Abstract
Accumulating evidence has revealed that survivin expression is associated with a malignant phenotype and poor prognosis in glioma. Survivin is also a potential target of microRNA (miRNA/miR)-218. The aim of the present study was to investigate the expression and function of survivin in glioblastoma, and to examine the association between survivin and miR-218. For that purpose, survivin mRNA levels were analyzed in 144 frozen samples of glioblastoma using whole-genome RNA sequencing. In vitro cell proliferation, migration, invasion and apoptosis assays were performed, and survivin expression was detected by western blotting. The results revealed that the mRNA expression levels of survivin were negatively and significantly associated with overall survival in glioblastoma. Further in vitro analyses suggested that miR-218 may inhibit the expression of survivin. Expression of miR-218 in the LN229 cell line was significantly lower than that in the immortalized human gliocyte HEB cell line. miR-218 markedly inhibited tumor cell proliferation, migration and invasion capacities, and decreased apoptosis. miR-218 also inhibited the expression of survivin. These results indicated that survivin may be a target of miR-218 and could serve as a predictive biomarker.
Keywords: glioblastoma, survivin, miRNA-218, prognosis, proliferation
Introduction
Glioblastoma multiforme (GBM) is one of the most common and malignant primary brain tumors (1), and is characterized by diffuse infiltration, uncontrolled proliferation and chemoresistance. Even following tumor resection and radio-chemotherapy, improvement in progression-free survival (PFS) and overall survival (OS) is limited and usually accompanied by malignant progression (2–4).
MicroRNAs (miRNA/miRs) are small, non-coding RNAs that regulate the expression of multiple genes at the post-transcriptional level by inducing degradation of target mRNAs or by inhibiting translation (5,6). Increasing evidence indicates that miRNAs regulate a variety of tumor cell processes, including proliferation, invasion, apoptosis and chemoresistance (7–9). miR-218 has been reported to be downregulated in several neoplasms and serves critical roles in tumorigenesis and metastasis. It exhibits low expression levels in numerous tumor tissues and is associated with patient prognosis (10–13).
Survivin, encoded by and also known as baculoviral inhibitor of apoptosis repeat-containing 5 (BIRC5), is a member of the inhibitor of apoptosis (IAP) family (14). Survivin is strongly expressed in a majority of tumors and is overexpressed during embryonic development, whereas it is absent in normal differentiated tissues (15). Survivin regulates cell-cycle progression, inhibits apoptosis and induces chromosomal instability (16,17). It is also associated with poor prognosis in ovarian cancer, breast cancer and leukemia (18,19). miR-218 directly binds to survivin mRNA, and the miR-218/survivin axis has been reported in a variety of tumors and regulates cancer cells migration, invasion and lymph node metastasis (20,21). A recent study predicted that miR-218 targets the 3′UTR of survivin mRNA and suggested that miR-218 may play an important role in the regulation of surviving (19). However, the association between miR-218 and survivin mRNA in GBM remains unknown. The present study investigated the miR-218/survivin axis in GBM and observed the effects of miR-218 overexpression on the proliferation, migration and invasion of glioma cells.
Materials and methods
Patients and clinical specimens
The data of 144 patients with GBM and genome RNA sequencing were collected from the Chinese Glioma Genome Atlas (CGGA) (http://www.cgga.org.cn). The clinical characteristics of the patients are listed in Table I. In total, 22 patients received radiotherapy only, 25 received temozolomide (TMZ) only, 59 were treated with radiotherapy plus TMZ and 38 received no treatment. Tumor specimens and adjacent tissues were obtained from six patients (36–58 years old; 2 women and 4 men) who underwent surgery between January 15th and February 20th, 2018) in the Department of Neurosurgery of Bejing Tiantan Hospital (Bejing, China). The adjacent tissues were defined as the area between the tumor and the normal brain tissue. The present study was approved by the Ethics Committee of The Capital Medical University (Bejing, China), and all patients provided informed consent.
Table I.
Clinical information for 20% of patients with longer survival times in low-survivin expression group.
| Sample ID | Gender | Age, years | OS, days | TCGA subtype | IDH mutation | MGMT methylation | RT | TMZ | TP53 mutation | EGFR mutation | ATRX mutation |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 494 | F | 59 | 2,072 | Neural | 0 | 1 | 1 | 1 | 1 | 0 | 0 |
| 680 | M | 44 | 1,832 | Neural | 0 | 0 | 1 | 1 | 1 | 0 | 0 |
| 761 | M | 45 | 1,744 | Proneural | 1 | 1 | 1 | 0 | 0 | 1 | 0 |
| 822 | M | 46 | 1,324 | Proneural | 1 | 1 | 1 | 1 | 1 | 0 | 0 |
| 491 | F | 37 | 1,074 | Proneural | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| D37 | M | 55 | 965 | Mesenchymal | 0 | 0 | 1 | 1 | 1 | 0 | 0 |
| 1026 | M | 59 | 875 | Classical | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| D57 | M | 40 | 766 | Classical | 0 | NA | 1 | 1 | 1 | 0 | 0 |
| 802 | M | 43 | 681 | Mesenchymal | 0 | 0 | 1 | 1 | 1 | 0 | 0 |
| 1023 | F | 58 | 681 | Classical | 0 | 0 | NA | NA | 0 | 0 | 0 |
| 700 | F | 25 | 669 | Proneural | 0 | 1 | 1 | 1 | 0 | 0 | 0 |
| D35 | F | 68 | 661 | Mesenchymal | 0 | 1 | 1 | 0 | 1 | 0 | 1 |
| 710 | M | 42 | 660 | Mesenchymal | 1 | 1 | 0 | 1 | 0 | 0 | 0 |
F, female; M, male; OS, overall survival; TCGA, the cancer genome atlas; IDH, isocitrate dehydrogenase; MGMT, O6-alkylguanine DNA alkyltransferase; RT, radiotherapy; TMZ, temozolomide; TP53, tumor suppressor p53; EGFR, epidermal growth factor receptor; 0, no; 1, yes; NA, not available.
