Abstract
Objective
To measure postmortem burden of frontotemporal lobar degeneration (FTLD) with TDP-43 (FTLD-TDP) or tau (FTLD-Tau) proteinopathy across hemispheres in primary progressive aphasia (PPA) using digital histopathology, and identify clinicopathological correlates of these distinct proteinopathies.
Methods
In an autopsy cohort of PPA (FTLD-TDP=13, FTLD-Tau=14), we analyzed laterality and regional distribution of postmortem pathology, quantified using a validated digital histopathological approach, in available brain tissue from up to eight cortical regions bilaterally. We related digital pathology to antemortem structural neuroimaging and specific clinical language features.
Results
Postmortem cortical pathology was left-lateralized in both FTLD-TDP (beta=−0.15,SE=0.05,p=0.007) and FTLD-Tau (beta=−0.09,SE=0.04,p=0.015), but the degree of lateralization decreased with greater overall dementia severity before death (beta=−8.18,SE=3.22,p=0.015). Among five core pathology regions sampled, we found greatest pathology in left orbitofrontal cortex (OFC) in FTLD-TDP, which was greater than in FTLD-Tau (F=47.07,df=1,17,p<0.001), and in left mid-frontal cortex (MFC) in FTLD-Tau, which was greater than in FTLD-TDP (F=19.34,df=1,16,p<0.001). Postmortem pathology was inversely associated with antemortem MRI cortical thickness (beta=−0.04,SE=0.01,p=0.007) in regions matching autopsy sampling. Irrespective of PPA syndromic variant, single-word comprehension impairment was associated with greater left OFC pathology (t=−3.72,df=10.72,p=0.004), while nonfluent speech with greater left MFC pathology (t=−3.62,df=12.00,p=0.004) among the five core pathology regions.
Interpretation
In PPA, FTLD-TDP and FTLD-Tau have divergent anatomic distributions of left-lateralized postmortem pathology that relate to antemortem structural imaging and distinct language deficits. While other brain regions may be implicated in neural networks supporting these complex language measures, our observations may eventually help to improve antemortem diagnosis of neuropathology in PPA.
Introduction
In primary progressive aphasia (PPA), neurodegeneration of the perisylvian language network is most often caused by frontotemporal lobar degeneration (FTLD) pathologies1,2. Clinical syndromic variants of PPA have been associated statistically with specific neuropathological substrates: the nonfluent/agrammatic variant (naPPA) with tauopathies (FTLD-Tau), the logopenic variant (lvPPA) with Alzheimer’s disease (AD) pathology, and the semantic variant (svPPA) with FTLD with transactive response DNA binding protein of ~43 kDa (TDP-43) inclusions (FTLD-TDP)2,3. However, these syndromic variants do not predict pathology with sufficient reliability for clinical trials, as FTLD-Tau with clinical svPPA or FTLD-TDP with clinical naPPA are not uncommon3–5. Moreover, many patients have features that overlap with different PPA variants3,4, making implementation of clinical criteria challenging even at specialized centers. Finally, AD pathology may mimic any of the PPA syndromes3,4,6. These factors limit the utility of current clinical variant criteria7 for predicting underlying neuropathology.
The anatomic distribution of disease is highly influential for the clinical manifestations of PPA. Recent work has studied hemispheric and regional brain involvement in PPA variants using in vivo techniques of neuroimaging8,9, but postmortem studies characterizing regional pathology are very rare, and antemortem imaging is seldom cross-validated with postmortem pathologic burden. Left-hemisphere lateralization of cortical disease has been demonstrated in vivo10,11 and confirmed qualitatively postmortem3, yet right-hemisphere disease appears to contribute to language deficits in antemortem imaging of autopsy-confirmed PPA12. Postmortem lateralization of pathology has been measured in limited PPA cases with FTLD-TDP13–16, while distribution of pathology in PPA with FTLD-Tau is understudied. Further, despite the limited reliability of clinicopathological correlations using PPA syndromic variants, individual clinical features of PPA have only rarely been related to specific anatomic distributions of disease in patients with presumed proteinopathies during life17,18, or with a categorical neuropathological diagnosis4,19. Here we use a digital approach to perform a fine-grained comparative study of postmortem FTLD-TDP and FTLD-Tau proteinopathies in PPA, and integrate antemortem clinical features and structural MRI to digital pathology. We test the hypotheses that (1) postmortem pathology in PPA is lateralized to the left hemisphere regardless of molecular pathology, (2) FTLD-TDP and FTLD-Tau have divergent, pathology-specific patterns of disease, and (3) the anatomic distribution of pathology is related to distinct antemortem linguistic and imaging features in PPA.
Materials and Methods
Patients
Experienced cognitive neurologists (MG, DAW, DJI) evaluated patients at the Penn Frontotemporal Degeneration Center or Alzheimer’s Disease Center, and selected cases meeting criteria from the Penn Integrated Neurodegenerative Disease Database20. We identified patients with FTLD pathologies that meet modern clinical PPA criteria1 based on systematic chart review and extraction of clinical features from a consensus panel of experienced investigators (CTM, DAW, DJI, KR, LAAG, MG, SA) established prospectively and prior to neuropathological diagnosis. Details on patient inclusion/exclusion are described in Fig 1. Patients with primary AD pathology or medium/high-level secondary AD co-pathology were excluded. Our final cohort consisted of 27 PPA patients with autopsy-confirmed FTLD-TDP (n=13) or FTLD-Tau (n=14). We previously reported clinical and qualitative pathology data for 12 of these patients as a reference group for a study of PPA with AD pathology4. All procedures were performed with informed consent in accordance with the regulations of the Penn Institutional Review Board.
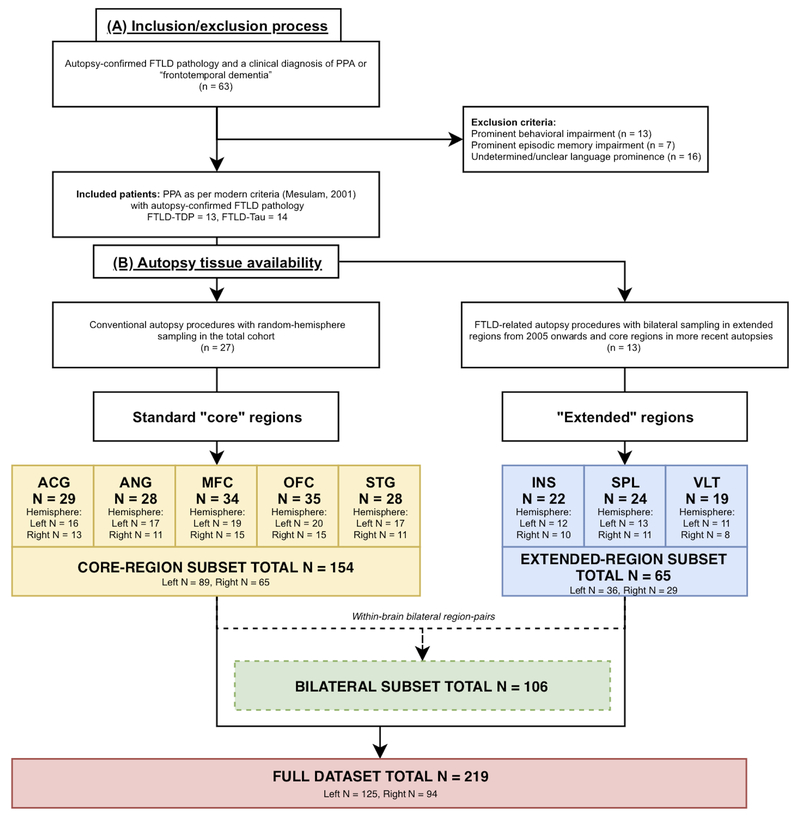
Figure 1. Flow-chart depicting patient inclusion/exclusion and pathology data availability.
Flow-chart shows (A) our inclusion/exclusion process and (B) the final availability of autopsy tissue. A: Of all patients with autopsy-confirmed FTLD pathology and a clinical diagnosis of PPA or “frontotemporal dementia” (n = 63) for those evaluated prior to modern criteria, we excluded those who did not qualify as PPA1 because of prominent behavioral (n = 13) or episodic memory (n = 7) impairments, or insufficient evidence to verify prominence of language impairment at onset (n = 16). Our final cohort consisted of 27 patients, of whom 13 patients with FTLD-TDP and 14 patients with FTLD-Tau. B: Available autopsy tissue included five standard “core” regions (i.e. ACG, ANG, MFC, OFC, STG), i.e. regions that were sampled at autopsy in the total cohort following conventional autopsy procedures with random hemisphere sampling24 (i.e. core-region subset). Additionally, our lab recently expanded pathology protocols to improve the neuropathological characterization of patients with FTLD25, by collecting tissue from both hemispheres in three “extended” regions (i.e. INS, SPL, VLT) in 13 recent PPA brains (i.e. extended-region subset), and more recently implemented bilateral sampling in core regions as well. In total, we collected 219 tissue samples (i.e. full dataset), comprising tissue from core (N = 154) and extended regions (N = 65), and including the subset of bilateral data with 106 matched left-right tissue samples (i.e. bilateral subset). Legend: ACG = anterior cingulate gyrus; ANG = angular gyrus; FTLD = frontotemporal lobar degeneration; FTLD-Tau = frontotemporal lobar degeneration with inclusions of the tau protein; FTLD-TDP = frontotemporal lobar degeneration with inclusions of the transactive response DNA-binding protein 43; INS = anterior insular cortex; MFC = mid-frontal cortex; n = number of patients; N = number of slides; OFC = orbitofrontal cortex; PPA = primary progressive aphasia; SPL = superior parietal lobule; STG = superior temporal gyrus; VLT = ventrolateral temporal cortex.
