Abstract
Background
Embryo incubation and assessment is a vital step in assisted reproductive technology (ART). Traditionally, embryo assessment has been achieved by removing embryos from a conventional incubator daily for quality assessment by an embryologist, under a microscope. In recent years time‐lapse systems (TLS) have been developed which can take digital images of embryos at frequent time intervals. This allows embryologists, with or without the assistance of embryo selection software, to assess the quality of the embryos without physically removing them from the incubator.
The potential advantages of a TLS include the ability to maintain a stable culture environment, therefore limiting the exposure of embryos to changes in gas composition, temperature, and movement. A TLS has the potential advantage of improving embryo selection for ART treatment by utilising additional information gained through continuously monitoring embryo development. Use of a TLS often adds significant extra cost to ART treatment.
Objectives
To determine the effect of a TLS compared to conventional embryo incubation and assessment on clinical outcomes in couples undergoing ART.
Search methods
We used standard methodology recommended by Cochrane. We searched the Cochrane Gynaecology and Fertility (CGF) Group Trials Register, CENTRAL, MEDLINE, Embase, CINAHL, and two trials registers on 7 January 2019 and checked references of appropriate papers.
Selection criteria
We included randomised controlled trials (RCTs) comparing TLS, with or without embryo selection software, versus conventional incubation with morphological assessment; and TLS with embryo selection software versus TLS without embryo selection software among couples undergoing ART.
Data collection and analysis
We used standard methodological procedures recommended by Cochrane. The primary review outcomes were live birth or ongoing pregnancy, miscarriage and stillbirth, and cumulative live birth or ongoing pregnancy rate. The secondary outcomes were clinical pregnancy and cumulative clinical pregnancy. We assessed the quality of the evidence using GRADE methodology. We made the following comparisons.
TLS with conventional morphological assessment of still TLS images versus conventional incubation and assessment
TLS utilising embryo selection software versus TLS with conventional morphological assessment of still TLS images
TLS utilising embryo selection software versus conventional incubation and assessment
Main results
We included nine RCTs (N = 2955 infertile couples). The quality of the evidence ranged from very low to low. The main limitations were high risk of bias in the included studies, imprecision, indirectness, and inconsistency. There were no data on cumulative live birth or ongoing pregnancy rate or cumulative clinical pregnancy rate.
TLS with conventional morphological assessment of still TLS images versus conventional incubation and assessment
It is unclear whether there is any difference between interventions in rates of live birth or ongoing pregnancy (odds ratio (OR) 0.91, 95% confidence interval (CI) 0.67 to 1.23, 3 RCTs, N = 826, I2 = 33%, low‐quality evidence) or in miscarriage rates (OR 1.90, 95% CI 0.99 to 3.61, 3 RCTs, N = 826, I2 = 0%, low‐quality evidence). The evidence suggests that if the rate of live birth or ongoing pregnancy associated with conventional incubation and assessment is 35%, the rate with the use of TLS with conventional morphological assessment of still TLS images would be between 27% and 40%, and if the miscarriage rate with conventional incubation is 4%, the rate associated with conventional morphological assessment of still TLS images would be between 4% and 14%. It is unclear whether there is a difference between the interventions in rates of stillbirth (OR 1.00, 95% CI 0.13 to 7.49, 1 RCT, N = 76, low‐quality evidence) or clinical pregnancy (OR 1.06, 95% CI 0.79 to 1.41, 4 RCTs, N = 875, I2 = 0%, low‐quality evidence).
TLS utilising embryo selection software versus TLS with conventional morphological assessment of still TLS images
All findings for this comparison were very uncertain due to the very low‐quality of the evidence. No data were available on live birth, but one RCT reported ongoing pregnancy. It is unclear whether there is any difference between the interventions in rates of ongoing pregnancy (OR 0.61, 95% CI 0.32 to 1.20, 1 RCT, N = 163); miscarriage (OR 1.39, 95% CI 0.64 to 3.01, 2 RCTs, N = 463, I2 = 0%); or clinical pregnancy (OR 0.97, 95% CI 0.67 to 1.42, 2 RCTs, N = 463, I2 = 0%). The evidence suggests that if the rate of ongoing pregnancy associated with TLS with conventional morphological assessment of still TLS images is 47%, the rate associated with TLS utilising embryo selection software would be between 22% and 52%, and if the miscarriage rate associated with conventional morphological assessment of still TLS images is 5%, the rate associated with TLS utilising embryo selection software would be between 4% and 15%. No studies reported stillbirth.
TLS utilising embryo selection software versus conventional incubation and assessment
The findings for this comparison were also very uncertain due to the very low quality of the evidence. It is unclear whether there is any difference between the interventions in rates of live birth (OR 1.12, 95% CI 0.92 to 1.36, 3 RCTs, N = 1617, I2 = 84%). There was very low‐quality evidence that TLS might reduce miscarriage rates (OR 0.63, 95% CI 0.45 to 0.89, 3 RCTs, N = 1617, I2 = 0%). It is unclear whether there is any difference between the interventions in rates of clinical pregnancy (OR 0.95, 95% CI 0.78 to 1.16, 3 RCTs, N = 1617, I2 = 89%). The evidence suggests that if the rate of live birth associated with conventional incubation and assessment is 48%, the rate with TLS utilising embryo selection software would be between 46% and 55%, and if the miscarriage rate with conventional incubation and assessment is 11%, the rate associated with TLS would be between 5% and 10%. No stillbirths occurred in the only study reporting this outcome.
Authors' conclusions
There is insufficient good‐quality evidence of differences in live birth or ongoing pregnancy, miscarriage and stillbirth, or clinical pregnancy to choose between TLS, with or without embryo selection software, and conventional incubation. As the evidence is of low or very low‐quality, our findings should be interpreted with caution.
Plain language summary
Time‐lapse systems for embryo incubation and embryo assessment for couples undergoing in vitro fertilisation and intracytoplasmic sperm injection
Review question
Does a time‐lapse system (TLS) improve the chances of a pregnancy and live‐born baby, and reduce the risk of miscarriage and stillbirth?
Background
In vitro fertilisation (IVF) and intracytoplasmic sperm injection (ICSI) are processes whereby a woman's eggs and a man's sperm are combined to achieve fertilisation outside of the body. Embryos are stored in an incubator and replaced into the woman between day 2 and 5 of development. Usually, embryos are removed from an incubator for assessment, under a microscope, of their quality and stage of development. A TLS can take images of embryos at frequent time intervals, which allows assessment without removing the embryos from the incubator. A TLS can also apply software that assists the embryologist in selecting the best‐quality embryo for replacement, potentially improving the chance of a baby.
Study characteristics
The evidence is current to January 2019. We included nine studies (randomised controlled trials, that is studies in which participants are assigned to one of two or more treatment groups using a random method) of 2955 infertile couples undergoing IVF or ICSI. There were three different study designs: (1) TLS with conventional assessment of still TLS images versus conventional incubation and assessment; (2) TLS utilising embryo selection software versus TLS with conventional assessment of still TLS images; and (3) TLS utilising embryo selection software versus conventional incubation and assessment.
What the review found
TLS with conventional assessment of still TLS images versus conventional incubation and assessment
All the evidence for this comparison was low‐quality. It is unclear whether there is any difference between the interventions in rates of livebirth or ongoing pregnancy or miscarriage. The evidence suggests that if the rate of livebirth or ongoing pregnancy associated with conventional incubation and assessment is 35%, the rate with use of TLS with conventional morphological assessment of still TLS images would be between 27% and 40%, and if the miscarriage rate with conventional incubation is 4%, the rate associated with conventional morphological assessment of still TLS images would be between 4% and 14%. It is unclear whether there is a difference between interventions in rates of stillbirth or clinical pregnancy.
TLS utilising embryo selection software versus TLS with conventional assessment of still TLS images
All findings for this comparison were very uncertain due to very low‐quality evidence. No data were available on livebirth, but one study reported ongoing pregnancy. It is unclear whether there is any difference between interventions in rates of ongoing pregnancy, miscarriage, or clinical pregnancy. The evidence suggests that if the rate of ongoing pregnancy associated with TLS with conventional morphological assessment of still TLS images is 47%, the rate associated with TLS utilising embryo selection software would be between 22% and 52%, and if the miscarriage rate associated with conventional morphological assessment of still TLS images is 5%, the rate associated with TLS utilising embryo selection software would be between 4% and 15%. No studies reported stillbirth.
TLS utilising embryo selection software versus conventional incubation and assessment
All findings for this comparison were very uncertain due to the very low‐quality of the evidence. It is unclear whether there is any difference between interventions with respect to rates of livebirth or clinical pregnancy. The evidence suggests lower rates of miscarriage in the TLS group for the outcome of miscarriage. The evidence suggests that if the livebirth rate associated with conventional incubation is 48%, the rate with the use of TLS would be between 46% and 55%, and if the miscarriage rate with conventional incubation is 11%, the rate associated with TLS would be between 5% and 10%.
Overall conclusions
There is no good evidence showing that TLS is more or less effective than conventional methods of embryo incubation. Patients may wish to take part in randomised controlled trials on TLS in order to add to the existing evidence base and to help guide assisted reproductive technology patients in the future.
Quality of the evidence
The quality of the evidence ranged from very low to low. The main limitations were high risk of bias in the included studies, imprecision, indirectness, and inconsistency.
Summary of findings
Background
Description of the condition
Embryo incubation is a critical step in all in vitro fertilisation (IVF) procedures. Embryo development within media in culture dishes in an incubator is a dynamic process, moving through the fertilisation stage to cleavage stage and then to the blastocyst stage in some cases. Throughout the incubation period, embryos are usually inspected at specific time points to provide a brief 'snapshot' assessment of the way the embryo is developing (morphological features). Embryologists apply a tiered grading system based on the morphology of the embryo in order to predict the potential for implantation and a successful pregnancy (Cummins 1986; Neuber 2003; Scott 2003; Scott 2003a; Shoukir 1997). A consensus on the minimum data set required for the accurate description of embryo morphology was established by Alpha Scientists in Reproductive Medicine and European Society of Human Reproduction and Embryology (ESHRE) Special Interest Group of Embryology (Alpha & ESHRE SIG 2011). A consensus on timings of observation of fertilised oocytes and embryos was established and deemed critical to the ability to compare results between different laboratories. The recommended checks, in hours, following insemination are:
a fertilisation check at 17 hours, a syngamy (fusion of gametes) check at 23 hours;
an early cleavage check at 26 hours post‐intracytoplasmic sperm injection (ICSI) or 28 hours post‐IVF;
day 2 embryo assessment at 44 hours;
day 3 embryo assessment at 68 hours;
day 4 embryo assessment at 92 hours;
day 5 embryo assessment at 116 hours.
Traditionally, the checks have been achieved by physically removing embryos from the controlled environment of the incubator to analyse them under a light microscope for assessment of embryo development and quality. This practice exposes the embryos to the potentially suboptimal conditions of the environment outside of the incubator and human handling (Meseguer 2012a). Time‐lapse systems (TLSs) have evolved over recent years to increase the frequency of morphological observations whilst minimising the impact of the external environment and human handling on embryo development.
Description of the intervention
A TLS is a device that takes digital images of embryos at set time intervals, for example every 5 to 15 minutes. The system can be installed into an existing embryo incubator or can exist as a combined time‐lapse incubation system. The images are compiled using software to create a time‐lapse sequence of embryo development. Images can be digitally displayed as a time‐lapse sequence on an external monitor to allow embryologists to assess the dynamic morphology of embryos, thus negating the need for the embryologist to remove the embryos from the incubator. Some TLSs also utilise computer‐assisted assessment of developmental milestones of embryos, also known as morphokinetic parameters, to offer a semiquantitative process of embryo evaluation (Conaghan 2013). These cell‐tracking software algorithms utilise data such as the timing of embryonic development events, and have evolved as a non‐invasive, non‐subjective way of attempting to improve the selection of embryos with the highest implantation potential. Some clinics have developed their own algorithms to adapt the standardised one that comes with the TLS device (Petersen 2016).
There are a number of commercially available TLSs developed by various manufacturers. Time‐lapse systems are available as devices that can be placed within existing conventional incubators, and some exist with an integrated incubator. The integrated TLS combines both the time‐lapse cameras and the incubator in one device.
How the intervention might work
There are two potential benefits of a TLS. Firstly, an advantage may lie with the undisturbed nature of the culture conditions, whereby images for embryo assessment can be obtained without removing embryos from the incubator environment for conventional benchtop light microscopy (which usually includes heated microscope stages). This minimises the exposure of embryos to both human handling and changes in air temperature and gas composition, which may lead to improved culture conditions.
A second potential advantage may be the ability of a TLS to accumulate detailed time‐lapse images of embryo development at regular time intervals. This includes the timing of cell divisions, intervals between cell cycles, and other development factors (e.g. dynamic pronuclei patterns, presence of multinucleation and fragmentation, and blastomere symmetry). Many of these features that are transient events may be missed by using standard morphological assessment at set time intervals. These detailed time‐lapse sequences can be utilised with or without cell‐tracking software algorithms as an adjunct to standard morphological assessment, to select the embryo with the highest implantation potential for transfer. This is important because there is a clear correlation between embryo morphology and viability (Finn 2010; Neuber 2006). The ability to select the highest‐quality embryo at an optimal stage of development for replacement in an assisted reproductive technology (ART) cycle may lead to a reduction in time to pregnancy and a reduced need for subsequent embryo transfers. It is worth noting that the different models of TLS follow the same basic principles but vary in technical detail such as gas mixture, temperature, group or single culture, and dark‐ or light‐field microscopy.
In order to assess the potential advantage of TLSs (i.e. the stable culture environment or the time‐lapse sequence of images which can be assessed with cell‐tracking algorithms, or both), studies can be grouped into the following three comparisons.
Trial design 1: TLS with conventional morphological assessment of still TLS images versus conventional incubation and assessment
These studies control for how the embryos are selected for transfer, but the incubation differs. This will help to establish whether the culture conditions of the TLS potentially impact on favourable outcomes such as pregnancy and live birth.
Trial design 2: TLS utilising embryo selection software versus TLS with conventional morphological assessment of still TLS images
These studies control for the culture environment, with both arms of the trial being incubated in a TLS, and the way in which embryos are selected for transfer is tested. This study design will help to establish whether embryo selection software improves the selection of top‐quality embryos and increases the pregnancy and live‐birth rate.
Trial design 3: TLS utilising embryo selection software versus conventional incubation and assessment
These studies aim to establish whether a combination of both the stable culture environment and the embryo selection software is superior to conventional embryo incubation and assessment at improving pregnancy and live birth rates.
Why it is important to do this review
New interventions such as TLSs should be evaluated by randomised controlled trials in order to establish their safety, clinical effectiveness, and cost‐effectiveness (Campbell 2000; Harper 2012). Countering the potential benefits outlined above, a TLS involves exposing embryos to light during image acquisition, at predetermined intervals. Furthermore, the authorities responsible for the regulation of fertility clinics and research involving human embryos have a responsibility to provide impartial and authoritative information to prospective and current patients on fertility treatments to aid them in making informed decisions about their care (ACART; HFEA). It is therefore vital that up‐to‐date and thorough systematic reviews that are accessible to patients and healthcare workers are published on the topic. This will enable information on the technology's success rates in terms of live birth or ongoing pregnancy rate, and safety in terms of adverse events, to be accessible and help guide informed decision making.
This is the third update of this Cochrane Review published under the same title initially 2015, Armstrong 2015, and again in 2018 (Armstrong 2018a). This update captures all newly available trial data and corrects an error in Analysis 3.1 in Armstrong 2018a.
We aimed with this updated review to establish whether there is evidence of any overall benefit of culturing embryos in a TLS with or without embryo selection software, over current conventional embryo incubation and assessment.
Objectives
To determine the effect of a time‐lapse system (TLS) compared to conventional embryo incubation and assessment on clinical outcomes in couples undergoing assisted reproductive technology (ART).
Methods
Criteria for considering studies for this review
Types of studies
Inclusions: any randomised controlled trial (RCT), whether published or not, which in principle could answer questions regarding clinical (postimplantation) outcomes.
Exclusions: quasi‐randomised and other concurrently controlled studies were excluded. We excluded trials that randomised oocytes or embryos, as it would not be possible to compare clinical outcomes. We excluded cross‐over trials as the design is not valid in this context.
Types of participants
Couples of any age undergoing assisted reproduction where embryo incubation was required.
Types of interventions
Time‐lapse system (TLS) with conventional morphological assessment of still TLS images versus conventional incubation and assessment (trial design 1)
TLS utilising embryo selection software versus TLS with conventional morphological assessment of still TLS images (trial design 2)
TLS utilising embryo selection software versus conventional incubation and assessment (trial design 3)
Any type of TLS, using any type of embryo selection software and any type of conventional incubator, was eligible.
Types of outcome measures
Primary outcomes
Live‐birth or ongoing pregnancy rate
Miscarriage and stillbirth
Cumulative live birth or ongoing pregnancy rate
Secondary outcomes
Clinical pregnancy, defined as evidence of a gestational sac, confirmed by ultrasound
Cumulative clinical pregnancy rate
Search methods for identification of studies
Three review authors (SA, PB, and JM) searched databases (from inception to 7 January 2019) for all published and unpublished RCTs of TLSs, without language restrictions and in consultation with the Cochrane Gynaecology and Fertility Group (CGFG) Information Specialist. We used both electronic searches of bibliographic databases and handsearching as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Electronic searches
We searched the following electronic databases, trial registers and websites.
Cochrane Gynaecology and Fertility Group Specialised Register, ProCite platform (searched 7 January 2019) (Appendix 1)
Cochrane Central Register of Controlled Studies (CENTRAL) (CRSO), web platform (searched 7 January 2019) (Appendix 2)
MEDLINE In‐Process & Other Non‐Indexed Citations, Ovid platform (searched from 1946 to 7 January 2019 (Appendix 3)
Embase, Ovid platform (searched from 1980 to 7 January 2019) (Appendix 4)
Cumulative Index to Nursing and Allied Health Literature (CINAHL), EBSCO platform (searched from 1961 to 7 January 2019) (Appendix 5)
For MEDLINE, we used the Cochrane Highly Sensitive Search Strategy for identifying randomised controlled trials: sensitivity and precision maximising version (2008 revision), Ovid format (Higgins 2011).
We also searched the following other electronic sources of trials (web platforms, all searched 7 January 2019).
Trial registers for ongoing and registered trials: World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.apps.who.int/trialsearch/) (Appendix 6) and US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov)
Web of Knowledge (wokinfo.com/)
ProQuest Dissertations and Theses (search.proquest.com/)
Grey literature through the System for Information on Grey Literature in Europe 'OpenGrey' (www.opengrey.eu/).
Searching other resources
We used the following methods to identify additional relevant RCTs:
contact with authors of all RCTs identified by other methods;
contact with manufacturers of TLSs;
handsearching of selected journals in obstetrics, gynaecology and reproductive medicine, as well as conference proceedings (for abstracts) of the European Society for Human Reproduction and Embryology (ESHRE) and the American Society for Reproductive Medicine (ASRM);
contacting known experts and personal contacts regarding unpublished materials;
searching the citation lists of all identified articles for any relevant references.
Data collection and analysis
Selection of studies
We used the software program Covidence to manage the screening of titles and abstracts and to generate the PRISMA flow diagram (Covidence). All review authors took part in independently scanning the titles and abstracts of the articles retrieved by the search. Three review authors (SA, PB, and JM) then obtained the full texts of potentially eligible studies and independently examined these against the inclusion criteria for their eligibility. In the case of doubt between the review authors, a fourth review author (CF) was consulted to establish consensus on whether to include the trial or not. We documented the selection process with a PRISMA flow chart.
Data extraction and management
Three review authors (SA, PB, and JM) independently obtained and extracted data. Any disagreements between review authors were resolved by consulting a fourth review author (CF) to achieve consensus. We extracted data using a data extraction form designed and piloted by the review authors. If studies were reported in multiple publications, we extracted data from the different publications and then combined these into a single data extraction form so that no data were omitted. We included the following characteristics of included studies in the data extraction form:
methods;
participants;
interventions;
outcomes, including adverse events;
funding source for studies.
Assessment of risk of bias in included studies
Three review authors (SA, PB, and JM) independently assessed the risk of bias in included studies using the Cochrane 'Risk of bias' assessment tool. We evaluated all included studies for the following: adequacy of sequence generation and allocation concealment; adequacy of blinding of couples, providers, and outcome assessors; completeness of outcome data; risk of selective outcome reporting; and risk of other potential sources of bias (Higgins 2011).
Any disagreements between authors were resolved by consulting a fourth review author (VJ) to achieve consensus. The results of the 'Risk of bias' assessment are presented in the 'Characteristics of included studies' table.
Measures of treatment effect
For dichotomous data (e.g. live birth or not), we calculated Mantel‐Haenszel odds ratios (ORs) and 95% confidence intervals (CIs).
Unit of analysis issues
We analysed the data per couple randomised. We excluded studies randomising oocytes or embryos.
Dealing with missing data
If relevant data were missing from an included study, we contacted the original investigators of the trial to request the missing data. All original investigators were contacted. In particular, we obtained clinical pregnancy, live‐birth, and stillbirth data from Park 2015; live‐birth and stillbirth data from Yang 2018; miscarriage and clinical pregnancy data per woman randomised for Goodman 2016; live‐birth and stillbirth data from Kahraman 2013; miscarriage data from Kaser 2017; and updated ongoing pregnancy and miscarriage data from Barberet 2018. If participants were described as 'lost to follow‐up' without a specified reason, we assumed the participant did not experience the event or outcome (i.e. did not become pregnant).
Assessment of heterogeneity
We considered whether the clinical and methodological characteristics of the included studies were sufficiently similar for meta‐analysis to provide a clinically meaningful summary. We assessed statistical heterogeneity by measuring the I2 statistic. We assumed that there was substantial heterogeneity when I2 was calculated as greater than 50% (Higgins 2011).
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, the we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert to duplication of data. We assessed within‐study reporting bias, which we judged as low risk if all of the study's prespecified primary outcomes were reported as outlined in the study's protocol.
