Abstract
EEG studies in healthy humans have highlighted that alpha-band activity is relatively reduced over the occipital–parietal areas of the hemisphere contralateral to the direction of spatial attention. Here, we investigated the hemispheric distribution of alpha during orienting of attention in male and female right brain-damaged patients with left spatial neglect. Temporal spectral evolution showed that in patients with neglect alpha oscillations over the damaged hemisphere were pathologically enhanced both during the baseline-fixation period that preceded cued orienting (capturing tonic alpha changes) and during orienting with leftward, rightward, or neutral-bilateral spatial cues (reflecting phasic alpha changes). Patients without neglect showed a similar though significantly less enhanced hemispheric asymmetry. Healthy control subjects displayed a conventional decrease of alpha activity over the hemisphere contralateral to the direction of orienting. In right-brain-damaged patients, neglect severity in the line bisection task was significantly correlated both with tonic alpha asymmetry during the baseline period and with phasic asymmetries during orienting of attention with neutral-bilateral and leftward cues. Asymmetries with neutral-bilateral and leftward cues were correlated with lesion of white matter tracts linking frontal with parietal–occipital areas. These findings show that disruption of rostrocaudal white matter connectivity in the right hemisphere interferes with the maintenance of optimal baseline tonic levels of alpha and the phasic modulation of alpha activity during shifts of attention. The hemispheric distribution of alpha activity can be used as a diagnostic tool for acquired pathological biases of spatial attention due to unilateral brain damage.
SIGNIFICANCE STATEMENT Alpha desynchronization over the hemisphere contralateral to the attended side of space is a reliable marker of attentional orienting in the healthy human brain: can the same marker be used to spot and quantify acquired disturbances of spatial attention after unilateral brain injuries? Are pathological modifications in the hemispheric distribution of alpha specifically linked to attentional neglect for one side of space? We show that in patients with right brain damage the pathological enhancement of alpha oscillations over the parietal and occipital areas of the injured hemisphere is correlated with reduced awareness for the left side of space and with the lesion of white matter pathways that subserve frontal modulation of alpha activity in posterior brain areas.
Keywords: alpha band, attention, awareness, EEG, spatial neglect, stroke
Introduction
Since its discovery (Berger, 1929) the oscillatory alpha-band (∼8 to 14 Hz) parietal–occipital EEG activity has been used extensively as a marker of vigilance, attention, cognitive processing, and cortical communication both in healthy humans and patients with brain damage (Klimesch et al., 2007; Jensen and Mazaheri, 2010). EEG studies that have monitored preparatory orienting of spatial attention ahead of the occurrence of lateral visual targets, have pointed out decreased alpha activity over the hemisphere contralateral to the attended side of space and increased alpha activity over the ipsilateral hemisphere (Worden et al., 2000; Yamagishi et al., 2003; Sauseng et al., 2005; Kelly et al., 2006; Thut et al., 2006; Capotosto et al., 2009; Rihs et al., 2009; Grent-'t-Jong et al., 2011; Rajagovindan and Ding, 2011). The decrease in alpha activity reflects enhanced cortical excitability that favors the processing of upcoming inputs at attended spatial positions. An increase in alpha activity reflects inhibition in the processing of upcoming inputs at unattended positions. In the healthy brain, the degree of interhemispheric alpha asymmetry during preparatory orienting predicts faster detection of visual targets in the attended side of space (Thut et al., 2006). Similarly, MEG investigations showed that spontaneous oscillatory alpha activity before stimulus onset predict the visual discrimination and conscious processing of ensuing target stimuli (van Dijk et al., 2008; Wyart and Tallon-Baudry, 2009).
The syndrome of left spatial neglect identifies a disabling condition that is more frequent after cortical–subcortical right brain damage (RBD) and is characterized by defective attentional processing of sensory events and defective programming of motor actions in the contralesional space (Bartolomeo et al., 2007; Doricchi et al., 2008; Gainotti et al., 2009; but see Doricchi et al., 2007, for neglect in imagery/dreaming space). Orienting of attention in spatial neglect has been extensively studied with behavioral methods that have identified a specific deficit in reorienting attention toward targets in the contralesional left side of space once attention is directed in the ipsilesional right side (Posner et al., 1984). In contrast to this, only a few studies have explored the EEG correlates of attentional orienting in neglect. In addition, with the notable exception of one recent investigation with event-related potentials (Lasaponara et al., 2018), all previous studies were focused on the early electrophysiological correlates of target detection (Di Russo et al., 2007) rather than on the activity that characterizes preparatory orienting of spatial attention ahead of target occurrence. Here we investigate the lateralization of oscillatory alpha activity during cued orienting of attention, in RBD patients with (N+) and without (N−) left spatial neglect and in a group of age-matched healthy control subjects (HCs). Based on EEG observations in healthy participants (Worden et al., 2000; Thut et al., 2006), one might expect that the pathological rightward attentional bias that characterizes the performance of N+ is associated with a pathological increment of preparatory alpha activity over the damaged right hemisphere and a corresponding pathological reduction of the same activity over the intact left hemisphere.
A number of recent EEG studies have highlighted that in healthy observers, changes in lateral bias of spatial attention are matched both with changes in the interhemispheric distribution of baseline/tonic alpha activity linked to nonspatial factors such as the level of arousal and alertness and with changes in phasic alpha activity that are elicited by orienting of attention (Newman et al., 2013, 2017; Benwell et al., 2014, 2018). Therefore, to clarify whether the pathological attentional bias experienced by patients with neglect is associated with changes in tonic (Sturm and Willmes, 2001; Husain and Rorden, 2003) and/or phasic factors affecting alpha oscillations, we evaluated both tonic alpha asymmetry during the baseline-fixation period that preceded the presentation of directional cues and phasic asymmetry during orienting of spatial attention with the same cues. Verifying the presence of an altered hemispheric distribution in alpha activity during orienting of attention and evaluating its relationship with the severity of neglect can provide a useful diagnostic/prognostic tool for neglect patients, who usually experience poor clinical outcome and must cope with longer and socially expensive rehabilitation treatments.
Materials and Methods
Participants
Twelve N+ patients, 13 N− patients, and 15 HCs of either sex were examined. N+ and N− patients did not differ in time elapsed from stroke onset (F(1,11) = 3, p = 0.23; mean time, 46 d). Age was equivalent among N+ patients, N− patients, and HCs (F(2,22) = 2.6, p = 0.32; mean age: HCs = 53.2 years; N+ patients = 62.6 years; N− patients = 61.9 years). Neglect evaluation scores of N+ and N− groups are reported in Table 1.
Table 1.
Demographic and clinical data from RBD patients (N− and N+ patients) and HCs
| Patient group | Sex | Age (years) | Stroke onset (months) | Line bisection (200 mm) rightward deviation (mm) | Letter cancellation |
Line cancellation |
Star cancellation |
Sentence reading test | Wundt–Jastrow illusion (unexpected responses) |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Left | Right | Left | Right | Left | Right | Left | Right | |||||||
| N− (n = 13) | M = 10 | Mean | 61.9 | 1.3 | −0.25 | 51 (53) | 48.6 (51) | 10.9 (11) | 10 (10) | 26.2 (27) | 25.7 (27) | 5.9 (6) | 0.2 (20) | 0.1 (20) |
| F = 3 | SD | 9.3 | 0.47 | 2.8 | 2.7 | 5.5 | 0.2 | 0 | 1 | 1.8 | 0.2 | 0.5 | 0.5 | |
| N+ (n = 12) | M = 8 | Mean | 62.6 | 1.7 | 23.2 | 19 (53) | 28 (51) | 6.2 (11) | 8.3 (10) | 9.2 (27) | 15.5 (27) | 3.1 (6) | 10.1 (20) | 0.5 (20) |
| F = 4 | SD | 10.4 | 0.36 | 19.9 | 20.5 | 21.2 | 5.1 | 2.4 | 11.1 | 8.6 | 2.9 | 8.1 | 1.1 | |
| HC (n = 15) | M = 8 | Mean | 53.2 | |||||||||||
| F = 7 | SD | 11.1 | ||||||||||||
F, Female; M, male. Please note that, compared with N− patients, N+ patients have stronger rightward bias/left side omissions in line bisection (t(23) = −4.1, p = 0.0003, unpaired t test), sentence reading (t(23) = 3.3, p = 0.002, unpaired t test), letter cancellation (F(1,23) = 16.5, p = 0.0004, ηp2 = 0.41), Line cancellation (F(1,23) = 10.4, p = 0.003, ηp2 = 0.31), star cancellation (F(1,23) = 22.8, p = 0.0000, ηp2 = 0.49), and in the Wundt–Jastrow area illusion task (F(1,23) = 18.3, p = 0.0002, ηp2 = 0.44).
All patients experienced vascular stroke. Patients with bilateral strokes, surgical interventions, signs of dementia, or history of previous neurological illness were excluded. All patients and participants were right handed and had normal or corrected-to-normal visual acuity. At the time of clinical and experimental examination, all patients were free from confusion and from temporal or spatial disorientation. Visual fields were tested with standard kinetic Goldmann perimetry. All patients had intact visual fields, with the exception of one N+ patient who experienced restriction of the left inferior quadrant with sparing of 10° around central fixation. Patients and control subjects gave their written informed consent for participating in the study, which was approved by the Institutional Ethical Committee of the Fondazione Santa Lucia Istituto di Ricovero e Cura a Carattere Scientifico (Rome, Italy).
