Abstract
Decline of tactile sensation associated with ageing depends on modifications in skin and both central and peripheral nervous systems. At present, age‐related changes in the periphery of the somatosensory system, particularly concerning the effects on mechanoreceptors, remain unknown. Here we used immunohistochemistry to analyse the age‐dependent changes in Meissner's and Pacinian corpuscles as well as in Merkel cell‐neurite complexes. Moreover, variations in the neurotrophic TrkB‐BDNF system and the mechanoprotein Piezo2 (involved in maintenance of cutaneous mechanoreceptors and light touch, respectively) were evaluated. The number of Meissner's corpuscles and Merkel cells decreased progressively with ageing. Meissner's corpuscles were smaller, rounded in morphology and located deeper in the dermis, and signs of corpuscular denervation were found in the oldest subjects. Pacinian corpuscles generally showed no relevant age‐related alterations. Reduced expression of Piezo2 in the axon of Meissner's corpuscles and in Merkel cells was observed in old subjects, as well was a decline in the BDNF‐TrkB neurotrophic system. This study demonstrates that cutaneous Meissner's corpuscles and Merkel cell‐neurite complexes (and less evidently Pacinian corpuscles) undergo morphological and size changes during the ageing process, as well as a reduction in terms of density. Furthermore, the mechanoprotein Piezo2 and the neurotrophic TrkB‐BDNF system are reduced in aged corpuscles. Taken together, these alterations might explain part of the impairment of the somatosensory system associated with ageing.
Keywords: ageing, BDNF‐TrkB system, glabrous skin innervation, human, Piezo2, sensory corpuscles
Introduction
The sensory decline in the main sensory modalities is a consequence of the ageing process. Nevertheless, sensory ageing is not homogeneous across sensory systems, and large differences exist among these systems (Andersen, 2012; Jayakody et al. 2018). The experimental and clinical findings regarding the ageing of the somatosensory system (involving mechanoreception, thermoreception and nociception) are not conclusive, probably because of the involvement of the central nervous system, the peripheral nervous system and skin (Shaffer & Harrison, 2007; Goble et al. 2009; Tseng et al. 2013; Decorps et al. 2014; Heft & Robinson, 2017). In any case, elderly people have reduced fine touch discrimination ability (Skedung et al. 2018).
Age‐dependent changes in the structure and physiology of the somatosensory cortex are now rather well known (Blatow et al. 2007; Brodoehl et al. 2013; Cheng & Lin, 2013; Gröschel et al. 2013; Hagiwara et al. 2014). Additionally, the occurrence of morphological and histological changes in aged skin due to genetic and environmental factors is widely acknowledged (Khavkin & Ellis, 2011; Rittie & Fisher, 2015; Kanaki et al. 2016; Lephart, 2016; Krutmann et al. 2017). Conversely, the effects of ageing on the somatosensory peripheral nervous system (dorsal root ganglia, large nerve fibres and sensory corpuscles; Vega et al. 1993; Ulfhake et al. 2002; see Rittie & Fisher, 2015), especially at the periphery, are largely unknown. Skin ageing is associated with an overall reduction in nerve fibre endings in the epidermis and dermis (Besné et al. 2002; Panoutsopoulou et al. 2009; Fromy et al. 2010; Namer, 2010), although photoaging is characterized by increased sensory nerves in skin (Toyoda et al. 2005).
Tactile sensation is one of the most important components of mechanosensation and is carried out by specific sensory formations localized in the skin and known collectively as cutaneous sensory corpuscles or receptors (Zimmerman et al. 2014). Functionally, these receptors fall into two categories: rapidly adapting (RA) mechanoreceptors and slowly adapting (SA) mechanoreceptors, each of which have two variants, type I and type II (Jones & Smith, 2014). SAI mechanoreceptors are associated with epidermal Merkel cell‐neurite complexes, and SAII mechanoreceptors are thought to be located in dermal Ruffini's corpuscles, although the function of SAII can be carried out by other sensory corpuscles (Wu, 1998; Paré et al. 2003; Olson et al. 2016). Merkel cells are specialized epidermal cells (Van Keymeulen et al. 2009) functionally connected to Aβ nerve fibres that act as SAI and express mechanoproteins (Ikeda et al. 2014; Maksimovic et al. 2014; García‐Mesa et al. 2017). In mammals, RA mechanoreceptors are found in Meissner's and Pacinian sensory corpuscles (Zimmerman et al. 2014). Meissner's corpuscles represent RAI mechanoreceptors (Vega et al. 2012), whereas cutaneous Pacinian corpuscles represent RAII mechanoreceptors (Vega et al. 2009; García‐Piqueras et al. 2017).
Because age‐dependent impairments in mechanosensory function have been reported and limited information is available about age‐dependent changes in mechanosensors, we conducted research on human digital skin to evaluate the quantitative and qualitative changes that occur in Merkel cells, Meissner's corpuscles and Pacinian corpuscles with ageing. We have recently studied the development of these structures until maturity (Feito et al. 2018), demonstrating that they are not stable but are dynamic structures that undergo permanent age‐related changes. On the other hand, because the ion channel Piezo2 is at the basis of mechanotransduction (see for a review Anderson et al. 2017) and is located in both Meissner's corpuscles and Merkel cells of human digital skin (García‐Mesa et al. 2017), we investigated whether the pattern of expression of this protein changes with ageing. Furthermore, it is well known that cutaneous mechanoreceptors depend on the neurotrophin system of the brain‐derived neurotrophic factor (BDNF) and its high‐affinity receptor TrkB for development, growth and maintenance (Botchkarev et al. 1999; LeMaster et al. 1999; González‐Martínez et al. 2004; Perez‐Pinera et al. 2008; Reed‐Geaghan et al. 2016). Additionally, both BDNF and TrkB have been detected in developing and adult sensory corpuscles in different species, including humans (Stark et al. 2001; Sedy et al. 2004; Calavia et al. 2010a; Cabo et al. 2015a). Therefore, we analysed whether the localization of BDNF and TrkB in mechanoreceptors varies with age. Reduced expression of BDNF and TrkB has been observed in the central nervous system of old subjects compared with young and adult subjects (Erickson et al. 2010; Forlenza et al. 2015), suggesting that these molecules have a role in normal ageing.
The present study aimed to add information about the mechanisms underlying sensory deficits in elderly subjects and is focused on the peripheral touch components of somatosensory perception, with analysis of the effects of ageing on the structural and neurotrophic features of cutaneous sensory corpuscles.
Methods
Skin samples were obtained from the palmar aspect of the distal phalanx of the first and second fingers during autopsy at the Servicio de Anatomía Patológica of the Hospital Universitario Central de Asturias, Oviedo, Spain (n = 6) and the Servicio de Anatomía Patológica of the Complejo Hospitalario Universitaro de Salamanca (n = 9) and from incidental amputation at the Service of Plastic Surgery of the Hospital Universitario Central de Asturias (n = 6). The samples were collected within 12 h after demise or incident from subjects free of neurological diseases and without a clinical history of fibromatosis or labour‐induced repeated trauma. The age range was 23–90 years and at least one case per decade of life was analysed. These materials were all obtained in compliance with Spanish Law (RD 1301/2006; Ley 14/2007; DR 1716/2011; Orden ECC 1414/2013) and were deposited in our laboratory collection (Registro Nacional de Biobancos, Sección colecciones, Ref. C‐0001627; the responsibility of O.G.‐S.). The study was approved by the Ethical Committee for Biomedical Research of the Principality of Asturias, Spain (Cod. CElm, PAst: Proyecto 266/18).
Immunohistochemistry and immunofluorescence
The specimens were fixed in 4% formaldehyde in 0.1 m phosphate‐buffered saline (pH 7.4) for 24 h, dehydrated and routinely embedded in paraffin. The pieces were cut to 7 μm thickness in sections perpendicular to the skin surface and mounted on gelatine‐coated microscope slides. The presence of Pacinian and Meissner's corpuscles in the skin samples was evaluated by staining with haematoxylin and eosin. The occurrence of Merkel cells was assessed with immunohistochemistry.
The automated diagnostic platform Leica Bond III was used for immunohistochemistry with the Leica Bond™ Polymer Refine Detection Kit (Leica Biosystems™, Newcastle upon Tyne, UK) according to the manufacturer's instructions. The primary antibodies were directed against the main corpuscular constituents: axons (neurofilament, PGP 9.5) and Schwann cell‐derived lamellar and inner core cells (S100 protein). Moreover, to identify Merkel cells, an antibody against cytokeratin 20 (CK20) was used. The BDNF‐TrkB neurotrophin system was detected using specific antibodies against specific amino acid sequences within these molecules. Indirect immunohistochemistry included several negative and positive controls, as well as the internal positive and negative controls. Additionally, representative sections were processed in the same way as described above using non‐immune rabbit or mouse sera instead of the primary antibodies or by omitting the primary antibodies during the incubation. Furthermore, when available, additional controls were carried out using specifically preabsorbed antisera. Under these conditions, no positive immunostaining was observed.
