Abstract
The n-butanolic extract, from an Iranian specimen of Nepeta asterotricha Rech. f. (NABE), displayed anti-inflammatory effects on lipopolysaccharide (LPS)-stimulated J774A.1 macrophages, which reduced nitrites and cytokines production. Bioassay guided fractionation of the extract led to the isolation of four iridoid glycosides, including a new one known as nepetamoside (1), one hexenyl-diglycoside, and some polyphenol and flavonoid components. None of the isolated iridoid components displayed significant effects on nitrites formation in an in vitro LPS-induced model of inflammation, thus suggesting that the plant anti-inflammatory effect is probably due to a synergistic action among its constituents.
Keywords: Nepeta asterotricha Rech. f., Labiatae, secondary metabolites, iridoid glycosides, nepetamoside, NMR, anti-inflammatory effect, nitrites, cytokines
1. Introduction
Nepeta L. (tribe Mentheae: subtribe Nepetoideae), is one of the largest genera in the Lamiaceae family. It represents more than 280 species, displaying a significant diversity in growth forms, pollination biology, floral morphology, and secondary metabolites [1] Nepeta is represented in the flora of Iran by 79 species, 42 of which, such as Nepeta asterotricha Rech. f., are endemic [2,3].
Decoctions and infusions from the aerial parts of Nepeta species have been used in traditional and folk medicines as antioxidant, anticonvulsant, tonic, antipyretic, antitussive, sedative, antiasthmatic, antispasmodic and anti-inflammatory medicines [4,5]. Some of these therapeutic actions were recently comprehensively reviewed [6] and received a modern pharmacological validation [7,8,9].
So far, the phytochemical study of the extracts from Nepeta species has mainly been devoted to the analysis of the volatile constituents in the essential oil [10,11]. Although the chemical composition of the volatile oils has been found to be influenced by external factors such as climatic and ecological conditions, plant organ and vegetative cycle stage, there were roughly two different chemotypes of Nepeta spp., the first one containing nepetalactone diastereoisomers as main constituents, whereas the second one contains 1,8-cineole (eucaliptol) as the predominant component. The chemical composition of the essential oils from root, leaf and aerial part of Nepeta asterotricha Rech. f. has been investigated and 1,8-cineole and nepetalactone have been identified as the major oil components of the leaf/aerial and root, respectively [12]. Moreover, biological studies revealed that the essential oil possesses antimicrobial and antifungal activities.
In this paper, we report the phytochemical study of the methanol extract from Nepeta asterotricha Rech. f. from Iran. Moreover, in order to validate the plant use in folk medicine, the anti-inflammatory potential of the Nepeta asterotricha extract as well as of the enriched fractions and isolated compounds was also investigated.
Bioassay guided fractionation of the crude methanol extract led to the isolation of four iridoid components, including a new one which we named nepetamoside, and to the identification of several known polyphenols and flavonoids components.
In vitro studies on LPS-stimulated macrophages revealed that the methanol extract of Nepeta asterotricha possessed anti-inflammatory effects by reducing the formation of nitrites and cytokines induced by lipopolysaccharide (LPS) in macrophages.
2. Results
Phytochemical Investigation and Evaluation of Biological Activity
Air-dried leaves of Nepeta asterotricha were ground and extracted with MeOH. Removal of the solvent under reduced pressure yielded a crude extract which was subjected to solvent partitioning using a Kupchan’s modified procedure to obtain four different fractions (n-hexane, CCl4, CHCl3 and n-BuOH). Among the fractions, the Nepeta asterotricha n-BuOH extract (NABE, 9.2 g) showed anti-inflammatory activity in the lipopolysaccharide (LPS)-stimulated J774A.1 macrophages.
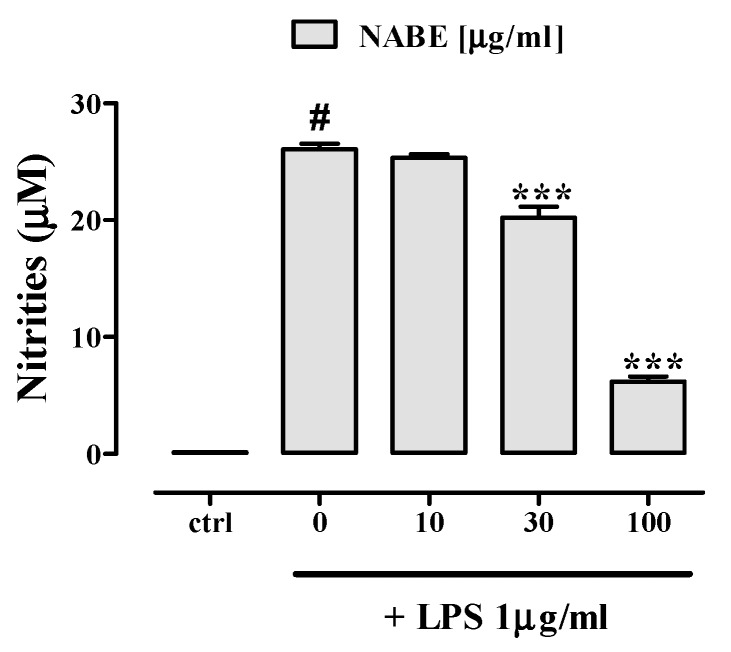
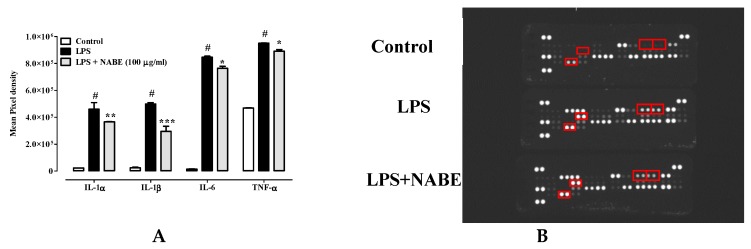
Macrophages, a heterogenous population of innate immune cells, are the key actors in the inflammation pathogenesis, by playing a crucial role in host defence and by controlling tissue-specific signals [13]. Their activation, mainly by LPS, is associated with the transcription of genes that encode for the production of inflammation-related mediators such as nitric oxide and cytokines [e.g., tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), interleukin-1β (IL-1β)] [14,15]. Therefore, we investigated the effect of NABE, its fractions and isolated compounds in J774A.1 macrophages stimulated by LPS. Treatment of J774A.1 macrophages with the inflammatory agent LPS (1 μg/mL for 24 h) caused a significant increase in nitrite (the stable form of nitric oxide) levels which was significantly reduced, in a concentration-dependent manner, by a pre-treatment with NABE at the concentration of 10–100 μg/mL, 30 min before LPS addition (Figure 