Abstract
A total of 85 strains of lactic acid bacteria were isolated from corn silage in this study and analyzed in vitro for their cholesterol removal, NPC1L1 protein down-regulation and bile salt deconjugation ability, respectively. Nineteen strains were selected for further analysis for their probiotic potential. Finally, 3 strains showing better probiotic potential were evaluated for their cholesterol-lowering activity in hamsters. The strains showing the greater cholesterol removal and NPC1L1 protein down-regulation activity had no significant effects on serum and hepatic cholesterol levels in hamsters (p > 0.05). However, Lactobacillus plantarum CAAS 18008 (1 × 109 CFU/d) showing the greater bile salt deconjugation ability significantly reduced serum low-density lipoprotein cholesterol, total cholesterol, and hepatic total cholesterol levels by 28.8%, 21.7%, and 30.9%, respectively (p < 0.05). The cholesterol-lowering mechanism was attributed to its bile salt hydrolase activity, which enhanced daily fecal bile acid excretion levels and thereby accelerated new bile acid synthesis from cholesterol in liver. This study demonstrated that the strains showing greater cholesterol removal and NPC1L1 protein down-regulation activity in vitro hardly reveal cholesterol-lowering activity in vivo, whereas the strains showing greater bile salt deconjugation ability in vitro has large potential to decrease serum cholesterol levels in vivo.
Keywords: cholesterol removal, NPC1L1 protein, bile salt deconjugation, lactic acid bacteria, probiotic, hypocholesterolemic activity
1. Introduction
Cardiovascular disease has become the leading cause of death in many countries worldwide and elevated levels of serum cholesterol have been demonstrated to be the principal cause of this disease [1]. Due to some undesirable side effects from the most commonly used cholesterol-lowering drugs, functional foods and nutraceuticals have recently received more attention to reduce serum cholesterol levels [2]. In the last decade, studies have been focused on cholesterol-lowering effects of probiotics [3], which are live microorganisms that when administered in adequate amounts confer a health benefit on the host [4].
Lactic acid bacteria, especially lactobacilli, are the most widely used probiotic microorganisms [5,6]. Several lactic acid bacterial strains have shown significant cholesterol-cholesterol activity in animals [7,8] and humans [9,10]. However, the exact mechanisms responsible for the cholesterol-lowering activity remain unclear. Three main possible mechanisms have been proposed, which include removing intestinal cholesterol by probiotic cells [11,12], inhibiting small-intestinal cholesterol absorption by the down-regulation of intestinal NPC1L1 protein levels [13], and increasing fecal bile acid excretion levels by bile salt deconjugation that is catalyzed by bile salt hydrolase (BSH) of probiotic cells [10,14].
Those probiotic strains of lactic acid bacteria showing cholesterol-lowering activity in vivo mainly originated from fermented vegetables [15], fermented dairy products [13], fermented meat products [16], fermented seafood [17], and human or animal feces [18]. However, to our knowledge, no information has been reported on the cholesterol-lowering potential of lactic acid bacterial strains originated from silage. Silage is fermented and high-moisture stored fodder that was widely used to fed to ruminants [19]. During silage fermentation, predominant lactic acid bacteria utilize water-soluble carbohydrates to produce lactic and acetic acids that decreased the pH in silage [20]. The lactic acid bacteria isolated from silage have shown greater antibacterial activity against clinical pathogenic bacteria [21] and gastrointestinal transit tolerance ability [22]. Inoculated silages improved cattle performance, possibly because of probiotic effects of lactic acid bacteria inoculants [23].
The aim of this study was to screen for potential cholesterol-lowering probiotic strains from 85 lactic acid bacterial strains isolated from corn silage based on the three hypothesized pathways in vitro and then evaluate their hypocholesterolemic activity in hamsters.
2. Results
2.1. Cholesterol Removal
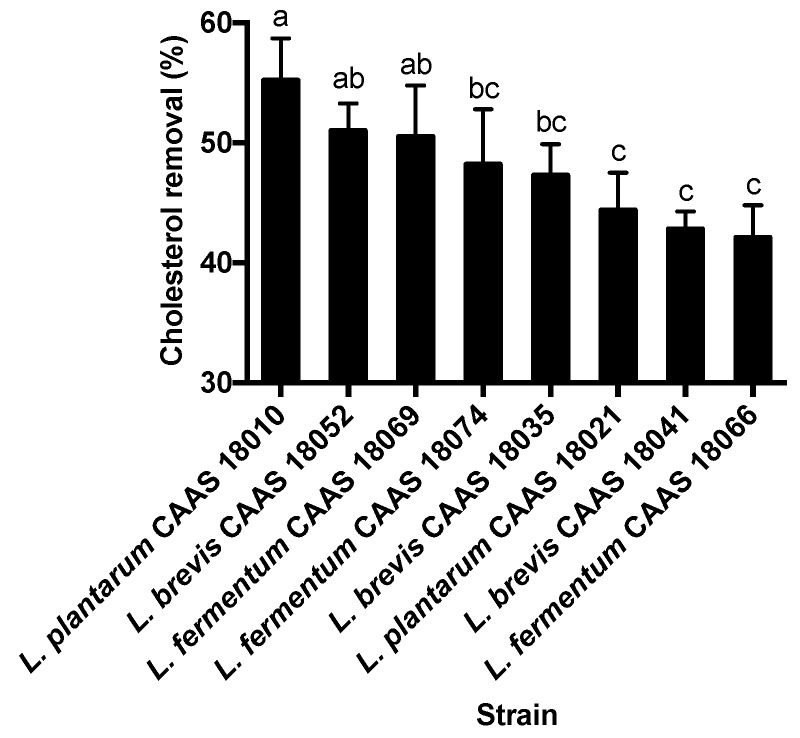
All the 85 strains of lactic acid bacteria were able to remove cholesterol from the fermentation broth during the 18-h incubation. Cholesterol removal rates varied among the strains (p < 0.05) and ranged from 3.8% to 55.2%. In general, E. faecium strains showed lower cholesterol removal rates, and none of the strains were able to remove more than 10% cholesterol from the fermentation broth. The 8 strains showing cholesterol removal rates of more than 40% were selected for further analysis. These strains are listed in Figure 1 in order of decreasing cholesterol removal rates. L. plantarum CAAS 18010, L. brevis CAAS 18052 and L. fermentum CAAS 18069 showed the greatest cholesterol removal ability and removed significantly more cholesterol from the fermentation broth than did L. plantarum CAAS 18021, L. brevis CAAS 18041 and L. fermentum CAAS 18066 (p < 0.05).
Figure 1.
Comparison of lactic acid bacterial strains for cholesterol removal ability. Data are represented as the mean ± SD (n = 3). Means not sharing a common letter differ significantly from each other (p < 0.05).