Cell culture and transfection
Immortalized human gliocyte HEB cells (cat. no. 001398) and human GBM LN229 cells (cat. no. 001397) were obtained from Shanghai Bioleaf Biotech Co., Ltd. The cell lines were maintained in Dulbecco's modified Eagle's medium (DMEM; Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) at 37°C in a 95% relative humidified incubator with 5% CO2. LN229 cells in the logarithmic growth phase were transfected with miR-218 mimics (10 µl/ml) using Lipofectamine™ 2000 (Invitrogen; Thermo Fisher Scientific, Inc.) and cultured at 37°C in a 5% CO2 humidified atmosphere for 6 h. Medium was then changed and cells were left in incubator for 48 h. The sequences transfected were as follows: Sense 5′-UUCUCCGAACGUGUCACGUTT-3′, anti-sense 5′-ACGUGACACGUUCGGAGAATT-3′; and negative control (NC) mimics, sense 5′-UUGUGCUUGAUCUAACCAUGU-3′, and anti-sense 5′-AUGGUUAGAUCAAGCACAAUU-3′ (Shanghai GenePharma Co., Ltd.).
Reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
The mRNA expression levels of miR-218 and survivin were detected in tumor specimens, adjacent tissues and the HEB and LN229 cell lines. In addition, mRNA levels of miR-218 and survivin were detected in the control, NC and mimics groups. The primers were provided by Tsingke Biological Technology, Co., Ltd. and were designed as follows: miR-218, loop primer 5′-GTCGTATCCAGTGCAGGGTCCGAGGTATTCGCACTGGATACGACACATGGTT-3′, forward 5′-TGCGCTTGTGCTTGATCTAAC-3′; U6, forward 5′-CGCTTCGGCAGCACATATAC-3′, reverse, 5′-AAATATGGAACGCTTCACGA-3′; survivin, forward 5′-ACCACCGCATCTCTACATTCA-3′, reverse 5′-CTTTGCATGGGGTCGTCATC-3′; and β-actin, forward 5′-AGCGAGCATCCCCCAAAGTT-3′ and reverse 5′-GGGCACGAAGGCTCATCATT-3′. U6 and β-actin were used as internal control for miR-218 and surviving, respectively. Total RNA (1 µg) was isolated using Trizol reagent (cat. no. 252250AX, Aidalb Biotechnologies Co., Ltd.) and reversely transcribed to cDNA using Hiscript Reverse Transcriptase Reagent Kit (cat. no. R101-01/02; Vazyme Ltd.) according to the manufacturer's instructions. The PCR amplification process was detection on an ABI 7900 or Viia7 Fast Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc., Waltham, MA, USA). The PCR conditions for amplification were as follows: Pre-denaturation at 95°C for 10 min, denaturation at 95°C for 30 sec, 40 cycles, annealing at 60°C for 30 sec and extension at 72°C for 30 sec. The relative expression levels were normalized to endogenous controls and were calculated using 2−ΔΔCq method (22).
Cell Counting Kit-8 (CCK-8) proliferation assay
Cell proliferation was evaluated using a CCK-8 proliferation assay (cat. no. C0037; Beyotime Institute of Biotechnology), which is a sensitive colorimetric assay for the determination of the number of viable cells based on the production of formazan (23). Prior to proliferation assays, the numbers of viable cells were equal between the experimental and NC group. LN229 cells were seeded into 96-well plates (2,000 cells/well) and cultured at 37°C in a 5% CO2 humidified atmosphere for 3 days. Next, 10 µl CCK-8 was added to each well and the cells were incubated at 37°C for 4 h. The absorbance value of each well was measured using a microplate reader 450 nm (24).
Transwell migration and invasion assays
Following transfection, an equal number of viable cells were added to the upper chambers (3-µm pore size) of a 24-well Transwell plate. Suspensions of cells (200 µl, 5×105 cells/ml) in DMEM from different groups were added to the upper chambers, which were pretreated with Matrigel for the invasion assay but untreated for the migration assay. The lower chambers contained 800 µl DMEM plus 10% FBS. After 24 h of incubation at 37°C and 5% CO2, the upper chambers were detached from the plates and the cells remaining on the upper surface of the filters were removed with cotton swabs. The cells on the lower surface were fixed with 10% methyl alcohol at 25°C for 30 min, and stained with Giemsa at 25°C for 20 min. Three high-power fields were selected randomly to examine the invading and migrating cells under light microscopy.
Apoptosis assay
Flow cytometry was used to analyze apoptosis in LN229 cells transfected with miR-218 mimics or the negative control (NC). According to the manufacturer's instructions, 48 h after transfection, cells were stained with Annexin V-allophycocyanin/7-amino-actinomycin D apoptosis detection kit (cat. no. KA3807; Abnova). Apoptotic fractions were detected using flow cytometry (AccuriC6; BD Biosciences) and CELL Quest 3.0 software.
Western blot analysis
Extraction of total protein from the tissues and cells was achieved with RIPA Lysis Buffer (cat. no. P0013B; Beyotime Institute of Biotechnology). Protein concentrations were determined using a BCA protein detection kit (cat. no. P0010; Beyotime Institute of Biotechnology). In total, 40 µg protein was subjected to electrophoresis on 10% SDS-polyacrylamide gels and then transferred to polyvinylidene difluoride membranes (cat. no. IPVH00010; EMD Millipore). Membranes were blocked with 5% fat-free milk diluted in TBST containing 0.1% Tween-20 for 2 h at 25°C. Membranes were then incubated with a 1:800-diluted anti-survivin antibody (cat. no. bs-0615R; BIOSS) or a 1:1,000-diluted anti-GAPDH antibody (cat. no. AB-P-R001; Hangzhou Xianzhi Biotechnology Co., Ltd.) as the loading control, for 24 h at 4°C. They were then incubated with the 1:5,000-diluted horseradish peroxidase (HRP) labeled goat anti-rabbit secondary antibody (cat. no. BA1045; Boster Biological Technology) for 60 min at 37°C. The immune imprint was visualized with an enhanced chemiluminescence western blot detection system (Bio-Rad Laboratories, Inc.). Relative expression level of survivin was normalized to endogenous control GAPDH ChemImager 5,500 V2.03 software.