Data synthesis
Where sufficient data were available, we combined the data for the primary outcomes by using a fixed‐effect model in the following comparisons.
TLS with conventional morphological assessment of still TLS images versus conventional incubation and assessment (trial design 1)
TLS utilising embryo selection software versus TLS with conventional morphological assessment of still TLS images (trial design 2)
TLS utilising embryo selection software versus conventional incubation and assessment (trial design 3)
Subgroup analysis and investigation of heterogeneity
Where sufficient data were available, we aimed to conduct the following subgroup analyses to determine the potential causes of heterogeneity for the live‐birth and clinical pregnancy outcomes:
donor oocytes (from donors of any age) versus autologous oocytes (from women of any age);
fresh cycles (where embryos were replaced either at cleavage stage (day 3) or blastocyst (day 5)) versus frozen cycles (where frozen embryos were replaced in an ART cycle).
If we detected substantial heterogeneity, we planned to explore it by employing the random‐effects model. We aimed to take any statistical heterogeneity into account when interpreting the results, especially if there was any variation in the direction of effect.
Sensitivity analysis
We planned to undertake sensitivity analyses for the review outcomes to determine whether the results were robust to decisions made during the review process. These analyses would have included consideration of whether the review conclusions would have differed if:
the summary effect measure had been risk ratio rather than odds ratio;
eligibility had been restricted to studies with low risk of bias for randomisation and allocation concealment;
the primary outcome had been live birth only (i.e. not including ongoing pregnancy).
Overall quality of the body of evidence: 'Summary of findings' table
We prepared 'Summary of findings' tables using GRADEpro GDT and Cochrane methods (GRADEpro GDT 2015). These tables evaluate the overall quality of the body of evidence for the main review outcomes (live birth or ongoing pregnancy, miscarriage and stillbirth, and clinical pregnancy) for the review comparisons:
TLS with conventional morphological assessment of still TLS images versus conventional incubation and assessment (trial design 1);
TLS utilising embryo selection software versus TLS with conventional morphological assessment of still TLS images (trial design 2); and
TLS utilising embryo selection software versus conventional incubation and assessment (trial design 3).
We assessed the quality of the evidence using GRADE criteria: risk of bias, consistency of effect, imprecision, indirectness, and publication bias. Two review authors (SA and PB) independently assessed the quality of the evidence as high, moderate, low, or very low, resolving any disagreements by discussion. Judgements were justified, documented, and incorporated into the reporting of results for each outcome.
Results
Description of studies
Results of the search
The most recent search took place in January 2019. We imported the 190 retrieved references into Covidence, and after removal of duplicates, all review authors screened 178 studies. We assessed 27 full‐text articles for eligibility, of which one was a new RCT eligible for inclusion (Barberet 2018); two were excluded because they did not meet our inclusion criteria for study design (Alhelou 2018; Hardarson 2016); 11 were ongoing (ChiCTR1800017127; ChiCTR‐IIR‐16008758; ISRCTN17792989; NCT01760278; NCT02222831; NCT02417441; NCT02657811; NCT02852356; NCT02965222; NCT03164551; NCT03445923); and one is awaiting classification (Hulme 2014). The other 12 articles were conference abstracts from existing studies in the review, and have been listed under the main study references.
Taking into account the studies found in previous iterations of the review (described below), the review now has a total of nine included studies, 22 excluded studies, 13 ongoing studies and one study awaiting assessment (Figure 1, Included studies, Excluded studies, Studies awaiting classification; Ongoing studies).
1.
Study flow diagram.
The first iteration of this review included three parallel‐design RCTs from a search that retrieved 33 articles in total (Kahraman 2013; Kovacs 2019; Rubio 2014). Two further searches in 2016 and 2017 retrieved 82 and 293 articles, respectively. We retrieved a further four articles through handsearching. We screened the titles and abstracts of 266 articles after removal of duplicates. Of these 25 articles were potentially eligible for inclusion in the review, and we retrieved these in full text. Five new studies met our inclusion criteria (Goodman 2016; Kaser 2017; Park 2015; Wu 2016; Yang 2018). We excluded the remaining 20 studies for the following reasons: three studies were not RCTs; three were systematic reviews; two were letters; nine randomised embryos or oocytes; two were pseudo‐randomised; and for one study we were unable to determine the nature of the control group despite our attempts to contact the authors.
Included studies
Study design and setting
We included nine RCTs in this review. The largest study was a multicentre RCT conducted in Spain, which was included in the first iteration of this review (Rubio 2014). The first iteration also included a single‐centre RCT conducted in Turkey (Kahraman 2013), and a further multicentre RCT conducted in Hungary for which the completed results are now available (Kovacs 2019). The second iteration of the review added three single‐centre studies conducted in the USA (Goodman 2016; Kaser 2017; Wu 2016), one single‐centre study conducted in Sweden (Park 2015), and one single‐centre study conducted in China (Yang 2018). This third iteration of the review includes completed study data from Yang 2018 and a completed single‐centre RCT conducted in France (Barberet 2018)
Participants
The studies included 2955 infertile couples undergoing assisted reproductive technology (ART). Four studies included couples undergoing intracytoplasmic sperm injection (ICSI) alone (Barberet 2018; Kahraman 2013; Park 2015; Rubio 2014). One study included couples undergoing in vitro fertilisation (IVF) (Goodman 2016). The remaining studies included couples undergoing both IVF and ICSI (Kaser 2017; Kovacs 2019; Wu 2016; Yang 2018).
The largest study was Rubio 2014, with 856 participants; the second largest study had 600 participants (Yang 2018), followed by Barberet 2018 with 386 participants, and Park 2015 with 364 participants. The next‐largest study had 300 participants (Goodman 2016), followed by Kaser 2017, with 163 participants. Kovacs 2019 had 161 participants, and the remaining two studies were relatively small, with 76 and 49 participants, respectively (Kahraman 2013; Wu 2016).
All studies utilised the autologous oocytes of the women randomised into their study, with the exception of Rubio 2014, which included couples undergoing ART with autologous or donor oocytes. The proportion of couples receiving donor oocytes in this study is unknown. Most donor oocytes in this study were used in fresh cycles, however some donor oocytes were obtained from an oocyte bank and were therefore vitrified.
All studies included women undergoing fresh embryo transfer, hence no cumulative cycle results were available. The majority of studies undertook single embryo transfer (Kahraman 2013; Kaser 2017; Kovacs 2019; Park 2015; Yang 2018). One study describes use of one or two embryos (Barberet 2018), and one study reports replacing between one and three embryos based on published American Society for Reproductive Medicine (ASRM) committee guidance and patient preferences (Goodman 2016). Another study undertook multiple embryo transfer (Rubio 2014), and a further study did not disclose the number of embryos transferred (Wu 2016).
The reported causes of infertility varied between studies. Some studies specifically described their participants as "good prognosis patients" (e.g. Rubio 2014 and Yang 2018). One study specifically described their participants as "poor prognosis patients", but provided no further information (Wu 2016). One study described "tubo‐peritoneal factor" as the cause of infertility (Kahraman 2013), and another described male‐factor infertility being present in more than 99% of participants in both arms, and female‐factor infertility being present in approximately 20% of participants in both arms (Park 2015). Kovacs 2019 described various causes of infertility in participants ("male, tubal, unexplained etc."). One study described "a combination of anovulation, diminished ovarian reserve, endometriosis, male factor, tubal, unknown, and uterine" as causes of infertility (Kaser 2017). Barberet 2018 included male‐factor, female‐factor, mixed, and idiopathic indications. Goodman 2016 described a range of infertility diagnoses ("unexplained, ovulatory dysfunction, male factor, tubal factor, low ovarian reserve, AMA [advanced maternal age], endometriosis, mixed factors and other").
Interventions
We sought to divide studies into three comparisons depending on the nature of the intervention and the control, in order to truly assess if, and where, the benefit of a TLS lies.
TLS with conventional morphological assessment of still TLS images versus conventional incubation and assessment (trial design 1)
Four studies undertook this comparison (Barberet 2018; Kahraman 2013; Park 2015; Wu 2016). All studies utilised an integrated TLS, and all had two arms. Embryo transfer (ET) was undertaken at blastocyst in Kahraman 2013, day three in Wu 2016, day two in Park 2015, and day 2, day 3, or day 5‐6 in Barberet 2018. Correspondence with the authors of one study confirmed that no embryo selection software was utilised in the intervention arm (Kahraman 2013). Embryos were left undisturbed in the TLS in the intervention arm in all three studies. In the control arm, embryos in all studies were assessed by conventional morphology using a benchtop microscope.
TLS utilising embryo selection software versus TLS with conventional morphological assessment of still TLS images (trial design 2)
Two studies undertook this comparison (Goodman 2016; Kaser 2017). One study utilised an integrated TLS (Goodman 2016), and the other utilised a TLS that was placed inside a conventional incubator (Kaser 2017). The embryos in the intervention arms were selected for transfer according to the information obtained from the embryo selection software, however the embryos of the women randomised to the intervention arm in one study were removed from the incubator for conventional benchtop morphology in addition to TLS selection (Kaser 2017). In addition, the embryos in the control arm of this study were assessed with conventional morphological assessment using a benchtop microscope. Time‐lapse system images were not utilised for the selection of embryos for replacement in the control arm.
One study had three arms (Kaser 2017). There were two intervention arms: both were TLS utilising embryo selection software, but one arm undertook ET on day 3, and the other undertook ET on day 5. The control arm undertook ET on day 5. The other study had two arms, with ET undertaken on day 3 or day 5 (Goodman 2016).
We conducted in‐depth discussions with the authors of Kaser 2017, and decided that trial design 2 was the most appropriate comparison, given that embryo selection software was utilised, and the trial design tested the embryo‐selection element of the TLS software.
TLS utilising embryo selection software versus conventional incubation and assessment (trial design 3)
Three studies undertook this comparison (Kovacs 2019; Rubio 2014; Yang 2018). Two of these studies utilised a TLS that was placed inside a conventional incubator (Kovacs 2019; Yang 2018), whilst the third study utilised an integrated TLS (Rubio 2014). In Rubio 2014, ET was undertaken on days 3 and 5 in both arms; in Kovacs 2019, blastocyst transfer was undertaken in both arms. One study undertook ET on day 3 in the intervention arm and day 5 (blastocyst) in the control arm (Yang 2018). We took methodological advice on Yang 2018, and made the decision to keep the study in our review despite the differing days of ET. We gave this study a rating of high risk of bias due to this within‐study imbalance.
Outcomes
All nine studies reported clinical pregnancy rates per couple. Miscarriage data were available for all included studies except for Wu 2016. Miscarriage data were confirmed to be loss of a clinical pregnancy (not biochemical) in six studies (Barberet 2018; Kahraman 2013; Kaser 2017; Kovacs 2019; Park 2015; Yang 2018). In two studies the miscarriage data were a mixture of biochemical and clinical pregnancy losses (Goodman 2016; Rubio 2014). Unfortunately, the authors of these two studies were unable to provide only miscarriage data from clinical pregnancies. In these cases we have taken the pragmatic view to include these data, as according to the authors of these studies the majority of the pregnancy losses were from clinical pregnancies.
Either live birth or ongoing pregnancy was reported in all the studies except Goodman 2016 and Wu 2016. We obtained unpublished live‐birth data for three studies following communication with the authors (Kahraman 2013; Park 2015; Yang 2018). For Rubio 2014, we obtained data from a related publication and conference abstract pertaining to the same study (Insua 2015; Insua 2017). We obtained stillbirth data from three studies following communication with the authors (Kahraman 2013; Park 2015; Yang 2018).
Excluded studies
We excluded 22 studies from the review because they did not meet our inclusion criteria for study design. For details see Characteristics of excluded studies.
Risk of bias in included studies
For details of the 'Risk of bias' assessments see Figure 2 and Figure 3.
2.
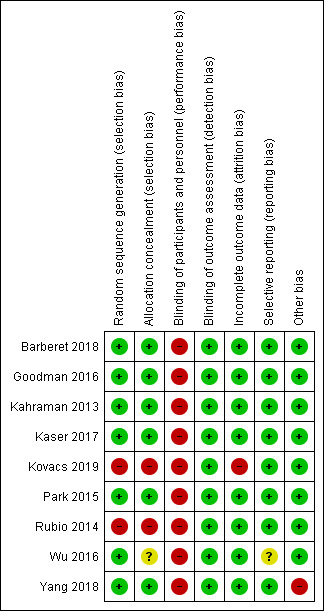
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
3.
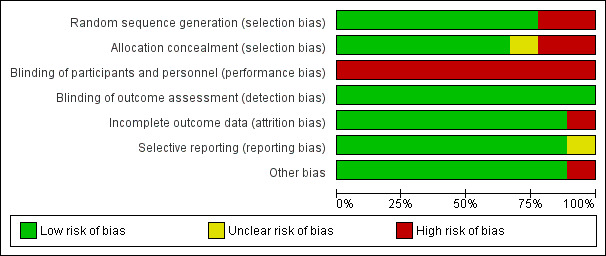
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Sequence generation
Seven of the nine studies were at low risk of selection bias related to sequence generation. Six used a computer‐generated randomisation list (Barberet 2018; Goodman 2016; Kahraman 2013; Kaser 2017; Park 2015; Wu 2016). One study utilised a random number table (Yang 2018).
We deemed two studies to have a high risk of bias for this domain (Rubio 2014; Kovacs 2019 ). In one study, although adequate random sequence generation was undertaken, some women were able to request the intervention, and in some cases this request was granted (Rubio 2014). The authors of this study assured us that this preferential allocation occurred in a minority of occasions and that the vast majority of participants were truly randomised, therefore we have maintained that this is an RCT. One study undertook paired randomisation whereby two envelopes containing time‐lapse or control group assignments were prepared, and the first patient was randomly assigned to one of the groups and the next patient received the other assignment (Kovacs 2019). This was repeated with patient numbers three and four, and so on.
Allocation concealment
Six studies described methods of allocation concealment that resulted in a judgement of low risk of selection bias (Barberet 2018; Goodman 2016; Kahraman 2013; Kaser 2017; Park 2015; Yang 2018). In each of these studies, the randomisation list or numbered, opaque, sealed envelopes were held and administered by personnel not directly involved in the recruitment of participants, or else the allocation was conducted remotely (Barberet 2018).
We deemed two studies to be at high risk of bias for this domain (Kovacs 2019; Rubio 2014). In Kovacs 2019, randomisation was carried out by the principal investigator who was involved in the study. In Rubio 2014, it was reported that in some cases the allocation was non‐random.
We judged one study for which there was limited description of randomisation to be at unclear risk of bias for this domain (Wu 2016). We understand that randomisation was undertaken by a member of the team not associated with the treatment cycle, and then subsequently the designation was reported to the embryology staff who processed the participant's oocytes/embryos. However, it was unclear how the randomisation list was stored, at what point the participants were randomised, and whether the person undertaking randomisation was responsible for recruitment.
Blinding
Blinding of participants and personnel (performance bias)
Three studies blinded their couples, and this blinding was not broken unless participants withdrew from the study (Goodman 2016; Kahraman 2013; Park 2015). Clinicians involved in the study were also blinded until after embryo transfer. One study described blinding the embryologist to the Eeva rating for the morphological assessment of embryos (Kaser 2017). The participants and physicians were all blinded to the TLS ratings. In addition, the sonographer was blinded in Goodman 2016, and the statistician was blinded in Park 2015.
Three studies did not blind or maintain blinding of their participating couples (Kovacs 2019; Rubio 2014; Yang 2018). In two of these studies, the clinical staff were also not blinded (Kovacs 2019; Yang 2018). The gynaecologist and statistician were blinded in Rubio 2014. We assessed these three studies as being at high risk of this bias.
Barberet 2018 did not discuss performance bias in detail or report who was blinded, but noted that it was not possible to blind investigators to the allocations. However, in this study embryos were selected for vitrification according to their morphology, which was graded in unblinded embryo assessments.
We deemed one study as having a high risk of performance bias as blinding was not described, and it would have been impossible to blind the embryologist (Wu 2016). We have been unable to contact the authors for further clarification.
None of the included studies blinded the embryologists, but this would have been impossible. We considered a lack of blinding of embryologists as reason for a judgement of high risk of performance bias. This renders all included studies as having a high risk of performance bias. In some studies, the lack of blinding may have influenced the number or day of transfer. In addition, it is impossible to remove the risk of performance bias when the person selecting the embryo for transfer is unblinded.
Blinding of outcome assessors (detection bias)
We judged all nine studies to be at low risk of detection bias because the outcomes (live birth or ongoing pregnancy, clinical pregnancy, miscarriage and stillbirth) are objective, and therefore cannot be influenced by knowledge of the intervention. Two studies described how staff performing the ultrasounds were blinded to the intervention (Goodman 2016; Rubio 2014). The remaining studies did not blind their outcome assessors, however we still deemed these studies as having a low risk of bias due to the reason described above.
Incomplete outcome data
We deemed the following studies to be at low risk of attrition bias:
Barberet 2018, because outcomes were reported for all participants, using intention‐to‐treat analysis;
Goodman 2016, because we were able to obtain the outcome data from the five women excluded after randomisation;
Kahraman 2013, because the 12 couples who dropped out after randomisation were accounted for, and the reasons were clearly stated;
Kaser 2017, because all data were presented in their paper as intention‐to‐treat;
Park 2015, because there was only one woman excluded from analysis due to having been accidentally randomised twice;
Wu 2016, because the small number of excluded participants were accounted for according to predetermined grounds for exclusion;
Rubio 2014, because the 13 couples who were excluded following randomisation were accounted for and were a very small proportion of the total number of couples randomised; and
Yang 2018, because the 15 couples who were excluded following randomisation were accounted for with clearly stated reasons for exclusion that were predetermined.
We judged one study to be at high risk of attrition bias because a large proportion of the couples recruited were excluded from the trial (22 out of 161 couples randomised) (Kovacs 2019). The reasons for dropout were provided, however not all of the reasons were specified in the predetermined exclusion criteria, and given the high attrition rate, we assessed this study at high risk of attrition bias.
We undertook an intention‐to‐treat analysis on all dichotomous outcomes, using data from those women excluded postrandomisation where possible.
Selective reporting
We considered eight studies to be at low risk of reporting bias because they reported and published all outcomes they had set out to investigate (Barberet 2018; Goodman 2016; Kahraman 2013; Kaser 2017; Kovacs 2019; Park 2015; Rubio 2014; Yang 2018). This was confirmed on communication with authors and by referencing against information in online trials registers if it was available.
We considered one study to be at unclear risk of reporting bias because access to their protocol was not available and we could not contact the authors to ask whether they had published all prespecified outcomes (Wu 2016).
Other potential sources of bias
We found no potential sources of within‐study bias in Barberet 2018, Goodman 2016, Kahraman 2013, Kaser 2017, Kovacs 2019, Park 2015, Rubio 2014, and Wu 2016. We assessed these studies as having a low risk of this form of bias.
We assessed one study, Yang 2018, as having a high risk of within‐study bias. This was due to the difference in day of embryo transfer between study arms (day 3 for intervention and day 5 for control). This difference in maturity of the embryo could have had an impact on the likelihood of an ongoing pregnancy.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. TLS with conventional morphological assessment of still TLS images compared to conventional incubation and assessment for embryo incubation and assessment in assisted reproduction.
| TLS with conventional morphological assessment of still TLS images compared to conventional incubation and assessment for embryo incubation and assessment in assisted reproduction | |||||
| Patient or population: couples undergoing assisted reproductive technology Setting: fertility clinic Intervention: TLS with conventional morphological assessment of still TLS images Comparison: conventional incubation and assessment | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with conventional incubation and assessment | Risk with TLS with conventional morphological assessment of still TLS images | ||||
| Live birth or ongoing pregnancy | 353 per 1000 | 332 per 1000 (268 to 402) | OR 0.91 (0.67 to 1.23) | 826 (3 RCTs) | ⊕⊕⊕⊝ Lowa |
| Miscarriage | 42 per 1000 | 77 per 1000 (42 to 137) | OR 1.90 (0.99 to 3.61) | 826 (3 RCTs) | ⊕⊕⊝⊝ Lowb |
| Stillbirth | 12 per 1000 | 12 per 1000 (2 to 86) | OR 1.00 (0.13 to 7.49) | 76 (1 RCT) | ⊕⊕⊝⊝ Lowc |
| Clinical pregnancy | 374 per 1000 | 388 per 1000 (321 to 458) | OR 1.06 (0.79 to 1.41) | 875 (4 RCTs) | ⊕⊕⊕⊝ Lowd |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial; TLS: time‐lapse system | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aWe downgraded our assessment of the quality of the evidence for live birth or ongoing pregnancy once for serious risk of performance bias and once for serious imprecision due to wide confidence intervals, compatible with a benefit in either group. bWe downgraded our assessment of the evidence for miscarriage once for serious risk of performance bias and once for serious imprecision due to wide confidence intervals and small number of events (total of 48). cWe downgraded our assessment of the quality of the evidence for stillbirth once for serious risk of performance bias and once for serious imprecision. Although two studies examined this outcome, one had no events in either arm and was therefore removed from meta‐analysis in accordance with Cochrane guidance. This left a single small study with very wide confidence intervals and only four events. dWe downgraded our assessment of the quality of the evidence for clinical pregnancy once for serious risk of performance bias and once for serious imprecision, due to wide confidence intervals compatible with a benefit in either group.
