Abstract
Biomass harvesting is one of the most expensive steps of the whole microalgal production pipeline. Therefore, the present work aimed to understand the effect of salinity on the growth performance, biochemical composition and sedimentation velocity of Tetraselmis sp. CTP4, in order to establish an effective low-cost pilot-scale harvesting system for this strain. At lab scale, similar growth performance was obtained in cultures grown at salinities of 5, 10 and 20 g L-1 NaCl. In addition, identical settling velocities (2.4–3.6 cm h-1) were observed on all salinities under study, regardless of the growth stage. However, higher salinities (20 g L-1) promoted a significant increase in lipid contents in this strain compared to when this microalga was cultivated at 5 or 10 g L-1 NaCl. At pilot-scale, cultures were cultivated semi-continuously in 2.5-m3 tubular photobioreactors, fed every four days, and stored in a 1-m3 harvesting tank. Upon a 24-hour settling step, natural sedimentation of the microalgal cells resulted in the removal of 93% of the culture medium in the form of a clear liquid containing only vestigial amounts of biomass (0.07 ± 0.02 g L-1 dry weight; DW). The remaining culture was recovered as a highly concentrated culture (19.53 ± 4.83 g L-1 DW) and wet microalgal paste (272.7 ± 18.5 g L-1 DW). Overall, this method provided an effective recovery of 97% of the total biomass, decreasing significantly the harvesting costs.
Keywords: Biotechnology, Plant biology, Microbiology
1. Introduction
Microalgae are microscopic photosynthetic microorganisms currently regarded as a promising feedstock for several biotechnological applications such as biofuels, bioremediation, human and animal nutrition, as well as a source of high value compounds (Huerlimann et al., 2010; Sing et al., 2014; Pereira et al., 2016). Although recent reports suggested their wide biotechnological potential, the current production costs of microalgal biomass are still the main hindrance for large-scale commercialization. Therefore, to decrease production costs, it is necessary to address and optimize the whole microalgal production pipeline, from strain selection to the effective establishment of cost-effective harvesting and downstream processes.
Environmental factors (e.g., light, temperature, culture medium and salinity) strongly influence culture productivity and biomass composition (Lananan et al., 2013; BenMoussa-Dahmen et al., 2016). Relatively high salinities (“high salt”) usually have a significant effect on microalgal cells, causing lower growth rates or even growth arrest (Ho et al., 2014; Zhu et al., 2016). In addition, salinity shifts may induce oxidative stress in the culture and alter its physiological and biochemical composition (Campenni' et al., 2013; Sing et al., 2014). However, some microalgae, namely euryhaline and/or osmotolerant strains, are able to thrive on a wide range of salinities, which might be essential in industrial facilities when valuable metabolites (e.g., polyunsaturated fatty acids and carotenoids) need to be produced or when culture management techniques are needed to control contaminants (von Alvensleben et al., 2013; Skjanes et al., 2013; Zhu et al., 2016).
Apart from cultivation costs, harvesting and biomass dewatering processes are the most expensive steps in the whole production pipeline. In fact, the costs associated with harvesting and water removal can easily reach 30% of the total cost. Therefore, any cost savings in these steps can be a key factor in the economic profitability of the whole process (Chen et al., 2011; Acien et al., 2016; Show et al., 2017).
There are several biomass concentration techniques available. Novel lab-scale technologies are emerging with promising application in industrial purposes, such as electrocoagulation, bio-flocculation, electro-flocculation (electrolytic process), ozonation-dispersed flotation, among others (Lananan et al., 2016; Singh and Patidar, 2018). However, nowadays, industrial production units mainly use centrifugation, ultrafiltration (membrane) or a combination of both methods in order to process large-scale culture volumes (e.g. Allmicroalgae and Necton S.A.). Although both techniques are highly efficient in microalgal biomass recovery, they have high CAPEX and significant advantages and disadvantages associated. Flocculation and flotation are described as more suitable for large-scale processing due to lower costs and energy demands, but require the use of chemical products (Bilad et al., 2014; t Lam et al., 2018; Yellapu et al., 2018). Natural sedimentation would be the perfect solution for the industry, however, most small size microalgae do not sediment or the sedimentation velocity restrains their recovery in a feasible period.
The Tetraselmis genus is considered as highly promising for different biotechnological applications, namely as a source of high value compounds (Pignolet et al., 2013; Sansone et al., 2017). In addition, Tetraselmis sp. CTP4 has previously been reported as a euryhaline, fast growing and robust microalgal strain, which holds high potential for scale-up in industrial production facilities (Pereira et al., 2018) as well as high sedimentation rates (Pereira et al., 2016).
Therefore, in this study we aim to expand our knowledge in order to understand the influence of salt concentration on growth and productivity rates, biomass composition, and sedimentation velocity of Tetraselmis sp. CTP4. After a preliminary laboratory assay, the results of production and sedimentation were validated at pilot-scale, in a 2.5-m3 photobioreactor and 1-m3 sedimentation tank.
2. Materials and methods
2.1. Microalgae strain
All experiments described in the present work were performed at the facilities of CMP (Secil Group, Portugal), between the 15th of September 2016 and 15th of August 2017. Tetraselmis sp. CTP4 was isolated from Ria Formosa (Portugal), as described in Pereira et al. (2016).