Experimental design and statistical analysis
Procedure and stimuli.
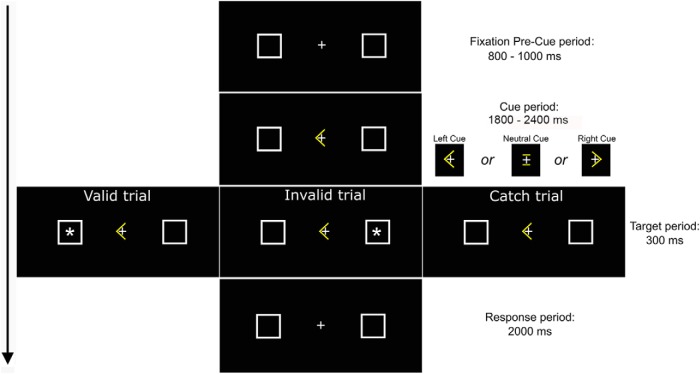
Participants performed a Posner task with central directional arrow cues that pointed leftward or rightward and with nondirectional cues that pointed to no lateral side of space (neutral cues, an “=” symbol; Fig. 1). Directional left cues and right cues were predictive of lateral target location on 70% of trials, while in trials with neutral cues targets appeared with equal probability in the left or in the right side of space. In each trial an initial central fixation period (800–1000 ms) was followed by the presentation of a cue (1800–2400 ms) and then by the presentation of a target asterisk (300 ms; size, 0.6° × 0.6°) in one of two lateral boxes (size, 1° × 1°), one centered 4.5° to the left and one to the right of the central fixation. Participants signaled the detection of lateral targets by pressing as fast as possible the central spacebar of the computer keyboard. Two thousand milliseconds were allowed for response. A total of 280 valid trials and 120 invalid trials with directional cues (200 left cues and 200 right cues) and 160 trials with neutral cues were presented (for further details, see Lasaponara et al., 2018).
Figure 1.
Time course of events during experimental task with the example of a trial with the cue pointing to the left. Duration of events is in ms.
EEG recording and preprocessing.
The EEG was recorded from 64 electrodes (Brain Vision LLC) placed according to the 10–10 system. All scalp channels were referenced online to the left mastoid (M1). Horizontal eye movements were monitored with bipolar recording from electrodes placed at the left and right outer canthi. Blinks and vertical eye movements were recorded with an electrode positioned below the left eye, which was referenced to site Fp1. The EEG from each electrode site was digitized at 250 Hz with an amplifier bandpass of 0.01–80 Hz, including a 50 Hz notch filter, and was stored for off-line averaging. Continuous EEG was recalculated against the average reference and then segmented in experimental epochs that lasted 2400 ms and covered the trial period ranging from 700 ms before cue onset (baseline-fixation period) to 1700 ms after cue onset (cue period). EEG activity recorded during the last 200 ms of the central fixation period was used for baseline correction. Before computerized artifact rejection, ocular correction was performed according to the independent component analysis (ICA) algorithm (Jung et al., 2000). Artifact rejection was performed before signal averaging to discard epochs in which deviations in eye position, blinks, or amplifier blocking occurred. All epochs in which EOG amplitudes and EEG amplitudes were greater than ±60 mV were excluded from further analysis. On average, 4.9%, 3.8%, and 4.2% of the trials were rejected in HC, N−, and N+ groups, respectively.
Determination of alpha frequency.
To evaluate the individual alpha frequency (IAF) for each cue-related EEG epoch and electrode, we computed the power spectrum during the baseline-fixation period using fast Fourier transform (Hanning window, 10%; maximal resolution, 0.5 Hz), and averaged the spectrum across all posterior electrodes (P7, P5, P3, P1, P8, P6, P4, P2, Pz, PO7, PO3, PO8, PO4, POz, O1, O2, and Oz; Thut et al., 2006; Capotosto et al., 2009). The individual alpha band was then defined as IAF −2 Hz to IAF +2 Hz (Capotosto et al., 2009). Average IAF values were as follows: HCs = 9.8 Hz (0.28 SE); N− patients = 9.3 Hz (0.33 SE); and N+ patients = 9.9 Hz (0.44 SE). According to these values, our alpha frequency window ranged from 7.5 to 11.9 Hz. IAF values were comparable among the three experimental groups (two-tailed t test: HC vs N−: t(28) = 1.2, p = 0.23; HC vs N+: t(25) = −0.2, p = 0.81; N− vs N+: t(25) = −1.2, p = 0.24).
Temporal spectral evaluation.
The alpha power in cue-related EEG activity was computed using temporal spectral evolution (TSE; Worden et al., 2000; Thut et al., 2006; Rihs et al., 2009). To this aim, each cue-related EEG epoch was as follows: (1) filtered into alpha band (8–12 Hz); (2) rectified; (3) smoothed through averaging within a moving time window (width, 100 ms); (4) trimmed of 200 ms at the beginning and at the end of experimental epochs to eliminate filter warm up artifacts; and (5) averaged across different epochs to get the alpha TSE for each cue direction (left, right), electrode, and participant. Finally, alpha TSE was averaged across the three electrodes considered in each pool of derivations (Parietal Left (PL): PO7-PO1-P5; Parietal Right (PR): PO8-PO2-P6). These derivations were selected as follows: (1) based on their use in previous electrophysiological studies that have documented alpha power lateralization during the orienting of spatial attention (Worden et al., 2000; Sauseng et al., 2005; Kelly et al., 2006; Thut et al., 2006; Rihs et al., 2009; Grent-'t-Jong et al., 2011); and (2) through the selection of posterior electrodes where the grand average showed the highest attentional modulation of alpha by cued orienting in the control group of healthy participants. In addition to this, we also verified that alpha power recorded at selected derivations during the baseline fixation was not different among the three experimental groups (F(2,37) = 1.1, p = 0.3; HC, 0.75 μV; N−, 1.01 μV; N+, 0.77 μV). This latter control was run using only derivations over the left hemisphere that was spared in all participants.
In line with recent studies (Thut et al., 2006; Hong et al., 2015), the average TSE was calculated both during the 500 ms that preceded cue onset (baseline-fixation period) and within the 300–1500 ms that followed cue onset (cue period). TSE values were calculated separately in the first half (300–900 ms after cue onset) and the second half of the cue period (900–1500 ms after cue onset). Individual data during the baseline-fixation period were analyzed through a group (HC, N−, N+) × hemisphere (H; left H, right H) × cue direction (left cue, right cue) repeated-measures ANOVA: the cue direction factor was included to check baseline levels of alpha in trials that included left cues or right cues. Cue period data were analyzed through a group (HC, N−, N+) × hemisphere (left H, right H) × cueing phase (first half vs second half of the cue period) × cue direction (left cue, right cue) ANOVA.
Event-related desynchronization/synchronization.
To partial out the potential influence of alpha asymmetries recorded during the baseline-fixation period on alpha asymmetries recorded during the cue period, we also computed the event-related synchronization (ERS)/event-related desynchronization (ERD) index using ERD% = (E − R)/R × 100% (Pfurtscheller and Lopes da Silva, 1999), where E indicates the alpha power at each time point after the cue onset, and R indicates the mean value of alpha power during the same baseline-fixation period used in the TSE analysis (i.e., 500 ms before cue onset). In this way, analyses of ERS/ERD allow to correct alpha asymmetries recorded during the cue period as a function of baseline asymmetries that are present before the presentation of cues. As for the case of TSE, individual ERS/ERD were calculated for the first half (300–900 ms postcue) and the second half of this period (900–1500 ms postcue). We then entered individual data in a group (C, N−, N+) × cueing phase (first half vs second half of the cue period) × cue direction (left cue, right cue) × hemisphere (ipsilateral, contralateral) repeated-measures ANOVAs. Time windows and electrode pools were the same as for the TSE analysis.
Alpha and behavioral lateralization indexes.
According to the method proposed by Thut et al. (2006), individual TSE data in response to left, right, and neutral cues, recorded over posterior regions of interest during the baseline-fixation period and during the first and second half of the cue period, were used to calculate a hemispheric lateralization index of the alpha band that incorporated the relative distribution of activity over both hemispheres in one value. These alpha asymmetry indexes were calculated according to the following formula:
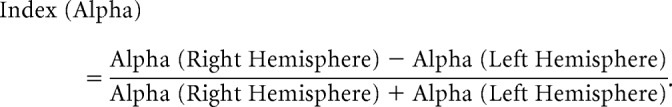 |
Using the same procedure, we calculated lateralization indices in behavioral responses to left- versus right-sided targets. Separate indexes for reaction times (RTs) and detection rates (DRs) were calculated using the following formulas:
 |
Since neglect patients can experience a high number of omissions in the detection of targets on the left side of space, asymmetry indexes of RTs were calculated with two different procedures. First, uncorrected (uncorr) indexes were computed taking into account only hit RTs provided by patients. Second, to allow comparison with other recent RTs investigations in neglect (Rengachary et al., 2011), we also calculated corrected (corr) asymmetry indexes in which omitted RTs were replaced with the maximum time that was allowed for response (2000 ms).
