Abstract
Resveratrol increases the production of nitric oxide (NO) in endothelial cells by upregulating the expression of endothelial NO synthase (eNOS), stimulating eNOS enzymatic activity, and preventing eNOS uncoupling. At the same time, resveratrol inhibits the synthesis of endothelin-1 and reduces oxidative stress in both endothelial cells and smooth muscle cells. Pathological stimuli-induced smooth muscle cell proliferation, vascular remodeling, and arterial stiffness can be ameliorated by resveratrol as well. In addition, resveratrol also modulates immune cell function, inhibition of immune cell infiltration into the vascular wall, and improves the function of perivascular adipose tissue. All these mechanisms contribute to the protective effects of resveratrol on vascular function and blood pressure in vivo. Sirtuin 1, AMP-activated protein kinase, and estrogen receptors represent the major molecules mediating the vascular effects of resveratrol.
Keywords: resveratrol, endothelium, endothelial nitic oxide synthase, sirtuin 1, cardiovascular disease, vascular function
1. Resveratrol and its Molecular Targets
The polyphenolic phytoalexin 3,5,4′-trihydroxy-trans-stilbene, better known under its trivial name resveratrol, can be found in numerous plants, such as white hellebore (Veratrum grandiflorum), mulberry (Morus rubra), peanut (Archis hypogaea), and grapes (Vitis vinifera) [1,2,3].
Since resveratrol gained popularity in 1992 [4], many targets responsible for its pharmacological effects have been identified [5,6]. These targets can be divided into those which directly interact with resveratrol (over 20) and those whose effects are indirectly changed, e.g., by modulation of their expression level [6]. Regarding vascular function, especially the estrogen receptor (ER), the NAD+-dependent, class III histone deacetylase sirtuin 1 (SIRT1), the nuclear factor-erythroid-derived 2-related factor-2 (Nrf2), and the AMP-activated protein kinase (AMPK) are of particular importance [7].
Resveratrol can directly activate SIRT1 on certain substrates [8,9]. Indirectly, resveratrol increases SIRT1 activity either through elevation of intracellular NAD+ concentration, which is dependent on an inhibition of phosphodiesterase (PDE) [10,11], or through an enhancement of the binding of SIRT1 to lamin A, an endogenous SIRT1 activator [12]. In addition, the SIRT1-dependent effects of resveratrol in vivo are partially attributable to an upregulation of SIRT1 expression by the compound [13,14,15].
Nrf2 is an indirect target protein of resveratrol. For its stimulation, concentrations of resveratrol lower than 1 µM are sufficient (lower than that for SIRT1) [16], making its activation possible by using resveratrol as a dietary supplement [2]. Nrf2 interacts with antioxidant-response elements after its translocation to the nucleus. Here, it triggers the expression of phase II and antioxidant defense enzymes, like heme oxygenase-1 (HO-1) [16].
As mentioned before, resveratrol has been linked to PDE inhibition. One result of this effect is the phosphorylation of AMPK [11]. Another suggested pathway for AMPK activation is based on LKB1 stimulation, e.g., by reduction of the intracellular ATP level [17,18] or its deacetylation by SIRT1 [19,20]. Interestingly, AMPK and SIRT1 seem to function synergistically and bolster one another [21,22].
Resveratrol can directly bind the estrogen receptors (ERs) [23]. Very low (nanomolar) concentrations of resveratrol are enough for the ER-mediated stimulation of endothelial NO synthase (eNOS) [24,25]. Additionally, resveratrol-mediated ER-stimulation has also been linked to HO-1 upregulation as well as to Nox downregulation [26].
Also, the effects of resveratrol in vivo may also involve its actions on potassium channels [27,28,29], gut microbiota [30,31], and circadian gene expression [32].
2. Effects of Resveratrol on Endothelial Cells
The endothelium, consisting of a single layer of flat, longish endothelial cells, covers the inner walls of blood vessels. Vascular endothelial cells are unique in their property of holding Weibel-palade bodies, the depot for the Von Willebrand factor which is crucial for hemostasis maintenance. Apart from its function in maintaining blood coagulation and serving as starting point for angiogenesis, the endothelium also provides a semi-permeable barrier to regulate the transfer of electrolytes, macromolecules, and fluid between the intravascular and the extravascular space. Endothelial cells synthesize important vasoactive substances, including prostacyclin, NO, and the vasocontractile endothelin-1 (ET-1). Therefore, endothelial cells are key regulators of blood pressure and vascular tone [33].
2.1. Resveratrol Enhances Endothelial NO Production
Under physiological conditions, the endothelial NO synthase (eNOS) is the main producer of vascular NO [34]. It confers antithrombotic, antihypertensive, and anti-atherosclerotic effects [34,35]. Endothelial NO reaches the smooth muscle cells (SMC) by diffusion and causes vasodilation [36]. In the blood, eNOS-produced NO prevents platelet aggregation and adhesion. The anti-atherosclerotic properties of NO include the prevention of leukocyte adhesion to and migration into the vascular wall as well as the repression of low-density lipoprotein oxidation and the proliferation of vascular smooth muscle cells [34,37,38,39]. Suppression of the eNOS gene in mice leads to blood pressure elevation [40] and atherosclerosis aggravation [41,42]. Recently, it has been shown that eNOS-produced NO might be involved in mitochondrial biogenesis boosting [13] and could be partly responsible for the observed antiaging effects in calorie restriction studies [43]. In addition, it has been demonstrated that eNOS-knockout mice exhibit insulin resistance as well as hyperinsulinemia [44], while overexpression of eNOS protects mice fed with a high-fat diet (HFD) from pathological weight gain [45].
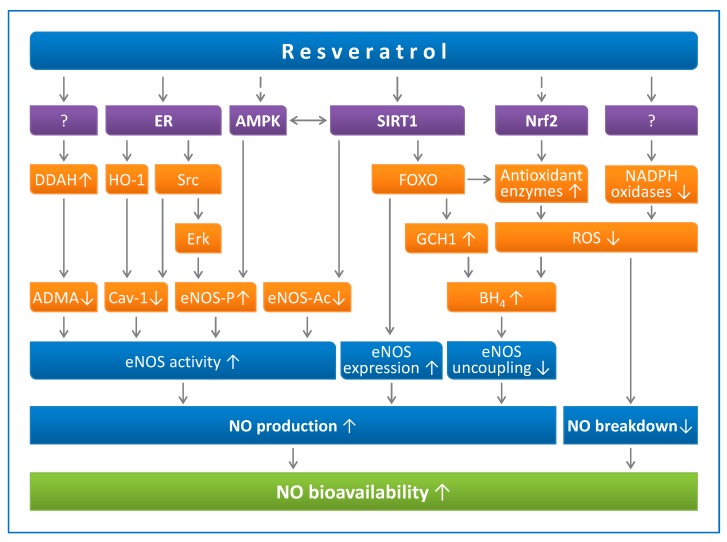
Resveratrol increases endothelial NO production through multiple mechanisms (Figure 1), including upregulation of eNOS expression, enhancement of eNOS enzymatic activity, and prevention of eNOS uncoupling [46].
Figure 1.
Resveratrol enhances NO production and prevents NO breakdown. Resveratrol can activate sirtuin 1 (SIRT1) directly (in a substrate-dependent manner) or indirectly (by either inhibiting phosphodiesterases or enhancing the effect of lamin A). SIRT1 stimulates endothelial NO synthase (eNOS) activity through deacetylation, enhances eNOS expression by deacetylating Forkhead box O (FOXO) transcription factors, and prevents eNOS uncoupling by upregulating GTP cyclohydrolase 1 (GCH1), the rate-limiting enzyme in tetrahydrobiopterin (BH4) biosynthesis. AMP-activated protein kinase (AMPK) and nuclear factor-erythroid-derived 2-related factor-2 (Nrf2) are indirect targets of resveratrol. AMPK phosphorylates eNOS at serine 1177. eNOS can also be phosphorylated by Erk1/2, which is stimulated by a pathway involving estrogen receptors (ER) and the tyrosine kinase Src. Caveolin-1 (Cav-1) is an eNOS-interacting protein that negatively regulates eNOS activity. Asymmetric dimethylarginine (ADMA) is an endogenous eNOS inhibitor that is degraded by dimethylarginine dimethylaminohydrolase (DDAH). The resveratrol targets for DDAH upregulation or for NADPH oxidase downregulation have not been identified so far. Reproduced from Xia et al. Molecules. 2014 [46], under the terms of the Creative Commons Attribution-Noncommercial License CC BY-NC.
2.1.1. Resveratrol Upregulates eNOS Expression
Resveratrol increases eNOS expression in endothelial cells. eNOS is constitutively expressed in the endothelium; its expression level changes in response to various stimuli and compounds [35,47]. We have previously shown that resveratrol itself [48] as well as resveratrol-containing red wines [49,50] enhance eNOS expression in endothelial cells (Table 1). Interestingly, this phenomenon is not dependent on ERs but associates with transcriptional and posttranscriptional mechanisms [48]. In endothelial cells, knock-down of the SIRT1 gene via siRNA inhibits the up-regulation of eNOS by resveratrol treatment [13]. Correspondingly, an endothelium-specific overexpression of SIRT1 leads to elevated eNOS expression [51]. Therefore, resveratrol-induced up-regulation of eNOS is likely to be SIRT1-dependent. Strengthening this suggestion, we were recently able to show the involvement of FOXO factors as downstream targets of SIRT1 in resveratrol effects [14].
Table 1.