Neuropathological Examination
Fresh tissue was sampled at autopsy in standardized regions for diagnosis and fixed overnight in 10% neutral buffered formalin21. Tissue was processed as described22, embedded in paraffin blocks and cut into 6 μm sections for immunohistochemical staining for tau, Aβ, TDP-43 and alpha-synuclein with well-characterized antibodies21. Neuropathological diagnosis was performed by expert neuropathologists (EBL, JQT) using established criteria23,24. Patients were classified based on primary neuropathological diagnosis as FTLD-TDP (i.e. subtypes A, B or C) or FTLD-Tau (i.e. Pick’s disease [PiD], progressive supranuclear palsy [PSP] or corticobasal degeneration [CBD]).
Genetic Analysis
Patients were genotyped for pathogenic mutations in GRN, C9orf72 and MAPT as described25 based on family history risk from structured pedigree analysis26.
Digital Image Analysis
For this study, we used tissue fixed in formalin in an identical manner as outlined above21, except for a minority of slides (n=10) fixed in 70% ethanol with 150 mmol NaCl to supplement regions missing formalin-fixed tissue as previously validated22. Tissue was immunostained for phosphorylated TDP-43 (rat monoclonal TAR5P-1D3, p409/410; Ascenion)27 or tau (AT8; Millipore)28 as described22. Digital image analysis was performed with Halo software v1.90 (Indica Labs, Albuquerque NM) with validated sampling and thresholding algorithms for FTLD-TDP and FTLD-Tau22. We measured percentage of area occupied (%AO) by TDP-43 or tau inclusions in grey matter regions of interest (ROI) as described22,25. To reduce batch effects of immunohistochemical staining, all slides were stained in the same batch or %AO measurements were transformed using linear regression derived from a subset of slides run in duplicate. We validated %AO scores by comparison to traditional ordinal scores (i.e. 0-3), obtained blinded to quantitative pathology measurements, as previously done22.
Pathology data (see Fig 1) included five standard “core” regions, which were sampled from a random hemisphere at autopsy according to standardized NIA/AA diagnostic guidelines24 in the total cohort. These core regions include: anterior cingulate gyrus (ACG, Brodmann area [BA] 24), angular gyrus (ANG, BA 39), mid-frontal cortex (MFC, BA 46), orbitofrontal cortex (OFC, BA 11), and superior-temporal gyrus (STG, BA 22). Additional data were available from “extended” regions sampled from both hemispheres in autopsies since 2005 (FTLD-TDP=5, FTLD-Tau=8) to capture anatomic substrates associated with language and behavior in FTLD as described25, i.e. anterior insular cortex (INS, BA 13), ventrolateral temporal cortex (VLT, BA 20), and a control region less involved in FTLD, i.e. superior parietal lobule (SPL, BA 5). We have 29 within-brain bilateral region-pairs from extended regions, and more recently we collected bilateral sampling in standard core regions as well, obtaining an additional set of 24 within-brain bilateral region-pairs. To analyze pathology burden between hemispheres across brain regions, we used all available bilateral data uniquely collected at our center (i.e. bilateral subset), comprising 53 bilateral region-pairs for a total of 106 individual tissue samples. To analyze pathology burden between brain regions within each hemisphere, we used data from core regions sampled in all autopsies (i.e. core-region subset), including 89 left-hemisphere and 65 right-hemisphere samples. Our total dataset including tissue from both standard core (N=154) and extended regions (N=65) amounted to 219 samples (i.e. full dataset). Please see Fig 1 for an overview of available sampled tissue. Missing data and damaged tissues were excluded from analyses.
Clinical Data
Available clinical records were reviewed blinded to neuropathological diagnosis. All patients had >1 visit with a comprehensive, formal language evaluation performed by an experienced cognitive neurologist at our outpatient cognitive neurology clinic using a validated protocol29,30, or similar bedside assessment in patients evaluated prior to modern PPA criteria. We abstracted the presence of clinical features systematically tested and reported in the record by the clinician as binary variables (i.e. presence/absence of specific features) using a standardized chart review and discussed in a weekly consensus panel as described4,25,31. Nonfluent speech was defined as the presence of slowed and/or effortful speech with reduced word output as described4,31. Clinical data were categorized into early disease (0-3 years after onset) and late disease (>3 years after onset). Patients with missing data in early (n=6) or late (n=7) disease periods were excluded from these analyses. Patients were classified into clinical PPA variants when consistent with current criteria7, or defined as unclassifiable due to concurrent semantic and nonfluent/agrammatic features. Classification details for individual patients are specified in Table 1. Additionally, we retrospectively scored the sum-of-boxes of the extended clinical dementia rating (CDR) scale including FTD-related behavior and language subfields32, at first and last available visits.
Table 1.
Cohort characteristics and demographics
| ID | Sex | Edu (Y) | Handedness | PPA variant | Path subtype | Gene mut | Brain Wt (gr) | PMI (hrs) | Braak | CERAD | Age at onset (Y) | Disease dur (Y) | CDR^ first visit | CDR^ last visit | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FTLD-TDP | 1 | M | 12 | R | svPPA¥ | C | 1053 | 4 | 0 | B | 62 | 18.8 | 1.5 | 19 | |
| 2 | F | 12 | R | svPPA | C | 1003 | 20 | 0 | 0 | 62 | 6.7 | 2.5 | na | ||
| 3 | M | 20 | L | svPPA | C | 1359 | 6 | 1 | 0 | 55 | 8.0 | 6.5 | 20 | ||
| 4§, ‡ | F | 18 | R | svPPA† | A | GRN | 790 | 8 | 0 | 0 | 56 | 5.7 | 14 | 23 | |
| 5‡ | F | 18 | R | Unclas | A | GRN | 742 | 4.5 | 1 | 0 | 52 | 10.5 | 10.5 | 17.5 | |
| 6§, ‡ | F | 12 | R | Unclas | A | GRN | 1055 | 8.5 | 1 | 0 | 65 | 3.5 | 4.5 | 23 | |
| 7 | M | 16 | R | svPPA | C | 1390 | 16.5 | 1 | A | 62 | 10.3 | 4.5 | 9.5 | ||
| 8 | M | 16 | R | svPPA | C | 1060 | 20 | 0 | 0 | 65 | 11.4 | 3 | 21 | ||
| 9§ | M | 18 | R | naPPA | A | GRN | 866 | 18 | 1 | 0 | 56 | 10.3 | 4 | 22 | |
| 10§ | M | 22 | R | svPPA | A | 1090 | 16 | 1 | 0 | 59 | 11.6 | 3 | 13.5 | ||
| 11 | M | 16 | R | naPPA | A | 1160 | 6.5 | 0 | 0 | 52 | 3.4 | 3.5 | 21 | ||
| 12 | M | na | R | svPPA | C | 1157 | 12.5 | 0 | 0 | 50 | 13.5 | 1 | 11.5 | ||
| 13§ | F | 18 | L | naPPA# | B | 1069 | 17 | 1 | A | 69 | 1.4 | 9 | 17.5 | ||
| FTLD-Tau | 14§ | F | 12 | R | naPPA | CBD | 902 | 14 | 0 | A | 66 | 11.1 | 2 | 19 | |
| 15 | F | 16 | R | naPPA | CBD | 1149 | 19.5 | 0 | 0 | 52 | 4.8 | 4.5 | 16 | ||
| 16‡ | F | 12 | R | naPPA | PSP | 1035 | 17 | 2 | A | 63 | 7.7 | 11.5 | na | ||
| 17 | M | 20 | R | Unclas | CBD | 1085 | 17 | 1 | A | 63 | 5.6 | 4 | 20 | ||
| 18 | M | 12 | R | naPPA | PSP | 1100 | 13 | 0 | 0 | 71 | 10.0 | 3.5 | 20 | ||
| 19§ | F | 16 | R | naPPA | CBD | 906 | 4 | 1 | 0 | 58 | 8.3 | 2 | 22 | ||
| 20§ | M | 20 | R | svPPA | PiD | 1049 | 4 | 0 | 0 | 54 | 17.2 | 6 | 24 | ||
| 21 | F | 12 | R | naPPA | CBD | 1340 | 19 | 0 | 0 | 74 | 5.9 | 2 | 14 | ||
| 22§ | F | 16 | R | Unclas | PiD | 1053 | 14 | 0 | 0 | 57 | 9.1 | 3.5 | 21 | ||
| 23§ | M | na | R | naPPA | PSP | 1310 | 4 | 2 | 0 | 76 | 7.9 | 4.5 | 20.5 | ||
| 24 | F | na | R | Unclas | CBD | 1080 | 15 | 0 | 0 | 65 | 5.7 | 2 | 20 | ||
| 25 | M | 16 | R | naPPA | PSP | 1014 | 15.5 | 1 | A | 67 | 5.7 | 4.5 | na | ||
| 26‡ | M | 16 | R | naPPA | PSP | 1297 | 5 | 1 | 0 | 70 | 9.6 | 5 | 15.5 | ||
| 27§ | F | 16 | R | naPPA | CBD | 1162 | 10 | 1 | A | 58 | 9.5 | 2.5 | 8 | ||
A-C = FTLD-TDP subtypes according to current neuropathological criteria23; CBD = corticobasal degeneration; CDR = Clinical Dementia Rating including FTD subfields32; dur = duration; edu = education; F = female; FTLD-Tau = frontotemporal lobar degeneration with inclusions of the tau protein; FTLD-TDP = frontotemporal lobar degeneration with inclusions of the transactive response DNA-binding protein 43; gr = grams; GRN = progranulin gene; hrs = hours; L = left-handed; M = male; na = not available; naPPA = nonfluent/agrammatic variant of primary progressive aphasia; path = pathology; PiD = Pick’s disease; PMI = postmortem interval; PSP = progressive supranuclear palsy; R = right-handed; svPPA = semantic variant of primary progressive aphasia; Unclas = unclassifiable primary progressive aphasia with concurrent nonfluent/agrammatic and semantic features; Wt = weight; Y = years.