Negative index values indicate higher alpha activity over the left hemisphere compared with the right hemisphere in EEG data and better processing of targets in the left relative to the right side of space (leftward bias) in behavioral data. In both cases, positive values indicate opposite trends (Thut et al., 2006). Alpha lateralization indexes were analyzed with a group (C, N−, N+) × cueing phase (baseline-fixation period, first half cue period, second half cue period) × cue direction (left cue, right cue, neutral cue) repeated-measures ANOVA. Behavioral asymmetry indexes (RTs and DR) were analyzed with a group (C, N−, N+) × cue direction (left cue, right cue) repeated-measures ANOVA. In an additional series of analyses, we performed multiple correlations between the indexes of baseline fixation and cue-related alpha asymmetry and the indexes of asymmetry in behavioral measures (RTs, DR). In the case of patient groups, we also checked for correlations between the indexes of baseline fixation and cue-related alpha asymmetry and the clinical scores in neglect tests. All p values were corrected for multiple comparisons.
Voxel-based lesion–symptom mapping.
Using the voxel-based lesion–symptom mapping (VLSM) technique (Bates et al., 2003), we investigated the anatomical correlates of changes in the alpha-hemispheric lateralization index in the whole sample of RBD (N+ and N−). VLSM allows analyzing the anatomical correlates of continuous behavioral data or, as in the present case, electrophysiological data on a voxel-by-voxel basis. Following the mapping of individual lesions based on 1.5 T MRI scans (Lasaponara et al., 2018), we performed a VLSM analysis to produce anatomical maps representing the z statistics of the voxelwise comparison between the average alpha asymmetry scores of the groups of patients with versus without a lesion in a given voxel. This allows for the isolation of lesioned voxels that predict the alpha-hemispheric lateralization index. We used the nonparametric Brunner–Munzel test (Brunner and Munzel, 2000) to perform statistical comparisons on a voxelwise basis, as implemented in the Niistat (Matlab Toolbox) and MRIcron software (Rorden et al., 2007). Brunner–Munzel tests were performed at each voxel using the alpha asymmetry scores as the dependent variable. To avoid producing inflated z-scores, tests were run using permutation-derived correction (Kimberg et al., 2007). This procedure is assumption free and more powerful compared with other procedures, such as the Bonferroni correction (Kimberg et al., 2007). As recommended for the analyses of data gathered from samples of medium size (Kimberg et al., 2007; Medina et al., 2010), correction for multiple comparisons was achieved by using the nonparametric permutation test and the significance level was set at p = 0.05. The localization of VLSM lesion peaks was determined in MNI space. The localization of lesion peaks on white matter pathways was made using the diffusion tensor imaging-based atlases by Catani and Thiebaut de Schotten (2012); see also Thiebaut de Schotten et al., 2011). White matter pathways were visualized using MRIcron software (Rorden et al., 2007).
Results
TSE
Baseline-fixation period tonic alpha activity
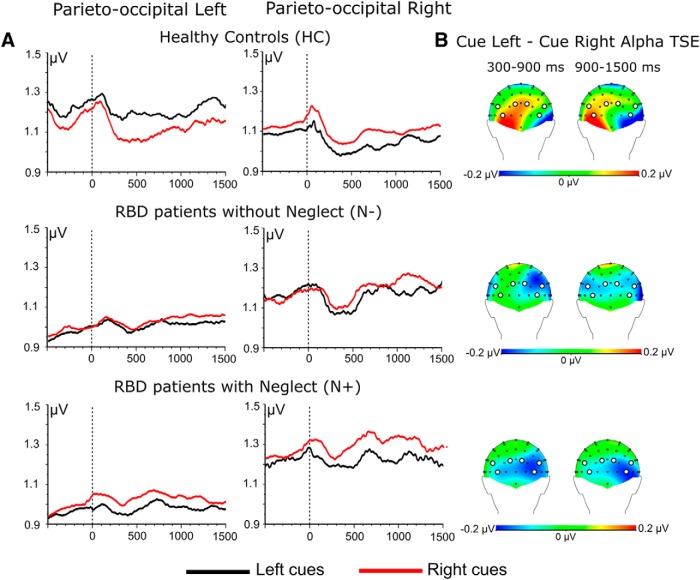
A significant group × hemisphere interaction (F(2,37) = 7.03, p = 0.002) pointed out no significant interhemispheric difference in HCs and N− patients (all p > 0.2). In contrast, in N+ patients tonic alpha activity was higher in the right compared with the left hemisphere [left H (0.95 μV) vs right H (1.24 μV), p = 0.0004]. Most important, there was no significant group × hemisphere × cue direction interaction (F(2,37) = 0.07, p = 0.49). This shows that during the baseline-fixation period the distribution of alpha activity over the two hemispheres across groups was independent of cue direction, as was expected (Fig. 2).
Figure 2.
A, Time course of grand-averaged alpha-band oscillatory activity (TSE) recorded as a function of cue direction (left cue, right cue) in the three experimental groups (HCs, N− patients, and N+ patients). In HCs, differences in TSE between left cues and right cues recorded ahead of cue onset are not significant (see Results). B, Topographical maps of alpha power differences (attend-left − attend-right) averaged across 300–900 ms and 900–1500 ms postcue onsets. White circles on scalp topographies highlight pooled derivations in each hemisphere.
Cue period phasic alpha activity
The ANOVA highlighted a significant group × hemisphere × cue direction interaction (F(2,37) = 6.3, p = 0.004). This interaction pointed out that in HCs, left cues produced higher levels of alpha activity over the left hemisphere with respect to right cues [left cues (1.21 μV) vs right cues (1.08 μV), p = 0.0001], while right cues produced higher levels of alpha activity over the right hemisphere with respect to left cues [right cues (1.07 μV) vs left cues (0.98 μV), p = 0.003]. In HCs, left cues also produced higher alpha activity over the left hemisphere (1.21 μV) rather than over the right hemisphere (left cues, 0.98 μV; p = < 0.001), while right cues produced comparable levels of alpha activity over the left hemisphere (1.08 μV) and the right hemisphere (1.07 μV; p = n.s.; Fig. 2) In N− patients, left cues and right cues produced comparable levels of alpha activity over the left hemisphere [left cues (1.01 μV) vs right cues (1.05 μV), p = n.s.], while over the right hemisphere right cues elicited higher alpha activity compared with left cues [right cues (1.17 μV) vs left cues (1.11 μV), p = 0.04]. N− patients showed higher levels of alpha activity over the right hemisphere than the left hemisphere, both with left cues [right hemisphere (1.11 μV) vs left hemisphere (1.01 μV), p = 0.004] and right cues [right hemisphere (1.17 μV) vs left hemisphere (1.05 μV), p = 0.002]. In N+ patients, right cues produced higher alpha activity with respect to left cues over the right hemisphere [right cues (1.28 μV) vs left cues (1.19 μV), p = 0.008], while left cues did not elicit higher alpha activity with respect to right cues over the left hemisphere [right cues (1.00 μV) vs left cues (0.93 μV), p = 0.05; Fig. 2]. This latter finding highlights that in N+ patients there is no effective preparatory inhibition of the right side of space when cues drive endogenous attention in the leftward direction. Finally, N+ patients showed a generalized enhancement of alpha activity over the right hemisphere both with left cues [right hemisphere (1.19 μV) vs left hemisphere (0.93 μV), p < 0.001] and right cues [right hemisphere (1.28 μV) vs left hemisphere (1.00 μV), p < 0.001].
ERS/ERD
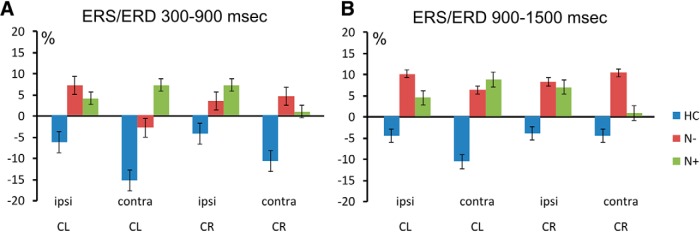
A significant group × cue direction × hemisphere interaction (F(2,37) = 4.06, p = 0.02) showed that in HCs there was stronger desynchronization over the right hemisphere with left cues [left hemisphere (−5.5%) vs right hemisphere (−13.2%), p = 0.01], while with right cues stronger desynchronization over the left hemisphere did not reach statistical significance [left hemisphere (−7.7%) vs right hemisphere (−4.2%), p = 0.26]. Similar results were also found in N− patients with left cues [left hemisphere (8.6%) vs right hemisphere (1.6%), p = 0.04] and right cues [left hemisphere (7.4%) vs right hemisphere (5.8%), p = 0.62; Fig. 3]. In N+ patients, with left cues no significant hemispheric difference was found in alpha synchronization [left hemisphere (4.2%) vs right hemisphere (7.9%), p = 0.28], while with right cues alpha synchronization was close to be significantly lower over the left hemisphere; that is, over the hemisphere that was contralateral to the side of space pointed by the cue [left hemisphere (0.8%) vs right hemisphere (7.1%), p = 0.07; Fig. 3].