2.2. Growth under different salinities
Cultures were grown in laboratory conditions, in 5-L glass airlift reactors at ∼25 °C, under continuous lighting (100 μmol photons m−2 s−1), aerated with filtered compressed air (0.2 μm) supplemented with CO2. Guillard's F2 medium was used as culture medium in all experiments. Synthetic seawater was prepared using commercial sodium chloride at the following concentrations: 5, 10 and 20 g L-1. All experiments were carried out in triplicate.
2.3. Growth assessment
Microalgal growth was measured by optical density at 540 nm in a Thermo Scientific Genesis 10S UV-Vis spectrophotometer. Biomass dry weight (DW) was determined by filtration of samples through a 0.45-μm cellulose filter, washed with ammonium formate (25 g L-1) and dried in an AnD MS-70 moisture analyzer at 120 °C. Cultures were monitored daily by means of microscopic observation (CX31RBSF, Olympus).
2.4. Sedimentation rate
Gravity-induced natural sedimentation was measured in cultures grown under different salinities and at different growth stages, following the guidelines used by Nollet and Dre Geldert (2000). The sedimentation rate was calculated in 100-mL measuring cylinders with a height of 16 cm. After the introduction of the culture in the sedimentation systems, they were kept in separate chamber without vibration. The height of settled culture was measured every 30 minutes, for 6 hours. Results are presented in cm h−1.
After the sedimentation process, the supernatant was removed using a glass pipette and the remaining concentrated cultures were centrifuged for 5 min at 2000 g and later freeze-dried for biochemical analysis.
2.5. Evaluation of biochemical composition
2.5.1. Total lipids
Total lipids were extracted according to a modified Bligh and Dyer (1959) protocol described in Pereira et al. (2011). Briefly, lipid extraction was performed with a mixture of chloroform and methanol (1:2) and homogenized for 1 minute using an IKA Ultra-Turrax disperser. Afterwards, 1 mL of chloroform was added, and samples were further homogenized for 30s. At a later stage, this step was repeated with 1 mL of water instead. Extracts were then centrifuged and the organic phase (chloroform) was transferred to pre-weighed tubes and dried overnight. Upon solvent evaporation, the extracted lipids were weighed and the lipid fraction was estimated by gravimetry.
2.5.2. Protein content
Total protein was estimated by Elemental analysis of C, H and N in the obtained biomass, using a Vario el III (Vario EL, Elementar Analyser systeme, GmbH, Hanau, Germany) according to the procedure provided by the manufacturer. Total protein was estimated by multiplying the nitrogen content by a factor of 6.25.
2.5.3. Ash content
The determination of ash content was performed by burning 1 g of biomass for 8 hours at 550 °C in a muffle furnace (J. P. Selecta, Sel horn R9-L).
2.6. Pilot-scale production of biomass
Outdoor pilot-scale 2.5-m3 tubular photobioreactors (PBRs) were used to grow Tetraselmis sp. CTP4 at a salt concentration of 10 g L-1 (n = 3). The pH was maintained at 8, by an automatic CO2 injection system, while the temperature of cultures was kept between 25-30 °C, using a water sprinkling thermoregulation system. The flow rate of cultures (9 m3 h-1) was measured using a Dynasonics DXN (Portable Ultrasonic Measurement System). PBRs were inoculated at DW of approximately 0.2 g L-1 and were allowed to grow until a DW of 2.3 g L-1 (12 days; based on previous results, the beginning of stationary phase). At this stage, a semi-continuous approach was implemented and 25% of the culture volume (∼600 L) was harvested (1st harvest) and fresh culture medium was added. The renewed culture was allowed to grow for 4 days and again 25% of the culture volume was replaced (2nd harvest). Finally, the cultures were allowed to grow for an additional 4-day period (3rd harvest, end of the trial) and the resulting culture was fully harvested. From the total volume of culture harvested at every step of the semi-continuous growth, only 250 L of each PBR was introduced in the pilot-scale sedimentation tank (see below for further details). Growth performance was daily assessed by means of optical density and DW. A RM Young meteorological station and an Apogee Logan UT SP-110 pyranometer registered the local temperature and radiation, respectively.
2.7. Pilot-scale sedimentation of cultivated biomass
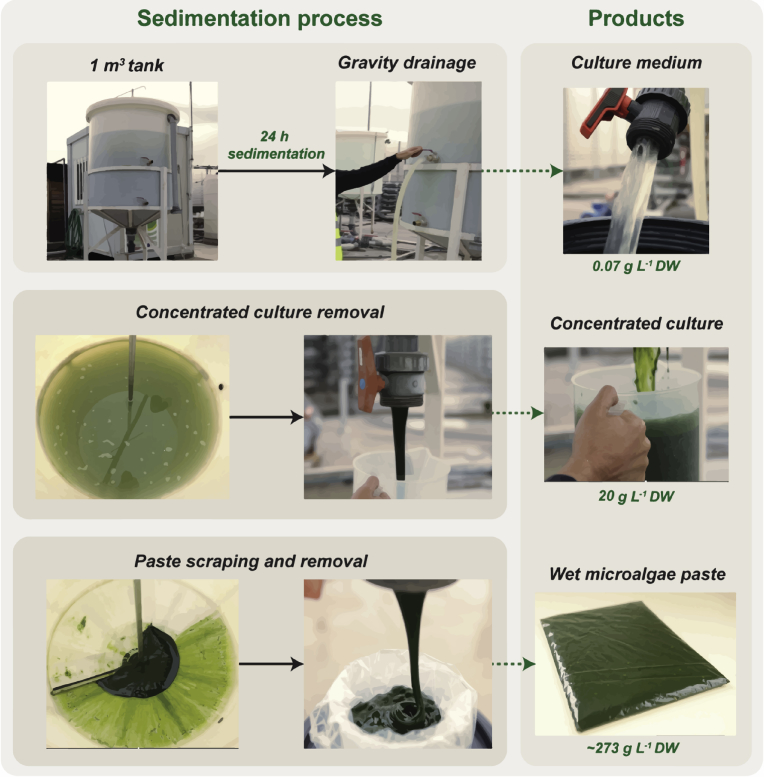
The pilot-scale sedimentation experiment was performed in a 1-m3 cylindrical-conical tank with a working volume of 0.75 m3, at the end of each semi-continuous growth (Fig. 1; Video 1). As previously stated, a volume of approximately 0.25 m3 of each 2.5-m3 PBR was transferred to the sedimentation tank and the culture was allowed to naturally sediment for 24 hours. Afterwards, the supernatant was drained by gravity using the two taps located on the side of the tank, which were connected to hoses to better direct the liquid into storage vessels. The remaining concentrated culture was then removed through the valve located on the bottom of the tank. Since a thick microalgal paste remained attached to the lower part of the sedimentation tank, a squeegee was assembled at the bottom of the tank to scrap and recover the rest of the biomass via the bottom valve. All streams of the process (culture medium, concentrated culture and wet paste) recovered from the sedimentation tank were immediately analysed for their DW.
