Abstract
Natural biopesticide development for invasive populations of red palm weevils is mainly responsible for the destruction of date palms and demands an extensive screening program of plant secondary metabolites. In the current study, the pesticidal potential of sesquiterpenes (C15 H24), an important class of plant secondary metabolites primarily composed of three isoprene units, was evaluated by laboratory toxicity, feeding performance bioassays, and host detoxification gene expression patterns. Dose-mortality response bioassays performed against mid-aged eighth-instar red palm weevil larvae revealed dose-dependent mortality. Only three sesquiterpenes, including Farnesol (LD50 = 6559 ppm) and Farnesyl acetate (LD50 = 7867 ppm), are considered to have significant toxicity, with Picrotoxin (LD50 = 317 ppm) being the most toxic. Furthermore, highly toxic sesquiterpene (Picrotoxin) established in the current study tremendously reduced the feeding performance indices, including the efficacy of conversion of digested food (ECD) (81.74%) and the efficacy of conversion of ingested food (ECI) (73.62%). The least toxic sesquiterpenes, including β-Caryophyllene, (+)-Cedrol, Nerolidol, (+)-Nootkatone, and Parthenolide, observed in the current study failed to impart significant reductions of ECI and ECD indices. Lethality of the least toxic sesquiterpenes was overcome by greatly inducing gene expressions of Glutathione S transferase (GST) and Cytochrome P450. These encouraging results enabled us to suggest Picrotoxin as a promising biopesticide for the control of red palm weevil infestations.
Keywords: sesquiterpenes, plant secondary metabolites, Rhynchophorus ferrugineus, biopesticides, natural products, host defense, detoxification mechanism
1. Introduction
Red palm weevil, Rhynchophorus ferrugineus (Olivier) (Coleoptera, Curculionidae), is considered the most devastating invasive pest and is mainly responsible for the destruction of date palms in the Arabian Peninsula. The cryptic lifestyle of red palm weevil grubs in the trunk exhibits serious management challenges. Red palm weevil control mainly depends on the intensive use of synthetic pesticides as a soil treatment, tree fumigation, trunk injection, wound dressing, and crown drenching of infested palms [1]. However, the indiscriminate use of synthetic pesticides is mainly responsible for pesticide residues in dates [2], environmental pollution [3], resistance development [4], and detrimental effects on wildlife [5]. Therefore, the use of pesticides is discouraged, and the concept of eco-friendly natural pesticide development is gaining attention and popularity.
Plant secondary metabolites are an untapped reservoir of chemicals with numerous potential uses [6,7]. They have expressed deleterious effects, including (1) reduced fecundity, (2) molt-inhibitory, (3) toxic, (4) respiratory inhibition, (5) growth retarding, (6) oviposition deterrence, (7) suppression of calling behavior, and (8) repellency against target pest species [8,9,10,11]. The incorporation of plant secondary metabolites into the Integrated Pest Management strategy is advantageous due to their broad spectrum of activity [12], and the fact that they pose the least risk to the environment [7] and biodegradability [12]. In addition, sesquiterpenes trigger a host defense mechanism to reduce pest damage [13].
Using plant secondary metabolites in controlling red palm weevils was neglected in the past. Therefore, little data are available on this aspect. A great deal of focus remained on the insecticidal potential of crude plant extracts against red palm weevils [14,15,16,17]. Little focus remained on the insecticidal potential of plant secondary metabolites, including rotenone, limonine, coumarin, and piperine, against red palm weevils [18,19,20]. A recent study screened the insecticidal potential of a wide range of acyclic monoterpenes against red palm weevil larvae [20]. Their results revealed insecticidal and growth inhibitory potential of Coumarin against red palm weevil larvae [20,21]. Insecticidal potential of sesquiterpenes (an important class of terpenes comprising three isoprenes) is well known against other pest species, including Aedes aegypti, Coptotermes formosanus, Dermacentor variabilis, Musca domestica, Nilaparvata lugens, Pediculus capitis, Periplaneta americana, Sitophilus zeamais, S. oryzae, Stephanitis pyrioides, and Tribolium castaneum [22,23,24,25,26,27,28]. Surprisingly, there has not been a single study on the insecticidal potential of sesquiterpenes against red palm weevils. The current study was designed to explore, for the first time, the pesticidal potential of red palm weevil larvae by (1) screening the most potent sesquiterpene among β-Caryophyllene, (+)-Cedrol, Farnesol, Farnesyl acetate, Nerolidol, (+)-Nootkatone, Parthenolide, and Picrotoxin by dose-mortality response laboratory bioassays; (2) evaluating the impacts of selected sesquiterpenes on the growth and development of red palm weevil larvae by dietary feeding performance bioassays; (3) exploring the impact of the red palm weevil detoxification mechanism against sesquiterpenes by quantifying detoxification-related genes including Glutathione S transferase (GST), Cytochrome P450, and Esterase by quantitative real-time PCR (qRT-PCR) in order to pave the way for safer natural biopesticides development against red palm weevil infestations.