Immunohistochemical (IHC) staining
IHC analysis of survivin expression was performed on section fixed for 24 h at 25°C with 10% formalin and paraffin-embedded using standard procedures. Briefly, sections (4-µm thick) were dried for 12 h at 37°C, deparaffinized in xylene for 10 min, rehydrated in an alcohol series, and then placed in 3% hydrogen peroxide for 10 min to block endogenous peroxidase activity. Sections were incubated with a 1:100 anti-survivin antibody at 4°C for 12 h. Subsequently, sections were washed in PBS three times for 5 min and then incubated for 50 min at 25°C with 100 µl 1:5,000-diluted HRP labeled goat anti-rabbit secondary antibody (cat. no. BA1045; Boster Biological Technology). Finally, immunostaining was visualized with diaminobenzidine, and sections were counterstained with hematoxylin and eosin for 25 sec at 25°C prior to washing again with PBS.
Fluorescence in situ hybridization (FISH)
The expression status of miR-218 in GBM samples was evaluated by FISH. Homo sapiens (hsa)-miR-218-5p probes (red-labeled; Sangon Biotech Co., Ltd.) (5′-ACATGGTTAGATCAAGCACAA-3′) directed against the mature miR-218 sequence were used. Sections (4-µm thick) were dried at 37°C for 12 h, deparaffinized in xylene for 10 min, rehydrated in an alcohol series, washed 3 times with PBS (5 min/wash), and then digested using proteinase K for 30 min at 37°C. After washing again 3 times using PBS (5 min/wash), the sections were hybridized with the probes (3 ng/ml) for 24 h at 45°C. Subsequently, the tissue sections were washed in 5X saline sodium citrate (SSC) at 45°C for 15 min, 4X SSC at 37°C for 15 min, 2X SSC (containing 50% deionized formamide) at 37°C for 15 min, 2X SSC (containing 20 µg/ml RNaseA) at 37°C for 15 min and 0.5X SSC for 15 min, and then washed 3 times for 5 min each with 0.01 mol/l PBS. Finally, sections were counterstained with DAPI (cat. no. C1002; Beyotime Institute of Biotechnology) for 5 min and examined with an Olympus BX53 microscope.
Statistical analysis
According to the median expression level of survivin, the samples were divided into two groups. Each group was assessed by the Student's t-test and χ2 test using R language 3.2.5 (https://cran.r-project.org/bin/windows/base/old/3.2.5/) and SPSS software 16.0 (SPSS, Inc.). OS was defined as the time following surgery until mortality or last follow-up date. Using the Kaplan-Meier method protract survival analysis by GraphPad Prism 7 (GraphPad Software, Inc.), the differences between the low- and high-survivin expression groups were calculated using the log-rank test. For continuous variables, comparisons of mean values between multiple groups were assessed by ANOVA, and then Holm-Sidak's multiple comparisons test was used. All data are presented as the mean ± standard error. P<0.05 was considered to indicate a statistically significant difference. Factors with P<0.05 in univariate analysis were incorporated into a multivariate Cox analysis.
Results
Survivin expression is associated with poor prognosis in patients with GBM
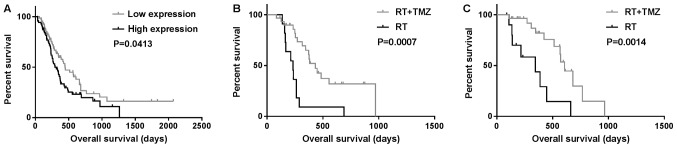
Data from 144 patients with GBM and whole-genome RNA sequencing were collected from the CGGA database and used in the present study. The prognosis of the group with high survivin expression was poor on Student's t-test and univariate analysis (P<0.05) (Fig. 1A; Table III). Survivin was highly expressed in classical subtype gliomas, while all neural subtype gliomas exhibited low expression of survivin (P=0.013 and P<0.01, respectively; Table II). In multivariate Cox analysis, the expression of survivin was observed to be associated with OS (P=0.006; Table III). In total, 81 patients with GBM received radiation plus TMZ chemotherapy or radiation only. OS in the radiotherapy alone group (358±82 days) was significantly different from that in the radiation plus TMZ chemotherapy group (885±116 days; P=0.0007 and P=0.0014, respectively) independent of the expression levels of survivin (Fig. 1B and C).
Figure 1.
Kaplan-Meier survival curves showing OS. (A) OS according to the expression of survivin; (B and C) OS of patients treated with RT + TMZ or RT only in the (B) high and (C) low-survivin expression groups. OS, overall survival; RT, radiotherapy; TMZ, temozolomide.
Table III.
Prognostic factors of overall survival for 144 patients with glioblastoma multiforme.