Fig. 1.
Pilot-scale sedimentation process for low-cost harvesting of Tetraselmis sp. CTP4 biomass. Microalgal culture was settled by natural sedimentation (24 hours). Thereafter, the culture medium was recovered via taps connected to hoses, located on the side section of the tank. Afterwards the concentrated culture was removed using the bottom valve of the tank. The paste deposited in the bottom of the tank was retrieved by a homemade scraping system into the lower valve directly into plastic bags.
2.8. Statistical analysis
Experiments were performed at least in triplicate and results are expressed as mean ± standard deviation. Significance of differences was assessed by ANOVA using SPSS v24.0.
3. Results and discussion
3.1. Effect of salt concentration on growth and sedimentation performance
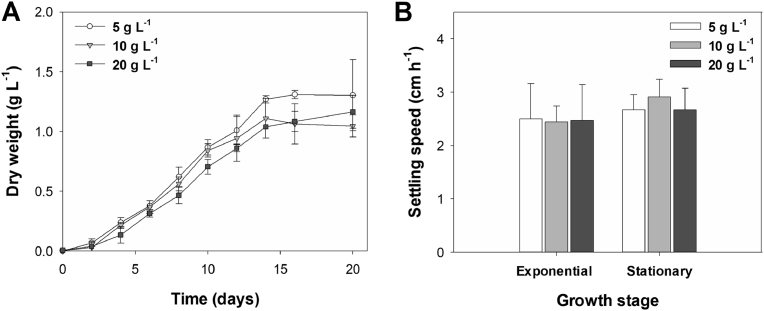
Tetraselmis sp. CTP4 was cultivated in laboratory conditions using three different salinities, namely, 5, 10 and 20 g L-1 (Fig. 2A), for 20 days. All cultures showed a growth curve similar to those previously obtained for this strain (Pereira et al., 2016). The lag phase took place for about 2 days and the stationary phase was reached at day 15. Cultures displayed similar growth without significant differences (p > 0.05) among the three salinities tested, reaching a final biomass DW of 1.2–1.5 g L-1. These results confirm the euryhaline properties of this strain and its capability to easily adapt to different salt conditions (Sing et al., 2014; Das et al., 2016; Fon-Sing and Borowitzka, 2016; Pereira et al., 2016). This is a key feature for the successful growth of microalgae in large-scale industrial facilities. In open production systems (e.g. raceways), environmental factors, such as evaporation and local precipitation, can significantly increase and decrease, respectively, the salt concentration in the medium (Fon-Sing and Borowitzka, 2016). On the other hand, in closed systems (photobioreactors), salinity up- and downshifts can be crucial to manage natural occurring contaminants with lower halotolerance (Pereira et al., 2018). In addition, growing microalgal cultures in low-salt media might decrease production downstream costs related to the management of saltwater discharges.
Fig. 2.
(A) Batch growth of Tetraselmis sp. CTP4 in 5 L reactors under three different salinities (5, 10 and 20 g L-1). (B) Sedimentation rate of cultures grown using different salinities at different growth stages (exponential and stationary), expressed in cm h−1. Error bars represent the standard deviation calculated from three replicates.
The sedimentation rate of Tetraselmis sp. CTP4 cultures grown under three salinities in the exponential and stationary growth stages is shown in Fig. 2B. The obtained data revealed no significant differences in the sedimentation rate of cultures grown within the range of salinities under study (5–20 g L-1), at either exponential or stationary phase (2–3 cm h-1; p > 0.05). Although all cultures grown at different salinities displayed similar sedimentation rates, the concentration of salt is known to affect the growth and physiology of several microalgae species and should, therefore, be considered as a variable influencing cell buoyancy and hence algal sedimentation rates (Roik et al., 2016). On the one hand, it could be hypothesized that a medium of higher density, provided by increasing salt concentrations, would cause a lower sedimentation rate due to a higher buoyancy of the cell. On the other hand, higher NaCl concentrations might induce faster settling velocities in microalgae, because the ionic strength of saline solutions could affect the negative charges at the cell surface. This would decrease the zeta potential associated to the plasma membrane, cell coverings (e.g., cell wall) and other extracellular materials (Church et al., 2017; Wen et al., 2017). Overall, more experiments are needed to clarify the relation between salinity and sedimentation rate, perhaps with a wider range of concentrations, and also under different growth stages. These studies would also be of great importance to unravel the significance of this culture parameter on culture sedimentation, which can be a key factor for decreasing the harvesting costs in microalgal production.
