Abstract
A common bioengineering strategy to add function to a given molecule is by conjugation of a new moiety onto that molecule. Adding multiple functions in this way becomes increasingly challenging and leads to composite molecules with larger molecular weights. In this review, we attempt to gain a new perspective by looking at this problem in reverse, by examining nature’s strategies of multiplexing different functions into the same pleiotropic molecule using emerging analysis techniques such as machine learning. We concentrate on examples from the innate immune system, which employs a finite repertoire of molecules for a broad range of tasks. An improved understanding of how diverse functions are multiplexed into a single molecule can inspire new approaches for the deterministic design of multifunctional molecules.
Graphical Abstract

1. INTRODUCTION
Synthetically modified molecules are known to have a broad range of functions, from targeted drug delivery to biomarker imaging to tracking of intracellular events.1 A common strategy to add function to a given molecule is by conjugation of a new moiety onto that molecule. As an index of the impressive progress, the repertoire of conjugation techniques used to attach synthetic chemical groups to proteins has grown drastically in recent years.2 As with all fields, there exist both contingent and structural difficulties. For instance, aside from the chemistry of bioconjugation, care must usually be taken to ensure that there is minimal interference between different parts of the resultant composite molecule. Moreover, adding multiple functions in this way leads to progressively more difficult chemistry and larger molecular weights. In this review, we attempt to gain a new perspective by looking at this problem in reverse, by examining so-called pleiotropic proteins, proteins that have multiple functions encoded into the same structure.
Over the past few decades, multifunctionality has been discovered in an increasing number of natural proteins. These so-called pleiotropic proteins with multiple coexisting roles can be considered products of natural bioconjugation, through which the introduction of covalent structural changes confers new functions.3 Due to the prevalence of proteins simultaneously involved in several cellular processes, multifunctionality is now believed to be the rule rather than the exception. In fact, over 60% of the proteins in archaea and bacteria, and over 80% of eukaryotic proteins, contain more than one functional domain.4 In addition, hundreds of “moonlighting” proteins, a subclass of multifunctional proteins, have been identified to perform two or more distinct functions within a single domain and are not the result of gene fusions, alternative splicing, or multiple proteolytic fragments.5–7 One example is phosphoglucose isomerase, which is a cytosolic glycolytic enzyme involved in energy metabolism. When secreted from cells, it also plays a dual role as the extracellular cytokine neuroleukin.6 While incorporating multiple functions into one protein may be nature’s method of doing more with less, protein multi-functionality introduces additional levels of complexity that can complicate efforts in understanding physiological processes and molecular mechanisms of disease.
With the significant implications of protein multifunctionality for human health, in both normal and pathological contexts, expanding on our current knowledge of multifunctional proteins can aid in the identification of other proteins and protein families that may also harbor additional functions, and provide insight on how these additional functions evolve. Furthermore, an improved understanding of how multifunctional proteins orchestrate their diverse roles also can enable the design and development of new therapeutics that can perform a multitude of actions, as well as more accurately target the specific roles of multifunctional proteins.
In this review, we present examples from the innate immune system, where multifunctionality is a matter of survival, given that a finite repertoire of molecules must organize against diverse and often unanticipated threats. Specifically, as a baseline example, we will start with a distinct function that seems simple enough, that of membrane remodeling and permeation. The point of the review is not to offer an exposition of current ideas on membrane remodeling or attempt a definitive and comprehensive account of the historical development of these ideas. Rather, the intention behind our choice of tracking this singular function is to adduce a classic case of a seemingly isolated function that is thought to be reasonably well understood in terms of peptide structure, before proceeding to an alternative, if not precisely opposite generalized conception of pleiotropic engineering, in which multiple functions are simultaneously optimized into a single peptide sequence. We first examine how membrane activity is encoded into a variety of peptides and proteins: In each of these molecules, membrane interactions underlie their roles in a variety of biological processes that involve membrane remodeling, including antimicrobial activity, membrane fusion and fission, budding and scission, and direct membrane translocation. In an extreme illustrative case of how membrane activity can be combined with other activities, we show how some of the structural requirements for membrane remodeling in innate immunity peptides, such as amphipathicity, can also allow self-assembly without membranes into scaffolds that organize immune ligands for multivalent presentation to immune receptors, leading to massive immune amplification. We expand the scope of inquiry and describe how machine- learning-based methods can be used to train a classifier that can discover unanticipated membrane activity in other proteins, especially proteins or peptides that have been annotated to have other primary functions (such as neuropeptides in signaling or molecular motors for force generation). Not only do we see how nature combines membrane activity with other functions, but we can also see specifically how membrane activity comes into being via evolution by combining machine learning and classical evolutionary biology methods. Finally, with these vignettes as a backdrop, we look at some engineering possibilities where we add membrane activity into existing molecules to make synthetic multifunctional molecules. Within the compass of this review, we use techniques that are not usually applied to bioconjugation problems, such as machine learning and synchrotron X-ray diffraction. The hope is that by using some of these techniques, we can learn by example from nature’s strategies of multiplexing different functions into the same molecule.
2. MULTIFUNCTIONALITY IN INNATE IMMUNITY PROTEINS
2.1. AMPs.
Antimicrobial peptides (AMPs) constitute a major component of the innate host defense system.8–13 Collectively, they display broad-spectrum antimicrobial activity and though widely diverse in sequence and structure, they share some common features. Most AMPs are short (<50 amino acids), cationic, and adopt an amphipathic structure that is characterized by segregated polar/cationic and hydrophobic regions.8–12 They are usually classified based on their secondary structures, with a large subgroup consisting of linear peptides that adopt an amphipathic -helix upon contact with a membrane, such as most cathelicidins14 and magainins.15 Another subgroup includes AMPs that consist of β-sheet structures that are stabilized by disulfide bonds, such as defensins16–18 and protegrins.19 Other peptides form extended structures that are dominated by a few amino acids, like the tryptophan-rich indolicidin20 and the proline- and arginine-rich PR-39.21
In general, AMPs function by disrupting the integrity of bacterial cell membranes, which leads to depolarization, leakage, and cell death.8,9 Membrane destabilization by AMPs is believed to be a consequence of their cationic and amphipathic nature, which promotes interactions between the peptide and the cell membrane. More specifically, electrostatic interactions between the polar/cationic residues of the AMP and the anionic membrane surface cause peptide–membrane attraction and binding. After adsorption of the AMP onto the membrane, hydrophobic interactions between the hydrophobic residues of the peptide and the bilayer interior perturb packing of the membrane lipids.8–10,22,23 Together, this combination of interactions leads to membrane disruption that can manifest in a variety of ways, including pore formation,22,24–26 blebbing,27,28 budding,29 and vesicularization.30 It is important to note that peptide concentrations in vivo can be several orders of magnitude greater than those shown to induce membrane disruption in vitro.16,31 Accordingly, these peptides potentially have greater capacity for membrane disruption than what has been demonstrated in experiments. In addition, increasing evidence indicates that certain AMPs utilize alternative modes of action, such as translocating across the cell membrane to interact with intracellular targets and consequently interfering with cellular processes. For example, indolicidin is able to permeabilize membranes without causing cell lysis. Instead, it kills bacteria by binding to DNA and inhibiting DNA synthesis.32 Although in such cases, membrane permeabilization alone may not achieve bacterial killing, it is still necessary in order for the AMP to reach its cytosolic targets.
While the membrane-disruptive function of AMPs is critical in defending against bacterial infections, more recent studies have also found that the role of AMPs in host defense is not limited to operating as direct microbicides. In fact, many AMPs have been discovered to also function in modulating the immune response, which can occur through activities such as inducing chemotaxis and chemokine production, inhibiting pro-inflammatory cytokine production, and modulating TLR-dependent inflammatory responses.
2.1.1. Chemotactic Activity.
Some AMPs have exhibited functions outside of membrane activity, and can directly recruit leukocytes or induce the expression of cytokines/chemokines, which can in turn indirectly recruit effector cells, including neutrophils, monocytes, macrophages, immature dendritic cells (DCs), and T cells. For instance, both human - and β- defensins are chemotactic for memory T cells and immature DCs, which is believed to occur through interactions with the G-protein-coupled receptor CCR6.33,34 Human -defensins HNP-1 and HNP-2 display chemotactic activity that mediates recruitment of monocytes to sites of inflammation,35 while human β-defensins HBD-3 and HBD-4 are reported to be chemotactic for monocytes and macrophages,36 and HBD-2 has chemoattractant activity for mast cells.37 Cathelicidins have similarly been found to exert chemotactic activity. For humans, LL-37 is chemotactic for neutrophils, monocytes, and T cells. In some cases, its chemotactic activity, such as for human blood-derived monocytes and T cells, is mediated by the G-protein-coupled formyl peptide receptor-like 1 (FPRL-1).38 Cathelicidins from bovine, human, mouse, and pig are chemotactic for nearly all subsets of peripheral blood cells.39–41 Granulysin, an AMP that is released by cytotoxic T cells and NK cells, exhibits potent broad-spectrum antimicrobial activity against both Gram-positive and Gram-negative bacteria.42,43 In addition to its microbicidal activity, granulysin also has demonstrated chemotactic activity toward monocytes, memory T cells, NK cells, and mature dendritic cells.44
AMPs have also been shown to have indirect chemotactic effects by stimulating the production of cytokines and chemokines from a range of cell types via receptor-dependent mechanisms.33,45 LL-37 induces release of IL-6, IL-8, TNF-, and granulocyte-macrophage colony-stimulating factor, and IL-1β in human keratinocytes,46 and enhances the IL-1β-induced secretion of cytokines IL-6, IL-10, and chemokines MCP-1 and MCP-3 in human peripheral blood monocytes.47 Its induction of IL-8 secretion then promotes the chemotaxis of neutrophils and release of LL-37 at high concentrations.48 HBD-2, HBD-3, HBD-4, and LL-37 interact with G-protein-coupled receptors and increase expression of IL-6, IL-10, IP-10, MCP-1, MIP-3, and RANTES in human keratinocytes.49 Furthermore, despite their structural differences, both human β-defensins and LL-37 have been found to exhibit similar effects on the activation of the ERK mitogen activated protein kinase (MAPK) pathway to stimulate pro-inflammatory cytokine production in epithelial cells.49,50 Likewise, granulysin activates monocytes and U937 cells to secrete proinflammatory cytokines, such as TNF-α, MCP-1, MCP-3, MIP-1β, RANTES, IL-1, IL-6, IL-10, and IFN-γ.44
2.1.2. Inhibition of Pro-Inflammatory Cytokines.
AMPs can also suppress certain cellular responses to inflammatory stimuli in order to offer protection against potentially lethal effects. For example, LL-37 can inhibit the secretion of pro-inflammatory cytokines induced by bacterial endotoxins, such as lipopolysaccharide (LPS) and lipoteichoic acid (LTA), to protect against endotoxemia.51–53 It has been further proposed that LL-37 is able to bind and neutralize the endotoxins before they can trigger inflammation.53–56 Similar protective effects against LPS-induced cytokine production have been observed for defensins and other cationic peptides.57
