Abstract
The columnar epithelium of the alimentary tract, extending from stomach to colon, is constantly renewed by proliferation of stem and progenitor cells, which give rise to the various differentiated cell types as required by the regional specification of the gut tube. Proliferation occurs in specific zones, which in the intestine form crypts that reach into the underlying stroma. Cellular replication in the crypt is supported by an intestinal stem cell niche, the identity of which has long been controversial. Multiple recent studies have identified subepithelial telocytes, marked by expression of the winged helix transcription factor Foxl1 and the hedgehog signaling mediator Gli1, as the critical source of pro-proliferative Wnt signals to the stem/progenitor cell compartment. This review attempts to summarize and integrate these findings.
Keywords: Stem Cell Niche, Telocyte, Foxl1, Winged Helix Transcription Factors
Abbreviations used in this paper: BMP, bone morphogenetic protein; CBC, crypt base columnar cell; EGF, epidermal growth factor; SMA, smooth muscle actin
Summary.
The nature of the intestinal stem cell niche has long been controversial. Recent evidence from multiple laboratories demonstrates convincingly that subepithelial are a critical source of growth factors that maintain stem and progenitor cell proliferation.
The adult mammalian intestinal epithelium has the highest turnover rate of any tissue in the body and is replaced within 3–5 days, depending on the species and the anterior-posterior location within the gut tube.1 This rapid cellular turnover is dependent on stem and progenitor cells, which rely on signals and growth factors provided by local niche cells to support their function and self-renewal. At least 2 types of intestinal stem cells have been identified. The first are the crypt base columnar (CBC) stem cells, marked by Lgr5 and Olfm4 expression, and through genetic lineage tracing they were shown to be able to give rise to all cell types in the intestinal epithelium.2, 3 CBCs are considered the active population of intestinal stem cells and cycle about once per day. Normally quiescent stem cells residing above the crypt base around the “+4” region, ie, 4 cell diameters distal to the crypt base, are marked by reporter alleles driven by Bmi1, Tert, and Hopx promoters4, 5, 6 and appear to constitute a reserve cell population.7 When the CBC population is ablated in mice, reserve stem cells produce new CBC stem cells and repopulate the intestinal epithelium.8
Intestinal stem cells, in turn, are dependent on a complex and only partially defined stem cell niche. This is most clearly illustrated by the expansion and growth of Lgr5-positive stem cells into organoids containing proliferating and differentiated epithelial cells in vitro for both mouse and human cells.9 Although originally presented as building crypt-villus structures in vitro “without a mesenchymal niche,”9 this culture system does in fact support growth and expansion of stem cells only if factors such as R-spondin, Wnt3a, Noggin, epidermal growth factor (EGF) (factors provided in vivo in part by the mesenchymal niche), and Matrigel are provided. Matrigel is an undefined mixture of extracellular matrix proteins and growth factors that provides mechanical and adhesion cues to the organoid system, without which organoid formation does not occur. Thus, the original organoid culture systems did not establish that intestinal stem cells self-renew and differentiate by using epithelial cell-autonomous or paracrine signaling in the absence of support by signaling and structural molecules provided by mesenchymal cells; it simply provided these key molecules in the culture system.
Among the factors provided by the niche to the intestinal stem and progenitor compartment, the Wnt/R-spondin system is the major mitogen. Wnt signaling is the key driver for proliferation of intestinal stem and progenitor cells, as shown, for instance, by the rapid loss of proliferation when the Wnt inhibitor Dickkopf-1 is overexpressed in the gut epithelium.10, 11 The Wnt/β-catenin pathway is activated by binding of palmitoylated Wnt ligands to Frizzled-LRP5/6 receptor complexes, which initiates a signaling cascade leading to stabilization of cytoplasmic β-catenin, its translocation into the nucleus, and action as a transcriptional co-activator with the Tcf/Lef transcription factors.12 The medical relevance of perturbations in this system was demonstrated more than 25 years ago through the identification of the gene mutated in patients with familial adenomatous polyposis, an inherited cancer predisposition syndrome.13, 14 The gene, termed APC, encodes a large protein that functions in the control of nuclear β-catenin activity as part of the cytoplasmic “destruction complex”.15 Thus, unbridled β-catenin activity leads to uncontrolled cell division in the stem cell compartment and, eventually, development of colonic adenomas and carcinomas. Likewise, activating mutations in β-catenin itself, although less frequent, can also lead to colorectal cancer.16 Multiple genetic studies in mice have shown the key role for intracellular mediators of the Wnt signaling in stem and progenitor proliferation and stem cell maintenance.10, 17 In addition, Valenta et al18 showed recently that when Wnt production is blocked globally through the conditional ablation of Wntless, a transmembrane protein required for the secretion of mature Wnt proteins, using a ubiquitously expressed Cre driver, homeostatic renewal of the intestinal epithelium was greatly impaired. However, the critical source of the Wnt ligands remained controversial until recently.
