Abstract
Adult neurogenesis is the process by which neural stem cells give rise to new functional neurons in specific regions of the adult brain, a process that occurs throughout life. Significantly, neurodegenerative and psychiatric disorders present suppressed neurogenesis, activated microglia, and neuroinflammation. Traffic-related air pollution has been shown to adversely affect the central nervous system. As the cardinal effects of air pollution exposure are microglial activation, and ensuing oxidative stress and neuroinflammation, we investigated whether acute exposures to diesel exhaust (DE) would inhibit adult neurogenesis in mice. Mice were exposed for 6 h to DE at a PM25 concentration of 250–300 μg/m3, followed by assessment of adult neurogenesis in the hippocampal subgranular zone (SGZ), the subventricular zone (SVZ), and olfactory bulb (OB). DE impaired cellular proliferation in the SGZ and SVZ in males, but not females. DE reduced adult neurogenesis, with male mice showing fewer new neurons in the SGZ, SVZ, and OB, and females showing fewer new neurons only in the OB. To assess whether blocking microglial activation protected against DE-induced suppression of adult hippocampal neurogenesis, male mice were pre-treated with pioglitazone (PGZ) prior to DE exposure. The effects of DE exposure on microglia, as well as neuroinflammation and oxidative stress, were reduced by PGZ. PGZ also antagonized DE-induced suppression of neurogenesis in the SGZ. These results suggest that DE exposure impairs adult neurogenesis in a sex-dependent manner, by a mechanism likely to involve microglia activation and neuroinflammation.
Keywords: Diesel exhaust, Adult neurogenesis, Microglia, Pioglitazone, Neuroinflammation
Introduction
Air pollution derives from a variety of sources, including industrial and vehicular emissions and biomass burning, and contains several components such as organic and inorganic particulates, metals, volatile organic compounds, and gases (Monks et al. 2009). Long associated with the development of chronic respiratory conditions and later with cardiovascular disorders and metabolic dysfunction, elevated air pollution has been associated recently with increased risk of adverse effects in the central nervous system (CNS) (Calderón-Garcidueñas et al. 2008, 2012; Costa et al. 2014a, 2017; Costa 2017; Genc et al. 2012). For example, epidemiological studies have shown an association between air pollution and cognitive impairment, dementia, and other neurodegenerative diseases (Chen et al. 2017; Weuve et al. 2012). One of the air pollution components of most concern is PM2.5, i.e., particulate matter having an aerodynamic diameter of 2.5 micrometers or less. PM2.5 is capable entering the circulatory system through the pulmonary or olfactory mucosa, and can also enter the brain through the olfactory nerve (Oberdörster et al. 2004). Concentration of PM2.5 routinely exceeds 100 μg/m3 for extended periods of time in some parts of the world, especially in certain areas of China and India (Kandlikar and Ramachandran 2000; Sun et al. 2004). Traffic-related air pollution is a major contributor to global air pollution, and diesel exhaust (DE) is one of the predominant components (Ghio et al. 2012).
Oxidative stress and inflammation are the two important processes by which air pollution exerts its systemic and central nervous system toxicity (Genc et al. 2012; Costa et al. 2017). Experimental exposure of mice to DE causes priming and activation of microglia and subsequent neuroinflammation and oxidative stress (Levesque et al. 2011; Cole et al. 2016). In vitro experiments have also shown that the toxicity of DE particulates (DEP) is dependent upon the activity of microglia, with monocultured neurons showing none of the neurotoxic response that the neurons co-cultured with microglia displayed. In addition, blocking microglial activation with the PPAR-γ agonist pioglitazone (PGZ) attenuated DEP neurotoxicity in vitro (Roqué et al. 2016).
Neuroinflammation and microglial activation can adversely influence regeneration of neurons in the brain (Carpentier and Palmer 2009; Ekdahl et al. 2003). The birth of new neurons in the adult brain, and their survival and functional integration into existing neural circuitries, known as adult neurogenesis, is restricted in rodents to two regions: the subgranular zone (SGZ) in the dentate gyrus of the hippocampus, and the area adjacent to the lateral ventricles and striatum, known as the subventricular zone (SVZ) (Alvarez-Buylla et al. 2001). The SVZ is also the point of origin for immature neuroblasts that migrate along the rostral migratory stream to the olfactory bulb (OB), where they develop into local interneurons (Pignatelli and Belluzzi 2010). Neurogenesis occurs in the adult brain constantly, though at a rate that decreases with age (Ming and Song 2011), possibly because of increased neuroinflammation and microglial activation (Schuitemaker et al. 2012). Microglial activation resulting from lipopolysaccharide administration, or increased levels of pro-inflammatory cytokines such as interleukin-6 (IL-6), or tumor necrosis factor-α (TNF-α) can impair hippocampal neurogenesis (Ekdahl et al. 2003; Iosif et al. 2006; Vallières et al. 2002). Even chronic peripheral inflammation may have an adverse effect on the CNS, as increased permeability of the endothelium of the blood–brain barrier allows activated macrophages to migrate into the relatively restricted cerebrospinal compartment, triggering microglial activation and neuroinflammation (Raghavendra et al. 2004; Takeshita and Ransohoff 2012).
Disruption of adult neurogenesis may affect cognitive and olfactory function (Frankland and Miller 2008), and may lead to severe cognitive impairment in neurodegenerative diseases. Indeed, even in the presence of characteristic Alzheimer’s disease (AD) neuropathology, cognitive function is equal to that of healthy individuals when neurogenesis is preserved (Briley et al. 2016). The gradual loss of neurons, coupled with a reduction in young adult-born neurons, plays a role in normal diminution of cognitive function due to the unique role of young neurons in memory (Bishop et al. 2010). Young neurons are very excitable, having a low threshold of depolarization, and they appear to be preferentially activated during the formation of new memories (Bischofberger 2007). Short-term memory also appears to be dependent on the activities of young neurons, and impairment of short-term memory is one of the first symptoms to emerge in the earliest stages of AD (Baudic et al. 2006). The ability to remember and forget is related to synaptic plasticity, or the weakening and strengthening of neural pathways over time, which also appears to be affected by neurogenesis (Saxe et al. 2006). Decreased neurogenesis, and the resulting impaired synaptic plasticity, may also be involved in depression and anxiety, and explain the therapeutic effectiveness of anti-depressants that increase neurogenesis (Hayley and Litteljohn 2013).
Based on our studies indicating that acute DE exposure induces neuroinflammation, microglial activation, and oxidative stress (Cole et al. 2016), we hypothesized that (1) mice acutely exposed to DE would show compromised neurogenesis relative to control animals and (2) given that neuroinflammation induced by DE was more pronounced in male mice (Cole et al. 2016), neurogenesis would be impaired to a greater extent in male animals. We further hypothesized that a pre-treatment with PGZ would protect against DE-induced inhibition of adult neurogenesis by attenuating microglial activation, neuroinflammation, and oxidative stress.
Materials and methods
Animals
Male and female 8-week-old C57BL6/J mice were purchased from Jackson Laboratories (Bar Harbor, ME). All animals were housed in specific pathogen-free facilities with a 12-h dark–light cycle, with feed and water available ad libitum. The mice were assigned randomly to either filtered air (FA) or DE exposures.
Bromodeoxyuridine treatment
A stock solution of bromodeoxyuridine (BrdU) was prepared using 20-mg/mL BrdU (Sigma-Aldrich, St. Louis, Missouri) and 0.007-N sodium hydroxide to facilitate dissolution in sterile normal saline (0.9% sodium chloride). The solution was buffered to pH 7.4 and sterile-filtered prior to use. Each animal was weighed and given an initial dose of 100-mg/kg BrdU by intraperitoneal injection. Mice received four more doses of 100-mg/kg BrdU given at 2-h intervals, resulting in a cumulative dose of 500 mg/kg/day (Pan et al. 2013a; Taupin 2007).
Exposure to diesel exhaust
Individually housed mice were exposed for 6 h to FA or DE (at a PM2.5 concentration of 250–300 μg/m3). Exposures to either FA or DE were conducted simultaneously in the University of Washington Controlled Exposure Laboratory’s Northlake Diesel Facility, which includes an SPF mouse housing room with Allentown caging systems (Allentown, NJ), with the housing racks modified to ventilate cages with either diluted DE or FA. DE was derived from a Yanmar YDG5500 diesel generator, with a load bank maintaining 75% of rated capacity, using No. 2 undyed, ultra-low sulfur on-highway fuel and Royal Purple Duralec 15W-40 Synthetic crankcase oil to lubricate moving parts, as previously described (Fox et al. 2015; Gould et al. 2008). During exposures, DE concentrations were continuously measured and maintained at steady levels using a feedback controller monitoring fine particulate levels (Fox et al. 2015; Gould et al. 2008). DE was limited to PM2.5 or smaller, having a mean aerodynamic diameter of 100 nm. Characterization of DE is described in detail in a previous publication (Fox et al. 2015). For assays of lipid peroxidation and of TNFα mRNA levels, mice were euthanized by CO2 asphyxiation within 2 h after the end of the exposure, and brain regions were dissected immediately, flash-frozen in liquid nitrogen, and stored at − 80 °C. For Iba1 and Ki67 immunohistochemistry, mice were euthanized 18 h after the end of exposure, and their brains fixed by transcardial perfusion and then embedded, sectioned, and stored at − 80 °C in cryoprotectant medium. For NeuN/BrdU immunohistochemistry, animals were pre-treated with 500-mg/kg BrdU the day before exposure, exposed for 6 h, then euthanized 21 days following exposure, to label adult-born cells that are still alive. Their brains were fixed, frozen, and sectioned as described above, and stored at − 80 °C in cryoprotectant medium (Pan et al. 2013a).