2.1.3. Modulation of TLR-Dependent Inflammatory Responses.
Toll-like receptors (TLRs) are widely expressed pattern-recognition receptors (PRRs) that detect signature pathogen-associated molecular patterns (PAMPs) from microorganisms and trigger inflammatory responses. These TLR-dependent inflammatory responses have been shown to be modulated by AMPs. Previous work has found that in allergic contact dermatitis, cathelicidins downregulate inflammation by inhibiting TLR4-mediated induction of cytokines in DCs, as well as inhibiting TLR4-induced DC maturation, which includes inhibiting the upregulation of costimulatory molecules CD40, CD80, and CD86.58 As described above, LL-37 can suppress the endotoxin-stimulated production of pro-inflammatory cytokines. This anti-inflammatory effect has been attributed to the inhibition of LTA-induced TLR2 activation and LPS-induced TLR4 activation.51,57
Alternatively, the effects of LL-37 exposure on TLR responses can also be pro-inflammatory. For instance, LL-37 is able to significantly enhance the flagellin activation of TLR5 in keratinocytes,59 as well as TLR activation by nucleic acids, namely TLR9 by DNA,60–62 TLR3 by double-stranded RNA (dsRNA),63,64 and TLR7/TLR8 by single-stranded RNA (ssRNA).65 In particular, LL-37 has been found to complex with the DNA and RNA ligands via electrostatic interactions, which triggers TLR activation and secretion of pro-inflammatory cytokines. In fact, this phenomenon has been observed to occur across a wide range of molecules, including other AMPs. High-resolution structural studies using small-angle X-ray scattering (SAXS) showed that cationic molecules can form columnar nanocrystalline complexes with dsDNA and dsRNA that amplify TLR9 and TLR3 activation, respectively, the degree of which is quantitatively correlated with the measured spacing between the nucleic acid columns of the complexes.64,66,67 Depending on the identity of the cationic molecule, this spacing parameter will vary. However, an optimal spacing was found to exist for both DNA and RNA that resulted in the strongest amplification of TLR-dependent cytokine production. Most strikingly, this spacing approximately matches the steric size of the TLR studied, suggesting that multivalent intercalative ligand–TLR binding is involved in amplifying TLR activation and cytokine production.68
2.2. Immune-Signaling Molecules.
While AMPs in general can exert their antimicrobial effects through direct membrane-disruptive activity, we have highlighted a number of complementary alternative functions of AMPs that can selectively modulate the immune defense responses against bacterial infections. The next question to ask is whether the opposite also applies, in which traditionally recognized immune-signaling molecules function as antimicrobial agents via membrane destabilization. Increasing evidence over recent years indicates that many of such molecules do indeed display direct bactericidal activity, which then suggests that they could have been classified as AMPs if this was their initially identified function. Interestingly, many of these immune molecules feature structural and compositional attributes that are common to AMPs.
For instance, the “kinocidins” constitute a class of chemokines, small secretory cytokines involved in chemotaxis, that have been discovered to possess direct microbicidal activity complementary to their ability to modulate inflammatory responses.69–73 In fact, many chemokines contain a β-sheet rich “γ-core” motif that also exists in classical AMPs such as defensins,71,73,74 as well as cationic charge and amphipathicity.72,74 Moreover, many of these immune signaling molecules have structures that also integrate modular domains of -helices and β-sheets, which parallel those of AMPs. Collectively, these similarities point to evolutionary conservation of membranedisruptive motifs that provide additional functionality and increased efficacy of host defense against pathogens. In addition, the chemotactic activity of certain AMPs further suggests functional reciprocity between chemokines and AMPs. Chemokines are classified into four groups based on conserved N-terminal cysteine motifs: CXC, CC, C, and CX3C.75 Examples of kinocidins include platelet factor 4 (PF-4), also known as platelet chemokine (CXC motif) ligand 4 (CXCL4),72,76 platelet basic protein (PBP),77 several IFN-γ- inducible CXC chemokines,78 and interleukin-8 (IL-8), also called chemokine (CXC motif) ligand 8 (CXCL8), one of the most well-characterized and recognized for its role in neutrophil chemotaxis. Despite its similarity to other kinocidins, IL-8 was only recently identified to have direct antimicrobial activity.71,79 Although most cytokines that have been found to possess direct antimicrobial activity are chemokines, bactericidal interleukins and interferons also exist. For instance, interleukin-26 (IL-26), a human Th17 cell-derived cytokine, was shown to kill bacteria by porating their membranes, while also being able to form complexes with both bacterial and self-DNA to activate TLR9 and induce production of pro-inflammatory cytokines.80,81 Another study demonstrated that type I interferon-β (IFN-β), which is most known for its role in the immune response against viral infections,82 can also directly kill Gram-positive bacteria, such as Staphylococcus aureus, via membrane permeabilization.83 This result supports the accumulating evidence that points to the involvement of type I IFNs in host response to bacterial infections,84–86 along with viral infections. Most striking is that the structures of both IL-2681 and IFN-β83 contain domains with amphipathic -helices rich in cationic residues, which are classic attributes of many membrane-disruptive AMPs, and thus believed to confer antimicrobial activity to the two cytokines. Taken together, these findings reveal a previously unrecognized role for cytokines in host defense against bacterial infections that involves direct membrane activity, distinct from their established immune signaling functions.
3. DIVERSITY OF MEMBRANE-ACTIVE MULTIFUNCTIONAL PROTEINS
The selected examples above illustrate the involvement of immunity molecules in multiple biological functions. In general, one of these is often direct membrane disruption that ultimately results in antimicrobial effects. However, protein multi-functionality that encompasses such direct membrane activity is not limited to immune proteins, and certain proteins may also exert membrane-disruptive and immunomodulatory effects in addition to their other known functions (Figure 1).
Figure 1.

Diagram illustrating diversity among membrane-active proteins and peptides. While bactericidal (red region), cell-penetrating (blue region), and membrane fusion- and fission-mediating (violet region) proteins and peptides are highly diverse, their functions often involve the ability to directly destabilize membranes (gold region). Of particular interest are membrane-active multifunctional proteins (green hashed region), which are those generally recognized for having other main functions (green region) in addition to roles associated with membrane-destabilizing activity. Thus far, the relatively low number of proteins identified as multifunctional has been attributed to the serendipitous nature of their discovery. However, newly developed machine-learning tools allow for systematic large-scale exploration of new and existing protein taxonomies to uncover additional membrane-active multifunctional proteins. Proteins and peptides in bold have been shown to generate negative Gaussian curvature (NGC) using SAXS experiments. Specific cytokines (dashed magenta circle) and neuropeptides (dashed cyan circle) that have been identified to be antimicrobial are also indicated. Table S1 provides references for the proteins and peptides shown here.
3.1. S100 Family.
The S100 family of EF-hand calcium-binding proteins regulate a wide range of intracellular and extracellular functions, including proliferation, differentiation, apoptosis, metabolism, and autocrine and paracrine signaling.87 Collectively, they have been discovered to exert alternative functionality as immunomodulators and direct microbicides. Several members of the family, namely S100A8, S100A9, and S100A12, have been reported to produce pro-inflammatory effects and are implicated in a variety of inflammatory conditions, such as psoriasis, arthritis, and chronic inflammatory bowel disease.88–90 Studies have specifically found that both S100A891 and S100A1292 can modulate host immune responses through TLR4 activation, and that S100A12 also exhibits chemotactic activity for monocytes.93 Furthermore, members of the S100 family are characterized by helix-rich structures and have been described to interact with lipids. With structural similarity to membrane-active antimicrobial molecules, it is not surprising that S100A794 and S100A1292 have the capacity to kill bacteria through membrane permeabilization.
3.2. RNase A Superfamily.
The ribonuclease (RNase) A superfamily has been intensively studied and consists of vertebrate RNase homologues to bovine pancreatic ribonu- clease (RNase A).95 Despite a wealth of information on the structural and chemical properties of RNase A family members, most of their biological functions remain unclear. In addition, members of the RNase A superfamily exhibit considerable sequence divergence, with identities that range from 20% to nearly 100%.96 In humans, eight RNases have been identified, including several that are antimicrobial.96 Among these, RNase 3 and RNase 7 are two representative members with well-characterized bactericidal activity against a variety of pathogens. These antimicrobial RNases are small, highly cationic, and share the common RNase A structure and key residues required for their enzymatic function. However, evidence indicates that their bactericidal activity is associated with membrane disruption and do not depend on their conserved catalytic RNase activity.96–99
Eosinophil cationic protein (ECP), a member of the RNase A superfamily and also known as RNase 3, is one of the major granule proteins secreted from activated eosinophils. Eosinophils have been implicated in bacterial infections and inflammation, as well as in immunoregulation and tissue remodeling.100,101 Remarkably, ECP is a single-chain cationic polypeptide that displays potent, broad-spectrum bactericidal activity against both Gram-positive and Gram-negative strains, which is believed to occur through a mechanism of action involving membrane disruption and pore formation.102–104 ECP has specifically been shown to permeabilize the membranes of Escherichia coli, S. aureus, and Mycobacterium vaccae and create pores in lipid vesicles.97,102–106 Recent studies have attributed its antimicrobial activity to an N-terminal domain that bears striking structural and chemical similarity to AMPs, such as adopting an -helical conformation and having a net positive charge due to a high number of arginine residues.105 In fact, both hydrophobic and cationic residues have been found to play key roles in the membrane-disruptive bactericidal activity of ECP by promoting protein binding and insertion into lipid bilayers.104,107 Aside from exerting antimicrobial effects, ECP has also been reported to participate in immunomodulation and inflammation in asthma and allergic diseases.108,109
RNase 7 is expressed in epithelial tissues and has similarly been described to exhibit both broad-spectrum antimicrobial activity and RNase activity. Along with other skin-derived AMPs, RNase 7 is considered one of the main components of the host defense against cutaneous infections.96,110 Notably, RNase 7 has been shown to be effective against both Grampositive (S. aureus, Staphylococcus saprophyticus, Propionibacterium acnes, Enterococcus faecalis, vancomycin-resistant Enterrococcus faecium) and Gram-negative bacteria (E. coli, Pseudomonas aeruginosa, Klebsiella pneumonia, Proteus mirabilis).110,111 Like ECP, the bactericidal activity of RNase 7 has been associated with its capacity to bind and permeate membranes, with surface clusters of positively charged lysine residues playing a crucial role.98 Interestingly, the exposure of keratinocytes to bacteria induces RNase 7 expression, a response that has been observed for other epithelial AMPs such as HBD-2, HBD-3, and HBD-4.110
These findings together suggest that ECP and RNase 7 share a common bactericidal mechanism of action involving membrane disruption. While both proteins are cationic with an abundance of arginine and lysine surface-exposed residues and display broad antimicrobial activity against Gram-positive and Gram-negative bacteria, they only share 40% sequence identity. Indeed, members of the RNase A superfamily exhibit extensive diversity, despite all having conserved catalytic RNase activity. The antimicrobial properties of ECP and RNase 7 therefore not only support the role of RNase A family members in host defense, but also underscore the multifunctional diversity that exists within the superfamily.
4. GENERAL PHYSICOCHEMICAL AND STRUCTURAL PROPERTIES OF MEMBRANE-ACTIVE PEPTIDES
While the antimicrobial activity of AMPs via membrane destabilization has been ascribed to their shared fundamental amphipathic motif, a large body of work has further examined how specific physiochemical properties and amino acid sequence composition can contribute to their bactericidal activity. In general, these factors influence the electrostatic and hydrophobic peptide–membrane interactions that overall determine AMP activity.