In 2011, Sato et al19 proposed that postmitotic, differentiated Paneth cells intercalated between the CBC cells at the crypt base constitute the niche for Lgr5+ stem cells in intestinal crypts. This conclusion was based on the observation that Paneth cells express signaling molecules such as Wnt3 and EGF. Furthermore, they found that co-culture with a Paneth cell-enriched population stimulated in vitro organoid formation by Lgr5+ CBC stem cells. This model was quickly challenged by the findings in 2 independent studies showing that loss of Paneth cells in mice lacking the transcription factor Math1 (Atoh1) had no effect on intestinal stem cell maintenance or proliferation or in the regenerative response of the epithelium to injury.20, 21 Furthermore, loss of all Wnt production in the epithelium using cell type-specific gene ablation of the obligate Wnt processing enzyme Porcupine (Porcn),22, 23 or the deletion of Wnt3 in Paneth cells, had no effect on crypt health.24 The latter study was consistent with earlier work by Gordon and colleagues who used 2 independent methods to ablate mature Paneth cells and found no effect on stem cell function in intestinal crypts.25 Together, these studies suggested the existence of an alternate or additional source of Wnt ligands. Whether this second source provides the primary niche Wnt signal to CBC stem cells or acts redundantly with Paneth cell-derived Wnt signals was uncertain until recently.
One possible and obvious alternative source of mitogenic Wnt signals is the mesenchymal compartment or stroma, which contains myofibroblasts and other cell types that are located in close proximity to intestinal epithelial stem cells.26 San Roman et al23 investigated the possibility that myofibroblasts represent the alternate niche Wnt source through inhibition of all Wnt ligand production via ablation of Porcn in Myh11-expressing myofibroblasts and smooth muscle cells. Somewhat surprisingly at the time, ablating Wnt signaling in the Myh11-Cre model had no effect on intestinal morphogenesis and stem cell function. Initially, these studies seemed to favor the notion that redundant Wnt signaling from both epithelial and mesenchymal compartments is responsible for crypt maintenance.
Recently, we discovered that a rare population of submucosal cells expressing the transcription factor Foxl1 is required for crypt maintenance.27, 28 Foxl1 is a DNA-binding transcription factor of the forkhead class that is characterized by an evolutionarily conserved 100 amino acid winged helix DNA binding domain.29, 30 In the gut, Foxl1-expressing cells appear first during midgestation (embryonic day 12.5 in the mouse),31 just before the epithelial transition. The epithelial transition, or formation of villi, refers to the transformation of the pseudo-stratified epithelium of the primitive gut tube, which is radially surrounded by mesenchymal cells, to the columnar epithelium structured into villi with mesenchymal cores that are characteristic of the mature intestine.32 This epithelial transition, which occurs on embryonic day 14.5 in mice and during gestational weeks 8–10 in humans, coincides with the first appearance of bone morphogenetic protein (BMP)–expressing mesenchymal clusters that accompany the formation of nascent villi.32 In fact, Walton et al32 showed that multiple BMPs (2, 4, 5, and 7) are expressed in these clusters, and furthermore, that inhibiting BMP signaling caused the merging of clusters and the formation of fewer villi.32 The relationship between Foxl1 activity, BMP signaling, and villus formation is supported by the finding that villus formation is delayed in Foxl1 null embryos, and that expression of Bmp 2 and 4 is reduced.33 Foxl1, in turn, is activated by hedgehog signaling from the epithelium via the binding of Gli transcription factors to multiple ultra-conserved Gli binding sites in the Foxl1/Foxf1 locus.34
In adulthood, Foxl1-expressing cells remain intimately associated with the basolateral membranes of all alimentary columnar epithelial cells (Figure 1) and expand through the elaboration of long, thin cytoplasmic extensions, reaching several hundred micrometers in size, compared with the approximately 10-μm width of a columnar epithelial cell.28 Cells with these properties were described as early as 1964, when Deane35 published the first ultrastructural analysis of the lamina propria and called these cells fibrocytes. Kaye and colleagues used transmission electron microscopy to determine that these cells form a “pericryptal fibroblast sheath” and are distinct from other mesenchymal cells, including the previously studied myofibroblasts.36, 37 They showed that these cells are closely appositioned to the epithelial cells of the colonic crypt, with only the 60-nm basal lamina separating the 2 cell types, and engage all epithelial cells in the proliferative region of the crypt. They estimated that each pericryptal fibroblast contacts 50–70 epithelial cells, depending on the position along the colonic crypt. Using autoradiography of 3H-thymidine pulse-labeled colon, they demonstrated further that pericryptal fibroblasts are 35 times less likely to be in the cell cycle than crypt epithelial cells.37 Very recently, genetic pulse-labeling using Gremlin1-CreER::Lox-Stop-Lox-GFP mice confirmed the slow turnover of the pericryptal fibroblast, because it took about 12 months after a tamoxifen pulse until all sheath cells had been replaced by cells expressing the GFP lineage label.38
Figure 1.
