Abstract
The present study assessed whether estrogen receptor (ER)β1 is associated with the survival of patients with advanced lung adenocarcinoma, with or without mutations of the epidermal growth factor receptor (EGFR) following treatment with EGFR-tyrosine kinase inhibitors (TKIs). Pathologically confirmed stage IV lung adenocarcinomas were assessed for EGFR mutations and ERβ1 expression. Progression-free survival (PFS) and overall survival (OS) were estimated using the Kaplan-Meier method and the log-rank test. A total of 122 out of the 201 (60.7%) patients had EGFR mutations, 64 (31.8%) of which were EGFR Del19 and 58 mutations (28.9%) were EGFR exon 21 L858R mutation. The presence of EGFR mutations was significantly increased in female patients compared with male patients (P<0.001) and in non-smokers compared with smokers (P<0.001). Patients with EGFR mutations had a significantly improved PFS and OS compared with patients without EGFR mutations treated with EGFR-TKIs. Furthermore, ERβ1 expression was significantly increased in patients with EGFR mutations compared with patients without EGFR mutations (P=0.001). However, the median PFS (P=0.005) and OS (P=0.002) of patients carrying the EGFR exon 21 L858R mutation was significantly decreased in patients with tumors where ERβ1 cytoplasmic expression was high. The multivariate analysis demonstrated that ERβ1 expression was the only independent predictor of PFS (P=0.002) and OS (P=0.003) in patients carrying the EGFR exon 21 L858R mutation. The data demonstrated that ERβ1 expression may predict outcomes of patients with lung adenocarcinoma treated with EGFR-TKI.
Keywords: estrogen receptor β1, epidermal growth factor receptor mutation, lung adenocarcinoma, tyrosine kinase inhibitor, prognostic marker
Introduction
Lung cancer is the leading cause of cancer-associated mortality worldwide and non-small cell lung cancer (NSCLC) accounts for 80–85% of all lung cancer cases (1). Mutations and gene amplifications of epidermal growth factor receptor (EGFR) frequently occur in NSCLC, and EGFR-tyrosine kinase inhibitors (EGFR-TKIs) are regularly used to treat patients with NSCLC and prolong progression-free survival (PFS) (2,3). Thus, it is recommended to screen for EGFR mutations in patients with NSCLC and that patients with sensitizing EGFR mutations are treated with first-line EGFR-TKIs, whereas patients with NSCLC wihtout EGFR mutation or unknown mutational status are treated with platinum-based chemotherapy (4,5). In the clinic, the majority of patients with EGFR mutations initially respond very well to EGFR-TKIs; however, a large proportion of them eventually develop drug resistance (6). The underlying molecular mechanisms of drug resistance may be due to receptor tyrosine-protein kinase erbB-2 (HER2) amplification and EGFR T790M mutation, MET proto-oncogene, receptor tyrosine kinase (c-MET) amplification, phosphatidylinositol-4,5-bisphosphate 3-kinase catalytic subunit α (PIK3CA) mutation or B-Raf proto-oncogene, serine/threonine kinase (BRAF) mutations in tumor tissues (7). However, the specific molecular mechanisms require further investigation. For example, previous studies demonstrated that estrogen receptor (ER)β was overexpressed in NSCLC tissue specimens, which may be associated with resistance to treatment with EGFR-TKIs in patients with NSCLC (8,9). ERβ is the most commonly observed subtype of ER expressed in lung cancer (8,9). Previous studies have suggested cross-talk between the ERβ and EGFR signaling pathways in lung cancer (10–12). ERβ is frequently overexpressed in NSCLC with EGFR mutations, particularly in lung adenocarcinoma (13,14), and ERβ expression was reported to be associated with the prognosis of NSCLC with EGFR mutations subsequent to treatment with EGFR-TKIs (15–17). There are five known isoforms of ERβ, and ERβ1 is the only known full-length and functional ERβ isoform expressed in various cells and tissues (18), and is the most relevant prognostic factor of all ERβ isoforms for patients with NSCLC (9).
