Abstract
Objective:
The objective was to assess risk of pelvic inflammatory disease (PID) among women with current asymptomatic undiagnosed cervical infection or who are at high risk of sexually transmitted infections (STIs), comparing those who have a copper-bearing (Cu–) or levonorgestrel (LNG–) intrauterine device (IUD) placed with women who do not.
Study design:
We searched PubMed and Cochrane Library for articles from January 1984 through January 2016 addressing our objective. We assessed study quality using the United States Preventive Services Task Force evidence grading system.
Results:
Our search strategy yielded 2220 articles, of which 10 met inclusion criteria. Two studies provided direct evidence of PID rates in women with undiagnosed gonococcal or chlamydial (GC/CT) infection or at high risk for STIs initiating IUDs versus other contraceptive methods (level II–2, fair to poor), and neither study found a difference. Eight studies provided indirect evidence (II–2 to II–3, fair to poor). One study found no difference in PID rates between initiators of Cu–versus LNG-IUDs. Five studies compared algorithms based on patient factors with laboratory GC/CT screening to predict cervical infection. Based on likelihood ratios, none of these algorithms adequately identified women at high risk of asymptomatic cervical infection who should not undergo IUD placement. Two studies compared IUD placement on the same day as STI screening with delayed placement after screening and found no difference in PID rates.
Conclusion:
Limited evidence suggests that IUD placement does not increase the risk of PID compared with no IUD placement among women with asymptomatic undiagnosed cervical infection or at high risk of STIs. Algorithms based on patient characteristics to identify women with asymptomatic GC/CT may be overly restrictive, leading to missed opportunities for IUD initiation. Historical concerns about higher PID risk among women at risk for STIs who use IUDs may not be relevant with modern devices and STI screening and treatment practices.
Keywords: Intrauterine device, Cervical infection, Pelvic inflammatory disease, Gonorrhea, Chlamydia
1. Introduction
Intrauterine devices (IUDs) offer highly effective contraception and are considered safe or generally safe for most women [1,2]. The use of IUDs has increased, and currently 10.3% of contraceptive users in the United States (US) use IUDs [containing either copper (Cu) or levonorgestrel (LNG)] [3]. However, IUD use remains low for younger women; only 2.8% of those aged 15–19 years who received services at Title X sites in 2013 used IUDs [4]. Women perceived as being at higher risk of sexually transmitted infections (STIs) or pelvic inflammatory disease (PID), such as young women or women with multiple partners, often face barriers to obtaining IUDs, including provider bias, which may partially explain this lower rate of use [5,6].
The risk of PID among IUD initiators is elevated in the first weeks following placement; however, the absolute risk of PID is very low [7]. US guidelines presently recommend that women should not have an IUD placed if they currently have purulent cervicitis, gonococcal or chlamydial (GC/CT) infection, or PID [2]. The Centers for Disease Control and Prevention (CDC) Sexually Transmitted Diseases (STD) Treatment Guidelines, 2015, recommend annual gonorrhea and chlamydia screening by nucleic acid amplification test (NAAT) for sexually active women aged less than 25 years, for women aged 35 years and under in correctional facilities and for other women at high risk based on history, sexual practices and the prevalence of disease in the community [10]. Most women who have been screened according to current CDC STD Treatment Guidelines do not need additional screening prior to IUD placement, and those who screen positive may be treated without IUD removal [8]. However, the risk of PID from an ascending asymptomatic cervical infection, which most commonly is caused by GC/CT infection, following IUD placement compared with no IUD placement is unknown [9]. This systematic review aimed to assess risk of PID among women with current asymptomatic undiagnosed cervical infection or who are high risk of STIs, comparing those who had a Cu–or LNG–IUD placed with those who did not have an IUD placed.
2. Materials and methods
We conducted this systematic review according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines [11]. We searched PubMed for studies conducted from 1984 (date of Cu–T380A IUD approval in the US) to January 2016 using the following search strategy: ((“Pelvic Inflammatory Disease”[MESH] OR pelvic inflammatory disease OR PID OR Salpingitis) OR (“Sexually Transmitted Diseases”[Mesh] OR “sexually transmitted diseases” OR “sexually transmitted disease” OR “sexually transmitted infections” OR “sexually transmitted infection”) OR (gonorrhea OR gonorrhea OR chlamydia) OR (“Uterine Cervicitis”[Mesh] OR cervicitis)) AND (((levonorgestrel AND intrauterine devices[mesh] OR iud OR iucd OR ius OR iuc OR intrauterine system OR intra-uterine system OR intrauterine device OR intra-uterine device OR intrauterine contraceptive OR intrauterine contraception) OR mirena) OR ((“Intrauterine Devices” [Mesh] OR “Intrauterine Devices, Medicated”[Mesh] OR “Intrauterine Devices, Copper”[Mesh]) OR iud OR iucd OR ius OR iuc OR intrauterine system OR intrauterine system OR intrauterine device OR intra-uterine device OR intrauterine contraceptive OR intrauterine contraception)). We also searched the Cochrane Library for any reviews examining IUDs and PID.
We included primary research articles in all languages that directly answered the question, “Among women with asymptomatic GC/CT or at high risk of STIs, is the risk of PID increased for women who undergo CuT380A or LNG–IUD placement compared with women who do not undergo IUD placement?” We included indirect evidence that examined algorithms for identifying women at increased risk of STIs prior to Cu– or LNG–IUD placement. We also considered indirect evidence studies that compared PID rates among LNG–and Cu–IUD initiators to examine whether PID risk differs between IUD types, and studies that reported PID rates in women screened versus not screened for GC/CT infection before Cu–or LNG–IUD placement.
