Abstract
Nanomaterials are attracted a great deal of attention from scientific community due its unique properties and applications. The small size ferrites have opened up the door for intensive research to utilize their properties for biomedical applications. Ferrite nanomaterials like MgFe2O4 and its silver doped nanocomposites (Ag– MgFe2O4) have been prepared using solid state combustion method using polyvinyl alcohol (PVA) as a fuel. The structure of as prepared ferrites and its silver doped nanocomposites were characterized using X-ray diffraction (XRD) tool and morphology by Scanning Electron Micrograph (SEM) tool respectively. Presence of the metals in the ferrite and its composite was confirmed by EDX pattern. Bonding nature in the composite is well studied by Fourier transform infrared (FT-IR) tool. Antibacterial activity study of the nanocomposite is carried out against various bacteria. Ag doped magnesium ferrite shows moderate activity against bacteria.
Keyword: Materials chemistry
1. Introduction
Nanotechnology is based on the science that deals with tweaking of the matter at the atomic and molecular scale in the size range between 1- 100 nm [1]. Nanomaterials and Nanotechnologies are enticed and magnetize in recent and upcoming research. The latest physical properties and advance technologies both in sample preparation and device fabrication evoke on account of the progress of nanoscience. The man in his quest for knowledge has been conceiving and developing physical world and its components in bigger than the biggest and smaller than the smallest dimensions of mass, length and time [2]. sometimes the changes in particles size are in such extent that, the completely new transpiration are dig up which helps in flowering of the world [3]. However the title is know about how the biological activity takes place for certain materials when it is reduced into nanoscale dimensions. In this world of elaboration nanotechnology, one of the main primary concerns should be the potential environment impact of nanoparticles (Nps). A proper way of estimating the nanotoxicity is to monitor the response of the bacteria against these nanoparticles [4].
Resistance of bacteria to bactericides and the higher generation antibiotics has increased in the latest years due to the flowering of resistance strains. Recently, it has been demonstrated that highly reactive metal oxide nanoparticles exhibit exoticbiocidal action against Gram-positive and Gram-negative bacteria [5]. This is the preparation, characterization, surface modification, and implementation of a very blooming generation of bactericidal materials. The toxicity is calculate on both the exposure and the size of the metal nanoparticles like alumina, copper, gold, magnesium, cobalt, barium, sliver, titanium, and zinc. Metal oxide is not only stable but generally it is said to be as safe to human beings [6]. There are several investigations that have found and are also being focused on the investigation of their properties of metal oxide nanoparticles. The toxicity of these nanoparticles is tested or can be recognized by two methods firstly, culturing in liquid media containing nanoparticles, and electro spraying the nanoparticles directly onto the bacteria surface. Ferrites are chemical compounds which come across as powder or ceramic bodies with ferrimagnetic properties achieved by iron oxides as their main component, Fe2O3 and FeO, which can be partly replaced by other transition metal oxides. Among the different ferrites, Magnesium ferrite (MgFe2O4) has special magnetic and physical properties which lead to its wide application in medicine. Bacterial pollution is a great risk for human health. Nanotechnology offers a way to develop new inorganic antibacterial agents. Nano-inorganic metal oxide has a potential to reduce bacterial contamination. The search for new good antibacterial material and study of the new properties of conventional material has become an active research field. Iron oxide has various applications regarding biomedicine due to its different properties [7]. It is reported that considerable antibacterial activity of magnesium based nanoparticles is attributed to the generation of reactive oxygen species. Magnesium ferrite (MgFe2O4) is one of the important functional magnetic materials with a cubic normal spinel structure type. It is used as a catalyst and humidity sensor. Among various materials used for sensing application, ferrite is used as a good class of sensing materials, but they suffer a drawback of being at higher temperature [8, 9].
The ferrites can be differentiated according to their crystalline structure: hexagonal (MFe12O19), garnet (M3Fe5O12) and spinel (MFe2O4), where M represents one or more bivalent transition metals (Mn, Fe, Co, Ni, Cu, and Zn). Transition metal ferrites, both doped and undoped, are magnetic candidates in a huge range of applications considering catalysis, sustainable hydrogen production application and electronic and magnetic devices, with others [10, 11, 12, 13, 14]. It is well known fact that, antibacterial activity depends on the size of the nanoparticles. The confluence of magnetic materials towards antibacterial properties can make this material important for application in biomedicine. Therefore, in the present study, attempts have been carried out to synthesized Ag doped magnesium ferrite nanoparticles improvement in the antibacterial activity. Thus the silver doped ferrite nanoparticles with good magnetic and antibacterial properties can offer great promises in biomedical and pharmaceutical applications and also wide application in the health and hygiene will constitute the basis for the next generation drug development.