Among -helical AMPs, for example, which constitute the largest and most extensively studied group of AMPs, those with higher cationic charge typically have greater affinity to more negatively charged bacterial membranes and therefore tend to be more selective for bacterial over mammalian cells.9,112 Accordingly, increased cationic charge, to a certain degree, has been demonstrated to enhance both antimicrobial activity and selectivity.113–115 Studies have also similarly shown that increased hydrophobicity can increase bactericidal activity, however, at the expense of selectivity.116,117 These findings suggest that an optimal balance of charge and hydrophobicity exists for maximal antimicrobial efficacy. Not only is the overall hydrophobicity important for antimicrobial activity, but additional properties such as the hydrophobic moment, often described as amphipathicity, and the angle subtended by hydrophobic helix face also correlate with bactericidal activity.118,119 For instance, helical AMPs generally feature a wide hydrophobic face and a narrow polar face, which allow them to penetrate into the membrane interior and disrupt lipid packing.120 In fact, increasing the bulkiness or the angle subtended by the hydrophobic face enhances the ability of the AMP to disrupt membranes and induce lysis.113,118,121
Further investigation into the relationship between charge and hydrophobicity included a compositional analysis of over 1080 cationic helical AMPs from the antimicrobial database.122 This study identified a positive correlation between Nk/(Nk + NR) (the ratio of the number of lysines to total number of lysines and arginines) and the average peptide hydrophobicity based on the Eisenberg consensus scale.112,123 Because membrane disruption is the general mechanism for AMPs, this relationship is informative about the relative amounts of arginine, lysine, and hydrophobicity that are needed on average to confer membrane activity to a helical peptide. We point out that a corollary of this argument is that membrane activity underdetermines the full sequence of an AMP, so that additional functions can be encoded into the same sequence.
Although the actual process of membrane disruption by an AMP depends on the specific peptide and target membrane, AMPs have been found to destabilize membranes through a range of modes such as pore formation,22,24–26 blebbing, 27,28 budding,29 and vesicularization.30 Interestingly, the generation of saddle-splay membrane curvature, also known as negative Gaussian curvature (NGC), is topologically required for all of these membrane-destabilizing processes (Figure 2).124 This type of membrane curvature is characterized by a surface that bends upward in one direction and bends downward in the orthogonal direction. Recent work using SAXS measurements reported that the ability of AMPs, both -helical and β-sheet peptides, to disrupt and permeabilize bacterial membranes is correlated with their capacity to induce NGC in lipid bilayers.112,123,125 The generation of NGC by AMPs is believed to arise from the combination of distinct membrane curvature effects produced by the electrostatic and hydrophobic peptide– membrane interactions.112,123 While the ability to generate NGC has also been observed for AMP mutants112,125 and synthetic AMP mimics,126–129 it is not limited to antimicrobial agents. For many other molecules with functions that involve membrane destabilization, a strong correlation has also been found between NGC generation and their activity. For instance, the induction of NGC is also exhibited by a range of cell-penetrating peptides (CPPs), a cognate class of molecular pore formers.130–132 CPPs are short peptide sequences capable of efficient translocation across cell membranes, while remaining nonlytic.133,134 Due to this unique ability, CPPs are routinely utilized for mediating the intracellular delivery of attached macromolecules. Most CPPs are cationic, with the arginine-rich CPPs, such as the peptide derived from the human immunodeficiency virus (HIV-1) transactivator of transcription (TAT) protein,131,135,136 being the most widely studied. Some CPPs can also adopt amphipathic structures, as seen with penetratin.132,137 While their exact mechanism remains controversial, evidence indicates that cellular uptake of CPPs can involve more than one pathway,138–140 such as direct translocation141–143 and endocytosis.144,145 Nonetheless, for any of these modes of action, membrane destabilization must occur for CPPs to enter cells. Consistent with the trend identified for AMPs, CPPs can also generate NGC in lipid membranes.130–132,146 In addition to AMPs and CPPs, other peptides directly involved in membrane-remodeling processes have also been shown to induce this specific type of destabilizing membrane curvature, including viral budding peptides147 and viral fusion peptides.148
Figure 2.
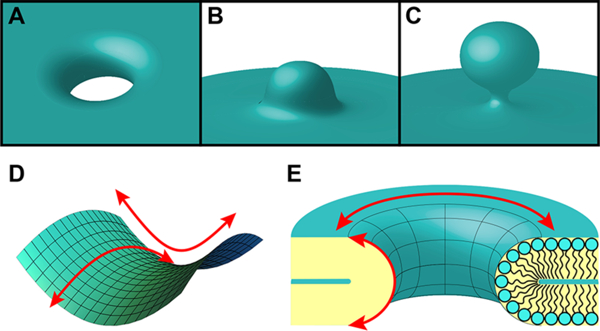
Negative Gaussian curvature (NGC). NGC manifests in a variety of membrane-remodeling processes, including (A) along the interior of a transmembrane pore, (B) at the bases of blebs, and (C) at the necks of buds. (D) NGC is also known as saddle-splay curvature due to the shape of its surface, which bends upward in one direction and downward in the orthogonal direction (red arrows). (E) A cross-section of a transmembrane pore depicts its curved inner surface that is characterized by NGC. The directions of the curvatures along the membrane surface that together create NGC are represented by red arrows.
5. DISCOVERY OF COMPLEMENTARY MEMBRANE ACTIVITY IN DIVERSE PROTEINS AND PEPTIDES
The ability to generate NGC has been observed for a diverse range of proteins and peptides associated with membrane-remodeling activity, which collectively suggests that the generation of NGC is a common root mechanism for membrane-destabilizing processes.
Furthermore, the detection of NGC-generating ability in other proteins and peptides can point to the existence of complementary membrane-destabilizing activity that has not been previously recognized or associated with their established function. Indeed, some proteins are now described as multifunctional due to recent discoveries of their additional antimicrobial activity. IFN-β, a cytokine known for its role in antiviral defense,82 was found to be directly antimicrobial against Gram-positive bacteria.83 Experiments demonstrated that the microbicidal activity of IFN-β stems from its ability to bind and permeabilize bacterial membranes. Remarkably, an α-helical domain of the cytokine closely resembles classical membrane-disruptive AMPs with respect to amino acid composition, amphipathicity, cationic charge, hydrophobicity, and the ability to generate NGC in membranes. S100A12 is another example in which AMP-like sequences exhibit bactericidal activity. As a member of the S100 family of calcium-binding proteins, this host-defense protein is associated with the inflammatory response and mediates a variety of cellular processes.87 S100A12 has been reported to display antimicrobial activity against bacteria.92,149 Interestingly, the structure of S100A12 contains multiple -helices characterized by cationic charge and amphipathicity. Compositional analysis further revealed that the amino acid content of S100A12 follows the sequence trend of known membrane-disruptive helical AMPs and fulfills the criterion for NGC generation.92,123 The newly discovered antimicrobial activities of IFN-β and S100A12 are only a sample of the functional diversity that exists within the realm of immunity proteins, and together suggest that far more proteins with multiplexed functionalities remain unidentified.
As we have observed, the membrane-disruptive bactericidal activity of AMPs depends on a variety of factors, many of which are closely related and may be interdependent.150 Due to this complexity, simple correlations between AMP activity and individual peptide properties are unlikely. In light of this, we expanded upon our earlier work, which identified correlations between amino acid composition and hydrophobicity in membrane-disruptive AMPs as well as between membrane-destabilizing effects and NGC generation, to develop a machine-learning algorithm that effectively predicts the membrane-destabilizing and NGC-generating capacities of a peptide based on its sequence and associated physicochemical properties.
6. DISCOVERING MEMBRANE-ACTIVE PEPTIDES AND PROTEINS USING MACHINE LEARNING
Given the sequence and structural diversity of membrane-active peptides and proteins, it is difficult to detect hidden membrane activity without direct empirical testing, apart from homology searches and sequence/structural alignments. However, the emergence of quantitative structure–activity relationship (QSAR) computational tools has enabled large scale in silico screening of genomes for membrane-active peptides and proteins. For example, many QSAR methods have been applied to discover and design membrane-active AMPs, a prototypical class of membrane-active peptides.151–155 Recently, a support vector machine (SVM)-based machine-learning tool was developed to identify peptide sequences that generate NGC in membranes.156–158 The SVM was trained on a database of experimentally validated -helical AMPs122,159,160 and decoy non-AMP -helical peptides.161 Based on 12 physicochemical descriptors most predictive of antimicrobial activity, the machine-learning tool takes a peptide sequence as input, and outputs a prediction of antimicrobial activity. Additional calibrating SAXS experiments on model membranes identified a relationship between the machine-learning classification metric (σ) and the ability of a peptide sequence to induce negative Gaussian membrane curvature.156 This machine-learning tool not only can guide the design of new synthetic membrane-active peptides for therapeutic purposes, but also allows us to probe the sequence space for previously unrecognized underlying membrane activity in new and existing protein taxonomies (Figure 3). In fact, the machine-learning classifier has successfully predicted membrane activity in neuropeptides, viral fusion proteins, endocytosis/exocytosis machinery, and mitochondrial fission proteins, among many others.156 Because of the serendipitous nature of multifunctional protein discovery, the number of proteins that have been identified and characterized as such is not high. However, this newly developed machine-learning tool now enables a systematic large-scale in silico approach for discovering protein sequences with membrane activity.
Figure 3.

Machine learning discovers hidden NGC-generating activity in proteins with diverse primary functions. (A) A machine-learning-based Monte Carlo search of the undiscovered peptide sequence space reveals sequences in existing peptides and proteins with the ability to remodel membranes.156 (B) Dnml is a molecular motor GTPase that also generates NGC to facilitate mitochondrial fission.162 (C) IFN-β is a pleiotropic, immunomodulatory cytokine that exhibits direct antimicrobial activity and NGC generation.83 (D) Influenza A M2 protein is a proton-selective ion channel that also generates NGC to enable viral budding and scission.147 All figure panels are reproduced with permission from their respective sources.
The machine-learning classifier has correctly predicted membrane-destabilizing activity in sequences contained within proteins that have been generally associated with other activities. These include cytokine IFN-β,83 mitochondrial fission protein Dnm1,162 and the influenza A virus M2 protein. 147 IFN-β consists of a six-helix bundle tertiary structure stabilized by cysteines. Electrostatic surface potential calculations revealed that several component helices of IFN-β are cationic and amphipathic, similar to canonical helical AMPs.83 Using a moving window scan, the component helices of IFN-β were screened for membrane activity using the machine-learning classifier, which predicted helix 4 of IFN-β to possess antimicrobial activity with high probabilities (σ = 0.95, P(+1) = 0.960). This finding is consistent with SAXS measurements that showed the ability of the helix to induce NGC in model membranes and antimicrobial assays that demonstrated its membrane-disruptive microbicidal activity against Gram-positive bacteria.83
Dnml is a cytosolic dynamin-related GTPase that plays a key role in the regulation of yeast mitochondrial fission.163 The current model describes Dnml purely as a molecular motor, forming spirals that wrap around mitochondria and constrict upon GTP hydrolysis to ultimately cause pinching and scission of the mitochondrial membrane. Using the machine-learning classifier, an N-terminal helix of Dnm1 was predicted to have membrane-destabilizing activity.162 SAXS experiments also found this specific helix to have the capacity to generate NGC in model mitochondrial membranes and promote the formation of fission necks narrower than could be achieved with simply mechanical pinching.162 These results together suggest that Dnm1 catalyzes mitochondrial fission through the combination of mechanical constriction and synergistic membrane-remodeling activity. Machine-learning analysis of 33 phylogenetically clustered Dnm1 relatives further revealed that many members within the dynamin superfamily had evolved the capacity to generate NGC and destabilize membranes.
