Abstract
The ligament of the head of femur (LHF), or ligamentum teres, is believed to provide blood supply to the head of femur and mechanical stability to the hip joint. But these functions in the adult are often debated. The existence and distribution of neurovascular structures within the ligament are not widely documented. This study examined the blood vessels and nervous tissue within the LHF to determine whether the ligament may have a vascular and proprioceptive function at the hip joint. Histological sections from the LHF from 10 embalmed hips (six female, four male; mean age 80.4 ± 8.7 years) were cut at three levels: the foveal attachment, mid‐length and its base where it attaches to the transverse acetabular ligament. Sections were stained with haematoxylin and eosin to study general tissue architecture or with von Willebrand factor and neurofilament to identify blood vessels and nervous tissue, respectively. The proportion of the ligament's cross‐sectional area occupied by blood vessels was expressed as a vascularity index (VI). Nerve endings within the ligament were identified and morphologically classified. Comparisons between the VI at the three levels, or between the tissue layers of the ligament, were made using 95% confidence intervals; statistical significance was set P < 0.05. The ligament tissue comprised three distinct layers: a synovial lining with cuboidal cells, a sub‐synovial zone formed of loose connective tissue and the ligament proper composed of dense collagen bundles. Patent blood vessels and nerve fibres were present both in the sub‐synovial zone and the ligament proper; Pacinian corpuscles and free nerve endings were found scattered only in the sub‐synovial zone. The VI of the ligament proper at the fovea was significantly higher than its middle (P = 0.01) and basal levels (P = 0.04); it was also higher than that of the sub‐synovial layer (P = 0.04). The LHF has three histologically distinct zones, and blood vessels and nerves are distributed both in the sub‐synovial layer and ligament proper. Higher vascularity within the ligament proper at its foveal insertion suggests a possible nutritive role of the LHF to the adult head of femur. The presence of nerves and nerve receptors indicates the ligament is involved in the perception of pain and proprioception, thereby contributing to mechanical stability of the joint.
Keywords: blood vessels, femur head, free nerve endings, hip, ligament, mechanoreceptors, proprioception
Introduction
The ligament of the head of femur (LHF), often referred to as the ligamentum teres in clinical literature, is an intra‐articular structure which runs between the acetabulum and fovea capitis femoris (FCF) of the head of femur (HOF). The ligament is believed partly to provide mechanical stability to the hip joint (Crelin, 1976; Rao et al. 2001; Sierra & Trousdale, 2009) and to carry blood vessels to the HOF (Wertheimer & Fernandes, 1971; Chung, 1976; Byrd & Jones, 2004; Kirici et al. 2010).
The presence of blood vessels within the ligament was first reported in the 19th century (Paletta, 1820, cited in Chandler & Kreuscher, 1932). The ligamental arteries usually arise from the obturator or the medial circumflex femoral artery (Weathersby, 1959; Crock, 1965; Standring, 2016; Perumal et al. 2018), branch in the sub‐synovial connective tissue of the acetabular fossa and supply fibrofatty tissues in that region, with some vessels reaching the femoral head along the LHF (Standring, 2016). There is agreement on the ligament's vascular contribution to the developing and juvenile HOF (Gardner & Gray, 1950; Crelin, 1976; Brewster, 1991; Bardakos & Villar, 2009). However, in adults, although vascular foramina are clearly visible on the foveal floor (Perumal et al. 2017), it is less clear whether the blood vessels directly contribute to the supply of the HOF (Garcia et al. 1956; Catto, 1965; Wertheimer & Fernandes, 1971; Chung, 1976; Ganz et al. 2001; Notzli et al. 2002; Zlotorowicz & Czubak, 2014).
Recently, the LHF has attracted attention from clinicians interested in ligament reconstruction (Chandrasekaran et al. 2016; Garabekyan et al. 2016; Pergaminelis et al. 2017; White et al. 2018) or in using the LHF as a graft (Sierra & Trousdale, 2009; Weidner et al. 2018). Clinical studies have also reported a higher prevalence of injuries in females and on the right side (Byrd & Jones, 2004; Haviv & O'Donnell, 2011; Amenabar & O'Donnell, 2013; Domb et al. 2013), the explanation for which is uncertain. The evolving clinical procedures for the LHF have resulted in the need for a detailed analysis of both gross and microscopic structure of the ligament that could help understand its potential functions in the hip joint (Chen et al. 1996; Leunig et al. 2000; Kirici et al. 2010; Philippon et al. 2014).
Current literature addressing the microstructure of the LHF provides information on general tissue architecture, the presence of blood vessels and nerve fibres within the ligament, and their role in the ligament's mechanical and vascular functions (Leunig et al. 2000; Sarban et al. 2007; Kirici et al. 2010; Shinohara et al. 2014; Dehao et al. 2015). However, the exact location of the neurovascular structures is unknown, and it is not clear whether the vessels solely supply the synovial tissue or run through the connective tissue core of the ligament to supply the adult HOF. The aims of this histological study were (1) to define the general microarchitecture of the LHF, (2) to quantify the distribution of blood vessels within the ligament, thereby providing evidence of the ligament's potential vascular contribution to the HOF, and (3) to describe the types of nerve endings present in the tissues of the LHF.
