Highlights
-
•
There has been no consensus on the optimal therapy for isolated superior mesenteric arterial dissection (ISMAD).
-
•
ISMAD patients can be treated conservatively if there are no signs of an aneurysm, ulcer-like projection (ULP), and mesenteric ischemia.
-
•
When an aneurysm or ULP sign exists, endovascular stenting was able to preserve superior mesenteric artery blood flow with improvement of the dissection.
Abbreviations: CECT, contrast-enhanced computed tomography; ISMAD, isolated superior mesenteric arterial dissection; SMA, superior mesenteric artery; ULP, ulcer-like projection
Keywords: Dissection, Mesenteric artery, Stents
Abstract
Objectives
Isolated superior mesenteric arterial dissection (ISMAD) is an uncommon type of arterial dissection and treated with surgery, stenting, or conservative management. This study aimed to evaluate the criteria for conservative therapy for ISMAD patients based on imaging findings.
Methods
Eighteen consecutive ISMAD patients without peritoneal irritation at onset were retrospectively studied. The decision to perform stenting was based on the emergence of peritoneal irritation, aneurysm, or mesenteric ischemia. Clinical manifestations, follow-up contrast-enhanced computed tomography (CECT) findings, and patient outcome were evaluated.
Results
Most patients (16, 89%) were successfully treated conservatively; two patients (11%) required endovascular stenting because of an aneurysm or ulcer-like projection (ULP) sign. The median duration of fasting and hospital stays was 3 (range, 1–8) and 9 (range, 4–34) days, respectively. On CECT, the median distance from the superior mesenteric artery (SMA) origin to the entry site was 12 mm (range, 5–35 mm), and the median length of dissection was 87.5 mm (range, 20–150 mm). Among 16 patients treated conservatively, serial imaging was obtained in 11 patients (69%), and disappearance of the dissection within 4 months occurred in five patients. Two patients treated with endovascular stent underwent follow-up CECT 1 year after onset, and there were no complications.
Conclusions
ISMAD patients without peritoneal irritation can be treated conservatively if there are no signs of an aneurysm, ULP, or mesenteric ischemia. When an aneurysm or ULP sign exists, endovascular stenting was able to preserve SMA blood flow with the improvement of the dissection.
1. Introduction
Isolated superior mesenteric arterial dissection (ISMAD) is an uncommon arterial event first reported by Bauersfeld et al. in 1947 [1]. Foord et al. reported an ISMAD incidence of 0.06% in autopsy cases [2]. There are various treatment options for ISMAD including surgery, endovascular stenting, and conservative management. Although most cases with mild symptoms can be successfully managed with conservative regimens [[3], [4], [5], [6]], endovascular stenting [[7], [8], [9], [10], [11], [12]] and surgical intervention [13] may be required. Invasive treatment is highly recommended in patients with the following characteristics: abdominal pain lasting for more than 5–7 days [7,8,12,13], true lumen occlusion greater than 80% [7], aneurysm dilatation more than 20 mm [7], signs of ruptured aneurysm [14] or mesenteric ischemia [13,14], and evidence of peritonitis [8,10].
Thus far, there has been no consensus on the optimal therapy for ISMAD, and its management is highly dependent on physician experience. The aim of this single-institution, retrospective study was to assess the border of conservative treatment and endovascular treatment. Herein, we report a consecutive series of 18 ISMAD cases and discuss therapeutic strategies based on clinical manifestations and contrast-enhanced computed tomography (CECT) findings.
2. Materials and methods
Ethical approval for the study was obtained from the institutional review board at our hospital (#TGE00991-012), and participants provided informed consent. From January 2009 to December 2017, 19 patients were diagnosed with ISMAD by CECT at Sapporo Higashi Tokushukai Hospital. One patient who was not hospitalized at our institution due to a lack of vacancy was excluded. Finally, we retrospectively reviewed 18 patients. All patients were treated conservatively following the diagnosis. Patient characteristics such as past and current medical history and manifestations were assessed.