These patients (n = 11) had available antemortem MRI data.
Patient 1 had a logopenic presentation in early disease (lvPPA-, i.e. meeting ancillary clinical features of lvPPA but not core features due to preserved repetition4), and progressed to an svPPA phenotype as per clinical criteria.
Patient 4 met core criteria for svPPA; but there was insufficient data to determine whether this patient fulfilled ancillary svPPA criteria.
Patient 13 had naPPA at onset and thereafter developed motor neuron disease features leading to a diagnosis of amyotrophic lateral sclerosis (ALS).
Low levels of secondary co-pathologies were present in a minority of patients with no or rare cortical involvement. Patients 4 and 5 had hippocampal sclerosis. Patient 6 had secondary argyrophilic grain disease with rare cortical pathology. Patients 16 and 26 had secondary Lewy Body disease (limbic stage).
The Clinical Dementia Rating (CDR) scale was scored at first available visit for all patients, and at last visit for patients with >1 available visits.
Neuroimaging
A subset of patients (n=11) had research-quality T1 structural MRI data. Quantitative MRI data were processed using ANTS33,34. We measured cortical thickness in each hemisphere using multi-atlas segmentation with joint label fusion35 in ROIs approximating regions sampled at autopsy, as described22,25. To determine cortical thinning in each ROI, we derived z-scores based on a demographically comparable healthy control cohort without psychiatric or neurological history (male=47, female=66, average education=16.0 years, average age=65.2 years).
Statistical Analysis
Categorical clinical features were compared between groups using Fisher’s Exact test, while non-normally distributed demographic variables were compared using Mann Whitney U test. Neuropathology and neuroimaging data were tested using parametric statistics as described below. We used linear mixed-effects (LME) modeling with random intercepts for individual patients to account for interdependency of multiple measurements from the same patient and for missing data36. To assess the effect of a multilevel categorical variable on the model, type III ANOVA with Satterthwaite approximation was employed. Planned post-hoc comparisons for LME outcomes were performed on LME-derived least-square means with Tukey correction for multiple comparisons. All analyses were two-sided with significance level set at 0.05, and were performed using R Statistical Software 3.4.1.
Since the absolute %AO varied due to differences in morphological inclusions25, %AO scores were scaled to a comparable range [0;1] (i.e. normalized %AO) using min-max normalization: x’=(x–min)/(max–min). To maximize biological accuracy, this normalization step was performed within pathology subtypes (FTLD-TDP: type A/B, type C; FTLD-Tau: CBD, PiD, PSP).
Lateralization of pathology was tested using an LME analysis of normalized %AO across all available bilaterally sampled region-pairs (i.e. bilateral subset) in FTLD-TDP and FTLD-Tau, covarying for disease duration at autopsy, and mutation status in FTLD-TDP. For a direct measure of laterality, an asymmetry index (AI) was calculated in bilateral region-pairs using raw %AO scores25; AI=(Left %AO – Right %AO)/(average Left %AO & Right %AO)*100. We tested regional AI with a one-sample t-test against the null-hypothesis that mean AI=0 (i.e. no lateralization), and we assessed the relationship between CDR score at last visit and AI using an LME model across all regions.
Within-group analysis of regional pathology was performed using a LME model testing the effect of region on normalized %AO in left- and right-hemisphere standard core regions (i.e. core-region subset), covarying for disease duration at autopsy, and mutation status in FTLD-TDP. We defined greatest core-region pathology as the region with the highest least-square mean derived from LME modeling in each hemisphere in FTLD-TDP and FTLD-Tau. Between-group comparisons of pathology burden were performed using analysis of covariance (ANCOVA) with normalized %AO as outcome variable, covarying for disease duration at autopsy.
We tested the relationship between normalized %AO and MRI cortical thickness in the full dataset with an LME model comprising ROIs matching available regional pathology data, covarying for time from scan to autopsy. We used independent samples t-tests to compare normalized %AO measurements in greatest core-pathology regions between patients with and without clinical language features characteristic of naPPA and svPPA.
Results
Clinical Characterization
The cohort comprised 13 patients with FTLD-TDP and 14 with FTLD-Tau (Table 1), who did not differ in demographic characteristics. All but two patients were right-handed. FTLD-TDP and FTLD-Tau differed in the frequencies of clinical variants (p=0.007, Fisher’s Exact test). FTLD-TDP was associated with svPPA in 8/13 patients (61.5%), while 3/13 (23.1%) patients had naPPA and 2/13 (15.4%) had an unclassifiable phenotype with mixed semantic and nonfluent/agrammatic features. FTLD-Tau was associated with naPPA in 10/14 patients (71.4%), while 1/14 (7.1%) had svPPA, and 3/14 (21.4%) were unclassifiable. Four FTLD-TDP patients carried a GRN mutation. Likewise, 8/9 (88.9%) svPPA patients had FTLD-TDP pathology, while 10/13 (76.9%) naPPA patients had FTLD-Tau pathology. Thus, clinical syndromes did not consistently predict expected pathology, and 5/27 (18.5%) patients could not be classified due to mixed features.
We compared clinical language features between FTLD-TDP and FTLD-Tau independently of clinical syndrome (Table 2; statistics: Fisher’s Exact test). In early disease (0-3 years after onset), 9/10 (90.0%) FTLD-Tau patients had nonfluent speech, which was more frequent than 4/11 (36.4%) patients with FTLD-TDP (p=0.024), while impairment of single-word comprehension in 7/10 (70.0%) patients with FTLD-TDP approached significance when compared to 2/9 (22.2%) patients in FTLD-Tau (p=0.070). In late disease (>3 years after onset), 11/12 (91.7%) of FTLD-Tau had nonfluent speech compared to 2/8 (25.0%) in FTLD-TDP (p=0.004). Additionally, FTLD-Tau had higher frequency of agrammatism (p=0.005), dysarthria (p=0.028), phonemic paraphasias (p=0.019) and impaired sentence repetition (p=0.018) than FTLD-TDP. Conversely, 7/7 (100.0%) patients with FTLD-TDP in late disease had single-word comprehension difficulties, greater than 5/12 (41.7%) in FTLD-Tau (p=0.017), and were more often impaired in object knowledge (p=0.009) and meaningful content of speech (p=0.019) than FTLD-Tau. Thus, distinctive language features, i.e. nonfluent speech in FTLD-Tau and impaired single-word comprehension in FTLD-TDP, were relatively more reliable than syndromic variants.
Table 2.