Methods
Specimen selection
Ten paired hip joints (five left, five right) from five embalmed cadavers (three female and two male cadavers; mean age 80.4 ± 8.7 years) were used. All cadavers were from a New Zealand European population and bequeathed to the Department of Anatomy, in accordance with the New Zealand Human Tissues Act (2008). Departmental ethical approval was received for this study.
Harvesting of tissue and sectioning
Dissection of the hip joint included sequentially removing the surrounding soft tissues and articular capsule. The LHF, with its intact synovial covering, was then removed en bloc from its attachment sites: the fovea capitis, transverse acetabular ligament (TAL), acetabular notch and the acetabular fossa. For the purpose of orientating and sectioning the ligament, horizontal lines were marked along its apex (foveal attachment), at its mid‐length and base (TAL attachment) using permanent tissue marking ink (Davidson Marking systems, Bardley Products Inc., Minneapolis, MN, USA). The ligaments were post‐fixed in 10% neutral buffered formalin and embedded in paraffin wax; they were then sectioned (4 μm thick) at each of the three marked levels of interest mentioned above.
Histology and immunohistochemistry
The tissues were stained with haematoxylin and eosin (H&E) to enable examination of general histological features of the ligament and its attachment sites; a Verhoeff–van Gieson's (VVG) stain was used to identify the presence of elastin and collagen fibres (Kazlouskaya et al. 2013). Immunohistochemistry was then undertaken to facilitate identification of vascular and nervous tissues within the LHF.
To identify selectively the blood vessels within the ligament, immunohistochemistry for vonWillebrand factor (vWF) was performed. vWF is a glycoprotein present in the endothelial cells of blood vessels but not lymphatic vessels (Jiang et al. 2008). A heat‐mediated antigen retrieval method (microwave heating at 95 °C for 25 min) was performed using Tris ethylenediaminetetraacetic acid (EDTA) buffer (pH 9.0). Immunohistochemistry was undertaken using a polyclonal antibody raised to vWF in rabbits (Covance SM1‐312R;; 1/600 dilution), which was replaced with 2% bovine serum antibody in the negative control. Cell Marque DAB chromagen was used with Mayer's Dako Agilent haematoxylin as a counterstain.
A similar technique of antigen retrieval (stove heating at 95° for 25 min) using citrate buffer (pH 6.0) was undertaken to enable identification of both myelinated and unmyelinated nerve fibres within the ligament. A polyclonal antibody raised to neurofilament in rabbits (pan‐neuronal, cocktail; BioLegend; 1/3000 dilution) was used, alongside the same negative control and counterstain applied to identify VWF. Sections of sciatic nerve with its intrinsic blood vessels served as control tissues for both vascular and nervous tissues.
From the H&E stained sections, the general histological features and different tissue layers of the LHF and the attachment sites were observed using an Olympus CX21 light microscope (Olympus Corporation, Tokyo, Japan). In each immunohistochemistry slide, five random rectangular fields from both the sub‐synovial layer and the ligament proper were photographed under a 20× objective using the random points imaging feature in the BX61 montaging microscope (Olympus Corporation).
These photomicrograph images were then exported to imagej software (National Institute of Health, Bethesda, MD, USA) to quantify the number, diameter, cross‐sectional area (CSA) and distribution of the vascular structures. All measurements were carried out by the first author and the mean of three separate measurements was used for calculations. Smaller vessels and those with completely collapsed walls that did not show the lumen were included in the number count but were excluded from measurements of luminal CSA. To quantify the regional vascular distribution, the total luminal CSA of the blood vessels in each image was measured. A vascularity index (VI) of the LHF was then calculated as a percentage of that LHF area occupied by the blood vessels in the image (Perumal & Stringer, 2014).
The nerve fibres within the ligament were identified using immunoreactivity to neurofilament. The location and size of the nerve bundles were documented and any nerve endings present were classified (Rein et al. 2013, 2015; Stecco et al. 2018).
Statistical analysis
The mean and standard deviation of each parameter was calculated in Microsoft excel (Version 16.16.5, Microsoft Corporation., Redmond, WA, USA), after which GraphPad prism (Version 7.0b, GraphPad software Inc., CA, USA) was used for further calculations. Considering the small sample size, non‐parametric tests were performed to compare the variables. Comparisons between the VI of the sub‐synovial layer and the ligament proper at three levels (apex, mid‐length and base of the ligament) and between the left and right sides, were made using a Wilcoxon matched‐pair signed ranks test. A Mann–Whitney U‐test was undertaken to determine sex differences in the vascular indices at the three selected levels, and a Friedman test used to examine whether there was significant variation in the vascular distribution along the length of the ligament. For all statistical tests, 95% confidence intervals were reported; statistical significance was set at P < 0.05. Where the mean of three measurements was used for calculations, the intra‐class correlation coefficient (ICC) was calculated to determine intra‐rater reliability, assessed using the criteria of Portney & Watkins (2015).
Results
General microarchitecture of the LHF
At all three levels of the LHF, the ligament showed three histologically distinct layers: (1) a synovial lining, (2) sub‐synovial tissue (loose connective tissue layer beneath the synovial membrane) and (3) the ligament proper (dense connective tissue core).
Synovial lining
The synovial lining uniformly covered the surface of the ligament and was composed of a continuous epithelium of cuboidal cells, one or two cells thick. The synovial membrane covered the basal regions of the ligament very loosely, whereas it was closely applied to the connective tissue core towards the apical regions. Sections of synovial plicae (folds) were also seen; connective tissue of these folds was continuous with the loose connective tissue of the sub‐synovial layer (Fig. 1).