Resveratrol increases NO production in endothelial cells.
| Cell Type | Effective Concentration | Effects | Reference |
|---|---|---|---|
| HUVEC | 10–100 µM | eNOS↑; NO↑ | [48] |
| EA.hy 926 | 10–100 µM | eNOS (via SIRT1/FOXO)↑; NO↑ | [14,48] |
| HCAEC | 1–100 µM | eNOS (via SIRT1)↑ | [13] |
| HUVEC | 0.1 µM | eNOS↑; VEGF↑; ET-1↓ | [33] |
| BAEC, HUVEC | 1–100 nM | p-eNOS↑ (via ERα & Erk1/2) | [24] |
| HUVEC | 1–100 µM | p-eNOS↑ (via AMPK) | [54] |
| STA | 50 µM | p-eNOS↑ (via AMPK) | [55] |
| RAEC | 100 µM | Ac-eNOS↓ | [56] |
Ac-eNOS; acetylated eNOS; BACE, bovine aortic endothelial cells; HCAEC, human coronary arterial endothelial cells; HUVEC, human umbilical vein endothelial cells; p-eNOS, phosphorylation of eNOS at serine 1177; STA, superior thyroid artery; RAEC, rat aortic endothelial cells; VEGF, vascular endothelial growth factor.
2.1.2. Resveratrol Increases eNOS Activity
In addition to eNOS expressional upregulation, resveratrol also enhances enzymatic activity of eNOS through post-translational modifications. Phosphorylation of eNOS at Ser-1177 is an activating modification [52,53]. Resveratrol has been shown to increase eNOS Ser-1177 phosphorylation in cultured endothelial cells [24,25]. For this effect, resveratrol concentrations of the nanomolar range are sufficient. The underlying signaling pathway includes the estrogen receptor ERα, G-protein Gα, caveolin-1 (Cav-1), the tyrosine kinase c-Src, and the MAP kinase Erk1/2 [24,25]. In concentrations of the micromolar range, phosphorylation of eNOS at the Ser-1177 by resveratrol also involves the activation of the AMPK [54,55]. Additionally, resveratrol enhances eNOS activity by inducing SIRT1-mediated deacetylation of eNOS at Lys-496 and Lys-506 [56,57].
An established endogenous eNOS inhibitor is asymmetric dimethylarginine (ADMA) [58]. Its degrading enzyme dimethylarginine dimethylaminohydrolase (DDAH) was found to be down-regulated by high concentrations of glucose, resulting in intracellular ADMA accumulation. This development, however, can be prevented by pre-treating of the cells with resveratrol [59].
Another negative regulator of eNOS is Cav-1 [60]. It was shown in vitro (endothelial cells [25,61]) and in vivo (rat heart [62,63]) that resveratrol targets these protein–protein interactions both by reduction of the Cav-1 expression level and by restriction of the Cav-1/eNOS association [62,63].
2.1.3. Resveratrol Prevents eNOS Uncoupling
Importantly, resveratrol also prevents eNOS uncoupling. This terminology describes the phenomenon of superoxide production by eNOS under pathological conditions [64,65,66]. Major mechanisms underlying eNOS uncoupling are the lack of the eNOS substrate l-arginine or the eNOS cofactor BH4 as well as eNOS S-glutathionylation [38,64,66]. Causes for BH4 insufficiency are its oxidation by potent oxidizing agents, such as peroxynitrite and superoxide [67,68,69,70]. This event and the following eNOS uncoupling though, can be prevented by resveratrol due to its antioxidant effects. Additionally, resveratrol boosts BH4 biosynthesis by enhancing the expression of GTP cyclohydrolase 1, an essential enzyme for BH4 production [71]. We previously described the vicious circle resulting from eNOS uncoupling leading to a potentiation of oxidative stress as a central mechanism adding to cardiovascular diseases [64,65,66]. Resveratrol intervenes in this circle by avoiding eNOS uncoupling and excess oxidative stress (Table 2).
Table 2.
Resveratrol reduces oxidative stress in endothelial cells.
| Cell Type | Effective Concentration | Effects | Reference |
|---|---|---|---|
| HUVEC | 1–100 µM | NADPH oxidase activity↓ | [72] |
| HUVEC | 10–100 µM | SOD1↑; GPx1↑; Nox4↓ | [73] |
| EA.hy 926 | 100 µM | SOD1↑; SOD2↑; SOD3↑; GPx1↑ catalase↑ | [71] |
| RAS | 1–100 µM | GPx1↑ catalase↑ | [74] |
| HCAEC | 1–10 µM | SOD2↑; SIRT1↑; GSH↑; mtROS↓ | [75] |
| HCAEC | 0.1–100 µM | Nrf2↑; NQO1↑; GCLC↑; HO-1↑ | [16] |
GCLC, γ-glutamylcysteine synthetase; GPx1, glutathione peroxidase 1; HCAEC, human coronary arterial endothelial cells; HO-1, heme oxygenase-1; HUVEC, human umbilical vein endothelial cells; NQO1, NAD(P)H:quinone–oxidoreductase 1; RAS, rat aortic segments; mtROS, mitochondrial reactive oxygen species.
2.2. Resveratrol Reduces Endothelial Oxidative Stress
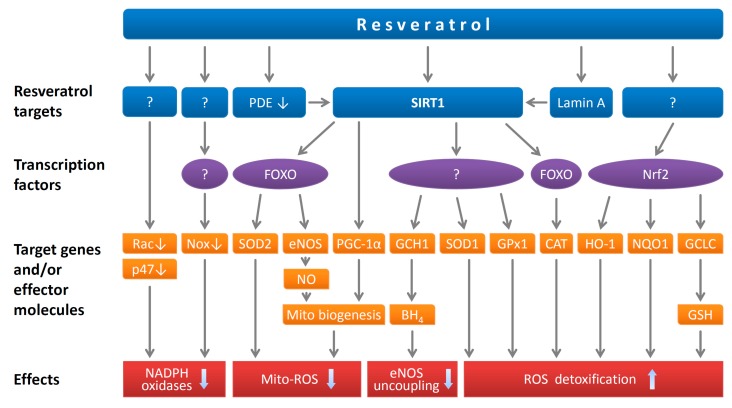
Since the capacity of resveratrol to directly eliminate free radicals is lower than that of already established antioxidant reagents, the means by which it diminishes oxidative stress in vivo are more likely due to its ability to regulate gene expression (Figure 2) [15]. Indeed, resveratrol is a potent gene regulator [76]. At a concentration of 0.1 µM, resveratrol has been shown to upregulate 127 genes and downregulate 233 genes in cultured human umbilical vein endothelial cells (HUVEC) [33].
Figure 2.
Antioxidant effects of resveratrol. Resveratrol inhibits NADPH oxidase-mediated reactive oxygen species (ROS) production by downregulation of the catalytic subunits (NOX proteins) and by inhibiting membrane translocation of Rac1 and inhibiting phosphorylation of p47phox. Resveratrol directly activates SIRT1 on certain substrates. It can also activate SIRT1 indirectly by potentiating the activation effect of lamin A or via a pathway involving phosphodiesterase (PDE) inhibition that leads to elevation of cellular NAD+. Among the established SIRT1 targets, FOXO transcription factors contribute to the antioxidant effects of resveratrol by upregulating antioxidant enzymes (e.g., SOD1, SOD2, GPx1 and catalase, CAT) and eNOS. SIRT1 inhibits mitochondrial superoxide production by stimulating mitochondrial biogenesis, which is mediated by PGC-1α deacetylation and by NO-dependent mechanisms. The upregulation of GCH1 leads to enhancement of BH4 biosynthesis and prevention of eNOS uncoupling. In addition, resveratrol upregulates a number of antioxidant enzymes by activating Nrf2. Reproduced from Xia et al. Br. J. Pharmacol. 2017 [15] with permission. Copyright © 2017 John Wiley and Sons (Hoboken, NJ, USA).
In cultured endothelial cells, resveratrol enhances the expression of phase 2 enzymes and antioxidant enzymes, including NAD(P)H:quinone–oxidoreductase 1 (NQO1), γ-glutamylcysteine synthetase (GCLC), HO-1, SOD1, SOD2, glutathione peroxidase 1 (GPx1), and catalase [16,71,73,74]. The expression [71,73] and activity [72] of NADPH oxidases, major ROS-producing enzymes in endothelial cells, are reduced by resveratrol (Table 2). Furthermore, resveratrol improves mitochondrial biogenesis and thus diminishes mitochondrial superoxide production [75].
SIRT1 and Nrf2 play important roles in the expressional regulation of redox genes by resveratrol [15]. Resveratrol-induced upregulation of SOD1, SOD2, GPx1, and catalase requires SIRT1, whereas Nrf2 is responsible for the upregulation of HO-1, NQO1, and GCLC by resveratrol [16,71,73,74,75].
2.3. Resveratrol Reduces Endothelin-1 Synthesis
ET-1 is a highly potent vasoconstrictor [33]. Overproduction of ET-1 is implicated in the development of vascular disease and atherosclerosis [77]. Interestingly, resveratrol has been shown to reduces ET-1 synthesis in endothelial cells and smooth muscle cells (Table 3), as well as in the plasma of resveratrol-treated rabbits [78]. It is conceivable that both the enhanced production of NO and reduced synthesis of ET-1 are involved in the vasodilation and blood pressure reduction by resveratrol in vivo.
Table 3.