To date, the presence of a number of EGFR mutations have been demonstrated to occur in NSCLC (19), each of which may contribute to different outcomes of patients treated with EGFR-TKIs; however, two EGFR mutations, exon 19 deletion (Del19) and the substitution L858R in exon 21, account for 80–90% of all EGFR mutations in NSCLC (20), and patients with NSCLC carrying these mutations respond favourably to treatment with EGFR-TKI (2,3). In contrast, other EGFR mutations, including G719X, L861Q and a de novo exon 20 T790M mutation, account for ~10% of the known EGFR mutations in NSCLC, and only certain patients responded favourably to treatment with EGFR-TKI (21). In addition, patients with the T790M mutation demonstrated resistance to treatment with first generation EGFR-TKIs (22). Previous studies have demonstrated that treatment of patients with NSCLC carrying Del19 mutation with EGFR-TKIs improved outcomes compared with the patients carrying the L858R mutation (23,24). In the present study, ERβ1 expression was retrospectively assessed in 201 lung adenocarcinoma tissue specimens, and the ERβ1 expression and survival of patients with lung adenocarcinoma carrying the EGFR Del19 or L858R mutation subsequent to treatment with EGFR-TKIs were examined. The present study was designed to confirm data from previous studies (23,24), and additionally provide useful information regarding treatment of patients with NSCLC with EGFR-TKIs or a combination of other drugs.
Patients and methods
Patients and treatment
Patients who were pathologically diagnosed with stage IV TNM lung adenocarcinoma were evaluated for eligibility (25). The inclusion criteria were: i) Patients with data pertaining to EGFR mutations; and ii) treatment with EGFR-TKIs or chemotherapy. The exclusion criteria were: i) Patients that had left hospital; ii) patients that had refused any chemotherapy or an EGFR test, or iii) there was no sufficient tissue specimen for the EGFR and immunohistochemistry (IHC) tests. Thus, 201 patients were eligible for the present study. Tissue samples from patients, for EGFR mutation analysis, were retrospectively collected from The Department of Thoracic Oncology, Anhui Provincial Cancer Hospital (Hefei, China) between January 2012 and June 2014. The cohort of patients had stage IV disease; thus, there were no patients that underwent tumor resection. The median age was 65 years (range, 27–84 years) and 72.1% were females. Lung cancer tissues were obtained through transbronchial biopsy or fine needle aspiration for histological diagnosis of NSCLC. The present study was approved by The Ethics Committee of Anhui Provincial Cancer Hospital, which waived patient consent due to mortality of all the individuals.
In terms of treatment options, patients with lung adenocarcinoma with no evidence of EGFR mutations were administered pemetrexed in combination with two 4-week cycles of cisplatin/carboplatin (area under the curve=5). From the cohort, two patients with a relatively uncommon EGFR 19Del plus T790M mutation, which may not have responded well to treatment with EGFR-TKI (20,21), also received chemotherapy as it was in doubt whether such patients would respond to the first generation of EGFR-TKIs. Patients with common EGFR mutations and two patients with uncommon EGFR mutations (one each of S768I/L858R and 19Del/G719X mutation) were administered the first-line therapy of gefitinib (250 mg/day), erlotinib (150 mg/day) or icotinib (125 mg, three times a day) for between 4 and 17.6 months (discontinued after occurrence of drug resistance). Treatment with EGFR-TKIs were discontinued when CT scans identified enhanced disease progression or if treatment toxicity was deemed unacceptable. For these patients, chemotherapy or the best supportive care were the options considered thereafter.
Patient assessment and follow-up
The clinicopathological data of the patients, including age, sex, smoking history, TNM stage and brain metastasis, were retrieved from their medical records and are presented in Table I. Clinically, all patients were evaluated on a monthly basis and their follow-up consisted of a physical examination, including the Eastern Cooperative Oncology Group performance status (26), laboratory tests and electrocardiography, whereas tumor burdens were assessed monthly or bimonthly using CT. Non-smokers were defined as individuals who had smoked <100 cigarettes in their lifetime, whereas others were defined as smokers. The effectiveness of chemotherapy or targeted therapy was evaluated with the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 (27) and disease progression was defined as ≥20% increase in the diameter of a tumor lesion following treatment with EGFR-TKI or chemotherapy according to RECIST 1.1 (27). The PFS was calculated as the interval from initial treatment to the progression of the disease, mortality of any cause or the last follow-up. The overall survival (OS) was calculated as the interval from initial treatment to mortality from any cause or the last follow-up. The survival data were obtained through the review of medical records, telephone follow-up or contact with the local Household Registration Department.