Figure 1.
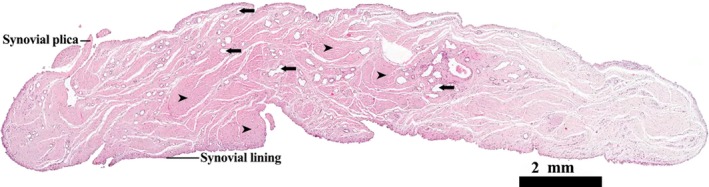
Transverse profile of the LHF mid‐way along its length. H&E stain. The full profile of the ligament is lined in synovial membrane, sections through synovial plicae are also evident. Scattered blood vessels (arrows) are visible both in the sub‐synovial layer and connective tissue of the ligament proper (arrowheads).
Sub‐synovial tissue
The sub‐synovial tissue layer contained loose connective and adipose tissue, with scattered fibroblasts (Fig. 2). Fine elastic fibres were observed, being randomly dispersed in this layer. Larger blood vessels and nerve bundles were evident in all specimens and usually co‐located to form neurovascular bundles. The mean (± standard deviation, SD) diameter of the larger arteries was 476 ± 156 μm (range: 201–733 μm), and the nerve bundles were 89 ± 46 μm (range: 37–196 μm). A few diseased blood vessels (showing occlusion, hyalinization, medial thickening) and recanalization of previously occluded vessels were seen; otherwise, the vessels appeared patent with a normal morphology.
Figure 2.
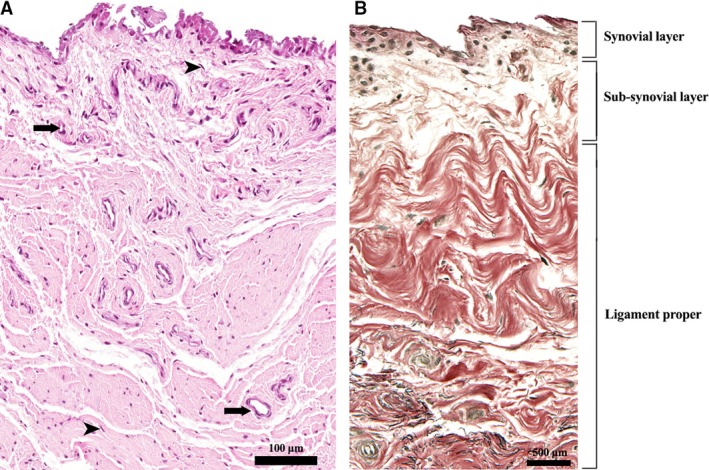
Tissue layers of the LHF. (A) H&E stain. Cuboidal synovial cells are visible on the surface (synovial layer); fibroblasts (arrowheads) and blood vessels (arrows) are present both in the sub‐synovial tissue and ligament proper. (B) VVG stain. Collagen fibres are stained in red and elastin in black.
The ligament proper
The connective tissue core of the ligament was composed of bundles of dense collagen fibres. These bundles originated from various parts of the acetabular fossa, being dispersed at the base of the ligament and converging towards its apex. Loose connective tissue from the sub‐synovial layer extended into the spaces between the bundles. Small blood vessels were contained between, and within, the collagen bundles, along with scattered fibroblasts. Fine elastin fibres running parallel to the collagen fibres arranged along the length of the ligament were also identified.
Ligament attachment sites
The acetabular and foveal attachment sites of the LHF showed a zonal transition of connective tissue from bone, fibrocartilage and collagen fascicles. A tidemark separated the bony and fibrocartilaginous tissue zones. Distally these features were apparent only at the ligament attachment zone of the fovea capitis; the receptacle zone devoid of articular cartilage was covered by fatty and synovial tissue. Patent blood vessels entered the fovea at the ligament attachment zone only (Fig. 3).
Figure 3.
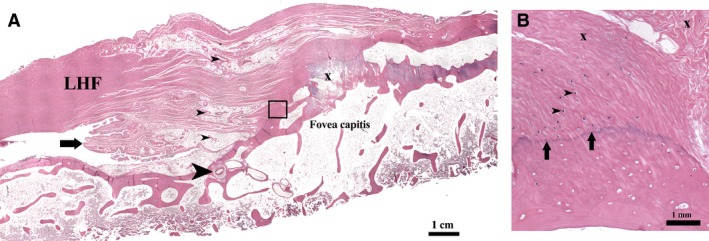
Histology of the LHF at its foveal attachment (H&E stain). (A) Ligamental blood vessels near the fovea capitis (small arrowheads). Patent vessels seen entering the foveal floor (large arrowhead). Section shows synovial plicae (arrow) and a prominent osteophyte (x). (B) Magnified view of the boxed area in (A): The enthesis shows a transition of collagen bundles (x) to fibrocartilage and to bone tissue at the foveal insertion. Chondrocytes are visible in the fibrocartilage (arrowheads). The tidemark (arrows) separates the fibrocartilage from bone.
