Abstract
Sodium and potassium are two alkali cations abundant in the biosphere. Potassium is essential for plants and its concentration must be maintained at approximately 150 mM in the plant cell cytoplasm including under circumstances where its concentration is much lower in soil. On the other hand, sodium must be extruded from the plant or accumulated either in the vacuole or in specific plant structures. Maintaining a high intracellular K+/Na+ ratio under adverse environmental conditions or in the presence of salt is essential to maintain cellular homeostasis and to avoid toxicity. The baker’s yeast, Saccharomyces cerevisiae, has been used to identify and characterize participants in potassium and sodium homeostasis in plants for many years. Its utility resides in the fact that the electric gradient across the membrane and the vacuoles is similar to plants. Most plant proteins can be expressed in yeast and are functional in this unicellular model system, which allows for productive structure-function studies for ion transporting proteins. Moreover, yeast can also be used as a high-throughput platform for the identification of genes that confer stress tolerance and for the study of protein–protein interactions. In this review, we summarize advances regarding potassium and sodium transport that have been discovered using the yeast model system, the state-of-the-art of the available techniques and the future directions and opportunities in this field.
Keywords: potassium transport, sodium transport, plant ion channels, yeast, functional complementation, protein-protein interaction, heterologous expression
1. Introduction
Potassium (K+) and sodium (Na+) are two nutrients essential for plant life. They are absorbed from the soil through the roots, translocated to the rest of plant organs through movement into the xylem/phloem, to be finally compartmentalized in the organs where they exert their specific functions.
In particular, K+ is a macronutrient involved in fundamental processes required for plant growth and development. It is required for the activity of numerous enzymes involved in the generation of adenosine triphosphate (ATP) and for cellular turgor maintenance [1,2]. It also participates in the regulation of the opening and closing of stomata [3,4], facilitates protein and starch synthesis and is involved in the neutralization of inorganic and organic anions and macromolecules, thus maintaining pH homeostasis and controlling the electrical potential across the plasma membrane [5].
The respiration rate of plants constitutes the determining factor for proper K+ uptake and transport by plants [6,7]. In fact, it has been observed that the ion concentration in the xylem vessels, which is directly determined by the transpiration rate, limits the rate of transfer of K+ from the root cells to the xylem vessels [8]. It has also been observed that low K+ concentrations in the soil during a long growth period will produce plants with a higher transpiration rate and increased stomata frequency per leaf area; while, higher internal K+ is correlated with a lower transpiration rate and fewer stomata per leaf area [6].
Na+ is considered to be a micronutrient since it is not necessary in high amounts for plant growth. It is not required for C3 plants, but is essential for C4 species, where it participates in the carbon cycle, chlorophyll synthesis and photosystem II activity [9]. Nevertheless, Na+ ions become important in small amounts for C3 species when exogenous K+ is present at low concentrations in the soil. It has been shown that Na+ can replace K+-specific functions due to the fact that in their hydrated forms, Na+ and K+ are chemically and structurally very similar [10,11]. On the other hand, K+ homeostasis is severely affected by salt stress and high salt concentrations are toxic for plants. For this reason, plants have developed specific mechanisms to sense, transport and store both K+ and Na+ [12].
In order to avoid and/or limit Na+ toxicity, plants have evolved several uptake systems consisting of multiple channel types able to discriminate and coordinate the influx of these two different cations. For instance, it has been shown that Na+-permeable channels present certain flexibility in their Na+:K+ selectivity depending on the external concentration of salt, cooperating with other inward cation channels at the plasma membrane [10]. Under saline conditions, reducing the activity of these non-selective channels is thought to increase salt tolerance by impeding Na+ accumulation.
In addition, plants have evolved uptake mechanisms able to adapt to large variations in the external concentrations of K+. In 1973, Epstein and collaborators proposed the presence of two mechanisms of K+ uptake that work simultaneously [1]. The first is a high-affinity system, mediated by carriers that are active when K+ concentrations are low (micromolar range), which was subsequently shown to operate through the coupled movement of K+ with other cations, such as H+ or Na+ [13,14]. The second is a low-affinity system that functions as a “passive” transporter, working when K+ concentrations are relatively high (millimolar range).
Since then, many other studies have been published describing the molecular details regarding different K+ channels and transporters, which have been cloned and characterized in several plant species [15,16,17,18,19,20,21]. Most of our current knowledge on the physiological impact of K+ channel activities derives from studies carried out in model plants, such as Arabidopsis thaliana. Other organisms have been successfully used as tools for the functional characterization of these ion transport proteins, such as Xenopus oocytes and the baker’s yeast, Saccharomyces cerevisiae.
The power of the budding yeast, S. cerevisiae in these kinds of studies resides not only in the multiple properties that make it an efficient, easy and attractive system in which to screen and select genes conferring certain phenotypes, but also in the fact that the majority of the subcellular processes governing cellular ion homeostasis in yeast cells are largely conserved in higher eukaryotes. Thus, insights from yeast can be easily translated to other organisms. In addition, S. cerevisiae allows for large-scale, genome-wide analyses in a fast and economically efficient manner. Work with S. cerevisiae allows for the discovery and/or characterization of many aspects of ion transporter function and regulation, but obviously the final physiological proof of yeast-based hypotheses need to be validated in planta. However, yeast remains an incredibly productive system to discover and study a multitude of aspects related plant ion transporter function and provides a platform for the generation of knowledge that can serve as the basis for experimental designs in more complex and time-consuming plant model systems.
For details on the studies of the physiological characterization of plant K+ channels both in vivo and in the Xenopus oocyte model, we refer the reader to other thorough reviews [22,23,24,25]. In this review, we will describe and summarize results obtained using four general experimental approaches employing S. cerevisiae that have been successfully applied to identify and/or characterize plant K+ and Na+ transport proteins and their regulators: Functional complementation using mutants, high-throughput protein–protein interaction assays, reconstitution of functional transport systems and identification of plant genes able to confer salt tolerance upon overexpression.
2. Functional Complementation as an Approach to Identify and Characterize Plant K+/Na+ Channels and Transporters
The functional complementation approach has been extremely successful for the identification and molecular cloning of plant ion channels. In 1992, the first two inward rectifying plant K+ channels (KAT1 and AKT1) were isolated by functional complementation of a yeast mutant devoid of its high affinity K+ transporter genes [15,16]. This seminal work set the paradigm for this experimental approach. Since then, several K+ transporters and regulators have been characterized, not only from plants, but also from mammals, viruses and bacteria [20,21,26,27,28,29,30,31,32,33].
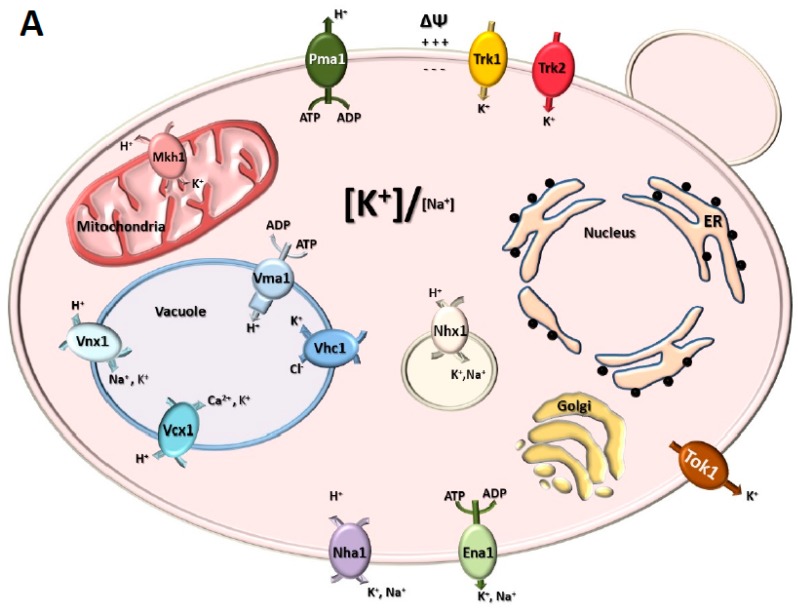
A brief summary of the major contributors to K+ uptake and Na+ extrusion in yeast will be useful for understanding the details of the genetic backgrounds that are exploited in the identification and subsequent functional studies of heterologous ion channels and transporters (Figure 1). For an extended description of the mechanisms and regulation of Na+ and K+ transport and homeostasis in yeast, we refer the reader to a comprehensive review [34].
Figure 1.
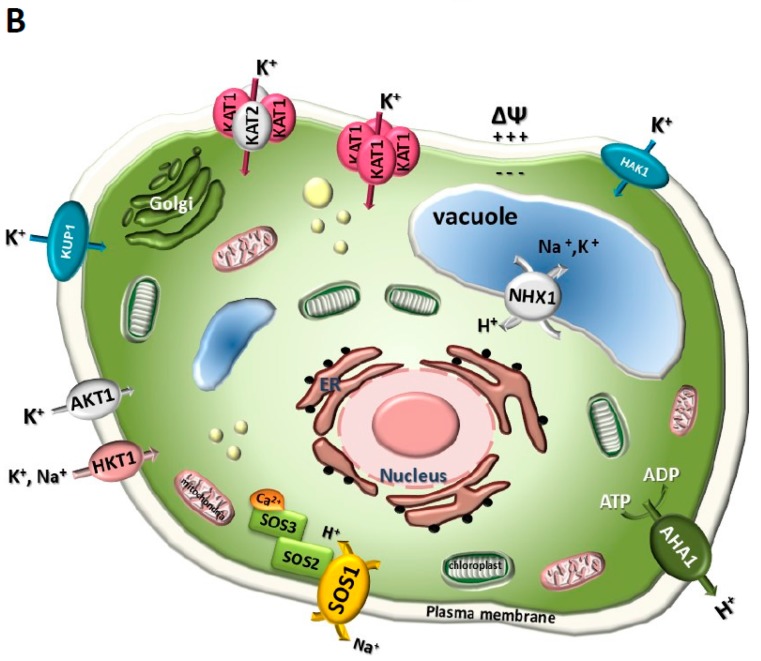
Schematic representation of the main monovalent channels and transporters in yeast and plant cells. (A) In a yeast cell, channels and transporters are present in almost all the organelles and cellular compartments. The introduction of positively charged ions and the expulsion of the negative ones maintains the negative plasma membrane potential. All the ion transporter proteins cited in the main text are represented. Inward/outward ion traffic is represented by arrows. (B) A schematic representation of a plant cell (without the cell wall). The KAT1 channel is represented in the known forms of homo-tetramer and hetero-tetramers with KAT2. All the transporters and channels cited in the text are represented. Organelle size is not to scale.
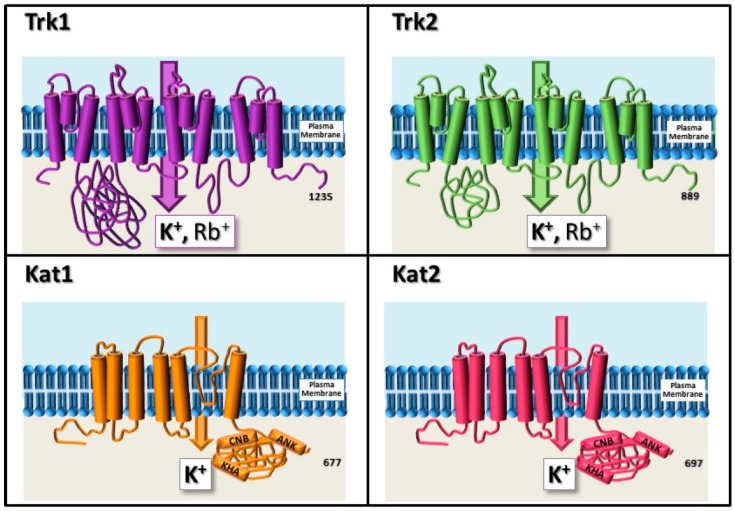
Nutritional uptake of K+ in S. cerevisiae depends mainly on two K+ transporters, named Trk1 and Trk2 [35,36,37]. These transporters use the electrochemical gradient generated by the plasma membrane H+-ATPase encoded by the PMA1 gene to mediate high affinity uptake against the concentration gradient accumulating concentrations of approximately 200 mM in the cytosol even when the external concentration is as low as 10 µM. Trk1 contains 1235 amino acids and has been proposed to contain four repetitions of an M1PM2 motif based on its homology to the KcsA K+ channel from Streptomyces lividans [38]. M1 and M2 are transmembrane segments that are connected by the P helix (Figure 2). Residues in the second transmembrane helix (M2) of the fourth M1PM2 repetition (M2D) have been shown to be crucial for Trk1-mediated K+ transport [39]. Structural prediction models suggest that the Trk1 monomer assembles into a dimer or possibly a tetramer, which would lead to the formation of a “metapore” that could be responsible for Cl− currents that have been observed in electrophysiology experiments [38,40,41]. Trk2 encodes a protein that is 55% identical to Trk1 [37], sharing the same topology, but differing in the length of the second cytosolic segment, which is considerably shorter in Trk2 (Figure 2). Trk1 and Trk2 allow yeast cells to grow under low K+ conditions and low pH. Trk1 is largely responsible for high affinity K+ influx, but is not considered as essential since the trk1 simple mutant and even the trk1 trk2 double mutant can grow in media supplemented with millimolar concentrations of K+. Deletion of the TRK2 gene has little effect on yeast growth on its own but increases by 10-fold the concentration of K+ required for growth of the trk1 trk2 double mutant [37]. Thus, the trk1 trk2 mutant cannot grow in limiting K+ concentrations (below 1 mM). This phenotype can be rescued by the heterologous expression of different types of functional K+ channels, as will be discussed below.
Figure 2.
Schematic representation of selected K+ transporter proteins from yeast and plants. Top row: Yeast Trk1 and Trk2 transporters. The 4 M1PM2 structure is depicted. Bottom row: KAT1 and KAT2 channel monomers. CNB: cyclic nucleotide binding domain; KHA: KHA domain; ANK: Ankyrin repeat.
As previously described, Trk1 and Trk2 are responsible for high affinity K+ uptake. These transporters have been shown to be relatively specific for K+ vs. Na+ ions [42]. However, under conditions of salt stress, Na+ can enter the cell through these transporters and through other non-specific transporters (reviewed in [43]). Under conditions of high external K+ or Na+ concentrations, two major transporters are responsible for the extrusion of these ions across the plasma membrane. The ENA1 gene encodes for a P-type ATPase whose expression is markedly induced upon salt or alkaline stress (reviewed in [44]). ENA transporters are localized to the plasma membrane and use the energy generated from ATP hydrolysis to extrude K+ or Na+ out of the cell [45,46,47]. Most yeast genomes contain three to five tandem copies of the ENA ATPases, thus requiring the deletion of the entire cluster to eliminate this cation extrusion function (for reviews, see [43,48,49]). At acidic pH, a second Na+/K+ extrusion system becomes important for yeast salt tolerance, the Nha1 antiporter. Nha1 is localized at the plasma membrane and acts as a dimeric, electrogenic proton antiporter with similar affinity for both K+ and Na+ [50,51,52]. Therefore, strains lacking the ENA cluster and the NHA1 gene are highly salt sensitive and have been used extensively to identify and characterize plant genes involved in cation extrusion and salt tolerance in general, as will be discussed in the upcoming sections.
Tok1 is the only known outward rectifying K+ channel in yeast [53]. It is a plasma membrane protein and its activity contributes significantly to the maintenance of the membrane potential [54]. However, the tok1 simple mutant does not have obvious growth phenotypes in high or low K+ media, but the mutant is tolerant to killer toxins [55].
Additional K+ transporters are present in the yeast vacuolar membrane. This category of transporters includes Vnx1 and Vhc1. Vnx1 works as an antiporter, exchanging vacuolar protons for cytosolic K+ or Na+. Accordingly, Vnx1 is involved in Na+ compartmentalization and thus, cytosol detoxification [56]. The VHC1 gene encodes for a vacuolar transporter that participates in K+ homeostasis [57]. It functions as a vacuolar K+/Cl− symporter, contributing to the maintenance of intracellular K+ concentrations and vacuole morphology [57,58].
In addition, there are several ion transport proteins in other compartments and organelles. For example, in the endosome membrane of yeast cells, there is a K+/Na+ transporter, Nhx1. It is a H+ antiporter that operates specifically compartmentalizing the cations and extruding H+ to the cytosol. Together with Vnx1, the Nhx1 transporter contributes to the vacuolar and endosomal sequestration of excess cations present in the cytosol, thus maintaining luminal pH of the vacuole and endosomes, respectively [59]. Kha1, works as a K+/H+ antiporter in the Golgi apparatus [60] and Mkh1 constitutes the system of exchanging protons for K+ in the mitochondria. Mkh1 is essential for mitochondrial homeostasis and consequently for respiratory growth of yeast cells [61].
3. Milestones in the Identification of K+ Channels and Transporters in Plants
The trk1 trk2 mutant has been extensively used in several studies involving K+ channel identification, characterization and regulation, not only for plant genes, but also for mammalian and bacterial ion transporters. As mentioned, Trk1 transporters use the electrochemical gradient generated by the Pma1 H+-ATPase to mediate high affinity K+ uptake. In fact, these transporters are the major consumers of this electrochemical gradient [62]. The membrane potential of the trk1 trk2 double mutant is more negative than the wild type strain, presumably because the positive charges in the form of protons extruded by Pma1 are not efficiently replaced by K+ ions via the Trk transporters [63]. Thus, this increased negative potential allows for intracellular K+ accumulation through functional heterologous K+ channels, which can be easily monitored by growth assays in low K+ media.
The identification of new genes involved in K+ uptake (K+ channels and transporters) has been mainly based on yeast mutant complementation assays. Briefly, in this procedure a cDNA library (or a candidate gene) from a specific organism is cloned into a yeast plasmid and subsequently transformed into a yeast strain that can be used to select for the function of the transport protein. Table 1 lists the yeast strains lacking the TRK1 and TRK2 genes that have been used in this type of approach. The identification of positive clones is rapid and with minimal bias. The expression of a functional, heterologous K+ channel rescues the poor growth phenotype of the mutant strain under limiting K+ concentrations. In the specific case of plasma membrane localized transporters, the genetic background preferentially used for the screening is the trk1 trk2 double mutant. This strain has also been employed in other studies, whenever the slow growth phenotype readout in low K+ media is useful.
Table 1.
Yeast stains used for cloning and functional complementation of plant K+ channels. All strains lack the TRK1 and TRK2 gene.
| Mutant Name | Genetic Background | Relevant Genotype |
|---|---|---|
| WΔ3 | W303-1A | MAT a ade2-1 canl-100 trpl-1 ura3-1 trk1::LEU2 trk2::HIS3 |
| CY162 | R757 | MAT a ura3–52 his3Δ200 his44–15 trk1Δtrk2::pCK64 |
| 9.3 | W303-1A | MAT a ena1-4Δ:HIS3::ena4Δ leu2 ura3–1 trp1–1 ade2–1 trk1Δ trk2::pCK64 |
| SGY1528 | W303-1A | MAT a ade2-1 canl-100 his3-11,15 leu2-3,112 trpl-1 ura3-1 trk1::HIS3 trk2::TRP1 |
| BYT12 | BY4741 | MATa his3 Δ1 leu2 Δ0 met15 Δ0 ura3 Δ0 trk1 Δ::loxP trk2 Δ::loxP |
| PLY240 | JRY379 | MAT a his3-Δ200 leu2-3,112 trp1-Δ901 ura3-52 suc2-Δ9 trk1Δ51 trk2Δ50::kanMX |
| PLY246 | JRY379 | MAT a his3-Δ200 leu2-3,112 trp1-Δ901 ura3-52 suc2-Δ9 trk1Δ51 trk2Δ50::kanMX tok1Δ1::HIS3 |
Here, we will describe in more detail a few major examples of plant K+ channels and transporters identified by yeast complementation. As previously mentioned, the first plant K+ channel cloned was KAT1, an inward rectifying channel primarily involved in stomatal opening. KAT1 was cloned by functional complementation of the high affinity K+ transport-defective trk1 trk2 mutant by screening an Arabidopsis cDNA library. The expression of AtKAT1 in the yeast rescued growth on 50 µM KCl, where the mutant normally requires at least 50 mM KCl for growth [64]. Subsequently, the injection of rRNA encoding AtKAT1 into Xenopus oocytes confirmed an inward K+ current, displaying a pattern characteristic of an inward rectifying channel with a high selectivity for K+ ions [27]. KAT1 belongs to the Shaker-family of transporters and resides in the plasma membrane of guard cells. This category of K+-selective channels typically consists of six trans-membrane domains, a cyclic nucleotide binding domain, a KHA domain rich in hydrophobic and acidic residues and some have an ankyrin repeat domain (Figure 2) [4,15,27,65,66,67,68]. Based on the structural data obtained from bacterial and animal Shaker channels, this family has been proposed to adopt a tetrameric pore forming structure, which can be composed of homo or hetero-tetramers [69,70]. In particular, KAT1 is able to form hetero-tetrameric channels at the guard cell plasma membrane with KAT2 [71]. Further characterization of the key KAT1 structural components has been carried out by random mutagenesis and complementation of the yeast trk1 trk2 mutant. These studies helped to define the positions in the pore that are determinant for K+ selectivity [72,73,74,75,76]. KAT1 isoforms from other plant species, such as potato [21], maize [77] and rice [78] have also been identified and functionally characterized.
