Abstract
Presynaptic Ca2+ entry occurs through voltage-gated Ca2+ (CaV) channels which are activated by membrane depolarization. Depolarization accompanies neuronal firing and elevation of Ca2+ triggers neurotransmitter release from synaptic vesicles. For synchronization of efficient neurotransmitter release, synaptic vesicles are targeted by presynaptic Ca2+ channels forming a large signaling complex in the active zone. The presynaptic CaV2 channel gene family (comprising CaV2.1, CaV2.2, and CaV2.3 isoforms) encode the pore-forming α1 subunit. The cytoplasmic regions are responsible for channel modulation by interacting with regulatory proteins. This article overviews modulation of the activity of CaV2.1 and CaV2.2 channels in the control of synaptic strength and presynaptic plasticity.
Keywords: Ca2+ channels, synaptic transmission, G-proteins, synaptic proteins, Ca2+ binding proteins
1. Introduction
Presynaptic Ca2+ entry into the active zone (AZ) occurs through voltage-gated Ca2+ (CaV) channels which are activated membrane depolarization and triggers synchronous neurotransmitter release from synaptic vesicles (SVs). Multiple mechanisms regulate the function of presynaptic Ca2+ channels [1,2,3,4]. The channel activity for opening, closing, or inactivation in response to membrane depolarization changes every few milliseconds during and after neuronal firing, resulting in control of synaptic strength [3,4]. Following a brief overview of Ca2+ channel structure/function, this article reviews the molecular and cellular mechanisms that modulate the activity of presynaptic Ca2+ channels in the regulation of neurotransmitter release and in the induction of short-term synaptic plasticity. To understand the physiological role of Ca2+ channel modulation in the regulation of synaptic transmission, a model synapse formed between sympathetic, superior cervical ganglion (SCG) neurons in culture was employed for functional study of channel interaction with G proteins, SNARE proteins, and Ca2+-binding proteins which sense residual Ca2+ in the AZ after the arrival of an action potential (AP).
2. Presynaptic Ca2+ Channels
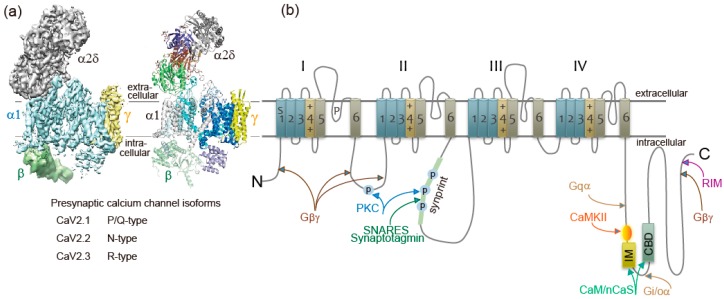
Ca2+ currents have diverse physiological roles and different pharmacological properties. Early investigations revealed distinct classes of Ca2+ currents which were identified with an alphabetical nomenclature [5]. P/Q-type, N-type, and R-type Ca2+ currents are observed primarily in neurons, require strong depolarization for activation [6], and are blocked by specific polypeptide toxins from snail and spider venoms [7]. P/Q-type and N-type Ca2+ currents initiate neurotransmitter release at most fast synapses [1,8,9]. The Ca2+ channels are composed of four or five distinct subunits (Figure 1a) [8,10]. The α1 subunit incorporates the conduction pore, the voltage sensors and gating apparatus, and target sites of toxins and intracellular regulators. The α1 subunit is composed of about 2000 amino acid residues and is organized in four homologous domains (I–IV) (Figure 1b). Each domain consists of six transmembrane α helices (S1 through S6) and a membrane-associated P loop between S5 and S6. The S1 through S4 segments serve as the voltage sensor module, whereas transmembrane segments S5 and S6 in each domain and the P loop between them form the pore module [11]. The intracellular segments serve as a signaling platform for Ca2+-dependent regulation of neurotransmission, as discussed below.
Figure 1.
Ca2+ channel structure and organization. (a) The subunit composition and structure of high-voltage-activated Ca2+ channels. The cryo-EM structure of the rabbit voltage-gated Ca2+ channel Cav1.1 complex at a nominal resolution of 4.2 Å. The overall EM density map on the left is colored according to different subunits. The structure model on the right is color-coded for distinct subunits. Reproduced from [12]. (b) The α1 subunit consists of four homologous domains (I-IV), each consisting of six transmembrane segments (S1-S6). S1–S4 represents the voltage-sensing module. S5–S6 represents the pore-forming unit. The large intracellular loops linking the different domains of the α1 subunit serve as sites of interaction of different regulatory proteins important for channel regulation, including G-protein (Gβγ, Gα), RIM, SNARE proteins, and synaptotagmin at the synprint site (shown in green bar), calmodulin (CaM), and neuronal Ca2+ sensor proteins (nCaS) at the IQ-like motif, which begins with the sequence isoleucine-methionine (IM) instead of isoleucine-glutamine (IQ) and the nearby downstream CaM-binding domain (CBD), calmodulin kinase II (CaMKII), and protein kinase C (PKC). Adapted from [4].
Ca2+ channel α1 subunits are encoded by ten distinct genes in mammals, which are divided into three subfamilies by sequence similarity [2,8,13]. The CaV2 subfamily members CaV2.1, CaV2.2, and CaV2.3 channels conduct P/Q-type, N-type, and R-type Ca2+ currents, respectively [2,8,9,13].
CaV channels are complexes of a pore-forming α1 subunit and auxiliary subunits. Skeletal muscle CaV channels have three distinct auxiliary protein subunits [8] (Figure 1a), the intracellular β subunit, the disulfide-linked α2δ subunit complex, and the γ subunit having four transmembrane segments. In contrast, brain neuron CaV2 channels are composed of the pore-forming α1 and the auxiliary β subunit [14]. The auxiliary subunits of Ca2+ channels have an important influence on their function [15,16]. The CaVβ subunit shifts their kinetics and voltage dependence of activation and inactivation [15,16]. Cell surface expression of the α1 subunits is enhanced by the CaVβ subunit [15,16]. The α2δ subunits are potent modulators of synaptic transmission. The α2δ subunits increase not only Cav1.2 but also Cav2.2, Cav2.1 currents, suggesting that the α2δ subunits enhance trafficking of the CaV channel complex [17]. Expression of α2δ subunits also appears to play a role in setting release probability [18]. Further details of these regulatory interactions are discussed below.
3. Intracellular Molecules Modulate Presynaptic Ca2+ Channels Activity
3.1. G Proteins
Presynaptic Ca2+ currents are reduced in magnitude by activation of G protein-coupled receptors for neurotransmitters at nerve terminals [19,20]. Gβγ subunits released from heterotrimeric G proteins of the Gi/Go class [19,20] bind directly to α1 subunits of the N-type Ca2+ channel [21,22] at the N terminus [23], the intracellular loop connecting domains I and II [21,24], and at the C terminus [25] (Figure 1b). Gβγ causes a positive shift in the voltage dependence of activation of the Ca2+ current [26,27,28]. The Gβγ-induced reduction of Ca2+ currents can be reversed by strong positive depolarization [26,27,28]. Reversal of this inhibition by depolarization provides a point of intersection between chemical and electrical signal transduction at the synapse and can potentially provide novel forms of short-term synaptic plasticity that do not rely on residual Ca2+.
The subtype of CaVβ can influence the extent and kinetics of Gβγ mediated inhibition and this regulation also depends on the subtype of Gβ involved [29,30]. Gβγ interacts with multiple sites on the N-terminus, I–II linker, and the C-terminus of the α1 subunit. Binding of Gβγ causes a conformational shift that promotes interaction of the N-terminus “inhibitory module” with the initial one-third of the I–II-linker. Strong membrane depolarization leads to unbinding of Gβγ and loss of interaction between the N-terminus and the I–II linker. This depends upon binding of CaVβ subunit to the α interaction domain (AID) on the I–II linker. In the absence of CaVβ1 subunit binding with tryptophan mutation in the AID (W391) of the CaV2.2 α1 subunit, Ca2+ channel inhibition still occurs but cannot be reversed by strong depolarization. CaVβ2a, that is palmitoylated at two N-terminal cysteine residues, can still bind to the α1 subunit and permit voltage-dependent relief of the inhibition [31]. It is possible that binding of CaVβ1 to the AID induces a rigid α-helical link with domain IS6, and this transmits the movement of the voltage-sensor and activation gate to the I–II linker to alter the Gβγ binding pocket at depolarized potentials [32].
Specific Gβ subunits have been shown to be responsible for the CaV2 channel modulation in different neurons. In rat SCG neurons CaV2.2 channels are differentially modulated by different types of Gβ subunits, with Gβ1 and Gβ2 being most effective, Gβ5 showing weaker modulation, and Gβ3 and Gβ4 being ineffective [33,34,35]. In contrast, in rat stellate ganglion neurons, Gβ2 and Gβ4 but not Gβ1 subunit are responsible for the coupling of CaV2.2 channels with noradrenaline receptors [36]. In the transfected human embryonic kidney tsA-201 cell line, CaV2.2 channel inhibition, with Gβ1 and Gβ3 being more effective than Gβ4 and Gβ2, and no significant modulation being induced by Gβ5 [37]. Gβ subunit-induced inhibition of CaV2.1 channel differed from those observed with the CaV2.2 channel. CaV2.1 channels exhibited more rapid rates of recovery from inhibition than those observed with CaV2.2 channels, on average, twice as rapidly for the CaV2.1 channels, indicating that Gβ binding to this channel subtype is less stable [37].
Regulation of the CaV2.2 channels also involves the interplay between Ca2+ channels and G protein interaction. Syntaxin-1A, a presynaptic plasma membrane protein, is required for G protein inhibition of presynaptic Ca2+ channels [38]. Physical interaction between syntaxin-1A and Ca2+ channels is a prerequisite for tonic Gβγ modulation of CaV2.2 channels, suggesting that syntaxin-1A mediates a colocalization of Gβγ subunits and CaV2.2 channels, thus resulting in a more effective G protein coupling to, and regulation of, the channel. The interactions between syntaxin, G proteins, and CaV2.2 channels are part of the structural specialization of the presynaptic terminal [39].
G proteins also induce voltage-independent inhibition of CaV2 channels through intracellular signaling pathways [1,19,40]. This often involves the Gq family of G proteins, which regulate the levels of phosphatidylinositide lipids by inducing hydrolysis of phosphatidylinositol bisphosphate via activation of phospholipase C enzymes [41]. Acetylcholine release from rat sympathetic neurons is reduced through this pathway via presynaptic muscarinic receptors activation [42].