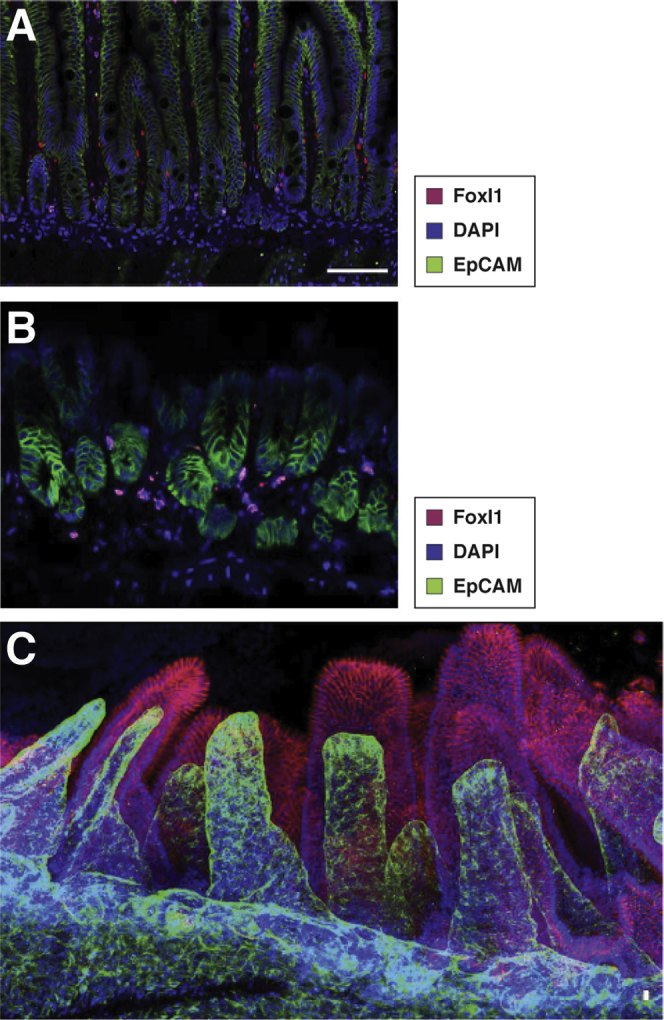
Foxl1+telocytes establish a subepithelial plexus immediately underneath the gastrointestinal epithelium. (A) Nuclei of telocytes in the mouse jejunum are labeled by Foxl1 immunostaining (red). EpCAM (green) outlines epithelial cells, and DAPI (blue) outlines all nuclei. Reprinted with permission from Aoki et al.27 (B) Nuclei of telocytes in the mouse glandular stomach are labeled by Foxl1 immunostaining (red). EpCAM (green) outlines epithelial cells, and DAPI (blue) outlines all nuclei. (C) Confocal imaging of X-Clarity (Logos Biosystems, Anyang-si, Gyeonggi-do, South Korea) cleared mouse jejunum demonstrates the telocyte plexus, here visualized by platelet-derived growth factor receptor-alpha staining (green) that underlies all epithelial cells. EpCAM (red) was used to label epithelial cells. Reprinted with permission from Shoshkes-Carmel et al.28
The first immunocytochemical stain for colonic pericryptal sheath cells was developed by the Bodmer group, although their PR 2D3 antibody also reacted with vascular smooth muscle and the smooth muscle cells in the muscularis mucosae.39 Bodmer and colleagues argued that pericryptal sheath cells belong to the smooth muscle or myofibroblast lineage, based on staining of the cells for smooth muscle actin (SMA) and myosin. We demonstrated previously that Foxl1-positive subepithelial cells are negative for α-smooth muscle actin (αSMA) and myosin heavy chain 11 (Myh11) immunoreactivity and express critical crypt signaling proteins such as Wnt2b, Wnt5a, and R-spondin3 as well as the BMP antagonists Gremlin 1 and Gremlin 2.27 Shortly thereafter, Stzepourginski et al40 identified Cd34+gp38+ mesenchymal cells as crypt stromal cells and showed that these cells support proliferation of intestinal stem cells in the organoid culture system. Although Foxl1+ cells form a continuous plexus from the crypt bottom to the villus tip (Figure 1C), Cd34+gp38+ are restricted to the crypt region.40 The notion that Foxl1+ cells are distinct from myofibroblasts is supported further by the aforementioned finding from the Shivdasani group that ablating all Wnt signaling in myofibroblasts using Myh11-CreER;PorcnloxP mice had no effect on epithelial health.23 Thus, Foxl1+ cells are not classic myofibroblasts.
Popescu coined the term telocytes for these pericryptal sheath cells, which had previously been known as interstitial Cajal-like cells but later found in 2010 to be completely distinct from Cajal cells, which are pacemaker cells involved in controlling smooth muscle contractions.41, 42 Telocytes are a distinct type of interstitial cells characterized by a small cell body and extremely long and thin extensions termed telopodes, and we have adopted this term for the Foxl1-positive cells for brevity and because it better distinguishes this cell type with unique morphologic features from other interstitial cells such as fibroblasts, myofibroblasts, and smooth muscle cells.
