Abstract
OBJECTIVE:
Benzodiazepines and anticholinergics are risk factors for delirium in the intensive care unit (ICU). We tested the impact of a deprescribing intervention on short-term delirium outcomes.
DESIGN:
Multi-site randomized clinical trial
SETTING:
ICU’s of three large hospitals
PARTICIPANTS:
Two hundred adults aged ≥ 18 years admitted to an ICU with delirium according to the Richmond Agitation Severity Scale and the Confusion Assessment Method for the ICU (CAM-ICU). Participants had a contraindication to haloperidol (seizure disorder or prolonged QT interval) or preference against haloperidol as a treatment for delirium, and were excluded for serious mental illness, stroke, pregnancy or alcohol withdrawal. Participants were randomized to a deprescribing intervention or usual care. The intervention included electronic alerts combined with pharmacist support to deprescribe anticholinergics and benzodiazepines.
MEASUREMENTS:
Primary outcomes were delirium duration measured by the CAM-ICU, and severity measured by the Delirium Rating Scale Revised-98 (DRS-R-98) and the CAM-ICU-7; secondary outcomes included adverse events and mortality.
RESULTS:
Participants had a mean age of 61.8 (standard deviation: 14.3) years, 59% female, and 52% African American with no significant differences in baseline characteristics between groups. No differences between groups were identified in the number exposed to anticholinergics (p=0.219) or benzodiazepines (p=0.566), the median total anticholinergic score (p=0.282), or the median total benzodiazepine dose in lorazepam equivalents (p=0.501). Neither median delirium/coma-free days (p=0.361) nor median change in delirium severity scores (p=0.582 for DRS-R-98; p=0.333 for CAM-ICU-7) were different between groups. No differences in adverse events or mortality were identified.
CONCLUSIONS:
When added to state-of-the-art clinical services, this deprescribing intervention had no impact on medication use in ICU participants. Given the age of the population, results of clinical outcomes may not be easily extrapolated to older adults. Nonetheless, improved approaches for deprescribing or preventing anticholinergics and benzodiazepines should be developed to determine the impact on delirium outcomes.
Keywords: delirium, deprescribing, anticholinergic, benzodiazepine
Introduction
Deprescribing interventions are hypothesized to reduce the future risk of drug-induced adverse events.1,2 We previously tested the impact of a deprescribing intervention in an electronic medical record (EMR) system to reduce exposure to anticholinergics among older adults with delirium or dementia in a general medical ward.3 In hospitalized patients, anticholinergics and benzodiazepines worsen neurotransmitter imbalances of cholinergic deficiency, and dopaminergic and gamma-aminobutyric acid (GABA) excess leading to higher risk of delirium.4–8 While our initial EMR-based deprescribing intervention did not reduce new orders for anticholinergics, it modestly increased discontinuation orders; however, no impact on clinical outcomes was identified.3
Feedback from providers exposed to the EMR-based intervention suggested the intervention failed to change prescribing habits due to a non-interruptive nature of the alert, and lack of human decision support.3 Based on this feedback, we enhanced the electronic deprescribing intervention by designing an interruptive alert that offered recommendations for alternative (non-anticholinergic) medications with easy-to-order keystrokes, and a pharmacist supporting the intervention through direct clinical support to medical and surgical teams.9
Delirium in critically ill adults has been associated with longer hospital stays and higher mortality, and higher rates of long-term cognitive impairment.10–18 Because no medication is FDA-approved for the treatment of delirium, we developed a multi-component approach to delirium treatment in the intensive care unit (ICU) based on the neurotransmitter models described above, called the pharmacological management of delirium (PMD).8,9,19 PMD includes deprescribing anticholinergics and benzodiazepines, and prescribing low dose haloperidol for 7 days. However, some patients in the ICU possess contraindications to haloperidol, such as prolonged QT intervals or seizure disorder, or have personal preference against using haloperidol for an off-label indication. Therefore, we performed two parallel, pragmatic randomized trials of the PMD intervention, one that employed all three components of the intervention (PMD),19 and this trial that employed only the deprescribing interventions (de-PMD). Our hypothesis for both trials was that participants receiving the intervention would have (1) higher number of days without delirium or coma, and (2) lower delirium severity.
Methods:
The Institutional Review Board of Indiana University (IU) Purdue University Indianapolis approved the study. Participants’ legally authorized representatives provided informed consent prior to enrollment.9
Study Setting:
The study was conducted within the ICU’s of Eskenazi Health, Indiana University (IU) Methodist Hospital, and IU University Hospital. Eskenazi hospital hosts three intensive care units: an 8-bed surgical ICU (SICU), a 14-bed medical ICU (MICU) and a 29-bed progressive ICU (PICU, a step-down unit). IU Methodist Hospital includes 65 mixed MICU and SICU beds, and IU Health University hospital included 36 MICU and SICU beds.
Enrollment, Eligibility and Randomization (See figure 1):
Figure 1: Primary Outcomes of de-PMD: Coma/Delirium Free Days (a) and Change in Delirium Severity Scores (b).