Lipid peroxidation assay
Lysates were prepared from frozen brain region samples homogenized in CLB lysis buffer (10-mM HEPES; 150mM NaCl; 1-mM CaCl2; 0.5-mM MgCl2; 10-μg/ml leupeptin; 10-μg/ml aprotinin; 1-mM PMSF; 50-mM NaF). The homogenate was incubated on ice for 10-min, centrifuged at 4 °C and 2000 × g for 5 min, and the resulting supernatant samples were stored at − 80 °C until ready for analysis. The protein content of each sample was determined using the Pierce bicinchoninic acid (BCA) assay (Thermo Scientific, Waltham, MA), with bovine serum albumin (BSA) as a standard, according to the manufacturer’s protocol. Lipid peroxidation was measured by quantifying levels of malondialdehyde (MDA), a byproduct of lipid peroxidation, using the Thiobarbituric Acid Reactive Substances (TBARS) assay (Cayman Chemical, Ann Arbor, MI) according to the manufacturer’s instructions, as previously described (Giordano et al. 2013). MDA content was normalized to the amount of protein loaded per well.
RNA extraction and real time PCR analysis
Prior to extraction, all working surfaces and instruments were cleaned with an RNAse inhibitor. Tissue homogenates were prepared using TRIzol RNA extraction reagent (Invitrogen, Carlsbad, California) and purified according to the manufacturer’s protocol. Concentration and quality of RNA were determined using a NanoDrop ND-100 Spectrophotometer (NanoDrop Technologies, Wilmington, Delaware). RNA samples (1 μg) were reverse transcribed using the iScript™ cDNA Synthesis Kit (Bio-Rad, Hercules, California) according to the manufacturer’s protocol. The resulting cDNAs were used for PCR amplification in the presence of primers specific to tumor necrosis factor (TNFα) (forward: GTCGTA GCAAACCACCAAGTG; reverse: CTTTGAGATCCATGC CGTTGG; 21 bp) and the housekeeping gene hypoxanthine phosphoribosyltransferase (HPRT1) (forward: GAGGAG TCCTGTTGATGTTGCCAAG; reverse: GGCTGGCCT ATAGGCTCATAGTGC; 25 bp). Amplification was carried out in a SimpliAmp Thermal Cycler (Applied Biosystems, Foster City, California) and quantification was carried out in a Bio-Rad CF384 Real-timer System thermal cycler, using iTaq Universal SYBR Green Supermix.
Pioglitazone treatment
A stock suspension of 1.25-mg/mL pioglitazone hydrochloride (PGZ; 98% pure; Sigma-Aldrich, St. Louis, Missouri) was prepared in a vehicle (VEH) of 0.5% carboxymethylcelluose sodium dissolved in PBS. For 4 days, animals were treated with 10 μL/g of PGZ (12.5 mg/kg) or 10-μL/g VEH by oral gavage, using a 20G stainless-steel curved feeding needle, each morning, up to and including the day of DE exposure (Drew et al. 2015; Maeda et al. 2008).
Transcardial perfusion and post-fixation
Mice were euthanized by CO2 asphyxiation, and the thoracic cavity was opened to expose the heart. A small incision was cut into the left ventricle of the heart, into which a blunted 20-gauge needle attached to a line containing saline was inserted and secured with a bulldog clamp. A small incision was cut into the right atrium to provide an outlet for the blood and perfusion fluids. First, 15 ml of 0.9% normal saline were pumped through the vasculature using a Minipuls 2 peristaltic pump (Gilson, Middleton, Wisconsin) to clear out the blood, followed by the same volume of 4% paraformaldehyde (PFA) in PBS (Santa Cruz Biotech, Santa Cruz, California) to achieve thorough fixation of tissues. Brains were resected intact from the perfused animals, placed in 50-mL conical tubes containing ice-cold 4% PFA in PBS, and post-fixed overnight at 4 °C. They were then removed from the PFA and placed in a solution of 30% sucrose in PBS at 4 °C until negative buoyancy was reached. The brains were rinsed in PBS and then embedded individually in O.C.T. Compound Embedding Medium (Sakura Finetek, Torrance, California), frozen, and stored at − 80 °C prior to sectioning (Pan et al. 2013a).
Olfactory bulb immunohistochemistry
Frozen, OCT-embedded, OB tissue was cut at a thickness of 14 μm using a Reichert-Jung Cryocut-1800 cryostat (Leica, Wetzlar, Germany) and every eighth section was placed directly on VWR SuperFrost-Plus Microslides. Slides were stored at − 20° until ready for processing. Slides destined for processing were removed from storage and allowed to sit at RT for 20 min to allow for adhesion of tissue to the slide. A Pap-Pen (Electron Microscopy) was used to draw around the sections to confine reagents to the surface of the slide. Slides were placed in a humidification chamber and washed with PBS to rehydrate the tissues. Sections were rinsed in water briefly, and then treated with 2-N hydrochloric acid for 30 min at 38 °C. Acid was neutralized with 0.1-M borate buffer (pH 8.5); sections were then permeabilized, first with 1% sodium dodecyl sulfate, then with PBS containing 0.25% Triton X-100 (PBST). Sections were blocked overnight using a blocking solution containing 10% goat serum and 1% bovine serum albumin (BSA) in PBST. Following blocking, sections were probed for BrdU and NeuN using monoclonal antibodies specific to the markers (BrdU (1:1000): MCA2060, AbD Serotec/Bio-Rad, Hercules, California; NeuN (1:1000): MAB377, EMD Millipore, Temecula, California). Following 48-h incubation in primary antibodies at 4 °C, the slides were rinsed in PBST and then probed with AlexaFluor 594 goat anti-rat and AlexaFluor goat 488 anti-mouse antibodies [(1:1000 dilutions) Invitrogen/ThermoFisher, Waltham, Massachusetts] for 48 h. Following nuclear staining with Hoechst 33,342, sections were rinsed in PBS, mounted using VectaShield Fluorescence Mounting Medium (H-1000, VectaShield, Burlingame), and cover slips were secured using nail polish (Pan et al. 2013a).
SGZ and SVZ immunohistochemistry
Every eighth coronal section of the subventricular and hippocampal region of each brain was cut at a thickness of 30 μm using a Reichert-Jung Cryocut-1800 cryostat (Leica) with temperature set at − 25 °C. Sections were stored at − 20 °C in 24-well plates containing cryprotectant medium until processed. Prior to staining, sections were rinsed in PBS to remove cryoprotectant and embedding medium. For Ki67 and Iba1 immunohistochemistry, no antigen retrieval method was used prior to permeabilization. For NeuN/BrdU double-staining, a 30-min DNA-denaturing step with 2N hydrochloric acid and subsequent neutralization with 0.1-M borate buffer was required to improve immunolabeling of BrdU. The sections were then permeabilized with PBST and blocked overnight in a buffer containing 1% BSA (w/v) and 10% normal goat serum (v/v) in PBST. Sections were then incubated for 48 h with the appropriate primary antibodies [BrdU (1:1000): MCA2060, AbD Serotec/Bio-Rad; NeuN (1:1000): MAB377, EMD Millipore; Iba1 (1:1000): ab107159, and Ki67 (1:1000): ab15580, Abcam, Cambridge, Massachusetts]. Sections were rinsed in PBST then incubated with AlexaFluor 594 goat anti-rat and AlexaFluor 488 goat anti-mouse antibodies (1:1000), AlexaFluor 568 donkey anti-goat (1:1000), or AlexaFluor 555 donkey anti-rabbit antibody (1:1000) for 48 h. The sections were rinsed in PBST and incubated in Hoechst 33,342 to identify nuclei. Following nuclear staining, sections were washed in PBS, and then mounted on gelatin-coated slides. VectaShield Fluorescence Mounting Medium (H-1000, VectaShield, Burlingame, California) was used to mount the sections and prevent photobleaching. No. 1 coverslips (VWR, South San Francisco, California) were placed on top of the sections and secured using nail polish. The slides were then placed in slide boxes and kept in the dark at 4 °C until ready for imaging (Jongbloets et al. 2017; Pan et al. 2012, 2013a).
Image acquisition and analysis
For assessment of adult neurogenesis, images were captured in three channels using a Marianas Imaging System, which included a Zeiss 200M Axiovert microscope with a motorized stage, a 175-W xenon lamp, and a Roper HQ Cool Snap digital camera. The software SlideBook 6.0 (3i Intelligent Imaging Innovations, Denver, Colorado) was used to set parameters of image capture. In the SGZ and SVZ, 40× 3D images of all BrdU-positive cells were collected. Images were uniformly adjusted for brightness and contrast. All BrdU-positive cells that contained a well-defined nucleus were counted, and BrdU-positive nuclei immunoreactive to NeuN were counted as double-labeled cells. The ratio of BrdU+/NeuN+ cells to all BrdU+ cells was calculated for each specimen and expressed as a percentage (Pan et al. 2012, 2013a, b).