Comparison of clinical language features between FTLD-TDP and FTLD-Tau groups
| Language features | EARLY (0-3 years) | LATE (> 3 years) | ||||
|---|---|---|---|---|---|---|
| FTLD-TDP | FTLD-Tau | Sig. | FTLD-TDP | FTLD-Tau | Sig. | |
| Imp single-word retrieval | 11/11 (100) | 10/10 (100.0) | na | 8/8 (100.0) | 12/12 (100.0) | na |
| Imp naming | 9/10 (90) | 8/9 (88.9) | 1.000 | 8/8 (100.0) | 12/12 (100.0) | na |
| Imp sentence repetition | 5/9 (55.6) | 3/8 (37.5) | 0.637 | 2/5 (40.0) | 11/11 (100.0) | 0.018* |
| Nonfluent speech | 4/11 (36.4) | 9/10 (90.0) | 0.024* | 2/8 (25.0) | 11/12 (91.7) | 0.004* |
| Agrammatism | 4/11 (36.4) | 7/10 (70.0) | 0.198 | 1/8 (12.5) | 10/12 (83.3) | 0.005* |
| Dysarthria | 3/11 (27.3) | 5/10 (50.0) | 0.387 | 1/8 (12.5) | 8/12 (66.7) | 0.028* |
| Empty speech content | 2/11 (18.2) | 2/10 (20.0) | 1.000 | 6/8 (75.0) | 2/12 (16.7) | 0.019* |
| Semantic paraphasias | 5/11 (45.5) | 3/10 (30.0) | 0.659 | 5/8 (62.5) | 7/12 (58.3) | 1.000 |
| Phonemic paraphasias | 2/11 (18.2) | 5/10 (50.0) | 0.183 | 2/8 (25.0) | 10/12 (83.3) | 0.019* |
| Imp gramm compr | 6/7 (85.7) | 5/10 (50.0) | 0.304 | 3/8 (37.5) | 10/12 (83.3) | 0.062 |
| Imp single-word compr | 7/10 (70) | 2/9 (22.2) | 0.070 | 7/7 (100.0) | 5/12 (41.7) | 0.017* |
| Imp object knowledge | 5/9 (55.6) | 2/9 (22.2) | 0.335 | 6/6 (100.0) | 3/12 (25.0) | 0.009* |
| Surface dyslexia | 1/8 (12.5) | 1/9 (11.1) | 1.000 | 4/4 (100.0) | 2/7 (28.6) | 0.061 |
compr = comprehension; FTLD-Tau = frontotemporal lobar degeneration with inclusions of the tau protein; FTLD-TDP = frontotemporal lobar degeneration with inclusions of the transactive response DNA-binding protein 43; gramm = grammatical; Imp = impaired; Sig. = significance.
Categorical binary clinical features were compared between FTLD-TDP and FTLD-Tau groups using the Fisher’s Exact test independently of clinical phenotype. Significance values are reported in the table and significant results (p < 0.05) are indicated with an asterisk (*).
Neuropathological Analysis: Lateralization
We studied the inter-hemispheric distribution of TDP-43 and tau across all bilaterally sampled region-pairs. Cortical pathology was lateralized to the left hemisphere in both FTLD-TDP (beta=−0.15,SE=0.05,p=0.007) and FTLD-Tau (beta=−0.09,SE=0.04,p=0.015). The two left-handed patients of our cohort did not have bilateral sampling and were not included in this analysis.
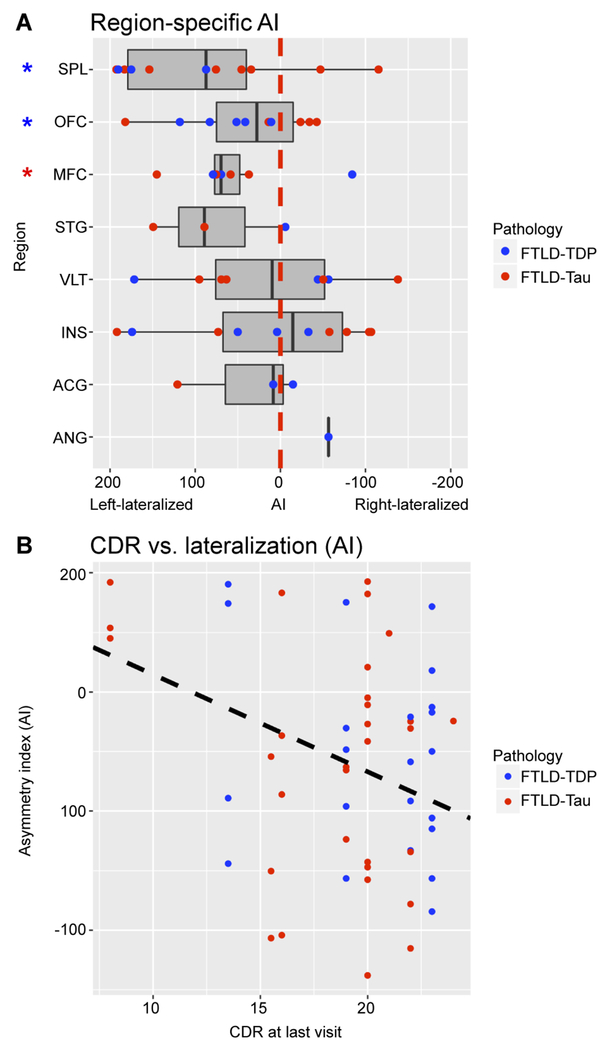
We used the AI as a direct measure of laterality in each region (Fig 2A). FTLD-TDP showed region-specific left lateralization of OFC (mean AI=60.9±41.0,t=3.32,df=4,p=0.029) and SPL (mean AI=150.9±55.6,t=4.70,df=2,p=0.042), whereas FTLD-Tau had region-specific left lateralization of MFC (mean AI=79.0±46.8,t=3.37,df=3,p=0.043). Further, AI was negatively associated with full CDR at last visit (beta=−8.18,SE=3.22,p=0.015) across all regions and irrespective of pathology group (Fig 2B), suggesting that increased overall clinical dementia severity before death was associated with reduced left lateralization of pathology.
Figure 2. Region-specific lateralization of cortical disease and association with clinical dementia severity.
A: Box-plots depict asymmetry index (AI) values calculated in available bilaterally sampled region-pairs. An AI greater than zero (on the left side of the red dotted line) indicates left lateralization of pathology burden. Overall we find left lateralization of cortical pathology in both FTLD-TDP and FTLD-Tau, but individual and regional patient data may have variable degree of hemispheric laterality. In FTLD-TDP, OFC and SPL are significantly left-lateralized (i.e. asymmetry index > 0; p < 0.05); in FTLD-Tau, MFC is significantly left-lateralized (p < 0.05). In the figure, blue asterisks indicate significant findings in FTLD-TDP, whereas the red asterisk indicates a significant finding in FTLD-Tau. B: Scatterplot shows an inverse linear relationship between clinical dementia severity proximal to autopsy and degree of lateralization (i.e. AI) across all bilaterally sampled regions (beta = −8.18, SE = 3.22, p = 0.015). Data were analyzed using LME analysis to account for interdependency of multiple measurements from the same individuals. Legend: ACG = anterior cingulate gyrus; AI = asymmetry index; ANG = angular gyrus; CDR = Clinical Dementia Rating including FTD subfields32; FTLD-Tau = frontotemporal lobar degeneration with inclusions of the tau protein; FTLD-TDP = frontotemporal lobar degeneration with inclusions of the transactive response DNA-binding protein 43; INS = anterior insular cortex; MFC = mid-frontal cortex; OFC = orbitofrontal cortex; PPA = primary progressive aphasia; SPL = superior parietal lobule; STG = superior temporal gyrus; VLT = ventrolateral temporal cortex.
Neuropathological Analysis: Within-Group Regional Burden
We analyzed within-group regional burden of pathology in the five standard core regions. In left-hemisphere FTLD-TDP, we found a significant effect of region on pathology burden (F=9.46,df=4,29,p<0.001). Among the five core regions sampled, left OFC had the greatest core-region pathology (Fig 3A). Planned post-hoc comparisons showed significantly higher burden in left OFC compared to left MFC (p<0.001) and ANG (p<0.001), and in left STG compared to left MFC (p=0.027). In left-hemisphere FTLD-Tau, we found a significant effect of region on cortical pathology (F=7.92,df=4,33,p<0.001). Among the five core regions sampled, left MFC had greatest core-region pathology (Fig 3A). Planned post-hoc comparisons showed significantly higher burden in left MFC compared to left OFC (p<0.001), STG (p=0.004) and ANG (p=0.024), and in left ACG compared to left OFC (p=0.006) and STG (p=0.022).
Figure 3. Regional distribution of pathology in five standard core regions in FTLD-TDP and FTLD-Tau in left and right hemispheres.
Heat-map portrays regional distribution of cortical pathology in left and right hemispheres in FTLD-TDP (blue) and FTLD-Tau (red). Here we display regional least-square means of normalized %AO from LME within-group regional analysis in five standard core regions. A: In the left hemisphere, we found greatest core-region pathology in left OFC in FTLD-TDP and left MFC in FTLD-Tau. B: In the right hemisphere, we found similar findings of greatest core-region pathology in right OFC in FTLD-TDP and right MFC in FTLD-Tau. Regions of greatest core-region pathology in each hemisphere are marked with an asterisk (*). Legend: ACG = anterior cingulate gyrus; ANG = angular gyrus; FTLD-Tau = frontotemporal lobar degeneration with inclusions of the tau protein; FTLD-TDP = frontotemporal lobar degeneration with inclusions of the transactive response DNA-binding protein 43; MFC = mid-frontal cortex; normalized %AO = percentage area occupied by pathology after normalization [0;1]; OFC = orbitofrontal cortex; STG = superior temporal gyrus.