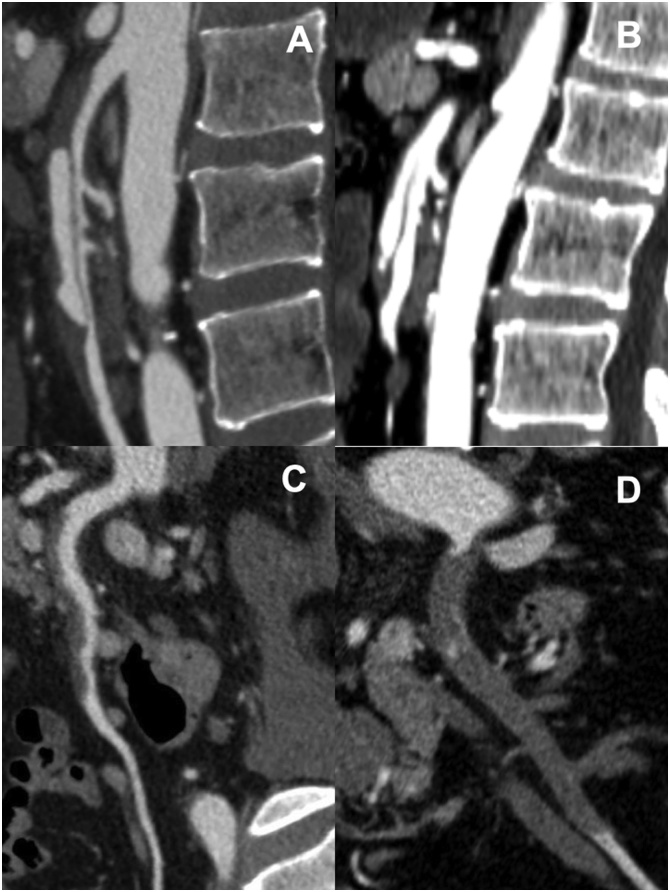
In the analysis of CECT data, distance from the superior mesenteric artery (SMA) origin to the entry site and length of the dissection were measured on both axial and sagittal views. The presence of an aneurysm or ulcer-like projection (ULP) sign [9], which is defined as a small blood sac sticking out from the true lumen into the thrombosed false lumen, was determined using the sagittal view. ISMAD cases were categorized into four types according to Yun’s classification (Fig. 1) [4], which consists of simple and commonly used criteria [15]. Three radiologists evaluated all CECT images.
Fig. 1.
Contrast-enhanced computed tomography findings of isolated superior mesenteric arterial dissection. A. Type I, patent true and false lumen revealing entry and re-entry sites. B. Type IIa, patent true and false lumen without re-entry. C. Type IIb, patent true lumen but thrombosed false lumen. D. Type III, superior mesenteric artery occlusion.
Conservative treatment included bowel rest and fluid resuscitation but not antiplatelet or antithrombotic therapy. This is because many authors concluded that antiplatelet or antithrombotic therapy was not effective [4,7,13,16]. After the disappearance of abdominal pain, water intake was permitted. Antihypertensive treatment was administered to patients who had hypertension to reduce systolic blood pressure below 120 mmHg. Follow-up CECT (1 week, 2 weeks, 1 month, 6 months, and 1 year after onset) was planned for all 18 patients. In cases involving an endovascular procedure, to prevent a stent stenosis, antiplatelet therapy with clopidogrel 75 mg per day for 1 month and aspirin 100 mg per day for 6 months postoperatively was prescribed.
3. Results
Clinical characteristics of the 18 patients are summarized in Table 1. All patients were men, with a median age of 51 years (range, 42–66 years). The rates of smoking history (13 patients, 72%) and hypertension (12 patients, 67%) were high. All patients complained of abdominal pain without peritoneal irritation symptoms such as muscular defense or rebound tenderness, and six patients (33%) had back pain. The patients were classified into four types according to Yun’s classification [4]: type I (3, 17%), IIa (1, 6%), IIb (10, 56%), and III (4, 22%). The median distance from the SMA origin to the entry site was 12 mm (range, 5–35 mm), and the median length of dissection was 87.5 mm (range, 20–150 mm).
Table 1.