For adult neurogenesis in the OB, a 20× montage of each section was taken. The granular cell layer of each section was then defined and stereologically sampled at 12.5% of the cross-sectional area. As with the SGZ and SVZ, three-channel 40× images were uniformly adjusted for color and brightness. The number of BrdU+ cells and the number of newborn neurons (NeuN+/BrdU+ cells) were expressed as density (number per cubic mm of region of interest) rather than a total number per region. Newborn neurons were also expressed as a percentage of all BrdU+ cells.
To assess proliferation in the SGZ and SVZ, two-channel images of all Ki67+ cells were taken in the SGZ and SVZ. Images were uniformly adjusted for contrast and brightness, and all Ki67+ cells with a well-defined nucleus were counted and expressed as a total number per region.
To assess microglial morphology, two-channel 40× images of the hippocampal dentate gyrus were captured using the Marianas Imaging System for imaging and Slide-Book 6.0 to set parameters of image capture. Two-channel 40× images were adjusted uniformly for contrast and brightness, and perimeter of microglial somata on the plane with the sharpest resolution of the nucleus were traced using the irregular drawing tool and analyzed to evaluate shape descriptor parameters using the open-source software FIJI. In addition to the microglial soma area, three other parameters were assessed: circularity (C = 4π*area/perimeter2), roundness (R = 4*area/π(major axis)2), and aspect ratio (AR = major axis/minor axis) (Jonas et al. 2012; Morrison and Filosa 2013; Torres-Platas et al. 2014).
Statistical analysis
Statistical analysis of all data was by one-way ANOVA with Bonferroni’s post-test. All graphs show the mean and SEM. Prism 5.02 (GraphPad, San Diego, California) was used for the preparation of graphs as well as statistical analysis of data.
Results
As observed previously with this paradigm of DE exposure (Cole et al. 2016), no morbidity was observed in animals exposed to 250–300-μg/m3 DE for 6 h, and there were no salient behavioral or physical differences between DE-and FA-exposed animals of either sex.
Fluorescence immunohistochemistry was used to analyze the neurogenic regions of the brain (OB, SVZ, and SGZ). Cellular proliferation was assessed by immunohistochemistry using Ki67 in the SGZ and SVZ. In male mice, DE exposure was associated with a significant reduction in the number of Ki67-immunopositive cells in both the SGZ (Fig. 1a) and the SVZ (Fig. 1b). In contrast, no significant differences were seen between DE- and FA-exposed female mice in the number of Ki67-immunopositive cells in either SGZ (Fig. 1a) or SVZ (Fig. 1b).
Fig. 1.
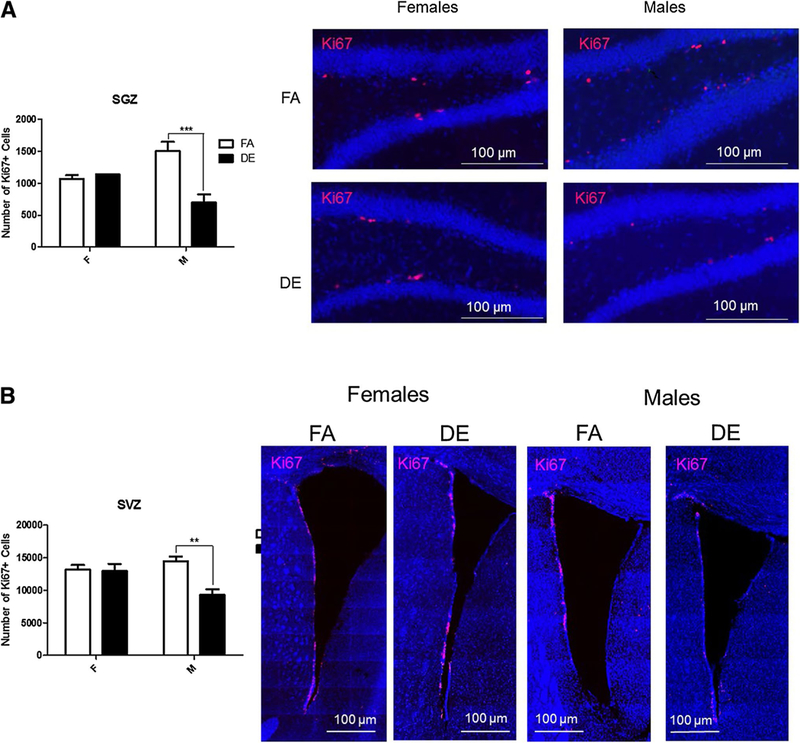
Acute DE exposure decreases proliferation in the SGZ (a) and SVZ (b) of male mice. Eight-week-old male and female C57BL/6J mice were exposed to DE (250 μg/m3) or FA for 6 h, and then sacrificed 18 h later. Images shown are representative micrographs of Ki67 immunohistochemistry in female and male mice. Results represent the mean (± SE) with n = 3 per group. Significantly different from FA, **p < 0.01, ***p < 0.001. SGZ subgranular zone of the hippocampus, SVZ subventricular zone
For assessment of adult neurogenesis, tissue sections containing the OB, SVZ, and SGZ were probed with antibodies specific to the cellular proliferation marker BrdU and the neuronal nuclear marker NeuN. BrdU+ and double-labeled cells (NeuN+/BrdU+) were counted and expressed as a total number and as a percentage of all BrdU+ cells. In male mice, the total number of BrdU+ cells was significantly reduced following DE exposure in both the SGZ (Fig. 2a) and the OB (Fig. 4a), but not in the SVZ (Fig. 3a). The number of double-positive NeuN+/BrdU+ cells was reduced in DE-exposed males in all three regions (Figs. 2b, 3b, 4b) compared to FA-exposed males. NeuN+/BrdU+ double-labeled cells expressed as a percentage of all BrdU+ cells were also significantly reduced in the SGZ (Fig. 2c), SVZ (Fig. 3c), and OB (Fig. 4c) of DE-exposed males compared to FA controls. No significant differences were seen between DE- and FA-exposed female mice in the SGZ (Fig. 2) or SVZ (Fig. 3) in either the number of BrdU+ cells (Figs. 2a, 3a) or the number or percentage of double-labeled NeuN+/BrdU+ cells (Figs. 2b, c, 3b, c). However, in the OB (Fig. 4), the total number of double-labeled NeuN+/BrdU+ cells was significantly reduced in DE-exposed females compared to FA-exposed controls (Fig. 4b).
Fig. 2.
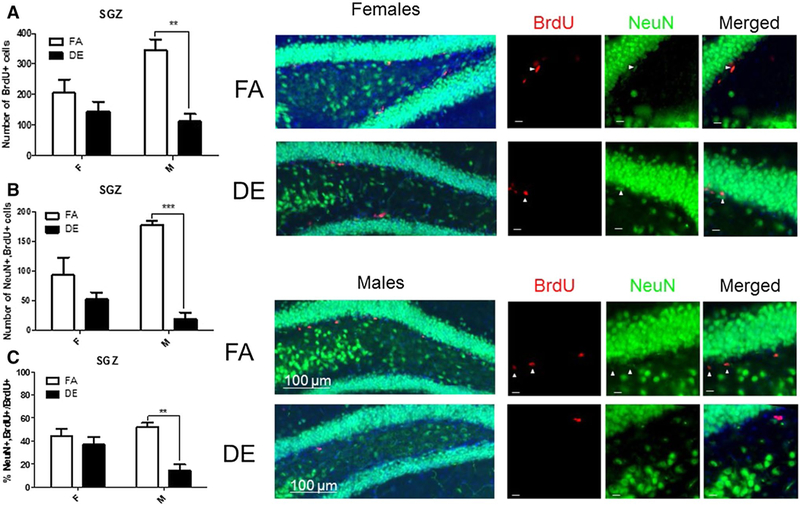
Effect of acute DE exposure on adult hippocampal neurogenesis in mice. Eight-week-old male and female C57BL/6J mice were given five 100-mg/kg doses of BrdU at 2-h intervals. On the following day they were exposed to DE (250 μg/m3) or FA for 6 h and then sacrificed 21 days later. The images shown are representative micrographs of NeuN/BrdU immunohistochemistry in the SGZ of FA- and DE-exposed mice. Scale bars in detail images represent 10 μm. The number of BrdU-stained cells (a) indicates surviving cells that been born since the beginning of the experiment. Double-stained NeuN/BrdU cells indicate surviving adult-born neurons, which are expressed both as a total number per brain region (b), and as a percentage of all BrdU-positive cells (c). Results represent the mean (± SE) with n = 3 per group. Significantly different from FA, **p < 0.01, ***p < 0.001
Fig. 4.