The AKT1 channel was isolated almost simultaneously to KAT1 [16]. The strategy adopted was the same: Functional complementation screening of an Arabidopsis cDNA library in the trk1 trk2 yeast strain. Neither KAT1 nor AKT1 are homologous to the yeast TRK1 gene product, but both are able to rescue the slow growth phenotype of the mutant under limiting K+ conditions. AKT1 also belongs to the Shaker family and shares sequence homology with KAT1, but it is mainly expressed in roots and has been shown to be largely responsible for K+ uptake from the soil. The complex kinetics of K+ uptake observed in the complemented yeast mutant were in good agreement with those observed for K+ root transporters, corroborating this conclusion [16,79,80,81,82]. The selective pore positions in this channel were later characterized by random mutagenesis and functional complementation in yeast, again using the trk1 trk2 mutant [83]. AKT1 homologues were subsequently isolated from higher plant species, such as barley (HvAKT1), maize (ZMK1), wheat (TaAKT1), potato (SKT1) and tomato (LKT1), and it was demonstrated that the function, and to varying extents, the sequence, are conserved, as well as the plasma membrane localization in root cells. In these cases, the characterization experiments were carried out in other heterologous non-yeast systems [28,84,85,86,87,88].
HAK genes encode for high-affinity K+ transporters expressed in root cells, where they contribute to the K+ uptake from the soil. The first HAKs isolated in plants were obtained from barley root cDNA, using an RT-PCR approach based on amino acid homology between the sequences of two K+ transporters known to belong to the HAK family, Kup of Escherichia coli [89] and HAK1 of Schwanniomyces occidentalis [90]. The characterization of these genes (HvHAK1 and HvHAK2) was carried out in trk1 trk2 yeast mutants, where the authors concluded that only HvHAK1 was able to rescue the growth phenotype, confirming the high affinity K+ transporter function [91].
AtHAK5 shares structural and functional characteristics with AKT1 [13,92]. This gene encodes a transporter cloned from Arabidopsis using degenerate primers deduced from the conserved regions DNG(D/E)GGTFA and FADLGHF, respectively present in the HAK1 sequence previously cloned from Hordeum vulgaris, HvHAK1 [91]. After cloning by RT-PCR, the full-length cDNA was inserted into a yeast expression vector and its functionality was tested by complementation of the growth defect of the trk1 trk2 mutant [93]. Other AtHAK family members have been cloned and tested for yeast complementation, but they do not rescue the growth phenotype of the yeast K+-deficient mutant.
Another category of K+ transporters identified using the yeast model system is represented by the KT/KUP family. They include AtKUP1 [26,94], AtKT2/KUP2 [95] and AtKT3/KUP4 [96], which were isolated by screening an Arabidopsis cDNA library in the trk1 trk2 mutant strain and selecting for growth under low K+ conditions. Analysis of the tissue expression of these transporters revealed that KT/KUP transporters are expressed in roots and in aerial parts of the plant [80].
KUP1 seems to work in both high- and low-affinity modes, which is characteristic of plant root K+ transporters, such as AKT1. The transition from the high-affinity to the low-affinity mode occurs at 100 µM to 200 µM external KCl. Given that K+ uptake via AtKUP1 was inhibited by NaCl and other K+ channel blockers, the authors suggested that KUP1 was possibly participating in K+ uptake from the soil. However, AtKUP1 could not be expressed in Xenopus oocytes, leaving the molecular characterization of the currents mediated by this channel unresolved [94]. In addition, we were not able to find any other study on this transporter in literature, thus the mechanism of K+ uptake by AtKUP1 seems to be still undefined.
AtKT2 mediates low-affinity K+ transport, likely facilitating passive diffusion of K+, while AtKUP4 mediates high-affinity K+ uptake. Recently, it has been reported that KUP4 is also involved in the process of embryo development during seed maturation [97].
High-affinity K+ transporters (HKT) transporters are structurally related to fungal and bacterial K+ transporters from the Trk/Ktr families. Phylogenetic and functional analyses indicate that HKT transporters can be classified into two sub-groups: Group I HKT transporters that are mainly Na+ selective, while group II HKT transporters can operate as Na+-K+ symporters or as K+-selective uniporters. Selective permeability to K+ relies on the glycine residues present in the middle of the pore of HKT/Trk/Ktr transporters. In fact, a substitution of at least one of the conserved glycine residues for a serine abolishes the selectivity to K+ and renders these transporters Na+-selective [98,99]. The HKT proteins are of particular interest to plant biologists, as some members of this class play an important role in salinity tolerance [100]. The first HKT1 gene characterized as a Na+-K+ symporter using the yeast functional complementation strategy in the trk1 trk2 mutant was from wheat [101]. Detailed molecular studies revealed that the transporter presents specific binding sites for each of the ions. In particular, when K+ and Na+ are present at similar concentrations, HKT1 functions as a Na+-coupled K+ transporter. Nevertheless, when Na+ is present at toxic concentrations (millimolar), the ion binds to the high-affinity K+-coupling sites, resulting in a low-affinity Na+-Na+ uptake [102,103,104]. The fact that Na+ competes with K+ when it is present at high concentrations suggests that HKT1 may be one of the Na+ transporters in plant roots, which is relevant for salt toxicity in plants. A genetic selection of mutations of HKT1 revealed some relevant positions conferring salt resistance to yeast and oocytes; for instance, N365S and Q270L are the most effective. The approach used in this study was based on yeast complementation of trk1 trk2 and ena1-4 trk1 trk2 strains [105].
In plants, it was shown that HKT1 is present in the plasma membrane of xylem parenchyma cells and participates in the extraction of Na+ ions from xylem vessels, thus contributing to the salt tolerance response [106]. The majority of the HKT transporters characterized later from other plant species (i.e., eucalyptus, rice, wheat) are all permeable to Na+ as well [107,108,109]. It is interesting to note that not all the HKTs isolated are able to rescue the yeast K+-deficiency phenotype. For instance, two out of the five HKT cDNAs isolated from rice (OsHKT1 and OsHKT4) were confirmed to be Na+-specific by functional complementation of the trk1 trk2 mutant [110].
More recently, an Arabidopsis AKT1-like channel was cloned from Lilium longiflorum pollen (LilKT1). The authors described the identification of this sequence from a pollen cDNA library by PCR amplification using degenerate primers derived from conserved regions of K+-channels. Ion currents are extremely relevant for pollen tube growth and thus for ovule fertilization. LilKT1 was subsequently characterized using the yeast functional complementation assay [111]. LilKT1 was expressed in two different yeast mutant strains lacking the TRK1 and TRK2 genes. Although overexpression of this channel did not fully complement the growth of these mutant strains, the authors demonstrated that this was because the K+ channel was unable to efficiently reach the plasma membrane in this heterologous system. Until the appearance of this report, the only inward rectifying K+ channel characterized in pollen grain was AtAKT6, which was characterized using the classical patch-clamp approach [22,112].
For studies of K+ channels that are not localized at the plasma membrane, alternative yeast mutant strains can also be employed. For example, yeast strains lacking the YVC1 gene have been used to study the function of TPKs (also known as KCO), which are tonoplast K+ channels [113,114]. The TPKs are a two-pore K+-channel family, with four transmembrane and two pore domains. They localize mainly in the vacuolar membrane in yeast and in the tonoplast in plants [115,116], with the exception of AtTPK4, which has been localized in the pollen tube plasma membrane. This channel contributes to K+ conductance, as demonstrated by the complementation of the K+-transport deficient yeast mutant PLY246, carrying trk1, trk2 and tok1 mutations [117]. In the case of the vacuolar channels, to use the yeast functional complementation approach, plant genes are expressed in yvc1 mutants and their activity is analyzed in isolated vacuoles. Using this strategy, the AtTPK1 gene product showed ion channel activity expected for an SV-type channel, with a strong selectivity for K+ over Na+ [113]. In addition, a TPK2-like transporter from Nicotiana tabaccum (NtTPK1), isolated by querying a tobacco database for sequences homologous to the AtTPK-family, was functionally characterized by whole cell patch clamp recordings on isolated vacuolar membranes from the yeast strain SH1006 lacking the YVC1 gene [114].
By using yeast strains defective in nhx1, the A. thaliana NHX1 homologue, AtNHX1, was characterized. The sequence was deduced from homologies with ScNHX1 and cloned using degenerate primers [118]. AtNHX1 complemented the yeast nhx1 mutant as shown by suppression of its extreme sensitivity to hygromycin and NaCl under conditions in which the K+ availability was reduced [119]. Subsequently, the Na+/H+ antiporters AtNHX1/2/5 and AtNHX6 were cloned and have been described as regulators of growth, flower development and reproduction, through the control of vacuolar pH and K+ homeostasis [120,121].
A few other examples of NHX genes identified in higher plants using yeast mutants have been described in the literature. For example, in 2002, based on the sequence homologies of Nhx1 cloned in yeast, rat, human, nematode and Arabidopsis, BvNHX1 (from Beta vulgaris) was cloned [122]. It was able to complement the nhx1 mutation in yeast, recovering the salt sensitive phenotype of the strain. In planta, mRNA transcript and protein levels correlated with an increase in vacuolar Na+/H+ antiporter activity in response to salinity treatment. With the same approach, LeNHX2 was cloned from tomato [18]. The gene was able to rescue the salt and hygromycin sensitivity phenotype of the yeast nhx1 mutant. It was confirmed that LeNHX2 mediates K+/H+ exchange, and to a lesser extent, Na+/H+ exchange in vitro. In addition, the NHX1 protein from Eutrema salsugineum, formerly Thellungiella halophilae, has also been functionally expressed in yeast [123].
The utility of yeast in the study of K+ channels not only includes the discovery of new heterologous K+ channels. In fact, there are examples in which the recovery of the phenotype of a yeast mutant defective in high affinity K+ transport has been used as a “proof of concept” for newly engineered K+ channels. This is the case of BLINK1, a blue light-gated K+ channel engineered by connecting the light sensor module of Avena sativa phototropin LOV2 to the N-terminus of Kcv, the smallest K+ channel known from chlorella virus [124,125]. The authors created a new method to combine a yeast-based screening system with light-activated K+ conductance. Finally, yeast has also been used as a chassis to improve the salt vacuolar accumulation capabilities of NHX1 by directed evolution using random mutagenesis and DNA shuffling [126].
4. High-Throughput and Directed Protein-Protein Interaction Assays Used to Identify Plant K+/Na+ Transporter Regulators
As reviewed in the previous section, yeast represents a powerful tool to study plant ion channels and transporters. Yeast can also be used as a high-throughput platform for the identification and subsequent study of protein–protein interactions (PPIs). Here we describe the state-of-the-art of the available PPI techniques and discuss the K+Na+ transporters/channels interactors identified using these approaches.
5. State-of-the-Art of the Available Techniques for Detecting Protein–Protein Interactions in Yeast
Since the classic yeast two-hybrid (Y2H) assay, new techniques to detect PPIs have been developed. These new techniques solve some of the limitations of the previous ones and offer a broader spectrum of possible baits or proteins that can be analyzed, mainly, according to their topology or subcellular localization. A summary of the important features of these techniques is shown in Table 2.
Table 2.
Techniques for detecting protein–protein interaction in yeast (* Cellular compartment where the interaction takes place).
| Possible Baits | Year | Technique | Response | Cellular Compartment * | References | |
|---|---|---|---|---|---|---|
| Proteins capable of entering nucleus | Non-transactivating proteins | 1989 | Classic Y2H system (Y2H) | Transcriptional activation | Nucleus | [127] |
| 1996 | Reverse Y2H system (rY2H) | Transcriptional activation | Nucleus | [128,129] | ||
| 1996 | Yeast three-hybrid system (Y3H) | Transcriptional activation | Nucleus | [130] | ||
| 1999 | Dual-bait system | Transcriptional activation | Nucleus | [131] | ||
| Transactivating proteins | 2001 | Repressed transactivator system (RTA) | Inhibition of transcriptional activation | Nucleus | [132] | |
| 2001 | RNA polymerase III system (Pol III) | Transcriptional activation | Nucleus | [133] | ||
| Membrane proteins | 1998 | Membrane split-ubiquitin system (MbY2H) | Transcriptional activation | Membrane periphery | [134,135,136] | |
| 2000 | Heterotrimeric G-protein fusion system | Inhibition of protein G signaling | Membrane periphery | [137] | ||
| 2001 | Reverse Ras recruitment system (rRRS) | Ras signaling | Membrane periphery | [138] | ||
| Cytosolic proteins | 1997 | SOS recruitment system (SRS) | Ras signaling | Membrane periphery | [139] | |
| 1998 | Ras recruitment system (RRS) | Ras signaling | Membrane periphery | [140] | ||
| 2007 | Cytosolic split-ubiquitin system (CytoY2H) | Transcriptional activation | Endoplasmic reticulum membrane periphery | [134,141] | ||
| Extracellular and secretory pathway proteins | 1997 | Yeast surface system (YS2H) | Extracellular surface | [142] | ||
| 2003 | SCINEX-P system | Downstream signaling & transcriptional activation | Endoplasmic reticulum | [143] | ||
| 2010 | Golgi Y2H system (GY2H) | Och1 activity | Golgi lumen | [144] | ||
| Nuclear, membrane and cytosolic proteins | 1994 | Generally applicable split-ubiquitin system | Uracil auxotrophy and 5-FOA resistance | Cytosol | [134,145] | |
| 1998 | Split-mDHFR system | DHFR activity | Native compartment | [146] | ||
| 2001 | Split-luciferase system | Luminescent signal | Native compartment | [147] | ||
| 2004 | Split-Trp system | Trp1 activity | Cytosol; Native compartment | [148] | ||
| 2005 | Split-FP system | Fluorescent signal | Native compartment | [149,150] | ||
Basically, all these techniques are different variants of functional reconstitution assays, where a reporter protein is separated into domains or fragments and upon interaction of the test proteins, the function of the reporter protein is reconstituted. The Y2H system has, without doubt, represented a revolution in the field, allowing for the detection of interactions in vivo in a true cellular environment. The classic Y2H technique is currently the most commonly used method for investigating PPIs.
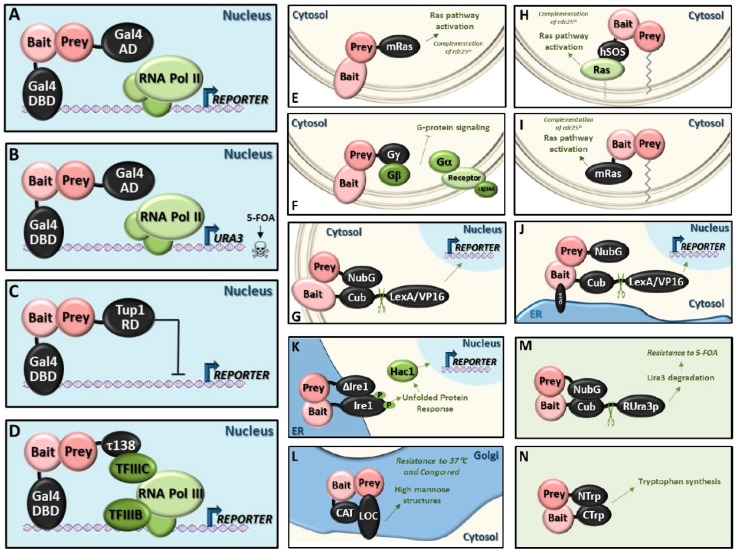
Among the different variants, the ones that are preferentially used in the plant sciences are the classic Y2H and the membrane split-ubiquitin system (reviewed in [151]). Below, we describe briefly selected Y2H variants. More detailed descriptions are provided in several publications [152,153,154]. A schematic diagram of these techniques is shown in Figure 3.
Figure 3.
Diagram of the techniques for detecting protein–protein interactions in yeast. (A) Classic Y2H system. (B) Reverse Y2H system. (C) Repressed transactivator system. (D) RNA polymerase III system (the τ138 subunit is encoded by the TFC3 gene). (E) Reverse Ras recruitment system. (F) Heterotrimeric G-protein fusion system. (G) Membrane split-ubiquitin system. (H) SOS recruitment system. (I) Ras recruitment system. (J) Cytosol split-ubiquitin system. (K) SCINEX-P system. (L) Golgi complex system (CAT: Catalytic domain Och1; LOC: Och1 domain required for membrane attachment). (M) Generally applicable split-ubiquitin system. (N) Split-Trp system. The subcellular location within a yeast cell and the operating mode (at the moment of bait-prey interaction) is represented. See text for more details.
5.1. Classic Y2H System (Y2H)
In this system, one protein is fused to the DNA-binding domain of a transcription factor, while the other protein is fused to a transcriptional activation domain [127]. Interaction between the bait and prey proteins reconstitutes the function of the transcription factor, leading to transcriptional activation of a reporter gene. Interacting proteins are identified by growing the yeast cells under conditions where cell survival is dependent on the transcription of the reporter gene. The most common reporter genes are HIS3 and ADE2, which reconstitute histidine and adenine biosynthesis, respectively. The bacterial lacZ gene is also commonly used as a reporter gene. In this case, upon interaction expression of the lacZ gene leads to the accumulation of the β-galactosidase enzyme that produces a blue product when X-gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) is added to the media.
It is also possible to first transform a set of different bait constructs in one mating type and a set of preys in the other mating type; then, by generating diploids by mating the two haploid strains, large numbers of binary interactions can be evaluated by growing in selective media (reviewed in [152]).
Limitations: Proteins have to enter the nucleus in order to induce the expression of a reporter gene; many false positives and negatives; lack of interaction dynamics and transactivating proteins cannot be properly analyzed. The reasons behind these shortcomings and some minor modifications to solve them have been recently reviewed and some of them will be discussed below [152].
5.2. Reverse Y2H System (rY2H)
This system is based on the classic Y2H assay, but in this case the interaction of two proteins leads to expression of a gene that confers toxicity to the yeast cells. The most commonly used is the URA3 gene that encodes a decarboxylase which produces a toxic intermediate in media containing 5-Fluoroorotic acid (5-FOA) leading to yeast cell death. This assay is used to identify inhibitors of protein–protein interactions or to select for mutants encoding proteins that have lost their ability to interact, thus providing a high-throughput system to identify inhibitors and key residues mediating protein–protein interactions. Removal of 5-FOA converts this assay to classic Y2H (reviewed in [152]).
Two additional systems using CYH2 or GAL1 instead of URA3 plus 5-FOA have been developed (reviewed in [152] and [153]). In the first case, a mutant background containing a cyclohexamide-resistant cyh2 allele is used. The interacting proteins drive the expression of the wild type copy of CYH2, which promotes sensitivity to cycloheximide. In the second system, the screening takes place in a strain that lacks the GAL7 gene, which catalyzes the conversion of galactose-1-phosphate into glucose-1-phosphate. The protein–protein interaction drives the expression of the galactokinase-encoding GAL1 reporter gene. In this system, when the protein–protein interaction occurs, galactose accumulates and reaches toxic concentrations [155].
Application: Detection of inhibitors or essential amino acids for the PPI.
5.3. Repressed Transactivator System (RTA)
The principle is similar to the rY2H system, however, instead of a transcriptional activation domain, the prey protein is fused to a transcriptional repression domain. Interaction of the two proteins leads to repression of the URA3 gene and consequently resistance to media containing 5-FOA [132]. This system was extended to screen for inhibitors of protein–protein interactions or to select for mutants encoding proteins that have lost their ability to interact. In this case, interaction of the two proteins leads to repression of a positive selection marker (reviewed in [154]). For example, upon interaction of bait and prey, the transcriptional repression domain of Tup1 would lead to repression of HIS3 and consequent growth deficiency on medium without histidine.
Advantages: The original version eliminates false positives by proteins that are transcriptional activators. The alternative versions can be used to identify inhibitors or essential amino acids for the PPI.