The present study reports the synthesis of Ag metal, MgFe2O3 and Ag doped magnesium ferrite using combustion method through metal carboxylate as precursor. PVA is used as a fuel for the combustion process. Prepared samples are well characterised by various characterisation techniques such as XRD, SEM, IR, EDX etc. Antibacterial activity study of the A doped MgFe2O3 nanocomposite is carried out against various bacteria.
2. Experimental
2.1. Materials and methods
The chemicals like AgNO3, oxalic acid, iron oxide, polyvlinyalcohol, magnesium chloride chemicals are used, which are of AR grade. Combustion synthesis method is used for synthesis of sliver metal, MgFe2O3 and Ag– MgFe2O3 nanocomposites materials. Polyvinylalcohol is used as fuel for combustion reaction.
2.2. Preparation of metal oxalates
The magnesium oxalate is prepared by dissolving known quantity of magnesium chloride and oxalic acid in 100 ml of double distilled water in a separate container with the molar ration of 1:1 respectively. These solutions are mixed together in a separate container and kept it on the magnetic stirrer for 1 hour to complete conversion of magnesium chloride into its oxalate by reacting with oxalic acid. Finally, as formed magnesium oxalate precipitation is washed with distilled water and dried by passing hot air with minimum pressure. Similar procedure is adopted for the preparation of iron oxalate and silver oxalate using respective metal salts such as ferrous ammonium sulphate and silver nitrate respectively by reacting with oxalic acid.
2.3. Synthesis of nanosized silver (Ag) metal
In the typical combustion process, the silver oxalate is burned with the poly (vinyl alcohol) (PVA) as a fuel with the molar ratio of 1:5; the combustion reaction is arrested at the particular temperature for the phase formation. The impurities present in the final product are separated by treating with acetone and dried.
2.4. Synthesis of MgFe2O4, nanoparticles
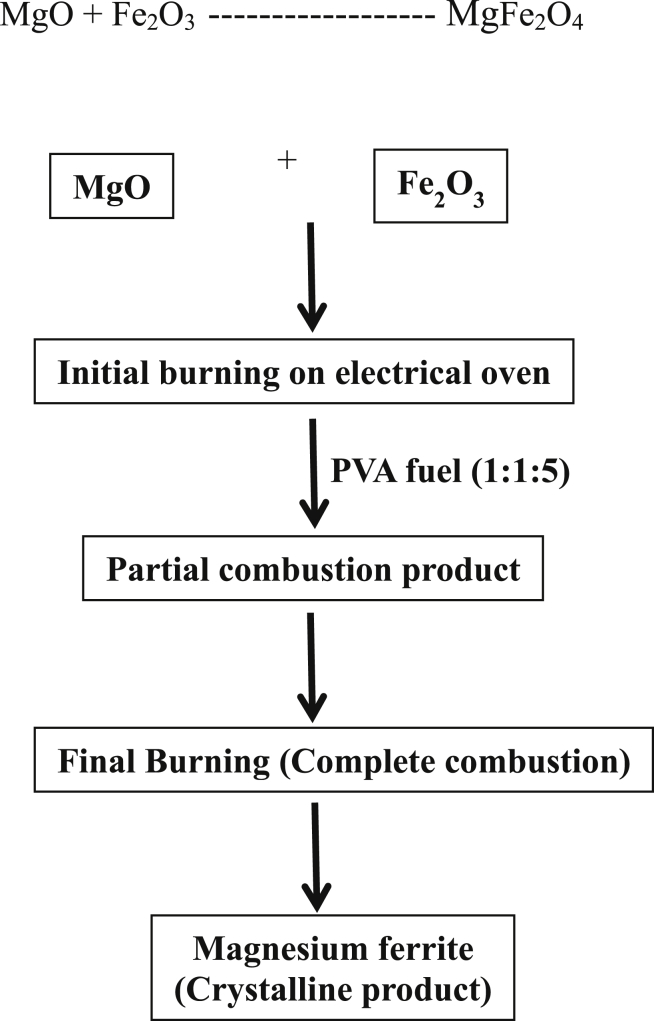
Magnesium ferrites nanomaterials are prepared by burning magnesium oxalate with iron oxalate using poly (vinyl alcohol) as a fuel for the combustion reaction in 1:5 ratio. The burning process takes place in a separate container to the particular temperature which results into the possible formation of partial metal oxides (MgO and Fe2O3). These oxides are mixed properly with PVA, grinded and burned again in a separate container in the ratio 1:1:5 respectively. This process starts with formation of froth, evolution fumes and burns with flame; finally it was completed at particular temperature. The product magnesium ferrite obtained after the complete combustion process and is grinded in the pestle and motor for half an hour. It is washed with acetone and concentrated action to get rid of carbon particles in the final product Following is the possible reaction taking place during combustion and synthetic scheme is given in Fig. 1.