Distribution of blood vessels in the LHF
The endothelial cells of arteries, veins, arterioles and venules demonstrated immunoreactivity for vWF. Blood vessels of varying sizes were identified in both the sub‐synovial zone and the ligament proper; the largest vessels were usually in the sub‐synovial tissue (Fig. 4). The total number of vessels present and their CSA at each level is documented in Table 1. There was a significant increase in the luminal CSA of the blood vessels of the ligament proper between the base and apex of the LHF (P = 0.04); however, this difference in CSA did not reach significance between the base and the mid‐length or the mid‐length and the apex (P = 0.26 and 0.11, respectively).
Figure 4.
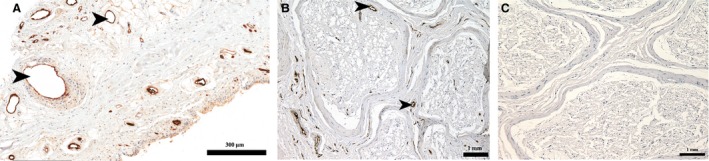
Immunoreactivity for vWF showing blood vessels (arrowheads) in the LHF and the positive control tissue. (A) LHF tissue; (B) positive and (C) negative control using blood vessels within sciatic nerve.
Table 1.
The number of blood vessels and the total CSA of the blood vessel lumen at each level of the LHF. All values presented are mean ± SD
| Level of LHF section | Sub‐synovial layer | Ligament proper | ||
|---|---|---|---|---|
| Number of blood vessels | Luminal CSA (μ2) | Number of blood vessels | Luminal CSA (μ2) | |
| Apex | 18 ± 9 | 6243 ± 5233 | 14 ± 6 | 9134 ± 8884 |
| Mid‐length | 12 ± 3 | 9490 ± 6267 | 10 ± 3 | 2723 ± 3809 |
| Base | 10 ± 6 | 15 680 ± 12 630 | 16 ± 2 | 669 ± 963 |
There was a significant difference between the VI of the sub‐synovial tissue and the ligament proper (Fig. 5). At the base and mid‐length of the ligament, the index was higher in the sub‐synovial connective tissue compared with the ligament proper (P = 0.002 and 0.037, respectively). This distribution was reversed at the apex of the ligament (P = 0.037).
Figure 5.
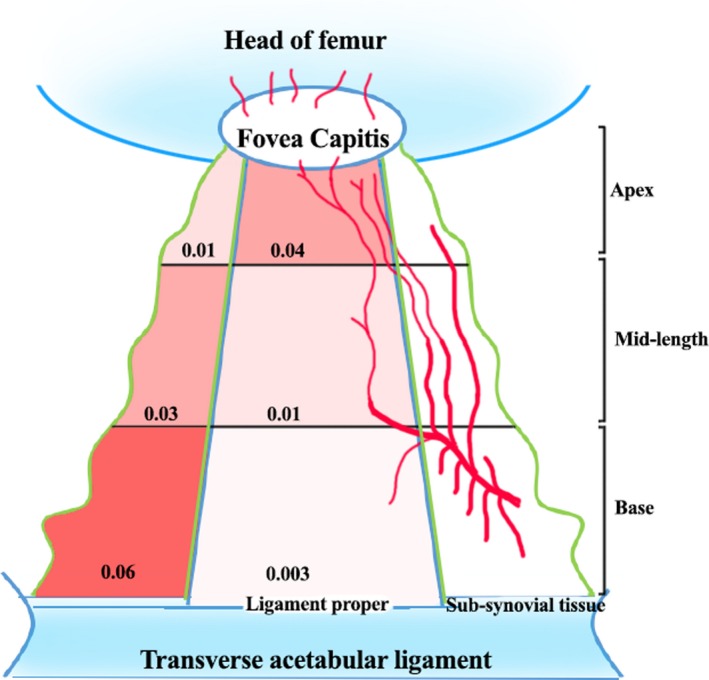
Schema of blood vessel distribution within the LHF. The ligamental vessels from the sub‐synovial tissue entering the ligament proper are illustrated. The VI at various levels are represented in numbers and also as colour gradients.
The distribution of blood vessels differed along the length of the ligament. The VI in the sub‐synovial tissue was significantly reduced towards the foveal attachment compared with the basal and mid‐length levels (P = 0.04 and 0.08, respectively). In contrast, in the ligament proper, there was a marked increase in the index towards the fovea (P = 0.002 and 0.02, respectively). There was no significant difference in the total VI of the LHF at any of the three levels for sex or side (P > 0.05). The ICC for these measurements was between 0.82 and 1.0, meaning the intra‐rater reliability was good (0.76–0.9) to excellent (> 0.9).
Distribution of nervous tissue in the LHF
Antibodies against neurofilament identified scattered nervous tissue throughout the ligament. Large nerve bundles were embedded in the sub‐synovial connective tissue, some close to the synovial membrane (Fig. 6A). Fine nerve fibres were located within the ligament proper and also associated with the walls of larger blood vessels. Several encapsulated Pacinian corpuscles (Fig. 6B,C) and free nerve endings (Fig. 6D) were identified, being confined to the sub‐synovial layer.
Figure 6.
