Abstract
Objective
The aim of this systematic review was to investigate the methods used for estimating the population attributable fraction (PAF) to leisure-time physical inactivity (PI) of coronary artery diseases, hypertension and stroke in order to provide the best available estimate for PAF.
Design
Systematic review.
Data sources
Four electronic databases (MEDLINE/PubMed, EMBASE, SPORTDiscus, and Cumulative Index to Nursing and Allied Health Literature) were searched from inception to August 2018.
Eligibility criteria for selecting studies
This review included prospective cohort studies, with men and women aged ≥18 years old, investigating the PAF attributable to leisure-time PI related to coronary artery diseases, hypertension and stroke.
Results
The PAF estimates of the three studies included were 13% (3%–22%) for ‘stage-1 hypertension’ subtype incidence due to ‘non-regular exercise’; 25% (10.4%–35.8%) for ‘stage-2 hypertension’ subtype incidence due to ‘activity of daily living’ and ‘vigorous-intensity sports’; and 8.5% (1.7%–16.7%) for ‘total: fatal and non-fatal’ cardiovascular events of ‘incidence and mortality’ endpoints due to non-accumulation of 550 kcal/week (subsets not specified).
Conclusions
The PAF estimate exhibited a protective dose–response relationship between hypertension and an increased amount of energy expenditure of leisure-time PI. In order to enhance accuracy of PAF estimates, the following steps are recommended: (1) to clearly define and state the working definition of leisure-time PI and dose using a reliable and valid objective measurement tool; (2) use a clear definition of outcome subtypes and endpoints using reliable and valid objective measures; and (3) estimate PAF using modelling techniques based on prospective data and ensuring to report 95% CI.
Keywords: exercise, evaluation, chronic
What is already known?
The population attributable fraction (PAF) is an epidemiological tool widely used to assess public health impact of exposures in population.
Recent literature suggests that prospective cohort studies are preferable to estimate the PAF for common chronic diseases.
What are the new findings?
To date, there is a lack of rigorous studies investigating PAF attributable to leisure-time physical inactivity (PI) in relation to highly prevalent cardiovascular diseases such as coronary artery disease, hypertension and stroke.
There is a methodological heterogeneity estimating the PAF, with a high degree of variability in the PAF estimates due to leisure-time PI across coronary artery disease and hypertension.
It is suggested that while the Levin’s equation, with the application of crude relative risk, might provide an unbiased estimate when there are no confounding factors, the Miettinen’s equation, with the application of adjusted relative risk, may be more suitable to resolve the confounding variables effect but not the interaction between risk factors.
Introduction
Physical inactivity (PI) has been recognised as a global pandemic,1 representing one of the most pressing public health problems of the 21st century.2 3 Further, taking into account recently published data, trends do not indicate the situation into the future. In this regard, by 2030, the average American population is projected to be two times more physically inactive, or sedentary, than when compared with the average American population in 1965. Unfortunately, a similar alarming trend is also being observed globally.4 It is, therefore, reasonable to suggest that PI is one of the main threats to worldwide population health and well-being.5 Physical activity (PA), or exercise, includes different domains, such as leisure-time, sport practice, physically active occupations, housework and transportation.6–8 In addition, the general consensus is to define leisure-time PI as the failure to achieve the following three criteria: (1) to perform between 150 and 300 min/week of PA at moderate intensity (3.0–5.9 metabolic equivalents [METs]); (2) to perform between 75 and 150 min/week of PA at vigorous intensity (≥6 METs); or (3) any combination of energy expenditure (EE) equivalent performing bouts of at least 10 min duration.9 Currently, there is enough evidence-based knowledge that shows that, when following these PA guidelines, there are unquestionable benefits in relation to providing better health outcomes.10 Despite this and the continuing efforts of global institutions promoting PA, approximately 31% of the world population aged ≥15 years old currently do not follow the minimum PA guidelines required for good health.11 In addition, it is important to highlight that leisure-time PI has been identified as an independent risk factor for multiple chronic non-communicable cardiovascular diseases (CVDs).11–15 More specifically, high blood pressure has been identified as one of the main risk factors for CVDs. This particular risk factor linked to other risk factors, such as high cholesterol, overweight or obesity, smoking and PI, have been associated with ~85% of coronary heart disease and ~73% of stroke cases worldwide.14 In this regard, currently more than 50% of worldwide premature deaths are caused by CVDs,14 with the most prevalent non-communicable CVDs of premature deaths worldwide being hypertension (13.5%, 7.6 million), coronary artery disease (13.2%, 7.4 million) and stroke (11.9%, 6.7 million).14 15 It is well known that regular PA is related to lower rates of CVDs especially when guidelines such as those previously mentioned are followed.14 16 For these reasons, this present systematic review has been focused on these three particular CVDs (ie, hypertension, coronary artery disease and stroke).
Given the wide-ranging burden, including on healthcare services, imposed by leisure-time PI, it is essential to establish a metric to accurately estimate leisure-time PI that may lead to specific cardiovascular conditions. The population attributable fraction (PAF) is an epidemiological tool widely used to assess public health impact of exposures in population.17 More specifically, the PAF is an impact measure of disease burden (ie, cardiovascular conditions such as coronary artery disease, hypertension and stroke) attributable to defined risk factors (ie, leisure-time PI).18 Briefly, the PAF integrates the relative risk (RR) of a disease and the prevalence of a risk factor (ie, leisure-time PI) at the population level, and it quantifies how a risk factor contributes to the outcome of interest compared with other risk factors.19 Thus, the PAF holds immense promise as a method for tracking the effectiveness of population-based leisure-time PI interventions. Despite the potential attractiveness of the PAF, from a public health and policy perspective, there are methodological and statistical inconsistencies limiting the usefulness and accuracy of the PAF estimation.20 Recent literature suggests that prospective cohort studies are preferable to estimate the PAF for common chronic conditions.21 22 Additionally, there are two published statistical techniques (piecewise constant hazards model and Cox model) to estimate the PAF from prospective data.23–25 To the authors’ knowledge, the methods (study design, exposure and outcome definitions, measurements and classifications) and the statistics used for estimating the PAF of leading non-communicable CVDs attributable to leisure-time PI have not been systematically explored.
Thus, the aim of this systematic review was to examine the methodology used to estimate the PAF of leading non-communicable CVD outcomes (coronary artery diseases, hypertension and stroke) attributable to leisure-time PI and to establish a tool to provide future methodological recommendations. Examining the most valid and reliable methods and statistics for estimating the PAF is critical for policy makers to identify the burden of non-communicable diseases, resulting from leisure-time PI, so that resource allocation can be improved based on enhanced, informed decisions.
Methods
This present systematic review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement.26
Eligibility criteria
Adult men and women aged ≥18 years old were included in this systematic review. The primary outcome investigated was the PAF attributed to leisure-time PI, which was either self-reported (ie, subjectively) or directly measured by accelerometer (ie, objectively). Studies were excluded if they (1) did not contain a PAF estimate, (2) used an exposure unrelated to leisure-time PI, (3) used an inappropriate study design for estimating the PAF (ie, cross-sectional, case–control or retrospective studies), (4) were not related to coronary artery diseases, hypertension or stroke, (5) were not reported in the English language, and (6) were duplicates.