Figure 3.
Grand-averaged alpha-band ERS/ERD values within pools of electrodes (Fig. 2) ipsilateral (ipsi) and contralateral (contra) to cue direction (CL = left cues; CR = right cues). A, B, values recorded during the 300–900 ms (A) and during 900–1500 ms (B) time periods that followed cue onset. Vertical bars indicate SE.
Alpha-EEG and behavioral asymmetry indexes
Alpha-EEG asymmetry index
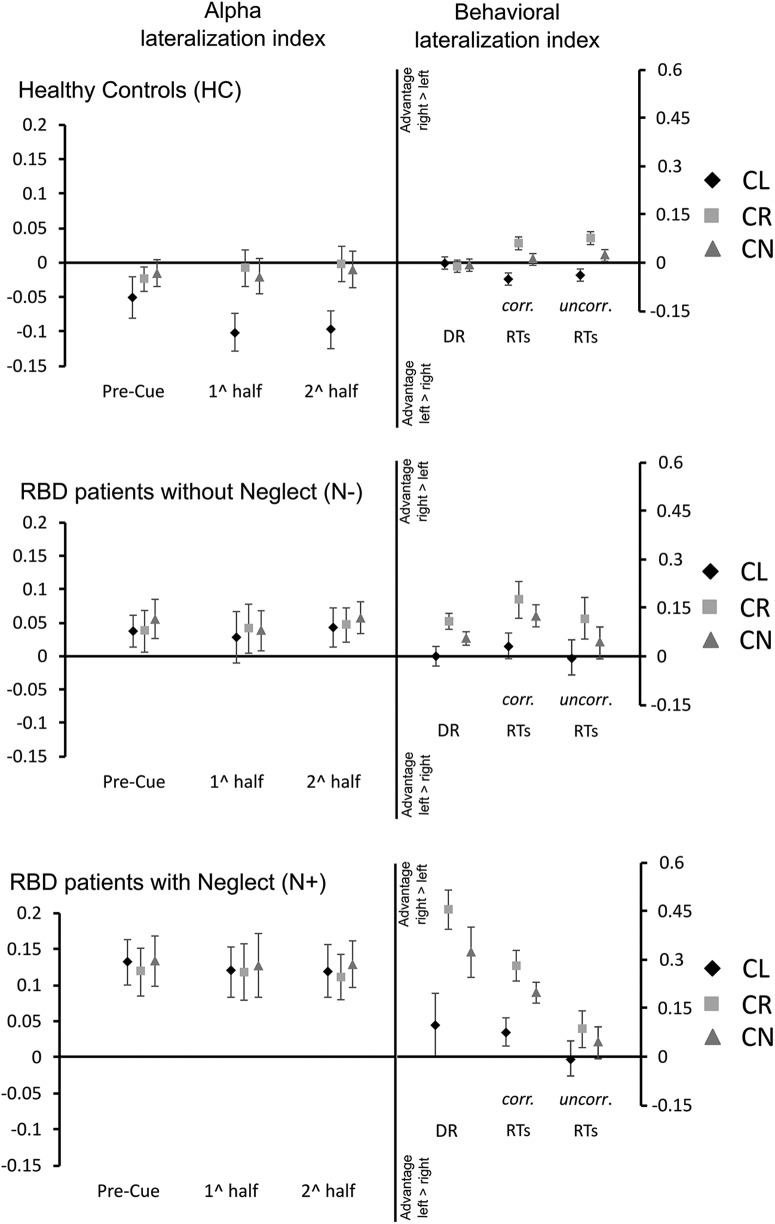
A main group effect (F(2,37) = 10.3, p < 0.001) pointed out that alpha asymmetry was higher in N+ patients (0.12) compared with both N− patients (0.04; p = 0.03) and HCs (−0.03; p < 0.001) and that N− patients had higher asymmetry than HCs (p = 0.02; Fig. 4). This main effect highlights a significant enhancement in alpha activity over the damaged right hemisphere in N+ patients with respect to N− patients and HCs, and in N− patients with respect to HCs. Importantly, the lack of significant group × cue direction and group × cueing phase × cue direction interactions (p >0.12 in both cases) showed that this enhancement was independent from cue direction. In HCs, there was a significant cueing phase (baseline-fixation, first half, second half) × cue direction (left cue, right cue, neutral cue) interaction (F(4,56) = 3.38, p = 0.01). This interaction highlighted that, compared with the baseline-fixation period, during the first and second half of the cue period, orienting with left cues produced a relative enhancement of alpha activity over the left hemisphere (Fig. 4), while no similar effect was present with right cues and neutral cues. This result is in line with previous observations in healthy participants (Thut et al., 2006). In N+ and N− patients, no change in the asymmetrical lateralization of alpha activity over the right hemisphere was present as a function of cue direction or cuing phase (all p > 0.37).
Figure 4.
Hemispheric lateralization index of alpha activity (left vs right hemisphere) and behavioral lateralization index in target processing (left vs right hemispace). Data are reported separately for HCs, N− patients, and N+ patients. For all experimental groups, panels on the left side show the evolution of alpha-lateralization along the baseline-fixation period, the first and the second half of cue presentation. Positive values in the alpha index indicate the higher alpha power over the right hemisphere, and negative values indicate the higher alpha power over the left hemisphere. Panels on the right side represent asymmetries in DRs and RTs between targets presented on the left and the right side of space. Positive values indicate better performance with targets on the right side of space, and negative values indicate better performance with targets on the left side. For RTs, the figure reports both uncorr indices calculated by considering only hits and corr indices that were calculated by replacing misses with the maximal time allowed for response (i.e., 2000 ms). Bars indicate SE. CL, Left cue; CN, neutral cue; CR, right cue.
Behavioral asymmetry index
A significant group (HC, N−, N+) × cue direction (left, right, neutral) interaction (F(4,74) = 9.06, p < 0.001) showed that, independent of cue direction, HCs and N− patients had comparable asymmetries in the DR of lateral targets (all p >0.13). In contrast, N+ patients showed a pathologically enhanced positive asymmetry in DR (Fig. 4) that corresponds to poorer detection of targets on the left side of space, when these were preceded by right cues (DR asymmetry = 0.45) or neutral cues (DR asymmetry = 0.32). This latter finding highlights the conventional deficit experienced by N+ patients in reorienting attention toward the contralesional space (Posner et al., 1984). Among groups, no differences in DRs was found with left cues (HCs = −0.002; N− patients = 0.006; N+ patients = 0.09; all p > 0.12). ANOVAs run on RT asymmetry indexes revealed no significant group × cue direction interaction both with corr and uncorr indexes (corr: F(4,74) = 1.09, p < 0.36; uncorr: F(4,74) = 0.1, p < 0.97). In contrast to this, there were highly significant main group (corr: F(2,37) = 11, p = 0.0001; uncorr: F(2,37) = 4.7, p < 0.01) and cue direction effects (corr: F(2,74) = 24, p < 0.0001; uncorr: F(2,74) = 35.6, p < 0.001). Post hoc comparisons showed that asymmetry in HCs (0.0007) was lower than in N+ and N− patients (corr: N−, 0.1; N+, 0.18; both, p < 0.001; uncorr: N−, 0.052; N+, 0.035; both, p < 0.04). These results point out that, when compared with HCs, right brain-damaged patients are generally slower in responding to targets on the left side of space than to targets on the right side (Fig. 4). Finally, post hoc tests showed that, compared with other types of cues, responses with right cues were relatively faster for targets presented on the right side of space (corr: both p < 0.0001; asymmetry indexes: right cues = 0.17; neutral cues = 0.11; left cues = 0.01; uncorr: both p < 0.0001; asymmetry indexes: right cues = 0.08; neutral cues = 0.03; left cues = −0.02). A similar advantage was also found when neutral cues were compared with left cues (p < 0.0001 both for corr and uncorr data). Average DRs and RTs of the three experimental groups are reported in Table 2.
Table 2.