Two coauthors (T.C.J. and K.B.S.) independently graded the articles according to the United States Preventive Services Task Force evidence grading system [12]. Quality factors assessed for randomized controlled trials (RCTs) included adequate randomization, allocation, concealment and blinding. Quality factors for cohort studies included exposure or intervention definitions/assessment (diagnosis of GC/CT infection by culture or NAATs or other defined screening, IUD type specified), outcome assessment (whether presence of PID was assessed by a provider using criteria consistent with CDC STD Treatment Guidelines [10] or by medical record validation), assessment of potential confounders (e.g., age, condom use, sexual behavior, history of STIs or PID, contraceptive method) at baseline and in modeling, loss to follow-up, and sample size and power. We did not include case–control studies that examined PID and IUD use if they could not assess the timing of PID diagnosis after IUD placement or assess whether a cervical infection was present at the time of IUD initiation. Due to the heterogeneity of study designs, we did not compute summary measures.
3. Results
This search identified 2220 articles of which 2 met our inclusion criteria for direct evidence and 8 for indirect evidence.
3.1. Direct evidence
A secondary data analysis (level II–2, fair) of a US prospective cohort study examined the rate of PID over 6 months among 4371 Cu–IUD or LNG–IUD initiators compared with 3240 non–IUD contraception initiators (Table 1) [13]. All women were screened for GC/CT at baseline with NAAT, and asymptomatic women initiated the IUD on the same day as screening. PID was assessed by self-report and confirmed through chart review. Among the 215 asymptomatic women testing positive for GC/CT at baseline, 1 of 91 women initiating an IUD developed PID compared to none of 124 women who did not initiate an IUD (rates of 1.1 vs. 0.0, respectively; p=.42). Most of these women began antibiotics according to national guidelines within 2–3 weeks of enrollment. Across all women regardless of baseline GC/CT status, rates of PID were low but were significantly higher among IUD users at 6 months[0.46%; 95% confidence interval (CI), CI 0.26–0.66] compared with non–IUD users (0.09%; 95% CI, 0–0.20; p=.005).
Table 1.
Direct evidence for safety of IUD initiation among women with current asymptomatic cervical infection or at increased risk of STIs
| Author, year, location, funding source | Study design, follow-up duration | Population, intervention | Comparison groups | Outcome | Results | Strengths | Weaknesses | Grade |
|---|---|---|---|---|---|---|---|---|
| Birgisson, 2013 [13] United States Anonymous foundation; Washington University Institute ofClinical and Translational Science grants, National Center for Advancing Translational Sciences grant; NICHD award K23HD070979 |
Secondary data analysis from prospective cohort (CHOICE project [27]) | N=7611 contraceptive initiators with baseline GC/CT testing GC/CT positive (n=215) GC/CT negative (n=7396) |
IUD users Non-IUD users (OCP, patch, ring, implant and DMPA) |
Rate of PID among IUD and non-IUD users by self-report at 3 and 6 months and confirmed by chart review | 23 cases PID (“likely” or “possible”) among 7611 women (0.30%) PID rates, 95% CI: IUD users: 0.46 (0.26–0.66) Non-IUD users: 0.09 (0–0.20) p=.005 Among GC/CT positive (n=215): IUD users: 1.1 (0.0–3.28) Non-IUD users: 0.0 (0.00–0.00); p=.42 Among GC/CT negative (n=7396): IUD users: 0.44 (0.24–0.64) Non-IUD users: 0.10 (0.00–0.21); p=.008 |
Large sample size Most patients testing positive were treated with antibiotics within 2–3 weeks of screening Mixed risk population, 40% had history of STI Stratified by GC/CT status at initiation of method | Groups differed at baseline by multiple potential confounders Screening test type not reported Results not reported by IUD type (LNG vs. Cu) or other potential confounders due to small number ofPID cases (crude analysis only) Power calculation not performed PID diagnosis by self-report, confirmed by chart review in all but 4 cases; classified as “likely PID”, “possible PID” or “not PID” by defined criteria Unclear if people assessing PID outcome were blind to contraceptive type or GC/CT status LTFU 18% fom primary study (this sample excludes all LTFU participants) |
II-2, Fair |
| Cropsey, 2010 [14] United States Source of funding NR |
Retrospective cohort Follow-up period not reported |
Women at tertiary care inner city clinic initiation IUD or DMPA (n=385) | IUD (n = 194) DMPA (n = 191) |
Rates of STI PID by chart review |
Odds of STI after contraception initiation for DMPA vs. IUD: aOR 2.67 (95% CI, 1.05–6.78) adjusted for marital status, race, duration of use, prior STI history, age; no longer significant with interaction terms for age of birth control by birth control type and length of birth control use by type After initiation: No dfmce in PID rate betwem groups (2.1% DMPAvs. 22% IUD, p=97) Rate of endometritis significantly higher among IUD group (2.2% vs. 0%, p < .05) | Some potential confounders assessed in multivariable model PID diagnostic criteria described |
Single site Groups differed at baseline by potential confounders No standard STI screening before contraception initiation, no diagnostic criteria/testing/treatment for STIs after initiation reported Did not assess sexualbehavior, condom use after initiation All data from chart review; outcomes may be underestimated if women sought care from other sites Unclear follow-up time and attrition Power calculation not performed Cu- and LNG-IUD results combined |
II-2,Poor |
Abbreviations: NICHD=Eunice Kennedy Shriver National Institute of Child Health and Human Development; GC/CT=gonorrhea/chlamydia; OCP=oral contraceptive pills; DMPA=depot medroxyprogesterone acetate; PID=pelvic inflammatory disease; IUD=intrauterine device; STI=sexually transmitted infection; LNG=levonorgestrel; Cu=copper; LTFU=loss to follow=up; aOR=adjusted odds ratio
One US retrospective cohort study (level II–2, poor) at a tertiary care inner city clinic reported PID rates among women undergoing either Cu–or LNG–IUD placement compared with initiation of depot medroxyprogesterone acetate (DMPA) [14]. STI screening and treatment practices and follow-up duration were not reported. PID was assessed using clear diagnostic criteria through chart review [14]. There was no significant difference in PID rates between 191 DMPA initiators and 194 IUD initiators after contraception initiation (2.1% vs. 2.2%, p=.97), although history of PID was more common at baseline among DMPA initiators compared with IUD initiators (4.7% vs. 0.5%, p=.01) [14]. Odds of STI were higher after contraception initiation among DMPA initiators compared with IUD initiators [adjusted odds ratio (OR), 2.67; 95% CI, 1.05–6.78, adjusted for several covariates].