3.2. Active Zone Proteins
Rab-interacting molecule (RIM), an AZ protein required for SVs docking and priming [43,44,45,46,47,48], and synaptic plasticity [49], interacts with the C-terminal cytoplasmic tails of CaV2.1 and CaV2.2 channels [46,48,50,51] (Figure 1b). The interaction is essential for recruiting Ca2+ channels to the presynaptic AZ [46] and determines channel density and SVs docking at the presynaptic AZ [48]. RIM-binding proteins, RIM-BPs, also interact with CaV2.1 and CaV2.2 channels [51], and are selectively required for high-fidelity coupling of AP-induced Ca2+ influx to Ca2+-stimulated SVs exocytosis [52]. The tripartite complex of RIM, RIM-BPs, and C-terminal tails of the CaV2 channels regulate the recruitment of CaV2 channels to AZs. Interaction of RIM with CaVβ subunits shifts the voltage dependence of inactivation to more positive membrane potentials, increasing Ca2+ channel activity [53]. In contrast, CaVβ subunits interaction with CAST/ERC2 shifts the voltage dependence of activation to more negative membrane potentials [54]. Positive regulation of presynaptic Ca2+ channel activity by RIM and CAST/ERC2, in addition to their function in SVs docking, increase the release probability of SVs docked close to CaV2 channels. Furthermore, Munc13, required for SVs priming, controls CaV2 channels shortly after AP firing to guarantee transmitter release for continuous neural activity [55].
3.3. t-SNAREs and Synaptotagmin-1
SV (v)-SNARE synaptobrevin 2 and presynaptic plasma membrane (t)-SNAREs syntaxin-1 and SNAP-25 are required for fusion of SVs with a plasma membrane to release neurotransmitters [56]. Both CaV2.1 and CaV2.2 channels at the presynaptic nerve terminals colocalize densely with syntaxin-1A [57,58,59], and also form a complex of with SNARE proteins [60,61,62] dependent on Ca2+ with maximal binding at 20 μM and reduced binding at lower or higher concentrations of Ca2+ [63]. The t-SNARE proteins syntaxin-1A and SNAP-25, but not the v-SNARE synaptobrevin, bind to the intracellular loop between domains II and III of the α1 subunit of CaV2.2 (amino acid residues 718-963) named as the synprint site (Figure 1b) [64,65]. CaV2.1 channels have an analogous synprint site, and different channel isoforms have distinct interactions with syntaxin and SNAP-25 [66,67], suggesting specialized regulatory properties for synaptic modulation.
t-SNAREs interacting with presynaptic CaV2.1 and CaV2.2 channels regulate channel activity (Figure 3a). Syntaxin-1A or SNAP-25 shifts the voltage dependence of inactivation toward more negative membrane potentials and reduces the availability of the channels to open [68,69,70]. Coexpression of SNAP-25 can reverse the inhibitory effects of syntaxin-1A [69,71]. The transmembrane region of syntaxin-1A and only a short segment within the H3 helix are critical for channel modulation [72], whereas the synprint site binds to the entire H3 helix in the cytoplasmic domain of syntaxin-1A [63,64,72]. Deletion of the synprint site weakened the modulation of the channels by syntaxin-1A, but did not abolish it, arguing that the synprint site acts as an anchor in facilitating channel modulation but is not required absolutely for modulatory action.
Dependent on Ca2+ concentration, syntaxin-1 interacts with either the synprint site or synaptotagmin-1; at low Ca2+ concentrations, syntaxin-1 binds synprint, while at higher concentrations (>30 μM) it associates with synaptotagmin-1 [63]. Synaptotagmin-1, -2, and -9 serve as the Ca2+ sensors for the fast, synchronous neurotransmitter release [56,73,74]. The Ca2+ binding site C2B domain of synaptotagmin-1 interacts with the synprint sites of both CaV2.1 and CaV2.2 channels (Figure 1b) [75]. Synaptotagmin-1 can relieve the inhibitory effects of SNAP-25 on CaV2.1 channels [70,76]. Relief of Ca2+ channel inhibition by the formation of the synaptotagmin/SNARE complex favors Ca2+ influx. This is a potential mechanism to increase the release probability of SVs docked close to CaV2 channels [4].
Interaction of syntaxin-1A and SNAP-25 with the synprint site is controlled by phosphorylation of the synprint site with protein kinase C (PKC) (Figure 1b) [65] and Ca2+/calmodulin-dependent protein kinase II (CaMKII) [77]. The negative shift of steady-state inactivation of CaV2.2 channels caused by syntaxin is blocked by PKC phosphorylation [65,71]. Thus, phosphorylation of the synprint site may serve as a biochemical switch controlling the SNARE-synprint interaction.
3.4. Ca2+-Sensor Proteins
Ca2+ elevation regulates CaV2.1 channels activity by its binding to CaM [8,78,79,80,81] and related neuron-specific Ca2+-binding proteins, CaBP1, VILIP-2 [82,83,84], and NCS-1 (frequenin) [85]. The presynaptic CaV2.1 channel proteins consist of a pore-forming α1 subunit associated with β, and possibly α2δ subunits (Figure 1a) [86]. The intracellular C terminus of the α1 subunit [81] called the IQ-like motif, which begins with the sequence isoleucine-methionine (IM) instead of isoleucine-glutamine (IQ), and the nearby downstream CaM-binding domain (CBD) are the interacting sites with these Ca2+-binding proteins (Figure 1b). Displacement with alanine in the IQ-like domain inhibited Ca2+-dependent CaV2.1 channels facilitation [78,81], whereas deletion of CBD inhibited Ca2+-dependent CaV2.1 channels inactivation [79,80,81,83,84]. Ca2+/CaM-dependent inactivation of CaV2.1 channels, dependent on global elevations of Ca2+, is observed in transfected cells overexpressing CaV2.1 channels [78,79,80] and in the nerve terminals of the calyx of Held [87,88] where CaV2.1 channels are densely localized. In contrast, the large neuronal cell bodies of Purkinje neurons [89] or SCG neurons [90] rarely show Ca2+-dependent CaV2.1 channels inactivation.
4. Negative Regulation of Neurotransmitter Release by Gβγ protein/CaV2 Channel Complex
Receptor-activated Gβγ modulation of presynaptic CaV2 channels is a potent negative regulation of neurotransmitter release. Electrophysiological recordings of Ca2+ currents and synaptic transmission at the calyx of Held demonstrated this type of negative regulation by activation of GABA-B receptors or metabotropic glutamate receptors [91,92]. Optical measurements of Ca2+ transients at the nerve terminals of the parallel fibers of cerebellar granule cells innervating Purkinje neurons has also demonstrated similar modulation by activation of CB1 receptors [93]. This Gβγ-mediated inhibition of Ca2+ channels is relieved by depolarization. At autapses formed by single hippocampal pyramidal neurons, trains of AP-like stimuli relieve the inhibition of synaptic transmission caused by activation of GABA-B receptors, resulted in facilitation of synaptic transmission, which was blocked by inhibition of CaV2.1 channels with neurotoxins [94]. Thus, presynaptic firing could reverse the neurotransmitter-mediated G protein inhibition of synaptic transmission. Regulator of G protein signaling-2 (RGS-2), which speeds GTPase activity of the α subunit of the activated G protein α-GTP, determines short-term plasticity in hippocampal neurons by regulating Gi/o-mediated inhibition of presynaptic Ca2+ channels. RGS-2 relieves the inhibition, resulting in a higher basal probability of release and synaptic facilitation [95]. However, at parallel fibers synapses onto Purkinje cells, this form of facilitation is not responsible for short-term synaptic plasticity [96].
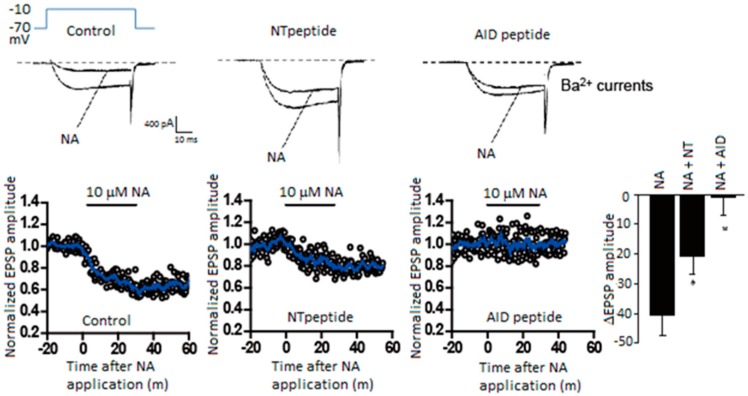
In SCG neurons noradrenaline shortens AP duration by reducing Ca2+ entry through CaV2.2 channels, resulting in a reduction of transmitter release [97]. Purified Gβγ microinjected into presynaptic SCG neurons in culture reduced synaptic transmission, and the Gβγ introduced neurons caused no further reduction of synaptic transmission with noradrenaline [97]. Thus, Gβγ is a potent negative regulator of neurotransmission inhibiting presynaptic CaV2.2 channels activity. The α1 subunit contains several Gβγ interaction sites, including the amino-terminal (NT) and I–II loop (Figure 1b). The “NT peptide” and an I–II loop α interaction domain “AID peptide” microinjected into presynaptic SCG neurons under long-term culture attenuated noradrenaline-induced G protein modulation (Figure 2) and inhibited synaptic transmission [98]. In acutely dissociated SCG neurons, NT and AID peptides reduced whole-cell Ba2+ current amplitude, modified voltage dependence of Ca2+ channel activation, and attenuated noradrenaline-induced G protein modulation (Figure 2) [98]. Co-application of NT and AID peptide negated inhibitory actions. Furthermore, a mutation within NT abolished inhibitory effects of the NT peptide [98]. Effects of CaV2.2 channel peptides demonstrate that the CaV2.2 amino-terminal and I–II loop serve as molecular determinants for Ca2+ channel function to inhibit synaptic transmission and to attenuate G protein modulation.
Figure 2.
Gβγ-mediated noradrenaline inhibition of transmitter release and N-terminal/I-II loop AID peptides of CaV2.2 α1-subunit. Noradrenaline (NA) induced Ba2+ current inhibition (upper traces) and transmitter release (lower graphs) were attenuated in the presence of Gβγ-interaction site of N-terminal peptide (CaV2.245-55, YKQSIAQRART) or AID peptide (CaV377-393, RQQQIEREL NGYLEWIF) (See Figure 1b). Ba2+ currents were recorded from superior cervical ganglion (SCG) neurons acutely dissociated from 3- to 6-week-old Wistar rats, while the synaptic transmission was recorded from long-term cultured SCG neurons isolated from p7 rat. NA was bath-applied 30 min after injection of the peptide at 1 mM in the injection pipette. EPSPs were normalized to amplitude prior to NA application at time = 0 min. Bar graph summarizing NA effects, *p < 0.05 vs. NA effects in controls (Student’s t-test). Adapted from [98].