Double immunofluorescence was performed on deparaffinized and rehydrated sections. Endogenous peroxidase activity and non‐specific binding were reduced by incubating sections with 3% H2O2 and a solution of 10% bovine serum albumin in Tris buffer solution (TBS), respectively. The sections were then incubated overnight at 4 °C in a humid chamber with a 1 : 1 mixture of a polyclonal antibody against S100‐protein (diluted 1 : 1000), PGP 9.5 (diluted 1 : 1000), BDNF (diluted 1 : 200) or TrkB (diluted 1 : 200) and a monoclonal antibody against NFP (diluted 1 : 1000) or CK20 (prediluted and 1 : 200 in the blocking solution). After rinsing with TBS, the sections were incubated for 1 h with Alexa Fluor 488‐conjugated goat anti‐rabbit IgG (Serotec, Oxford, UK), diluted 1 : 1000 in TBS containing 5% mouse serum (Serotec), rinsed again and incubated for another hour with Cy3‐conjugated donkey anti‐mouse antibody (Jackson‐Immuno Research, Baltimore, MD, USA) diluted 1 : 50 in TBS. Both steps were performed at room temperature in a dark humid chamber. Finally, to ascertain structural details, sections were counterstained and mounted with 4′,6‐diamidino‐2‐phenylindole (DAPI) diluted in glycerol medium (10 ng mL−1). Triple fluorescence was detected using a Leica DMR‐XA automatic fluorescence microscope (Photonic Microscopy Service, University of Oviedo) with Leica Confocal Software, version 2.5 (Leica Microsystems, Heidelberg GmbH, Germany) and the captured images were processed using the software imagej, version 1.43 g Master Biophotonics Facility, McMaster University Ontario (www.macbiophotonics.ca). Controls of the specificity of the reaction were performed as for indirect immunohistochemistry.
Data on case material and the characteristics of the primary antibodies used are summarized in Tables 1 and 2, respectively.
Table 1.
Data about the subjects and materials
| Case | Age in years | Gender M/F | Hand/finger |
|---|---|---|---|
| 1 | 23 | M | L/1/D |
| 2 | 30 | M | L/1/D |
| 3 | 35 | F | L/1/D |
| 4 | 37 | M | L/2/D |
| 5 | 38 | F | R/1/D |
| 6 | 40 | M | L/1/D |
| 7 | 42 | M | L/1/D |
| 8 | 46 | M | R/2/D |
| 9 | 47 | M | L/1/D |
| 10 | 55 | F | R/1/D |
| 11 | 58 | F | L/1/D |
| 12 | 58 | M | L/2/D |
| 13 | 63 | F | R/2/D |
| 14 | 66 | M | L/1/D |
| 16 | 69 | M | L/1/D |
| 16 | 72 | M | R/1/D |
| 17 | 74 | M | L/1/D |
| 18 | 81 | F | L/1/D |
| 19 | 85 | M | L/2/D |
| 20 | 88 | M | L/1/D |
| 21 | 90 | M | L/1/D |
F, female; M, male; L, left; R, right; 1 or 2, first or second fingers, respectively; D, distal phalanx.
Table 2.
Primary antibodies used in the study
| Antigen (clone) | Origin | Dilution | Supplier | Catalogue number |
|---|---|---|---|---|
| S100P | Rabbit | 1 : 1000 | DAKO, Glostrup, Denmark | Z 0311 |
| P200 kDa NF (RT‐97) | Mouse | 1 : 1000 | Boehringer‐Mannheim, Mannheim, Germany | 1178709 |
| PGP 9.5 | Rabbit | 1 : 1000 | Abcam, Hamburg, Germany | ab10404 |
| Cytokeratin 20 | Mouse | Prediluted | Leica, Newcastle, UK | PA0022 |
| Piezo2 | Rabbit | 1 : 500 | Sigma Aldrich, St. Louis, MO, USA | HPA040616 |
| BDNF | Rabbit | 1 : 200 | Chemicon Int, Temecula, CA, USA | AB1534SP |
| TrkB | Rabbit | 1 : 200 | Santa Cruz Biotechnology, Santa Cruz, CA, USA | SC12 |
BDNF, brain‐derived neurotrophic factor; NF, neurofilament; NSE, neuron‐specific enolase.
The antibody against BDNF is directed against the sequence H2N‐HSDPARRGEL‐COOH (manufacturer's note); the antibody anti‐TrkB was directed against the residues 794‐808 of the intracytoplasmic domain of human TrkB (manufacturer's note); the amino acid sequence recognized by the antibody against Piezo2 is FEDENKAAVRIMAGDNVEICMNLDAASFSQHNP (manufacturer's information).
Quantitative analyses
Quantitative analyses were performed to determine the density of cutaneous digital Meissner's corpuscles and Merkel cells at different ages but not the density of Pacinian corpuscles because of their irregular distribution in the skin dermis. To quantify Meissner's corpuscles, we used the method proposed by Verendeev et al. (2015) to determine its density in the fingertips of primates. The samples included in the study were divided into three age groups: 20–39 years, 40–59 years, and 60 years or older (see Table 1). Briefly, six 7‐μm‐thick sections of each skin sample, 200 μm apart, were processed for S100 protein immunohistochemistry to identify Meissner's corpuscles. The sections were then scanned by an SCN400F scanner (Leica, Leica Biosystems™), and the scans were computerized using SlidePath Gateway LAN software (Leica, Leica Biosystems™). Then, in each section, Meissner's corpuscles were identified and counted by two independent observers (J.G.‐P. and J.A.V.). The average numerical values were corrected by applying Abercrombie's formula: N = n*T/(T + H), where N is the corrected average number of Meissner's corpuscles, n is the counted average number of Meissner's corpuscles in all sections of a fingertip, T is the average section thickness, and H is the average diameter of the counted Meissner's corpuscles. Through a specific tool of the previously mentioned software, the average Meissner's corpuscle's diameter was determined by measuring its horizontal axis drawing a straight line approximately in the central region of each corpuscle. The epidermal length of each section (mm) was measured with the same tool, and the average length was multiplied by the section's thickness (mm) to give the measured surface area (mm2). Finally, the average number of Meissner's corpuscles (N) was divided by the surface area (mm2) to calculate the density of Meissner's corpuscles per squared millimetre of skin (number of Meissner's corpuscles/mm2). Afterward, the average density for each pre‐established age group was calculated from the individual densities. To determine the density of digital Merkel's cells, we used the same Merkel cell immunostaining method for CK20.
Moreover, in the same sections, the density of Meissner's corpuscles (Meissner Index) and Merkel's cells (Merkel Cells Index) was determined using the methodology proposed by Paré et al. (2007) and Bhat et al. (2008). These methods calculated the number of Meissner's corpuscles and Merkel's cells with respect to the number of dermal papillae and epidermal pegs, respectively. To compare age groups, the measurements were standardized for the length of skin analysed.
Significant differences among the three pre‐established age groups were assessed with the Kruskal–Wallis H‐test; P‐values < 0.05 were considered statistically significant (marked in the figures as *P < 0.05, **P < 0.01).
Results
Ageing of digital Meissner's corpuscles
Meissner's corpuscles were identified at all ages investigated (Figs 1, 2, 3), but differences in the morphology, size, number, placement within the dermis, and intensity of immunostaining for the evaluated markers were noted between the three pre‐established age groups. In the age group 20–39 years, Meissner's corpuscles were elongated and were always localized in the apex of the dermal papillae. The lamellar cells were packed, arranged in parallel, and displayed strong S100 protein immunoreactivity (Figs 1A and 2B,D); within the corpuscle, the axon showed a tortuous trajectory (Fig. 2A,C). By 40–59 years, the predominant localization of Meissner's corpuscles was also within the dermal papillae, but some were also highly displaced (Fig. 1B); the corpuscles showed no reduction in size, and the architecture and immunohistochemical profile of the axon and lamellar cells did not vary (Fig. 2–H). The scenario changed dramatically in the older subjects (Fig. 1C). In this group, the size of the Meissner's corpuscles was reduced, the morphology was rounded (only a few showed a typical elongated morphology) and most were localized behind rete pegs instead of inside the dermal papillae. In those corpuscles, the lamellar cells showed reduced immunostaining for S100 protein, and the axon was sometimes undistinguishable (Figs 2I–l and 3). This picture applied to approximately 70% of the corpuscles, whereas in the remaining 30%, the size, morphology and localization were similar to those of the younger subjects.
Figure 1.