Recent efforts have identified an additional function of the influenza A virus M2 protein in directly inducing membrane curvature that promotes viral budding activity. During the viral replication cycle, viral components assemble to form progeny virions that bud at the plasma membrane of the infected host cell, thereby spreading infection. The budding of enveloped viruses is a complex process that involves the extrusion of the membrane and scission of the bud to release the virion from the former host, which are steps that require organized membrane remodeling with localized regions of high membrane curvature. While many enveloped viruses, such as HIV-1 and the Ebola virus, hijack the host endosomal sorting complex required for transport (ESCRT) machinery to facilitate membrane scission and budding virion release,164–166 studies have suggested that the influenza virus is able to undergo budding via an ESCRT-independent mechanism.165,167 The M2 protein from the influenza A virus is a multifunctional protein that forms a homotetramer in the membrane to function as a proton-selective ion channel168,169 and plays a variety of roles during the life cycle of the virus.170–172 For instance, in vitro and in vivo experiments have revealed that the M2 protein mediates budding and virion release from cells.173,174 Interestingly, the predominant localization of M2 at the necks of budding virions points to its ability to directly induce the specific types of membrane curvature that facilitate budding and membrane scission.173,175 Indeed, results from SAXS measurements of M2-induced membrane deformations showed that the protein generates NGC in model membranes.147 Consistent with its role in promoting the final pinching off of a budding virion, the quantitative amount of NGC induced by M2 can produce a scission neck with a diameter that is ten times smaller than the diameter of a spherical budding virion.147 Remarkably, the generation of NGC by M2 is largely conferred by its cytoplasmic C-terminal amphipathic helix, which has been shown using electron microscopy,173 SAXS measurements,147 and machine-learning classifier156 predictions (σ = 1.63, P(+1) = 0.996). More specifically, the C-terminal amphipathic helical domain of M2 alone is sufficient to generate NGC, however, to a lesser extent than the full-length protein.147 This finding indicates that other regions of the M2 protein affect curvature generation, which further suggests that cross-talk between different domains within multifunctional proteins is likely involved in achieving maximal protein activity.
The examples presented here demonstrate that the machine-learning classifier is a powerful tool that can efficiently and accurately detect membrane activity, and therefore, can enable the discovery of membrane-active multifunctional proteins that have so far been primarily recognized for other functions.
7. DESIGN OF SYNTHETIC MULTIFUNCTIONAL MOLECULES
The ability to design in membrane-disruptive activity allows for the creation of new multifunctional molecules and provides many opportunities for future therapeutics. It is important to note that the potential influence of cross-talk between domains on the activity of a whole protein presents significant implications for engineering multifunctional proteins, peptides, and their derivatives. On these grounds, we look to nature for guidance. What can we learn from existing multifunctional proteins as products of natural bioconjugation? It would make sense that the most efficient and optimized synthetic designs would be those that best emulate nature’s approach to incorporating multifunctionality into proteins: maximizing usage of the whole molecule to integrate each individual functionality, rather than simply joining multiple modular domains that each have a discrete function (Figure 4).
Figure 4.

Design of multifunctional molecules. A simple approach to creating a multifunctional molecule is through joining two (or more) components that each have a separate function. For example, component X, depicted here as a red sphere, can be directly conjugated to a second component Y, represented by a blue cylinder, to form the multifunctional compound X–Y (upper right) with modular domains. Alternatively, hybridizing the two components X and Y to create one compound XY (violet spherocylinder, lower right) that integrates the dual functionalities of X and Y within the same domain is a strategy that more closely mimics the design of natural multifunctional molecules.
Taking these factors into consideration, we aimed to create a multifunctional molecule by hybridizing two separate compounds with different functions. More specifically, this strategy was applied in re-engineering conventional antibiotics to target bacteria that have low metabolic activity, known as persisters, which are often tolerant to common antibiotic treatments and play a role in antibiotic resistance.176–181 Aminoglycoside antibiotics, such as tobramycin, target bacterial ribosomes to block protein synthesis. This class of antibiotics has limited effectiveness against persisters, which have diminished membrane permeability due in part to decreased uptake activity in their low metabolic state. Reduced uptake activity limits the cellular accumulation of drug and consequently decreases susceptibility of the cell to the antibiotic, in turn promoting the development of bacterial resistance to aminoglycosides. Augmenting the membrane permeability of aminoglycosides provides a means to improve their efficacy against persister cells and resistant strains of bacteria. This can be accomplished by conjugating the antibiotic with a short peptide transporter sequence that promotes rapid membrane penetration. The specific design of the transporter sequence is informed by previously established sequence principles for peptides that disrupt and permeate membranes via destabilizing curvature generation.112,123,131,132 For example, the conjugation of a 12-amino-acid peptide sequence derived from the CPP penetratin182 with the aminoglycoside tobramycin formed the antibiotic-peptide hybrid Pentobra.179–181 The compositional contributions from both tobramycin and peptide components of Pentobra together achieve the appropriate proportions of cationic and hydrophobic groups to effectively generate NGC and penetrate cell membranes.179 By enabling the efficient cellular delivery of aminoglycoside tobramycin, which alone can have limited cell uptake, this hybrid molecule thus displays multifunctionality by integrating two mutually amplifying bactericidal functionalities: disruption of membrane integrity and inhibition of protein synthesis.
8. CONCLUSIONS AND OUTLOOK
In principle, antimicrobial/membrane activity can be grafted onto existing proteins with other functions in a number of ways. The first order solution is to simply conjugate a known membrane-active peptide onto another domain with a different primary function using a covalent linker with conventional bioconjugate chemistry. However, we believe this process can be improved by combining a machine-learning approach with directed evolution or computational protein design methodologies, such as Rosetta. A recent paper utilized a computational method to combine cell-penetrating, DNA-binding, pheromone, and antimicrobial activities into one domain.183
The growing number and diversity of identified proteins with multifunctionality suggests that it is a general phenomenon that exists in all kingdoms of life. Expanding our knowledge of protein multifunctionality and its evolution may potentially allow for the selective inhibition or introduction of distinct functions, and thus presents new opportunities for future molecular engineering. The examples shown here illustrate that methods based on machine learning and artificial intelligence can be helpful for informing our understanding of how diverse functions are multiplexed into a single sequence, with implications for the deterministic design of multifunctional molecules.
Supplementary Material
ACKNOWLEDGMENTS
M.W.L. and G.C.L.W. acknowledge support from NIH Grant 1R21AI122212. E.Y.L. acknowledges support from the Systems and Integrative Biology Training Program (T32GM008185), the Medical Scientist Training Program (T32GM008042), and the Dermatology Scientist Training Program (T32AR071307) at UCLA. E.Y.L. and G.C.L.W. also acknowledge an Early Career Research Grant and a Discovery Grant from the National Psoriasis Foundation, respectively. X-ray research was conducted at Stanford Synchrotron Radiation Lightsource, SLAC National Laboratory, supported by the US DOE Office of Basic Energy Sciences under Contract no. DE-AC02–76SF00515.
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.bioconj-chem.8b00176.
Supplementary table of molecules and references (PDF)
The authors declare no competing financial interest.
REFERENCES
- (1).Francis MB, and Carrico IS (2010) New frontiers in protein bioconjugation. Curr. Opin. Chem. Biol. 14, 771–773. [DOI] [PubMed] [Google Scholar]
- (2).Stephanopoulos N, and Francis MB (2011) Choosing an effective protein bioconjugation strategy. Nat. Chem. Biol. 7, 876–884. [DOI] [PubMed] [Google Scholar]
- (3).Jeffery CJ (2016) Protein species and moonlighting proteins: Very small changes in a protein’s covalent structure can change its biochemical function. J. Proteomics 134, 19–24. [DOI] [PubMed] [Google Scholar]
- (4).Apic G, Gough J, and Teichmann SA (2001) Domain combinations in archaeal, eubacterial and eukaryotic proteomes. J. Mol. Biol. 310, 311–325. [DOI] [PubMed] [Google Scholar]
- (5).Jeffery CJ (1999) Moonlighting proteins. Trends Biochem. Sci. 24, 8–11. [DOI] [PubMed] [Google Scholar]
- (6).Jeffery CJ (2003) Multifunctional proteins: examples of gene sharing. Ann. Med. 35, 28–35. [DOI] [PubMed] [Google Scholar]
- (7).Huberts DHEW, and van der Klei IJ (2010) Moonlighting proteins: An intriguing mode of multitasking. Biochim. Biophys. Ada, Mol. Cell Res. 1803, 520–525. [DOI] [PubMed] [Google Scholar]
- (8).Zasloff M (2002) Antimicrobial peptides of multicellular organisms. Nature 415, 389–395. [DOI] [PubMed] [Google Scholar]