3.2. Proximate composition
The proximate composition of the biomass produced under the different salinities was further determined to assess the effect of salt on the biochemical composition. Overall, the content of protein (ranging from 40.5 to 42.7% of DW) and ashes (7.5–8.2% of DW) in the biomass were quite similar across all salinities under study (p > 0.05). On the other hand, lipid and carbohydrate contents differed significantly (p < 0.05). Low and intermediate salt concentrations (5 and 10 g L-1, respectively) showed lower total lipid contents (4.9 and 5.6% of DW), as compared to that obtained at high salt (8.5% of DW, at 20 g L-1; p < 0.05). The increase in lipid contents occurred at the expense of carbohydrates (p < 0.05), which decreased from 46.5 to 41.2% of DW as the salinity increased from 5 to 20 g L-1.
The metabolism of microalgal cells is highly affected by environmental factors, such as salinity, light, pH, temperature and nutrient availability. In turn, metabolic fluctuations influence growth and biochemical composition of the biomass produced (Dammak et al., 2016). High salt combined with low salt growth conditions was previously reported to contribute to lipid and protein enrichment in the final biomass (Ho et al., 2014). Moreover, accumulation of lipids and proteins, induced by salinity shifts, might also promote higher CO2 mitigation by microalgae due to the high carbon content of these biomolecules (BenMoussa-Dahmen et al., 2016).
Regarding the Tetraselmis genus, the effect of increasing salinities leading to higher lipid content has been previously reported (Khatoon et al., 2014; Dammak et al., 2016), as well as for other microalgal strains (Salama et al., 2013; Karpagam et al., 2015). The rise of salinity in the medium might lead to an increment in osmotic pressure in the microalgal cells, which involves changes in cell metabolism and activation of several molecular physiological responses (Dammak et al., 2016). For example, upon a salinity upshift, cells usually accumulate osmoprotectant solutes, also known as osmolytes, such as glycerol (Salama et al., 2013; Talebi et al., 2013) and mannitol (Fon-Sing and Borowitzka, 2016). In addition, cells produce stress proteins to maintain stability and normal growth. However, it is noteworthy that the opposite effect has also been previously described by other authors (Renaud and Parry, 1994; Das et al., 2016). This difference might be explained by species-/strain-specific effects, as well as through differences in their metabolism, biochemical composition and molecular responses. In accordance with the results of this study, a higher starch accumulation under low salinity has been previously reported for the Tetraselmis genus, which has been suggested to be associated or even enhanced by other factors, such as nitrogen deprivation (Yao et al., 2013). Lower salt concentrations force cells to increase their osmotic potential in order to reach an equilibrium with that of the surrounding medium. To this end, cells restrict the biosynthesis and accumulation of small osmolytes and channel the carbon flux to starch synthesis. Unlike other storage polysaccharides, such as glycogen, and because of its crystalline structure and poor solubility in water, starch granules are osmotically inert (Ball et al., 2011). Therefore, the fact that this polysaccharide does not depress the osmotic potential might explain the observed trend for higher carbohydrate contents as salinity is decreased (Table 1).
Table 1.
Biomass composition of batch cultures grown in 5 L reactors under different salinities (5, 10 and 20 g L-1). Values are the mean and corresponding standard deviation of three replicates. Different letters within each biochemical component indicate significant differences.
| Salt (g/L) | Proteins (%) | Lipids (%) | Carbohydrates (%) | Ashes (%) |
|---|---|---|---|---|
| 5 | 40.49 ± 1.34a | 4.86 ± 1.00a | 46.52 ± 1.12a | 8.04 ± 0.15a |
| 10 | 41.10 ± 0.09a | 5.58 ± 0.06a | 45.10 ± 0.27a | 8.22 ± 0.30a |
| 20 | 42.69 ± 0.42a | 8.54 ± 0.09b | 41.23 ± 1.44b | 7.53 ± 0.52a |
3.3. Pilot-scale growth
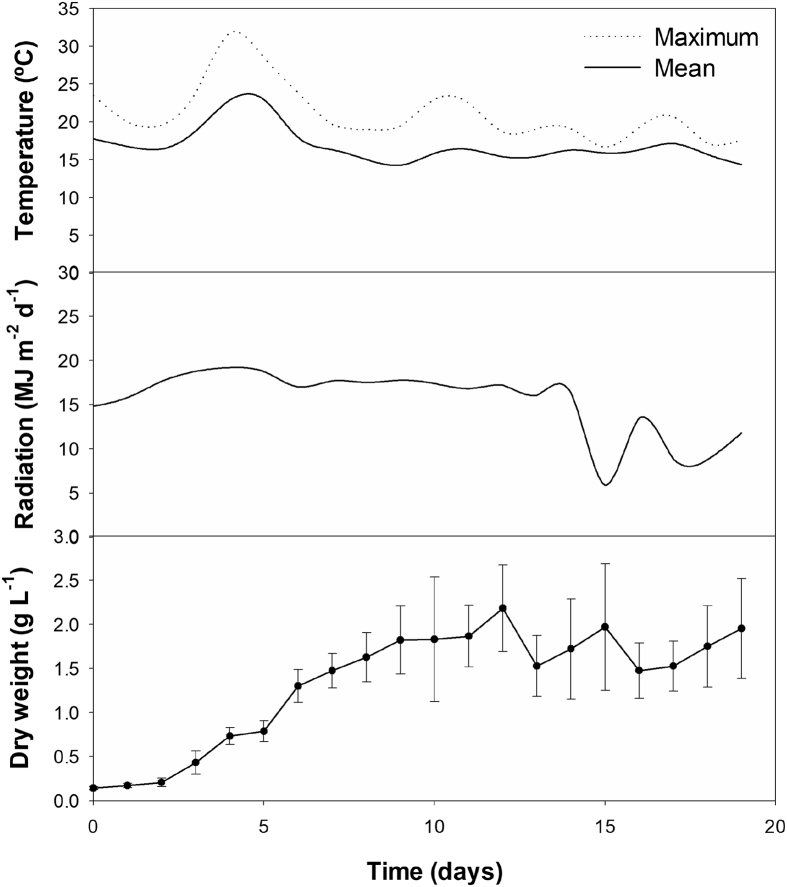