Each Foxl1+ telocyte is in close contact with dozens of epithelial cells, and together the Foxl1+ cells form a subepithelial plexus that extends from the glandular stomach to the colon and thus is in contact with all columnar epithelia of the alimentary canal (Figure 1).28 The first functional evidence that Foxl1-expressing telocytes are a critical component of the intestinal stem cell niche came from experiments in mice published in 2016 where diphtheria toxin–mediated ablation in Foxl1-DTA mice resulted in cessation of epithelial proliferation within a few days after administration of the toxin.27 Remarkably, just 3 days after loss of Foxl1+ telocytes, the length of both small and large bowel was shortened, jejunal villus length was cut in half, and the number of cycling cells per crypt was reduced by more than 95%. This study established an absolute requirement for Foxl1-expressing cells in crypt maintenance. Although Wnt signaling to the epithelium, as assessed by nuclear β-catenin staining, was clearly reduced, this study did not unequivocally address whether the effect of Foxl1+ telocyte ablation was mediated solely by Wnt proteins or whether additional signaling or support molecules produced by these cells act to maintain CBC self-renewal and crypt-villus fidelity.
Using the genetic handle of the Foxl1-Cre driver, Shoshkes-Carmel et al28 sorted intestinal telocytes from Foxl1-Cre::Rosa26-mTmG mice and performed transcriptome profiling. From this experiment, 2 important themes emerged. First, Foxl1+ telocytes have an expression profile clearly distinct from Foxl1-negative mesenchymal cells (eg, myofibroblasts) or epithelial cells, with high expression of specific Wnt molecules such as the canonical Wnt ligand Wnt2b and the non-canonical ligand Wnt5a, as well as the key Bmp molecules 4, 5, 6 and 7, the receptors and downstream mediators of the hedgehog pathway, as well as other key growth factors. Second and initially puzzling, Foxl1 telocytes express not just the Wnt ligands as expected but also multiple antagonists of the canonical Wnt pathway, such as Dkk2 and 3, and the decoy receptor Sfrp1. This apparently paradoxical observation was resolved by using single molecule RNA-FISH, which showed that telocytes partition the production of Wnt activators and inhibitors such that the former are enriched near the crypt base, whereas the latter are more abundant near the crypt-villus junction. It will be fascinating to investigate how Foxl1 cells control the localized production of these key signaling molecules to ensure that proliferation only occurs in the crypt.
Answering the question of whether Wnt signals emanating from telocytes are indeed required for crypt function, Shoshkes-Carmel et al went on to develop an inducible Foxl1-CreER line and crossed these with mice carrying a loxP-flanked allele of Porcn.28, 43 Porcn encodes a Golgi-resident enzyme that is required for the maturation of all Wnt proteins, and thus its ablation eliminates all Wnt production from a Porcn–/– cell. Remarkably, the consequences for intestinal and organismal health were striking and immediate. Within 24 hours after tamoxifen administration, DNA replication in the crypt compartment had ceased, and by 72 hours crypts had collapsed, jejunal villus length was reduced by 90%, and epithelial integrity had been lost. In keeping with the loss of crypt function, expression of the stem cell marker Olfm4 was eliminated, and the Wnt/β-catenin pathway was shut off. This finding provided clear evidence that the Wnt proteins produced by epithelial cells including the Paneth cells are not able to compensate for the loss of Wnt signals from Foxl1+ telocytes, and neither were myofibroblasts or other Foxl1-negative stromal cells. This study therefore established Foxl1-expressing subepithelial telocytes as the critical source of Wnt signals that are necessary to maintain the proliferation and function of intestinal stem and transit amplifying cells. Although other intestinal cell types, including epithelial Paneth cells, express multiple Wnt proteins, they are unable to compensate for the loss of Wnt ligands produced by Foxl1-positive cells. Further support for this notion comes from a study by Degirmenci et al44 that ablated Wntless – like Porcupine required for the production of all Wnt proteins – in Gli1-producing cells in the mesenchyme.44 Colonic, but not small intestinal, crypts collapsed in this model, albeit with much slower kinetics. This difference compared with the very rapid crypt collapse in the Foxl1-CreER::PorcnloxP/loxP model is likely the result of the very long half-life of the Wntless protein. Previously, Valenta et al18 had ablated Wntless by using the ubiquitously expressed Rosa26-CreER driver and showed that whereas Wntless mRNA was eliminated 6 days after tamoxifen treatment as expected, Wntless protein was still detectable a full 10 days after the onset of gene deletion. Foxl1 and the related and genetically linked gene Foxf1 had been shown almost 10 years ago to be targets of the Gli proteins,34 responding to hedgehog proteins secreted by the gastrointestinal epithelium; thus it fits well that ablation of Wnt secretion from Gli1-expressing cells results in a phenotype similar to its elimination from Foxl1+ telocytes. A striking difference between the model by Degirmenci et al,44 ie, ablation of Wntless using an inducible Gli1-CreER driver, and ablation of Porcupine using the Foxl1-CreER transgene28 is the fact that only the latter shows a dramatic phenotype in the small intestine. Wntless ablation by Gli1-CreER only caused small intestinal crypt collapse when combined with a Villin-CreER driver, which thus removed any Wnt proteins emanating from the intestinal epithelium.44 Why epithelial Wnt proteins can compensate for loss of Wntless in Gli1+ stromal cells, although they cannot overcome the loss of Porcn in Foxl1+ telocytes, is presently unknown, although it is possible that the differences in the kinetics of Wnt signaling removal might be at least part of the cause.