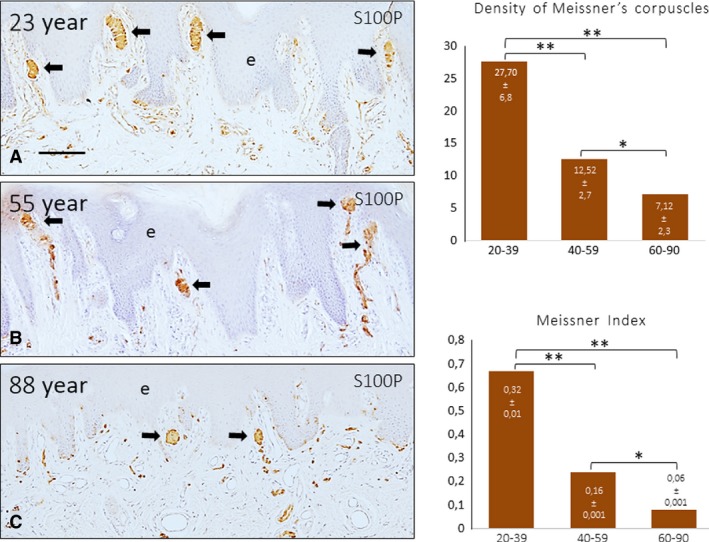
Sections of glabrous digital skin from 3 subjects with age of 23 (A), 55 (B) and 88 (C) years; immunostained was performed to evaluate S100 protein (S100P). Nerves and Meissner's corpuscles were immunolabelled (arrows). e, epidermis. Scale bar: 100 μm. The density of Meissner's corpuscles in the three pre‐established age ranges is shown in the graphs, determined as number of Meissner's corpuscles/mm2 (upper) and the number of Meissner's corpuscles/dermal papillae. A significant reduction in the density of Meissner's corpuscles was observed with ageing. *P < 0.05, **P < 0.01.
Figure 2.
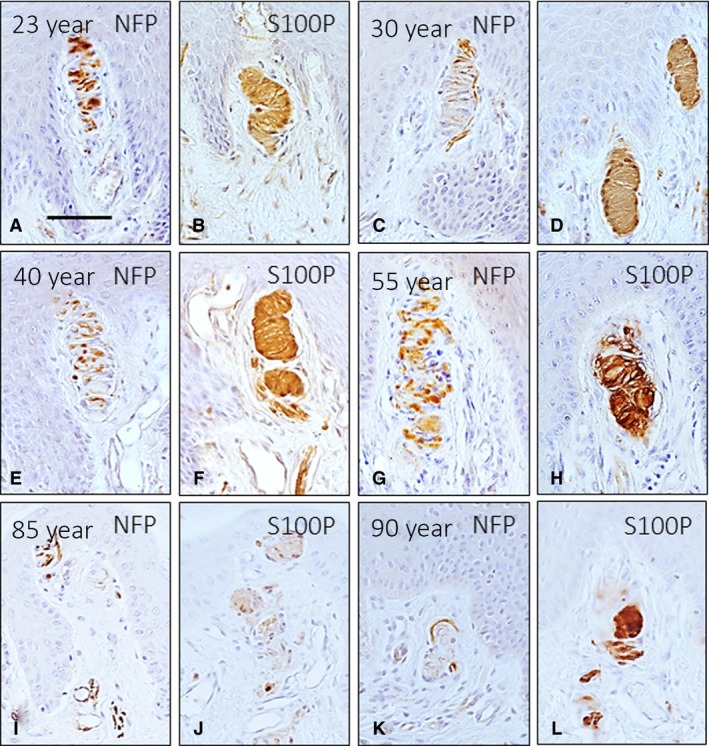
Immunohistochemical localization of neurofilament protein (NFP) and S100 protein (S100P) in the axon and lamellar cells, respectively, of human digital Meissner's corpuscles of subjects of different ages: 23 (A, B), 30 (C, D), 40 (E, F), 55 (G, H), 85 (I, J) and 90 (K, L) years. The localization, size, morphology and arrangement of corpuscular constituents varied with ageing. Moreover, the Meissner's corpuscles from old subjects showed a marked decrease in the intensity of the immunoreaction for S100P, and in some of these corpuscles, it was impossible to identify the axon. e, epidermis. Scale bar: 60 μm.
Figure 3.
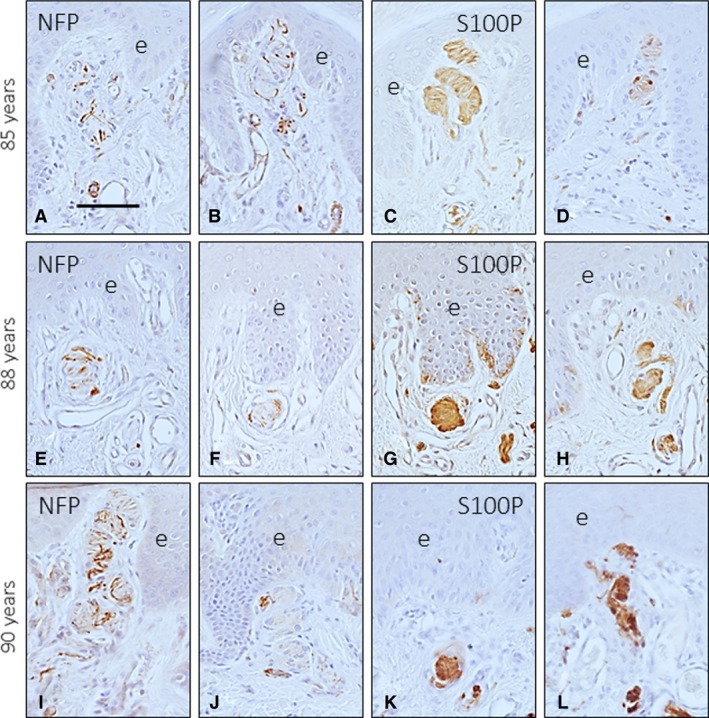
Digital Meissner's corpuscles from three subjects with ages of 85 (A–D), 88 (E–H) and 90 (I–L) years. Meissner's corpuscles were localized in the dermal papillae, but most were found in the reticular dermis. Those found at this location were smaller in the old subjects than in the adults, showed a rounded morphology and lacked the typical immunohistochemical profile. e, epidermis. Scale bar: 60 μm.
All these findings were confirmed by confocal microscopy of the Meissner's corpuscles of the three age groups, which showed that ageing induced changes in the size, morphology and immunohistochemical profile of these structures (Fig. 4). Of particular interest was the absence of axon profiles in a large percentage of Meissner's corpuscles from older subjects (Fig. 4I).
Figure 4.
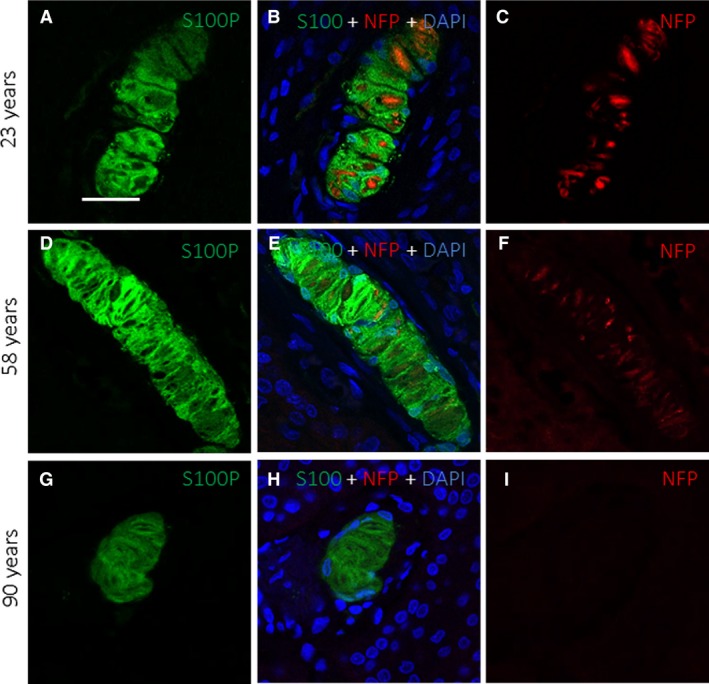
Double immunofluorescence for S100 protein (green fluorescence) and neurofilament protein (red fluorescence) in human digital Meissner's corpuscles of subjects with ages of 23 (A–C), 58 (D–F) and 90 (G–I) years. Sections were counterstained with DAPI to ascertain structural details. Objective 63 × /1.40 oil; pinhole 1.37; XY resolution 139.4 nm and Z resolution 235.8 nm. Scale bar: 20 μm.
In addition to the above‐described qualitative changes, ageing was accompanied by a progressive reduction in the number of Meissner's corpuscles with both methods utilized. Significant differences were observed between age groups, especially between young subjects and adult and old subjects (Fig. 1).
Ageing of digital Pacinian corpuscles
No evident variations in the Pacinian corpuscles were found between the three established age groups (Fig. 5). The arrangement of all corpuscular components was almost identical and no apparent age‐related changes were found in the number of concentric lamellae forming the outer core and the capsule (ranging between 19 and 41), although large differences were noted among corpuscles. The axon displayed NFP immunoreactivity (Fig. 5A,C,E,G), whereas the lamellae of the inner core were positive for S100 protein (Fig. 5B,D,F,H,J). Nevertheless, in some Pacinian corpuscles of older subjects (approximately 15–25%), no immunoreactivity for NFP was detected, and S100 protein immunoreactivity was restricted to the outer lamellae of the inner core (Fig. 5I‐l). Additionally, in some other corpuscles, the neural compartment of the corpuscles (i.e. the axon and the inner core) was disarranged compared with the typical organization of these structures (Fig. 6).