To verify whether these findings were consistent within pathology subtypes, we performed a sub-analysis comparing regions of greatest core-region pathology, and found that left OFC was more affected than left MFC in both FTLD-TDP type A/B (beta=0.24,SE=0.02,p<0.001) and type C (beta=0.41,SE=0.08,p=0.008); whereas left MFC was more affected than left OFC in both CBD (beta=0.21,SE=0.05,p=0.023) and PSP (beta=0.23,SE=0.07,p=0.010). We could not perform this sub-analysis in PiD because of limited sample (n=1).
Considering the well-established association between FTLD-TDP and svPPA2–4 with focal anterior temporal atrophy37, we examined limited left VLT data available in FTLD-TDP (n=4) and found relatively high pathology burden (0.76±0.12).
Although the right hemisphere had less pathology, the findings of greatest core-region pathology were similar to the left hemisphere (Fig 3B). In FTLD-TDP, region had a significant effect on right-hemisphere pathology (F=4.39,df=4,26,p=0.008), and OFC was the region of greatest core-region pathology in the right hemisphere. Planned post-hoc comparisons showed greater burden in right OFC compared to right ANG (p=0.009), and in right ACG compared to right ANG (p=0.019). In FTLD-Tau, right-hemisphere pathology differed by region (F=3.51,df=4,17,p=0.029), and MFC was the region of greatest core-region pathology in the right hemisphere. While planned post-hoc comparisons failed to show significant pairwise contrasts between regions, we observed a trend for greater pathology in right MFC compared to right OFC (p=0.059).
Neuropathological Analysis: Between-Group Comparisons of Pathology Burden
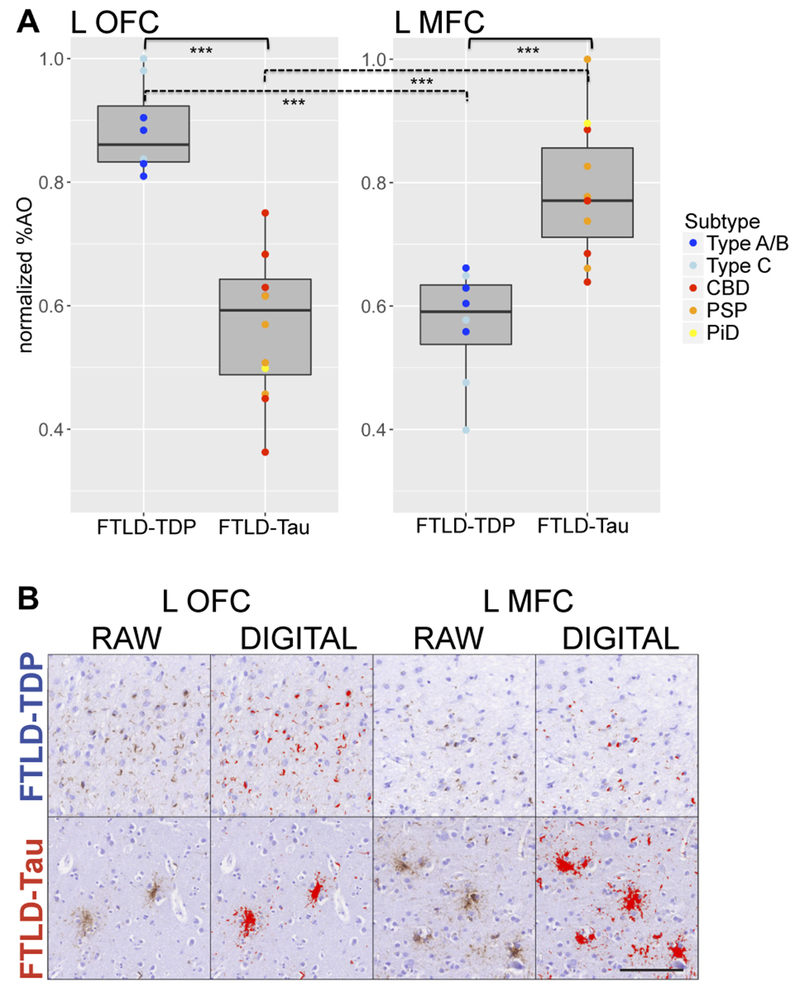
We directly compared burden in left-hemisphere core-regions with greatest pathology (i.e. OFC, MFC) between FTLD-TDP and FTLD-Tau (Fig 4). FTLD-TDP had significantly higher pathology than FTLD-Tau in left OFC (FTLD-TDP=0.88±0.07, FTLD-Tau=0.57±0.11; F=47.07,df=1,17,p<0.001), whereas FTLD-Tau had higher pathology in left MFC (FTLD-TDP=0.57±0.09, FTLD-Tau=0.79±0.11; F=19.34,df=1,16,p<0.001). In a sub-analysis in pathology subtypes, we found that both FTLD-TDP type A/B and type C had greater pathology in left OFC than CBD (p<0.006) and PSP (p<0.004), while CBD and PSP had greater pathology in left MFC than FTLD-TDP type A/B (p<0.03) and type C (p<0.02). In the single PiD patient with left-hemisphere sampling, left MFC burden (=0.90) was greater and left OFC burden (=0.50) was smaller compared to all individual measurements in FTLD-TDP subtypes. Thus, there was a double-dissociation of left-hemisphere pathology, distinguishing OFC as greatest core-region pathology in FTLD-TDP and MFC as greatest core-region pathology in FTLD-Tau, consistent across morphological subtypes of these proteinopathies.
Figure 4. Regional comparisons of cortical neuropathological burden in greatest core-region pathology regions.
A: Boxplots portray direct within-group and between-group comparisons of cortical pathology (i.e. normalized %AO) in greatest core-region pathology regions. Within FTLD-TDP, left OFC had greater pathology than left MFC (p < 0.001), whereas within FTLD-Tau, left MFC had greater pathology than left OFC (p < 0.001). Between pathologies, FTLD-TDP had greater pathology in left OFC than FTLD-Tau (mean FTLD-TDP = 0.88 ± 0.07; mean FTLD-Tau = 0.57 ± 0.11; F = 47.07, df = 1,17, p < 0.001), whereas FTLD-Tau had greater pathology in left MFC than FTLD-TDP (mean FTLD-TDP = 0.57 ± 0.09; mean FTLD-Tau = 0.79 ± 0.11; F = 19.34, df = 1,16, p < 0.001). Sub-analyses showed consistent results across pathology subtypes. Here, digital pathology measurements are color-coded by pathology subtypes. Significant findings with p < 0.001 are marked with three asterisks (***). B: Images show pathology burden in left OFC and left MFC comparatively in a case of FTLD-TDP type A with a GRN mutation and clinical svPPA and in a case of FTLD-Tau PSP with naPPA (scale bar = 100 μm). We include both raw images with immunohistochemical staining of TDP-43 (rat monoclonal TAR5P-1D3, p409/410; Ascenion)27 and tau (AT8; Millipore)28, as well as digital images with thresholding parameters for digital detection of TDP-43 and tau inclusions. While the FTLD-TDP case has relatively high burden of TDP-43 inclusions in OFC and relatively low burden in MFC, the FTLD-Tau case has relatively low burden of tau inclusions in OFC and relatively high burden in MFC. Legend: CBD = corticobasal degeneration; FTLD-Tau = frontotemporal lobar degeneration with inclusions of the tau protein; FTLD-TDP = frontotemporal lobar degeneration with inclusions of the transactive response DNA-binding protein 43; L = left; MFC = mid-frontal cortex; normalized %AO = percentage area occupied by pathology after normalization [0;1]; OFC = orbitofrontal cortex; PiD = Pick’s disease; PSP = progressive supranuclear palsy; Type A/B, Type C = FTLD-TDP subtypes.
Clinicopathological Association: Neuroimaging
We tested the association of postmortem pathology with antemortem cortical thinning in corresponding ROIs in 11 patients with available MRI (FTLD-Tau=6, FTLD-TDP=5). Pathology burden was inversely associated with MRI cortical thickness across all available tissue regions and corresponding MRI ROIs (beta=−0.04,SE=0.01,p=0.007) (Fig 5A). Next, we examined cortical thinning in left-hemisphere ROIs corresponding to the five standard core regions to mirror our within-group regional analysis. Among these five core ROIs, left OFC was the region of greatest core-region atrophy in FTLD-TDP (mean=−3.78±2.25), while left MFC was the region of greatest core-region atrophy in FTLD-Tau (mean=−2.10±1.53). Thus, the antemortem distribution of disease on MRI matched postmortem findings in core regions (Fig 5B). Similarly, in the right hemisphere, OFC and MFC were the regions of greatest core-region atrophy in FTLD-TDP and FTLD-Tau, respectively (FTLD-TDP right OFC=−2.89±0.80; FTLD-Tau right MFC=−2.06±1.26), consistent with postmortem findings.
Figure 5. MRI cortical thinning is reflective of cortical neuropathological burden at autopsy.