5.4. RNA Polymerase III System (Pol III)
This technique also derives from the classic Y2H system, however, the second protein is fused to Tfc3 (aka τ138), a subunit of the multimeric protein complex TFIIIC (one of the two transcription factors involved in RNA polymerase III-mediated transcription). After interaction of both proteins, the TFIIIC complex is bound to DNA and recruits a second transcription factor (TFIIIB) and Pol III. This will activate transcription of the reporter gene (reviewed in [153,154]).
Advantages: Elimination of false positives by proteins that are able to autoactivate RNA pol II on their own.
5.5. Small-G-Protein-Based Methods
These methods make use of the Ras pathway, which is essential in yeast. They are based on the reconstitution of the function of the Ras pathway by bringing the Ras-GEF protein, SOS or Ras itself to the membrane for activation.
-
-
SOS recruitment system (SRS): One protein is fused to a modified human Sos protein (hSos), which can functionally replace its yeast counterpart Cdc25, but only if it is targeted to the plasma membrane. The other protein is fused to the C-terminus of the v-Src myristoylation sequence, which targets proteins to the membrane. Interactions between both proteins leads to the recruitment of hSos to the membrane and activation of the yeast Ras pathway that complements the temperature‑sensitive cdc25 mutation at the restrictive temperature (36 °C) [139].
Advantages: Can be applied to cytosolic proteins that are unable to enter the nucleus or that require post-translational modifications in the cytoplasm.
-
-
Ras recruitment system (RRS): The principle is similar to the SRS, however, hSos is substituted by a mutant form of mammalian Ras (mRas). This Ras protein (Ras(61)ΔF), is a constitutively active form of mammalian Ras, which lacks the CAAX box required for its lipid modification and subsequent localization to the plasma membrane [140]. The bait protein is fused to this version of mRas. The prey proteins are fused to a membrane localization sequence. In this way, if the bait and prey proteins interact, the constitutively active form of Ras is recruited to the plasma membrane and can complement the cdc25 temperature-sensitive mutant.
Advantages: Reduction of false positives; furthermore, the smaller size of mRas compared with hSOS reduces the steric hindrance problem observed with the large hSos protein.
-
-
Reverse Ras recruitment system (rRRS): The principle is similar to the RRS, however, mRas is fused to the prey protein and the bait protein contains its own membrane localization signal or is an intrinsic membrane protein [138].
Advantages: Can be applied to membrane proteins.
5.6. Heterotrimeric G-Protein Fusion System
Interaction between integral membrane bait and a soluble prey protein, fused to the γ-subunit (Ste18) of a heterotrimeric G-protein, will sequester the β-subunit, thus disrupting formation of heterotrimeric G-protein complex and subsequent downstream signaling, leading to growth arrest in a pheromone-dependent growth inhibition assay (halo assay) and reduced expression of a pheromone-controlled reporter in a ste18 strain [137].
Advantages: Can be applied to membrane proteins; furthermore, only one of the two proteins is a fusion protein.
5.7. Screening for Interactions Between Extracellular Proteins (SCINEX-P)
The two proteins to be tested are N-terminally fused to the endoplasmic reticulum (ER) transmembrane protein mutants Ire1K702R and Ire1∆tail, respectively. Interaction between the two proteins, leads to dimerization of both Ire1 mutant forms at the ER membrane and functional complementation of the Ire1 dimer, which is the principle mediator of the unfolded protein response (UPR) in yeast. Upon Ire1 activation, the Hac1 transcription factor is activated and, in this system, leads to the transcriptional activation of reporter genes containing Hac1-responsive promoters [143].
Advantages: Can be applied to proteins located in the lumen of the ER, including extracellular and secreted proteins.
5.8. Golgi Y2H System (GY2H)
The two proteins of interest are fused to both modular domains of the Golgi-resident glycosyltransferase Och1: The N-terminal LOC domain for membrane attachment and the C-terminal CAT domain that performs the mannose transfer reaction within the Golgi lumen. This is an essential reaction for the production of the high mannose structure that covers the cell wall of wild-type yeast. The absence of this mannose structure renders yeast cells sensitive to high temperatures and to cell wall damaging agents, such as the benzidine-type dye Congo red. Upon interaction of both proteins, the function of the Och1 protein is reconstituted and the och1 mutant will be able to grow at the non-permissive temperature (37 °C) or in the presence of Congo red [144].
Advantages: Can be applied to proteins located in the Golgi lumen. It has been used to characterize interactions between transactivating transcription factors that cannot be studied using traditional Y2H approaches.
5.9. Dual-Bait System
The principle is similar to the classic Y2H system, however, two known interactors of a protein of interest are each fused to a different DNA-binding domain, each of which targets the promoter of a different reporter, while the (mutated) prey protein is attached to an activation domain [131]. With this system, mutations that specifically target only one interaction, leaving the other interaction intact, can be identified.
Application: Analysis of specific binding sites for proteins with multiple interactors.
5.10. Split-Ubiquitin System
In this system, the protein that is reconstituted is ubiquitin. The carboxy-terminal fragment of ubiquitin (Cub) is fused in frame to a fully functional yeast transcription factor, which is in turn fused to a protein that is excluded from the nucleus (usually an integral membrane protein). The prey protein is fused to a mutated version (I13G) of the N-terminal region of ubiquitin (NubG), which has very low propensity to bind to the Cub fragment. Upon protein–protein interaction, the ubiquitin protein is reconstituted, and ubiquitin-specific proteases cleave the transcription factor from the Cub fusion, which then travels to the nucleus to activate the transcription of reporter genes.
-
-
Membrane split-ubiquitin system (MbY2H): The bait protein needs to be excluded from the nucleus and the topology must be such that the fusions are in the cytosol. This system has been successfully applied to integral membrane proteins. It can also be used for proteins that are resident in other membrane systems or those that have lipid modifications [135].
Advantages: Can be applied to membrane proteins.
Moreover, mating type a and alpha two-hybrid strains have been developed that enable very efficient mating-based screenings using yeast strains already containing high coverage cDNA libraries from different organisms [156]. The prey protein is cloned into the appropriate Cub vector and transformed into the correct mating type, which can then be mated with high efficiency to a strain of the opposite mating type containing Nub-fused cDNA libraries.
-
-
Cytosolic split-ubiquitin system (CytoY2H): The same strategy as that used for the MbY2H system is employed but, in this case, the bait protein does not have to be a membrane-bound protein by itself, because the integral ER membrane protein Ost4 is added at the N-terminal end of the bait to impede its entry into the nucleus (reviewed in [152,153,154]).
Advantages: Can be applied to cytosolic proteins that require post-translational modifications in the cytoplasm.
-
-
Generally applicable split-ubiquitin system: In this case, the transcription factor is replaced by the reporter protein Ura3, with an arginine residue (R-Ura3) between Ura3 and Cub. After interaction of bait and prey, which can reside in membranes or the cytosol, Ura3 is cleaved off and it is quickly degraded due to the exposed N-terminal arginine residue. Consequently, the cells become resistant to 5-FOA. The R-Ura3 method is especially suitable for finding transcription factor partners, both activators and repressors (reviewed in [153]).
Advantages: Careful optimization of 5-FOA levels reduces false discovery rates.
Application: Studies on protein conformations changes. Significant changes in the conformation of a protein can be expected to alter the distance between the N-terminus and the C-terminus of most proteins. By attaching Nub to the N terminus and Cub to the C terminus of a single polypeptide, changes in the time-averaged distance between the N and the C terminus can be measured according to the efficiency of the Nub-Cub reconstitution [157].
5.11. Split-Trp System
The two proteins are fused to the C-terminal (CTrp) and the N-terminal (NTrp) fragments of the Trp1 protein required for tryptophan biosynthesis. Upon interaction, a quasi-native Trp1 protein is reconstituted and allows trp1 deficient yeast strains to grow on medium lacking tryptophan (reviewed in [154]).
Advantages: Can be applied to all types of proteins, independently of their subcellular localization.
5.12. Split-mDHFR System
The two proteins are fused to two dihydrofolate reductase (DHFR) fragments. After interaction of both proteins, DHFR is reconstituted and catalyzes the reduction of dihydrofolate into tetrahydrofolate, which is essential for cell proliferation, growth and survival.
The murine DHFR (mDHFR) can serve as a reporter protein in bacterial and fungal DHFR systems in which a PPI is detected by survival of the cell in the presence of methotrexate or trimethoprim, with mDHFR taking over the function of the host DHFR protein [146].
Advantages: Can be applied to all types of proteins, independently of their subcellular localization.
As reviewed in [152], new challenges are to devise novel yeast systems which can provide real-time data and more reliable outputs. It may be useful to design alternatives that can report PPIs more specifically and sensitively than yeast growth phenotypes, but with the same level of simplicity. In this sense, fluorescence-based systems for high-throughput screening in yeast could be an alternative, although adaptation for single cell detection and automation will be required for these types of techniques to compete with current growth-based selection systems [158].
6. Interactors of K+/Na+ Transporters/Channels Detected Using Protein–Protein Interaction Techniques in Yeast
Many PPIs have been described for K+/Na+ transporters/channels in plants (reviewed in [159]). In this review, we will focus on those detected using the yeast-based methods described above (Table 3).
Table 3.
Interactors of K+/Na+ transporters/channels detected using protein–protein interaction techniques in yeast.
| Na+/K+ Transporter/Channel | Interactors | Technique | References |
|---|---|---|---|
| AKT1 | KAT1, AtKC1 | MbY2H, mating-based | [156] |
| KDC1 | MbY2H | [160] | |
| AKT2 | MRH1/MDIS2 | MbY2H, mating-based | [161] |
| SLAC1 | MbY2H, mating-based | [162] | |
| OsHKT1 | OsCNIH1 | MbY2H, mating-based | [163] |
| KAT1 | KAT1, AKT1, PUP11 | MbY2H, mating-based | [156] |
| SLAC1 | MbY2H, mating-based | [162] | |
| VAMP721 | MbY2H, mating-based | [164] | |
| KAT2 | SLAC1 | MbY2H, mating-based | [162] |
| AtKC1 | AKT1, NRT2.7, ROP1 | MbY2H, mating-based | [156] |
| SLAC1 | MbY2H, mating-based | [162] | |
| SYP121 | MbY2H, mating-based | [165,166] | |
| VAMP721 | MbY2H, mating-based | [164] | |
| KDC1 | AKT1 | MbY2H | [160] |
| KUP6 | SnRK2.6 , SnRK2.2 | MbY2H, mating-based | [167] |
| AKT1 | AIP1, CIPK6, CIPK16 | Y2H | [168] |
| AKT1, AKT2, AtKC1 | Y2H | [169] | |
| CBL10 (CBL5, CBL7) | Y2H | [170] | |
| CIPK23 | Y2H | [171,172,173] | |
| Y2H competition assay | [170] | ||
| AKT1, OsAKT1, PutAKT1 | KPutB1, OsKOB1 | Y2H | [174] |
| AKT2 | AKT1, AKT2, AtKC1 | Y2H | [169] |
| CIPK6 | Y2H | [175] | |
| MRH1/MDIS2 | Y2H Matchmaker Gold | [161] | |
| PP2CA | Y2H | [176] | |
| AKT3 | AtPP2CA | Y2H | [177] |
| GORK | AtPP2CA | Y2H | [178] |
| GORK, SKOR | Y2H | [179] | |
| OsHAK1 | OsRUPO | Y2H | [180] |
| KAT1 | KDC1 | Y2H | [181] |
| VvKAT1 | VvSnRK2.4 | Y2H | [182] |
| OsKAT2 | OsKAT2, OsKAT3 | Y2H | [183] |
| OsKAT3 | OsKAT2, OsKAT3 | Y2H | [183] |
| AtKC1 | AKT1 | Y2H | [169] |
| KDC1 | KAT1 | Y2H | [181] |
| OsKOB1 | AKT1, OsAKT1, PutAKT1 | Y2H | [174] |
| KPutB1 | AKT1, OsAKT1, PutAKT1 | Y2H | [174] |
| KST1 | SKT2, SKT3 | Y2H | [184] |
| SKOR | SKOR, GORK | Y2H | [179] |
| SKT2 | KST1 | Y2H | [184] |
| SKT3 | KST1 | Y2H | [184] |
| TRH1 | TRH1 | Y2H | [185] |
Briefly, interactors have been detected for the three families of genes encoding plant plasma membrane K+ transporters: The HKT family, the HAK/KUP/KT K+ transporter family and the Shaker K+ channel family. Within the Shaker K+ channel family, interactors have been detected for almost all of the members. Specifically, interactors have been detected for HKT1, HAK1, KUP6, AKT1/2/3, KAT1/2 AtKC1, GORK, SKOR, as well as carrot KDC1, potato SKT2/3, OsKAT3 and subunits OsKOB1, KPutB1KST1 and TRH1.
Among the detected interactors of these K+ transporters/channels, several homo- or hetero-oligomerization events have been detected and are summarized in Table 4.
Table 4.
Oligomers of K+/Na+ transporters/channels detected using protein–protein interaction techniques in yeast.
| Oligomer | References | |
|---|---|---|
| AKT1 | AKT1, AKT2 | [169] |
| KAT1 | [156] | |
| AtKC1 | [156,169] | |
| KDC1 | [160] | |
| AKT1, OsAKT1, PutAKT1 | KPutB1, OsKOB1 | [174] |
| AKT2 | AKT1, AKT2 | [169] |
| AtKC1 | [169] | |
| GORK | GORK, SKOR | [179] |
| KAT1 | AKT1 | [156] |
| KAT1 | [156] | |
| KDC1 | [181] | |
| OsKAT2 | OsKAT2, OsKAT3 | [183] |
| OsKAT3 | OsKAT2, OsKAT3 | [183] |
| AtKC1 | AKT1 | [156,169] |
| KDC1 | AKT1 | [160] |
| KAT1 | [181] | |
| OsKOB1 | AKT1, OsAKT1, PutAKT1 | [174] |
| KPutB1 | AKT1, OsAKT1, PutAKT1 | [174] |
| KST1 | SKT2, SKT3 | [184] |
| SKOR | SKOR, GORK | [179] |
| SKT2 | KST1 | [184] |
| SKT3 | KST1 | [184] |
| TRH1 | TRH1 | [185] |
Moreover, putative regulatory proteins such as kinases, phosphatases, GTPases, as well as anion channels, purine and nitrate transporters, targeting-related proteins and calcium sensors have been identified. The detected interactors and their proposed regulatory function are detailed in Table 5.
Table 5.
Regulatory proteins of K+/Na+ transporters/channels detected using protein–protein interaction techniques in yeast. Light gray, kinases and phosphatases; medium gray, channels and transporters; dark gray targeting-related proteins.
| Na+/K+ Transporter/Channel | Regulatory Protein | Na+/K+ Transporter/Channel Regulation | References |
|---|---|---|---|
| AKT1 | AIP1 | Reduces AKT1 activity | [168] |
| CBL10 (CBL5, CBL7) | Impairs AKT1 activity | [170] | |
| CIPK6, CIPK16 | Phosphorylates and activates AKT1 | [168] | |
| CIPK23 | Phosphorylates and activates AKT1 | [170,171,172,173] | |
| AKT2 | CIPK6 | Upon interaction with CIPK6, CBL4 mediates ER-to-PM translocation of AKT2 and enhances AKT2 activity | [175] |
| MRH1/MDIS2 | [161] | ||
| PP2CA | Dephosphorylates and inhibits AKT2, regulated by ABA signaling | [176] | |
| SLAC1 | [162] | ||
| AKT3 | AtPP2CA | [177] | |
| GORK | AtPP2CA | Dephosphorylation-independent inactivation of GORK | [178] |
| OsHAK1 | OsRUPO | Disruption of RUPO leads to K+ over-accumulation in pollen | [180] |
| OsHKT1 | OsCNIH1 | Golgi-localization of OsHKT1 | [163] |
| KAT1 | PUP11 | [156] | |
| SLAC1 | Inhibits KAT1 activity | [162] | |
| VAMP721 | Suppresses KAT1 and KC1 activity | [164] | |
| VvKAT1 | VvSnRK2.4 | [182] | |
| KAT2 | SLAC1 | [162] | |
| AtKC1 | NRT2.7 | K+ is known to increase nitrate (NO3− ) uptake from soil | [156] |
| ROP1 | Actin filament reorganization affects K+ channel activities in stomata. ROP1 regulates pollen tip growth | [156] | |
| SLAC1 | [162] | ||
| SYP121 | Promotes KAT1 activity, in the presence of KC1 | [165,166] | |
| VAMP721 | Suppresses KAT1 and KC1 activity | [164] | |
| KUP6 | SnRK2.6, SnRK2.2 | SnRK2.6 phosphorylates KUP6, regulated by ABA signaling (drought stress) | [167] |
Indeed, at least two classes of kinases have been implicated in KAT1 channel regulation. For example, Open Stomata 1 (OST1, also called SnRK2.6 or SRK2E) is a Snf1-related kinase involved in abscisic acid (ABA) signaling that has been shown to phosphorylate KAT1 leading to channel inhibition in oocytes and yeast model systems [186]. These observations are in agreement with a more recent study showing a physical interaction between Ost1 and KAT1 (but not KAT2) in planta and Ost1-dependent regulation of ABA-mediated Kin currents in Arabidopsis guard cells [187].
Finally, two types of SNARE (soluble N-ethylmaleimide-sensitive factor protein attachment protein receptor) proteins, the Q-SNARE SYP121 and the R-SNARE VAMP721, have been shown to physically interact with KC1 and KAT1 and to modulate channel activity [164,165,166,188,189]. In this case, these proteins have been postulated to directly regulate KAT1 gating in opposing manners (SYP121 activates/VAMP721 inhibits) and, in fact, mutational studies have indicated that the trafficking and gating functions of SYP121 can be physically separated [164,189]. Moreover, primary evidence for the functional coupling of the SNARE SYP121 with Ca2+ channels underscores a mechanism for integration of SNARE- and Ca2+-mediated control of K+ channel currents in guard cells [189]. Furthermore, calcineurin B-like calcium sensors (CBLs) and a target kinase (CBL-interacting protein kinase 23 or CIPK23) appear to phosphorylate and activate AKT1 [168], and CBL4 mediates ER-to-PM translocation of AKT2 and enhances AKT2 activity upon interaction with CIPK6 although in a phosphorylation-independent manner [175].
As shown in Table 3, only classic Y2H and membrane split-ubiquitin techniques have been used as yeast-based techniques for the detection of PPIs of K+/Na+ transporters/channels in plants. Both techniques are limited to proteins capable of entering the nucleus and membrane proteins, respectively. In the future, the use of yeast-based techniques for detection of PPI of other type of proteins, such as those listed in Table 2, may reveal new interactors and expand the knowledge of K+/Na+ transporters/channel structure and their regulation.
7. Reconstitution of Functional Plant Ion Transport Systems in Yeast: The SOS Pathway Paradigm
In previous sections, we have summarized the use of yeast to clone and characterize ion transport proteins and yeast-based protein-protein interaction assays that have been employed to identify regulators of these channels and transporters. Another very powerful approach is the reconstitution of ion transport systems in yeast. In these cases, the transporter alone may have limited function and the co-expression of regulatory proteins is required to observe more robust phenotypes.
One canonical example of the use of yeast as a system to reconstitute a functional ion transport system is the SOS signaling pathway. The Arabidopsis sos (salt overly sensitive) genes were isolated in a genetic screen for plant mutants hypersensitive to NaCl [190]. SOS1 was the first plasma membrane Na+/H+ antiporter described in plants [191]. SOS2 is a Ser/Thr protein kinase, which belongs to the SnRK3 family and contains two functional domains [192]. SOS2 can also be found in the literature as calcineurin B-like protein-interacting protein kinase (CIPKs). SOS3, on the other hand, is a calcium sensor [193]. There is also a SOS4, involved in vitamin B6 metabolism [194] important for root hair development [195] and for vitamin-B6 mediated processes in the chloroplast [196].
Heterologous expression of SOS1-3 in yeast was instrumental in the characterization of the mechanism of action and the interplay among these proteins. SOS3 is a calcium sensor and was the first member characterized of the SCaBPs (SOS3-like calcium-binding protein) family, also named as calcineurin B-like/CBL. SOS3 recruits the SOS2 protein kinase to the plasma membrane upon activation by calcium. SOS2, once activated by SOS3, is able to phosphorylate SOS1 and increase its Na+ transport activity [197].