Summary of findings 2. TLS utilising embryo selection software compared to TLS with conventional morphological assessment of still TLS images for embryo incubation and assessment in assisted reproduction.
| TLS utilising embryo selection software compared to TLS with conventional morphological assessment of still TLS images for embryo incubation and assessment in assisted reproduction | ||||||
| Patient or population: couples undergoing assisted reproductive technology Setting: fertility clinic Intervention: TLS utilising embryo selection software Comparison: TLS with conventional morphological assessment of still TLS images | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with TLS with conventional morphological assessment of still TLS images | Risk with TLS utilising embryo selection software | |||||
| Live birth or ongoing pregnancy | 472 per 1000 | 353 per 1000 (222 to 517) | OR 0.61 (0.32 to 1.20) | 163 (1 RCT) | Very lowa | The outcome was ongoing pregnancy; no live‐birth data were available. |
| Miscarriage | 54 per 1000 | 74 per 1000 (35 to 147) | OR 1.39 (0.64 to 3.01) | 463 (2 RCTs) | Very lowb | |
| Stillbirth | No studies reported this outcome. | ‐ | ‐ | ‐ | ‐ | |
| Clinical pregnancy | 537 per 1000 | 529 per 1000 (437 to 622) | OR 0.97 (0.67 to 1.42) | 463 (2 RCTs) | Very lowc | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial; TLS: time‐lapse system | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | ||||||
aWe downgraded our assessment of the quality of the evidence for live birth or ongoing pregnancy once for serious risk of performance bias and twice for very serious imprecision due to there being only one RCT with a small number of events (64) and wide confidence intervals compatible with a benefit in either group. bWe downgraded our assessment of the quality of the evidence for miscarriage once for serious risk of performance bias; once for serious indirectness (heterogeneity between the study designs: one included study involved removing embryos for benchtop microscopy daily in both the intervention and control arms, whereas the other study left embryos in the intervention and control arms undisturbed); and once for serious imprecision (wide confidence intervals compatible with a benefit in either group and a low number of events overall (N = 29)). cWe downgraded our assessment of the quality of the evidence for clinical pregnancy once for serious risk of performance bias, once for serious indirectness (as described above), and once for serious imprecision (wide confidence intervals compatible with a benefit in either group).
Summary of findings 3. TLS utilising embryo selection software compared to conventional incubation and assessment for embryo incubation and assessment in assisted reproduction.
| TLS utilising embryo selection software compared to conventional incubation and assessment for embryo incubation and assessment in assisted reproduction | |||||
| Patient or population: couples undergoing ART Setting: fertility clinic Intervention: TLS utilising embryo selection software Comparison: conventional incubation and assessment | |||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with conventional incubation and assessment | Risk with TLS utilising embryo selection software | ||||
| Live birth or ongoing pregnancy | 475 per 1000 | 504 per 1000 (455 to 554) | OR 1.12 (0.92 to 1.36) | 1617 (3 RCTs) | Very lowa |
| Miscarriage | 108 per 1000 | 71 per 1000 (52 to 98) | OR 0.63 (0.45 to 0.89) | 1617 (3 RCTs) | Very lowb |
| Stillbirth | No events occurred in the only study reporting this outcome. | ‐ | 600 (1 RCT) |
‐ | |
| Clinical pregnancy | 605 per 1000 | 593 per 1000 (545 to 640) | OR 0.95 (0.78 to 1.16) | 1617 (3 RCTs) | Very lowc |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio; RCT: randomised controlled trial; TLS: time‐lapse system | |||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||
aWe downgraded our assessment of the quality of the evidence for live birth twice for very serious risk of bias (high risk of both performance bias and selection bias in two studies, and of other bias in the third study). In one study, the randomisation of participants was undertaken by the principal investigator, and allocation concealment was not described. In another study, some participants could request the intervention, and this request was granted. In the third study, the day of transfer varied between the two study arms. We also downgraded our assessment of the quality of the evidence once for serious indirectness, as one included study undertook multiple embryo transfers per woman and included women receiving donor oocytes from younger women. Although further downgrading was not possible, there was also serious inconsistency (I2 = 86%), possibly secondary to differing embryo transfer policies across the studies: one study had blastocyst transfers, one had varied days of transfer, and one had day 3 transfer for the intervention arm and day 5 transfer for the control arm. bWe downgraded our assessment of the quality of the evidence for miscarriage twice for very serious risk of bias (as outlined above) and once for serious indirectness secondary to one included study including miscarriages of biochemical pregnancies as well as clinical pregnancies. The authors of the study were unable to separate these miscarriage data. cWe downgraded our assessment of the quality of the evidence for clinical pregnancy twice for very serious risk of bias and once for serious indirectness, as one included study undertook multiple embryo transfers per woman and included women receiving donor oocytes from younger women. Although further downgrading was not possible, there was also serious inconsistency (I2 = 89%), possibly secondary to differing embryo transfer policies across the studies: one study had blastocyst transfers, one had varied days of transfer, and one had day 3 transfer for the intervention arm and day 5 transfer for the control arm.
1. TLS with conventional morphological assessment of still TLS images versus conventional incubation and assessment (trial design 1)
Four studies undertook this comparison (Barberet 2018; Kahraman 2013; Park 2015; Wu 2016), with a total of 875 participants.
Primary outcomes
1.1 Live birth or ongoing pregnancy
Two studies provided live‐birth data following correspondence with their authors (Kahraman 2013; Park 2015; N = 440), and one study provided data on ongoing pregnancy (Barberet 2018; N = 386). There were 141 events reported among the 469 women randomised to the TLS arm, and 124 events among the 357 women randomised to the control arm (conventional incubation and embryo assessment).
It is unclear whether there is any difference between interventions in rates of live birth or ongoing pregnancy (odds ratio (OR) 0.91, 95% confidence interval (CI) 0.67 to 1.23, 3 RCTs, N = 826, I2 = 33%, low‐quality evidence, Analysis 1.1, Figure 4). The evidence suggests that if the rate of live birth or ongoing pregnancy associated with conventional incubation and assessment is 35%, the rate with the use of TLS with conventional morphological assessment of still TLS images would be between 27% and 40%.
1.1. Analysis.
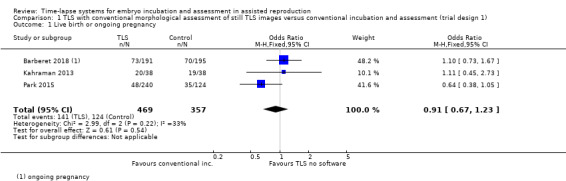
Comparison 1 TLS with conventional morphological assessment of still TLS images versus conventional incubation and assessment (trial design 1), Outcome 1 Live birth or ongoing pregnancy.
4.
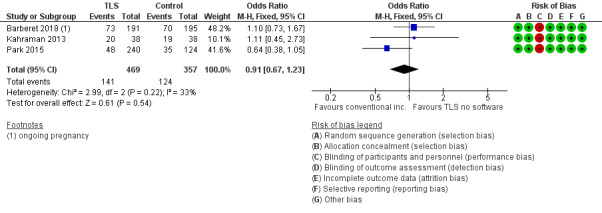
Forest plot of comparison: 1 TLS with conventional morphological assessment of still TLS images versus conventional incubation and assessment (trial design 1), outcome: 1.1 Live birth or ongoing pregnancy.
A sensitivity analysis restricting the analysis to studies reporting live birth did not influence this finding substantially.
1.2 ‐ 1.3 Miscarriage and stillbirth
Three studies provided data on miscarriage (Barberet 2018; Kahraman 2013; Park 2015; N = 826), and two studies also provided data on stillbirth (Kahraman 2013; Park 2015; N = 440). The data on stillbirth were made available following communication with the authors of Park 2015.
Out of 469 women randomised to the intervention arm, 33 experienced a miscarriage, whereas out of 357 randomised to the control arm, 15 experienced a miscarriage. It is unclear whether there is any difference between interventions in rates of miscarriage (OR 1.90, 95% CI 0.99 to 3.61, 3 RCTs, N = 826; I2 = 0%, low‐quality evidence, Analysis 1.2). The evidence suggests that if the miscarriage rate with conventional incubation is 4%, the rate associated with the use of TLS with conventional morphological assessment of still TLS images would be between 4% and 14%.
1.2. Analysis.
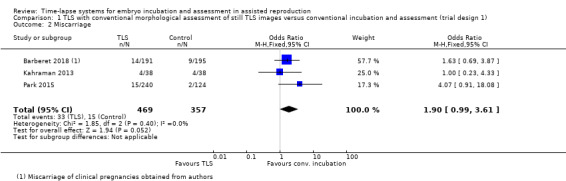
Comparison 1 TLS with conventional morphological assessment of still TLS images versus conventional incubation and assessment (trial design 1), Outcome 2 Miscarriage.
Regarding stillbirth, there were 2 stillbirths out of 38 women randomised to the intervention arm, and 2 stillbirths out of 38 women randomised to the control arm in Kahraman 2013. There were no stillbirths recorded in either arm in Park 2015, meaning that a result is inestimable. In accordance with Cochrane methodological guidance, we have removed Park 2015 from meta‐analysis. Results from the single study, Kahraman 2013, suggest that it is unclear whether there is any difference between interventions in rates of stillbirth (OR 1.00, 95% CI 0.13 to 7.49, 1 RCT, N = 76, low‐quality evidence, Analysis 1.3).
1.3. Analysis.
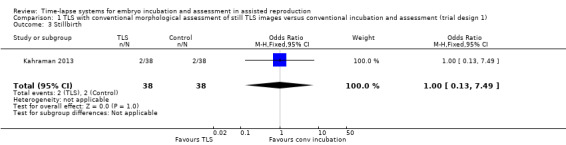
Comparison 1 TLS with conventional morphological assessment of still TLS images versus conventional incubation and assessment (trial design 1), Outcome 3 Stillbirth.
Cumulative live birth or ongoing pregnancy
No data were provided for this outcome.
Secondary outcomes
1.4 Clinical pregnancy
All four studies provided clinical pregnancy data (Barberet 2018; Kahraman 2013; Park 2015; Wu 2016; N = 875). There were 178 clinical pregnancies among the 493 women randomised to the intervention arm, and 143 clinical pregnancies among the 382 women randomised to the control arm.
It is unclear whether there is any difference between interventions in rates of clinical pregnancy (OR 1.06, 95% CI 0.79 to 1.41, 4 RCTs, N = 875, I2 = 0%, low‐quality evidence, Analysis 1.4).
1.4. Analysis.
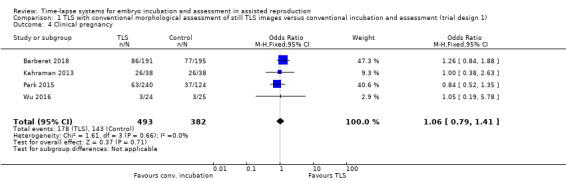
Comparison 1 TLS with conventional morphological assessment of still TLS images versus conventional incubation and assessment (trial design 1), Outcome 4 Clinical pregnancy.
Cumulative clinical pregnancy
No data were provided for this outcome.
2. TLS utilising embryo selection software versus TLS with conventional morphological assessment of still TLS images (trial design 2)
Two studies undertook this comparison (Goodman 2016; Kaser 2017), with a total of 463 participants. It is worth noting that in Kaser 2017 there were two intervention groups: one involved day 3 embryo transfer and the other day 5 embryo transfer. The two intervention groups are represented as separate entities at meta‐analysis, and the single control group has been split to share between the two intervention groups in order to avoid artificially doubling the effect of the control group.
Primary outcomes
2.1 Live birth or ongoing pregnancy
Neither study collected live‐birth data. This was confirmed on correspondence with the authors of both studies. One RCT reported ongoing pregnancy (Kaser 2017).
There were 39 ongoing pregnancies among the 110 women randomised to the intervention arm, and 25 ongoing pregnancies among the 53 women randomised to the control arm. It is unclear whether there is any difference between interventions for this outcome (OR 0.61, 95% CI 0.32 to 1.20, 1 RCT, N = 163, very low‐quality evidence, Analysis 2.1, Figure 5). The evidence suggests that if the rate of ongoing pregnancy associated with TLS with conventional morphological assessment of still TLS images is 47%, the rate associated with TLS utilising embryo selection software would be between 22% and 52%.
2.1. Analysis.
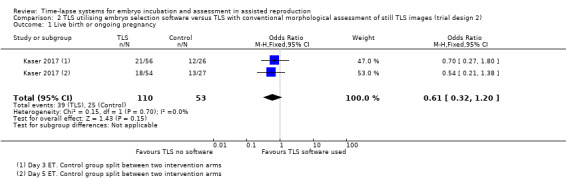
Comparison 2 TLS utilising embryo selection software versus TLS with conventional morphological assessment of still TLS images (trial design 2), Outcome 1 Live birth or ongoing pregnancy.
5.
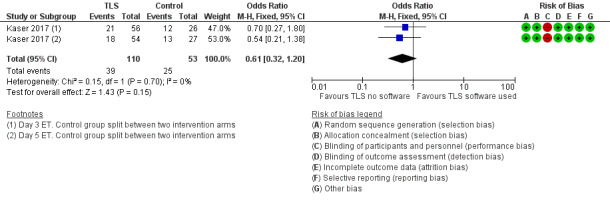
Forest plot of comparison: 2 TLS utilising embryo selection software versus TLS with conventional morphological assessment of still TLS images (trial design 2), outcome: 2.1 Live birth or ongoing pregnancy.
2.2 Miscarriage and stillbirth
Neither study collected data on stillbirth.
We obtained miscarriage data for all randomised women following correspondence with the authors of both studies. For Goodman 2016, the miscarriage data include a combination of biochemical and clinical pregnancy losses. Unfortunately, these data could not be separated for our review. For Kaser 2017, the data include miscarriages from clinical pregnancy losses.
There were 18 miscarriages out of 260 women randomised to the intervention arm, and 11 miscarriages out of 203 women randomised to the control arm. We are uncertain whether TLS utilising embryo selection software influences miscarriage rates (OR 1.39, 95% CI 0.64 to 3.01, 2 RCTs, N = 463, I2 = 0%, very low‐quality evidence, Analysis 2.2). The evidence suggests that if the miscarriage rate associated with assessment of still TLS images is 5%, the rate with embryo selection software would be between 4% and 14%.
2.2. Analysis.
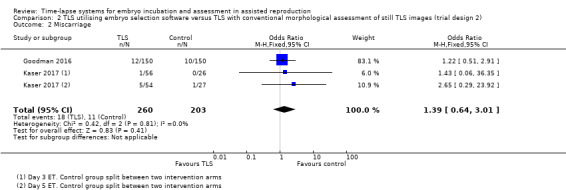
Comparison 2 TLS utilising embryo selection software versus TLS with conventional morphological assessment of still TLS images (trial design 2), Outcome 2 Miscarriage.
Cumulative live birth or ongoing pregnancy
No data were provided for this outcome.
Secondary outcomes
2.3 Clinical pregnancy
Both studies reported this outcome. There were 132 clinical pregnancies out of the 260 women randomised to the intervention group, and 109 pregnancies out of the 203 women randomised to the control group. It is unclear whether there is any difference between interventions in clinical pregnancy rates (OR 0.97, 95% CI 0.67 to 1.42, 2 RCTs, N = 463, I2 = 0%, very low‐quality evidence, Analysis 2.3).
2.3. Analysis.
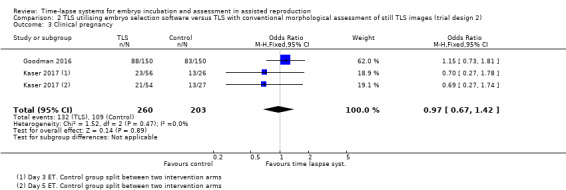
Comparison 2 TLS utilising embryo selection software versus TLS with conventional morphological assessment of still TLS images (trial design 2), Outcome 3 Clinical pregnancy.
Cumulative clinical pregnancy
No data were provided for this outcome.
3. TLS utilising embryo selection software versus conventional incubation and assessment (trial design 3)
Three studies undertook this comparison (Kovacs 2019; Rubio 2014; Yang 2018), with a total of 1351 participants. There were marked methodological differences between two of these studies, Kovacs 2019; Rubio 2014, and the third study, Yang 2018, with respect to study design as well as internal validity. In contrast to the other two studies, Yang 2018 had differing days of embryo transfer in the intervention and the control arms of the study. Moreover, Yang 2018 was at low risk of selection bias, whereas the other two studies were at high risk of selection bias relating to both sequence generation and allocation concealment. As noted below, there was high heterogeneity when these three studies were combined, which may be attributable to differences in design, differences in risk of bias, or both.
Primary outcomes
3.1 Live birth or ongoing pregnancy
Live‐birth data were available for all three studies (Kovacs 2019; Rubio 2014; Yang 2018). For Rubio 2014, we obtained data from a recently published paper and a published conference abstract (the references for these are provided as subreferences under Rubio 2014). Yang 2018 (N = 600) provided data on live birth following email communication. As noted above, the study design of Yang 2018 was very different from that of the other two studies in this comparison owing to the fact that it has differing days of embryo transfer in the intervention and the control arms of the study.
There were 412 events among the 824 women randomised to the intervention arm, and 376 events among the 793 women randomised to the control arm. It is unclear whether there is any difference between interventions in rates of live birth (OR 1.12, 95% CI 0.92 to 1.36, 3 RCTs, N = 1617, I2 = 84%, very low‐quality evidence, Analysis 3.1, Figure 6). There was high statistical heterogeneity for this finding, possibly due to the above mentioned differing study designs. The evidence suggests that if the rate of live birth or ongoing pregnancy associated with conventional incubation is 48%, the rate with TLS utilising embryo selection software would be between 46% and 55%.
3.1. Analysis.
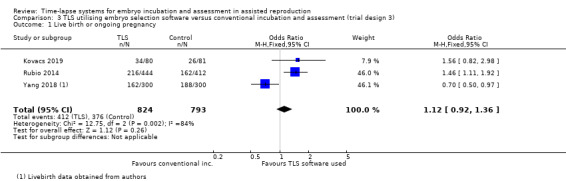
Comparison 3 TLS utilising embryo selection software versus conventional incubation and assessment (trial design 3), Outcome 1 Live birth or ongoing pregnancy.
6.
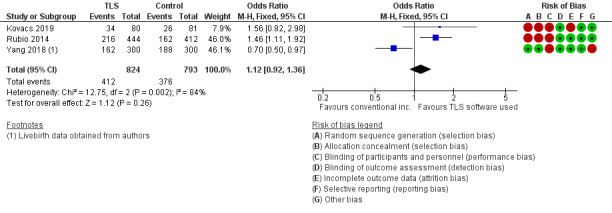
Forest plot of comparison: 3 TLS utilising embryo selection software versus conventional incubation and assessment (trial design 3), outcome: 3.1 Live birth or ongoing pregnancy.
3.2 ‐ 3.3 Miscarriage and stillbirth
Two studies defined miscarriage data as loss of clinical pregnancies (Kovacs 2019; Yang 2018). The other study reported a combination of biochemical and clinical pregnancy losses (Rubio 2014). Stillbirth data were made available following email correspondence with Yang 2018. There were no stillbirths in either arm of this study.
There were 60 miscarriages among 824 women randomised to the intervention arm, and 86 miscarriages among 793 women randomised to the control arm. The evidence suggests that TLS utilising embryo selection software may reduce miscarriage rates, but this finding is very uncertain as the evidence is of very low quality (OR 0.63, 95% CI 0.45 to 0.89, 3 RCTs, N = 1617, I2 = 0%, Analysis 3.2). The evidence suggests that if the miscarriage rate with conventional incubation is 11%, the rate associated with TLS would be between 5% and 10%.
3.2. Analysis.
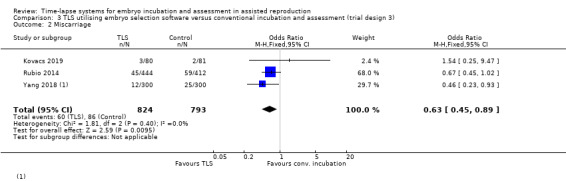
Comparison 3 TLS utilising embryo selection software versus conventional incubation and assessment (trial design 3), Outcome 2 Miscarriage.
Cumulative live birth or ongoing pregnancy
No data were provided for this outcome.
Secondary outcomes
3.4 Clinical pregnancy
Three studies reported this outcome (Kovacs 2019; Rubio 2014; Yang 2018; N = 1617). There were 489 clinical pregnancies among 824 women randomised to the intervention arm, and 480 clinical pregnancies among 793 women randomised to the control arm. It is unclear whether there is any difference between interventions for this outcome (OR 0.95, 95% CI 0.78 to 1.16, 3 RCTs, N = 1617, I2 = 89%, Analysis 3.4). This finding is very uncertain due to the high risk of bias in the included studies and the high level of heterogeneity in study design.
3.4. Analysis.
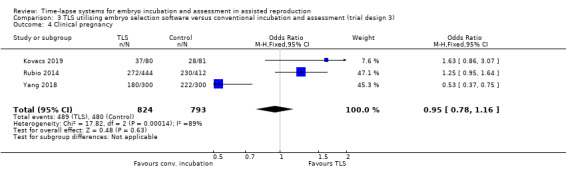
Comparison 3 TLS utilising embryo selection software versus conventional incubation and assessment (trial design 3), Outcome 4 Clinical pregnancy.
Cumulative clinical pregnancy
No data were provided for this outcome.
Subgroup and sensitivity analysis
We did not perform any other planned subgroup or sensitivity analyses as there were insufficient included studies for any specific comparison.
Discussion
Summary of main results
Trial design 1
The comparison 'TLS with conventional morphological assessment of still TLS images versus conventional incubation and assessment' aims to assess the potential advantages of a stable incubator environment. The embryo selection software is not utilised, and the embryos are left undisturbed until transfer. The four relevant studies included participants with a variety of infertility diagnoses. One study described its participants as "poor prognosis", with no further details (Wu 2016). Another study described women with "tubo‐peritoneal factor" (Kahraman 2013), and the third study described over 99% male‐factor infertility, with 20% female‐factor in both arms (Park 2015). One study included women with a variety of diagnoses (Barberet 2018). This variety adds to the broad applicability of results to common clinical practice. Two studies undertook embryo transfer at day 2 or 3 (Park 2015; Wu 2016), whereas one study undertook blastocyst transfer (Kahraman 2013), and the fourth study undertook embryo transfer on a variety of days from day 2 to blastocyst (Barberet 2018). All oocytes were autologous.
The evidence is of low quality, and it is unclear whether there is any difference between interventions in rates of live birth or ongoing pregnancy, miscarriage and stillbirth, or clinical pregnancy.