Fig. 1.
Schematic representation for synthesis of magnesium ferrite synthesis.
2.5. Synthesis of Ag doped magnesium ferrite nanomaterials
Magnesium ferrite nanomaterials are doped with the silver metal nanoparticles using the combustion method. The process starts with burning of magnesium ferrite with silver metal nanoparticles, and PVA keeping the molar ratio as 2:1:5 respectively. Initially, it burns with sooty flame followed by reduced non sooty flame for complete reaction. This reaction was arrested at a particular temperature around 500 °C and the sample is heated at the same temperature for about 30 minutes to phase formed Ag doped magnesium ferrite nanoparticles. Later it is washed with acetone and concentrated action to remove the carbon particles and other organic impurity.
2.6. Antibacterial activity
The chemically synthesized nanocomposite is examined for antibacterial activity against MDR isolates using agar well diffusion method. Clinically isolated pathogens were inoculated in Luria-Bertani Broth and incubated at 37 °C for 6 hrs, 100μl of each microorganism were inoculated on Muller Hinton Agar (MHA) plates: agar wells of 5mm diameter were prepared with the help of a sterilized steel cork borer. Different concentrations of the synthesized nanoparticles were used to investigate the antibacterial activity.
3. Results and discussion
3.1. X-ray diffraction (XRD)
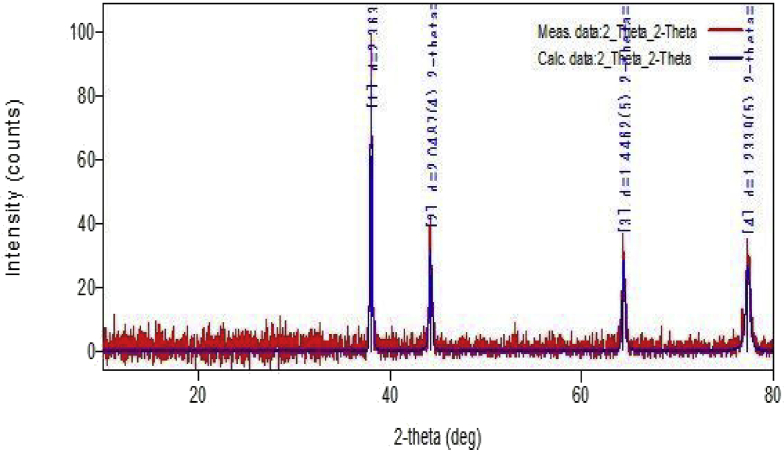
The crystalline nature of the synthesized nanoparticles was analyzed by X-ray diffraction tool. Fig. 2 shows XRD pattern of the synthesized Ag metal nanoparticles after the reduction of silver nitrate. Intense peaks corresponding to (111), (200), (220) and (311) were observed for the silver nanoparticles in accordance with JCPDS files no. 03–0921. This confirms the formation of crystalline silver metal nanoparticles [15].
Fig. 2.
XRD pattern for silver nanoparticles.