A: Scatterplot portrays a significant negative relationship between pathology burden (i.e. normalized %AO) and cortical thickness z-scores from antemortem MRI in regions matching autopsy sampling across pathology groups (beta = −0.04, SE = 0.01, p = 0.007). The fit line represents the linear association between cortical thickness z-scores (x-axis) and normalized %AO (y-axis) in corresponding regions, and has been derived in a linear mixed effects model36 accounting for multiple measurements from the same patient as well as one covariate (i.e. time from scan to autopsy), which may confound the linear association between the two measurements. Data points are color-coded by pathology (i.e. FTLD-TDP = blue, FTLD-Tau = red). B: Heat-map shows relative MRI cortical thinning in left-hemisphere ROIs matching five standard core regions sampled at autopsy in FTLD-TDP and FTLD-Tau. Regions are color-coded by relative severity of cortical thinning as compared to healthy controls (scale bars = z-scores) within pathology groups. Group means are obtained from 11 patients (5 FTLD-TDP, 6 FTLD-Tau) with available antemortem structural MRI. Among these core ROIs, FTLD-TDP has greatest core-region atrophy in left OFC (mean = −3.78 ± 2.25), while FTLD-Tau has greatest core-region atrophy in left MFC (mean = −2.10 ± 1.53), validating our postmortem findings. Regions of greatest core-region atrophy are marked with an asterisk (*). Legend: FTLD-Tau = frontotemporal lobar degeneration with inclusions of the tau protein; FTLD-TDP = frontotemporal lobar degeneration with inclusions of the transactive response DNA-binding protein 43; normalized %AO = percentage area occupied by pathology after normalization [0;1].
Clinicopathological Association: Language Features
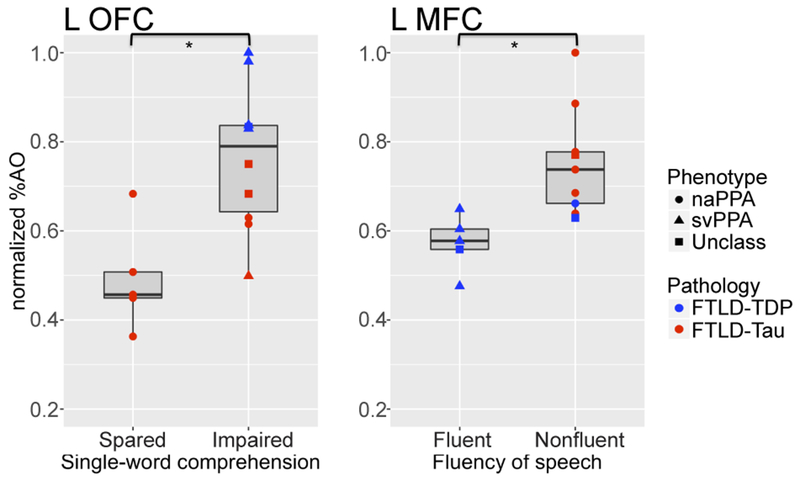
Finally, we related the most robust clinical language features, i.e. nonfluent speech and single-word comprehension, to pathology in core-regions with greatest pathology (Fig 6). Across the cohort, patients with early nonfluent speech had higher left MFC burden than patients without such impairment (t=−3.62,df=12.00,p=0.004). Patients with early impairment of single-word comprehension showed a trend for greater burden in left OFC (t=−2.01,df=7.89,p=0.080). As several patients developed comprehension difficulties only later in the disease (Table 2), we looked at late impairment (i.e. >3 years after onset) of single-word comprehension, and found that it significantly associated with left OFC pathology (t=−3.72,df=10.72,p=0.004). We did not find an association of single-word comprehension impairment with left STG pathology (p>0.5), nor did we find an association of right-hemisphere OFC or MFC pathology with single-word comprehension or nonfluent speech, respectively (p>0.05).
Figure 6. Clinicopathological associations of left-hemisphere greatest core-region pathology regions with clinical language features.
Boxplots show correlations of cortical pathology burden (i.e. normalized %AO) in greatest core-region pathology regions with clinical language features. Data points are color-coded by pathology group (i.e. FTLD-TDP = blue, FTLD-Tau = red) and shape-coded by clinical phenotype (i.e. circle = naPPA, triangle = svPPA, square = unclassifiable with mixed features). Irrespective of PPA clinical variant, patients with single-word comprehension impairment in late disease (i.e. >3 years after onset) had more severe cortical disease in left OFC than patients with sparing of this language function (t = −3.72, df = 10.72, p = 0.004); patients with nonfluent speech in early disease (i.e. 0-3 years after onset) had more severe cortical disease in left MFC than patients with fluent speech (t = −3.62, df = 12.00, p = 0.004). Statistical significance (p < 0.05) is indicated with an asterisk (*). Legend: FTLD-Tau = frontotemporal lobar degeneration with inclusions of the tau protein; FTLD-TDP = frontotemporal lobar degeneration with inclusions of the transactive response DNA-binding protein 43; MFC = mid-frontal cortex; naPPA = nonfluent/agrammatic variant of primary progressive aphasia; normalized %AO = percentage area occupied by pathology after normalization [0;1]; OFC = orbitofrontal cortex; svPPA = semantic variant of primary progressive aphasia; Unclass = unclassifiable primary progressive aphasia with concurrent nonfluent/agrammatic and semantic features.
Since the left anterior temporal lobe has been implicated in semantic deficits in svPPA37,38 and we had limited availability of autopsy tissue in this region, we performed an exploratory analysis of a left-hemisphere anterior temporal ROI in antemortem MRI. We found that FTLD-TDP had prominent cortical thinning in left anterior temporal cortex (FTLD-TDP=−4.49±1.53), which did not differ from left OFC cortical thinning in FTLD-TDP (t=1.16,df=4,p=0.310). Left anterior temporal cortical thinning was greater in FTLD-TDP than FTLD-Tau (FTLD-Tau=−2.11±1.66; t=−2.47,df=8.86,p=0.036). Finally, MRI cortical thinning was associated with early single-word comprehension deficits in both anterior temporal (t=3.03,df=5.88,p=0.024) and orbitofrontal (t=5.05,df=5.08,p=0.004) cortices.
Discussion
This comparative, bi-hemispheric, digital pathology study of FTLD-TDP and FTLD-Tau in PPA presents several novel and important findings. First, our digital approach provides expected evidence of left-lateralized pathology in PPA, but some of the observed heterogeneity in laterality may partly be explained by advancing overall dementia severity at end-stage disease (Fig 2). Next, we find a double-dissociation in patterns of cortical pathology in ventral-frontal and temporal regions in FTLD-TDP as opposed to dorsolateral frontal regions in FTLD-Tau (Fig 3–4), and these appear to be common across morphological subtypes of each proteinopathy. Moreover, we find converging evidence for these regional patterns in antemortem MRI, with a direct association between antemortem cortical thinning and postmortem digital pathology (Fig 5). Finally, this regional susceptibility to distinct proteinopathies directly relates to clinical language features, irrespective of PPA syndromic variants (Fig 6). We conclude that divergent patterns of cortical pathology in PPA with underlying FTLD-TDP or FTLD-Tau may be helpful to improve antemortem diagnosis of neuropathology based on specific regional burden and associated language features.
Histopathological comparisons across hemispheres in FTLD are very rare due to the lack of bilateral sampling in standard neuropathological protocols23,24. Lateralization of postmortem disease has been shown in rare prior postmortem studies, either qualitatively3 or quantitatively in a small sample of FTLD-TDP13–16. Imaging studies suggest that there may be regional specificity to language-related areas in the rate of atrophy progression and lateralization in PPA10,11, but these studies lack autopsy data. In our rare bilateral dataset, we found overall left lateralization of cortical pathology in FTLD-TDP and FTLD-Tau, consistent with previous reports3,14. Moreover, our digital approach enabled us to detect novel evidence of regional and individual-patient variability (Fig 2). We observed region-specific lateralization of postmortem pathology in regions with high burden, i.e. OFC in FTLD-TDP and MFC in FTLD-Tau, and in a relatively spared region in FTLD-TDP (i.e. SPL). In our recent postmortem study in bvFTD, our digital approach also revealed that lateralization varied depending on region25. While autopsy data are inherently cross-sectional, our examination of overall dementia severity before autopsy suggests that pathology may become more evenly distributed across hemispheres with advancing disease in PPA. Since all patients had severe language impairment at last visit (score=3/3 of CDR language subscale), we could not relate laterality to end-stage language impairment specifically, and decreasing lateralization at end-stage disease may reflect the emergence and progression of non-language features captured by the CDR contributing to global disease severity. Although we observed this relationship across FTLD-TDP and FTLD-Tau, FTLD proteinopathies may differ in the rate of neurodegeneration39, and future work will help clarify the progression of lateralization in these pathologies.