- (9).Shai Y (1999) Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by a-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim. Biophys. Acta, Biomembr. 1462, 55–70. [DOI] [PubMed] [Google Scholar]
- (10).Brogden KA (2005) Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 3, 238–250. [DOI] [PubMed] [Google Scholar]
- (11).Hancock REW, and Lehrer R (1998) Cationic peptides: a new source of antibiotics. Trends Biotechnol. 16, 82–88. [DOI] [PubMed] [Google Scholar]
- (12).Hancock REW, and Sahl H-G (2006) Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotechnol. 24, 1551–1557. [DOI] [PubMed] [Google Scholar]
- (13).Yeaman MR, and Yount NY (2003) Mechanisms of Antimicrobial Peptide Action and Resistance. Pharmacol. Rev. 55, 27–55. [DOI] [PubMed] [Google Scholar]
- (14).Durr UHN, Sudheendra US, and Ramamoorthy A (2006) LL-37, the only human member of the cathelicidin family of antimicrobial peptides. Biochim. Biophys. Acta, Biomembr. 1758, 1408–1425. [DOI] [PubMed] [Google Scholar]
- (15).Zasloff M (1987) Magainins, a class of antimicrobial peptides from Xenopus skin: isolation, characterization of two active forms, and partial cDNA sequence of a precursor. Proc. Natl. Acad. Sci. U. S. A. 84, 5449–5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Ganz T (2003) Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3, 710–720. [DOI] [PubMed] [Google Scholar]
- (17).Lehrer RI (2004) Primate defensins. Nat. Rev. Microbiol. 2, 727–738. [DOI] [PubMed] [Google Scholar]
- (18).Selsted ME, and Ouellette AJ (2005) Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 6, 551–557. [DOI] [PubMed] [Google Scholar]
- (19).Kokryakov VN, Harwig SSL, Panyutich EA, Shevchenko AA, Aleshina GM, Shamova OV, Korneva HA, and Lehrer RI (1993) Protegrins: leukocyte antimicrobial peptides that combine features of corticostatic defensins and tachyplesins. FEBS Lett. 327, 231–236. [DOI] [PubMed] [Google Scholar]
- (20).Selsted ME, Novotny MJ, Morris WL, Tang YQ, Smith W, and Cullor JS (1992) Indolicidin, a novel bactericidal tridecapeptide amide from neutrophils. J. Biol. Chem. 267, 4292–4295. [PubMed] [Google Scholar]
- (21).Agerberth B, Lee J-Y, Bergman T, Carlquist M, Boman HG, Mutt V, and Jörnvall H (1991) Amino acid sequence of PR-39. Eur. J. Biochem. 202, 849–854. [DOI] [PubMed] [Google Scholar]
- (22).Matsuzaki K, Sugishita K. i., Ishibe N, Ueha M, Nakata S, Miyajima K, and Epand RM (1998) Relationship of Membrane Curvature to the Formation of Pores by Magainin 2. Biochemistry 37, 11856–11863. [DOI] [PubMed] [Google Scholar]
- (23).Matsuzaki K (1999) Why and how are peptide-lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim. Biophys. Acta, Biomembr. 1462, 1–10. [DOI] [PubMed] [Google Scholar]
- (24).Spaar A, Munster C, and Salditt T (2004) Conformation of Peptides in Lipid Membranes Studied by X-Ray Grazing Incidence Scattering. Biophys. J. 87, 396–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (25).Yang L, Harroun TA, Weiss TM, Ding L, and Huang HW (2001) Barrel-Stave Model or Toroidal Model? A Case Study on Melittin Pores. Biophys. J. 81, 1475–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (26).Tang M, Waring AJ, and Hong M (2007) Phosphate-Mediated Arginine Insertion into Lipid Membranes and Pore Formation by a Cationic Membrane Peptide from Solid-State NMR. J.Am. Chem. Soc. 129, 11438–11446. [DOI] [PubMed] [Google Scholar]
- (27).Saiman L, Tabibi S, Starner TD, San Gabriel P., Winokur PL, Jia HP, McCray PB, and Tack BF (2001) Cathelicidin Peptides Inhibit Multiply Antibiotic-Resistant Pathogens from Patients with Cystic Fibrosis. Antimicrob. Agents Chemother. 45, 2838–2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Kalfa VC, Jia HP, Kunkle RA, McCray PB, Tack BF, and Brogden KA (2001) Congeners of SMAP29 Kill Ovine Pathogens and Induce Ultrastructural Damage in Bacterial Cells. Antimicrob. Agents Chemother. 45, 3256–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Yu Y, Vroman JA, Bae SC, and Granick S (2010) Vesicle Budding Induced by a Pore-Forming Peptide. J. Am. Chem. Soc. 132, 195–201. [DOI] [PubMed] [Google Scholar]
- (30).Falagas ME, Kasiakou SK, and Saravolatz LD (2005) Colistin: The Revival of Polymyxins for the Management of Multidrug-Resistant Gram-Negative Bacterial Infections. Clin. Infect. Dis. 40, 1333–1341. [DOI] [PubMed] [Google Scholar]
- (31).Ayabe T, Satchell DP, Wilson CL, Parks WC, Selsted ME, and Ouellette AJ (2000) Secretion of microbicidal α-defensins by intestinal Paneth cells in response to bacteria. Nat. Immunol. 1, 113–118. [DOI] [PubMed] [Google Scholar]
- (32).Hsu C-H, Chen C, Jou M-L, Lee AY-L, Lin Y-C, Yu YP, Huang W-T, and Wu S-H (2005) Structural and DNA-binding studies on the bovine antimicrobial peptide, indolicidin: evidence for multiple conformations involved in binding to membranes and DNA. Nucleic Acids Res. 33, 4053–4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Yang D, Chertov O, and Oppenheim JJ (2001) Participation of mammalian defensins and cathelicidins in anti-microbial immunity: receptors and activities of human defensins and cathelicidin (LL-37). J. Leukocyte Biol. 69, 691–697. [PubMed] [Google Scholar]
- (34).Yang D, Chertov O, Bykovskaia SN, Chen Q, Buffo MJ, Shogan J, Anderson M, Schroder JM, Wang JM, Howard OMZ, et al. (1999) β-Defensins: Linking Innate and Adaptive Immunity Through Dendritic and T Cell CCR6. Science 286, 525–528. [DOI] [PubMed] [Google Scholar]
- (35).Territo MC, Ganz T, Selsted ME, and Lehrer R (1989) Monocyte-chemotactic activity of defensins from human neutrophils. J. Clin. Invest. 84, 2017–2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Yang D, Biragyn A, Kwak LW, and Oppenheim JJ (2002) Mammalian defensins in immunity: more than just microbicidal. Trends Immunol. 23, 291–296. [DOI] [PubMed] [Google Scholar]
- (37).Niyonsaba F, Iwabuchi K, Matsuda H, Ogawa H, and Nagaoka I (2002) Epithelial cell-derived human β-defensin-2 acts as a chemotaxin for mast cells through a pertussis toxin-sensitive and phospholipase C-dependent pathway. Int. Immunol. 14, 421–426. [DOI] [PubMed] [Google Scholar]
- (38).Yang D, Chen Q, Schmidt AP, Anderson GM, Wang JM, Wooters J, Oppenheim JJ, and Chertov O (2000) LL-37, the Neutrophil Granule- And Epithelial Cell-Derived Cathelicidin, Utilizes Formyl Peptide Receptor-Like 1 (Fprl1) as a Receptor to Chemo-attract Human Peripheral Blood Neutrophils, Monocytes, and T Cells. J. Exp. Med. 192, 1069–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Bowdish DME, Davidson DJ, Scott MG, and Hancock REW (2005) Immunomodulatory Activities of Small Host Defense Peptides. Antimicrob. Agents Chemother. 49, 1727–1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (40).Kurosaka K, Chen Q, Yarovinsky F, Oppenheim JJ, and Yang D (2005) Mouse Cathelin-Related Antimicrobial Peptide Chemoattracts Leukocytes Using Formyl Peptide Receptor-Like 1/ Mouse Formyl Peptide Receptor-Like 2 as the Receptor and Acts as an Immune Adjuvant. J. Immunol. 174, 6257–6265. [DOI] [PubMed] [Google Scholar]
- (41).Ohgami K, Ilieva IB, Shiratori K, Isogai E, Yoshida K, Kotake S, Nishida T, Mizuki N, and Ohno S (2003) Effect of Human Cationic Antimicrobial Protein 18 Peptide on Endotoxin-Induced Uveitis in Rats. Invest. Ophthalmol. Visual Sci. 44, 4412–4418. [DOI] [PubMed] [Google Scholar]
- (42).Stenger S, Hanson DA, Teitelbaum R, Dewan P, Niazi KR, Froelich CJ, Ganz T, Thoma-Uszynski S, MeMn A, Bogdan C, et al. (1998) An Antimicrobial Activity of Cytolytic T Cells Mediated by Granulysin. Science 282, 121–125. [DOI] [PubMed] [Google Scholar]
- (43).Wei H-M, Lin L-C, Wang C-F, Lee Y-J, Chen Y-T, and Liao Y-D (2016) Antimicrobial Properties of an Immunomodulator - 15 kDa Human Granulysin. PLoS One 11, e0156321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Deng A, Chen S, Li Q Lyu S.-c., Clayberger C, and Krensky AM (2005) Granulysin, a Cytolytic Molecule, Is Also a Chemoattractant and Proinflammatory Activator. J. Immunol. 174, 5243–5248. [DOI] [PubMed] [Google Scholar]
- (45).Elssner A, Duncan M, Gavrilin M, and Wewers MD (2004) A Novel P2X7 Receptor Activator, the Human Cathelicidin-Derived Peptide LL37, Induces IL-1β Processing and Release. J. Immunol. 172, 4987–4994. [DOI] [PubMed] [Google Scholar]
- (46).Braff MH, Hawkins M. i. A., Nardo AD, Lopez-Garcia B, Howell MD, Wong C, Lin K, Streib JE, Dorschner R, Leung DYM, et al. (2005) Structure-Function Relationships among Human Cathelicidin Peptides: Dissociation of Antimicrobial Properties from Host Immunostimulatory Activities. J. Immunol. 174, 4271–4278. [DOI] [PubMed] [Google Scholar]
- (47).Yu J, Mookherjee N, Wee K, Bowdish DME, Pistolic J, Li Y, Rehaume L, and Hancock REW (2007) Host Defense Peptide LL-37, in Synergy with Inflammatory Mediator IL-1β, Augments Immune Responses by Multiple Pathways. J. Immunol. 179, 7684–7691. [DOI] [PubMed] [Google Scholar]
- (48).Brown KL, and Hancock REW (2006) Cationic host defense (antimicrobial) peptides. Curr. Opin. Immunol. 18, 24–30. [DOI] [PubMed] [Google Scholar]
- (49).Niyonsaba F, Ushio H, Nagaoka I, Okumura K, and Ogawa H (2005) The Human β-Defensins (–1, –2, –3, –4) and Cathelicidin LL-37 Induce IL-18 Secretion through p38 and ERK MAPK Activation in Primary Human Keratinocytes. J. Immunol. 175, 1776–1784. [DOI] [PubMed] [Google Scholar]
- (50).Niyonsaba F, Ushio H, Nakano N, Ng W, Sayama K, Hashimoto K, Nagaoka I, Okumura K, and Ogawa H (2007) Antimicrobial Peptides Human β-Defensins Stimulate Epidermal Keratinocyte Migration, Proliferation and Production of Proinflammatory Cytokines and Chemokines. J. Invest. Dermatol. 127, 594–604. [DOI] [PubMed] [Google Scholar]
- (51).Mookherjee N, Brown KL, Bowdish DME, Doria S, Falsafi R, Hokamp K, Roche FM, Mu R, Doho GH, Pistolic J, et al. (2006) Modulation of the TLR-Mediated Inflammatory Response by the Endogenous Human Host Defense Peptide LL-37. J. Immunol. 176, 2455–2464. [DOI] [PubMed] [Google Scholar]
- (52).Mookherjee N, Wilson HL, Doria S, Popowych Y, Falsafi R, Yu J, Li Y, Veatch S, Roche FM, Brown KL, et al. (2006) Bovine and human cathelicidin cationic host defense peptides similarly suppress transcriptional responses to bacterial lipopolysaccharide. J. Leukocyte Biol. 80, 1563–1574. [DOI] [PubMed] [Google Scholar]
