Key Points
The NKG2D ligand ULBP-4 is expressed on healthy monocytes.
Monocyte ULBP-4 expression regulates NKG2D expression by NK cells.
Introduction
Natural killer group 2 D (NKG2D) is an activating receptor on natural killer (NK) cells. NKG2D signaling is induced when this receptor is ligated by one of multiple proteins that function as NKG2D ligands. These are the major histocompatibility complex class I chain-related (MIC) proteins and the UL16 binding proteins (ULBP1-6).1 NKG2D ligands are considered “stress ligands,” with expression induced by stressors, including viral infection or DNA damage.2,3 Nevertheless, there are cells that are not considered stressed that also express NKG2D ligands.1 The function of this expression is largely unknown.1
Monocytes from healthy donors have been reported to express little to no NKG2D ligands.4-7 However, an omission in these previous studies is measurement of ULBP-4 protein. Here, we demonstrate that ULBP-4 is expressed by monocytes from healthy individuals. This expression resulted in the downregulation of NKG2D from the cell surface of NK cells in monocyte–NK cell cocultures.
Methods
Cell purification
Blood was harvested from healthy volunteers with institutional review board approval. Peripheral blood mononuclear cells were isolated using Histopaque (Sigma-Aldrich). Monocytes were purified using the Untouched Human Monocyte Kit (Invitrogen) or anti-CD14 magnetic particles (BD Biosciences). NK cells were purified using the Untouched Human NK cells kit (Invitrogen) or anti-CD56 magnetic particles (BD Biosciences).
Antibodies, cytokines, and recombinant proteins
Anti-CD14, anti-CD3, and anti-CD56 were purchased from BD Biosciences. Anti-NKG2D and mouse immunoglobulin G1 (IgG1) were purchased from BioLegend. Anti-MICA/B, anti-ULBP1, anti-ULBP2/5/6, anti-ULBP3, anti-ULBP4 (clone 709116), mouse IgG2A, mouse IgG2B, hNKG2D-Fc, control Fc, rULBP-4-Fc, and rhIL-18 were purchased from R&D Systems. rhIL-2, rhIL-12, and rhIL-15 were purchased from Peprotech.
Immunoblotting
Cells were lysed in Laemmli buffer (Bio-Rad). ULBP-4 was detected with clone 709116 (R&D Systems).
Enzyme-linked immunosorbent assay (ELISA)
Monocytes were cultured in RPMI 1640 (Sigma-Aldrich) supplemented with 10% fetal calf serum (Hyclone), penicillin, and streptomycin (Life Technologies). Eighteen to 20 hours later, ULBP-4 present in the culture supernatant was detected using an ULBP-4–specific ELISA (MyBiosource).
Monocyte-NK coculture
Cells were cultured in a 24-well plate in RPMI 1640 supplemented with 10% fetal calf serum, penicillin, and streptomycin for 18 to 20 hours. In some experiments, the following cytokines were added: interleukin-12 (IL-12), IL-15, and IL-18 (10 ng/mL each). In other experiments, Human BD Fc Block (25 μg/mL; BD Biosciences) was added and the cells incubated at room temperature for 15 minutes; then, hNKG2D-Fc or control Fc (300 ng/mL) was added. NKG2D surface expression was measured using flow cytometry.
Jurkat killing assay
Jurkat cells were labeled with 1 μM carboxyfluorescein diacetate succinimidyl ester (Invitrogen). Carboxyfluorescein diacetate succinimidyl ester–labeled Jurkats (105 per well) and NK cells (105 per well) were plated in a 96-well round-bottom plate, spun, and cultured for 4 hours at 37°C. The number of live Jurkats in each well was then determined by flow cytometry.