Search strategy
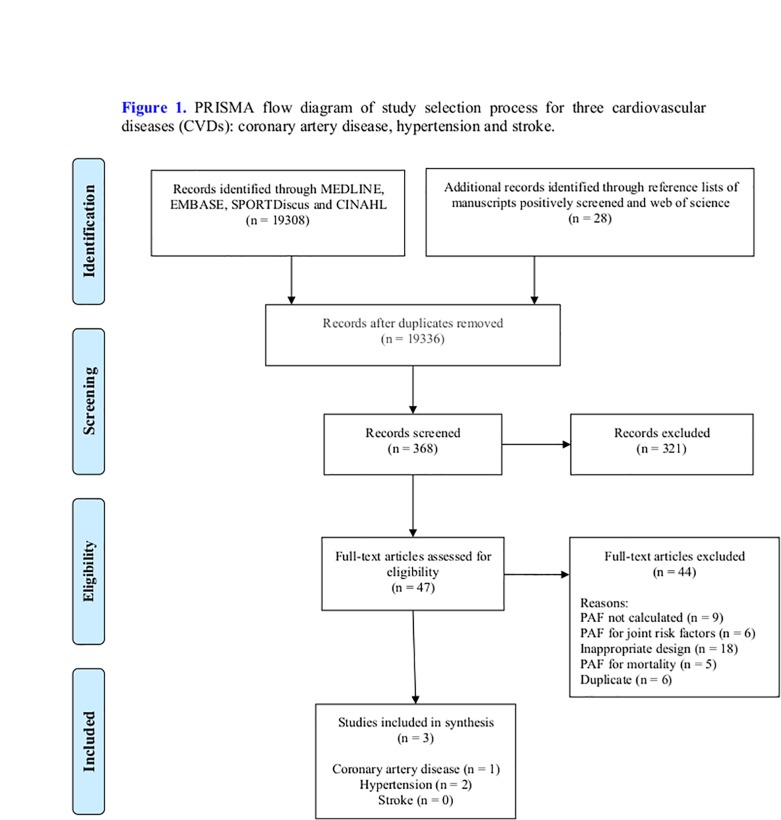
A systematic literature comprehensive search was undertaken using MEDLINE/PubMed, EMBASE, SPORTDiscus, and Cumulative Index to Nursing and Allied Health Literature databases from their inception to August 2018. The different search terms (table 1) were adapted for use with each database. The search strategy keywords related to three components: (1) participants (eg, men and women aged ≥18 years old), and (2) the primary outcome measure PAF attributed to PI in three non-communicable CVDs, as mentioned previously. The unique search restriction was that the studies should be written in English language. Peer-reviewed, published studies estimating the PAF using modelling on raw data using a prospective cohort study design23 were included. The title and abstracts of retrieved studies were independently screened by two reviewers (JCD, HAT) to identify studies that met the eligibility criteria. After this initial screening, the same two reviewers independently assessed the full texts of the included studies, and they manually searched all references of the articles selected for full-text review to identify any additional relevant papers. Disagreements on inclusion of studies were resolved through discussion with a third reviewer (KMK) to reach a consensus. When the required data (eg, operational definition of exercise) were not reported in the original article, such as Suka et al27 study, the authors of this present study emailed the authors of the identified article to acquire further details. At the end of this process, three studies were included in this systematic review (Figure 1).
Table 1.
Search strategy (from inception to 15 August 2018)
| Search ID | Search keywords |
| 1 | Physical activity$.mp. |
| 2 | Physical inactivity.mp. |
| 3 | Fitness.mp. |
| 4 | Physical fitness/ |
| 5 | Sedentary lifestyle/ |
| 6 | Cardiorespiratory fitness.mp. |
| 7 | Motor activity/ |
| 8 | Exp sports/ OR running/ OR jogging/ OR walking/ OR weight lifting/ |
| 9 | Exp exercise/ OR muscle stretching exercises/ OR plyometric exercise/ OR resistance training/ OR physical fitness |
| 10 | *Physical exertion/ |
| 11 | 1 OR 2 OR 3 OR 4 OR 5 OR 6 OR 7 OR 8 OR 9 OR 10 |
| 12 | Population attributable fraction.mp. |
| 13 | Excess risk.mp. |
| 14 | Attributable risk.mp. |
| 15 | Exp risk/ |
| 16 | Population attributable risk.mp. |
| 17 | Exp morbidity/ OR incidence/ OR pevalence/ |
| 18 | Exp risk/ OR logic models/ OR risk assessment/ OR risk factors/ |
| 19 | 12 OR 13 OR 14 OR 15 OR 16 OR 17 OR 18 |
| 20 | Exp cardiovascular diseases/ |
| 21 | Exp heart diseases/ |
| 22 | Exp myocardial infarction/ |
| 23 | Exp death, sudden, cardiac/ |
| 24 | Exp coronary disease/ |
| 25 | Exp coronary artery disease/ |
| 26 | Exp vascular diseases/ |
| 27 | 20 OR 21 OR 22 OR 23 OR 24 OR 25 OR 26 |
| 28 | 11 AND 19 AND 27 |
| 29 | High blood pressure.mp. OR exp hypertension/ |
| 30 | 11 AND 19 AND 29 |
| 31 | Exp stroke/ |
| 32 | Exp cerebrovascular disorders/ |
| 33 | Exp brain ischemia/ |
| 34 | Exp cerebral infarction/ OR exp brain infarction/ |
| 35 | Exp infarction, middle cerebral artery/ |
| 36 | Exp intracranial aneurysm/ |
| 37 | Exp subarachnoid hemorrhage/ |
| 38 | Exp cerebral hemorrhage/ |
| 39 | Exp ischemic attack, transient/ |
| 40 | 31 OR 32 OR 33 OR 34 OR 35 OR 36 OR 37 OR 38 OR 39 |
| 41 | 11 AND 19 AND 40 |
| 42 | Limit S28 to english language |
| 43 | Limit 29 to “all adult (18 plus years)” |
Figure 1.