Average percentage DRs and RTs in the three experimental groups (HCs, N− patients, and N+ patients with neglect N+), in response to Valid, Neutral and Invalid targets presented in the Left and in the Right side of space
| Group | Left targets |
Right targets |
||||
|---|---|---|---|---|---|---|
| Valid | Neutral | Invalid | Valid | Neutral | Invalid | |
| HC | ||||||
| RTs corr | 479.5 (48.7) | 517.7 (86.9) | 536.5 (100.8) | 472.8 (47.1) | 511.8 (141.5) | 538.9 (117.1) |
| RTs uncorr | 428.1 (46.3) | 448.7 (54.3) | 463.3 (60.5) | 424.2 (55.4) | 443.1 (56.3) | 459.2 (49.1) |
| DRs | 95.7 (4.4) | 96.2 (3.9) | 96.6 (5.7) | 94.4 (5.5) | 95.4 (7.0) | 95.4 (6.4) |
| N− | ||||||
| RTs corr | 756.1 (192.1) | 835.1 (255.5) | 939.2 (313.9) | 593.4 (122.3) | 638.5 (154.9) | 738.7 (239.5) |
| RTs uncorr | 522.1 (102.4) | 563.6 (120.4) | 593.3 (130.1) | 474.8 (82.7) | 503.9 (88.7) | 522.4 (99.9) |
| DRs | 85.4 (8.6) | 83.4 (10.8) | 77.1 (17.6) | 93.3 (5.3) | 92.1 (6.3) | 86.4 (9.6) |
| N+ | ||||||
| RTs corr | 1140.9 (447.6) | 1349.5 (503.1) | 1480.1 (513.5) | 804.3 (301.8) | 894.9 (354.4) | 998.1 (446.2) |
| RTs uncorr | 558.4 (136.2) | 605.9 (154.5) | 609.8 (126.3) | 509.6 (117.1) | 548.1 (124.8) | 568.1 (137.9) |
| DRs | 61.7 (27.9) | 47.2 (31.9) | 37.7 (32.8) | 80.7 (13.0) | 77.3 (17.3) | 71.3 (24.0) |
SDs are reported in parentheses.
Correlations among baseline-fixation and cue-period alpha asymmetries, behavioral–RTs asymmetry and clinical data
Baseline-fixation period (tonic alpha asymmetry)
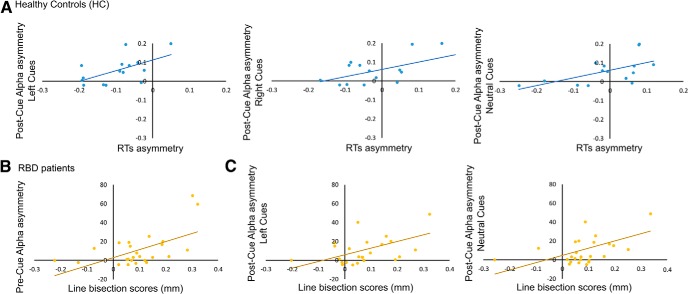
In HCs, no significant correlation was found between interhemispheric asymmetries in baseline-fixation tonic alpha and behavioral asymmetries in DRs or RTs (all r < 0.25, all p = n.s.). In contrast, in the entire group of RBD patients (i.e., N− and N+ patients together), higher levels of alpha activity over the right hemisphere were positively correlated with neglect severity in the line bisection task (r = 0.57, p = 0.02; Fig. 5). Most important, in RBD patients tonic alpha asymmetry was not correlated with lesion size (r = 0.29, p = 0.2).
Figure 5.
A, Scatterplots of significant correlations between alpha interhemispheric asymmetries and asymmetries in RTs in HCs during the cue period. Positive alpha values indicate higher alpha power over the right hemisphere, and positive RT values indicate faster responses to targets on the right side of space. B, Scatterplots of significant correlations between alpha interhemispheric asymmetries during the baseline-fixation period (Pre-Cue) and line bisection performance (in mm) in the entire sample of RBD patients (i.e., N+ and N− patients together). C, Scatterplots of significant correlations between alpha interhemispheric asymmetries during the cue period (Post-Cue) and line bisection performance (in mm) in the entire sample of RBD patients (i.e., N+ and N− patients together). Positive values in line bisection scores indicate rightward deviation from the true line center.
Cue period (phasic alpha asymmetry)
In HCs, a series of significant positive correlations highlighted that, independent of cue type, the more alpha activity was lateralized over the right hemisphere, the greater was the RT advantage to targets presented on the right side of space (all r > 0.57; all p < 0.02; Fig. 5).
In the entire group of patients, higher levels of alpha activity over the right hemisphere during the presentation of neutral cues and left cues (first half of the cue period) were significantly correlated with neglect severity in the line bisection task (neutral cues: r = 0.453; p = 0.023; left cues: r = 0.42; p = 0.03; Fig. 5). In the case of neutral cues, the right hemispheric lateralization of alpha activity was also significantly correlated with lesion size (r = 0.458; p = 0.021), while the right hemispheric lateralization of alpha activity recorded during the presentation of left cues was not correlated with lesion size (r = 0.1; p = 0.3).
VLSM results
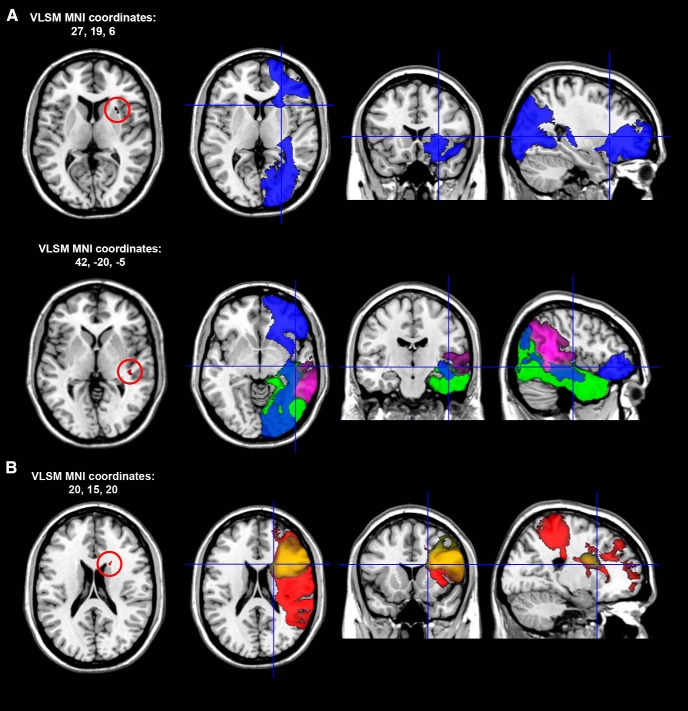
The VLSM analysis highlighted no significant anatomical correlate for tonic alpha asymmetry in the baseline-fixation period. In contrast, the analysis showed that the hemispheric lateralization of phasic alpha activity during the presentation of neutral cues was correlated with two subcortical lesion peaks (Fig. 6). The first was located in the frontal white matter below the Rolandic opercular gyrus [MNI coordinates: 28, 15, 4; Brodmann area (BA) 48]. The second peak was located in the white matter of the temporo-parietal junction (TPJ; MNI coordinates: 42, −20, −5; BA 48). The first lesion peak causes disconnection of inferior occipito-frontal fasciculus (IFOF), while the second one involves the posterior branches of the IFOF, the inferior longitudinal fasciculus (ILF), and the posterior segment of the arcuate fasciculus.
Figure 6.
A, Representative slices showing the anatomical correlates of hemispheric lateralization in alpha oscillatory activity recorded during the presentation of bidirectional neutral cues in the entire sample of RBD patients. The localization of the anterior lesion peak (A, top row) and posterior peak (A, bottom row) is defined in MNI coordinates. The first map on the left of each row shows Z statistics calculated with Brunner–Munzel rank order statistics with permutation-derived correction (Brunner and Munzel, 2000; Medina et al., 2010). All peaks are significant at the p < 0.05 level. In each row, the second, third, and fourth slice show the localization of VLSM anatomical peaks in white matter pathways according to the atlas by Catani and Thiebaut De Schotten (2012): blue, IFOF; green, ILF; purple, posterior segment of the arcuate fasciculus. B, Anatomical correlates of hemispheric lateralization in alpha oscillatory activity recorded during the presentation of cues pointing to the left side of space: red (SLF III), yellow (frontal inferior longitudinal fasciculus).
A second significant correlation was found between the hemispheric lateralization of phasic alpha activity during orienting with left cues and a lesion peak in the white matter of the frontal lobe close to the head of the caudate nucleus (MNI coordinates: 42, −20, −5). This lesion overlaps with and likely disconnects the third branch of the superior longitudinal fasciculus (SLF III) and the frontal ILF (Fig. 6). All VLSM correlations resisted correction for lesion size that, on average, was higher in the N+ group (N+ = 123.353 voxels, N− 23.531 = voxels; F(1,23) = 12.7, p = 0.002).
Discussion
Here we report the first electrophysiological study that, to our knowledge, investigates the hemispheric distribution of oscillatory alpha activity (∼8 to 13 Hz) during orienting of spatial attention in RBD patients affected by left spatial neglect.
Tonic alpha (baseline-fixation period)
In regard to tonic alpha activity related to the baseline-fixation period that preceded orienting of attention with spatial cues, analyses with TSE and alpha lateralization indexes pointed out no significant hemispheric asymmetry in healthy control subjects. In contrast, RBD patients showed a tonic enhancement of alpha activity over the damaged hemisphere. Compared with healthy control subjects, this asymmetry was higher both in N− and N+ patients, and, most importantly, it was significantly higher in N+ patients than in N− patients. In the healthy observer, nonspatial factors like attentional load and decrease in alertness produce a relative enhancement of tonic alpha activity over the right hemisphere and a corresponding rightward drift of spatial attention (Newman et al., 2013; Benwell et al., 2018). In our study, analyses of hemispheric lateralization indexes showed that in the whole group of RBD patients (i.e., N+ and N− patients), the enhancement of tonic alpha activity over the damaged hemisphere was significantly correlated with neglect severity in line bisection (i.e., with the pathological rightward drift of the subjective line midpoint). It is important to note that tonic alpha asymmetry was instead not correlated with lesion size that, as it often happens, was larger in N+ patients. This result shows that hemispheric asymmetries in tonic alpha activity that are not related to episodes of lateral orienting, can provide a measure of neglect severity. Since concomitant hemianopia can have a relevant influence on the line bisection performance of N+ patients (Doricchi and Angelelli, 1999; Doricchi et al., 2005), and since in our study only N+ patients without hemianopia were considered, further investigations are needed to test whether tonic alpha asymmetry can be an index of neglect severity also in N+ patients who suffer concomitant hemianopia.