Resveratrol reduces endothelin-1 synthesis.
| Cell Type | Effective Concentration | Effects | Reference |
|---|---|---|---|
| HUVEC | 1–100 µM | ROS↓; p-Erk1/2↓; strain-induced ET-1↓ | [72] |
| HUVEC | 0.1 µM | ET-1↓; eNOS↑; VEGF↑ | [79] |
| HUVEC | 30 µM | ET-1↓; ECE-1↓ | [80] |
| HASMC | 100 µM | H2O2-induced ET-1↓; | [81] |
| RASMC | 10–100 µM | AngII-induced ET-1↓; proliferation↓ | [82] |
AngII, angiotensin II; ECE-1, endothelin-converting enzyme-1; HASMC, human aortic smooth muscle cells; HUVEC, human umbilical vein endothelial cells; RASMC, rat aortic smooth muscle cells; ROS, reactive oxygen species.
3. Effects of Resveratrol on Vascular Smooth Muscle Cells
3.1. Resveratrol Reduces Oxidative Stress in Smooth Muscle Cells
Resveratrol has been shown to produce biphasic effects on HO-1 expression in human aortic SMC in a NF-κB-dependent manner, with a HO-1 induction at low concentrations of resveratrol (1–10 μM) and a reduction at high concentrations of resveratrol (≥20 μM) [83]. This phenomenon has not been observed in later studies. In in rat aortic SMC, resveratrol (1–30 µM) causes a concentration-dependent upregulation of HO-1 mediated by Nrf2 [84].
Treatment of cultured rat aortic SMC with resveratrol (25–100 µM) leads to upregulation of SOD, catalase, glutathione, glutathione reductase, glutathione peroxidase, glutathione S-transferase (GST) and NOQ1 [85]. GST and NQO1 are typical phase 2 enzymes responsible for detoxification of both ROS and electrophilic xenobiotics [85]. The induction of such cellular antioxidants and phase 2 enzymes by resveratrol protects the cells from oxidative stress and oxidants-induced cytotoxicity (Table 4).
Table 4.
Effects of resveratrol in vascular smooth muscle cells (VSMC).
| Cell Type | Effective Concentration | Effects | Reference |
|---|---|---|---|
| VSMC | 50–100 µM | Serum- and PDGF-induced proliferation↓ | [94] |
| RASMC | 0.1–1 µM | AGEs-stimulated proliferation↓ | [95] |
| RASMC | 25–50 µM | AngII-induced proliferation↓; p-Akt↓ | [96] |
| HASMC | 1–100 µM | Proliferation↓; p53↑; cell cycle arrest without apoptosis at 6.25–12.5 µM; apoptosis at 25 µM | [97] |
| RASMC | 50–100 µM | Serum-induced proliferation↓; cell cycle arrest | [98] |
| RASMC | 10–100 µM | AngII-induced proliferation↓; ET-1↓ | [82] |
| BASMC | 10–100 µM | Serum-induced proliferation↓; cell cycle arrest | [99] |
| HASMC | 20–100 µM | TNF-α-induced proliferation↓; cell cycle arrest | [100] |
| HASMC | 10–50 µM | Proliferation↓; p53↑; HSP27↑ | [101] |
| RFSMC | 25–50 µM | oxLDL-induced proliferation↓; PI3K/Akt/mTOR/p70S6K↓ | [102] |
| HASMC | 5–20 µM | PI3K activity↓; proliferation↓ | [103] |
| RASMC | 3–100 µM | Nrf2↑, HO-1↑; cyclin D↓, proliferation↓ | [84] |
| HVSMC | 3–100 µM | Differentiation of de-differentiated VSMC to the contractile phenotype | [86] |
| RASMC | 50 µM | TGF-β-stimulated SMC de-differentiation↓; p-Akt↓; p-mTOR↓; KLF5↓ | [93] |
AGEs, advanced glycation end-products; AngII, angiotensin II; BASMC, bovine aortic smooth muscle cells; HASMC, human aortic smooth muscle cells; HVSMC, human vascular smooth muscle cells; RASMC, rat aortic smooth muscle cells; RFSMC, rabbit femoral smooth muscle cells.
3.2. Resveratrol Inhibits Smooth Muscle Cell Proliferation
Normally differentiated vascular smooth muscle cells (VSMC) express a specific set of contractile proteins, have low synthetic activity, and proliferate slowly [86]. Upon stimulation by growth factor or in response to vascular injury, VSMC can change their phenotype through de-differentiation, proliferation, and migration to the site of injury [86]. While the phenotypic plasticity in VSMC is essential for the maintenance and repair of the vasculature, excessive proliferation of VSMC promotes the development of atherosclerosis, restenosis, and pulmonary hypertension [86,87].
Resveratrol has been shown to inhibit VSMC proliferation induced by various mitogens (Table 4). Depending on the mitogenic stimuli, the molecular mechanisms include inhibition of PI3K/Akt/mTOR pathway or cell cycle arrest (Table 4). In addition, antiproliferative effects in VSMC have also been reported for the resveratrol tetramer vitisin B [88] and for pterostilbene, a natural dimethylated analog of resveratrol [89]
Oral administration of resveratrol has been shown to significantly suppress intimal hyperplasia in mouse models of wire-injured arteries [84,90]. Intraperitoneal injection of resveratrol also inhibited the development of intimal hyperplasia in the rat carotid artery injury model [91].
Because of the low bioavailability of systemically administrated resveratrol, local delivery may represent a promising method improving the in vivo efficacy of the compound. Resveratrol coated on a stent reduced stenosis in rat carotid artery by 65% [92]. In a recent study of rat carotid balloon angioplasty, resveratrol was applied in Pluronic gel around the injured artery [93]. Strikingly, periadventitial application of resveratrol significantly improved all three major pathologies contributing to restenosis: VSMC hyperplasia in the intima, impairment of re-endothelialization, and constrictive remodeling [93]. Periadventitial delivery of resveratrol produced a much greater neointima-inhibiting effect (86%) than systemic resveratrol administration reported in previous studies. Moreover, post-surgery endothelial recovery was accelerated by resveratrol without causing constrictive remodeling [93]. These are compelling advantages over drug-eluting stents currently used in clinical settings.
3.3. Resveratrol Prevents Arterial Stiffness and Vascular Remodeling
In addition to inhibition of VSMC proliferation, resveratrol also prevents vascular remodeling and arterial stiffness. In cultured rat aortic SMC stimulated with advanced glycation end-products (AGEs), resveratrol reduces TGF-β1 expression and collagen synthesis [95]. In human VSMC, TNF-α-induced expression of matrix metalloproteinase (MMP)-9 is inhibited by resveratrol [100].
Resveratrol has been shown to prevent high-fat, high-sucrose diet-induced arterial stiffness in mice [104]. This effect is likely to be mediated by SIRT1, because similar effects can be achieved with SIRT1 activators or by global SIRT1 overexpression [104]. Interestingly, overnight fasting acutely decreased arterial stiffness in wildtype mice but not in mice lacking SIRT1 in VSMC. Conversely, VSMC-specific SIRT1 overexpression prevented diet-induced arterial stiffness. The antistiffness effect of SIRT1 has been attributable to its anti-inflammatory and antioxidant properties mediated by NF-κB inhibition and downregulation of VCAM-1 and p47phox [104].
Resveratrol downregulates angiotensin II (AngII) type 1 receptor expression in VSMC through SIRT1 activation [105]. Moreover, resveratrol treatment decreases serum AngII level and the aortic expression of prorenin receptor (PRR) and angiotensin converting enzyme (ACE) and increases serum Ang(1–7) level and the expression of ACE2, AngII type 2 receptor (AT2R), and Mas receptor (MasR). These mechanisms (modulation of the renin–angiotensin system) have been made responsible for the protective effects of resveratrol on aging-induced vascular fibrosis [106] and kidney fibrosis [107].
4. Effects of Resveratrol on Immune Cells
Although leukocytes do not belong to the resident cells of the vascular wall, they contribute to the pathogenesis of cardiovascular disease [38]. Activated endothelial cells express adhesion molecules on their surface, leading to cell–cell interaction and immune cell infiltration into the vascular wall [108]. Resveratrol modulates the function of immune cells, including CD4+/CD8+ T cells [109], regulatory T cells [110], natural killer (NK) cells [111], polymorphonuclear leukocytes [109], and monocytes [110,112].
Resveratrol prevents the interaction between immune cells and endothelial cells. On the one hand, resveratrol (10–100 µM) inhibits the expression and binding activity of chemokine receptors on leukocytes, e.g., CCR2 on monocytes [110]. On the other hand, resveratrol (0.1–1 µM) also reduces the expression of adhesion molecules on endothelial cells [113]. Reduced macrophage infiltration and ameliorated vascular inflammation by resveratrol have also been demonstrated in vivo [114].
5. Effects of Resveratrol on PVAT
The perivascular adipose tissue (PVAT) is now considered an important regulator of vascular function by secreting a large number of vasoactive substances, including NO [115,116]. We have recently seen that the importance of PVAT-eNOS may even exceed that of endothelial eNOS under certain conditions [117]. In the thoracic aorta of diet-induced obese mice, for example, vascular dysfunction can only be observed if PVAT is kept in place. In aortae without PVAT of mice with obesity, vascular function remains normal [117,118]. This observation has led to the hypothesis that the reduction of NO-dependent vasodilation in obese mice is not due to a dysfunction of eNOS in the endothelium but in the PVAT [117]. Indeed, vascular function of the obese mice can be normalized by pharmacological improvements of PVAT-eNOS function [117,118].
Interestingly, resveratrol has been shown to improve PVAT function [119,120]. The acetylcholine-induced relaxation of aortae from normal rats is inhibited by conditioned media derived from PVAT of rats fed with fructose [119] or HFD [120]. The fructose- and HFD-induced PVAT dysfunction is largely reversed by an oral treatment with resveratrol (20 mg/kg for 8 weeks) [119,120]. Similar PVAT dysfunction can be induced by an in vitro incubation of PVAT with palmitic acid. The palmitic acid-induced PVAT dysfunction is normalized by resveratrol (10 µM), an effect that is prevented by either the AMPK inhibitor compound C or the SIRT1 inhibitor nicotinamide [119]. Because these experiments are performed using PVAT-derived conditioned media, the observed protective effects of resveratrol can be clearly attributed to PVAT.