Figure 5.
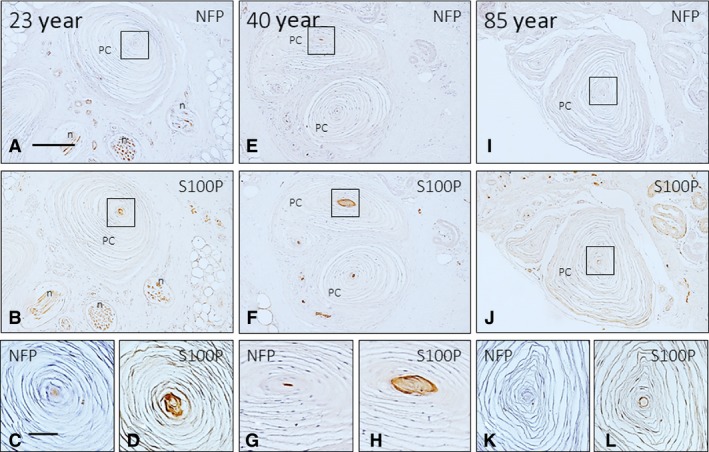
Immunohistochemical localization of neurofilament protein (NFP) and S100 protein (S100P) in the axon and the inner core cells, respectively, of human digital Pacinian corpuscles of subjects with ages of 23 (A–D), 55 (E–H)., and 88 (I–L) years. The axon supplying the corpuscles was identifiable in all corpuscles of 23‐ and 55‐year‐old subjects and was not identified in the 88‐year‐old subject. The inner core cells displayed S100P immunoreactivity in all lamellae in the 23‐ and 55‐year‐old subjects, whereas in the older subjects, S100P immunoreactivity was restricted to the outer core lamellae. Scale bar: 50 μm.
Figure 6.
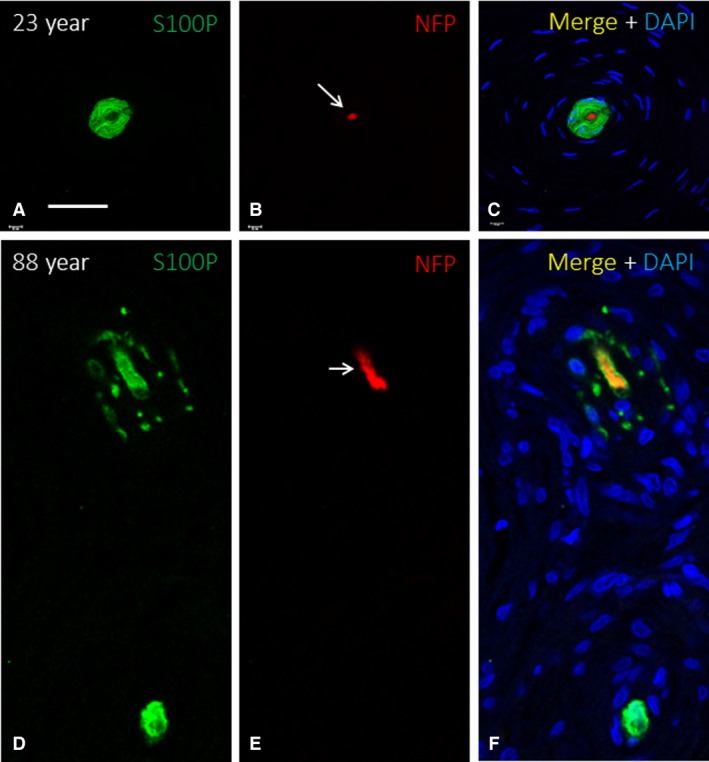
Double immunofluorescence for S100 protein (green fluorescence) and neurofilament protein (red fluorescence; arrows in B and E) in human digital Pacinian corpuscles of subjects with age of 23 (A–C) and 88 (D–F) years. Sections were counterstained with DAPI to ascertain structural details. The axon and the inner core of the 88‐year‐old subject are disarranged, although the corpuscle retained the general organization. Objective 63 × /1.40 oil; pinhole 1.37; XY resolution 139.4 nm and Z resolution 235.8 nm. Scale bar: 50 μm.
Ageing of cutaneous Merkel's cells
Digital Merkel's cells are epidermal cells primarily localized in the basal strata that selectively express the intermediate filament CK20. Using the expression of this protein as a marker, Merkel's cells were found in the epidermal rete pegs of all subjects, and the number of cells progressively decreased with age (Fig. 7). The quantitative analysis showed significant differences between the young subjects and the adult and old subjects (graphs in Fig. 7). Interestingly, clusters of up to four Merkel's cells were observed in young subjects, whereas they were less frequent in middle‐aged subjects, and almost absent in the older ones, in which only isolated Merkel's cells were found.
Figure 7.
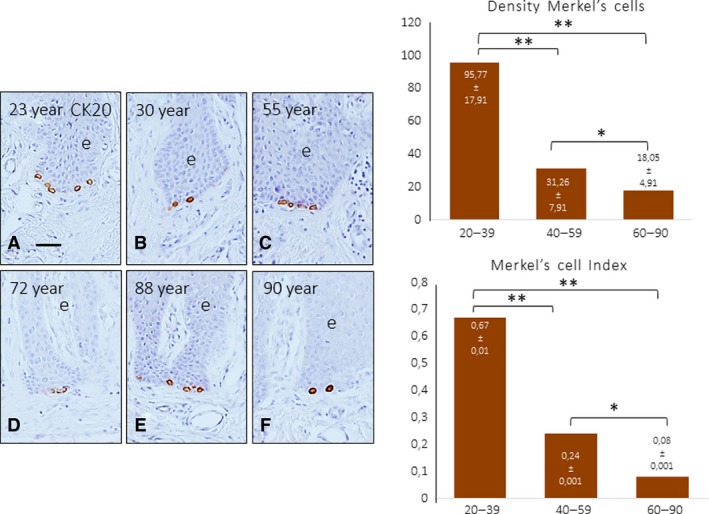
Immunohistochemical characterization of CK20 in human digital cells, identified based on their localization as Merkel's cells, in subjects of different ages: 23 (A), 30 (B), 55 (C), 72 (D), 88 (E) and 90 (F) years. Scale bar: 50 μm. The density of Merkel's cells in the three pre‐established age ranges is shown in the graph. A significant reduction in the density of Merkel's cells was observed with ageing determined as number of Merkel's cells/mm2 (upper) and the number of Merkel's cells/rete peg. *P < 0.05, **P < 0.01.
A graphic summary of the age‐related changes in Meissner's corpuscles and Merkel's cells described above is presented in Fig. 8.
Figure 8.
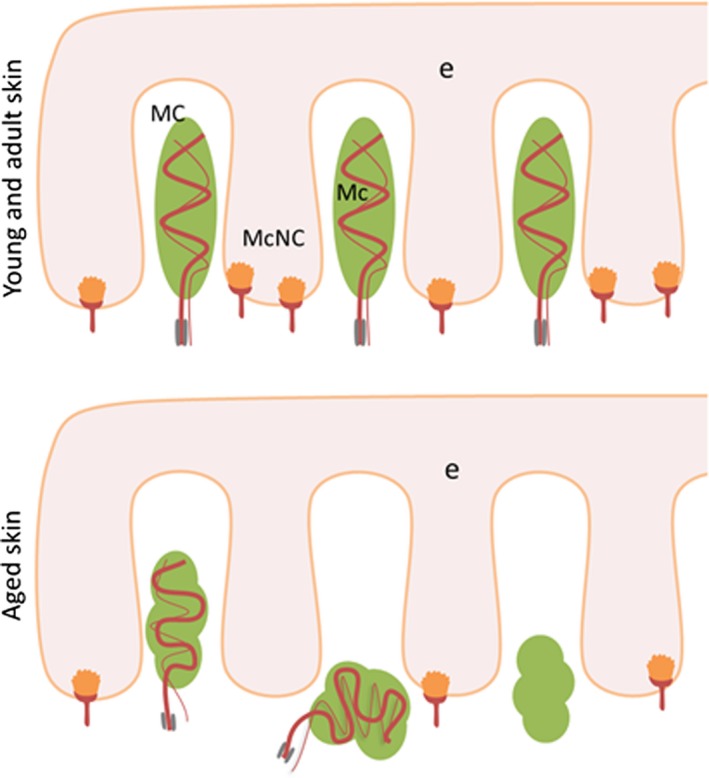
Schematic representation of Meissner's corpuscles (MC) and Merkel's cell‐neurite complexes (McNc) in the human digital glabrous skin of young‐adult (top) and old (bottom) subjects. Ageing results in a reduction in the density of both types of mechanoreceptors as well as changes in the placement, size, morphology and immunohistochemical profile of Meissner's corpuscles. e, epidermis.