In our study, FTLD-TDP and FTLD-Tau had divergent regional distributions of postmortem pathology, which were consistent across hemispheres and among morphological subtypes of each proteinopathy. FTLD-TDP had greatest core-region pathology in left OFC. Postmortem work identifies OFC as a likely site of early TDP-43 pathology in bvFTD40. We are unaware of any large-scale study of regional TDP-43 distribution specifically in PPA, yet one reported PPA patient with GRN mutation showed relatively high TDP-43 counts in OFC13. TDP-43 co-pathology may occur in aged AD or cognitively normal patients, and relatively mild TDP-43 pathology is found in OFC41, as opposed to our data in PPA and in our previous study of bvFTD25. This suggests that TDP-43 co-pathology in advanced aging may be distinct from primary TDP-43 pathology in FTLD. While our findings point to OFC as a key disease area in PPA with FTLD-TDP, we had limited tissue outside of our core-regions, and we cannot exclude anterior temporal regions to have high TDP-43 burden. Indeed, we found preliminary evidence of relatively high pathology in left VLT (0.76±0.12) in our limited sample with available tissue (n=4) that was similar to mean TDP-43 burden in left OFC (Fig 3), but a larger sample would be necessary to more reliably assess pathology burden in this area. Relatively high temporal burden in FTLD-TDP was also found in left STG (Fig 3A); yet this region is more posterior compared to areas typically associated with semantic knowledge in PPA38,42. Nevertheless, with increasing severity, the distribution of pathology may spread from anterior temporal to more posterior temporal regions9.
FTLD-Tau is known to affect frontal areas43, but regional burden of FTLD-Tau in PPA is understudied. In a cohort of PiD, mostly with clinical bvFTD, we found highest levels of pathology in frontal and limbic regions31. Further, significant frontal disease in PSP has been linked to cognitive features44. Here, FTLD-Tau showed greatest core-region pathology in MFC bilaterally, greater in the left hemisphere than the right hemisphere, consistent with the idea that both frontal cortices may contribute to language deficits in naPPA12. However, only left-hemisphere MFC burden directly associated with nonfluent speech. We cannot, however, rule out other indirect contributions of right-hemisphere disease to language deficits, or the involvement of other regions outside of our sampling.
Regional patterns of specific proteinopathies likely influence the clinical manifestations of PPA. In our cohort, 8/9 svPPA patients had FTLD-TDP pathology, whereas 10/13 naPPA patients had FTLD-Tau pathology. While this is consistent with previous associations2, a considerable subset of the cohort (19%) was unclassifiable due to concurrent nonfluent/agrammatic and semantic features (2/5=FTLD-TDP, 3/5=FTLD-Tau), similar to previous reports of PPA with mixed features3,4. Due to these ambiguities of PPA syndromic variants7, we compared specific language features between proteinopathies irrespective of clinical syndromes. We found a double-dissociation, with greater single-word comprehension difficulties in FTLD-TDP as opposed to nonfluent speech in FTLD-Tau (Table 2), and this was associated with increased burden in specific neuroanatomical regions, i.e. left OFC and left MFC, respectively. Distinct regional patterns of FTLD-TDP and FTLD-Tau (Fig 3–4) thus directly relate to language deficits (Fig 6).
Consider first semantic deficits in FTLD-TDP. Left OFC burden, prominent in FTLD-TDP, was associated with antemortem semantic difficulties across the cohort. OFC may play a specific role in the semantic network such as lexical search45; orbitofrontal areas have been implicated in semantic studies in svPPA and bvFTD37,46, and atrophy in svPPA has been shown to extend to OFC9. Additionally, the left uncinate fasciculus connecting anterior temporal and ventral-frontal areas may contribute to semantic retrieval and semantic association tasks47. While we had limited anterior temporal tissue (i.e. VLT), in antemortem MRI we found an association of anterior temporal and orbitofrontal atrophy, greater in FTLD-TDP, with single-word comprehension difficulties. Thus, alongside the anterior temporal cortex, OFC appears intimately associated with TDP-43 pathology and may contribute to semantic deficits in PPA. By comparison, patients with nonfluent speech had greater left MFC pathology than patients with intact fluency, consistent with previous antemortem work12,48,49. We could not test the reported association between nonfluent speech and left INS pathology12 in our dataset due to limited data in this region. Yet, our findings of a double-dissociation in language features between FTLD-TDP and FTLD-Tau highlight the potential role of these linguistic features that can serve as inexpensive screening tools for diagnostic markers that predict pathology in PPA, while avoiding some of the ambiguities associated with syndromic variants50.
This is, to our knowledge, the first attempt to integrate evidence from antemortem MRI with postmortem pathology in PPA. We found that pathology measurements are reflected in the degree of antemortem cortical thinning during life in corresponding regions, while accounting for the time period between scanning and autopsy. Indeed, greatest core-region pathology regions in FTLD-TDP and FTLD-Tau corresponded to areas of greatest core-region atrophy on MRI, in both left and right hemispheres. Our study in bvFTD also associated regional pathologic burden with antemortem MRI cortical thinning25; this was achievable partly because of our digital histopathological approach. These novel findings of concordance between postmortem pathology and antemortem atrophy are an important first step for pathologic-imaging validation in PPA, and provide “proof-of-concept” evidence that tissue-guided imaging approaches may eventually help establish diagnostic biomarkers during life.
Some caveats should be considered when interpreting these data. We had insufficient harmonized neuropsychological assessments for quantitative analysis of language and had missing data for some patients; however, we used a validated chart extraction method including a consensus panel, most patients had detailed longitudinal assessments with structured language evaluations, and >75% were seen within three years of onset. Comprehensive standardized neuropsychological assessments in PPA patients followed to autopsy are needed to confirm our clinicopathological associations. While we have strong rationale to compare FTLD-TDP and FTLD-Tau based on current nomenclature23 and shared genetic risk51,52, our cohort was neuropathologically and genetically diverse. The few GRN carriers and left-handed individuals did not appear to deviate substantially from typical PPA with FTLD-TDP and FTLD-Tau. We carefully identified patients with a monoproteinopathy, not confounded by vascular or significant AD co-pathology. While we were underpowered to evaluate all genetic and pathological subgroups, we accounted for morphological differences by normalizing %AO measurements within proteinopathy subtypes, and sub-analyses in these subtypes showed consistent findings (Fig 4). We performed rigorous validation of our digital algorithms and sampling approach22 to minimize (pre-)analytical bias. Due to the rarity of antemortem research-quality MRI in autopsy cohorts, our MRI subset was relatively small (n=11). Finally, although we sampled several “extended” regions important for FTLD, this represents only an approximation of the widespread language network described in whole-brain neuroimaging8,9,12. Therefore, other regions in the language network, such as the left anterior temporal lobe, may show stronger associations with semantic comprehension and TDP-43 pathology in future studies.
With these limitations in mind, we demonstrate that postmortem pathology is left-lateralized in PPA regardless of underlying pathology, that lateralization diminishes with increasingly severe dementia, that FTLD-TDP and FTLD-Tau have doubly-dissociated, pathology-specific patterns of disease, and that the anatomic distribution of pathology is related to distinct antemortem linguistic and imaging features in FTLD-TDP and FTLD-Tau with clinical PPA. We thus propose that distinct FTLD proteinopathies underlying PPA may eventually be detected during life with the help of tissue-guided imaging and neuropsychological linguistic markers reflecting divergent microscopic patterns of TDP-43 and tau.
Acknowledgements
We are very thankful to the patients and their families for their participation in medical research. We thank Manuela Neumann and Elisabeth Kremmer for providing the phosphorylation-specific TDP-43 antibody p409/410. We thank Mary Leonard for her assistance in the creation of Figure 3. We thank Chris Olm and Pilar Ferraro for their assistance in the creation of Figure 5B. We thank Alzheimer Nederland and the Royal Netherlands Academy of Arts and Sciences (Van Walree Grant) for supporting author Lucia A.A. Giannini with student travel funding. This study was supported by NIH grants AG017586, AG038490, AG052943, AG054519, AG010124, AG043503, NS088341, Penn Institute on Aging, an anonymous donor, and the Wyncote Foundation.
Footnotes
Potential Conflicts of Interest
Nothing to report.