1). Modulation of NO synthesis represents an important approach for the treatment of inflammatory conditions [16]. Of note, in macrophages not treated with LPS, NABE did not modify, per se, basal nitrite levels (data not shown). NABE, at concentrations ranging from 10 to 100 μg/mL, did not affect the cell vitality after 24 h of exposure, thus suggesting an absence of cytotoxic effects at these concentrations. (Control 100 ± 2.5; NABE 10 µg/mL 99.8 ± 3.2; NABE 30 µg/mL 99.6 ± 3.3; NABE 100 µg/mL 98.9 ± 3.8. Mean ± SEM of three independent experiments, each performed in octuplicate). By contrast, NABE at a concentration of 300 µg/mL induced a significant inhibition of macrophages viability (Control 100 ± 2.5; NABE 300 µg/mL 86.8 ± 2.3; p < 0.05. Mean ± SEM of three independent experiments, each performed in octuplicate). DMSO 20% was used as a positive control and significantly reduced cell viability (Control 100 ± 2.5; DMSO 20% 20.8 ± 1.2; *** p < 0.001. Mean ± SEM of three independent experiments, each performed in octuplicate). The anti-inflammatory effect of NABE was corroborated by quantification of a cytokines panel as assessed by a Proteome Profiler Mouse Cytokine Array Kit that is a membrane-based sandwich immunoassay. By using this assay, we measured, at the same time, the levels of 40 pro-inflammatory and anti-inflammatory secreted cytokine proteins in LPS-stimulated J774. According to literature [14], LPS (1 µg/mL) determined a significant increase of several cytokines, including interleukin (IL)-1α, IL-1β, IL-6 and TNF-α in macrophages (Figure 2). Among the screened cytokines, NABE (100 μg/mL) significantly decreased the production of these key pro-inflammatory cytokines in macrophages stimulated by LPS (Figure 2). However, NABE (100 μg/mL) did not affect the production of anti-inflammatory cytokines (e.g., IL-10) (data not shown).
Figure 1.
Inhibitory effect of Nepeta asterotricha n-butanol extract (NABE) on nitrite levels in the cell medium of J774A.1 macrophages stimulated with lipopolysaccharide (LPS, 1 μg/mL) for 24 h. NABE, at the concentration range of 10–100 μg/mL, were added to the cell media 30 min before LPS stimulus. Results are expressed as mean ± SEM of three experiments (in triplicates). # p < 0.001 vs control (ctrl); *** p < 0.001 vs. LPS alone.
Figure 2.
(A) Inhibitory effect of Nepeta asterotricha n-butanolic extract (NABE) on inflammatory cytokines levels [i.e., IL-1α, IL-1β, IL-6 and TNF-α] in the cell medium of J774A.1 macrophages stimulated with lipopolysaccharide (LPS, 1 μg/mL) for 24 h. NABE, used at the concentration of 100 μg/mL, were added to the cell media 30 min before LPS stimulus. (B) Representative images of each array (untreated, treated with LPS and LPS+ NABE). Results are expressed as mean ± SEM of three experiments (in triplicates). # p < 0.001 vs control; * p < 0.05, ** p < 0.01 and *** p < 0.001 vs LPS alone.
In order to identify the substance/s responsible for the NABE anti-inflammatory effect, an aliquot of NABE (2.4 g) was fractionated by DCCC to afford 12 fractions. Among the fractions, fractions 5, 6, 7, 11 and 12 (100 µg/mL) were able to significantly reduce the formation of nitrites induced by LPS (Control 5.6 ± 0.06; LPS 25.9 ± 0.31#; fraction 5 22.8 ± 0.32 *; fraction 6 21.3 ± 0.93 **; fraction 7 21.8 ± 0.94 **; fraction 11 18.5 ± 0.18 ***; fraction 12 7.98 ± 0.28 ***, # p < 0.001 vs. control, * p < 0.05, ** p < 0.01 and *** p < 0.001 vs. LPS, n = 3) being most effective on fraction 12 (% of inhibition: 88.3 %). All these fractions at the concentration of 100 µg/mL did not affect cell viability (data not shown).
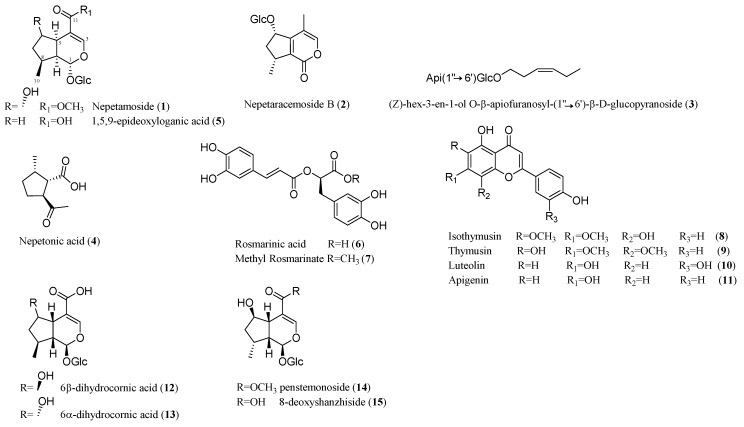
However, for a complete phytochemical investigation, all fractions were subjected to further purification (see Materials and Methods for details) by reversed phase HPLC to afford a new iridoid glucoside (1) together with know nepetaracemoside B (2) [17], (Z)-hex-3-en-1-ol O-β-apiofuranosyl-(1′′→6′)-β-d-glucopyranoside (3) [18] nepetonic acid (4) [19], and 1,5,9-epi-deoxyloganic acid glucosyl ester (5) [20] (Figure 3).
Figure 3.
New (1) and known (2–11) compounds isolated from Nepeta asterotricha Rech. f. and compounds 12–15 as reference compounds reported in Table 1 and Table 2.
Compound 1 was isolated as a colorless oil. C17H26O10 molecular formula was deduced from the sodiated molecular ion peak at m/z 413.1411 (calcd. 413.1418) observed in the HRESIMS, together with the 13C-NMR spectroscopic data (see Supplementary Materials). The 1H spectrum (Table 1) displayed the diagnostic carbinol proton signals relative to a β-glucopyranose unit, including the anomeric proton signal at δH 4.56 (1H, d, J = 7.9 Hz), and the signals relative to the diasteroisomeric methylene protons at C-6′: 3.84 (1H, d, J = 11.8 Hz) and 3.68 (1H, dd, J = 4.0, 11.8 Hz). The 13C-NMR chemical shifts, with one anomeric carbon at δC 104.2, four methine signals between 71.2 and 78.3 ppm and one methylene at δC 62.5 confirmed the presence of the above monosaccharide unit.