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| Variable | P-value | P-value | HR | 95% CI lower | 95% CI upper |
| Sex | 0.353 | ||||
| Age, ≤48 vs. >48 | 0.88 | ||||
| ATRX mutation | 0.519 | ||||
| EGFR mutation | 0.241 | ||||
| TERT mutation | 0.752 | ||||
| IDH mutation | 0.069 | 0.01 | 0.204 | 0.061 | 0.686 |
| MGMT methylation | 0.009 | 0.12 | 0.533 | 0.241 | 1.178 |
| Survivin high expression | 0.041 | 0.006 | 2.898 | 1.355 | 6.198 |
| KPS | <0.001 | 0.005 | 0.343 | 0.162 | 0.723 |
| RT vs. RT+TMZ | <0.001 | 0.005 | 0.311 | 0.139 | 0.697 |
| Resection, GTR vs. STR | 0.174 | 0.151 | 1.704 | 0.824 | 3.522 |
HR, hazard ratio; CI, confidence interval; EGFR, epidermal growth factor receptor; TERT, telomerase reverse transcriptase; IDH, isocitrate dehydrogenase; MGMT, O6-alkylguanine DNA alkyltransferase; KPS, Karnofsky performance status; GTR, gross total resection; STR, subtotal resection; RT, radiotherapy; TMZ, temozolomide.
Table II.
Clinical and molecular characteristics of 144 patients with glioblastoma multiforme.
| Survivin expression | ||||
|---|---|---|---|---|
| Variables | Total | High | Low | P-value |
| Sex | ||||
| Female | 51 | 23 | 28 | 0.384 |
| Male | 93 | 49 | 44 | |
| Age, years | ||||
| >48 | 71 | 33 | 38 | 0.405 |
| ≤48 | 73 | 39 | 34 | |
| IDH mutation | ||||
| Mutation | 39 | 20 | 19 | 0.851 |
| Wild type | 105 | 52 | 53 | |
| TP53 mutation | ||||
| Mutation | 77 | 36 | 41 | 0.404 |
| Wild type | 67 | 36 | 31 | |
| EGFR mutation | ||||
| Mutation | 39 | 19 | 20 | 0.851 |
| Wild type | 105 | 53 | 52 | |
| ATRX mutation | ||||
| Mutation | 13 | 4 | 9 | 0.146 |
| Wild type | 131 | 68 | 63 | |
| TERT promoter mutation | ||||
| Mutation | 36 | 21 | 15 | 0.305 |
| Wild type | 65 | 31 | 34 | |
| MGMT methylation | ||||
| Yes | 61 | 34 | 27 | 0.368 |
| No | 71 | 34 | 37 | |
| KPS | ||||
| >70 | 60 | 27 | 33 | 0.317 |
| ≤70 | 36 | 20 | 16 | |
| Resection | ||||
| GTR | 90 | 44 | 46 | 0.717 |
| STR | 46 | 24 | 22 | |
| Treatment | ||||
| RT | 22 | 11 | 11 | 0.946 |
| RT+TZM | 59 | 30 | 29 | |
| TCGA subtype | ||||
| Proneural | 33 | 20 | 13 | 0.165 |
| Neural | 12 | 0 | 12 | <0.001 |
| Classical | 48 | 31 | 17 | 0.013 |
| Mesenchymal | 51 | 21 | 30 | 0.117 |
IDH, isocitrate dehydrogenase; TP53, tumor suppressor p53; EGFR, epidermal growth factor receptor; TERT, telomerase reverse transcriptase; MGMT, O6-alkylguanine DNA alkyltransferase; KPS, Karnofsky performance status; GTR, gross total resection; STR, subtotal resection; RT, radiotherapy; TMZ, temozolomide; TCGA, The Cancer Genome Atlas.
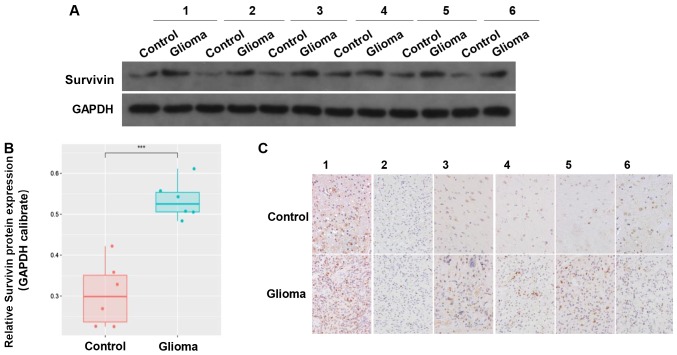
Expression of survivin is reduced in tumor samples compared with in adjacent tissues
The clinical data of 6 cases of GBM tumor samples were collected. The results revealed that there was higher expression of survivin in the glioma tissues compared with in the adjacent control tissues (Fig. 2A and B). IHC indicated that survivin was mainly located in the cytoplasm and nucleus (Fig. 2C). These results suggested that survivin was highly expressed in gliomas.
Figure 2.
Expression of survivin in clinical tissue samples. (A and B) Protein expression of survivin in glioma and adjacent tissues. (C) Immunohistochemistry showing the expression of survivin in gliomas and adjacent tissues. ***P<0.001.
