Abstract
Antimicrobial resistance is considered one of the greatest threats to global and public health today. The World Health Organization, the Food and Agriculture Organization, and the World Organisation for Animal Health, known as the Tripartite Collaboration, have called for urgent action. We have previously published a systematic review of 181 studies, demonstrating that interventions that restrict antibiotic use in food-producing animals are associated with a reduction in antibiotic resistant bacterial isolates in both animals and humans. What remains unknown, however, are whether (and what) unintended consequences may arise from such interventions. We therefore undertook a sub-analysis of the original review to address this research question. A total of 47 studies described potential consequences of antibiotic restrictions. There were no consistent trends to suggest clear harm. There may be increased bacterial contamination of food products, the clinical significance of which remains unclear. There is a need for rigorous evaluation of the unintended consequences of antibiotic restrictions in human health, food availability, and economics, given their possible widespread implications.
Keywords: Antimicrobial resistance, One health, Antimicrobial use
1. Context
With increasing attention paid to the rapid rise in antimicrobial resistance and its resulting health and economic consequences, there is mounting pressure to develop strategies to promote prudent use of antibiotics in humans and in agriculture [1]. Though the World Health Organization (WHO) has made recommendations on prudent use of antimicrobials in food-producing animals as early as 1997 [2], they recently undertook a rigorous process, following international standards, to develop and publish formal guidelines on this topic [3]. These WHO Guidelines recommended both a reduction and restriction of antibiotics in food-producing animals, and were informed by our recent systematic review and meta-analysis showing that such measures likely reduce antibiotic resistance in animals and also in certain human populations (particularly those having direct contact with animals) [4]. Evidence though of potential unintended consequences is less clear. There are concerns that restrictions of antibiotic use in food-producing animals may negatively impact animal health and welfare, resulting in increased rates of infection and a paradoxical increase in antibiotic use for therapy [[5], [6], [7]]. Furthermore, antibiotic growth promoters have been used to maximize growth, production, and feed efficiency, resulting in some hesitation in response to complete bans of these products.[8] Increasing evidence suggests though, that the benefit of antibiotics for productivity is likely minimal in industrialized production,[[9], [10], [11]] with no significant long-term negative impacts seen when antibiotic growth promoters are eliminated.[[11], [12], [13], [14]].
McEwen et al. conducted a narrative review of 14 studies that examined unintended consequences of national-level restrictions of antibiotic use in food-producing animals [15]. Five studies reported no adverse consequences, while the others reported increases in certain diseases in the animals, increased antibiotic use for therapeutic purposes, and decreased feed efficiency. These effects tended to be small, temporary and likely to be mitigated by improved biosecurity, hygiene, and animal housing and husbandry practices. The authors concluded that the implementation of strategies to restrict antibiotic use in food-producing animals should not be delayed.
To add to this evidence base, we present here a sub-analysis of our previously published systematic review [4]. The methods have been described in detail in that publication. [4] In summary, we searched electronic databases Agricola (1970-present), AGRIS (http://agris.fao.org), BIOSIS Previews (1980-present), CAB Abstracts (1910-present), MEDLINE (1946-present), EMBASE (1974-present), Global Index Medicus (http://www.globalhealthlibrary.net; non-MEDLINE indices included AIM [AFRO], LILACS [AMRO/PAHO], IMEMR [EMRO], IMSEAR [SEARO], WPRIM [WPRO], WHOLIS [KMS], and SciELO), ProQuest Dissertations, and Science Citation Index (1899-present), in July 2016 with an update in January 2017. Inclusion criteria were original studies describing interventions to reduce antibiotic use in food-producing animals, and that compared proportions of antibiotic-resistant bacterial isolates in animals or humans between intervention and comparator groups. Any interventions that reduced or restricted one or more antibiotics, to any extent, were considered; these included mandatory or voluntary bans, antibiotic-free or organic production systems, national reduction targets, or requiring veterinary consultation or culture and sensitivity testing prior to antibiotic use. For this sub-analysis, we specifically identified the subset of studies that report unintended consequences of interventions that restrict antibiotic use in food-producing animals; the key findings from this sub-analysis are summarized below.