3.2. Indirect evidence (Table 2)
Table 2.
Indirect evidence for safety of IUD among women at increased risk of STIs
| Author, year, location, funding source | Study design, follow-up duration | Population, intervention | Comparison groups | Outcome | Results | Strengths | Weaknesses | Grade |
|---|---|---|---|---|---|---|---|---|
| Grentzer, 2015 [16] United States The Susan Thompson Buffett Foundation; NICHD award K23HD070979; Washington University Institute of Clinical and Translational Science grants, National Center for Advancing Translational Sciences grant |
Secondary data analysis from prospective cohort (CHOICE project [27]) |
N =5087 women aged 14–45 years with Cu- (n =1057) and LNG-IUD (n =4035) placement and NAAT for gonorrhea and chlamydia 140/5087 (2.8%) + chlamydia 16/5087 (0.3%) + gonorrhea | Algorithm: 1: age 2: age, 1 + sex partners 3: age, 1 + sex partners, condom use or STI history |
For each algorithm compared with NAAT results: Sensitivity Specificity PPV NPV NLR |
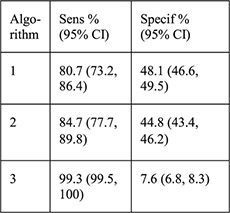 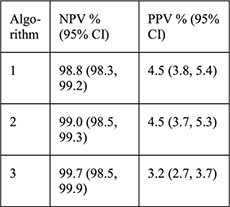 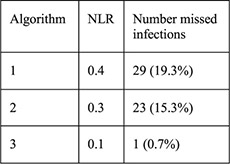 PID rates not reported for this sample, for CHOICE IUD users self-report rate was 0.46% at 6 months [28] |
Large sample size all undergoing STI screening by algorithms and NAAT Screening tests described with credible reference Treatment provided if testing+ Models based on 2010 CDC STD treatment guidelines [23] for STI screening except for survival sex Risk factors assessed by interview not records |
Groups with testing + and − differed at baseline by multiple potential confounders Results not separated by IUD type (LNG vs. Cu) Did not examine PID rates for women with screening + vs. − Unclear generalizability for large study within small geographic area NAAT has high false-positive rate in low-prevalence areas, so misclassification possible among low-risk groups |
II-2 (diagnostic accuracy study), fair |
| Papic, 2015 [22] United States R01PG000859 from Department of Health and Human Services, Office of Population Affairs |
Secondary data analysis from prospective cohort [29] 3 months |
Women aged 15–45 years seeking pregnancy testing or EC who elected same-day Cu- or LNG-IUD placement or no IUD (n=1060), all underwent STI | For those with survey data: 1. Same-day IUD placement (n=28) 2. Delayed IUD placement (n=17) 3. No IUD placement (n=227) |
PID rate within 3 months by self-report, chart review or diagnostic code STI treatment | Among survey data group: No difference in PID rates by self-report between 3 groups (4.6%, 11.8%, 4.9%, p = .54 for 1 vs. 2, p=1.00 for 1 vs. 3) No difference in being treated for an STI within 3 months (14.3%, 5.9%, 17.0%; p=.64 for 1 vs. 2, p= 1.00 for 1 vs. 3) |
3 groups from survey data sample similar at baseline, potential confounders assessed at baseline Authors assessed women with survey data and women | Small proportion of total sample with demographic baseline data, small samples of women having IUDs placed Excluded women with signs of cervicitis from same-day placement only; potentially |
II-2, poor |
| screening with 3-month follow-up survey data (n=272, with chart review for n=51) or chart review by EMR for 3 months (n=947) 0.8% chlamydia prevalence |
For EMR data: 1. Same-day IUD placement (n =31) 2. Delayed IUD placement (n =40) 3. Used hormonal contraceptives within 3 months (n=312) 4. Used no prescription contraception within 3 months (n=564) |
Among EMR group: No difference in PID rates (95% CI) between 4 groups: 1. 6.5% (0.8–21) 2. 5.0% (0.6–16.9) 3. 1.9% (0.7–4.1) 4. 0.9% (0.3–2.1) |
without, and assessed women with EMR data available and women without for total sample n=1060 and reported no differences by limited demographic information available Treatment provided iftesting+ | included in other groups PID by self-report or EMR and when cross-referenced did not always match PID diagnosis criteria not defined Did not report STI screening results at baseline by group or treatment details Did not assess outcomes by potential confounders High LTFU of surveys |
||||
| Murphy, 2012 [17] United States NICHD award R21HD063028 |
Secondary data analysis from prospective cohort and pilot study of Cu-IUD vs. LNG for EC [30,31] |
Women aged 18–30 years seeking EC who chose Cu-IUD and had same-day STI screening with adequate results (n = 197) 8/197 (4.1%) + chlamydia 0/197 (0%) + gonorrhea |
1: History of STI 2: Aged < 25y 3: 2 + partners 4: Aged <25 years and history of STI 5: Aged <25 years and 2 + partners 6: History of STI and 2 + partners 7: Aged <25 years and history of STI and 2 + partners | For each screening type compared with laboratory testing: Sensitivity Specificity PPV NPV PLR NLR |
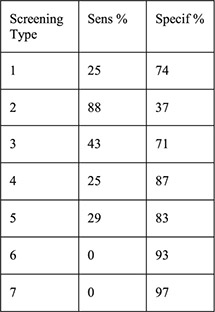 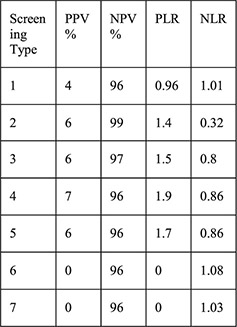 No cases of upper genital tract infection |
Entire sample had STI screening by algorithms and laboratory STI screening for gonorrhea and chlamydia Screening tests by algorithms described with credible reference Treatment provided if testing+ Risk factors assessed by interview not records |
Small sample, 2 sites with only 8 cases testing+ Women at high risk of PID were excluded from prospective cohort Unclear generalizability for small geographic area with low prevalence of cervical infections Screening test type not reported |
I I - 2 (diagnostic accuracy study), poor |
| Sufrin, 2012[21] United States Kaiser Permanente Northern California Residency Program, Kaiser Foundation Hospitals |
Retrospectivecohort 12 months data prior to and 90 days after IUD placement |