S. cerevisiae has demonstrated to be a very useful tool to reveal the molecular mechanisms governing the SOS system and their targets and specificities. As explained above, the complete system SOS1-3 can be reconstituted in yeast. SOS1 confers a weak phenotype of Na+ tolerance when expressed in yeast mutants lacking the endogenous Na+ extrusion systems (Ena1-4 and Nha1, see above). Overexpression of SOS2 and SOS3 in the yeast ena1-4 nha1 mutant strain overexpressing SOS1 dramatically rescues the Na+ sensitivity phenotype, while overexpression of SOS2 or SOS3 in yeast in the absence of SOS1 does not confer any phenotype [197]. This explains why the SOS proteins have never been identified in a heterologous expression screening of plant genes in yeast. SOS1 overexpression confers a very weak phenotype and salt tolerance conferred by SOS2 and SOS3 requires SOS1. Nevertheless, yeast is an ideal platform that provides a fast and easy way to test putative SOS proteins. In the Arabidopsis genome, there are nine genes that encode putative SOS3 calcium sensors and 24 that encode putative SOS2 protein kinases [198]. This reconstitution system has facilitated the characterization of some of them as bona fide participants in the Na+ homeostasis machinery and has provided a system to determine their varying degrees of specificity and the combinatorial diversity of the complex. For instance, SOS2 may be activated by SOS3 or by its homologue SCaBP8/CBL10 [199], after the Ca2+-dependent binding to the FISL motif in the SOS2 NAF domain, which probably acts as an autoinhibitory domain. SOS3 is also phosphorylated by SOS2 [200] and this stabilizes the interaction and increases salt tolerance [201]. The SOS2/SOS3 module also activates the antiporter activity of the tonoplast NHX K+-Na+/H+ antiporters [202] and the H+/Ca2+ antiporter CAX1 [203]. The yeast model system also allows the cartography of the functional domains by using deletions or to analyze the effect of post-translational modifications like phosphorylation [204]. Therefore, the yeast reconstitution strategy has been a useful tool to identify and characterize plant proteins, which are upstream regulators of the SOS system, and therefore participate in the molecular response to salt stress [205].
Arabidopsis is the most popular model system for plant molecular biology, but yeast has also been used to characterize SOS proteins from other plants. In Table 6, the SOS orthologues whose function has been shown in yeast are summarized.
Table 6.
SOS genes from various plant species characterized in yeast.
| Plant Species | Genes Characterized | Reference |
|---|---|---|
| Arabidopsis thaliana | SOS1-3 | [197] |
| Chrysanthemum crassum | SOS1 | [206] |
| Cymodocea nodosa | SOS1 | [207] |
| Eutrema salsugineum | SOS1 | [208] |
| Glycine max | SOS1 | [209] |
| Oryza sativa | SOS1-3 | [210] |
| Phragmites australis Trinius | SOS1 | [211] |
| Physcomitrella patens | SOS1 | [212] |
| Populus trichocarpa | SOS1-3 | [213] |
| Schrenkiella parvula | SOS1 | [208] |
| Sesuvium portulacastrum | SOS1 | [214] |
| Solanum lycopersicum | SOS2 | [215] |
| Triticum aestivum | SOS1 | [216] |
| Triticum durum | SOS1 | [217] |
It is also important to note that yeast is an optimal system to study the SOS pathway as the sodium extrusion ability of the SOS system, when heterologously expressed in yeast, is dependent on the proton gradient generated by the yeast proton pump Pma1 [218]. The SOS system would not likely be functional in an animal-based system, given that animal cells use sodium to energize the plasma membrane. In fact, yeast is also a good system to investigate the plant plasma membrane proton ATPase. In plants, the formation of the proton gradient depends on a multigene family [219]. In Arabidopsis, there are three major isoforms (AHA1–3). AHA2 was the first to be functionally expressed in yeast. The full version of the protein was retained in internal membranes and weakly complemented the growth defect of a conditional pma1 mutant, but a truncated version in which the last 94 amino acids of AHA2 were deleted was able to partially localize to the plasma membrane and fully complemented the growth defect, suggesting an autoinhibitory function for this region [220]. The functional evaluation of the three isoforms in yeast indicates that the major differences among them are quantitative rather than qualitative, although the heterologous expression is not as efficient as other plant membrane proteins and most of it is retained in the endoplasmic reticulum [221]. The fact that AHA proteins are properly expressed and are functional in yeast enables the design of mutational approaches in order to identify versions with improved transport coupling efficiency [222].
Another example of reconstitution of a plant signaling pathway is related to ABA signaling. Upon abiotic stress, plants produce ABA. This hormone enters the cell and elicits plant responses by binding to soluble PYRABACTIN RESISTANCE1 (PYR1)/PYR1-LIKE (PYL)/REGULATORY COMPONENTS OF ABA RECEPTORS (RCAR) receptors, a family of proteins with 14 members. PYR/PYL/RCAR receptors perceive ABA intracellularly and, as a result, form ternary complexes and inactivate clade A PP2C phosphatases [223,224]. This activates downstream targets of the PP2Cs, like proteins belonging to the sucrose non-fermenting 1-related protein kinase (SnRK) subfamily 2, which regulate the transcriptional response to ABA and stomatal aperture. Additional targets of clade A PP2Cs include SOS2 members such as SnRK1, SnRK3s/calcineurin B-like (CBL)-interacting protein kinases (CIPKs), calcium-dependent protein kinases (CDPKs/CPKs), ion transporters such as the K+ channel AKT1 and AKT2 or the slow anion channel 1 (SLAC1) and SLAC1 homolog 3 (SLAH3), and transcriptional regulators, such as bZIP transcription factors and chromatin-remodeling complexes [225]. The point is that there is a multiplicity of possible interactions, as not all PYR/PYL/RCAR receptors interact with PP2C phosphatases and not all the interactions are ABA-dependent. In addition, C2-domain abscisic acid-related (CAR) proteins regulate the interaction of the hormone receptors with the plasma membrane [226]. Many of these interactions have been characterized by yeast two-hybrid, and the presence or the absence of the hormone in the yeast growth media can modulate this binding, therefore these ternary complexes (two proteins and ABA) can be reconstituted using yeast [227]. This system has been employed to carry out screenings to identify chemicals that can act as ABA agonists. These novel molecules may induce plant tolerance to abiotic stress by increasing ABA-mediated response [228].
8. Identification of Plant Genes Involved in K+/Na+ Homeostasis by Heterologous Expression
As described above, a classical approach utilizing the yeast model system is to use mutant strains deficient in potassium uptake or in sodium extrusion and introducing plant cDNAs to complement a given phenotype, a strategy which is still in use [229]. This strategy is a way to identify transporters, to confirm their function and/or to perform molecular characterization by carrying out structure-function studies. This is possible because in yeast the quantification of the transport activity can be easily assayed by growth tests or defined in more detail by measuring ion transport and ion content. Another highly exploited approach is the use of yeast as a heterologous system to screen for plant genes involved in potassium and sodium homeostasis by screening for tolerance to salt stress induced by NaCl. The predecessor that set the paradigm for this strategy was a very successful screen carried out in the Serrano laboratory. In this case, instead of plant genes, yeast halotolerant (HAL) genes were identified by overexpressing a yeast genomic library and selecting clones able to grow in media with 1.2 M NaCl or 0.2 M or 0.4 M LiCl. In this screening procedure, the investigators identified Hal1 [230], Hal3, a regulator of the Ppz1 protein phosphatase, which regulates Trk1 and Trk2 [231], Hal4 and Hal5, two protein kinases that regulate Trk1 [232], Crz1 the calcineurin-regulated transcription factor, which controls the expression of ENA1 [233] and Qdr2, an ABC multidrug transporter able to transport monovalent and divalent cations [234], among others. Some of these genes proved to confer salt tolerance when overexpressed in plants [235,236].
The key point regarding this technique is that it allows for the identification of genes related to K+/Na+ homeostasis. The main advantage of using this kind of approach is that it is fast and can identify genes previously unrelated to ion homeostasis. Since these initial studies using a yeast genomic library, this approach has been carried out by several groups using plant cDNA libraries (Table 7). There are several strategies to increase the chances of success, for instance, the cDNA library can be obtained from plants after a stress treatment to increase the number of transcripts related to the abiotic stress response. The screening can be performed in a wild type yeast or in a stress-sensitive mutant, such as those described above. The use of mutants is a way to enhance the sensitivity of the screening and increase the chances of identifying plant genes conferring tolerance. For genes conferring weak or mild phenotypes, the yeast stress defense system could mask the effect, but by using stress-sensitive mutants, functional genes can be isolated. The most typical strain for performing screenings is the ena1-4 mutant that harbors a deletion in the genes encoding the Na+ extrusion pumps.
Table 7.
A summary of the heterologous overexpression screenings performed in yeast using plant cDNA libraries.
| Plant Species | cDNA Library [Reference] | Yeast Strain | Screening Conditions | Isolated Genes | Reference |
|---|---|---|---|---|---|
| Arabidopsis thaliana | Leaves from Arabidopsis seedlings [239] | ena1-4 (W303-1A) | 50 mM LiCl | AtRCY1 (K/T-cyclin with SR domain of splicing proteins) AtSRL1 (SR domain of splicing proteins) |
[237] |
| AtLTL1 (GDSL-motif lipase) | [240] | ||||
| [241] | cna1cna2YPH499 | 200 mM NaCl | AtSTZ1 (Zinc finger protein) AtSTO1 (B-Box domain protein 24) |
[242] | |
| Atriplex canescens | Young leaves and stems treated with 400 mM NaCl [243] | WT (INVSc1) | 2 M NaCl |
KJ026992 (Cyclophilin) KJ027014 (Glycine-rich protein) KJ027023 (Cytochrome P450) KJ027035 (Temperature-induced lipocalin) KJ027049 (Cysteine proteinase A494) KJ027057 (Alanine aminotransferase 2) KJ027061 (Hexose transporter) KJ027088 (RNA-binding family protein) KJ027102 (Cysteine proteinases) KJ027110 (calmodulin1) |
[244] |
| Beta vulgaris | Leafs from salt stressed plants [245] | ena1-4 nha1(W303-1A) | 150 mM NaCl | BvCK2 (catalytic subunit of the casein kinase) | [245] |
| BveIF1A (Translation initiation factor) | [246] | ||||
| BvSATO1 (RNA binding protein with RGG and RE/D motifs) BvSATO2 (homologous to SATO1) BvSATO4 (RNA binding protein) BvSATO5 (RNA binding protein) BvU2AF (U2snRNP AF protein) |
[247] | ||||
| gpd1(W303-1A) | 1 M Sorbitol | BvSAT1 (Serine acetyl trasferase 1) | [248] | ||
| BvGLB2 (Type II non symbiotic plant hemoglobin) | [249] | ||||
| WT (W303-1A) | 10 °C | BvCOLD1 (TIP-like aquaporin) | [250] | ||
| Ipomoea pes-caprae | Growing leaves, shoots and roots [251] | ena1-4 nha1 nhx1(W303-1A) | 75 mM NaCl |
MF680587 (putative abscisic acid, stress, and ripening-induced protein (ASR)) MF680589 (nudix hydrolase, chloroplastic) MF680592 (F-box protein At5g46170-like) MF680597 (fructokinase) MF680602 (adenosylhomocysteinase 1) MF680603 (peptide upstream protein) MF680604 (catalase) MF680608 (40S ribosomal protein S25) MF680611 (phosphomannomutase) MF765747 (dnaj protein-like protein) MF680614 (phytosulfokines-like) MF680616 (sugar carrier protein C) MF680619 (abscisic acid 8′-hydroxylase 4) KX426069 (dehydrin) |
[251] |
| Jatropha curcas | 3-4 week seedlings treated with 150 mM NaCl [252] | shs-2 (UV-generated mutant in the BY4741 background) | 750 mM NaCl |
FJ489601 (Allene oxide cyclase) FJ489602 (Thioredoxin H-type (TRX-h)) FJ489603 (Metallothionein) FJ489604 (Heterotrophic ferredoxin) FJ489605 (Defensin) FJ489606 (Calmodulin-7 (CAM-7)) FJ489608 (S18.A ribosomal protein) FJ489609 (60S ribosomal protein L18a) FJ489611 (Unknown protein) FJ619041 (Membrane protein -2) FJ619045 (Profilin-like protein) FJ619048 (Copper chaperone) FJ619052 (Annexin-like protein) FJ619053 (Al-induced protein) FJ619055 (60S ribosomal protein L39) FJ619056 (Ribosomal protein L37) FJ619057 (Ribosomal protein L15) FJ623457 (40S ribosomal protein S15) FJ623458 (40S ribosomal S18) |
[252] |
| Oryza sativa | Leaves from seedlings treated with different abiotic stresses [253] | WT (AH109) | 900 mM NaCl | OsMPG1 (mannose-1-phosphate guanyl transferase gene) | [253] |
| Paspalum vaginatum | Cultivated stolons treated with 250 mM NaCl [254] | ena1-4(G19) | 500 mM NaCl |
KT203435 (Uncharacterized protein) KT203436 (Iron-regulated transporter) KT203439 (Early light-induced protein) KT203440 (14-3-3-Like protein) KT203441 (Class 1 HSP) KT203442 (Cysteine synthase) KT203443 (Aldo-ketoreductase) KT203444 (L-Ascorbate peroxidase 2) KT203447 (Nop14-like family protein) KT203450 (Protein IQ-DOMAIN 14-like) KT203451 (Metacaspase-5-like) |
[254] |
| Phoenix datilifera | Salt-treated roots [255] | WT (INvSc1) | 2 M NaCl |
XM_008806660.2 (11S globulin seed storage protein 2-like) XM_008805834.2 (ABC transporter) XM_008780694.2 (Aquaporin PIP1-2) XM_008793314.1 (Aquaporin PIP2-4-like) XM_008779330.1 (Cysteine desulfurase) XM_008802947.2 (Cytochrome b5-like) XM_008783561.2 (Cytospin-A-like 1) XM_008797620.2 (Hexokinase-2-like) XM_008780092.2 (Mavicyanin-like 1) XM_008814440.2 (Peroxidase 3-like) XM_008786338.2 (Peroxidase 3-like 2) XR_604439.2 (Uncharacterized) XM_008800513.2 (Uncharacterized) XM_017846106.1 (Uncharacterized) XM_017846788.1 (Uncharacterized) |
[255] |
| Salicornia europaea | 3-month-old plants | WT (BY4741) | 1.6 M NaCl | SeNN8 (Similar to FKBP5) SeNN24 (Thaumatin like protein) SeNN43 (Unknown protein) |
[256] |
| Solanum tuberosum | Plants grown in vitro and subjected to heat shock at 35 °C [257] | WT (BY4741) | 39 °C | StnsLTP1 (Non-specific Lipid Transfer Protein-1) | [258] |
| Zea mays | Maize kernels | Not indicated | Not indicated | MBF1a (Multiprotein bridging factor 1a transcriptional coactivator) | [259] |
| Zoysia matrella | Cultivated stolons treated with 300 mM NaCl | ena1-4 (G19) | 500 mM NaCl |
KM265171 (Uncharacterized protein) KM265174 (C2H2-type Zinc finger protein) KM265176 (Unknown protein) KM265177 (Alcohol dehydrogenase 1) KM265179 (Protein disulfide isomerase) KM265182 (Glyoxylate reductase) KM265183 (Serine carboxypeptidase) |
[260] |
One striking feature of this kind of screening is that most of the isolated genes are not conserved in yeast. One would expect that plant genes could confer salt stress tolerance in yeast by increasing K+ uptake, Na+ extrusion or Na+ accumulation in the vacuole, but what happens instead is that in many cases there is no change in the ion content. The tolerance is attained by complementing a molecular process, which becomes limiting upon a decrease in the K+/Na+ ratio, such as gene splicing, or by counteracting some of the side effects of the salt stress, like the osmotic stress induced by high concentrations of Na+ in the medium or the concomitant oxidation [237]. In Table 7, we have compiled the results found in the literature for plant genes isolated in heterologous screenings in yeast that confer salt tolerance upon overexpression, with information on the origin of the cDNA library, the yeast strain used and the screening conditions. This review is focused on the use of the baker’s yeast S. cerevisiae, but some laboratories have also used the fission yeast, Schizosaccharomyces pombe, in a similar manner with positive results [238].
The most represented category are genes related to protein post-translational modifications, including protein folding, sorting and degradation. This suggests that processes related to protein sorting or protein regulation are inhibited by salt stress or that plant proteins are regulating the yeast salt stress response proteins. For instance, among the genes identified in this type of screen is a cyclophilin [244] and an FKBP protein [256]. These proteins have peptidyl-prolyl cis-trans isomerase activity, and have been described to regulate the H+-ATPase in plants [261]. Another major category is RNA binding proteins. One of the few commercialized GMO crops with increased salt tolerance is the Droughtgard® maize by BASF. The gene that confers this tolerance is a bacterial cold shock protein (CSPb from Bacillus subtilis), which also is an RNA binding protein. Therefore, this protein is likely to be a target of salt toxicity and overexpression of genes related to this process may complement the growth defect induced by salt stress [262]. Proteins related to protein translation, mainly ribosomal proteins, and photosynthesis-related proteins are also among the most common genes identified (Table 7). These are pivotal processes for plant cell physiology, so genes of both categories are abundant in cDNA libraries. It is likely that even cDNAs that confer a minor phenotype may be isolated due to the large number of individual cDNAs in the libraries. Finally, it is also interesting to point out that although some membrane transporters have been identified, Na+, K+ or Cl− channels have never been recovered in these screenings. The chances of identifying a complex multimeric integral membrane protein are low, as not all plant membrane proteins are properly folded, processed, assembled or localized in yeast due to the absence of the specific machinery [263,264]. In addition, they may lose activity due to the different composition of the membrane lipids [265]. Among the identified membrane transporters there is only one protein annotated as a cation/calcium exchanger [260], PIP and TIP aquaporins [250,255] and an ABC multidrug transporter, among others [255]. In most cases, the phenotype is probably due to non-specific Na+ or K+ transport.
It is also important to mention that heterologous expression of plant genes in yeast also has several limitations. For instance, not all plant genes are properly expressed in yeast due to codon-usage bias [266]. Another possible drawback of these kinds of screenings is the possibility of a dominant negative effect, so the phenotype observed may not be an indication of the function of the gene in plants, but represent an indirect effect due to interference with a yeast pathway that induces a phenotype similar to the one being evaluated [267]. These circumstances could explain the fact that in some screenings proteins related to signal transduction pathways, such as protein kinases [245] or ABA signaling-related proteins [260] also appear. Finally, it is also important to recall that at least some plant ion transport systems may also require multiple genes for complete function of the channel or transporter. The quintessential example here is the SOS system discussed above, which has never been identified by a cDNA library screening because only one gene is overexpressed at a time. Despite these shortcomings, the yeast heterologous expression strategy has made many important contributions to our understanding of plant K+ and Na+ homeostasis and will surely continue to provide valuable information in the future.
Acknowledgements
This work was supported by the Spanish Ministry of Economy and Competitiveness (BIO201677776-P and BIO2016-81957-REDT) and the Valencian Government (AICO/2018/300).