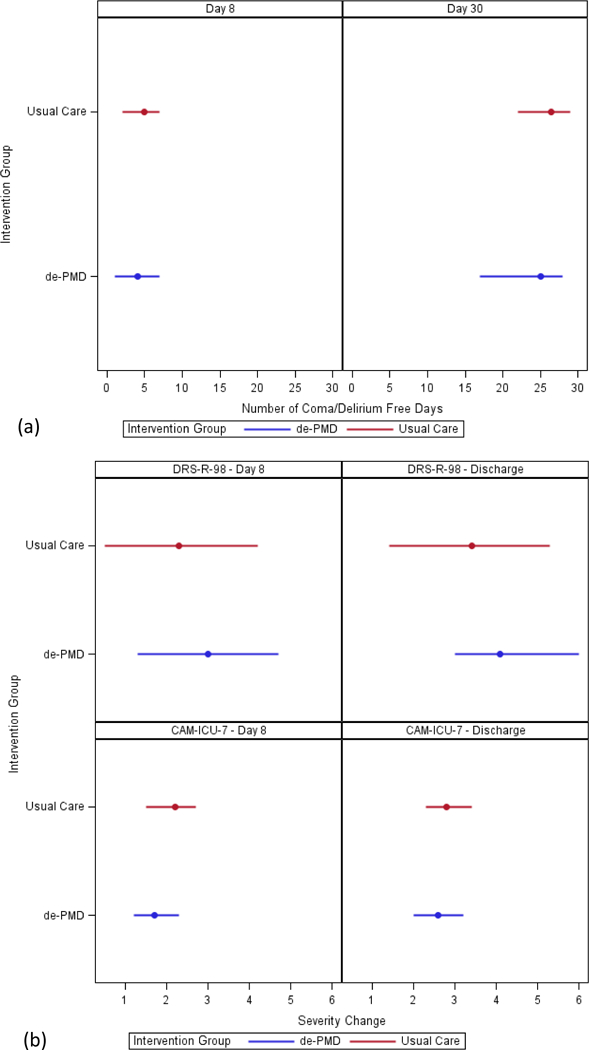
Reporting of primary outcomes of median (interquartile range) number of days alive without delirium or coma at day 8 and day 30 (a), and the mean (standard deviation) reduction from baseline in delirium severity scores at day 8 and discharge according to the Delirium Rating Scale-Revised-1998 and the Confusion Assessment Method for the Intensive Care Unit-7 (b).
Inclusion criteria consisted of the following: (1) patients admitted to the ICU ward for ≥ 24 hours, (2) age ≥ 18 years, (3) screen positive for delirium based on the Richmond Agitation-Sedation Scale (RASS)20 and the Confusion Assessment Method for the ICU (CAM-ICU)21,22 on any day during the ICU stay, (4) had a contraindication to haloperidol (such as QT prolongation or seizure disorder) or personal preference to avoid exposure to haloperidol as a delirium treatment, and (5) are English-speaking. Patients were excluded if they had 1) history of severe mental illness, 2) delirium due to alcohol intoxication, 3) aphasic stroke, 4) were pregnant or nursing, or 5) previously been enrolled in the study. Randomization of eligible patients occurred in a 1:1 ratio between the intervention and usual care groups utilizing computer-generated allocation in random blocks of four.
Intervention content and delivery:
The intervention consisted of a multi-component decision aid preventing or deprescribing definite anticholinergic medications and benzodiazepines (including the benzodiazepine-receptor agonist zolpidem).9 Briefly, two methods were employed: 1) a computerized decision support intervention to interrupt orders for strong anticholinergics, and 2) human (pharmacist) decision support that included twice-daily surveillance of medication orders and administration records. After randomization to the intervention, computerized alerts were triggered with new or renewal orders for definite anticholinergics identified according to the Anticholinergic Cognitive Burden (ACB) scale and included in the hospital formularies (see supplemental material for screenshots of computerized alerts and alternatives).7,23–26 Alerts were interruptive, provided brief education of the risk of anticholinergics, and recommended a non-anticholinergic alternative. The human decision support (intervention pharmacist performing twice daily medication reviews) identified anticholinergics prescribed despite the computerized alerts and communicated directly with the primary medical and surgical ICU teams to offer alternatives.9 The intervention intended to align prescribing practices with state-of-the-art care for anticholinergics and benzodiazepines in the ICU.27–29
Usual Care:
Those randomized to the usual care group received no electronic or human decision support for pharmacologic management of delirium throughout their hospital stay. Low dose haloperidol was not included as part of this intervention, however providers could prescribe haloperidol without restriction on dose, frequency, or duration to those enrolled in either group.
At all study sites, and over the course of the trial, implementation of new standards of care were introduced at each study site.27 These care bundles include the ABCDE bundle (Awakening and Breathing Coordination, Delirium monitoring and management, and Early mobility). These practices have been shown in prior studies to reduce the duration of mechanical ventilation, length of ICU stay, and mortality.30–33
Primary Outcomes:
a) Delirium Duration.
We identified delirium using the Richmond Agitation Sedation Scale (RASS)20 and the Confusion Assessment Method for the ICU (CAM-ICU).21,22 The CAM-ICU was chosen based on the recommendation of national guidelines,27 its practical use in the ICU and its acceptable psychometric properties.22 The CAM-ICU score was determined by examining (a) acute or fluctuating changes in mental status, (b) inattention, (c) altered level of consciousness, and (d) disorganized or incoherent thinking. CAM-ICU was considered to be positive if the patient displayed both (a) and (b), plus (c) and/or (d). RASS was utilized as a sister instrument to measure level of arousal alongside the CAM-ICU.20 Patients with a RASS score of −3 to +4 were eligible for delirium assessment, while scores of −4 and −5 were characterized as comatose and not eligible for CAM-ICU assessment. Blinded research assistants conducted twice daily RASS and CAM-ICU assessments after 24 hours of ICU admission and until discharge from the hospital, death, or 30 days after enrollment.
b) Delirium Severity:
Delirium severity was assessed using the Delirium Rating Scale-revised (DRS-R-98)34,35 and the CAM-ICU-7.36 The DRS-R-98 is a 16-item clinician-rated scale (rated 0 to 3, maximum 39 points) with higher scores indicating greater severity of delirium. The CAM-ICU-7 is a seven point rating scale (0–7, higher scores more severe) that has been derived from the RASS and the CAM-ICU. Severity assessments were performed by trained and blinded research assistants in a similar frequency reported above.