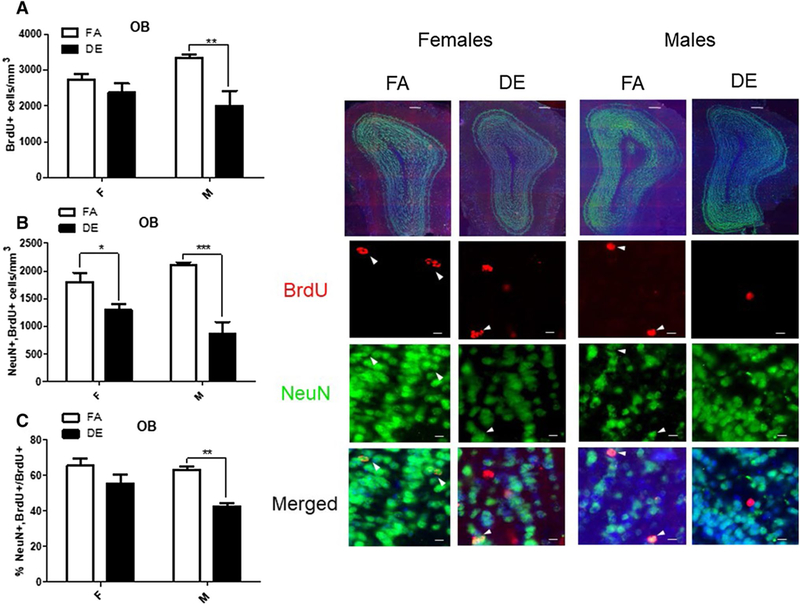
Effect of acute DE exposure on adult neurogenesis in the OB of mice. Eight-week-old male and female C57BL/6J mice were given five 100-mg/kg doses of BrdU at 2-h intervals. On the following day, they were exposed to DE (250 μg/m3) or FA for 6 h and then sacrificed 21 days later. The images shown are representative micrographs of NeuN/BrdU immunohistochemistry in the OB of FA- and DE-exposed mice. Scale bars in detail images represent 10 μm. a Number of BrdU-positive cells indicates surviving cells that been born since the beginning of the experiment. Double-stained NeuN/BrdU cells indicate surviving adult-born neurons, which are expressed both as a total number per brain region (b), and as a percentage of all BrdU-positive cells (c). Data shown represent the mean (± SE) with n = 3 per group. Significantly different from FA, *p < 0.05, **p < 0.01, ***p < 0.001. OB olfactory bulb
Fig. 3.
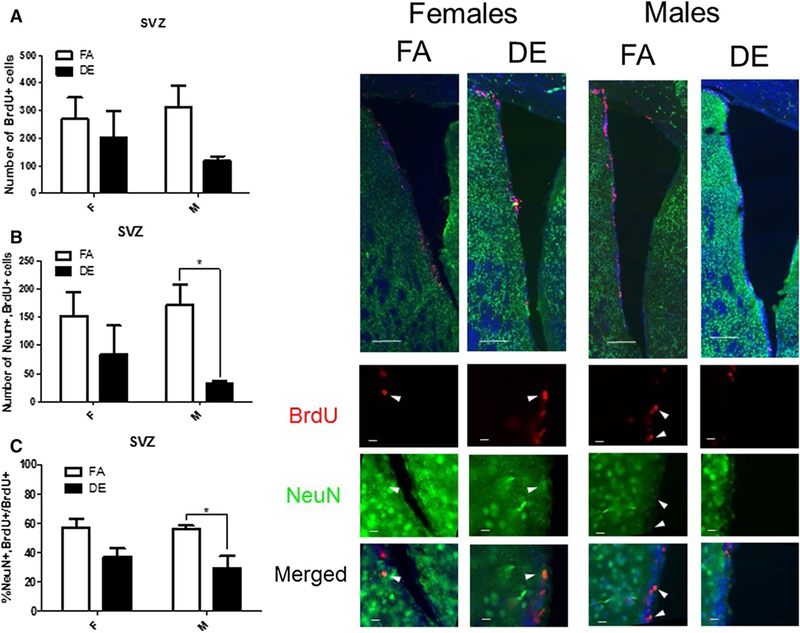
Effect of acute DE exposure on adult neurogenesis in the SVZ of mice. Eight-week-old male and female C57BL/6J mice were given five 100-mg/kg doses of BrdU at 2-h intervals. On the following day, they were exposed to DE (250 μg/m3) or FA for 6 h, and then sacrificed 21 days later. Images shown are representative micrographs of NeuN/BrdU immunohistochemistry in the SVZ of FA- and DE-exposed mice. Scale bars in montage images represent 100 microns, while scale bars in detail images represent 10 μm. The number of BrdU-stained cells (a) indicates surviving cells that been born since the beginning of the experiment. Double-stained NeuN/BrdU cells indicate surviving adult-born neurons, which are expressed both as a total number per brain region (b) and as a percentage of all BrdU-positive cells (c). Data shown represent the mean (± SE) with n = 3 per group. Significantly different from FA, *p < 0.05
Pioglitazone (PGZ) has been shown to suppress DE-induced microglial activation in vitro (Roque et al. 2016). Since microglial activation and ensuing neuroinflammation has been shown to affect adult neurogenesis, we assessed the effect of pre-treatment with PGZ on DE-induced inhibition of adult neurogenesis in the SGZ. Male mice were used in this experiment, because they were more sensitive than females to the effects of DE exposure. Mice were pre-treated with PGZ or VEH for 4 days, then exposed to DE or FA for 6 h, and sacrificed 18 h later for assessment of microglial activation, or 3 weeks later for assessment of adult neurogenesis.
Iba1 immunohistochemistry was used to define the microglial somata in the dentate gyrus of the hippocampus, and shape descriptors associated with reactive microglial pheno-types were used to assess the degree of microglial activation associated with DE exposure. Acute DE exposure resulted in changes in microglial morphology typically associated with microglial activation, and these changes were antagonized by PGZ pre-treatment (Fig. 5). Specifically, microglial soma area was increased significantly with DE exposure (Fig. 5a), while circularity of microglial soma defined as 4π(area/perimeter2) and roundness of the soma defined as 4*area/π(major axis)2 were significantly reduced by DE exposure (Fig. 5b, c). Finally, aspect ratio (Fig. 5d), the quotient of the major and the minor axis of the best-fit ellipse, was significantly increased in microglia of the DE-exposed mice compared to FA controls. PGZ decreased the effects of DE exposure on all parameters of microglial morphology (Fig. 5a–d), indicating that the treatment was effective at inhibiting microglial activation. This was further confirmed by the findings that the increases in the levels of TNF-α mRNA and of lipid peroxidation in cortex and hippocampus caused by DE exposure were antagonized by pre-treatment with PGZ (Fig. 6a, b).
Fig. 5.
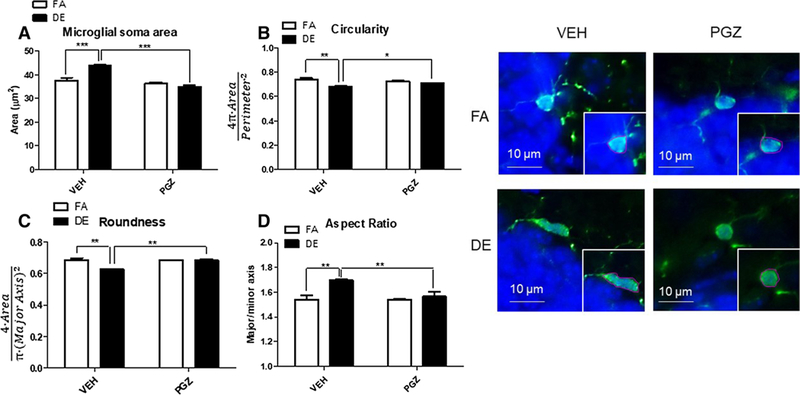
Pioglitazone (PGZ) pre-treatment suppresses microglial activation in the hippocampus of male mice. Eight-week-old male C57BL/6J mice were pre-treated with 12.5 mg/kg/day PGZ or vehicle (VEH) for 4 days up to and including the day of exposure, and were then exposed to DE (250 μg/m3) or FA for 6 h and then sacrificed 18 h later. Representative micrographs (on the right) of Iba1 immunohistochemistry of FA- and DE-exposed mice show microglia from the hippocampus, both as originally captured, and then as defined using tracing tool (inset) to allow for shape descriptor analysis using FIJI. Increases in area of the microglial soma (a) and aspect ratio (d) and decreases in circularity (b) and roundness (c) were indicators of microglial activation. Microglial soma area (a) is measured in μm2, circularity (b) is calculated as 4π(area/perimeter2), roundness (c), a measure of inverse aspect ratio, is calculated by R = 4*area/π(major axis)2. Aspect ratio (d) is defined as major axis/minor axis. Results represent the mean (± SE) with n = 3 per group. VEH/FA significantly different from VEH/DE; VEH/DE significantly different from PGZ/DE; *p < 0.05, **p < 0.01, ***p < 0.001
Fig. 6.