Table I.
Clinicopathological characteristics of 201 patients with stage IV lung carcinoma.
| Epidermal growth factor receptor mutations, n (%) | ||||
|---|---|---|---|---|
| Clinicopathological characteristic | n | Mutation | No mutation | P-value |
| Age (years) | 0.314 | |||
| ≥65 | 103 | 66 (64.1) | 37 (36.9) | |
| <65 | 98 | 56 (57.1) | 42 (42.9) | |
| Sex | <0.001a | |||
| Male | 56 | 22 (39.3) | 34 (60.7) | |
| Female | 145 | 100 (69.0) | 45 (31.0) | |
| Smoking status | <0.001a | |||
| Smoker | 62 | 20 (32.3) | 42 (67.7) | |
| Never-smoker | 139 | 102 (73.4) | 37 (26.6) | |
| ECOG performance status | 0.675 | |||
| 0 | 108 | 67 (62.0) | 41 (38.0) | |
| ≥1 | 93 | 55 (59.1) | 38(40.9) | |
| Brain metastasis | 0.605 | |||
| Yes | 115 | 64 (55.6) | 51 (44.4) | |
| No | 86 | 51 (59.3) | 35 (40.7) | |
P<0.001. ECOG, Eastern Cooperative Oncology Group.
DNA extraction and detection of EGFR mutations
Genomic DNA was extracted from formalin-fixed, paraffin-embedded tumor tissue specimens using the Cobas® DNA Sample Preparation Kit according to the manufacturer's protocol (Roche Molecular Diagnostics, Pleasanton, CA, USA). The presence of EGFR mutations was assessed using a Cobas z 480 real-time PCR system (Roche Molecular Diagnostics) which is capable of detecting 42 EGFR mutations (28).
IHC
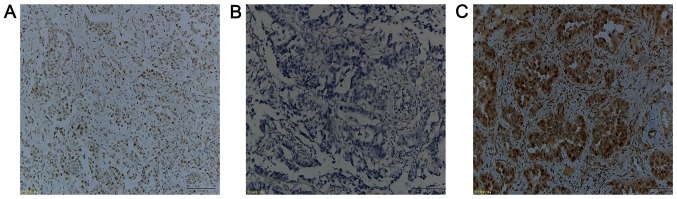
All IHC steps were performed on a BenchMark XT system (Ventana Medical Systems, Inc., Tucson, AZ, USA), according to the manufacturer's protocol. Briefly, paraffin-embedded tissue sections of a 4-µm thickness were fixed in 10% neutral-buffered formalin for 24 h at room temperature and deparaffinized and subsequently placed into a 60°C oven for 2 h. To block endogenous peroxidase activity, 3% hydrogen peroxide was used for 8 min at 37°C. A mouse monoclonal anti-human ERβ1 antibody PPG5/10 (1:50; cat. no. M7292; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA) was incubated with the tissue sections for 32 min at 37°C. Subsequently, the slides were incubated with a ultraView universal HRP Multimer, which contains a cocktail of HRP labeled antibodies (goat anti-mouse IgG, goat anti-mouse IgM and goat anti-rabbit) (prediluted; cat. no. 760-500; Ventana Medical Systems, Inc.) for 8 min at 37°C. Finally, the tissue sections were counterstained with hematoxylin for 16 min at 37°C and assessed under a light microscope (Olympus Corporation, Tokyo, Japan) at ×10 magnification using the Allred scoring system according to previous studies (14,28). ERβ expression was divided into nuclear or cytoplasmic staining and scored based on the proportion of positive cells (0, no staining at all; 1, ≤1%; 2, 2–10%; 3, 11–33%; 4, 34–66%; and 5, >67%) and the staining intensity (0, no staining; 1, weak; 2, moderate; and 3, strong staining). The evaluation of the IHC staining was performed independently by two pathologists who were blinded to the identity of the patients. The staining index of the cytoplasmic or nuclear score was subsequently reached by the addition of the staining proportion and intensity to provide a score between 0 and 8 (Fig. 1). Any disagreements were resolved by reviewing the immunostained sections to reach a consensus. High or low ERβ expression was defined according to a previous study (29). The median of the cytoplasmic scores of ERβ1 immunostaining was 4; thus, the low level of the cytoplasmically immunostaining was defined as ≤4, whereas >4 was classified as a high level of cytoplasmic ERβ1 expression.