2. Results
2.1. Toxicity of Sesquiterpenes against Red Palm Weevil Larvae
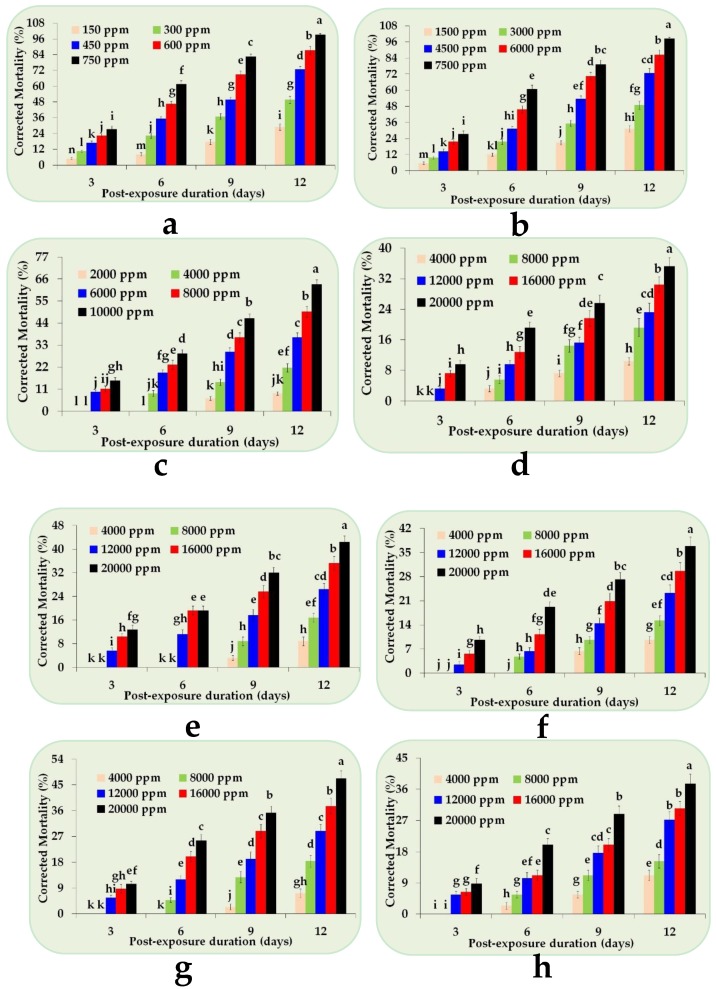
The insecticidal potential of all tested sesquiterpenes against red palm weevil larvae, evaluated here by diet-incorporated dose-mortality response bioassays, exhibited dose-dependent mortality response, as shown in Figure 1a–h. In the current study, Picrotoxin (Figure 1a) was found to be the most toxic compound against red palm weevil larvae resulting in the least LD50 value of 317 ppm (Table 1). Feeding the eighth-instar red palm weevil larvae a diet incorporated with different doses of Picrotoxin imparted significant mortality at all studied time intervals (F = 126.05; df = 3, 64; p < 0.0001) with different doses (F = 1730.14; df = 4, 64; p < 0.0001) and their interaction (F = 20.52; df = 12, 64; p < 0.0001).
Figure 1.
Corrected cumulative (%) dose mortality response of red palm weevil larvae against (a) Picrotoxin; (b) Farnesol; (c) Farnesyl acetate; (d) β-Caryophyllene; (e) (+)-Cedrol; (f) Nerolidol; (g) (+)-Nootkatone; and (h) Parthenolide. Each bar (mean ± SE) is the mean of five replicates. The bars followed by different letter(s) are significantly different (Fisher’s Least Significant Difference test, α = 0.05).
Table 1.
Susceptibility of red palm weevils to different sesquiterpenes.
| Treatment | LD50 (95% CI) (ppm) | χ2 | Slope ± Standard Error |
|---|---|---|---|
| Farnesyl acetate | 7867 (6911–8956) | 1.18 | 2.45 ± 0.29 |
| Farnesol | 6559 (5543–7761) | 4.09 | 2.07 ± 0.28 |
| Picrotoxin | 317 (538–709) | 1.05 | 2.39 ± 0.29 |
| β-Caryophyllene * | n/a | n/a | n/a |
| (+)-Cedrol * | n/a | n/a | n/a |
| Nerolidol * | n/a | n/a | n/a |
| (+)-Nootkatone * | n/a | n/a | n/a |
| Parthenolide * | n/a | n/a | n/a |
* Failed to calculate LD50 values due to <50% mortality.
Similarly, Farnesyl acetate also revealed significant differences in mortality at all the tested doses (F = 1262.73; df = 4, 64; p < 0.0001) at different time intervals (F = 95.39; df = 3, 64; p < 0.0001) and their interaction (F = 15.99; df = 12, 64; p < 0.0001), as shown in Figure 1c. In contrast, LD50 values for the compounds such as β-Caryophyllene, (+)-Cedrol, Nerolidol, (+)-Nootkatone, and Parthenolide could not be calculated, because these sesquiterpenes failed to impart 50% mortality even at the highest dose (20000 ppm) during the course of experimentation (Table 1). Despite this, the interaction of the doses of these least potent sesquiterpenes at different time intervals revealed significant differences in mortality for β-Caryophyllene (F = 5.24; df = 12, 64; p < 0.0001), (+)-Cedrol (F = 14.02; df = 12, 64; p < 0.0001), Nerolidol (F = 5.45; df = 12, 64; p < 0.0001), (+)-Nootkatone (F = 11.99; df = 12, 64; p < 0.0001), and Parthenolide (F = 4.12; df = 12, 64; p < 0.0001), as shown in Figure 1d–h.
2.2. Growth Retarding Activities of Sesquiterpens
Red palm weevil eighth-instar larvae fed on a diet incorporated with sesquiterpenes revealed significant variations in their feeding indices, including efficacy of conversion of ingested food (ECI), efficacy of conversion of digested food (ECD), and approximate digestibility (AD). We recorded the highest reduction in ECD (81.74%) from red palm weevil larvae fed on a diet incorporated with the most toxic, Picrotoxin. Furthermore, we recorded significant variations among all treatments (F = 118; df = 8, 36; p < 0.0001). However, the lowest reduction (<11%) was observed from the diets incorporated with β-Caryophyllene (6.95%), (+)-Cedrol (10.32%), Nerolidol (7.29%), and Parthenolide (4.53%), as shown in Table 2.
Table 2.
Impact of different sesquiterpenes on the growth indices of red palm weevil larvae.
| Treatments | Approximate Digestibility (AD) | ECI | ECD |
|---|---|---|---|
| Picrotoxin | 74.49 ± 0.26a | 5.07 ± 0.34e | 6.81 ± 0.47e |
| Farnesol | 62.70 ± 0.39b | 14.09 ± 0.28d | 22.49 ± 0.57d |
| Farnesyl acetate | 60.70 ± 0.26c | 15.79 ± 0.51 | 26.01 ± 0.85c |
| β-Caryophyllene | 53.85 ± 0.28de | 18.68 ± 0.15ab | 34.70 ± 0.46b |
| (+)-Cedrol | 55.13 ± 0.75d | 18.40 ± 0.48ab | 33.44 ± 1.32b |
| Nerolidol | 53.85 ± 0.28de | 18.61 ± 0.15ab | 34.57 ± 0.46b |
| (+)-Nootkatone | 63.89 ± 0.50b | 17.83 ± 0.54b | 27.95 ± 1.06c |
| Parthenolide | 52.82 ± 0.88ef | 18.75 ± 0.48ab | 35.60 ± 1.50ab |
| Control | 51.55 ± 0.23f | 19.22 ± 0.16a | 37.29 ± 0.48a |
ECI stands for efficacy of conversion of ingested food; ECD stands for efficacy of conversion of digested food.