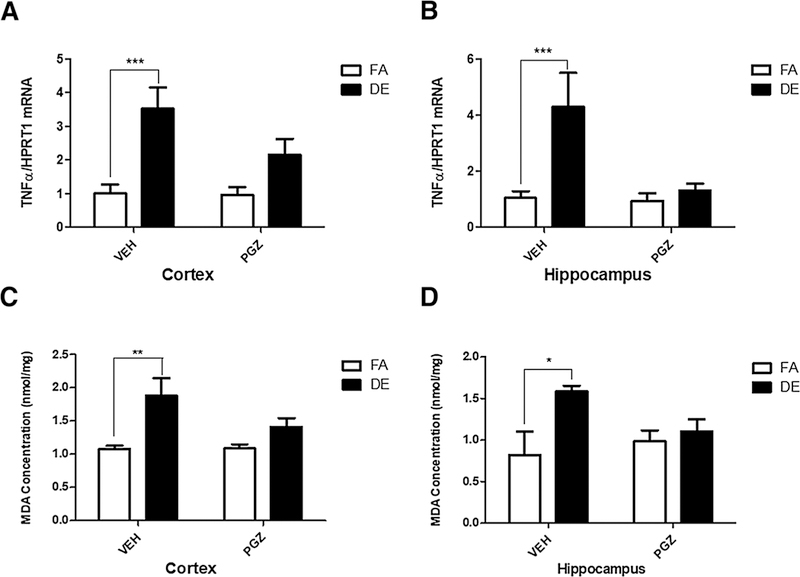
Effect of pioglitazione on oxidative stress and cytokine expression. Lipid peroxidation (levels of malondialdehyde) and levels of TNF-α mRNA were assessed in the cerebral cortex (a, c), and the hippocampus (b, d) of male mice following 4 days of pre-treatment with either 12. 5-mg/kg/day PGZ or with VEH, followed by a 6-h exposure to DE (250 μg/m3) or FA. Results represent the mean (± SE) with n = 6 per group. Significantly different from FA control, *p < 0.05, **p < 0.01, ***p < 0.001
As expected, DE exposure significantly reduced cellular proliferation in the SGZ of the VEH-treated mice (Fig. 7). PGZ pre-treatment antagonized this effect of DE. In animals pre-treated with PGZ DE exposure did not affect proliferation (Fig. 7). With regard to neurogenesis, in VEH-treated control animals, the total number of BrdU+ cells in the SGZ was reduced by DE exposure, and this effect was antagonized by PGZ (Fig. 8a). Similarly, NeuN+/BrdU+ cells expressed both as a total number and as percentage of all BrdU+ cells were reduced by DE exposure in VEH-treated controls, and this was also antagonized by pre-treatment with PGZ (Fig. 8b, c).
Fig. 7.
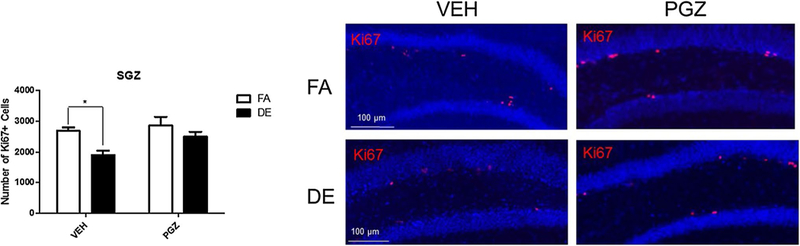
Effect of PGZ pre-treatment on DE-induced inhibition of proliferation in the SGZ of male mice. Eight-week-old male C57BL/6J mice were pre-treated with 12.5-mg/kg/day PGZ or VEH for 4 days up to and including the day of exposure, and were then exposed to DE (250 μg/m3) or FA for 6 h and then sacrificed 18 h later. Images shown are representative micrographs of Ki67+ cells in the hippocampus of FA- and DE-exposed mice, without and with PGZ pre-treatment. Significantly different from FA control, *p < 0.05
Fig. 8.
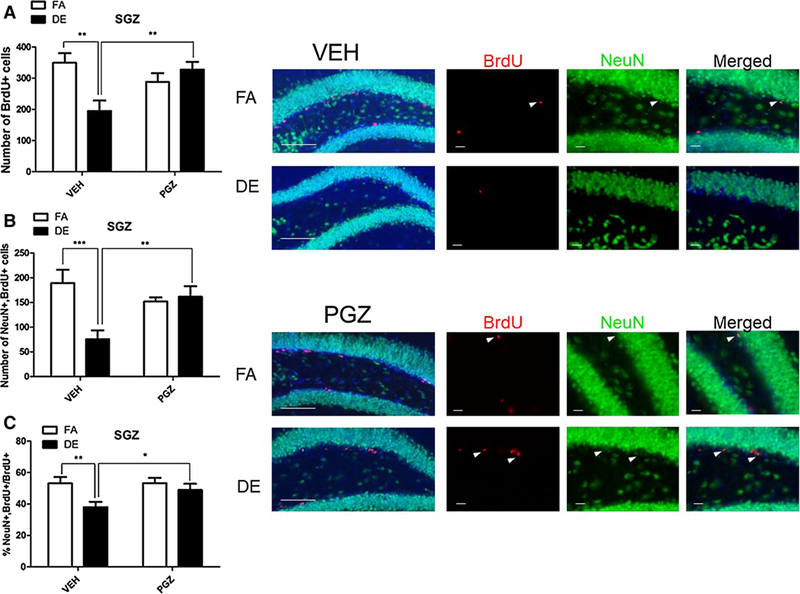
Effect of PGZ pre-treatment on DE-induced inhibition of adult hippocampal neurogenesis in male mice. Eight-week-old male C57BL/6J mice were pre-treated with 12.5-mg/kg/day PGZ or VEH for 4 days. On day 3 of pre-treatment, they were given five 100-mg/kg doses of BrdU at 2-h intervals. On day 4, mice were exposed to DE (250 μg/m3) or FA for 6 h, then sacrificed 21 days later. Images shown are representative micrographs of NeuN/BrdU immunohistochemistry in the SGZ of FA- and DE-exposed mice. a BrdU+nuclei indicate surviving cells that been born since the beginning of the experiment. Double-stained NeuN/BrdU cells indicate surviving adult-born neurons, which are expressed both as a total number per brain region (b) and as a percentage of all BrdU-positive cells (c). Results represent the mean (± SE) with n = 3 per group. Significantly different from FA control or between VEH-pre-treatment and PGZ pre-treatment, *p < 0.05, **p < 0.01, ***p < 0.001
Discussion
The main findings of this study are that acute exposure to DE causes an impairment of adult neurogenesis in mice, which is likely secondary to neuroinflammation and oxidative stress that follow microglial activation, and that this effect is more pronounced in male animals. Young adult mice (8 weeks) were used in this study, and the exposure was for 6 h to a moderate/high concentration of DE (250–300-μg/m3 PM2.5). Such levels of PM2.5 can often be reached and even exceeded in several cities worldwide, particularly in India and China (Allen et al. 2013; Kandlikar and Ramachandran 2000; Sun et al. 2004; Costa et al. 2017). Traffic-related air pollution is a major contributor of PM2.5 levels and DE is the major source of PM2.5 and ultrafine particles (Ghio et al. 2012; Calderón-Garcidueñas et al. 2015). In some megacities such as New Delhi, where diesel fuel powers many passenger vehicles in addition to heavy machinery and public transportation conveyances, the contribution of traffic to air pollution is estimated to be as high as 72% (Goyal et al. 2006). The use of diesel fuel to power heavy machinery and vehicles of mass transit results in high daily exposures for those who work in certain occupations; miners and mechanics in bus garages regularly experience some of the very highest levels of particulate air pollution, where PM2.5 may range from 300 to 1000 μg/m3 (Pronk et al. 2009). It should also be noted that DE contains several other components that were not measured in this study, and their potential contribution to neurotoxicity cannot be discounted. In future studies, it would be of much interest to also measure PM deposition in brain over time.
A short-term (6 h) exposure to DE was sufficient to affect adult neurogenesis in different brain regions. We had previously shown that the same DE exposure caused activation of microglia, increased oxidative stress (elevated lipid peroxidation), and neuroinflammation (increased levels of pro-inflammatory cytokines; Cole et al. 2016). These findings were confirmed in the present study, as lipid peroxidation and TNF-α mRNA levels were increased in cerebral cortex and hippocampus of DE-exposed mice (Fig. 6) and hippocampal microglia were activated (Fig. 5). Our earlier findings (Cole et al. 2016) also indicated that male mice are more sensitive to DE-induced oxidative stress and neuroinflammation. We had formulated the hypothesis of a possible higher susceptibility of male mice on the basis of a series of findings related to the levels of expression of the intracellular enzyme paraoxonase-2 (PON2). This enzyme has antioxidant and anti-inflammatory properties (Giordano et al. 2011; Schweikert et al. 2012), and is believed to have a neuroprotective role (Costa et al. 2014b). In vitro and in vivo studies have shown that males express lower levels of PON2 in brain and other tissues (Giordano et al. 2011, 2013); as such, they are more susceptible to oxidative stress and neuroinflammation than female mice (Giordano et al. 2011, 2013; Costa et al. 2014b), though additional mechanisms may also be involved in the differential susceptibility of male and female mice to DE-neurotoxic effects (Cole et al. 2016).
Since it has been shown that microglial activation and increased levels of pro-inflammatory cytokines such as IL-6 or TNF-α impair hippocampal neurogenesis (Ekdahl et al. 2003; Iosif et al. 2006; Vallières et al. 2002), we hypothesized that DE exposure would inhibit adult neurogenesis. Furthermore, based on our previous findings, we also hypothesized that DE-induced inhibition of neurogenesis would be more pronounced in male mice. Results of this study confirm both hypotheses. DE exposure impaired proliferation in the SGZ and SVZ only in male mice (Fig. 1); in the same two regions, adult neurogenesis was also impaired only in males (Figs. 2, 3). In the OB, the total number of adult-born neurons was reduced in males (Fig. 4), and to a minor extent was reduced in females (Fig. 4).