5. Synchronous Neurotransmitter Release Regulated by Ca2+ Channel/SNARE Proteins Complex
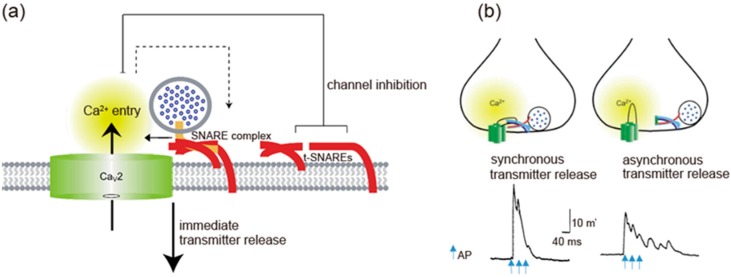
Synprint peptides derived from CaV2.2 channels reduced transmitter release from the microinjected presynaptic SCG neurons in culture, due to competitive uncoupling of the endogenous Ca2+ channel-SNARE proteins interaction in nerve terminals [99]. Synprint peptides selectively inhibited fast synchronous synaptic transmission, while they increased late asynchronous release (Figure 3b). Similarly, synprint peptides reduced transmitter release from embryonic Xenopus spinal neurons [100]. Increasing the external Ca2+ concentration effectively rescued this inhibition, implying that synprint peptides competitively displaces docked SVs away from Ca2+ channels, and this effect can be overcome by increasing Ca2+ influx into presynaptic terminals [100].
Figure 3.
Spatial regulation of transmitter release by the I-II loop interaction with SNAREs. (a) The I-II loop interacts with t-SNAREs, resulting in inhibition of Ca2.2 channels opening. Once AP opens the channels, an increase in Ca2+ mediates interaction with SNAREs complex and induces transmitter release. Adapted from [4]. (b) Triple APs induces a large synchronous transmitter release from the first AP. In contrast, asynchronous transmitter release was observed in the presence of 130 μM synprint peptide (see Figure 1b). Adapted from [99].
At the calyx of Held, presynaptic neurons express P/Q-, N- and R-type Ca2+ currents in postnatal day 7 rats. P/Q-type Ca2+ currents are more effective than N-type Ca2+ currents and R-type Ca2+ currents in eliciting neurotransmitter release [101,102,103]. The high efficiency of P/Q-type Ca2+ currents to initiate neurotransmitter release is correlated with the close localization of CaV2.1 channels near docked SVs [104], as shown by immunocytochemistry [105], suggesting localization of CaV2 channels determines the efficiency of neurotransmitter release in response to neural activity.
CaV2 channels interaction with SNARE proteins, that is dependent on Ca2+ concentration [63], have two opposing effects: at the pre-firing state synaptic transmission is blocked by enhancing CaV2 channels inactivation, whereas immediately after AP firing tethering SVs near the point of Ca2+ entry enhances synaptic transmission. The overexpression of a syntaxin mutant that is unable to regulate CaV2.2 channels, but still binds to them [72], increased the efficiency of synaptic transmission at Xenopus neuromuscular junctions, as reflected in increased quantal content [106]. In contrast, injected synprint peptides reduced the basal efficiency of synaptic transmission, as reflected in reduced quantal content of synaptic transmission [106]. These results demonstrate a bidirectional regulation of synaptic transmission in vivo by interactions of CaV2.2 channels with SNARE proteins.
6. Presynaptic Plasticity Induced by Ca2+-Sensors-Mediated CaV2.1 Channel Modulation
At most fast synapse in the central nervous system, CaV2 channels are expressed diversely. In contrast, synaptic transmission of long-term cultured sympathetic SCG neurons, forming a well-characterized cholinergic synapse [107,108], is mediated by CaV2.2 channels [109,110]. The physiological role of presynaptic CaV2.1 channel modulation by Ca2+-sensors was explored by exogenously expressed α1 subunit derived from the brain CaV2.1 channel that functionally generates P/Q type currents with other endogenous subunits in SCG neuron [111]. Section 6 describes presynaptic plasticity induced by modulation of the CaV2.1 channel that is mediated by CaM or expression of neuron-specific Ca2+-sensor proteins, monitoring excitatory postsynaptic potentials (EPSPs) evoked by various patterns of presynaptic APs firing in the presence of the blocker of endogenous CaV2.2 channels [109].
6.1. Ca2+/CaM Mediates Synaptic Depression and Facilitation
Modulation of presynaptic Ca2+ channels has a powerful influence on synaptic transmission [90]. The cytoplasmic regions of the α1 subunit are the target of regulatory proteins for channel modulation (Figure 1B). Brain-derived α1 subunit of the CaV2.1 channel mediates transmitter release from the transfected SCG neurons [111]. The transmitter release changes after AP firing due to modulation of CaV2.1 channel interacting with Ca2+ bound CaM (Figure 4) [90]. CaM has two Ca2+ binding sites, N and C robes. The N-robe sensing rapid and higher increase in Ca2+ concentration [112] initiates synaptic depression, and following facilitation is mediated by the C-robe sensing lower Ca2+ concentration. EPSPs recorded by pairs of APs with varied stimulation intervals show paired-pulse depression (PPD) and facilitation (PPF) (Figure 4a). PPD with a short interval (<50 ms) was blocked by deletion of the CBD, while PPF with intermediate interval (50–100 ms) was blocked by mutation of the IQ-like motif. Thus, the decline in Ca2+ elevation after the first AP causes temporal regulation of the CaV2.1 channel interacting with CaM, resulting in a change in the transmitter release efficacy (Figure 4b). The time-dependent opposing modulation of the CaV2.1 channel activity may support a stable synaptic transmission.
Figure 4.
Temporal regulation of Ca2+ channel activity by CaM and nCaS after AP(s) firing modulates synaptic transmission. (a) Regulation of transmitter release (lower trace) after an AP firing (upper trace). Dependent on the inter-stimulus interval the second AP induces paired-pulse depression (PPD) and facilitation (PPF). The PPD was prevented by ΔCBD, while PPF was prevented by IM-AA mutation of CaV2.1 channels. (b) Model for Ca2+/CaM-dependent inactivation and facilitation of Ca2+ channels and neurotransmitter release. (c) Biphasic synaptic transmission during 1-s train of APs at 30 Hz changed to synaptic depression by the IM-AA mutation or to synaptic facilitation by the ΔCBD. (d) Overexpression of CaBP1 (blue) blocks synaptic facilitation, while overexpression of VILIP-2 (red) blocks synaptic depression, during 1-s train of APs at 10 Hz. Adapted from [90] (a–c) and [113] (d).
Neural information in vivo is encoded in bursts of AP firing. Short-term presynaptic plasticity caused by APs bursts involves the CaM-dependent regulation of CaV2.1 channel. Mutation of the IQ-like motif potentiated reduction of the release efficacy, whereas the deletion of the CBD increased the release efficacy (Figure 4c, IM-AA/ΔCBD). Thus, during APs bursts, CaM binding to the CBD controls negatively the release efficacy, whereas CaM binding to IQ-like motif controls it positively. At a higher frequency of APs burst over 20 Hz, the release efficacy of SCG neurons mediated by CaV2.1 channels reduced gradually (Figure 4c, WT), suggesting that the CaM-dependent inactivation of CaV2.1 channels shapes the time course of short-term synaptic plasticity by determining the timing of the peak of synaptic facilitation during APs bursts as well as the steady-state level of synaptic depression at the end of the APs bursts.
6.2. Neuron-Specific Ca2+-Sensor Proteins Mediate Synaptic Depression and Facilitation
CaBP1, VILIP-2, and NCS-1 are members of a subfamily of neuron-specific Ca2+-sensor proteins (nCaS) that possess four EF-hand Ca2+-binding motifs. CaBP-1, VILIP-2, and NCS-1 bind to the same site as CaM, and modulate CaV2.1 channel activity. CaBP1, highly expressed in the brain and retina [114], causes rapid inactivation of CaV2.1 channels, binding to the CBD [84]. VILIP-2, highly expressed in the neocortex and hippocampus [115], increases Ca2+-dependent facilitation of CaV2.1 channels but inhibits Ca2+-dependent inactivation of CaV2.1 channels, binding to both IQ-like motif and CBD [83]. NCS-1, the classical example of facilitation of synaptic activity by nCaS, reduces Ca2+-dependent inactivation of P/Q-type Ca2+ currents through interaction with the IQ-like motif and CBD without affecting peak current or activation kinetics [85].
Synaptic transmission of SCG neurons transfected with CaBP1 and VILIP-2 changed by their modulation of CaV2.1 channels with binding residual Ca2+ [113]. APs burst at 10 Hz induces synaptic facilitation followed by synaptic depression due to endogenous CaM. CaBP1 coexpressed with CaV2.1 channels, significantly reduced the synaptic facilitation and enhanced the synaptic depression (Figure 4d) [113]. In contrast, VILIP-2 coexpressed with CaV2.1 reduced the synaptic depression and enhanced the synaptic facilitation (Figure 4d) [113]. CaBP1 and VILIP-2 have opposing effects on short-term synaptic plasticity, either favoring synaptic depression or facilitation, suggesting that nCaS via regulation of presynaptic Ca2+ channels may play a critical role in determining the diversity of short-term synaptic plasticity at CNS synapses.
The expression of NCS-1 in presynaptic SCG neurons does not affect synaptic transmission, eliminating effects of this nCaS on endogenous N-type Ca2+ currents [85]. However, in SCG neurons expressing CaV2.1 channels, coexpression of NCS-1 induces facilitation of synaptic transmission in response to paired APs and trains of APs, and this effect is lost in CaV2.1 channels with mutations in the IQ-like motif and CBD [85]. These results reveal that NCS-1 directly modulates CaV2.1 channels to induce short-term synaptic facilitation, and further demonstrate that nCaS are crucial in fine-tuning short-term synaptic plasticity.
6.3. Temporal Regulation of Release Efficacy by Ca2+-Sensor Proteins
The opening of Ca2+ channel creates a steep gradient of Ca2+ elevation in the AZ, where each nCaS has a different affinity and binding speed to Ca2+ [112]. The affinity is CaM (5–10 μM) > CaBP1 (2.5 μM) >VILIP-2 (~1 μM) [116]. CaM has a lower affinity and a higher binding speed to Ca2+ than nCaS, suggesting a temporal regulation of CaV2.1 channel activity by CaM versus nCaS. Their affinity and binding speed to Ca2+ determinate timing of the CaV2.1 channel modulation. Thus differential effects of CaM and nCaS on facilitation and inactivation of the presynaptic CaV2.1 channels would substantially change the encoding of the synaptic properties in response to bursts of APs firing [117].