The results obtained in the laboratory were followed by a pilot-scale experiment using an outdoor 2.5-m3 tubular PBR in semi-continuous mode at the intermediate salinity (10 g L-1). The choice of this intermediate salinity for the scale-up step arose from two main factors, namely preventing the proliferation of possible contaminants, commonly found when the salinities are low, and limiting the use of salt when the growth medium is prepared. This balance is important in order to increase overall productivity and decrease production costs. Growth in the pilot-scale PBR was carried out at a stable mean temperature around 16 °C, with the exception of day 4, 5 and 6, where higher temperatures were registered (∼23 °C; Fig. 3). Total radiation was stable during the first 14 days of the growth period (∼17 MJ m-2 d−1), while in the last 6 days a decrease in total radiation was observed. All PBRs were inoculated at a concentration of ∼0.2 g L-1 DW and reached the early stationary phase in 12 days, with a biomass concentration of 2.3 g L-1 DW (1st growth stage). During this stage, cultures presented mean volumetric and areal productivities of 0.17 g L-1 d−1 and 16.09 g m-2 d−1, respectively. Upon feeding the culture with fresh medium, the decrease in radiation led to lower growth rates of cultures. In this 2nd growth stage, cultures took 4 days to reach ∼2.0 g L-1 and displayed mean volumetric and areal productivities of 0.15 g L-1 d−1 and 13.91 g m-2 d−1, respectively. A similar pattern was seen in the 3rd growth stage, with a lower radiation observed on-site. Fed cultures grew slower, so that 5 days were necessary to reach ∼2.0 g L-1, whereas the mean volumetric and areal productivities were 0.12 g L-1 d−1 and 11.23 g m-2 d−1, respectively. These biomass productivities are quite similar to the results previously reported for this strain in the same production system (Pereira et al., 2018).
Fig. 3.
Mean and maximum temperature and radiation registered on site during the growth of Tetraselmis sp. CTP4 in outdoor pilot scale photobioreactors (2.5 m3) for 18 days. Error bars represent the standard deviation calculated from three replicates.
3.4. Pilot-scale sedimentation process
The biomass recovered using the semi-continuous cultivation approach was allowed to sediment in a pilot-scale tank; upon which the culture was fed three times, namely on the 6th, 9th and 13th of October. The initial DW of cultures (process input) used to perform the sedimentation tests in the three distinct harvesting points was 2.18, 1.97 and 1.95 g L-1 (Table 2). The performance of the sedimentation process (biomass removal efficiency) was quantified by measuring the DW of the culture medium removed via lateral taps connected to hoses, the concentrated culture recovered from the lower valve, and the microalgal paste that settled at the bottom of the tank (Fig. 1). Therefore, after the sedimentation period (24 hours), approximately 0.712 m3 of culture medium were easily removed from the sedimentation tank by gravity drainage. This medium had the appearance of a clear liquid containing only vestigial biomass (0.07 ± 0.02 g L-1). This volume corresponded to 93% of the total culture volume (Table 2). The culture volume that remained in the conical section of the sedimentation tank thus represented only 7% of the total culture volume. Afterwards, this highly concentrated culture was transferred to an appropriate container via the tap located at the bottom section of the tank (Fig. 1), reaching a concentration of 19.53 ± 4.83 g L-1. However, as part of the microalgal biomass settled at the bottom of the tank, this fraction was recovered from the lower section of the tank in the form of a microalgal paste using a scraping device, with a mean biomass 272.7 ± 18.5 g L-1. Interestingly, this paste can thus be packed immediately, should this be the intended final product. Overall, these values represent a removal efficiency of 97% of the total biomass introduced in the sedimentation tank. In other words, this low-cost, gravity-dependent harvesting method only led to a biomass loss of 3% upon culture medium removal, without the use of any additional energy input.
Table 2.