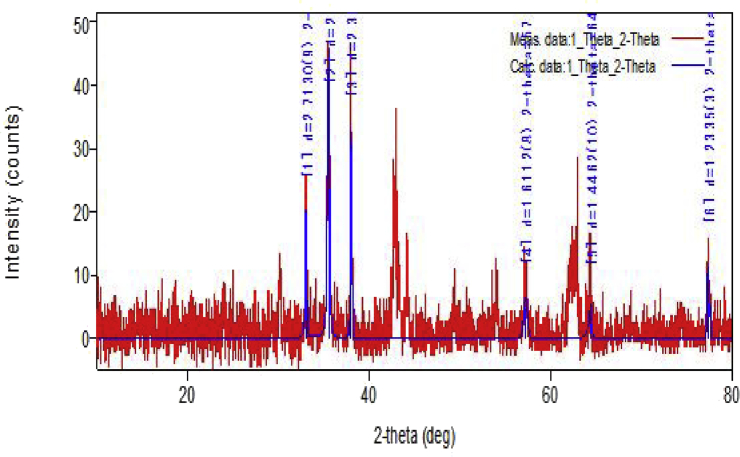
Fig. 3 shows the XRD pattern of the as prepared Ag–MgFe2O4, nanocomposite materials. This pattern shows the Bragg's reflection which indicates the presence of the crystalline nature of the above samples. Peaks obtained in the pattern are matched with standard JCPDS file of the silver metal and MgFe2O4 (22–1086) nanomaterials. Some silver peaks are identified and some other unidentified peaks are due the presence magnesium ferrite in accordance to its JCPDS file. It is combustion derived doping process; shows shifting of the some peaks in the pattern are observed. The above pattern represents the presence of the silver doped in MgFe2O4. These results are supported by the EDX results [16].
Fig. 3.
XRD pattern of Ag–MgFe2O4 nanocomposite.
3.2. Scanning electron microscope (SEM)
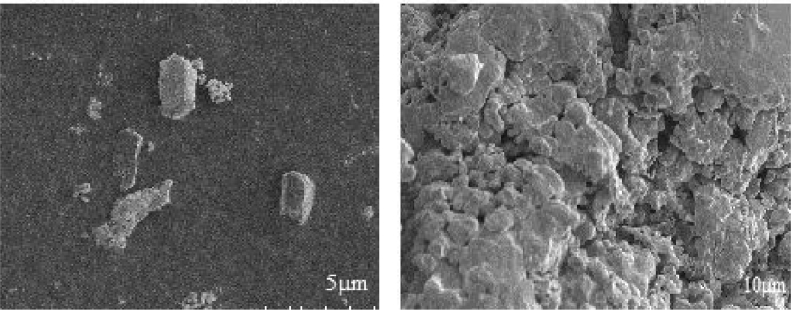
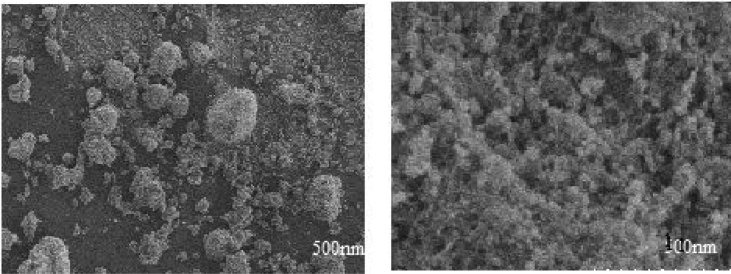
Field Emission Scanning Electron Microscope has been used to study the particle morphology. Fig. 4 shows SEM image of silver metal nanoparticles at low and high resolution. The image indicating the size ranging from 20-90 nm and has the irregular shaped particles forms a compact sheet like arrangement and the clear image can be observed in higher resolution image. Fig. 5 shows SEM images of Ag doped magnesium ferrite at low and high resolution. The image shows almost particles are spherical in nature and same particles shows micro-self-doping forms a microneting. This kind of morphology will be highly applicable in the catalysis. Micro-self doped can be viewed properly in the high resolution image [17].
Fig. 4.
SEM image of silver nanoparticles with high and low resolution.
Fig. 5.
SEM image of Ag–MgFe2O4 nanocomposite with high and low resolution.