Picrotoxin, established in the current study as the most potent compound, tremendously reduced the ECI (73.62%) of red palm weevil larvae (Table 2). The sesquiterpenes including β-Caryophyllene (2.81%), (+)-Cedrol (4.27%), Nerolidol (3.17%), (+)-Nootkatone (7.23%), and Parthenolide (2.45%) failed to impart tremendous reduction of ECI compared to the control treatment diet (Table 2). Overall, all the treatments showed significant variations (F = 144; df = 8, 36; p < 0.0001). Furthermore, the ECI index of β-Caryophyllene, (+)-Cedrol, Nerolidol, and Parthenolide remained statistically at the same level of significance (Table 2). Overall, we recorded the directly proportional relationship of toxicity of the sesquiterpenes with growth indices (ECI and ECD). Potent sesquiterpenes revealed the highest reduction of ECI and ECD.
To the contrary, we recorded an inversely proportional relationship between AD and toxicity (Table 2). Picrotoxin, the most potent reported here, significantly enhanced (30.80%) red palm weevil larval AD compared with the control. In addition, AD significantly increased upon the exposure to different sesquiterpenes (F= 238; df = 8, 36; p < 0.0001).
2.3. Regulation of Detoxification Genes of Red Palm Weevils in Response to Sesquiterpenes
2.3.1. Cytochrome P450 Gene Expression of Red Palm Weevil Larvae
Toxicity of tested sesquiterpenes induced different levels of the Cytochrome P450 gene (Table 3). The quantitative expression of Cytochrome P450 revealed significant differences (F = 197; df = 7, 32; p < 0.0001). Larvae fed on a diet incorporated with Picrotoxin and Farnesol tremendously reduced the expression of tested detoxification genes such as Cytochrome P450. Among all the sesquiterpenes, the least toxic Parthenolide greatly enhanced P450 expression of red palm weevil larvae and remained significant at the highest level. However, β-Caryophyllene and (+)-Cedrol also greatly enhanced the expression of P450 genes and remained significant at the same level of significance (Table 3).
Table 3.
Relative fold change in the expression patterns of Rhynchophorus ferruginous detoxification genes in the mid-gut of eighth-instar larvae using quantitative real-time PCR (qRT-PCR).
| Treatments | Cytochrome P450 | GST | Esterase |
|---|---|---|---|
| Picrotoxin | 2.01 ± 0.12g | 1.97 ± 0.11g | 1.09 ± 0.06d |
| Farnesol | 3.96 ± 0.34f | 4.63 ± 0.41f | 1.16 ± 0.07d |
| Farnesyl acetate | 5.58 ± 0.71e | 7.62 ± 0.69e | 1.26 ± 0.08cd |
| β-Caryophyllene | 15.71 ± 0.38c | 18.73 ± 0.36c | 2.54 ± 0.17a |
| (+)-Cedrol | 16.26 ± 0.46c | 19.20 ± 0.38c | 2.37 ± 0.08ab |
| Nerolidol | 17.87 ± 0.47b | 21.96 ± 0.55b | 2.18 ± 0.06b |
| (+)-Nootkatone | 10.53 ± 0.65d | 15.78 ± 0.87d | 1.51 ± 0.05c |
| Parthenolide | 21.41 ± 0.69a | 25.25 ± 0.49a | 2.20 ± 0.07b |
GST stands for Glutathione S transferase.
2.3.2. Glutathione S-Transferase Gene Expression of Red Palm Weevil Larvae
Artificial diet incorporated with different sesquiterpenes induced different levels of GST expressions, resulting in significant differences (F = 266; df = 7, 32; p < 0.0001) in their quantitative expressions. The least toxic sesquiterpenes established in the current study against red palm weevil larvae, such as β-Caryophyllene, (+)-Cedrol, Nerolidol, (+)-Nootkatone, and Parthenolide, showed higher expressions of GST (>15%) compared with potent sesquiterpenes. Among all the treatments, Picrotoxin failed to induce GST expression of red palm weevil larvae and remained significant at the lowest level (Table 3).
2.3.3. Esterase Gene Expression of Red Palm Weevil Larvae
Quantitative expression of the detoxification gene, Esterase, also showed significant differences in the expression (F = 45; df = 7, 32; p < 0.0001) from the mid-gut of red palm weevil larvae. Overall, all the sesquiterpenes failed to induce higher expressions of Esterase among red palm weevil larvae (Table 3).
3. Discussion
Development of insecticide resistance demands the exploration of newer, plant-based, eco-friendly biopesticides for controlling the infestations of red palm weevils. Our drafted screening program to find the most toxic sesquiterpenes reported here—for the first time—the toxicity of Picrotoxin against red palm weevils. The most potent sesquiterpene, Picrotoxin, established in the current study tremendously disturbed the larval growth and evaded the host detoxification mechanism. The red palm weevil detoxification mechanism displayed a massive and rapid reprogramming of gene expressions in response to the least toxic sesquiterpenes for their survival.
Higher larval mortality of red palm weevils was recorded from the artificial diet incorporated with Picrotoxin. The exposed red palm weevil larvae failed to tolerate Picrotoxin. Furthermore, Picrotoxin-fed larvae became sluggish, which ultimately led toward the death of > 96% of the larvae during the course of experimentation. Picrotoxin, a known natural plant toxin isolated from flowing plants (Menispermaceae), is the representative of non-competitive antagonists (NCAs) [29]. In addition to use as ectoparasiticides and insecticides, they also play very important roles as molecular tools in the studies of γ-aminobutyrate (GABA) receptors at physiological and biochemical levels [30]. Therefore, Picrotoxin as GABAergic receptors, which act by modulating and blocking chlorine channels [31,32], are listed as antidotes against barbiturate poisoning [33], convulsants [34], memory deficiency in animals [32], and they can speed the mammalian circadian clock [35]. In insects, Picrotoxin tested against many insect species showed various activities, such as acting as a pesticide [36,37], acting as a modulator [38], abolishing the oscillatory synchronization [39], and causing a loss in the ability to perceive odor mixtures of configural stimulants [40]. In the past, another neurotoxic toxic compound, Fipronil, with the antagonism of GABA as the mechanism of toxicity was tested against red palm weevils as quarantine treatment [41], Endotherapic injection [42], and laboratory toxicity bioassays [43]. However, Picrotoxin (LD50 of 317 ppm) in the current study was found to be the most potent compound against red palm weevil larvae compared with other neurotoxins, including Fipronil, which showed 773.78 ppm of red palm weevil larval LC50 [43]. The toxicity of this compound coincides with AlJabr et al., who reported the toxicity (LD50 of 0.672 g/L) of the plant secondary metabolite, Coumarin, against red palm weevil larvae [20]. In the past, the toxicity of Picrotoxin against red palm weevils had never been tested. This is the first study evaluating and reporting the toxicity of Picrotoxin against red palm weevil larvae. These findings enable us to suggest Picrotoxin as a great alternate option for red palm weevil management.