Lesions in ventral parietal and frontal areas like the TPJ and the inferior and middle frontal gyri are considered to be responsible for nonspatial symptoms in neglect, like reduced alertness and the pathological prolongation of the attentional blink (Sturm and Willmes, 2001; Husain and Rorden, 2003). According to the hypothesis advanced by Corbetta and Shulman (2011), because of anatomical contiguity ventral lesions would produce functional hypoactivation in adjacent dorsal superior parietal lobule (SPL) and intraparietal sulcus (IPS) areas. Dorsal areas in the right hemisphere would be responsible for leftward orienting and, vice versa, those in the left hemisphere for rightward orienting. Due to interhemispheric competition, hypoactivation of SPL/IPS in the right hemisphere would cause a corresponding hyperactivation of homologous areas in the left hemisphere and a pathologically rightward attentional bias (i.e., the spatial symptoms of neglect). Though providing a plausible explanation of the functional interaction between damaged ventral and intact dorsal attentional areas, this view should probably be revised in the light of more recent findings suggesting that lesions in ventral areas can also be responsible for spatially lateralized deficits, as are the pathological enhancement of novelty reaction and the contextual updating for events in the ipsilesional space and reduction of the same responses for events occurring in the contralesional space (Lasaponara et al., 2018). In addition, we have provided both univariate and multivariate fMRI evidence showing that the SPL and TPJ areas in the left hemisphere display a selective response to visually invalid targets on the right side of space, while the same areas in the right hemisphere respond to invalid targets on both sides of space (Dragone et al., 2015; Silvetti et al., 2016). This hemispheric organization of the reorienting network, which two MEG studies have also documented in the auditory domain (Kaiser et al., 2000; Dietz et al., 2014), provides a good account for the leftward-reorienting deficit experienced by N+ patients. The involvement of SPL in the spatial remapping of invalid target locations is also supported by neurophysiological studies in the monkey (Constantinidis and Steinmetz, 2001) and by lesion studies in patients with unilateral brain damage (Ptak and Schnider, 2011; Vandenberghe et al., 2012).
Phasic alpha (cue period)
In keeping with results from the study by Thut et al. (2006), the assessment of the temporal evolution of EEG spectral frequency (i.e., TSE) showed that in healthy control subjects phasic alpha activity was relatively suppressed over the right hemisphere and enhanced over the left hemisphere during orienting toward the left side of space. In contrast, when attention was directed toward the right side, no marked hemispheric lateralization in alpha activity was found. As previously considered (Thut et al., 2006), these patterns in the hemispheric distribution of alpha activity can be due to a basic leftward attentional bias (i.e., “pseudo-neglect”; Jewell and McCourt, 2000) that is linked to the general dominance of the right hemisphere in the control of spatial attention. The same results are also compatible with the hypothesis that the right hemisphere can represent and attend both the left and the right side of space, while the left hemisphere is only able to attend the right side (Mesulam, 1981; Dragone et al., 2015; Silvetti et al., 2016). This pattern of hemispheric control would in fact predict, in keeping with our results, stronger alpha asymmetry with cues driving attention to the left side of space, because in this case the right hemisphere would be predominantly involved in orienting. In contrast, with cues directing attention rightward, both hemispheres would be involved in orienting and no alpha asymmetry would be evoked.
In RBD patients, the pathological increase in alpha power that was present over the damaged hemisphere during the baseline-fixation period was also maintained during orienting with spatial cues and was independent of the direction of orienting. As for the case of tonic alpha activity, the hemispheric imbalance in phasic alpha activity was significantly higher in N+ patients than in N− patients. TSE analysis also showed that the suppression of alpha activity over the left hemisphere during rightward orienting was significantly stronger in N+ patients. This pathological enhancement seems therefore to specifically reflect the pathological ipsilesional attentional bias experienced by N+ patients. All of these results were confirmed by analyses run with alpha lateralization index based on TSE data.
To gain further insights into the hemispheric imbalance in phasic alpha activity, we run analyses of EEG ERS/ERD that allow the evaluation of changes in cue-related alpha activity with respect to corresponding baseline values recorded during the baseline-fixation period. These analyses showed that in healthy control subjects alpha power was significantly desynchronized over the hemisphere contralateral to the direction of attention, although this was not matched with a corresponding enhancement in synchronization over the ipsilateral hemisphere (Hong et al., 2015). This finding suggests a degree of independence between the desynchronization of alpha activity in one hemisphere and the synchronization of alpha activity in the other hemisphere during lateral orienting of attention. Most important, ERS/ERD analyses showed that while during leftward orienting N− patients had significant alpha desynchronization over the damaged right hemisphere, N+ patients had no similar desynchronization, a result that points at no preparatory facilitation for stimuli arriving on the left side of space in N+ patients. In contrast, during rightward orienting N+ patients showed a pathological enhancement of alpha synchronization over the right hemisphere, a result that suggests enhanced preparatory inhibition of stimuli arriving on the left side of space (Vanni et al., 1997; Foxe et al., 1998; Worden et al., 2000; Fu et al., 2001; Pfurtscheller, 2001; Kelly et al., 2006).
The evaluation of asymmetries in reaction times and detection rates of targets on the left and right sides of space showed that N+ patients experienced a pathological reduction in the detection of left targets both when cues directed attention to the right, which points to a conventional reorienting deficit in the contralesional direction (Posner et al., 1984), and when neutral cues directed attention toward both sides of space (but see also Lasaponara et al., 2018). Together, EEG and behavioral results suggest that the higher values of phasic alpha activity over the lesioned hemisphere in RBD patients are associated with a stronger bias in the detection of ipsilesional targets. We also found that, similar to tonic alpha, hemispheric asymmetries in phasic alpha activity during orienting with bilateral–neutral and left cues were positively correlated with neglect severity in the line bisection task. Interestingly, the line bisection task depends on the activity of parietal areas (Binder et al., 1992; Fink et al., 2000; Verdon et al., 2010) and taps on mechanisms that regulate the simultaneous distribution of attention along the entire horizontal space (Binder et al., 1992). Therefore, our findings might suggest a functional link between the interhemispheric imbalance of phasic alpha activity and the dysfunction of neural circuits that regulate the performance in the line bisection task in patients with acquired right brain damage.
Anatomical correlates of alpha asymmetry
VLSM analyses showed that hemispheric asymmetries in phasic alpha activity during orienting with neutral and left cues were correlated with damage in two specific brain areas. A first lesion peak was found in the white matter of the frontal lobe close to the anterior insula and to the frontal operculum. According to the white matter atlas by Catani and Thiebaut de Schotten (2012), this lesion should produce a disconnection of the inferior frontal-occipital fasciculus, which provides a direct link between frontal areas and the visual cortex. The second lesion peak was located in the white matter of the parietal lobe and overlapped with three fasciculi: (1) the IFOF; (2) the ILF that links the parietal cortex with ventral occipital-temporal visual areas; and (3) the posterior segment of the arcuate fasciculus that bridges the temporal-parietal cortex with the insular and inferior frontal cortex. In addition to confirming the role played by the lesion of white matter parietal–frontal pathways in spatial neglect (Doricchi and Tomaiuolo, 2003; Thiebaut de Schotten et al., 2005; Bartolomeo et al., 2007; Doricchi et al., 2008; Verdon et al., 2010), these results suggest an anatomical–functional explanation for the pathological alpha asymmetry experienced by RBD patients. Recent findings have in fact highlighted that occipital/parietal alpha activity correlates significantly with EEG activity in the lateral prefrontal cortex and that posterior alpha activity receives a top-down modulation from the frontal eye fields and the inferior frontal gyri (Mathewson et al., 2014; Clayton et al., 2015; Liu et al., 2016; Wang et al., 2016). Direct evidence for the frontal modulation of posterior alpha activity is provided by studies showing that transcranial magnetic stimulation over the lateral prefrontal cortex reduces the alpha-phase synchronization between prefrontal and occipital/parietal areas (Sauseng et al., 2011) and the lateralization of occipital alpha activity during lateral shifts of visuospatial attention (Mazaheri et al., 2009, 2010). Together, this evidence suggests that parietal–frontal disconnection might specifically contribute to the interhemispheric imbalance of alpha activity in right brain damage. The finding that in our patients, higher levels of alpha activity over the damaged right hemisphere were not merely linked to lesion size supports this view. Alpha asymmetries recorded during the baseline-fixation period and during the presentation of neutral or left cues were in fact all correlated with neglect severity, while only asymmetry with neutral cues was also correlated with lesion size. In addition, all of the neural correlates of phasic alpha asymmetries highlighted in VLSM analyses, including those related to neutral cues, resisted correction for lesion size.