Secondary & Other Outcomes:
Secondary outcomes were collected through direct observation and the EMR, and included mortality rates (up to 30 days after discharge), length of stay (both ICU and total hospital stay) falls, use of physical restraints, pulling out intravenous lines or urinary catheters, re-intubation, and pressure ulcers.13,37
We collected baseline demographic and clinical information from the EMR as well as each participant’s legally authorized representative. Prior cognitive function was obtained by legally authorized representatives using the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE).38,39 Activities of daily living and instrumental activities of daily living prior to ICU admission were assessed through Katz40 and Lawton41 scales, completed with input from participant’s legally authorized representatives. Electronic medical records were used to collect reasons for admission, severity of illness (APACHE II)42 within 24 hours of admission to the ICU, daily mechanical ventilation status, and the Charlson Comobidity Index.43 Medication data included drug, dose, time administered, and route was collected manually from hospital administration records. Cumulative or total use by medication or class is reported as a sum of each dose administered.
Sample Size:
Since this trial was conducted in ICU patients with contraindications to haloperidol, we did not set a sample size a priori. Instead, enrollment to the trial occurred concurrently with the PMD trial19 and was stopped at the end of funding support. Using the two-sample t-test for continuous outcomes, we estimate that the sample size of 100 in each group provides 80% power for detecting a group difference with effect size of 0.4 SD or greater.
Drug-related adverse events:
Adverse effects were monitored using EMRs and direct observation throughout the hospital stay or up to 30 days after enrollment. Likelihood for causality with study interventions was assessed using the Naranjo scale.44 All adverse events were reported to an independent Data Safety Monitoring Board (DSMB).
Data Analyses:
Baseline differences between groups were assessed using Fisher’s exact tests for categorical outcomes and Wilcoxon-Rank sum tests for continuous measures with skewed data. Additionally, we used Fisher’s exact test to compare the percentage of patients who received targeted medications, complications, and adverse events. To test for differences in the number of adverse events and total and daily medication doses between groups, we used the Wilcoxon-Rank sum test.
Delirium/coma free days and length of stay were compared using the nonparametric Wilcoxon Rank-Sum test. Two time points were used for delirium/coma free days and for delirium severity: day 8 post-randomization as this was the end of the haloperidol course in the intervention group, and at discharge. Patients who died before day 8 or discharge had their subsequent delirium/coma free days counted as 0. Patients who were discharged before day 8 had the remaining days counted as delirium/coma free. To adjust for length of stay when comparing delirium/coma free days at discharge, we used a Poisson regression model that included an offset equal to the log of length of stay post randomization.
Since delirium severity measured by DRS-R-98 had substantial missing values, we used multiple imputation methods to compare change in DRS-R-98 scores from baseline to day 8 or discharge. We used both regression and propensity-based methods for multiple imputation. The MI procedure in SAS was used to create a set of 30 imputed datasets using the following variables: day post randomization, morning/afternoon assessment, mechanical ventilation, RASS score, coma, delirium, and 4 variables obtained from chart review (hallucinations, delusions, confusion, and disorientation). We used the MIANALYZE procedure in SAS to compare DRS-R-98 scores at baseline, day 8, discharge, and change scores between the two groups. Results were similar for both imputation procedures, therefore, we report the regression-based imputation. A mixed effects model with mean daily CAM-ICU-7 scores as the dependent variable was used to compare the difference in CAM-ICU-7 change from baseline to day 8 or to discharge. The mixed model included randomization group, time, group and time interaction as independent variables and a random effect for patients.
We used Fisher’s exact tests to examine differences in mortality at ICU and hospital discharge, and 30 days post discharge. The Wilcoxon-Rank sum test was used to compare length of stay, ICU length of stay, and days on mechanical ventilation post randomization.
All analyses were calculated on an intention-to-treat basis. The analyses were conducted in all patients (including those who withdrew) using available data until time of death, discharge, withdrawal or 30 days after enrollment. Analyses were conducted using SAS v9.4.
Results:
Participants:
The supplemental file (Supplemental Figure) contains the CONSORT diagram shows the flow of participants through screening and study completion. The study screened 12,402 ICU patients for eligibility, with 6,653 never experiencing delirium (53.6%), and 4,183 (33.7%) meeting other exclusion criteria and not eligible for this trial. As described above, this trial occurred in parallel with the PMD delirium treatment trial that included low-dose haloperidol in addition to deprescribing benzodiazepines and anticholinergics; eight participants with prolonged QT intervals were screened for the parallel trail (PMD) but before this trial was initiated and were therefore excluded. Among those eligible, 351 were enrolled in the PMD trial and 200 in this de-PMD trial. Supplemental table 3 reports the distribution of contraindications to haloperidol and the proportions who received haloperidol through routine clinical care.
Participant Characteristics:
As seen in table 1, the mean age of participants was 61.8 (standard deviation (SD) 14.3 years), 59% were female and 52% were African American. Study groups did not differ significantly with respect to age, gender, race, education, comorbidities, acute severity of illness, and discharge diagnosis. The majority of participants were admitted to the medical ICU services (74%) and received mechanical ventilation for at least one day (72%). Almost half were admitted with acute respiratory failure and/or sepsis (49%), while 24% were admitted for altered mental status or other neurological diagnoses.