PRISMA flow diagram of study selection process for three cardiovascular diseases: coronary artery disease, hypertension and stroke. CINAHL, Cumulative Index to Nursing and Allied Health Literature; PAF, population attributable fraction; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Data extraction and analysis
The authors of this present study developed a list of data extraction template, based on the prospective cohort studies guidelines provided by Hammoudeh et al,28 for the studies included in this systematic review. The criteria were developed by an expert in the field after reviewing potentially relevant checklists such as PRISMA26 and Consolidated Standards of Reporting Trials,29 30 due to their best association with journal impact indices.31 These key details were publication details (author’s name, year of publication, country, journal name and study design), outcomes (definition, ascertainment, activity level classification for adjusted relative risk [RRadj], endpoints and subtypes for hypertension, coronary artery disease and stroke), study details (age, total number of participants, study sample, data collection year and number of follow-up years), exposure (definition, measurement, categorisation, per cent of PI domains, subgroups and country), RRadj subgroup (95% CI), level of adjustment for confounders, PI subset-specific %PAF (95% CI, subgroup, country and outcome subtype) and statistics used to estimate the PAF. This information was recorded and listed in table format (tables 2–4), where we characterised the effects of the exposure on patient outcomes providing the conceptual framework and times of latency, and with further information on confounder variables associated with each study.32 All outcomes were extracted, and for those studies where EE was not expressed in kcal/week it was assessed as explained in the Methodological assessment section. In addition, due to the small number of studies included in the review and to their heterogeneous methodology, a meta-analysis was considered inappropriate for this systematic review.
Table 2.
Study characteristics and outcome measured for two cardiovascular conditions (coronary artery diseases and hypertension)
| Publication (author, year, country, journal, study design) |
Outcome (definition, ascertainment, activity level classification for RRadj) |
Exposure (leisure-time PA and PI: definition, measurement, categorisation) |
Study details (age, total number of participants, study sample, data collection year, number of follow-up years) |
Confounders |
| Coronary artery disease | ||||
| Grau et al,45
2010, Spain, Preventive Medicine, prospective cohort study. |
Definition: non-fatal and fatal events (acute myocardial infarction, angina pectoris or death). Ascertainment: non-fatal cardiovascular events ascertained by telephone questionnaire and medical record review, fatal cardiovascular events ascertained and identified from regional and national mortality registers. Activity level classification for RRadj: light-intensity (≤4.0 METs), moderate-intensity (4.5–5.5 METs) and heavy-intensity (≥6.0 METs) leisure-time PA. |
Definition: sedentary (average weekly EE in moderate-intensity to vigorous-intensity leisure-time PA <1000 kcal). Measurement: trained interviewer used validated Spanish version of Minnesota Leisure Time Physical Activity Questionnaire during the previous year. Categorisation: light-intensity (≤4.0 METs), moderate-intensity (4.5–5.5 METs), heavy-intensity (≥6.0 METs) leisure-time PA. |
Pe source: 18–75 years old, n=3734 participants (n=1802 men and n=1932 women), Spain, 1996–2012. RRadj source: 35–74 years old, n=3734 participants (n=1802 men and n=1932 women), prospective cohort study, Spain, 2005, follow-up time: 10 years (average 6.9 years). |
Age, sex, hypertension, LDL cholesterol, HDL cholesterol, diabetes and smoking. |
| Hypertension | ||||
| Paffenbarger et al,44
1983, USA, American Journal of Epidemiology, prospective cohort study. |
Definition: systolic blood pressure >160 mm Hg. Ascertainment: self-reported, physician-diagnosed hypertension. Activity level classification for RRadj: stair-climbing (<50 or >50), block-walking (<5 or >5), leisure-time sport (none, light, moderate and vigorous intensity), PA index (<2000 or >2000 kcal/week). |
Definition: stair-climbing (<50), block-walking (<5) and absence of vigorous-intensity leisure-time sport, PA index (<2000 kcal/week) Measurement: PA index was computed as EE in kcal/week from current self-reported PA levels mainly in three types of activities: stair-climbing (number of steps), block-walking (number of blocks) and leisure-time sports (light, moderate and vigorous intensity). Categorisation: stair-climbing (<50 or >50), block-walking (<5 or >5) and leisure-time sport (none, light, moderate and vigorous intensity), PA index (<2000 or >2000 kcal/week). |
Pe source: 35–74 years old, n=14 998 men, mailed questionnaire, 1962–1972, USA, >70% response rate. RRadj source: 35–74 years old, n=14 998 men, prospective cohort study, USA, 1962–1972, follow-up time: 6–10 years. |
Adjusted for a variety of important confounders or intermediary factors including age, BMI and family history. |
| Suka et al,27
2002, Japan, Environmental Health and Preventive Medicine, prospective cohort study. |
Definition: systolic blood pressure ≥140 mm Hg and/or diastolic blood pressure ≥90 mm Hg. Ascertainment: initiation of antihypertensive therapy or systolic blood pressure ≥140 mm Hg using annual health examination and questionnaire. Activity level classification for RRadj: active (regular exercise) and inactive (no regular exercise). |
Definition: no regular exercise (<20 min, 2 days/week)*. Measurement: annual health questionnaire. Categorisation: active (regular exercise) and inactive (no regular exercise). |
Pe source: 30–59 years old, n=6306 men, annual health questionnaire, 1991–1998, Japan. RRadj source: 30–59 years old, n=6306 men, prospective cohort study, Japanese, 1991–1998, follow-up time: 7 years. |
Age, BMI, blood pressure, glucose intolerance and alcohol intake. |
*Personal communication with the author.
BMI, body mass index; EE, energy expenditure; HDL, high-density lipoprotein; LDL, low-density lipoprotein; METs, metabolic equivalents; PA, physical activity; PI, physical inactivity; Pe, prevalence of exposure; RRadj, adjusted relative risk.
Table 3.
Summary estimate of prevalence of exposure (Pe), adjusted relative risk (RRadj) and the PAF for two cardiovascular conditions (coronary artery diseases and hypertension), and the methods for calculating the PAF attributable to leisure-time PA domain and/or subsets
| Publication (author, year, country, journal, design) |
Outcome (endpoint, subtype) |
Prevalence of exposure (%Pe) |
RRadj
subgroup HR (95% CI) RRadj (95% CI) |
Domain and/or subset-specific %PAF (95% CI) |
PAF (calculation method) |
| Coronary artery disease | |||||
| Grau et al,45
2010, Spain, Preventive Medicine, prospective cohort study. |
Coronary artery disease. Endpoint: incidence and mortality. Subtype: overall acute myocardial infarction, angina pectoris. |
Age group: 35–74 years old 1995: 67.8 2000: 56.0 2005: 50.2 Age group: 35–54 years old 1995: 69.2 2000: 52.9 2005: 47.1 Age group: 55–74 years old 1995: 66.5 2000: 59.5 2005: 53.4 |
Subgroup age: Age group: 35–74 years old HR: 0.91 (0.32 to 2.60) Age group: 35–54 years old HR: 0.95 (0.56 to 1.58) Age group 55–74 years old HR: 0.96 (0.60 to 1.51) |
Moderate-intensity, vigorous-intensity leisure activity (subsets not specified): 35–74 years old: −5 (−63 to 58) 35–54 years old: −7 (−39 to 24) 55–74 years old: −5 (−32 to 22) |
Estimated HR and PI prevalence (Pe) were entered into crude Levin’s equation: Bootstrap method was used to estimate CI. |
| Hypertension | |||||
| Paffenbarger et al,44
1983, USA, American Journal of Epidemiology, prospective cohort study. |
Hypertension. Endpoint: incident. Subtype: stage 2 hypertension. |
Men: 65% | Subgroup sex: Men: 1.52 (1.18 to 1.86) |
Activity of daily living: stair-climbing and block-walking. + Vigorous-intensity sports: men: 25.3 (10.4 to 35.8)* |
Estimated RRadj and PI prevalence (Pe) were entered into crude Levin’s equation: |
| Suka et al,27
2002, Japan, Environmental Health and Preventive Medicine, prospective cohort study |
Hypertension. Endpoint: incident. Subtype: stage 1 hypertension. |
Men. All subjects: 86.5%. Age group: 30–39 years old: 78.7% Age group: 40–49 years old: 86.4% Age group: 50–59 years old: 85.9% |
Subgroup sex-age: Men. All subjects: 1.17 (1.03 to 1.33) Age group: 30–39 years old: 1.68 (0.89 to 3.22) Age group: 40–49 years old: 1.20 (1.04 to 1.38) Age group: 50–59 years old: 0.82 (0.56 to 1.22) |
Exercise subset: ≥20 min, ≥2 days/week (intensity not specified) All subjects: 13 (3 to 22) Age group: 30–39 years old: 37 (0 to 66) Age group: 40–49 years old: 15 (4 to 25) Age group: 50–59 years old: 0 (0 to 16) |
Estimated RRadj and PI prevalence (Pe) were entered into crude Levin’s equation: |
*Substitution method was used to construct 95% CI for the PAF.