Harvesting by sedimentation of Tetraselmis sp. CTP4, under a pilot-scale semi-continuous cultivation. Values are the mean and corresponding standard deviation of three replicates.
| Sedimentation process | Unit | Mean ± SD |
|---|---|---|
| Inputs | ||
| Initial culture dry weight | g L−1 | 2.03 ± 0.13 |
| Outputs | ||
| Culture medium | g L−1 | 0.07 ± 0.02 |
| Concentrated culture | g L−1 | 19.53 ± 4.83 |
| Microalgal paste | g L−1 | 272.7 ± 18.5 |
| Biomass | ||
| Settling velocity | cm h−1 | 3.44 ± 0.10 |
| Removal | % | 96.64 ± 0.86 |
Biomass harvesting is considered one of the main costing steps of the whole microalgal production pipeline (Chen et al., 2011; Acien et al., 2016; Show et al., 2017). Microalgae harvesting requires high energy inputs, because of the small size of cells, low density (similar to that of water) and low cell concentration of autotrophic cultures (Bilad et al., 2014). Therefore, the method developed in the present work, based solely in the natural settling capacity of this strain, represents a major decrease in harvesting costs in the pipeline of biomass production. There are several authors that have corroborated this proof of concept although in different backgrounds. Yu et al. (2012) obtained 97.9% recovery of the microalga Monoraphidium sp. FXY-10 in 24 hours by natural sedimentation, but only in lab-scale experiments. Meanwhile, Hom-Diaz et al. (2017), in spite of working with different PBRs and sedimentation systems, reported a large-scale gravity sedimentation harvesting method with an 88% biomass recovery within 24 hours. Interestingly, there are other experiments reported for different species, namely Tetraselmis suecica, in which the microalgae per se were applied as bioflocculants in order to increase the sedimentation rate of non-flocculant cultures and decrease the energy requirements and costs of downstream processing (Salim et al., 2012).
4. Conclusions
Taking into account the robustness, stress tolerance and biochemical properties of Tetraselmis sp. CTP4 previously reported, here the authors report that the growth and sedimentation rate are not affected within the range of salinities studied. This work also shows that cultures can be easily harvested via a simple, cost-effective, gravity-dependent process, using cylindrical-conical reservoirs fitted with lateral taps, a scraper, and a bottom valve. This low-cost approach takes into account the specific properties of the strain in order to improve the profitability and sustainability of biomass harvesting. Nevertheless, further optimization of this method using a secondary sedimentation system and different tank geometries will most likely lead to improved reduction costs.
Declarations
Author contribution statement
Mafalda Trovão, Hugo Pereira: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Joana Silva, João Varela: Conceived and designed the experiments; Wrote the paper.
Jaime Páramo: Conceived and designed the experiments; Performed the experiments.
Pedro Quelhas, Tamára Santos, Joana T. Silva, Adriana Machado: Performed the experiments; Analyzed and interpreted the data.
Luísa Gouveia, Luísa Barreira: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by the Portuguese national budget P2020 and the CMAR/Multi/04326/2013 grant of the Foundation for Science and Technology (FCT), the ALGARED+ 1398 EP - INTERREG V-A España Portugal project, the ALGACO2 project (nº 023310; Industrial cultivation of microalgae as a green technology for atmospheric CO2 capture), and the COST Action 1408 - European Network for Bio-products. H.P. (SFRH/BD/105541/2014) was funded by a PhD grant from FCT.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors would like to acknowledge all members of CMP for the kind support and help throughout this work.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
Title: Low-cost pilot scale harvesting procedure. Legend: Low-cost harvesting procedure optimized at pilot-scale for Tetraselmis sp. CTP4 biomass using a 1 m3 cylinder conical tank. The process relies in a 24-hour sedimentation step, followed by the removal of the culture medium by gravity drainage through the lateral openings of the tank, while the concentrated culture and wet microalgal paste are removed by the bottom using the lower valve of the tank.
References
- Acien F.G., Gomez-Serrano C., Morales-Amaral M.M., Fernandez-Sevilla J.M., Molina-Grima E. Wastewater treatment using microalgae: how realistic a contribution might it be to significant urban wastewater treatment? Appl. Microbiol. Biotechnol. 2016;100:9013–9022. doi: 10.1007/s00253-016-7835-7. [DOI] [PubMed] [Google Scholar]
- Ball S., Colleoni C., Cenci U., Raj J.N., Tirtiaux C. The evolution of glycogen and starch metabolism in eukaryotes gives molecular clues to understand the establishment of plastid endosymbiosis. J. Exp. Bot. 2011;62:1775–1801. doi: 10.1093/jxb/erq411. [DOI] [PubMed] [Google Scholar]
- BenMoussa-Dahmen I., Chtourou H., Rezgui F., Sayadi S., Dhouib A. Salinity stress increases lipid, secondary metabolites and enzyme activity in Amphora subtropica and Dunaliella sp for biodiesel production. Bioresour. Technol. 2016;218:816–825. doi: 10.1016/j.biortech.2016.07.022. [DOI] [PubMed] [Google Scholar]