Figure 1.
Immunohistochemical assessment of ERβ1 expression in lung adenocarcinoma tissue specimens. (A) The Allred scoring system of ERβ1 expression in tissue specimens. Cytoplasmic score of 8 + nuclear score of 8=total score of 16 (tissue specimen from a patient with EGFR Del19 mutation). (B) The cytoplasmic score of 3 + nuclear score of 7=total score of 10 (tissue specimen from a patient with EGFR exon 21 L858R mutation). (C) The cytoplasmic score 0 + nuclear score 0=total score 0 (tissue specimen from a patient without any EGFR mutation). Scale bar, 100 µm. ERβ1, estrogen receptor β1; EGFR, epidermal growth factor.
Statistical analysis
All statistical analyses were performed using SPSS 20.0 (IBM Corporation, Armonk, NY, USA). The primary endpoint of the treatment responses was the PFS, whereas the secondary endpoint was the OS. The median PFS and OS were estimated using the Kaplan-Meier curves and statistically analyzed using the log-rank test. Where appropriate, the data were additionally presented as the 95% confidence intervals (CI) and the hazard ratios (HRs) with associated 95% CI. The variables significantly associated with survival in a univariate analysis using the log-rank test were further assessed using a multivariate analysis with the Cox proportional model. P<0.05 was considered to indicate a statistically significant difference.
Results
Characteristics of patients
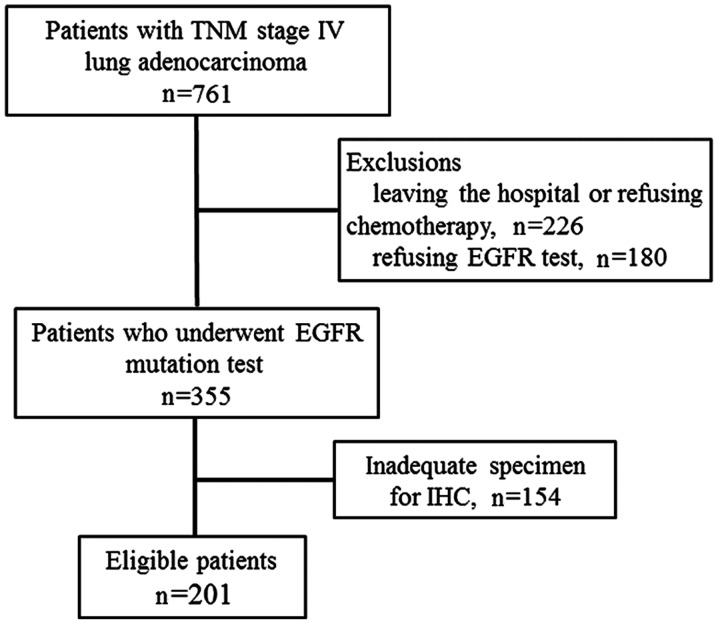
In the present study, a total of 761 patients diagnosed with lung adenocarcinoma between January 2012 and June 2014 (Fig. 2) were retrospectively reviewed. Due to the nature of the study, 201 patients with TNM stage IV with a known EGFR mutation status were included in the analysis. The demographic and baseline characteristics are listed in Table I. The EGFR mutation frequency was significantly higher in female patients (69.0%) compared with male patients (39.3%; P<0.001) and also higher in non-smokers (73.4%) compared with smokers (32.3%; P<0.001). Other clinicopathological data did not demonstrate statistical significance between patients with or without EGFR mutations (Table I).