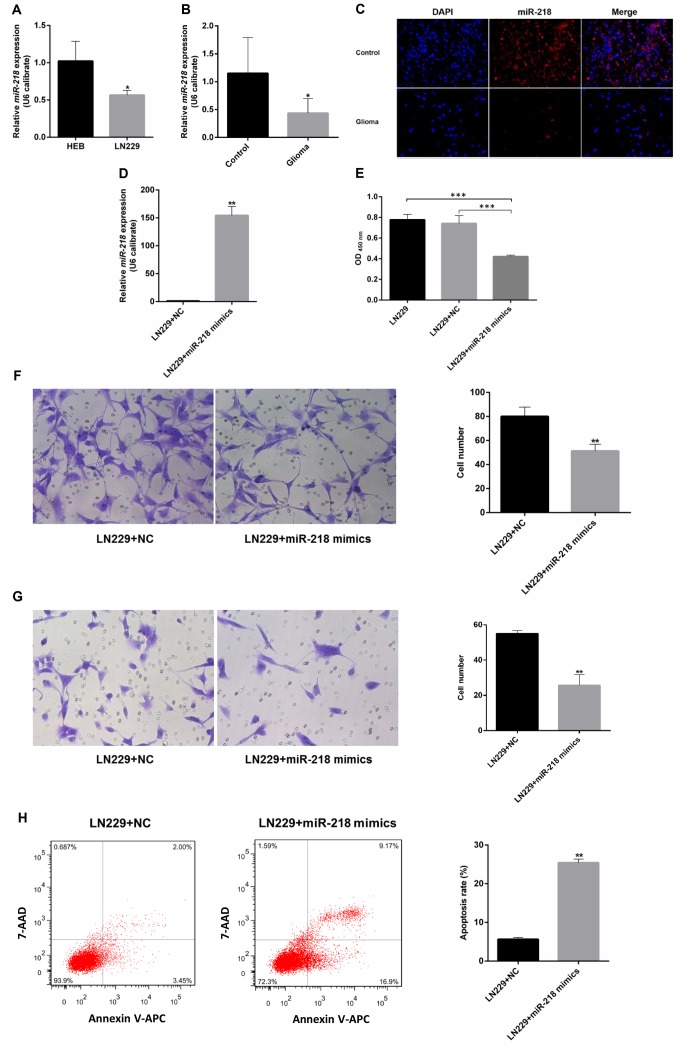
miR-218 expression in the LN229 GBM cell line is significantly lower compared with that in the control cell line. Compared with immortalized human gliocyte HEB cells (25,26), GBM cells had lower miR-218 expression (Fig. 3A). The same pattern was observed in glioma and adjacent samples from 6 patients with GBM. Compared with in the adjacent tissues, the level of miR-218 expression in glioma was reduced (Fig. 3B). A red-labeled hsa-miR-218-5p probe was used for FISH. The results revealed that miR-218-5p expression in glioma tissues was low compared with that in adjacent tissues (Fig. 3C).
Figure 3.
Function of miR-218 in glioblastoma. miR-218 expression in (A) cell lines and (B) clinical samples, as detected by RT-qPCR and (C) fluorescence in situ hybridization. (D) Expression of miR-218 in LN229 cells transfected with miR-218 mimics or NC. (E-H) Proliferation, migration, invasion and apoptosis of LN229 cells transfected with miR-218 mimics or NC. *P<0.05, **P<0.01 and ***P<0.001. miR, microRNA; NC, negative control; 7-AAD, 7-amino-actinomycin D; APC, allophycocyanin.
miR-218 inhibits the proliferation, migration and invasion of GBM cells, and contributes to apoptosis
Upon transfection with miR-218 mimics, the expression of miR-218 in LN229 cells increased significantly compared with that of the LN229+NC group (Fig. 3D). A CCK-8 proliferation assay revealed that, upon transfection with miR-218 mimics, the optical density values of LN229 cells decreased significantly compared with the LN229+NC group, suggesting that overexpression of miR-218 can inhibit the proliferation of LN229 cells (Fig. 3E). Transwell migration and invasion assays revealed that miR-218 mimics can inhibit the invasion and migration of glioma cells (Fig. 3F and G). The results of flow cytometry demonstrated that the apoptosis rate of LN229 cells markedly increased upon transfection with miR-218 mimics compared with that of the LN229+NC group, suggesting that high expression of miR-218 can promote the apoptosis of LN229 cells (Fig. 3H).
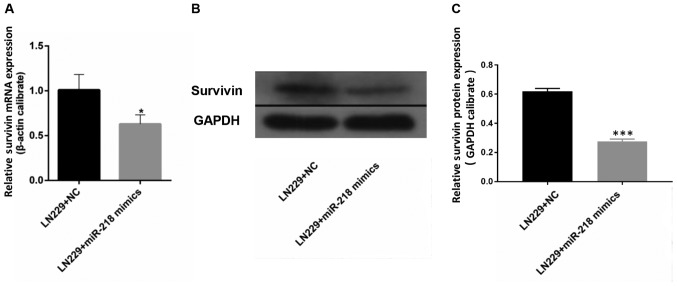
miR-218 overexpression is associated with reduced expression of survivin. The results of RT-qPCR demonstrated that the mRNA expression of survivin in LN229 cells was significantly reduced upon transfection with miR-218 mimics compared with that in the LN229+NC group (Fig. 4A). Western blotting revealed a similar outcome: Expression of survivin in LN229 cells was lower following transfection with miR-218 mimics compared with that in the LN229+NC group (Fig. 4B and C).
Figure 4.
Survivin is a target of miR-218. Upon transfection with miR-218 mimics, (A) survivin mRNA was assessed by RT-qPCR and (B and C) survivin protein was determined by western blot analysis in LN229 cells. *P<0.05 and ***P<0.001. miR, microRNA; NC, negative control.