Patient characteristics and clinical features.
| Features (n = 18) | ||
|---|---|---|
| Median age (range, years) | 51 (42-66) | |
| Male | 18 (100%) | |
| Coecisting medical conditions | ||
| Smoking | 13 (72.2%) | |
| Hypertension | 12 (66.7%) | |
| Dyslipidemia | 4 (22.2%) | |
| Diabetes mellitus | 1 (5.6%) | |
| Symptoms | ||
| Sudden onset | 14 (77.8%) | |
| Insidious onset | 4 (22.2%) | |
| Upper abdominal pain | 18 (100%) | |
| Back pain | 6 (33.3%) | |
| Other symptoms | ||
| Vomiting | 1 (5.6%) | |
| Diarrhea | 2 (11.1%) | |
| Bloody stool | 1 (5.6%) | |
| Treatments | ||
| Conservation only | 16 (88.9%) | |
| Conservation and stenting | 2 (11.1%) | |
| Median time | ||
| Symptoms duration (range, hr) | 29.5 (6-190) | |
| Fasting time (range, day) | 3 (1-8) | |
| Length of stay (range, day) | 9 (4-34) | |
| ISMAD features on CECT (Yun's classification) | ||
| I | 3 (16.7%) | |
| IIa | 1(5.6%) | |
| IIb | 10 (55.6%) | |
| III | 4 (22.2%) | |
| Median distance from the SMA origin to the entry site (range, mm) | 12 (5-35) | |
| Median length of dissection (range, mm) | 87.5 (20-150) | |
ISMAD, isolated superior mesenteric arterial dissection.
CECT, contrast-enhanced computed tomography.
SMA, superior mesenteric artery.
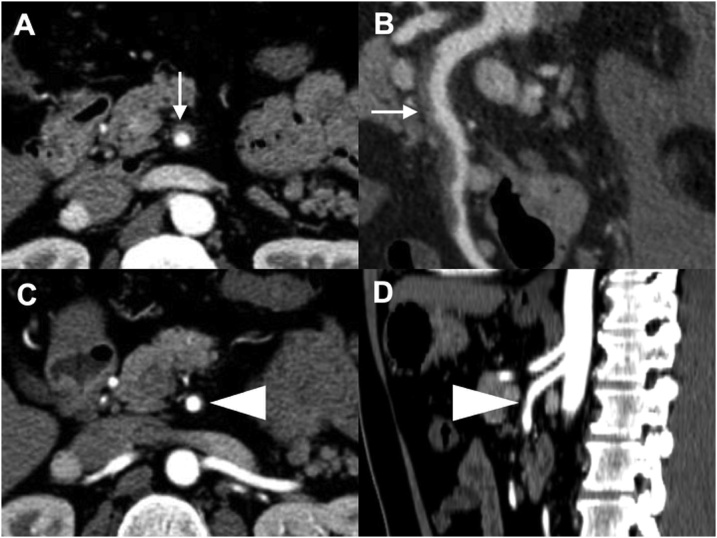
The median duration of abdominal pain was 29.5 (range, 6–190) hours. The median durations of fasting and hospital stay were 3 (range, 1–8) and 9 (range, 4–34) days, respectively. Among 16 cases treated conservatively, serial imaging was obtained in 11 cases (69%) during 4–116 months of follow-up, and complete disappearance of the dissection was observed in 5 cases, all type IIb, within 4 months (Fig. 2, Table 2).
Fig. 2.
(Case no. 12) Type IIb isolated superior mesenteric arterial dissection (ISMAD). A & B. Contrast-enhanced computed tomography showing ISMAD with patent true lumen and thrombosed false lumen at the time of onset (white arrows). C & D. The thrombosed false lumen had completely disappeared 82 days later (white arrowheads).
Table 2.
Follow-up CECT results of 11 patients after conservative treatment.
| Yun's classification | Before treatment (n = 11) | Complete disappearance (n = 5) |
|---|---|---|
| I | 2 | NA |
| IIa | 0 | NA |
| IIb | 7 | 5 |
| III | 2 | NA |
CECT, contrast-enhanced computed tomography.
NA, not available.
Cases observed by serial CECT imaging over more than 4 months.