Table 1:
Baseline Characteristics of enrolled participants (n=200)
| Overall | de-PMD (N=99) |
Usual Care (N=101) |
|
|---|---|---|---|
| Age | 61.8 (14.3) | 61.3 (14.6) | 62.4 (14.1) |
| Female n (%) | 118 (59.0) | 65 (65.7) | 53 (52.5) |
| African-American n (%) | 102 (52.0) | 54 (55.1) | 48 (49.0) |
| Education (years) | 11.6 (2.1) | 11.5 (2.1) | 11.6 (2.1) |
| APACHEa II | 21.2 (8.3) | 20.6 (8.2) | 21.7 (8.3) |
| Charlson Comorbidity Index | 3.2 (2.5) | 3.2 (2.5) | 3.2 (2.4) |
| Activities of Daily Living (ADL)b | 5.3 (1.4) | 5.3 (1.4) | 5.3 (1.5) |
| Instrumental Activities of Daily Living (IADL)c | 5.6 (2.8) | 5.5 (2.8) | 5.7 (1.5) |
| IQCODEd | 3.3 (0.5) | 3.3 (0.4) | 3.3 (0.5) |
| Mechanically Ventilated n (%) | 143 (71.9) | 73 (73.7) | 70 (70.0) |
| ICU Location | |||
| Medical ICUe n (%) | 147 (73.9) | 72 (72.7) | 75 (75.0) |
| Surgical ICU n (%) | 35 (17.6) | 20 (20.2) | 15 (15.0) |
| Progressive/step-down ICU n (%) | 17 (8.5) | 7 (7.1) | 10 (10.0) |
| Diagnoses | |||
| Acute Respiratory Failure/Sepsis | 98 (49.0) | 50 (50.5) | 47 (47.5) |
| Neurologic/Altered Mental Status | 47 (23.5) | 23 (23.2) | 24 (23.8) |
| Trauma | 16 (8.0) | 6 (6.1) | 10 (9.9) |
| Otherf | 39 (19.5) | 20 (20.2) | 19 (18.8) |
Data presented as mean (standard deviation) unless otherwise specified
APACHE: Acute Physiology and Chronic Health evaluation
ADLs assessed by Katz Scale
IADLs assessed by Lawton Scale
IQCODE: Informant Questionnaire on Cognitive Decline in the Elderly
ICU: Intensive Care Unit
Others: Include cardiovascular and gastrointestinal diagnoses
Primary Outcomes:
No difference in the median number of days without delirium or coma was identified between those randomized to the intervention compared to usual care groups at either day 8 [p=0.361] or 30 days after randomization [p=0.108] (Figure and Supplemental Table 2). Similarly, no difference in the mean reduction in delirium severity scores was seen between groups as measured by either the DRS-R98 or CAM-ICU-7 from baseline to day 8 or discharge (Figure and Supplemental Table 2).
Secondary Outcomes:
No differences in secondary outcomes, including ICU mortality [p=0.794], in-hospital mortality [p=0.478], 30-day mortality [p=0.291], number of days ventilated [p=0.242], number of hospital days post-randomization [p=0.246], and number discharged to home [p=0.447] were identified between groups (Supplemental Table 2). Those randomized to intervention had a higher median number of ICU days after randomization [p=0.019].
Measures of Medication Use:
Table 2 and Supplemental Table 1 describe characteristics of exposure to analgosedatives used in the study population, with benzodiazepines and anticholinergics reported as lorazepam equivalents and anticholinergic burden scores, respectively. Target medication exposure is reported by group and time (defined as prior to enrollment, day-of randomization, and post-randomization periods). The deprescribing intervention did not change the number exposed or median total dose of lorazepam equivalents between groups [intervention: 38.3 mg (IQR 98.6mg) vs. usual care: 37.7 (IQR 115.9mg); p=0.501]. Although the intervention group had a lower mean total ACB score (a cumulative measure of exposure), the difference was not statistically significant [intervention: 4.3 (SD 8.8) vs. usual care: 6.1 (SD 16.6); p=0.282]. The median number of days an anticholinergic was administered among those receiving any anticholinergic was lower but not significantly different between groups [intervention: 3.5 (IQR 1–7) vs. usual care: 4 (IQR 1–15); p=0.32]. To better identify potential subgroups in which the de-PMD intervention reduced anticholinergic use, we explored differences between study group and age, service, and time since enrollment, though we note the study was not powered to detect differences in subgroups. Participants in SICU service teams showed differences approaching statistical significance; the median (IQR) number of anticholinergic doses among SICU participants in the intervention group receiving at least one dose of anticholinergics was 5 (2–8.5) compared to 6 (1–19) in usual care [p=0.114]. The median (IQR) number of doses among those age 65 and over and receiving at least one anticholinergic dose was 4.5 (1–12) in the intervention group and 8 (2–15) [p=0.604] in the usual care group.