2.2. NPC1L1 Protein Down-Regulation
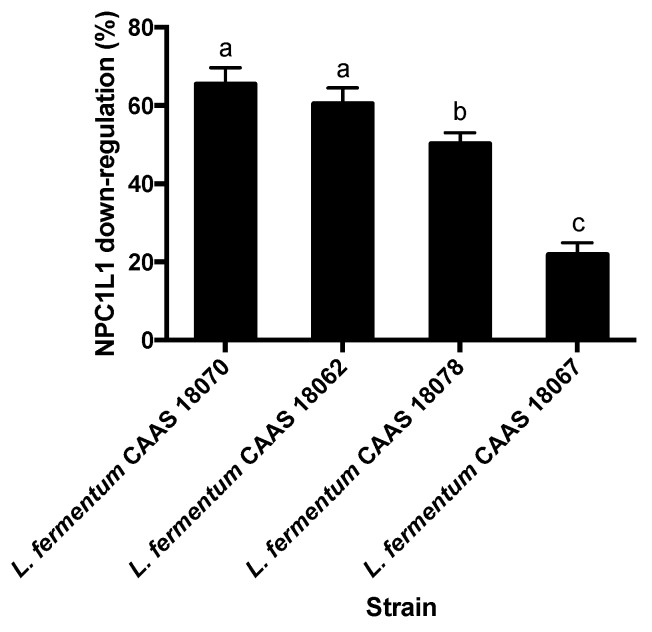
None of the 85 strains of lactic acid bacteria significantly up-regulated NPC1L1 protein levels of Caco-2 cells (p > 0.05). However, 4 strains of L. fermentum significantly down-regulated NPC1L1 protein levels of Caco-2 cells (p < 0.05) (Figure 2). NPC1L1 protein down-regulation rates varied among the strains (p < 0.05) and ranged from 22.5% to 65.5%. L. fermentum CAAS 18070 and CAAS 18062 showed the greatest.
Figure 2.
Comparison of lactic acid bacterial strains for NPC1L1 down-regulation ability. Data are represented as the mean ± SD (n = 3). Means not sharing a common letter differ significantly from each other (p < 0.05).
2.3. Bile Salt Deconjugation
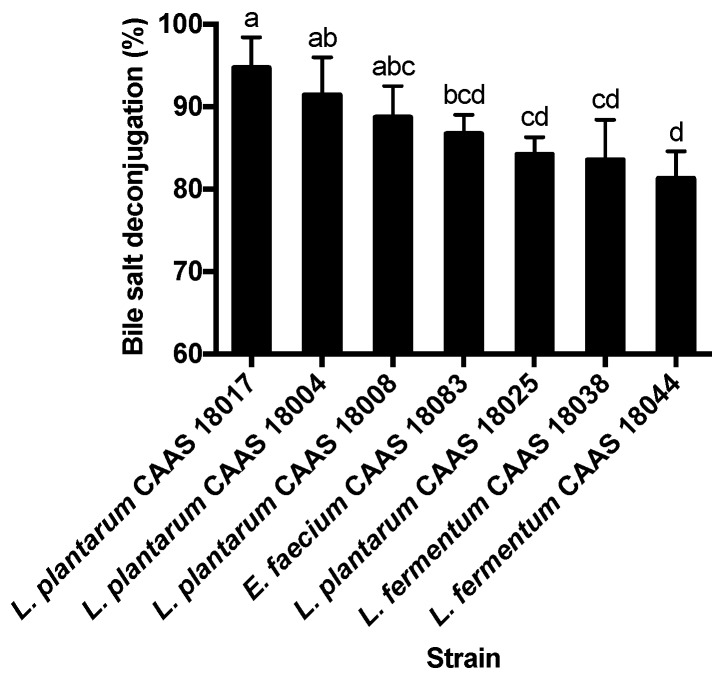
Fifty-eight strains of the 85 strains of lactic acid bacteria showed bile salt deconjugation ability. These BSH-positive strains consisted of 24 L. plantarum strains, 25 L. brevis strains, 4 L. fermentum strains, and 5 E. faecium strains. Bile salt deconjugation ability varied among the 58 BSH-positive strains (p < 0.05) and ranged from 8.7 % to 94.7%. The 7 strains showing bile salt deconjugation rates of more than 80% were selected for further analysis. These strains are listed in Figure 3 in order of decreasing bile salt deconjugation rates. L. plantarum CAAS 18017, CAAS 18004, and CAAS 18008 showed the greatest bile salt deconjugation ability, while E. faecium CAAS 18083, L. plantarum CAAS 18025 and L. brevis CAAS 18038 and CAAS 18044 showed the least bile salt deconjugation ability.
Figure 3.
Comparison of lactic acid bacterial strains for bile salt deconjugation ability. Data are represented as the mean ± SD (n = 3). Means not sharing a common letter differ significantly from each other (p < 0.05).
2.4. Basic Probiotic Properties
The basic probiotic properties, including acid tolerance, bile tolerance, and adhesion ability to Caco-2 cells [24], were further determined for the 8 strains showing the greater cholesterol removal ability, the 4 strains showing NPC1L1 down-regulation activity, and the 7 strains showing the greater bile salt deconjugation ability (Table 1).
Table 1.
Acid tolerance, bile tolerance, and adhesion ability of the selected strains.
| Hypothesized Pathway | Strain | Acid Tolerance (%) | Bile Tolerance (%) | Adhesion (Bacterial Counts /100 cells) |
|---|---|---|---|---|
| Greater cholesterol removal | L. plantarum CAAS 18010 | 61.6 ± 2.2 b | NG | 282 ± 30 a |
| L. plantarum CAAS 18021 | 33.6 ± 4.1 f | 83.6 ± 5.5 a | 281 ± 19 a | |
| L. brevis CAAS 18035 | 66.3 ± 2.0 ab | 41.5 ± 3.8 h | 251 ± 10 ab | |
| L. brevis CAAS 18041 | 65.9 ± 3.7 ab | 81.9 ± 5.6 ab | 228± 34 bc | |
| L. brevis CAAS 18052 | 65.2 ± 3.9 ab | 75.8 ± 2.8 bc | 224 ± 23 bc | |
| L. fermentum CAAS 18066 | 22.5 ± 1.0 h | 54.6 ± 4.1 g | 213± 28 bc | |
| L. fermentum CAAS 18069 | 55.5 ± 3.2 c | NG | 107 ± 19 e | |
| L. fermentum CAAS 18074 | 21.7 ± 3.0 h | 76.0 ± 5.1 bc | 241 ± 26 ab | |
| NPC1L1 protein down-regulation |
L. fermentum CAAS 18062 | 30.8 ± 2.4 fg | 75.5 ± 4.2 bc | 245 ± 27 ab |
| L. fermentum CAAS 18067 | 49.9 ± 3.8 cd | 54.6 ± 3.6 g | 106 ± 19 e | |
| L. fermentum CAAS 18070 | 63.4± 2.9 ab | NG | 151 ± 23 d | |
| L. fermentum CAAS 18078 | 61.2 ± 3.4 b | 65.8 ± 2.3 de | 240 ± 22 abc | |
| Greater bile salt deconjugation | L. plantarum CAAS 18004 | 61.5 ± 3.4 b | NG | 205 ± 26 bc |
| L. plantarum CAAS 18008 | 68.5 ± 3.0 a | 75.7 ± 4.2 bc | 241± 14 ab | |
| L. plantarum CAAS 18017 | 55.5 ± 4.3 c | 80.7 ± 4.7 ab | 93 ± 20 e | |
| L. plantarum CAAS 18025 | 40.2 ± 3.0 e | 63.5 ± 4.2 ef | 101 ± 24 e | |
| L. brevis CAAS 18038 | 27.0 ± 2.6 gh | 71.8 ± 2.9 cd | 207± 29 bc | |
| L. brevis CAAS 18044 | 63.9 ± 3.9 ab | 74.8 ± 4.3 bc | 194 ± 34 c | |
| E. faecium CAAS 18083 | 45.5 ± 3.3 de | 57.5 ± 1.7 fg | 121 ± 26 de |
a–g Means in the same column not sharing a common superscript letter differed significantly (p < 0.05). A NG sign indicates that the strains did not grow in the presence of 0.3% oxgall.