Women aged 14–49 years with Cu- or LNG-IUD placement with continuous membership with Kaiser system for given follow-up time (n=57,728) | 1. Same-day STI screening (n=5633) 2. Screening 1 day up to 8 weeks before placement (n=11,041) 3. Screening 8 weeks up to 1 year before placement (n=13,662) 4. No screening within 1 year (n=27,392) In models: Any screen: groups 1, 2, 3 Any prescreen: groups 2, 3 |
Risk of PID within 90 days placement Risk difference between groups (a priori margin of equivalence set: -0.006 to 0.006 based on prior studies of PID risk in IUD users | Risk of PID in sample: 0.0054 (95% CI 0.0048–0.0060) Risk of PID (95% CI): Group 1: 0.0044 (0.0029–0.0066) Group 2: 0.0099 (0.0082–0.0120) p < .001 comparing groups 1 and 2 Group 3: 0.0056 (0.0044–0.0070) Group 4: 0.0036 (0.0029–0.0044) p<.01 comparing groups 3 and 4 p < .0001 comparing groups 2 and 4 Adjusted risk differences (for age, race and ethnicity) were not significant comparing 1) no screen to any screen; 2) group 1 to any prescreen; 3) group 1 with group 2; 4) group 1 with group 4 Sensitivity analysis expanding PID diagnosis criteria did not change results Adjusted risk difference by age (younger than 26 years and 26 years or older) did not find differences between groups |
Large sample size in closed health care system Exposure: screen timing clearly defined and objective PID diagnosis criteria clearly described with sensitivity analyses performed Power calculation performed to detect risk difference between groups Screening performed according to CDC STD treatment guidelines Stratified by age |
Information regarding treatment for screening + results not reported Women excluded from l ogistic regression (n=1221) due to demographic information not available Multivariable analysis could not adjust for potential confounders that were not available in records Unclear if assessors of outcome blinded to exposure status Selection bias: screening determination based on risk factors which are the same as outcome risk factors |
II-2, fair |
| Campbell, 2007 [15] United States Source of funding NR |
Retrospective cohort Follow-up period not reported |
Women at urban, university-based clinic with Cu- or LNG-IUD placement (n = 194) | LNG-IUD (n=155) Cu-IUD (n=39) |
STI rates PID rates (by types of IUD, and by history of STI at time of IUD placement) by chart review |
History of STI before initiation: 31.7% STI diagnosed after initiation: 5.4% Rates of PID were similar before and after initiation (0.05%, 0.22%, respectively; p=.38) for all IUD users Rates of gonorrhea and herpes ½ infection were higher among Cu-IUD users (5.4% for both) than LNG-IUD users (0%, 0.7%, respectively) (p=.004, p=.04, respectively) Rates of PID did not differ between Cu-IUD (2.7%) and LNG-IUD (2.0%) users (p=.80); rates of endometritis did not differ between IUD types (5.4% vs. 1.3%, p=.13) Among women with history of STIs before initiation, rates of PID were similar to women with no history of STIs before initiation (1.7% vs. 2.4%, p=.79) but had higher rates of STIs after initiation (11.9% vs. 2.4%, p=.007) |
Groups similar at baseline, some potential confounders assessed PID diagnostic criteria defined Low attrition | Single site, small sample size No standard STI screening before placement and diagnosis criteria/testing/ treatment for STIs and PID not reported Did not assess sexual behavior, condom use Unclear follow-up time Power calculations not performed All data from chart review; outcomes may be underestimated if women sought care from other sites Coding of exposure and outcome done at same time; no blinding to exposure status |
II-2, poor |
| Morrison 2007 [18] Kenya, Zimbabwe, Jamaica, United States, Uganda, Thailand USAID, FHI |
Secondary data analysis from 4 data sets (diagnostic accuracy study of algorithms validated with 2 additional data sets) | Family planning clinics in Kenya, Zimbabwe, Jamaica, US for development of algorithms (samples ranged from 615 to around 1400 women); Thailand (n=1525), Uganda (n=1731) for validation (all moderate to high STI prevalence) excluding women with cervical mucopus, cervical/vaginal ulcer or clinical diagnosis PID | Women grouped into low (score 0), moderate (score 1–2), and high risk (score 3+) for cervical infection based on: Historical variables: age under 25 years, not living with partner, low education for the population, bleeding between periods, recent condom use, number of sex partners Clinical signs: cervical abnormality (includes cervical edema, erythema or friability, trawberry cervix) |
Likelihood ratio (aim to identify ideal algorithm with low LR < 0.75 for low risk of current cervical infection group and high LR > 2.0 for high risk of current cervical infection group) | Clinical signs did not improve the history-based algorithm—did not improve identification of low vs. high risk women—in original 4 countries Validation (historical only):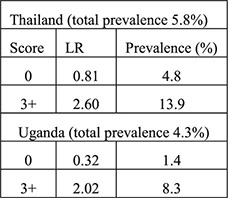 Validation (historical plus clinical): 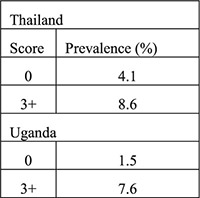 Algorithms performed differently in certain countries when certain variables were included (e.g., ethnicity, education) |
Validated in diverse populations at family planning clinics: generalizable Risk factors assessed by interview not records | Results limited by existing data sets, unclear who performed interviews, how and when GC/CT tested | II-2, fair |
| Morrison, 1999 [19] Kenya Source of fundingNR |