2. Findings
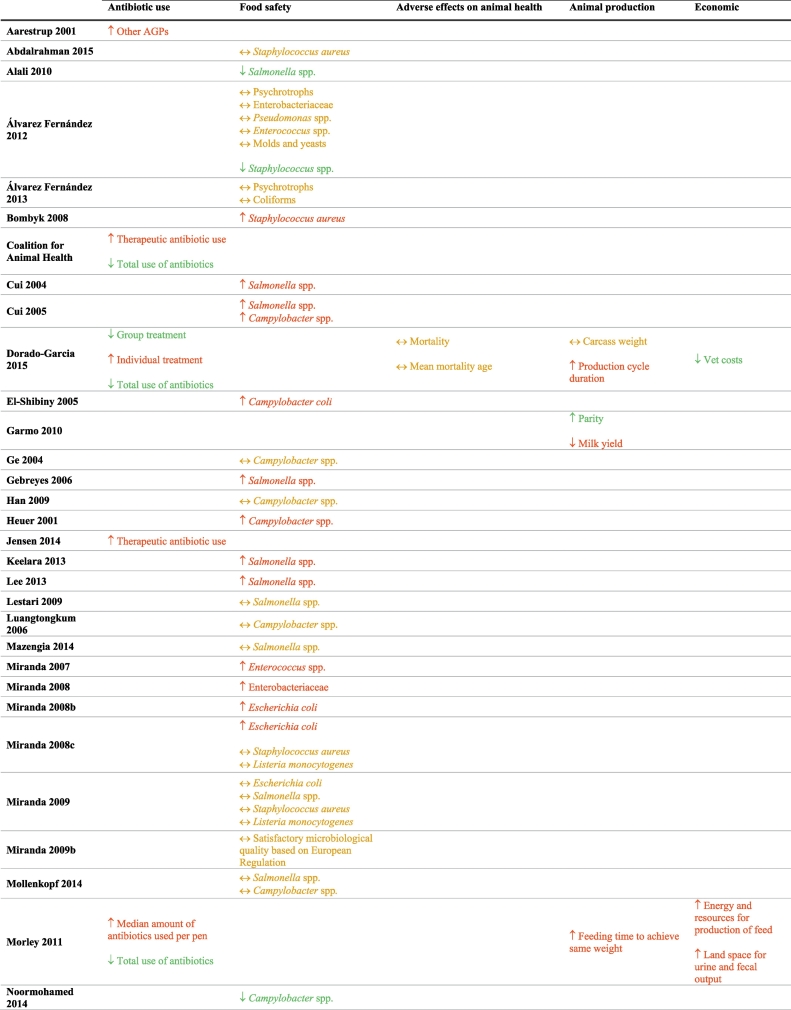
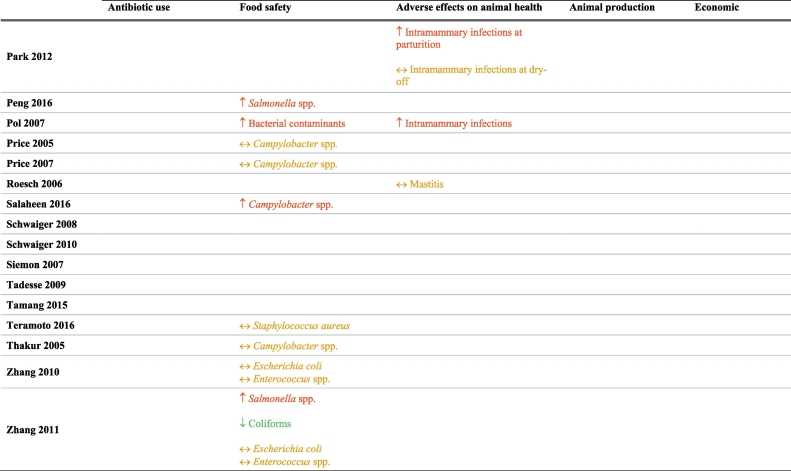
Of the 181 studies included in the original systematic review, 47 were included in this sub-analysis, on the basis of the studies explicitly reporting information on potential unintended consequences associated with antibiotic restriction strategies (Table 1). Detailed characteristics and quality assessments of the individual studies can be found in our prior publication [4]. The unintended consequence that was most frequently examined in this subset of studies was bacterial contamination and/or food safety. None explored adverse effects on human health or decrease in food availability for human consumption.