- Bilad M.R., Arafat H.A., Vankelecom I.F.J. Membrane technology in microalgae cultivation and harvesting: a review. Biotechnol. Adv. 2014;32:1283–1300. doi: 10.1016/j.biotechadv.2014.07.008. [DOI] [PubMed] [Google Scholar]
- Bligh E.G., Dyer W.J. A rapid method for the total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Campenni’ L., Nobre B.P., Santos C.A., Oliveira A.C., Aires-Barros M.R., Palavra A.F., Gouveia L. Carotenoids and lipids production of autotrophic microalga Chlorella protothecoides under nutritional, salinity and luminosity stress conditions. Appl. Microbiol. Biotechnol. 2013;97:1383–1393. doi: 10.1007/s00253-012-4570-6. [DOI] [PubMed] [Google Scholar]
- Chen C.Y., Yeh K.L., Aisyah R., Lee D.J., Chang J.S. Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: a critical review. Bioresour. Technol. 2011;102:71–81. doi: 10.1016/j.biortech.2010.06.159. [DOI] [PubMed] [Google Scholar]
- Church J., Hwang J.H., Kim K.T., McLean R., Oh Y.K., Nam B. Effect of salt type and concentration on the growth and lipid content of Chlorella vulgaris in synthetic saline wastewater for biofuel production. Bioresour. Technol. 2017;243:147–153. doi: 10.1016/j.biortech.2017.06.081. [DOI] [PubMed] [Google Scholar]
- Dammak M., Haase S.M., Miladi R., Ben Amor F., Barkallah M., Gosset D. Enhanced lipid and biomass production by a newly isolated and identified marine microalga. Lipids Health Dis. 2016;15:13. doi: 10.1186/s12944-016-0375-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das P., Thaher M.I., Hakim M., Al-Jabri H., Alghasal G. A comparative study of the growth of Tetraselmis sp in large scale fixed depth and decreasing depth raceway ponds. Bioresour. Technol. 2016;216:114–120. doi: 10.1016/j.biortech.2016.05.058. [DOI] [PubMed] [Google Scholar]
- Fon-Sing S., Borowitzka M.A. Isolation and screening of euryhaline Tetraselmis spp. suitable for large-scale outdoor culture in hypersaline media for biofuels. J. Appl. Phycol. 2016;28:1–14. [Google Scholar]
- Ho S.H., Ye X.T., Hasunuma T., Chang J.S., Kondo A. Perspectives on engineering strategies for improving biofuel production from microalgae - a critical review. Biotechnol. Adv. 2014;32:1448–1459. doi: 10.1016/j.biotechadv.2014.09.002. [DOI] [PubMed] [Google Scholar]
- Hom-Diaz A., Jaen-Gil A., Bello-Laserna I., Rodriguez-Mozaz S., Vicent T., Barcelo D. Performance of a microalgal photobioreactor treating toilet wastewater: pharmaceutically active compound removal and biomass harvesting. Sci. Total Environ. 2017;592:1–11. doi: 10.1016/j.scitotenv.2017.02.224. [DOI] [PubMed] [Google Scholar]
- Huerlimann R., de Nys R., Heimann K. Growth, lipid content, productivity, and fatty acid composition of tropical microalgae for scale-up production. Biotechnol. Bioeng. 2010;107:245–257. doi: 10.1002/bit.22809. [DOI] [PubMed] [Google Scholar]
- Karpagam R., Raj K.J., Ashokkumar B., Varalakshmi P. Characterization and fatty acid profiling in two fresh water microalgae for biodiesel production: lipid enhancement methods and media optimization using response surface methodology. Bioresour. Technol. 2015;188:177–184. doi: 10.1016/j.biortech.2015.01.053. [DOI] [PubMed] [Google Scholar]
- Khatoon H., Rahman N.A., Banerjee S., Harun N., Suleiman S.S., Zakaria N.H. Effects of different salinities and pH on the growth and proximate composition of Nannochloropsis sp and Tetraselmis sp isolated from South China Sea cultured under control and natural condition. Int. Biodeterior. Biodegrad. 2014;95:11–18. [Google Scholar]
- Lananan F., Jusoh A., Ali N., Lam S.S., Endut A. Effect of Conway Medium and f/2 Medium on the growth of six genera of South China Sea marine microalgae. Bioresour. Technol. 2013;141:75–82. doi: 10.1016/j.biortech.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Lananan F., Yunos F.H.M., Nasir N.M., Abu Bakar N.S., Lam S.S., Jusoh A. Optimization of biomass harvesting of microalgae, Chlorella sp utilizing auto-flocculating microalgae, Ankistrodesmus sp as bio-flocculant. Int. Biodeterior. Biodegrad. 2016;113:391–396. [Google Scholar]
- Nollet L.M., Dre Geldert L.S. CRC Press; Ghent: 2000. Handbook of Water Analysis. [Google Scholar]
- Pereira H., Barreira L., Mozes A., Florindo C., Polo C., Duarte C.V., Custódio L. Microplate-based high throughput screening procedure for the isolation of lipid-rich marine microalgae. Biotechnol. Biofuels. 2011;4:1. doi: 10.1186/1754-6834-4-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira H., Gangadhar K.N., Schulze P.S.C., Santos T., de Sousa C.B., Schueler L.M. Isolation of a euryhaline microalgal strain, Tetraselmis sp. CTP4, as a robust feedstock for biodiesel production. Sci. Rep. 2016;6 doi: 10.1038/srep35663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira H., Páramo J., Silva J., Marques A., Barros A., Maurício D. Scale-up and large-scale production of Tetraselmis sp. CTP4 (Chlorophyta) for CO 2 mitigation: from an agar plate to 100-m 3 industrial photobioreactors. Sci. Rep. 2018;8(1):5112. doi: 10.1038/s41598-018-23340-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignolet O., Jubeau S., Vaca-Garcia C., Michaud P. Highly valuable microalgae: biochemical and topological aspects. J. Ind. Microbiol. Biotechnol. 2013;40:781–796. doi: 10.1007/s10295-013-1281-7. [DOI] [PubMed] [Google Scholar]