Secondary data analysis from prospective cohort 4-month follow-up with 1-month visit | Women with IUD placement and baseline HIV testing (n=615) with endocervical specimen testing for chlamydia antigen test and gonorrhea culture (n=580) | Women with + testing Women with – testing Algorithms: 1a. (any): age ≤ 24 years, single/divorced/widowed,≥2 sex partners in last 3 months, STD symptoms in past year 1b. (any risk from 1a or any of following): abnormal vaginal or cervical discharge, ulcerations, pelvic/adnexal/cervical motion tenderness 2. (any): age <20 years, cervical discharge, age 20–24 years and≥2 sex partners or no condom use in last 3 months, age > 25 years and ≥2 sex partners and no condom use in last 3 months 3a. (any): Age ≤ 24 years, single/divorced/widowed, Luhya ethnicity, live births ≤ 2 3b: (sum of ≥2): age ≤ 24 years (1), single/divorced/widowed (2), Luhya ethnicity (2), live births ≤ 2 (1) |
PID rates Sensitivity Specificity PPV NPV PLR NLR |
Among women with cervical infection at 1 month (n=32, 5.5%), PID rate 3.1% Among women without infection at 1 month (n=548) PID rate 0.4%; no p value reported 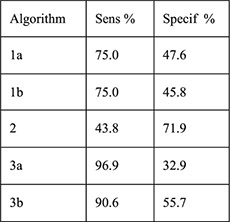 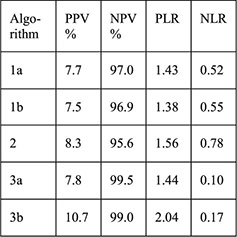
|
Large sample size Screening tests described with credible reference PID diagnosis criteria defined Low attrition (5%) Risk factors assessed by interview not records |
Laboratory screening not performed at time of IUD initiation No statistical testing comparing women with infection and women without infection and risk of PID Treatment for cervical infection not reported Algorithms 3a and 3b calculated for study population and not generalizable |
II-2 (diagnostic Accuracy study), poor |
| Faundes, 1998 [20] Brazil Population Council's Robert H. Ebert Program for Critical Issues in Reproductive Health and Population; John D. and Catherine T. MacArthur Foundation; UNDP/UNFPA/WHO/World Bank Special Program of Research, Development and Research Training in Human Reproduction |
Cross-sectional and follow-up study without a comparison group 1 month | Women attending family planning clinic for contraception initiation, screened for chlamydial or gonococcal infection by history/clinical signs and laboratory screening (CT antibody and GC culture) (n=407) | CT positive by laboratory screening (n=27, 6.7%) CT negative and GC negative (n=380) Presumptive clinical diagnosis of GC or CT infection: history of multiple partners, purulent cervical secretion, hyperemia, bleeding of cervix at touch or pelvic pain during bimanual exam (n=29); no IUDs inserted Presumptive clinical diagnosis negative (n=378) |
Correlation of demographic and history variables with CT infection Findings on pelvic exam (pain, vulvar hyperemia, inguinal lymph node, vulvar lesions, vaginal or cervical hyperemia, excessive or foul smelling discharge, purulent cervical mucus, cervical bleeding at touch Sensitivity, specificity, false positive and false negative of presumed clinical diagnosis |
No correlation of race, education, coitarche, use of condoms, lifetime partners (1 vs. more than 1) STI history to CT infection. Longer years of current partnership associated with higher prevalence of CT (χ2=13.0, p=.0046). No significant differences in any exam findings between CT+ and CT− groups (p values all >.1). Presumptive clinical diagnosis had 7.4% sensitivity for CT, 92.8% specificity, 93.1% false positive and 6.6% false negative 19/327 IUD acceptors were CT positive 2/19 were diagnosed with PID within 2 weeks and treated; one had IUD removed and one did not 17/19 were treated at 1 month and did not have PID |
No discussion of why CT associated with longer partnership: suspicious result Outcome assessment done by investigator not involved in interviews Risk factors assessed by interview not records All CT positive were treated |
Single site Small sample size. CT diagnosed by antibody not culture: potential for false positives Symptoms, exam and CT results only available for 261 women CT negative PID diagnosis criteria not clearly defined Follow-up unclear for CT negative women |
II-3, fair |
Abbreviations: NICHD=Eunice Kennedy Shriver National Institute of Child Health and Human Development; LNG=levonorgestrel; Cu=copper; IUD=intrauterine device; STI=sexually transmitted infection; NAAT=nucleic acid amplification test; PPV=positive predictive value; NPV=negative predictive value; NLR=negative likelihood ratio; Sens=sensitivity; Specif=specificity; CI=confidence interval; CDC= Centers for Disease Control and Prevention; STD=sexually transmitted disease; PID=pelvic inflammatory disease; EC=emergency contraception; EMR=electronic medical record; LTFU=loss to follow-up; PLR= positive likelihood ratio; USAID=United States Agency for International Development; FHI=Family Health International; LR=likelihood ratio; GC=gonorrhea; CT=chlamydia; NR=not reported; UNDP=United Nations Development Programme; UNFPA=United Nations Population Fund; WHO=World Organization.
One US retrospective cohort study (level II–2, poor) compared PID rates among women initiating LNG–IUD (n= 149) or Cu–IUD (n=37) in a high-risk urban clinic with31.7% baseline STI history [15]. STI screening and treatment practices and follow-up duration were not reported. PID was assessed through chart review; however, diagnostic criteria were not reported. This study reported similar rates of PID in the two IUD groups (2.0% for LNG–IUD vs. 2.7% for Cu–IUD, p=.80) after placement[15]. Rates of PID among all IUD users did not differ before and after placement. Those with prior STI history were more likely to have another STI after placement but not more likely to have PID.
Five studies (level II–2 to II–3, fair to poor) examined algorithms to predict current asymptomatic cervical infection based on patient history and/or clinical factors. These studies reported sensitivities, specificities, negative and positive predictive values (NPVs and PPVs) and/or likelihood ratios (LRs) for current infection using laboratory testing as the gold standard [16–20].