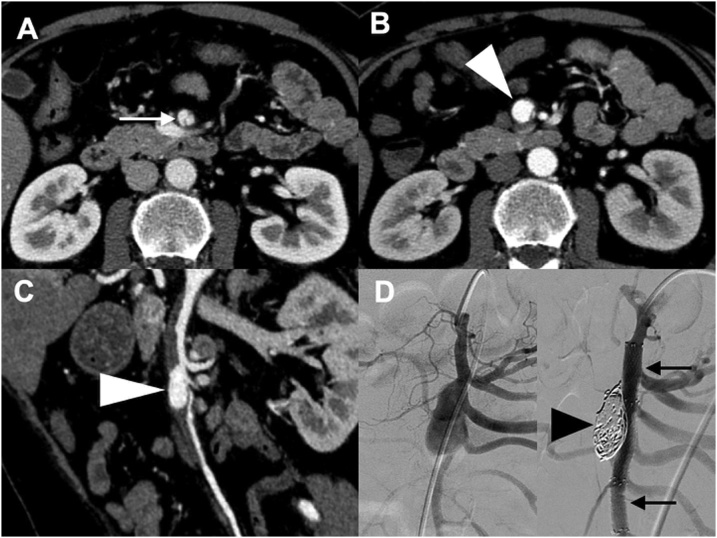
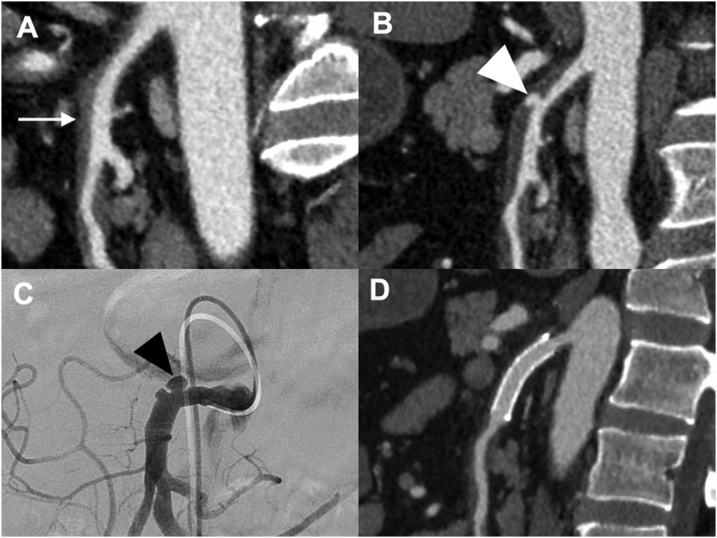
Two patients (11%) newly developed an aneurysm or ULP sign and consequently underwent endovascular stenting. One patient had type IIa ISMAD associated with a newly developed 15 mm × 9 mm aneurysm at day 11. The aneurysm increased in size, reaching 21 mm × 14 mm at day 23. Considering the risk of rupture [9,17], we performed endovascular treatment followed by antiplatelet therapy. Following two stenting procedures on the SMA via femoral access, we performed coil embolization by inserting a micro-catheter from a gap of the stent into the aneurysm (Fig. 3 and Table 3). Another patient had type IIb ISMAD with a newly developed 4 mm × 2 mm ULP sign at day 10. At day 24, it increased to 8 mm × 4 mm in size. We therefore performed endovascular treatment on the SMA through a trans-femoral artery approach followed by antiplatelet therapy (Fig. 4 and Table 3). In both patients, endovascular treatment succeeded, and the aneurysm was thrombosed and ULP sign disappeared. Postoperatively, complications or recurrence did not occur during the 14- and 12-month follow-up periods. Mesenteric ischemia did not occur in any patients during the follow-up period.
Fig. 3.
(Case no. 17) Contrast-enhanced computed tomography and angiography images of a patient with type IIa isolated superior mesenteric arterial dissection. A. Axial view at day 1. The white arrow indicates patent false lumen. B. An aneurysm emerged at day 11 (white arrowhead). C. At day 23, the aneurysm size increased during conventional therapy (white arrowhead). D. Angiography image of the aneurysm before (left panel) and after treatment (right panel; black arrows indicate two stents and black arrowhead indicates the endovascular coil for embolization).