Table 2:
de-PMD Trial Medication Exposures
| Pre-Randomization | Randomization Day | Post-Randomization | |||||||
|---|---|---|---|---|---|---|---|---|---|
| de-PMD* (N=99) |
Usual Care (N=100) |
P- value |
de-PMD (N=99) |
Usual Care (N=100) |
P- value |
de-PMD (N=99) |
Usual Care (N=100) |
P- value |
|
| Haloperidol | |||||||||
| Patients† n (%) | 7 (7.1) | 13 (13.0) | 0.238 | 6.1 (6) | 8.0 (8) | 0.783 | 29 (29.3) | 20 (20.0) | 0.141 |
| Median daily Dose (IQR) | 0 (0–0) | 0 (0–0) | 0.176 | 0 (0–0) | 0 (0–0) | 0.575 | 0 (0–0.2) | 0 (0–0) | 0.117 |
| Benzodiazepines‡ | |||||||||
| Patients† n (%) | 69 (69.7) | 58 (58.0) | 0.105 | 37 (37.4) | 35.0 (35) | 0.769 | 60 (60.6) | 56 (56.0) | 0.566 |
| Median daily Dose (IQR) | 1.1 (0–10.0) | 0.3 (0–3.7) | 0.044 | 0 (0–4) | 0 (0–2) | 0.550 | 0.1 (0–1.2) | 0.1 (0–1.1) | 0.634 |
| Anticholinergic Burden§ | |||||||||
| Patients† n (%) | 16 (16.2) | 15 (15.0) | 0.847 | 11 (11.1) | 12 (12.0) | 1.000 | 34 (34.3) | 26 (26.0) | 0.219 |
| Median daily score (IQR) | 0 (0–0) | 0 (0–0) | 0.832 | 0 (0–0) | 0 (0–0) | 0.823 | 0 (0–0.3) | 0 (0–0.1) | 0.266 |
| Olanzapine | |||||||||
| Patients† n (%) | 3 (3.0) | 1 (1.0) | 0.369 | 3 (3.0) | 1 (1.0) | 0.369 | 4 (4.0) | 2 (2.0) | 0.445 |
| Median daily Dose (IQR) | 0 (0–0) | 0 (0–0) | 0.306 | 0 (0–0) | 0 (0–0) | 0.306 | 0 (0–0) | 0 (0–0) | 0.387 |
| Quetiapine | |||||||||
| Patient† s n (%) | 4 (4.0) | 6 (6.0) | 0.748 | 3 (3.0) | 5 (5.0) | 0.721 | 8 (8.1) | 9 (9.0) | 1.000 |
| Median daily Dose (IQR) | 0 (0–0) | 0 (0–0) | 0.509 | 0 (0–0) | 0 (0–0) | 0.461 | 0 (0–0) | 0 (0–0) | 0.776 |
| Risperidone | |||||||||
| Patients† n (%) | 0 (0.0) | 1 (1.0) | 1.000 | 0 (0.0) | 0 (0.0) | 1 (1.0) | 1 (1.0) | 1.000 | |
| Median daily Dose (IQR) | 0 (0–0) | 0 (0–0) | 0.320 | 0 (0–0) | 0 (0–0) | 1.000 | 0 (0–0) | 0 (0–0) | 0.989 |
| Dexmedetomidine | |||||||||
| Patients† n (%) | 10 (10.1) | 5 (5.0) | 0.191 | 2 (2.0) | 2 (2.0) | 1.000 | 6 (6.1) | 11 (110) | 0.311 |
| Median daily Dose (IQR) | 0 (0–0) | 0 (0–0) | 0.175 | 0 (0–0) | 0 (0–0) | 0.992 | 0 (0–0) | 0 (0–0) | 0.218 |
| Opioids ∥ | |||||||||
| Patients† n (%) | 86 (86.9) | 76 (76.0) | 0.068 | 68 (68.7) | 62 (62.0) | 0.372 | 83 (83.8) | 70 (70.0) | 0.028 |
| Median daily Dose (IQR) | 35.9 (2.5–77.8) | 27.5 (0.6–87.2) | 0.522 | 36.8 (0–107.5) | 24.1 (0–88.4) | 0.460 | 6.3 (0.8–44.1) | 3.5 (0–34.6) | 0.078 |
| Propofol | |||||||||
| Patients† n (%) | 41 (41.4) | 45 (45.0) | 0.668 | 22 (22.2) | 28 (28.0) | 0.414 | 38 (38.4) | 27 (27.0) | 0.098 |
| Median daily Dose (IQR) | 0 (0–144) | 0 (0–906) | 0.304 | 0 (0–0) | 0 (0–229) | 0.508 | 0 (0–199) | 0 (0–1) | 0.088 |
| Clonidine | |||||||||
| Patients† n (%) | 2 (2.0) | 3 (3.0) | 1.000 | 1 (1.0) | 3 (3.0) | 0.621 | 6 (6.1) | 7 (7.0) | 1.000 |
| Median daily Dose (IQR) | 0 (0–0) | 0 (0–0) | 0.653 | 0 (0–0) | 0 (0–0) | 0.321 | 0 (0–0) | 0 (0–0) | 0.780 |
| Propranolol | |||||||||
| Patients† n (%) | 2 (2.0) | 0 (0.0) | 0.246 | 0 (0.0) | 0 (0.0) | 3 (3.0) | 3 (3.0) | 1.000 | |
| Median daily Dose (IQR) | 0 (0–0) | 0 (0–0) | 0.154 | 0 (0–0) | 0 (0–0) | 1.000 | 0 (0–0) | 0 (0–0) | 0.974 |
de-PMD: deprescribing in the Pharmacological Management of Delirium
Patients who received the drug during hospitalization
Benzodiazepine data presented as Lorazepam equivalents and includes zolpidem
Anticholinergic burden measured by Anticholinergic Burden (ACB) scale
Opioids data presented as morphine equivalents
Haloperidol dose is presented in milligrams (mg)
Adverse Events:
Twenty-seven participants in the intervention group and twenty-two in the usual care group experienced a serious adverse event (p=0.413). Table 3 describes the distribution of all adverse events recorded by study assessors with no differences between groups observed. Intervention participants experienced a higher rate of pulling out respiratory, gastrointestinal, or intravenous access hardware compared to usual care [p=0.033]. Supplemental Table 4 reports no differences in the distribution of delirium psychomotor subtypes by study group based on RASS scores.