6. Effects of Resveratrol on Vascular Function and Blood Pressure in Vivo
Antihypertensive effects of resveratrol have been observed in several animal models [7,121,122]. These include genetic hypertension (e.g., spontaneously hypertensive rats [SHR]) [123,124], AngII-induced hypertension [124], renal models of hypertension [125,126], DOCA salt hypertension [127], programmed hypertension [128] and diet-induced metabolic syndromes [129,130,131]. Increased endothelial NO production, reduced vascular oxidative stress, and the resulting improvement of vascular function are likely the major molecular mechanisms underlying the blood pressure-lowering effects of resveratrol (Table 5).
Table 5.
Resveratrol reduces blood pressure in animal models.
| Model | Resveratrol dose | Effects | Reference |
|---|---|---|---|
| SHR | 5 mg/kg (50 mg/L in drinking water) for 10 weeks | BP↓; ROS↓; 3-NT↓; EF↑; eNOS↑; eNOS uncoupling↓ | [123] |
| SHR | 146 mg/kg (4 g/kg mixed in chow) for 5 weeks | BP↓; FMD↑; p-AMPK↑; p-eNOS↑; 4-HNE↓ | [124] |
| AngII-infused mouse | 320 mg/kg (4 g/kg mixed in chow) for 2 weeks | BP↓; FMD↑; p-AMPK↑; p-eNOS↑; 4-HNE↓ | [124] |
| Partially nephrectomized rats | 50 mg/kg/day mixed in diet for 4 weeks | BP↓; NO↑; ET-1↓; AngII↓ | [125] |
| Two-kidney, one-clip rats | 10 mg/kg i.p. for 6 weeks | BP↓; EF↑; plasma TAC ↑; NO↑; tissue SOD↑, catalase↑, GSH↑, MDA↓ cardiac hypertrophy↓ | [126] |
| DOCA salt | 1 mg/kg by gavage for 32 days | BP↓; EF↑ | [127] |
| Zucker rats | 10 mg/kg by gavage for 8 weeks | BP↓; eNOS↑; TG↓; TC↓; insulin↓; leptin↓ | [132] |
| HFD-fed female rats | 20 mg/kg/day mixed with diet for 8 weeks | BP↓; EF↑ | [129] |
| Fructose-fed rats | 10 mg/kg by gavage for 45 days | BP↓; cardiac hypertrophy↓ eNOS↑; TBARS↓ |
[130] |
| HFCS-induced MetS in rats | 5 mg/day (50 mg/L in drinking water) for 10 weeks | BP↓; TG↓; EF↑; p-eNOS↑; ROS↓ | [131] |
| Ovariectomized rats | 5 mg/kg by gavage for 3 weeks | BP↓; EF↑ | [133] |
| Obese rats programmed by early weaning | 30 mg/kg/day for 30 days | BP↓; TG↓; LDL↓; plasma MDA↓, SOD↑, catalase↑ | [128] |
3-NT, 3-nitrotyrosine; 4-HNE, 4-hydroxy-2-nonenal; BP, blood pressure; EF, endothelial function; HFCS, high-fructose corn syrup; HFD, high-fat diet; LDL, low-density lipoprotein; MDA, malondialdehyde; MetS, metabolic syndrome; SHR, spontaneously hypertensive rats; TAC, total antioxidant capacity; TBARS, thiobarbituric acid reactive substances; TC, total cholesterol; TG, triglycerides.
Interestingly, fecal microbiome transplants from resveratrol-treated healthy mice reduce the systolic blood pressure and left ventricular mass of hypertensive mice [30].
7. Conclusions
Resveratrol increases endothelial NO production, decreases ET-1 synthesis, reduces vascular oxidative stress, and prevents smooth muscle proliferation, vascular remodeling, and arterial stiffness. In addition, resveratrol also inhibits immune cells infiltration into the vascular wall and mitigates vascular inflammation. All these mechanisms contribute to the in vivo effects of resveratrol on vascular function and blood pressure.
Author Contributions
H.L. and A.D. drafted the manuscript. N.X. and S.H. made critical revisions.
Funding
Original works from the authors’ laboratory contributing to this review were supported by grants LI-1042/1-1 and LI-1042/3-1 from the Deutsche Forschungsgemeinschaft (DFG) and the intramural fund (Stufe I) of the Johannes Gutenberg University Medical Center Mainz. A.D. and H.L. were supported by research grants from the Boehringer Ingelheim Foundation for the collaborative research consortium “Novel and neglected cardiovascular risk factors: molecular mechanisms and therapeutic implications”.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Aggarwal B.B., Bhardwaj A., Aggarwal R.S., Seeram N.P., Shishodia S., Takada Y. Role of resveratrol in prevention and therapy of cancer: Preclinical and clinical studies. Anticancer Res. 2004;24:2783–2840. [PubMed] [Google Scholar]
- 2.Baur J.A., Sinclair D.A. Therapeutic potential of resveratrol: The in vivo evidence. Nat. Rev. Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- 3.Gupta S.C., Kannappan R., Reuter S., Kim J.H., Aggarwal B.B. Chemosensitization of tumors by resveratrol. Ann. N. Y. Acad. Sci. 2011;1215:150–160. doi: 10.1111/j.1749-6632.2010.05852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catalgol B., Batirel S., Taga Y., Ozer N.K. Resveratrol: French paradox revisited. Front. Pharmacol. 2012;3:141. doi: 10.3389/fphar.2012.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pirola L., Frojdo S. Resveratrol: One molecule, many targets. IUBMB Life. 2008;60:323–332. doi: 10.1002/iub.47. [DOI] [PubMed] [Google Scholar]
- 6.Harikumar K.B., Aggarwal B.B. Resveratrol: A multitargeted agent for age-associated chronic diseases. Cell Cycle. 2008;7:1020–1035. doi: 10.4161/cc.7.8.5740. [DOI] [PubMed] [Google Scholar]
- 7.Li H., Xia N., Forstermann U. Cardiovascular effects and molecular targets of resveratrol. Nitric Oxide. 2012;26:102–110. doi: 10.1016/j.niox.2011.12.006. [DOI] [PubMed] [Google Scholar]
- 8.Hubbard B.P., Gomes A.P., Dai H., Li J., Case A.W., Considine T., Riera T.V., Lee J.E., E S.Y., Lamming D.W., et al. Evidence for a common mechanism of SIRT1 regulation by allosteric activators. Science. 2013;339:1216–1219. doi: 10.1126/science.1231097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Howitz K.T., Bitterman K.J., Cohen H.Y., Lamming D.W., Lavu S., Wood J.G., Zipkin R.E., Chung P., Kisielewski A., Zhang L.L., et al. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- 10.Alexander S.P., Fabbro D., Kelly E., Marrion N., Peters J.A., Benson H.E., Faccenda E., Pawson A.J., Sharman J.L., Southan C., et al. The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br. J. Pharmacol. 2015;172:6024–6109. doi: 10.1111/bph.13354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park S.J., Ahmad F., Philp A., Baar K., Williams T., Luo H., Ke H., Rehmann H., Taussig R., Brown A.L., et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell. 2012;148:421–433. doi: 10.1016/j.cell.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu B., Ghosh S., Yang X., Zheng H., Liu X., Wang Z., Jin G., Zheng B., Kennedy B.K., Suh Y., et al. Resveratrol rescues SIRT1-dependent adult stem cell decline and alleviates progeroid features in laminopathy-based progeria. Cell Metab. 2012;16:738–750. doi: 10.1016/j.cmet.2012.11.007. [DOI] [PubMed] [Google Scholar]
- 13.Csiszar A., Labinskyy N., Pinto J.T., Ballabh P., Zhang H., Losonczy G., Pearson K., de Cabo R., Pacher P., Zhang C., et al. Resveratrol induces mitochondrial biogenesis in endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H13–H20. doi: 10.1152/ajpheart.00368.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xia N., Strand S., Schlufter F., Siuda D., Reifenberg G., Kleinert H., Forstermann U., Li H. Role of SIRT1 and FOXO factors in eNOS transcriptional activation by resveratrol. Nitric Oxide. 2013;32:29–35. doi: 10.1016/j.niox.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 15.Xia N., Daiber A., Forstermann U., Li H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017;174:1633–1646. doi: 10.1111/bph.13492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ungvari Z., Bagi Z., Feher A., Recchia F.A., Sonntag W.E., Pearson K., de Cabo R., Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H18–H24. doi: 10.1152/ajpheart.00260.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hawley S.A., Ross F.A., Chevtzoff C., Green K.A., Evans A., Fogarty S., Towler M.C., Brown L.J., Ogunbayo O.A., Evans A.M., et al. Use of cells expressing gamma subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 2010;11:554–565. doi: 10.1016/j.cmet.2010.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dasgupta B., Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc. Natl. Acad. Sci. USA. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou X., Xu S., Maitland-Toolan K.A., Sato K., Jiang B., Ido Y., Lan F., Walsh K., Wierzbicki M., Verbeuren T.J., et al. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J. Biol. Chem. 2008;283:20015–20026. doi: 10.1074/jbc.M802187200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lan F., Cacicedo J.M., Ruderman N., Ido Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J. Biol. Chem. 2008;283:27628–27635. doi: 10.1074/jbc.M805711200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ruderman N.B., Xu X.J., Nelson L., Cacicedo J.M., Saha A.K., Lan F., Ido Y. AMPK and SIRT1: A long-standing partnership? Am. J. Physiol. Endocrinol. Metab. 2010;298:E751–E760. doi: 10.1152/ajpendo.00745.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haigis M.C., Sinclair D.A. Mammalian sirtuins: Biological insights and disease relevance. Annu. Rev. Pathol. 2010;5:253–295. doi: 10.1146/annurev.pathol.4.110807.092250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bowers J.L., Tyulmenkov V.V., Jernigan S.C., Klinge C.M. Resveratrol acts as a mixed agonist/antagonist for estrogen receptors alpha and beta. Endocrinology. 2000;141:3657–3667. doi: 10.1210/endo.141.10.7721. [DOI] [PubMed] [Google Scholar]