Author Contributions
A.L., N.A.-C., J.M.M. and L.Y. wrote the manuscript and prepared the figures and tables.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Epstein E. Mechanisms of Ion Transport through Plant Cell Membranes. Int. Rev. Cytol. 1973;34:123–168. [Google Scholar]
- 2.Kochian L.V., Lucas W.J. Potassium Transport in Roots. Adv. Bot. Res. 1989;15:136–151. [Google Scholar]
- 3.Schroeder J.I. Knockout of the guard cell K+out channel and stomatal movements. Proc. Natl. Acad. Sci. USA. 2003;100:4976–4977. doi: 10.1073/pnas.1031801100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hurst A.C., Meckel T., Tayefeh S., Thiel G., Homann U. Trafficking of the plant potassium inward rectifier KAT1 in guard cell protoplasts of Vicia faba. Plant J. 2004;37:391–397. doi: 10.1046/j.1365-313X.2003.01972.x. [DOI] [PubMed] [Google Scholar]
- 5.Nieves-Cordones M., Al Shiblawi F.R., Sentenac H. The Alkali Metal Ions: Their Role for Life. Metal Ions in Life Science. Springer International Publishing; Zurich, Switzerland: 2016. Roles and Transport of Sodium and Potassium in Plants; pp. 291–324. [DOI] [PubMed] [Google Scholar]
- 6.BRAG H. The Influence of Potassium on the Transpiration Rate and Stomatal Opening in Triticum aestivum andPisum sativum. Physiol. Plant. 1972;26:250–257. doi: 10.1111/j.1399-3054.1972.tb08553.x. [DOI] [Google Scholar]
- 7.Mohd Zain N.A., Ismail M.R. Effects of potassium rates and types on growth, leaf gas exchange and biochemical changes in rice (Oryza sativa) planted under cyclic water stress. Agric. Water Manag. 2016;164:83–90. doi: 10.1016/j.agwat.2015.09.022. [DOI] [Google Scholar]
- 8.Hooymans J.J.M. The influence of the transpiration rate on uptake and transport of potassium ions in barley plants. Planta. 1969;88:369–371. doi: 10.1007/BF00387465. [DOI] [PubMed] [Google Scholar]
- 9.Ohnishi J.-I., Flugge U.-I., Heldt H.W., Kanai R. Involvement of Na+ in Active Uptake of Pyruvate in Mesophyll Chloroplasts of Some C4 Plants : Na+/Pyruvate Cotransport. Plant Physiol. 1990;94:950–959. doi: 10.1104/pp.94.3.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amtmann A., Sanders D. Mechanisms of Na+ Uptake by Plant Cells. Adv. Bot. Res. 1998;29:75–112. [Google Scholar]
- 11.Horie T., Costa A., Kim T.H., Han M.J., Horie R., Leung H.Y., Miyao A., Hirochika H., An G., Schroeder J.I. Rice OsHKT2;1 transporter mediates large Na+ influx component into K+-starved roots for growth. EMBO J. 2007;26:3003–3014. doi: 10.1038/sj.emboj.7601732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu H. Plant salt tolerance and Na+ sensing and transport. Crop J. 2018;6:215–225. doi: 10.1016/j.cj.2018.01.003. [DOI] [Google Scholar]
- 13.Pyo Y.J., Gierth M., Schroeder J.I., Cho M.H. High-affinity K(+) transport in Arabidopsis: AtHAK5 and AKT1 are vital for seedling establishment and postgermination growth under low-potassium conditions. Plant Physiol. 2010;153:863–875. doi: 10.1104/pp.110.154369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maathuis F.J., Sanders D. Mechanism of high-affinity potassium uptake in roots of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA. 1994;91:9272–9276. doi: 10.1073/pnas.91.20.9272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anderson J.A., Huprikar S.S., Kochian L.V., Lucas W.J., Gaber R.F. Functional expression of a probable Arabidopsis thaliana potassium channel in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 1992;89:3736–3740. doi: 10.1073/pnas.89.9.3736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sentenac H., Bonneaud N., Minet M., Lacroute F., Salmon J.M., Gaymard F., Grignon C. Cloning and expression in yeast of a plant potassium ion transport system. Science. 1992;256:663–665. doi: 10.1126/science.1585180. [DOI] [PubMed] [Google Scholar]
- 17.Rodríguez-Rosales M.P., Gálvez F.J., Huertas R., Aranda M.N., Baghour M., Cagnac O., Venema K. Plant NHX cation/proton antiporters. Plant Signal. Behav. 2009;4:265–276. doi: 10.4161/psb.4.4.7919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Venema K., Belver A., Marín-Manzano M.C., Rodríguez-Rosales M.P., Donaire J.P. A novel intracellular K+/H+ antiporter related to Na+/H+ antiporters is important for K+ ion homeostasis in plants. J. Biol. Chem. 2003;278:22453–22459. doi: 10.1074/jbc.M210794200. [DOI] [PubMed] [Google Scholar]
- 19.Gassmann W., Schroeder J.I. Inward-rectifying K+ channels in root hairs of wheat. Plant Physiol. 1994;105:1399–1408. doi: 10.1104/pp.105.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cao Y., Ward J.M., Kelly W.B., Ichida A.M., Gaber R.F., Anderson J.A., Uozumi N., Schroeder J.I., Crawford N.M. Multiple genes, tissue specificity, and expression-dependent modulationcontribute to the functional diversity of potassium channels in Arabidopsis thaliana. Plant Physiol. 1995;109:1093–1106. doi: 10.1104/pp.109.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Müller-Röber B., Ellenberg J., Provart N., Willmitzer L., Busch H., Becker D., Dietrich P., Hoth S., Hedrich R. Cloning and electrophysiological analysis of KST1,an inward rectifying K+ channel expressed in potato guard cells. EMBO J. 1995;14:2409–2416. doi: 10.1002/j.1460-2075.1995.tb07238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lebaudy A., Véry A.A., Sentenac H. K+ channel activity in plants: Genes, regulations and functions. FEBS Lett. 2007;581:2357–2366. doi: 10.1016/j.febslet.2007.03.058. [DOI] [PubMed] [Google Scholar]
- 23.Véry A.A., Nieves-Cordones M., Daly M., Khan I., Fizames C., Sentenac H. Molecular biology of K+ transport across the plant cell membrane: What do we learn from comparison between plant species? J. Plant Physiol. 2014;171:748–769. doi: 10.1016/j.jplph.2014.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Dreyer I., Uozumi N. Potassium channels in plant cells. FEBS J. 2011;278:4293–4303. doi: 10.1111/j.1742-4658.2011.08371.x. [DOI] [PubMed] [Google Scholar]
- 25.Zimmermann S., Sentenac H. Plant ion channels: From molecular structures to physiological functions. Curr. Opin. Plant Biol. 1999;2:477–482. doi: 10.1016/S1369-5266(99)00020-5. [DOI] [PubMed] [Google Scholar]
- 26.Fu H.H., Luan S. AtKuP1: A dual-affinity K+ transporter from Arabidopsis. Plant Cell. 1998;10:63–73. doi: 10.1105/tpc.10.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schachtman D.P., Schroeder J.I., Lucas W.J., Anderson J.A., Gaber R.F. Expression of an inward-rectifying potassium channel by the Arabidopsis KAT1 cDNA. Science. 1992;258:1654–1658. doi: 10.1126/science.8966547. [DOI] [PubMed] [Google Scholar]
- 28.Gaymard F., Cerutti M., Horeau C., Lemaillet G., Urbach S., Ravallec M., Devauchelle G., Sentenac H., Thibaud J.B. The baculovirus/insect cell system as an alternative to Xenopus oocytes. First characterization of the AKT1 K+channel from Arabidopsis thaliana. J. Biol. Chem. 1996;271:22863–22870. doi: 10.1074/jbc.271.37.22863. [DOI] [PubMed] [Google Scholar]
- 29.Su H., Balderas E., Vera-Estrella R., Golldack D., Quigley F., Zhao C., Pantoja O., Bohnert H.J. Expression of the cation transporter McHKT1 in a halophyte. Plant Mol. Biol. 2003;52:967–980. doi: 10.1023/A:1025445612244. [DOI] [PubMed] [Google Scholar]
- 30.Paynter J.J., Andres-Enguix I., Fowler P.W., Tottey S., Cheng W., Enkvetchakul D., Bavro V.N., Kusakabe Y., Sansom M.S.P., Robinson N.J., et al. Functional complementation and genetic deletion studies of KirBac channels: Activatory mutations highlight gating-sensitive domains. J. Biol. Chem. 2010;285:40754–40761. doi: 10.1074/jbc.M110.175687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jan Y.N., Bichet D., Lin Y.-F., Ibarra C.A., Huang C.S., Yi B.A., Jan Y.N., Jan L.Y. Evolving potassium channels by means of yeast selection reveals structural elements important for selectivity. Proc. Natl. Acad. Sci. USA. 2004;101:4441–4446. doi: 10.1073/pnas.0401195101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zaks-Makhina E., Kim Y., Aizenman E., Levitan E.S. Novel neuroprotective K+ channel inhibitor identified by high-throughput screening in yeast. Mol. Pharmacol. 2004;65:214–219. doi: 10.1124/mol.65.1.214. [DOI] [PubMed] [Google Scholar]
- 33.Paynter J.J., Sarkies P., Andres-Enguix I., Tucker S.J. Genetic selection of activatory mutations in KcsA. Channels. 2008;2:413–418. doi: 10.4161/chan.2.6.6874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yenush L. Potassium and Sodium Transport in Yeast. In: Ramos J., Sychrová H., Kschischo M., editors. Yeast Membrane Transport, Advances in Experimental Medicine and Biology. Springer International Publishing; Cham, Switzerland: 2016. pp. 187–228. [DOI] [PubMed] [Google Scholar]
- 35.Gaber R.F., Styles C.A., Fink G.R. TRK1 encodes a plasma membrane protein required for high-affinity potassium transport in Saccharomyces cerevisiae. Mol. Cell. Biol. 1988;8:2848–2859. doi: 10.1128/MCB.8.7.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ko C.H., Gaber R.F. TRK1 and TRK2 encode structurally related K+ transporters in Saccharomyces cerevisiae. Mol. Cell. Biol. 1991;11:4266–4273. doi: 10.1128/MCB.11.8.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ko C.H., Buckley A.M., Gaber R.F. TRK2 is required for low affinity K+transport in Saccharomyces cerevisiae. Genetics. 1990;125:305–312. doi: 10.1093/genetics/125.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Durell S.R., Guy H.R. Structural models of the KtrB, TrkH, and Trk1,2 symporters based on the structure of the KcsA K(+) channel. Biophys. J. 1999;77:789–807. doi: 10.1016/S0006-3495(99)76932-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Haro R., Rodríguez-Navarro A. Functional analysis of the M2(D) helix of the TRK1 potassium transporter of Saccharomyces cerevisiae. Biochim. Biophys. Acta. 2003;1613:1–6. doi: 10.1016/S0005-2736(03)00132-9. [DOI] [PubMed] [Google Scholar]
- 40.Kuroda T., Bihler H., Bashi E., Slayman C.L., Rivetta A. Chloride channel function in the yeast TRK-potassium transporters. J. Membr. Biol. 2004;198:177–192. doi: 10.1007/s00232-004-0671-1. [DOI] [PubMed] [Google Scholar]
- 41.Zayats V., Stockner T., Pandey S.K., Wörz K., Ettrich R., Ludwig J. A refined atomic scale model of the Saccharomyces cerevisiae K+-translocation protein Trk1p combined with experimental evidence confirms the role of selectivity filter glycines and other key residues. Biochim. Biophys. Acta Biomembr. 2015;1848:1183–1195. doi: 10.1016/j.bbamem.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Haro R., Rodríguez-Navarro A. Molecular analysis of the mechanism of potassium uptake through the TRK1 transporter of Saccharomyces cerevisiae. Biochim. Biophys. Acta. 2002;1564:114–122. doi: 10.1016/S0005-2736(02)00408-X. [DOI] [PubMed] [Google Scholar]
- 43.Ariño J., Ramos J., Sychrová H. Alkali Metal Cation Transport and Homeostasis in Yeasts. Microbiol. Mol. Biol. Rev. 2010;74:95–120. doi: 10.1128/MMBR.00042-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ruiz A., Arino J. Function and regulation of the Saccharomyces cerevisiae ENA sodium ATPase system. Eukaryot. Cell. 2007;6:2175–2183. doi: 10.1128/EC.00337-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Haro R., Garciadeblas B., Rodríguez-Navarro A. A novel P-type ATPase from yeast involved in sodium transport. FEBS Lett. 1991;291:189–191. doi: 10.1016/0014-5793(91)81280-L. [DOI] [PubMed] [Google Scholar]
- 46.Wieland J., Nitsche A.M., Strayle J., Steiner H., Rudolph H.K. The PMR2 gene cluster encodes functionally distinct isoforms of a putative Na+ pump in the yeast plasma membrane. EMBO J. 1995;14:3870–3882. doi: 10.1002/j.1460-2075.1995.tb00059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Benito B., Quintero F.J., Rodríguez-Navarro A. Overexpression of the sodium ATPase of Saccharomyces cerevisiae: Conditions for phosphorylation from ATP and Pi. Biochim. Biophys. Acta. 1997;1328:214–226. doi: 10.1016/S0005-2736(97)00098-9. [DOI] [PubMed] [Google Scholar]
- 48.Benito B., Garciadeblás B., Rodríguez-Navarro A. Potassium- or sodium-efflux ATPase, a key enzyme in the evolution of fungi. Microbiology. 2002;148:933–941. doi: 10.1099/00221287-148-4-933. [DOI] [PubMed] [Google Scholar]
- 49.Palmgren M.G., Nissen P. P-type ATPases. Annu. Rev. Biophys. 2011;40:243–266. doi: 10.1146/annurev.biophys.093008.131331. [DOI] [PubMed] [Google Scholar]
- 50.Bañuelos M.A., Sychrová H., Bleykasten-Grosshans C., Souciet J.L., Potier S. The Nha1 antiporter of Saccharomyces cerevisiae mediates sodium and potassium efflux. Microbiology. 1998;144 (Pt 1):2749–2758. doi: 10.1099/00221287-144-10-2749. [DOI] [PubMed] [Google Scholar]
- 51.Nakamura N., Tanaka S., Teko Y., Mitsui K., Kanazawa H. Four Na+/H+ exchanger isoforms are distributed to Golgi and post-Golgi compartments and are involved in organelle pH regulation. J. Biol. Chem. 2005;280:1561–1572. doi: 10.1074/jbc.M410041200. [DOI] [PubMed] [Google Scholar]
- 52.Ohgaki R., Nakamura N., Mitsui K., Kanazawa H. Characterization of the ion transport activity of the budding yeast Na+/H+ antiporter, Nha1p, using isolated secretory vesicles. Biochim. Biophys. Acta. 2005;1712:185–196. doi: 10.1016/j.bbamem.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 53.Ketchum K.A., Joiner W.J., Sellers A.J., Kaczmarek L.K., Goldstein S.A. A new family of outwardly rectifying potassium channel proteins with two pore domains in tandem. Nature. 1995;376:690–695. doi: 10.1038/376690a0. [DOI] [PubMed] [Google Scholar]
- 54.Maresova L., Urbankova E., Gaskova D., Sychrova H. Measurements of plasma membrane potential changes in Saccharomyces cerevisiae cells reveal the importance of the Tok1 channel in membrane potential maintenance. FEMS Yeast Res. 2006;6:1039–1046. doi: 10.1111/j.1567-1364.2006.00140.x. [DOI] [PubMed] [Google Scholar]
- 55.Ahmed A., Sesti F., Ilan N., Shih T.M., Sturley S.L., Goldstein S.A. A molecular target for viral killer toxin: TOK1 potassium channels. Cell. 1999;99:283–291. doi: 10.1016/S0092-8674(00)81659-1. [DOI] [PubMed] [Google Scholar]
- 56.Cagnac O., Leterrier M., Yeager M., Blumwald E. Identification and characterization of Vnx1p, a novel type of vacuolar monovalent cation/H+ antiporter of Saccharomyces cerevisiae. J. Biol. Chem. 2007;282:24284–24293. doi: 10.1074/jbc.M703116200. [DOI] [PubMed] [Google Scholar]
- 57.Petrezselyova S., Kinclova-Zimmermannova O., Sychrova H. Vhc1, a novel transporter belonging to the family of electroneutral cation-Cl(−) cotransporters, participates in the regulation of cation content and morphology of Saccharomyces cerevisiae vacuoles. Biochim. Biophys. Acta. 2013;1828:623–631. doi: 10.1016/j.bbamem.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 58.André B., Scherens B. The yeast YBR235w gene encodes a homolog of the mammalian electroneutral Na(+)-(K+)-C1- cotransporter family. Biochem. Biophys. Res. Commun. 1995;217:150–153. doi: 10.1006/bbrc.1995.2757. [DOI] [PubMed] [Google Scholar]
- 59.Nass R., Rao R. Novel localization of a Na+/H+ exchanger in a late endosomal compartment of yeast. Implications for vacuole biogenesis. J. Biol. Chem. 1998;273:21054–21060. doi: 10.1074/jbc.273.33.21054. [DOI] [PubMed] [Google Scholar]
- 60.Maresova L., Sychrova H. Physiological characterization of Saccharomyces cerevisiae kha1 deletion mutants. Mol. Microbiol. 2005 doi: 10.1111/j.1365-2958.2004.04410.x. [DOI] [PubMed] [Google Scholar]
- 61.Nowikovsky K., Froschauer E.M., Zsurka G., Samaj J., Reipert S., Kolisek M., Wiesenberger G., Schweyen R.J. The LETM1/YOL027 gene family encodes a factor of the mitochondrial K+ homeostasis with a potential role in the Wolf-Hirschhorn syndrome. J. Biol. Chem. 2004;279:30307–30315. doi: 10.1074/jbc.M403607200. [DOI] [PubMed] [Google Scholar]
- 62.Rodríguez-Navarro A. Potassium transport in fungi and plants. Biochim. Biophys. Acta. 2000;1469:1–30. doi: 10.1016/S0304-4157(99)00013-1. [DOI] [PubMed] [Google Scholar]
- 63.Madrid R., Gómez M.J., Ramos J., Rodríguez-Navarro A. Ectopic potassium uptake in trk1 trk2 mutants of Saccharomyces cerevisiae correlates with a highly hyperpolarized membrane potential. J. Biol. Chem. 1998;273:14838–14844. doi: 10.1074/jbc.273.24.14838. [DOI] [PubMed] [Google Scholar]
- 64.Anderson J.A., Nakamura R.L., Gaber R.F. Heterologous expression of K+ channels in Saccharomyces cerevisiae: Strategies for molecular analysis of structure and function. Symp. Soc. Exp. Biol. 1994;48:85–97. [PubMed] [Google Scholar]
- 65.Lebaudy A., Pascaud F., Véry A.A., Alcon C., Dreyer I., Thibaud J.B., Lacombe B. Preferential KAT1-KAT2 heteromerization determines inward K+ current properties in Arabidopsis guard cells. J. Biol. Chem. 2010;285:6265–6274. doi: 10.1074/jbc.M109.068445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ache P., Becker D., Ivashikina N., Dietrich P., Roelfsema M.R.G., Hedrich R. GORK, a delayed outward rectifier expressed in guard cells of Arabidopsis thaliana, is a K+-selective, K+-sensing ion channel. FEBS Lett. 2000;486:93–98. doi: 10.1016/S0014-5793(00)02248-1. [DOI] [PubMed] [Google Scholar]
- 67.Schroeder J.I., Allen G.J., Hugouvieux V., Kwak J.M., Waner D. GUARD CELL SIGNAL TRANSDUCTION. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001;52:627–658. doi: 10.1146/annurev.arplant.52.1.627. [DOI] [PubMed] [Google Scholar]
- 68.Choe H., Sackin H., Palmer L.G. Permeation Properties of Inward-Rectifier Potassium Channels and Their Molecular Determinants. J. Gen. Physiol. 2000;115:391–404. doi: 10.1085/jgp.115.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang Y., Lee A., Chen J., Ruta V., Cadene M., Chait B.T., MacKinnon R. X-ray structure of a voltage-dependent K+ channel. Nature. 2003;423:33–41. doi: 10.1038/nature01580. [DOI] [PubMed] [Google Scholar]
- 70.Long S.B., Campbell E.B., Mackinnon R. Crystal structure of a mammalian voltage-dependent Shaker family K+ channel. Science. 2005;309:897–903. doi: 10.1126/science.1116269. [DOI] [PubMed] [Google Scholar]
- 71.Pilot G., Lacombe B., Gaymard F., Chérel I., Boucherez J., Thibaud J.B., Sentenac H. Guard Cell Inward K+ Channel Activity in Arabidopsis Involves Expression of the Twin Channel Subunits KAT1 and KAT2. J. Biol. Chem. 2001;276:3215–3221. doi: 10.1074/jbc.M007303200. [DOI] [PubMed] [Google Scholar]