Trial design 2
The comparison 'TLS utilising embryo selection software versus TLS with conventional morphological assessment of still TLS images' aims to assess the potential advantages of the embryo selection software over conventional morphology. In this comparison, both arms of the study are housed in a TLS, but the embryo selection software is utilised in only one arm. The incubator environment is therefore identical in both arms. Two studies were eligible for this comparison. One study had two intervention arms: embryo transfer on day 3 and embryo transfer on day 5 (Kaser 2017). The control arm had embryo transfer on day 5 only. The other study, Goodman 2016, undertook a combination of embryo transfer on day 3 or 5. It is worth noting that the embryos were left undisturbed in Goodman 2016, however in Kaser 2017, the embryos in both intervention arms and in the control arm underwent daily conventional morphological assessment, in addition to the application of embryo selection software in the intervention arms. There was a broad variety of infertility diagnoses in both studies, which adds to the overall applicability of results to broad clinical practice.
All findings for this comparison were very uncertain due to the very low quality of the evidence. No data were available on live birth, but one study reported ongoing pregnancy: it is uncertain whether there is any difference between interventions in rates of ongoing pregnancy, miscarriage, or clinical pregnancy. No evidence for stillbirth was available.
Trial design 3
The comparison 'TLS utilising embryo selection software versus conventional incubation and assessment' aims to assess the potential advantages of a combination of the stable incubator environmentand the embryo selection software versus conventional incubation and assessment. Three studies undertook this comparison. One of these studies utilised a combination of autologous and donor oocytes; the proportion of each is unknown (Rubio 2014). The remaining two studies used autologous oocytes. One study undertook embryo transfer on day 3 in the intervention group and day 5 in the control group (Yang 2018). Another study undertook transfer on day 5 (Kovacs 2019), and in the third study there was a combination of transfer on day 3 and day 5 (Rubio 2014). A variety of infertility diagnoses were recorded in the women in these studies. Two studies described their participants as "good prognosis" (Rubio 2014; Yang 2018).
All findings for this comparison were very uncertain due to the very low quality of the evidence. It is unclear whether there is any difference between interventions in live‐birth rates. It is suggested that TLS might reduce miscarriage rates, but it is unclear whether there is any difference between interventions in clinical pregnancy rates. One study examined stillbirth, but as there were no events in either arm, it was not possible to reach any conclusions regarding this outcome.
Overall completeness and applicability of evidence
This updated systematic review on time‐lapse systems now includes nine RCTs. Data from 2955 women have gone towards formulating the findings of this review, but unfortunately all the evidence is of low or very low quality.
Approximately 50% of participants were included in trials that assessed TLS utilising embryo selection software versus conventional incubation and assessment (trial design 3). This is mainly due to the largest included trial undertaking this comparison (Rubio 2014). Trial designs 1 and 2 (TLS with conventional morphological assessment of still TLS images versus conventional incubation and assessment, and TLS utilising embryo selection software versus TLS with conventional morphological assessment of still TLS images) include the remaining 33% and 17% of participants, respectively, but there were no women available to inform live‐birth findings in trial design 2, meaning there are profound gaps in evidence for TLS in this comparison. In addition, there were no stillbirth data for trial design 2. This may be because stillbirth is so rare that it is not considered to be an important outcome, but it is important that future trials report this outcome, as it is a measure of safety.
Trial designs 1 and 2 included 875 and 463 women, respectively, in comparison to the 1617 women included in trial design 3. Despite the additional information from previous and newly incorporated trials, the results of the review remain unclear. Further trials of each design are required to bolster participant numbers and to interrogate the robustness of the finding of insufficient evidence of differences in live‐birth, miscarriage, clinical pregnancy, and stillbirth rates to choose between TLS with or without embryo selection software versus conventional incubation and assessment. The largest trial that informs trial design 3 has a number of biases arising from the non‐randomised approach for some participants, the subsequent lack of blinding, the use of donor oocytes in a number of women, and the routine use of multiple embryo transfer.
There was heterogeneity between trials in the diagnosis of infertility, the day of embryo transfer, the use of IVF or ICSI, and the make and model of TLS. All of these factors help to make the results of this review more applicable to clinical practice in the real world, where there is naturally this variation in clinical practices.
All included studies excluded women who underwent frozen embryo transfer, except Kahraman 2013, whose investigators were able to provide data for these women. The investigators of Rubio 2014 were unable to provide data specifically for women who underwent donor oocyte IVF/ICSI. Consequently, in order to subgroup autologous, donor, and frozen oocytes, future studies will need to present their results under these subgroups and state explicitly how many couples underwent these interventions.
Most studies undertook elective single embryo transfer (Kahraman 2013; Kaser 2017; Kovacs 2019; Park 2015; Yang 2018). However, three studies undertook multiple embryo transfers (Barberet 2018; Goodman 2016; Rubio 2014). We were unable to obtain from the authors of Rubio 2014 the exact proportion of couples who received multiple embryo transfer in each arm of the study. Given that this study contributed a large proportion of the data in trial design 3, it is important to recognise that the results presented here may reflect rates of clinical outcomes in keeping with multiple embryo transfer as opposed to single embryo transfer. One study did not disclose the number of embryos transferred per woman (Wu 2016).
Quality of the evidence
The quality of the evidence ranged from very low to low. The main limitations were high risk of bias in the included studies, imprecision, indirectness, and inconsistency. Risk of bias was most commonly associated with performance bias (lack of blinding of participants or those involved in the study) and selection bias (failure to use reliable methods of sequence generation and allocation concealment).
Inconsistency is evident across the comparisons. In particular, the point estimates of meta‐analyses of comparison 3 suggest some benefit from TLS in its entirety compared to conventional incubation and assessment, whereas most of the point estimates from comparisons 1 and 2 suggest a reduction in benefit from using TLS without the embryo selection software compared to control. This finding is difficult to explain scientifically given the difference in direction of results in comparisons that assess the stable incubator environment of the TLS, and ability of embryo selection software to help select the best embryo. Despite differences between the interventions, we would anticipate a consistent direction of effect across the three comparisons.
The inconsistency in the totality of the evidence relates to two studies in comparison 3 that found a benefit for TLS (Kovacs 2019; Rubio 2014). Both these studies were at high risk of selection bias (relating to both sequence generation and allocation concealment), which reduces our confidence in their findings. We rated the evidence for comparison 3 as very low (lower than for comparisons 1 and 2), denoting very little confidence in the effect estimate. With respect to inconsistency within comparison 3, there are two plausible explanations for the high statistical heterogeneity: in contrast to the other two studies, Yang 2018 had differing days of embryo transfer in the intervention and the control arms of the study. Moreover, Yang 2018 was at low risk of selection bias, whereas (as noted above) the other two studies were at high risk of selection bias.
The quality of the evidence for trial design 1 (TLS with conventional morphological assessment of still TLS images versus conventional incubation and assessment) is low, the main limitations being performance bias and imprecision (Table 1).
The quality of the evidence for trial design 2 (TLS utilising embryo selection software versus TLS with conventional morphological assessment of still TLS images) is very low, the main limitations being performance bias, indirectness, and imprecision (Table 2).
The quality of the evidence for trial design 3 (TLS utilising embryo selection software versus conventional incubation and assessment) is also very low, the main limitations being performance bias, selection bias, indirectness, and inconsistency (Table 3).
Potential biases in the review process
We aimed to identify all eligible studies for inclusion in this review, and contacted authors of the included studies on many occasions in an effort to include as much information as possible. The authors of most studies were forthcoming with further study information, which helped us to accrue a full picture of the study outcomes, as well as providing information needed to assess and establish risk of bias.
Agreements and disagreements with other studies or reviews
There are four published systematic reviews to date using the same inclusion and exclusion criteria on the topic of TLS versus conventional incubation (Chen 2017; Kaser 2014; Polanski 2014; Pribenszky 2017). Two of these are now out of date, with new studies published since their reporting (Kaser 2014; Polanski 2014). Both reviews reported no evidence of a difference between TLS and control.
One systematic review, Kaser 2014, included 13 eligible studies after systematic searching, however the majority of these were retrospective cohort studies, and none of them were RCTs. Kaser 2014 concluded that there is currently limited evidence to support the routine clinical use of TLS for selection of human pre‐implantation embryos.
Chen 2017 included six eligible studies, but it missed two further eligible RCTs that are included in this review. Chen 2017 does not include all the potential live‐birth data, including data from Kahraman 2013, Kovacs 2019, and Park 2015. It concludes that there is currently "insufficient evidence to support that time‐lapse imaging is superior to conventional methods for embryo incubation and selection".
The authors of Pribenszky 2017 undertook a systematic review of TLS utilising TLS embryo selection software. They concluded that TLS using embryo selection software was associated with a significantly higher ongoing pregnancy rate, a significantly lower early pregnancy loss, and a significantly higher live‐birth rate in comparison to control. However, we have detected a number of problems with this review that have been published as a letter (Armstrong 2018). The issues outlined are as follows.
They have combined trials with different intervention and control arms. For example, three of the five included trials are study design 3, but one is study design 1 and one is study design 2.
They have also included a trial that describes itself as a prospective cohort study, not an RCT. On closer investigation, this trial is pseudo‐randomised (randomisation based on patient record number). This is not considered methodologically sound for systematic reviews of RCTs.
The authors describe applying an intention‐to‐treat analysis (which is considered the gold standard in fertility research), however the early pregnancy loss, live‐birth, and stillbirth data are analysed per woman that became pregnant. This is known to skew the results toward showing a larger intervention effect.
It appears that full data from the included trials have not been entered into the review. For example, live‐birth data are not included from Rubio 2014, despite being published as an abstract in 2015.
We note that all three authors declared in this review that they work for Vitrolife, a biotechnology company that manufactures and promotes TLS.
Authors' conclusions
Implications for practice.
Overall, there is insufficient good‐quality evidence of differences in rates of live birth or ongoing pregnancy, miscarriage and stillbirth, or clinical pregnancy to choose between time‐lapse systems (TLS), with or without embryo selection software, and control.
Women need to be aware, especially in view of the cost of TLS, that there is no good evidence that TLS with or without embryo selection software is more effective than conventional methods of embryo incubation and assessment. They may wish to take part in randomised controlled trials (RCTs) on TLS so as to add to the existing evidence base, and help guide assisted reproductive technology patients of the future.
Implications for research.
Randomised controlled trials that randomise couples or women, not embryos or oocytes, to either TLS or conventional incubation should be designed and conducted to add to the currently limited RCT evidence. These studies should be large enough to answer the clinical questions that are important in fertility research, such as live birth, clinical and ongoing pregnancy, and adverse events. Cumulative clinical pregnancy rates should be reported in future studies in order to determine the impact of a TLS on embryo selection.
Suggested designs of RCTs which seek to differentiate the unique advantages of TLS are as follows.
Trial design 1) TLS utilising routine morphological assessment of TLS images versus conventional incubation and assessment
Trial design 2a) TLS utilising embryo selection software versus TLS utilising routine morphological assessment of TLS images
Trial design 2b) TLS utilising one type of embryo selection software versus TLS utilising a different type of embryo selection software
Trial design 3) TLS utilising embryo selection software versus conventional incubation and assessment
These study designs will help to differentiate between: the potential advantages of the stable culture environment TLS provides (trial design 1); the potential advantage of embryo selection software (trial design 2); and the potential advantage of TLS in its entirety utilising embryo selection software versus conventional incubation and assessment (trial design 3).
In addition, it would be useful for future trials to include a cost analysis element, which may help patients to balance the costs and benefits of using this technology. It may also be helpful to explore patient satisfaction and quality of life with TLS versus with control. Some clinics are sharing TLS images with patients during the incubation period. It would be useful to explore whether this helps or worsens treatment anxiety.
Feedback
Feedback on review protocol
Summary
Summary of a letter sent to David Tovey, Editor in Chief at the Cochrane Editorial Unit, London on 2nd February 2015:
1. The title of the review does not reflect the protocol and would better read "Time‐lapse systems for embryo monitoring/assessment in assisted reproduction".
2. To the best of our knowledge several studies are ongoing in the field of time‐lapse, while only one RCT has been published and this has as primary endpoint clinical pregnancy rate. There is not a single peer‐reviewed published RCT that currently would fulfil the primary outcome measure of this intended time‐lapse review.
3. Totally, two intervention studies of this time‐lapse review emphasize the concept of cell‐tracking algorithms. The cell‐tracking algorithm per definition is an inherent and patented feature of a commercial product and hence only applied to a small fraction of patients in published time‐lapse studies. Focusing on this could bias the neutrality of an evaluation. Hence we propose that the concept of cell‐tracking algorithms should be re‐phrased including morphokinetic evaluation models, clearly stating the aim of the model ( e.g. blastocyst prediction or implantation prediction based on day 2/3/4/5 parameters), and how the model was applied in relation to the actual transfer day.
4. We would like to ensure that studies comparing "standard morphology" provide an exact definition of the standard evaluation. We particularly note several studies, which refer to a single day 3 observation as a "standard" evaluation when comparing to effectiveness against an automated cell‐tracking algorithm.
5. The protocol mentions light exposure as a potential negative aspect of time‐lapse imaging. In view of this we like to bring to the attention to the authors that there is a recent publication on this topic. This study has investigated the light exposure in a time‐lapse system and concludes, that the overall exposure even in a 5 day culture period is much lower compared to standard observation as currently practiced. Similar findings were reported earlier.
Reply
Summary of the reply sent by the review authors to Professors Pribenzky and Montag on 20th February 2015:
1. The title of the review includes the words ‘embryo incubation’ because it is currently not possible to detect whether the potential advantage of time‐lapse lies in its capacity for embryo monitoring/assessment or as a method of achieving a stable culture/incubation environment. For that reason we would defend keeping the title as it is.
2. Your second point surrounds the use of live‐birth as a primary outcome despite the paucity of trials assessing this outcome. The Cochrane Menstrual Disorders and Subfertility Group provide guidelines on appropriate outcomes in reviews that are pertinent to both patients and clinicians. In the case of fertility interventions, live‐birth is accepted as a suitable primary outcome. We currently have one study that reports this outcome, and in the future, more eligible studies assessing this outcome will be added to the review making this an important primary outcome.
3. Your third point raises the question on authors’ neutrality when describing ‘cell‐tracking algorithms’, which is considered by you to be an inherent and patented feature of a commercial product. We consider the phrase ‘cell‐tracking algorithms’ to have no connection to any particular commercial product and adequately explains a process to the reader. The review does not name the manufacturer of time‐lapse technology used in each study. Sadly information from published studies does not provide detailed information on the aims of the cell‐tracking algorithm model, making it impossible to comment on what basis the information was applied. We will look to include this information in future updates of this review.
4. Your fourth point similarly outlines the importance of describing ‘standard morphology’ in each study. We agree that where this information is available, it should be described as part of the characteristics of each study.
5. Finally, you question the sentence in the protocol surrounding the potential negative aspect of light exposure on embryos associated with time‐lapse systems given the recent findings of two studies. However, this review merely aims to establish both the potential benefits and harms of time‐lapse, and offers up possible suggestions as to areas of potential harm. Potential harm is being assessed through adverse events; in this case miscarriage rate.
The authors thanked Professors Pribenzky and Montag for taking the time to carefully consider the protocol.
Contributors
Associate Professor Csaba Pribenszky, St Istvan Univ. Faculty of Vet. Sci., Budapest, Hungary
Professor Markus Montag, Ilabcomm GmbH, St. Augustin, Germany
Review authors: Sarah Armstrong, Nicola Arroll, Lynsey M Cree, Vanessa Jordan, Cindy Farquhar
Associate Professor Csaba Pribenszky is a senior scientist in Vitrolife AB and Dr Markus Montag is consulting for Vitrolife AB
Feedback on review received 2015
Summary
1. Cochrane guidelines state that in case of fertility interventions, live birth has to be the primary outcome. In the view of this we have looked carefully at the data that were given by Cochrane on live birth from the one study for which the authors provided live birth data. As these data were not available in the original publication – because it was not a primary outcome ‐ we contacted the authors ourselves to access the data, The study by Kahraman et al. reported to Cochrane for the time‐lapse system (TLS) group 18 term live births, 2 miscarriages after 5 months of pregnancy and for the conventional incubation (CI) group 17 term live births, 1 preterm still birth and 1 induced abortion. This leaves the fact that Cochrane reported two pregnancies more for each group than the authors of the study did. The authors reported in their response letter to Cochrane that 5 patients were excluded in the TLS group and 7 in the CI group. The reason for exclusion was, that these patients did not meet the inclusion criteria and received two embryos for transfer (which was not mentioned in the response letter to Cochrane) due to bad embryo development, freeze‐all after OHSS or no selection on day 5. From these excluded patients, 2 patients in the TLS group and 3 patients in the CI group became pregnant, resulting in 2 live births in each arm (one in a fresh day 3 transfer and one in a freeze‐thaw transfer for the TLS group; one in a fresh day 5 transfer and one in a freeze‐thaw transfer for the CI group). From this, we assume that the 2 live births from patients excluded from the study were added in each arm to give the final 20 live births for the TLS group and 19 for the CI group. But we clearly consider that this is not an adequate evaluation and presentation of the data, unless these details are made available to the readers. It is also highly questionable, if using outcome details from patients that were excluded or dropped out is in general a proper way to evaluate the effectiveness of a new treatment technology. Since this is unpublished and thus information that has not been verified we are interested in knowing what Cochrane guidelines are for such decisions and what the decision making process and control mechanisms are to guarantee correctness of such modifications? 2. We agree that it is a challenge to distinguish in some published studies if a cell‐tracking algorithm has been used or not. ..Whether a cell‐tracking algorithm has been used or not, it is always the embryologist (or physician) who makes the final choice for transfer, because every algorithm may have flaws and embryos may not develop as nicely as expected, despite an algorithm was applied. For the studies that were used for the Cochrane review, one study applied only standard morphology on day 5 for the decision on which embryo to transfer (Kahraman et al., 2013). However, the study by Kovacs clearly uses an algorithm that combines scores based on kinetics and morphology, all scored on the time‐lapse sequences: these scores add up for each embryo, where the one with the highest is selected for transfer. The same holds true for the study by Rubio et al. (2014). One of the authors of this letter to Cochrane (CP) was involved in and is a co‐author to the study by Kovacs et al.; therefore this statement is a fact and not fiction. Consequently the subgrouping done in the current Cochrane review is wrong and the evaluation shown in Figure 6 of the review not correct.
3. In the Cochrane review the number of positive ß‐hCG pregnancies from the Rubio paper (so called “biochemical pregnancies”) and the clinical pregnancies from the Kahraman & Kovacs paper are used to evaluate the Clinical Pregnancy Rate. As Rubio et al. defined “pregnancies” as those having a positive ß‐hCG serum level; the Cochrane report includes a mix of different definitions. Biochemical pregnancy rate is not the same as clinical pregnancy rate unless it is confirmed by the authors that the number is the same. Therefore, stating a mix of biochemical pregnancies and clinical pregnancies as THE CPR in the Cochrane review without further explanation to the readers is unacceptable and wrong. The number of pregnant patients is related to the number of patients included in the study (Intention to treat; TLS / CI: 444/412 for Rubio; 30/32 for Kovacs; 38/38 for Kahraman). Although this may be standard according to Cochrane guidelines, it is a bit odd that patients that dropped out or were excluded are still considered. Taking these into account the real number of patients treated and analysed would be different (TLS / CI: 438/405 for Rubio; 24/25 for Kovacs; 33/31 for Kahraman). The dropouts mentioned by Kahraman (TLS: 5, CI: 7) were re‐added by Cochrane to the total numbers, however; also the clinical outcome was re‐added. The problem is that patients with unusual bad embryo development on D3 and D4, as well as frozen‐thawed transfer cycles, received 2 embryos for transfer and not a single embryo as initially planned for the study. Therefore it is a point for discussion to include the outcome of these (excluded) cycles in the evaluation.
4. In the fifth point of your reply you refer to miscarriage rates. The different studies included in the report used different time‐points to define ongoing pregnancy: ‐ Rubio et al. define ongoing pregnancy as presence of fetal heart beat in week 12 of pregnancy ‐ Kahraman et al. consider ongoing pregnancy as being beyond week 5 after pick up (positive gestational sac) ‐ for the study by Kovacs et al. no definition has been given by the authors. A lack of uniform definition and different interpretation of terms such as miscarriage, clinical pregnancy or ongoing pregnancy makes assessment of miscarriage difficult. Considering the different end points defined by the studies included in this report, we think it is not possible to make a clear assessment of miscarriage rate. Also, there is no information for the readers that the respective papers use different definitions. The Early pregnancy loss in the Kahraman paper is a mix of pregnancy losses such as ectopic pregnancy or a presence of a gestational sac without fetus or fetal heartbeat. Since Rubio et al define ongoing pregnancy as presence of fetal heart in week 12 of pregnancy; it is not possible to distinguish between biochemical pregnancy loss and clinical pregnancy loss before week 12. We think that either more information should be provided to the readers allowing for a correct interpretation of the results or that this calculation should be excluded due to heterogeneity of the data from the different studies. We understand that for the primary end point the number of patients treated is used for comparison of results. However, what strikes us, is the fact that in the Cochrane review the number of the “miscarriages” is put in relation to the number of intention to treat ‐ instead of relating these to real clinical pregnancies (which is difficult as discussed on the previous topic on definition of clinical pregnancy). Based on this there are considerable flaws in the calculation presented in the Cochrane review for the miscarriage rate! In clinical embryology miscarriage rates are always seen in relation to the pregnancies achieved and considered as an important indicator for embryo viability beyond implantation. 5. We do agree that more studies are important for all aspects mentioned in the Cochrane review, but we do not agree with the presentation and evaluation of the data as they are presented right now. We would therefore like to ask the authors of this Cochrane review: ‐ to withdraw the current review ‐ to state the reason for withdrawal ‐ to reassess the data, provide more information for the readers allowing correct interpretation and, where necessary, redo the calculations ‐ to include experts with deeper knowledge in clinical embryology and time‐lapse imaging for a revised version of the Cochrane review
Reply
1. We thank you for your feedback and confirm that the data from Kahraman et al. reported in the Cochrane review includes two additional live births per group and the denominator includes the patients who were excluded post randomisation. The reason for these additions are that we have applied the intention to treat principle, which we stated we would utilise in our protocol. This is in line with Menstrual Disorders and Subfertility Cochrane group (MDSG) guidelines and the CONSORT statement. The intention to treat principle is a standard, uncontroversial, and well recognized protocol. Item 16 of the CONSORT statement states for example “Intention‐to‐treat analysis is generally favoured because it avoids bias associated with non‐random loss of participants”. The MDSG guideline states under unit of analysis issues that “the primary analysis should be per woman randomized and that data will be analysed on an intention to treat basis and attempts will be made to obtain missing data from original trialists.” We believe that the reasons for drop‐outs were clearly described in the characteristics of study table and risk of bias tables in addition to describing the unpublished nature of the data.