Conclusions
To summarize, the results of the present study provide advances in the understanding of the pathological anatomical and functional correlates of the neglect syndrome and suggest an opportunity for using measures of interhemispheric lateralization of alpha activity as a diagnostic and prognostic tool for the evaluation of attentional biases in patients with right brain damage. Due to their potential clinical relevance, future studies run in large normative samples of patients should test further the reliability of our findings and conclusions.
Footnotes
This study was supported by the “Ministero della Salute” (Grant Ricerca Finalizzata #RF10.091”) and the “Fondazione Roma Terzo Pilastro” to F.D. and the Fondazione Santa Lucia Istituto di Ricovero e Cura a Carattere Scientifico to S.L.
The authors declare no competing financial interests.
References
- Bartolomeo P, Thiebaut de Schotten M, Doricchi F (2007) Left unilateral neglect as a disconnection syndrome. Cereb Cortex 17:2479–2490. 10.1093/cercor/bhl181 [DOI] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF (2003) Voxel-based lesion–symptom mapping. Nat Neurosci 6:448–450. 10.1038/nn1050 [DOI] [PubMed] [Google Scholar]
- Benwell CS, Harvey M, Thut G (2014) On the neural origin of pseudoneglect: EEG-correlates of shifts in line bisection performance with manipulation of line length. Neuroimage 86:370–380. 10.1016/j.neuroimage.2013.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benwell CSY, Keitel C, Harvey M, Gross J, Thut G (2018) Trial-by-trial co-variation of pre-stimulus EEG alpha power and visuospatial bias reflects a mixture of stochastic and deterministic effects. Eur J Neurosci 48:2566–2584. 10.1111/ejn.13688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger H. (1929) Ueber das elektroencephalogramm des menschen. Arch Psychiatr Nervenkrankheiten 87:527–570. 10.1007/BF01797193 [DOI] [Google Scholar]
- Binder J, Marshall R, Lazar R, Benjamin J, Mohr JP (1992) Distinct syndromes of hemineglect. Arch Neurol 49:1187–1194. 10.1001/archneur.1992.00530350109026 [DOI] [PubMed] [Google Scholar]
- Brunner E, Munzel U (2000) The nonparametric behrens-fisher problem: asymptotic theory and a small-sample approximation. Biom J 42:17–25. [DOI] [Google Scholar]
- Capotosto P, Babiloni C, Romani GL, Corbetta M (2009) Frontoparietal cortex controls spatial attention through modulation of anticipatory alpha rhythms. J Neurosci 29:5863–5872. 10.1523/JNEUROSCI.0539-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Thiebaut de Schotten M (2012) Atlas of human brain connections. Oxford: Oxford UP. [Google Scholar]
- Clayton MS, Yeung N, Cohen Kadosh R (2015) The roles of cortical oscillations in sustained attention. Trends Cogn Sci 19:188–195. 10.1016/j.tics.2015.02.004 [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Steinmetz MA (2001) Neuronal responses in area 7a to multiple stimulus displays: II. Responses are suppressed at the cued location. Cereb Cortex 11:592–597. 10.1093/cercor/11.7.592 [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL (2011) Spatial neglect and attention networks. Annu Rev Neurosci 34:569–599. 10.1146/annurev-neuro-061010-113731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietz MJ, Friston KJ, Mattingley JB, Roepstorff A, Garrido MI (2014) Effective connectivity reveals right-hemisphere dominance in audiospatial perception: implications for models of spatial neglect. J Neurosci 34:5003–5011. 10.1523/JNEUROSCI.3765-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Russo F, Aprile T, Spitoni G, Spinelli D (2007) Impaired visual processing of contralesional stimuli in neglect patients: a visual-evoked potential study. Brain 131:842–854. 10.1093/brain/awm281 [DOI] [PubMed] [Google Scholar]
- Doricchi F, Angelelli P (1999) Misrepresentation of horizontal space in left unilateral neglect role of hemianopia. Neurology 52:1845–1852. 10.1212/WNL.52.9.1845 [DOI] [PubMed] [Google Scholar]
- Doricchi F, Tomaiuolo F (2003) The anatomy of neglect without hemianopia: a key role for parietal–frontal disconnection? Neuroreport 14:2239–2243. 10.1097/00001756-200312020-00021 [DOI] [PubMed] [Google Scholar]
- Doricchi F, Guariglia P, Figliozzi F, Silvetti M, Bruno G, Gasparini M (2005) Causes of cross-over in unilateral neglect: between-group comparisons, within-patient dissociations and eye movements. Brain 128:1386–1406. 10.1093/brain/awh461 [DOI] [PubMed] [Google Scholar]
- Doricchi F, Iaria G, Silvetti M, Figliozzi F, Siegler I (2007) The “ways” we look at dreams: evidence from unilateral spatial neglect (with an evolutionary account of dream bizarreness). Exp Brain Res 178:450–461. 10.1007/s00221-006-0750-x [DOI] [PubMed] [Google Scholar]
- Doricchi F, Thiebaut de Schotten M, Tomaiuolo F, Bartolomeo P (2008) White matter (dis) connections and gray matter (dys) functions in visual neglect: gaining insights into the brain networks of spatial awareness. Cortex 44:983–995. 10.1016/j.cortex.2008.03.006 [DOI] [PubMed] [Google Scholar]
- Dragone A, Lasaponara S, Silvetti M, Macaluso E, Doricchi F (2015) Selective reorienting response of the left hemisphere to in- valid visual targets in the right side of space: relevance for the spatial neglect syndrome. Cortex 65:31–35. 10.1016/j.cortex.2014.12.009 [DOI] [PubMed] [Google Scholar]
- Fink GR, Marshall JC, Shah NJ, Weiss PH, Halligan PW, Grosse-Ruyken M, Ziemons K, Zilles K, Freund HJ (2000) Line bisection judgments implicate right parietal cortex and cerebellum as assessed by fMRI. Neurology 54:1324–1331. 10.1212/WNL.54.6.1324 [DOI] [PubMed] [Google Scholar]
- Foxe JJ, Simpson GV, Ahlfors SP (1998) Parieto-occipital ∼10 Hz activity reflects anticipatory state of visual attention mechanisms. Neuroreport 9:3929–3933. 10.1097/00001756-199812010-00030 [DOI] [PubMed] [Google Scholar]
- Fu KMG, Foxe JJ, Murray MM, Higgins BA, Javitt DC, Schroeder CE (2001) Attention-dependent suppression of distracter visual input can be cross-modally cued as indexed by anticipatory parieto–occipital alpha-band oscillations. Cogn Brain Res 12:145–152. 10.1016/S0926-6410(01)00034-9 [DOI] [PubMed] [Google Scholar]
- Gainotti G, De Luca L, Figliozzi F, Doricchi F (2009) The influence of distracters, stimulus duration and hemianopia on first saccade in patients with unilateral neglect. Cortex 45:506–516. 10.1016/j.cortex.2007.12.008 [DOI] [PubMed] [Google Scholar]
- Grent-'t-Jong T, Boehler CN, Kenemans JL, Woldorff MG (2011) Differential functional roles of slow-wave and oscillatory-alpha activity in visual sensory cortex during anticipatory visual–spatial attention. Cereb Cortex 21:2204–2216. 10.1093/cercor/bhq279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong X, Sun J, Bengson JJ, Mangun GR, Tong S (2015) Normal aging selectively diminishes alpha lateralization in visual spatial attention. Neuroimage 106:353–363. 10.1016/j.neuroimage.2014.11.019 [DOI] [PubMed] [Google Scholar]
- Husain M, Rorden C (2003) Non-spatially lateralized mechanisms in hemispatial neglect. Nat Rev Neurosci 4:26–36. 10.1038/nrn1005 [DOI] [PubMed] [Google Scholar]
- Jensen O, Mazaheri A (2010) Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front Hum Neurosci 4:186. 10.3389/fnhum.2010.00186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell G, McCourt ME (2000) Pseudoneglect: a review and meta-analysis of performance factors in line bisection tasks. Neuropsychologia 38:93–110. 10.1016/S0028-3932(99)00045-7 [DOI] [PubMed] [Google Scholar]
- Jung TP, Makeig S, Humphries C, Lee TW, McKeown MJ, Iragui V, Sejnowski TJ (2000) Removing electroencephalographic artifacts by blind source separation. Psychophysiology 37:163–178. 10.1111/1469-8986.3720163 [DOI] [PubMed] [Google Scholar]
- Kaiser J, Lutzenberger W, Preissl H, Ackermann H, Birbaumer N (2000) Right-hemisphere dominance for the processing of sound-source lateralization. J Neurosci 20:6631–6639. 10.1523/JNEUROSCI.20-17-06631.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly SP, Lalor EC, Reilly RB, Foxe JJ (2006) Increases in alpha oscillatory power reflect an active retinotopic mechanism for distracter suppression during sustained visuospatial attention. J Neurophysiol 95:3844–3851. 10.1152/jn.01234.2005 [DOI] [PubMed] [Google Scholar]