Time window of the CaM- and nCaS-induced CaV2.1 channel modulation after AP firing can be estimated by the paired-pulse protocol applying to SCG neurons transfected with CaV2.1 channels. CaM mediated PPD with a short interval (<100 ms), and PPF with intermediate interval (20–100 ms) (Figure 2a). In contrast, NCS-1 induced PPF with a shorter interval (30–50 ms) [85]. CaBP1 induced PPD with interval <150 ms, while VILIP-2 induced PPF with an interval of 50–250 ms [113]. These data suggest that CaM modulates CaV2.1 channels shortly after Ca2+ entry and lasts 100 ms, while NCS-1 acts much shorter and CaBP1 and VILIP-2 actions last longer than CaM effects. The time-dependent action of CaM and nCaS reflects the decline rate of Ca2+ concentration at the CaV2.1 channels after an AP firing. The divergent actions of CaM and nCaS on CaV2.1 channels fine-tune the function and regulatory properties of presynaptic P/Q-type Ca2+ currents, allowing a greater range of input-output relationships and causing various short-term plasticity at different synapses [4].
6.4. CaMKII Saves as Effector Checkpoint for Ca2+ Entry
CaMKII is the most prominent Ca2+/CaM-dependent regulator of postsynaptic response [118,119,120,121] and presynaptic function [122,123,124,125]. The autophosphorylated form of CaMKII [7], which does not require the catalytic activity of the enzyme [126], binds to the α1 subunit of CaV2.1 channels upstream of the IQ-like motif, and enhances the activity by slowing inactivation and positively shifting the voltage dependence of inactivation [126]. The dephosphorylation of CaMKII does not reverse the binding [127]. The presence of a competing peptide that blocks the interaction of CaMKII with presynaptic CaV2.1 channels of SCG neurons prevented both PPD and PPF, suggesting that binding of CaMKII to CaV2.1 channels is required for the expression of this regulatory effect. Similarly, the expression of the brain-specific CaMKII inhibitor CaMKIIN [128], which prevents CaMKII binding to CaV2.1 channels [126], also prevented PPD and PPF. Thus, the noncatalytic regulation of CaV2.1 channels by bound CaMKII controls the activity of those channels that have the effector of the Ca2+ signal (i.e., CaMKII) in position to bind the entering Ca2+ and respond to it [126]. SNARE proteins and RIM similarly serve as effectors of the Ca2+ signal for initiation of SVs exocytosis increasing the activity of the CaV2.1 channels by the formation of a complete SNAREs complex with synaptotagmin and RIM bound [53,70]. This “effector checkpoint” mechanism serves to focus Ca2+ entry through those Ca2+ channels whose effectors are bound and ready to respond.
Furthermore, autophosphorylated CaMKII bound to CaV2.1 channels also binds to synapsin-1, a phosphoprotein of the SVs, increases its phosphorylation and induces oligomers of synapsin-1 [127]. Synapsin-1 is a major presynaptic phosphoprotein that is a prominent substrate for CaMKII, and phosphorylation by CaMKII regulates the effects of synapsin-1 on the trafficking of SVs [129]. The phosphorylation of synapsin-1 by CaMKII increases synaptic transmission at the squid giant synapse [122,123]. Formation of the ternary complex of CaV2.1 and synapsin-1 bound to CaMKII would modulate the dynamics of SV function in AZs containing these proteins [127].
7. Neuronal Firing and Presynaptic Short-Term Plasticity
Neuronal firing regulates presynaptic Ca2+ channels by Ca2+ bound CaM and nCaS and causes facilitation and inactivation of neurotransmitter release. The differential expression of these Ca2+-dependent regulatory proteins may provide a means of cell-type-specific regulation of presynaptic Ca2+ channels and short-term synaptic plasticity. The short-term plasticity of neurotransmitter release shapes the postsynaptic response to bursts of impulses and is crucial for the fine-grained encoding of information in the nervous system [117,130].
7.1. Presynaptic Short-Term Facilitation
The Calyx of Held, the large presynaptic terminal enabling to record directly presynaptic Ca2+ current by voltage-clamp methods, suggests that neuronal firing controls P/Q- and N-type currents to modulate differentially synaptic transmission. Presynaptic Ca2+ current consists of a combination of P/Q- and N-type currents in young mice and shows activity-dependent facilitation that predicts the amount of synaptic facilitation according to the power law [131,132]. CaV2.1 knockout lost both facilitation of the presynaptic Ca2+ current and synaptic facilitation [101,131,132]. The remaining N-type Ca2+ currents are less efficient in mediating synaptic transmission and do not support facilitation of synaptic transmission, but they are more sensitive to modulation by G protein-coupled receptors [101]. These results suggest that CaV2.1 channels are responsible for neuronal activity-dependent synaptic facilitation, while CaV2.2 channels have strong G protein regulation.
Presynaptic short APs bursts generate augmentation and longer APs bursts generate post-tetanic potentiation (PTP) relying on residual Ca2+. The optical measurement of presynaptic Ca2+ transients with the induction of PTP in the calyx of Held showed an increase in the Ca2+ influx to the extent that predicted PTP when the power law of neurotransmission was applied, and the Ca2+ transient decayed with a time course of the decay of PTP [133]. In CaV2.1-transfected SCG neurons, PTP was not significantly affected by mutations at the IQ-like motif [90]. In contrast, PPF and augmentation share a common mechanism involving an increase in instantaneous Ca2+ entry through CaV2.1 channels by CaM- and nCaS-binding in an activity-dependent manner, which in turn facilitates neurotransmitter release. It is likely that facilitation of presynaptic Ca2+ currents may contribute to short-term facilitation [90,132], and the augmentation and the PTP represent overlapping processes caused by differential combinations of mechanisms at different synapses [130].
The expression of CaVβ subunits has a strong influence on synaptic facilitation in hippocampal synapses through their effects on Ca2+ channel function [134]. Cavβ2 and Cavβ4 subunits distribute in clusters and localize to synapses. Caβ2 induces depression, whereas Cavβ4 induces PPF followed by synaptic depression during longer stimuli trains. The induction of PPF by Cavβ4 correlates with a reduction in the release probability and cooperativity of the transmitter release. These results suggest that Cavβ subunits determine the gating properties of the presynaptic Ca2+ channels within the presynaptic terminal in a subunit-specific manner and may be involved in the organization of the Ca2+ channel relative to the release machinery [134].
The mutation of CaV2.1 channels at the IQ-like motif in hippocampal neurons confirmed the mechanism of short-term synaptic facilitation dependent nCaS regulation of CaV2.1 channels with brief and local Ca2+ elevation [135]. In addition, long-term potentiation of synaptic transmission at the Schaffer collateral-CA1 synapse, that is thought to be primarily generated postsynaptically, is substantially weakened by the mutation. Furthermore, the impairments in short-term and long-term plasticity due to CaV2.1 channel mutation at the IQ-like motif are associated with pronounced deficits in spatial learning and memory in context-dependent fear conditioning and in the Barnes circular maze. Thus, regulation of CaV2.1 channels by CaM and nCaS is required for not only presynaptic facilitation but also induction of postsynaptic long-term potentiation, and spatial learning and memory [136].
7.2. Presynaptic Short-Term Depression
At the calyx of Held, presynaptic stimulation at 100 Hz induces robust synaptic depression [88]. Synaptic depression during high-frequency APs bursts in presynaptic neurons is generally thought to be a result of SVs depletion [130]. In a prominent feature of synaptic transmission, the depression is caused by a decrease in release probability [103]. The release probability is determined by docked SVs and Ca2+ current in the AZ. Presynaptic loading of peptides that disrupt CaM interactions reduced both Ca2+-dependent inactivation of the P/Q-type Ca2+ current and PPD [88]. The Ca2+-dependent inactivation of the presynaptic Ca2+ current, rather than SVs depletion, causes rapid synaptic depression for stimuli ranging from 2 to 30 Hz [87,88].
The transfection of SCG neurons with CaV2.1 channels lacking the CBD, a mutation reducing Ca2+-dependent inactivation in heterologous expression systems [80,81], blocked PPD, and reduced synaptic depression during APs burst up to 40 Hz [90]. CaBP1 expression, which blocks Ca2+-dependent facilitation of P/Q-type Ca2+ current, induced PPD, and synaptic depression during APs burst. However, the synaptic depression was absent in the presynaptic neuron coexpressed with CaBP1 and CaV2.1 channels lacking the CBD [113]. These results further demonstrate that rapid synaptic depression is caused by inactivation of presynaptic CaV2.1 channel bound with CaM or CaBP1. During APs burst at 30 Hz and 40 Hz, a slower phase of synaptic depression is likely caused by SVs depletion.
Data from the calyx of Held and CaV2.1-transfected SCG neurons suggest a conserved mechanism for generating rapid synaptic depression evoked by physiological rate and duration (at 40 Hz for 1 s) of APs bursts in multiple synapses where neuronal activity elevates presynaptic Ca2+ transient, and such a Ca2+ rise dependent binding of nCaS to CaV2 channels inactivates presynaptic Ca2+ channels. Studies of β subunits within cultured hippocampal neurons also support an important role for CaV2 channels modulation in synaptic plasticity: the overexpression of CaVβ4 favors facilitation whereas the overexpression of CaVβ2 favors depression [134].
7.3. CaMKII Regulates Short-Term Synaptic Plasticity
The binding of CaMKII to CaV2.1 channels enhances their functional activity by inhibiting their inactivation [126] and enhances the activity of CaMKII by increasing its autophosphorylation [127]. SCG neurons introduced a competing peptide that blocks the interaction of CaMKII with CaV2.1 channels or SCG neurons transfected the brain-specific CaMKII inhibitor CaMKIIN [128] which prevents CaMKII binding to CaV2.1 channels [126] prevented not only PPF and PPD but also synaptic depression during APs burst and augmentation after a conditioning APs burst. It is unlikely that the basal release probability is affected by competing for peptide injection or CaMKIIN expression because the mean amplitudes of the first EPSPs are unchanged. Binding of CaMKII to the CaV2.1 channel is required for both up-regulation of channel activity in presynaptic facilitation and for Ca2+-independent activation of CaMKII by CaV2.1, and one or both of these effects is necessary for normal short-term synaptic plasticity.
7.4. Ca2+-Binding Molecules Regulate Short-Term Synaptic Plasticity
Synaptotagmin-1, 2, and 9 serve as Ca2+ sensors to mediate the fast synchronous transmitter release as discussed above [56,73,74]. In contrast, synaptotagmin-7 that binds slowly to Ca2+ via its C2A domain [137] is not required for the synchronous synaptic transmission but mediates asynchronous transmitter release [111]. Synaptotagmin-7 is also required for the short-term facilitation, such as PPF and synaptic facilitation during APs burst, at several synapses [138]. Synaptotagmin-7 has a stronger contribution to membrane binding, and perhaps to bridging the vesicle and plasma membranes [111] that may enhance the fast transmitter release in response to repetitive APs firing.
In the presynaptic terminal Ca2+ buffers such as parvalbumin, calbindin, and related Ca2+-binding proteins control Ca2+ homeostasis [139] and synaptic strength [140,141,142]. A slow Ca2+ buffer parvalbumin [143] controls decay rate of short-term plasticity [144]. In contrast, a rapid Ca2+ buffer calbindin [145] alters short-term synaptic facilitation in multiple ways at different synapses [146].