Table 3.
Stents and coils used at the endovascular treatment.
| Case | Stent | Coil |
|---|---|---|
| No.17 | LIFE STENT φ 6 mm × 40 mm (1) | Target coil 14 mm ×50 cm (1) |
| SMART CONTROL STENT φ 6 mm × 60 mm (1) | Target coil 10 mm ×40 cm (1) | |
| Target coil 9 mm ×30 cm (2) | ||
| Target coil 7 mm ×20 cm (1) | ||
| No.18 | INNOVA STENT φ 8 mm × 40 mm | no coils |
Fig. 4.
(Case no. 18) Contrast-enhanced computed tomography (CECT) and angiography images of a patient with type IIb isolated superior mesenteric arterial dissection. A. Sagittal view at day 1. The white arrow indicates thrombosed false lumen. B. CECT indicated an ulcer-like projection (ULP) sign at day 24 (white arrowhead). C. Angiography showed ULP sign at day 28 (black arrowhead). D. CECT was performed 1 month later after endovascular stenting. The ULP sign had disappeared.
4. Discussion
In our retrospective study, most patients (16, 89%) were successfully treated conservatively; two patients (11%) required endovascular stenting because of an aneurysm or ULP sign. All 18 patients were alive and had no complications during the follow-up period.
ISMAD may be complicated by aneurysms [7,12,14,16,18,19], ruptured aneurysms [9,16,20], mesenteric ischemia [9], and mesenteric necrosis [13,16]; therefore, several treatments may be considered. As the primary treatment for symptomatic ISMAD, some authors have recommended conservative treatment [[3], [4], [5], [6],21], while others endovascular treatment [16,[22], [23], [24]]. However, there is no consensus regarding decision-making for the appropriate therapy.
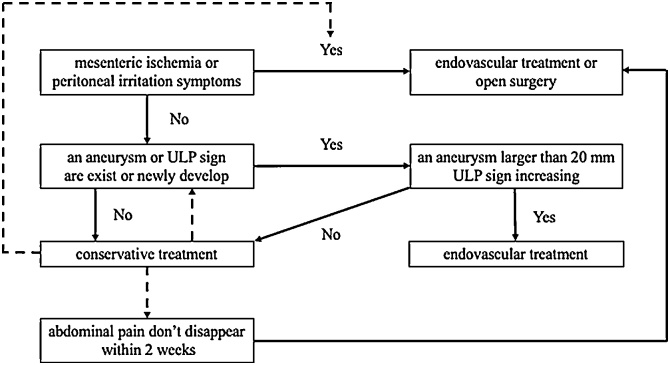
In our series, with conservative management, abdominal pain disappeared within 8 days in all patients. Another study of 27 patients demonstrated that abdominal pain could be relieved by conservative therapy within 2 weeks [5]. Thus, these studies indicate that conservative treatment may be appropriate for 2 weeks in cases without an aneurysm or ULP sign, mesenteric ischemia, or peritoneal irritation. We therefore propose an algorithm for decision-making in patients with ISMAD based on symptoms and radiographic appearance (Fig. 5); patients without mesenteric ischemia or peritoneal irritation can be treated conservatively, whereas presence of aneurysm or ULP sign requires endovascular stenting. Invasive treatment may be useful in cases involving an aneurysm larger than 20 mm or ULP sign.
Fig. 5.
Algorithm of the treatment for isolated superior mesenteric arterial dissection.
Even cases with true lumen occlusion exceeding 80% are not always candidates for invasive treatment. Kim et al. reported increased stenosis of the true lumen 1 week after the diagnosis of ISMAD in 16 patients including 9 with SMA occlusion, all of whom were treated conservatively [5]. Another study demonstrated that conservative treatment could be applied even in cases involving complete compression of the true lumen by the false lumen, as long as distal blood flow was preserved [6]. In our series, although the true lumen was completely occluded in four type III patients, the distal blood flow was maintained, and these patients were treated conservatively. Hence, conservative treatment may be possible as long as distal blood flow can be detected, even if stenosis of the true lumen is severe.