In addition, ablation of Porcupine using a PDGFRα-Cre line in a study by Grecius et al45 caused a marked decrease in the number of proliferating cells in neonatal crypts, again consistent with the findings of Shoshkes-Carmel et al.28 This study also established that R-spondin 3 is a more potent activator of the Wnt pathway than R-spondin 1 and confirmed that R-spondin 3 is expressed in pericryptal fibroblast, or intestinal telocytes. However, PDGFRα-Cre driven ablation of R-spondin 3 did not perturb normal intestinal homeostasis; effects were only seen in dextran sodium sulfate–induced colitis. Greicius et al showed further that pericryptal fibroblasts genetically labeled by a PDGFRα-Cre driver support the growth of organoids deficient for Porcupine. It should be noted that although all Foxl1+ cells expressed PDGFRα, most PDGFRα cells in the gut stroma are Foxl1-negative (ie, Foxl1+ telocytes are a small subset of PDGFRα+ cells),28 making the Foxl1-CreER model more specific for gastrointestinal telocytes. Table 1 summarizes the observations from the relevant mouse models discussed here. Taking the findings from multiple laboratories together, it appears that the debate on the identity of the intestinal stem cell niche is settled.46 A schematic summarizing the current view of the intestinal stem cell niche is shown in Figure 2.
Table 1.
Mouse Models and Their Phenotypes
| Gene targeted | Timing of gene ablation | Targeted cell types | Phenotype | Reference |
|---|---|---|---|---|
| Porcupine (Porcn) | Adulthood | Foxl1+ telocytes, conditionally ablated in adult mice | Loss of active Wnt signaling in the crypt, loss of all crypt proliferation | 28 |
| Porcupine (Porcn) | Fetal life | Intestinal epithelial cells (VillinCre) | Normal | 23 |
| Porcupine (Porcn) | Fetal life | Myofibroblasts (Myh11-Cre) | Normal | 23 |
| Porcupine (Porcn) | Fetal life | PDGFRalpha expressing submucosal cells | Early postnatal lethality; reduced epithelial proliferation | 45 |
| R-spondin 3 (Rspo3) | Fetal life | PDGFRalpha expressing submucosal cells | Epithelial homeostasis normal, increased sensitivity to DSS colitis | 45 |
| Wntless (Wls) | Adulthood | Gli1-expresssing submucosal cells | Loss of active Wnt signaling in colonic crypts, loss of colonic crypt proliferation. Small intestinal crypts appear normal | 44 |
DSS, dextran sodium sulfate; PDGFR, platelet-derived growth factor.
Figure 2.
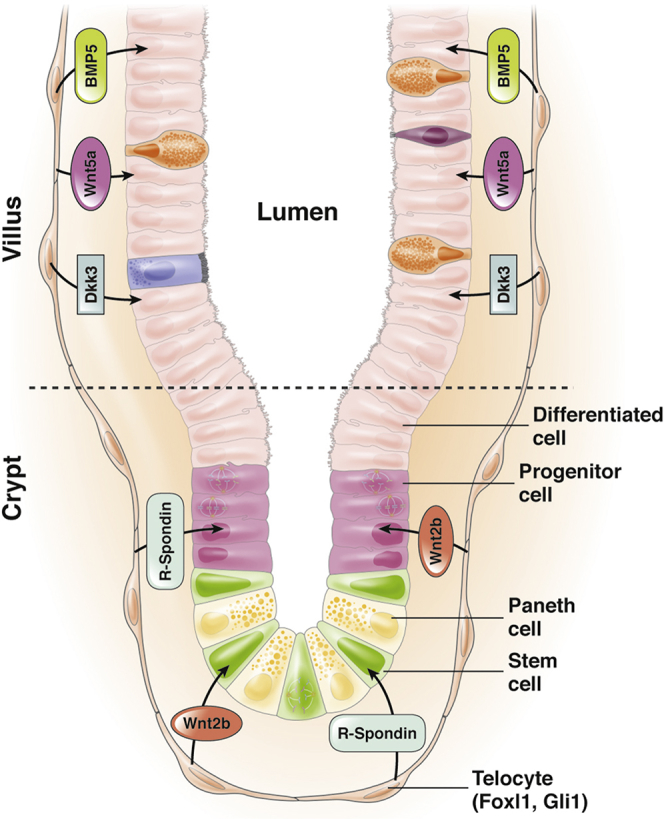
Foxl1+telocytes are closely appositioned to the intestinal epithelium and compartmentalize production of key signaling molecules along the crypt-villus axis. Foxl1/Gli1 positive telocytes are large, thin cells that form a continuous plexus underneath the gastric and intestinal epithelium. Telocytes compartmentalize production of signaling molecules such that expression of pro-proliferative factors such as R-Spondin and Wnt2b is highest in the crypt, and that of pro-differentiation factors such as BMP5 and Wnt5a is highest in the villi, thus supporting both intestinal regeneration as well as differentiation.