Gender differences in adult neurogenesis have been observed in some studies. Male rodents show higher hippocampal neurogenesis (as we also observed, see Fig. 2), accompanied by better performance in tests of spatial memory (Perfilieva et al. 2001). Anxiety disorders, which are also associated with reduced neurogenesis, are more prevalent and disabling in women than in men (McLean et al. 2011). These differences may, in part, be due to the effect of the differential levels of androgens; indeed, androgens increase adult hippocampal neurogenesis by promoting survival of young neurons in the dentate gyrus (Hamson et al. 2013). Gender differences also appear to extend to the effect of environmental factors on adult neurogenesis; for example, hippocampal neurogenesis is impaired by high-fat diet and stress in male, but not female, rodents (Lindqvist et al. 2006).
To test the hypothesis that inhibition of adult neurogenesis was secondary to microglia activation and ensuing oxidative stress and neuroinflammation, we utilized the PPAR-γ agonist PGZ. PGZ and other thiazolidinediones are recognized for their antidiabetic and hypolipidemic properties (Smith 2001), effects that may be mediated by the modulation of different parameters of mitochondrial function, and the promotion of mitochondrial biogenesis (Smith 2001; Bogacka et al. 2005; Ghosh et al. 2007; Corona and Duchen 2016; Miglio et al. 2009). PGZ also has neuroprotective activities that are ascribed to attenuation of microglial activation (Ji et al. 2010). Indeed, PGZ can reduce inflammation and microglial activation by activating the PPAR-γ of microglia, inhibiting the release of a number of pro-inflammatory substances (Carta and Pisanu 2013; Ji et al. 2010; Drew et al. 2015). We had previously shown that PGZ inhibits the neurotoxicity of DE particles in a microglia-neuron co-culture (Roqué et al. 2016). In the present study, we found that administration of PGZ to male mice antagonizes DE-induced microglial activation, as well as increases in lipid peroxidation and neuroinflammation in the hippocampus and the cerebral cortex (Figs. 5, 6). Upon PGZ pre-treatment, DE-induced inhibition of proliferation and adult neurogenesis in the SGZ was also inhibited (Figs. 7, 8).
The precise mechanisms by which DE exposure may inhibit adult neurogenesis are still elusive, though activation of microglia appears to play a relevant role. Microglia undergoes extensive cytoskeleton remodeling to better carry out certain functions such as phagocytosis (Arcuri et al. 2017; Perry and Teeling 2013). While the default state of microglia is silent vigilance, in which they survey their territory with fine, highly branched processes (Ito et al. 1998), certain chemical signals (e.g., extracellular ATP and subtle changes in concentration of potassium), that may indicate damaged or stressed neurons, can induce chemotaxis, and may activate microglia, triggering them to release TNF-α (Gehrmann et al. 1995; Hide et al. 2000). Reactive microglia have greater cell body area and thicker, shorter processes (Jonas et al. 2012; Morrison and Filosa 2013; Torres-Platas et al. 2014). It should be noted that microglia may either promote or inhibit adult neurogenesis (Sato 2015), depending on the activation state (Aarum et al. 2003; Xu et al. 2017). Notably, the M2 activation state, which is induced by anti-inflammatory cytokines such as IL-4, favors proliferation, and can direct neural progenitor cells (NPC) towards neurogenesis (Cherry et al. 2014; Choi et al. 2017; Zhao et al. 2017). In contrast, the inflammatory cytokines such as interferon-γ and TNFα, as well as oxidative stress, induce the M1 (classical activation) state, provoking killing behavior in microglia and other macrophages (Colton 2009). Thus, the two polarities of microglial activation have drastically different effects upon neurogenesis in the adult brain. Specific assessment of M1 (classical) and M2 (alternative) polarization upon exposure to DE would be useful to elucidate this issue. The mode of microglial activation may promote or inhibit NPC proliferation, cause them to embark upon a glial rather than a neuronal lineage, or favor either survival or apoptosis (Yuan et al. 2017). Another possibility is the phagocytosis of stressed but viable developing neurons by microglia, in a process called “phagoptosis” (Brown and Neher 2014). The ability of PGZ to inhibit both microglial activation and the effects of DE on neurogenesis suggests that this event is critical to DE-induced inhibition of neurogenesis. However, PGZ may also have other mechanisms of neuroprotection (e.g., improving the number and efficiency of mitochondria); hence, additional or alternative mechanisms for the effects of DE on neurogenesis are possible.
Another potential mechanism relates to the ability of traffic-related air pollution to suppress production of brain-derived neurotrophic factor (BDNF) (Bos et al. 2011, 2012). BDNF is a neurotrophin with stimulant effects on proliferation of NSCs, favoring differentiation of NSCs into neuro-blasts rather than glial cells, and promoting the survival of adult-born neurons (Binder and Scharfman 2004). Factors such exercise or dietary polyphenols may increase BDNF signaling, and thus enhance neurogenesis (Sleiman et al., 2016; Zhang et al. 2012). However, the increase in BDNF levels caused by exercise may be abrogated by exposure to particulate air pollution in both human and animal models (Bos et al. 2011, 2012). Increased lipid peroxidation and reduced BDNF resulting from a high-fat diet impair proliferation in the SGZ, and direct treatment of NPCs with malondialdehyde in vitro also significantly reduced proliferation, which was then restored by treatment with BDNF (Park et al. 2010).
Independent of the specific mechanism, suppression of neurogenesis by DE is of interest with regard to the reported reduced cognitive function and depression associated with traffic-related air pollution that has been observed in elderly adults (Chen et al. 2017; Lim et al. 2012; Weuve et al. 2012). Notably, impaired neurogenesis is an early event in the etiology of Alzheimer’s disease (AD) (Demars et al. 2010).In particular, older adults with high levels of exposure to particulate air pollution show poorer cognitive outcomes than non-exposed individuals (Ailshire and Clarke 2015). Aberrant neurogenesis is associated with poor cognitive functioning, and even individuals having characteristic AD neuropathology show no impairment of cognitive functions when neurogenesis is conserved (Briley et al. 2016). These considerations should, however, be tempered by the fact that in the present study, the persistence over time of the effect of DE on neurogenesis was not assessed and that an assessment of cognitive behavior was not carried out. A follow-up of this study should also likely investigate neuroinflammation at timepoints beyond what done here, to determine whether persistent recurrent neuroinflammation remains, or waves of neuroinflammatory responses are seen.
In conclusion, results of this study show that short-term exposures to moderate/high concentrations of DE induce neuroinflammation, oxidative stress, and microglial activation, and suppress adult neurogenesis and cellular proliferation in male mice, as evidenced by a reduced number of Ki67+ cells in the SVZ and SGZ, and a reduced number of surviving NeuN/BrdU double-labeled cells in the OB, SVZ, and SGZ. The effects were significantly less pronounced in female mice. When microglial activation was blocked by pre-treatment with the PPAR-γ agonist PGZ, DE-induced suppression of adult neurogenesis was significantly antagonized. These findings underscore the importance of considering sex/gender when measuring neurotoxic effects, including alterations in neurogenesis, and reinforce the hypothesis that traffic-related air pollution may contribute to cognitive decline and perhaps also neurodegenerative diseases (Cacciottolo et al. 2017).
Acknowledgements
This study was supported in part by grants from the National Institute of Environmental Health Sciences (R01ES22949, R01ES28273, P30ES07033), the National Institute of Child Health and Human Development (U54HD083091), and by funds from the Department of Environmental and Occupational Health Sciences, University of Washington. Thanks are due to Mr. James Stewart, who oversaw diesel exhaust exposures, the members of Dr. Lucio G. Costa’s and Dr. Zhengui Xia’s laboratory for their assistance in troubleshooting and helpful discussions, and Dr. Jennifer Stone and Mr. Glen McDonald at the Center on Human Development and Disability for their help with fluorescence microscopy and immunohistochemistry.
Footnotes
Compliance with ethical standards
Ethical statement C57BL6/J mice of both sexes were used in these studies. All animal procedures were pre-approved by the University of Washington Institutional Animal Care and Use Committee (IACUC), Protocol 2077-14. Experiments were carried out in accordance with the National Research Council Guide for the Care and Use of Laboratory Animals, as adopted by the National Institutes of Health.
Conflict of interest The authors declare that they do not have any conflict of interest.