In the current study, similarly high ECI (73.62%) and ECD (81.74%) reductions were calculated among larvae fed on a diet incorporated with Picrotoxin. The reduced indices of ECI and ECD in response to the most potent Picrotoxin might have been because of the shortfall in food reserves, which might have appeared because of (1) the intake of less food due to toxicity, and (2) the utilization of most of the energy for the host defense in place of the host growth and development. The previous research findings also claimed reductions in ECI and ECD in response to toxins, suspensions, and plant extracts. In this regard, Hussain et al. [44] reported tremendous reduction in ECI and ECD indices of Ocinara varians Walker larvae against suspensions of entomopathogenic fungi. The findings of Koul et al. [45] reported the biological activity of limonoids against Helicoverpa armigera (Hubner) and Spodoptera litura (Fabricius). Their results showed larval growth inhibition in response to three tested limonoids. However, they suggested that growth inhibition might have been due to feeding deterrence. Our results are in line with Silva et al. [46], who reported similar growth patterns of Anagasta kuehniella (Zeller) larvae fed on an artificial diet incorporated with the extract of Croton urucurana. The findings of [21] also reported a tremendous reduction in ECI and ECD indices of red palm weevil larvae fed on a diet incorporated with a plant secondary metabolite. They suggested that the reduced growth in the form of ECI and ECD indices might have been due to the utilization of most of the energy reserves to combat the host defense instead of being used for growth and development. Such energy-deficient larvae after slow growth ultimately led to death [20,21]. Another study conducted on the toxicity of insecticides against red palm weevil larvae also revealed similar growth patterns from the most toxic insecticides [4]. In addition to Picrotoxin, Farnesol (LD50 of 6559 ppm) also showed favorable results in terms of toxicity. Similar to Picrotoxin, Farnesol also tremendously reduced ECD (39.69%) and ECI (26.91%) indices of red palm weevil larvae. Such reductions in ECI and ECD indices are in agreement with the previous study on the susceptibility exploration of red palm weevils towards fungal infections [47]. They reported the highest reductions in ECI and ECD indices upon infections with the most pathogenic isolates of entomopathogenic fungi. Their findings suggested that pathogen virulence regulated the growth and defense mechanism of red palm weevils. Furthermore, they suggested that the least virulent isolate of Isaria fumosorosea 03011 failed to impart significant reductions, as depicted in the current study in the case of the least toxic sesquiterpenes, including β-Caryophyllene, (+)-Cedrol, Nerolidol, and Parthenolide. Similarly, conidial suspension of Beauveria Bassiana isolate B8465 also revealed low inhibition of ECI and ECD indices of red palm weevil different instar (fourth, eighth, and twelfth) larvae [48]. They suggested that entomopathogenic fungal isolate lacking a right set of virulence traits could not impart pathogenesis in the target host, resulting in the failure to impart growth reductions. Our results are also in line with AlJabr et al. [20], who reported the similar negligible response of plant secondary metabolites such as methyl isoeugenol and methyl eugenol on the growth indices of red palm weevil larvae. Based on these findings, we might suggest that a reduction in feeding performance indices (ECI and ECD) is directly proportional to the toxicity of sesquiterpenes. Such a situation might arise because of the shortfall of energy reserves. These energy-deficient larvae utilize most of their energy reserves in physiological activities. Under such circumstances, exposed larvae grow very slowly, which ultimately leads towards death.
AD is also an important feeding performance index recorded in the current study. Feeding performance bioassays revealed a 30.80% increase in the AD of Picrotoxin compared with the control treatment. A similar increase in AD value is in line with the findings of AlJar et al. [20], who reported that the toxic, Coumarin, significantly enhanced the digestibility of energy-deficient larvae of red palm weevils in order to meet their energy demands. Enhanced AD response has also been reported among red palm weevil larvae to fungal suspensions [48], plant secondary metabolites [21,49], and plant extracts [18], as well as Ocinara varians Walker larvae infected with conidial suspensions of entomopathogenic fungi [44,50]. A similarly enhanced response of AD in response to labramin among A. kuehniella was reported by Martinez et al. [51]. On the other hand, the least toxic compound, Parthenolide, revealed in the current study that it remained statistically near the control treatment. In the past, a similar response of AD from non-toxic treatments was highlighted by AlJabr et al. [20]. They reported Methyl eugenol as the least toxic compound that failed to regulate abnormal growth of red palm weevil larvae. In contrast, a potent treatment, especially Coumarin, greatly affected growth, resulting in a significant increase in the AD of exposed larvae, which is in agreement with the current study in the case of Picrotoxin. Our results are also in line with the previous study on the insecticidal and growth retarding potential of black pepper extracts and their constituent, Piperine, against red palm weevil larvae [18]. Results revealed the highest AD index from the most potent Piperine-fed larvae. Such a tremendous increase in AD reported here and claimed before is mainly because of the fact that exposed larvae demand extra energy to regulate the host defense mechanism. In order to meet emerging energy demands, exposed larvae utilized their intrinsic capabilities, which ultimately enhanced the AD of their limited foodstuff.
Toxicity of the compounds expedites the regulation of detoxification-related genes by increasing the host metabolism. In this regard, host defense-related genes, especially Glutathione S Transferase, Cytochrome P 450, and Esterase, are known for their importance in host detoxification mechanisms. Previous studies revealed the increase in GST expression in resistant red palm weevil populations provided with a cypermethrin-incorporated artificial diet [4]. In the current study, we quantified the expression patterns of GST, Cytochrome P450, and Esterase. Results revealed tremendous expression of all tested detoxification-related genes in response to the least potent compounds, including β-Caryophyllene, (+)-Cedrol, Nerolidol, (+)-Nootkatone, and Parthenolide. Among all the tested detoxification genes, GST showed the highest response against the least toxic compounds. The enhanced GST response of red palm weevils fed on a diet incorporated with less toxic compounds corroborates a recently published study on the modulation of the host detoxification system in response to an important plant secondary metabolite [20]. Our results are also in line with the previous enhanced response of toxin-pathogen application against date palm dust mites [49]. Furthermore, their study suggested that host detoxification genes such as GST, Cytochrome P450, and Esterase detoxify the toxin by increasing their solubility, which leads towards the toxin degradation and removal from the host body. The enhanced detoxification genes expression patterns greatly reduced the lethality of the tested compound. These findings enable us to suggest that Picrotoxin, the most potent compound established in the current study, disguised the expressions of detoxification genes, which ultimately imparted the least LD50 value (317 ppm). However, expression of Esterase, which we found very little of in the current study, does not mean that they are not engaged in the detoxification mechanism. We might suggest that low expression of the Esterase gene might have been due to low specificity with the tested sesquiterpenes. These encouraging results pave the way towards the development of eco-friendly red palm weevil management option.