Table 1.
1H-NMR data of Nepetamoside a (1), 6β-dihydrocornic acid (12) b, 6α-dihydrocornic acid (13) b, penstemonoside (14) c and 8-deoxyshanzhiside (15) c.
| C | 1 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|
| 1 | 5.39 br s | 5.25 d (5) | 5.21 d (9) | 5.58 d (2.5) | 5.44 d (2) |
| 3 | 7.41 s | 7.41 s | 7.62 s | 7.48 d (0.9) | 7.34 s |
| 4 | - | - | - | - | - |
| 5 | 2.85 t (8.5) | 2.79 t (6) | 2.82 dd (4, 9) | 2.88 br d | 2.70 d (9) |
| 6 | 4.19 br s | 4.05 m | 4.47 t (4) | 4.23 m | 4.11 t (2) |
| 7 | 1.40 ddd (4.6, 10.2, 13.7) 1.76 dd (7, 13.1) |
1.25 m 2.17 m |
1.38 ddd (4, 10, 13) 1.92 dd (8, 13) |
1.80 m 1.50 ddd (4.2, 9.8, 14) |
1.38 ddd (4, 10, 13) 1.65 dd (8, 13) |
| 8 | 2.58 m | 1.96 q (7) | 2.30 m | 2.58 m | 2.43 m |
| 9 | 2.80 m | 2.03 dt (5, 6, 7) | 1.70 dt (4, 8) | 2.71 td (2.5, 9.3, 11.7) | 2.56 dt (2, 9.2, 9,2) |
| 10 | 1.05 d (7.2) | 1.15 d (7) | 1.12 d (8) | 1.02 d (7.2) | 0.87 d (7) |
| 11 | - | - | - | - | - |
| OMe | 3.71 s | - | - | 3.75 s | - |
| Glc | |||||
| 1′ | 4.56 d (7.9) | 4.65 d (8) | 4.70 d (8) | 4.76 d (8.1) | 4.63 d (8) |
| 2′ | 3.20 t (8.2) | 3.20 t (8) | 3.24 dd (8, 9) | 3.25 dd (8.1, 9.3) | 3.10 t (9) |
| 3′ | 3.35 ovl | 3.37 m | 3.40 t (9) | 3.30-3.51 m | 3.33 t (9) |
| 4′ | 3.31 ovl | 3.37 m | 3.31 m | 3.30-3.51 m | 3.23 t (9) |
| 5′ | 3.29 ovl | 3.30 m | 3.29 m | 3.30-3.51 m | 3.33 t (9) |
| 6′ | 3.68 dd (4, 11.8) 3.84 d (11.8) |
3.67 dd (6, 12) 3.89 dd (2, 12) |
3.67 dd (6, 12) 3.86 dd (2, 12) |
3.72 dd (5.7, 12.3) 3.92 dd (2, 12.3) |
3.62 dd (6, 12) 3.77 d (12) |
Besides the sugar unit, 13C-NMR spectrum also showed eleven carbons, one methyl (δC 16.7), three sp3 methines (δC 33.5, 42.1 and 42.7), one sp3 methylene (δC 41.9), one α,β-unsaturated methyl ester group (δC 169.5, 154.4, 110.9, 51.8), one ketal carbon (δC 100.7), and one carbinol secondary carbon (δC 77.7) that were reminiscent of an iridoid aglycone [21,22,23]. The analysis of 1H-1H COSY allowed us to assign the spin system C-1-C-9/C-5 (bold in Figure 4) and to place the hydroxyl group at C-6, on the basis of the chemical shift of the H-6 proton (δH 4.19) and the methyl group at C-8 (δH 2.58). The functionalization of the pyrane ring as well as the glycosidation at C-1 were determined by the diagnostic HMBC correlations in Figure 4. The assigned planar structure was found to be identical to that of penstemonoside, originally [24] isolated from Penstemon barbatus, and then reported from Castilleja rhexifolia [25].
Figure 4.
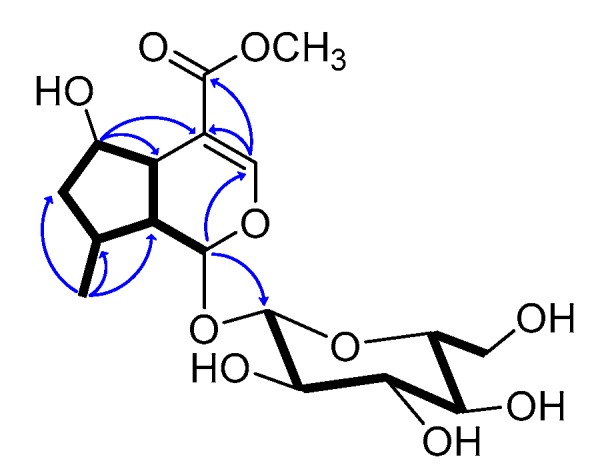
COSY connectivities (bold bonds) and key HMBC correlations (blue arrows) for nepetamoside (1).
As reported in the literature [26], due to the high flexibility of the iridoid skeleton, the dipolar couplings cannot be used as parameters for the configurational assignment. We assign a cis-fused ring structure [27] on the basis of the 1H and 13C NMR resonances of C-1, C-5 and C-9, together with the relative scalar coupling constants. The careful comparison of the NMR spectral data (Table 1 and Table 2) of 6β-dihydrocornic acid (12) and 6α-dihydrocornic acid (13) [28], penstemonoside (14) [24], 8-dihydroshanzhiside (15) [29], (Figure 3), indicated a relative disposition of the hydroxyl and methyl groups at C-6 and C-8 respectively as in penstemonoside and 8-dihydroshanzhiside, the stereostructure of which has been secured by X-ray investigation.
Table 2.