Discussion
Survivin is a member of the IAP protein family, and is encoded by the BIRC5 gene. Survivin is associated with inhibition of apoptosis and regulation of cell-cycle progression (17) and acts as an inhibitor of programmed cell death by binding to caspase-3/7 in the G2/M phase (27). The expression of survivin is higher during fetal development and in the majority of tumors, whereas it is completely absent in adults and in normal cells (17,28). Overexpression of survivin has been confirmed in breast, lung, ovarian, prostate and colon cancer (21,29). Expression of survivin has also been associated with poor prognosis and chemotherapy resistance (21,30,31). A previous study have demonstrated that high expression of survivin may increase chemotherapy resistance, and that survivin inhibition represents an approach to overcoming drug resistance (32). However, no relevant genome atlas studies have been reported in GBM. Survivin is also a potential target of miR-218, which is involved in the proliferative, migratory and invasive behavior of various tumors (20,21). Our previous study, which included 220 gliomas from the CGGA database, demonstrated that the expression of survivin is associated with tumor grade, and that it may be a novel prognostic factor in gliomas (10). Using whole-genome RNA sequencing data of 144 patients with GBM in the present study, it was observed that patients with high expression of survivin had shorter OS times than those with low expression. However, patients who received radiation plus TMZ chemotherapy had a better prognosis compared with patients receiving radiation only, regardless of their survivin expression levels. This may be due to the limited number of cases included in the present study and the heterogeneity of gliomas. Cheng et al (13) demonstrated that miR-218 has a significantly decreased expression level in gliomas, which was also associated with poor PFS and OS. It was also reported that miR-218 may regulate tumor progression (33–36). HEB cells are not fully ‘normal’ astrocytes, as the cell line has been immortalized, which may reduce the differences in proliferative ability between astrocytes and GBM cells (25,26). Therefore, patient samples were used in the present study to verify the results. The current study indicated that the expression of miR-218 in the LN229 cell line was significantly lower than that in HEB cells, which was also validated in clinical samples. In vitro, the results of the present study confirmed that miR-218 could inhibit the proliferation, migration and invasion of GBM cells, and contribute to their apoptosis. As indicated by Gao and Jin (37), miR-218 can influence cancer-related processes by targeting a series of genes, including survivin. However, the role of the miR-218/survivin axis in GBM remains unknown. Compared with previous studies, the present study explored the association between miR-218 and survivin in GBM, and demonstrated that the expression of survivin in LN229 cells decreased significantly upon transfection with miR-218 mimics. Combined with luciferase assay results from previous studies, these observations suggest that miR-218 may directly target surviving (19–21,38). These results indicate that survivin may serve as a potential predictive biomarker targeting miR-218.
However, there are several limitations in the present study. LN229 is a typical glioma cell line, and when compared with other GBM cells, there are no significant differences in adherence and proliferation capacity (39–41). However, in the current study, only one GBM cell line was used and there was no verification using animal models. Furthermore, the sample size was limited. In addition, the association between survivin protein expression and patient survival is unknown. Thus, multiple cell lines, animal models, and larger multicenter sites for patient recruitment will be considered in future studies. In the laboratory, a stably overexpressing cell line will be set up and used to determine relative proliferation and migration. Patients or their relatives will be followed up to obtain extra prognostic information. Associated pathways will also be investigated, such as SLIT2-ROBO1 pathway. Furthermore, survivin has been reportedly associated with mitotic catastrophe (16,17), which should be further explored in glioma. Cell cycle analysis will also be performed to assess the impact of miR-218 overexpression on LN229 cells. Experiments using a caspase inhibitor, such as z-VAD-fmk, to identify the type of cell death are required to confirm the involvement of the apoptotic cascade (42). A luciferase assay will be used to demonstrate that miR-218 directly targets survivin. These remaining questions will be addressed in future studies.
Acknowledgements
The authors would like to thank Ms. Hua Huang from the Beijing Neurosurgical Institute (Beijing, China) for technical support with experiments and her contribution to the CGGA database.
Glossary
Abbreviations
- GBM
glioblastoma multiforme
- PFS
progression-free survival
- OS
overall survival
- miR-218
microRNA-218
- IAP
inhibitor of apoptosis
- BIRC5
baculoviral inhibitor of apoptosis repeat-containing 5
- miRNAs/miRs
microRNAs
- CGGA
Chinese Glioma Genome Atlas
- TMZ
temozolomide
- DMEM
Dulbecco's modified Eagle's medium
- FBS
fetal bovine serum
- NC
negative control
- CCK-8
Cell Counting Kit-8
- IHC
immunohistochemistry
- FISH
fluorescence in situ hybridization
- RT-qPCR
reverse transcription-quantitative polymerase chain reaction
- SSC
saline sodium citrate
Funding
The present study was supported by the Natural Science Foundation of Beijing (Beijing, China; grant no. 7152052), the Natural Science Foundation of China Capital Medical University, (Beijing, China; grant no. PYZ2017145), and the Miaopu Project of Beijing Tiantan Hospital (Beijing, China; grant no. 2017MP05).
Availability of data and materials
The datasets generated and/or analyzed during the current study are available in the Chinese Glioma Genome Atlas (CGGA) (http://www.cgga.org.cn).
Authors' contributions
XT, PY and KW performed most of the experiments and drafted the manuscript. YL and XL helped with data analysis. XS, RH and KZ helped with tumor samples collection, designed figures and revised the manuscript. JW designed the study and revised the manuscript. All authors read and approved the final manuscript.
Ethics approval and consent to participate
This study was approved by the Ethics Committee of The Capital Medical University (Beijing, China). All patients provided informed consent.
Patient consent for publication
Written informed consent was obtained from each patient involved in this study.
Competing interests
The authors declare that they have no conflicts of interest.