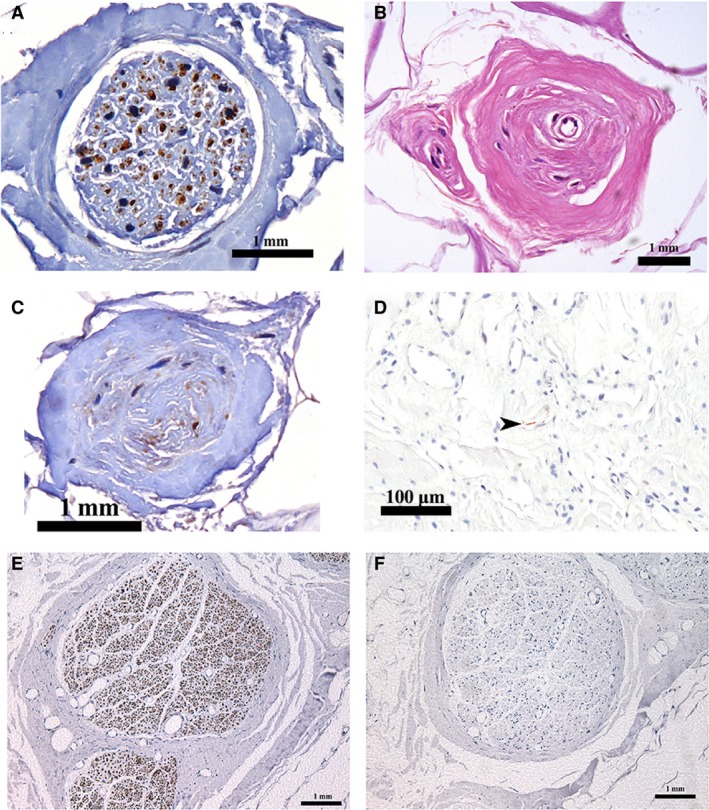
Neurofilament immunoreactivity in the LHF. (A) Nerve bundle; Pacinian corpuscle stained with (B) H&E and (C) neurofilament; (D) a free nerve ending (arrowhead); (E) positive and (F) negative control from the sciatic nerve.
Discussion
This study identifies patent blood vessels throughout the LHF tissue in elderly cadavers. It demonstrates differences in the cross‐sectional area of the lumen of blood vessels at different levels of the ligament, and the presence of nerve endings including mechanoreceptors that are mainly situated in the sub‐synovial layer of the ligament. The deeper vessels and nerves appear to run within a well‐protected elastin and collagenous core before they penetrate the foveal floor. In addition to the neurovascular structures, identification of fibrocartilage at both the proximal and distal attachment sites of the ligament suggests a weight‐transmitting role of the ligament (Shinohara et al. 2014).
The general architecture of the LHF, comprising three distinct layers, is similar to previous reports (Chen et al. 1996; Kirici et al. 2010; Dehao et al. 2015). Numerous vessels of variable calibre were distributed both in the sub‐synovial layer and ligament proper, a finding that has been debated earlier (Chandler & Kreuscher, 1932; Garcia et al. 1956; Weathersby, 1959; Calandriello & Mignani, 1962; Crelin, 1976; Byrd & Jones, 2004). Using light microscopy and immunohistochemistry, we were able to identify these types of vessels in all specimens, whereas other techniques including contrast injection studies sometimes failed to demonstrate small blood vessels within the ligament, possibly due to issues with inadequate filling of contrast material (Garcia et al. 1956; Crock, 1965; Wertheimer & Fernandes, 1971; Zlotorowicz & Czubak, 2014).
Histological observations at the level of the foveal attachment confirmed the presence of larger patent blood vessels, and a relatively higher VI within the ligament proper. The increase in VI at this level could possibly be due to (1) close adherence of the synovial layer to the ligament proper towards the FCF, and/or (2) anastomosis of these ligamental vessels with other vessels that supply the HOF (Wertheimer & Fernandes, 1971; Chung, 1976). At the base of the ligament, it is possible that the stem of the ligamental blood vessels ramifying within the loose synovial covering at the acetabular fossa (Standring, 2016) could have resulted in a significantly higher VI in the sub‐synovial layer compared with the ligament proper. The blood vessels enter the ligament proper along the mid‐length of the LHF (Perumal et al. 2018); this may explain why the vascularity of the ligament proper increases towards the apex of the ligament.
The mean data relating to luminal CSA of the ligament proper had a large standard deviation at some levels, indicating a wide range in the size of ligamental blood vessels; this could reflect the difference in the luminal size of distended arteries and veins that are not maintained at physiological pressure ex vivo. In spite of this variation, the mean luminal CSA and VI of the vessels at the apex were significantly higher (P = 0.04 and 0.02, respectively) than the basal level of LHF. This significant increase in the concentration of vasculature towards the FCF compared with basal levels, and larger foveal vessels might suggest that the vessels are directed distally to supply the foveal region. Also, with luminal patency and fine nerve filaments identified in the walls, the ligamental blood vessels seem to be functional (Lang & Balint, 1952; Dehao et al. 2015), even when harvested from elderly cadavers. However, as the direction of blood flow within the ligament was not known, clarification is required to confirm whether these deeper vessels do vascularise the HOF.