Among the 8 strains showing the greater cholesterol removal ability, L. plantarum CAAS 18021, L. fermentum CAAS 18066, and L. fermentum CAAS 18074 were significantly less acid tolerant than the other strains (p < 0.05); L. plantarum CAAS 18010, L. fermentum CAAS 18069 and L. brevis CAAS 18035 were significantly less bile tolerant than the other strains (p < 0.05). Therefore, these strains were eliminated. The remaining two strains (L. brevis CAAS 18041 and CAAS 18052) did not differ significantly in their acid and bile tolerance and adhesion ability (p > 0.05), but the latter showed significantly greater cholesterol removal ability than the former (p < 0.05) (Figure 1). Therefore, L. brevis CAAS 18052 was selected for further animal feeding trail.
Among the 4 strains showing NPC1L1 protein down-regulation activity, L. fermentum CAAS 18062 was the least acid tolerant; L. fermentum CAAS 18067 was the least adherent to Caco-2 cells; L. fermentum CAAS 18070 was the least bile tolerant. Therefore, these strains were eliminated. The remaining 1 strain (L. fermentum CAAS 18078) was selected for further animal feeding trail.
Among the 7 strains showing the greater bile salt deconjugation ability, L. plantarum CAAS 18004 was the least bile tolerant; L. plantarum CAAS 18017, CAAS 18025 and E. faecium CAAS 18083 were the least adherent to Caco-2 cells; L. brevis CAAS 18038 was the least acid tolerant. Therefore, these strains were eliminated. The remaining 2 strains (L. plantarum CAAS 18008 and L. brevis CAAS 18044) did not differ significantly in their acid and bile tolerance (p > 0.05), but the former showed greater adhesion to Caco-2 cells and bile salt deconjugation than the latter. For this reason, L. plantarum CAAS 18008 was selected for further animal feeding trail.
2.5. Serum and Hepatic Cholesterol
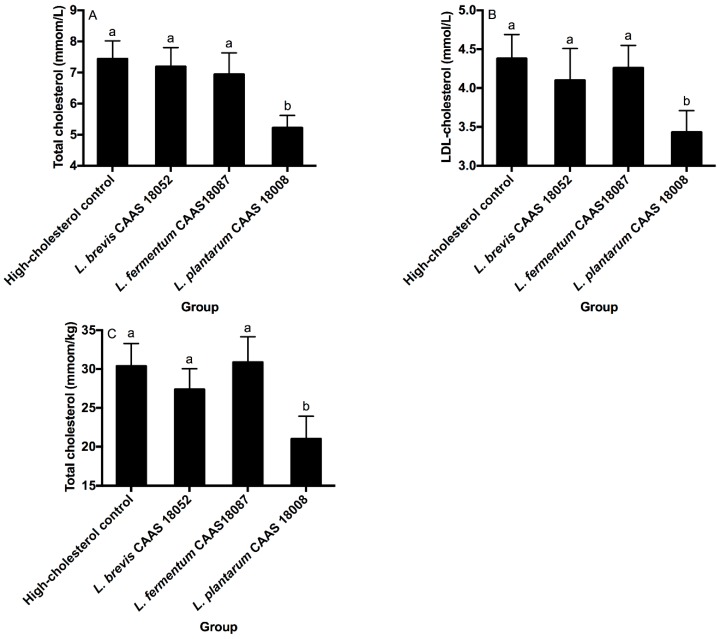
L. brevis CAAS 18052, L. fermentum CAAS 18078, and L. plantarum CAAS 18008 did not significantly affect serum HDL-cholesterol levels in hamsters (p > 0.05, data not shown). L. brevis CAAS 18052 and L. fermentum CAAS 18078 also did not significantly affect serum LDL-cholesterol, total cholesterol, and hepatic total cholesterol levels in hamsters (p > 0.05) (Figure 4A–C). However, L. plantarum CAAS 18008 significantly decreased serum LDL-cholesterol, total cholesterol and hepatic total cholesterol levels in hamsters by 28.8%, 21.7%, and 30.9%, respectively (p < 0.05).
Figure 4.
Serum total cholesterol (A), LDL-cholesterol (B) and hepatic total cholesterol (C) levels of the different groups. Data are represented as the mean ± SD (n = 8). Means not sharing a common letter differ significantly from each other (p < 0.05).
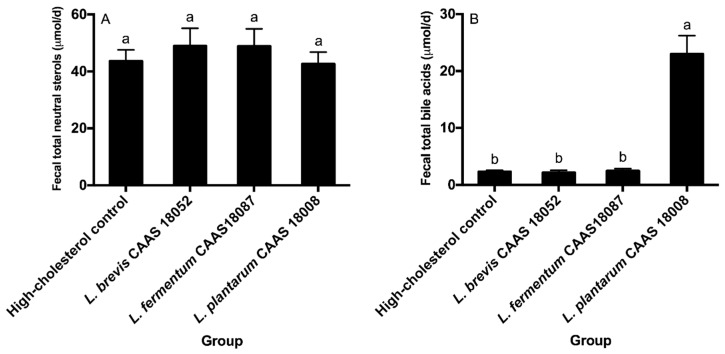
2.6. Fecal Sterol Excretion
Daily fecal excretion levels of total neutral sterols and total bile acids of the different groups are shown in Figure 5. L. brevis CAAS 18052, L. fermentum CAAS 18078, and L. plantarum CAAS 18008 did not significantly affect daily fecal total neutral sterol excretion levels in hamsters (p > 0.05) (Figure 5A). L. brevis CAAS 18052 and L. fermentum CAAS 18078 also did not significantly affect daily fecal total bile acid excretion levels in hamsters (p > 0.05) (Figure 5B). However, L. plantarum CAAS 18008 significantly enhanced daily fecal total bile acid excretion level in hamsters by 9.0 times (p < 0.05).