Figure 2.
Flow diagram of inclusion and exclusion criteria for cohort selection. TNM, tumor node metastasis; EGFR, epidermal growth factor receptor; IHC, immunohistochemistry.
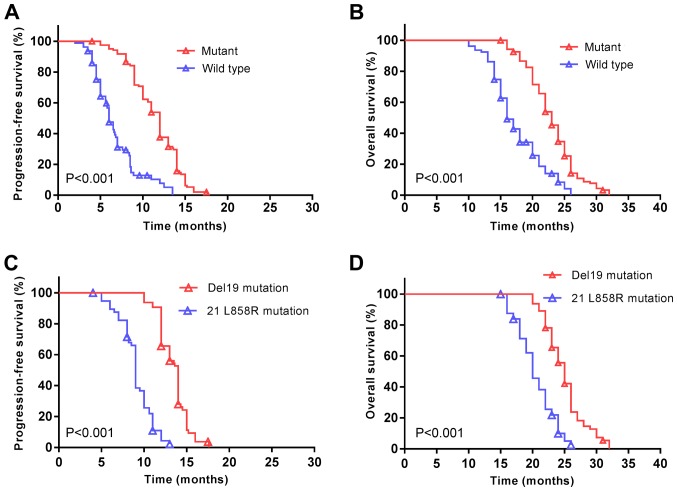
In the present cohort, the median PFS was 10 months (95% CI; 9.5–10.5 months) and the median OS was 21 months (95% CI; 20.1–21.9 months). Patients with EGFR mutations demonstrated a significantly increased median PFS (12 vs. 6 months, P<0.001; Fig. 3A), and a longer median OS (23 vs. 16 months, P<0.001; Fig. 3B) compared with patients without EGFR mutations subsequent to treatment with EGFR-TKIs. The median PFS was additionally significantly increased among patients with EGFR Del19 compared with the EGFR exon 21 L858R mutation (14 vs. 9 months, P<0.001; Fig. 3C). A similar trend was observed for the median OS (25 vs. 20 months, P<0.001; Fig. 3D).
Figure 3.
An exon 21 L858R EGFR mutation is associated with a decreased survival rate of patients with non-small cell lung cancer. (A) PFS and (B) OS were significantly decreased in patients with an EGFR mutation. P<0.001. (C) PFS and (D) OS were significantly decreased in patients carrying the 21 L858R EGFR mutation compared with patients carrying the exon Del19 mutation. P<0.001. Symbols represent censored observations. EGFR, epidermal growth factor receptor; PFS, progression free survival; OS, overall survival.
ERβ1 expression is associated with survival of patients with EGFR mutations
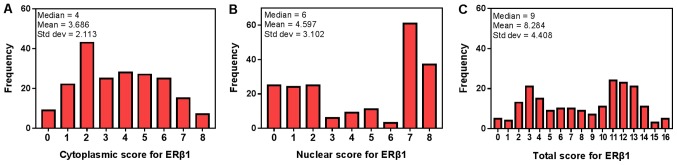
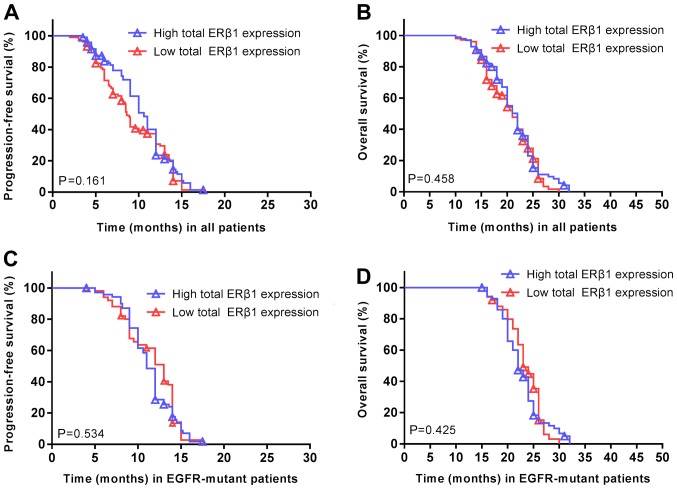
The median nuclear ERβ1 score was 6, which was set as the cut-off value to distinguish between low and high expression of nuclear ERβ1. In addition, the median total score of cytoplasmic and nuclear ERβ1 immunostaining was 9, which was used to distinguish between the low and high levels of total cytoplasmic and nuclear ERβ1 immunostaining in tumor tissues (Fig. 4). ERβ1 was expressed in the cytoplasm and nuclei of 98 patients with lung adenocarcinoma, expressed in the cytoplasm in 74 patients and expressed in the nucleus in 99 patients (Table II). ERβ1 expression was significantly increased in patients with EGFR mutations (71 patients, 58.2%) compared with patients without EGFR mutations (27 patients, 34.1%; P=0.001). However, no significant difference in ERβ1 expression