In this case, the numbers of drop‐outs and additional pregnancies is similar in both arms of the study therefore we can confirm that the inclusion of the additional data would not have affected the overall results. Whilst we value your thoughts we are of the view that this is the most methodologically correct approach and therefore it will remain unchanged in this review.
2. Thank you for the additional information and clarification of the design and therefore classification of the Kovacs study. We consider that it is difficult to assess whether the algorithms have been used to make clinical decisions or not. This additional information can be incorporated at the next scheduled update and we will add footnotes indicating this in the meantime. It does not change the overall analysis or the results for either of the subgroups.
3. We agree that mixing biochemical and clinical pregnancy rate is not the ideal study design. In the case of the Rubio study, raw data on ongoing pregnancy rate was not provided to us despite a number of requests to the study authors. Therefore the best available data was utilized. We acknowledge that we should have made this clear in the characteristics of study table, and we will add a footnote to this effect. In the updated review, we will contact the Rubio study authors again to request data on ongoing clinical pregnancies.
You briefly touch on the challenge of various studies using single or dual embryo transfer, as well as fresh and frozen‐thawed transfer cycles and different days of transfer. You highlight this in conjunction with the intention to treat principle which includes outcomes from patients with a variety of these procedures. In our protocol we outlined that we would include studies that utilize any of these variations in treatment, as occurs in real life. We have detailed the number of embryos transferred, including details on planned day of transfer in the characteristics of studies table for each included study.
4. We acknowledge the heterogeneity in the definition of miscarriage between the included studies. Unfortunately this is an unresolved academic issue in the field of fertility research, where there is a lack of uniform definition, not only amongst journals, but also between countries. As you have highlighted, often papers do not provide a definition. In our protocol we described that miscarriage and stillbirth would be expressed per woman randomized. If we were to report per pregnancy, there is a risk of unbalancing the groups and adding bias to the analysis.
5. The review will be updated with new data when it becomes available. We do not consider that the points raised justify withdrawing the review. We would like to assure you that as part of the publication process this review has been through a rigorous peer review process. This included peer review from embryologists within the field.
The authors thanked Professors Pribenzky and Montag for taking the time to carefully consider the review.
Contributors
Associate Professor Csaba Pribenszky, St Istvan Univ. Faculty of Vet. Sci., Budapest, Hungary
Professor Markus Montag, Ilabcomm GmbH, St. Augustin, Germany
Review authors: Sarah Armstrong, Nicola Arroll, Lynsey M Cree, Vanessa Jordan, Cindy Farquhar
Associate Professor Csaba Pribenszky and Prof Markus Montag were asked to disclose their conflicts of interest. They stated that CP is a senior scientist in Vitrolife AB and MM is consulting for Vitrolife AB.
Feedback received November 2018
Summary
To the Editor,
The undersigned, as the authors of a meta‐analysis recently published in RBMO (1) and opinions to the former Cochrane review on time‐lapse systems (TLS) for ART (2) are raising a number of concerns about the information issued in the latest Cochrane review on this topic (3). Time‐lapse, with several systems available, and being used in the clinical practice, was introduced a decade ago. In 2014 only two papers could serve as a basis for evaluation and neither had information regarding live birth. In 2018 there are 5 clinical studies that discuss time‐lapse as a technique. These studies demonstrate the benefits for both incubation and embryo evaluation and show live birth data (4). Regardless of the best methodology and intention, information gained from personal communications and assessment of methods, execution and biases of different studies might sometimes be derived differently between investigators. We believe that the correct dissemination of opposing views serves the scientific community and highlights the essence and usefulness of an intervention better.
Primarily we would like to highlight that the only meaningful clinical comparison with and without time‐lapse is when the intervention is used in its intended entirety: for incubation and evaluation (1,4). Using TLS only for incubation or for checking still images does not represent the intended full use of time‐lapse to gain clinically relevant information about embryo development which is otherwise missed with static evaluation.
Furthermore, we have discovered some essential errors in the manuscript:
1: In the results summary section it says “The evidence suggests that if the live birth rate associated with no TLS is 38%, the rate with use of conventional incubation would be between 36% and 58%, and that if miscarriage rate with conventional incubation is 9%, the rate associated with TLS would be between 4% and 10%.” The comparison groups have been referred to incorrectly. The proper sentence should be this: “…with use of conventional incubation is 38%, the rate with TLS would be between 36% and 58%.”
The plain language summary erroneously states the same: “The evidence suggests that if the live birth rate associated with no TLS is 38%, the rate with use of conventional incubation would be between 36% and 58%.” So again, the proper phrasing should be the following: “The evidence suggests that if the live birth rate associated with no TLS is 38%, the rate with use of TLS would be between 36% and 58%.”
2: Park et al. is included in the “TLS utilising embryo selection software”, however Park did not use an algorithm. The study reported D2 transfer results and time‐lapse information was not used for embryo evaluation. This is significant as erroneously including Park et al. into the analysis alters the results and leads to a wrong conclusion. The results are shown incorrectly in the Analysis 3.1. (Comparison 3. TLS utilising embryo selection software versus conventional incubation and assessment (trial design 3)), for outcome “1 Livebirth”. There are further inconsistencies with regards to the use of Park et al.: the study is used for Comparison 1. (Fig 4. LBR and Fig 5. Miscarriage) and for Comparison 3. In comparison 3, it is only used for LBR (Fig 6) but not for Miscarriage (Fig 7). The authors refer to Park et al. as having used published data only (page 30.), however, LBR was not published. Moreover, why did the authors not include Park for the CPR in Fig 8.? If 3.1 comparison is executed correctly, without the inclusion of Park et al., the analysis would show an effect for the clear benefit of time‐lapse.
3: Acquiring LBR data carries issues as well. On Page 42 the authors state that they acquired data from Park et al. (contradicting information on Page 30. ) via communication with the authors. However, authors of other publications were not contacted. Neither Kovacs et al. or Rubio et al. were contacted for LBR data (personal communication to KP). The data is in fact publicly available. Kovacs has published LBR at ESHRE 2015 and data is available on ClinicalTrials.gov. LBR of the Rubio study has been published in ASRM, 2015 6 and was published as full paper in 2017 September (Insua et al.), reporting the same numbers as in the congress abstract in 2015. Insua et al. 2017 was left out from the Cochrane review. The review states that the data collection was closed in 2017 August. Nevertheless the protocol history on https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD011320.pub3/information #whatsNew states that on the 23rd of January, 2018 a new search had been performed, at that time Insua et al. was available. Moreover, the authors of the Cochrane review actually advised us in their “letter to the Editor” of RBMOnline (published online in December 29, 2017) to use the data of Insua et al. Including Insua et al into the analysis would further strengthen the positive effect of time‐lapse, as was described formerly by Pribenszky et al.
4 . 4: The study by Wu et al. (2016) had been excluded from the analysis due to allocation bias. Allocation bias existed for Rubio et al. and also for Kovacs et al. (both gained high risk), but these studies were actually included. Including data from Wu et al., (2016) would have further strengthened the positive conclusions towards the effect of time‐lapse. There are also some minor points worth mentioning:
1. In the background section it says, that “Some clinics have developed their own algorithms to adapt the standardised one that comes with the TLS device (Petersen 2016).” Nevertheless, the standardised algorithms as mentioned in the quote can not be modified by the users, moreover it does not automatically come with the TLS device.
2. In the Methods it states that “…completed results of a single‐centre RCT in Hungary (Kovacs 2013).” whereas that study was a two‐center RCT. Based on the above discussion points, the analysis is misleading and points to an erroneous conclusion with regards to the benefit of using time‐lapse incubation and embryo evaluation in clinical practice.
CP is a senior scientist in Vitrolife AB MM is consulting for Vitrolife AB
References:
1. Pribenszky C, Nilselid AM, Montag M. (2017) Time‐lapse culture with morphokinetic embryo selection improves pregnancy and live birth chances and reduces early pregnancy loss: a meta‐analysis. Reproductive BioMedicine Online, Vol. 35, Issue 5, p511–520.
2. Armstrong S, Arroll N, Cree LM, Jordan V, Farquhar C. (2015) Time‐lapse systems for embryo incubation and assessment in assisted reproduction. Cochrane Database of Systematic Reviews 2015, Issue 2.
3. Armstrong S, Bhide P, Jordan V, Pacey A, Farquhar C. (2018) Time‐lapse systems for embryo incubation and assessment in assisted reproduction. (Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No.: CD011320
4. Pribenszky C, Nilselid AM, Montag M. (2018) Response: time‐lapse systems for ART. Reproductive BioMedicine Online, Vol. 36, Issue 3, p290–292.
5. Armstrong S, Bhide P, Jordan V, Pacey A, Farquhar C. (2017) Time‐lapse systems for ART. Reproductive BioMedicine Online, Vol. 36, Issue 3, p288–289 (Published online: December 29, 2017)
6. Insua MF, Cobo A, Larreategui Z, Ferrando M, Remohi J, Meseguer M. (2015) Obstetric and perinatal outcomes of singleton newborns using time lapse monitoring. Fertil. Steril. 2015; 104: Issue 3, Supplement, Pages e212–e213.
Reply
Dear Dr Montag and Prof Pribenszky,
Re: Armstrong S, Bhide P, Jordan V, Pacey A, Farquhar C. Time‐lapse systems for embryo incubation and assessment in assisted reproduction. Cochrane Database of Systematic Reviews 2018, Issue 5. Art. No.: CD011320. DOI: 10.1002/14651858.CD011320.pub3.
Thank you for your comments regarding our Cochrane review submitted 21st November 2018. We would like to take the opportunity to respond to your comments.
You remark that time‐lapse systems (TLS) have been in clinical use for over a decade and reference your own systematic review of 5 studies on the topic, to demonstrate evidence of improvement in clinical outcomes when using TLS (1). We uphold our belief that novel interventions and medical devices should be rigorously tested for clinical efficacy and safety through RCTs prior to widespread clinical use, and that Cochrane systematic reviews offer a robust way of distilling all available evidence. This Cochrane review includes a further 4 RCTs not included in your review, and discounts a non‐randomized study you included. Additional methodological flaws in your systematic review were outlined in our published response at the time (2). This Cochrane review highlights how there remains insufficient evidence of differences in clinical outcomes to choose between TLS and conventional incubation and assessment and that the available evidence is at high risk of bias for randomization and allocation concealment.
You comment that the only meaningful comparison to undertake in systematic reviewing of TLS is to use TLS in its entirety (utilizing embryo selection software) as the intervention, versus conventional embryo incubation and assessment. However, TLS are a complex technology and combining all trial designs together is unhelpful and is prone to inconsistency. The potential benefit of TLS may lie in either the undisturbed culture environment, the ability for the software to help choose the best quality embryo for replacement, or indeed a combination of the two (3). Our three comparisons enable us to attempt to scientifically answer this question. Indeed, the trial designs allow us to easily split studies in this manner.
Regarding points 1 and 2 of your letter, we would like to thank you for highlighting an error in our manuscript. We accidentally put the data for Park 2015 in comparison 3.1 instead of that for Kovacs 2013 (livebirth for TLS utilizing embryo selection software versus conventional embryo incubation and assessment). This was a genuine mistake and we are sincerely sorry. As a result of finding this error, we have updated the review to ensure we correct any errors and update it with all newly available data.
Regarding point 3 of your letter, we did indeed contact all authors for livebirth data, including Peter Kovacs of Kovacs 2013 and Marcos Meseguer of Rubio 2014. We have records of our correspondence with them. We utilized data from Insua 2017, the reference can be found as a sub‐reference under the primary study Rubio 2014.
Point 4 of your letter states that we excluded data from Wu 2016 in our analysis due to ‘allocation bias’. This is incorrect. We included all available data from this study, comprising clinical pregnancy rates in comparison 1.4.
Thank you for taking the time to thoroughly critique our review and we welcome the scientific debate over the clinical efficacy of novel ART technologies and devices. We have updated your disclosed conflicts of interest in our review.
Yours sincerely
Dr Sarah Armstrong
Dr Priya Bhide
Dr Vanessa Jordan
Prof Allan Pacey
Prof Cindy Farquhar
1 Pribenszky, C., Nilselid, A.‐M., and Montag, M. Time‐lapse culture with morphokinetic embryo selection improves pregnancy and live birth chances and reduces early pregnancy loss: a meta‐analysis. Reprod. Biomed. Online. 2017; 35: 512–520
2 Armstrong S, Bhide P, Jordan V, Pacey A, Farquhar C. Time lapse systems for ART. Reproductive Biomedicine Online 2017. DOI: 10.1016/j.rbmo.2017.12.012
3 Armstrong S, Vail A, Mastenbroek S, Jordan V, Farquhar C. Time‐lapse in the IVF lab: how should we assess potential benefit? Human Reproduction 2015, 30 (1):3‐8. DOI: 10.1093/humrep/deu250
Contributors
Associate Professor Csaba Pribenszky, St Istvan Univ. Faculty of Vet. Sci., Budapest, Hungary
Professor Markus Montag, Ilabcomm GmbH, St. Augustin, Germany
Review authors: Sarah Armstrong, Priya Bhide, Jane Marjoribanks, Vanessa Jordan, Allan Pacey and Cindy Farquhar
Associate Professor Csaba Pribenszky and Prof Markus Montag were asked to disclose their conflicts of interest. They stated that CP is a senior scientist in Vitrolife AB and MM is consulting for Vitrolife AB.
What's new
| Date | Event | Description |
|---|---|---|
| 7 January 2019 | New search has been performed | Updated with the addition of one new randomised controlled trial, Barberet 2018, and two further publications with new data relating to two previously included randomised controlled trials (Kovacs 2019; Yang 2018). Correction made to data in Analysis 3.1 (Kovacs 2019). The primary outcome has been changed from live birth to live birth or ongoing pregnancy (composite outcome). |
| 7 January 2019 | New citation required but conclusions have not changed | The addition of one new trial and two publications with new data has not changed our conclusions. |
History
Protocol first published: Issue 9, 2014 Review first published: Issue 2, 2015
| Date | Event | Description |
|---|---|---|
| 2 August 2018 | Amended | Correction of a typo in Characteristics of studies |
| 23 January 2018 | New citation required but conclusions have not changed | The addition of five new studies has not changed our conclusions. The comparisons have been restructured. |
| 23 January 2018 | New search has been performed | We added five new studies for this update (Goodman 2016; Kaser 2017; Park 2015; Wu 2016; Yang 2018). Kovacs 2019 had new data published in 2017. |
| 5 October 2015 | Feedback has been incorporated | Feedback on the review was received in April 2015. Feedback and the review authors' response to the feedback have been summarised in the 'Feedback' section. Footnotes were added to 'Summary of findings' tables 1 and 3 and to the 'Characteristics of included studies' table for Rubio 2014. |
| 5 March 2015 | Feedback has been incorporated | Feedback applicable to the review protocol was received in February 2015. Feedback and the review authors' response to the feedback have been summarised in the 'Feedback' section. No changes were made to the review. |
Acknowledgements
Our heartfelt thanks go to:
Marian Showell for her assistance with search strategies and re‐running searches;
Helen Nagels for her editorial advice and assistance;
the editorial review team, who dedicated their valued time and effort to this review;
the women/couples who joined the randomised controlled trials included here, whose altruism has helped to guide evidence;
Ms Nicola Arroll and Dr Lynsey Cree, who contributed to the first iteration of this review;
Roger Hart, Andy Vail, and Jeanette MacKenzie for their invaluable peer review.
Appendices
Appendix 1. Cochrane Gynaecology and Fertility Specialised Register search strategy
ProCite platform
Searched 7 January 2019
Keywords CONTAINS "time lapse monitoring" or "time lapse" or "embryoscope" or Title CONTAINS "time lapse monitoring" or "time lapse" or "embryoscope" (55 hits)
Appendix 2. CENTRAL CRSO search strategy
Web platform
Searched 7 January 2019
#1 MESH DESCRIPTOR Fertilization in Vitro EXPLODE ALL TREES 1952
#2 MESH DESCRIPTOR Sperm Injections, Intracytoplasmic EXPLODE ALL TREES 509
#3 MESH DESCRIPTOR Embryo Transfer EXPLODE ALL TREES 1025
#4 (in vitro fertili?ation):TI,AB,KY 2476
#5 (ivf or icsi):TI,AB,KY 4768
#6 (intracytoplasmic sperm injection*):TI,AB,KY 1468
#7 embryo*:TI,AB,KY 5563
#8 blastocyst*:TI,AB,KY 938
#9 MESH DESCRIPTOR Ectogenesis EXPLODE ALL TREES 11
#10 MESH DESCRIPTOR Embryonic Development EXPLODE ALL TREES 548
#11 MESH DESCRIPTOR Reproductive Techniques, Assisted EXPLODE ALL TREES 2989
#12 (assisted reproduct*):TI,AB,KY 1009
#13 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 9096
#14 Eeva*:TI,AB,KY 19
#15 (Primo Vision*):TI,AB,KY 12
#16 Embryoviewer*:TI,AB,KY 3
#17 Embryoscope*:TI,AB,KY 51
#18 timelapse*:TI,AB,KY 7
#19 (time lapse*):TI,AB,KY 232
#20 (sequential embryo*):TI,AB,KY 4
#21 #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 254
#22 #13 AND #21 148
Appendix 3. MEDLINE search strategy
Ovid platform
Searched from 1946 to 7 January 2019
1 exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ (39146) 2 in vitro fertili?ation.tw. (21435) 3 ivf‐et.tw. (2188) 4 icsi.tw. (7626) 5 intracytoplasmic sperm injection$.tw. (6614) 6 ivf.tw. (21740) 7 (embryo or embryos).tw. (172905) 8 blastocyst$.tw. (20675) 9 exp ectogenesis/ or exp embryonic development/ (55562) 10 exp Reproductive Techniques, Assisted/ (65007) 11 assisted reproduct$.tw. (13347) 12 or/1‐11 (261098) 13 time lapse.tw. (10391) 14 timelapse.tw. (127) 15 Embryoscope$.tw. (57) 16 Embryoviewer.tw. (0) 17 Eeva$.tw. (55) 18 Primo Vision$.tw. (6) 19 (sequential embryo$ adj2 scor$).tw. (2) 20 (sequential embryo$ adj2 assess$).tw. (2) 21 or/13‐20 (10567) 22 randomized controlled trial.pt. (473863) 23 controlled clinical trial.pt. (92838) 24 randomized.ab. (430801) 25 randomised.ab. (85959) 26 placebo.tw. (199703) 27 clinical trials as topic.sh. (185645) 28 randomly.ab. (302952) 29 trial.ti. (192194) 30 (crossover or cross‐over or cross over).tw. (78777) 31 or/22‐30 (1249782) 32 exp animals/ not humans.sh. (4532405) 33 31 not 32 (1149881) 34 12 and 21 and 33 (52)
Appendix 4. Embase search strategy
Ovid platform
Searched from 1980 to 7 January 2019
1 exp embryo transfer/ or exp fertilization in vitro/ or exp intracytoplasmic sperm injection/ (66279) 2 in vitro fertili?ation.tw. (29005) 3 ivf‐et.tw. (3035) 4 icsi.tw. (16058) 5 intracytoplasmic sperm injection$.tw. (9451) 6 ivf.tw. (39332) 7 (embryo or embryos).tw. (186387) 8 blastocyst$.tw. (27731) 9 exp ectogenesis/ (124) 10 exp embryo development/ (149582) 11 exp infertility therapy/ (94639) 12 assisted reproduct$.tw. (21367) 13 or/1‐12 (350365) 14 time lapse$.tw. (14196) 15 timelapse.tw. (516) 16 Embryoscope$.tw. (531) 17 Eeva$.tw. (159) 18 Primo Vision$.tw. (42) 19 (sequential adj2 embryo$ scor$).tw. (3) 20 (sequential adj2 embryo$ assess$).tw. (3) 21 or/14‐20 (14742) 22 Clinical Trial/ (943095) 23 Randomized Controlled Trial/ (525520) 24 exp randomization/ (80582) 25 Single Blind Procedure/ (33489) 26 Double Blind Procedure/ (153616) 27 Crossover Procedure/ (57605) 28 Placebo/ (314683) 29 Randomi?ed controlled trial$.tw. (193503) 30 Rct.tw. (30758) 31 random allocation.tw. (1845) 32 randomly allocated.tw. (31235) 33 allocated randomly.tw. (2383) 34 (allocated adj2 random).tw. (798) 35 Single blind$.tw. (21833) 36 Double blind$.tw. (186587) 37 ((treble or triple) adj blind$).tw. (868) 38 placebo$.tw. (276887) 39 prospective study/ (492047) 40 or/22‐39 (1961581) 41 case study/ (58345) 42 case report.tw. (359641) 43 abstract report/ or letter/ (1041120) 44 or/41‐43 (1449925) 45 40 not 44 (1911901) 46 13 and 21 and 45 (228)
Appendix 5. CINAHL search strategy
EBSCO platform
Searched from 1961 to 7 January 2019
| # | Query | Results |
| S34 | S21 AND S33 | 26 |
| S33 | S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 | 1,297,598 |
| S32 | TX allocat* random* | 9,662 |
| S31 | (MH "Quantitative Studies") | 21,626 |
| S30 | (MH "Placebos") | 11,084 |
| S29 | TX placebo* | 54,815 |
| S28 | TX random* allocat* | 9,662 |
| S27 | (MH "Random Assignment") | 52,588 |
| S26 | TX randomi* control* trial* | 163,344 |
| S25 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 997,917 |
| S24 | TX clinic* n1 trial* | 237,741 |
| S23 | PT Clinical trial | 86,729 |
| S22 | (MH "Clinical Trials+") | 254,280 |
| S21 | S12 AND S20 | 183 |
| S20 | S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 | 984 |
| S19 | TX sequential embryo* N2 assess* | 3 |
| S18 | TX sequential embryo* N2 scor* | 2 |
| S17 | TX Primo Vision | 17 |
| S16 | TX timelapse | 7 |
| S15 | TX Eeva* | 308 |
| S14 | TX Embryoscope* | 0 |
| S13 | TX time lapse | 653 |
| S12 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 | 21,263 |
| S11 | TX ectogenesis | 13 |
| S10 | TX assisted reproduct* | 3,396 |
| S9 | (MM "Reproduction Techniques+") | 8,104 |
| S8 | (MM "Fetal Development") | 3,165 |
| S7 | TX blastocyst* | 1,976 |
| S6 | TX(embryo or embryos) | 8,229 |
| S5 | TX intracytoplasmic sperm injection* | 787 |
| S4 | TX IVF or TX ICSI | 4,476 |
| S3 | (MM "Fertilization in Vitro") | 3,120 |
| S2 | TX vitro fertilization | 6,316 |
| S1 | TX vitro fertilisation | 6,316 |
Appendix 6. ClinicalTrials.gov trial registry and the WHO ICTRP portal
Web platform
Searched 9 January 2019
'Timelapse'
'Time lapse
'Embryoscope'
Data and analyses
Comparison 1. TLS with conventional morphological assessment of still TLS images versus conventional incubation and assessment (trial design 1).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth or ongoing pregnancy | 3 | 826 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.67, 1.23] |
| 2 Miscarriage | 3 | 826 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.90 [0.99, 3.61] |
| 3 Stillbirth | 1 | 76 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.13, 7.49] |
| 4 Clinical pregnancy | 4 | 875 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.79, 1.41] |
Comparison 2. TLS utilising embryo selection software versus TLS with conventional morphological assessment of still TLS images (trial design 2).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth or ongoing pregnancy | 1 | 163 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.32, 1.20] |
| 2 Miscarriage | 2 | 463 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.39 [0.64, 3.01] |
| 3 Clinical pregnancy | 2 | 463 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.67, 1.42] |
Comparison 3. TLS utilising embryo selection software versus conventional incubation and assessment (trial design 3).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth or ongoing pregnancy | 3 | 1617 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.92, 1.36] |
| 2 Miscarriage | 3 | 1617 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.45, 0.89] |
| 3 Stillbirth | 1 | 600 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Clinical pregnancy | 3 | 1617 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.78, 1.16] |
3.3. Analysis.
Comparison 3 TLS utilising embryo selection software versus conventional incubation and assessment (trial design 3), Outcome 3 Stillbirth.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Barberet 2018.