3.3. Transmission electron microscope (TEM)
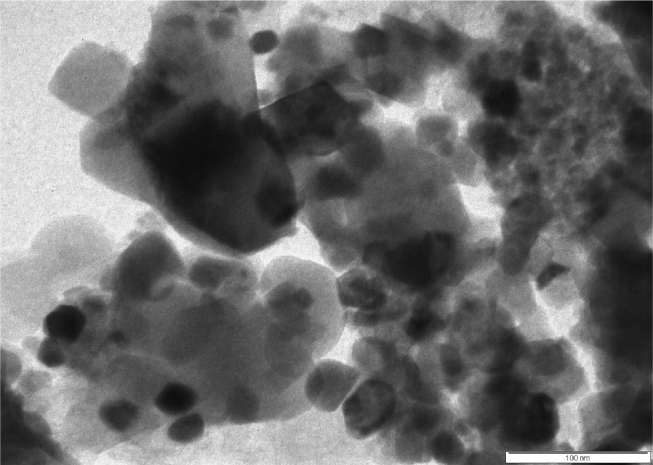
Fig. 6 depicts the TEM image of Ag–MgFe2O4 nanocomposite. The image shows some spherical particles having diameter of 100 nm. It has given us further insight into the nanostructure and crystallinity of the sample. The clear and uniform lattice fringes confirmed that, the high crystalline nature of the Ag doped ferrite. One can find the reduced compactness in the image supports the high crystallinity of the sample. Some sintered particles show irregular particles and narrow distributed particles having approximately particle size 100 nm.
Fig. 6.
TEM image of Ag–MgFe2O4 nanocomposite.
3.4. Energy dispersive X-Rays (EDX)
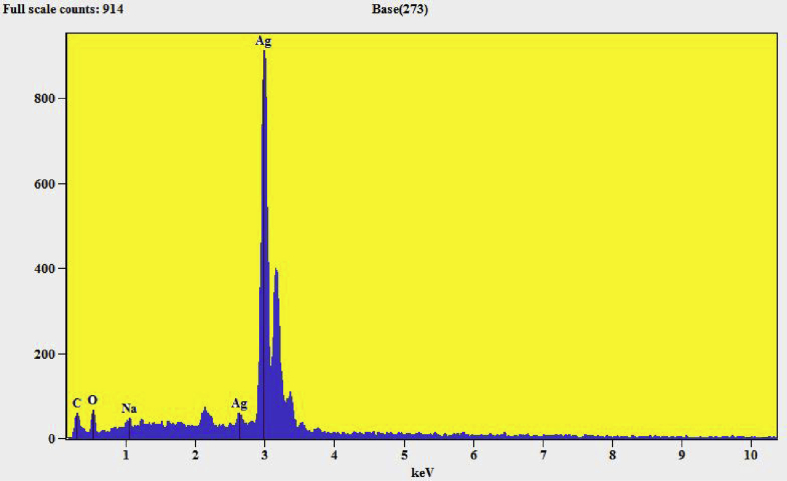
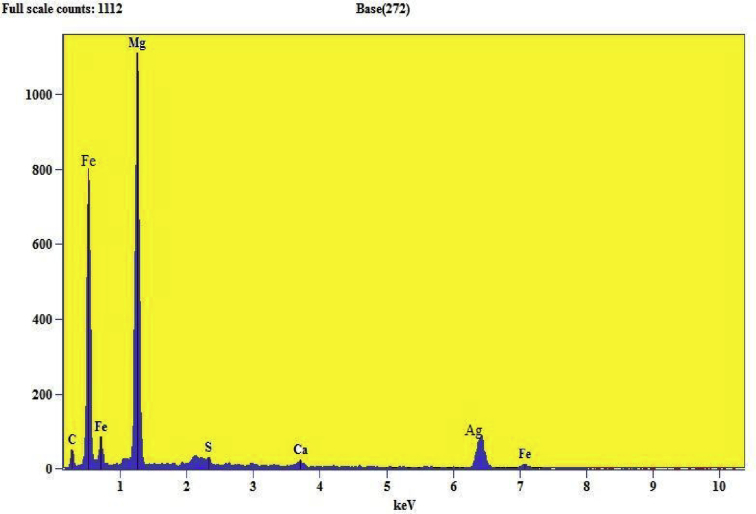
EDX (energy-dispersive X-ray) report confirms a chemical composition or contaminants for the synthesized silver doped magnesium ferrite nanomaterials. Fig. 7 shows EDX pattern of as prepared Ag metal [18]. The pattern shows Ag highest percentage signals at respective position confirms the formation of Ag metal. Fig. 8 shows the EDX pattern of Ag–MgFe2O4 nanocomposite. The EDX spectrum gives the highest percentage signals of magnesium, silver and iron for contaminants in nanoparticles. Both patterns shows small peaks of metals and carbon may be due to trace amount of impurities by synthesis of samples. These peaks may call internal fluorescence peaks or by artefact of the grid on which the sample was coated. Results of our study prove that there are some contaminants which are showed in Table 1. This confirms the doping of Ag metal with magnesium ferrite.