- 24.Klinge C.M., Blankenship K.A., Risinger K.E., Bhatnagar S., Noisin E.L., Sumanasekera W.K., Zhao L., Brey D.M., Keynton R.S. Resveratrol and estradiol rapidly activate MAPK signaling through estrogen receptors alpha and beta in endothelial cells. J. Biol. Chem. 2005;280:7460–7468. doi: 10.1074/jbc.M411565200. [DOI] [PubMed] [Google Scholar]
- 25.Klinge C.M., Wickramasinghe N.S., Ivanova M.M., Dougherty S.M. Resveratrol stimulates nitric oxide production by increasing estrogen receptor alpha-Src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J. 2008;22:2185–2197. doi: 10.1096/fj.07-103366. [DOI] [PubMed] [Google Scholar]
- 26.Yu H.P., Hwang T.L., Hwang T.L., Yen C.H., Lau Y.T. Resveratrol prevents endothelial dysfunction and aortic superoxide production after trauma hemorrhage through estrogen receptor-dependent hemeoxygenase-1 pathway. Crit. Care Med. 2010;38:1147–1154. doi: 10.1097/CCM.0b013e3181cd124e. [DOI] [PubMed] [Google Scholar]
- 27.Nagaoka T., Hein T.W., Yoshida A., Kuo L. Resveratrol, a component of red wine, elicits dilation of isolated porcine retinal arterioles: Role of nitric oxide and potassium channels. Investig. Ophthalmol. Vis. Sci. 2007;48:4232–4239. doi: 10.1167/iovs.07-0094. [DOI] [PubMed] [Google Scholar]
- 28.Gojkovic-Bukarica L., Novakovic A., Kanjuh V., Bumbasirevic M., Lesic A., Heinle H. A role of ion channels in the endothelium-independent relaxation of rat mesenteric artery induced by resveratrol. J. Pharmacol. Sci. 2008;108:124–130. doi: 10.1254/jphs.08128FP. [DOI] [PubMed] [Google Scholar]
- 29.Protic D., Radunovic N., Spremovic-Radenovic S., Zivanovic V., Heinle H., Petrovic A., Gojkovic-Bukarica L. The Role of Potassium Channels in the Vasodilatation Induced by Resveratrol and Naringenin in Isolated Human Umbilical Vein. Drug. Dev. Res. 2015;76:17–23. doi: 10.1002/ddr.21236. [DOI] [PubMed] [Google Scholar]
- 30.Kim T.T., Parajuli N., Sung M.M., Bairwa S.C., Levasseur J., Soltys C.M., Wishart D.S., Madsen K., Schertzer J.D., Dyck J.R.B. Fecal transplant from resveratrol-fed donors improves glycaemia and cardiovascular features of the metabolic syndrome in mice. Am. J. Physiol. Endocrinol. Metab. 2018;315:E511–E519. doi: 10.1152/ajpendo.00471.2017. [DOI] [PubMed] [Google Scholar]
- 31.Chaplin A., Carpene C., Mercader J. Resveratrol, Metabolic Syndrome, and Gut Microbiota. Nutrients. 2018;10:1651. doi: 10.3390/nu10111651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Park I., Lee Y., Kim H.D., Kim K. Effect of Resveratrol, a SIRT1 Activator, on the Interactions of the CLOCK/BMAL1 Complex. Endocrinol. Metab. 2014;29:379–387. doi: 10.3803/EnM.2014.29.3.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nicholson S.K., Tucker G.A., Brameld J.M. Effects of dietary polyphenols on gene expression in human vascular endothelial cells. Proc. Nutr. Soc. 2008;67:42–47. doi: 10.1017/S0029665108006009. [DOI] [PubMed] [Google Scholar]
- 34.Li H., Forstermann U. Nitric oxide in the pathogenesis of vascular disease. J. Pathol. 2000;190:244–254. doi: 10.1002/(SICI)1096-9896(200002)190:3<244::AID-PATH575>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 35.Li H., Wallerath T., Forstermann U. Physiological mechanisms regulating the expression of endothelial-type NO synthase. Nitric Oxide. 2002;7:132–147. doi: 10.1016/S1089-8603(02)00127-1. [DOI] [PubMed] [Google Scholar]
- 36.Zhao Y., Vanhoutte P.M., Leung S.W. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015;129:83–94. doi: 10.1016/j.jphs.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 37.Li H., Forstermann U. Prevention of atherosclerosis by interference with the vascular nitric oxide system. Curr. Pharm. Des. 2009;15:3133–3145. doi: 10.2174/138161209789058002. [DOI] [PubMed] [Google Scholar]
- 38.Forstermann U., Xia N., Li H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017;120:713–735. doi: 10.1161/CIRCRESAHA.116.309326. [DOI] [PubMed] [Google Scholar]
- 39.Li H., Horke S., Forstermann U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis. 2014;237:208–219. doi: 10.1016/j.atherosclerosis.2014.09.001. [DOI] [PubMed] [Google Scholar]
- 40.Huang P.L., Huang Z., Mashimo H., Bloch K.D., Moskowitz M.A., Bevan J.A., Fishman M.C. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0. [DOI] [PubMed] [Google Scholar]
- 41.Kuhlencordt P.J., Gyurko R., Han F., Scherrer-Crosbie M., Aretz T.H., Hajjar R., Picard M.H., Huang P.L. Accelerated atherosclerosis, aortic aneurysm formation, and ischemic heart disease in apolipoprotein E/endothelial nitric oxide synthase double-knockout mice. Circulation. 2001;104:448–454. doi: 10.1161/hc2901.091399. [DOI] [PubMed] [Google Scholar]
- 42.Liu V.W., Huang P.L. Cardiovascular roles of nitric oxide: A review of insights from nitric oxide synthase gene disrupted mice. Cardiovasc. Res. 2008;77:19–29. doi: 10.1016/j.cardiores.2007.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nisoli E., Tonello C., Cardile A., Cozzi V., Bracale R., Tedesco L., Falcone S., Valerio A., Cantoni O., Clementi E., et al. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 44.Duplain H., Burcelin R., Sartori C., Cook S., Egli M., Lepori M., Vollenweider P., Pedrazzini T., Nicod P., Thorens B., et al. Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation. 2001;104:342–345. doi: 10.1161/01.CIR.104.3.342. [DOI] [PubMed] [Google Scholar]
- 45.Sansbury B.E., Cummins T.D., Tang Y., Hellmann J., Holden C.R., Harbeson M.A., Chen Y., Patel R.P., Spite M., Bhatnagar A., et al. Overexpression of endothelial nitric oxide synthase prevents diet-induced obesity and regulates adipocyte phenotype. Circ. Res. 2012;111:1176–1189. doi: 10.1161/CIRCRESAHA.112.266395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Xia N., Forstermann U., Li H. Resveratrol and endothelial nitric oxide. Molecules. 2014;19:16102–16121. doi: 10.3390/molecules191016102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Li H., Wallerath T., Munzel T., Forstermann U. Regulation of endothelial-type NO synthase expression in pathophysiology and in response to drugs. Nitric Oxide. 2002;7:149–164. doi: 10.1016/S1089-8603(02)00111-8. [DOI] [PubMed] [Google Scholar]
- 48.Wallerath T., Deckert G., Ternes T., Anderson H., Li H., Witte K., Forstermann U. Resveratrol, a polyphenolic phytoalexin present in red wine, enhances expression and activity of endothelial nitric oxide synthase. Circulation. 2002;106:1652–1658. doi: 10.1161/01.CIR.0000029925.18593.5C. [DOI] [PubMed] [Google Scholar]
- 49.Wallerath T., Poleo D., Li H., Forstermann U. Red wine increases the expression of human endothelial nitric oxide synthase: A mechanism that may contribute to its beneficial cardiovascular effects. J. Am. Coll. Cardiol. 2003;41:471–478. doi: 10.1016/S0735-1097(02)02826-7. [DOI] [PubMed] [Google Scholar]
- 50.Wallerath T., Li H., Godtel-Ambrust U., Schwarz P.M., Forstermann U. A blend of polyphenolic compounds explains the stimulatory effect of red wine on human endothelial NO synthase. Nitric Oxide. 2005;12:97–104. doi: 10.1016/j.niox.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 51.Zhang Q.J., Wang Z., Chen H.Z., Zhou S., Zheng W., Liu G., Wei Y.S., Cai H., Liu D.P., Liang C.C. Endothelium-specific overexpression of class III deacetylase SIRT1 decreases atherosclerosis in apolipoprotein E-deficient mice. Cardiovasc. Res. 2008;80:191–199. doi: 10.1093/cvr/cvn224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fleming I. Molecular mechanisms underlying the activation of eNOS. Pflugers Arch. 2010;459:793–806. doi: 10.1007/s00424-009-0767-7. [DOI] [PubMed] [Google Scholar]