13C-NMR data of Nepetamoside a (1), 6β-dihydrocornic acid (12) b, 6α-dihydrocornic acid (13) b, penstemonoside (14) b and 8-deoxyshanzhiside (15) c.
| C | 1 | 12 | 13 | 14 | 15 |
|---|---|---|---|---|---|
| 1 | 100.7 | 97.5 | 101.2 | 96.1 | 96.0 |
| 3 | 154.4 | 153.6 | 155.9 | 153.7 | 153.3 |
| 4 | 110.9 | 110.8 | 107.4 | 111.0 | 110.3 |
| 5 | 42.7 | 43.7 | 43.5 | 43.0 | 41.1 |
| 6 | 77.7 | 78.8 | 75.1 | 77.8 | 77.1 |
| 7 | 41.9 | 42.7 | 43.2 | 41.7 | 40.8 |
| 8 | 33.5 | 34.3 | 35.2 | 33.8 | 32.5 |
| 9 | 42.1 | 47.9 | 47.0 | 42.5 | 40.5 |
| 10 | 16.7 | 21.1 | 21.9 | 16.6 | 15.7 |
| 11 | 169.5 | 171.0 | 171.1 | 169.5 | 171.1 |
| OMe | 51.8 | - | - | 51.8 | - |
| Glc | |||||
| 1′ | 104.2 | 100.2 | 100.4 | 99.7 | 98.6 |
| 2′ | 75.2 | 74.8 | 74.9 | 74.5 | 72.9 |
| 3′ | 78.0 | 78.1 | 78.1 | 78.1 | 75.9 |
| 4′ | 71.2 | 71.7 | 71.7 | 71.5 | 69.9 |
| 5′ | 78.3 | 78.4 | 78.5 | 77.8 | 76.5 |
| 6′ | 62.5 | 62.8 | 63.0 | 62.7 | 61.0 |
However, the 13C-NMR data of compound 1, albeit similar to that of penstemonoside, were not superimposable. The most significant difference is related to C-1 and C-1′ chemical shifts.
By analogy with previous literature data [30], we envisioned an enantiomeric configuration in the aglycone portion of 1, with respect to penstemonoside. This presumption was confirmed by consistent evidence. First, the specific rotation values of the iridoid glycosides with “classic” (1R, 5R, 9S) stereostructure were reported as invariably negative, whereas the iridoid glycosides from plants of Nepeta genus with a distinctive enantiomeric (1S, 5S, 9R) stereostructure displayed positive optical rotation values. The positive value of specific rotation of compound 1 was in agreement with a (1S,5S,9R) stereostructure. Analogously, the CD spectrum of 1 displayed a positive Cotton effect, as reported for iridoids from Nepeta [31]. Moreover, the enzymatic hydrolysis of 1 led to a C-1 epimeric mixture of aglycones, whose 1H-NMR data were superimposable to those reported for the aglycone of penstemonoside [25].
Therefore, compound 1 was determined to be ent-1,5,6,8,9-penstemonoside for which we propose the trivial name nepetamoside.
The fraction 12 from DCCC, the more active fraction in the nitrite assay, containing a complex mixture of flavonoids and polyphenol components, was first further purified by Sephadex® LH-20 and then by reversed phase HPLC to afford rosmarinic acid (6), methyl rosmarinate (7), 8-hydroxycirsimaritin (also named isothymusin) (8), thymusin (9), luteolin (10) and apigenin (11) (Figure 3). The structure of the known compounds was elucidated by comparison of the spectral data with those reported in the literature.
Several studies reported the determination of flavonoids and phenolic acids profile [32] of aerial parts of Nepeta species although in most cases the identification of the individual components was mainly performed by HPLC analysis. In particular, a comprehensive study on 38 species of Nepeta [33], including Nepeta asterotricha, analyzed some common features in the composition of flavonoids. Our analysis gave results similar to those reported for the same species, with 8-hydroxycirsimaritin (8), which represents a chemotaxonomic marker of the Nepeta genus as a major flavonoid component.
Our analysis didn’t reveal the presence of genkwanin and cirsimaritin usually reported as additional major components of this fraction.
Concerning the phenolic acid components, whereas rosmarinic acid (6) was reported as a major component in some Nepeta species [30], its methyl ester (7) has so far been isolated only from a Chinese specimen of Nepeta pratii [34].
All the phenolic compounds isolated in the present study are very common chemical constituents of several plants and, as single components, have been subjected to extensive pharmacological investigation for their anti-inflammatory potential. Only 8-hydroxycirsimaritin (8) has not been pharmacologically characterized. In our screening 8-hydroxycismaritin didn’t affect the nitrite production in LPS-stimulated J774A.1 macrophages. However, since 8-hydroxycirsimaritin is a high chemically unstable compound [35], we could assume that the observed inactivity may be caused by chemical decomposition during acquisition of the data.
The anti-inflammatory effects of rosmarinic acid (6) and apigenin (11) (the main components found in NABE) have been extensively demonstrated [36,37,38]. Therefore, it has been presumed that the anti-inflammatory effect of NABE could be due to the presence of these compounds. However, the amount of these compounds found in NABE is too low to exert anti-inflammatory effects, therefore, it is believed that the overall effect of NABE on nitrites and cytokines productions is probably due to a synergistic action of all components (found in NABE) rather than due to a single compound.
3. Materials and Methods
3.1. General Information
Specific rotations were measured on a Perkin Elmer 243 B polarimeter (Waltham, MA, USA). Micromass Q-TOF mass spectrometer was used to perform high-resolution ESI-MS spectra (Q-TOF premier, Waters Co., Milford, MA, USA).
NMR spectra were recorded on Varian Inova 400 and 500 NMR spectrometers (Palo Alto, CA, USA) (1H at 400 and 13C at 100, 1H at 500 MHz and 13C at 125 MHz, respectively), equipped with a SUN microsystem ultra 5 hardware. Coupling constants (J values) are reported in Hertz (Hz), and chemical shifts (δ) in ppm, referred to CHD2OD (δH 3.31 and δC 49.0). Spin multiplicities are given as s (singlet), br s (broad singlet), d (doublet), t (triplet) or m (multiplet). Through-space 1H connectivities were obtained using a ROESY experiment with mixing times of 150, 200 and 250 ms.
DCCC was performed using a DCC-A (Rakakikai Co. Di Tokio, Japan) equipped with 250 columns (internal diameter 3 mm). Silica gel (200–400 mesh) from Macherey-Nagel Company and Sephadex® LH-20 from Sigma Aldrich (St. Louis, MO, USA) was used for chromatography.
HPLC was performed using a Waters Model 510 pump equipped with Waters Rheodine injector and a differential refractometer, model 401 experiment (Waters Co., Milford, MA, USA).
3.2. Plant Material
The aerial parts of Nepeta asterotricha were collected at full flowering time in Deh-Bala, Yazd, Iran. The sample were identified and placed in Herbarium of Medicinal Plants and Drugs Research Institute, Shahid Beheshti University, Tehran, Iran. The sample code in the Herbarium is MPH-2569.