- 72.Saito S., Hoshi N., Zulkifli L., Widyastuti S., Goshima S., Dreyer I., Uozumi N. Identification of regions responsible for the function of the plant K+ channels KAT1 and AKT2 in Saccharomyces cerevisiae and Xenopus laevis oocytes. Channels. 2017;11:510–516. doi: 10.1080/19336950.2017.1372066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nakamura R.L., Anderson J.A., Gaber R.F. Determination of key structural requirements of a K+ channel pore. J. Biol. Chem. 1997;272:1011–1018. doi: 10.1074/jbc.272.2.1011. [DOI] [PubMed] [Google Scholar]
- 74.Nakamura R.L., Gaber R.F. Ion selectivity of the Kat1 K+ channel pore. Mol. Membr. Biol. 2009;26:293–308. doi: 10.1080/09687680903188332. [DOI] [PubMed] [Google Scholar]
- 75.Kochian L.V., Garvin D.F., Shaff J.E., Chilcott T.C., Lucas W.J. Towards an understanding of the molecular basis of plants K+ transport: Characterization of cloned K+ transport cDNAs. Plant Soil. 1993;155:115–118. doi: 10.1007/BF00024997. [DOI] [Google Scholar]
- 76.Lai H.C., Grabe M., Yuh N.J., Lily Y.J. The S4 voltage sensor packs against the pore domain in the KAT1 voltage-gated potassium channel. Neuron. 2005;47:395–406. doi: 10.1016/j.neuron.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 77.Su Y.-H. Regulation by External K+ in a Maize Inward Shaker Channel Targets Transport Activity in the High Concentration Range. PLANT CELL ONLINE. 2005;17:1532–1548. doi: 10.1105/tpc.104.030551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Obata T., Kitamoto H.K., Nakamura A., Fukuda A., Tanaka Y. Rice Shaker Potassium Channel OsKAT1 Confers Tolerance to Salinity Stress on Yeast and Rice Cells. Plant Physiol. 2007;144:1978–1985. doi: 10.1104/pp.107.101154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hirsch R.E., Lewis B.D., Spalding E.P., Sussman M.R. A role for the AKT1 potassium channel in plant nutrition. Science. 1998;280:918–921. doi: 10.1126/science.280.5365.918. [DOI] [PubMed] [Google Scholar]
- 80.Ahn S.J., Shin R., Schachtman D.P. Expression of KT/KUP Genes in Arabidopsis and the Role of Root Hairs in K+ Uptake. Plant Physiol. 2004;134:1135–1145. doi: 10.1104/pp.103.034660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang Y., Wu W.-H. Potassium transport and signaling in higher plants. Annu. Rev. Plant Biol. 2013;64:451–476. doi: 10.1146/annurev-arplant-050312-120153. [DOI] [PubMed] [Google Scholar]
- 82.Gierth M., Mäser P., Schroeder J.I. The potassium transporter AtHAK5 functions in K(+) deprivation-induced high-affinity K(+) uptake and AKT1 K(+) channel contribution to K(+) uptake kinetics in Arabidopsis roots. Plant Physiol. 2005;137:1105–1114. doi: 10.1104/pp.104.057216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ros R., Lemaillet G., Fonrouge A.G., Daram P., Enjuto M., Salmon J.M., Thibaud J.B., Sentenac H. Molecular determinants of the Arabidopsis AKT1 K+ channel ionic selectivity investigated by expression in yeast of randomly mutated channels. Physiol. Plant. 2002;105:459–468. doi: 10.1034/j.1399-3054.1999.105310.x. [DOI] [Google Scholar]
- 84.Marten I., Gaymard F., Lemaillet G., Thibaud J.B., Sentenac H., Hedrich R. Functional expression of the plant K+ channel KAT1 in insect cells. FEBS Lett. 1996;380:229–232. doi: 10.1016/0014-5793(96)00042-7. [DOI] [PubMed] [Google Scholar]
- 85.Zimmermann S., Talke I., Ehrhardt T., Nast G., Müller-Röber B. Characterization of SKT1, an Inwardly Rectifying Potassium Channel from Potato, by Heterologous Expression in Insect Cells. Plant Physiol. 1998;116:879–890. doi: 10.1104/pp.116.3.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Philippar K., Fuchs I., Lüthen H., Hoth S., Bauer C.S., Haga K., Thiel G., Ljung K., Sandberg G., Böttger M., et al. Auxin-induced K+ channel expression represents an essential step in coleoptile growth and gravitropism. Proc. Natl. Acad. Sci. USA. 1999;96:12186–12191. doi: 10.1073/pnas.96.21.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hartje S., Zimmermann S., Klonus D., Mueller-Roeber B. Functional characterisation of LKT1, a K+ uptake channel from tomato root hairs, and comparison with the closely related potato inwardly rectifying K+ channel SKT1 after expression in Xenopus oocytes. Planta. 2000;210:723–731. doi: 10.1007/s004250050673. [DOI] [PubMed] [Google Scholar]
- 88.Boscari A., Clément M., Volkov V., Golldack D., Hybiak J., Miller A.J., Amtmann A., Fricke W. Potassium channels in barley: Cloning, functional characterization and expression analyses in relation to leaf growth and development. Plant Cell Environ. 2009;32:1761–1777. doi: 10.1111/j.1365-3040.2009.02033.x. [DOI] [PubMed] [Google Scholar]
- 89.Schleyer M., Bakker E.P. Nucleotide sequence and 3′-end deletion studies indicate that the K+- uptake protein kup from Escherichia coli is composed of a hydrophobic core linked to a large and partially essential hydrophilic C terminus. J. Bacteriol. 1993;175:6925–6931. doi: 10.1128/jb.175.21.6925-6931.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bañuelos M.A., Klein R.D., Alexander-Bowman S.J., Rodríguez-Navarro A. A potassium transporter of the yeast Schwanniomyces occidentalis homologous to the Kup system of Escherichia coli has a high concentrative capacity. EMBO J. 1995;14:3021–3027. doi: 10.1002/j.1460-2075.1995.tb07304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Santa-María G.E., Rubio F., Dubcovsky J., Rodríguez-Navarro A. The HAK1 gene of barley is a member of a large gene family and encodes a high-affinity potassium transporter. Plant Cell. 1997;9:2281–2289. doi: 10.1105/tpc.9.12.2281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nieves-Cordones M., Alemán F., Martínez V., Rubio F. The Arabidopsis thaliana HAK5 K+ transporter is required for plant growth and K+ acquisition from low K+ solutions under saline conditions. Mol. Plant. 2010;3:326–333. doi: 10.1093/mp/ssp102. [DOI] [PubMed] [Google Scholar]
- 93.Rubio F., Santa-María Guillermo E., Rodríguez-Navarro A. Cloning of Arabidopsis and barley cDNAs encoding HAK potassium transporters in root and shoot cells. Physiol. Plant. 2001;109:34–43. doi: 10.1034/j.1399-3054.2000.100106.x. [DOI] [Google Scholar]
- 94.Kim E.J., Kwak J.M., Uozumi N., Schroeder J.I. AtKUP1: An Arabidopsis gene encoding high-affinity potassium transport activity. Plant Cell. 1998;10:51–62. doi: 10.1105/tpc.10.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Quintero F.J., Blatt M.R. A new family of K+ transporters from Arabidopsis that are conserved across phyla. FEBS Lett. 1997;415:206–211. doi: 10.1016/S0014-5793(97)01125-3. [DOI] [PubMed] [Google Scholar]
- 96.Rigas S., Debrosses G., Haralampidis K., Vicente-Agullo F., Feldmann K.A., Grabov A., Dolan L., Hatzopoulos P. TRH1 encodes a potassium transporter required for tip growth in Arabidopsis root hairs. Plant Cell. 2001;13:139–151. doi: 10.1105/tpc.13.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Tenorio-Berrío R., Pérez-Alonso M.M., Vicente-Carbajosa J., Martín-Torres L., Dreyer I., Pollmann S. Identification of two auxin-regulated potassium transporters involved in seed maturation. Int. J. Mol. Sci. 2018;19:2132. doi: 10.3390/ijms19072132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Su Y., Luo W., Lin W., Ma L., Kabir M.H. Model of cation transportation mediated by high-affinity potassium transporters (HKTs) in higher plants. Biol. Proced. Online. 2015;17:1. doi: 10.1186/s12575-014-0013-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Maser P., Hosoo Y., Goshima S., Horie T., Eckelman B., Yamada K., Yoshida K., Bakker E.P., Shinmyo A., Oiki S., et al. Glycine residues in potassium channel-like selectivity filters determine potassium selectivity in four-loop-per-subunit HKT transporters from plants. Proc. Natl. Acad. Sci. USA. 2002;99:6428–6433. doi: 10.1073/pnas.082123799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hamamoto S., Horie T., Hauser F., Deinlein U., Schroeder J.I., Uozumi N. HKT transporters mediate salt stress resistance in plants: From structure and function to the field. Curr. Opin. Biotechnol. 2015;32:113–120. doi: 10.1016/j.copbio.2014.11.025. [DOI] [PubMed] [Google Scholar]
- 101.Schachtman D.P., Schroeder J.I. Structure and transport mechanism of a high-affinity potassium uptake transporter from higher plants. Nature. 1994;370:655–658. doi: 10.1038/370655a0. [DOI] [PubMed] [Google Scholar]
- 102.Rubio F., Gassmann W., Schroeder J.I. Sodium-driven potassium uptake by the plant potassium transporter HKT1 and mutations conferring salt tolerance. Science. 1995;270:1660–1663. doi: 10.1126/science.270.5242.1660. [DOI] [PubMed] [Google Scholar]
- 103.Gassmann W., Rubio F., Schroeder J.I. Alkali cation selectivity of the wheat root high-affinity potassium transporter HKT1. Plant J. 1996;10:869–882. doi: 10.1046/j.1365-313X.1996.10050869.x. [DOI] [PubMed] [Google Scholar]
- 104.Uozumi N., Kim E.J., Rubio F., Yamaguchi T., Muto S., Tsuboi A., Bakker E.P., Nakamura T., Schroeder J.I. The Arabidopsis HKT1 gene homolog mediates inward Na(+) currents in xenopus laevis oocytes and Na(+) uptake in Saccharomyces cerevisiae. Plant Physiol. 2000;122:1249–1259. doi: 10.1104/pp.122.4.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rubio F., Schwarz M., Gassmann W., Schroeder J.I. Genetic selection of mutations in the high affinity K+ transporter HKT1 that define functions of a loop site for reduced Na+ permeability and increased Na+ tolerance. J. Biol. Chem. 1999;274:6839–6847. doi: 10.1074/jbc.274.11.6839. [DOI] [PubMed] [Google Scholar]
- 106.Sunarpi, Horie T., Motoda J., Kubo M., Yang H., Yoda K., Horie R., Chan W.Y., Leung H.Y., Hattori K., et al. Enhanced salt tolerance mediated by AtHKT1 transporter-induced Na unloading from xylem vessels to xylem parenchyma cells. Plant J. 2005;44:928–938. doi: 10.1111/j.1365-313X.2005.02595.x. [DOI] [PubMed] [Google Scholar]
- 107.Fairbairn D.J., Liu W., Schachtman D.P., Gomez-Gallego S., Day S.R., Teasdale R.D. Characterisation of two distinct HKT1-like potassium transporters from Eucalyptus camaldulensis. Plant Mol. Biol. 2000;43:515–525. doi: 10.1023/A:1006496402463. [DOI] [PubMed] [Google Scholar]
- 108.Horie T., Yoshida K., Nakayama H., Yamada K., Oiki S., Shinmyo A. Two types of HKT transporters with different properties of Na+ and K+ transport in Oryza sativa. Plant J. 2001;27:129–138. doi: 10.1046/j.1365-313x.2001.01077.x. [DOI] [PubMed] [Google Scholar]
- 109.Wang T.B., Gassmann W., Rubio F., Schroeder J.I., Glass A.D. Rapid Up-regulation of HKT1, a high-affinity potassium transporter gene, in roots of barley and wheat following withdrawal of potassium. Plant Physiol. 1998;118:651–659. doi: 10.1104/pp.118.2.651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Garciadeblás B., Senn M.E., Bañuelos M.A., Rodríguez-Navarro A. Sodium transport and HKT transporters: The rice model. Plant J. 2003;34:788–801safi. doi: 10.1046/j.1365-313X.2003.01764.x. [DOI] [PubMed] [Google Scholar]
- 111.Safiarian M.J., Pertl-Obermeyer H., Lughofer P., Hude R., Bertl A., Obermeyer G. Lost in traffic? The K+ channel of lily pollen, LilKT1, is detected at the endomembranes inside yeast cells, tobacco leaves, and lily pollen. Front. Plant Sci. 2015;6:47. doi: 10.3389/fpls.2015.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mouline K., Véry A.A., Gaymard F., Boucherez J., Pilot G., Devic M., Bouchez D., Thibaud J.B., Sentenac H. Pollen tube development and competitive ability are impaired by disruption of a Shaker K+ channel in Arabidopsis. Genes Dev. 2002;16:339–350. doi: 10.1101/gad.213902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bihler H. TPK1 Is a Vacuolar Ion Channel Different from the Slow-Vacuolar Cation Channel. Plant Physiol. 2005;139:417–424. doi: 10.1104/pp.105.065599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hamamoto S., Marui J., Matsuoka K., Higashi K., Igarashi K., Nakagawa T., Kuroda T., Mori Y., Murata Y., Nakanishi Y., et al. Characterization of a tobacco TPK-type K+ channel as a novel tonoplast K+ channel using yeast tonoplasts. J. Biol. Chem. 2008;283:1911–1920. doi: 10.1074/jbc.M708213200. [DOI] [PubMed] [Google Scholar]
- 115.Goldstein S.A.N., Bockenhauer D., O’Kelly I., Zilberberg N. Potassium leak channels and the KCNK family of two-p-domain subunits. Nat. Rev. Neurosci. 2001;2:175–184. doi: 10.1038/35058574. [DOI] [PubMed] [Google Scholar]
- 116.Patel A.J., Honoré E. Properties and modulation of mammalian 2P domain K+ channels. Trends Neurosci. 2001;24:339–346. doi: 10.1016/S0166-2236(00)01810-5. [DOI] [PubMed] [Google Scholar]
- 117.Becker D., Geiger D., Dunkel M., Roller A., Bertl A., Latz A., Carpaneto A., Dietrich P., Roelfsema M.R., Voelker C., et al. AtTPK4, an Arabidopsis tandem-pore K+ channel, poised to control the pollen membrane voltage in a pH- and Ca2+-dependent manner. Proc. Natl. Acad. Sci. USA. 2004;101:15621–15626. doi: 10.1073/pnas.0401502101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Apse M.P., Aharon G.S., Snedden W.A., Blumwald E. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science. 1999;285:1256–1258. doi: 10.1126/science.285.5431.1256. [DOI] [PubMed] [Google Scholar]
- 119.Gaxiola R.A., Rao R., Sherman A., Grisafi P., Alper S.L., Fink G.R. The Arabidopsis thaliana proton transporters, AtNhx1 and Avp1, can function in cation detoxification in yeast. Proc. Natl. Acad. Sci. USA. 1999;96:1480–1485. doi: 10.1073/pnas.96.4.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bassil E., Blumwald E., Nakano R., Ohto M., Liang Y.-C., Ushijima K., Esumi T., Tajima H., Belmonte M., Coku A. The Arabidopsis Na+/H+ Antiporters NHX1 and NHX2 Control Vacuolar pH and K+ Homeostasis to Regulate Growth, Flower Development, and Reproduction. Plant Cell. 2011;23:3482–3497. doi: 10.1105/tpc.111.089581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bassil E., Ohto M.A., Esumi T., Tajima H., Zhu Z., Cagnac O., Belmonte M., Peleg Z., Yamaguchi T., Blumwald E. The Arabidopsis intracellular Na+/H+ antiporters NHX5 and NHX6 are endosome associated and necessary for plant growth and development. Plant Cell. 2011;23:224–239. doi: 10.1105/tpc.110.079426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Xia T., Apse M.P., Aharon G.S., Blumwald E. Identification and characterization of a NaCl-inducible vacuolar Na+/H+antiporter in Beta vulgaris. Physiol. Plant. 2002;116:206–212. doi: 10.1034/j.1399-3054.2002.1160210.x. [DOI] [PubMed] [Google Scholar]
- 123.Wu C., Gao X., Kong X., Zhao Y., Zhang H. Molecular Cloning and Functional Analysis of a Na+/H+ Antiporter Gene ThNHX1 from a Halophytic Plant Thellungiella halophila. Plant Mol. Biol. Report. 2009;27:1–12. doi: 10.1007/s11105-008-0048-1. [DOI] [Google Scholar]
- 124.Cosentino C., Alberio L., Thiel G., Moroni A. Yeast-based screening system for the selection of functional light-driven K+ channels. Methods Mol. Biol. 2017;1596:271–285. doi: 10.1007/978-1-4939-6940-1_17. [DOI] [PubMed] [Google Scholar]
- 125.Plugge B., Gazzarrini S., Nelson M., Cerana R., Van Etten J.L., Derst C., DiFrancesco D., Moroni A., Thiel G. A potassium channel protein encoded by chlorella virus PBCV-1. Science. 2000;287:1641–1644. doi: 10.1126/science.287.5458.1641. [DOI] [PubMed] [Google Scholar]
- 126.Xu K., Zhang H., Blumwald E., Xia T. A novel plant vacuolar Na+/H+ antiporter gene evolved by DNA shuffling confers improved salt tolerance in yeast. J. Biol. Chem. 2010;285:22999–23006. doi: 10.1074/jbc.M109.073783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Fields S., Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 128.Vidal M., Brachmann R.K., Fattaey A., Harlow E., Boeke J.D. Reverse two-hybrid and one-hybrid systems to detect dissociation of protein-protein and DNA-protein interactions. Proc. Natl. Acad. Sci. USA. 1996;93:10315–10320. doi: 10.1073/pnas.93.19.10315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Leanna C.A., Hannink M. The reverse two-hybrid system: A genetic scheme for selection against specific protein/protein interactions. Nucleic Acids Res. 1996;24:3341–3347. doi: 10.1093/nar/24.17.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Licitra E.J., Liu J.O. A three-hybrid system for detecting small ligand-protein receptor interactions. Proc. Natl. Acad. Sci. USA. 1996;93:12817–12821. doi: 10.1073/pnas.93.23.12817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Serebriiskii I., Khazak V., Golemis E.A. A two-hybrid dual bait system to discriminate specificity of protein interactions. J. Biol. Chem. 1999;274:17080–17087. doi: 10.1074/jbc.274.24.17080. [DOI] [PubMed] [Google Scholar]
- 132.Hirst M., Ho C., Sabourin L., Rudnicki M., Penn L., Sadowski I. A two-hybrid system for transactivator bait proteins. Proc. Natl. Acad. Sci. USA. 2001;2001:8726–8731. doi: 10.1073/pnas.141413598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Petrascheck M., Castagna F., Barberis A. Two-hybrid selection assay to identify proteins interacting with polymerase II transcription factors and regulators. Biotechniques. 2001;30:296–298,300,302. doi: 10.2144/01302st02. [DOI] [PubMed] [Google Scholar]
- 134.Johnsson N., Varshavsky A. Split ubiquitin as a sensor of protein interactions in vivo. Proc. Natl. Acad. Sci. USA. 1994;91:10340–10344. doi: 10.1073/pnas.91.22.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stagljar I., Korostensky C., Johnsson N., te Heesen S. A genetic system based on split-ubiquitin for the analysis of interactions between membrane proteins in vivo. Proc. Natl. Acad. Sci. USA. 1998;95:5187–5192. doi: 10.1073/pnas.95.9.5187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Gisler S.M., Kittanakom S., Fuster D., Wong V., Bertic M., Radanovic T., Hall R.A., Murer H., Biber J., Markovich D., et al. Monitoring Protein-Protein Interactions between the Mammalian Integral Membrane Transporters and PDZ-interacting Partners Using a Modified Split-ubiquitin Membrane Yeast Two-hybrid System. Mol. Cell. Proteomics. 2008;7:1362–1377. doi: 10.1074/mcp.M800079-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ehrhard K.N., Jacoby J.J., Fu X.Y., Jahn R., Dohlman H.G. Use of G-protein fusions to monitor integral membrane protein-protein interactions in yeast. Nat. Biotechnol. 2000;18:1075–1079. doi: 10.1038/80274. [DOI] [PubMed] [Google Scholar]
- 138.Hubsman M., Yudkovsky G., Aronheim A. A novel approach for the identification of protein-protein interaction with integral membrane proteins. Nucleic Acids Res. 2001;29:E18. doi: 10.1093/nar/29.4.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Aronheim A., Zandi E., Hennemann H., Elledge S.J., Karin M. Isolation of an AP-1 repressor by a novel method for detecting protein-protein interactions. Mol. Cell. Biol. 1997;17:3094–3102. doi: 10.1128/MCB.17.6.3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Broder Y.C., Katz S., Aronheim A. The ras recruitment system, a novel approach to the study of protein-protein interactions. Curr. Biol. 1998;8:1121–1124. doi: 10.1016/S0960-9822(98)70467-1. [DOI] [PubMed] [Google Scholar]