PA, physical activity; PAF, population attributable fraction; PI, physical inactivity.
Table 4.
Summary of weekly EE assigned to leisure-time PA (domain and/or subsets) for prevention of two cardiovascular conditions (coronary artery diseases and hypertension) at the population level
| Publication (author, year, country) |
Outcome subtype |
PAF %PAF (95% CI) |
Leisure-time domain (subsets) |
EE (kcal/week) |
Assigned metabolic equivalents (METs) |
| Coronary artery disease | |||||
| Grau et al,45
2010, Spain. |
Acute myocardial infarction and angina pectoris incidence and mortality. | Not significant. | Moderate-intensity, vigorous-intensity leisure activity: subsets not specified. | >1000 | – |
| Hypertension | |||||
| Paffenbarger et al,44
1983, USA. |
Stage 2 hypertension incidence. | All subjects (men): 25.3% (10.4 to 35.8)* | Activity of daily living: stair-climbing and block-walking. + Vigorous-intensity sports. |
>2000 | – |
| Suka et al,27
2002, Japan. |
Stage 1 hypertension incidence. | All subjects (men): 13% (3 to 22) | Exercise subset: regular exercise ≥40 min/week, 20 min/day, 2 days/week (intensity not specified). |
150–300†
>400† |
Moderate intensity (3–6 METs), vigorous intensity (8 METs). |
*Substitution method was used to estimate 95% CI for the PAF.
†EE calculated and rounded37: EE=METs × (t/week) x BM (expressed in kcal/week), where EE is the energy expenditure expressed in kcal/week, t is the exercise duration expressed in hours, and BM is the body mass expressed in kg.
EE, energy expenditure; PA, physical activity; PAF, population attributable fraction.
Methodological assessment
As this present systematic review consists of prospective cohort studies, a published and consensual quality assessment checklist suitable for this study was not available. Thus, Al Tunaiji et al’s20 modified checklist was used to assess the quality of the primary studies included in this systematic review (box 1).
Box 1. Quality assessment (questions) checklist.
Question 1: Was a clear definition provided for the exposure (leisure-time physical inactivity)?
Leisure-time physical inactivity (PI) was defined as failure to meet the following three criteria: (1) to perform between 150 and 300 min/week of physical activity (PA) to moderate intensity (3.0–5.9 metabolic equivalent [METs]) (ie, equivalent to energy expenditure [EE] of 550–1100 kcal/week)9 17 37 39; (2) to perform between 75 and 150 min/week of PA to vigorous intensity (≥6 METs) (ie, equivalent to EE of 550–2200 kcal/week); or (3) any combination of EE equivalent, performing bouts of at least 10 min duration.9 17 57 Exercise and sport are considered subsets of the leisure-time domain.8
Question 2: Was the exposure (leisure-time PI) measured objectively?
Check whether PI was self-reported (ie, subjectively) or directly measured by accelerometer (ie, objectively).
Question 3: Was a clear clinical definition provided for the outcomes (coronary artery disease, hypertension and stroke)?
The primary cardiovascular disease outcomes of interest for the population attributable fraction (PAF) estimates were incidence endpoints45: (1) coronary artery disease is defined as a reduced blood supply to the heart muscle that is either asymptomatic or manifests as angina pectoris or myocardial infarction subtypes; (2) hypertension subtypes, defined as normal blood pressure (systolic <120 mm Hg, diastolic <80 mm Hg), prehypertension (systolic 120–139 mm Hg, diastolic 80–89 mm Hg), stage 1 hypertension (systolic 140–159 mm Hg, diastolic 90–99 mm Hg) and stage 2 hypertension (systolic ≥160 mm Hg, diastolic ≥100 mm Hg)40; and (3) stroke or cerebrovascular disease, which involves an interruption to the blood supply of the brain when the cerebral artery is clogged by a blood clot (ischaemic stroke subtype: 87% of all strokes), a blood clot quickly dislodges (transient ischaemic attack subtype), or a blood clot ruptures (haemorrhagic stroke subtype).11–14
Question 4: Was the outcome ascertained by objective measures or, if self-reported, confirmed by other measures?
(1) Coronary artery disease was ascertained by resting and/or treadmill stress exercise ECG, and (2) hypertension was categorised as normal blood pressure (systolic <120 mm Hg, diastolic <80 mm Hg), prehypertension (systolic 120–139 mm Hg, diastolic 80–89 mm Hg), stage 1 hypertension (systolic 140–159 mm Hg, diastolic 90–99 mm Hg) or stage 2 hypertension (systolic ≥160 mm Hg, diastolic ≥100 mm Hg).40
Question 5: Was the follow-up time provided?
The PAF is subject to follow-up time bias.26 Specifically, a shorter follow-up time is associated with an overestimated PAF, while a longer follow-up time is associated with an underestimated PAF.
Question 6: Was the PAF fully adjusted?