Results and discussion
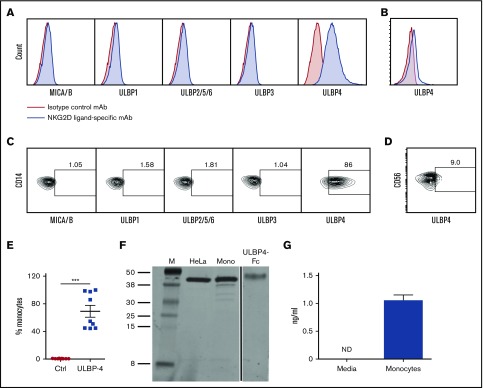
We analyzed monocytes harvested from the blood of healthy individuals for surface expression of NKG2D ligands. As reported in previous studies,4-7 little MICA, MICB, ULBP-1, ULBP-2, ULBP-3, ULBP-5, or ULBP-6 was detected (Figure 1A,C). However, ULBP-4 was detected on >40% of monocytes in all individuals tested (Figure 1A,C,E). This is in contrast to the low ULBP-4 expression on NK cells (Figure 1B,D) that we previously reported.8 We confirmed that ULBP-4 was the protein detected by performing immunoblotting (Figure 1F). NKG2D ligands can be shed from cells.9 Therefore, we looked for ULBP-4 in the supernatant of monocyte cultures, and we found ULBP-4 was present (Figure 1G). Finally, similar to what was published previously,6 we found that NKG2D ligand expression was increased on monocytes by lipopolysaccharide stimulation (supplemental Figure 1A-B). These data demonstrate that unlike other ligands that require stimulation for expression, monocytes in healthy individuals express ULBP-4 on the cell surface and shed this NKG2D ligand.
Figure 1.
Monocytes in healthy individuals express the NKG2D ligand ULBP-4. Monocytes or NK cells were purified from the peripheral blood of healthy volunteers and analyzed for expression of NKG2D ligands. (A-D) Example flow cytometry histograms (A-B) and contour plots (C-D) of NKG2D ligand staining of monocytes (4′,6-diamidino-2-phenylindole-negative −CD14+ cells) (A,C) or NK cells (4′,6-diamidino-2-phenylindole–negative CD3−CD56+ cells) (B,D) from 1 individual. The numbers shown are the percent of cells with staining with the given NKG2D ligand-specific antibody above control antibody staining. (E) The percent (average [AVG] ± standard error of the mean [SEM]) of monocytes that expressed ULBP-4 on the surface in all healthy individuals tested (n = 9). (F) Immunoblotting with ULBP-4–specific antibody with HeLa cells, monocytes purified from a healthy individual, and rULBP-Fc. These results are representative of 4 individual experiments. (G) Monocytes were cultured for 20 hours, and ULBP-4 was measured in the supernatant by ELISA. These results are representative of 4 individual experiments. ***P < .001 in 2-tailed Mann-Whitney U test. Ctrl, control; M, marker; mAb, monoclonal antibody; mono, monocytes; ND, not detected.
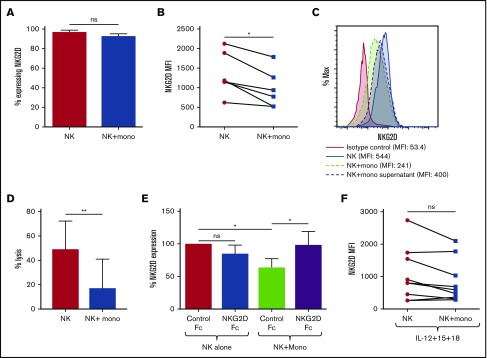
NKG2D expression on NK cells can be negatively affected by interaction with NKG2D ligand-expressing cells or soluble ligands.9 Therefore, we tested whether monocyte ULBP-4 expression reduced NKG2D on NK cells. We cultured NK cells with or without autologous monocytes and compared NKG2D expression. Although the percentage of NK cells expressing NKG2D was unaltered, the NK cells cocultured with monocytes had decreased NKG2D expression (Figure 2A-B). Although to a lesser extent, monocyte culture supernatant also reduced NKG2D (Figure 2C). ULBP-4 expression was also reduced on monocytes after coculture with NK cells, whereas soluble ULBP-4 levels were unchanged (supplemental Figure 1C-D), indicating ULBP-4 may be internalized along with NKG2D. Coculture did not induce ULBP-4 on NK cells (supplemental Figure 2A). To determine whether NKG2D expression could recover, after 20 hours of coculture, we separated the NK cells and cultured them alone. As demonstrated in other systems,10,11 within 24 hours NKG2D expression recovered (supplemental Figure 2B).
Figure 2.