Table 1.
Unintended consequences of interventions restricting antibiotic use in food-producing animals.
Abbreviations: AGP – Antibiotic growth promoters; ↑ = increased in the intervention compared to the comparator group; ↓ = decreased in the intervention compared to the comparator group; and ↔ = no difference between the intervention and comparator groups.
Where Red = favors comparator group; Green = favors intervention group; Yellow = no difference between intervention and comparator group.
2.1. Antibiotic use (n = 5)
One study found an increase in the use of non-restricted antibiotic growth promoters (AGPs) after the ban of one specific AGP [16]. Four studies reported that though there was an increase in the use of therapeutic antibiotics to treat individual animals, there remained a reduction in the total amount of antibiotics used [5,[17], [18], [19]].
2.2. Food safety (n = 34)
Fifteen studies found an increased rate of bacterial contamination in retail meats when antibiotic restrictions were applied [[20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34]]. Eighteen studies reported either no difference in contamination rates or less contamination in the intervention group when the use of antibiotics was restricted [[35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52]]. One study showed variable results depending on the bacteria in question [53].
2.3. Animal health (n = 4)
Two studies in dairy herds reported increased prevalence of intramammary infections and mastitis pathogens with restriction of antibiotic use (due to organic production) [33,54], while a third study showed no difference in mastitis between groups [55]. The single study that examined mortality reported no difference in either mortality rate or mean age at mortality in intervention versus comparator groups [17].
2.4. Animal production (n = 3)
Two studies reported adverse effects on animal production with increased feeding time to achieve target weight and increased production cycle duration [17,19]. One study showed variable results, with increased parity but lower milk yield in dairy cows [56]. The effects of antibiotic restrictions on animal production vary likely as they depend upon concurrent management changes implemented to promote animal health. For example, when Denmark banned antibiotic growth promoters, productivity improved likely due to a multimodal strategy that included increased veterinary oversight and changes to feed composition to include whole wheat and feeding enzymes.[14,57].
2.5. Costs and economics (n = 2)
One study estimated increased costs in animal production due to increased feeding time to reach target weight, when antibiotic use is restricted [19]. Another study reported decreased veterinary costs with antibiotic restriction; the specific cost inputs and drivers of this cost difference were not reported [17].
3. Interpretation of findings
This sub-analysis of our comprehensive systematic review suggests that unintended consequences are uncommonly reported in studies that are designed to examine the effect of antibiotic restrictions in food-producing animals on antibiotic resistance. Of the 181 studies included in our original systematic review, only 47 reported any unintended consequences. Of these, nearly one-third reported unintended consequences in the discussion section of the publication, without specifying these in a research question or objective.
Despite theoretical concerns that restrictions in antibiotic use in food-producing animals may result in numerous harms to both animal and human health, these are not borne out in our sub-analysis. The associations between unintended consequences and antibiotic restrictions are mixed across all outcome domains, with no clear or consistent trend. Half of the studies reporting on safety of retail food products suggest increased contamination when antibiotic restriction measures are in place. Because no study examined human health outcomes, the clinical significance of this is unclear.
We recognize that unintended consequences were not specifically the focus of our systematic review. As a result, this sub-analysis does not comprehensively capture all studies on this topic. Furthermore, all but two of the studies were undertaken in the United States of America or in Europe. Generalizability of our findings may therefore be limited, especially to low and lower-middle-income countries where management and hygiene practices may be less developed. However, our study complements the previously-mentioned paper on this topic by McEwen et al. [15], by virtue of our identification of a number of additional studies not covered by their recent review. Together, our two reviews provide value in summarizing an informative, though small, body of literature examining potential harms of interventions that restrict antibiotic use in food-producing animals. We demonstrate that future research on antibiotic restrictions in agriculture should more specifically consider their impact on unintended consequences. The increasing global efforts to reduce and restrict antibiotic use in food-producing animals present the perfect opportunity to conduct rigorous evaluations of potential harms and to provide insight regarding the role of local context in the relationship between antibiotic restriction and unintended consequences.