Luan et al. considered endovascular stenting the best option for ISMAD because it could provide immediate symptom relief with shorter fasting time and good long-term results [16]. A significant benefit of endovascular stenting was also demonstrated by several other reports (Table 4) [7,10,12]. However, negative effects of the interventional approach, such as failure to place the stent in the right position [8,10] and late restenosis [4,13,22], may emerge. Therefore, although stenting has a significant therapeutic effect, it requires long-term antiplatelet administration, resulting in a high medical cost [6].
Table 4.
Comparison of fasting time and length of hospital stay between conservative treatment and endovascular stenting.
| Authors | Conservative treatment successfulas a primary treatment | Endovascular stenting successful as a primary treatment |
|
|---|---|---|---|
| Min et al. [9] | n | 7 | 4 |
| Median fasting time (range, days) | 8.0 (2–18) | 2.5 (1–4) | |
| Pang et al. [12] | n | 3 | 7 |
| Median fasting time (range, days) | 9 | 3 | |
| Median length of stay (range, days) | 14 | 5.5 | |
| Jia et al. [14] | n | 12 | 3 |
| Median fasting time (range, days) | 8.5 (4–14) | 3.5 (2–5) |
n, number of patients.
Follow-up is necessary not only during the acute stage but also in the long term. There have been reports of dissection disappearance [8,9,13,25], which occurred more often in thrombosed false lumen than in patent false lumen [9,13,25]. Park et al. found that most dissection disappearance on CT occurred within 6 months [13]. In our series, ISMAD completely disappeared by conservative treatment in five type IIb patients within 4 months. On the contrary, there have been cases of new aneurysm development 4 months later [19] and of the development of bowel necrosis after 6 months that was treated with open surgery [13], suggesting a requirement of careful surveillance even after symptom relief.
This study has several limitations. First, it is a retrospective study in a single institution with a limited number of patients. Although we confirmed that all 18 patients were alive and had no complications during the follow-up period, data from blood tests and serial imaging of several patients were insufficient. Second, our series did not include severe cases with peritoneal irritation or mesenteric ischemia. Hence, we could not establish a definitive conclusion regarding the cases for which invasive treatment is highly recommended at the time of diagnosis. For future investigations, we intend to perform blood tests including lactate at the time of diagnosis and follow-up serial imaging in all ISMAD patients even at least for 1 year.
In conclusion, based on our 9-year single-institution experience with ISMAD, patients without peritoneal irritation can be treated conservatively when signs of an aneurysm, ULP, or mesenteric ischemia are not evident. However, endovascular stenting must be considered when radiographic signs of an aneurysm or ULP are observed to preserve SMA blood flow with the improvement of the dissection.
Author agreement
All authors agree submission of this article.
Competing financial interests
The authors declare no competing financial interests.
Funding
The authors state that this work has not received any funding.
Guarantor
Tomoyuki Ohta, Director of Sapporo Higashi Tokushukai Hospital.
Conflict of interest
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Ethical approval for the study was obtained from the institutional review board at our hospital (#TGE00991-012), and participants provided informed consent.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
Study subjects or cohorts have not been previously reported.
Methodology
-
•
retrospective
-
•
diagnostic or prognostic study / observational
-
•
performed at one institution
Acknowledgments
No funding was obtained for this study. We would like to thank Koji Arashiro, Ryosuke Kurosawa, and Yasuyuki Okino (Department of Radiology, Sapporo Higashi Tokushukai Hospital) for CT imaging reconstruction and Ayumu Sugitani (Sapporo Higashi Tokushukai Hospital) for his valuable advice concerning the statistical analysis. We are also very grateful to Drs. Yusuke Ono and Toru Kono (Sapporo Higashi Tokushukai Hospital) for helpful discussions during the project.
