00105-18. doi: 10.1128/JVI.00105-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Elphick GF, Querbes W, Jordan JA, Gee GV, Eash S, Manley K, Dugan A, Stanifer M, Bhatnagar A, Kroeze WK, Roth BL, Atwood WJ. 2004. The human polyomavirus, JCV, uses serotonin receptors to infect cells. Science 306:1380–1383. doi: 10.1126/science.1103492. [DOI] [PubMed] [Google Scholar]
- 9.Stroh LJ, Maginnis MS, Blaum BS, Nelson CD, Neu U, Gee GV, O'Hara BA, Motamedi N, DiMaio D, Atwood WJ, Stehle T. 2015. The greater affinity of JC polyomavirus capsid for alpha2,6-linked lactoseries tetrasaccharide c than for other sialylated glycans is a major determinant of infectivity. J Virol 89:6364–6375. doi: 10.1128/JVI.00489-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haley SA, O'Hara BA, Nelson CDS, Brittingham FLP, Henriksen KJ, Stopa EG, Atwood WJ. 2015. Human polyomavirus receptor distribution in brain parenchyma contrasts with receptor distribution in kidney and choroid plexus. Am J Pathol 185:2246–2258. doi: 10.1016/j.ajpath.2015.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Feng Z, Hensley L, McKnight KL, Hu F, Madden V, Ping L, Jeong SH, Walker C, Lanford RE, Lemon SM. 2013. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature 496:367–371. doi: 10.1038/nature12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geoghegan EM, Pastrana DV, Schowalter RM, Ray U, Gao W, Ho M, Pauly GT, Sigano DM, Kaynor C, Cahir-McFarland E, Combaluzier B, Grimm J, Buck CB. 2017. Infectious entry and neutralization of pathogenic JC polyomaviruses. Cell Rep 21:1169–1179. doi: 10.1016/j.celrep.2017.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skotland T, Sandvig K, Llorente A. 2017. Lipids in exosomes: current knowledge and the way forward. Prog Lipid Res 66:30–41. doi: 10.1016/j.plipres.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 14.Toscano MG, de Haan P. 2018. How simian virus 40 hijacks the intracellular protein trafficking pathway to its own benefit … and ours. Front Immunol 9:1160. doi: 10.3389/fimmu.2018.01160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yin X, Ambardekar C, Lu Y, Feng Z. 2016. Distinct entry mechanisms for nonenveloped and quasi-enveloped hepatitis E viruses. J Virol 90:4232–4242. doi: 10.1128/JVI.02804-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kondo Y, Windrem MS, Zou L, Chandler-Militello D, Schanz SJ, Auvergne RM, Betstadt SJ, Harrington AR, Johnson M, Kazarov A, Gorelik L, Goldman SA. 2014. Human glial chimeric mice reveal astrocytic dependence of JC virus infection. J Clin Invest 124:5323–5336. doi: 10.1172/JCI76629. [DOI] [PMC free article] [PubMed] [Google Scholar]