Contributors
Each of the 12 authors meets the authorship requirements as established by the International Committee of Medical Journal Editors in the Uniform Requirements for Manuscripts Submitted to Biomedical Journals. All authors were involved in the design and development of the study. HG created the search strategy and conducted the literature search in electronic databases. DN conducted the grey literature search. KT and NC screened all studies for inclusion into the original systematic review and performed all study quality assessments. SC, PR, and HB provided input on studies where consensus could not be reached. KT, NC, DN, AP, and NS, performed data extraction. All authors contributed to data interpretation and data analysis. KT drafted the manuscript and all authors revised it critically for content. All authors have full access to all data and can take responsibility for the integrity of the data and accuracy of the data analysis. All authors have read and approved the manuscript.
Role of the funding source
The WHO was involved in both the original systematic review and meta-analysis, as well as this sub-study. They were involved in developing the research question, the study design and the study protocol. They had no involvement in data extraction or interpretation of findings. The authors have been given permission by the WHO to publish this article. All had full access to all of the data and can take responsibility for the integrity of the data and the accuracy of the data analysis.
Acknowledgments
Acknowledgements
This study was commissioned and paid for by the World Health Organization (WHO). They were involved in developing the research question, the study design and the study protocol. They had no involvement in data extraction or interpretation of findings. The authors have been given permission by the WHO to publish this article. The corresponding author had full data and had final responsibility for the decision to submit for publication. Copyright in the original work on which this article is based belongs to the WHO. The authors have been given permission to publish this article. The authors alone are responsible for the views expressed in this publication and do not necessarily represent the views, decisions, or policies of the World Health Organization.
Conflict of interest
JK is the principal investigator on an unrestricted grant in aid to conduct an epidemiological study of invasive pneumococcal disease in humans, including impact of pneumococcal vaccines (Pfizer Canada). He is also the local co-investigator on contract of a clinical trial of a maternal pertussis vaccine (GSK Canada). All other authors declare no conflicts of interest other than the WHO funding of this study.
References
- 1.United Nations General Assembly of the United Nations: President of the 71st Session. 2016. http://www.un.org/pga/71/event-latest/high-level-meeting-on-antimicrobial-resistance/
- 2.World Health Organization . Report of a WHO Meeting, Berlin, Germany, 13–17 October 1997. 1997. The medical impact of antimicrobial use in food animals.https://apps.who.int/iris/bitstream/handle/10665/64439/WHO_EMC_ZOO_97.4.pdf?sequence=1 [Google Scholar]
- 3.World Health Organization WHO Guidelines on Use of Medically Important Antimicrobials in Food-Producing Animals. 2017. http://apps.who.int/iris/bitstream/handle/10665/258970/9789241550130-eng.pdf;jsessionid=FC6969336920B3D0DA150C31EF36D25B?sequence=1 [PubMed]
- 4.Tang K.L., Caffrey N.P., Nóbrega D.B., Cork S.C., Ronksley P.E., Barkema H.W. Restricting the use of antibiotics in food-producing animals and its associations with antibiotic resistance in food-producing animals and human beings: a systematic review and meta-analysis. The Lancet Planetary Health. 2017;1 doi: 10.1016/S2542-5196(17)30141-9. (e316-e27) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coalition for Animal Health . European Test Case: Increased Animal Disease, Mixed Human Health Benefit. 2019. Political bans on antibiotics are counterproductive. [Google Scholar]