The PAFs are subject to confounding bias.24 A popular method of calculating the PAF is the use of published RRadj and prevalence of exposure in Miettinen’s formula:43 44
This method can yield biased PAF estimates44 because the confounding variables are not adequately adjusted.24 In the full adjustment method, the PAF is calculated from prospective data using modelling techniques (including piecewise constant hazard models or Cox model) that account for known confounders.24
Although this approach has not been standardised yet, it has been published in a peer-reviewed journal.20 The methodological quality assessment of the studies was performed independently by two reviewers (JCD, HAT), and discrepancies were resolved through discussion to achieve consensus; however, failing agreement, a third-party reviewer (KMK) arbitrated. Six items were rated either as ‘yes’ (=1) or ‘no/unable to determine’ (=0). The maximum achievable score was 6, with higher scores indicating better methodological quality of the study. Results were categorised adapting the most used checklist.33–35 Interpretation of results was as follows: ‘strong quality’ (≥4.5) represented the top 75%; ‘moderate quality’ (4.4–3.0) represented 50%–74%; ‘limited quality’ (2.9–1.5) represented 25%–49%; and ‘poor quality’ (<1.5) represented <25%. In addition, to allow integration of activities differing in intensity and duration accumulated over a week, leisure-time PI was expressed as EE, in kcal/week. For studies not reporting accumulated activity expressed as EE, MET values of 3.0–5.9 (‘moderate-intensity’) or ≥6.0 (‘vigorous-intensity’) were assigned,36 taking into account that 1 MET is equal to 1 kcal/kg/hour, and calculated using the following equation37:
| (1) |
where EE is the energy expenditure expressed in kcal/week, t is the exercise duration expressed in hours, and BM is the body mass expressed in kg.
The primary outcome measure statistic, the PAF, was defined as the excess number of cases attributable to leisure-time PI or avoidable by leisure-time PA that is estimated by fully adjusted modelling techniques (such as piecewise constant hazards model or Cox model) from prospective data.38–40 The Miettinen’s equation (equation 2) or one of its variants from published data was used to estimate the PAF.23 41–43
| (2) |
where Pe is the population prevalence of exposure and RRadj is the adjusted relative risk. The 95% CI for the PAF was estimated using the substitution method when these data were not reported.
Results
The search strategy and selection process are summarised in figure 1, with 19 336 records initially identified. A total of three studies published from inception to August 2018 met the criteria and were included in this systematic review, one for coronary artery diseases and two for hypertension. However, none of the stroke studies met the inclusion criteria. The included studies, in the review, were prospective cohort studies exploring the PAF attributable to leisure-time PI. The key study findings are summarised in tables 2–4.
Types of outcome measures used and methodological assessment
Based on the six-item list of criteria, there were distinct variations in quality across studies with respect to defining and measuring leisure-time PI, defining and ascertaining cardiovascular conditions, and adjusting for confounders and length of follow-up time. Both leisure-time PI and cardiovascular conditions were self-reported. None of the included prospective studies used modelling techniques (either piecewise constant hazards model or Cox model) to estimate the PAF. One study of hypertension44 did not report the CI. According to the checklist for quality assessment (box 1), the mean methodological quality score was 4.0 (SD 0.0) out of a total score of 6 or 67%, giving an overall quality score of ‘moderate quality’ (figure 2).
Figure 2.
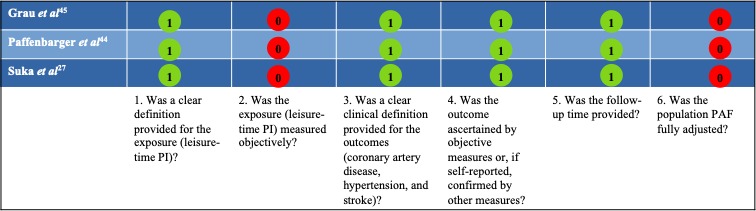
Methodological quality score (yes=1; no/unable to determine=0). PAF, population attributable fraction; PI, physical inactivity.
Study characteristics
A total of 24 948 participants (n=23 016 men, n=1932 women) (range of mean ages 33–69 years) were included in this review. Participant characteristics varied in age, gender, medical history, socioeconomic background, cultures and exposure to leisure-time attributable to leisure-time PI. One of the studies included was related to coronary diseases and two were related to hypertension. In addition, three out of the studies included prospective cohort designs (tables 2–4).
Coronary artery diseases
Grau et al45 estimated the PAF of coronary artery diseases due to lack of participation in moderate-intensity to vigorous-intensity (<1000 kcal/week) leisure-time PI (subset not specified) in Spanish men and women. This was not found to be of significance (negative values) for ‘overall: acute myocardial infarction and angina pectoris’ and cardiovascular ‘incidence and mortality’ endpoints for different age groups. The outcome (overall acute myocardial infarction and angina pectoris) and exposure (leisure-time PI) were both self-reported. The PAF was estimated from a prospective cohort using the crude Levin’s equation (equation 3).46 The HR (95% CI) for age groups ranged from 0.91 (0.32 to 2.60) to 0.96 (0.60 to 1.51), and the prevalence of leisure-time PI for different years and age groups ranged from 47.1% to 69.2% (table 3).
| (3) |
where Pe is the population prevalence of exposure and RRcrude is the crude relative risk.
Hypertension
Suka et al27 estimated the PAF (%PAF [95% CI]) of the ‘stage-1 hypertension’ subtype of incidence endpoints among Japanese men due to the ‘non-regular exercise’ subset of leisure-time domain (intensity not specified, but EE calculated as either <150 kcal/week for moderate intensity or <400 kcal/week for vigorous intensity), which was 13% (3% to 22%) for all age groups, 37% (0% to 66%) for the age group 30–39 years old, and 15% (4% to 25%) for the age group 40–49 years old. The RRadj (95% CI) ranged from 1.17 (1.03 to 1.33) to 1.52 (1.18 to 1.86), and the prevalence of non-exercisers (intensity not specified) ranged from 65% to 86% (table 3). In addition, Paffenbarger et al44 estimated the PAF (%PAF [95% CI]) of the ‘stage-2 hypertension’ subtype of incidence endpoints among US men due to ‘activity in daily living, including stairs climbed and blocks walked’ and ‘vigorous-intensity sports’ subset of leisure-time domain (<2000 kcal/week), which was 25% (10.4% to 35.8%).44 The RRadj (95% CI) was 1.52 (1.18 to 1.86) and the prevalence of PI was 65% (table 3). Both studies27 44 estimated the PAF from prospective cohorts using the crude Levin’s equation (equation 3).46 In both studies, the outcome ‘stage-1 hypertension’ subtype17 and ‘stage-2 hypertension’ subtype,47 and exposure (leisure-time domain based on exercise subset),17 ‘activity of daily living’ and ‘vigorous-intensity sports’ subset of leisure-time domain47 were self-reported.
Discussion
This systematic review evaluated the methods used for estimating the PAF of three leading non-communicable cardiovascular conditions—coronary artery diseases, hypertension and stroke—attributable to leisure-time PI in adult men and women. Four main findings have emerged from this review: (1) there was a lack of rigorous study investigating the PAF attributable to leisure-time PI related to the aforementioned CVDs; (2) there were PAF methodological heterogeneity (conceptual, operational definition of exposure [domain and/or subsets of leisure-time PI]), outcome (subtypes and endpoints of CVDs and hypertension) and statistical levels; (3) there was a large degree of variability in the PAF estimates across the coronary artery disease and hypertension attributable to leisure-time PI; and (4) there was no presence of a standardised quality assessment tool (for prospective cohort study designs) relevant for this systematic review, and as such the authors developed a list of methodological flaws in the PAF estimates to provide an indication of study quality; thus, the validity of these questions remains to be established.