Earlier anatomical and clinical reports have provided evidence of ligamental vessels supplying the perifoveal region of the adult HOF, and the possibility of avascular necrosis or degenerative arthritis in this zone following damage to these vessels (Watson‐Jones, 1946; Lang & Balint, 1952; Garcia et al. 1956; Wertheimer & Fernandes, 1971; Chung, 1976; Byrd & Jones, 2004; Kaya et al. 2014). Arthroscopic diagnosis of LHF tears is often associated with articular cartilage damage, arthritis and microtrauma to the joint (Gray & Villar, 1997; Domb et al. 2013). Reports of haemorrhage following ligament injury (Bochhi et al. 1987; Byrd & Jones, 2004; Dehao et al. 2015), spontaneous healing of the LHF (Schaumkel & Villar, 2009; O'Donnell et al. 2014; Davarinos et al. 2017) and revascularization of the femoral head following fracture of the neck of femur (Catto, 1965; Chen et al. 1996) are attributed to the dense vascular distribution within the ligamental tissue. It has been suggested that surgical procedures for LHF injuries that demand removal of ligamental tissue should be minimised, and any ligamental remnants during debridement should be preserved to protect any remaining blood supply (Byrd & Jones, 2004; Dehao et al. 2015). Our observations of patent ligamental vessels even in the elderly support these clinical reports. From our results, it appears that while superficial ligamental vessels of the sub‐synovial layer could supply the synovial tissue, those in the deeper plane could potentially reach the HOF.
The LHF is believed to provide mechanical stability to the hip joint, but only secondary to that of the capsular ligaments (Rao et al. 2001; Sierra & Trousdale, 2009; Philippon et al. 2014; van Arkel et al. 2015); this stabilising function could partly be due to the nervous tissue within the LHF (Leunig et al. 2000; Dehao et al. 2015). Earlier studies have explored the presence of free nerve endings in the LHF in both normal and dysplastic hips (Leunig et al. 2000; Muratli et al. 2004; Sarban et al. 2007; Haversath et al. 2013). However, no proprioceptive nerve endings in the ligament tissue have previously been reported. The mechanoreceptors identified in this study suggest a possible proprioceptive role of the LHF during normal hip joint movement, supporting its mechanical function. In addition to free nerve endings sensing nociception, damage to these mechanoreceptors could alter proprioception (Leunig et al. 2000; Dehao et al. 2015), which could predispose to minor hip instability that is noted clinically following LHF injury (Leunig et al. 2000; Simpson et al. 2011; Lindner et al. 2013).
Limitations
This study has some limitations which require consideration. First, the sample size was comparatively small and the samples were collected from elderly embalmed cadavers. Shrinkage artefact is usually reported for formalin‐based fixatives and tissues that have been embedded in paraffin wax (Chatterjee, 2014); this could have altered the architecture of the ligament and affected the CSA of blood vessels.
The blood vessels studied were not maintained at physiological pressure, which means there is a chance of compression or dilation, again affecting the measurement of the luminal CSA of these vessels. The VI provided information about the overall vascular distribution within the ligament, but did not separate arterial supply from venous drainage. As the lymphatic vessels do not contain vWF, they were neither stained nor analysed in this study.
Although arteries are clearly identified at the foveal zone of the ligament and HOF, it could not be concluded whether they originated from the ligamental arteries or from other vessels supplying the HOF. Identification of valves within the ligamental veins by microdissection might have provided information on the directional flow but, other than a report on valves in a 14‐week‐old embryo (Brewster, 1991), no other evidence exists in the literature, and identification of valves was beyond the scope of this study. The presence of nerve bundles and mechanoreceptors in the tissues of the LHF was verified, but their distribution was not quantified; this approach is warranted in future investigations to better understand the contribution of neural elements to joint stability.
Conclusion
This study shows that the LHF has three distinct histological zones. Blood vessels run in the LHF both in the sub‐synovial layer and ligament proper, and are focused towards the fovea capitis. The findings support a potential nutritive role of the ligamental vessels to the sub‐foveal zone of the adult HOF. Neural elements identified in the ligament also suggest the LHF has a nociceptive and proprioceptive role. These findings, in addition to the distribution of fibrocartilage at the ligament attachment zones, suggest that mechanically the LHF could contribute to joint stability.
Conflict of interest
The authors have no conflict of interests.
Author contributions
Author 1 (V.P.): Study design, dissections, data collection and analysis, photomicrographs and illustrations, drafting the manuscript. Author 2 (S.J.W.): Supervising the work, commenting on drafts and the final version of the manuscript. Author 3 (H.D.N.): Supervising the work, commenting on drafts and the final version of the manuscript.
Acknowledgements
We would like to thank the staff at the Otago Histology Services Unit, Department of Pathology, University of Otago, New Zealand, for their assistance with the immunohistochemistry techniques; and Dr Arijit Bishnu from Kasturba Medical College, Manipal, India, for his opinion on the pathology of blood vessels identified in the slides.