- (53).Rosenfeld Y, Papo N, and Shai Y (2006) Endotoxin (Lipopolysaccharide) Neutralization by Innate Immunity Host-Defense Peptides: Peptide Properties and Plausible Modes of Action. J. Biol. Chem. 281, 1636–1643. [DOI] [PubMed] [Google Scholar]
- (54).Tydell CC, Yuan J, Tran P, and Selsted ME (2006) Bovine Peptidoglycan Recognition Protein-S: Antimicrobial Activity, Localization, Secretion, and Binding Properties. J. Immunol. 176, 1154–1162. [DOI] [PubMed] [Google Scholar]
- (55).Yibin G, Jiang Z, Hong Z, Gengfa L, Liangxi W, Guo W, and Yongling L (2005) A synthesized cationic tetradecapeptide from hornet venom kills bacteria and neutralizes lipopolysaccharide in vivo and in vitro. Biochem. Pharmacol. 70, 209–219. [DOI] [PubMed] [Google Scholar]
- (56).Chen X, Dings RPM, Nesmelova I, Debbert S, Haseman JR, Maxwell J, Hoye TR, and Mayo KH (2006) Topomimetics of Amphipathic β-Sheet and Helix-Forming Bactericidal Peptides Neutralize Lipopolysaccharide Endotoxins. J. Med. Chem. 49, 7754–7765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (57).Scott MG, Vreugdenhil ACE, Buurman WA, Hancock REW, and Gold MR (2000) Cutting Edge: Cationic Antimicrobial Peptides Block the Binding of Lipopolysaccharide (LPS) to LPS Binding Protein. J. Immunol. 164, 549–553. [DOI] [PubMed] [Google Scholar]
- (58).Di Nardo A, Braff MH, Taylor KR, Na C, Granstein RD, McInturff JE, Krutzik S, Modlin RL, and Gallo RL (2007) Cathelicidin Antimicrobial Peptides Block Dendritic Cell TLR4 Activation and Allergic Contact Sensitization. J. Immunol. 178, 1829–1834. [DOI] [PubMed] [Google Scholar]
- (59).Nijnik A, Pistolic J, Filewod NCJ, and Hancock R E. W. (2012) Signaling Pathways Mediating Chemokine Induction in Keratinocytes by Cathelicidin LL-37 and Flagellin. J. Innate Immun. 4, 377–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang Y-H, Homey B, Cao W, Wang Y-H, Su B, Nestle FO, et al. (2007) Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature 449, 564–569. [DOI] [PubMed] [Google Scholar]
- (61).Hurtado P, and Au Peh C. (2010) LL-37 Promotes Rapid Sensing of CpG Oligodeoxynucleotides by B Lymphocytes and Plasmacytoid Dendritic Cells. J. Immunol. 184, 1425–1435. [DOI] [PubMed] [Google Scholar]
- (62).Nakagawa Y, and Gallo RL (2015) Endogenous Intracellular Cathelicidin Enhances TLR9 Activation in Dendritic Cells and Macrophages. J. Immunol. 194, 1274–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (63).Lai Y, Adhikarakunnathu S, Bhardwaj K, Ranjith-Kumar CT, Wen Y, Jordan JL, Wu LH, Dragnea B, Mateo LS, and Kao CC (2011) LL37 and Cationic Peptides Enhance TLR3 Signaling by Viral Double-stranded RNAs. PLoS One 6, e26632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (64).Lee EY, Takahashi T, Curk T, Dobnikar J, Gallo RL, and Wong GCL (2017) Crystallinity of Double-Stranded RNA-Antimicrobial Peptide Complexes Modulates Toll-Like Receptor 3-Mediated Inflammation. ACS Nano 11, 12145–12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (65).Ganguly D, Chamilos G, Lande R, Gregorio J, Meller S, Facchinetti V, Homey B, Barrat FJ, Zal T, and Gilliet M (2009) Self-RNA-antimicrobial peptide complexes activate human dendritic cells through TLR7 and TLR8. J. Exp. Med. 206, 1983–1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (66).Schmidt NW, Jin F, Lande R, Curk T, Xian W, Lee C, Frasca L, Frenkel D, Dobnikar J, Gilliet M, et al. (2015) Liquid- crystalline ordering of antimicrobial peptide-DNA complexes controls TLR9 activation. Nat. Mater. 14, 696–700. [DOI] [PubMed] [Google Scholar]
- (67).Lee EY, Lee CK, Schmidt NW, Jin F, Lande R, Curk T, Frenkel D, Dobnikar J, Gilliet M, and Wong GCL (2016) A review of immune amplification via ligand clustering by self-assembled liquid-crystalline DNA complexes. Adv. Colloid Interface Sci. 232, 17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (68).Lee EY, Lee MW, and Wong GCL (2018) Modulation of toll-like receptor signaling by antimicrobial peptides. Semin. Cell Dev. Biol, DOI: DOI: 10.1016/j.semcdb.2018.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (69).Yount NY, and Yeaman MR (2004) Multidimensional signatures in antimicrobial peptides. Proc. Natl. Acad. Sci. U. S. A. 101, 7363–7368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (70).Yount NY, Gank KD, Xiong YQ, Bayer AS, Pender T, Welch WH, and Yeaman MR (2004) Platelet Microbicidal Protein 1: Structural Themes of a Multifunctional Antimicrobial Peptide. Antimicrob. Agents Chemother. 48, 4395–4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (71).Yount NY, Waring AJ, Gank KD, Welch WH, Kupferwasser D, and Yeaman MR (2007) Structural correlates of antimicrobial efficacy in IL-8 and related human kinocidins. Biochim. Biophys. Acta, Biomembr. 1768, 598–608. [DOI] [PubMed] [Google Scholar]
- (72).Yeaman MR, and Yount NY (2007) Unifying themes in host defence effector polypeptides. Nat. Rev. Microbiol. 5, 727–740. [DOI] [PubMed] [Google Scholar]
- (73).Yount NY, Kupferwasser D, Spisni A, Dutz SM, Ramjan ZH, Sharma S, Waring AJ, and Yeaman MR (2009) Selective reciprocity in antimicrobial activity versus cytotoxicity of hBD-2 and crotamine. Proc. Natl. Acad. Sci. U. S. A. 106, 14972–14977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (74).Yount NY, and Yeaman MR (2006) Structural congruence among membrane-active host defense polypeptides of diverse phylogeny. Biochim. Biophys. Ada, Biomembr. 1758, 1373–1386. [DOI] [PubMed] [Google Scholar]
- (75).Nguyen L, and Vogel H (2012) Structural perspectives on antimicrobial chemokines. Front. Immunol. 3, 384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (76).Yeaman MR, Yount NY, Waring AJ, Gank KD, Kupferwasser D, Wiese R, Bayer AS, and Welch WH (2007) Modular determinants of antimicrobial activity in platelet factor-4 family kinocidins. Biochim. Biophys. Acta, Biomembr. 1768, 609–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Tang Y-Q, Yeaman MR, and Selsted ME (2002) Antimicrobial Peptides from Human Platelets. Infect. Immun. 70, 6524–6533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (78).Cole AM, Ganz T, Liese AM, Burdick MD, Liu L, and Strieter RM (2001) Cutting Edge: IFN-Inducible ELR- CXC Chemokines Display Defensin-Like Antimicrobial Activity. J. Immunol. 167, 623–627. [DOI] [PubMed] [Google Scholar]
- (79).Björstad Å, Fu H, Karlsson A, Dahlgren C, and Bylund J Interleukin-8-Derived Peptide Has Antibacterial Activity. Antimicrob. Agents Chemother. 49, 3889–3895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).Meller S, Di Domizio J, Voo KS, Friedrich HC, Chamilos G, Ganguly D, Conrad C, Gregorio J, Le Roy D, Roger T, et al. (2015) TH17 cells promote microbial killing and innate immune sensing of DNA via interleukin 26. Nat. Immunol. 16, 970–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Poli C, Augusto JF, Dauve J, Adam C, Preisser L, Larochette V, Pignon P, Savina A, Blanchard S, Subra JF, et al. (2017) IL-26 Confers Proinflammatory Properties to Extracellular DNA. J. Immunol. 198, 3650–3661. [DOI] [PubMed] [Google Scholar]
- (82).Platanias LC (2005) Mechanisms of type-I- and type-II- interferon-mediated signalling. Nat. Rev. Immunol. 5, 375–386. [DOI] [PubMed] [Google Scholar]
- (83).Kaplan A, Lee MW, Wolf AJ, Limon JJ, Becker CA, Ding M, Murali R, Lee EY, Liu GY, Wong GCL, et al. (2017) Direct Antimicrobial Activity of IFN-ß. J. Immunol. 198, 4036–4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Boxx GM, and Cheng G (2016) The Roles of Type I Interferon in Bacterial Infection. Cell Host Microbe 19, 760–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Kovarik P, Castiglia V, Ivin M, and Ebner F (2016) Type I Interferons in Bacterial Infections: A Balancing Act. Front. Immunol. 7, 652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (86).Decker T, Muller M, and Stockinger S (2005) The Yin and Yang of type I interferon activity in bacterial infection. Nat. Rev. Immunol. 5, 675–687. [DOI] [PubMed] [Google Scholar]
- (87).Donato R, Cannon BR, Sorci G, Riuzzi F, Hsu K, Weber J, and Geczy CL (2013) Functions of S100 Proteins. Curr. Mol Med. 13, 24–57. [PMC free article] [PubMed] [Google Scholar]
- (88).Elsbach P (2003) What is the real role of antimicrobial polypeptides that can mediate several other inflammatory responses? J. Clin. Invest. 111, 1643–1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Eckert RL, Broome A-M, Ruse M, Robinson N, Ryan D, and Lee K (2004) S100 Proteins in the Epidermis. J. Invest. Dermatol. 123, 23–33. [DOI] [PubMed] [Google Scholar]
- (90).Donato R (2001) S100: a multigenic family of calcium-modulated proteins of the EF-hand type with intracellular and extracellular functional roles. Int. J. Biochem. Cell Biol. 33, 637–668. [DOI] [PubMed] [Google Scholar]
- (91).Vogl T, Tenbrock K, Ludwig S, Leukert N, Ehrhardt C, van Zoelen MAD, Nacken W, Foell D, van der Poll T, Sorg C, et al. (2007) Mrp8 and Mrp14 are endogenous activators of Toll-like receptor 4, promoting lethal, endotoxin-induced shock. Nat. Med. 13, 1042–1049. [DOI] [PubMed] [Google Scholar]
- (92).Realegeno S, Kelly-Scumpia KM, Dang AT, Lu J, Teles R, Liu PT, Schenk M, Lee EY, Schmidt NW, Wong GCL, et al. (2016) S100A12 Is Part of the Antimicrobial Network against Mycobacterium leprae in Human Macrophages. PLoS Pathog. 12, e1005705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (93).Yang Z, Tao T, Raftery MJ, Youssef P, Di Girolamo N, and Geczy CL (2001) Proinflammatory properties of the human S100 protein S100A12. J. Leukocyte Biol. 69, 986–994. [PubMed] [Google Scholar]
- (94).Michalek M, Gelhaus C, Hecht O, Podschun R, Schröder JM, Leippe M, and Grotzinger J (2009) The human antimicrobial protein psoriasin acts by permeabilization of bacterial membranes. Dev. Comp. Immunol. 33, 740–746. [DOI] [PubMed] [Google Scholar]
- (95).Raines RT (1998) Ribonuclease A. Chem. Rev. 98, 1045–1066. [DOI] [PubMed] [Google Scholar]
- (96).Boix E, and Nogues MV (2007) Mammalian antimicrobial proteins and peptides: overview on the RNase A superfamily members involved in innate host defence. Mol. BioSyst. 3, 317–335. [DOI] [PubMed] [Google Scholar]
- (97).Rosenberg HF (1995) Recombinant Human Eosinophil Cationic Protein: Ribonuclease Activity Is Not Essential for Cytotoxicity. J. Biol. Chem. 270, 7876–7881. [DOI] [PubMed] [Google Scholar]