References
- 1.Weller M, van den Bent M, Hopkins K, Tonn JC, Stupp R, Falini A, Cohen-Jonathan-Moyal E, Frappaz D, Henriksson R, Balana C, et al. EANO guideline for the diagnosis and treatment of anaplastic gliomas and glioblastoma. Lancet Oncol. 2014;15:e395–e403. doi: 10.1016/S1470-2045(14)70011-7. [DOI] [PubMed] [Google Scholar]
- 2.Thakkar JP, Dolecek TA, Horbinski C, Ostrom QT, Lightner DD, Barnholtz-Sloan JS, Villano JL. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev. 2014;23:1985–1996. doi: 10.1158/1055-9965.EPI-14-0275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ostrom QT, Gittleman H, Stetson L, Virk SM, Barnholtz-Sloan JS. Epidemiology of gliomas. Cancer Treat Res. 2015;163:1–14. doi: 10.1007/978-3-319-12048-5_1. [DOI] [PubMed] [Google Scholar]
- 4.Omuro A, DeAngelis LM. Glioblastoma and other malignant gliomas: A clinical review. JAMA. 2013;310:1842–1850. doi: 10.1001/jama.2013.280319. [DOI] [PubMed] [Google Scholar]
- 5.Li N, Wang L, Tan G, Guo Z, Liu L, Yang M, He J. MicroRNA-218 inhibits proliferation and invasion in ovarian cancer by targeting Runx2. Oncotarget. 2017;8:91530–91541. doi: 10.18632/oncotarget.21069. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 6.Shi D, Zhang Y, Lu R, Zhang Y. The long non-coding RNA MALAT1 interacted with miR-218 modulates choriocarcinoma growth by targeting Fbxw8. Biomed Pharmacother. 2018;97:543–550. doi: 10.1016/j.biopha.2017.10.083. [DOI] [PubMed] [Google Scholar]
- 7.Mohr AM, Mott JL. Overview of microRNA biology. Semin Liver Dis. 2015;35:3–11. doi: 10.1055/s-0034-1397344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hammond SM. An overview of microRNAs. Adv Drug Deliv Rev. 2015;87:3–14. doi: 10.1016/j.addr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen P, Zhao Y, Li Y. MiR-218 inhibits migration and invasion of lung cancer cell by regulating Robo1 expression. Zhongguo Fei Ai Za Zhi. 2017;20:452–458. doi: 10.3779/j.issn.1009-3419.2017.07.03. (In Chinese) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bao ZS, Li MY, Wang JY, Zhang CB, Wang HJ, Yan W, Liu YW, Zhang W, Chen L, Jiang T. Prognostic value of a nine-gene signature in glioma patients based on mRNA expression profiling. CNS Neurosci Ther. 2014;20:112–118. doi: 10.1111/cns.12171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen W, Yu Q, Chen B, Lu X, Li Q. The prognostic value of a seven-microRNA classifier as a novel biomarker for the prediction and detection of recurrence in glioma patients. Oncotarget. 2016;7:53392–53413. doi: 10.18632/oncotarget.10534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Y, Ding L, Hu Q, Xia J, Sun J, Wang X, Xiong H, Gurbani D, Li L, Liu Y, Liu A. MicroRNA-218 functions as a tumor suppressor in lung cancer by targeting IL-6/STAT3 and negatively correlates with poor prognosis. Mol Cancer. 2017;16:141. doi: 10.1186/s12943-017-0710-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng MW, Wang LL, Hu GY. Expression of microRNA-218 and its clinicopathological and prognostic significance in human glioma cases. Asian Pac J Cancer Prev. 2015;16:1839–1843. doi: 10.7314/APJCP.2015.16.5.1839. [DOI] [PubMed] [Google Scholar]
- 14.Mobahat M, Narendran A, Riabowol K. Survivin as a preferential target for cancer therapy. Int J Mol Sci. 2014;15:2494–2516. doi: 10.3390/ijms15022494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaskoll T, Chen H, Min Zhou Y, Wu D, Melnick M. Developmental expression of survivin during embryonic submandibular salivary gland development. BMC Dev Biol. 2001;1:5. doi: 10.1186/1471-213X-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Conde M, Michen S, Wiedemuth R, Klink B, Schröck E, Schackert G, Temme A. Chromosomal instability induced by increased BIRC5/Survivin levels affects tumorigenicity of glioma cells. BMC Cancer. 2017;17:889. doi: 10.1186/s12885-017-3932-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sheng L, Wan B, Feng P, Sun J, Rigo F, Bennett CF, Akerman M, Krainer AR, Hua Y. Downregulation of Survivin contributes to cell-cycle arrest during postnatal cardiac development in a severe spinal muscular atrophy mouse model. Hum Mol Genet. 2018;27:486–498. doi: 10.1093/hmg/ddx418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yoshikawa K, Shimada M, Higashijima J, Nakao T, Nishi M, Takasu C, Kashihara H, Eto S, Bando Y. Ki-67 and survivin as predictive factors for rectal cancer treated with preoperative chemoradiotherapy. Anticancer Res. 2018;38:1735–1739. doi: 10.21873/anticanres.12409. [DOI] [PubMed] [Google Scholar]
- 19.Li PL, Zhang X, Wang LL, Du LT, Yang YM, Li J, Wang CX. MicroRNA-218 is a prognostic indicator in colorectal cancer and enhances 5-fluorouracil-induced apoptosis by targeting BIRC5. Carcinogenesis. 2015;36:1484–1493. doi: 10.1093/carcin/bgv145. [DOI] [PubMed] [Google Scholar]
- 20.Kogo R, How C, Chaudary N, Bruce J, Shi W, Hill RP, Zahedi P, Yip KW, Liu FF. The microRNA-218~Survivin axis regulates migration, invasion, and lymph node metastasis in cervical cancer. Oncotarget. 2015;6:1090–1100. doi: 10.18632/oncotarget.2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu Y, Xu K, Yagüe E. miR-218 targets survivin and regulates resistance to chemotherapeutics in breast cancer. Breast Cancer Res Treat. 2015;151:269–280. doi: 10.1007/s10549-015-3372-9. [DOI] [PubMed] [Google Scholar]