Short-term plasticity may be a combination of the three molecular mechanisms, Ca2+ channel modulation, synaptotagmin-7 action and Ca2+ buffering, activated by Ca2+ elevation with neuronal firing. Ca2+ channel modulation with CaM and nCaS is a response to millisecond Ca2+ dynamics. The slower synaptotagmin-7 action integrates local and global Ca2+ entry, and Ca2+ buffering may control the spread Ca2+ accumulation [146].
8. Conclusions
In response to presynaptic AP firing, Ca2+ binding proteins triggers SVs exocytosis and regulate the probability. Thus, modulation of presynaptic Ca2+ channels has a powerful influence on synaptic transmission. At the pre-firing state, Ca2+ channels activity is inhibited by interaction with AZ proteins. AP firing relieves the inhibition by switching to interact with SNAREs and synaptotagmin, the effectors for Ca2+-dependent exocytosis. During and post firing, the activity of the CaV2.1 channel is regulated by interaction with CaM and nCaS dependent on individual speed and affinity of binding to residual Ca2+. Interacting with CaMKII, the CaV2.1 channel increases the binding to CaM and nCaS and their interaction causes short-term facilitation and depression of synaptic transmission. Fine-tuning the function and regulatory properties of presynaptic P/Q-type Ca2+ currents allow a greater range of input-output relationships and short-term plasticity. In contrast, tonic inhibition of N-type Ca2+ currents is activated by G-protein coupled-autoreceptors and retrograde signaling receptors.
Acknowledgments
The author expresses her sincere thanks to all the collaborators concerned with the studies described in this review.
Abbreviations
| APs | action potentials |
| AZ | active zone |
| CaV2 channels | voltage-gated Ca2+ channels |
| SVs | synaptic vesicles |
| SCG | superior cervical ganglion |
| EPSPs | excitatory postsynaptic potentials |
| RIM | Rab-interacting molecule |
| RIM-BPs | RIM-binding proteins |
| nCaS | neuron specific Ca2+ sensor proteins |
| CaM | calmodulin |
| CaBP1 | Ca2+-binding protein-1 |
| VILIP-2 | Visinin-like protein-2 |
| NCS-1 | neuronal calcium sensor-1 |
| CaMKII | Ca2+/CaM-dependent protein kinase II |
| PPF | paired-pulse facilitation |
| PPD | paired-pulse depression |
| ISI | inter-stimulus interval |
| PTP | post-tetanic potentiation |
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Dunlap K., Luebke J.I., Turner T.J. Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci. 1995;18:89–98. doi: 10.1016/0166-2236(95)80030-6. [DOI] [PubMed] [Google Scholar]
- 2.Snutch T.P., Reiner P.B. Ca2+ channels: Diversity of form and function. Curr. Opin. Neurobiol. 1992;2:247–253. doi: 10.1016/0959-4388(92)90111-W. [DOI] [PubMed] [Google Scholar]
- 3.Tedford H.W., Zamponi G.W. Direct G protein modulation of Cav2 calcium channels. Pharm. Rev. 2006;58:837–862. doi: 10.1124/pr.58.4.11. [DOI] [PubMed] [Google Scholar]
- 4.Catterall W.A., Few A.P. Calcium channel regulation and presynaptic plasticity. Neuron. 2008;59:882–901. doi: 10.1016/j.neuron.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 5.Tsien R.W., Lipscombe D., Madison D.V., Bley K.R., Fox A.P. Multiple types of neuronal calcium channels and their selective modulation. Trends Neurosci. 1988;11:431–438. doi: 10.1016/0166-2236(88)90194-4. [DOI] [PubMed] [Google Scholar]
- 6.Tsien R.W., Ellinor P.T., Horne W.A. Molecular diversity of voltage-dependent Ca2+ channels. Trends Pharm. Sci. 1991;12:349–354. doi: 10.1016/0165-6147(91)90595-J. [DOI] [PubMed] [Google Scholar]
- 7.Miljanich G.P., Ramachandran J. Antagonists of neuronal calcium channels: Structure, function, and therapeutic implications. Annu. Rev. Pharm. Toxicol. 1995;35:707–734. doi: 10.1146/annurev.pa.35.040195.003423. [DOI] [PubMed] [Google Scholar]
- 8.Catterall W.A. Structure and regulation of voltage-gated Ca2+ channels. Annu. Rev. Cell Dev. Biol. 2000;16:521–555. doi: 10.1146/annurev.cellbio.16.1.521. [DOI] [PubMed] [Google Scholar]
- 9.Olivera B.M., Miljanich G.P., Ramachandran J., Adams M. Calcium channel diversity and neurotransmitter release: The ω-conotoxins and ω-agatoxins. Annu. Rev. Biochem. 1994;63:823–867. doi: 10.1146/annurev.bi.63.070194.004135. [DOI] [PubMed] [Google Scholar]
- 10.Takahashi M., Seagar M.J., Jones J.F., Reber B.F., Catterall W.A. Subunit structure of dihydropyridine-sensitive calcium channels from skeletal muscle. Proc. Natl. Acad. Sci. USA. 1987;84:5478–5482. doi: 10.1073/pnas.84.15.5478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank H.Y., Yarov-Yarovoy V., Gutman G.A., Catterall W.A. Overview of molecular relationships in the voltage-gated ion channel superfamily. Pharmacol. Rev. 2005;57:387–395. doi: 10.1124/pr.57.4.13. [DOI] [PubMed] [Google Scholar]
- 12.Wu J., Yan Z., Li Z., Yan C., Lu S., Dong M., Yan N. Structure of the voltage-gated calcium channel Cav1.1 complex. Science. 2015;350:aad2395. doi: 10.1126/science.aad2395. [DOI] [PubMed] [Google Scholar]
- 13.Ertel E.A., Campbell K.P., Harpold M.M., Hofmann F., Mori Y., Perez-Reyes E., Schwartz A., Snutch T.P., Tanabe T., Birnbaumer L., et al. Nomenclature of voltage-gated calcium channels. Neuron. 2000;25:533–535. doi: 10.1016/S0896-6273(00)81057-0. [DOI] [PubMed] [Google Scholar]
- 14.Müller C.S., Haupt A., Bildl W., Schindler J., Knaus H.G., Meissner M., Meissner B., Striessnig J., Flockerzi V., Fakler B., et al. Quantitative proteomics of the Cav2 channel nano-environments in the mammalian brain. Proc. Natl. Acad. Sci. USA. 2010;107:14950–14957. doi: 10.1073/pnas.1005940107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dolphin A.C. Beta subunits of voltage-gated calcium channels. J. Bioenerg. Biomembr. 2003;35:599–620. doi: 10.1023/B:JOBB.0000008026.37790.5a. [DOI] [PubMed] [Google Scholar]
- 16.Hofmann F., Lacinova L., Klugbauer N. Voltage-dependent calcium channels: From structure to function. Rev. Physiol. Biochem. Pharm. 1999;139:33–87. doi: 10.1007/BFb0033648. [DOI] [PubMed] [Google Scholar]
- 17.Davies A., Hendrich J., Van Minh A.T., Wratten J., Douglas L., Dolphin A.C. Functional biology of the α2δ subunits of voltage-gated calcium channels. Trends Pharm. Sci. 2007;28:220–228. doi: 10.1016/j.tips.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 18.Hoppa M.B., Lana B., Margas W., Dolphin A.C., Ryan T.A. α2δ a expression sets presynaptic calcium channel abundance and release probability. Nature. 2012;486:122–125. doi: 10.1038/nature11033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hille B. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci. 1994;17:531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 20.Ikeda S.R., Dunlap K. Voltage-dependent modulation of N-type calcium channels: Role of G protein subunits. Adv. Second Messenger Phosphoprot. Res. 1999;33:131–151. doi: 10.1016/s1040-7952(99)80008-1. [DOI] [PubMed] [Google Scholar]
- 21.Hertlitze S., Garcia D.E., Mackie K., Hille B., Scheuer T., Catterall W.A. Modulation of Ca2+ channels by G-protein βγ subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda S.R. Voltage-dependent modulation of N-type calcium channels by G-protein βγ subunits. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 23.Cantı C., Page K.M., Stephens G.J., Dolphin A.C. Identification of residues in the N terminus of α1B critical for inhibition of the voltage-dependent calcium channel by Gβγ. J. Neurosci. 1999;19:6855–6864. doi: 10.1523/JNEUROSCI.19-16-06855.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zamponi G.W., Bourinet E., Nelson D., Nargeot J., Snutch T.P. Crosstalk between G proteins and protein kinase C mediated by the calcium channel α1 subunit. Nature. 1997;385:442–446. doi: 10.1038/385442a0. [DOI] [PubMed] [Google Scholar]
- 25.Li B., Zhong H., Scheuer T., Catterall W.A. Functional role of a C-terminal G βγ-binding domain of Cav2.2 channels. Mol. Pharm. 2004;66:761–769. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 26.Bean B.P. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989;340:153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- 27.Marchetti C., Carbone E., Lux H.D. Effects of dopamine and noradrenaline on Ca channels of cultured sensory and sympathetic neurons of chick. Pflug. Arch. 1986;406:104–111. doi: 10.1007/BF00586670. [DOI] [PubMed] [Google Scholar]
- 28.Tsunoo A., Yoshii M., Narahashi T. Block of calcium channels by enkephalin and somatostatin in neuroblastoma-glioma hybrid NG108-15 cells. Proc. Natl. Acad. Sci. USA. 1986;83:9832–9836. doi: 10.1073/pnas.83.24.9832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Canti C., Bogdanov Y., Dolphin A.C. Interaction between G proteins and accessory subunits in the regulation of 1B calcium channels in Xenopus oocytes. J. Physiol. 2000;527 Pt. 3:419–432. doi: 10.1111/j.1469-7793.2000.t01-1-00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Feng Z.P., Arnot M.I., Doering C.J., Zamponi G.W. Calcium channel beta subunits differentially regulate the inhibition of N-type channels by individual Gβ isoforms. J. Biol. Chem. 2001;276:45051–45058. doi: 10.1074/jbc.M107784200. [DOI] [PubMed] [Google Scholar]
- 31.Dresviannikov A.V., Page K.M., Leroy J., Pratt W.S., Dolphin A.C. Determinants of the voltage dependence of G protein modulation within calcium channel beta subunits. Pflug. Arch. 2009;457:743–756. doi: 10.1007/s00424-008-0549-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zamponi G.W., Currie K.P. Regulation of CaV2 calcium channels by G protein coupled receptors. Biochim. Biophys. Acta. 2013;1828:1629–1643. doi: 10.1016/j.bbamem.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.García D.E., Li B., García-Ferreiro R.E., Hernández-Ochoa E.O., Yan K., Gautam N., Catterall W.A., Mackie K., Hille B. G-protein beta-subunit specificity in the fast membrane-delimited inhibition of Ca2+ channels. J. Neurosci. 1998;18:9163–9170. doi: 10.1523/JNEUROSCI.18-22-09163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Reyes-Vaca A., de la Cruz L., Garduño J., Arenas I., Garcia D.E. Fast Inactivation of CaV2.2 Channels Is Prevented by the Gβ1 Subunit in Rat Sympathetic Neurons. J. Mol. Neurosci. 2017;63:377–384. doi: 10.1007/s12031-017-0988-8. [DOI] [PubMed] [Google Scholar]