A recent analysis from a US prospective cohort study evaluated three algorithms using risk factors for GC/CT to predict current infection among women choosing Cu–IUD or LNG–IUDs (n=5087) [16]. Factors included age ≤25 years alone; age ≤25 years and multiple sexual partners; and age ≤25 years, multiple sex partners, inconsistent condom use and/or history of an STI. GC/CT NAAT screening results were positive for 2.8% of chlamydia tests and 0.3% of gonorrhea tests. Groups positive for either test were significantly different from the group with negative tests with regards to age, race, education, marital status, insurance, multiple sex partners and IUD chosen at baseline. The algorithm that included the most risk factors was most sensitive (99.3%) for detecting GC/CT infection with the least number of missed infections (n=1, 0.7%), lowest negative LR (0.1) and a high NPV (99.7%); however, the specificity was extremely low (7.5%), and PPV was also extremely low (3.2%). No positive LR was reported. Thus, most of the women identified as at risk for infection by this algorithm did not have an infection and could have been denied an IUD unnecessarily.
A secondary data analysis from the US examined 197 women choosing the Cu–IUD within a prospective cohort of women seeking emergency contraception (EC) [17]. Seven algorithms that included history of STI, age b25 years, multiple sexual partners and various combinations of these factors were compared with GC/CT screening results at the time of IUD placement. Laboratory results were positive for4.1% of chlamydia tests and 0% of gonorrhea tests. When examining the seven algorithms, sensitivities ranged from 0% to 88%, specificities from 37% to 97%, PPV from 0% to 7%, NPV from 96% to 99%, positive LR from 0 to 1.9 and negative LR from 0.8 to 1.08, suggesting that none of these algorithms would adequately differentiate women with GC/CT who should not undergo placement and women without infection who could safely undergo placement.
In a third secondary data analysis from a prospective study, authors evaluated algorithms to predict cervical infection and subsequent IUD–related complications among new Cu–IUD users in Kenya where laboratory STI screening is not routinely performed [19]. The five algorithms included age, marital status, STI symptoms, condom use, Luhya ethnicity, live births, and symptoms of discharge or tenderness on exam. The primary outcome was GC/CT infection (by gonorrhea culture or chlamydial antigen testing 1 month after placement) among 580 women. Thirty-two women had a diagnosed cervical infection during follow-up(5.0% chlamydia and 0.5% gonorrhea), and three cases of PID were diagnosed (one woman with cervical infection and two without). None of the 5 algorithms accurately predicted these 32 cervical infections. An algorithm containing age, marital status, ethnicity and parity performed best but would have identified 46.9% of the 580 women as high risk of cervical infection, potentially denying 272 women from getting an IUD to prevent 3 cases of PID.
In an expanded analysis from this study, authors identified risk factors for cervical infection using data from Kenya, Zimbabwe, Jamaica and the US and then validated the algorithms based on these risk factors using data and laboratory testing for cervical infections from Uganda and Thailand [18]. All settings were considered moderate to high prevalence of cervical infections (range, 4%–14%). An algorithm containing variables obtained by patient history performed best with negative LRs of 0.8 and 0.3 to identify low-risk women in Uganda and Thailand, respectively, and positive LRs of 2.6 and 2.0 to identify high-risk women. Addition of clinical signs to the algorithms did not improve LRs. If all women in Thailand and Uganda considered as having low to moderate risk of infection were considered acceptable for IUD placement, 98% and 87% of women would have been referred for IUDs according to this algorithm.
The final algorithm study of 407 sexually active women in Brazil examined how a clinical diagnosis based on demographic and historical variables compared to antibody testing of cervical specimens for Chlamydia trachomatis and culture for Neisseria gonorrhoeae [20]. There were no gonococcal infections and 27 chlamydial infections. The only variable significantly associated with chlamydial infection was years of current partnership, with a higher prevalence of infection reported for partnerships ≥12 months compared with those b12 months. There were no significant differences in pelvic exam findings between women with laboratory confirmed infection and those without. Twenty–nine women met a clinical diagnosis for infection (by history of multiple partners, purulent cervical secretion, hyperemia, bleeding of cervix at touch or pelvic pain during bimanual exam), and no IUD was inserted. This diagnosis resulted in 7.4% sensitivity, 92.8% specificity, 93.1% false positive and 6.6% false negative for chlamydial infection. Of the 327 women who had an IUD inserted, 19 had positive chlamydia screening, and of these, 2 were diagnosed with PID within 2 weeks and treated. Authors did not provide information on PID cases among non–IUD acceptors or among women with negative chlamydia testing.
Finally, two studies (level II–2, fair to poor) examined the risk of PID among women who had GC/CT screening at the time of IUD insertion [21,22]. A retrospective cohort study examined nearly 60,000 women who had an IUD inserted at a large, integrated managed care consortium in Northern California where women were screened for GC/CT according to 2010 CDC guidelines [21,23]. PID was assessed by chart review, and a sensitivity analysis did not find a difference between two different methods of outcome ascertainment for PID. Among women not screened prior to IUD placement (women considered low risk for STIs by screening guidelines) compared with women screened on the day of IUD placement (women with risk factors for STIs), there was no difference in risk of PID (adjusted OR, 1.16; 95% CI, 0.69–1.96). The overall risk of PID diagnosis within 90 days of IUD placement in the total sample was very low (absolute risk, 0.0054; 95% CI, 0.0048–0.0060). The authors did not report how many women were diagnosed with and treated for GC/CT infections in both groups, although they stated that prompt treatment was common.
A secondary data analysis examined PID outcomes among a prospective cohort study of women attending a Title X clinic (chlamydia prevalence, 8%) for pregnancy testing or EC (including the Cu–IUD). Survey data were available for 272 women, of whom 51 had an IUD placed[22]. PID was assessed by diagnosis codes in electronic medical records (EMRs), by chart review for antibiotic treatment or by self-report. All women were screened for GC/CT at enrollment, but no information was provided on diagnosis and treatment. At 3-month follow-up, there were no differences in self-reported PID between women who had same-day IUD placement, IUD placement at a later date or no IUD placement. Results were the same when the authors examined a larger population using IUD placement timing and PID diagnosis information extracted solely from EMR or chart review data (n=947).
4. Discussion
This systematic review identified two studies providing direct evidence (quality II–2, fair to poor) that women with asymptomatic gonococcal or chlamydial infection or at high risk of STIs do not have higher rates of PID when initiating a Cu–IUD or LNG–IUD compared to other contraceptive methods [13,14].