Ageing of the BDNF‐TrkB system in cutaneous mechanoreceptors
The development and maintenance of Meissner's corpuscles and, to a lesser extent, Merkel's cells and Merkel's cell‐axon complexes are under the control of the BDNF‐TrkB neurotrophin system. Thus, we investigated whether the components of that system are present in those sensory structures and whether these components undergo age‐dependent variations. In human digital Meissner's corpuscles from adult subjects, immunoreactivities for BDNF and TrkB were detected in the lamellar cells (Supporting Information Fig. S1a–c) and the axon (Fig. S1d–f), respectively. In the Pacinian corpuscles, BDNF was found in the inner core cells (Supporting Information Fig. S2), and TrkB was found in the axons (data not shown). In Merkel's cells, BDNF was undetectable (Supporting Information Fig. S3A–C), whereas TrkB immunoreactivity regularly occurred in the cytoplasm (Fig. S3D–F).
The pattern of localization of both BDNF and TrkB with age was as described above. However, in younger subjects, BDNF immunoreactivity also showed localization in axons (Fig. 9A). With ageing, the intensity of immunostaining for BDNF (Fig. 9B–D) and TrkB (Fig. 9E–H) progressively decreased, and TrkB immunoreactivity was undetectable in older subjects (Fig. 9H). These results were found in approximately 85% of Meissner's corpuscles, and no apparent age‐dependent changes were observed in the remaining corpuscles (15%). The percentage of TrkB‐positive Merkel's cells also declined with age, with a reduction of approximately 60% in the old age group compared with the young and adult age groups (data not shown).
Figure 9.
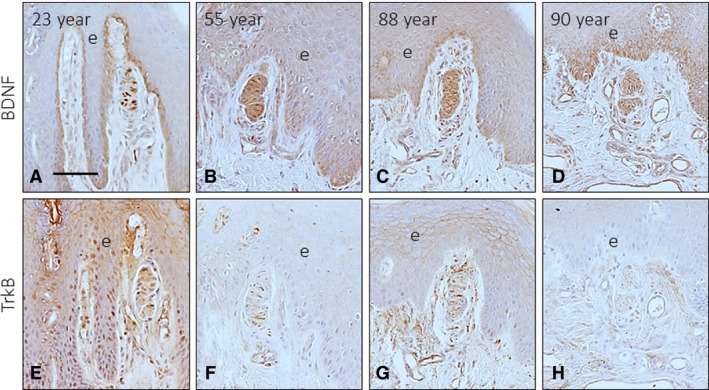
Immunohistochemical detection of brain‐derived neurotrophic factor (BDNF) and its signalling receptor TrkB in Meissner's corpuscles of subjects with ages of 23 (A, E), 55 (B, F), 88 (C, G) and 90 (D, H) years. Scale bar: 60 μm.
Piezo2 levels in Meissner's corpuscles and Merkel's cells decrease with age
The occurrence of Piezo2 immunoreactivity in the axons of human cutaneous Meissner's corpuscles and the cytoplasm of Merkel's cells has been demonstrated recently by our research group (García‐Mesa et al. 2017). Here, we observed that the axons of most of the Meissner's corpuscles of the younger subjects displayed Piezo2 positivity (Fig. 10A,B), which remained unchanged in the middle‐aged group and was markedly reduced in the older subject age group (Fig. 10D,E). No Piezo2 immunostaining was noted in the lamellar cells. In agreement with the above data regarding the age‐dependent decrease in the density of Merkel's cells, there was a parallel decrease in the density of the Piezo2‐positive ones (Fig. 10C,F). On the other hand, although no direct contacts between Merkel's cells and axons were observed, the density of axon in the vicinity or apparently contacting Merkel's cell also decreased with age (Fig. 11).
Figure 10.
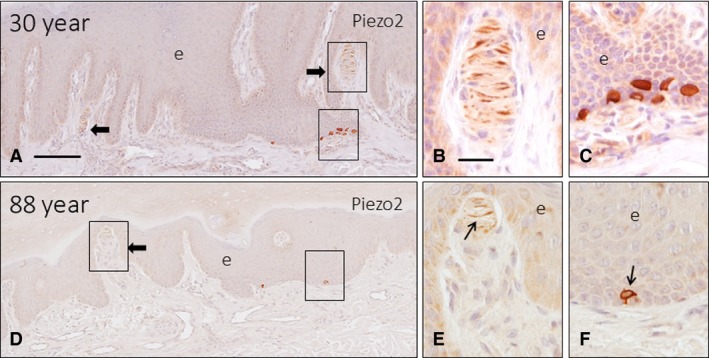
Sections of glabrous digital skin from 2 subjects with age of 30 (A) and 88 (D) years immunostained to detect Piezo2. The axon of most Meissner's corpuscles displayed Piezo2 immunoreactivity in young individuals (arrows in A, B), whereas this immunoreactivity was restricted to segments of the corpuscle, or even absent in the older subjects (E). The density of Piezo2‐positive Merkel cells in young subjects (C) was higher than that in the old subjects (f, arrow). e, epidermis. Scale bar: 100 μm (A,D), 20 μm (B–C,E–F).
Figure 11.
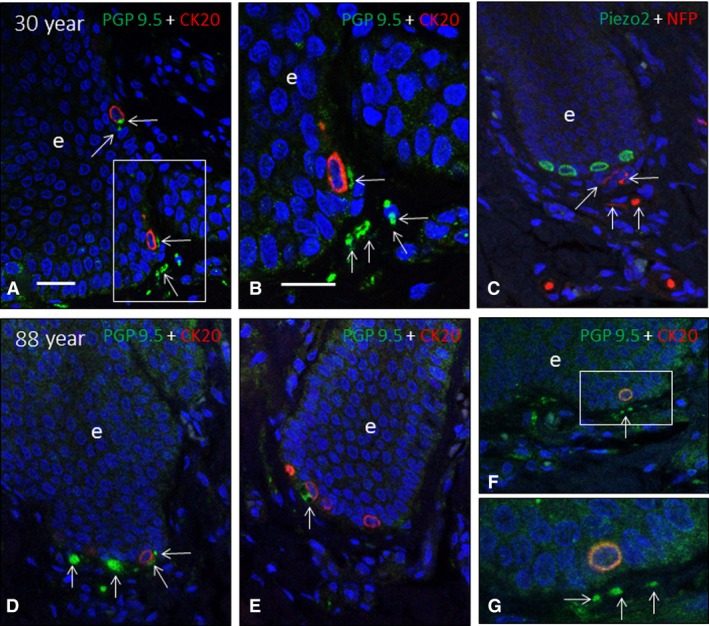
Double immunofluorescence for neuron‐specific enolase (green fluorescence in A and B), neurofilament proteins (green fluorescence in C–F; red fluorescence in G), cytokeratin 20 (red fluorescence in A–F) and Piezo2 (green fluorescence in) in human Merkel's cell‐neurite complexes of subjects with ages of 30), and 88 ) years. Axonal profiles are indicated by arrows. e: epidermis. Objective 63 × /1.40 oil; pinhole 1.37; XY resolution 139.4 nm and Z resolution 235.8 nm. Scale bar: 30 μm (A‐F), 20 μm (B,G).
Discussion
In human skin, a series of sensory formations, collectively known as sensory corpuscles, are able to detect different qualities of somatosensation (Zimmerman et al. 2014). In particular, Merkel's cell‐neurite complexes, Meissner's corpuscles and Pacinian corpuscles discriminate unique aspects of touch, acting as SAI and SAII mechanoreceptors (Jones & Smith, 2014; Zimmerman et al. 2014). Ageing is accompanied by a progressive decrease in the somatosensory system that affects the quality of life of elderly subjects (Skedung et al. 2018). This deterioration presumably involves all levels of the somatosensory pathways from the skin to the cerebral cortex, including the cutaneous receptors in which the mechanical input is transduced into electrical energy. The present study was designed to investigate the age‐dependent changes in some types of cutaneous sensory corpuscles and in one mechanoprotein directly involved in mechanotransduction (Piezo2), as well as the variations in a neurotrophic system (BDNF‐TrkB) that is essential for their development and maintenance.
The effects of ageing on touch have been reviewed in detail by Wickremaratchi & Llewelyn (2006), Decorps et al. (2014) and Heft & Robinson (2017). Ageing is also associated with a progressive decline in cutaneous thermic (Guergova & Dufour, 2011) and mechanical (Wickremaratchi & Llewelyn, 2006; Wu et al. 2011) perception, as well as a marked degradation of tactile spatial acuity (Kalisch et al. 2009; Skedung et al. 2018). This impairment could be due at least in part to a reduction in the density and distribution of Meissner's and Pacinian corpuscles (Wickremaratchi & Llewelyn, 2006), as well as Merkel's disc (Lumpkin et al. 2003).
Age‐dependent changes in cutaneous Meissner's and Pacinian corpuscles
Classic studies have demonstrated that Pacinian and Meissner's corpuscles decrease in number and undergo structural deterioration upon ageing in humans (Bolton et al. 1966; Kennedy et al. 2011), monkey (Paré et al. 2007) and mice (Mathewson & Nava, 1985).