4. Materials and Methods
4.1. Insects Rearing
The population of red palm weevil was obtained from an already developed culture maintained under controlled conditions in the growth chamber at 30 ± 1ºC, 75% ± 5% relative humidity (RH) at the Laboratory of Bio-control and Molecular Biology, King Faisal University, Saudi Arabia. Third-instar red palm weevil larvae were reared on an artificial diet standardized in our previous study [48].
4.2. Sesquiterpenes
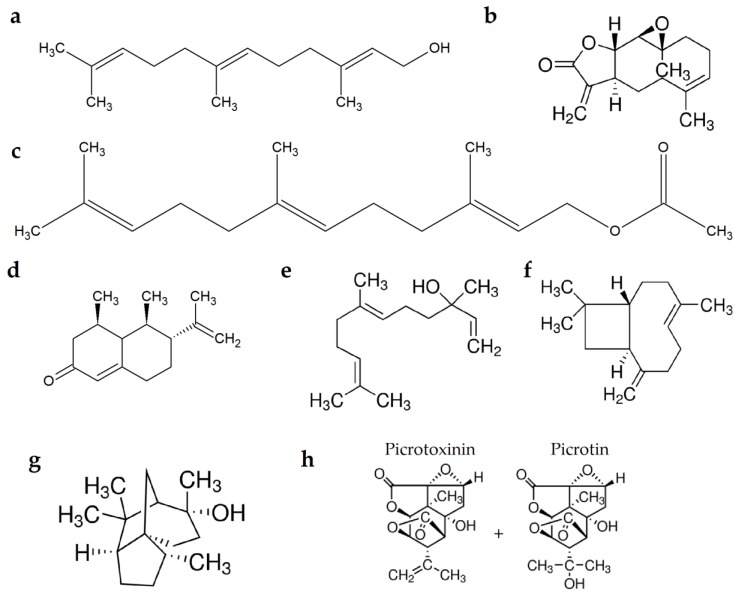
Eight sesquiterpenes including β-Caryophyllene (Cat # W225207), (+)-Cedrol (Cat # W521418-1KG-K), Farnesol (Cat # F203-100G), Farnesyl acetate (Cat # 45895-10ML-F), Nerolidol (Cat # W277207-1KG-K), (+)-Nootkatone (Cat # 74437-2.5G), Parthenolide (Cat # P0667-25MG), and Picrotoxin (Cat # P1675-5G), as shown in Figure 2, were purchased from Sigma Aldrich, UK.
Figure 2.
Structures of studied sesquiterpenes, (a) Farnesol, (b) Parthenolide, (c) Farnesyl acetate, (d) (+)-Nootkatone, (e) Nerolidol, (f) β-Caryophyllene, (g) (+)-Cedrol, and (h) Picrotoxin.
4.3. Laboratory Toxicity Testing of Sesquiterpenes against Red Palm Weevil Larvae
The sesquiterpenes selected for the current study were never previously tested against red palm weevils. Therefore, preliminary range-finding dose-mortality bioassays were conducted to finalize the doses for all tested sesquiterpenes. The finalized five doses of each tested sesquiterpene including β-Caryophyllene, (+)-Cedrol; Farnesol, Farnesyl acetate; Nerolidol, (+)-Nootkatone; Parthenolide, and Picrotoxin were prepared by dissolving each tested sesquiterpene in 0.05% of the appropriate solvent. All the components of the artificial diet disclosed in our previous studies were mixed in 500 mL of each tested sesquiterpene at the required dose [18,20]. The control treatment diets were prepared using the respective solvent at a dose of 0.05%. Twenty-five mid-aged (eighth-instar) red palm weevil larvae were singly-fed on the treated artificial diet for 12 days in perforated plastic bowls. Overall, five replicates, each from a different generation, were prepared. Bioassays were repeated over time. Each experimental unit was kept in the growth chamber at 30 ± 1 °C, 75% ± 5% RH. Mortality data were recorded after 24 h feeding on the diets. Control mortality data were subtracted from the treatment mortality data using Abbott’s formula [52].
where n stands for “insect population”; T stands for “treated”; and Co stands for “control”.
Cumulative corrected larval mortality data were angularly transformed for data homogenization. Homogenized cumulative corrected mortality data were analyzed by repeated measures ANOVA at different time intervals with Fisher’s LSD test [53]. Probit analysis was performed to determine the lethal dose (LD50) of sesquiterpenes showing 50% red palm weevil larval mortality.
4.4. Larval Growth and Development Retarding Abilities of Tested Sesquiterpenes
Eighth-instar red palm weevils were used to evaluate the impact of sesquiterpenes on the larval feeding performance. The larvae were provided with an artificial diet incorporated with either sesquiterpenes β-Caryophyllene, (+)-Cedrol, Farnesol, Farnesyl acetate, Nerolidol, (+)-Nootkatone, and Parthenolide at a single dose of 317 ppm. The dose 317 ppm was the LD50 of the most potent sesquiterpene (Picrotoxin). A measured amount of the artificial diet incorporated with each tested sesquiterpene was separately provided in perforated plastic bowls. Each treatment comprised 25 singly-fed eighth-instar red palm weevil larvae. Five replicates were prepared similarly using different generations of red palm weevils. Each experimental unit was kept for 72 h at 30 ± 1 °C, 75% ± 5% RH. After 72 h post feeding, ECD, ECI, and AD indices were calculated on the basis of dry frass weight, food consumed, and weight gained basis, as described in previous studies [47,48,50]. Variations in growth indices as a result of feeding on the diet incorporated with different sesquiterpenes were calculated by one-way ANOVA and Fisher’s LSD test using Statistical Analysis Software (SAS) [54].