References
- Amenabar T, O'Donnell J (2013) Successful treatment of isolated, partial thickness ligamentum teres tears with debridement and capsulorrhaphy. Hip Int 23, 576–582. [DOI] [PubMed] [Google Scholar]
- van Arkel RJ, Amis AA, Cobb JP, et al. (2015) The capsular ligaments provide more hip rotational restraint than the acetabular labrum and the ligamentum teres. Bone Joint J 97B, 484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardakos NV, Villar RN (2009) The ligamentum teres of the adult hip. J Bone Joint Surg Br 91, 8–15. [DOI] [PubMed] [Google Scholar]
- Bochhi L, Orso CA, Passarello F, et al. (1987) Atherosclerosis of the vessels in the ligamentum teres. Optical and electron microscopy findings in elderly patients with femoral neck fractures. Ital J Orthop Traumatol 13, 365–369. [PubMed] [Google Scholar]
- Brewster SF (1991) The development of the ligament of the head of the femur. Clin Anat 4, 245–255. [Google Scholar]
- Byrd JWT, Jones KS (2004) Traumatic rupture of the ligamentum teres as a source of hip pain. Arthroscopy 20, 385–391. [DOI] [PubMed] [Google Scholar]
- Calandriello B, Mignani G (1962) The role of ligamentum teres in congenital dislocation of the hip. Clin Orthop Relat Res 22, 60–72. [Google Scholar]
- Catto M (1965) A histological study of avascular necrosis of the femoral head after transcervical fracture. J Bone Joint Surg 47B, 749–776. [PubMed] [Google Scholar]
- Chandler SB, Kreuscher PH (1932) A study of the blood supply of the ligamentum teres and its relation to the circulation of the head of the femur. J Bone Joint Surg 14, 834–846. [Google Scholar]
- Chandrasekaran S, Martin TJ, Close MR, et al. (2016) Arthroscopic reconstruction of the ligamentum teres: a case series in four patients with connective tissue disorders and generalized ligamentous laxity. J Hip Preserv Surg 3, 358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S (2014) Artefacts in histopathology. J Oral Maxillofac Pathol 18, 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen HH, Li AF, Li KC, et al. (1996) Adaptations of ligamentum teres in ischemic necrosis of human femoral head. Clin Orthop Relat Res 328, 268–275. [DOI] [PubMed] [Google Scholar]
- Chung SMK (1976) The arterial supply of the developing proximal end of the human femur. J Bone Joint Surg Am 58, 961–970. [PubMed] [Google Scholar]
- Crelin ES (1976) An experimental study of the hip stability in human newborn cadavers. Yale J Biol Med 49, 109–121. [PMC free article] [PubMed] [Google Scholar]
- Crock H (1965) A revision of the anatomy of the arteries supplying the upper end of the human femur. J Anat 99, 77–88. [PMC free article] [PubMed] [Google Scholar]
- Davarinos N, Bonvin A, Christofilopoulos P (2017) Ligamentum teres reattachment post‐surgical dislocation of the hip: a case report. Regenerative capacity affirming its greater role in hip stability and function? J Hip Preserv Surg 4, 337–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dehao BW, Bing TK, Young JLS (2015) Histology of the ligamentum teres of the hip: a basic science study. Acta Orthop Bras 23, 29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domb BG, Martin DE, Botser IB (2013) Risk factors for ligamentum teres tears. Arthroscopy 29, 64–73. [DOI] [PubMed] [Google Scholar]
- Ganz R, Gill TJ, Gautier E, et al. (2001) Surgical dislocation of the adult hip: a technique with full access to the femoral head and acetabulum without the risk of avascular necrosis. J Bone Joint Surg 83B, 1119–1124. [DOI] [PubMed] [Google Scholar]
- Garabekyan T, Chadayammuri V, Pascual‐garrido C, et al. (2016) All‐arthroscopic ligamentum teres reconstruction with graft fixation at the femoral head‐neck junction. Arthrosc Tech 5, 143–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia AV, McDonald JR, Coventry MB (1956) Vascular changes of the ligamentum teres femoris in disease of the hip. Proc Staff Meet Mayo Clin 31, 599–604. [PubMed] [Google Scholar]
- Gardner E, Gray DJ (1950) Prenatal development of the human hip joint. Am J Anat 87, 163–211. [DOI] [PubMed] [Google Scholar]
- Gray AJR, Villar RN (1997) The ligamentum teres of the hip: an arthroscopic classificationof its pathology. Arthroscopy 13, 575–578. [DOI] [PubMed] [Google Scholar]
- Haversath M, Hanke J, Landgraeber S, et al. (2013) The distribution of nociceptive innervation in the painful hip. Bone Joint J 95B, 770–776. [DOI] [PubMed] [Google Scholar]
- Haviv B, O'Donnell J (2011) Arthroscopic debridement of the isolated ligamentum teres rupture. Knee Surg Sports Traumatol Arthrosc 19, 1510–1513. [DOI] [PubMed] [Google Scholar]
- Jiang S, Bailey AS, Goldman DC, et al. (2008) Hematopoietic stem cells contribute to lymphatic endothelium. PLoS One 3, e3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya M, Suziki T, Minowa T, et al. (2014) Ligamentum teres injury is associated with the articular damage pattern in adults with femoroacetabular impingement. Arthroscopy 30, 1582–1587. [DOI] [PubMed] [Google Scholar]
- Kazlouskaya V, Malhotra S, Lambe J, et al. (2013) The utility of elastic Verhoeff‐Van Gieson staining in dermatopathology. J Cutan Pathol 40, 211–225. [DOI] [PubMed] [Google Scholar]