- 53.Heiss E.H., Dirsch V.M. Regulation of eNOS enzyme activity by posttranslational modification. Curr. Pharm. Des. 2014;20:3503–3513. doi: 10.2174/13816128113196660745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu Q., Hao X., Yang Q., Si L. Resveratrol prevents hyperglycemia-induced endothelial dysfunction via activation of adenosine monophosphate-activated protein kinase. Biochem. Biophys. Res. Commun. 2009;388:389–394. doi: 10.1016/j.bbrc.2009.08.021. [DOI] [PubMed] [Google Scholar]
- 55.Carrizzo A., Puca A., Damato A., Marino M., Franco E., Pompeo F., Traficante A., Civitillo F., Santini L., Trimarco V., et al. Resveratrol improves vascular function in patients with hypertension and dyslipidemia by modulating NO metabolism. Hypertension. 2013;62:359–366. doi: 10.1161/HYPERTENSIONAHA.111.01009. [DOI] [PubMed] [Google Scholar]
- 56.Mattagajasingh I., Kim C.S., Naqvi A., Yamamori T., Hoffman T.A., Jung S.B., DeRicco J., Kasuno K., Irani K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA. 2007;104:14855–14860. doi: 10.1073/pnas.0704329104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Arunachalam G., Yao H., Sundar I.K., Caito S., Rahman I. SIRT1 regulates oxidant- and cigarette smoke-induced eNOS acetylation in endothelial cells: Role of resveratrol. Biochem. Biophys. Res. Commun. 2010;393:66–72. doi: 10.1016/j.bbrc.2010.01.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maas R., Boger R., Luneburg N. ADMA and the role of the genes: Lessons from genetically modified animals and human gene polymorphisms. Pharmacol. Res. 2009;60:475–480. doi: 10.1016/j.phrs.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 59.Frombaum M., Therond P., Djelidi R., Beaudeux J.L., Bonnefont-Rousselot D., Borderie D. Piceatannol is more effective than resveratrol in restoring endothelial cell dimethylarginine dimethylaminohydrolase expression and activity after high-glucose oxidative stress. Free Radic. Res. 2011;45:293–302. doi: 10.3109/10715762.2010.527337. [DOI] [PubMed] [Google Scholar]
- 60.Feron O., Dessy C., Moniotte S., Desager J.P., Balligand J.L. Hypercholesterolemia decreases nitric oxide production by promoting the interaction of caveolin and endothelial nitric oxide synthase. J. Clin. Investig. 1999;103:897–905. doi: 10.1172/JCI4829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tian C., Zhang R., Ye X., Zhang C., Jin X., Yamori Y., Hao L., Sun X., Ying C. Resveratrol ameliorates high-glucose-induced hyperpermeability mediated by caveolae via VEGF/KDR pathway. Genes Nutr. 2013;8:231–239. doi: 10.1007/s12263-012-0319-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Penumathsa S.V., Koneru S., Samuel S.M., Maulik G., Bagchi D., Yet S.F., Menon V.P., Maulik N. Strategic targets to induce neovascularization by resveratrol in hypercholesterolemic rat myocardium: Role of caveolin-1, endothelial nitric oxide synthase, hemeoxygenase-1, and vascular endothelial growth factor. Free Radic. Biol. Med. 2008;45:1027–1034. doi: 10.1016/j.freeradbiomed.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Penumathsa S.V., Thirunavukkarasu M., Zhan L., Maulik G., Menon V.P., Bagchi D., Maulik N. Resveratrol enhances GLUT-4 translocation to the caveolar lipid raft fractions through AMPK/Akt/eNOS signalling pathway in diabetic myocardium. J. Cell. Mol. Med. 2008;12:2350–2361. doi: 10.1111/j.1582-4934.2008.00251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Forstermann U., Munzel T. Endothelial nitric oxide synthase in vascular disease: From marvel to menace. Circulation. 2006;113:1708–1714. doi: 10.1161/CIRCULATIONAHA.105.602532. [DOI] [PubMed] [Google Scholar]
- 65.Li H., Forstermann U. Pharmacological Prevention of eNOS Uncoupling. Curr. Pharm. Des. 2014;20:3595–3606. doi: 10.2174/13816128113196660749. [DOI] [PubMed] [Google Scholar]
- 66.Li H., Forstermann U. Uncoupling of endothelial NO synthase in atherosclerosis and vascular disease. Curr. Opin. Pharmacol. 2013;13:161–167. doi: 10.1016/j.coph.2013.01.006. [DOI] [PubMed] [Google Scholar]
- 67.Laursen J.B., Somers M., Kurz S., McCann L., Warnholtz A., Freeman B.A., Tarpey M., Fukai T., Harrison D.G. Endothelial regulation of vasomotion in apoE-deficient mice: Implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–1288. doi: 10.1161/01.CIR.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 68.Landmesser U., Dikalov S., Price S.R., McCann L., Fukai T., Holland S.M., Mitch W.E., Harrison D.G. Oxidation of tetrahydrobiopterin leads to uncoupling of endothelial cell nitric oxide synthase in hypertension. J. Clin. Investig. 2003;111:1201–1209. doi: 10.1172/JCI200314172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Alp N.J., McAteer M.A., Khoo J., Choudhury R.P., Channon K.M. Increased endothelial tetrahydrobiopterin synthesis by targeted transgenic GTP-cyclohydrolase I overexpression reduces endothelial dysfunction and atherosclerosis in ApoE-knockout mice. Arterioscler. Thromb. Vasc. Biol. 2004;24:445–450. doi: 10.1161/01.ATV.0000115637.48689.77. [DOI] [PubMed] [Google Scholar]
- 70.Zou M.H., Shi C., Cohen R.A. Oxidation of the zinc-thiolate complex and uncoupling of endothelial nitric oxide synthase by peroxynitrite. J. Clin. Investig. 2002;109:817–826. doi: 10.1172/JCI0214442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xia N., Daiber A., Habermeier A., Closs E.I., Thum T., Spanier G., Lu Q., Oelze M., Torzewski M., Lackner K.J., et al. Resveratrol reverses endothelial nitric-oxide synthase uncoupling in apolipoprotein E knockout mice. J. Pharmacol. Exp. Ther. 2010;335:149–154. doi: 10.1124/jpet.110.168724. [DOI] [PubMed] [Google Scholar]
- 72.Liu J.C., Chen J.J., Chan P., Cheng C.F., Cheng T.H. Inhibition of cyclic strain-induced endothelin-1 gene expression by resveratrol. Hypertension. 2003;42:1198–1205. doi: 10.1161/01.HYP.0000103162.76220.51. [DOI] [PubMed] [Google Scholar]
- 73.Spanier G., Xu H., Xia N., Tobias S., Deng S., Wojnowski L., Forstermann U., Li H. Resveratrol reduces endothelial oxidative stress by modulating the gene expression of superoxide dismutase 1 (SOD1), glutathione peroxidase 1 (GPx1) and NADPH oxidase subunit (Nox4) J. Physiol. Pharmacol. 2009;60(Suppl. 4):111–116. [PubMed] [Google Scholar]
- 74.Ungvari Z., Orosz Z., Rivera A., Labinskyy N., Xiangmin Z., Olson S., Podlutsky A., Csiszar A. Resveratrol increases vascular oxidative stress resistance. Am. J. Physiol. Heart Circ. Physiol. 2007;292:H2417–H2424. doi: 10.1152/ajpheart.01258.2006. [DOI] [PubMed] [Google Scholar]
- 75.Ungvari Z., Labinskyy N., Mukhopadhyay P., Pinto J.T., Bagi Z., Ballabh P., Zhang C., Pacher P., Csiszar A. Resveratrol attenuates mitochondrial oxidative stress in coronary arterial endothelial cells. Am. J. Physiol. Heart Circ. Physiol. 2009;297:H1876–H1881. doi: 10.1152/ajpheart.00375.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xia N., Forstermann U., Li H. Resveratrol as a gene regulator in the vasculature. Curr. Pharm. Biotechnol. 2014;15:401–408. doi: 10.2174/1389201015666140711114450. [DOI] [PubMed] [Google Scholar]
- 77.Corder R., Douthwaite J.A., Lees D.M., Khan N.Q., Viseu Dos Santos A.C., Wood E.G., Carrier M.J. Endothelin-1 synthesis reduced by red wine. Nature. 2001;414:863–864. doi: 10.1038/414863a. [DOI] [PubMed] [Google Scholar]
- 78.Zou J.G., Wang Z.R., Huang Y.Z., Cao K.J., Wu J.M. Effect of red wine and wine polyphenol resveratrol on endothelial function in hypercholesterolemic rabbits. Int. J. Mol. Med. 2003;11:317–320. doi: 10.3892/ijmm.11.3.317. [DOI] [PubMed] [Google Scholar]
- 79.Nicholson S.K., Tucker G.A., Brameld J.M. Physiological concentrations of dietary polyphenols regulate vascular endothelial cell expression of genes important in cardiovascular health. Br. J. Nutr. 2010;103:1398–1403. doi: 10.1017/S0007114509993485. [DOI] [PubMed] [Google Scholar]