- 6.Jensen V.F., de Knegt L.V., Andersen V.D., Wingstrand A. Temporal relationship between decrease in antimicrobial prescription for Danish pigs and the "yellow card" legal intervention directed at reduction of antimicrobial use. Prev Vet Med. 2014;117:554–564. doi: 10.1016/j.prevetmed.2014.08.006. [DOI] [PubMed] [Google Scholar]
- 7.Karavolias J., Salois M.J., Baker K.T., Watkins K. Raised without antibiotics: impact on animal welfare and implications for food policy. Translational Animal Science. 2018;2:337–348. doi: 10.1093/tas/txy016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hao H., Cheng G., Iqbal Z., Ai X., Hussain H.I., Huang L. Benefits and risks of antimicrobial use in food-producing animals. Front. Microbiol. 2014;5:288. doi: 10.3389/fmicb.2014.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aarestrup F.M. The livestock reservoir for antimicrobial resistance: a personal view on changing patterns of risks, effects of interventions and the way forward. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2015;370:20140085. doi: 10.1098/rstb.2014.0085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Collignon P., Wegener H.C., Braam P., Butler C.D. The routine use of antibiotics to promote animal growth does little to benefit protein undernutrition in the developing world. Clin. Infect. Dis. 2005;41:1007–1013. doi: 10.1086/433191. [DOI] [PubMed] [Google Scholar]
- 11.Graham J.P., Boland J.J., Silbergeld E. Growth promoting antibiotics in food animal production: an economic analysis. Public Health Rep. 2007;122:79–87. doi: 10.1177/003335490712200111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aarestrup F.M., Jensen V.F., Emborg H.D., Jacobsen E., Wegener H.C. Changes in the use of antimicrobials and the effects on productivity of swine farms in Denmark. Am. J. Vet. Res. 2010;71:726–733. doi: 10.2460/ajvr.71.7.726. [DOI] [PubMed] [Google Scholar]
- 13.Emborg H., Ersboll A.K., Heuer O.E., Wegener H.C. The effect of discontinuing the use of antimicrobial growth promoters on the productivity in the Danish broiler production. Prev Vet Med. 2001;50:53–70. doi: 10.1016/s0167-5877(01)00218-5. [DOI] [PubMed] [Google Scholar]
- 14.Schlundt J., Aarestrup F.M. Commentary: benefits and risks of antimicrobial use in food-producing animals. Front. Microbiol. 2017;8:181. doi: 10.3389/fmicb.2017.00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McEwen S.A., Angulo F.J., Collignon P.J., Conly J.M. Unintended consequences associated with national-level restrictions on antimicrobial use in food-producing animals. The Lancet Planetary Health. 2018;2 doi: 10.1016/S2542-5196(18)30138-4. (e279-e82) [DOI] [PubMed] [Google Scholar]
- 16.Aarestrup F.M., Seyfarth A.M., Emborg H.D., Pedersen K., Hendriksen R.S., Bager F. Effect of abolishment of the use of antimicrobial agents for growth promotion on occurrence of antimicrobial resistance in fecal enterococci from food animals in Denmark. Antimicrob. Agents Chemother. 2001;45:2054–2059. doi: 10.1128/AAC.45.7.2054-2059.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dorado-Garcia A., Graveland H., Bos M.E., Verstappen K.M., Van Cleef B.A., Kluytmans J.A. Effects of reducing antimicrobial use and applying a cleaning and disinfection program in veal calf farming: experiences from an intervention study to control livestock-associated MRSA. PLoS One. 2015;10 doi: 10.1371/journal.pone.0135826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jensen H.H., Hayes D.J. Impact of Denmark's ban on antimicrobials for growth promotion. Curr. Opin. Microbiol. 2014;19:30–36. doi: 10.1016/j.mib.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 19.Morley P.S., Dargatz D.A., Hyatt D.R., Dewell G.A., Patterson J.G., Burgess B.A. Effects of restricted antimicrobial exposure on antimicrobial resistance in fecal Escherichia coli from feedlot cattle. Foodborne Pathog. Dis. 2011;8:87–98. doi: 10.1089/fpd.2010.0632. [DOI] [PubMed] [Google Scholar]