Figure 5.
Daily excretion levels of fecal total neutral sterols (A) and total bile acids (B) of the different groups. Data are represented as the mean ± SD (n = 8). Means not sharing a common letter differ significantly (p < 0.05).
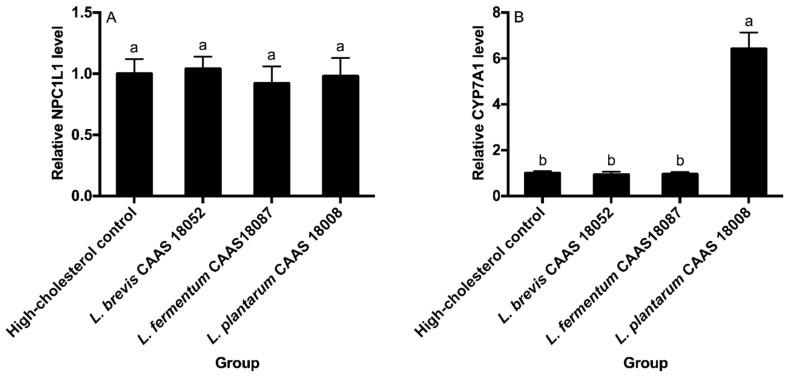
2.7. Small Intestinal NPC1L1 Protein and Hepatic Cholesterol-7α-Hydroxylase (CYP7A1)
The small intestinal NPC1L1 protein and hepatic CYP7A1 levels of the different groups are shown in Figure 6. L. brevis CAAS 18052, L. fermentum CAAS 18078, and L. plantarum CAAS 18008 did not significantly affect the small intestinal NPC1L1 protein levels (p > 0.05) (Figure 6A). L. brevis CAAS 18052 and L. fermentum CAAS 18078 also did not significantly affect hepatic CYP7A1 levels in hamsters (p > 0.05) (Figure 6B). However, L. plantarum CAAS 18008 significantly increased the hepatic CYP7A1 level in hamsters by 5.4 times (p < 0.05).
Figure 6.
Small intestinal NPC1L1 protein (A) and hepatic CYP7A1 (B) levels of the different groups. Data are represented as the mean ± SD (n = 8). Means not sharing a common letter differ significantly from each other (p < 0.05).
3. Discussion
Cholesterol removed from media by lactobacilli could be bound to surface of lactobacilli [11], incorporated into cellular membrane [25], or transferred into the cytoplasm of lactobacilli [26]. However, the removed cholesterol was mainly incorporated into the phospholipid bilayers of the cellular membrane of lactobacilli [27]. The incorporation of cholesterol has been reported to strengthen the cell envelope by increasing ratio of C to P and thereby enhance cellular resistance to enzymatic hydrolysis and ultrasonic damage [12]. Therefore, the incorporation of cholesterol into cellular membrane may benefit survival of lactobacilli in the gastrointestinal tract. However, up to now, no research has investigated whether the ingestion of lactobacilli capable of removing cholesterol will have a significant influence on serum cholesterol levels.
Cholesterol removal ability of lactobacilli markedly depends on their own growth status. Lactobacilli are able to remove a significant amount of cholesterol from media only in the growing status, and the resting and dead cells of lactobacilli generally have a very low ability to remove cholesterol [28]. Cholesterol absorption mainly occurs in the duodenum and upper jejunum, where dietary and biliary cholesterol is available for uptake from the intestinal lumen [29]. However, a higher concentration of bile salts occurs in these regions of the small intestine [30], which significantly inhibits the growth of intestinal bacteria, including lactobacilli. For these reasons, L. brevis CAAS 18052 struggled to remove a significant amount of cholesterol in the duodenum and upper jejunum of hamsters, although it showed greater cholesterol removal ability in vitro. This was an important reason why L. brevis CAAS 18052 did show significant cholesterol-lowering activity in hamsters.
NPC1L1 protein is a predicted polytopic membrane protein that plays a key role in the absorption of intestinal cholesterol [31]. NPC1L1 protein transfers free cholesterol into cells through vesicular endocytosis and it is highly expressed in the duodenum and upper jejunum of the small intestine [32]. In theory, the down-regulation of the small intestinal NPC1L1 protein has a potential to decrease the amount of cholesterol that is absorbed from the small intestine, thereby affecting serum cholesterol levels. The ingestion of L. acidophilus ATCC 4356 at a dose of 1 × 109 CFU per day has been reported to lead to a significant decrease in serum LDL-cholesterol and total cholesterol levels in rats through the down-regulation of the small intestinal NPC1L1 protein by the strain ingested [33]. In contrast to this study, L. fermentum CAAS 18078 at the same dose did show a significant cholesterol-lowering activity in hamsters, although it showed the greater NPC1L1 protein down-regulation activity in vitro. This conflicting result may be due to the different properties of the strains and animal models used.
The down-regulation of NPC1L1 protein of Caco-2 observed in this study should be attributed to extracellular metabolites (soluble effector molecules) secreted by L. fermentum CAAS 18078 during the incubation [34]. Under the in vitro static incubation conditions, these soluble effector molecules could fully interact with the Caco-2 cells. However, under in vivo dynamic conditions, these soluble effector molecules struggled to fully interact with the epithelial cells of the duodenum and upper jejunum of hamster due to the intestinal peristalsis and interference of food components and intestinal bacteria. In addition, due to the growth inhibition of L. fermentum CAAS 18078 in the duodenum and upper jejunum of hamster by a higher concentration of bile salts, the ability of the strain to secrete the soluble effector molecules had to decline. These were also important reasons why L. fermentum CAAS 18078 did not show significant hypocholesterolemic activity in hamsters.
The catalysis of BSH is responsible for bile salt deconjugation by strains of lactobacilli. The bile salt deconjugation has become a profound mechanism on hypocholesterolemic effects of probiotic lactobacilli [35,36]. Fermented milk of BSH-active L. reuteri NCIMB 30242 has shown a significant hypocholesterolemic effect in hypercholesterolemic adults [10]. Oral administration of the immobilized BSH derived from L. buchneri ATCC 4005 led to a significant decrease in serum total cholesterol level by 58% in rats fed a cholesterol-rich diet [37]. In addition, the fermented milk prepared by wild-type L. casei F0822 significantly decrease serum LDL-cholesterol and total cholesterol levels in hamsters, whereas the fermented milk prepared by BSH-deficient mutant of L. casei F0822 did not showed the hypocholesterolemic effects in hamsters [38].