- (98).Huang Y-C, Lin Y-M, Chang T-W, Wu S-J, Lee Y-S, Chang MD-T, Chen C, Wu S-H, and Liao Y-D (2007) The Flexible and Clustered Lysine Residues of Human Ribonuclease 7 Are Critical for Membrane Permeability and Antimicrobial Activity. J. Biol. Chem. 282, 4626–4633. [DOI] [PubMed] [Google Scholar]
- (99).Köten B, Simanski M, Gläser R, Podschun R, Schröder JM, and Harder J (2009) RNase 7 Contributes to the Cutaneous Defense against Enterococcus faecium. PLoS One 4, e6424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (100).Hogan SP, Rosenberg HF, Moqbel R, Phipps S, Foster PS, Lacy P, Kay AB, and Rothenberg ME (2008) Eosinophils: Biological Properties and Role in Health and Disease. Clin. Exp. Allergy 38, 709–750. [DOI] [PubMed] [Google Scholar]
- (101).Gomes I, Mathur SK, Espenshade BM, Mori Y, Varga J, and Ackerman SJ (2005) Eosinophil-fibroblast interactions induce fibroblast IL-6 secretion and extracellular matrix gene expression: Implications in fibrogenesis. J. Allergy Clin. Immunol. 116, 796–804. [DOI] [PubMed] [Google Scholar]
- (102).Young JD-E, Peterson CGB, Venge P, and Cohn ZA (1986) Mechanism of membrane damage mediated by human eosinophil cationic protein. Nature 321, 613–616. [DOI] [PubMed] [Google Scholar]
- (103).Lehrer RI, Szklarek D, Barton A, Ganz T, Hamann KJ, and Gleich GJ (1989) Antibacterial properties of eosinophil major basic protein and eosinophil cationic protein. J. Immunol. 142, 4428–4434. [PubMed] [Google Scholar]
- (104).Carreras E, Boix E, Rosenberg HF, Cuchillo CM, and Nogues MV (2003) Both Aromatic and Cationic Residues Contribute to the Membrane-Lytic and Bactericidal Activity of Eosinophil Cationic Protein. Biochemistry 42, 6636–6644. [DOI] [PubMed] [Google Scholar]
- (105).Torrent M, de la Torre BG, Nogues VM, Andreu D, and Boix E (2009) Bactericidal and membrane disruption activities of the eosinophil cationic protein are largely retained in an N-terminal fragment. Biochem. J. 421, 425–434. [DOI] [PubMed] [Google Scholar]
- (106).Pulido D, Torrent M, Andreu D, Nogués MV, and Boix E(2013) Two Human Host Defense Ribonucleases against Mycobacteria, the Eosinophil Cationic Protein (RNase 3) and RNase 7. Antimicrob. Agents Chemother. 57, 3797–3805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (107).Torrent M, Cuyás E, Carreras E, Navarro S, López O, de la Maza A, Nogues MV, Reshetnyak YK, and Boix E (2007) Topography Studies on the Membrane Interaction Mechanism of the Eosinophil Cationic Protein. Biochemistry 46, 720–733. [DOI] [PubMed] [Google Scholar]
- (108).Kato M, Yamada Y, Maruyama K, and Hayashi Y (2010) Serum Eosinophil Cationic Protein and 27 Cytokines/Chemokines in Acute Exacerbation of Childhood Asthma. Int. Arch. Allergy Immunol. 152, 62–66. [DOI] [PubMed] [Google Scholar]
- (109).Venge, Carlson, Karawacjzyk, and Trulson (1999) Eosinophil cationic protein (ECP): molecular and biological properties and the use of ECP as a marker of eosinophil activation in disease. Clin. Exp. Allergy 29, 1172–1186. [DOI] [PubMed] [Google Scholar]
- (110).Harder J, and Schröder J-M (2002) RNase 7, a Novel Innate Immune Defense Antimicrobial Protein of Healthy Human Skin. J. Biol. Chem. 277, 46779–46784. [DOI] [PubMed] [Google Scholar]
- (111).Spencer JD, Schwaderer AL, Wang H, Bartz J, Kline J, Eichler T, DeSouza KR, Sims-Lucas S, Baker P, and Hains DS Ribonuclease 7, an antimicrobial peptide upregulated during infection, contributes to microbial defense of the human urinary tract. Kidney Int. 83, 615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Schmidt NW, and Wong GCL (2013) Antimicrobial peptides and induced membrane curvature: Geometry, coordination chemistry, and molecular engineering. Curr. Opin. Solid State Mater. Sci 17, 151–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Dathe M, and Wieprecht T (1999) Structural features of helical antimicrobial peptides: their potential to modulate activity on model membranes and biological cells. Biochim. Biophys. Acta, Biomembr. 1462, 71–87. [DOI] [PubMed] [Google Scholar]
- (114).Dathe M, Nikolenko H, Meyer J, Beyermann M, and Bienert M (2001) Optimization of the antimicrobial activity of magainin peptides by modification of charge. FEBS Lett. 501, 146–150. [DOI] [PubMed] [Google Scholar]
- (115).Matsuzaki K, Nakamura A, Murase O, Sugishita K. i., Fujii N, and Miyajima K (1997) Modulation of Magainin 2–Lipid Bilayer Interactions by Peptide Charge. Biochemistry 36, 2104–2111. [DOI] [PubMed] [Google Scholar]
- (116).Wieprecht T, Dathe M, Beyermann M, Krause E, Maloy WL, MacDonald DL, and Bienert M (1997) Peptide Hydrophobicity Controls the Activity and Selectivity of Magainin 2 Amide in Interaction with Membranes. Biochemistry 36, 6124–6132. [DOI] [PubMed] [Google Scholar]
- (117).Kustanovich I, Shalev DE, Mikhlin M, Gaidukov L, and Mor A (2002) Structural Requirements for Potent Versus Selective Cytotoxicity for Antimicrobial Dermaseptin S4 Derivatives. J. Biol. Chem. 277, 16941–16951. [DOI] [PubMed] [Google Scholar]
- (118).Tytler EM, Segrest JP, Epand RM, Nie SQ, Epand R, Mishra VK, Venkatachalapathi YV, and Anantharamaiah GM (1993) Reciprocal effects of apolipoprotein and lytic peptide analogs on membranes. Cross-sectional molecular shapes of amphipathic alpha helixes control membrane stability. J. Biol. Chem. 268, 22112–22118. [PubMed] [Google Scholar]
- (119).Drin G, and Antonny B (2010) Amphipathic helices and membrane curvature. FEBS Lett. 584, 1840–1847. [DOI] [PubMed] [Google Scholar]
- (120).Epand RM, Shai Y, Segrest JP, and Anantharamiah GM (1995) Mechanisms for the modulation of membrane bilayer properties by amphipathic helical peptides. Biopolymers 37, 319–338. [DOI] [PubMed] [Google Scholar]
- (121).Segrest JP, De Loof H, Dohlman JG, Brouillette CG, and Anantharamaiah GM (1990) Amphipathic helix motif: Classes and properties. Proteins: Struct. Fund. Genet. 8, 103–117. [DOI] [PubMed] [Google Scholar]
- (122).Wang G, Li X, and Wang Z (2016) APD3: the antimicrobial peptide database as a tool for research and education. Nucleic Acids Res. 44, D1087–D1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (123).Schmidt NW, Mishra A, Lai GH, Davis M, Sanders LK, Tran D, Garcia A, Tai KP, McCray PB, Ouellette AJ, et al. (2011) Criterion for Amino Acid Composition of Defensins and Antimicrobial Peptides Based on Geometry of Membrane Destabilization. J. Am. Chem. Soc. 133, 6720–6727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (124).Gelbart WM, Ben-Shaul A, and Roux D (1994) Micelles Membranes, Microemulsionss and Monolayers, p 608, Springer-Verlag, New York, NY. [Google Scholar]
- (125).Schmidt NW, Tai KP, Kamdar K, Mishra A, Lai GH, Zhao K, Ouellette AJ, and Wong GCL (2012) Arginine in α-Defensins: Differential Effects on Bactericidal Activity Correspond to Geometry of Membrane Curvature Generation and Peptide–Lipid Phase Behavior. J. Biol. Chem. 287, 21866–21872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (126).Yang L, Gordon VD, Trinkle DR, Schmidt NW, Davis MA, DeVries C, Som A, Cronan JE, Tew GN, and Wong GL (2008) Mechanism of a prototypical synthetic membrane-active antimicrobial: Efficient hole-punching via interaction with negative intrinsic curvature lipids. Proc. Natl. Acad. Sci. U. S. A. 105, 20595–20600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (127).Hu K, Schmidt NW, Zhu R, Jiang Y, Lai GH, Wei G, Palermo EF, Kuroda K, Wong GCL, and Yang L (2013) A Critical Evaluation of Random Copolymer Mimesis of Homogeneous Antimicrobial Peptides. Macromolecules 46, 1908–1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (128).Lee MW, Chakraborty S, Schmidt NW, Murgai R, Gellman SH, and Wong GCL (2014) Two interdependent mechanisms of antimicrobial activity allow for efficient killing in nylon-3-based polymeric mimics of innate immunity peptides. Biochim. Biophys. Acta, Biomembr. 1838, 2269–2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Xiong M, Lee MW, Mansbach RA, Song Z, Bao Y, Peek RM, Yao C, Chen L-F, Ferguson AL, Wong GCL, et al. (2015) Helical antimicrobial polypeptides with radial amphiphilicity. Proc. Natl. Acad. Sci. U. S. A. 112, 13155–13160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).Schmidt NW, Lis M, Zhao K, Lai GH, Alexandrova AN, Tew GN, and Wong GCL (2012) Molecular Basis for Nanoscopic Membrane Curvature Generation from Quantum Mechanical Models and Synthetic Transporter Sequences. J. Am. Chem. Soc. 134, 19207–19216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Mishra A, Lai GH, Schmidt NW, Sun VZ, Rodriguez AR, Tong R, Tang L, Cheng J, Deming TJ, Kamei DT, et al. (2011) Translocation of HIV TAT peptide and analogues induced by multiplexed membrane and cytoskeletal interactions. Proc. Natl. Acad. Sci. U. S. A. 108, 16883–16888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Schmidt N, Mishra A, Lai GH, and Wong GCL (2010) Arginine-rich cell-penetrating peptides. FEBS Lett. 584, 1806–1813. [DOI] [PubMed] [Google Scholar]
- (133).Milletti F (2012) Cell-penetrating peptides: classes, origin, and current landscape. Drug Discovery Today 17, 850–860. [DOI] [PubMed] [Google Scholar]
- (134).Bechara C, and Sagan S (2013) Cell-penetrating peptides: 20 years later, where do we stand? FEBS Lett. 587, 1693–1702. [DOI] [PubMed] [Google Scholar]
- (135).Frankel AD, and Pabo CO (1988) Cellular uptake of the tat protein from human immunodeficiency virus. Cell 55, 1189–1193. [DOI] [PubMed] [Google Scholar]
- (136).Ziegler A, Nervi P, Durrenberger M, and Seelig J (2005) The Cationic Cell-Penetrating Peptide CPPTAT Derived from the HIV-1 Protein TAT Is Rapidly Transported into Living Fibroblasts: Optical, Biophysical, and Metabolic Evidence. Biochemistry 44, 138–148. [DOI] [PubMed] [Google Scholar]
- (137).Deshayes S, Morris MC, Divita G, and Heitz F (2006) Interactions of amphipathic CPPs with model membranes. Biochim. Biophys. Acta, Biomembr. 1758, 328–335. [DOI] [PubMed] [Google Scholar]
- (138).Ziegler A (2008) Thermodynamic studies and binding mechanisms of cell-penetrating peptides with lipids and glycosaminoglycans. Adv. Drug Delivery Rev. 60, 580–597. [DOI] [PubMed] [Google Scholar]