Fig. 7.
EDX images of silver metal nanoparticles.
Fig. 8.
EDX images of Ag–MgFe2O4 nanocomposite.
Table 1.
Metal indexing of the Ag metal and Magnesium ferrite by EDX pattern.
| Samples | Metal indexing (keV) |
|---|---|
| Ag | Ag(2.8), Ag(3.0) |
| Ag–MgFe2O4 | Ag(6.4), Mg(1.3), Fe(0.5), Fe(0.7), Fe(7.0) |
3.5. Infrared study (FT-IR)
Table 2 shows vibrational frequencies of as prepared Ag–MgFe2O4 nanocomposite. The sample shows peaks at 3160, 1550, 1150, 660, 540, 420 cm−1. The metal-oxygen bonding and nature of the synthesized oxide sample was carried out by infrared study. Metal oxides generally give absorption bands below 1000 cm−1 arising from inter-atomic vibrations [19]. The peak 3160 cm−1 corresponds to water of absorption. Vibrational frequency at 1550 cm−1 is due to the presence of carbon dioxide. Frequency at 1150 cm−1 is due to some overtones. Peaks below 1000 cm−1 corresponds to Metal-oxygen (M-O) vibrational modes of the samples conform the formation of Ag doping magnesium ferrite [20, 21, 22].
Table-2.
Vibrational frequencies of Ag–MgFe2O4 nanocomposite.
| Sl. No |
Frequencies (cm−1) |
| 1 | 3160 |
| 2 | 1550 |
| 3 | 1150 |
| 4 | 660 |
| 5 | 540 |
| 6 | 420 |
3.6. Antibacterial activity
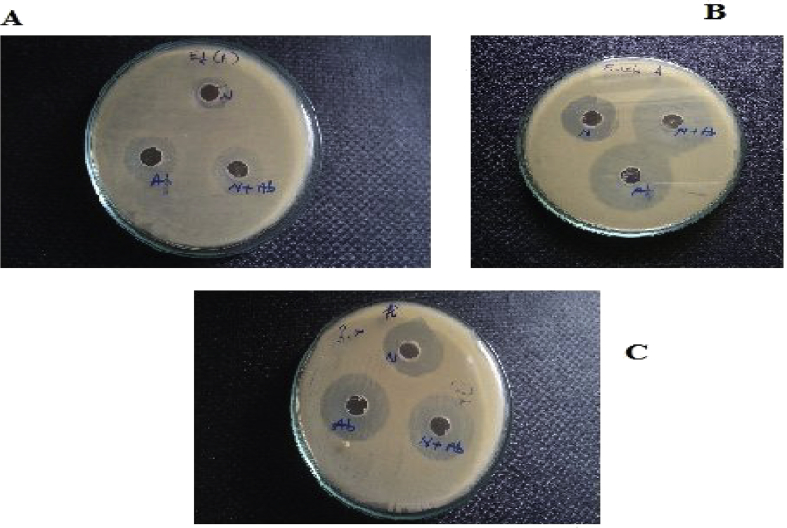
The as-synthesized Ag–MgFe2O4 nanocomposite was tested for their antibacterial effect against the strain E coli, E faecal is and pseudonomonasareginosa by agar well diffusion method. The results of the antibacterial activity of are illustrated in Fig. 9 the figure shows that nanocomposite has good antibacterial activity and the bacteria cells have been killed at the respective concentration. Table 3 shows the inhibition zone of these bacteria. Investigation of antibacterial activity against MDR pathogens using Ag–MgFe2O4 nanocomposite and higher generation antibiotics. According to these results, it can be found that Ag plays a determinant role in the antibacterial effect. The fundamental understanding on the influence of magnetic iron oxide nanoparticle on cellular growth and functions is very important for further biological applications. The comparative study on growth of different bacteria the influences of magnetic iron oxide were revealed the effect of magnetic iron oxide nanoparticle on bacterial growth. Many researchers reported that silver nanoparticles show better activity towards pathogens. The present article reporting the effect of Ag doped ferrite towards the activity of the pathogens. In general, the samples efficiently inhibit the growth of the microbes. It can be seen that, the contact biocidal property of the pure ferrite samples are insignificant compared to that of silver doped samples. The efficacy of the nanoparticles increased with silver content. The biocidal activity exhibited by the samples is observed to depend on both the particle size and the silver content. It is well known that in the nano-regime, the bactericidal activity exhibited by the smaller particles is higher than bigger particles.