Studies in humans are limited and do not use specific immunohistochemistry assays for each corpuscular component. Here, we observed that the size, morphology and structure of Meissner's corpuscles remained essentially unaltered until 60 years of age, and these corpuscles then progressively shrank, changed morphology and topographical localization, and lost their immunohistochemical profile. Interestingly, the absence of immunoreactivity for axonal markers in older subjects suggests denervation of those corpuscles, which is supported by the decrease in the expression of S100 protein by the lamellar cells. In fact, it is well known that lamellar cells of denervated Meissner corpuscles lack S100 protein (Del Valle et al. 1993; Marquez et al. 1997; Albuerne et al. 1998). Globally, our results are in good agreement with the data reported by Nava & Mathewson (1996) in Meissner‐like corpuscles of murine forepaw digital pads. These authors observed that in mice aged to their maximum life expectancy, Meissner‐like corpuscles decrease and become disorganized and lobulated, and these changes are attributed to distal axonopathy and atrophy of the sensory neurons. A study carried out in the Meissner‐like corpuscles of the murine palatine mucosa showed that only rudiments of corpuscles were encountered in old animals due to an atrophy of the axon and lamellae (Iida & Tachibana, 1996). On the other hand, these changes in Meissner's corpuscles we observed here affect most, but not all the corpuscles, suggesting that tactile texture discrimination remains partially intact with age, irrespective of cutaneous condition. A recent study by Skedung et al. (2018) evaluated the density of Meissner's corpuscles in two groups of aged subjects with high and low performance, showing that the number was reduced approximately 50% in the subjects with lower performance. Thus, the lower performance of this group can be confidently linked to a neural decline.
Regarding Pacinian corpuscles, the effects of ageing were not evident in either the number or the structure. However, a small population of Pacinian corpuscles from the older subjects seemed to be denervated (no labelling of the axon and absence of S100 protein in the whole inner core) or the neural compartment was disarranged. Pacinian corpuscles have been related to the detection of vibration (Bell et al. 1994; Zelená, 1994; Zimmerman et al. 2014) and these morphological variations might be correlated with the decreased vibration sensitivity reported in elderly people (see Shaffer & Harrison, 2007; Landelle et al. 2018).
The exhaustive review on the Pacinian corpuscles carried out by Zelená (1994) in the book ‘Nerves and Mechanoreceptors’ does not mention the age‐dependent changes in Pacinian corpuscles in humans or other mammalian species.
Age‐related changes in the expression of basic corpuscular antigens
We observed that in some Meissner's corpuscles from the aged subjects, there was a reduction in the immunoreactivity for S100 protein along with an absence of immunohistochemically detectable axons. These two findings suggest denervation of these corpuscles because the expression of S100 protein by lamellar cells depends on the functional integrity of the axon (Marquez et al. 1997; Albuerne et al. 1998).
Age‐dependent changes in Merkel's cells and Merkel's cell‐neurite complexes
In the present study we have found a marked reduction of the density of Merkel's cells in aged subjects. As far as we know, these age‐related changes hae note been reported earlier. Deterioration of the discs of Merkel was observed with ageing in rats (Fundin et al. 1997), but deterioration was not observed in Merkel's cell‐neurite complexes either in humans or in rodents (Bolton et al. 1966; Mathewson & Nava, 1985; Paré et al. 2007). Recently, Moayedi et al. (2018) reported that Merkel's cells undergo a dramatic reduction in density with ageing in murine palatine mucosa.
Piezo2 expression is reduced in aged cutaneous mechanoreceptors
Activation of mechanically gated ion channels is at the origin of the detection of low‐ or high‐threshold mechanical stimuli. Different candidates have been proposed to be the mechanotransducers in human sensory corpuscles and Merkel's cells (Calavia et al. 2010b; Cabo et al. 2015b; Alonso‐González et al. 2017). However, only Piezo2 has been demonstrated to be an essential component of distinct stretch‐activated ion channels involved in mechanotransduction (see Roudaut et al. 2012; Delmas & Coste, 2013; Wu et al. 2017;. Piezo2 is present in mechanosensory neurons (Coste et al. 2010; Ranade et al. 2014) and different low‐threshold cutaneous mechanoreceptors, including human Meissner's corpuscles and Merkel's cells (García‐Mesa et al. 2017). Although the ageing effects on the structure and/or the function of these mechanosensitive ion channels have not been described, one can speculate that their reduced expression in the axons supplying RAI and SAI could contribute to age‐related tactile defects.
BDNF‐TrkB neurotrophin complex is altered in the skin of aged subjects
The development, survival and maintenance of cutaneous sensory corpuscles are controlled by different neurotrophins and their Trk signalling receptors (see Montano et al. 2010). The BDNF‐TrkB system is involved in different modalities of touch because it controls Meissner's corpuscles and, to a lesser extent, Pacinian corpuscles and Merkel's cell‐neurite complexes (González‐Martínez et al. 2004; Sedy et al. 2004; De Carlos et al. 2006; Perez‐Pinera et al. 2008). BDNF‐TrkB continues to be expressed in some neurons of adult sensory ganglia and in some types of sensory corpuscles throughout the entire lifespan (see Montano et al. 2010). The present study and previous studies (Calavia et al. 2010a) demonstrate that human cutaneous sensory corpuscles contain immunohistochemically detectable BDNF and TrkB, and confirm that TrkB immunoreactivity in Meissner's corpuscles decreases in subjects older than 50 years. Our results demonstrate that the ageing of sensory corpuscles is accompanied by a decrease in the immunohistochemically detectable BDNF and TrkB, which were absent in some Meissner's corpuscles. Whether the absence of BDNF‐TrkB is responsible, at least in part, for the size and morphological changes observed in Meissner's corpuscles and in the density of Merkel cells remains to be investigated. In any case, our results could explain the reduction in sensory neurons of the dorsal root ganglia that occurs with ageing (Vega et al. 1993). In the human central nervous system, low levels of BDNF and reduced expression of TrkB are related to ageing and the related diseases (Erickson et al. 2010; Forlenza et al. 2015; Nunes et al. 2018).
Overall, the present study demonstrates that the cutaneous Meissner's corpuscles and Merkel's cell‐neurite complexes and, less evidently, Pacinian corpuscles, which represent the most peripheral part of the sensory nervous system, undergo age‐dependent morphological and size changes as well as a decrease in their density. The mechanoprotein Piezo2 involved in fine touch is also reduced in aged corpuscles, as is the trophic system responsible for their maintenance. These changes partially explain the impairment of the somatosensory with ageing.
Author contributions
J.G.‐P., Y.G‐M. and I.T.P. performed the experiments. O.G.‐S. and J.A.V. designed the study and analysed the data. J.F., J.C. and B.M.‐B. collected the material follwing legal conditions and performed part of the experiments. J.G.‐.P and J.A.V. wrote the manuscript.
Supporting information
Fig. S1. Double immunofluorescence for BDNF (a) and TrkB (d) (green fluorescence) and S100 protein (red fluorescence) in human digital Meissner's corpuscles of one 30‐year‐old subject. BDNF and S100 protein were colocalized, suggesting that BDNF was present in the lamellar cells. Conversely, no colocalization of TrkB and S100 protein was detected, indicating that TrkB is mainly located in axons. Objective 63 × /1.40 oil; pinhole 1.37; XY resolution 139.4 nm and Z resolution 235.8 nm. Scale bar: 30 μm.
Fig. S2. Double immunofluorescence for BDNF (green fluorescence) and NFP (b) and S100 protein (e) (red fluorescence) in human digital Meissner's corpuscles of one 30‐year‐old subject. BDNF and S100 protein were colocalized, suggesting that BDNF is present in inner core cells. Conversely, no colocalization of BDNF and NFP was detected, indicating that BDNF is not present in the axon. Objective 63 × /1.40 oil; pinhole 1.37; XY resolution 139.4 nm and Z resolution 235.8 nm. ic, inner core; arrow indicates the axon. Scale bar: 50 μm.
Fig. S3. Double immunofluorescence for BDNF (a) and TrkB (d) (green fluorescence) and CK20 (red fluorescence) in human digital Merkel cells (arrows). BDNF and CK20 never colocalized, whereas TrkB and CK20 colocalized in the cytoplasm of Merkel cells. Objective 63 × /1.40 oil; pinhole 1.37; XY resolution 139.4 nm and Z resolution 235.8 nm. ic: inner core; arrow indicates the axon. Scale bar: 30 μm.
Acknowledgements
This study was supported in part by a grant from Gerencia Regional de Salud de Castilla y León (GRS 1615/A/17). The authors thank Dr Marta Guervos (Servicios Comunes de Investigación, Microscopia Confocal, Universidad de Oviedo) and Marta Sánchez‐Pitiot (Grupo de Histopatología Molecular, Instituto Universitario de Oncología del Principado de Asturias) for technical assistance.