L. plantarum CAAS 18008 showed the greater BSH activity in this study, which suggests that this strain was able to hydrolyze glycine- and/or taurine-conjugated bile salts in the intestinal tract of hamsters to release amino acids and free bile acids. The free bile acids are less soluble and poorly absorbed from the small intestine compared with their conjugated forms [39], which would increase fecal bile acid excretion levels [40]. To replace the lost bile acids, more new bile acids would be synthesized from cholesterol in the hepatic tissue of hamsters through catalysis of CYP7A1 and thereby would cause a significant decrease in the serum cholesterol levels in hamsters. In addition, the greater acid and bile tolerance ability also promoted survival of L. plantarum CAAS 18008 in the gastrointestinal tract and thereby enhanced its cholesterol-lowering activity.
4. Materials and Methods
4.1. Source and Maintenance of Lactic Acid Bacteria Strains
Twenty corn silage samples were collected from Shaanxi provine, China. The samples were serially diluted in sterile distilled water and the diluents (100 µL) were spread onto MRS agar plates (Oxoid, Basingstoke, Hampshire, UK). After anaerobic incubation for 72 h at 37 °C, typical colonies were selected from the plates and identified by both 16S rDNA sequencing and carbohydrate fermentation pattern using an API 50 CHL system (BioMeriéx, France) [41]. The isolates were genetically differentiated at the strain level by random amplification of polymorphic DNA-PCR using the primers M13, AB111, and AB106 [42]. A total of 85 strains of lactic acid bacteria were obtained and they consisted of 32 L. plantarum strains, 28 L. brevis strains, 20 L. fermentum strains, and 5 E. faecium strains. The cultures were stored in 30% glycerol at −86 °C. They were activated three times in MRS broth (Oxoid, Basingstoke, Hampshire, UK) prior to use.
4.2. Cholesterol Removal
Cholesterol removal ability of the lactic acid bacterial strains was measured according to the previous method [28] with minor modification. Briefly, water-soluble cholesterol (cholesteryl-polyethylene glycol 600 sebacate, Sigma-Aldrich, St. Louis, MO, USA) was added to the sterile MRS broth at a final concentration of approximately100 mg/L by filter sterilization (0.22 µm, Millipore, Bedford, MA, USA). The overnight cultures of lactic acid bacterial strains were inoculated into the broth with 1% (v/v) inoculum size and incubated anaerobically at 37 °C for 18 h. The cultures were centrifuged at 10,000 g for 15 min, and supernatants were taken for determining cholesterol concentrations by capillary gas chromatography [43].
4.3. NPC1L1 Proteiin Down-Regulation
NPC1L1 down-regulation by lactic acid bacterial strains was determined by the previous method [34] with several modifications. Briefly, the overnight cultures (16 h) of lactic acid bacterial strains were centrifuged at 10,000 g for 15 min, washed once with distilled water, and resuspended in Dulbecco’s Modified Eagle’s Medium (DMEM, Life Technologies, Carlsbad, CA, USA) at 1 × 108 CFU/mL. The bacterial suspension (2 mL) was inoculated into monolayer Caco-2 cells, which were cultured on glass slide placed in six-well tissue culture plates, and incubated at 37 °C for 2 h. The medium was dumped out and the Caco-2 cells were washed twice with DMEM, and resuspended in phosphate buffer saline (PBS) after trypsinization to an absorbance of 1.0 at 600 nm. The cell suspension was mixed with the same volume of RIPA lysis buffer (Beyotime Institute of Biotechnology, Shanghai, China) and homogenized at 10,000 r/min for 10 min with an Ultra-Turrax T25 high-speed homogenizer (IKA Labortechnik, Staufen, Germany). The homogenates were used to determine NPC1L1 protein levels with a human NPC1L1 ELISA kit from AVIVA Systems Biology (San Diego, California, USA).
4.4. Bile Salt Deconjugation
The overnight cultures (16 h) of lactic acid bacterial strains were centrifuged at 10,000 g for 15 min, washed once with distilled water, and resuspended in MRS broth supplemented with a human bile salt mixture [44] (Sigma-Aldrich, total concentration of 4 mmol/L) at 1 × 108 CFU/mL. The cultures were incubated anaerobically for 2 h at 37 °C, centrifuged at 10,000 g for 15 min, and the supernatants (1 mL) were drawn for determining the conjugated bile salt concentrations using ion-pair high-performance liquid chromatography [45].
4.5. Determination of Acid Tolerance
The overnight cultures (16 h) of lactic acid bacterial strains were centrifuged at 10,000 g for 15 min, washed once with distilled water, and resuspended in acidified MRS broth (pH 2.0, hydrochloric acid) at 1 × 108 CFU/mL. The cultures were incubated for 2 h at 37 °C and one-milliliter sample was taken, and enumerated viable counts by plate pouring method. The acid tolerance ability of strains was calculated according to the following equation [46]:
| (1) |
where N0 is the total viable count before the incubation (CFU/mL) and N1 is the total viable count after the 2-h incubation (CFU/mL).
4.6. Determination of Bile Tolerance
Bile tolerance ability of the cultures of lactic acid bacterial strains was measured according to the previous method [45]. Briefly, the overnight cultures (16 h) of lactic acid bacterial strains were inoculated (0.01‰, v/v) into 1/2 strength buffered MRS broth (pH 7.3, 0.1 mol/L sodium phosphate) supplemented with and without 0.3% (w/v) oxgall (BD Difco, Sparks, MD, USA), and incubated for 12 h at 37 °C under anaerobic conditions. One-milliliter sample was taken, and enumerated viable counts by plate pouring method. The bile tolerance ability of the cultures was calculated based on the propagation rate in the presence of bile according to the following equation:
| (2) |
where N0 is the viable counts before the incubation in the broth (CFU/mL) and N1 and N2 are the viable counts after the 12-h incubation in the broth with and without oxgall, respectively.
4.7. Determination of Adhesion Ability
The overnight cultures (16 h) of lactic acid bacterial strains were centrifuged at 10,000 g for 15 min, washed once with distilled water, and resuspended in DMEM at 1108 CFU/mL. The suspensions (2 mL) were inoculated into monolayer Caco-2 cells, which were cultured on glass slide placed in six-well tissue culture plates, and incubated at 37 °C for 1 h. The cells were washed three times with DMEM to remove unbound bacteria, fixed with 2 mL of methanol, stained with 2 mL of Giemsa stain solution (1:20) (Sigma-Aldrich) [47]. The adhesion ability of lactic acid bacterial strains was expressed as the adherent bacterial counts per 100 Caco-2 cells.