Fig. 9.
Antibacterial activity results of Ag–MgFe2O4 nanocomposite against A) E Coli B) Enterococcus faecal C) Pseudonomonasareginosa.
Table 3.
Results of antibacterial study of Ag–MgFe2O4 nanocomposite.
| Pathogenic bacteria | Zone of inhibition (mm) | ||
|---|---|---|---|
| E.coli | Antibacterial activity | Synergistic effect | |
| N (120μl) | Ab (16μl) | N + Ab (120μl:10μl) | |
| 16 | 25 (ceftriaxone) | 22 | |
| Enterococcus faecalis | 15 | 22 (vancomicin) | 18 |
| Pseudonomonasareginosa | 13 | 25 (ceftazidime) | 16 |
4. Conclusions
Recent studies show that, the pathogens are growing resistant toward the antibiotics and these antibiotics have their own side effects which may cause laziness and low downs the immunity in the body and also make the body week. So the alternative part of this problem is to replace the nanoparticles instead of using higher generation antibiotics. Antibacterial activity of ferrite nanoparticles has received huge significant and the great interest over the worldwide. The implementation is particularly with the help of nanotechnology for the synthesis or the preparation of the nanoparticles with the high techniques. These nanoparticles exhibit attractive antibacterial properties due to increased specific surface area as the reduced particle size leading to enhanced particle surface reactivity. Which concludes that the ferrite with sliver doped nanoparticles plays very important role in the inhibition of the multi drug resistant bacteria. Following are the conclusions made by our study.
-
1)
Combustion method for the synthesis of Ag metal, magnesium ferrite and Ag doped magnesium ferrite are very simple and needs simple equipments.
-
2)
This method may be used for the preparation of other metals and metal oxides.
-
3)
Synthesis via oxalate promotes the formation of particles under controlled stage leads nanosize.
-
4)
PVA is acting as a good fuel for the combustion process. Initially it froths and forms the expected solid product after complete combustion.
-
5)
This method insists for the doping of other metal to the any ferrites and other nanomaterials.
Declarations
Author contribution statement
Arunkumar Lagashetty, Amruta Pattar, Sangappa K Ganiger: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
Authors are gratefully acknowledging the support by Dr. A. Venkataraman, Professor, Department of Chemistry, Gulbarga University, Kalaburagi for spectral analysis. Thanks are due to President and Principal, Reshmi Degree College, Kalaburagi for constant support and encouragement.
References
- 1.Prakash Bharti Satya, Singh Ekta, Kumar Utkarsh. Synthesis and characterization of nickel doped tin oxide nanoparticles by hydrothermal method. Nanosci. Nanotech. Res. 2017;4(3):115–119. [Google Scholar]
- 2.Mahboubeh H., Fatemeh Z., Zahra J.Razi., Ali A., Zohreh A. Synthesis of cobalt ferrite (CoFe2O4) nanoparticles using combustion, co precipitation, and precipitation methods A comparison study of size, structural, and magnetic properties. J. Mag. Mater. 2014;371:43–48. [Google Scholar]
- 3.Raveendra R.S., Prashanth P.A., Prasad B Daruka, Chandra Nayaka S., Suresha G.P., Nagabhushana B.M., Nagabhushana H., Bhagya N.P. Synthesis, characterization and antibacterial activity of zinc ferrite nanopowder. Int. Jour. of Sci. Resc. 2013;1(4):543–547. [Google Scholar]
- 4.Soaresjr F.H., Pinheiro A.V.B., Torres Marco Antonic Morales, Soares Joao Maria. Novel one-pot preparation of CoFe2O4–Ag nanocrystalline powders. J. Material Letters. 2013;113:67–70. [Google Scholar]
- 5.Sanpo Noppakun, Wen1 Cuie, Berndt Christopher C., Wang James. Microatructural and antibacterial properties of zinc-substituted cobalt ferrite nanopowders synthesized by sol-gel methods. J. Appl. Phys. 2012:112–118. [Google Scholar]