- 20.Bombyk R.A., Bykowski A.L., Draper C.E., Savelkoul E.J., Sullivan L.R., Wyckoff T.J. Comparison of types and antimicrobial susceptibility of staphylococcus from conventional and organic dairies in west-Central Minnesota, USA. J. Appl. Microbiol. 2008;104:1726–1731. doi: 10.1111/j.1365-2672.2007.03681.x. [DOI] [PubMed] [Google Scholar]
- 21.Cui S. University of Maryland, College Park; Ann Arbor: 2004. Detection and Characterization of Escherichia Coli O157:H7 and Salmonella in Food [Ph.D.] [Google Scholar]
- 22.Cui S., Ge B., Zheng J., Meng J. Prevalence and antimicrobial resistance of campylobacter spp. and salmonella serovars in organic chickens from Maryland retail stores. Appl. Environ. Microbiol. 2005;71:4108–4111. doi: 10.1128/AEM.71.7.4108-4111.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Shibiny A., Connerton P.L., Connerton I.F. Enumeration and diversity of campylobacters and bacteriophages isolated during the rearing cycles of free-range and organic chickens. Appl. Environ. Microbiol. 2005;71:1259–1266. doi: 10.1128/AEM.71.3.1259-1266.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gebreyes W.A., Thakur S., Morrow W.E.M. Comparison of prevalence, antimicrobial resistance, and occurrence of multidrug-resistant salmonella in antimicrobial-free and conventional pig production. J. Food Prot. 2006;69:743–748. doi: 10.4315/0362-028x-69.4.743. [DOI] [PubMed] [Google Scholar]
- 25.Heuer O.E., Pedersen K., Andersen J.S., Madsen M. Prevalence and antimicrobial susceptibility of thermophilic campylobacter in organic and conventional broiler flocks. Lett. Appl. Microbiol. 2001;33:269–274. doi: 10.1046/j.1472-765x.2001.00994.x. [DOI] [PubMed] [Google Scholar]
- 26.Keelara S., Scott H.M., Morrow W.M., Gebreyes W.A., Correa M., Nayak R. Longitudinal study of distributions of similar antimicrobial-resistant salmonella serovars in pigs and their environment in two distinct swine production systems. Appl. Environ. Microbiol. 2013;79:5167–5178. doi: 10.1128/AEM.01419-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee S.K., Chon J.W., Song K.Y., Hyeon J.Y., Moon J.S., Seo K.H. Prevalence, characterization, and antimicrobial susceptibility of salmonella Gallinarum isolated from eggs produced in conventional or organic farms in South Korea. Poult. Sci. 2013;92:2789–2797. doi: 10.3382/ps.2013-03175. [DOI] [PubMed] [Google Scholar]
- 28.Miranda J.M., Guarddon M., Mondragon A., Vazquez B.I., Fente C.A., Cepeda A. Antimicrobial resistance in enterococcus spp. strains isolated from organic chicken, conventional chicken, and Turkey meat: a comparative survey. J. Food Prot. 2007;70:1021–1024. doi: 10.4315/0362-028x-70.4.1021. [DOI] [PubMed] [Google Scholar]
- 29.Miranda J.M., Guarddon M., Vázquez B.I., Fente C.A., Barros-Velázquez J., Cepeda A. Antimicrobial resistance in Enterobacteriaceae strains isolated from organic chicken, conventional chicken and conventional Turkey meat: a comparative survey. Food Control. 2008;19:412–416. [Google Scholar]
- 30.Miranda J.M., Vázquez B.I., Fente C.A., Barros-Velázquez J., Cepeda A., Abuín C.M.F. Antimicrobial resistance in Escherichia coli strains isolated from organic and conventional pork meat: a comparative survey. Eur. Food Res. Technol. 2008;226:371–375. [Google Scholar]
- 31.Miranda J.M., Vazquez B.I., Fente C.A., Calo-Mata P., Cepeda A., Franco C.M. Comparison of antimicrobial resistance in Escherichia coli, Staphylococcus aureus, and listeria monocytogenes strains isolated from organic and conventional poultry meat. J. Food Prot. 2008;71:2537–2542. doi: 10.4315/0362-028x-71.12.2537. [DOI] [PubMed] [Google Scholar]