4.8. Animal Feeding Trial
Six-week-old male Syrian hamsters were obtained from Beijing Vital River Laboratory Animal Technology Company (China). Hamsters were individually housed in a room kept at a 22 ± 2 °C temperature, a 60 ± 5% humidity, and a 12-h light-dark cycle. All animal experiments were conducted under the Guide for Care and Use of Laboratory Animals [48] and the procedures involving animals were approved by Animal Ethical Committee of China Agricultural University (No. CAU20171020-3, 20 October, 2017).
Animals were randomly divided into 4 groups of 8 hamsters. All animals were fed a high-cholesterol diet (0.4 % cholesterol in AIN 93 M diet) during a 28-d feeding period [49]. First group (high-cholesterol control) was given 1-mL distilled water daily by gavage, and the other three groups were given 1-mL bacterial cell suspensions of L. brevis CAAS 18052, L. fermentum CAAS18087 and L. plantarum CAAS 18008 (1 × 109 CFU/mL each) daily by gavage, respectively. All animals were allowed free access to feed and water during the feeding period.
4.9. Determination of Serum and Hepatic Cholesterol Levels
Hamsters were fasted for 12 h and whole blood was collected from the retro-orbital plexus, centrifuged at 3000 g for 15 min for separating serum. The obtained serum samples were used to determine low-density lipoprotein (LDL)-cholesterol, high-density lipoprotein (HDL)-cholesterol and total cholesterol levels by enzymatic colorimetry using a Synchron LX20 automated biochemical analyzer (Beckman Coulter, Fullerton, CA, USA) with commercial kits from Nanjing Jiancheng Bioengineering Institute (China). For hepatic cholesterol analysis, liver homogenates were extracted twice with a binary mixed solvent of chloroform and methanol (2:1, v/v) and the lower-layer organic phase (chloroform) was combined for determining total cholesterol levels by gas chromatography [42] using a 7890 A gas chromatograph equipped with a flame ionization detector and a HP-5 fused silica capillary column (30 m × 0.25 mm; film thickness 0.25 μm) (Agilent Technologies, Inc., Wilmington, Delaware, USA) set at a flow rate of carrier gas (N2) of 1 mL/min.
4.10. Determination of Fecal Neutral and Acidic Sterols
Feces were collected over the last 3 days and dehydrated by lyophilization. The dried fecal samples (100 mg) were extracted twice with 2-mL absolute ethyl alcohol at 50 °C. The combined extracts were used to determine enzymatically total bile acid levels using a Synchron LX20 automated biochemical analyzer with a commercial kit from Nanjing Jiancheng Bioengineering Institute (China) and determine fecal neutral sterols (cholesterol, coprostanol, and cholestane) by gas chromatography-mass spectrometry [18] using a 7890 A gas chromatograph fitted with a 5975-C mass spectrometry detector (electron impact ion source) and a HP-5 MS fused silica capillary column (30 m × 0.25 mm; film thickness 0.25 μm) (Agilent Technologies, Inc., Wilmington, Delaware, USA) set at a flow rate of carrier gas (He) of 1 mL/min.
4.11. Determination of Small Intestinal NPC1L1 Protein and Hepatic Cholesterol-7α-Hydroxylase (CYP7A1)
The whole small intestine and liver were homogenized in ice-cold RIPA lysis buffer with an Ultra-Turrax T25 homogenizer for 10 min at 9000 r/min. The homogenates were centrifuged at 8000 g for 15 min and the supernatants were drawn for analyzing small intestinal NPC1L1 protein and hepatic CYP7A1 levels with commercial hamster NPC1L1 and CYP7A1 ELISA kits from Haling Biological Technology Company (Shanghai, China), respectively [38].
4.12. Statistical Analysis
All experiments were repeated three times except the animal feeding trial (n = 8) and data were expressed as the mean ± standard deviation. Statistical analysis was performed using SPSS software (version 24.0, IBM Corporation, Armonk, NY, USA). Statistical differences between the means were analyzed by one-way analysis of variance followed by Duncan’s multiple-range test. A difference of p < 0.05 was considered statistically significant.
5. Conclusions
The strains showing the greater cholesterol removal and NPC1L1 protein down-regulation activity in vitro had no significant effects on serum cholesterol levels in hamsters, whereas L. plantarum CAAS 18008 showing the greater bile salt deconjugation ability in vitro significantly decreased serum HDL-cholesterol, total cholesterol, and hepatic total cholesterol levels in hamsters.
Author Contributions
J.L. (Jiaping Lv) conceived and designed the experiments; C.M. performed the experiments and wrote the paper; C.Z. analyzed the data; S.Z. and J.L. (Jing Lu) contributed materials; X.P. revised the paper. All authors read and approved the final manuscript.
Funding
This study was financially partially supported by the National Key R&D Program of China (No. 2017YFC1600903), the National Natural Science Foundation of China (No. 31871833) and the Science and Technology Project of Beijing Education Committee (No. KM201812448003).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.DiRienzo D.B. Effect of probiotics on biomarkers of cardiovascular disease: implications for heart-healthy diets. Nutr. Rev. 2014;72:18–29. doi: 10.1111/nure.12084. [DOI] [PubMed] [Google Scholar]
- 2.Bartley G.E., Yokoyama W., Young S.A., Anderson W.H.K., Hung S.-C., Albers D.R., Langhorst M.L., Kim H. Hypocholesterolemic effects of hydroxypropyl methylcellulose are mediated by altered gene expression in hepatic bile and cholesterol pathways of male hamsters. J. Nutr. 2010;140:1255–1260. doi: 10.3945/jn.109.118349. [DOI] [PubMed] [Google Scholar]
- 3.Wang C.-Y., Wu S.-C., Ng C.-C., Shyu Y.-T. Effect of Lactobacillus-fermented adlay-based milk on lipid metabolism of hamsters fed cholesterol-enriched diet. Food Res. Int. 2010;43:819–824. doi: 10.1016/j.foodres.2009.11.020. [DOI] [Google Scholar]