- Renaud S.M., Parry D.L. Microalgae for use in tropical aquaculture .2. effect of salinity on growth, gross chemical-composition and fatty-acid composition of 3 species of marine microalgae. J. Appl. Phycol. 1994;6:347–356. [Google Scholar]
- Roik A., Rothig T., Roder C., Ziegler M., Kremb S.G., Voolstra C.R. Year-long monitoring of physico-chemical and biological variables provide a comparative baseline of coral reef functioning in the central red sea. PLoS One. 2016;11:34. doi: 10.1371/journal.pone.0163939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salama E.S., Kim H.C., Abou-Shanab R.A.I., Ji M.K., Oh Y.K., Kim S.H. Biomass, lipid content, and fatty acid composition of freshwater Chlamydomonas mexicana and Scenedesmus obliquus grown under salt stress. Bioproc. Biosyst. Eng. 2013;36:827–833. doi: 10.1007/s00449-013-0919-1. [DOI] [PubMed] [Google Scholar]
- Salim S., Vermue M.H., Wijffels R.H. Ratio between autoflocculating and target microalgae affects the energy-efficient harvesting by bio-flocculation. Bioresour. Technol. 2012;118:49–55. doi: 10.1016/j.biortech.2012.05.007. [DOI] [PubMed] [Google Scholar]
- Sansone C., Galasso C., Orefice I., Nuzzo G., Luongo E., Cutignano A. The green microalga Tetraselmis suecica reduces oxidative stress and induces repairing mechanisms in human cells. Sci. Rep. 2017;7 doi: 10.1038/srep41215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Show P.L., Tang M.S.Y., Nagarajan D., Ling T.C., Ooi C.W., Chang J.S. A holistic approach to managing microalgae for biofuel applications. Int. J. Mol. Sci. 2017;18:34. doi: 10.3390/ijms18010215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sing S.F., Isdepsky A., Borowitzka M.A., Lewis D.M. Pilot-scale continuous recycling of growth medium for the mass culture of a halotolerant Tetraselmis sp. in raceway ponds under increasing salinity: a novel protocol for commercial microalgal biomass production. Bioresour. Technol. 2014;161:47–54. doi: 10.1016/j.biortech.2014.03.010. [DOI] [PubMed] [Google Scholar]
- Singh G., Patidar S.K. Microalgae harvesting techniques: a review. J. Environ. Manag. 2018;217:499–508. doi: 10.1016/j.jenvman.2018.04.010. [DOI] [PubMed] [Google Scholar]
- Skjanes K., Rebours C., Lindblad P. Potential for green microalgae to produce hydrogen, pharmaceuticals and other high value products in a combined process. Crit. Rev. Biotechnol. 2013;33:172–215. doi: 10.3109/07388551.2012.681625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- t Lam G.P., Vermue M.H., Eppink M.H.M., Wijffels R.H., van den Berg C. Multi-product microalgae biorefineries: from concept towards reality. Trends Biotechnol. 2018;36:216–227. doi: 10.1016/j.tibtech.2017.10.011. [DOI] [PubMed] [Google Scholar]
- Talebi A.F., Tabatabae M., Mohtashami S.K., Tohidfar M., Moradi F. Comparative salt stress study on intracellular ion concentration in marine and salt-adapted freshwater strains of microalgae. Not. Sci. Biol. 2013;5:309–315. [Google Scholar]
- von Alvensleben N., Stookey K., Magnusson M., Heimann K. Salinity tolerance of Picochlorum atomus and the use of salinity for contamination control by the freshwater cyanobacterium Pseudanabaena limnetica. PLoS One. 2013;8 doi: 10.1371/journal.pone.0063569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen H., Li Y.P., Shen Z., Ren X.Y., Zhang W.J., Liu J. Surface characteristics of microalgae and their effects on harvesting performance by air flotation. Int. J. Agric. Biol. Eng. 2017;10:125–133. [Google Scholar]
- Yao C.H., Ai J.N., Cao X.P., Xue S. Salinity manipulation as an effective method for enhanced starch production in the marine microalga Tetraselmis subcordiformis. Bioresour. Technol. 2013;146:663–671. doi: 10.1016/j.biortech.2013.07.134. [DOI] [PubMed] [Google Scholar]
- Yellapu S.K., Bharti Kaur. R., Kumar L.R., Tiwari B., Zhang X.L. Recent developments of downstream processing for microbial lipids and conversion to biodiesel. Bioresour. Technol. 2018;256:515–528. doi: 10.1016/j.biortech.2018.01.129. [DOI] [PubMed] [Google Scholar]
- Yu X.Y., Zhao P., He C., Li J.J., Tang X.H., Zhou J.P. Isolation of a novel strain of Monoraphidium sp and characterization of its potential application as biodiesel feedstock. Bioresour. Technol. 2012;121:256–262. doi: 10.1016/j.biortech.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Zhu L.D., Li Z.H., Hiltunen E. Strategies for lipid production improvement in microalgae as a biodiesel feedstock. BioMed Res. Int. 2016;8 doi: 10.1155/2016/8792548. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Title: Low-cost pilot scale harvesting procedure. Legend: Low-cost harvesting procedure optimized at pilot-scale for Tetraselmis sp. CTP4 biomass using a 1 m3 cylinder conical tank. The process relies in a 24-hour sedimentation step, followed by the removal of the culture medium by gravity drainage through the lateral openings of the tank, while the concentrated culture and wet microalgal paste are removed by the bottom using the lower valve of the tank.