The three studies included in this systematic review demonstrated a large degree of variability in the PAF estimates across the two conditions: coronary artery disease and hypertension due to leisure-time PI. There are two contributing explanations for the observed variability in the PAF estimates: heterogeneous study methods (ie, study design, definition of exposures and outcomes, measurement, and classification differences) and partially adjusted statistical analyses used to estimate the PAF. Specifically, further variation is notable across countries (Spanish, Japanese and US studies/participants) and age. Hereafter, it is elaborated on how two categories related to calculation methods and study methodology contributed to the observed variation in the PAF estimates according to two conditions: coronary artery disease and hypertension. One coronary artery disease study met the inclusion criteria.45 The Grau et al45 study, including male and female participants, showed that the PAF of ‘total: fatal and non-fatal’ cardiovascular events of ‘incidence and mortality’ endpoints due to non-accumulation of <1000 kcal/week from moderate-intensity to vigorous-intensity leisure-time PA domain (subsets not specified) failed to reach a level of significance (negative values). In a non-prospective cohort study, Lee et al48 reported the PAF of ‘total: fatal and non-fatal’ cardiovascular events of ‘incidence and mortality’ endpoints due to non-accumulation of 550 kcal/week from moderate leisure-time PA domain (subsets not specified) for Spanish male participants to be 8.3% (1.7%–16.7%). Different study designs (cohort vs non-cohort) and endpoints (moderate intensity vs moderate intensity to vigorous intensity) used for leisure-time PI most likely played a role in the variation of the PAF estimates.20 49 50
In regard to hypertension, two prospective studies27 44 yielded the best available country-specific PAF estimates for hypertension. Regarding the Suka et al27 study, with Japanese male participants, the PAF of stage 1 hypertension subtype of incidence endpoints among participants due to ‘non-regular exercise’ subset of leisure-time domain (intensity not specified, but EE calculated as either <150 kcal/week of moderate intensity or <400 kcal/week vigorous intensity [ie, 550 kcal/week recommended weekly dose of leisure-time PA]) was 13% (3%–22%) for all age groups, 37% (0%–66%) for the age group 30–39 years old, and 15% (4%–25%) for the age group 40–49 years old. In addition, the Sattelmair et al40 study, on US male participants, showed the PAF estimate of the ‘stage-2 hypertension’ subtype of incidence endpoints among men due to ‘activity of daily living including stairs climbed and blocks walked’ and ‘vigorous-intensity sports’ subset of leisure-time PA domain (<2000 kcal/week [ie, >1100 kcal/week] of the recommended weekly dose of leisure-time PA) at 25% (10.4%–35.8%).
The PAF estimates varied widely from 13% (3%–22%) for non-regular moderate-intensity to vigorous-intensity (550 kcal/week) to 25.3% (10.4%–35.8%) for non-participation in ‘activities of daily living including stairs climbed and blocks walked’ and the ‘vigorous-intensity sports’ subset of the leisure-time PA domain (>1100 kcal/week). This is partially explained by the use of different definitions of leisure-time domain and the subsets included. Therefore, acknowledging the distinction between domains and subsets of leisure-time PI is essential in the interpretation of results. In addition, the use of different definitions and measurement methods to estimate the exposure (leisure-time PI) and outcomes (coronary artery disease and hypertension), and self-reporting of leisure-time PA,51 52 could explain the observed degree of variation in the PAF.38 Self-reporting of leisure-time PI is prone to measurement error (ie, is often an underestimation of leisure-time PI), and consequently the PAF estimates are biased up to 44% (ie, overestimation).52 Additionally, studies in this review used different instruments to measure leisure-time PI, and this could be a further source of variability. Different questionnaires have different properties and concomitant variation in the level of validity and reliability. Furthermore, leisure-time PI was categorised over differing reference time periods (eg, the previous year, last week or a typical week). For example, Suka et al27 dichotomised PI as ‘regular exercise’ and ‘non-regular exercise’, and such customisation of a risk factor might lead to non-differential miss-classification.7 53 Different classification of disease endpoints, particularly when disease subtypes exist within an outcome, could also contribute to the observed variation in the PAF estimates. In the case of coronary heart disease, there is sufficient information to suggest that increasing leisure-time PA reduces the risk incidence, mortality and morbidity.7 However, the relationship between dose and response may be different for incidence, morbidity and mortality for different subtypes of a condition.7 50 The three studies in this systematic review ascertained the outcome of the two conditions (coronary artery disease and hypertension) using self-reported methods. Self-reported outcomes are subject to reporting errors. For example, there is a discrepancy between self-reported and objectively measured prevalence of hypertension (37% vs 64%, respectively)54 and (7% vs 34%, respectively) with low correlation (r=0.17).55 In addition, the asymptomatic nature of most chronic conditions could be problematic. For instance, asymptomatic hypertension might be underestimated with a reporting error biased towards the upper bound.55 This underestimation of incidence can lead to an underestimate of RR and the PAF. Therefore, objective measurement of CVD is desirable for accurate PAF estimates. Finally, regarding stroke diseases, none of the stroke studies met the inclusion criteria.
The PAF estimates at the population level exhibited a dose–response relationship. In the Suka et al27 study, the PAF estimate of the ‘stage-1 hypertension’ subtype among Japanese men due to a ‘non-regular exercise’ subset (<150 kcal/week exercise of moderate intensity or <400 kcal/week of vigorous intensity [ie, not accumulating 550 kcal/week, the lower bound of recommended guidelines]) was 13% (3%–22%). Furthermore, in the Sattelmair et al40 study on US men, the PAF estimate of ‘stage-2 hypertension’ due to ‘activities of daily living including stairs climbed and blocks walked’ and ‘leisure-time subset: vigorous-intensity sports’ (<2000 kcal/week [ie, accumulating >1100 kcal/week, the upper bound of recommended guidelines with additional health benefits]) was 25% (10.4%–35.8%). These PAF estimates suggest that the number of hypertension cases can be reduced by 13% (3%–22%) if the general population is engaged in even light-intensity PA (<550–150 kcal/week). This preventative effect can be increased to 25% (10.4%–35.8%) and even provide additional health benefits if the general population is encouraged to accumulate >1100 kcal/week of equivalent leisure-time PA. The findings of two recent systematic meta-analyses40 56 on RR support this dose–response relationship. Some PA is better than none, and additional benefits are produced as the amount of PA increases for both conditions of CVD (coronary artery disease56 and hypertension57). Interestingly, the best evidence-based method for estimating the PAF is the fully adjusted PAF method using modelling techniques such as piecewise constant hazards model or Cox model.20 Despite scoring high in the quality assessment, none of the included prospective studies for coronary artery disease and hypertension used these modelling techniques. All the three studies included in this review used the crude Levin’s equation (equation 3).46 The results of this systematic review suggested that while the Levin’s equation46 (using the RRcrude) might provide an unbiased estimate when there is no confounding variables,42 43 the Miettinen’s equation (using the RRadj) therefore may resolve the confounding effect but not the interaction between risk factors (ie, the PAF is partially adjusted).42 43
Conclusion
The PAF estimates exhibited a protective dose–response relationship between hypertension and an increased amount of leisure-time PA, expressed as EE (kcal/week), accumulated along a continuum of intensities (light, moderate, moderate-vigorous and vigorous intensity). Country-specific PAF estimates need to be considered and interpreted by domain and/or subsets of leisure-time PI. In order to obtain the most accurate estimate of the PAF attributable to leisure-time PI, the following steps are required: (1) clearly define and state the working definition of leisure-time PI (domain and/or subsets) and dose (duration, frequency with referent time frame and intensity) using a reliable and valid objective measurement tool; (2) use a clear definition of outcome subtypes and endpoints using reliable and valid objective measures; and (3) estimate the PAF using a modelling technique on prospective data and report the 95% CI.