- 141.Mockli N., Deplazes A., Hassa P.O., Zhang Z., Peter M., Hottiger M.O., Stagljar I., Auerbach D. Yeast split-ubiquitin-based cytosolic screening system to detect interactions between transcriptionally active proteins. Biotechniques. 2007;42:725–730. doi: 10.2144/000112455. [DOI] [PubMed] [Google Scholar]
- 142.Boder E.T., Wittrup K.D. Yeast surface display for screening combinatorial polypeptide libraries. Nat. Biotechnol. 1997;15:553–557. doi: 10.1038/nbt0697-553. [DOI] [PubMed] [Google Scholar]
- 143.Urech D.M., Lichtlen P., Barberis A. Cell growth selection system to detect extracellular and transmembrane protein interactions. Biochim. Biophys. Acta. 2003;1622:117–127. doi: 10.1016/S0304-4165(03)00133-8. [DOI] [PubMed] [Google Scholar]
- 144.Dube D.H., Li B., Greenblatt E.J., Nimer S., Raymond A.K., Kohler J.J. A two-hybrid assay to study protein interactions within the secretory pathway. PLoS ONE. 2010;5:e15648. doi: 10.1371/journal.pone.0015648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Laser H., Bongards C., Schuller J., Heck S., Johnsson N., Lehming N. A new screen for protein interactions reveals that the Saccharomyces cerevisiae high mobility group proteins Nhp6A/B are involved in the regulation of the GAL1 promoter. Proc. Natl. Acad. Sci. USA. 2000;97:13732–13737. doi: 10.1073/pnas.250400997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Pelletier J.N., Campbell-Valois F.X., Michnick S.W. Oligomerization domain-directed reassembly of active dihydrofolate reductase from rationally designed fragments. Proc. Natl. Acad. Sci. USA. 1998;95:12141–12146. doi: 10.1073/pnas.95.21.12141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ozawa T., Kaihara A., Sato M., Tachihara K., Umezawa Y. Split luciferase as an optical probe for detecting protein-protein interactions in mammalian cells based on protein splicing. Anal. Chem. 2001;73:2516–2521. doi: 10.1021/ac0013296. [DOI] [PubMed] [Google Scholar]
- 148.Tafelmeyer P., Johnsson N., Johnsson K. Transforming a (beta/alpha)8--barrel enzyme into a split-protein sensor through directed evolution. Chem. Biol. 2004;11:681–689. doi: 10.1016/S1074-5521(04)00112-7. [DOI] [PubMed] [Google Scholar]
- 149.Hu C.-D., Chinenov Y., Kerppola T.K. Visualization of interactions among bZIP and Rel family proteins in living cells using bimolecular fluorescence complementation. Mol. Cell. 2002;9:789–798. doi: 10.1016/S1097-2765(02)00496-3. [DOI] [PubMed] [Google Scholar]
- 150.Magliery T.J., Wilson C.G.M., Pan W., Mishler D., Ghosh I., Hamilton A.D., Regan L. Detecting protein-protein interactions with a green fluorescent protein fragment reassembly trap: Scope and mechanism. J. Am. Chem. Soc. 2005;127:146–157. doi: 10.1021/ja046699g. [DOI] [PubMed] [Google Scholar]
- 151.Xing S., Wallmeroth N., Berendzen K.W., Grefen C. Techniques for the Analysis of Protein-Protein Interactions in Vivo. Plant Physiol. 2016;171:727–758. doi: 10.1104/pp.16.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Moosavi B., Mousavi B., Yang W.-C., Yang G.-F. Yeast-based assays for detecting protein-protein/drug interactions and their inhibitors. Eur. J. Cell Biol. 2017;96:529–541. doi: 10.1016/j.ejcb.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 153.Stynen B., Tournu H., Tavernier J., Van Dijck P. Diversity in genetic in vivo methods for protein-protein interaction studies: From the yeast two-hybrid system to the mammalian split-luciferase system. Microbiol. Mol. Biol. Rev. 2012;76:331–382. doi: 10.1128/MMBR.05021-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bruckner A., Polge C., Lentze N., Auerbach D., Schlattner U. Yeast two-hybrid, a powerful tool for systems biology. Int. J. Mol. Sci. 2009;10:2763–2788. doi: 10.3390/ijms10062763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gunde T., Tanner S., Auf der Maur A., Petrascheck M., Barberis A. Quenching accumulation of toxic galactose-1-phosphate as a system to select disruption of protein-protein interactions in vivo. Biotechniques. 2004;37:844–852. doi: 10.2144/04375PT03. [DOI] [PubMed] [Google Scholar]
- 156.Obrdlik P., El-Bakkoury M., Hamacher T., Cappellaro C., Vilarino C., Fleischer C., Ellerbrok H., Kamuzinzi R., Ledent V., Blaudez D., et al. K+ channel interactions detected by a genetic system optimized for systematic studies of membrane protein interactions. Proc. Natl. Acad. Sci. USA. 2004;101:12242–12247. doi: 10.1073/pnas.0404467101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Raquet X., Eckert J.H., Muller S., Johnsson N. Detection of altered protein conformations in living cells. J. Mol. Biol. 2001;305:927–938. doi: 10.1006/jmbi.2000.4239. [DOI] [PubMed] [Google Scholar]
- 158.Kojima T., Karasawa S., Miyawaki A., Tsumuraya T., Fujii I. Novel screening system for protein-protein interactions by bimolecular fluorescence complementation in Saccharomyces cerevisiae. J. Biosci. Bioeng. 2011;111:397–401. doi: 10.1016/j.jbiosc.2010.12.013. [DOI] [PubMed] [Google Scholar]
- 159.Cherel I., Gaillard I. The Complex Fine-Tuning of K(+) Fluxes in Plants in Relation to Osmotic and Ionic Abiotic Stresses. Int. J. Mol. Sci. 2019;20 doi: 10.3390/ijms20030715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Bregante M., Yang Y., Formentin E., Carpaneto A., Schroeder J.I., Gambale F., Lo Schiavo F., Costa A. KDC1, a carrot Shaker-like potassium channel, reveals its role as a silent regulatory subunit when expressed in plant cells. Plant Mol. Biol. 2008;66:61–72. doi: 10.1007/s11103-007-9252-x. [DOI] [PubMed] [Google Scholar]
- 161.Sklodowski K., Riedelsberger J., Raddatz N., Riadi G. The receptor-like pseudokinase MRH1 interacts with the voltage- gated potassium channel AKT2. Nat. Publ. Gr. 2017:1–12. doi: 10.1038/srep44611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Zhang A., Ren H.-M., Tan Y.-Q., Qi G.-N., Yao F.-Y., Wu G.-L., Yang L.-W., Hussain J., Sun S.-J., Wang Y.-F. S-type Anion Channels SLAC1 and SLAH3 Function as Essential Negative Regulators of Inward K+ Channels and Stomatal Opening in Arabidopsis. Plant Cell. 2016;28:949–955. doi: 10.1105/tpc.15.01050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Rosas-Santiago P., Lagunas-Gomez D., Barkla B.J., Vera-Estrella R., Lalonde S., Jones A., Frommer W.B., Zimmermannova O., Sychrova H., Pantoja O. Identification of rice cornichon as a possible cargo receptor for the Golgi-localized sodium transporter OsHKT1;3. J. Exp. Bot. 2015;66:2733–2748. doi: 10.1093/jxb/erv069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhang B., Karnik R., Wang Y., Wallmeroth N., Blatt M.R., Grefen C. The Arabidopsis R-SNARE VAMP721 Interacts with KAT1 and KC1 K+ Channels to Moderate K+ Current at the Plasma Membrane. Plant Cell. 2015;27:1697–1717. doi: 10.1105/tpc.15.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Honsbein A., Sokolovski S., Grefen C., Campanoni P., Pratelli R., Paneque M., Chen Z., Johansson I., Blatt M.R. A tripartite SNARE-K+ channel complex mediates in channel-dependent K+ nutrition in Arabidopsis. Plant Cell. 2009;21:2859–2877. doi: 10.1105/tpc.109.066118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Grefen C., Chen Z., Honsbein A., Donald N., Hills A., Blatt M.R. A novel motif essential for SNARE interaction with the K(+) channel KC1 and channel gating in Arabidopsis. Plant Cell. 2010;22:3076–3092. doi: 10.1105/tpc.110.077768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Osakabe Y., Arinaga N., Umezawa T., Katsura S., Nagamachi K., Tanaka H., Ohiraki H., Yamada K., Seo S.U., Abo M., et al. Osmotic stress responses and plant growth controlled by potassium transporters in Arabidopsis. Plant Cell. 2013;25:609–624. doi: 10.1105/tpc.112.105700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Lee S.C., Lan W., Kim B., Li L., Cheong Y.H., Pandey G.K., Lu G., Buchanan B.B., Luan S. A protein phosphorylation / dephosphorylation network regulates a plant potassium channel. Proc. Natl. Acad. Sci. USA. 2007;104:15959–15964. doi: 10.1073/pnas.0707912104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pilot G., Gaymard F., Mouline K., Chérel I., Sentenac H. Regulated expression of Arabidopsis Shaker K+ channel genes involved in K+ uptake and distribution in the plant. Plant Mol. Biol. 2003;51:773–787. doi: 10.1023/A:1022597102282. [DOI] [PubMed] [Google Scholar]
- 170.Ren X., Qi G., Feng H., Zhao S., Zhao S., Wang Y., Wu W. Calcineurin B-like protein CBL10 directly interacts with AKT1 and modulates K + homeostasis in Arabidopsis. Plant J. 2013;74:258–266. doi: 10.1111/tpj.12123. [DOI] [PubMed] [Google Scholar]
- 171.Li L., Kim B.G., Cheong Y.H., Pandey G.K., Luan S. A Ca(2)+ signaling pathway regulates a K(+) channel for low-K response in Arabidopsis. Proc. Natl. Acad. Sci. USA. 2006;103:12625–12630. doi: 10.1073/pnas.0605129103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Xu J., Li H.D., Chen L.Q., Wang Y., Liu L.L., He L., Wu W.H. A protein kinase, interacting with two calcineurin B-like proteins, regulates K+ transporter AKT1 in Arabidopsis. Cell. 2006;125:1347–1360. doi: 10.1016/j.cell.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 173.Geiger D., Becker D., Vosloh D., Gambale F., Palme K., Rehers M., Anschuetz U., Dreyer I., Hedrich R. Heteromeric At KC1·AKT1 Channels in Arabidopsis Roots Facilitate Growth under K + -limiting Conditions. J. Biol. Chem. 2009;284:21288–21295. doi: 10.1074/jbc.M109.017574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Ardie S.W., Nishiuchi S., Liu S., Takano T. Ectopic expression of the K+ channel beta subunits from Puccinellia tenuiflora (KPutB1) and rice (KOB1) alters K+ homeostasis of yeast and Arabidopsis. Mol. Biotechnol. 2011;48:76–86. doi: 10.1007/s12033-010-9349-3. [DOI] [PubMed] [Google Scholar]
- 175.Held K., Pascaud F., Eckert C., Gajdanowicz P., Hashimoto K. Calcium-dependent modulation and plasma membrane targeting of the AKT2 potassium channel by the CBL4 / CIPK6 calcium sensor / protein kinase complex. Nat. Publ. Gr. 2011;21:1116–1130. doi: 10.1038/cr.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cherel I. Physical and Functional Interaction of the Arabidopsis K+ Channel AKT2 and Phosphatase AtPP2CA. PLANT CELL ONLINE. 2002;14:1133–1146. doi: 10.1105/tpc.000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Vranova E., Tahtiharju S., Sriprang R., Willekens H., Heino P., Palva E.T., Inze D., Van Camp W. The AKT3 potassium channel protein interacts with the AtPP2CA protein phosphatase 2C. J. Exp. Bot. 2001;52:181–182. doi: 10.1093/jexbot/52.354.181. [DOI] [PubMed] [Google Scholar]
- 178.Lefoulon C., Boeglin M., Moreau B., Véry A., Szponarski W., Dauzat M., Michard E., Gaillard I., Chérel I. The Arabidopsis AtPP2CA Protein Phosphatase Inhibits the GORK K+ Efflux Channel and Exerts a Dominant Suppressive Effect on Phosphomimetic-activating Mutations. J. Biol. Chem. 2016;291:6521–6533. doi: 10.1074/jbc.M115.711309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Dreyer I., Poree F., Schneider A., Mittelstadt J., Bertl A., Sentenac H., Thibaud J.-B., Mueller-Roeber B. Assembly of plant Shaker-like K(out) channels requires two distinct sites of the channel alpha-subunit. Biophys. J. 2004;87:858–872. doi: 10.1529/biophysj.103.037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Liu L., Zheng C., Kuang B., Wei L., Yan L., Wang T. Receptor-Like Kinase RUPO Interacts with Potassium Transporters to Regulate Pollen Tube Growth and Integrity in Rice. PLoS Genet. 2016;12:e1006085. doi: 10.1371/journal.pgen.1006085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Naso A., Dreyer I., Pedemonte L., Testa I., Gomez-porras J.L., Usai C., Mueller-rueber B., Diaspro A., Gambale F., Picco C. The Role of the C-Terminus for Functional Heteromerization of the Plant Channel KDC1. Biophys. J. 2009;96:4063–4074. doi: 10.1016/j.bpj.2009.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Boneh U., Biton I., Schwartz A., Ben-Ari G. Characterization of the ABA signal transduction pathway in Vitis vinifera. Plant Sci. 2012;187:89–96. doi: 10.1016/j.plantsci.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 183.Hwang H., Yoon J., Kim H.Y., Min M.K., Kim J.-A., Choi E.-H., Lan W., Bae Y.-M., Luan S., Cho H., et al. Unique Features of Two Potassium Channels, OsKAT2 and OsKAT3, Expressed in Rice Guard Cells. PLoS ONE. 2013;8:e72541. doi: 10.1371/journal.pone.0072541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Ehrhardt T., Zimmermann S., Muller-Rober B. Association of plant K+(in) channels is mediated by conserved C-termini and does not affect subunit assembly. FEBS Lett. 1997;409:166–170. doi: 10.1016/S0014-5793(97)00502-4. [DOI] [PubMed] [Google Scholar]
- 185.Daras G., Rigas S., Tsitsekian D., Iacovides T.A., Hatzopoulos P. Potassium transporter TRH1 subunits assemble regulating root-hair elongation autonomously from the cell fate determination pathway. Plant Sci. 2015;231:131–137. doi: 10.1016/j.plantsci.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 186.Sato A., Sato Y., Fukao Y., Fujiwara M., Umezawa T., Shinozaki K., Hibi T., Taniguchi M., Miyake H., Goto D.B., et al. Threonine at position 306 of the KAT1 potassium channel is essential for channel activity and is a target site for ABA-activated SnRK2/OST1/SnRK2.6 protein kinase. Biochem. J. 2009;424:439–448. doi: 10.1042/BJ20091221. [DOI] [PubMed] [Google Scholar]
- 187.Acharya B.R., Jeon B.W., Zhang W., Assmann S.M. Open Stomata 1 (OST1) is limiting in abscisic acid responses of Arabidopsis guard cells. New Phytol. 2013;200:1049–1063. doi: 10.1111/nph.12469. [DOI] [PubMed] [Google Scholar]
- 188.Honsbein A., Blatt M.R., Grefen C. A molecular framework for coupling cellular volume and osmotic solute transport control. J. Exp. Bot. 2011;62:2363–2370. doi: 10.1093/jxb/erq386. [DOI] [PubMed] [Google Scholar]
- 189.Sokolovski S., Hills A., Gay R.A., Blatt M.R. Functional interaction of the SNARE protein NtSyp121 in Ca2+ channel gating, Ca2+ transients and ABA signalling of stomatal guard cells. Mol. Plant. 2008;1:347–358. doi: 10.1093/mp/ssm029. [DOI] [PubMed] [Google Scholar]
- 190.Zhu J.K., Liu J., Xiong L. Genetic analysis of salt tolerance in arabidopsis. Evidence for a critical role of potassium nutrition. Plant Cell. 1998;10:1181–1191. doi: 10.1105/tpc.10.7.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Shi H., Quintero F.J., Pardo J.M., Zhu J.-K. The putative plasma membrane Na(+)/H(+) antiporter SOS1 controls long-distance Na(+) transport in plants. Plant Cell. 2002;14:465–477. doi: 10.1105/tpc.010371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Guo Y., Halfter U., Ishitani M., Zhu J.K. Molecular characterization of functional domains in the protein kinase SOS2 that is required for plant salt tolerance. Plant Cell. 2001;13:1383–1400. doi: 10.1105/tpc.13.6.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Liu J., Zhu J.K. A calcium sensor homolog required for plant salt tolerance. Science. 1998;280:1943–1945. doi: 10.1126/science.280.5371.1943. [DOI] [PubMed] [Google Scholar]
- 194.Shi H., Xiong L., Stevenson B., Lu T., Zhu J.-K. The Arabidopsis salt overly sensitive 4 mutants uncover a critical role for vitamin B6 in plant salt tolerance. Plant Cell. 2002;14:575–588. doi: 10.1105/tpc.010417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Shi H., Zhu J.-K. SOS4, A Pyridoxal Kinase Gene, Is Required for Root Hair Development in Arabidopsis. Plant Physiol. 2002;129:585–593. doi: 10.1104/pp.001982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Rueschhoff E.E., Gillikin J.W., Sederoff H.W., Daub M.E. The SOS4 pyridoxal kinase is required for maintenance of vitamin B6-mediated processes in chloroplasts. Plant Physiol. Biochem. 2013;63:281–291. doi: 10.1016/j.plaphy.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 197.Quintero F.J., Ohta M., Shi H., Zhu J.-K., Pardo J.M. Reconstitution in yeast of the Arabidopsis SOS signaling pathway for Na+ homeostasis. Proc. Natl. Acad. Sci. USA. 2002;99:9061–9066. doi: 10.1073/pnas.132092099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Gong D., Guo Y., Schumaker K., Zhu J., Zhang J., Fuglsang A.T., Palmgren M.G., Wu W., Guo Y. The SOS3 Family of Calcium Sensors and SOS2 Family of Protein Kinases in Arabidopsis. Plant Physiol. 2011;134:919–926. doi: 10.1104/pp.103.037440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Quan R., Lin H., Mendoza I., Zhang Y., Cao W., Yang Y., Shang M., Chen S., Pardo J.M., Guo Y. SCABP8/CBL10, a Putative Calcium Sensor, Interacts with the Protein Kinase SOS2 to Protect Arabidopsis Shoots from Salt Stress. PLANT CELL ONLINE. 2007;19:1415–1431. doi: 10.1105/tpc.106.042291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Du W., Lin H., Chen S., Wu Y., Zhang J., Fuglsang A.T., Palmgren M.G., Wu W., Guo Y. Phosphorylation of SOS3-like calcium-binding proteins by their interacting SOS2-like protein kinases is a common regulatory mechanism in Arabidopsis. Plant Physiol. 2011;156:2235–2243. doi: 10.1104/pp.111.173377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Lin H., Yang Y., Quan R., Mendoza I., Wu Y., Du W., Zhao S., Schumaker K.S., Pardo J.M., Guo Y. Phosphorylation of SOS3-LIKE CALCIUM BINDING PROTEIN8 by SOS2 protein kinase stabilizes their protein complex and regulates salt tolerance in Arabidopsis. Plant Cell. 2009;21:1607–1619. doi: 10.1105/tpc.109.066217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Qiu Q.-S., Guo Y., Quintero F.J., Pardo J.M., Schumaker K.S., Zhu J.-K. Regulation of vacuolar Na+/H+ exchange in Arabidopsis thaliana by the salt-overly-sensitive (SOS) pathway. J. Biol. Chem. 2004;279:207–215. doi: 10.1074/jbc.M307982200. [DOI] [PubMed] [Google Scholar]
- 203.Cheng N.-H., Pittman J.K., Zhu J.-K., Hirschi K.D. The protein kinase SOS2 activates the Arabidopsis H(+)/Ca(2+) antiporter CAX1 to integrate calcium transport and salt tolerance. J. Biol. Chem. 2004;279:2922–2926. doi: 10.1074/jbc.M309084200. [DOI] [PubMed] [Google Scholar]