| Methods | Study: completed single‐centre RCT of couples with infertility undergoing ICSI Country: France Cause and length of infertility: male factor (76% to 77%), female factor (42% to 46%). Mixed (64% to 74%), idiopathic (3%). Oocytes: autologous oocytes Embryo transfer: 1 or 2 fresh embryos on day 2, day 3 or day 5‐6. Informed consent: not mentioned Total study duration: March 2016 to December 2016 Funding sources: not mentioned |
|
| Participants | A total of 386 couples with infertility undergoing ICSI with autologous oocytes were randomised: 191 to TLS selection (closed system, Embryoscope incubator) and 195 to conventional selection (benchtop G185 incubator). There were 4 misallocations (1 in G185 group and 3 in the TLI group) and 1 participant not fulfilling the inclusion criteria (only 2 injected oocytes). Data analysed by intention‐to‐treat as well as per‐protocol. Age (years, mean ± SD, time‐lapse selection versus conventional selection): 32.1 ± 4.8 versus 32.3 ± 4.6 BMI (kg/m2, mean ± SD, time‐lapse selection versus conventional selection): 23.5 ± 3.8 versus 24 ± 4.3 Ethnicity: not reported Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | TLS with conventional morphological assessment of still TLS images (intervention) Conventional incubation and assessment (control) |
|
| Outcomes | Ongoing pregnancy per couple randomised (defined as the presence of a gestational sac with a foetal heartbeat at >/= 12 weeks) (obtained from email communication with author) Miscarriage of clinical pregnancy per couple randomised (updated ongoing‐pregnancy and detailed miscarriage rates obtained from authors following email communication) Clinical pregnancy (with at least 1 intrauterine gestational sac visible on the ultrasound examination 4 to 5 weeks after ET) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The allocation algorithm, which relied on a minimization approach, was established by the statistician of the coordinating centre before the start of the trial. This allocation was stratified on woman's age (<orR37 years), day of oocyte retrieval (Friday [leading to ET at day 3] or not [leading to ET at day 2]), and rank of attempts (rank 1–2 or 3‐4)." |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization was performed online by the investigator (embryologist) using the secure Tenalea platform (Formsvision BV), after identification through a personal password and after a final check of the eligibility criteria" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Due to the nature of the study intervention, it was not possible to blind investigators to the embryo morphology assessments. However, for the analyses the data manager, statistician, and embryologists were blinded to the allocation." "Embryos were selected for vitrification according to their morphology, which was graded in unblinded embryo assessments" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Due to the nature of the study intervention, it was not possible to blind investigators to the embryo morphology assessments. However, for the analyses the data manager, statistician, and embryologists were blinded to the allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | Primary outcome (embryo implantation) is as per registered protocol. Pregnancy outcomes not mentioned in protocol. |
| Other bias | Low risk | No other sources of bias identified. |
Goodman 2016.
| Methods | Study: completed single‐centre RCT of couples with infertility undergoing IVF Country: USA Cause and length of infertility: infertility diagnosis included unexplained, ovulatory dysfunction, male factor, tubal factor, low ovarian reserve, AMA, endometriosis, mixed factors, and other. Mean length of infertility in both groups was approximately 31.5 months. Oocytes: autologous oocytes Embryo transfer: between 1 and 3 fresh embryos on day 3 or day 5. The number of embryos transferred was based on published ASRM committee guidance and patient preferences. Informed consent: yes Total study duration: March 2014 to May 2015 (14 months) Funding sources: quote: "no external funding for the study" |
|
| Participants | A total of 300 couples with infertility undergoing IVF with autologous oocytes were recruited: 150 randomised to TLS selection (cell‐tracking algorithm of TLS utilised) and 150 randomised to conventional selection (TLS with conventional once‐daily morphologic embryo screening). 5 couples did not receive the allocated intervention: 2 from the time‐lapse selection arm due to lack of fertilisation, and 3 from the conventional selection group, 2 due to no fertilisation and 1 due to no sperm. Age (years, mean ± SD, time‐lapse selection versus conventional selection): 33.6 ± 4.0 versus 33.2 ± 3.9 BMI (kg/m2, mean ± SD, time‐lapse selection versus conventional selection): 26.3 ± 6.7 versus 26.9 ± 7.4 Ethnicity: combination of white, black, Asian, Middle Eastern, and other Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | TLS utilising cell‐tracking algorithm (intervention) TLS with conventional assessment of morphological parameters from still TLS images (control) |
|
| Outcomes | Clinical pregnancy rate per couple randomised (defined by the presence of foetal cardiac activity on transvaginal ultrasonography at >= 6 weeks gestational age)Miscarriage per couple randomised | |
| Notes | Data on clinical pregnancy from women excluded following randomisation and miscarriage data were obtained following communication with the authors. Live‐birth and stillbirth data were requested, but this information was not available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomized 1:1 to conventional embryo selection versus Embryoscope time‐lapse morphokinetic selection with the use of a computer‐generated random number sequence" |
| Allocation concealment (selection bias) | Low risk | Quote: "The list was housed in the laboratory, where it was accessible only by research personnel not involved with the recruitment of patients" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Patients, physicians and staff, and sonographers were blinded to how embryos were selected". However, the embryologist who was responsible for deciding on day of embryo transfer (day 3 or day 5) was unblinded, therefore deemed high risk. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "sonographers were blinded" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | We have obtained all relevant data from women who were excluded postrandomisation from the authors. |
| Selective reporting (reporting bias) | Low risk | We confirmed with the authors that all outcomes the study set out to assess were published. |
| Other bias | Low risk | No other sources of bias identified. |
Kahraman 2013.
| Methods | Study: completed single‐centre RCT of couples with infertility undergoing ICSI Country: Turkey Cause and length of infertility: tubo‐peritoneal factor. Length of infertility not reported. Oocytes: autologous oocytes Embryo transfer: single embryo transfer at blastocyst Informed consent: yes Total study duration: December 2011 to June 2012 (6 months) Funding sources: none |
|
| Participants | A total of 76 couples with infertility undergoing ICSI with autologous oocytes were recruited: 38 were randomised to TLS and 38 were randomised to conventional incubation. In all, 12 couples withdrew from the study: 7 in the conventional incubation arm and 5 in the TLS arm. Reasons for withdrawal were documented and data for outcomes such as live birth, adverse events, and clinical pregnancy for these couples were included in this review. Age (years, mean ± SD, TLS versus conventional incubation): 28.5 ± 3.32 versus 28.5 ± 3.72; P = 0.83 BMI (kg/m2, mean ± SD, TLS versus conventional incubation): 23.92 ± 3.79 versus 23.92 ± 4.42; P = 0.77 Ethnicity: not reported Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | TLS with conventional morphological assessment of still TLS images (intervention) Conventional incubation and assessment (control) |
|
| Outcomes | Live‐birth rates per couple randomised Clinical pregnancy rate per couple randomised (clinical pregnancy was defined as the presence of a gestational sac detected on ultrasound 3 weeks after the first βhCG test, which was performed 14 days after oocyte retrieval) Stillbirth and miscarriage per couple randomised |
|
| Notes | Live‐birth and stillbirth information was available following communication with the author and was not published. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer based randomization list" Quote: "Randomisation was done according to a list generated on random.org" |
| Allocation concealment (selection bias) | Low risk | Communication with author. Quote: "Randomization list was held by one of the investigators who was not involved clinically with the patients. Also, he was not routinely working in the embryology laboratory. The randomization from random.org was printed out into sequentially numbered lists where the groups were masked and not revealed until the recruitment of each patient" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Communication with author. Quote: "Clinicians were blinded in the study up to the point after the embryo transfer was performed. Also the patients did not know to which group they were allocated. Only the discontinued patients received information about the incubation process once the drop‐out decision was made (Due to the need to inform the patients about their early/cancelled transfers)". It was impossible to blind the embryologist, therefore performance bias deemed high risk. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Communication with author. Quote: "Clinicians, those assessing the outcome were not necessarily blinded to the intervention as some of our ART patients prefer to have those controls outside our clinic and report the outcomes to us". The outcomes are objective and are therefore unlikely to be influenced by knowledge of the intervention, therefore detection bias deemed low risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 12 couples discontinued the trial following randomisation secondary to adverse events that were not reported as adverse events or analysed within the main publication. However, on communication with the author, the numbers of discontinued participants in each arm were disclosed, alongside reasons for dropouts. Quote: "embryos transferred day 3, 4 and 5 with single blastocyst developed; total freezing because of ovarian hyperstimulation syndrome (OHSS) risk" |
| Selective reporting (reporting bias) | Low risk | Communication with author. Quote: "As reported in our article, we have published all of the outcomes we aimed to assess. Unfortunately, we do not formally prepare a study protocol". On contacting the author, data on live birth and adverse events were made available, although this information was not published. |
| Other bias | Low risk | None detected. |
Kaser 2017.
| Methods | Study: completed RCT of couples with infertility undergoing a fresh SET Country: USA Cause and length of infertility: a combination of anovulation, diminished ovarian reserve, endometriosis, male factor, tubal, unknown, uterine, and other Oocytes: autologous oocytes Embryo transfer: single embryo transfer Informed consent: yes Total study duration: August 2014 to February 2016 (18 months) Funding sources: Progyny Inc |
|
| Participants | A total of 163 couples with infertility undergoing ART with autologous oocytes were recruited:
In all, 13 couples did not receive the allocated intervention:
Age (years, mean ± SD): Day 3 + TLS 34.6 ± 3.1, Day 5 + TLS 33.7 ± 3.4, Day 5 control 34.1 ± 3.1 BMI (kg/m2, mean ± SD): Day 3 + TLS 26 ± 6.9, Day 5 + TLS 25.5 ± 6.1, Day 5 control 25.5 ± 6.5 Ethnicity: a combination of white, Asian, black, Hispanic, and "other" ethnicities Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | TLS utilising conventional benchtop morphology and embryo selection software (2 intervention arms: day 3 and day 5 embryo transfer) TLS with conventional benchtop morphology (control). Embryo selection software or time‐lapse photography was not utilised. |
|
| Outcomes | Clinical pregnancy rate per couple randomised Miscarriage rate per couple randomised (data obtained from authors) |
|
| Notes | Wrote to authors August 2017 asking for further information. Note differing days of embryo transfer. Control group split between 2 intervention groups for purposes of this review. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were blocked according to age (<35, 35‐37, 38‐40 years) and randomised 1:1:1 at the fertilization check by an embryologist using computer‐generated, random number sequence cards enclosed in opaque, serially numbered envelopes" |
| Allocation concealment (selection bias) | Low risk | Quote: "random number sequence cards enclosed in opaque, serially numbered envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Embryologists were blinded to the Eeva (time lapse) ratings at the conventional morphology evaluation (i.e. one embryologist performed conventional morphology and a different embryologist reviewed the Eeva ratings, and patients and physicians were blinded to the Eeva ratings until a negative pregnancy test of the primary endpoint was reached". The embryologist was ultimately unblinded to the allocation, therefore high risk of performance bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and physicians were blinded to the Eeva ratings. Correspondence with author. Quote: "As patients were randomised to day 3 or day 5 transfer, blinding was not possible between groups 1 vs. group 2/3 (as the patient and physician knew which day the transfer was happening). For patients randomised to groups 2 or 3, both patients and physicians were blinded to study arm (so they knew a day 5 transfer was happening, but not how the embryo was selected for transfer)". The outcomes are objective and are therefore unlikely to be influenced by knowledge of the intervention, therefore detection bias deemed as low risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data presented as intention‐to‐treat and "as treated". |
| Selective reporting (reporting bias) | Low risk | Communication with authors. Quote: "All outcomes published" |
| Other bias | Low risk | None detected. |
Kovacs 2019.
| Methods | Study: completed multicentre RCT of couples with infertility undergoing IVF or ICSI Country: Hungary Cause and length of infertility: various causes (male, tubal, unexplained, etc.) of at least 1 year's duration Oocytes: autologous Embryo transfer: single embryo transfer at blastocyst Informed consent: yes Total study duration: July 2012 to April 2015 (33 months) Funding sources: none |
|
| Participants | 161 couples with infertility undergoing IVF or ICSI with single embryo transfer at blastocyst. 80 couples were randomised to TLS and 81 were randomised to conventional incubation. 22 couples dropped out of the study after randomisation: 12 dropped out from the TLS arm (2 dual embryo transfer requested; 1 no fertilisation; 7 fewer than 3 good embryos on day 3; 2 elective cryopreservation for OHSS risk), and 10 dropped out from the control arm (1 no fertilisation; 8 fewer than 3 good embryos on day 3; 1 elective cryopreservation for OHSS risk). Age: (years, mean ± SD, TLS versus conventional incubation): 31.2 ± 2.7 versus 32.1 ± 2.5 BMI: (kg/m2, mean ± SD, TLS versus conventional incubation): 22.3 ± 3.3 versus 22.2 ± 3.0 Ethnicity: Caucasian (understood to be white) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | TLS utilising cell‐tracking algorithm (intervention) Conventional incubation and assessment (control) |
|
| Outcomes | Clinical pregnancy rate per couple Miscarriage per couple randomised Live birth |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | On communication with author, paired randomisation sequence was explained: Quote: "Two envelopes containing time‐lapse or control group assignment were prepared. The first patient was randomly assigned to one of the groups and the next patient received the other assignment. This was repeated with patient number 3 and 4 and so on" |
| Allocation concealment (selection bias) | High risk | Communication with author. Quote: "The randomization is carried out by the principal investigator who is involved in the study" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Communication with author. Quote: "There was no blinding" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Communication with author. Quote: "There was no blinding". The outcomes are objective and are therefore unlikely to be influenced by knowledge of the intervention, therefore detection bias deemed as low risk. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Dropouts following randomisation and not included in intention‐to‐treat: 161 participants were randomised (80 TLS versus 81 standard monitoring), of which 22 participants dropped out. Reasons for dropouts were provided, however the reasons provided were not all predetermined exclusion criteria, and given the high attrition rate, we deemed this study as at high risk of attrition bias. |
| Selective reporting (reporting bias) | Low risk | None detected. |
| Other bias | Low risk | None detected. |
Park 2015.
| Methods | Study: single‐centre RCT, couples undergoing ICSI Country: Sweden Cause and length of infertility: male‐factor infertility was present in > 99% of participants in both study arms. Female‐factor infertility was present in approximately 20% of participants in both study arms. Duration of infertility was approximately 2.8 years in both study arms. Oocytes: autologous Embryo transfer: single embryo transfer at day 2 Informed consent: yes Total study duration: May 2010 to February 2014 (3 years, 9 months) Funding sources: Sahlgrenska Academy, Sahlgrenska University Hospital, LUA/ALF 70940, Ferring Research Infertility and Gynecology Grant, Hjalmar Svensson Grant, Unisense Fertilitech: Unisense provided the EmbryoScope free of charge during the study. |
|
| Participants | 364 couples with infertility undergoing their first IVF cycle with ICSI. 1 embryo (in a few cases 2 embryos, N = 12) of good quality, or in some cycles of less good quality (N = 27), was transferred on day 2, and supernumerary good‐quality embryos were frozen. 241 couples were randomised to TLS, and 124 were randomised to conventional incubation. 1 couple was excluded from the TLS arm as they had been randomised twice. Age: (years, mean ± SD, TLS versus conventional incubation): 31.8 ± 4.3 versus 31.8 ± 4.1; P = 0.90 BMI: (kg/m2, mean ± SD, TLS versus conventional incubation): 24.4 ± 3.9 versus 24.3 ± 4.0; P = 0.70 Ethnicity: not reported Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | TLS with conventional morphological assessment of still TLS images (intervention) Conventional incubation and assessment (control) |
|
| Outcomes | Clinical pregnancy rate per couple randomised Ongoing pregnancy rate defined as presence of the foetal heart at >= 8 weeks' gestation Miscarriage per couple randomised |
|
| Notes | Live‐birth, stillbirth, and clinical pregnancy data obtained on communication with study authors. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was undertaken using (quote): "a web‐based randomization programme and all the patients’ oocytes were allocated to culture in either a conventional incubator or in a closed system, in proportion 1:2" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was carried out by the embryologist after oocyte retrieval". On communication with the authors, they clarified that the embryologist undertaking the randomisation may have also undertaken the embryo assessment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The patients as well as the treating physician and the person performing the statistical analyses were blinded to which type of procedure was used until the outcome of transfer (pregnant versus not pregnant) was known". It was not possible to blind the embryologists, therefore performance bias deemed at high risk. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The patients as well as the treating physician and the person performing the statistical analyses were blinded to which type of procedure was used until the outcome of transfer (pregnant versus not pregnant) was known. Embryologists were not possible to blind" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only 1 woman was excluded from analysis in the intervention arm, as she was randomised twice. No women were excluded from the control arm. No dropouts. |
| Selective reporting (reporting bias) | Low risk | All predetermined outcomes were reported. |
| Other bias | Low risk | None detected. |
Rubio 2014.
| Methods | Study: completed multicentre RCT of couples with infertility undergoing ICSI Country: Spain Cause and length of infertility: not reported Oocytes: autologous and donor Embryo transfer: multiple embryo transfer (1.86 per couple, 95% CI 1.8 to 1.9) on day 3 and day 5 Informed consent: not reported Total study duration: February 2012 to July 2013 (17 months) Funding sources: the instrumentation, disposables, and utensils used in this study were fully paid for by IVI. IVI is a minor shareholder in Unisense FertiliTech A/S, but none of the authors have any economic affiliation with Unisense FertiliTech A/S. |
|
| Participants | A total of 856 couples with infertility undergoing IVF with autologous and donor oocytes: 444 couples were randomised to TLS and 412 to conventional incubation. In all, 13 couples were excluded from the study: 6 in the TLS arm (reasons: 2 had cancelled oocyte donation, and 4 had their embryos vitrified) and 7 in the conventional incubation arm (reasons: 1 woman had endometrial bleeding; 2 had cancelled oocyte donation; and 4 couples had their embryos vitrified). Age (years, mean ± SD, TLS versus conventional incubation): 34.7 ± 2.7 versus 34.6 ± 2.7 BMI (kg/m2, mean ± SD, TLS versus conventional incubation): 23.2 ± 3.7 versus 23.04 ± 2.8 Ethnicity: not reported Inclusion criteria: autologous or oocyte donation. Those receiving oocyte donation had 1 of the following diagnoses: failure to achieve pregnancy after at least 3 cycles of ART, genetic female or chromosomal disorders, or low response to controlled ovarian hyperstimulation. Donors were:
Inclusion criteria for both arms of study:
Exclusion criteria:
For autologous oocyte patients:
|
|
| Interventions | TLS utilising cell‐tracking algorithms (intervention) Conventional incubation and assessment (control) |
|
| Outcomes | Miscarriage per couple randomised Clinical pregnancy rate per couple randomised Live birth (obtained from Insua 2015 and Insua 2017) |
|
| Notes | October 2015: following clarification from authors of comments on this review, it has been made aware to us that the pregnancy data from this study are a combination of biochemical and ongoing pregnancy, therefore the miscarriage data may also include miscarriages from biochemical pregnancies. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Despite adequate random sequence generation, participants were able to request the intervention in some cases, and this was granted. See evidence below: Quote: "Patients were allocated to either TMS (study group) or SI (control group) using a computer generated randomization table which was handled by the embryologist at the laboratory in charge the day before the oocyte retrieval or oocyte donation. The randomization was not perfectly performed as the patient distribution to the two groups would have been expected to be 50:50 ratio than the reported 51.9:48.1. The main reason for this deviation was limited patient requests for TMS culture" |
| Allocation concealment (selection bias) | High risk | In some cases allocation was non‐random (see above). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Gynaecologist and statistician were blinded. Participants and embryologist were not blinded. Quote: "The study is considered double blind because 1) the gynaecologist (evaluating the primary effect) did not know to which group the patients had been assigned, and 2) the statistician evaluating the results only knew the incubators by a binary code, not by type" Communication with author. Quote: "The intention was to do triple blinded, but we discovered that some of our patients were informed (because they asked) of the group they were in. Therefore blinding failed in some of our patients. We then decided to describe it as double blind because patients blinding partially failed" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The gynaecologist evaluating the primary effect was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 13 participants were excluded from study after randomisation as they suffered adverse events (cancelled oocyte donation, embryos vitrified, and endometrial bleeding). Not included in intention‐to‐treat, but all excluded participants were accounted for, therefore low risk of attrition bias. |
| Selective reporting (reporting bias) | Low risk | Reported all outcomes declared on ClinicalTrials.gov On communication with the author: "We are currently collecting data on live birth and stillbirth" |
| Other bias | Low risk | None detected. |
Wu 2016.