4.5. Physiological Impacts of Sesquiterpenes on the Host Detoxification Defense Mechanism
The mid-gut portion of the alimentary canal of eighth-instar red palm weevil larvae was used to determine the expression patterns of detoxification genes, including GST, Esterase, and Cytochrome P450. Each treatment (sesquiterpenes) at a single dose of 317 ppm was incorporated in the artificial diet to feed eighth-instar red palm weevil larvae for 72 h in the incubator under controlled conditions. Five replicates were prepared by five different larval gut samples. After dissection in saline, the mid-gut portion of each larva was ground in liquid nitrogen for total RNA extraction using RNeasy® Universal Mini Kit (Qiagen Cat No 73404) following manufacturer recommendations. Each extracted total RNA was used to synthesize first strand cDNA using a commercially available kit (Clontech Cat # 6110A). The synthesized first strand cDNA product of each reaction was stored at −20 °C. The primers for GST (F 5′-ATAGCCAACCACCACTGTCG-3′ and R 5′-CGTTCCTCTTGCCGCTAGTT-3′) with accession number KR902496; Esterase (F 5′-ACCTACAAGAATCCGACGCC-3′ and R 5′-ACTCCGAAACTTTGGGCCAT-3′) with accession number KT748822; Cytochrome P450 (F 5′-TGGAGAAACACCCGCAAGAA-3′ and R 5′-CGGCGATTTTGCCTACCAAG-3′) with accession number KT748789; and β-Actin (F 5′-AAAGGTTCCGTTGCCCTGAA-3′ and R 5′-TGGCGTACAAGTCCTTCCTG-3′) with accession number KM438516, designed using the online tool on NCBI, were purchased from Macrogen, Korea (http://www.macrogen.com/en/company/summary.php). β-Actin was used as an internal control. Differential expressions of GST, Esterase, and Cytochrome P450 were determined using SYBR® Premix Ex Taq™ II kit (TaKaRa Clontech, France) in CFX96 TouchTM (Bio-Rad, UK) according to the manufacturer’s protocol. Numerical values obtained from each experimental unit were compared with those of the control by relative fold expression obtained by transforming the obtained results into absolute values using 2−ΔΔCt [55]. The relative expression of each gene was set to 1 for the uninfected (control) treatment. SAS Institute (2000) was used to determine variations among treatments by ANOVA and Fisher’s LSD test.
5. Conclusions
In conclusion, our results demonstrated Picrotoxin as the most potent sesquiterpene against red palm weevils. An artificial diet incorporated with Picrotoxin was proven to inhibit the growth activities and to reprogram the detoxification defense mechanism of red palm weevils. Picrotoxin, a natural plant neurotoxin, is an important addition in the list of red palm weevil controlling products. Future research should focus on various aspects including formulation, safety, and application in order to optimize the efficacy of Picrotoxin for managing red palm weevil infestations.
Acknowledgments
We would like to thank Master students for their assistance.
Author Contributions
A.H., and A.M.A. conceived the idea. A.H., A.M.A., H.A. and M.R.-u.-h. designed the study. A.H. and M.R.-u.-h. performed the experiments. A.H., A.M.A., H.A. and M.R.-u.-h. analyzed the data. A.M.A. provided the resources. A.H. wrote the original draft. A.M.A., H.A. and M.R.-u.-h. reviewed and edited the manuscript. A.H., A.M.A., M.R.-u.-h. and H.A. approved the manuscript for publication.
Funding
This research was funded by King Abdul Aziz City for Science and Technology (KACST); grant number MT-37-234.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Hussain A., Rizwan-ul-Haq M., Al-Jabr A.M., Al-Ayied H.Y. Managing invasive populations of red palm weevil: A worldwide perspective. J. Food Agric. Environ. 2013;11:456–463. [Google Scholar]
- 2.Abdallah O.I., Alamer S.S., Alrasheed A.M. Monitoring pesticide residues in dates marketed in Al-Qassim, Saudi Arabia using a QuEChERS methodology and Liquid Chromatography-Tandem Mass Spectrometry. Biomed. Chromatogr. 2018;32:e4199. doi: 10.1002/bmc.4199. [DOI] [PubMed] [Google Scholar]
- 3.Mahmood I., Imadi S.R., Shazadi K., Gul A., Hakeem K.R. Plant, Soil and Microbes. Springer International Publishing; Cham, Switzerland: 2016. Effects of Pesticides on Environment; pp. 253–269. [Google Scholar]
- 4.Al-Ayedh H., Hussain A., Rizwan-ul-Haq M., Al-Jabr A.M. Status of insecticide resistance in field-collected populations of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) Int. J. Agric. Biol. 2016;18:103–110. doi: 10.17957/IJAB/15.0070. [DOI] [Google Scholar]
- 5.Kohler H.-R., Triebskorn R. Wildlife ecotoxicology of pesticides: can we track effects to the population level and beyond? Science. 2013;341:759–765. doi: 10.1126/science.1237591. [DOI] [PubMed] [Google Scholar]
- 6.Balandrin M., Klocke J., Wurtele E., Bollinger W. Natural plant chemicals: sources of industrial and medicinal materials. Science. 1985;228:1154–1160. doi: 10.1126/science.3890182. [DOI] [PubMed] [Google Scholar]
- 7.Yang L., Wen K., Ruan X., Zhao Y., Wei F., Wang Q. Response of plant secondary metabolites to environmental factors. Molecules. 2018;23:762. doi: 10.3390/molecules23040762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khan Z.R., Saxena R.C. Effect of steam distillate extracts of resistant and susceptible rice cultivars on behavior of Sogatella furcifera (Homoptera: Delphacidae) J. Econ. Entomol. 1986;79:928–935. doi: 10.1093/jee/79.4.928. [DOI] [Google Scholar]
- 9.Zhao B., Grant G., Langevin D., MacDonald L. Deterring and inhibiting effects of quinolizidine alkaloids on spruce budworm (Lepidoptera: Tortricidae) oviposition. Environ. Entomol. 1998;27:984–992. doi: 10.1093/ee/27.4.984. [DOI] [Google Scholar]