Recommendations
There is an urgent need for prospective longitudinal studies examining the PAF attributable to leisure-time PI in patients with CVD, using standardised methodologies, in order to further increase evidence-based knowledge on what is the most useful methodology to estimate the PAF attributed to leisure-time PI.
Footnotes
Contributors: HAT and JCD searched for relevant literature and wrote the manuscript. HAT, JCD and KMK conceived the study idea. KMK helped with drafting and revisions. KMK has given the final approval of the version to be published. All the authors read and approved the final manuscript.
Funding: This work was supported by the Canadian Institutes of Health Research (CIHR) Emerging Teams grant (KMK) - Mobility in Aging (Institute of Aging). JCD is funded by CIHR and Michael Smith Foundation for Health Research (MSFHR) Postdoctoral Fellowships.
Competing interests: None declared.
Patient consent for publication: Not required.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1. Kohl HW, Craig CL, Lambert EV, et al. . The pandemic of physical inactivity: global action for public health. The Lancet 2012;380:294–305. 10.1016/S0140-6736(12)60898-8 [DOI] [PubMed] [Google Scholar]
- 2. Blair SN. Physical inactivity: the biggest public health problem of the 21st century. Br J Sports Med 2009;43:1–2. [PubMed] [Google Scholar]
- 3. Colditz GA. Economic costs of obesity and inactivity. Med Sci Sports Exerc 1999;31(11 Suppl):S663–7. 10.1097/00005768-199911001-00026 [DOI] [PubMed] [Google Scholar]
- 4. Ding D, Lawson KD, Kolbe-Alexander TL, et al. . The economic burden of physical inactivity: a global analysis of major non-communicable diseases. The Lancet 2016;388:1311–24. 10.1016/S0140-6736(16)30383-X [DOI] [PubMed] [Google Scholar]
- 5. SW N, Popkin BM. Time use and physical activity: a shift away from movement across the globe: declines in movement across the globe. Obes Rev 2012;13:659–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kandula NR, Lauderdale DS, time L. Leisure time, non-leisure time, and occupational physical activity in Asian Americans. Ann Epidemiol 2005;15:257–65. 10.1016/j.annepidem.2004.06.006 [DOI] [PubMed] [Google Scholar]
- 7. Bull FC, Armstrong TP, Dixon T, et al. . Physical inactivity : Lopez AD, Rodgers A, Murray CJL, et al., Comparative quantification of health risks. global and regional burden of disease attributable to selected major risk factors. Geneva: World Health Organization, 2004: 729–882. [Google Scholar]
- 8. Khan KM, Thompson AM, Blair SN, et al. . Sport and exercise as contributors to the health of nations. Lancet 2012;380:59–64. 10.1016/S0140-6736(12)60865-4 [DOI] [PubMed] [Google Scholar]
- 9. Arem H, Moore SC, Patel A, et al. . Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med 2015;175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Füzéki E, Banzer W. Physical activity recommendations for health and beyond in currently inactive populations. Int J Environ Res Public Health 2018;15 10.3390/ijerph15051042. [Epub ahead of print: 22 May 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. World Health Organization Global recommendations on physical activity for health. Geneva: World Health Organization, 2010. [PubMed] [Google Scholar]
- 12. Blair SN, Cheng Y, Holder JS. Is physical activity or physical fitness more important in defining health benefits? Med Sci Sports Exerc 2001;33(6 Suppl):S379–S399. discussion S419-420 10.1097/00005768-200106001-00007 [DOI] [PubMed] [Google Scholar]
- 13. Lee D-C, Pate RR, Lavie CJ, et al. . Leisure-time running reduces all-cause and cardiovascular mortality risk. J Am Coll Cardiol 2014;64:472–81. 10.1016/j.jacc.2014.04.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organization The top 10 causes of death. Available: www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death [Accessed 1 Jul 2018].
- 15. Lawes CMM, Hoorn SV, Rodgers A. Global burden of blood-pressure-related disease, 2001. The Lancet 2008;371:1513–8. 10.1016/S0140-6736(08)60655-8 [DOI] [PubMed] [Google Scholar]
- 16. US Department of Health and Human Services 2008 physical activity guidelines for Americans. Available: http://www.health.gov/paguidelines/pdf/paguide.pdf [Accessed 1 Jul 2018].