- 22.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 23.Ishiyama M, Miyazono Y, Sasamoto K, Ohkura Y, Ueno K. A highly water-soluble disulfonated tetrazolium salt as a chromogenic indicator for NADH as well as cell viability. Talanta. 1997;44:1299–1305. doi: 10.1016/S0039-9140(97)00017-9. [DOI] [PubMed] [Google Scholar]
- 24.Sun Y, Zhang Y, Chi P. Pirfenidone suppresses TGF-β1-induced human intestinal fibroblasts activities by regulating proliferation and apoptosis via the inhibition of the Smad and PI3K/AKT signaling pathway. Mol Med Rep. 2018;18:3907–3913. doi: 10.3892/mmr.2018.9423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dai Z, Wu J, Chen F, Cheng Q, Zhang M, Wang Y, Guo Y, Song T. CXCL5 promotes the proliferation and migration of glioma cells in autocrine- and paracrine-dependent manners. Oncol Rep. 2016;36:3303–3310. doi: 10.3892/or.2016.5155. [DOI] [PubMed] [Google Scholar]
- 26.Zhang T, Shao Y, Chu TY, Huang HS, Liou YL, Li Q, Zhou H. MiR-135a and MRP1 play pivotal roles in the selective lethality of phenethyl isothiocyanate to malignant glioma cells. Am J Cancer Res. 2016;6:957–972. [PMC free article] [PubMed] [Google Scholar]
- 27.Lechler P, Renkawitz T, Campean V, Balakrishnan S, Tingart M, Grifka J, Schaumburger J. The antiapoptotic gene survivin is highly expressed in human chondrosarcoma and promotes drug resistance in chondrosarcoma cells in vitro. BMC Cancer. 2011;11:120. doi: 10.1186/1471-2407-11-120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao G, Wang Q, Gu Q, Qiang W, Wei JJ, Dong P, Watari H, Li W, Yue J. Lentiviral CRISPR/Cas9 nickase vector mediated BIRC5 editing inhibits epithelial to mesenchymal transition in ovarian cancer cells. Oncotarget. 2017;8:94666–94680. doi: 10.18632/oncotarget.21863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Slattery ML, Mullany LE, Sakoda LC, Wolff RK, Samowitz WS, Herrick JS. Dysregulated genes and miRNAs in the apoptosis pathway in colorectal cancer patients. Apoptosis. 2018;23:237–250. doi: 10.1007/s10495-018-1451-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jingjing L, Wangyue W, Qiaoqiao X, Jietong Y. MiR-218 increases sensitivity to cisplatin in esophageal cancer cells via targeting survivin expression. Open Med (Wars) 2016;11:31–35. doi: 10.1515/med-2016-0007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zarogoulidis P, Petanidis S, Kioseoglou E, Domvri K, Anestakis D, Zarogoulidis K. MiR-205 and miR-218 expression is associated with carboplatin chemoresistance and regulation of apoptosis via Mcl-1 and Survivin in lung cancer cells. Cell Signal. 2015;27:1576–1588. doi: 10.1016/j.cellsig.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 32.Zheng HC. The molecular mechanisms of chemoresistance in cancers. Oncotarget. 2017;8:59950–59964. doi: 10.18632/oncotarget.19048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tu Y, Gao X, Li G, Fu H, Cui D, Liu H, Jin W, Zhang Y. MicroRNA-218 inhibits glioma invasion, migration, proliferation, and cancer stem-like cell self-renewal by targeting the polycomb group gene Bmi1. Cancer Res. 2013;73:6046–6055. doi: 10.1158/0008-5472.CAN-13-0358. [DOI] [PubMed] [Google Scholar]
- 34.Gu JJ, Gao GZ, Zhang SM. miR-218 inhibits the migration and invasion of glioma U87 cells through the Slit2-Robo1 pathway. Oncol Lett. 2015;9:1561–1566. doi: 10.3892/ol.2015.2904. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Xia H, Yan Y, Hu M, Wang Y, Wang Y, Dai Y, Chen J, Di G, Chen X, Jiang X. MiR-218 sensitizes glioma cells to apoptosis and inhibits tumorigenicity by regulating ECOP-mediated suppression of NF-κB activity. Neuro Oncol. 2013;15:413–422. doi: 10.1093/neuonc/nos296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu J, Xu W, Zhu J. Propofol suppresses proliferation and invasion of glioma cells by upregulating microRNA-218 expression. Mol Med Rep. 2015;12:4815–4820. doi: 10.3892/mmr.2015.4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao X, Jin W. The emerging role of tumor-suppressive microRNA-218 in targeting glioblastoma stemness. Cancer Lett. 2014;353:25–31. doi: 10.1016/j.canlet.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 38.Alajez NM, Lenarduzzi M, Ito E, Hui AB, Shi W, Bruce J, Yue S, Huang SH, Xu W, Waldron J, et al. MiR-218 suppresses nasopharyngeal cancer progression through downregulation of survivin and the SLIT2-ROBO1 pathway. Cancer Res. 2011;71:2381–2391. doi: 10.1158/0008-5472.CAN-10-2754. [DOI] [PubMed] [Google Scholar]
- 39.Yang X, Zhang C, Guo T, Feng Y, Liu Q, Chen Y, Zhang Q. Reduced expression of microRNA206 regulates cell proliferation via cyclinD2 in gliomas. Mol Med Rep. 2015;11:3295–3300. doi: 10.3892/mmr.2015.3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang H, Wang Y, Bao Z, Zhang C, Liu Y, Cai J, Jiang C. Hypomethylated Rab27b is a progression-associated prognostic biomarker of glioma regulating MMP-9 to promote invasion. Oncol Rep. 2015;34:1503–1509. doi: 10.3892/or.2015.4125. [DOI] [PubMed] [Google Scholar]
- 41.Liu Y, Tang K, Yan W, Wang Y, You G, Kang C, Jiang T, Zhang W. Identifying Ki-67 specific miRNA-mRNA interactions in malignant astrocytomas. Neurosci Lett. 2013;546:36–41. doi: 10.1016/j.neulet.2013.04.030. [DOI] [PubMed] [Google Scholar]
- 42.Zhou GS, Song LJ, Yang B. Isoliquiritigenin inhibits proliferation and induces apoptosis of U87 human glioma cells in vitro. Mol Med Rep. 2013;7:531–536. doi: 10.3892/mmr.2012.1218. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the Chinese Glioma Genome Atlas (CGGA) (http://www.cgga.org.cn).