References
- 1.Mesulam MM. Primary progressive aphasia. Ann. Neurol 2001;49(4):425–432. [PubMed] [Google Scholar]
- 2.Grossman M. Primary progressive aphasia: clinicopathological correlations. Nat. Rev 2010;6(2):88–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mesulam MM, Weintraub S, Rogalski EJ, et al. Asymmetry and heterogeneity of Alzheimer’s and frontotemporal pathology in primary progressive aphasia. Brain 2014;137(4):1176–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giannini LAA, Irwin DJ, Mcmillan CT, et al. Clinical marker for Alzheimer disease pathology in logopenic primary progressive aphasia. Neurology 2017;88(24):2276–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Spinelli EG, Mandelli ML, Miller ZA, et al. Typical and atypical pathology in primary progressive aphasia variants. Ann. Neurol 2017;81(3):430–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alladi S, Xuereb J, Bak T, et al. Focal cortical presentations of Alzheimer’s disease. Brain 2007;130(Pt 10):2636–45. [DOI] [PubMed] [Google Scholar]
- 7.Gorno-Tempini ML, Hillis AE, Weintraub S, et al. Classification of primary progressive aphasia and its variants. Neurology 2011;76(11):1006–1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mandelli ML, Vilaplana E, Brown JA, et al. Healthy brain connectivity predicts atrophy progression in non-fluent variant of primary progressive aphasia. Brain 2016;139(Pt 10):2778–2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins JA, Montal V, Hochberg D, et al. Focal temporal pole atrophy and network degeneration in semantic variant primary progressive aphasia. Brain 2017;140(2):457–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rohrer JD, Clarkson MJ, Kittus R, et al. Rates of hemispheric and lobar atrophy in the language variants of frontotemporal lobar degeneration. J. Alzheimers. Dis 2012;30(2):407–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rogalski E, Cobia D, Martersteck A, et al. Asymmetry of cortical decline in subtypes of primary progressive aphasia. Neurology 2014;83(13):1184–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grossman M, Powers J, Ash S, et al. Disruption of large-scale neural networks in non-fluent/agrammatic variant primary progressive aphasia associated with frontotemporal degeneration pathology. Brain Lang 2013;127(2):106–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gliebus G, Bigio EH, Gasho K, et al. Asymmetric TDP-43 distribution in primary progressive aphasia with progranulin mutation. Neurology 2010;74(20):1607–1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim G, Ahmadian SS, Peterson M, et al. Asymmetric pathology in primary progressive aphasia with progranulin mutations and TDP inclusions. Neurology 2016;86(7):627–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim G, Vahedi S, Gefen T, et al. Asymmetric TDP pathology in primary progressive aphasia with right hemisphere language dominance. Neurology 2018;90(5):e396–e403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim G, Bolbolan K, Gefen T, et al. Atrophy and microglial distribution in primary progressive aphasia with TDP-43. Ann. Neurol 2018;83(6):1096–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nevler N, Ash S, Irwin DJ, et al. Validated automatic speech biomarkers in primary progressive aphasia [Internet]. Ann. Clin. Transl. Neurol 2018;Available from: http://doi.wiley.com/10.1002/acn3.653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Josephs KA, Martin PR, Botha H, et al. [18F]AV-1451 tau-PET and primary progressive aphasia. Ann. Neurol 2018;83(3):599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Santos-Santos MA, Mandelli ML, Binney RJ, et al. Features of Patients With Nonfluent/Agrammatic Primary Progressive Aphasia With Underlying Progressive Supranuclear Palsy Pathology or Corticobasal Degeneration. JAMA Neurol 2016;73(6):733–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie SX, Baek Y, Grossman M, et al. Building an integrated neurodegenerative disease database at an academic health center. Alzheimers. Dement 2011;7(4):e84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Toledo JB, Van Deerlin VM, Lee EB, et al. A platform for discovery: The University of Pennsylvania Integrated Neurodegenerative Disease Biobank. Alzheimers. Dement 2014;10(4):477–84.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Irwin DJ, Byrne MD, McMillan CT, et al. Semi-Automated Digital Image Analysis of Pick’s Disease and TDP-43 Proteinopathy. J. Histochem. Cytochem 2016;64(1):54–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackenzie IR, Neumann M, Bigio EH, et al. Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol 2010;119(1):1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Montine TJ, Phelps CH, Beach TG, et al. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 2012;123(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Irwin DJ, McMillan CT, Xie SX, et al. Asymmetry of post-mortem neuropathology in behavioural-variant frontotemporal dementia. Brain 2018;141(1):288–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wood EM, Falcone D, Suh E, et al. Development and validation of pedigree classification criteria for frontotemporal lobar degeneration. JAMA Neurol 2013;70(11):1411–1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Neumann M, Kwong LK, Lee EB, et al. Phosphorylation of S409/410 of TDP-43 is a consistent feature in all sporadic and familial forms of TDP-43 proteinopathies. Acta Neuropathol 2009;117(2):137–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mercken M, Vandermeeren M, Lübke U, et al. Monoclonal antibodies with selective specificity for Alzheimer Tau are directed against phosphatase-sensitive epitopes. Acta Neuropathol 1992;84(3):265–272. [DOI] [PubMed] [Google Scholar]
- 29.Libon DJ, Rascovsky K, Gross RG, et al. The Philadelphia Brief Assessment of Cognition (PBAC): A validated screening measure for dementia. Clin. Neuropsychol 2011;25(8):1314–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Avants BB, Libon DJ, Rascovsky K, et al. Sparse canonical correlation analysis relates network-level atrophy to multivariate cognitive measures in a neurodegenerative population. Neuroimage 2014;84:698–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Irwin DJ, Brettschneider J, McMillan CT, et al. Deep clinical and neuropathological phenotyping of Pick disease. Ann. Neurol 2016;79(2):272–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knopman DS, Weintraub S, Pankratz VS. Language and behavior domains enhance the value of the clinical dementia rating scale. Alzheimer’s Dement 2011;7(3):293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Avants BB, Tustison NJ, Song G, et al. A reproducible evaluation of ANTs similarity metric performance in brain image registration. Neuroimage 2011;54(3):2033–2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tustison NJ, Cook PA, Klein A, et al. Large-scale evaluation of ANTs and FreeSurfer cortical thickness measurements. Neuroimage 2014;99:166–79. [DOI] [PubMed] [Google Scholar]
- 35.Wang H, Yushkevich PA. Multi-atlas segmentation with joint label fusion and corrective learning-an open source implementation. Front. Neuroinform 2013;7:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics 1982;38(4):963–974. [PubMed] [Google Scholar]
- 37.Mummery CJ, Patterson K, Price CJ, et al. A voxel-based morphometry study of semantic dementia: Relationship between temporal lobe atrophy and semantic memory. Ann. Neurol 2000;47(1):36–45. [PubMed] [Google Scholar]
- 38.Mesulam MM, Wieneke C, Hurley R, et al. Words and objects at the tip of the left temporal lobe in primary progressive aphasia. Brain 2013;136(2):601–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whitwell JL, Boeve BF, Weigand SD, et al. Brain atrophy over time in genetic and sporadic frontotemporal dementia: A study of 198 serial magnetic resonance images. Eur. J. Neurol 2015;22(5):745–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brettschneider J, Del Tredici K, Irwin DJ, et al. Sequential distribution of pTDP-43 pathology in behavioral variant frontotemporal dementia (bvFTD). Acta Neuropathol 2014;127(3):423–439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nag S, Yu L, Boyle PA, et al. TDP-43 pathology in anterior temporal pole cortex in aging and Alzheimer’s disease. Acta Neuropathol. Commun 2018;6(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bonner MF, Price AR, Peelle JE, Grossman M. Semantics of the visual environment encoded in parahippocampal cortex. J. Cogn. Neurosci 2016;28(3):361–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kovacs GG. Invited review: Neuropathology of tauopathies: Principles and practice. Neuropathol. Appl. Neurobiol 2015;41(1):3–23. [DOI] [PubMed] [Google Scholar]
- 44.Josephs KA, Boeve BF, Duffy JR, et al. Atypical progressive supranuclear palsy underlying progressive apraxia of speech and nonfluent aphasia. Neurocase 2005;11(4):283–96. [DOI] [PubMed] [Google Scholar]
- 45.Thompson-Schill SL. Neuroimaging studies of semantic memory: Inferring “how” from “where.” Neuropsychologia 2003;41(3):280–92. [DOI] [PubMed] [Google Scholar]
- 46.Cousins KA, York C, Bauer L, Grossman M. Cognitive and anatomic double dissociation in the representation of concrete and abstract words in semantic variant and behavioral variant frontotemporal degeneration. Neuropsychologia 2016;84:244–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Grossman M, McMillan C, Moore P, et al. What’s in a name: Voxel-based morphometric analyses of MRI and naming difficulty in Alzheimer’s disease, frontotemporal dementia and corticobasal degeneration. Brain 2004;127(3):628–649. [DOI] [PubMed] [Google Scholar]
- 48.Ash S, Moore P, Vesely L, et al. Non-fluent speech in frontotemporal lobar degeneration. J. Neurolinguistics 2009;22(4):370–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gunawardena D, Ash S, McMillan C, et al. Why are patients with progressive nonfluent aphasia nonfluent? Neurology 2010;75(7):588–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grossman M. Linguistic Aspects of Primary Progressive Aphasia. Annu. Rev. Linguist 2018;4:377–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Deerlin VM, Sleiman PMA, Martinez-Lage M, et al. Common variants at 7p21 are associated with frontotemporal lobar degeneration with TDP-43 inclusions. Nat. Genet 2010;42(3):234–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kouri N, Ross OA, Dombroski B, et al. Genome-wide association study of corticobasal degeneration identifies risk variants shared with progressive supranuclear palsy. Nat Commun 2015;6:7247. [DOI] [PMC free article] [PubMed] [Google Scholar]