References
- Aarum J, Sandberg K, Haeberlein SLB, Persson MAA (2003) Migration and differentiation of neural precursor cells can be directed by microglia. Proc Natl Acad Sci 100:15983–15988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ailshire JA, Clarke P (2015) Fine particulate matter air pollution and cognitive function among US older adults. J Gerontol B Psychol Sci Soc Sci 70:322–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen RW, Gombojav E, Barkhasragchaa B, Byambaa T, Lkhasuren O, Amram O, Takaro TK, Janes CR (2013) An assessment of air pollution and its attributable mortality in Ulaanbaatar, Mongolia. Air Qual Atmosphere Health 6:137–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez-Buylla A, García-Verdugo JM, Tramontin AD (2001) A unified hypothesis on the lineage of neural stem cells. Nat Rev Neurosci 2:287–293 [DOI] [PubMed] [Google Scholar]
- Arcuri C, Mecca C, Bianchi R, Giambanco I, Donato R (2017) The pathophysiological role of microglia in dynamic surveillance, phagocytosis and structural remodeling of the developing CNS. Front Mol Neurosci 10:191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baudic S, Barba GD, Thibaudet MC, Smagghe A, Remy P, Traykov L (2006) Executive function deficits in early Alzheimer’s disease and their relations with episodic memory. Arch Clin Neuropsychol 21:15–21 [DOI] [PubMed] [Google Scholar]
- Binder DK, Scharfman HE (2004) Brain-derived neurotrophic factor. Growth Factors Chur Switz 22:123–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischofberger J (2007) Young and excitable: new neurons in memory networks. Nat Neurosci 10:273–275 [DOI] [PubMed] [Google Scholar]
- Bishop NA, Lu T, Yankner BA (2010) Neural mechanisms of ageing and cognitive decline. Nature 464:529–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogacka I, Xie H, Bray GA, Smith SR (2005) Pioglitazone induces mitochondrial biogenesis in human subcutaneous adipose tissue in vivo. Diabetes 54:1392–1399 [DOI] [PubMed] [Google Scholar]
- Bos I, Jacobs L, Nawrot TS, de Geus B, Torfs R, Panis I, Degraeuwe LB, and Meeusen R (2011) No exercise-induced increase in serum BDNF after cycling near a major traffic road. Neurosci Lett 500:129–132 [DOI] [PubMed] [Google Scholar]
- Bos I, De Boever P, Int Panis L, Sarre S, Meeusen R (2012) Negative effects of ultrafine particle exposure during forced exercise on the expression of brain-derived neurotrophic factor in the hippocampus of rats. Neuroscience 223:131–139 [DOI] [PubMed] [Google Scholar]
- Briley D, Ghirardi V, Woltjer R, Renck A, Zolochevska O, Taglialatela G, Micci M-A (2016) Preserved neurogenesis in non-demented individuals with AD neuropathology. Sci Rep 6:27812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown GC, Neher JJ (2014) Microglial phagocytosis of live neurons. Nat Rev Neurosci 15:209–216 [DOI] [PubMed] [Google Scholar]
- Cacciottolo M, Wang X, Driscoll I, Woodward N, Saffari A, Reyes J et al. (2017) Particulate air pollutants, APOE alleles and their contributions to cognitive impairment in older women and to amyloidogenesis in experimental models. Transl Psychiat. 7, e1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Mora-Tiscareño A, Ontiveros E, Gómez-Garza G, Barragán-Mejía G, Broadway J, Chapman S, Valencia-Salazar G, Jewells V, Maronpot RR et al. (2008) Air pollution, cognitive deficits and brain abnormalities: a pilot study with children and dogs. Brain Cogn 68:117–127 [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Kavanaugh M, Block M, D’Angiulli A, Delgado-Chávez R, Torres-Jardón R, González-Maciel A, Reynoso-Robles R, Osnaya N, Villarreal-Calderon R et al. (2012) Neuroinflammation, hyperphosphorylated tau, diffuse amyloid plaques, and down-regulation of the cellular prion protein in air pollution exposed children and young adults. J Alzheimers Dis 28:93–107 [DOI] [PubMed] [Google Scholar]
- Calderón-Garcidueñas L, Kulesza RJ, Doty RL, D’angiulli A, Torres-Jardon R (2015) megacities air pollution problems: Mexico City Metropolitan Area critical issues on the central nervous system pediatric impact. Environ Res 137:157–169 [DOI] [PubMed] [Google Scholar]
- Carpentier PA, Palmer TD (2009) Immune influence on adult neural stem cell regulation and function. Neuron 64:79–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta AR, Pisanu A (2013) Modulating microglia activity with PPAR-γ agonists: a promising therapy for Parkinson’s disease? Neurotox Res 23:112–123 [DOI] [PubMed] [Google Scholar]
- Chen H, Kwong JC, Copes R, Tu K, Villeneuve PJ, van Donkelaar A, Hystad P, Martin RV, Murray BJ, Jessiman B et al. (2017) Living near major roads and the incidence of dementia, Parkinson’s disease, and multiple sclerosis: a population-based cohort study. Lancet 389:718–726 [DOI] [PubMed] [Google Scholar]
- Cherry JD, Olschowka JA, O’Banion MK (2014) Neuroinflammation and M2 microglia: the good, the bad, and the inflamed. J Neuroinflammation 11:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JY, Kim JY, Kim JY, Park J, Lee WT, Lee JE (2017) M2 phenotype microglia-derived cytokine stimulates proliferation and neuronal differentiation of endogenous stem cells in ischemic brain. Exp Neurobiol 26:33–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole TB, Coburn JL, Dao K, Roque PJ (2016) Sex and genetic differences in the effects of acute diesel exhaust exposure on inflammation and oxidative stress in mouse brain. Toxicology 374:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colton CA (2009) Heterogeneity of microglial activation in the innate immune response in the brain. J Neuroimmune Pharmacol 4:399–418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona JC, Duchen MR (2016) PPARγ as a therapeutic target to rescue mitochondrial function in neurological disease. Free Radic Biol Med 100:153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG (2017) Traffic-related air pollution and neurodegenerative diseases: epidemiological and experimental evidence In: Aschner M Costa LG (eds) In: Advances in neurotoxicology, vol 1,. Elsevier, New York, pp 1–47 [Google Scholar]
- Costa LG, Cole TB, Coburn J, Chang Y-C, Dao K, Roque P (2014a). Neurotoxicants are in the air: convergence of human, animal, and in vitro studies on the effects of air pollution on the brain. BioMed Res Int. 736385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, de Laat R, Dao K, Pellacani C, Cole TB, Furlong CE (2014b) Paraoxonase-2 (PON2) in brain and its potential role in neuroprotection. Neurotoxicology 43:3–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa LG, Cole TB, Coburn J, Chang Y-C, Dao K, Roqué PJ (2017) Neurotoxicity of traffic-related air pollution. Neurotoxicology 59:133–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demars M, Hu Y-S, Gadadhar A, Lazarov O (2010) Impaired neurogenesis is an early event in the etiology of familial Alzheimer’s disease in transgenic mice. J Neurosci Res 88:2103–2117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew PD, Johnson JW, Douglas JC, Phelan KD, Kane CJM (2015) Pioglitazone blocks ethanol induction of microglial activation and immune responses in the hippocampus, cerebellum, and cerebral cortex in a mouse model of fetal alcohol spectrum disorders. Alcohol Clin Exp Res 39:445–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekdahl CT, Claasen J-H, Bonde S, Kokaia Z, Lindvall O (2003) Inflammation is detrimental for neurogenesis in adult brain. Proc Natl Acad Sci 100:13632–13637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JR, Cox DP, Drury BE, Gould TR, Kavanagh TJ, Paulsen MH, Sheppard L, Simpson CD, Stewart JA, Larson TV et al. (2015) Chemical characterization and in vitro toxicity of diesel exhaust particulate matter generated under varying conditions. Air Qual Atmos Health 8:507–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankland PW, Miller FD (2008) Regenerating your senses: multiple roles for neurogenesis in the adult brain. Nat Neurosci 11:1124–1126 [DOI] [PubMed] [Google Scholar]
- Gehrmann J, Matsumoto Y, Kreutzberg GW (1995) Microglia: Intrinsic immuneffector cell of the brain. Brain Res Rev 20:269–287 [DOI] [PubMed] [Google Scholar]
- Genc S, Zadeoglurari Z, Fuss SH, Genc K (2012) The adverse effects of air pollution on the nervous system. J Toxicol 23:ID782472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghio AJ, Smith CB, Madden MC (2012) Diesel exhaust particles and airway inflammation. Curr Op Pulm Med 18:144–150 [DOI] [PubMed] [Google Scholar]
- Ghosh S, Patel N, Rahn D, McAllister J, Sadeghi S, Horwitz G, Berry D, Wang KX, Swerdlow RH (2007) The Thiazolidinedione pioglitazone alters mitochondrial function in human neuron-like cells. Mol Pharmacol 71:1695–1702 [DOI] [PubMed] [Google Scholar]
- Giordano G, Cole TB, Furlong CE, Costa LG (2011) Paraoxonase-2 (PON2) in the central nervous system: a neuroprotective role. Toxicol Appl Pharmacol 256:369–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giordano G, Tait L, Furlong CE, Cole TB, Kavanagh TJ, Costa LG (2013) Gender differences in brain susceptibility to oxidative stress are mediated by levels of paraoxonase 2 (PON2) expression. Free Radic Biol Med 58:98–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould T, Larson T, Stewart J, Kaufman JD, Slater D, McEwen N (2008) A controlled inhalation diesel exhaust exposure facility with dynamic feedback control of PM concentration. Inhal Toxicol 20:49–52 [DOI] [PubMed] [Google Scholar]
- Goyal SK, Ghatge SV, Nema P, Tamhane SM (2006) Understanding urban vehicular pollution problem vis-a-vis ambient air quality—case study of a megacity (Delhi, India). Environ Monit Assess 119:557–569 [DOI] [PubMed] [Google Scholar]