- 6.Sharma S.K., Socolovsky L.M., Pirota K.R., Knobel M. Synthesis of Ag–CoFe2O4 dimer colloidal nanoparticles and enhancement of their magnetic response. J. Appl. Phys. 2011;109:123–129. [Google Scholar]
- 7.Jeevanandam J., Barhoum A., Chen Y.S., Dufresne A., Danquan M.K. Bilstein, Review on nanoparticles and nanostructured materials: history, sources, toxicity and regulations. J. Nanotechnology. 2018;9:1050–1074. doi: 10.3762/bjnano.9.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y., Fan Z., Zhang Z., Niu W., Li C., Yang N., Chen B., Zhang H. Two-dimensional metal nanomaterials: synthesis, properties, and applications. Chem. Rev. 2018;13:6409–6455. doi: 10.1021/acs.chemrev.7b00727. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z., Wishen, Xue J., Liu Y., Liu Y., Yan P., Liu J., Tang J. Recent advances in synthetic methods and applications of silver nanostructures. Nanoscale Res. Lett. 2018;13:54. doi: 10.1186/s11671-018-2450-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balakrishnan P., Veluchamy P. Synthesis and characterization of CoFe2O4 magnetic nanoparticles using sol-gel method. Int. J. Chem. Tech. Res. 2015;8(1):271–276. [Google Scholar]
- 11.Anti-microbial activity of metal oxide Nps against Gram-positive and Gram-negative bacteria: a comparative study. Int. J. Nanomed. December 2012;4:38. doi: 10.2147/IJN.S35347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Deraz N.M., Fouda Moustafa M.G. Fabrication and magnetic properties of cobalt – copper nano composite. Int. J. Electrochem. Sci. 2013;8:2682–2690. [Google Scholar]
- 13.Rajput Namita. Nanotechnology in civil engineering and construction: a review. Int. J. Adv. Engg. Tech. Jan., 2015;7(5):127–133. [Google Scholar]
- 14.Kaur Navneet, Kaur Manpreet. Comparative studies on impact of synthesis methods on structural and magnetic properties of magnesium ferrite nanoparticles. Proces. Appl. Ceram. 2014;8(3):137–143. [Google Scholar]
- 15.Basavaraja S., Balaji Arunkumar Lagashetty S.D., Rajasab S., Venkataraman A. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium semitectum. Mater. Res. Bull. 2008;41:1162–1170. [Google Scholar]
- 16.Lagashetty Arunkumar, Havanoor Vijayanand, Basavaraj S., Balaji S.D., Venkataraman A. Microwave-assisted route for synthesis of nanosized metal oxides. Sci. Technol. Adv. Mater. 2008;8:484–493. [Google Scholar]
- 17.Omer Mohamed I.M., Elbadawi A.A., Yassin O.A. Synthesis and structural properties of MgFe2O4 ferrite nano-particles. J. Appl. Ind. Sci. 2013;1(4):20–23. [Google Scholar]
- 18.Amiri S., Shokrollahi H. The role of cobalt ferrite magnetic nanoparticles in medical science Materials. Sci. Eng. C. 2013;33:1–8. doi: 10.1016/j.msec.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 19.Hu Yawei, Zhang Wei, Pan Wei. Photo catalytic activities of hydrothermally synthesized In(OH)3 and In2O3 nanocubes. Mater. Res. 2013;16(3):668–671. [Google Scholar]
- 20.Ganiger Sangappa K., Murugendrappa M.V. Lab scale study on humidity sensing and D.C. Conductivity of polypyrrole/strontium arsenate (Sr3(AsO4)2) ceramic composites”. Polym. Sci. B. 2018;60(3):395–404. [Google Scholar]
- 21.Swamy Pratviraj, Basavaraj S., Lagashetty Arunkumar, Srinivas Rao N.V., Nijgunappa R., Venkataraman A. Synthesis and characterization of Zinc Ferrite nanoparticles obtained by self-propagating low temperature combustion method. Bull. Mater. Sci. 2011;34(7):1325–1330. [Google Scholar]
- 22.Lagashetty Arunkumar, Ganiger Sangappa K. Microwave-assisted synthesis, characterisation and thermal study of nano sized metal aluminates. J. Metallurgy & Mater. Sci. 2018;60(3):139–148. [Google Scholar]