3.3. Extraction and Isolation
The aerial parts of the plant (230 g) were extracted with methanol (3 × 1 L) at room temperature and the crude methanolic extract was subjected to a modified Kupchan’s partitioning procedure as follows. The methanol extract was dissolved in a mixture of MeOH/H2O containing 10% H2O and partitioned against n-hexane (3.5 g). The water content (% v/v) of the MeOH extract was adjusted to 30% and partitioned against CHCl3 (18.7 g). The aqueous phase was concentrated to remove MeOH and then extracted with n-BuOH (9.2 g). An aliquot of n-BuOH extract (2.4 g) was submitted to droplet counter-current chromatography (DCCC) with n-BuOH/Me2CO/H2O (3:1:5) in descending mode (the upper phase was the stationary phase). The obtained fractions were monitored by TLC on Silica gel plates with n-BuOH/AcOH/H2O (12:3:5) and CHCl3/MeOH/H2O (80:18:2) as eluents. Twelve fractions were obtained and purified by HPLC on a Nucleodur 100-5 C18 column (5 μm, 4.6 mm i.d × 250 mm) using MeOH/H2O (4:6) as eluent. An aliquot of fraction 7 (50 mg), containing exclusively nepetamoside (1), was purified in HPLC to give 17 mg of pure compound 1 (tR = 14.2 min). Fraction 9 (30.1 mg) afforded 3.8 mg of nepetaracemoside B (2) (tR = 6.5 min) and 4.2 mg of (Z)-hex-3-en-1-ol O-β-apiofuranosyl-(1″→6′)-β-d-glucopyranoside (3) (tR = 26 min), fraction 10 (270 mg) contained exclusively nepetonic acid (4) (62 mg; tR = 30 min) and fraction 11 (548 mg) afforded 23.4 mg of 1,5,9-epi-deoxyloganic acid (5) (tR = 24 min).
Fraction 12 was further chromatographed on Sephadex® LH-20 (MeOH eluent). Fractions were collected and combined according to TLC analysis into nine main fractions (A–I). Fraction G (37.6 mg) was further purified by HPLC with MeOH/H2O 1:1 (flow rate 1.0 mL/min) to give 3.6 mg of rosmarinic acid (6) (tR = 3.5 min), 1.0 mg of methyl rosmarinate (7) (tR = 12 min), 9.4 mg of 8-hydroxycirsimaritin (8) (tR = 31.5 min), 0.7 mg of thymusin (9) (tR = 50 min). Fraction H (7.7 mg) was separated by HPLC with MeOH/H2O 1:1 (flow rate 1.0 mL/min) obtaining 1.1 mg of luteolin (10) (tR = 28.5 min) and 0.8 mg of apigenin (11) (tR = 48.4 min).
3.3.1. Nepetamoside (1)
Yellow amorphous solid; [α + 81.4 (c 0.60, MeOH); 1H and 13C-NMR data in CD3OD given in Table 1 and Table 2; HRESIMS (positive-ion mode) m/z 413.1411 [M + Na]+ (calcd. for C17H26O10Na, 413.1418).
3.3.2. Nepetaracemoside B (2)
Yellow amorphous solid; [α − 4.0 (c 0.12, MeOH); 1H and 13C-NMR data were identical to those previously reported in literature [17]; HRESIMS (positive-ion mode) m/z 365.1212 [M + Na]+ (calcd. for C16H22O8Na, 365.1207).
3.3.3. (Z)-hex-3-en-1-ol O-β-apiofuranosyl-(1″→6′)-β-d-glucopyranoside (3)
White amorphous solid; 1H and 13C-NMR data were identical to those previously reported in literature [18]. HRESIMS (positive-ion mode) m/z 417.1729 [M + Na]+ (calcd. for C17H30O10Na, 417.1731).
3.3.4. Nepetonic Acid (4)
Yellow amorphous solid; [α + 16.6 (c 0.79, MeOH); 1H and 13C-NMR data were identical to those previously reported in literature [19]; HRESIMS (negative-ion mode) m/z 169.0869 [M − H]− (calcd. for C9H13O3, 169.0870).
3.3.5. 1.5.9-epi-deoxyloganic Acid (5)
Yellow amorphous solid; [α + 65.7 (c 1.61, MeOH); 1H and 13C-NMR data were identical to those previously reported in literature [20]; HRESIMS (negative-ion mode) m/z 359.1345 [M − H]− (calcd. for C16H23O9, 359.1348).
3.3.6. Rosmarinic Acid (6)
Yellow amorphous solid; 1H and 13C-NMR data were identical to those previously reported in literature [39,40]. HRESIMS (negative-ion mode) m/z 359.0773 [M − H]− (calcd. for C18H15O8, 359.0779).
3.3.7. Methyl Rosmarinate (7)
Yellow amorphous solid; Spectroscopic data were in agreement with those reported in literature [40]; HRESIMS (positive-ion mode) m/z 397.0902 [M + Na]+ (calcd. for C19H18O8Na, 397.0894).
3.3.8. 8-Hydroxycirsimaritin or Isothymusin (8)
Yellow amorphous solid; Spectroscopic data were in agreement with those reported in literature [41]; HRESIMS (positive-ion mode) m/z 353.0639 [M + Na]+ (calcd. for C17H14O7Na, 353.0632).
3.3.9. Thymusin (9)
Yellow amorphous solid; Spectroscopic data were in agreement with those reported in literature [41]; HRESIMS (positive-ion mode) m/z 353.0637 [M + Na]+ (calcd. for C17H14O7Na, 353.0632).
3.3.10. Luteolin (10)
Yellow amorphous solid; 1H and 13C-NMR data were identical to those previously reported in literature [42]; HRESIMS (positive-ion mode) m/z 309.0372 [M + Na]+ (calcd. for C15H10O6Na, 309.0370).
3.3.11. Apigenin (11)
Yellow powder;1H and 13C-NMR data were identical to those previously reported in literature [42]; HRESIMS (positive-ion mode) m/z 293.0426 [M + Na]+ (calcd. for C15H10O5Na, 293.0420).
3.4. Enzymatic Hydrolysis of Nepetamoside 1
A solution of 1 (2 mg) in H2O (1 mL) was hydrolyzed with β-glucosidase at 37 °C for 24 h. After reaction for 12 h, the TLC analysis (SiO2 with n-BuOH-AcOH-H2O 60:15:25) showed that the starting material has disappeared. The mixture was passed through a C-18 SEP-PACK cartridge, washed with H2O and eluted with MeOH. The MeOH eluate gave the aglycone epimeric mixture. Selected 1H-NMR data (400 MHz, CD3OD) of 1α and 1β epimers: δH 1.10 (d, J = 7.0 Hz, H3-10), 3.77 (s, OCH3), 4.07/4.24 (br singlets, H-6), 4.98/5.42 (br singlets H-l) and 7.44 (s, H-3). HRESIMS m/z 251.0893 [M + Na]+ (calcd. for C11H16O5Na, 251.0890).