- (139).Ludtke SJ, He K, Heller WT, Harroun TA, Yang L, and Huang HW (1996) Membrane Pores Induced by Magainin. Biochemistry 35, 13723–13728. [DOI] [PubMed] [Google Scholar]
- (140).Jiao C-Y, Delaroche D, Burlina F, Alves ID, Chassaing G, and Sagan S (2009) Translocation and Endocytosis for Cell-penetrating Peptide Internalization. J. Biol. Chem. 284, 33957–3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (141).Maiolo JR, Ferrer M, and Ottinger EA (2005) Effects of cargo molecules on the cellular uptake of arginine-rich cell-penetrating peptides. Biochim. Biophys. Acta, Biomembr. 1712, 161–172. [DOI] [PubMed] [Google Scholar]
- (142).Iwasa A, Akita H, Khalil I, Kogure K, Futaki S, and Harashima H (2006) Cellular uptake and subsequent intracellular trafficking of R8-liposomes introduced at low temperature. Biochim. Biophys. Acta, Biomembr. 1758, 713–720. [DOI] [PubMed] [Google Scholar]
- (143).Ter-Avetisyan G, Tunnemann G, Nowak D, Nitschke M, Herrmann A, Drab M, and Cardoso MC (2009) Cell Entry of Arginine-rich Peptides Is Independent of Endocytosis. J. Biol. Chem. 284, 3370–3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (144).Ferrari A, Pellegrini V, Arcangeli C, Fittipaldi A, Giacca M, and Beltram F (2003) Caveolae-Mediated internalization of extracellular HIV-1 tat fusion proteins visualized in real time. Mol. Ther. 8, 284–294. [DOI] [PubMed] [Google Scholar]
- (145).Richard JP, Melikov K, Brooks H, Prevot P, Lebleu B, and Chernomordik LV (2005) Cellular Uptake of Unconjugated TAT Peptide Involves Clathrin-dependent Endocytosis and Heparan Sulfate Receptors. J. Biol. Chem. 280, 15300–15306. [DOI] [PubMed] [Google Scholar]
- (146).Lee MW, Han M, Bossa GV, Snell C, Song Z, Tang H, Yin L, Cheng J, May S, Luijten E, et al. (2017) Interactions between Membranes and “Metaphilic” Polypeptide Architectures with Diverse Side-Chain Populations. ACS Nano 11, 2858–2871. [DOI] [PubMed] [Google Scholar]
- (147).Schmidt NW, Mishra A, Wang J, DeGrado WF, and Wong GCL (2013) Influenza Virus A M2 Protein Generates Negative Gaussian Membrane Curvature Necessary for Budding and Scission. J. Am. Chem. Soc. 135, 13710–13719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (148).Yao H, Lee MW, Waring AJ, Wong GCL, and Hong M (2015) Viral fusion protein transmembrane domain adopts β-strand structure to facilitate membrane topological changes for virus–cell fusion. Proc. Natl. Acad. Sci. U. S. A. 112, 10926–10931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (149).Cunden LS, Gaillard A, and Nolan EM (2016) Calcium ions tune the zinc-sequestering properties and antimicrobial activity of human S100A12. Chem. Sci. 7, 1338–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (150).Tossi A, Sandri L, and Giangaspero A (2000) Amphipathic, a-helical antimicrobial peptides. Biopolymers 55, 4–30. [DOI] [PubMed] [Google Scholar]
- (151).Fjell CD, Jenssen H, Hilpert K, Cheung WA, Panté N, Hancock REW, and Cherkasov A (2009) Identification of Novel Antibacterial Peptides by Chemoinformatics and Machine Learning. J. Med. Chem. 52, 2006–2015. [DOI] [PubMed] [Google Scholar]
- (152).Porto WF, Fernandes FC, and Franco OL (2010) In Advances in Bioinformatics and Computational Biology (Ferreira CE, Miyano S, and Stadler PF, Eds.) pp 59–62, Springer, Berlin, Heidelberg, 5th Brazilian Symposium on Bioinformatics, Rio de Janeiro, Brazil, August 31-September 3, 2010. [Google Scholar]
- (153).Porto WF, Pires ÁS, and Franco OL (2012) CS-AMPPred: An Updated SVM Model for Antimicrobial Activity Prediction in Cysteine-Stabilized Peptides. PLoS One 7, e51444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (154).Hilpert K, Fjell CD, and Cherkasov A (2008) Short Linear Cationic Antimicrobial Peptides: Screening, Optimizing, and Prediction Peptide-Based Drug Design (Otvos L, Ed.) pp 127–159, Humana Press, Totowa, NJ. [DOI] [PubMed] [Google Scholar]
- (155).Lata S, Sharma B, and Raghava G (2007) Analysis and prediction of antibacterial peptides. BMC Bioinf. 8, 263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (156).Lee EY, Fulan BM, Wong GCL, and Ferguson AL (2016) Mapping membrane activity in undiscovered peptide sequence space using machine learning. Proc. Natl. Acad. Sci. U. S. A. 113, 13588–13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (157).Lee EY, Wong GCL, and Ferguson AL (2018) Machine learning-enabled discovery and design of membrane-active peptides. Bioorg. Med. Chem. 26, 2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (158).Lee EY, Lee MW, Fulan BM, Ferguson AL, and Wong GCL (2017) What can machine learning do for antimicrobial peptides, and what can antimicrobial peptides do for machine learning? Interface Focus 7, 20160153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (159).Wang Z, and Wang G (2004) APD: the Antimicrobial Peptide Database. Nucleic Acids Res. 32, D590–D592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (160).Wang G, Li X, and Wang Z (2009) APD2: the updated antimicrobial peptide database and its application in peptide design. Nucleic Acids Res. 37, D933–D937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (161).Kozma D, Simon I, and Tusnady GE (2012) PDBTM: Protein Data Bank of transmembrane proteins after 8 years. Nucleic Acids Res. 41, D524–D529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (162).Lee MW, Lee EY, Lai GH, Kennedy NW, Posey AE, Xian W, Ferguson AL, Hill RB, and Wong GCL (2017) Molecular Motor Dnm1 Synergistically Induces Membrane Curvature To Facilitate Mitochondrial Fission. ACS Cent. Sci. 3, 1156–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (163).Bleazard W, McCaffery JM, King EJ, Bale S, Mozdy A, Tieu Q, Nunnari J, and Shaw JM (1999) The dynamin-related GTPase Dnm1 regulates mitochondrial fission in yeast. Nat. Cell Biol. 1 298–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (164).Carlton JG, and Martin-Serrano J (2009) The ESCRT machinery: new functions in viral and cellular biology. Biochem. Soc. Trans. 37, 195–199. [DOI] [PubMed] [Google Scholar]
- (165).Chen BJ, and Lamb RA (2008) Mechanisms for enveloped virus budding: Can some viruses do without an ESCRT? Virology 372, 221–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (166).Pornillos O, Garrus JE, and Sundquist WI (2002) Mechanisms of enveloped RNA virus budding. Trends Cell Biol. 12, 569–579. [DOI] [PubMed] [Google Scholar]
- (167).Bruce EA, Medcalf L, Crump CM, Noton SL, Stuart A, Wise HM, Elton D, Bowers K, and Digard P (2009) Budding of filamentous and non-filamentous influenza A virus occurs via a VPS4 and VPS28-independent pathway. Virology 390, 268–278. [DOI] [PubMed] [Google Scholar]
- (168).Stewart SM, and Pekosz A (2011) Mutations in the Membrane-Proximal Region of the Influenza A Virus M2 Protein Cytoplasmic Tail Have Modest Effects on Virus Replication. J. Virol. 85, 12179–12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (169).Schnell JR, and Chou JJ (2008) Structure and mechanism of the M2 proton channel of influenza A virus. Nature 451, 591–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (170).Takeda M, Pekosz A, Shuck K, Pinto LH, and Lamb RA (2002) Influenza A Virus M2 Ion Channel Activity Is Essential for Efficient Replication in Tissue Culture. J. Virol. 76, 1391–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (171).Watanabe T, Watanabe S, Ito H, Kida H, and Kawaoka Y (2001) Influenza A Virus Can Undergo Multiple Cycles of Replication without M2 Ion Channel Activity. J. Virol. 75, 5656–5662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (172).Iwatsuki-Horimoto K, Horimoto T, Noda T, Kiso M, Maeda J, Watanabe S, Muramoto Y, Fujii K, and Kawaoka Y (2006) The Cytoplasmic Tail of the Influenza A Virus M2 Protein Plays a Role in Viral Assembly. J. Virol. 80, 5233–5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (173).Rossman JS, Jing X, Leser GP, and Lamb R A. (2010) Influenza Virus M2 Protein Mediates ESCRT-Independent Membrane Scission. Cell 142, 902–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (174).Rossman JS, Jing X, Leser GP, Balannik V, Pinto LH, and Lamb R A. (2010) Influenza Virus M2 Ion Channel Protein Is Necessary for Filamentous Virion Formation. J. Virol. 84, 5078–5088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (175).Rossman JS, and Lamb R A. (2011) Influenza virus assembly and budding. Virology 411, 229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (176).Lewis K (2007) Persister cells, dormancy and infectious disease. Nat. Rev. Microbiol. 5, 48–56. [DOI] [PubMed] [Google Scholar]
- (177).Keren I, Shah D, Spoering A, Kaldalu N, and Lewis K (2004) Specialized Persister Cells and the Mechanism of Multidrug Tolerance in Escherichia coli. J. Bacteriol 186, 8172–8180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (178).Spoering AL, and Lewis K (2001) Biofilms and Planktonic Cells of Pseudomonas aeruginosa Have Similar Resistance to Killing by Antimicrobials. J. Bacteriol. 183, 6746–6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (179).Schmidt NW, Deshayes S, Hawker S, Blacker A, Kasko AM, and Wong GCL (2014) Engineering Persister-Specific Antibiotics with Synergistic Antimicrobial Functions. ACS Nano 8, 8786–8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (180).Schmidt NW, Agak GW, Deshayes S, Yu Y, Blacker A, Champer J, Xian W, Kasko AM, Kim J, and Wong GCL (2015) Pentobra: A Potent Antibiotic with Multiple Layers of Selective Antimicrobial Mechanisms against Propionibacterium Acnes. J. Invest. Dermatol. 135, 1581–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (181).Deshayes S, Xian W, Schmidt NW, Kordbacheh S, Lieng J, Wang J, Zarmer S, Germain SS, Voyen L, Thulin J, et al. (2017) Designing Hybrid Antibiotic Peptide Conjugates To Cross Bacterial Membranes. Bioconjugate Chem. 28, 793–804. [DOI] [PubMed] [Google Scholar]
- (182).Derossi D, Chassaing G, and Prochiantz A (1998) Trojan peptides: the penetratin system for intracellular delivery. Trends Cell Biol. 8, 84–87. [PubMed] [Google Scholar]
- (183).Diener C, Garza Ramos Martínez G., Moreno Blas D, Castillo González DA, Corzo G, Castro-Obregon S, and Del Rio G (2016) Effective Design of Multifunctional Peptides by Combining Compatible Functions. PLoS Comput. Biol. 12, e1004786. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