- 35.Hernández-Castellanos J.M., Vivas O., Garduño J., De la Cruz L., Arenas I., Elías-Viñas D., Mackie K., García D.E. Gβ2 mimics activation kinetic slowing of CaV2.2 channels by noradrenaline in rat sympathetic neurons. Biochem. Biophys. Res. Commun. 2014;445:250–254. doi: 10.1016/j.bbrc.2014.01.192. [DOI] [PubMed] [Google Scholar]
- 36.Mahmoud S., Yun J.K., Ruiz-Velasco V. Gβ2 and Gβ4 participate in the opioid and adrenergic receptor-mediated Ca2+ channel modulation in rat sympathetic neurons. J. Physiol. 2012;590:4673–4689. doi: 10.1113/jphysiol.2012.237644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Arnot M.I., Stotz S.C., Jarvis S.E., Zamponi G.W. Differential modulation of N-type 1B and P/Q-type 1A calcium channels by different G protein subunit isoforms. J. Physiol. 2000;527 Pt. 2:203–212. doi: 10.1111/j.1469-7793.2000.00203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stanley E.F., Mirotznik R.R. Cleavage of syntaxin prevents G-protein regulation of presynaptic calcium channels. Nature. 1997;385:340–343. doi: 10.1038/385340a0. [DOI] [PubMed] [Google Scholar]
- 39.Jarvis S.E., Magga J.M., Beedle A.M., Braun J.E., Zamponi G.W. G protein modulation of N-type calcium channels is facilitated by physical interactions between syntaxin 1A and Gβγ. J. Biol. Chem. 2000;275:6388–6394. doi: 10.1074/jbc.275.9.6388. [DOI] [PubMed] [Google Scholar]
- 40.Strock J., Diverse-Pierluissi M.A. Ca2+ channels as integrators of G protein-mediated signaling in neurons. Mol. Pharm. 2004;66:1071–1076. doi: 10.1124/mol.104.002261. [DOI] [PubMed] [Google Scholar]
- 41.Delmas P., Coste B., Gamper N., Shapiro M.S. Phosphoinositide lipid second messengers: New paradigms for calcium channel modulation. Neuron. 2005;47:179–182. doi: 10.1016/j.neuron.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Kubista H., Kosenburger K., Mahlknecht P., Drobny H., Boehm S. Inhibition of transmitter release from rat sympathetic neurons via presynaptic M1 muscarinic acetylcholine receptors. Br. J. Pharm. 2009;156:1342–1352. doi: 10.1111/j.1476-5381.2009.00136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Koushika S.P., Richmond J.E., Hadwiger G., Weimer R.M., Jorgensen E.M., Nonet M.L. A post-docking role for active zone protein Rim. Nat. Neurosci. 2001;4:997–1005. doi: 10.1038/nn732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schoch S., Mittelstaedt T., Kaeser P.S., Padgett D., Feldmann N., Chevaleyre V., Castillo P.E., Hammer R.E., Han W., Schmitz F., et al. Redundant functions of RIM1α and RIM2α in Ca2+-triggered neurotransmitter release. EMBO J. 2006;25:5852–5863. doi: 10.1038/sj.emboj.7601425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gracheva E.O., Hadwiger G., Nonet M.L., Richmond J.E. Direct interactions between C. elegans RAB-3 and Rim provide a mechanism to target vesicles to the presynaptic density. Neurosci. Lett. 2008;444:137–142. doi: 10.1016/j.neulet.2008.08.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kaeser P.S., Deng L., Wang Y., Dulubova I., Liu X., Rizo J., Südhof T.C. RIM proteins tether Ca2+ channels to presynaptic active zones via a direct PDZ-domain interaction. Cell. 2011;144:282–295. doi: 10.1016/j.cell.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Deng L., Kaeser P.S., Xu W., Südhof T.C. RIM proteins activate vesicle priming by reversing autoinhibitory homodimerization of Munc13. Neuron. 2011;69:317–331. doi: 10.1016/j.neuron.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Han Y., Kaeser P.S., Südhof T.C., Schneggenburger R. RIM determines Ca2+ channel density and vesicle docking at the presynaptic active zone. Neuron. 2011;69:304–316. doi: 10.1016/j.neuron.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Castillo P.E., Schoch S., Schmitz F., Südhof T.C., Malenka R.C. RIM1α is required for presynaptic long-term potentiation. Nature. 2002;415:327–330. doi: 10.1038/415327a. [DOI] [PubMed] [Google Scholar]
- 50.Coppola T., Magnin-Lüthi S., Perret-Menoud V., Gattesco S., Schiavo G., Regazzi R. Direct interaction of the Rab3 effector RIM with Ca2+ channels, SNAP-25, and synaptotagmin. J. Biol. Chem. 2001;276:32756–32762. doi: 10.1074/jbc.M100929200. [DOI] [PubMed] [Google Scholar]
- 51.Hibino H., Pironkova R., Onwumere O., Vologodskaia M., Hudspeth A.J., Lesage F. RIM binding proteins (RBPs) couple Rab3-interacting molecules (RIMs) to voltage-gated Ca2+ channels. Neuron. 2002;34:411–423. doi: 10.1016/S0896-6273(02)00667-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Acuna C., Liu X., Gonzalez A., Südhof T.C. RIM-BPs Mediate tight coupling of action potentials to Ca2+-triggered neurotransmitter release. Neuron. 2015;87:1234–1247. doi: 10.1016/j.neuron.2015.08.027. [DOI] [PubMed] [Google Scholar]
- 53.Kiyonaka S., Wakamori M., Miki T., Uriu Y., Nonaka M., Bito H., Beedle A.M., Mori E., Hara Y., De Waard M., et al. RIM1 confers sustained activity and neurotransmitter vesicle anchoring to presynaptic Ca2+ channels. Nat. Neurosci. 2007;10:691–701. doi: 10.1038/nn1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kiyonaka S., Nakajima H., Takada Y., Hida Y., Yoshioka T., Hagiwara A., Kitajima I., Mori Y., Ohtsuka T. Physical and functional interaction of the active zone protein CAST/ERC2 and the β-subunit of the voltage-dependent Ca2+ channel. J. Biochem. 2012;152:149–159. doi: 10.1093/jb/mvs054. [DOI] [PubMed] [Google Scholar]
- 55.Calloway N., Gouzer G., Xue M., Ryan T.A. The active-zone protein Munc13 controls the use-dependence of presynaptic voltage-gated calcium channels. Elife. 2015;4:1–15. doi: 10.7554/eLife.07728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sudhof T.C. The synaptic vesicle cycle. Annu. Rev. Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- 57.Cohen M.W., Jones O.T., Angelides K.J. Distribution of Ca2+ channels on frog motor nerve terminals revealed by fluorescent omega-conotoxin. J. Neurosci. 1991;11:1032–1039. doi: 10.1523/JNEUROSCI.11-04-01032.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Westenbroek R.E., Hell J.W., Warner C., Dubel S.J., Snutch T.P., Catterall W.A. Biochemical properties and subcellular distribution of an N-type calcium channel α1 subunit. Neuron. 1992;9:1099–1115. doi: 10.1016/0896-6273(92)90069-P. [DOI] [PubMed] [Google Scholar]
- 59.Westenbroek R.E., Sakurai T., Elliott E.M., Hell J.W., Starr T.V., Snutch T.P., Catterall W.A. Immunochemical identification and subcellular distribution of the α1A subunits of brain calcium channels. J. Neurosci. 1995;15:6403–6418. doi: 10.1523/JNEUROSCI.15-10-06403.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bennett M.K., Calakos N., Scheller R.H. Syntaxin: A synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- 61.Leveque C., el Far O.U.S.S.A.M.A., Martin-Moutot N., Sato K., Kato R., Takahashi M., Seagar M.J. Purification of the N-type calcium channel associated with syntaxin and synaptotagmin. A complex implicated in synaptic vesicle exocytosis. J. Biol. Chem. 1994;269:6306–6312. [PubMed] [Google Scholar]
- 62.Yoshida A., Oho C., Omori A., Kuwahara R., Ito T., Takahashi M. HPC-1 is associated with synaptotagmin and ω-conotoxin receptor. J. Biol. Chem. 1992;267:24925–24928. [PubMed] [Google Scholar]
- 63.Sheng Z.H., Rettig J., Cook T., Catterall W.A. Calcium-dependent interaction of N-type calcium channels with the synaptic core complex. Nature. 1996;379:451–454. doi: 10.1038/379451a0. [DOI] [PubMed] [Google Scholar]
- 64.Sheng Z.H., Rettig J., Takahashi M., Catterall W.A. Identification of a syntaxin-binding site on N-type calcium channels. Neuron. 1994;13:1303–1313. doi: 10.1016/0896-6273(94)90417-0. [DOI] [PubMed] [Google Scholar]
- 65.Yokoyama C.T., Myers S.J., Fu J., Mockus S.M., Scheuer T., Catterall W.A. Mechanism of SNARE protein binding and regulation of Cav2 channels by phosphorylation of the synaptic protein interaction site. Mol. Cell Neurosci. 2005;28:1–17. doi: 10.1016/j.mcn.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 66.Kim D.K., Catterall W.A. Ca2+-dependent and -independent interactions of the isoforms of the alpha1A subunit of brain Ca2+ channels with presynaptic SNARE proteins. Proc. Natl. Acad. Sci. USA. 1997;94:14782–14786. doi: 10.1073/pnas.94.26.14782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rettig J., Sheng Z.H., Kim D.K., Hodson C.D., Snutch T.P., Catterall W.A. Isoform-specific interaction of the alpha1A subunits of brain Ca2+ channels with the presynaptic proteins syntaxin and SNAP-25. Proc. Natl. Acad. Sci. USA. 1996;93:7363–7368. doi: 10.1073/pnas.93.14.7363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bezprozvanny I., Scheller R.H., Tsien R.W. Functional impact of syntaxin on gating of N-type and Q-type calcium channels. Nature. 1995;378:623–626. doi: 10.1038/378623a0. [DOI] [PubMed] [Google Scholar]
- 69.Wiser O., Bennett M.K., Atlas D. Functional interaction of syntaxin and SNAP-25 with voltage-sensitive L- and N-type Ca2+ channels. Embo J. 1996;15:4100–4110. doi: 10.1002/j.1460-2075.1996.tb00785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhong H., Yokoyama C.T., Scheuer T., Catterall W.A. Reciprocal regulation of P/Q-type Ca2+ channels by SNAP-25, syntaxin and synaptotagmin. Nat. Neurosci. 1999;2:939–941. doi: 10.1038/14721. [DOI] [PubMed] [Google Scholar]