3.5. Biological Assays
3.5.1. Cell Culture
J774A.1 macrophages (ATCC, from LGC Standards, Milan, Italy) were used for in vitro experiments. Macrophages were routinely maintained at 37 °C in a humidified atmosphere of 5% CO2 and were cultured in Dulbecco’s Modified Eagle’s medium (DMEM, Lonza Group, Milan, Italy) supplemented with 10% fetal bovine serum (FBS, Sigma Aldrich, Milan, Italy) 100 U/mL penicillin and 100 μg/L streptomycin, 2 mM l-glutamine, 20 mM Hepes [4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid] and 1 mM Na pyruvate. The medium was changed every 48 h in conformity with the manufacturer’s protocols.
3.5.2. Cell Viability
Cell viability was evaluated by measuring the incorporation of neutral red (NR), a weak cationic dye, in lysosomes (NR assay). Briefly, J774A.1 macrophages were seeded in 96-well plates at a density of 3 × 104 cells per well and were allowed to adhere for 24 h. Then, cells were treated with the Nepeta asterotricha n-butanolic extract (NABE, 10–100 µg/mL) for 24 h. Subsequently, macrophages were incubated with NR dye solution (50 μg/mL in medium) for 3 h at 37 °C and then lysed with 1% acetic acid. The absorbance was read at 532 nm (iMarkTM microplate reader, Bio-Rad, Milan, Italy). All experiments were performed in triplicate (including 8 replicates for each treatment).
3.5.3. Nitrite Measurement and Pharmacological Treatment In Vitro
The effect of NABE on the nitric oxide (NO) production was assessed by measuring nitrites, stable metabolites of NO, in macrophages medium via a colorimetric assay, as previously described [43]. J774A.1 macrophages (5 × 105 cells per well seeded in a 24-well plate) were incubated with NABE (10–100 µg/mL) for 30 min and subsequently with LPS (1 μg/mL) for 24 h. Then, the cell supernatant was collected and incubated with 100 µL of Griess reagent (0.2% naphthylethylenediamine dihydrochloride and 2% sulphanilamide in 5% phosphoric acid) at room temperature for 10 min in order to allowed the formation of a colored azo dye. The absorbance was read at 550 nm. Serial-diluted sodium nitrite (Sigma-Aldrich, Milan, Italy) was used to generate a standard curve. The data were expressed as µM of nitrite. Each sample was determined in triplicate.
3.5.4. Protein Array Analysis
In order to confirm the anti-inflammatory effect of NABE, we also measured the levels of proinflammatory and antinflammatory secreted cytokine proteins in LPS-stimulated macrophages by using the Proteome Profiler Mouse Cytokine Array Kit, Panel A (R&D system, Space import export Srl, Milan, Italy) following the manufacturer’s instructions. Briefly, J774A.1 macrophages were incubated with NABE at concentration of 100 µg/mL for 30 min and subsequently with LPS (1 μg/mL) for 24 h. Successively, cell supernatants were incubated with Mouse Cytokine Detection Antibody Cocktail for 1 h at room temperature. Then, the samples were incubated with a membrane containing 40 different anti-cytokine antibodies printed in duplicate at 4 °C overnight on a rocking platform shaker. The unbound proteins were removed and the membranes were washed three times with a washing buffer. The Streptavidin-HRP solution was then added to the membranes on a rocking platform shaker for 30 min. After being washed three times, the protein spots were visualized using the chemiluminescence detection reagents supplied in the Array Kits (R&D system, Space import export Srl, Milan, Italy). The intensity score of each duplicated array spot was measured with software ImageQuant Capture (GE Healthcare, Milan, Italy) and analyzed using Quantity One Software version 4.6.3 (Bio-Rad, Milan, Italy).
3.5.5. Statistical Analysis
Results are expressed as the mean ± standard error of the mean (S.E.M.) of 3 independent experiments. To determine statistical significance, a Student’s t-test was used for comparing a single treatment mean with a control mean, and a one-way analysis of variance followed by a Tukey–Kramer multiple comparisons test was used for analysis of multiple treatment means. A value of p < 0.05 was considered significant.
4. Conclusions
To the best of our knowledge, this is the first paper that has both analyzed the chemical composition of the aerial parts of Nepeta asterotricha and evaluated its anti-inflammatory action.
Phytochemical analysis revealed the presence of several already known iridoids, a new iridoid glycoside and several known polyphenols and flavonoids. As a result of the use of macrophages, we also demonstrated that the n-butanolic extract of Nepeta asterotricha exerted anti-inflammatory effects which are probably due to a synergistic action among the components present in the extract.
In conclusion, our results validate the traditional use of Nepeta asterotricha for the treatment of inflammatory conditions. Further pre-clinical studies should be performed in vivo to confirm the Nepeta asterotricha anti-inflammatory effect, as well as to assess its safety.
Supplementary Materials
The following are available online. Figures S1–S6: 1H, 13C-NMR, COSY, HSQC, HMBC and HRESIMS spectra of compound 1; Figures S7–S16: 1H-NMR spectra of compounds 2–11.
Author Contributions
Conceptualization and supervision, M.V.D.A.; Investigation, S.M.G., C.F. (Carmen Festa), S.D.M., C.F. (Claudia Finamore), E.P., A.S., O.A.P., F.B. Data curation, M.V.D.A., S.D.M., C.F. (Carmen Festa) and F.B. All authors contributed equally to this work. The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.
Funding
This work was supported by MIUR-ITALY PRIN2015 “Top-down and Bottom-up approach in the development of new bioactive chemical entities inspired on natural products scaffolds” (Project N. 2015MSCKCE_003).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 1–7 are available from the authors.