- 4.Howarth G.S., Wang H. Role of endogenous microbiota, probiotics and their biological products in human health. Nutrients. 2013;5:58–81. doi: 10.3390/nu5010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu C.-H., Hsueh Y.-H., Kuo J.-M., Liu S.-J. Characterization of a potential probiotic Lactobacillus brevis RK03 and efficient production of γ-aminobutyric acid in batch fermentation. Int. J. Mol. Sci. 2018;19:143. doi: 10.3390/ijms19010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pavli F., Tassou C., Nychas GJ E., Chorianopoulos N. Probiotic incorporation in edible films and coatings: bioactive solution for functional foods. Int. J. Mol. Sci. 2018;19:150. doi: 10.3390/ijms19010150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang Y., Xu N., Xi A., Ahmed Z., Zhang B., Bai X. Effects of Lactobacillus plantarum MA2 isolated from Tibet kefir on lipid metabolism and intestinal microflora of rats fed on high-cholesterol diet. Appl. Microbiol. Biotechnol. 2009;84:341–347. doi: 10.1007/s00253-009-2012-x. [DOI] [PubMed] [Google Scholar]
- 8.Ding W.R., Shi C., Chen M., Zhou J.W., Long R.J., Guo X.S. Screening for lactic acid bacteria in traditional fermented Tibetan yak milk and evaluating their probiotic and cholesterol-lowering potentials in rats fed a high-cholesterol diet. J. Funct. Foods. 2017;32:324–332. doi: 10.1016/j.jff.2017.03.021. [DOI] [Google Scholar]
- 9.Fuentes M.C., Lajo T., Carrioon J.M., Cune J. Cholesterol-lowering efficacy of Lactobacillus plantarum CECT 7527, 7528 and 7529 in hypercholesterolaemic adults. Br. J. Nutr. 2013;109:1866–1872. doi: 10.1017/S000711451200373X. [DOI] [PubMed] [Google Scholar]
- 10.Jones M.L., Martoni C.J., Parent M., Prakash S. Cholesterol-lowering efficacy of a microencapsulated bile salt hydrolase-active Lactobacillus reuteri NCIMB 30242 yoghurt formulation in hypercholesterolaemic adults. Br. J. Nutr. 2012;107:1505–1513. doi: 10.1017/S0007114511004703. [DOI] [PubMed] [Google Scholar]
- 11.Lye H.-S., Rahmat-Ali G.R., Liong M.-T. Mechanisms of cholesterol removal by lactobacilli under conditions that mimic the human gastrointestinal tract. Int. Dairy J. 2010;20:169–175. doi: 10.1016/j.idairyj.2009.10.003. [DOI] [Google Scholar]
- 12.Lye F.S., Alias K.A., Rusul G., Liong M.T. Ultrasound treatment enhances cholesterol removal ability of lactobacilli. Ultrason. Sonochem. 2012;19:632–641. doi: 10.1016/j.ultsonch.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 13.Huang Y., Wang J., Quan G., Wang X., Yang L., Zhong L. Lactobacillus acidophilus ATCC 4356 prevents atherosclerosis via inhibition of intestinal cholesterol absorption in apolipoprotein E-knockout mice. Appl. Environ. Microbiol. 2014;80:7496–7504. doi: 10.1128/AEM.02926-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones M.L., Tomaro-Duchesneau C., Martoni C.J., Prakash S. Cholesterol lowering with bile salt hydrolase-active probiotic bacteria, mechanism of action, clinical evidence, and future direction for heart health applications. Expert Opin. Biol. Ther. 2013;13:631–642. doi: 10.1517/14712598.2013.758706. [DOI] [PubMed] [Google Scholar]
- 15.Choi E.A., Chang H.C. Cholesterol-lowering effects of a putative probiotic strain Lactobacillus plantarum EM isolated from kimchi. Lwt-Food Sci. Technol. 2015;62:210–217. doi: 10.1016/j.lwt.2015.01.019. [DOI] [Google Scholar]
- 16.Mandal V., Sen S.K., Mandal N.C. Effect of prebiotics on bacteriocin production and cholesterol lowering activity of Pediococcus acidilactici LAB 5. World J. Microbiol. Biotechnol. 2009;25:1837–1847. doi: 10.1007/s11274-009-0085-4. [DOI] [Google Scholar]
- 17.Kuda T., Yazaki T., Ono M., Takahashi H., Kimura B. In vitro cholesterol-lowering properties of Lactobacillus plantarum AN6 isolated from aji-narezushi. Lett. Appl. Microbiol. 2013;57:187–192. doi: 10.1111/lam.12094. [DOI] [PubMed] [Google Scholar]
- 18.Guo C.-F., Zhao D., Yuan Y.-H., Yue T.-L., Liu B., Li J.-Y. Lactobacillus casei-fermented milk improves serum and hepatic lipid profiles in diet-induced hypercholesterolaemic hamsters. J. Funct Foods. 2016;26:691–697. doi: 10.1016/j.jff.2016.08.035. [DOI] [Google Scholar]
- 19.Ferraretto L.F., Shaver R.D., Luck B.D. Silage review: Recent advances and future technologies for whole-plant and fractionated corn silage harvesting. J. Dairy Sci. 2018;101:3937–3951. doi: 10.3168/jds.2017-13728. [DOI] [PubMed] [Google Scholar]
- 20.Pang H., Qin G., Tan Z., Li Z., Wang Y., Cai Y. Natural populations of lactic acid bacteria associated with silage fermentation as determined by phenotype, 16S ribosomal RNA and recA gene analysis. Syst. Appl. Microbiol. 2011;34:235–241. doi: 10.1016/j.syapm.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 21.Li D., Ni K., Pang H., Wang Y., Cai Y., Jin Q. Identification and antimicrobial activity detection of lactic acid bacteria isolated from corn stover silage. Asian Australas. J. Anim. Sci. 2015;28:620–631. doi: 10.5713/ajas.14.0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li B., Zhen H., Zhang X., Wang S., Zhang Y., Fang Z., Huang Z., Shi P. Probiotic properties of Enterococcus strains isolated from the silage. J. Food Saf. 2015;35:108–118. doi: 10.1111/jfs.12165. [DOI] [Google Scholar]
- 23.Weinberg Z.G., Chen Y., Gamburg M. The passage of lactic acid bacteria from silage into rumen fluid, in vitro studies. J. Dairy Sci. 2004;87:3386–3397. doi: 10.3168/jds.S0022-0302(04)73474-8. [DOI] [PubMed] [Google Scholar]
- 24.Dunne C., O’Mahony L., Murphy L., Thornton G., Morrissey D., O’Halloran S., Feeney M., Flynn S., Fitzgerald G., Daly C., et al. In vitro selection criteria for probiotic bacteria of human origin: correlation with in vivo findings. Am. J. Clin. Nutr. 2001;73:386S–392S. doi: 10.1093/ajcn/73.2.386s. [DOI] [PubMed] [Google Scholar]
- 25.Noh D.O., Kim S.H., Gilliland S.E. Incorporation of cholesterol into the cellular membrane of Lactobacillus acidophilus ATCC 43121. J. Dairy Sci. 1997;80:3107–3113. doi: 10.3168/jds.S0022-0302(97)76281-7. [DOI] [PubMed] [Google Scholar]