Eight studies provided indirect evidence (quality II–2 to II–3, fair to poor). One study found no difference in PID rates among Cu–IUD versus LNG–IUD initiators at high risk for STIs [15]. Five studies compared algorithms containing historical or demographic variables with laboratory testing for cervical infections (generally GC/CT), and all were unable to reliably differentiate women with infections from those without [16–20]. Algorithms to identify women with asymptomatic GC/CT do not have added benefit over appropriate laboratory screening at the time of IUD insertion and may be overly restrictive, leading to missed opportunities for IUD initiation. Two studies examined IUD placement on the same day as GC/CT screening compared with delayed placement after screening and found no differences in PID rates between groups [21,22]; one of these studies also compared same-day IUD placement with no placement and reported no difference in PID rates [22].
The body of evidence for this systematic review is limited by several factors. Diagnosis of PID is complex, and PID is frequently overdiagnosed (clinical diagnosis of PID has a PPV of 65%–90% compared with laparoscopy) or underdiagnosed (many cases have only subtle, vague symptoms or are asymptomatic) [9,10]. In addition, clinicians may be more likely to diagnose PID in an IUD user than a non-IUD user, leading to ascertainment bias [6]. In most included studies, PID was assessed through self-report or retrospectively through chart review without clear diagnostic criteria. Therefore, the accuracy of PID diagnosis and the likelihood of differential diagnosis between IUD users and nonusers are concerns. Most studies did not report detailed information on follow-up procedures and treatment regimens for women with positive GC/CT screening tests. Therefore, while our conclusions focus on the association between screening and risk of PID, this assumes that women had appropriate follow-up and treatment when necessary.
One of the major limitations of this body of evidence over time has been the use of inappropriate comparison groups, particularly the inclusion of hormonal contraceptive users in the comparison group, as hormonal contraceptive may exert a protective effect against PID [6,24]. While more recent studies have generally included more appropriate comparison groups, both direct evidence studies in this review included hormonal users in comparison groups, which may bias towards a larger difference in PID rates between IUD and non-IUD users [13,14]. Finally, as PID is a relatively rare event, many studies may not have been adequately powered to detect a difference between groups.
Two indirect studies that compared same-day IUD placements with delayed placements were also limited by differing baseline characteristics between groups; women were excluded from same-day placement if they had signs of cervicitis and were potentially included in other groups. These and other studies did not adequately assess high-risk sexual behaviors such as condom use or number of partners after IUD placement [14,15].
All but one of the five algorithm studies were secondary data analyses without consistent reporting of the type of laboratory testing used, rates of PID, PID diagnostic criteria and outcome ascertainment, and how treatment was handled for women with cervical infection. Generalizability was limited in all but one study [18]. Prevalence rates of STIs, GC/CT in particular, were not always reported. The laboratory testing varied by study, with two studies not reporting test type [17,18], one study using chlamydia antigen testing and gonorrhea culture [19], another using chlamydia antibody and gonorrhea culture and one study using NAAT [16]. NAAT has improved sensitivity and specificity over nonculture tests such as antigen or antibody testing, and it is easier to transport specimens and maintain specimen integrity in NAAT compared with culture [25]. However, like all screening tests, the PPV of NAAT is dependent on the prevalence of these infections in the population being studied; thus, for the studies included in this review, the PPV could vary from 50% with a 1% prevalence to 89% with an 8% prevalence of cervical infection. Basing an algorithm study on NAAT screening is therefore limited, and culture may have been a better comparison for these studies.
A systematic review previously conducted for the World Health Organization Medical Eligibility Criteria examined whether initiation or use of an IUD in women with STIs increased the risk of PID compared with non-IUD users [26]. The authors did not identify any direct evidence but reported indirect evidence from six studies comparing PID risk between women with and without an STI at the time of Cu–IUD initiation. Two of these studies were included in this review [19,20], while the remaining did not meet our inclusion criteria for IUD types or study design. Our study updates this previous review by including two studies with direct evidence and by restricting to IUDs currently available in the US, which provides more reliable risk estimates for current devices. Likewise, it is a strength of the current report that included studies had to address our specific research questions and therefore tended to be more recent (all published since 1998) with less bias than was common in older IUD studies.
In summary, poor to fair quality evidence from level II–2 to II–3 studies demonstrated that IUD placement in women with asymptomatic GC/CT infection or at high risk of STIs did not increase the risk of PID compared to initiating other contraceptive methods. Historical concerns of higher PID risk among women at risk for STIs who use IUDs may not be relevant with modern devices and STI screening and treatment practices. The information in this review was presented to an expert panel in August 2015 at a meeting held by the CDC and will be incorporated into the forthcoming update of the US Medical Eligibility Criteria for Contraceptive Use.
Footnotes
Financial disclosures: none.
Publisher's Disclaimer: Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the US Centers for Disease Control and Prevention.