References
- Albuerne M, López S, Naves FJ, et al. (1998) S100α and S100β proteins in human cutaneous sensory corpuscles: effects of nerve and spinal cord injury. Anat Rec 251, 351–359. [DOI] [PubMed] [Google Scholar]
- Alonso‐González P, Cabo R, José I, et al. (2017) Human digital Meissner corpuscles display immunoreactivity for the multifunctional ion channels Trpc6 and Trpv4. Anat Rec 300, 1022–1031. [DOI] [PubMed] [Google Scholar]
- Andersen GJ (2012) Aging and vision: changes in function and performance from optics to perception. Wiley Interdiscip Rev Dev Biol 3, 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EO, Schneider ER, Bagriantsev SN (2017) Piezo2 in cutaneous and proprioceptive mechanotransduction in vertebrates. Curr Top Membr 79, 197–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J, Bolanowski S, Holmes MH (1994) The structure and function of Pacinian corpuscles: a review. Prog Neurobiol 42, 79–128. [DOI] [PubMed] [Google Scholar]
- Besné I, Descombes C, Breton L (2002) Effect of age and anatomical site on density of sensory innervation in human epidermis. Arch Dermatol. 138, 1445‐1450. [DOI] [PubMed] [Google Scholar]
- Bhat GM, Bhat MA, Kour K, et al. (2008) Density and structural variations of Meissner's corpuscle at different sites in human glabrous skin. J Anar Soc India 57, 30–33. [Google Scholar]
- Blatow M, Nennig E, Durst A, et al. (2007) fMRI reflects functional connectivity of human somatosensory cortex. NeuroImage 37, 927–936. [DOI] [PubMed] [Google Scholar]
- Bolton CF, Winkelmann RK, Dyck PJ (1966) A quantitative study of Meissner's corpuscles in man. Neurology 16, 1–9. [DOI] [PubMed] [Google Scholar]
- Botchkarev VA, Kief S, Paus R, et al. (1999) Overexpression of brain‐derived neurotrophic factor increases Merkel cell number in murine skin. J Invest Dermatol 113, 691–692. [DOI] [PubMed] [Google Scholar]
- Brodoehl S, Klingner C, Stieglitz K, et al. (2013) Age‐related changes in the somatosensory processing of tactile stimulation – An fMRI study. Behav Brain Res 238, 259–264. [DOI] [PubMed] [Google Scholar]
- Cabo R, Alonso P, José IS, et al. (2015a) Brain‐derived neurotrofic factor and its receptor TrkB are present, but segregated, within mature cutaneous pacinian corpuscles of Macaca fascicularis . Anat Rec 298, 624–629. [DOI] [PubMed] [Google Scholar]
- Cabo R, Alonso P, Viña E, et al. (2015b) ASIC2 is present in human mechanosensory neurons of the dorsal root ganglia and in mechanoreceptors of the glabrous skin. Histochem Cell Biol 143, 267–276. [DOI] [PubMed] [Google Scholar]
- Calavia MG, Feito J, López‐Iglesias L, et al. (2010a) The lamellar cells in human Meissner corpuscles express TrkB. Neurosci Lett 468, 106–109. [DOI] [PubMed] [Google Scholar]
- Calavia MG, Montaño JA, García‐Suárez O, et al. (2010b) Differential localization of acid‐sensing ion channels 1 and 2 in human cutaneus pacinian corpuscles. Cell Mol Neurobiol 30, 841–848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng CH, Lin YY (2013) Aging‐related decline in somatosensory inhibition of the human cerebral cortex. Exp Brain Res 226, 145–152. [DOI] [PubMed] [Google Scholar]
- Coste B, Mathur J, Schmidt M, et al. (2010) Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 330, 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Carlos F, Cobo J, Germanà G, et al. (2006) Abnormal development of pacinian corpuscles in double trkB‐trkC knockout mice. Neurosci Lett 410, 157–161. [DOI] [PubMed] [Google Scholar]
- Decorps J, Saumet JL, Sommer P, et al. (2014) Effect of ageing on tactile transduction processes. Ageing Res Rev 13, 90–99. [DOI] [PubMed] [Google Scholar]
- Del Valle ME, Cabal A, Alvarez‐Mendez JC, et al. (1993) Effect of denervation on lamellar cells of Meissner‐like sensory corpuscles of the rat. An immunohistochemical study. Cell Mol Biol (Noisy‐le‐grand) 39, 801–807. [PubMed] [Google Scholar]
- Delmas P, Coste B (2013) Mechano‐gated ion channels in sensory systems. Cell 155, 278–284. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Prakash RS, Voss MW, et al. (2010) Brain‐derived neurotrophic factor is associated with age‐related decline in hippocampal volume. J Neurosci 30, 5368–5375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feito J, Garcia‐Suarez O, Garcia‐Piqueras J, et al. (2018) The development of human digital Meissner's and Pacinian corpuscles. Ann Anat 219, 8–24. [DOI] [PubMed] [Google Scholar]
- Forlenza OV, Miranda AS, Guimar I, et al. (2015) Decreased neurotrophic support is associated with cognitive decline in non‐demented subjects. J Alzheimers Dis 46, 423–429. [DOI] [PubMed] [Google Scholar]
- Fromy B, Sigaudo‐Roussel D, Gaubert‐Dahan ML, et al. (2010) Aging‐associated sensory neuropathy alters pressure‐induced vasodilation in humans. J Investig Dermatol 130, 849–855. [DOI] [PubMed] [Google Scholar]
- Fundin BT, Bergman E, Ulfhake B. (1997) Alterations in mystacial pad innervation in the aged rat. Exp Brain Res 117, 324–340. [DOI] [PubMed] [Google Scholar]
- García‐Mesa Y, García‐Piqueras J, García B, et al. (2017) Merkel cells and Meissner's corpuscles in human digital skin display Piezo2 immunoreactivity. J Anat 231, 978–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García‐Piqueras J, García‐Suárez O, Rodríguez‐González MC, et al. (2017) Endoneurial‐CD34 positive cells define an intermediate layer in human digital Pacinian corpuscles. Ann Anat 211, 55–60. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Coxon JP, Wenderoth N, et al. (2009) Proprioceptive sensibility in the elderly: degeneration, functional consequences and plastic‐adaptive processes. Neurosci Biobehav Rev 33, 271–278. [DOI] [PubMed] [Google Scholar]
- González‐Martínez T, Germana GP, Monjil DF, et al. (2004) Absence of Meissner corpuscles in the digital pads of mice lacking functional TrkB. Brain Res 1002, 120–128. [DOI] [PubMed] [Google Scholar]
- Gröschel S, Sohns JM, Schmidt‐Samoa C, et al. (2013) Effects of age on negative BOLD signal changes in the primary somatosensory cortex. NeuroImage 71, 10–18. [DOI] [PubMed] [Google Scholar]
- Guergova S, Dufour A (2011) Thermal sensitivity in the elderly: a review. Ageing Res Rev 10, 80–92. [DOI] [PubMed] [Google Scholar]
- Hagiwara K, Ogata K, Okamoto T, et al. (2014) Age‐related changes across the primary and secondary somatosensory areas: an analysis of neuromagnetic oscillatory activities. Clin Neurophysiol 125, 1021–1029. [DOI] [PubMed] [Google Scholar]
- Heft MW, Robinson ME (2017) Somatosensory function in old age. J Oral Rehabil 44, 327–332. [DOI] [PubMed] [Google Scholar]
- Iida S, Tachibana T (1996) Age‐related changes in Meissner corpuscles in the mouse palate: a histochemical and ultrastructural study. Arch Histol Cytol 59, 281–290. [DOI] [PubMed] [Google Scholar]
- Ikeda R, Cha M, Ling J, et al. (2014) Merkel cells transduce and encode tactile stimuli to drive Aβ‐afferent impulses. Cell 157, 664–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayakody DMP, Friedland PL, Martins RN, et al. (2018) Impact of aging on the auditory system and related cognitive functions: a narrative review. Front Neurosci 12, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LA, Smith AM (2014) Tactile sensory system: encoding from the periphery to the cortex. Wiley Interdiscip Rev Dev Biol 6, 279–287. [DOI] [PubMed] [Google Scholar]
- Kalisch T, Ragert P, Schwenkreis P, et al. (2009) Impaired tactile acuity in old age is accompanied by enlarged hand representations in somatosensory cortex. Cereb Cortex 19, 1530–1538. [DOI] [PubMed] [Google Scholar]
- Kanaki T, Makrantonaki E, Zouboulis CC (2016) Biomarkers of skin aging. Rev Endocr Metab Disord 17, 433–442. [DOI] [PubMed] [Google Scholar]
- Kennedy WR, Selim MM, Brink TS, et al. (2011) A new device to quantify tactile sensation in neuropathy. Neurology 76, 1642–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khavkin J, Ellis DAF (2011) Aging skin: histology, physiology, and pathology. Facial Plast Surg Clin North Am 19, 229–234. [DOI] [PubMed] [Google Scholar]
- Krutmann J, Bouloc A, Sore G, et al. (2017) The skin aging exposome. J Dermatol Sci 85, 152–161. [DOI] [PubMed] [Google Scholar]
- Landelle C, El Ahmadi A, Kavounoudias A (2018) Age‐related impairment of hand movement perception based on muscle proprioception and touch. Neuroscience 381, 91–104. [DOI] [PubMed] [Google Scholar]