- 10.Isman M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006;51:45–66. doi: 10.1146/annurev.ento.51.110104.151146. [DOI] [PubMed] [Google Scholar]
- 11.Ebadollahi A., Jalali Sendi J. A review on recent research results on bio-effects of plant essential oils against major Coleopteran insect pests. Toxin Rev. 2015;34:76–91. doi: 10.3109/15569543.2015.1023956. [DOI] [Google Scholar]
- 12.Rattan R.S. Mechanism of action of insecticidal secondary metabolites of plant origin. Crop Prot. 2010;29:913–920. doi: 10.1016/j.cropro.2010.05.008. [DOI] [Google Scholar]
- 13.Iason G. The role of plant secondary metabolites in mammalian herbivory: Ecological perspectives. Proc. Nutr. Soc. 2005;64:123–131. doi: 10.1079/PNS2004415. [DOI] [PubMed] [Google Scholar]
- 14.Nassar M., Abdulah A. Assessment of Boxus chinensis oil and precocene II for the control of the red palm weevil, Rhychophorus ferrugineus (Oliv.) (Coleoptera-Curculionidae) and the palm beetle, Pseudophilus testaceous. J. Entomol. 2005;2:1–8. [Google Scholar]
- 15.Salama H., Ismail I. Potential of certain natural extracts for the control of the red palm weevil, Rhynchophorus ferrugineus (Oliver) Arch. Phytopathol. Plant Prot. 2007;40:233–236. doi: 10.1080/03235400500383669. [DOI] [Google Scholar]
- 16.El-Bokl M.M., Baker R.F.A., El-Gammal H.L., Mahmoud M.Z. Biological and histopathological effects of some insecticidal agents against red palm weevil Rhynchophorus ferrugineus. Egypt. Acad. J. Biol. Sci. 2010;1:7–22. doi: 10.21608/eajbsd.2010.14151. [DOI] [Google Scholar]
- 17.Shukla P., Vidyasagar P.S.P.V., Aldosari S.A., Abdel-Azim M. Antifeedant activity of three essential oils against the red palm weevil, Rhynchophorus ferrugineus. Bull. Insectology. 2012;65:71–76. [Google Scholar]
- 18.Hussain A., Rizwan-ul-Haq M., Al-Ayedh H., Aljabr A.M. Toxicity and detoxification mechanism of black pepper and its major constituent in controlling Rhynchophorus ferrugineus Olivier (Curculionidae: Coleoptera) Neotrop. Entomol. 2017;46:685–693. doi: 10.1007/s13744-017-0501-7. [DOI] [PubMed] [Google Scholar]
- 19.Abdullah M.A.R. Identification of the biological active compounds of two natural extracts for the control of the red palm weevil, Rhynchophorus ferrugineus (Oliver) (Coleoptera: Curculionidae) Egypt Acad. J. Biol. Sci. 2009;2:35–44. doi: 10.21608/eajbsa.2009.15427. [DOI] [Google Scholar]
- 20.AlJabr A.M., Hussain A., Rizwan-ul-Haq M., Al-Ayedh H. Toxicity of plant secondary metabolites modulating detoxification genes expression for natural red palm weevil pesticide development. Molecules. 2017;22:169. doi: 10.3390/molecules22010169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.AlJabr A.M., Hussain A., Rizwan-ul-haq M., Al-Ayedh H. Method for Controlling Growth of Red Palm Weevil. 9,596,851. U.S. Patent. 2017 Mar 21;
- 22.Ahn Y.J., Kwon M., Park H.M., Han C.K. Potent insecticidal activity of Ginkgo biloba derived Trilactone terpenes against Nilaparvata lugens. Phytochem. Pest Control. 1997:90–105. doi: 10.1021/bk-1997-0658.ch007. [DOI] [Google Scholar]
- 23.Maistrello L., Henderson G., Laine R.A. Comparative effects of vetiver oil, nootkatone and disodium octaborate tetrahydrate on Coptotermes formosanus and its symbiotic fauna. Pest Manag. Sci. 2003;59:58–68. doi: 10.1002/ps.601. [DOI] [PubMed] [Google Scholar]
- 24.Mao L., Henderson G. Evaluation of potential use of nootkatone against maize weevil (Sitophilus zeamais Motschulsky) and rice weevil [S. oryzae (L.)](Coleoptera: Curculionidae) J. Stored Prod. Res. 2010;46:129–132. doi: 10.1016/j.jspr.2010.01.002. [DOI] [Google Scholar]
- 25.Anderson J.A., Coats J.R. Acetylcholinesterase inhibition by nootkatone and carvacrol in arthropods. Pestic. Biochem. Physiol. 2012;102:124–128. doi: 10.1016/j.pestbp.2011.12.002. [DOI] [Google Scholar]
- 26.Chaubey M. Acute, lethal and synergistic effects of some terpenes against Tribolium castaneum herbst (Coleoptera: Tenebrionidae) Ecol. Balk. 2012;4:53–62. [Google Scholar]
- 27.Di Campli E., Di Bartolomeo S., Delli Pizzi P., Di Giulio M., Grande R., Nostro A., Cellini L. Activity of tea tree oil and nerolidol alone or in combination against Pediculus capitis (head lice) and its eggs. Parasitol. Res. 2012;111:1985–1992. doi: 10.1007/s00436-012-3045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ali A., Murphy C.C., Demirci B., Wedge D.E., Sampson B.J., Khan I.A., Baser K.H.C., Tabanca N. Insecticidal and biting deterrent activity of rose-scented geranium (Pelargonium spp.) essential oils and individual compounds against Stephanitis pyrioides and Aedes aegypti. Pest Manag. Sci. 2013;69:1385–1392. doi: 10.1002/ps.3518. [DOI] [PubMed] [Google Scholar]
- 29.Olsen R.W. Picrotoxin-like channel blockers of GABAA receptors. Proc. Natl. Acad. Sci. USA. 2006;103:6081–6082. doi: 10.1073/pnas.0601121103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ozoe Y. γ-Aminobutyrate- and Glutamate-gated Chloride Channels as targets of insecticides. Adv. Insect Physiol. 2013;44:211–286. [Google Scholar]
- 31.Shin R., Ikemoto S. Administration of the GABAA receptor antagonist picrotoxin into rat supramammillary nucleus induces c-Fos in reward-related brain structures. Supramammillary picrotoxin and c-Fos expression. BMC Neurosci. 2010;11:101. doi: 10.1186/1471-2202-11-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Godinho A.F., de Oliveira Souza A.C., Carvalho C.C., Horta D.F., De Fraia D., Anselmo F., Chaguri J.L., Faria C.A. Memory impairment due to fipronil pesticide exposure occurs at the GABA A receptor level, in rats. Physiol. Behav. 2016;165:28–34. doi: 10.1016/j.physbeh.2016.06.035. [DOI] [PubMed] [Google Scholar]