- 32.Peng M., Salaheen S., Almario J.A., Tesfaye B., Buchanan R., Biswas D. Prevalence and antibiotic resistance pattern of salmonella serovars in integrated crop-livestock farms and their products sold in local markets. Environ. Microbiol. 2016;18:1654–1665. doi: 10.1111/1462-2920.13265. [DOI] [PubMed] [Google Scholar]
- 33.Pol M., Ruegg P.L. Relationship between antimicrobial drug usage and antimicrobial susceptibility of gram-positive mastitis pathogens. J. Dairy Sci. 2007;90:262–273. doi: 10.3168/jds.S0022-0302(07)72627-9. [DOI] [PubMed] [Google Scholar]
- 34.Salaheen S., Peng M., Biswas D. Ecological dynamics of campylobacter in integrated mixed crop-livestock farms and its prevalence and survival ability in post-harvest products. Zoonoses Public Health. 2016;13:13. doi: 10.1111/zph.12279. [DOI] [PubMed] [Google Scholar]
- 35.Abdalrahman L.S., Stanley A., Wells H., Fakhr M.K. Isolation, virulence, and antimicrobial resistance of methicillin-resistant Staphylococcus aureus (MRSA) and methicillin sensitive Staphylococcus aureus (MSSA) strains from Oklahoma retail poultry meats. Int. J. Environ. Res. Public Health. 2015;12:6148–6161. doi: 10.3390/ijerph120606148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alali W.Q., Thakur S., Berghaus R.D., Martin M.P., Gebreyes W.A. Prevalence and distribution of salmonella in organic and conventional broiler poultry farms. Foodborne Pathog. Dis. 2010;7:1363–1371. doi: 10.1089/fpd.2010.0566. [DOI] [PubMed] [Google Scholar]
- 37.Alvarez-Fernandez E., Cancelo A., Diaz-Vega C., Capita R., Alonso-Calleja C. Antimicrobial resistance in E. coli isolates from conventionally and organically reared poultry: a comparison of agar disc diffusion and Sensi test gram-negative methods. Food Control. 2013;30:227–234. [Google Scholar]
- 38.Alvarez-Fernandez E., Dominguez-Rodriguez J., Capita R., Alonso-Calleja C. Influence of housing systems on microbial load and antimicrobial resistance patterns of Escherichia coli isolates from eggs produced for human consumption. J. Food Prot. 2012;75:847–853. doi: 10.4315/0362-028X.JFP-11-182. [DOI] [PubMed] [Google Scholar]
- 39.Ge B., Zheng J., Meng J., editors. Abstracts of the Interscience Conference on Antimicrobial Agents and Chemotherapy. Oct-Nov, 2004. Antimicrobial susceptibility of campylobacter from retail organic and conventional chickens. [Google Scholar]
- 40.Han F., Lestari S.I., Pu S., Ge B. Prevalence and antimicrobial resistance among campylobacter spp. in Louisiana retail chickens after the enrofloxacin ban. Foodborne Pathog. Dis. 2009;6:163–171. doi: 10.1089/fpd.2008.0171. [DOI] [PubMed] [Google Scholar]
- 41.Lestari S.I., Han F., Wang F., Ge B. Prevalence and antimicrobial resistance of salmonella serovars in conventional and organic chickens from Louisiana retail stores. J. Food Prot. 2009;72:1165–1172. doi: 10.4315/0362-028x-72.6.1165. [DOI] [PubMed] [Google Scholar]
- 42.Luangtongkum T., Morishita T.Y., Ison A.J., Huang S., McDermott P.F., Zhang Q. Effect of conventional and organic production practices on the prevalence and antimicrobial resistance of campylobacter spp. in poultry. Appl. Environ. Microbiol. 2006;72:3600–3607. doi: 10.1128/AEM.72.5.3600-3607.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mazengia E., Samadpour M., Hill H.W., Greeson K., Tenney K., Liao G. Prevalence, concentrations, and antibiotic sensitivities of salmonella Serovars in poultry from retail establishments in Seattle, Washington. J. Food Prot. 2014;77:885–893. doi: 10.4315/0362-028X.JFP-13-394. [DOI] [PubMed] [Google Scholar]