Foxl1-expressing telocytes are not restricted to the mouse model but have recently been described in the human colon as well.47 Kinchen et al47 used single cell RNA-seq analysis of the colonic stroma from patients with ulcerative colitis and healthy controls. A population they termed stromal 2 closely resembles the murine telocytes described above, because it is Foxl1 positive and expresses Bmp2 and Wnt5a. Interestingly, there appeared to be a decrease in the proportion of stromal 2 cells in the patients with ulcerative colitis, raising the exciting possibility that these cells might play a role in the pathogenesis of inflammatory bowel disease. Because of the unique location of telocytes at the interface between epithelium and submucosa, it is tempting to speculate that these cells could mediate the interactions of luminal antigens with the immune system after failure of the intestinal barrier. Clearly, much work remains to be done to understand the full contribution of these important players in the intestinal stem cell niche.
Acknowledgments
The author thanks Avital Swisa, Ayana Kondo, and Chris Lengner for critical reading of the manuscript. Related work in the author’s lab was supported through NIDDK R37-DK053839.
Footnotes
Conflicts of interest The author discloses no conflicts.
References
- 1.Creamer B., Shorter R.G., Bamforth J. The turnover and shedding of epithelial cells: I—the turnover in the gastro-intestinal tract. Gut. 1961;2:110–118. doi: 10.1136/gut.2.2.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker N., van Es J.H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J., Clevers H. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 3.van der Flier L.G., Haegebarth A., Stange D.E., van de Wetering M., Clevers H. OLFM4 is a robust marker for stem cells in human intestine and marks a subset of colorectal cancer cells. Gastroenterology. 2009;137:15–17. doi: 10.1053/j.gastro.2009.05.035. [DOI] [PubMed] [Google Scholar]
- 4.Montgomery R.K., Carlone D.L., Richmond C.A., Farilla L., Kranendonk M.E., Henderson D.E., Baffour-Awuah N.Y., Ambruzs D.M., Fogli L.K., Algra S., Breault D.T. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci U S A. 2011;108:179–184. doi: 10.1073/pnas.1013004108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sangiorgi E., Capecchi M.R. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takeda N., Jain R., LeBoeuf M.R., Wang Q., Lu M.M., Epstein J.A. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334:1420–1424. doi: 10.1126/science.1213214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yousefi M., Li L., Lengner C.J. Hierarchy and plasticity in the intestinal stem cell compartment. Trends Cell Biol. 2017;27:753–764. doi: 10.1016/j.tcb.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tian H., Biehs B., Warming S., Leong K.G., Rangell L., Klein O.D., de Sauvage F.J. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sato T., Vries R.G., Snippert H.J., van de Wetering M., Barker N., Stange D.E., van Es J.H., Abo A., Kujala P., Peters P.J., Clevers H. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 10.Pinto D., Gregorieff A., Begthel H., Clevers H. Canonical Wnt signals are essential for homeostasis of the intestinal epithelium. Genes Dev. 2003;17:1709–1713. doi: 10.1101/gad.267103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kuhnert F., Davis C.R., Wang H.T., Chu P., Lee M., Yuan J., Nusse R., Kuo C.J. Essential requirement for Wnt signaling in proliferation of adult small intestine and colon revealed by adenoviral expression of Dickkopf-1. Proc Natl Acad Sci U S A. 2004;101:266–271. doi: 10.1073/pnas.2536800100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Logan C.Y., Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. doi: 10.1146/annurev.cellbio.20.010403.113126. [DOI] [PubMed] [Google Scholar]
- 13.Kinzler K.W., Nilbert M.C., Vogelstein B., Bryan T.M., Levy D.B., Smith K.J., Preisinger A.C., Hamilton S.R., Hedge P., Markham A. Identification of a gene located at chromosome 5q21 that is mutated in colorectal cancers. Science. 1991;251:1366–1370. doi: 10.1126/science.1848370. [DOI] [PubMed] [Google Scholar]
- 14.Nishisho I., Nakamura Y., Miyoshi Y., Miki Y., Ando H., Horii A., Koyama K., Utsunomiya J., Baba S., Hedge P. Mutations of chromosome 5q21 genes in FAP and colorectal cancer patients. Science. 1991;253:665–669. doi: 10.1126/science.1651563. [DOI] [PubMed] [Google Scholar]
- 15.Stamos J.L., Weis W.I. The beta-catenin destruction complex. Cold Spring Harb Perspect Biol. 2013;5:a007898. doi: 10.1101/cshperspect.a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fearon E.R. Molecular genetics of colorectal cancer. Annu Rev Pathol. 2011;6:479–507. doi: 10.1146/annurev-pathol-011110-130235. [DOI] [PubMed] [Google Scholar]