- Kimberg DY, Coslett HB, Schwartz MF (2007) Power in voxel-based lesion-symptom mapping. J Cogn Neurosci 19:1067–1080. 10.1162/jocn.2007.19.7.1067 [DOI] [PubMed] [Google Scholar]
- Klimesch W, Sauseng P, Hanslmayr S (2007) EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res Rev 53:63–88. 10.1016/j.brainresrev.2006.06.003 [DOI] [PubMed] [Google Scholar]
- Lasaponara S, D'Onofrio M, Pinto M, Dragone A, Menicagli D, Bueti D, Doricchi F, De Lucia M, Tomaiuolo F, Doricchi F (2018) EEG correlates of preparatory orienting, contextual updating and inhibition of sensory processing in left spatial neglect. J Neurosci 38:3792–3808. 10.1523/JNEUROSCI.2817-17.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Bengson J, Huang H, Mangun GR, Ding M (2016) Top-down modulation of neural activity in anticipatory visual attention: control mechanisms revealed by simultaneous EEG-fMRI. Cereb Cortex 26:517–529. 10.1093/cercor/bhu204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathewson KE, Beck DM, Ro T, Maclin EL, Low KA, Fabiani M, Gratton G (2014) Dynamics of alpha control: preparatory suppression of posterior alpha oscillations by frontal modulators revealed with combined EEG and event-related optical signal. J Cogn Neurosci 26:2400–2415. 10.1162/jocn_a_00637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaheri A, Nieuwenhuis IL, van Dijk H, Jensen O (2009) Prestimulus alpha and mu activity predicts failure to inhibit motor responses. Hum Brain Mapp 30:1791–1800. 10.1002/hbm.20763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazaheri A, Coffey-Corina S, Mangun GR, Bekker EM, Berry AS, Corbett BA (2010) Functional disconnection of frontal cortex and visual cortex in attention-deficit/hyperactivity disorder. Biol Psychiatry 67:617–623. 10.1016/j.biopsych.2009.11.022 [DOI] [PubMed] [Google Scholar]
- Medina J, Kimberg DY, Chatterjee A, Coslett HB (2010) Inappropriate usage of the brunner–munzel test in recent voxel-based lesion-symptom mapping studies. Neuropsychologia 48:341–343. 10.1016/j.neuropsychologia.2009.09.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesulam MM. (1981) A cortical network for directed attention and unilateral neglect. Ann Neurol 10:309–325. 10.1002/ana.410100402 [DOI] [PubMed] [Google Scholar]
- Newman DP, O'Connell RG, Bellgrove MA (2013) Linking time-on-task, spatial bias and hemispheric activation asymmetry: a neural correlate of rightward attention drift. Neuropsychologia 51:1215–1223. 10.1016/j.neuropsychologia.2013.03.027 [DOI] [PubMed] [Google Scholar]
- Newman DP, Loughnane GM, Kelly SP, O'Connell RG, Bellgrove MA (2017) Visuospatial asymmetries arise from differences in the onset time of perceptual evidence accumulation. J Neurosci 37:3378–3385. 10.1523/JNEUROSCI.3512-16.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G. (2001) Functional brain imaging based on ERD/ERS. Vis Res 41:1257–1260. 10.1016/S0042-6989(00)00235-2 [DOI] [PubMed] [Google Scholar]
- Pfurtscheller G, Lopes da Silva FH (1999) Event-related EEG/MEG synchronization and desynchronization: basic principles. Clin Neurophysiol 110:1842–1857. 10.1016/S1388-2457(99)00141-8 [DOI] [PubMed] [Google Scholar]
- Posner MI, Walker JA, Friedrich FJ, Rafal RD (1984) Effects of parietal injury on covert orienting of attention. J Neurosci 4:1863–1874. 10.1523/JNEUROSCI.04-07-01863.1984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak R, Schnider A (2011) The attention network of the human brain: relating structural damage associated with spatial neglect to functional imaging correlates of spatial attention. Neuropsychologia 49:3063–3070. 10.1016/j.neuropsychologia.2011.07.008 [DOI] [PubMed] [Google Scholar]
- Rajagovindan R, Ding M (2011) From prestimulus alpha oscillation to visual-evoked response: an inverted-U function and its attentional modulation. J Cogn Neurosci 23:1379–1394. 10.1162/jocn.2010.21478 [DOI] [PubMed] [Google Scholar]
- Rengachary J, He BJ, Shulman GL, Corbetta M (2011) A behavioral analysis of spatial neglect and its recovery after stroke. Front Hum Neurosci 5:29. 10.3389/fnhum.2011.00029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rihs TA, Michel CM, Thut G (2009) A bias for posterior α-band power suppression versus enhancement during shifting versus maintenance of spatial attention. Neuroimage 44:190–199. 10.1016/j.neuroimage.2008.08.022 [DOI] [PubMed] [Google Scholar]
- Rorden C, Karnath HO, Bonilha L (2007) Improving lesion-symptom mapping. J Cogn Neurosci 19:1081–1088. 10.1162/jocn.2007.19.7.1081 [DOI] [PubMed] [Google Scholar]
- Sauseng P, Freunberger R, Feldheim JF, Hummel FC (2011) Right prefrontal TMS disrupts interregional anticipatory EEG alpha activity during shifting of visuospatial attention. Front Psychol 2:241. 10.3389/fpsyg.2011.00241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Stadler W, Schabus M, Doppelmayr M, Hanslmayr S, Gruber WR, Birbaumer N (2005) A shift of visual spatial attention is selectively associated with human EEG alpha activity. Eur J Neurosci 22:2917–2926. 10.1111/j.1460-9568.2005.04482.x [DOI] [PubMed] [Google Scholar]
- Silvetti M, Lasaponara S, Lecce F, Dragone A, Macaluso E, Doricchi F (2016) The response of the left ventral attentional system to invalid targets and its implication for the spatial neglect syndrome: a multivariate fMRI investigation. Cereb Cortex 26:4551–4562. 10.1093/cercor/bhv208 [DOI] [PubMed] [Google Scholar]
- Sturm W, Willmes K (2001) On the functional neuroanatomy of intrinsic and phasic alertness. Neuroimage 14:S76–S84. 10.1006/nimg.2001.0839 [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Urbanski M, Duffau H, Volle E, Lévy R, Dubois B, Bartolomeo P (2005) Direct evidence for a parietal-frontal pathway subserving spatial awareness in humans. Science 309:2226–2228. 10.1126/science.1116251 [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Ffytche DH, Bizzi A, Dell'Acqua F, Allin M, Walshe M, Murray R, Murphy DG, Catani M (2011) Atlasing location, asymmetry and inter-subject variability of white matter tracts in the human brain with MR diffusion tractography. Neuroimage 54:49–59. 10.1016/j.neuroimage.2010.07.055 [DOI] [PubMed] [Google Scholar]
- Thut G, Nietzel A, Brandt SA, Pascual-Leone A (2006) α-Band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J Neurosci 26:9494–9502. 10.1523/JNEUROSCI.0875-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk H, Schoffelen JM, Oostenveld R, Jensen O (2008) Prestimulus oscillatory activity in the alpha band predicts visual discrimination ability. J Neurosci 28:1816–1823. 10.1523/JNEUROSCI.1853-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe R, Molenberghs P, Gillebert CR (2012) Spatial attention deficits in humans: the critical role of superior compared to inferior parietal lesions. Neuropsychologia 50:1092–1103. 10.1016/j.neuropsychologia.2011.12.016 [DOI] [PubMed] [Google Scholar]
- Vanni S, Revonsuo A, Hari R (1997) Modulation of the parieto-occipital alpha rhythm during object detection. J Neurosci 17:7141–7147. 10.1523/JNEUROSCI.17-18-07141.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdon V, Schwartz S, Lovblad KO, Hauert CA, Vuilleumier P (2010) Neuroanatomy of hemispatial neglect and its functional components: a study using voxel-based lesion-symptom mapping. Brain 133:880–894. 10.1093/brain/awp305 [DOI] [PubMed] [Google Scholar]
- Wang C, Rajagovindan R, Han SM, Ding M (2016) Top-down control of visual alpha oscillations: sources of control signals and their mechanisms of action. Front Hum Neurosci 10:15. 10.3389/fnhum.2016.00015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worden MS, Foxe JJ, Wang N, Simpson GV (2000) Anticipatory biasing of visuospatial attention indexed by retinotopically specific-band electroencephalography increases over occipital cortex. J Neurosci 20:RC63. 10.1523/JNEUROSCI.20-06-j0002.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyart V, Tallon-Baudry C (2009) How ongoing fluctuations in human visual cortex predict perceptual awareness: baseline shift versus decision bias. J Neurosci 29:8715–8725. 10.1523/JNEUROSCI.0962-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi N, Callan DE, Goda N, Anderson SJ, Yoshida Y, Kawato M (2003) Attentional modulation of oscillatory activity in human visual cortex. Neuroimage 20:98–113. 10.1016/S1053-8119(03)00341-0 [DOI] [PubMed] [Google Scholar]