- 204.Quintero F.J., Martinez-Atienza J., Villalta I., Jiang X., Kim W.-Y., Ali Z., Fujii H., Mendoza I., Yun D.-J., Zhu J.-K., et al. Activation of the plasma membrane Na/H antiporter Salt-Overly-Sensitive 1 (SOS1) by phosphorylation of an auto-inhibitory C-terminal domain. Proc. Natl. Acad. Sci. USA. 2011;108:2611–2616. doi: 10.1073/pnas.1018921108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.de Dios Barajas-Lopez J., Moreno J.R., Gamez-Arjona F.M., Pardo J.M., Punkkinen M., Zhu J.-K., Quintero F.J., Fujii H. Upstream kinases of plant SnRKs are involved in salt stress tolerance. Plant J. 2018;93:107–118. doi: 10.1111/tpj.13761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Song A., Lu J., Jiang J., Chen S., Guan Z., Fang W., Chen F. Isolation and characterisation of Chrysanthemum crassum SOS1, encoding a putative plasma membrane Na+/H+ antiporter. Plant Biol. 2012;14:706–713. doi: 10.1111/j.1438-8677.2011.00560.x. [DOI] [PubMed] [Google Scholar]
- 207.Garciadeblás B., Haro R., Benito B. Cloning of two SOS1 transporters from the seagrass Cymodocea nodosa. SOS1 transporters from Cymodocea and Arabidopsis mediate potassium uptake in bacteria. Plant Mol. Biol. 2007;63:479–490. doi: 10.1007/s11103-006-9102-2. [DOI] [PubMed] [Google Scholar]
- 208.Jarvis D.E., Ryu C.-H., Beilstein M.A., Schumaker K.S. Distinct Roles for SOS1 in the Convergent Evolution of Salt Tolerance in Eutrema salsugineum and Schrenkiella parvula. Mol. Biol. Evol. 2014;31:2094–2107. doi: 10.1093/molbev/msu152. [DOI] [PubMed] [Google Scholar]
- 209.Zhao X., Wei P., Liu Z., Yu B., Shi H. Soybean Na+/H+ antiporter GmsSOS1 enhances antioxidant enzyme activity and reduces Na+ accumulation in Arabidopsis and yeast cells under salt stress. Acta Physiol. Plant. 2017;39:19. doi: 10.1007/s11738-016-2323-3. [DOI] [Google Scholar]
- 210.Martínez-Atienza J., Jiang X., Garciadeblas B., Mendoza I., Zhu J.-K., Pardo J.M., Quintero F.J. Conservation of the salt overly sensitive pathway in rice. Plant Physiol. 2007;143:1001–1012. doi: 10.1104/pp.106.092635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Takahashi R., Liu S., Takano T. Isolation and characterization of plasma membrane Na+/H+ antiporter genes from salt-sensitive and salt-tolerant reed plants. J. Plant Physiol. 2009;166:301–309. doi: 10.1016/j.jplph.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 212.Fraile-Escanciano A., Kamisugi Y., Cuming A.C., Rodríguez-Navarro A., Benito B. The SOS1 transporter of Physcomitrella patens mediates sodium efflux in planta. New Phytol. 2010;188:750–761. doi: 10.1111/j.1469-8137.2010.03405.x. [DOI] [PubMed] [Google Scholar]
- 213.Tang R.-J., Liu H., Bao Y., Lv Q.-D., Yang L., Zhang H.-X. The woody plant poplar has a functionally conserved salt overly sensitive pathway in response to salinity stress. Plant Mol. Biol. 2010;74:367–380. doi: 10.1007/s11103-010-9680-x. [DOI] [PubMed] [Google Scholar]
- 214.Zhou Y., Yin X., Duan R., Hao G., Guo J., Jiang X. SpAHA1 and SpSOS1 Coordinate in Transgenic Yeast to Improve Salt Tolerance. PLoS ONE. 2015;10:e0137447. doi: 10.1371/journal.pone.0137447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Huertas R., Olías R., Eljakaoui Z., Gálvez F.J., Li J., De Morales P.A., Belver A., Rodríguez-Rosales M.P. Overexpression of SlSOS2 (SlCIPK24) confers salt tolerance to transgenic tomato. Plant Cell Environ. 2012;35:1467–1482. doi: 10.1111/j.1365-3040.2012.02504.x. [DOI] [PubMed] [Google Scholar]
- 216.Xu H., Jiang X., Zhan K., Cheng X., Chen X., Pardo J.M., Cui D. Functional characterization of a wheat plasma membrane Na+/H+ antiporter in yeast. Arch. Biochem. Biophys. 2008;473:8–15. doi: 10.1016/j.abb.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 217.Feki K., Quintero F.J., Pardo J.M., Masmoudi K. Regulation of durum wheat Na+/H+ exchanger TdSOS1 by phosphorylation. Plant Mol. Biol. 2011;76:545–556. doi: 10.1007/s11103-011-9787-8. [DOI] [PubMed] [Google Scholar]
- 218.Serrano R., Kielland-Brandt M.C., Fink G.R. Yeast plasma membrane ATPase is essential for growth and has homology with (Na+ + K+), K+- and Ca2+-ATPases. Nature. 1986;319:689–693. doi: 10.1038/319689a0. [DOI] [PubMed] [Google Scholar]
- 219.Harper J.F., Surowy T.K., Sussman M.R. Molecular cloning and sequence of cDNA encoding the plasma membrane proton pump (H+-ATPase) of Arabidopsis thaliana (cation pumps/nucleotide sequence/amino add homology/oligonucleotide screening/transmembrane segments) Proc. Natl. Acad. Sci. USA. 1989;86:1234–1238. doi: 10.1073/pnas.86.4.1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Palmgren M.G., Christensen G. Complementation in situ of the yeast plasma membrane H+-ATPase gene pmal by an H+-ATPase gene from a heterologous species. FEBS Lett. 1993;317:216–222. doi: 10.1016/0014-5793(93)81279-9. [DOI] [PubMed] [Google Scholar]
- 221.Palmgren M.G., Christensen G. Functional comparisons between plant plasma membrane H+-ATPase isoforms expressed in yeast. J. Biol. Chem. 1994;269:3027–3033. [PubMed] [Google Scholar]
- 222.Baunsgaard L., Venema K., Axelsen K.B., Villalba J.M., Welling A., Wollenweber B., Palmgren M.G. Modified plant plasma membrane H+-ATPase with improved transport coupling efficiency identified by mutant selection in yeast. Plant J. 1996;10:451–458. doi: 10.1046/j.1365-313X.1996.10030451.x. [DOI] [PubMed] [Google Scholar]
- 223.Santiago J., Dupeux F., Betz K., Antoni R., Gonzalez-Guzman M., Rodriguez L., Márquez J.A., Rodriguez P.L. Structural insights into PYR/PYL/RCAR ABA receptors and PP2Cs. Plant Sci. 2012;182:3–11. doi: 10.1016/j.plantsci.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 224.Cutler S.R., Rodriguez P.L., Finkelstein R.R., Abrams S.R. Abscisic Acid: Emergence of a Core Signaling Network. Annu. Rev. Plant Biol. 2010;61:651–679. doi: 10.1146/annurev-arplant-042809-112122. [DOI] [PubMed] [Google Scholar]
- 225.Pizzio G.A., Rodriguez L., Antoni R., Gonzalez-Guzman M., Yunta C., Merilo E., Kollist H., Albert A., Rodriguez P.L. The PYL4 A194T mutant uncovers a key role of PYR1-LIKE4/PROTEIN PHOSPHATASE 2CA interaction for abscisic acid signaling and plant drought resistance. Plant Physiol. 2013;163:441–455. doi: 10.1104/pp.113.224162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Rodriguez L., Gonzalez-Guzman M., Diaz M., Rodrigues A., Izquierdo-Garcia A.C., Peirats-Llobet M., Fernandez M.A., Antoni R., Fernandez D., Marquez J.A., et al. C2-Domain Abscisic Acid-Related Proteins Mediate the Interaction of PYR/PYL/RCAR Abscisic Acid Receptors with the Plasma Membrane and Regulate Abscisic Acid Sensitivity in Arabidopsis. Plant Cell. 2014;26:4802–4820. doi: 10.1105/tpc.114.129973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Belda-Palazon B., Rodriguez L., Fernandez M.A., Castillo M.-C., Anderson E.M., Gao C., Gonzalez-Guzman M., Peirats-Llobet M., Zhao Q., De Winne N., et al. FYVE1/FREE1 Interacts with the PYL4 ABA Receptor and Mediates Its Delivery to the Vacuolar Degradation Pathway. Plant Cell. 2016;28:2291–2311. doi: 10.1105/tpc.16.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Okamoto M., Cutler S.R. Chemical Control of ABA Receptors to Enable Plant Protection Against Water Stress. Methods Mol. Biol. 2018;1795:127–141. doi: 10.1007/978-1-4939-7874-8_11. [DOI] [PubMed] [Google Scholar]
- 229.Shen Y., Shen L., Shen Z., Jing W., Ge H., Zhao J., Zhang W. The potassium transporter OsHAK21 functions in the maintenance of ion homeostasis and tolerance to salt stress in rice. Plant Cell Env. 2015;38:2766–2779. doi: 10.1111/pce.12586. [DOI] [PubMed] [Google Scholar]
- 230.Gaxiola R., de Larrinoa I.F., Villalba J.M., Serrano R. A novel and conserved salt-induced protein is an important determinant of salt tolerance in yeast. EMBO J. 1992;11:3157–3164. doi: 10.1002/j.1460-2075.1992.tb05392.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Yenush L., Mulet J.M., Ariño J., Serrano R. The Ppz protein phosphatases are key regulators of K+ and pH homeostasis: Implications for salt tolerance, cell wall integrity and cell cycle progression. EMBO J. 2002;21:920–929. doi: 10.1093/emboj/21.5.920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mulet J.M., Leube M.P., Kron S.J., Rios G., Fink G.R., Serrano R. A novel mechanism of ion homeostasis and salt tolerance in yeast: The Hal4 and Hal5 protein kinases modulate the Trk1-Trk2 potassium transporter. Mol. Cell. Biol. 1999;19:3328–3337. doi: 10.1128/MCB.19.5.3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Mendizabal I., Rios G., Mulet J.M., Serrano R., de Larrinoa I.F. Yeast putative transcription factors involved in salt tolerance. FEBS Lett. 1998;425:323–328. doi: 10.1016/S0014-5793(98)00249-X. [DOI] [PubMed] [Google Scholar]
- 234.Rios G., Cabedo M., Rull B., Yenush L., Serrano R., Mulet J.M. Role of the yeast multidrug transporter Qdr2 in cation homeostasis and the oxidative stress response. FEMS Yeast Res. 2013;13:97–106. doi: 10.1111/1567-1364.12013. [DOI] [PubMed] [Google Scholar]
- 235.Bordas M., Montesinos C., Dabauza M., Salvador A., Roig L.A., Serrano R., Moreno V. Transfer of the yeast salt tolerance gene HAL1 to Cucumis melo L. cultivars and in vitro evaluation of salt tolerance. Transgenic Res. 1997;6:41–50. doi: 10.1023/A:1018453032336. [DOI] [PubMed] [Google Scholar]
- 236.Gisbert C., Rus A.M., Bolarín M.C., López-Coronado J.M., Arrillaga I., Montesinos C., Caro M., Serrano R., Moreno V. The yeast HAL1 gene improves salt tolerance of transgenic tomato. Plant Physiol. 2000;123:393–402. doi: 10.1104/pp.123.1.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Forment J., Naranjo M.A., Roldán M., Serrano R., Vicente O. Expression of Arabidopsis SR-like splicing proteins confers salt tolerance to yeast and transgenic plants. Plant J. 2002;30:511–519. doi: 10.1046/j.1365-313X.2002.01311.x. [DOI] [PubMed] [Google Scholar]
- 238.Liu N., Chen A.-P., Zhong N.-Q., Wang F., Wang H.-Y., Xia G.-X. Functional screening of salt stress-related genes from Thellungiella halophila using fission yeast system. Physiol. Plant. 2007;129:671–678. doi: 10.1111/j.1399-3054.2007.00857.x. [DOI] [Google Scholar]
- 239.Minet M., Dufour M.E., Lacroute F. Complementation of Saccharomyces cerevisiae auxotrophic mutants by Arabidopsis thaliana cDNAs. Plant J. 1992;2:417–422. doi: 10.1111/j.1365-313x.1992.00417.x. [DOI] [PubMed] [Google Scholar]
- 240.Naranjo M.A., Forment J., Roldán M., Serrano R., Vicente O. Overexpression of Arabidopsis thaliana LTL1, a salt-induced gene encoding a GDSL-motif lipase, increases salt tolerance in yeast and transgenic plants. Plant Cell Env. 2006;29:1890–1900. doi: 10.1111/j.1365-3040.2006.01565.x. [DOI] [PubMed] [Google Scholar]
- 241.Elledge S.J., Mulligan J.T., Ramer S.W., Spottswood M., Davis R.W. lambdaYES: A multifunctional cDNA expression vector for the isolation of genes by complmentation of yeast and E. coli mutations. Proc. Natl. Acad. Sci. USA. 1991;88:1731–1735. doi: 10.1073/pnas.88.5.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Lippuner V., Cyert M.S., Gasser C.S. Two classes of plant cDNA clones differentially complement yeast calcineurin mutants and increase salt tolerance of wild-type yeast. J. Biol. Chem. 1996;271:12859–12866. doi: 10.1074/jbc.271.22.12859. [DOI] [PubMed] [Google Scholar]
- 243.Li J., Sun X., Yu G., Jia C., Liu J., Pan H. Generation and Analysis of Expressed Sequence Tags (ESTs) from Halophyte Atriplex canescens to Explore Salt-Responsive Related Genes. Int. J. Mol. Sci. 2014;15:11172–11189. doi: 10.3390/ijms150611172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Yu G., Li J., Sun X., Liu Y., Wang X., Zhang H., Pan H. Exploration for the Salinity Tolerance-Related Genes from Xero-Halophyte Atriplex canescens Exploiting Yeast Functional Screening System. Int. J. Mol. Sci. 2017;18:2444. doi: 10.3390/ijms18112444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Kanhonou R., Serrano R., Palau R.R. A catalytic subunit of the sugar beet protein kinase CK2 is induced by salt stress and increases NaCl tolerance in Saccharomyces cerevisiae. Plant Mol. Biol. 2001;47:571–579. doi: 10.1023/A:1012227913356. [DOI] [PubMed] [Google Scholar]
- 246.Rausell A., Kanhonou R., Yenush L., Serrano R., Ros R. The translation initiation factor eIF1A is an important determinant in the tolerance to NaCl stress in yeast and plants. Plant J. 2003;34:257–267. doi: 10.1046/j.1365-313X.2003.01719.x. [DOI] [PubMed] [Google Scholar]
- 247.Serrano R., Montesinos C., Gaxiola R., Ríos G., Forment J., Leube M., Mulet J.M., Naranjo M.A., Roldán M., Vicente O., et al. Functional genomics of salt tolerance: The yeast overexpression approach. Acta Hortic. 2003;609:31–38. doi: 10.17660/ActaHortic.2003.609.2. [DOI] [Google Scholar]
- 248.Mulet J.M., Alemany B., Ros R., Calvete J.J., Serrano R. Expression of a plant serine O-acetyltransferase in Saccharomyces cerevisiae confers osmotic tolerance and creates an alternative pathway for cysteine biosynthesis. Yeast. 2004;21:303–312. doi: 10.1002/yea.1076. [DOI] [PubMed] [Google Scholar]
- 249.Mulet Salort J.M., Sanz Molinero A.I., Serrano Salom R. Comprising Expression of Haemoglobin from Arabidopsis Basel (CH) S371. U.S. Patent. 2010 Mar 9;
- 250.Porcel R., Bustamante A., Ros R., Serrano R., Mulet Salort J.M. BvCOLD1: A novel aquaporin from sugar beet (Beta vulgaris L.) involved in boron homeostasis and abiotic stress. Plant Cell Environ. 2018;41:2844–2857. doi: 10.1111/pce.13416. [DOI] [PubMed] [Google Scholar]
- 251.Zheng J.-X., Zhang H., Su H.-X., Xia K.-F., Jian S.-G., Zhang M. Ipomoea pes-caprae IpASR Improves Salinity and Drought Tolerance in Transgenic Escherichia coli and Arabidopsis. Int. J. Mol. Sci. 2018;19:2252. doi: 10.3390/ijms19082252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Eswaran N., Parameswaran S., Sathram B., Anantharaman B., Raja Krishna Kumar G., Tangirala S.J. Yeast functional screen to identify genetic determinants capable of conferring abiotic stress tolerance in Jatropha curcas. BMC Biotechnol. 2010;10:23. doi: 10.1186/1472-6750-10-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Kumar R., Mustafiz A., Sahoo K.K., Sharma V., Samanta S., Sopory S.K., Pareek A., Singla-Pareek S.L. Functional screening of cDNA library from a salt tolerant rice genotype Pokkali identifies mannose-1-phosphate guanyl transferase gene (OsMPG1) as a key member of salinity stress response. Plant Mol. Biol. 2012;79:555–568. doi: 10.1007/s11103-012-9928-8. [DOI] [PubMed] [Google Scholar]
- 254.Chen Y., Chen C., Tan Z., Liu J., Zhuang L., Yang Z., Huang B. Functional Identification and Characterization of Genes Cloned from Halophyte Seashore Paspalum Conferring Salinity and Cadmium Tolerance. Front. Plant Sci. 2016;7:102. doi: 10.3389/fpls.2016.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Patankar H.V., Al-Harrasi I., Al-Yahyai R., Yaish M.W. Identification of Candidate Genes Involved in the Salt Tolerance of Date Palm (Phoenix dactylifera L.) Based on a Yeast Functional Bioassay. DNA Cell Biol. 2018;37:524–534. doi: 10.1089/dna.2018.4159. [DOI] [PubMed] [Google Scholar]
- 256.Nakahara Y., Sawabe S., Kainuma K., Katsuhara M., Shibasaka M., Suzuki M., Yamamoto K., Oguri S., Sakamoto H. Yeast functional screen to identify genes conferring salt stress tolerance in Salicornia europaea. Front. Plant Sci. 2015;6:920. doi: 10.3389/fpls.2015.00920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Gangadhar B.H., Yu J.W., Sajeesh K., Park S.W. A systematic exploration of high-temperature stress-responsive genes in potato using large-scale yeast functional screening. Mol. Genet. Genomics. 2014;289:185–201. doi: 10.1007/s00438-013-0795-z. [DOI] [PubMed] [Google Scholar]
- 258.Gangadhar B.H., Sajeesh K., Venkatesh J., Baskar V., Abhinandan K., Yu J.W., Prasad R., Mishra R.K. Enhanced Tolerance of Transgenic Potato Plants Over-Expressing Non-specific Lipid Transfer Protein-1 (StnsLTP1) against Multiple Abiotic Stresses. Front. Plant Sci. 2016;7:1228. doi: 10.3389/fpls.2016.01228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Kim M.-J., Lim G.-H., Kim E.-S., Ko C.-B., Yang K.-Y., Jeong J.-A., Lee M.-C., Kim C.S. Abiotic and biotic stress tolerance in Arabidopsis overexpressing the multiprotein bridging factor 1a (MBF1a) transcriptional coactivator gene. Biochem. Biophys. Res. Commun. 2007;354:440–446. doi: 10.1016/j.bbrc.2006.12.212. [DOI] [PubMed] [Google Scholar]
- 260.Chen Y., Zong J., Tan Z., Li L., Hu B., Chen C., Chen J., Liu J. Systematic mining of salt-tolerant genes in halophyte-Zoysia matrella through cDNA expression library screening. Plant Physiol. Biochem. 2015;89:44–52. doi: 10.1016/j.plaphy.2015.02.007. [DOI] [PubMed] [Google Scholar]
- 261.Bissoli G., Niñoles R., Fresquet S., Palombieri S., Bueso E., Rubio L., García-Sánchez M.J., Fernández J.A., Mulet J.M., Serrano R. Peptidyl-prolyl cis-trans isomerase ROF2 modulates intracellular pH homeostasis in Arabidopsis. Plant J. 2012;70:704–716. doi: 10.1111/j.1365-313X.2012.04921.x. [DOI] [PubMed] [Google Scholar]
- 262.Wang C., Burzio L.A., Koch M.S., Silvanovich A., Bell E. Purification, characterization and safety assessment of the introduced cold shock protein B in DroughtGard maize. Regul. Toxicol. Pharmacol. 2015;71:164–173. doi: 10.1016/j.yrtph.2014.12.014. [DOI] [PubMed] [Google Scholar]
- 263.Cabral K.M., Almeida M.S., Valente A.P., Almeida F.C., Kurtenbach E. Production of the active antifungal Pisum sativum defensin 1 (Psd1) in Pichia pastoris: Overcoming the inefficiency of the STE13 protease. Protein Expr. Purif. 2003;31:115–122. doi: 10.1016/S1046-5928(03)00136-0. [DOI] [PubMed] [Google Scholar]
- 264.Sigoillot M., Brockhoff A., Lescop E., Poirier N., Meyerhof W., Briand L. Optimization of the production of gurmarin, a sweet-taste-suppressing protein, secreted by the methylotrophic yeast Pichia pastoris. Appl. Microbiol. Biotechnol. 2012;96:1253–1263. doi: 10.1007/s00253-012-3897-3. [DOI] [PubMed] [Google Scholar]
- 265.Opekarová M., Tanner W. Specific lipid requirements of membrane proteins--a putative bottleneck in heterologous expression. Biochim. Biophys. Acta. 2003;1610:11–22. doi: 10.1016/S0005-2736(02)00708-3. [DOI] [PubMed] [Google Scholar]
- 266.Farrokhi N., Hrmova M., Burton R.A., Fincher G.B. Heterologous and cell free protein expression systems. Methods Mol. Biol. 2009;513:175–198. doi: 10.1007/978-1-59745-427-8_10. [DOI] [PubMed] [Google Scholar]
- 267.Veitia R.A., Caburet S., Birchler J.A. Mechanisms of Mendelian dominance. Clin. Genet. 2018;93:419–428. doi: 10.1111/cge.13107. [DOI] [PubMed] [Google Scholar]