ULBP-4 expression by monocytes regulates NKG2D surface expression by autologous NK cells. Monocytes and NK cells purified from the same individual were cocultured for 18 to 20 hours. (A) The percent of NK cells expressing NKG2D cultured in the presence or absence of autologous monocytes (AVG ± SEM) (n = 6). (B) The mean fluorescent intensity (MFI) (AVG ± SEM) of anti-NKG2D staining on the surface of NK cells cultured in the presence or absence of autologous monocytes (n = 6). (C) NKG2D expression on NK cells cultured with autologous monocytes or monocyte culture supernatant. These results are representative of results from 4 independent experiments. (D) Lysis of Jurkats by NK cells cultured in the absence of monocytes, or isolated from NK-monocyte coculture at an effector:target cell ratio of 1:1. These results are combined results from 2 independent experiments. (E) NK cells were cultured in the presence or absence of autologous monocytes along with NKG2D-Fc or control Fc. Twenty hours later, NKG2D surface expression on the NK cells was determined by flow cytometry. The level of anti-NKG2D staining is expressed as percent of anti-NKG2D staining on NK cells cultured in the absence of monocytes and with control Fc, which is defined as 100% expression (AVG ± SEM) (n = 5). (F) NK cells were cultured in the presence or absence of autologous monocytes and IL-12 (10 ng/mL), IL-15 (10 ng/mL), and IL-18 (10 ng/mL). Twenty hours later, NKG2D surface expression on the NK cells was determined by flow cytometry. The MFI (AVG ± SEM) of anti-NKG2D staining on the surface of NK cells is shown (n = 9). *P < .04 in 2-tailed Wilcoxon matched-pairs signed rank test. **P < .03 in 1-tailed Student t test. ns, not statistically significant.
Under no condition was NKG2D-dependent lysis of monocytes observed (supplemental Figure 3). Therefore, to test the functional consequence of reduced NKG2D, we tested the ability of NK cells to lyse Jurkat cells. Jurkats express high levels of NKG2D ligands, and killing of these cells by NK cells involves NKG2D.12,13 Corresponding with the reduced NKG2D on NK cells, the NK cells that had been cocultured with monocytes exhibited reduced killing of Jurkats compared with those that had been cultured alone (Figure 2D).
There is no blocking ULBP-4 antibody available; therefore, we blocked ULBP-4 using human NKG2D-Fc. NKG2D-Fc inhibited the NKG2D decrease on NK cells induced by monocytes (Figure 2E). This confirmed that the reduction in NKG2D was mediated by ligands on the monocytes. To determine whether NKG2D was also decreased on activated NK cells, we performed cocultures in the presence of high-dose IL-2 or the combination of IL-12, IL-15, and IL-18. In contrast to nonactivated NK cells or those activated with IL-2 (supplemental Figure 2C), NKG2D expression was not significantly changed by coculture with monocytes when NK cells were activated with IL-12, IL-15, and IL-18 (Figure 2F). These cytokines did not induce expression of other NKG2D ligands or alter ULBP-4 expression on monocytes (supplemental Figure 1E).
In summary, we found that monocytes from healthy donors expressed ULBP-4. This expression correlated with decreased NKG2D expression on cocultured autologous NK cells, which was reversed by blockade of NKG2D ligands with NKG2D-Fc. NKG2D expression was recently demonstrated to be controlled on mature mouse NK cells by NKG2D ligands expressed on macrophages or endothelial cells.14,15 Similarly, our data indicate that at least 1 function of monocyte NKG2D ligand expression is to regulate NKG2D expression on NK cells. Of the currently known NKG2D ligands, the only one detectable on healthy monocytes is ULBP-4; therefore, ULBP-4 is likely the NKG2D ligand responsible for the decreased NKG2D expression on NK cells. Despite ULBP-4 expression, healthy monocytes were not killed via NKG2D by NK cells. This is likely due to high expression of ligands for NK cell inhibitory receptors, including major histocompatibility complex class I, expressed on healthy monocytes. The cytokines IL-12, IL-15, and IL-18 have well-known functions in NK cell activation.8,16 The ability of NK cells treated with these cytokines to overcome the ULBP-4–mediated NKG2D downregulation via interaction with monocytes demonstrates yet another role for these cytokines in increasing NK cell responsiveness. ULBP-4 has been understudied compared with other NKG2D ligands.17 The role of NKG2D ligands on monocytes in cancer progression in particular is an active area of research.5,7 The results of our study demonstrate that it is critical in these studies to analyze protein expression of all 8 ligands in order to draw a conclusion as to effects on overall NKG2D ligand expression.
Supplementary Material
The full-text version of this article contains a data supplement.
Acknowledgments
This work was supported by the American Association of Immunologists Careers in Immunology Fellowship Program (M.A.M. and N.S.), the University of Kansas Cancer Center’s Cancer Support Grant P30 CA168524 (Biospecimen Repository) from the National Institutes of Health, National Cancer Institute, and the National Institutes of Health, National Institute of General Medical Sciences grant P30 GMI103326 (Flow Cytometry Core).