Footnotes
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.ejro.2019.05.004.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- 1.Bauersfeld S.R. Dissecting aneurysm of the aorta; A presentation of 15 cases and A review of the recent literature. Ann. Intern. Med. 1947;26(6):873–889. doi: 10.7326/0003-4819-26-6-873. [DOI] [PubMed] [Google Scholar]
- 2.Foord A.G., Lewis R.D. Primary dissecting aneurysms of peripheral and pulmonary arteries: dissecting hemorrhage of media. Arch. Pathol. 1959;68:553–577. [PubMed] [Google Scholar]
- 3.Cho Y.P., Ko G.Y., Kim H.K., Moon K.M., Kwon T.W. Conservative management of symptomatic spontaneous isolated dissection of the superior mesenteric artery. Br. J. Surg. 2009;96(7):720–723. doi: 10.1002/bjs.6631. [DOI] [PubMed] [Google Scholar]
- 4.Yun W.S., Kim Y.W., Park K.B., Cho S.K., Do Y.S., Lee K.B., Kim D.I., Kim D.K. Clinical and angiographic follow-up of spontaneous isolated superior mesenteric artery dissection. Eur. J. Vasc. Endovasc. Surg. 2009;37(5):572–577. doi: 10.1016/j.ejvs.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 5.Kim H.K., Jung H.K., Cho J., Lee J.M., Huh S. Clinical and radiologic course of symptomatic spontaneous isolated dissection of the superior mesenteric artery treated with conservative management. J. Vasc. Surg. 2014;59(2):465–472. doi: 10.1016/j.jvs.2013.07.112. [DOI] [PubMed] [Google Scholar]
- 6.Okamura K., Morizumi S., Kawata M., Suematsu Y. Conservative therapy as a primary treatment for spontaneous isolated dissection of the superior mesenteric artery. Ann. Vasc. Surg. 2014;28(8):1939–1945. doi: 10.1016/j.avsg.2014.06.062. [DOI] [PubMed] [Google Scholar]
- 7.Min S.I., Yoon K.C., Min S.K., Ahn S.H., Jae H.J., Chung J.W., Ha J., Kim S.J. Current strategy for the treatment of symptomatic spontaneous isolated dissection of superior mesenteric artery. J. Vasc. Surg. 2011;54(2):461–466. doi: 10.1016/j.jvs.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Dong Z., Fu W., Chen B., Guo D., Xu X., Wang Y. Treatment of symptomatic isolated dissection of superior mesenteric artery. J. Vasc. Surg. 2013;57(Suppl. 2):69S–76S. doi: 10.1016/j.jvs.2012.07.060. [DOI] [PubMed] [Google Scholar]
- 9.Sakamoto I., Ogawa Y., Sueyoshi E., Fukui K., Murakami T., Uetani M. Imaging appearances and management of isolated spontaneous dissection of the superior mesenteric artery. Eur. J. Radiol. 2007;64(1):103–110. doi: 10.1016/j.ejrad.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 10.Pang P., Jiang Z., Huang M., Zhou B., Zhu K., Shan H. Value of endovascular stent placement for symptomatic spontaneous isolated superior mesenteric artery dissection. Eur. J. Radiol. 2013;82(3):490–496. doi: 10.1016/j.ejrad.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 11.Gobble R.M., Brill E.R., Rockman C.B., Hecht E.M., Lamparello P.J., Jacobowitz G.R., Maldonado T.S. Endovascular treatment of spontaneous dissections of the superior mesenteric artery. J. Vasc. Surg. 2009;50(6):1326–1332. doi: 10.1016/j.jvs.2009.07.019. [DOI] [PubMed] [Google Scholar]
- 12.Jia Z.Z., Zhao J.W., Tian F., Li S.Q., Wang K., Wang Y., Jiang L.Q., Jiang G.M. Initial and middle-term results of treatment for symptomatic spontaneous isolated dissection of superior mesenteric artery. Eur. J. Vasc. Endovasc. Surg. 2013;45(5):502–508. doi: 10.1016/j.ejvs.2013.01.039. [DOI] [PubMed] [Google Scholar]
- 13.Park Y.J., Park K.B., Kim D.I., Do Y.S., Kim D.K., Kim Y.W. Natural history of spontaneous isolated superior mesenteric artery dissection derived from follow-up after conservative treatment. J. Vasc. Surg. 2011;54(6):1727–1733. doi: 10.1016/j.jvs.2011.07.052. [DOI] [PubMed] [Google Scholar]