- 17.van Es J.H., Haegebarth A., Kujala P., Itzkovitz S., Koo B.K., Boj S.F., Korving J., van den Born M., van Oudenaarden A., Robine S., Clevers H. A critical role for the Wnt effector Tcf4 in adult intestinal homeostatic self-renewal. Mol Cell Biol. 2012;32:1918–1927. doi: 10.1128/MCB.06288-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Valenta T., Degirmenci B., Moor A.E., Herr P., Zimmerli D., Moor M.B., Hausmann G., Cantu C., Aguet M., Basler K. Wnt ligands secreted by subepithelial mesenchymal cells are essential for the survival of intestinal stem cells and gut homeostasis. Cell Rep. 2016;15:911–918. doi: 10.1016/j.celrep.2016.03.088. [DOI] [PubMed] [Google Scholar]
- 19.Sato T., van Es J.H., Snippert H.J., Stange D.E., Vries R.G., van den Born M., Barker N., Shroyer N.F., van de Wetering M., Clevers H. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–418. doi: 10.1038/nature09637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Durand A., Donahue B., Peignon G., Letourneur F., Cagnard N., Slomianny C., Perret C., Shroyer N.F., Romagnolo B. Functional intestinal stem cells after Paneth cell ablation induced by the loss of transcription factor Math1 (Atoh1) Proc Natl Acad Sci U S A. 2012;109:8965–8970. doi: 10.1073/pnas.1201652109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim T.H., Escudero S., Shivdasani R.A. Intact function of Lgr5 receptor-expressing intestinal stem cells in the absence of Paneth cells. Proc Natl Acad Sci U S A. 2012;109:3932–3937. doi: 10.1073/pnas.1113890109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kabiri Z., Greicius G., Madan B., Biechele S., Zhong Z., Zaribafzadeh H., Edison, Aliyev J., Wu Y., Bunte R., Williams B.O., Rossant J., Virshup D.M. Stroma provides an intestinal stem cell niche in the absence of epithelial Wnts. Development. 2014;141:2206–2215. doi: 10.1242/dev.104976. [DOI] [PubMed] [Google Scholar]
- 23.San Roman A.K., Jayewickreme C.D., Murtaugh L.C., Shivdasani R.A. Wnt secretion from epithelial cells and subepithelial myofibroblasts is not required in the mouse intestinal stem cell niche in vivo. Stem Cell Reports. 2014;2:127–134. doi: 10.1016/j.stemcr.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Farin H.F., Van Es J.H., Clevers H. Redundant sources of Wnt regulate intestinal stem cells and promote formation of Paneth cells. Gastroenterology. 2012;143:1518–1529 e7. doi: 10.1053/j.gastro.2012.08.031. [DOI] [PubMed] [Google Scholar]
- 25.Garabedian E.M., Roberts L.J., McNevin M.S., Gordon J.I. Examining the role of Paneth cells in the small intestine by lineage ablation in transgenic mice. J Biol Chem. 1997;272:23729–23740. doi: 10.1074/jbc.272.38.23729. [DOI] [PubMed] [Google Scholar]
- 26.Roulis M., Flavell R.A. Fibroblasts and myofibroblasts of the intestinal lamina propria in physiology and disease. Differentiation. 2016;92:116–131. doi: 10.1016/j.diff.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 27.Aoki R., Shoshkes-Carmel M., Gao N., Shin S., May C.L., Golson M.L., Zahm A.M., Ray M., Wiser C.L., Wright C.V., Kaestner K.H. Foxl1-expressing mesenchymal cells constitute the intestinal stem cell niche. Cell Mol Gastroenterol Hepatol. 2016;2:175–188. doi: 10.1016/j.jcmgh.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shoshkes-Carmel M., Wang Y.J., Wangensteen K.J., Toth B., Kondo A., Massasa E.E., Itzkovitz S., Kaestner K.H. Subepithelial telocytes are an important source of Wnts that supports intestinal crypts. Nature. 2018;557:242–246. doi: 10.1038/s41586-018-0084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hannenhalli S., Kaestner K.H. The evolution of Fox genes and their role in development and disease. Nat Rev Genet. 2009;10:233–240. doi: 10.1038/nrg2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaestner K.H., Lee K.H., Schlondorff J., Hiemisch H., Monaghan A.P., Schutz G. Six members of the mouse forkhead gene family are developmentally regulated. Proc Natl Acad Sci U S A. 1993;90:7628–7631. doi: 10.1073/pnas.90.16.7628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaestner K.H., Bleckmann S.C., Monaghan A.P., Schlondorff J., Mincheva A., Lichter P., Schutz G. Clustered arrangement of winged helix genes fkh-6 and MFH-1: possible implications for mesoderm development. Development. 1996;122:1751–1758. doi: 10.1242/dev.122.6.1751. [DOI] [PubMed] [Google Scholar]