- 33.Kohn R., Platt S., Saltman S.Y. The Picrotoxin-Barbiturate Antagonism. J. Am. Med. Assoc. 1938;111:387–390. doi: 10.1001/jama.1938.02790310009004. [DOI] [Google Scholar]
- 34.Coppola A., Moshé S.L. Animal models. Handbook Clin. Neurol. 2012;107:63–98. doi: 10.1016/B978-0-444-52898-8.00004-5. [DOI] [PubMed] [Google Scholar]
- 35.Freeman G.M., Nakajima M., Ueda H.R., Herzog E.D. Picrotoxin dramatically speeds the mammalian circadian clock independent of Cys-loop receptors. J. Neurophysiol. 2013;110:103–108. doi: 10.1152/jn.00220.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ozoe Y., Akamatsu M., Higata T., Ikeda I., Mochida K., Koike K., Ohmoto T., Nikaido T. Picrodendrin and related terpenoid antagonists reveal structural differences between ionotropic GABA receptors of mammals and insects. Bioorg. Med. Chem. 1998;6:481–492. doi: 10.1016/S0968-0896(98)00012-1. [DOI] [PubMed] [Google Scholar]
- 37.McGovran E.R., Mayer E.L., Clark E.P. The Toxicity of the Natural Bitter Substances, Quassin, Tenulin, Helenalin, and Picrotoxin, and Some of Their Derivatives to Certain Insects. US Department of Agriculture, Bureau of Entomology and Plant Quarantine; Washington, DC, USA: 1942. p. E572. [Google Scholar]
- 38.Amat C., Hue B. Activation of picrotoxin-resistant GABA receptors by GABA and related compounds induces modulation of cockroach dorsal paired median (DPM) neuron firing. J. Insect Physiol. 1997;43:1125–1131. doi: 10.1016/S0022-1910(97)00077-2. [DOI] [PubMed] [Google Scholar]
- 39.MacLeod K., Laurent G. Distinct mechanisms for synchronization and temporal patterning of odor-encoding neural assemblies. Science. 1996;274:976–979. doi: 10.1126/science.274.5289.976. [DOI] [PubMed] [Google Scholar]
- 40.Choudhary A.F., Laycock I., Wright G.A. γ-Aminobutyric acid receptor A-mediated inhibition in the honeybee’s antennal lobe is necessary for the formation of configural olfactory percepts. Eur. J. Neurosci. 2012;35:1718–1724. doi: 10.1111/j.1460-9568.2012.08090.x. [DOI] [PubMed] [Google Scholar]
- 41.Al-Shawaf A.M., Al-Shagag A., Al-Bagshi M., Al-Saroj S., Al-Bather S., Al-Dandan A.M., Abdallah A.B., Faleiro J.R. A quarantine protocol against red palm weevil Rhynchophorus ferrugineus (olivier) (Coleptera: Curculiondae) in date palm. J. Plant Prot. Res. 2013;53:409–415. doi: 10.2478/jppr-2013-0061. [DOI] [Google Scholar]
- 42.Nabawy M. Metwaly method Endotherapic Injection for palm trees – the new methods used to control the red palm weevil (Rhynchophorus ferrugineus Olivier) Acta Hortic. 2010:977–984. doi: 10.17660/ActaHortic.2010.882.113. [DOI] [Google Scholar]
- 43.Shawir M.S., Abbassy M.A., Salem Y.M. Laboratory evaluation of some insecticides against larval and adult stages of Red Palm Weevil’s Rhynchophorus ferrugineus (Olivier) Alex. Sci. Exch. J. 2014;35:75–79. [Google Scholar]
- 44.Hussain A., Tian M.Y., He Y.R., Ahmed S. Entomopathogenic fungi disturbed the larval growth and feeding performance of Ocinara varians (Lepidoptera: Bombycidae) larvae. Insect Sci. 2009;16:511–517. doi: 10.1111/j.1744-7917.2009.01272.x. [DOI] [Google Scholar]
- 45.Koul O., Singh G., Singh R., Singh J., Daniewski W.M., Berlozecki S. Bioefficacy and mode-of-action of some limonoids of salannin group from Azadirachta indica A. Juss and their role in a multicomponent system against lepidopteran larvae. J. Biosci. 2004;29:409–416. doi: 10.1007/BF02712112. [DOI] [PubMed] [Google Scholar]
- 46.Silva L.B., Silva W., Macedo M.L.R., Peres M.T.L.P. Effects of Croton urucurana extracts and crude resin on Anagasta kuehniella (Lepidoptera: Pyralidae) Braz. Arch. Biol. Technol. 2009;52:653–664. doi: 10.1590/S1516-89132009000300018. [DOI] [Google Scholar]
- 47.Hussain A., Rizwan-ul-Haq M., Al-Ayedh H., AlJabr A. Susceptibility and immune defence mechanisms of Rhynchophorus ferrugineus (Olivier) (Coleoptera: Curculionidae) against entomopathogenic fungal infections. Int. J. Mol. Sci. 2016;17:1518. doi: 10.3390/ijms17091518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hussain A., Rizwan-ul-Haq M., Al-Ayedh H., Ahmed S., Al-Jabr A.M. Effect of Beauveria bassiana infection on the feeding performance and antioxidant defence of red palm weevil, Rhynchophorus ferrugineus. BioControl. 2015;60:849–859. doi: 10.1007/s10526-015-9682-3. [DOI] [Google Scholar]
- 49.AlJabr A., Hussain A., Rizwan-ul-haq M. Toxin-Pathogen synergy reshaping detoxification and antioxidant defense mechanism of Oligonychus afrasiaticus (McGregor) Molecules. 2018;23:1978. doi: 10.3390/molecules23081978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hussain A., Ruan L., Tian M., He Y. Pathogenic effect of Metarhizium anisopliae on the larval growth and development of Ocinara varians Walker (Lepidoptera: Bombycidae) Pak. Entomol. 2009;31:116–121. [Google Scholar]
- 51.Martinez D.S., Freire M.d., Mazzafera P., Araujo-Júnior R.T., Bueno R.D., Macedo M.L. Insecticidal effect of labramin, a lectin-like protein isolated from seeds of the beach apricot tree, Labramia bojeri, on the Mediterranean flour moth, Ephestia kuehniella. J. Insect Sci. 2012;12:62. doi: 10.1673/031.012.6201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abbott W.S. A method of computing the effectiveness of an insecticide. J. Econ. Entomol. 1925;18:265–267. doi: 10.1093/jee/18.2.265a. [DOI] [Google Scholar]
- 53.Statistix Statistix 8.1 Tallahassee. Analytical Software; Orlando, FL, USA: 2003. [Google Scholar]
- 54.SAS Institute SAS User’s Guide: Statistics. SAS Institute; Cary, NC, USA: 2000. [Google Scholar]
- 55.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]