- 14.Cho B.S., Lee M.S., Lee M.K., Choi Y.J., Kim C.N., Kang Y.J., Park J.S., Ahn H.Y. Treatment guidelines for isolated dissection of the superior mesenteric artery based on follow-up CT findings. Eur. J. Vasc. Endovasc. Surg. 2011;41(6):780–785. doi: 10.1016/j.ejvs.2010.12.022. [DOI] [PubMed] [Google Scholar]
- 15.Luan J.Y., Li X. Computed tomography imaging features and classification of isolated dissection of the superior mesenteric artery. Eur. J. Vasc. Endovasc. Surg. 2013;46(2):232–235. doi: 10.1016/j.ejvs.2013.04.035. [DOI] [PubMed] [Google Scholar]
- 16.Luan J.Y., Li X., Li T.R., Zhai G.J., Han J.T. Vasodilator and endovascular therapy for isolated superior mesenteric artery dissection. J. Vasc. Surg. 2013;57(6):1612–1620. doi: 10.1016/j.jvs.2012.11.121. [DOI] [PubMed] [Google Scholar]
- 17.Stone W.M., Abbas M., Cherry K.J., Fowl R.J., Gloviczki P. Superior mesenteric artery aneurysms: is presence an indication for intervention? J. Vasc. Surg. 2002;36(2):234–237. doi: 10.1067/mva.2002.125027. discussion 237. [DOI] [PubMed] [Google Scholar]
- 18.Satokawa H., Takase S., Seto Y., Yokoyama H., Gotoh M., Kogure M., Midorikawa H., Saito T., Maehara K. Management strategy of isolated spontaneous dissection of the superior mesenteric artery. Ann. Vasc. Dis. 2014;7(3):232–238. doi: 10.3400/avd.oa.14-00071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim J.H., Roh B.S., Lee Y.H., Choi S.S., So B.J. Isolated spontaneous dissection of the superior mesenteric artery: percutaneous stent placement in two patients. Korean J. Radiol. 2004;5(2):134–138. doi: 10.3348/kjr.2004.5.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wagner W.H., Allins A.D., Treiman R.L., Cohen J.L., Foran R.F., Levin P.M., Cossman D.V. Ruptured visceral artery aneurysms. Ann. Vasc. Surg. 1997;11(4):342–347. doi: 10.1007/s100169900058. [DOI] [PubMed] [Google Scholar]
- 21.Nagai T., Torishima R., Uchida A., Nakashima H., Takahashi K., Okawara H., Oga M., Suzuki K., Miyamoto S., Sato R., Murakami K., Fujioka T. Spontaneous dissection of the superior mesenteric artery in four cases treated with anticoagulation therapy. Intern. Med. 2004;43(6):473–478. doi: 10.2169/internalmedicine.43.473. [DOI] [PubMed] [Google Scholar]
- 22.AbuRahma A.F., Stone P.A., Bates M.C., Welch C.A. Angioplasty/stenting of the superior mesenteric artery and celiac trunk: early and late outcomes. J. Endovasc. Ther. 2003;10(6):1046–1053. doi: 10.1177/152660280301000604. [DOI] [PubMed] [Google Scholar]
- 23.Chu S.Y., Hsu M.Y., Chen C.M., Yeow K.M., Hung C.F., Su I.H., Shie R.F., Pan K.T. Endovascular repair of spontaneous isolated dissection of the superior mesenteric artery. Clin. Radiol. 2012;67(1):32–37. doi: 10.1016/j.crad.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Li N., Lu Q.S., Zhou J., Bao J.M., Zhao Z.Q., Jing Z.P. Endovascular stent placement for treatment of spontaneous isolated dissection of the superior mesenteric artery. Ann. Vasc. Surg. 2014;28(2):445–451. doi: 10.1016/j.avsg.2013.01.028. [DOI] [PubMed] [Google Scholar]
- 25.Ahn H.Y., Cho B.S., Mun Y.S., Jang J.H., Kim C.N., Lee M.S., Kang Y.J. Treatment results for spontaneous isolated superior mesenteric artery dissection according to our previous guidelines and collective literature review. Ann. Vasc. Surg. 2014;28(7):1595–1601. doi: 10.1016/j.avsg.2014.04.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.