- 80.Coppa T., Lazze M.C., Cazzalini O., Perucca P., Pizzala R., Bianchi L., Stivala L.A., Forti L., Maccario C., Vannini V., et al. Structure-activity relationship of resveratrol and its analogue, 4,4’-dihydroxy-trans-stilbene, toward the endothelin axis in human endothelial cells. J. Med. Food. 2011;14:1173–1180. doi: 10.1089/jmf.2010.0272. [DOI] [PubMed] [Google Scholar]
- 81.Ruef J., Moser M., Kubler W., Bode C. Induction of endothelin-1 expression by oxidative stress in vascular smooth muscle cells. Cardiovasc. Pathol. 2001;10:311–315. doi: 10.1016/S1054-8807(01)00095-3. [DOI] [PubMed] [Google Scholar]
- 82.Chao H.H., Juan S.H., Liu J.C., Yang H.Y., Yang E., Cheng T.H., Shyu K.G. Resveratrol inhibits angiotensin II-induced endothelin-1 gene expression and subsequent proliferation in rat aortic smooth muscle cells. Eur. J. Pharmacol. 2005;515:1–9. doi: 10.1016/j.ejphar.2005.03.035. [DOI] [PubMed] [Google Scholar]
- 83.Juan S.H., Cheng T.H., Lin H.C., Chu Y.L., Lee W.S. Mechanism of concentration-dependent induction of heme oxygenase-1 by resveratrol in human aortic smooth muscle cells. Biochem. Pharmacol. 2005;69:41–48. doi: 10.1016/j.bcp.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 84.Kim J.W., Lim S.C., Lee M.Y., Lee J.W., Oh W.K., Kim S.K., Kang K.W. Inhibition of neointimal formation by trans-resveratrol: Role of phosphatidyl inositol 3-kinase-dependent Nrf2 activation in heme oxygenase-1 induction. Mol. Nutr. Food Res. 2010;54:1497–1505. doi: 10.1002/mnfr.201000016. [DOI] [PubMed] [Google Scholar]
- 85.Li Y., Cao Z., Zhu H. Upregulation of endogenous antioxidants and phase 2 enzymes by the red wine polyphenol, resveratrol in cultured aortic smooth muscle cells leads to cytoprotection against oxidative and electrophilic stress. Pharmacol. Res. 2006;53:6–15. doi: 10.1016/j.phrs.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 86.Thompson A.M., Martin K.A., Rzucidlo E.M. Resveratrol induces vascular smooth muscle cell differentiation through stimulation of SirT1 and AMPK. PLoS ONE. 2014;9:e85495. doi: 10.1371/journal.pone.0085495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wang D., Uhrin P., Mocan A., Waltenberger B., Breuss J.M., Tewari D., Mihaly-Bison J., Huminiecki L., Starzynski R.R., Tzvetkov N.T., et al. Vascular smooth muscle cell proliferation as a therapeutic target. Part 1: Molecular targets and pathways. Biotechnol. Adv. 2018;36:1586–1607. doi: 10.1016/j.biotechadv.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 88.Ong E.T., Hwang T.L., Huang Y.L., Lin C.F., Wu W.B. Vitisin B, a resveratrol tetramer, inhibits migration through inhibition of PDGF signaling and enhancement of cell adhesiveness in cultured vascular smooth muscle cells. Toxicol. Appl. Pharmacol. 2011;256:198–208. doi: 10.1016/j.taap.2011.08.013. [DOI] [PubMed] [Google Scholar]
- 89.Park E.S., Lim Y., Hong J.T., Yoo H.S., Lee C.K., Pyo M.Y., Yun Y.P. Pterostilbene, a natural dimethylated analog of resveratrol, inhibits rat aortic vascular smooth muscle cell proliferation by blocking Akt-dependent pathway. Vascul. Pharmacol. 2010;53:61–67. doi: 10.1016/j.vph.2010.04.001. [DOI] [PubMed] [Google Scholar]
- 90.Khandelwal A.R., Hebert V.Y., Kleinedler J.J., Rogers L.K., Ullevig S.L., Asmis R., Shi R., Dugas T.R. Resveratrol and quercetin interact to inhibit neointimal hyperplasia in mice with a carotid injury. J. Nutr. 2012;142:1487–1494. doi: 10.3945/jn.112.162628. [DOI] [PubMed] [Google Scholar]
- 91.Orozco-Sevilla V., Naftalovich R., Hoffmann T., London D., Czernizer E., Yang C., Dardik A., Dardik H. Epigallocatechin-3-gallate is a potent phytochemical inhibitor of intimal hyperplasia in the wire-injured carotid artery. J. Vasc. Surg. 2013;58:1360–1365. doi: 10.1016/j.jvs.2012.11.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kleinedler J.J., Foley J.D., Orchard E.A., Dugas T.R. Novel nanocomposite stent coating releasing resveratrol and quercetin reduces neointimal hyperplasia and promotes re-endothelialization. J. Control. Release. 2012;159:27–33. doi: 10.1016/j.jconrel.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 93.Zhu Y., Takayama T., Wang B., Kent A., Zhang M., Binder B.Y., Urabe G., Shi Y., DiRenzo D., Goel S.A., et al. Restenosis Inhibition and Re-differentiation of TGFbeta/Smad3-activated Smooth Muscle Cells by Resveratrol. Sci. Rep. 2017;7:41916. doi: 10.1038/srep41916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zou J., Huang Y., Chen Q., Wang N., Cao K., Hsieh T.C., Wu J.M. Suppression of mitogenesis and regulation of cell cycle traverse by resveratrol in cultured smooth muscle cells. Int. J. Oncol. 1999;15:647–651. doi: 10.3892/ijo.15.4.647. [DOI] [PubMed] [Google Scholar]
- 95.Mizutani K., Ikeda K., Yamori Y. Resveratrol inhibits AGEs-induced proliferation and collagen synthesis activity in vascular smooth muscle cells from stroke-prone spontaneously hypertensive rats. Biochem. Biophys. Res. Commun. 2000;274:61–67. doi: 10.1006/bbrc.2000.3097. [DOI] [PubMed] [Google Scholar]
- 96.Haider U.G., Sorescu D., Griendling K.K., Vollmar A.M., Dirsch V.M. Resveratrol suppresses angiotensin II-induced Akt/protein kinase B and p70 S6 kinase phosphorylation and subsequent hypertrophy in rat aortic smooth muscle cells. Mol. Pharmacol. 2002;62:772–777. doi: 10.1124/mol.62.4.772. [DOI] [PubMed] [Google Scholar]
- 97.Mnjoyan Z.H., Fujise K. Profound negative regulatory effects by resveratrol on vascular smooth muscle cells: A role of p53-p21(WAF1/CIP1) pathway. Biochem. Biophys. Res. Commun. 2003;311:546–552. doi: 10.1016/j.bbrc.2003.10.023. [DOI] [PubMed] [Google Scholar]
- 98.Haider U.G., Sorescu D., Griendling K.K., Vollmar A.M., Dirsch V.M. Resveratrol increases serine15-phosphorylated but transcriptionally impaired p53 and induces a reversible DNA replication block in serum-activated vascular smooth muscle cells. Mol. Pharmacol. 2003;63:925–932. doi: 10.1124/mol.63.4.925. [DOI] [PubMed] [Google Scholar]
- 99.Poussier B., Cordova A.C., Becquemin J.P., Sumpio B.E. Resveratrol inhibits vascular smooth muscle cell proliferation and induces apoptosis. J. Vasc. Surg. 2005;42:1190–1197. doi: 10.1016/j.jvs.2005.08.014. [DOI] [PubMed] [Google Scholar]
- 100.Lee B., Moon S.K. Resveratrol inhibits TNF-alpha-induced proliferation and matrix metalloproteinase expression in human vascular smooth muscle cells. J. Nutr. 2005;135:2767–2773. doi: 10.1093/jn/135.12.2767. [DOI] [PubMed] [Google Scholar]
- 101.Wang Z., Chen Y., Labinskyy N., Hsieh T.C., Ungvari Z., Wu J.M. Regulation of proliferation and gene expression in cultured human aortic smooth muscle cells by resveratrol and standardized grape extracts. Biochem. Biophys. Res. Commun. 2006;346:367–376. doi: 10.1016/j.bbrc.2006.05.156. [DOI] [PubMed] [Google Scholar]
- 102.Brito P.M., Devillard R., Negre-Salvayre A., Almeida L.M., Dinis T.C., Salvayre R., Auge N. Resveratrol inhibits the mTOR mitogenic signaling evoked by oxidized LDL in smooth muscle cells. Atherosclerosis. 2009;205:126–134. doi: 10.1016/j.atherosclerosis.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 103.Choi K.H., Kim J.E., Song N.R., Son J.E., Hwang M.K., Byun S., Kim J.H., Lee K.W., Lee H.J. Phosphoinositide 3-kinase is a novel target of piceatannol for inhibiting PDGF-BB-induced proliferation and migration in human aortic smooth muscle cells. Cardiovasc. Res. 2010;85:836–844. doi: 10.1093/cvr/cvp359. [DOI] [PubMed] [Google Scholar]
- 104.Fry J.L., Al Sayah L., Weisbrod R.M., Van Roy I., Weng X., Cohen R.A., Bachschmid M.M., Seta F. Vascular Smooth Muscle Sirtuin-1 Protects Against Diet-Induced Aortic Stiffness. Hypertension. 2016;68:775–784. doi: 10.1161/HYPERTENSIONAHA.116.07622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miyazaki R., Ichiki T., Hashimoto T., Inanaga K., Imayama I., Sadoshima J., Sunagawa K. SIRT1, a longevity gene, downregulates angiotensin II type 1 receptor expression in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2008;28:1263–1269. doi: 10.1161/ATVBAHA.108.166991. [DOI] [PubMed] [Google Scholar]