Table 3.
Comparison of Adverse Events between de-PMD and Usual Care Groups
| de-PMD (n=99) |
Usual Care (n=101) |
P-value | |
|---|---|---|---|
| Patients with Serious Adverse Event | 27 (27.3) | 22 (21.8) | 0.413 |
| Total Number of Serious Adverse Events | 37 | 35 | |
| Event Type* | |||
| Death | 11 (11.1) | 8 (7.9) | 0.478 |
| QT prolongation | 0 (0) | 0 (0) | N/A |
| Cardiac Arrhythmias | 1 (1.0) | 1 (1.0) | 1.000 |
| Extra Pyramidal Symptoms | 0 (0) | 0 (0) | N/A |
| Neuroleptic Malignant Syndrome | 0 (0) | 0 (0.0) | N/A |
| Prolonged Hospitalization | 5 (5.0) | 2 (2.0) | 0.277 |
| Other | 15 (15.2) | 14 (13.9) | 0.843 |
| Relationship to Protocol | |||
| Event Definitely related | 0 (0) | 0 (0) | N/A |
| Event Probably related | 0 (0) | 0 (0) | N/A |
| Event Possibly related | 0 (0) | 0 (0) | N/A |
| Organ System | |||
| Central Nervous System | 4 (4.0) | 2 (2.0) | 0.443 |
| Cardiovascular | 10 (10.1) | 9 (8.9) | 0.814 |
| Respiratory | 11 (11.1) | 12 (11.9) | 1.000 |
| Gastrointestinal | 3 (3.0) | 3 (3.0) | 1.000 |
| Genitourinary | 0 (0.0) | 4 (4.0) | 0.121 |
| Skin | 1 (1.0) | 0 (0.0) | 0.495 |
| Hepatic | 0 (0) | 0 (0) | N/A |
| Hematologic | 3 (3.0) | 3 (3.0) | 1.000 |
| Endocrine | 0 (0) | 0 (0) | N/A |
| Musculoskeletal | 1 (1.0) | 1 (1.0) | 1.000 |
| Delirium-related complications | |||
| Inappropriate attempt to get out of bed, N (%) | 8 (8.1) | 5 (5.0) | 0.407 |
| Verbal agitation, N (%) | 5 (5.0) | 5 (5.0) | 1.000 |
| Fall, N (%) | 2 (2.0) | 0 (0.0) | 0.246 |
| Delayed Procedure, N (%) | 0 (0.0) | 0 (0.0) | |
| Pulling Tubes, N (%) | 9 (9.1) | 2 (2.0) | 0.033 |
| Hospital Acquired Pressure Ulcers, N (%) | 18 (18.2) | 10 (10.0) | 0.107 |
Data presented as N (%) unless otherwise specified
Event types are presented as number of patients with the event. A single patient may have more than one adverse event
Discussion:
This randomized trial showed that our electronic and pharmacist-based deprescribing intervention had no significant impact on the number exposed, median or total dose of anticholinergics and benzodiazepines, and therefore did not change delirium, length of stay, or mortality outcomes among critically ill adults with delirium. Despite 58% of participants being exposed to at least one dose of benzodiazepines in the post-randomization period, and 30% of participants exposed to strong anticholinergics, the mean and median daily dose of benzodiazepines and anticholinergics was low (table 3). Our combined deprescribing intervention failed to further reduce measures of medication use and therefore could not determine whether deprescribing these medications can reduce delirium outcomes.
The failure of our intervention to influence anticholinergic and benzodiazepine exposure could be attributed to a number of factors related to the design of the intervention as well as the clinical environment in which the study was conducted. First, our intervention employed an interruptive alert and recommendation of alternatives within the EMR at the time of order entry. Our prior experience with approaches in preventing anticholinergic orders also failed to reduce exposure to anticholinergics.3 Although we included a pharmacist review of medications, our alert was not triggered for pre-existing orders, which may have inflated the number of participants ever exposed to a target medication. Additionally, the intervention did not intend to change sedation practices, which frequently employed benzodiazepines among ventilated participants. During the study, an updated clinical practice guideline for the management of pain, agitation, and delirium in critically ill adults recommended the minimization of benzodiazepines and anticholinergics.27 This may have resulted in contamination, compromising our ability to influence medication use. We note that medical and surgical ICU teams in each study site received support from clinical pharmacists who have post-graduate training in critical care, participate in rounds with ICU teams on a daily basis, have an influence in sedation and pain management practices, and have access to all clinical data relevant to the care of delirium and other medical illnesses. Therefore our results may be different in healthcare systems without comprehensive clinical pharmacy support.
While we believe the use of electronic medical records offer a scalable opportunity for deprescribing interventions, our experience in this and prior trials suggest that our approach should not be repeated.3,19 Future deprescribing trials should be improved with attention to the design and timing of alerts as well as the incorporation of behavior change theories including priming and default settings,45 A successful EMR-based deprescribing intervention could have scalable opportunities not only in acute care environments, but also primary care and rehabilitation or extended care facilities. We note, however, that given the age of the population studied, results of clinical outcomes may not be easily extrapolated to older adults.