- 17. Mansournia MA, Altman DG. Population attributable fraction. BMJ 2018;360 10.1136/bmj.k757 [DOI] [PubMed] [Google Scholar]
- 18. Porta M. A dictionary of epidemiology. Rev Esp Salud Publica 2008;82 10.1590/S1135-57272008000400008 [DOI] [Google Scholar]
- 19. Jenicek M. Epidemiolgy: the logic of modern medicine. Montreal: EPIMED International, 1995. [Google Scholar]
- 20. Al Tunaiji H, Davis JC, Mackey DC, et al. . Population attributable fraction of type 2 diabetes due to physical inactivity in adults: a systematic review. BMC Public Health 2014;14 10.1186/1471-2458-14-469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen YQ, Hu C, Wang Y. Attributable risk function in the proportional hazards model for censored time-to-event. Biostatistics 2006;7:515–29. 10.1093/biostatistics/kxj023 [DOI] [PubMed] [Google Scholar]
- 22. Samuelsen SO, Eide GE. Attributable fractions with survival data. Stat Med 2008;27:1447–67. 10.1002/sim.3022 [DOI] [PubMed] [Google Scholar]
- 23. Benichou J. A review of adjusted estimators of attributable risk. Stat Methods Med Res 2001;10:195–216. 10.1177/096228020101000303 [DOI] [PubMed] [Google Scholar]
- 24. Laaksonen M, Fraction PA. Population attributable fraction (PAF) in epidemiologic follow up studies. Helsinki: National Institute for Health and Welfare, 2010. [Google Scholar]
- 25. Laaksonen MA, Härkänen T, Knekt P, et al. . Estimation of population attributable fraction (PAF) for disease occurrence in a cohort study design. Stat Med 2010;29:860–74. 10.1002/sim.3792 [DOI] [PubMed] [Google Scholar]
- 26. Moher D, Liberati A, Tetzlaff J. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med 2009;151:264–9. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 27. Suka M, Sugimori H, Yoshida K. Preventive strategy for hypertension based on attributable risk measures. Environ Health Prev Med 2002;7:79–81. 10.1007/BF02897334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hammoudeh S, Gadelhaq W, Janahi I. Prospective cohort studies in medical research : Barria RM, Cohort studies in health sciences. Chile: IntechOpen, 2018: 11–28. [Google Scholar]
- 29. Moher D, Hopewell S, Schulz KF, et al. . Consort 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869 10.1136/bmj.c869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schulz KF, Altman DG, Moher D, et al. . Consort 2010 statement: updated guidelines for reporting parallel group randomised trials. Int J Surg 2011;9:672–7. 10.1016/j.ijsu.2011.09.004 [DOI] [PubMed] [Google Scholar]
- 31. Sharp MK, Tokalić R, Gómez G, et al. . A cross-sectional bibliometric study showed suboptimal Journal endorsement rates of STROBE and its extensions. J Clin Epidemiol 2019;107:42–50. 10.1016/j.jclinepi.2018.11.006 [DOI] [PubMed] [Google Scholar]
- 32. Velentgas P, Dreyer NA, Nourjah P, et al. . Developing a protocol for observational comparative effectiveness research: a user’s guide. Rockville. MD: Agency for Healthcare Research and Quality (US), 2013: 1–192. [PubMed] [Google Scholar]
- 33. Downs SH, Black N. The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non-randomised studies of health care interventions. J Epidemiol Community Health 1998;52:377–84. 10.1136/jech.52.6.377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Hartling L, Brison RJ, Crumley ET, et al. . A systematic review of interventions to prevent childhood farm injuries. Pediatrics 2004;114:e483–96. 10.1542/peds.2003-1038-L [DOI] [PubMed] [Google Scholar]
- 35. Hignett S. Systematic review of patient handling activities starting in lying, sitting and standing positions. J Adv Nurs 2003;41:545–52. 10.1046/j.1365-2648.2003.02566.x [DOI] [PubMed] [Google Scholar]
- 36. Ainsworth BE, Haskell WL, Herrmann SD, et al. . Compendium of physical activities: a second update of codes and Met values. Med Sci Sports Exerc 2011;2011:1575–81. [DOI] [PubMed] [Google Scholar]
- 37. Ford ES, Merritt RK, Heath GW, et al. . Physical activity behaviors in lower and higher socioeconomic status populations. Am J Epidemiol 1991;133:1246–56. 10.1093/oxfordjournals.aje.a115836 [DOI] [PubMed] [Google Scholar]
- 38. Bushman BA. Wouldn’t you like to know: how can I use METs to quantify the amount of aerobic exercise? ACSMs Health Fit J 2012;16. [Google Scholar]
- 39. Chobanian AV, Bakris GL, Black HR, et al. . Seventh report of the joint National Committee on prevention, detection, evaluation, and treatment of high blood pressure. Hypertension 2003;42:1206–52. 10.1161/01.HYP.0000107251.49515.c2 [DOI] [PubMed] [Google Scholar]
- 40. Sattelmair J, Pertman J, Ding EL, et al. . Dose response between physical activity and risk of coronary heart disease: a meta-analysis. Circulation 2011;124:789–95. 10.1161/CIRCULATIONAHA.110.010710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Daly LE. Confidence limits made easy: interval estimation using a substitution method. Am J Epidemiol 1998;147:783–90. 10.1093/oxfordjournals.aje.a009523 [DOI] [PubMed] [Google Scholar]
- 42. Hanley JA. A heuristic approach to the formulas for population attributable fraction. J Epidemiol Community Health 2001;55:508–14. 10.1136/jech.55.7.508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rockhill B, Newman B, Weinberg C. Use and misuse of population attributable fractions. Am J Public Health 1998;88:15–19. 10.2105/AJPH.88.1.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Paffenbarger RS, Wing AL, Hyde RT, et al. . Physical activity and incidence of hypertension in college alumni. Am J Epidemiol 1983;117:245–57. 10.1093/oxfordjournals.aje.a113537 [DOI] [PubMed] [Google Scholar]
- 45. Grau M, Subirana I, Elosua R, et al. . Why should population attributable fractions be periodically recalculated? Prev Med 2010;51:78–84. 10.1016/j.ypmed.2010.03.012 [DOI] [PubMed] [Google Scholar]
- 46. Levin ML. The occurrence of lung cancer in man. Acta Unio Int Contra Cancrum 1953;9:531–41. [PubMed] [Google Scholar]
- 47. Vandenbroucke JP, von Elm E, Altman DG, et al. . Strengthening the reporting of observational studies in epidemiology (STROBE): explanation and elaboration. Epidemiology 2007;18:805–35. [DOI] [PubMed] [Google Scholar]
- 48. Lee I-M, Shiroma EJ, Lobelo F, et al. . Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. The Lancet 2012;380:219–29. 10.1016/S0140-6736(12)61031-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Macera CA, Powell KE. Population attributable risk: implications of physical activity dose. Med Sci Sports Exerc 2001;33(6 Suppl):S635–S639. discussion 640-641 10.1097/00005768-200106001-00032 [DOI] [PubMed] [Google Scholar]
- 50. Powell KE, Blair SN. The public health burdens of sedentary living habits: theoretical but realistic estimates. Med Sci Sports Exerc 1994;26:851–6. [PubMed] [Google Scholar]
- 51. Janssen I. Health care costs of physical inactivity in Canadian adults. Appl Physiol Nutr Metab 2012;37:803–6. 10.1139/h2012-061 [DOI] [PubMed] [Google Scholar]
- 52. Prince SA, Adamo KB, Hamel M, et al. . A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int J Behav Nutr Phys Act 2008;5 10.1186/1479-5868-5-56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev 1987;9:1–30. 10.1093/oxfordjournals.epirev.a036298 [DOI] [PubMed] [Google Scholar]
- 54. Mosca I, Bhuachalla BN, Kenny RA. Explaining significant differences in subjective and objective measures of cardiovascular health: evidence for the socioeconomic gradient in a population-based study. BMC Cardiovasc Disord 2013;13 10.1186/1471-2261-13-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Johnston DW, Propper C, Shields MA. Comparing subjective and objective measures of health: evidence from hypertension for the income/health gradient. J Health Econ 2009;28:540–52. 10.1016/j.jhealeco.2009.02.010 [DOI] [PubMed] [Google Scholar]
- 56. Liu X, Zhang D, Liu Y, et al. . Dose-response association between physical activity and incident hypertension: a systematic review and meta-analysis of cohort studies. Hypertension 2017;69:813–20. [DOI] [PubMed] [Google Scholar]
- 57. Remington PL, Brownson RC, Wegner MV. Chronic disease epidemiology and control. 3rd edition Washington, USA: American Public Health Association Publications, 2010. [Google Scholar]