References
- 1.Rechinger K.H., Hedge I.C., Ietswaart J.H., Jalas J., Mennema J., Seybold S. Labiatae. In: Rechinger K.H., editor. Flora Iranica. Volume 150 ADEVA Akademische Druck- u. Verlagsanstalt; Graz, Austria: 1982. [Google Scholar]
- 2.Jamzad Z. A survey of Lamiaceae in the flora of Iran. Rostaniha. 2013;14:59–67. [Google Scholar]
- 3.Mozaffarian V. Identification of Medicinal and Aromatic Plants of Iran. Farhang Moaser Publishers; Tehran, Iran: 2013. p. 1444. [Google Scholar]
- 4.Süntar I., Nabavi S.M., Barreca D., Fischer N., Efferth T. Pharmacological and chemical features of Nepeta L. genus: Its importance as a therapeutic agent. Phytother. Res. 2018;32:185–198. doi: 10.1002/ptr.5946. [DOI] [PubMed] [Google Scholar]
- 5.Naghibi F., Mosaddegh M., Motamed S.M., Ghorbani A. Labiatae family in folk medicine in Iran: From ethnobotany to pharmacology. IJPR. 2005;4:63–79. [Google Scholar]
- 6.Salehi B., Valussi M., Jugran A.K., Martorell M., Ramirez-Alarcon K., Stojanovic-Radic Z.Z., Antolak H., Kregiel D., Ksenija S.M., Sharifi-Rad M. Nepeta species: From farm to food applications and phytotherapy. Trends Food Sci. Technol. 2018;80:104–122. doi: 10.1016/j.tifs.2018.07.030. [DOI] [Google Scholar]
- 7.Al-Taweel A.M., Raish M., Perveen S., Fawzy G.A., Ahmad A., Ansari M.A., Mudassar S., Ganaie M.A. Nepeta deflersiana attenuates isoproterenol-induced myocardial injuries in rats: Possible involvement of oxidative stress, apoptosis, inflammation through nuclear factor (NF)-κB down regulation. Phytomedicine. 2017;34:67–75. doi: 10.1016/j.phymed.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Rahmati B., Beik A. Prevention of morphine dependence and tolerance by Nepeta menthoides was accompanied by attenuation of nitric oxide overproduction in male mice. J. Ethnopharmacol. 2017;199:39–51. doi: 10.1016/j.jep.2017.01.032. [DOI] [PubMed] [Google Scholar]
- 9.Mahmood H., Chaudhry M.A., Masood Z., Saeed M.A., Adnan S. A mechanistic evaluation of the traditional uses of Nepeta ruderalis in gastrointestinal and airway disorders. Pharm. Biol. 2017;55:1017–1021. doi: 10.1080/13880209.2017.1285325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma A., Cannoo D.S. Phytochemical composition of essential oils isolated from different species of genus Nepeta of Labiatae family: A review. Pharmacophore. 2013;4:181–211. [Google Scholar]
- 11.Mamadalieva N.Z., Akramov D.K., Ovidi E., Tiezzi A., Nahar L., Azimova S.S., Sarker S.D. Aromatic medicinal plants of the Lamiaceae family from Uzbekistan: Ethnopharmacology, essential oils composition, and biological activities. Medicines. 2017;4:8. doi: 10.3390/medicines4010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ezzatzadeh E., Sofla S.F.I., Pourghasem E., Rustaiyan A., Zarezadeh A. Antimicrobial activity and chemical constituents of the essential oils from root, leaf and aerial part of Nepeta asterotricha from Iran. J. Essent. Oil Bear. Pl. 2014;17:415–421. doi: 10.1080/0972060X.2014.901624. [DOI] [Google Scholar]
- 13.Okabe Y., Medzhitov R. Tissue-specific signals control reversible program of localization and functional polarization of macrophages. Cell. 2014;157:832–844. doi: 10.1016/j.cell.2014.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meng F., Lowell C.A. Lipopolysaccharide (LPS)-induced macrophage activation and signal transduction in the absence of Src-family kinases Hck, Fgr, and Lyn. J. Exp. Med. 1997;185:1661–1670. doi: 10.1084/jem.185.9.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rossol M., Heine H., Meusch U., Quandt D., Klein C., Sweet M.J., Hauschildt S. LPS-induced cytokine production in human monocytes and macrophages. Crit. Rev. Immunol. 2011;31:379–446. doi: 10.1615/CritRevImmunol.v31.i5.20. [DOI] [PubMed] [Google Scholar]
- 16.Kolb H., Kolb-Bachofen V. Nitric oxide: A pathogenetic factor in autoimmunity. Immunol. Today. 1992;13:157–160. doi: 10.1016/0167-5699(92)90118-Q. [DOI] [PubMed] [Google Scholar]
- 17.Takeda Y., Yuuko K., Toshiya M., Hideaki O., Gisho H., Tagawa M., Sezik E., Yesilada E. Nepetaracemosides A and B, iridoid glucosides from Nepeta racemosa. Chem. Pharm. Bull. 1999;47:1433–1435. doi: 10.1248/cpb.47.1433. [DOI] [Google Scholar]
- 18.Sueyoshi E., Yu Q., Matsunami K., Otsuka H. Three new olefinic acetogenin glycosides from leaves of Staphylea bumalda DC. J. Nat. Med. 2009;63:61–64. doi: 10.1007/s11418-008-0279-3. [DOI] [PubMed] [Google Scholar]
- 19.Ahmed A.A., Hassan H.E., Hegazy M.F., Tzakou O., Couladis M., Mohamed A.E.H.H., Abdella M.A., Paré P. Argolic acid A and argolic methyl ester B, two new cyclopentano-monoterpenes diol from Nepeta argolica. Nat. Prod. Commun. 2006;1:523–526. doi: 10.1177/1934578X0600100701. [DOI] [Google Scholar]
- 20.Takeda Y., Ooiso Y., Masuda T., Honda G., Otsuka H., Sezik E., Yesilada E. Iridoid and eugenol glycosides from Nepeta cadmea. Phytochemistry. 1998;49:787–791. doi: 10.1016/S0031-9422(98)00125-3. [DOI] [Google Scholar]
- 21.Dinda B., Debnath S., Harigaya Y. Naturally occurring iridoids. A review, Part 1. Chem. Pharm. Bull. 2007;55:159–222. doi: 10.1248/cpb.55.159. [DOI] [PubMed] [Google Scholar]
- 22.Dinda B., Chowdhury D.R., Mohanta B.C. Naturally occurring iridoids, secoiridoids and their bioactivity. An updated review, Part 3. Chem. Pharm. Bull. 2009;57:765–796. doi: 10.1248/cpb.57.765. [DOI] [PubMed] [Google Scholar]