Our study had limitations worth noting: 1) this study was conducted in three urban academic hospitals with a diverse population, and consistent clinical pharmacy support and may not be generalizable to other institutions; 2) participants who were not users of benzodiazepine or anticholinergics did not receive any intervention which reduced our ability to measure impact; 3) our design called for randomization of the participant rather than provider or site, therefore contamination among both providers and clinical pharmacists may have compromised the impact of our intervention and results; and 4) the computerized intervention was not triggered for existing medication orders and may have been more effective in the prevention of medication use and ultimately delirium prevention.
Conclusion
This multi-component deprescribing intervention did not significantly reduce benzodiazepine or anticholinergic use compared to usual care among adults admitted to the ICU, and therefore could not influence delirium outcomes. Improving the efficacy of deprescribing interventions with more attention to the decision processes of deprescribing could improve future research in reducing delirium outcomes in ICU populations.
Supplementary Material
Acknowledgements:
Sponsor’s Role:
The National Institutes of Health/National Institute on Aging had no role in the study design, data collection, analysis, data interpretation, and the decision to submit the paper for publication. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Funding: Supported by a grant from the National Institute on Aging (R01AG034205). Dr. Campbell’s work on the project was also supported through an award from the National Institute on Aging (K23AG044440). Dr. Khan’s work on the project was supported through an award from the National Institute on Aging (K23AG043476).
We certify that this work is novel clinical research. The potential impact of this research on clinical care or health policy includes the following: Our results showed that a combination deprescribing intervention was ineffective in aligning prescribing habits with guideline recommendations in critical care environments, requiring additional research into effective deprescribing approaches.
Footnotes
| Elements of Financial/ Personal Conflicts |
Noll L. Campbell |
Anthony J. Perkins |
Babar A. Khan |
Sujuan Gao |
Mark O. Farber |
Sikandar Khan |
Sophia Wang |
Malaz A. Boustani |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | Yes | No | |
| Employment or Affiliation | X | X | X | X | X | X | X | X | ||||||||
| Grants/Funds | X | X | X | X | X | X | X | X | ||||||||
| Honoraria | X | X | X | X | X | X | X | X | ||||||||
| Speaker Forum | X | X | X | X | X | X | X | X | ||||||||
| Consultant | X | X | X | X | X | X | X | X | ||||||||
| Stocks | X | X | X | X | X | X | X | X | ||||||||
| Royalties | X | X | X | X | X | X | X | X | ||||||||
| Expert Testimony | X | X | X | X | X | X | X | X | ||||||||
| Board Member | X | X | X | X | X | X | X | X | ||||||||
| Patents | X | X | X | X | X | X | X | X | ||||||||
| Personal Relationship | X | X | X | X | X | X | X | X | ||||||||
References:
- 1.Scott IA, Hilmer SN, Reeve E, et al. Reducing inappropriate polypharmacy: the process of deprescribing. JAMA internal medicine. 2015;175(5):827–834. [DOI] [PubMed] [Google Scholar]
- 2.Campbell NL, Boustani MA. Adverse cognitive effects of medications: turning attention to reversibility. JAMA Intern Med. 2015;175(3):408–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boustani MA, Campbell NL, Khan BA, et al. Enhancing care for hospitalized older adults with cognitive impairment: a randomized controlled trial. J Gen Intern Med. 2012;27(5):561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Trzepacz PT. Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine. Semin Clin Neuropsychiatry. 2000;5(2):132–148. [DOI] [PubMed] [Google Scholar]
- 5.Trzepacz PT, Bourne R, Zhang S. Designing clinical trials for the treatment of delirium. J Psychosomatic Res. 2008;65(3):299–307. [DOI] [PubMed] [Google Scholar]
- 6.Khan BA, Zawahiri M, Campbell NL, Boustani MA. Biomarkers for delirium--a review. J Am Geriatr Soc. 2011;59 Suppl 2:S256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell N, Boustani M, Limbil T, et al. The cognitive impact of anticholinergics: a clinical review. Clin Interv Aging. 2009;4:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Campbell N, Boustani MA, Ayub A, et al. Pharmacological management of delirium in hospitalized adults--a systematic evidence review. J Gen Intern Med. 2009;24(7):848–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell NL, Khan BA, Farber M, et al. Improving delirium care in the intensive care unit: the design of a pragmatic study. Trials. 2011;12(1):139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941–948. [DOI] [PubMed] [Google Scholar]
- 11.Lin SM, Liu CY, Wang CH, et al. The impact of delirium on the survival of mechanically ventilated patients. Crit Care Med. 2004;32(11):2254–2259. [DOI] [PubMed] [Google Scholar]
- 12.Ely EW, Shintani A, Truman B, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291(14):1753–1762. [DOI] [PubMed] [Google Scholar]
- 13.Ely EW, Gautam S, Margolin R, et al. The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med. 2001;27(12):1892–1900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pisani MA, Kong SYJ, Kasl SV, Murphy TE, Araujo KL, Van Ness PH. Days of delirium are associated with 1-year mortality in an older intensive care unit population. American journal of respiratory and critical care medicine. 2009;180(11):1092–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shehabi Y, Riker R, Bokesch P, Wisemandle W, Shintani A, Ely EW. Delirium duration and mortality in lightly sedated, mechanically ventilated intensive care patients. Crit Care Med. 2010;38(12):2311–2318. [DOI] [PubMed] [Google Scholar]