- 106.Jang I.A., Kim E.N., Lim J.H., Kim M.Y., Ban T.H., Yoon H.E., Park C.W., Chang Y.S., Choi B.S. Effects of Resveratrol on the Renin-Angiotensin System in the Aging Kidney. Nutrients. 2018;10:1741. doi: 10.3390/nu10111741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim E.N., Kim M.Y., Lim J.H., Kim Y., Shin S.J., Park C.W., Kim Y.S., Chang Y.S., Yoon H.E., Choi B.S. The protective effect of resveratrol on vascular aging by modulation of the renin-angiotensin system. Atherosclerosis. 2018;270:123–131. doi: 10.1016/j.atherosclerosis.2018.01.043. [DOI] [PubMed] [Google Scholar]
- 108.Gresele P., Cerletti C., Guglielmini G., Pignatelli P., de Gaetano G., Violi F. Effects of resveratrol and other wine polyphenols on vascular function: An update. J. Nutr. Biochem. 2011;22:201–211. doi: 10.1016/j.jnutbio.2010.07.004. [DOI] [PubMed] [Google Scholar]
- 109.Rotondo S., Rajtar G., Manarini S., Celardo A., Rotillo D., de Gaetano G., Evangelista V., Cerletti C. Effect of trans-resveratrol, a natural polyphenolic compound, on human polymorphonuclear leukocyte function. Br. J. Pharmacol. 1998;123:1691–1699. doi: 10.1038/sj.bjp.0701784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cullen J.P., Morrow D., Jin Y., von Offenberg Sweeney N., Sitzmann J.V., Cahill P.A., Redmond E.M. Resveratrol inhibits expression and binding activity of the monocyte chemotactic protein-1 receptor, CCR2, on THP-1 monocytes. Atherosclerosis. 2007;195:e125–e133. doi: 10.1016/j.atherosclerosis.2007.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li Q., Huyan T., Ye L.J., Li J., Shi J.L., Huang Q.S. Concentration-dependent biphasic effects of resveratrol on human natural killer cells in vitro. J. Agric. Food Chem. 2014;62:10928–10935. doi: 10.1021/jf502950u. [DOI] [PubMed] [Google Scholar]
- 112.Pendurthi U.R., Williams J.T., Rao L.V. Resveratrol, a polyphenolic compound found in wine, inhibits tissue factor expression in vascular cells: A possible mechanism for the cardiovascular benefits associated with moderate consumption of wine. Arterioscler. Thromb. Vasc. Biol. 1999;19:419–426. doi: 10.1161/01.ATV.19.2.419. [DOI] [PubMed] [Google Scholar]
- 113.Ferrero M.E., Bertelli A.E., Fulgenzi A., Pellegatta F., Corsi M.M., Bonfrate M., Ferrara F., De Caterina R., Giovannini L., Bertelli A. Activity in vitro of resveratrol on granulocyte and monocyte adhesion to endothelium. Am. J. Clin. Nutr. 1998;68:1208–1214. doi: 10.1093/ajcn/68.6.1208. [DOI] [PubMed] [Google Scholar]
- 114.Guo R., Liu B., Wang K., Zhou S., Li W., Xu Y. Resveratrol ameliorates diabetic vascular inflammation and macrophage infiltration in db/db mice by inhibiting the NF-kappaB pathway. Diab. Vasc. Dis. Res. 2014;11:92–102. doi: 10.1177/1479164113520332. [DOI] [PubMed] [Google Scholar]
- 115.Xia N., Li H. The role of perivascular adipose tissue in obesity-induced vascular dysfunction. Br. J. Pharmacol. 2017;174:3425–3442. doi: 10.1111/bph.13650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xia N., Forstermann U., Li H. Effects of resveratrol on eNOS in the endothelium and the perivascular adipose tissue. Ann. N. Y. Acad. Sci. 2017;1403:132–141. doi: 10.1111/nyas.13397. [DOI] [PubMed] [Google Scholar]
- 117.Xia N., Horke S., Habermeier A., Closs E.I., Reifenberg G., Gericke A., Mikhed Y., Munzel T., Daiber A., Forstermann U., et al. Uncoupling of Endothelial Nitric Oxide Synthase in Perivascular Adipose Tissue of Diet-Induced Obese Mice. Arterioscler. Thromb. Vasc. Biol. 2016;36:78–85. doi: 10.1161/ATVBAHA.115.306263. [DOI] [PubMed] [Google Scholar]
- 118.Xia N., Weisenburger S., Koch E., Burkart M., Reifenberg G., Forstermann U., Li H. Restoration of perivascular adipose tissue function in diet-induced obese mice without changing bodyweight. Br. J. Pharmacol. 2017;174:3443–3453. doi: 10.1111/bph.13703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sun Y., Li J., Xiao N., Wang M., Kou J., Qi L., Huang F., Liu B., Liu K. Pharmacological activation of AMPK ameliorates perivascular adipose/endothelial dysfunction in a manner interdependent on AMPK and SIRT1. Pharmacol. Res. 2014;89:19–28. doi: 10.1016/j.phrs.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 120.Chen Y., Xu X., Zhang Y., Liu K., Huang F., Liu B., Kou J. Diosgenin regulates adipokine expression in perivascular adipose tissue and ameliorates endothelial dysfunction via regulation of AMPK. J. Steroid Biochem. Mol. Biol. 2016;155 Pt A:155–165. doi: 10.1016/j.jsbmb.2015.07.005. [DOI] [PubMed] [Google Scholar]
- 121.Zordoky B.N., Robertson I.M., Dyck J.R. Preclinical and clinical evidence for the role of resveratrol in the treatment of cardiovascular diseases. Biochim. Biophys. Acta. 2015;1852:1155–1177. doi: 10.1016/j.bbadis.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 122.Park E.J., Pezzuto J.M. The pharmacology of resveratrol in animals and humans. Biochim. Biophys. Acta. 2015;1852:1071–1113. doi: 10.1016/j.bbadis.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 123.Bhatt S.R., Lokhandwala M.F., Banday A.A. Resveratrol prevents endothelial nitric oxide synthase uncoupling and attenuates development of hypertension in spontaneously hypertensive rats. Eur. J. Pharmacol. 2011;667:258–264. doi: 10.1016/j.ejphar.2011.05.026. [DOI] [PubMed] [Google Scholar]
- 124.Dolinsky V.W., Chakrabarti S., Pereira T.J., Oka T., Levasseur J., Beker D., Zordoky B.N., Morton J.S., Nagendran J., Lopaschuk G.D., et al. Resveratrol prevents hypertension and cardiac hypertrophy in hypertensive rats and mice. Biochim. Biophys. Acta. 2013;1832:1723–1733. doi: 10.1016/j.bbadis.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 125.Liu Z., Song Y., Zhang X., Liu Z., Zhang W., Mao W., Wang W., Cui W., Zhang X., Jia X., et al. Effects of trans-resveratrol on hypertension-induced cardiac hypertrophy using the partially nephrectomized rat model. Clin. Exp. Pharmacol. Physiol. 2005;32:1049–1054. doi: 10.1111/j.1440-1681.2005.04299.x. [DOI] [PubMed] [Google Scholar]
- 126.Toklu H.Z., Sehirli O., Ersahin M., Suleymanoglu S., Yiginer O., Emekli-Alturfan E., Yarat A., Yegen B.C., Sener G. Resveratrol improves cardiovascular function and reduces oxidative organ damage in the renal, cardiovascular and cerebral tissues of two-kidney, one-clip hypertensive rats. J. Pharm. Pharmacol. 2010;62:1784–1793. doi: 10.1111/j.2042-7158.2010.01197.x. [DOI] [PubMed] [Google Scholar]
- 127.Chan V., Fenning A., Iyer A., Hoey A., Brown L. Resveratrol improves cardiovascular function in DOCA-salt hypertensive rats. Curr. Pharm. Biotechnol. 2011;12:429–436. doi: 10.2174/138920111794480552. [DOI] [PubMed] [Google Scholar]
- 128.Franco J.G., Lisboa P.C., Lima N.S., Amaral T.A., Peixoto-Silva N., Resende A.C., Oliveira E., Passos M.C., Moura E.G. Resveratrol attenuates oxidative stress and prevents steatosis and hypertension in obese rats programmed by early weaning. J. Nutr. Biochem. 2013;24:960–966. doi: 10.1016/j.jnutbio.2012.06.019. [DOI] [PubMed] [Google Scholar]
- 129.Aubin M.C., Lajoie C., Clement R., Gosselin H., Calderone A., Perrault L.P. Female rats fed a high-fat diet were associated with vascular dysfunction and cardiac fibrosis in the absence of overt obesity and hyperlipidemia: Therapeutic potential of resveratrol. J. Pharmacol. Exp. Ther. 2008;325:961–968. doi: 10.1124/jpet.107.135061. [DOI] [PubMed] [Google Scholar]
- 130.Miatello R., Vazquez M., Renna N., Cruzado M., Zumino A.P., Risler N. Chronic administration of resveratrol prevents biochemical cardiovascular changes in fructose-fed rats. Am. J. Hypertens. 2005;18:864–870. doi: 10.1016/j.amjhyper.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 131.Akar F., Uludag O., Aydin A., Aytekin Y.A., Elbeg S., Tuzcu M., Sahin K. High-fructose corn syrup causes vascular dysfunction associated with metabolic disturbance in rats: Protective effect of resveratrol. Food Chem. Toxicol. 2012;50:2135–2141. doi: 10.1016/j.fct.2012.03.061. [DOI] [PubMed] [Google Scholar]
- 132.Rivera L., Moron R., Zarzuelo A., Galisteo M. Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem. Pharmacol. 2009;77:1053–1063. doi: 10.1016/j.bcp.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 133.Mizutani K., Ikeda K., Kawai Y., Yamori Y. Resveratrol attenuates ovariectomy-induced hypertension and bone loss in stroke-prone spontaneously hypertensive rats. J. Nutr. Sci. Vitaminol. 2000;46:78–83. doi: 10.3177/jnsv.46.78. [DOI] [PubMed] [Google Scholar]