- Kirici Y, Kiliç C, Öztaş E (2010) The ligament of head of femur and its arteries. J Clin Anal Med 1, 22–25. [Google Scholar]
- Lang A, Balint J (1952) Contributions to the anatomy of the ligamentum teres femoris. Acta Morphol Acad Sci Hung 3, 275–285. [PubMed] [Google Scholar]
- Leunig M, Beck M, Stauffer E, et al. (2000) Free nerve endings in the ligamentum capitis femoris. Acta Orthop Scand 71, 452–454. [DOI] [PubMed] [Google Scholar]
- Lindner D, Sharp KG, Trenga AP, et al. (2013) Arthroscopic ligamentum teres reconstruction. Arthrosc Tech 2, 21–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muratli HH, Bicimoglu A, Tabak YA, et al. (2004) Mechanoreceptor evaluation of the hip joint capsule and ligamentum teres femoris in developmental dysplasia: a preliminary study. J Pediatr Orthop 13B, 299–302. [DOI] [PubMed] [Google Scholar]
- Notzli HP, Siebenrock KA, Hempfing A, et al. (2002) Perfusion of the femoral head during surgical dislocation of the hip: monitoring by laser doppler flowmetry. J Bone Joint Surg 84B, 300–304. [DOI] [PubMed] [Google Scholar]
- O'Donnell JM, Pritchard M, Salas AP, et al. (2014) The ligamentum teres – its increasing importance. J Hip Preserv Surg 1, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergaminelis N, Renouf J, Fary C, et al. (2017) Outcomes of arthroscopic debridement of isolated ligamentum teres tears using the iHOT‐33. BMC Musculoskelet Disord 18, 554–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perumal V, Stringer MD (2014) The intrinsic arterial vascularity and morphology of the median nerve within the carpal tunnel: a microscopic study. Anat Sci Int 89, 28–33. [DOI] [PubMed] [Google Scholar]
- Perumal V, Woodley SJ, Nicholson HD (2017) The morphology and morphometry of the fovea capitis femoris. Surg Radiol Anat 39, 791–798. [DOI] [PubMed] [Google Scholar]
- Perumal V, Woodley SJ, Nicholson HD (2018) Clinical anatomy of the ligament of the head of femur. Clin Anat 32, 90–98. [DOI] [PubMed] [Google Scholar]
- Philippon MJ, Rasmussen MT, Turnbull TL, et al. (2014) Structural properties of the native ligamentum teres. Orthop J Sports Med 2, 2325967114561962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Portney LG, Watkins MP (2015) Foundations of Clinical Research: Applications to Practice, 3rd edn, p. 892 Upper Saddle River, NJ: Pearson/Prentice Hall. [Google Scholar]
- Rao J, Zhou YX, Villar RN (2001) Injury to the ligamentum teres – mechanism, findings and results of treatment. Clin Sports Med 20, 791–799. [DOI] [PubMed] [Google Scholar]
- Rein S, Hagart E, Hanisch U, et al. (2013) Immunohistochemical analysis of sensory nerve endings in ankle ligaments: a cadaver study. Cells Tissues Organs 197, 64–76. [DOI] [PubMed] [Google Scholar]
- Rein S, Semisch M, Garcia‐Elias M, et al. (2015) Immunohistochemical mapping of sensory nerve endings in the human triangular fibrocartilage complex. Clin Orthop Relat Res 473, 3245–3253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarban S, Baba F, Kocabey Y, et al. (2007) Free nerve endings and morphological features of the ligamentum capitis femoris in developmental dysplasia of the hip. J Pediatr Orthop 16, 351–356. [DOI] [PubMed] [Google Scholar]
- Schaumkel JV, Villar RN (2009) Healing of the ruptured ligamentum teres after hip dislocation – an arthroscopic finding. Hip Int 19, 64–66. [DOI] [PubMed] [Google Scholar]
- Shinohara Y, Kumai T, Higashiyama I, et al. (2014) Histological and molecular characterisation of the femoral attachment of the human ligamentum capitis femoris. Scand J Med Sci Sports 24, 245–253. [DOI] [PubMed] [Google Scholar]
- Sierra RJ, Trousdale RT (2009) Labral reconstruction using the ligamentum teres capitis: report of a new technique. Clin Orthop Relat Res 467, 753–759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JM, Field RE, Villar RN (2011) Arthroscopic reconstruction of the ligamentum teres. Arthroscopy 27, 436–441. [DOI] [PubMed] [Google Scholar]
- Standring S (2016) Gray's Anatomy: The Anatomical Basis of Clinical Practice, 40th edn, pp. 1378–1379. Edinburgh: Elsevier. [Google Scholar]
- Stecco C, Macchi V, Barbieri A, et al. (2018) Hand fasciae innervation: the palmar aponeurosis. Clin Anat 31, 677–683. [DOI] [PubMed] [Google Scholar]
- Watson‐Jones R (1946) Fractures and Joint Injuries, 3rd edn, p. 79 Edinburgh: Livingstone. [Google Scholar]
- Weathersby H (1959) The origin of the artery of the ligamentum teres femoris. J Bone Joint Surg Am 41, 261–263. [PubMed] [Google Scholar]
- Weidner J, Wyatt M, Beck M (2018) Labral augmentation with ligamentum teres capitis femoris: presentation of a new technique and preliminary results. J Hip Preserv Surg 5, 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertheimer LG, Fernandes L (1971) Arterial supply of the femoral head. A combined angiographic and histological study. J Bone Joint Surg Am 53, 545–556. [PubMed] [Google Scholar]
- White BJ, Scoles AM, Herzog MM (2018) Simultaneous acetabular labrum and ligamentum teres reconstruction: a case report. J Hip Preserv Surg 5, 166–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotorowicz M, Czubak J (2014) Vascular anatomy and blood supply to the femoral head In: Osteonecrosis. (eds Koo K‐H, Mont MA, Jones LC.), pp. 19–25. Berlin: Springer‐Verlag. [Google Scholar]