- Hamson DK, Wainwright SR, Taylor JR, Jones BA, Watson NV, Galea LaM (2013) Androgens increase survival of adult-born neurons in the dentate gyrus by an androgen receptor-dependent mechanism in male rats. Endocrinology 154:3294–3304 [DOI] [PubMed] [Google Scholar]
- Hayley S, Litteljohn D (2013) Neuroplasticity and the next wave of antidepressant strategies. Front Cell Neurosci 7:218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hide I, Tanaka M, Inoue A, Nakajima K, Kohsaka S, Inoue K, Nakata Y (2000) Extracellular ATP triggers tumor necrosis factor-α release from rat microglia. J Neurochem 75:965–972 [DOI] [PubMed] [Google Scholar]
- Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJH, Bonde S, Kokaia Z, Jacobsen S-EW, Lindvall O (2006) Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J Neurosci 26:9703–9712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S (1998) Microglia-specific localisation of a novel calcium binding protein, Iba1. Mol Brain Res 57:1–9 [DOI] [PubMed] [Google Scholar]
- Ji H, Wang H, Zhang F, Li X, Xiang L, Aiguo S (2010) PPARγ agonist pioglitazone inhibits microglia inflammation by blocking p38 mitogen-activated protein kinase signaling pathways. Inflamm Res 59:921–929 [DOI] [PubMed] [Google Scholar]
- Jonas RA, Yuan T-F, Liang Y- X, Jonas JB, Tay DKC, Ellis-Behnke RG (2012) The spider effect: morphological and orienting classification of microglia in response to stimuli in vivo. PLoS One 7:e30763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongbloets BC, Lemstra S, Schellino R, Broekhoven MH, Parkash J, Hellemons AJCGM, Mao T, Giacobini P, van Praag H, De Marchis S et al. (2017) Stage-specific functions of Semaphorin7A during adult hippocampal neurogenesis rely on distinct receptors. Nat Commun 8:14666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandlikar M, Ramachandran G (2000) The causes and consequences of particulate air pollution in urban India: a synthesis of the science. Annu Rev Energy Environ 25:629–684 [Google Scholar]
- Levesque S, Taetzsch T, Lull ME, Kodavanti U, Stadler K, Wagner A, Johnson JA, Duke L, Kodavanti P, Surace MJ et al. (2011) Diesel exhaust activates and primes microglia: air pollution, neuroinflammation, and regulation of dopaminergic neurotoxicity. Environ Health Perspect 119:1149–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim Y-H, Kim H, Kim JH, Bae S, Park HY, Hong Y- C (2012) Air pollution and symptoms of depression in elderly adults. Environ Health Perspect 120:1023–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist A, Mohapel P, Bouter B, Frielingsdorf H, Pizzo D, Brundin P, Erlanson-Albertsson C (2006) High-fat diet impairs hippocampal neurogenesis in male rats. Eur J Neurol 13:1385–1388 [DOI] [PubMed] [Google Scholar]
- Maeda T, Kiguchi N, Kobayashi Y, Ozaki M, Kishioka S (2008) Pioglitazone attenuates tactile allodynia and thermal hyperalgesia in mice subjected to peripheral nerve injury. J Pharmacol Sci 108:341–347 [DOI] [PubMed] [Google Scholar]
- McLean CP, Asnaani A, Litz BT, Hofmann SG (2011) Gender differences in anxiety disorders: prevalence, course of illness, comorbidity and burden of illness. J Psychiatr Res 45:1027–1035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miglio G, Rosa AC, Rattazzi L, Collino M, Lombardi G, Fantozzi R (2009) PPARgamma stimulation promotes mitochondrial biogenesis and prevents glucose deprivation-induced neuronal cell loss. Neurochem Int 55:496–504 [DOI] [PubMed] [Google Scholar]
- Ming G, Song H (2011) Adult neurogenesis in the mammalian brain: significant answers and significant questions. Neuron 70:687–702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks PS, Granier C, Fuzzi S, Stohl A, Williams ML, Akimoto H, Amann M, Baklanov A, Baltensperger U, Bey I et al. (2009) Atmospheric composition change—global and regional air quality. Atmos Environ 43:5268–5350 [Google Scholar]
- Morrison HW, Filosa JA (2013) A quantitative spatiotemporal analysis of microglia morphology during ischemic stroke and reperfusion. J Neuroinflammation 10:782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberdörster G, Sharp Z, Atudorei V, Elder A, Gelein R, Kreyling W, Cox C (2004) Translocation of inhaled ultrafine particles to the brain. Inhal Toxicol 16:437–445 [DOI] [PubMed] [Google Scholar]
- Pan Y-W, Zou J, Wang W, Sakagami H, Garelick MG, Abel G, Kuo CT, Storm DR, Xia Z (2012) Inducible and conditional deletion of extracellular signal-regulated kinase 5 disrupts adult hippocampal neurogenesis. J Biol Chem 287:23306–23317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y-W, Wang W, Xia Z (2013a) Assessment of adult neurogenesis in mice. Curr Protoc Toxicol 12:12.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y-W, Storm DR, Xia Z (2013b) Role of adult neurogenesis in hippocampus-dependent memory, contextual fear extinction and remote contextual memory: new insights from ERK5 MAP Kinase. Neurobiol Learn Mem 105:81–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HR, Park M, Choi J, Park K-Y, Chung HY, Lee J (2010) A high-fat diet impairs neurogenesis: involvement of lipid peroxidation and brain-derived neurotrophic factor. Neurosci Lett 482:235–239 [DOI] [PubMed] [Google Scholar]
- Perfilieva E, Risedal A, Nyberg J, Johansson BB, Eriksson PS (2001) Gender and strain influence on neurogenesis in dentate gyrus of young rats. J Cereb Blood Flow Metab 21:211–217 [DOI] [PubMed] [Google Scholar]
- Perry VH, Teeling J (2013) Microglia and macrophages of the central nervous system: the contribution of microglia priming and systemic inflammation to chronic neurodegeneration. Semin Immunopathol 35:601–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignatelli A, Belluzzi O (2010) Neurogenesis in the adult olfactory bulb In: Menini A (ed) The neurobiology of olfaction. CRC Press/Taylor & Francis), Boca Raton (FL) (Chap. 11). [PubMed] [Google Scholar]
- Pronk A, Coble J, Stewart P (2009) Occupational exposure to diesel engine exhaust: a literature review. J Expo Sci Environ Epidemiol 19:443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavendra V, Tanga FY, DeLeo JA (2004) Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci 20:467–473 [DOI] [PubMed] [Google Scholar]
- Roqué PJ, Dao K, Costa LG (2016). Microglia mediate diesel exhaust particle-induced cerebellar neuronal toxicity through neuroinflammatory mechanisms. NeuroToxicology 56, 204–214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K (2015) Effects of microglia on neurogenesis. Glia 63:1394–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang J-W, Malleret G, David DJ, Monckton JE, Garcia ADR, Sofroniew MV, Kandel ER, Santarelli L et al. (2006) Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci 103:17501–17506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuitemaker A, van der Doef TF, Boellaard R, van der Flier WM, Yaqub M, Windhorst AD, Barkhof F, Jonker C, Kloet RW (2012) Microglial activation in healthy aging. Neurobiol Aging 33:1067–1072 [DOI] [PubMed] [Google Scholar]
- Schweikert EM, Amort J, Wilgenbus P, Forsetrman U, teiber JF, Horke S (2012) Paraoxonases-2 and −3 are important defense enzymes against Pseudomonas aeruginosa virulence factors due to their anti-oxidative and anti-inflammatory properties. J Lipids 2012:352857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith U (2001) Pioglitazone: mechanism of action. Int J Clin Pract Suppl 121:13–18 [PubMed] [Google Scholar]
- Sun Y, Zhuang G, Wang Y, Han L, Guo J, Dan M, Zhang W, Wang Z, Hao Z (2004) The air-borne particulate pollution in Beijing—concentration, composition, distribution and sources. Atmos Environ 38:5991–6004 [Google Scholar]
- Takeshita Y, Ransohoff RM (2012) Inflammatory cell trafficking across the blood-brain barrier (BBB): chemokine regulation and in vitro models. Immunol Rev 248:228–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin P (2007) BrdU immunohistochemistry for studying adult neurogenesis: paradigms, pitfalls, limitations, and validation. Brain Res Rev 53:198–214 [DOI] [PubMed] [Google Scholar]
- Torres-Platas SG, Comeau S, Rachalski A, Bo GD, Cruceanu C, Turecki G, Giros B, Mechawar N (2014) Morphometric characterization of microglial phenotypes in human cerebral cortex. J Neuroinflammation 11:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallières L, Campbell IL, Gage FH, Sawchenko PE (2002) Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. J Neurosci 22:486–492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weuve J, Puett RC, Schwartz J, Yanosky JD, Laden F, Grodstein F (2012) Exposure to particulate air pollution and cognitive decline in older women. Arch Intern Med 172:219–227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Wang Z, Li J, Wu H, Peng Y, Fan L, Chen J, Gu C, Yan F, Wang L et al. (2017). The polarization states of microglia in TBI: a new paradigm for pharmacological intervention. Neural Plast, 5405104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Ge H, Liu W, Zhu H, Chen Y, Zhang X, Yang Y, Yin Y, Chen W, Wu W et al. (2017) M2 microglia promotes neurogenesis and oligodendrogenesis from neural stem/progenitor cells via the PPARγ signaling pathway. Oncotarget 8:19855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Wang Y-Y, Liu H, Lu Y-F, Wu Q, Liu J, Shi J-S (2012). Resveratrol produces neurotrophic effects on cultured dopaminergic neurons through prompting astroglial BDNF and GDNF release. Evid BAsed Complement Alternat Med. 937605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S, Ma L, Chu Z, Xu H, Wu W, Liu F (2017) Regulation of microglial activation in stroke. Acta Pharmacol Sin 38:445–458 [DOI] [PMC free article] [PubMed] [Google Scholar]