- 44.Miranda J.M., Mondragon A., Vazquez B.I., Fente C.A., Cepeda A., Franco C.M. Influence of farming methods on microbiological contamination and prevalence of resistance to antimicrobial drugs in isolates from beef. Meat Sci. 2009;82:284–288. doi: 10.1016/j.meatsci.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 45.Miranda J.M., Mondragón A., Vázquez B.I., Fente C.A., Cepeda A., Franco C.M. Microbiological quality and antimicrobial resistance of Escherichia coli and Staphylococcus aureus isolated from conventional and organic "Arzúa-Ulloa" cheese. CyTA - Journal of Food. 2009;7:103–110. [Google Scholar]
- 46.Mollenkopf D.F., Cenera J.K., Bryant E.M., King C.A., Kashoma I., Kumar A. Organic or antibiotic-free labeling does not impact the recovery of enteric pathogens and antimicrobial-resistant Escherichia coli from fresh retail chicken. Foodborne Pathog. Dis. 2014;11:920–929. doi: 10.1089/fpd.2014.1808. [DOI] [PubMed] [Google Scholar]
- 47.Noormohamed A., Fakhr M.K. Prevalence and antimicrobial susceptibility of campylobacter spp. in Oklahoma conventional and organic retail poultry. The open microbiology journal. 2014;8:130–137. doi: 10.2174/1874285801408010130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Price L.B., Johnson E., Vailes R., Silbergeld E. Fluoroquinolone-resistant campylobacter isolates from conventional and antibiotic-free chicken products. Environ. Health Perspect. 2005;113:557–560. doi: 10.1289/ehp.7647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Price L.B., Lackey L.G., Vailes R., Silbergeld E. The persistence of fluoroquinolone-resistant campylobacter in poultry production. Environ. Health Perspect. 2007;115:1035–1039. doi: 10.1289/ehp.10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Teramoto H., Salaheen S., Debabrata B. Contamination of post-harvest poultry products with multidrug resistant Staphylococcus aureus in Maryland-Washington DC metro area. Food Control. 2016;65:132–135. [Google Scholar]
- 51.Thakur S., Gebreyes W.A. Prevalence and antimicrobial resistance of campylobacter in antimicrobial-free and conventional pig production systems. J. Food Prot. 2005;68:2402–2410. doi: 10.4315/0362-028x-68.11.2402. [DOI] [PubMed] [Google Scholar]
- 52.Zhang J., Wall S.K., Xu L., Ebner P.D. Contamination rates and antimicrobial resistance in bacteria isolated from "grass-fed" labeled beef products. Foodborne Pathog. Dis. 2010;7:1331–1336. doi: 10.1089/fpd.2010.0562. [DOI] [PubMed] [Google Scholar]
- 53.Zhang J., Massow A., Stanley M., Papariella M., Chen X., Kraft B. Contamination rates and antimicrobial resistance in enterococcus spp., Escherichia coli, and salmonella isolated from "no antibiotics added"-labeled chicken products. Foodborne Pathog. Dis. 2011;8:1147–1152. doi: 10.1089/fpd.2011.0852. [DOI] [PubMed] [Google Scholar]
- 54.Park Y.K., Fox L.K., Hancock D.D., McMahan W., Park Y.H. Prevalence and antibiotic resistance of mastitis pathogens isolated from dairy herds transitioning to organic management. J. Vet. Sci. 2012;13:103–105. doi: 10.4142/jvs.2012.13.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Roesch M., Perreten V., Doherr M.G., Schaeren W., Schallibaum M., Blum J.W. Comparison of antibiotic resistance of udder pathogens in dairy cows kept on organic and on conventional farms. J. Dairy Sci. 2006;89:989–997. doi: 10.3168/jds.S0022-0302(06)72164-6. [DOI] [PubMed] [Google Scholar]
- 56.Garmo R.T., Waage S., Sviland S., Henriksen B.I., Osteras O., Reksen O. Reproductive performance, udder health, and antibiotic resistance in mastitis bacteria isolated from Norwegian red cows in conventional and organic farming. Acta Vet. Scand. 2010;52 doi: 10.1186/1751-0147-52-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wielinga P.R., Jensen V.F., Aarestrup F.M., Schlundt J. Evidence-based policy for controlling antimicrobial resistance in the food chain in Denmark. Food Control. 2014;40:185–192. [Google Scholar]