| Methods | Study: completed single‐centre RCT of couples with infertility undergoing IVF and ICSI Country: USA Cause and length of infertility: "poor prognosis patients". Length of infertility not reported. Oocytes: autologous oocytes Embryo transfer: day 3 transfer of embryo. Number not disclosed. Informed consent: yes Total study duration: December 2014 to March 2015 (3.5 months) Funding sources: intramural funds from The Center for Human Reproduction and by grants from The Foundation for Reproductive Medicine. Vitrolife, Goteborg, Sweden, contributed a free EmbryoScope for the length of the study. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript. |
|
| Participants | A total of 49 couples with infertility undergoing IVF or ICSI with autologous oocytes: 24 couples were randomised to TLS and 25 to conventional incubation. In all, 18 couples were excluded from the study: 8 in the TLS arm (reasons: 6 had no mature oocytes or no fertilisation after ICSI, and 2 women had their embryos transferred on day 2), and 10 in the conventional incubation arm (reasons: 5 women had no mature oocytes or no fertilisation after ICSI, and 5 women had their embryos transferred on day 2). Age (years, mean ± SD, TLS versus conventional incubation): 38.8 ± 1.0 versus 40.4 ± 1.8 BMI (kg/m2, mean ± SD, TLS versus conventional incubation): not reported Ethnicity: not reported Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | TLS with conventional morphological assessment of still TLS images (intervention) Conventional incubation and assessment (control) |
|
| Outcomes | Clinical pregnancy rate per couple randomised (defined as ultrasound confirmation but no gestation was provided) | |
| Notes | Contacted authors August 2017 for further information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer randomization to either TLS or standard embryology was the responsibility of a member of the centre's Statistics Section (SKD) who was completely dissociated from the patient's IVF cycle" |
| Allocation concealment (selection bias) | Unclear risk | Randomisation was undertaken by a member of the team not associated with the treatment cycle. Quote: "The designation was then reported to the embryology staff which processed the patient's oocytes/embryos" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described. However, given that it would have been impossible to blind embryologists, performance bias deemed high risk. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Not possible to blind outcome assessors. The outcomes are objective and are therefore unlikely to be influenced by knowledge of the intervention, therefore detection bias deemed low risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Excluded participants were accounted for and were considered by trialists to be valid prespecified grounds for exclusion. |
| Selective reporting (reporting bias) | Unclear risk | No access to protocol |
| Other bias | Low risk | None detected. |
Yang 2018.
| Methods | Study: completed single‐centre RCT of couples with infertility undergoing IVF and ICSI Country: China Cause and length of infertility: quote: "good prognosis patients". Length of infertility not reported. Oocytes: autologous oocytes Embryo transfer: single embryo transfer; day 3 transfer of embryos in intervention group and day 5 transfer in control group Informed consent: obtained from all participants Total study duration: October 2015 to April 2017 (18 months) Funding sources: study funded by Ferring |
|
| Participants | A total of 600 couples with infertility undergoing IVF or ICSI with autologous oocytes: 300 couples were randomised to TLS utilising embryo selection software, and 300 couples were randomised to conventional incubation and morphology. In all, 15 couples were excluded from the study for the purpose of modified intention‐to‐treat analysis: 10 in the TLS arm (6 refused day 3 and time‐lapse algorithm; 3 had instrument breakdown; and 1 had an unforeseen medical condition) and 5 in the conventional incubation arm (3 refused day 5 and conventional morphological assessment, and 2 did not receive time‐lapse observation). Age (years, mean ± SD, TLS versus conventional incubation): not reported BMI (kg/m2, mean ± SD, TLS versus conventional incubation): not reported Ethnicity: not reported Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | TLS utilising embryo selection software (intervention) Conventional incubation and assessment (control) |
|
| Outcomes | Live birth per couple randomised (provided following email communication with authors) Miscarriage rate per couple randomised (clinical (gestational sac) pregnancy losses) Clinical pregnancy (defined as presence of gestational sac seen at 4 weeks after embryo transfer) Stillbirth (provided following email communication with authors) |
|
| Notes | Note differing days of embryo transfer (day 3 for intervention group and day 5 for control). All embryos cultured in TLS to day 3, then control embryos transferred to conventional incubator to day 5. Embryos in control arm evaluated by routine morphological assessment. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised in a 1:1 ratio via online‐generated blocks (www.random.org) once they had 2PN (>/=6 normally fertilized oocytes) on Day 1 of the cycle." |
| Allocation concealment (selection bias) | Low risk | Quote: "The study investigators (YLL and XYK) created the randomization list and study nurses who were unaware of the study protocol enveloped the randomised allocation in a consecutive order. The investigator (YLL) assessed the patient's eligibility and performed the randomization by opening the sealed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Communication with authors. Quote: "The study was not blinded because study participants and clinic staff were aware of which group they were following" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "clinic staff were not blinded" The outcomes are objective and are therefore unlikely to be influenced by knowledge of the intervention, therefore detection bias deemed as low risk. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Low number of dropouts, and reasons for attrition given. Quote: "The subject was excluded from the study post‐randomization if she did not undergo fresh transfer due to any unforeseen reason including ovarian hyper‐stimulation or uterine disorder." |
| Selective reporting (reporting bias) | Low risk | All study outcomes were published. |
| Other bias | High risk | Variation between arms of study in day of transfer (day 3 for intervention and day 5 for control). |
AMA: advanced maternal age
ASRM: American Society for Reproductive Medicine
ART: assisted reproductive technology
βhCG: beta human chorionic gonadotropin
BMI: body mass index
CI: confidence interval
ET: embryo transfer
FSH: follicle‐stimulating hormone
ICSI: intracytoplasmic sperm injection
IU: international units
IVF: in vitro fertilisation
OHSS: ovarian hyperstimulation syndrome
PCOS: polycystic ovarian syndrome
RCT: randomised controlled trial
SD: standard deviation
SET: single embryo transfer
TLS: time‐lapse system
2D: two‐dimensional
3D: three‐dimensional
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adamson 2016 | Not an RCT |
| Alhelou 2018 | Not an RCT |
| Arnesen 2014 | Pseudo‐randomised study ‐ this was established after discussion with the main author, who described allocation to intervention or control based on capacity of either incubator. |
| Belles 2014 | Randomised oocytes |
| Cruz 2011 | Randomised oocytes |
| Freour 2014 | Letter not containing study data |
| Hardarson 2016 | Randomised embryos, and study design not relevant |
| Huang 2014 | Unable to determine the nature of the control arm |
| Ingerslev 2011 | Randomised oocytes |
| Kaser 2014 | Systematic review |
| Kirkegaard 2012 | Randomised oocytes |
| Kirkegaard 2014 | Letter not containing study data |
| Kirkegaard 2015 | Systematic review |
| Loewke 2012 | Not an RCT |
| Lowen 2017 | Randomised embryos |
| Mara 2010 | Randomised oocytes |
| Meseguer 2012 | Not an RCT |
| Nakahara 2010 | Randomised oocytes |
| Polanski 2014 | Systematic review |
| Siristatidis 2015 | Non‐randomised study |
| Wu 2015 | Randomised embryos |
| Yang 2014 | Randomised oocytes |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Hulme 2014.
| Methods | RCT of embryos in uninterrupted culture (EmbryoScope (ES), Unisense Fertilitech, Denmark) versus standard incubation for ICSI cycles |
| Participants | Women undergoing ICSI |
| Interventions | TLS with conventional morphological assessment of still TLS images versus conventional incubation and assessment |
| Outcomes | Clinical pregnancy rate |
| Notes | Need to establish that women, not embryos, were randomised, as well as the nature of randomisation. Attempted to contact authors January 2019 but have received no response as of yet. |
ICSI: intracytoplasmic sperm injection
RCT: randomised controlled trial
TLS: time‐lapse system
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐IIR‐16008758.
| Trial name or title | Evaluating the implantation rate of GERI‐incubator‐cultured embryos |
| Methods | Multicentre randomised controlled trial |
| Participants | Projected sample size: 1214 Characteristics of women must be as follows:
|
| Interventions | Intervention: embryo cultured and assessed by the GERI time‐lapse system Control: embryo cultured in conventional incubator and assessed by benchtop microscope |
| Outcomes | Primary outcome: implantation rate Secondary outcomes: clinical pregnancy, ongoing pregnancy (week 10‐12), multiple pregnancy, ectopic pregnancy, spontaneous miscarriage (week 10‐12 confirmed by ultrasound) |
| Starting date | July 2016 |
| Contact information | Guoning Huang: guoning.huang@outlook.com |
| Notes | States that study will be completed by October 2017 and published within 6 months |
ChiCTR1800017127.
| Trial name or title | A prospective randomised controlled study of cleavage embryo transplantation using dynamic observation techniques |
| Methods | Randomised controlled trial |
| Participants | Women:
|
| Interventions | Intervention: time‐lapse observation Control: traditional embryo morphology assessment methods |
| Outcomes | Primary outcome: ongoing pregnancy Secondary outcomes: implantation, early abortion, ectopic pregnancy |
| Starting date | 15 January 2019 |
| Contact information | Yifan Gu: evangoo@163.com |
| Notes |
ISRCTN17792989.
| Trial name or title | A pragmatic, multi‐centre, three‐arm randomized controlled trial to assess the clinical effectiveness and safety of time lapse imaging in in‐vitro fertilisation treatment (also known as the TILT study) |
| Methods | Randomised controlled trial |
| Participants | Couples undergoing IVF or ICSI treatment where:
|
| Interventions | Intervention group 1: group embryos are grown in the time‐lapse incubator using time‐lapse imaging for embryo selection Intervention group 2: embryos are grown in the time‐lapse incubator using only standard assessment techniques Control group: embryos are grown in standard incubators |
| Outcomes | Live birth Pregnancy |
| Starting date | September 2017 |
| Contact information | Doris Lanz: d.lanz@qmul.ac.uk Dominic Baxter: cd.baxter@qmul.ac.uk |
| Notes |
Khan 2018 [pers comm].
| Trial name or title | TILT ‐ Time‐Lapse Imaging Trial |
| Methods | Multicentre randomised controlled trial |
| Participants | Participants undergoing IVF/ICSI treatment and:
|
| Interventions | Intervention 1: incubation and assessment of embryos using TLI systems (morphokinetic parameters + undisturbed culture + morphological assessment) Intervention 2: incubation of embryos in undisturbed culture and standard embryo assessment (undisturbed culture + morphological assessment) Control: standard care (morphological assessment alone) |
| Outcomes | Primary outcome: live‐birth rate Secondary outcomes: clinical pregnancy rate, elective single embryo transfer rate, multiple‐birth rate, miscarriage of clinical pregnancy rate, stillbirth rate, and major congenital abnormality rate |
| Starting date | February 2018 |
| Contact information | Professor Khalid Khan: k.s.khan@qmul.ac.uk |
| Notes | CPMS ID 37510. Undergoing ISRCTN registration |
NCT01760278.
| Trial name or title | Assessment of implantation potential of embryos by time‐lapse technology (EmbryoScope) |
| Methods | Randomised parallel trial |
| Participants | Women:
|
| Interventions | Intervention: the embryos of patients in this arm will be cultured in the Embryoscope. Embryos to be transferred will be identified by the Embryoviewer Control: the embryos of participants would be cultured in conventional culture environment, and analysis would be done using established subjective morphological criteria |
| Outcomes | Primary outcome: number of embryos produced Secondary outcomes include: clinical pregnancy |
| Starting date | December 2012 |
| Contact information | Hrishikesh D Pai, Bloom IVF and Fertility Centre |
| Notes | Due for completion 2013 |
NCT02222831.
| Trial name or title | Optimizing IVF treatment ‐ the impact of time‐lapse culture and preimplantation factor (PIF) on embryo development |
| Methods | Randomised controlled trial |
| Participants | Included:
|
| Interventions | Intervention:
Control:
|
| Outcomes | Primary outcome: embryo quality Secondary outcomes include: clinical pregnancy |
| Starting date | |
| Contact information | Betina Boel Povlsen, MSc, Laboratory Director, The Fertility Clinic Skive; Denmark, Regionshospitalet Viborg, Skive |
| Notes |
NCT02417441.
| Trial name or title | TiLE (Time Lapse Eeva) Clinical Trial (TiLE) |
| Methods | Randomised controlled trial |
| Participants | Included females aged 18 to 40, meeting the following criteria:
|
| Interventions | Intervention: embryos of consented participants will be assessed with Eeva system and morphological assessment and will be followed up after embryo transfer until achievement of implantation and confirmation of pregnancy Control: no Eeva |
| Outcomes | Primary outcome: implantation rate Secondary outcomes include: clinical pregnancy, multiple pregnancy, ongoing pregnancy, spontaneous miscarriage |
| Starting date | 30 June 2015 |
| Contact information | The Merck KGaA Communication Center, Darmstadt, Germany |
| Notes | Study completed February 2017. |
NCT02657811.
| Trial name or title | Time‐lapse incubation for embryo culture ‐ morphokinetics and environmental stability |
| Methods | Randomised controlled trial |
| Participants | Inclusion:
|
| Interventions | Intervention 1: time‐lapse incubation ‐ morphologic/morphokinetic assessment Intervention 2: time‐lapse incubation ‐ morphologic assessment Control: bench incubation ‐ morphologic assessment |
| Outcomes | Primary outcome: ongoing pregnancy Secondary outcomes include: cumulative ongoing pregnancy, live birth, spontaneous abortion |
| Starting date | October 2016 |
| Contact information | Contact: Ruth Ronn: RuthRonn@szmc.org.il; Talia Eldar‐Geva: gevat@szmc.org.il |
| Notes | Estimated completion: August 2018 |
NCT02852356.
| Trial name or title | Validation study using a time‐lapse morphometry MIRI imaging incubator (TiMMI) |
| Methods | Parallel randomised controlled trial |
| Participants | Inclusion criteria:
|
| Interventions | Intervention: MIRI‐TL time‐lapse incubator Control: standard incubator |
| Outcomes | Primary outcome: live birth |
| Starting date | July 2016 |
| Contact information | Matthew VerMilyea, Ovation Fertility, Austin, Texas, USA |
| Notes | Completed December 2017 |
NCT02965222.
| Trial name or title | A study select top‐grade embryo by time‐lapse imaging |
| Methods | Randomised single‐blinded controlled trial |
| Participants | Inclusion criteria:
|
| Interventions | Intervention: after injecting human chorionic gonadotrophin 38 to 40 hours, the participant's oocytes will be taken with ICSI, then placed in the time‐lapse. The oocytes will be IVF after 4 hours and will be stripped surrounding cumulus cell, then will be placed in the time‐lapse. The third day the top‐grade embryo will be selected by time‐lapse imaging. The cleavage pattern and time parameters for marker embryo development were recorded daily after insemination. Control: the development of the embryos at time‐lapse was recorded directly and was not disturbed by temperature changes. |
| Outcomes | Primary outcome: live birth Secondary outcomes include: clinical pregnancy |
| Starting date | December 2016 |
| Contact information | |
| Notes | Estimated completion: December 2018 |
NCT03164551.
| Trial name or title | TICON‐Day 3, Time lapse versus conventional method in day 3 embryo culture and assessment (TICON) |
| Methods | Parallel randomised controlled trial |
| Participants | Includes:
|
| Interventions | Intervention: GERI+ incubator Control: conventional incubator |
| Outcomes | Primary outcome: clinical pregnancy Secondary outcomes include: ongoing pregnancy, multiple pregnancy, ectopic pregnancy, spontaneous miscarriage |
| Starting date | April 2018 |
| Contact information | Merck KGaA Communication Center: service@merckgroup.com |
| Notes |
NCT03445923.
| Trial name or title | Can time‐lapse parameters be used to predict pregnancy of human embryos? |
| Methods | Randomised triple‐blinded parallel controlled trial |
| Participants | Eligible:
|
| Interventions | Intervention group: time‐lapse monitoring (KID score) Control: morphology only |
| Outcomes | Primary outcome: ongoing pregnancy Secondary outcomes include: early pregnancy loss |
| Starting date | May 2018 |
| Contact information | Thorir Hardarson: thorir.hardarson@livio.is; Kersti Lundin: kersti.lundin@vgregion.sev |
| Notes |
NTR5423.
| Trial name or title | Embryo SELECtion using TIme‐lapse MOnitoring in IVF and ICSI patients |
| Methods | Multicentre randomised controlled trial |
| Participants | Women scheduled for a SET during their first IVF or ICSI cycle at any of the participating IVF centres will be considered for inclusion. |
| Interventions | A) embryo selection based on Eeva results and continuous culture in GERI+ incubator (GERI+ Eeva complete), and B) routine embryo selection based on morphology and continuous culture in GERI+ incubator (GERI culture only), will be compared to C) routine embryo selection based on morphology and interrupted culture in GERI+ incubator (control). Embryos in all 3 groups will be cultured in the GERI+ time‐lapse incubator. |
| Outcomes | Primary outcomes are the ongoing pregnancy rate of the first fresh SET and the cumulative ongoing pregnancy rate including the first fresh SET and all subsequent cryo transfers from the same ovum pick up cycle within 1 year. Secondary outcomes are biochemical pregnancy rate and live‐birth rate after fresh SET, cumulative live‐birth rate, miscarriage rate, time to pregnancy, embryo morphology and number of useable embryos (i.e. embryos used for transfer or cryopreservation), morphokinetic parameters, pregnancy rates in 3 female age groups, cost efficiency, outcome of manual time‐lapse annotations. |
| Starting date | 1 March 2017 |
| Contact information | DC Kieslinger: d.kieslinger@vumc.nl |
| Notes |
COS: controlled ovarian stimulation E2: estradiol FSH: follicle‐stimulating hormone ICSI: intracytoplasmic sperm injection IU: international unit IVF: in vitro fertilisation mIU: milli‐international unit SET: single embryo transfer 2PN: 2 pronuclei
Differences between protocol and review
We have altered the review title to reflect both the assessment and culture capability of TLS.
We have altered the wording of the Types of interventions section in the Methods to clarify the comparisons made. We sought to divide studies into the following three comparisons based on the nature of the intervention and the control in order to truly assess if there is a clinical benefit to TLS, and where the benefit of TLS might lie.
TLS with conventional morphological assessment of still TLS images versus conventional incubation and assessment (trial design 1)
TLS utilising embryo selection software versus TLS with conventional morphological assessment of still TLS images (trial design 2)
TLS utilising embryo selection software versus conventional incubation and assessment (trial design 3)
We changed the wording of the outcome 'adverse events' to ‘miscarriage and stillbirth’.
We have removed 'alternative imputation strategies' from Sensitivity analysis.
In the 2019 update, we changed the primary outcome to 'live birth or ongoing pregnancy'. The rationale was that there are very few pregnancy losses after 12 weeks' gestation, and the inclusion of the additional data would increase the power of the analysis. We planned to conduct a sensitivity analysis to investigate the effect of using this composite outcome, however this was not needed because only one study was included in Analysis 2.1.
Contributions of authors
SA developed the protocol and wrote the first draft of the review. PB, VJ, AP, and CF commented on and made changes to the review. SA, PB, and JM screened the search titles and extracted data from the full‐text articles. SA and PB contacted authors for further information. VJ gave her methodological and content opinion on the full review.
Ms Nicola Arroll and Dr Lynsey Cree were both authors of the first iteration of this review, but have not participated in this review update.
Sources of support
Internal sources
No sources of support supplied
External sources
None, Other.
Declarations of interest
Dr Priya Bhide is a co‐investigator for the TILT trial, a randomised controlled trial of time‐lapse system versus undisturbed culture versus conventional incubation and assessment, which has recently obtained ethics approval. TILT is funded by the Barts Charity.