- 32.Walton K.D., Whidden M., Kolterud A., Shoffner S.K., Czerwinski M.J., Kushwaha J., Parmar N., Chandhrasekhar D., Freddo A.M., Schnell S., Gumucio D.L. Villification in the mouse: Bmp signals control intestinal villus patterning. Development. 2016;143:427–436. doi: 10.1242/dev.130112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaestner K.H., Silberg D.G., Traber P.G., Schutz G. The mesenchymal winged helix transcription factor Fkh6 is required for the control of gastrointestinal proliferation and differentiation. Genes Dev. 1997;11:1583–1595. doi: 10.1101/gad.11.12.1583. [DOI] [PubMed] [Google Scholar]
- 34.Madison B.B., McKenna L.B., Dolson D., Epstein D.J., Kaestner K.H. FoxF1 and FoxL1 link hedgehog signaling and the control of epithelial proliferation in the developing stomach and intestine. J Biol Chem. 2009;284:5936–5944. doi: 10.1074/jbc.M808103200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Deane H.W. Some electron microscopic observations on lamina propria of gut with comments on close association of macrophages plasma cells + eosinophils. Anat Rec. 1964;149:453–473. doi: 10.1002/ar.1091490315. [DOI] [PubMed] [Google Scholar]
- 36.Kaye G.I., Lane N., Pascal R.R. Colonic pericryptal fibroblast sheath: replication migration and cytodifferentiation of a mesenchymal cell system in adult tissue—2: fine structural aspects of normal rabbit and human colon. Gastroenterology. 1968;54:852–865. [PubMed] [Google Scholar]
- 37.Pascal R.R., Kaye G.I., Lane N. Colonic pericryptal fibroblast sheath: replication migration and cytodifferentiation of a mesenchymal cell system in adult tissue—1: autoradiographic studies of normal rabbit colon. Gastroenterology. 1968;54:835–851. [PubMed] [Google Scholar]
- 38.Worthley D.L., Churchill M., Compton J.T., Tailor Y., Rao M., Si Y., Levin D., Schwartz M.G., Uygur A., Hayakawa Y., Gross S., Renz B.W., Setlik W., Martinez A.N., Chen X., Nizami S., Lee H.G., Kang H.P., Caldwell J.M., Asfaha S., Westphalen C.B., Graham T., Jin G., Nagar K., Wang H., Kheirbek M.A., Kolhe A., Carpenter J., Glaire M., Nair A., Renders S., Manieri N., Muthupalani S., Fox J.G., Reichert M., Giraud A.S., Schwabe R.F., Pradere J.P., Walton K., Prakash A., Gumucio D., Rustgi A.K., Stappenbeck T.S., Friedman R.A., Gershon M.D., Sims P., Grikscheit T., Lee F.Y., Karsenty G., Mukherjee S., Wang T.C. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell. 2015;160:269–284. doi: 10.1016/j.cell.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Richman P.I., Tilly R., Jass J.R., Bodmer W.F. Colonic pericrypt sheath-cells: characterization of cell type with new monoclonal-antibody. J Clin Pathol. 1987;40:593–600. doi: 10.1136/jcp.40.6.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stzepourginski I., Nigro G., Jacob J.M., Dulauroy S., Sansonetti P.J., Eberl G., Peduto L. CD34+ mesenchymal cells are a major component of the intestinal stem cells niche at homeostasis and after injury. Proc Natl Acad Sci U S A. 2017;114:E506–E513. doi: 10.1073/pnas.1620059114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cretoiu D., Cretoiu S.M., Simionescu A.A., Popescu L.M. Telocytes, a distinct type of cell among the stromal cells present in the lamina propria of jejunum. Histol Histopathol. 2012;27:1067–1078. doi: 10.14670/HH-27.1067. [DOI] [PubMed] [Google Scholar]
- 42.Cretoiu S.M., Popescu L.M. Telocytes revisited. Biomol Concepts. 2014;5:353–369. doi: 10.1515/bmc-2014-0029. [DOI] [PubMed] [Google Scholar]
- 43.Liu W., Shaver T.M., Balasa A., Ljungberg M.C., Wang X., Wen S., Nguyen H., Van den Veyver I.B. Deletion of Porcn in mice leads to multiple developmental defects and models human focal dermal hypoplasia (Goltz syndrome) PLoS One. 2012;7:e32331. doi: 10.1371/journal.pone.0032331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Degirmenci B., Valenta T., Dimitrieva S., Hausmann G., Basler K. GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. Nature. 2018;558:449–453. doi: 10.1038/s41586-018-0190-3. [DOI] [PubMed] [Google Scholar]
- 45.Greicius G., Kabiri Z., Sigmundsson K., Liang C., Bunte R., Singh M.K., Virshup D.M. PDGFRalpha(+) pericryptal stromal cells are the critical source of Wnts and RSPO3 for murine intestinal stem cells in vivo. Proc Natl Acad Sci U S A. 2018;115:E3173–E3181. doi: 10.1073/pnas.1713510115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Samuelson L.C. Debate over the identity of an intestinal niche-cell population settled. Nature. 2018;558:380–381. doi: 10.1038/d41586-018-05281-z. [DOI] [PubMed] [Google Scholar]
- 47.Kinchen J., Chen H.H., Parikh K., Antanaviciute A., Jagielowicz M., Fawkner-Corbett D., Ashley N., Cubitt L., Mellado-Gomez E., Attar M., Sharma E., Wills Q., Bowden R., Richter F.C., Ahern D., Puri K.D., Henault J., Gervais F., Koohy H., Simmons A. Structural remodeling of the human colonic mesenchyme in inflammatory bowel disease. Cell. 2018;175:372–386 e17. doi: 10.1016/j.cell.2018.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]