- 26.Iranmanesh M., Ezzatpanah H., Mojgani N. Antibacterial activity and cholesterol assimilation of lactic acid bacteria isolated from traditional Iranian dairy products. Lwt-Food Sci. Technol. 2014;58:355–359. doi: 10.1016/j.lwt.2013.10.005. [DOI] [Google Scholar]
- 27.Lye H.S., Rusul G., Liong M.T. Removal of cholesterol by lactobacilli via incorporation and conversion to coprostanol. J. Dairy Sci. 2010;93:1383–1392. doi: 10.3168/jds.2009-2574. [DOI] [PubMed] [Google Scholar]
- 28.Liong M.T., Shah N.P. Acid and bile tolerance and cholesterol removal ability of lactobacilli strains. J. Dairy Sci. 2005;88:55–66. doi: 10.3168/jds.S0022-0302(05)72662-X. [DOI] [PubMed] [Google Scholar]
- 29.Kockx M., Kritharides L. Intestinal cholesterol absorption. Curr. Opin. Lipidol. 2018;29:484–485. doi: 10.1097/MOL.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 30.McConnell E.L., Fadda H.M., Basit A.W. Gut instincts: Explorations in intestinal physiology and drug delivery. Int. J. Pharm. 2008;364:213–226. doi: 10.1016/j.ijpharm.2008.05.012. [DOI] [PubMed] [Google Scholar]
- 31.Altmann S.W., Davis H.R., Zhu L.-J., Yao X., Hoos L.M., Tetzloff G., Iyer S.P.N., Maguire M., Golovko A., Zeng M., et al. Niemann-Pick C1 like 1 protein is critical for intestinal cholesterol absorption. Science. 2004;303:1201–1204. doi: 10.1126/science.1093131. [DOI] [PubMed] [Google Scholar]
- 32.Wang J., Chu B.-B., Ge L., Li B.-L., Yan Y., Song B.-L. Membrane topology of human NPC1L1, a key protein in enterohepatic cholesterol absorption. J. Lipid Res. 2009;50:1653–1662. doi: 10.1194/jlr.M800669-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang Y., Wang J.F., Cheng Y., Zheng Y.C. The hypocholesterolaemic effects of Lactobacillus acidophilus American Type Culture Collection 4356 in rats are mediated by the down-regulation of Niemann-Pick C1-Like 1. Br. J. Nutr. 2010;104:807–812. doi: 10.1017/S0007114510001285. [DOI] [PubMed] [Google Scholar]
- 34.Huang Y., Zheng Y. The probiotic Lactobacillus acidophilus reduces cholesterol absorption through the down-regulation of Niemann-Pick C1-like 1 in Caco-2 cells. Br. J. Nutr. 2010;103:473–478. doi: 10.1017/S0007114509991991. [DOI] [PubMed] [Google Scholar]
- 35.Chen Z.-Y., Ma K.Y., Liang Y., Peng C., Zuo Y. Role and classification of cholesterol-lowering functional foods. J Funct Foods. 2011;3:61–69. doi: 10.1016/j.jff.2011.02.003. [DOI] [Google Scholar]
- 36.Patel A.K., Singhania R.R., Pandey A., Chincholkar S.B. Probiotic bile salt hydrolase: current developments and perspectives. Appl. Biochem. Biotechnol. 2010;162:166–180. doi: 10.1007/s12010-009-8738-1. [DOI] [PubMed] [Google Scholar]
- 37.Sridevi N., Vishwe P., Prabhune A. Hypocholesteremic effect of bile salt hydrolase from Lactobacillus buchneri ATCC 4005. Food Res. Int. 2009;42:516–520. doi: 10.1016/j.foodres.2009.02.016. [DOI] [Google Scholar]
- 38.Guo C.-F., Zhang S., Yuan Y.-H., Li J.-Y., Yue T.-L. Bile salt hydrolase and S-layer protein are the key factors affecting the hypocholesterolemic activity of Lactobacillus casei-fermented milk in hamsters. Mol. Nutr. Food Res. 2018;62:1800728. doi: 10.1002/mnfr.201800728. [DOI] [PubMed] [Google Scholar]
- 39.Gu J.J., Hofmann A.F., Tonnu H.T., Schteingart C.D., Mysels K.J. Solubility of calcium salts of unconjugated and conjugated natural bile-acids. J. Lipid Res. 1992;33:635–646. [PubMed] [Google Scholar]
- 40.Ridlon J.M., Kang D.J., Hylemon P.B. Bile salt biotransformations by human intestinal bacteria. J. Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Ru X., Zhang C.-C., Yuan Y.-H., Yue T.-L., Guo C.-F. Bile salt hydrolase activity is present in nonintestinal lactic acid bacteria at an intermediate level. Appl. Microbiol. Biotechnol. 2019;103:893–902. doi: 10.1007/s00253-018-9492-5. [DOI] [PubMed] [Google Scholar]
- 42.Settanni L., Di Grigoli A., Tornambé G., Bellina V., Francesca N., Moschetti G., Bonanno A. Persistence of wild Streptococcus thermophilus strains on wooden vat and during the manufacture of a traditional Caciocavallo type cheese. Int. J. Food Microbiol. 2012;155:73–81. doi: 10.1016/j.ijfoodmicro.2012.01.022. [DOI] [PubMed] [Google Scholar]
- 43.Fletouris D.J., Botsoglou N.A., Psomas I.E., Mantis A.I. Rapid determination of cholesterol in milk and milk products by direct saponification and capillary gas chromatography. J Dairy Sci. 1998;81:2833–2840. doi: 10.3168/jds.S0022-0302(98)75842-4. [DOI] [PubMed] [Google Scholar]
- 44.Tanaka H., Hashiba H., Kok J., Mierau I. Bile salt hydrolase of Bifidobacterium longum - Biochemical and genetic characterization. Appl. Environ. Microbiol. 2000;66:2502–2512. doi: 10.1128/AEM.66.6.2502-2512.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu P.-L., Yuan Y.-H., Yue T.-L., Guo C.-F. A new method for the in vitro determination of the bile tolerance of potentially probiotic lactobacilli. Appl. Microbiol. Biotechnol. 2018;102:1903–1910. doi: 10.1007/s00253-018-8742-x. [DOI] [PubMed] [Google Scholar]
- 46.Han Q., Kong B., Chen Q., Sun F., Zhang H. In vitro comparison of probiotic properties of lactic acid bacteria isolated from Harbin dry sausages and selected probiotics. J. Funct. Foods. 2017;32:391–400. doi: 10.1016/j.jff.2017.03.020. [DOI] [Google Scholar]
- 47.Pan X., Chen F., Wu T., Tang H., Zhao Z. The acid, bile tolerance and antimicrobial property of Lactobacillus acidophilus NIT. Food Control. 2009;20:598–602. doi: 10.1016/j.foodcont.2008.08.019. [DOI] [Google Scholar]
- 48.NIH (National Institutes of Health) Guide for the Care and Use of Laboratory Animals. National Academy Press; Washington, DC, USA: 1996. [Google Scholar]
- 49.Guo C.-F., Yuan Y.-H., Yue T.-L., Li J.-Y. Hamsters are a better model system than rats for evaluating the hypocholesterolemic efficacy of potential probiotic strains. Mol. Nutr. Food Res. 2018;62:1800170. doi: 10.1002/mnfr.201800170. [DOI] [PubMed] [Google Scholar]