References
- [1].Trussell J Contraceptive failure in the United States. Contraception 2011;83:397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].U S medical eligibility criteria for contraceptive use, 2010 MMWR Recomm Rep 2010;59:1–6. [PubMed] [Google Scholar]
- [3].Institute G. Contraceptive use in the United States. Fact Sheet 2015. [Google Scholar]
- [4].Romero L, Pazol K, Warner L, Gavin L, Moskosky S, Besera G, et al. Vital signs: trends in use of long-acting reversible contraception among teens aged 15–19 years seeking contraceptive services—United States, 2005–2013. MMWR Morb Mortal Wkly Rep 2015;64:363–9. [PMC free article] [PubMed] [Google Scholar]
- [5].Tyler CP, Whiteman MK, Zapata LB, Curtis KM, Hillis SD, Marchbanks PA. Health care provider attitudes and practices related to intrauterine devices for nulliparous women. Obstet Gynecol 2012; 119:762–71. [DOI] [PubMed] [Google Scholar]
- [6].Grimes DA. Intrauterine device and upper-genital-tract infection. Lancet 2000;356:1013–9. [DOI] [PubMed] [Google Scholar]
- [7].Farley TM, Rosenberg MJ, Rowe PJ, Chen JH, Meirik O. Intrauterine devices and pelvic inflammatory disease: an international perspective. Lancet 1992;339:785–8. [DOI] [PubMed] [Google Scholar]
- [8].selected US practice recommendations for contraceptive use, 2013: adapted from the World Health Organization selected practice recommendations for contraceptive use, 2nd edition. MMWR Recomm Rep 2013;62:1–0. [PubMed] [Google Scholar]
- [9].Brunham RC, Gottlieb SL, Paavonen J. Pelvic inflammatory disease. Med 2015;372:2039–48. [DOI] [PubMed] [Google Scholar]
- [10].Workowski KA, Bolan GA, Centers for Disease Control, Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015;64:1–37. [PMC free article] [PubMed] [Google Scholar]
- [11].Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Harris RP, Helfand M, Woolf SH, Lohr KN, Mulrow CD, Teutsch SM, et al. Current methods of the US preventive services task force: a review of the process. Prev Med 2001;20:21–35. [DOI] [PubMed] [Google Scholar]
- [13].Birgisson NE, Zhao Q, Secura GM, Madden T, Peipert JF. Positive testing for Neisseria gonorrhoeae and Chlamydia trachomatis and the risk of pelvic inflammatory disease in IUD users. J Womens Health (Larchmt) 2015;24:354–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Cropsey KL, Matthews C, Campbel S, Ivey S, Adawadkar S. Long-term, reversible contraception use among high-risk women treated in a university-based gynecology clinic: comparison between IUD and Depo-Provera. J Womens Health (Larchmt) 2010;19:349–53. [DOI] [PubMed] [Google Scholar]
- [15].Campbell SJ, Cropsey KL, Matthews CA. Intrauterine device use in a high-risk population: experience from an urban university clinic. Obstet Gynecol 2007;197:193 [e1–6; discussion e6–7]. [DOI] [PubMed] [Google Scholar]
- [16].Grentzer JM, Peipert JF, Zhao Q, McNicholas C, Secura GM, Madden T. Risk-based screening for Chlamydia trachomatis and Neisseria gonorrhoeae prior to intrauterine device insertion. Contraception 2015;92:313–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Murphy PA, Jacobson J, Turok DK. Criterion-based screening for sexually transmitted infection: sensitivity, specificity, and predictive values of commonly used questions. J Midwifery Womens Health 2012;57:622–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Morrison CS, Murphy L, Kwok C, Weiner DH. Identifying appropriate IUD candidates in areas with high prevalence of sexually transmitted infections. Contraception 2007;75:185–92. [DOI] [PubMed] [Google Scholar]
- [19].Morrison CS, Sekadde-Kigondu C, Miller WC, Weiner DH, Sinei SK. Use of sexually transmitted disease risk assessment algorithms for selection of intrauterine device candidates. Contraception 1999; 59:97–06. [DOI] [PubMed] [Google Scholar]
- [20].Faundes A, Telles E, Cristofoletti ML, Faundes D, Castro S, Hardy E. The risk of inadvertent intrauterine device insertion in women carriers of endocervical chlamydia trachomatis. Contraception 1998;58:105–9. [DOI] [PubMed] [Google Scholar]
- [21].Sufrin CB, Postlethwaite D, Armstrong MA, Merchant M, Wendt JM, Steinauer JE. Neisseria gonorrhea and Chlamydia trachomatis screening at intrauterine device insertion and pelvic inflammatory disease. Obstet Gynecol 2012;120:1314–21. [DOI] [PubMed] [Google Scholar]
- [22].Papic M, Wang N, Parisi SM, Baldauf E, Updike G, Schwarz EB. Same-day intrauterine device placement is rarely complicated by pelvic infection. Womens Health Issues 2015;25:22–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Workowski KA, Berman S, Centers for Disease C, Prevention. Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep 2010;59:1–10. [PubMed] [Google Scholar]
- [24].Toivonen J, Luukkainen T, Allonen H. Protective effect of intrauterine release of levonorgestrel on pelvic infection: three years’ comparative experience of levonorgestrel- and copper-releasing intrauterine devices. Obstet Gynecol 1991;77:261–4. [DOI] [PubMed] [Google Scholar]
- [25].Centers for Disease Control, Prevention. Recommendations for the laboratory-based detection of Chlamydia trachomatis and Neisseria gonorrhoeae—2014. MMWR Recomm Rep 2014;63:1–9. [PMC free article] [PubMed] [Google Scholar]
- [26].Mohllajee AP, Curtis KM, Peterson HB. Does insertion and use of an intrauterine device increase the risk of pelvic inflammatory disease among women with sexually transmitted infection? A systematic review. Contraception 2006;73:145–53. [DOI] [PubMed] [Google Scholar]
- [27].Winner B, Peipert JF, Zhao Q, Buckel C, Madden T, Allsworth JE, et al. Effectiveness of long-acting reversible contraception. Med 2012; 366:1998–2007. [DOI] [PubMed] [Google Scholar]
- [28].McNicholas C, Peipert JF, Maddipati R, Madden T, Allsworth JE, Secura GM. Sexually transmitted infection prevalence in a population seeking no-cost contraception. Sex Transm Dis 2013;40:546–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Schwarz EB, Papic M, Parisi SM, Baldauf E, Rapkin R, Updike G. Routine counseling about intrauterine contraception for women seeking emergency contraception. Contraception 2014;90:66–71. [DOI] [PubMed] [Google Scholar]
- [30].Turok DK, Godfrey EM, Wojdyla D, Dermish A, Torres L, Wu SC. Copper T380 intrauterine device for emergency contraception: highly effective at any time in the menstrual cycle. Hum Reprod 2013;28: 2672–6. [DOI] [PubMed] [Google Scholar]
- [31].Turok DK, Gurtcheff SE, Handley E, Simonsen SE, Sok C, Murphy P. A pilot study of the copper T380a IUD and oral levonorgestrel for emergency contraception. Contraception 2010;82:520–5. [DOI] [PubMed] [Google Scholar]