- 71.Jarvis S.E., Zamponi G.W. Distinct molecular determinants govern syntaxin 1A-mediated inactivation and G-protein inhibition of N-type calcium channels. J. Neurosci. 2001;21:2939–2948. doi: 10.1523/JNEUROSCI.21-09-02939.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bezprozvanny I., Zhong P., Scheller R.H., Tsien R.W. Molecular determinants of the functional interaction between syntaxin and N-type Ca2+ channel gating. Proc. Natl. Acad. Sci. USA. 2000;97:13943–13948. doi: 10.1073/pnas.220389697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Geppert M., Goda Y., Hammer R.E., Li C., Rosahl T.W., Stevens C.F., Südhof T.C. Synaptotagmin I: A major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- 74.Xu J., Mashimo T., Sudhof T.C. Synaptotagmin-1, -2, and -9: Ca2+ sensors for fast release that specify distinct presynaptic properties in subsets of neurons. Neuron. 2007;54:567–581. doi: 10.1016/j.neuron.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 75.Sheng Z.H., Yokoyama C.T., Catterall W.A. Interaction of the synprint site of N-type Ca2+ channels with the C2B domain of synaptotagmin I. Proc. Natl. Acad. Sci. USA. 1997;94:5405–5410. doi: 10.1073/pnas.94.10.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wiser O., Tobi D., Trus M., Atlas D. Synaptotagmin restores kinetic properties of a syntaxin-associated N-type voltage sensitive calcium channel. FEBS Lett. 1997;404:203–207. doi: 10.1016/S0014-5793(97)00130-0. [DOI] [PubMed] [Google Scholar]
- 77.Yokoyama C.T., Sheng Z.H., Catterall W.A. Phosphorylation of the synaptic protein interaction site on N-type calcium channels inhibits interactions with SNARE proteins. J. Neurosci. 1997;17:6929–6938. doi: 10.1523/JNEUROSCI.17-18-06929.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.DeMaria C.D., Soong T.W., Alseikhan B.A., Alvania R.S., Yue D.T. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature. 2001;411:484–489. doi: 10.1038/35078091. [DOI] [PubMed] [Google Scholar]
- 79.Lee A., Scheuer T., Catterall W.A. Ca2+/calmodulin-dependent facilitation and inactivation of P/Q-type Ca2+ channels. J. Neurosci. 2000;20:6830–6838. doi: 10.1523/JNEUROSCI.20-18-06830.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Lee A., Wong S.T., Gallagher D., Li B., Storm D.R., Scheuer T., Catterall W.A. Ca2+/calmodulin binds to and modulates P/Q-type calcium channels. Nature. 1999;399:155–159. doi: 10.1038/20194. [DOI] [PubMed] [Google Scholar]
- 81.Lee A., Zhou H., Scheuer T., Catterall W.A. Molecular determinants of Ca2+/calmodulin-dependent regulation of CaV2.1 channels. Proc. Natl. Acad. Sci. USA. 2003;100:16059–16064. doi: 10.1073/pnas.2237000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Few A.P., Lautermilch N.J., Westenbroek R.E., Scheuer T., Catterall W.A. Differential regulation of CaV2.1 channels by calcium-binding protein 1 and visinin-like protein-2 requires N-terminal myristoylation. J. Neurosci. 2005;25:7071–7080. doi: 10.1523/JNEUROSCI.0452-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lautermilch N.J., Few A.P., Scheuer T., Catterall W.A. Modulation of CaV2.1 channels by the neuronal calcium-binding protein visinin-like protein-2. J. Neurosci. 2005;25:7062–7070. doi: 10.1523/JNEUROSCI.0447-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee A., Westenbroek R.E., Haeseleer F., Palczewski K., Scheuer T., Catterall W.A. Differential modulation of CaV2.1 channels by calmodulin and Ca2+-binding protein 1. Nat. Neurosci. 2002;5:210–217. doi: 10.1038/nn805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yan J., Leal K., Magupalli V.G., Nanou E., Martinez G.Q., Scheuer T., Catterall W.A. Modulation of CaV2.1 channels by neuronal calcium sensor-1 induces short-term synaptic facilitation. Mol. Cell Neurosci. 2014;63:124–131. doi: 10.1016/j.mcn.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu H., De Waard M., Scott V.E., Gurnett C.A., Lennon V.A., Campbell K.P. Identification of three subunits of the high affinity omega-conotoxin MVIIC-sensitive Ca2+ channel. J. Biol. Chem. 1996;271:13804–13810. doi: 10.1074/jbc.271.23.13804. [DOI] [PubMed] [Google Scholar]
- 87.Forsythe I.D., Tsujimoto T., Barnes-Davies M., Cuttle M.F., Takahashi T. Inactivation of presynaptic calcium current contributes to synaptic depression at a fast central synapse. Neuron. 1998;20:797–807. doi: 10.1016/S0896-6273(00)81017-X. [DOI] [PubMed] [Google Scholar]
- 88.Xu J., Wu L.G. The decrease in the presynaptic calcium current is a major cause of short-term depression at a calyx-type synapse. Neuron. 2005;46:633–645. doi: 10.1016/j.neuron.2005.03.024. [DOI] [PubMed] [Google Scholar]
- 89.Chaudhuri D., Alseikhan B.A., Chang S.Y., Soong T.W., Yue D.T. Developmental activation of calmodulin-dependent facilitation of cerebellar P-type Ca2+ current. J. Neurosci. 2005;25:8282–8294. doi: 10.1523/JNEUROSCI.2253-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mochida S., Few A.P., Scheuer T., Catterall W.A. Regulation of presynaptic CaV2.1 channels by Ca2+ sensor proteins mediates short-term synaptic plasticity. Neuron. 2008;57:210–216. doi: 10.1016/j.neuron.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 91.Kajikawa Y., Saitoh N., Takahashi T. GTP-binding protein βγ subunits mediate presynaptic calcium current inhibition by GABAB receptor. Proc. Natl. Acad. Sci. USA. 2001;98:8054–8058. doi: 10.1073/pnas.141031298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Takahashi T., Forsythe I.D., Tsujimoto T., Barnes-Davies M., Onodera K. Presynaptic calcium current modulation by a metabotropic glutamate receptor. Science. 1996;274:594–597. doi: 10.1126/science.274.5287.594. [DOI] [PubMed] [Google Scholar]
- 93.Brown S.P., Safo P.K., Regehr W.G. Endocannabinoids inhibit transmission at granule cell to Purkinje cell synapses by modulating three types of presynaptic calcium channels. J. Neurosci. 2004;24:5623–5631. doi: 10.1523/JNEUROSCI.0918-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Brody D.L., Yue D.T. Relief of G-protein inhibition of calcium channels and short-term synaptic facilitation in cultured hippocampal neurons. J. Neurosci. 2000;20:889–898. doi: 10.1523/JNEUROSCI.20-03-00889.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Han J., Mark M.D., Li X., Xie M., Waka S., Rettig J., Herlitze S. RGS2 determines short-term synaptic plasticity in hippocampal neurons by regulating Gi/o-mediated inhibition of presynaptic Ca2+ channels. Neuron. 2006;51:575–586. doi: 10.1016/j.neuron.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 96.Kreitzer A.C., Regehr W.G. Modulation of transmission during trains at a cerebellar synapse. J. Neurosci. 2000;20:1348–1357. doi: 10.1523/JNEUROSCI.20-04-01348.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Stephens G.J., Mochida S. G protein βγ subunits mediate presynaptic inhibition of transmitter release from rat superior cervical ganglion neurones in culture. J. Physiol. 2005;563:765–776. doi: 10.1113/jphysiol.2004.080192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bucci G., Mochida S., Stephens G.J. Inhibition of synaptic transmission and G protein modulation by synthetic CaV2.2 Ca2+ channel peptides. J. Physiol. 2011;589:3085–3101. doi: 10.1113/jphysiol.2010.204735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mochida S., Sheng Z.H., Baker C., Kobayashi H., Catterall W.A. Inhibition of neurotransmission by peptides containing the synaptic protein interaction site of N-type Ca2+ channels. Neuron. 1996;17:781–788. doi: 10.1016/S0896-6273(00)80209-3. [DOI] [PubMed] [Google Scholar]
- 100.Rettig J., Heinemann C., Ashery U., Sheng Z.H., Yokoyama C.T., Catterall W.A., Neher E. Alteration of Ca2+ dependence of neurotransmitter release by disruption of Ca2+ channel/syntaxin interaction. J. Neurosci. 1997;17:6647–6656. doi: 10.1523/JNEUROSCI.17-17-06647.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Inchauspe C.G., Forsythe I.D., Uchitel O.D. Changes in synaptic transmission properties due to the expression of N-type calcium channels at the calyx of Held synapse of mice lacking P/Q-type calcium channels. J. Physiol. 2007;584:835–851. doi: 10.1113/jphysiol.2007.139683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Iwasaki S., Momiyama A., Uchitel O.D., Takahashi T. Developmental changes in calcium channel types mediating central synaptic transmission. J. Neurosci. 2000;20:59–65. doi: 10.1523/JNEUROSCI.20-01-00059.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wu L.G., Borst J.G. The reduced release probability of releasable vesicles during recovery from short-term synaptic depression. Neuron. 1999;23:821–832. doi: 10.1016/S0896-6273(01)80039-8. [DOI] [PubMed] [Google Scholar]
- 104.Wadel K., Neher E., Sakaba T. The coupling between synaptic vesicles and Ca2+ channels determines fast neurotransmitter release. Neuron. 2007;53:563–575. doi: 10.1016/j.neuron.2007.01.021. [DOI] [PubMed] [Google Scholar]
- 105.Wu L.G., Westenbroek R.E., Borst J.G.G., Catterall W.A., Sakmann B. Calcium channel types with distinct presynaptic localization couple differentially to transmitter release in single calyx-type synapses. J. Neurosci. 1999;19:726–736. doi: 10.1523/JNEUROSCI.19-02-00726.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Keith R.K., Poage R.E., Yokoyama C.T., Catterall W.A., Meriney S.D. Bidirectional modulation of transmitter release by calcium channel/syntaxin interactions in vivo. J. Neurosci. 2007;27:265–269. doi: 10.1523/JNEUROSCI.4213-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mochida S., Nonomura Y., Kobayashi H. Analysis of the mechanism for acetylcholine release at the synapse formed between rat sympathetic neurons in culture. Microsc. Res. Tech. 1994;29:94–102. doi: 10.1002/jemt.1070290206. [DOI] [PubMed] [Google Scholar]
- 108.Ma H., Mochida S. A cholinergic model synapse to elucidate protein function at presynaptic terminals. Neurosci. Res. 2007;57:491–498. doi: 10.1016/j.neures.2006.12.015. [DOI] [PubMed] [Google Scholar]