- 23.Dinda B., Debnath S., Banik R. Naturally occurring iridoids, secoiridoids and their bioactivity. An updated review, Part 4. Chem. Pharm. Bull. 2011;59:803–833. doi: 10.1248/cpb.59.803. [DOI] [PubMed] [Google Scholar]
- 24.Chaudhuri R.K., Salarna O., Sticher O. Structures of two rare iridoid glucosides from Penstemon barbatus. Tetrahedron Lett. 1981;22:4061–4064. doi: 10.1016/S0040-4039(01)82065-7. [DOI] [Google Scholar]
- 25.Roby M.R., Stermitz F.R. Penstemonoside and other iridoids from Castilleja rhexifolia.Conversion of penstemonoside to the pyridine monoterpene alkaloid rhexifoline. J. Nat. Prod. 1984;47:854–857. doi: 10.1021/np50035a017. [DOI] [Google Scholar]
- 26.Jensen S.R., Çalış I., Gotfredsen C.H., Søtofte I. Structural revision of some recently published iridoid glucosides. J. Nat. Prod. 2007;70:29–32. doi: 10.1021/np060452a. [DOI] [PubMed] [Google Scholar]
- 27.Krull R.E., Stermitz F.R. Trans-fused iridoid glycosides from Penstemon mucronatus. Phytochemistry. 1998;49:2413–2415. doi: 10.1016/S0031-9422(98)00349-5. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka N., Tanaka T., Fujioka T., Fujii H., Mihashi K., Shimomura K., Ishimaru K. An ellagic compound and iridoids from Cornus capitata root cultures. Phytochemistry. 2001;57:1287–1291. doi: 10.1016/S0031-9422(01)00179-0. [DOI] [PubMed] [Google Scholar]
- 29.Li M., Jia Z., Zhang R., Hu Z., Tian X. The structure of an iridoid glycoside, 8-deoxyshanzhiside, from Lamiophlomis rotate. Carbohydrate Res. 2008;343:561–565. doi: 10.1016/j.carres.2007.11.020. [DOI] [PubMed] [Google Scholar]
- 30.Takeda Y., Morimoto Y., Matsumoto T., Honda G., Tabata M., Fujita T., Otsuka H., Sezik E., Yesilada E. Nepetanudoside, an iridoid glucoside with an unusual stereostructure from Nepeta nuda ssp. albiflora. J. Nat. Prod. 1995;58:1217–1221. doi: 10.1021/np50122a009. [DOI] [Google Scholar]
- 31.Nagy T., Kocsis A., Morvai M., Szabo L.F., Podanyi B., Gergely A., Jerkovich G. 2′-4′- and 6′-O-substituted 1,5,9-epideoxyloganic acids from Nepeta grandiflora. Phytochemistry. 1998;47:1067–1072. doi: 10.1016/S0031-9422(98)80074-5. [DOI] [Google Scholar]
- 32.Formisano C., Rigano D., Senatore F. Chemical constituents and biological activities of Nepeta species. Chem. Biodivers. 2011;8:1783–1818. doi: 10.1002/cbdv.201000191. [DOI] [PubMed] [Google Scholar]
- 33.Jamzad Z., Grayer R.J., Kite G.C., Simmonds M.S.J., Ingrouille M., Jalili A. Leaf surface flavonoids in Iranian species of Nepeta (Lamiaceae) and some related genera. Biochem. Syst. Ecol. 2003;31:587–600. doi: 10.1016/S0305-1978(02)00221-1. [DOI] [Google Scholar]
- 34.Hou Z.F., Tu Y.Q., Li Y. Three new phenolic compounds from Nepeta prattii. J. Chin. Chem. Soc. 2002;49:255–258. doi: 10.1002/jccs.200200039. [DOI] [Google Scholar]
- 35.Grayer R.J., Veitch N.C., Kite G.C., Price A.M., Kokubun T. Distribution of 8-oxygenated leaf surface flavones in the genus Ocimum. Phytochemistry. 2001;56:559–567. doi: 10.1016/S0031-9422(00)00439-8. [DOI] [PubMed] [Google Scholar]
- 36.Jin B.R., Chung K.S., Cheon S.Y., Lee M., Hwang S., Noh Hwang S., Rhee K.J., An H.J. Rosmarinic acid suppresses colonic inflammation in dextran sulphate sodium (DSS)-induced mice via dual inhibition of NF-κB and STAT3 activation. Sci. Rep. 2017;7:46252. doi: 10.1038/srep46252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen W.P., Jin G.J., Xiong Y., Hu P.F., Bao J.P., Wu L.D. Rosmarinic acid down-regulates NO and PGE(2) expression via MAPK pathway in rat chondrocytes. J. Cell Mol. Med. 2018;22:346–353. doi: 10.1111/jcmm.13322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Palacz-Wrobel M., Borkowska P., Paul-Samojedny M., Kowalczyk M., Fila-Danilow A., Suchanek-Raif R., Kowalski J. Effect of apigenin, kaempferol and resveratrol on the gene expression and protein secretion of tumor necrosis factor alpha (TNF-α) and interleukin-10 (IL-10) in RAW-264.7 macrophages. Biomed. Pharmacother. 2017;93:1205–1212. doi: 10.1016/j.biopha.2017.07.054. [DOI] [PubMed] [Google Scholar]
- 39.Exarchou V., Troganis A., Gerothanassis I.P., Tsimidou M., Boskou D. Identification and quantification of caffeic and rosmarinic acid in complex plant extracts by the use of variable-temperature two-dimensional nuclear magnetic resonance spectroscopy. J. Agric. Food Chem. 2001;49:2–8. doi: 10.1021/jf990928e. [DOI] [PubMed] [Google Scholar]
- 40.Abedini A., Roumy V., Mahieux S., Biabiany M., Standaert-Vitse A., Rivière C., Sahpaz S., Bailleul F., Neut C., Hennebelle T. Rosmarinic acid and its methyl ester as antimicrobial components of the hydromethanolic extract of Hyptis atrorubens Poit. (Lamiaceae) Evid Based Complement. Alternat. Med. 2013;2013:604536. doi: 10.1155/2013/604536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferreres F., Barberan F.A.T., Tomas F. 5,6,4′-Trihydroxy-7,8-dimethoxyflavone from Thymus membranaceus. Phymchemistry. 1985;24:1869–1871. doi: 10.1016/S0031-9422(00)82579-0. [DOI] [Google Scholar]
- 42.Alwahsh M.A.A., Khairuddean M., Chong W.K. Chemical constituents and antioxidant activity of Teucrium barbeyanum Aschers. Rec. Nat. Prod. 2015;9:159–163. [Google Scholar]
- 43.Marra R., Nicoletti R., Pagano E., Della Greca M., Salvatore M.M., Borrelli F., Lombardi N., Vinale F., Woo S.L., Andolfi A. Inhibitory effect of trichodermanone C, a sorbicillinoid produced by Trichoderma citrinoviride associated to the green alga Cladophora sp., on nitrite production in LPS-stimulated macrophages. Nat. Prod. Res. 2018;31:1–9. doi: 10.1080/14786419.2018.1479702. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.