There are no other conflicts of interest for any of the review authors.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Barberet 2018 {published data only}
- Barberet J, Chammas J, Bruno C, Valot E, Vuillemin C, Jonval L, et al. Randomized controlled trial comparing embryo culture in two incubator systems: G185 K‐System versus EmbryoScope. Fertility and Sterility 2018;109(2):302‐9. [DOI] [PubMed] [Google Scholar]
Goodman 2016 {published and unpublished data}
- Goodman LR, Goldberg J, Falcone T, Austin C, Desai N. Does the addition of time‐lapse morphokinetics in the selection of embryos for transfer improve pregnancy rates? A randomized controlled trial. Fertility and Sterility 2016;105(2):275‐85. [DOI] [PubMed] [Google Scholar]
- Goodman LR, Goldberg JM, Falcone T, Austin C, Desai N. Does use of time‐lapse microscopy in the selection of embryos for transfer improve pregnancy rates? A randomized controlled trial. Fertility and Sterility 2015;104(3):e96. [DOI] [PubMed] [Google Scholar]
Kahraman 2013 {published and unpublished data}
- Kahraman S, Cetinkaya M, Pirkevi C, Yelke H, Kumtepe Y. Comparison of blastocyst development and cycle outcome in patients with eSET using either conventional or time lapse incubators. A prospective study of good prognosis patients. Journal of Reproductive and Stem Cell Biotechnology 2013;3(2):55‐61. [Google Scholar]
Kaser 2017 {published data only}
- Kaser DJ, Bormann CL, Missmer SA, Farland LV, Ginsburg ES, Racowsky C. A pilot randomized controlled trial of Day 3 single embryo transfer with adjunctive time‐lapse selection versus Day 5 single embryo transfer with or without adjunctive time‐lapse selection. Human Reproduction 2017;32(8):1598‐603. [DOI] [PubMed] [Google Scholar]
- Kaser DJ, Bormann CL, Missmer SA, Farland LV, Ginsburg ES, Racowsky C. EEVA pregnancy pilot study: a randomized controlled trial of single embryo transfer (SET) on day 3 or day 5 with or without time‐lapse imaging (TLI) selection. Fertility and Sterility 2016;106(3):e312. [Google Scholar]
Kovacs 2019 {published and unpublished data}
- Kovacs P, Matyas S, Forgacs V, Sajgo A, Molnar L, Pribenszky C. Non‐invasive embryo evaluation and selection using time‐lapse monitoring: results of a randomized controlled study. European Journal of Obstetrics & Gynecology and Reproductive Biology 2019;233:58‐63. [DOI] [PubMed] [Google Scholar]
- Kovacs P, Matyas S, Forgacs V, Sajgo A, Rarosi F, Pribenszky C. Time‐lapse embryo selection for single blastocyst transfer ‐ results of a multicentre, prospective, randomized clinical trial. Fertility and Sterility 2013;100(3):S90. [Google Scholar]
- Kovacs P, Matyas SZ, Forgacs V, Reichart A, Rarosi F, Bernard A, et al. Can a composite score based on time lapse observation aid embryo selection for single embryo transfer; an interim report. Human Reproduction 2013;28(Suppl 1):169. [Google Scholar]
- Matyas SZ, Kovacs P, Forgacs V, Sajgo A, Pribenszky CS. Selection of single blastocyst for transfer using time‐lapse monitoring during in vitro fertilization in good prognosis patients: a randomized controlled trial. Human Reproduction 2015;30(Suppl 1):i119. [Google Scholar]
Park 2015 {published data only}
- Park H, Bergh C, Selleskog U, Thurin‐Kjellberg A, Lundin K. No benefit of culturing embryos in a closed system compared with a conventional incubator in terms of number of good quality embryos: results from an RCT. Human Reproduction 2015;30(2):268‐75. [DOI] [PubMed] [Google Scholar]
- Selleskog U, Park H, Bergh C, Lundin K. A prospective randomised controlled trial of the efficacy of using a closed time‐lapse system for embryo culture. Human Reproduction 2014;29(Suppl 1):i61. [Google Scholar]
Rubio 2014 {published and unpublished data}
- Ayerdi F, Rubio I, Galan A, Larreategui Z, Vidal C, Meseguer M. Clinical validation of embryo culture and selection by morphokinetic analysis; a randomized controlled trial by time‐lapse system. Human Fertility 2014;17(4):298. [DOI] [PubMed] [Google Scholar]
- Insua MF, Cobo A, Larreategui Z, Ferrando M, Remohi J, Meseguer M. Obstetric and perinatal outcomes of singleton newborns using time lapse monitoring. Fertility and Sterility 2015;104(Suppl 3):e212‐3. [DOI] [PubMed] [Google Scholar]
- Insua MF, Cobo AC, Larreategui Z, Ferrando M, Serra V, Meseguer M. Obstetric and perinatal outcomes of pregnancies conceived with embryos cultured in a time‐lapse monitoring system. Fertility and Sterility 2017;108(3):498‐504. [DOI] [PubMed] [Google Scholar]
- Perez S, Rubio I, Aparicio B, Beltran D, Garcia‐Laez V, Meseguer M. Prospective validation of a time‐lapse based algorithm for embryo selection. Fertility and Sterility 2014;102(Suppl 3):e322. [Google Scholar]
- Rubio I, Galan A, Larreategui Z, Ayerdi F, Bellver J, Herrero J, et al. Clinical validation of embryo culture and selection by morphokinetic analysis: a randomized, controlled trial of the EmbryoScope. Fertility and Sterility 2014;10(14):738. [DOI] [PubMed] [Google Scholar]
- Santos JM, Rubio I, Larreategui Z, Ayerdi F, Remohi J, Meseguer M. Clinical validation of embryo culture and selection by morphokinetic analysis; a randomized controlled trial by time‐lapse imaging. Fertility and Sterility 2014;102(Suppl 3):e87. [DOI] [PubMed] [Google Scholar]
Wu 2016 {published data only}
- Wu Y‐G, Lazzaroni‐Tealdi E, Wang Q, Zhang L, Barad DH, Kushnir VA, et al. Different effectiveness of closed embryo culture system with time‐lapse imaging (Embryoscope TM) in comparison to standard manual embryology in good and poor prognosis patients: a prospectively randomized pilot study. Reproductive Biology and Endocrinology 2016;14(49):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2018 {published data only}
- Yang L, Cai S, Zhang S, Kong X, Gu Y, Lu C, et al. Single embryo transfer by Day 3 time‐lapse selection versus Day 5 conventional morphological selection: a randomized, open‐label, non‐inferiority trial. Human Reproduction 2018;33(5):869‐76. [DOI] [PubMed] [Google Scholar]
- Yang L, Kong X, Zhang S, Dai J, Gong F, Lu G, et al. Single embryo transfer on cleavage‐stage(D3) using timelapse selection VS on blastocyst(D5) using traditional morphological selection in patients with good prognosis: a prospective randomized controlled trial. Human Reproduction 2017;32(Suppl 1):i102‐3. [Google Scholar]
References to studies excluded from this review
Adamson 2016 {published data only}
- Adamson GD, Abusief ME, Palao L, Witmer J, Palao LM, Gvakharia M. Improved implantation rates of day 3 embryo transfers with the use of an automated time‐lapse‐enabled test to aid in embryo selection. Fertility and Sterility 2016;105(2):369‐75. [DOI] [PubMed] [Google Scholar]
Alhelou 2018 {unpublished data only}
- Alhelou Y, Mat Adenan NA, Ali J. Embryo culture conditions are significantly improved during uninterrupted incubation: a randomized controlled trial. Reproductive Biology 2018;18:40‐5. [DOI: 10.1016/j.repbio.2017.12.003] [DOI] [PubMed] [Google Scholar]
Arnesen 2014 {published data only}
- Arnesen RE, McEvoy K, Critchlow D, Hunter HR, Lloyd AE, Wilson Y, et al. Comparison of clinical pregnancy rates following day 3 embryo transfer using a time‐lapse incubator compared to a flatbed incubator. Human Fertility 2014;17(4):299. [Google Scholar]
Belles 2014 {published data only}
- Belles M, Costa‐Borges N, Molina JM, Ballesteros A, Pellicer A, Florensa M, et al. Embryo quality and clinical outcomes using EmbryoscopeTM, MincTM and HeracellTM 150i incubators: preliminary results from a randomized study with donor oocytes. Human Reproduction 2014;29(Suppl 1):i160. [Google Scholar]
Cruz 2011 {published data only}
- Cruz M, Gadea B, Garrido N, Pedersen KS, Martinez M, Perez‐Cano I, et al. Embryo quality, blastocyst and ongoing pregnancy rates in oocyte donation patients whose embryos were monitored by time‐lapse imaging. Journal of Assisted Reproduction and Genetics 2011;28(7):569‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Freour 2014 {published data only}
- Freour T, Basile N, Barriere P, Meseguer M. Systematic review on clinical outcomes following selection of human preimplantation embryos with time‐lapse monitoring. Human Reproduction Update 2015;21(1):153‐4. [DOI] [PubMed] [Google Scholar]
Hardarson 2016 {published data only}
- Hardarson T, Bungum M, Conaghan J, Meintjes M, Chantilis SJ, Molnar L, et al. Noninferiority, randomized, controlled trial comparing embryo development using media developed for sequential or undisturbed culture in a time‐lapse setup. Fertility and Sterility 2015;104(6):1452‐9. [DOI] [PubMed] [Google Scholar]
Huang 2014 {published data only}
- Huang G, Wu LH. Randomized study assessing the impact of Primo Vision time‐lapse embryo monitoring system (tlm) on embryo culture and selection. Human Reproduction 2014;29(Suppl 1):i177. [Google Scholar]
Ingerslev 2011 {published data only}
- Ingerslev HJ, Kirkegaard K, Hindkjaer J, Grondahl ML, Kesmodel US, Agerholm I. Cleavage rates of human embryos randomized to culture in conventional incubator and culture in a time lapse instrument. Human Reproduction 2011;26(Suppl 1):P164. [Google Scholar]
Kaser 2014 {published data only}
- Kaser DJ, Racowsky C. Clinical outcomes following selection of human preimplantation embryos with time‐lapse monitoring: a systematic review. Human Reproduction Update 2014;20(5):617‐31. [DOI] [PubMed] [Google Scholar]
Kirkegaard 2012 {published data only}
- Kirkegaard K, Hindkjaer JJ, Grondahl ML, Kesmodel US, Ingerslec HJ. A randomized clinical trial comparing embryo culture in a conventional incubator with a time‐lapse incubator. Journal of Assisted Reproduction and Genetics 2012;29(6):565‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirkegaard 2014 {published data only}
- Kirkegaard K, Ingerslev HJ. Clinical outcomes following selection of human preimplantation embryos with time‐lapse monitoring: a systematic review. Human Reproduction Update 2014;20(5):617‐31. [DOI] [PubMed] [Google Scholar]
Kirkegaard 2015 {published data only}
- Kirkegaard K, Ahlstrom A, Ingerslev HJ, Hardson T. Choosing the best embryo by time lapse versus standard morphology. Fertility and Sterility 2015;103(2):323‐32. [DOI] [PubMed] [Google Scholar]
Loewke 2012 {published data only}
- Loewke K, Moussavi F, Maddah M, Ivani K, Behr B, Suraj V. Development and validation of an automated computer vision algorithm for real‐time embryo viability prediction. Fertility and Sterility 2012;98(Suppl 3):288. [Google Scholar]
Lowen 2017 {published data only}
- Lowen PK, Kermack AJ, Wellstead SJ, Calder PC, Houghton FD, Macklon NS. A prospective randomised trial comparing embryo development in the MINC incubator versus the EmbryoScope incubator. Human Reproduction 2017;32(Suppl 1):i210. [Google Scholar]
Mara 2010 {published data only}
- Mara C, Nicols G, Inmaculada P‐C, Niels R, Manuel M, Marcos M. Comparative study of embryo quality, blastocyst and ongoing pregnancy rates in oocyte donation patients sharing EmbryoScope and standard incubator. Fertility and Sterility 2010;94(4 Suppl 1):s78. [Google Scholar]
Meseguer 2012 {published data only}
- Meseguer M. Looking at embryo development ‐ clinical results from time‐lapse RCT. Human Reproduction 2012;27(Suppl 2):ii36. [Google Scholar]
Nakahara 2010 {published data only}
- Nakahara T, Iwase A, Goto M, Harata T, Suzuki M, Ienaga M, et al. Evaluation of the safety of time‐lapse observations for human embryos. Journal of Assisted Reproduction and Genetics 2010;27(2‐3):93‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Polanski 2014 {published data only}
- Polanski LT, Coelho Neto MA, Nastri CO, Navarro PA, Ferriani RA, Raine‐Fenning N, et al. Time‐lapse embryo imaging for improving reproductive outcomes: systematic review and meta‐analysis. Ultrasound in Obstetrics & Gynecology 2014;44(4):394‐401. [DOI] [PubMed] [Google Scholar]
Siristatidis 2015 {published data only}
- Siristatidis C, Komitopoulou MA, Makris A, Sialakouma A, Botzaki M, Mastorakos G, et al. Morphokinetic parameters of early embryo development via time lapse monitoring and their effect on embryo selection and ICSI outcomes: a prospective cohort study. Journal of Assisted Reproduction and Genetics 2015;32(4):563‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2015 {published data only}
- Wu Y, Lazzaroni‐Tealdi E, Wang Q, Albertini DF, Barad DH, Kushnir VA, et al. Randomized comparison of embryo development in closed time‐lapse photography system with traditional standard embryology culture with day‐3 embryo transfers. Fertility and Sterility 2015;104(Suppl 3):e319. [Google Scholar]
Yang 2014 {published data only}
- Yang Z, Zhang J, Salem S, Liu X, Kuang Y, Salem R, et al. Selection of competent blastocysts for transfer by combining time‐lapse monitoring and array CGH testing for patients undergoing preimplantation genetic screening: a prospective study with sibling oocytes. BMC Medical Genomics 2014;7(38):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Hulme 2014 {published data only}
- Hulme D, Jenner LJ, Campbell A, Fishel S. A randomised controlled comparison of clinical outcome, following time lapse and standard incubation. Human Fertility. Conference: ACE 9th Biennial Conference 2014;17. [Google Scholar]
References to ongoing studies
ChiCTR1800017127 {published data only}
- ChiCTR1800017127. A prospective randomized controlled study of cleavage embryo transplantation using dynamic observation techniques. www.chictr.org.cn/showprojen.aspx?proj=29089 (registered 16 January 2016).
ChiCTR‐IIR‐16008758 {published data only}
- ChiCTR‐IIR‐16008758. A phase IV, prospective, randomized, controlled, multiple‐center study to compare the implantation rate of embryos cultured and assessed in GERI? undisturbed incubation system vs conventional open incubator system. www.chictr.org.cn/showprojen.aspx?proj=14790 (registered 1 July 2016).
ISRCTN17792989 {published data only}
- ISRCTN17792989. A pragmatic, multi‐centre, three‐arm randomised controlled trial to assess the clinical effectiveness and safety of time lapse imaging in in‐vitro fertilisation treatment (also known as the TILT study). www.isrctn.com/ISRCTN17792989 (first received 4 April 2018).
Khan 2018 [pers comm] {published data only}
- Khan K. [personal communication]. Conversation with: Sarah Armstrong (University of Sheffield, UK) March 2018.
NCT01760278 {unpublished data only}
- NCT01760278. Assessment of implantation potential of embryos by time‐lapse technology (Embryoscope). www.clinicaltrials.gov/ct2/show/NCT01760278 (first received 4 January 2013).
NCT02222831 {unpublished data only}
- NCT02222831. Optimizing IVF treatment ‐ the impact of time‐lapse culture and preimplantation factor (PIF) on embryo development. www.clinicaltrials.gov/ct2/show/NCT02222831 (first received 21 August 2014).
NCT02417441 {unpublished data only}
- NCT02417441. TiLE (Time Lapse Eeva) Clinical Trial (TiLE). www.clinicaltrials.gov/ct2/show/NCT02417441 (first received 15 April 2015).
NCT02657811 {unpublished data only}
- NCT02657811. Time‐lapse incubation for embryo culture ‐ morphokinetics and environmental stability. www.clinicaltrials.gov/ct2/show/NCT02657811 (first received 18 January 2016).
NCT02852356 {unpublished data only}
- NCT02852356. Validation study using a time‐lapse morphometry MIRI imaging incubator (TiMMI). www.clinicaltrials.gov/ct2/show/NCT02852356 (first received 2 August 2016).
NCT02965222 {unpublished data only}
- NCT02965222. A study select top‐grade embryo by time‐lapse imaging. www.clinicaltrials.gov/ct2/show/NCT02965222 (first received 16 November 2016).
NCT03164551 {unpublished data only}
- NCT03164551. TICON‐Day 3, Time lapse versus conventional method in day 3 embryo culture and assessment (TICON). www.clinicaltrials.gov/ct2/show/NCT03164551 (first received 23 May 2017).
NCT03445923 {unpublished data only}
- NCT03445923. Can time‐lapse parameters be used to predict pregnancy of human embryos?. www.clinicaltrials.gov/ct2/show/NCT03445923 (first received 26 February 2018).
NTR5423 {published data only}
- NTR5423. Time‐lapse monitoring in IVF and ICSI patients [Embryo SELECtion using TIme‐lapse MOnitoring in IVF and ICSI patients]. www.trialregister.nl/trial/5314 (registered 8 September 2015).
Additional references
ACART
- ACART. Advisory Committee on Assisted Reproductive Technology: Guiding Principles. www.acart.health.govt.nz/about‐us (accessed 30 June 2014).
Alpha & ESHRE SIG 2011
- Alpha Scientists in Reproductive Medicine and ESHRE Special Interest Group of Embryology. The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Human Reproduction 2011;26(6):1270‐83. [1472‐6483] [DOI] [PubMed] [Google Scholar]
Armstrong 2018
- Armstrong S, Bhide P, Jordan V, Pacey A, Farquhar C. Time‐lapse systems for ART. Reproductive Biomedicine Online 2018;36(3):288‐9. [DOI] [PubMed] [Google Scholar]
Campbell 2000
- Campbell M, Fitzpatrick R, Haines A, Kinmouth AL, Sandercock P, Spiegelhalter D, et al. Framework for design and evaluation of complex interventions to improve health. BMJ 2000;321(7262):694‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2017
- Chen M, Wei S, Hu J, Yuan J, Liu F. Does time‐lapse imaging have favorable results for embryo incubation and selection compared with conventional methods in clinical in vitro fertilization? A meta‐analysis and systematic review of randomized controlled trials. PLOS ONE 2017;12(6):e0178720. [DOI] [PMC free article] [PubMed] [Google Scholar]
Conaghan 2013
- Conaghan J, Chen AA, Willman SP, Ivani K, Chenette PE, Boostanfar R, et al. Improving embryo selection using a computer‐automated time‐lapse image analysis test plus day 3 morphology: results from a prospective multicenter trial. Fertility and Sterility 2013; Vol. 100, issue 2:412‐9.e5. [0015‐0282] [DOI] [PubMed]
Covidence [Computer program]
- Veritas Health Innovation. Covidence. Version accessed 10 January 2019. Melbourne, Australia: Veritas Health Innovation.
Cummins 1986
- Cummins JM, Breen TM, Harrison KL, Shaw JM, Wilson LM, Hennessey JF. A formula for scoring human embryo growth rates in in vitro fertilization: its value in predicting pregnancy and in comparison with visual estimates of embryo quality. Journal of In Vitro Fertilization and Embryo Transfer 1986;3(5):284‐95. [DOI] [PubMed] [Google Scholar]
Finn 2010
- Finn A, Scott L, O’Leary T, Davies D, Hill J. Sequential embryo scoring as a predictor of aneuploidy in poor‐prognosis patients. Reproductive BioMedicine Online 2010;21(3):381‐90. [1472‐6483] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Version accessed 9 May 2018. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Harper 2012
- Harper J, Magli MC, Lundin K, Barratt CL, Brison D. When and how should new technology be introduced into the IVF laboratory?. Human Reproduction 2012;27(2):303‐13. [DOI] [PubMed] [Google Scholar]
HFEA
- HFEA. Human Fertilisation and Embryology Authority. www.hfea.gov.uk/ (accessed 30 June 2014).
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Meseguer 2012a
- Meseguer M, Rubio I, Cruz M, Basile N, Marcos J, Requena A. Embryo incubation and selection in a time‐lapse monitoring system improves pregnancy outcome compared with a standard incubator: a retrospective cohort study. Fertility and Sterility 2012;98(6):1481‐9.e10. [0015‐0282] [DOI] [PubMed] [Google Scholar]
Neuber 2003
- Neuber E, Rinaudo P, Trimarchi JR, Sakkas D. Sequential assessment of individually cultured human embryos as an indicator of subsequent good quality blastocyst development. Human Reproduction 2003;18(6):1307‐12. [DOI] [PubMed] [Google Scholar]
Neuber 2006
- Neuber E, Mahutte NG, Arici A, Sakkas D. Sequential embryo assessment outperforms investigator‐driven morphological assessment at selecting a good quality blastocyst. Fertility and Sterility 2006;85(3):794‐6. [0015‐0282] [DOI] [PubMed] [Google Scholar]
Petersen 2016
- Petersen BM, Boel M, Montag M, Gardner DK. Development of a generally applicable morphokinetic algorithm capable of predicting the implantation potential of embryos transferred on Day 3. Human Reproduction 2016;31(10):2231–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pribenszky 2017
- Pribenszky C, Nilselid A‐M, Montag M. Time‐lapse culture with morphokinetic embryo selection improves pregnancy and live birth chances and reduces early pregnancy loss: a meta‐analysis. Reproductive BioMedicine Online 2017;35(5):511‐20. [DOI: 10.1016/j.rbmo.2017.06.022] [DOI] [PubMed] [Google Scholar]
Scott 2003
- Scott L. The biological basis of noninvasive strategies for selection of human oocytes and embryos. Human Reproduction Update 2003;9(3):237‐49. [DOI] [PubMed] [Google Scholar]
Scott 2003a
- Scott L. Pronuclear scoring as a predictor of embryo development. Reproductive BioMedicine Online 2003;6(2):201‐14. [DOI] [PubMed] [Google Scholar]
Shoukir 1997
- Shoukir Y, Campana A, Farley T, Sakkas D. Early cleavage of in‐vitro fertilized human embryos to the 2‐cell stage: a novel indicator of embryo quality and viability. Human Reproduction 1997;12(7):1531‐6. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Armstrong 2014
- Armstrong S, Arroll N, Cree LM, Jordan V, Farquhar C. Time‐lapse systems for embryo incubation and assessment in assisted reproduction. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD011320] [DOI] [PubMed] [Google Scholar]
Armstrong 2015
- Armstrong S, Arroll N, Cree LM, Jordan V, Farquhar C. Time‐lapse systems for embryo incubation and assessment in assisted reproduction. Cochrane Database of Systematic Reviews 2015, Issue 2. [DOI: 10.1002/14651858.CD011320.pub2] [DOI] [PubMed] [Google Scholar]
Armstrong 2018a
- Armstrong S, Bhide P, Jordan V, Pacey A, Farquhar C. Time‐lapse systems for embryo incubation and assessment in assisted reproduction. Cochrane Database of Systematic Reviews 2018, Issue 5. [DOI: 10.1002/14651858.CD011320.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]