- 16.Marcantonio E, Ta T, Duthie E, Resnick N. Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. J Am Geriatr Soc. 2002;50:850–857. [DOI] [PubMed] [Google Scholar]
- 17.Kelly KG, Zisselman M, Cutillo-Schmitter T, Reichard R, Payne D, Denman SJ. Severity and course of delirium in medically hospitalized nursing facility residents. Am J Geriatr Psychiatry. 2001;9(1):72–77. [PubMed] [Google Scholar]
- 18.McCusker J, Cole M, Abrahamowicz M, Primeau F, Belzile E. Delirium predicts 12-month mortality. Arch Intern Med. 2002;162(4):457–463. [DOI] [PubMed] [Google Scholar]
- 19.Khan BA, Perkins AJ, Campbell NL, et al. Pharmacological Management of Delirium in the Intensive Care Unit: A Randomized Controlled Pragmatic Trial. Under Review. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344. [DOI] [PubMed] [Google Scholar]
- 21.Ely EW, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286(21):2703–2710. [DOI] [PubMed] [Google Scholar]
- 22.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med. 2001;29(7):1370–1379. [DOI] [PubMed] [Google Scholar]
- 23.Campbell NL, Perkins AJ, Bradt P, et al. Association of Anticholinergic Burden with Cognitive impairment and Healthcare Utilization Among a Diverse Ambulatory Older Adult Population. Pharmacotherapy. 2016;36(11):1123–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Campbell NL, Boustani MA, Lane KA, et al. Use of anticholinergics and the risk of cognitive impairment in an African American population. Neurology. 2010;75(2):152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cai X, Campbell N, Khan B, Callahan C, Boustani M. Long-term anticholinergic use and the aging brain. Alzheimer’s & Dementia. 2013;9(4):377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Campbell NL, Maidment I, Fox GC, Khan BA, Boustani MA. The 2012 update to the Anticholinergic Cognitive Burden Scale. J Am Geriatr Soc. 2013;61(S1):S142–143. [Google Scholar]
- 27.Barr J, Fraser GL, Puntillo K, et al. Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med. 2013;41(1):263–306. [DOI] [PubMed] [Google Scholar]
- 28.Khan BA, Zawahiri M, Campbell NL, et al. Delirium in hospitalized patients: implications of current evidence on clinical practice and future avenues for research--a systematic evidence review. J Hosp Med. 2012;7(7):580–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Khan BA, Gutteridge D, Campbell NL. Update on pharmacotherapy for prevention and treatment of post-operative delirium: a systematic evidence review. Curr Anesthesiol Rep. 2015;5(1):57–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schweickert WD, Pohlman MC, Pohlman AS, et al. Early physical and occupational therapy in mechanically ventilated, critically ill patients: a randomised controlled trial. Lancet (London, England). 2009;373(9678):1874–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vasilevskis EE, Ely EW, Speroff T, Pun BT, Boehm L, Dittus RS. Reducing iatrogenic risks: ICU-acquired delirium and weakness--crossing the quality chasm. Chest. 2010;138(5):1224–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morandi A, Brummel NE, Ely EW. Sedation, delirium and mechanical ventilation: the ‘ABCDE’ approach. Curr Opin Crit Care. 2011;17(1):43–49. [DOI] [PubMed] [Google Scholar]
- 33.Pandharipande P, Banerjee A, McGrane S, Ely EW. Liberation and animation for ventilated ICU patients: the ABCDE bundle for the back-end of critical care. Crit Care. 2010;14(3):157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N. Validation of the Delirium Rating Scale-revised-98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001;13(2):229–242. [DOI] [PubMed] [Google Scholar]
- 35.Trzepacz PT, Baker RW, Greenhouse J. A symptom rating scale for delirium. Psychiatry Res. 1988;23(1):89–97. [DOI] [PubMed] [Google Scholar]
- 36.Khan BA, Perkins AJ, Gao S, et al. The Confusion Assessment Method for the ICU-7 Delirium Severity Scale: a Novel Delirium Severity Instrument for Use in the ICU. Crit Care Med. 2017;45(5):851–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pandharipande P, Shintani A, Peterson J, et al. Lorazepam is an independent risk factor for transition to delirium in intensive care unit patients. Anesthesiology. 2006;104(1):21–26. [DOI] [PubMed] [Google Scholar]
- 38.Jorm A, Jacomb P. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychological medicine. 1989;19(4):1015–1022. [DOI] [PubMed] [Google Scholar]
- 39.Jorm AF. The Informant Questionnaire on cognitive decline in the elderly (IQCODE): a review. Int Psychogeriatr. 2004;16:275–293. [DOI] [PubMed] [Google Scholar]
- 40.Katz S FA, Moskowitz RW, Jackson BA, Jaffe MW. Studies of illness in the aged. The index of Adl: A standardized measure of biological and psychosocial function. J Am Med Assoc. 1963;185:914–919. [DOI] [PubMed] [Google Scholar]
- 41.Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Nursing Research. 1970;19(3):278. [PubMed] [Google Scholar]
- 42.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Critical care medicine. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 43.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. Journal of chronic diseases. 1987;40(5):373–383. [DOI] [PubMed] [Google Scholar]
- 44.Naranjo CA, Busto U, Sellers EM. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30(2):239–245. [DOI] [PubMed] [Google Scholar]
- 45.Thaler R, Sunstein C. Nudge: Improving decisions about health, wealth, and happiness. New Haven: Yale University Press; 2008. [Google Scholar]
- 46.Maldonado JR. Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry. 2013;21(12):1190–1222. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