- 109.Mochida S., Saisu H., Kobayashi H., Abe T. Impairment of syntaxin by botulinum neurotoxin C1 or antibodies inhibits acetylcholine release but not Ca2+ channel activity. Neuroscience. 1995;65:905–915. doi: 10.1016/0306-4522(94)00508-3. [DOI] [PubMed] [Google Scholar]
- 110.Mochida S., Westenbroek R.E., Yokoyama C.T., Itoh K., Catterall W.A. Subtype-selective reconstitution of synaptic transmission in sympathetic ganglion neurons by expression of exogenous calcium channels. Proc. Natl. Acad. Sci. USA. 2003;100:2813–2818. doi: 10.1073/pnas.262787299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Voleti R., Tomchick D.R., Südhof T.C., Rizo J. Exceptionally tight membrane-binding may explain the key role of the synaptotagmin-7 C2A domain in asynchronous neurotransmitter release. Proc. Natl. Acad. Sci. USA. 2017;114:E8518–E8527. doi: 10.1073/pnas.1710708114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Voleti R., Tomchick D.R., Südhof T.C., Rizo J. Calmodulin as a direct detector of Ca2+ signals. Nat. Neurosci. 2011;14:301–304. doi: 10.1038/nn.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Leal K., Mochida S., Scheuer T., Catterall W.A. Fine-tuning synaptic plasticity by modulation of CaV2.1 channels with Ca2+ sensor proteins. Proc. Natl. Acad. Sci. USA. 2012;109:17069–17074. doi: 10.1073/pnas.1215172109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Haeseleer F., Sokal I., Verlinde C.L., Erdjument-Bromage H., Tempst P., Pronin A.N., Benovic J.L., Fariss R.N., Palczewski K. Five members of a novel Ca2+-binding protein (CABP) subfamily with similarity to calmodulin. J. Biol. Chem. 2000;275:1247–1260. doi: 10.1074/jbc.275.2.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Burgoyne R.D., Weiss J.L. The neuronal calcium sensor family of Ca2+-binding proteins. Biochem. J. 2001;353:1–12. doi: 10.1042/bj3530001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mikhaylova M., Hradsky J., Kreutz M.R. Between promiscuity and specificity: Novel roles of EF-hand calcium sensors in neuronal Ca2+ signaling. J. Neurochem. 2011;118:695–713. doi: 10.1111/j.1471-4159.2011.07372.x. [DOI] [PubMed] [Google Scholar]
- 117.Abbott L.F., Regehr W.G. Synaptic computation. Nature. 2004;431:796–803. doi: 10.1038/nature03010. [DOI] [PubMed] [Google Scholar]
- 118.Kennedy M.B., Bennett M.K., Bulleit R.F., Erondu N.E., Jennings V.R., Miller S.G., Molloy S.S., Patton B.L., Schenker L.J. Structure and regulation of type II calcium/calmodulin-dependent protein kinase in central nervous system neurons. Cold Spring Harb. Symp. Quant. Biol. 1990;55:101–110. doi: 10.1101/SQB.1990.055.01.013. [DOI] [PubMed] [Google Scholar]
- 119.Lüscher C., Nicoll R.A., Malenka R.C., Muller D. Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat. Neurosci. 2000;3:545–550. doi: 10.1038/75714. [DOI] [PubMed] [Google Scholar]
- 120.Schulman H., Greengard P. Ca2+-dependent protein phosphorylation system in membranes from various tissues, and its activation by “calcium-dependent regulator”. Proc. Natl. Acad. Sci. USA. 1978;75:5432–5436. doi: 10.1073/pnas.75.11.5432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Shepherd J.D., Huganir R.L. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu. Rev. Cell Dev. Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 122.Llinas R., McGuinness T.L., Leonard C.S., Sugimori M., Greengard P. Intraterminal injection of synapsin I or calcium/calmodulin-dependent protein kinase II alters neurotransmitter release at the squid giant synapse. Proc. Natl. Acad. Sci. USA. 1985;82:3035–3039. doi: 10.1073/pnas.82.9.3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Llinas R., Gruner J.A., Sugimori M., McGuinness T.L., Greengard P. Regulation by synapsin I and Ca2+-calmodulin-dependent protein kinase II of the transmitter release in squid giant synapse. J. Physiol. 1991;436:257–282. doi: 10.1113/jphysiol.1991.sp018549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chapman P.F., Frenguelli B.G., Smith A., Chen C.M., Silva A.J. The α-Ca2+/calmodulin kinase II: A bidirectional modulator of presynaptic plasticity. Neuron. 1995;14:591–597. doi: 10.1016/0896-6273(95)90315-1. [DOI] [PubMed] [Google Scholar]
- 125.Lu F.M., Hawkins R.D. Presynaptic and postsynaptic Ca2+ and CamKII contribute to long-term potentiation at synapses between individual CA3 neurons. Proc. Natl. Acad. Sci. USA. 2006;103:4264–4269. doi: 10.1073/pnas.0508162103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jiang X., Lautermilch N.J., Watari H., Westenbroek R.E., Scheuer T., Catterall W.A. Modulation of CaV2.1 channels by Ca2+/calmodulin-dependent protein kinase II bound to the C-terminal domain. Proc. Natl. Acad. Sc. USA. 2008;105:341–346. doi: 10.1073/pnas.0710213105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Magupalli V.G., Mochida S., Yan J., Jiang X., Westenbroek R.E., Nairn A.C., Scheuer T., Catterall W.A. Ca2+-independent activation of Ca2+/calmodulin-dependent protein kinase II bound to the C-terminal domain of CaV2.1 calcium channels. J. Biol. Chem. 2013;288:4637–4648. doi: 10.1074/jbc.M112.369058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chang B.H., Mukherji S., Soderling T.R. Characterization of a calmodulin kinase II inhibitor protein in brain. Proc. Natl. Acad. Sci. USA. 1998;95:10890–10895. doi: 10.1073/pnas.95.18.10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Benfenati F., Valtorta F., Chieregatti E., Greengard P. Interaction of free and synaptic vesicle-bound synapsin I with F-actin. Neuron. 1992;8:377–386. doi: 10.1016/0896-6273(92)90303-U. [DOI] [PubMed] [Google Scholar]
- 130.Zucker R.S., Regehr W.G. Short-term synaptic plasticity. Annu. Rev. Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]
- 131.Inchauspe C.G., Martini F.J., Forsythe I.D., Uchitel O.D. Functional compensation of P/Q by N-type channels blocks short-term plasticity at the calyx of Held presynaptic terminal. J. Neurosci. 2004;24:10379–10383. doi: 10.1523/JNEUROSCI.2104-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ishikawa T., Kaneko M., Shin H.S., Takahashi T. Presynaptic N-type and P/Q-type Ca2+ channels mediating synaptic transmission at the calyx of Held of mice. J. Physiol. 2005;568:199–209. doi: 10.1113/jphysiol.2005.089912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Habets R.L., Borst J.G. Post-tetanic potentiation in the rat calyx of Held synapse. J. Physiol. 2005;564:173–187. doi: 10.1113/jphysiol.2004.079160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Xie M., Li X., Han J., Vogt D.L., Wittemann S., Mark M.D., Herlitze S. Facilitation versus depression in cultured hippocampal neurons determined by targeting of Ca2+ channel Cavbeta4 versus Cavβ2 subunits to synaptic terminals. J. Cell Biol. 2007;178:489–502. doi: 10.1083/jcb.200702072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nanou E., Sullivan J.M., Scheuer T., Catterall W.A. Calcium sensor regulation of the CaV2.1 Ca2+ channel contributes to short-term synaptic plasticity in hippocampal neurons. Proc. Natl. Acad. Sci. USA. 2016;113:1062–1067. doi: 10.1073/pnas.1524636113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nanou E., Scheuer T., Catterall W.A. Calcium sensor regulation of the CaV2.1 Ca2+ channel contributes to long-term potentiation and spatial learning. Proc. Natl. Acad. Sci. USA. 2016;113:13209–13214. doi: 10.1073/pnas.1616206113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Maximov A., Lao Y., Li H., Chen X., Rizo J., Sørensen J.B., Südhof T.C. Genetic analysis of synaptotagmin-7 function in synaptic vesicle exocytosis. Proc. Natl. Acad. Sci. USA. 2008;105:3986–3991. doi: 10.1073/pnas.0712372105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jackman S.L., Turecek J., Belinsky J.E., Regehr W.G. The calcium sensor synaptotagmin 7 is required for synaptic facilitation. Nature. 2016;529:88–91. doi: 10.1038/nature16507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Schwaller B. Cytosolic Ca2+ buffers. Cold Spring Harb. Perspect. Biol. 2010;2:a004051. doi: 10.1101/cshperspect.a004051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Gainey M.A., Feldman D.E. Multiple shared mechanisms for homeostatic plasticity in rodent somatosensory and visual cortex. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2017;372:1–7. doi: 10.1098/rstb.2016.0157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chevaleyre V., Piskorowski R. Modulating excitation through plasticity at inhibitory synapses. Front. Cell Neurosci. 2014;8:1–7. doi: 10.3389/fncel.2014.00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cheron G., Servais L., Dan B. Cerebellar network plasticity: From genes to fast oscillation. Neuroscience. 2008;153:1–19. doi: 10.1016/j.neuroscience.2008.01.074. [DOI] [PubMed] [Google Scholar]
- 143.Lee S.H., Schwaller B., Neher E. Kinetics of Ca2+ binding to parvalbumin in bovine chromaffin cells: Implications for Ca2+ transients of neuronal dendrites. J. Physiol. 2000;525:419–432. doi: 10.1111/j.1469-7793.2000.t01-2-00419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Müller M., Felmy F., Schwaller B., Schneggenburger R. Parvalbumin is a mobile presynaptic Ca2+ buffer in the calyx of Held that accelerates the decay of Ca2+ and short-term facilitation. J. Neurosci. 2007;27:2261–2271. doi: 10.1523/JNEUROSCI.5582-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nägerl U.V., Novo D., Mody I., Vergara J.L. Binding kinetics of calbindin-D(28k) determined by flash photolysis of caged Ca2+ Biophys. J. 2000;79:3009–3018. doi: 10.1016/S0006-3495(00)76537-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nanou E., Catterall W.A. Calcium Channels, Synaptic Plasticity, and Neuropsychiatric Disease. Neuron. 2018;98:466–481. doi: 10.1016/j.neuron.2018.03.017. [DOI] [PubMed] [Google Scholar]