- LeMaster AM, Krimm RF, Davis BM, et al. (1999) Overexpression of brain‐derived neurotrophic factor enhances sensory innervation and selectively increases neuron number. J Neurosci 19, 5919–5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lephart ED (2016) Skin aging and oxidative stress: Equol's anti‐aging effects via biochemical and molecular mechanisms. Ageing Res Rev 31, 36–54. [DOI] [PubMed] [Google Scholar]
- Lumpkin EA, Collisson T, Parab P, et al. (2003) Math1‐driven GFP expression in the developing nervous system of transgenic mice. Gene Expr Patterns 3, 389–395. [DOI] [PubMed] [Google Scholar]
- Maksimovic S, Nakatani M, Baba Y, et al. (2014) Epidermal Merkel cells are mechanosensory cells that tune mammalian touch receptors. Nature 509, 617–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez J, Perez‐Perez M, Naves FJ, et al. (1997) Effect of spinal cord and peripheral nerve injury on human cutaneous sensory corpuscles. An immunohistochemical study. J Peripher Nerv Syst 2, 49–59. [PubMed] [Google Scholar]
- Mathewson RC, Nava PB (1985) Effects of age on Meissner corpuscles: a study of silver‐impregnated neurites in mouse digital pads. J Comp Neurol 231, 250–259. [DOI] [PubMed] [Google Scholar]
- Moayedi Y, Duenas‐Bianchi LF, Lumpkin EA (2018) Somatosensory innervation of the oral mucosa of adult and aging mice. Sci Rep 8, 9975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montano JA, Perez‐Pinera P, Garcia‐Suarez O, et al. (2010) Development and neuronal dependence of cutaneous sensory nerve formations: lessons from neurotrophins. Microsc Res Tech 73, 513–529. [DOI] [PubMed] [Google Scholar]
- Namer B (2010) Age related changes in human C‐fiber function. Neurosci Lett 470, 185–187. [DOI] [PubMed] [Google Scholar]
- Nava PB, Mathewson RC (1996) Effect of age on the structure of Meissner corpuscles in murine digital pads. Microsc Res Tech 34, 376–389. [DOI] [PubMed] [Google Scholar]
- Nunes PV, Nascimento CF, Kim HK, et al. (2018) Low brain‐derived neurotrophic factor levels in post‐mortem brains of older adults with depression and dementia in a large clinicopathological sample. J Affect Disord 241, 176–181. [DOI] [PubMed] [Google Scholar]
- Olson W, Dong P, Fleming M, et al. (2016) The specification and wiring of mammalian cutaneous low‐threshold mechanoreceptors. Wiley Interdiscip Rev Dev Biol 5, 389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panoutsopoulou IG, Wendelschafer‐Crabb G, Hodges JS, et al. (2009) Skin blister and skin biopsy to quantify epidermal nerves: a comparative study. Neurology 72, 1205–1210. [DOI] [PubMed] [Google Scholar]
- Paré M, Behets C, Cornu O (2003) Paucity of presumptive ruffini corpuscles in the index finger pad of humans. J Comp Neurol 456, 260–266. [DOI] [PubMed] [Google Scholar]
- Paré M, Albrecht PJ, Noto CJ, et al. (2007) Differential hypertrophy and atrophy among all types of cutaneous innervation in the glabrous skin of the monkey hand during aging and naturally occurring type 2 diabetes. J Comp Neurol 501, 543–567. [DOI] [PubMed] [Google Scholar]
- Perez‐Pinera P, García‐Suarez O, Germanà A, et al. (2008) Characterization of sensory deficits in TrkB knockout mice. Neurosci Lett 433, 43–47. [DOI] [PubMed] [Google Scholar]
- Ranade SS, Woo SH, Dubin AE, et al. (2014) Piezo2 is the major transducer of mechanical forces for touch sensation in mice. Nature 516, 121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed‐Geaghan EG, Wright MC, See LA, et al. (2016) Merkel cell‐driven BDNF signaling specifies SAI neuron molecular and electrophysiological phenotypes. J Neurosci 36, 4362–4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittie L, Fisher GJ (2015) Natural and sun‐induced aging of human skin. Cold Spring Harb Perspect Med 5, a015370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roudaut Y, Lonigro A, Coste B, et al. (2012) Touch sense: functional organization and molecular determinants of mechanosensitive receptors. Channels 6, 234–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedy J, Szeder V, Walro JM, et al. (2004) Pacinian corpuscle development involves multiple Trk signaling pathways. Dev Dyn 231, 551–563. [DOI] [PubMed] [Google Scholar]
- Shaffer SW, Harrison AL (2007) Aging of the somatosensory system: a translational perspective. Phys Ther 87, 193–207. [DOI] [PubMed] [Google Scholar]
- Skedung L, El Rawadi C, Arvidsson M, et al. (2018) Mechanisms of tactile sensory deterioration amongst the elderly. Sci Rep 8, 5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark B, Risling M, Carlstedt T (2001) Distribution of the neurotrophin receptors p75 and trkB in peripheral mechanoreceptors; observations on changes after injury. Exp Brain Res 136, 101–107. [DOI] [PubMed] [Google Scholar]
- Toyoda M, Nakamura M, Nakada K, et al. (2005) Characteristic alterations of cutaneous neurogenic factors in photoaged skin. Br J Dermatol 153, 13–22. [DOI] [PubMed] [Google Scholar]
- Tseng MT, Chiang MC, Yazhuo K, et al. (2013) Effect of aging on the cerebral processing of thermal pain in the human brain. Pain 154, 2120–2129. [DOI] [PubMed] [Google Scholar]
- Ulfhake B, Bergman E, Fundin BT (2002) Impairment of peripheral sensory innervation in senescence. Auton Neurosci 96, 43–49. [DOI] [PubMed] [Google Scholar]
- Van Keymeulen A, Mascre G, Youseff KK, et al. (2009) Epidermal progenitors give rise to Merkel cells during embryonic development and adult homeostasis. J Cell Biol 187, 91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega JA, Calzada B, Del Valle ME (1993) Age‐induced changes in the mammalian autonomic and sensory ganglia In: Aging of the autonomic nervous system (ed. Amenta F.), pp. 37–67. Boca Raton, FL: CRC Press. [Google Scholar]
- Vega JA, García‐Suárez O, Montaño JA, et al. (2009) The Meissner and Pacinian sensory corpuscles revisited new data from the last decade. Microsc Res Tech 72, 299–309. [DOI] [PubMed] [Google Scholar]
- Vega JA, Lopez‐Muniz A, Calavia MG, et al. (2012) Clinical implication of Meissner's corpuscles. CNS Neurol Disord Drug Targets 11, 856–868. [DOI] [PubMed] [Google Scholar]
- Verendeev A, Thomas C, McFarlin SC, et al. (2015) Comparative analysis of Meissner's corpuscles in the fingertips of primates. J Anat 227, 72–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickremaratchi MM, Llewelyn JG (2006) Effects of ageing on touch. Postgrad Med J 82, 301–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G (1998) Clustering of slowly adapting type II mechanoreceptors in human peripheral nerve and skin. Brain 121, 265–279. [DOI] [PubMed] [Google Scholar]
- Wu M, Fannin J, Rice KM, et al. (2011) Effect of aging on cellular mechanotransduction. Ageing Res Rev 10, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Lewis AH, Grandl J (2017) Touch, tension, and transduction – the function and regulation of Piezo ion channels. Trends Biochem Sci 42, 57–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelená J (1994) Nerves and Mechanoreceptors. London: Chapman & Hall. [Google Scholar]
- Zimmerman A, Bai L, Ginty DD (2014) The gentle touch receptors of mammalian skin. Science 346, 950–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Double immunofluorescence for BDNF (a) and TrkB (d) (green fluorescence) and S100 protein (red fluorescence) in human digital Meissner's corpuscles of one 30‐year‐old subject. BDNF and S100 protein were colocalized, suggesting that BDNF was present in the lamellar cells. Conversely, no colocalization of TrkB and S100 protein was detected, indicating that TrkB is mainly located in axons. Objective 63 × /1.40 oil; pinhole 1.37; XY resolution 139.4 nm and Z resolution 235.8 nm. Scale bar: 30 μm.
Fig. S2. Double immunofluorescence for BDNF (green fluorescence) and NFP (b) and S100 protein (e) (red fluorescence) in human digital Meissner's corpuscles of one 30‐year‐old subject. BDNF and S100 protein were colocalized, suggesting that BDNF is present in inner core cells. Conversely, no colocalization of BDNF and NFP was detected, indicating that BDNF is not present in the axon. Objective 63 × /1.40 oil; pinhole 1.37; XY resolution 139.4 nm and Z resolution 235.8 nm. ic, inner core; arrow indicates the axon. Scale bar: 50 μm.
Fig. S3. Double immunofluorescence for BDNF (a) and TrkB (d) (green fluorescence) and CK20 (red fluorescence) in human digital Merkel cells (arrows). BDNF and CK20 never colocalized, whereas TrkB and CK20 colocalized in the cytoplasm of Merkel cells. Objective 63 × /1.40 oil; pinhole 1.37; XY resolution 139.4 nm and Z resolution 235.8 nm. ic: inner core; arrow indicates the axon. Scale bar: 30 μm.