Authorship
Contribution: N.S. and M.A.M. performed experiments, analyzed results, made figures, and were involved in manuscript editing; and M.A.M. designed the research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Mary A. Markiewicz, Department of Microbiology, Molecular Genetics & Immunology, University of Kansas Medical Center, 3901 Rainbow Blvd, MS 3029, Kansas City, KS 66160; e-mail: mmarkiewicz@kumc.edu.
References
- 1.Trembath AP, Markiewicz MA. More than decoration: roles for natural killer group 2 member D ligand expression by immune cells. Front Immunol. 2018;9:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raulet DH, Gasser S, Gowen BG, Deng W, Jung H. Regulation of ligands for the NKG2D activating receptor. Annu Rev Immunol. 2013;31(1):413-441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lanier LL. NKG2D receptor and its ligands in host defense. Cancer Immunol Res. 2015;3(6):575-582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Campbell AR, Duggan MC, Suarez-Kelly LP, et al. . MICA-expressing monocytes enhance natural killer cell Fc receptor-mediated antitumor functions. Cancer Immunol Res. 2017;5(9):778-789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crane CA, Austgen K, Haberthur K, et al. . Immune evasion mediated by tumor-derived lactate dehydrogenase induction of NKG2D ligands on myeloid cells in glioblastoma patients. Proc Natl Acad Sci USA. 2014;111(35):12823-12828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kloss M, Decker P, Baltz KM, et al. . Interaction of monocytes with NK cells upon Toll-like receptor-induced expression of the NKG2D ligand MICA. J Immunol. 2008;181(10):6711-6719. [DOI] [PubMed] [Google Scholar]
- 7.Nowbakht P, Ionescu MC, Rohner A, et al. . Ligands for natural killer cell-activating receptors are expressed upon the maturation of normal myelomonocytic cells but at low levels in acute myeloid leukemias. Blood. 2005;105(9):3615-3622. [DOI] [PubMed] [Google Scholar]
- 8.Sharma N, Trinidad CV, Trembath AP, Markiewicz MA. NKG2D signaling between Human NK Cells Enhances TACE-Mediated TNF-α Release. J Immunol. 2017;199(8):2865-2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chitadze G, Bhat J, Lettau M, Janssen O, Kabelitz D. Generation of soluble NKG2D ligands: proteolytic cleavage, exosome secretion and functional implications. Scand J Immunol. 2013;78(2):120-129. [DOI] [PubMed] [Google Scholar]
- 10.Coudert JD, Scarpellino L, Gros F, Vivier E, Held W. Sustained NKG2D engagement induces cross-tolerance of multiple distinct NK cell activation pathways. Blood. 2008;111(7):3571-3578. [DOI] [PubMed] [Google Scholar]
- 11.Coudert JD, Zimmer J, Tomasello E, et al. . Altered NKG2D function in NK cells induced by chronic exposure to NKG2D ligand-expressing tumor cells. Blood. 2005;106(5):1711-1717. [DOI] [PubMed] [Google Scholar]
- 12.Diefenbach A, Jamieson AM, Liu SD, Shastri N, Raulet DH. Ligands for the murine NKG2D receptor: expression by tumor cells and activation of NK cells and macrophages. Nat Immunol. 2000;1(2):119-126. [DOI] [PubMed] [Google Scholar]
- 13.Bae DS, Hwang YK, Lee JK. Importance of NKG2D-NKG2D ligands interaction for cytolytic activity of natural killer cell. Cell Immunol. 2012;276(1-2):122-127. [DOI] [PubMed] [Google Scholar]
- 14.Thompson TW, Jackson BT, Li PJ, et al. . Tumor-derived CSF-1 induces the NKG2D ligand RAE-1δ on tumor-infiltrating macrophages. eLife. 2018;7:e32919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson TW, Kim AB, Li PJ, et al. . Endothelial cells express NKG2D ligands and desensitize antitumor NK responses. eLife. 2017;6:e30881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fehniger TA, Cooper MA. Harnessing NK cell memory for cancer immunotherapy. Trends Immunol. 2016;37(12):877-888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zöller T, Wittenbrink M, Hoffmeister M, Steinle A. Cutting an NKG2D ligand short: cellular processing of the peculiar human NKG2D ligand ULBP4. Front Immunol. 2018;9:620. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.