was observed between females and males, irrespective of the localization of ERβ1. Furthermore, there were no significant associations between other clinical features and overall ERβ1 expression. No significant differences were observed between overall ERβ1 expression and the PFS (P=0.161; Fig. 5A) or OS (P=0.458; Fig. 5B) of all the patients. Similarly, there was no association between ERβ1 expression and PFS (P=0.534; Fig. 5C) or OS (P=0.425; Fig. 5D) in patients with EGFR mutations.
Figure 4.
Immunostaining scores of ERβ1 expression. A histogram of the (A) cytoplasmic scores, (B) nuclear scores (C) and total scores of ERβ1 expression in 201 cases of patients with lung adenocarcinoma. ERβ1, estrogen receptor-β1.
Table II.
Association of ERβ1 expression with clinicopathological parameters of 201 lung adenocarcinoma patients.
| Cytoplasmic expression of ERβ1 | Nuclear expression of ERβ1 | Total expression of ERβ1 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Characteristic | High (n) | Low (n) | P-value | High (n) | Low (n) | P-value | High (n) | Low (n) | P-value |
| Age (years) | 0.57 | 0.29 | 0.36 | ||||||
| ≥65 | 36 | 67 | 47 | 56 | 47 | 56 | |||
| <65 | 38 | 60 | 52 | 46 | 51 | 47 | |||
| Sex | 0.59 | 0.41 | 0.17 | ||||||
| Male | 19 | 37 | 25 | 31 | 23 | 33 | |||
| Female | 55 | 90 | 74 | 71 | 75 | 70 | |||
| Smoking status | 0.12 | 0.87 | 0.19 | ||||||
| Ever smoker | 18 | 44 | 30 | 32 | 26 | 36 | |||
| Never smoker | 56 | 83 | 69 | 70 | 72 | 67 | |||
| Performance status | 0.27 | 0.95 | 0.34 | ||||||
| 0 | 36 | 72 | 53 | 55 | 56 | 52 | |||
| ≥1 | 38 | 55 | 46 | 47 | 42 | 51 | |||
| Brain metastasis | 0.92 | 0.45 | 0.98 | ||||||
| Yes | 42 | 73 | 54 | 61 | 56 | 59 | |||
| No | 32 | 54 | 45 | 41 | 42 | 44 | |||
| EGFR-mutant status | 0.12 | 0.40 | 0.001a | ||||||
| Yes | 50 | 72 | 63 | 59 | 71 | 51 | |||
| No | 24 | 55 | 36 | 43 | 27 | 52 | |||
P<0.01. ERβ1, estrogen receptor β1.
Figure 5.
Total cellular ERβ1 expression is not associated with the survival of patients with NSCLC. (A) PFS and (B) OS were not significantly altered in patients with high ERβ1 expression compared with low expression. P=0.161; P=0.458, respectively. (C) PFS and (D) OS in patients with EGFR-mutant NSCLC were not significantly altered in patients with high ERβ1 expression compared with low expression. P=0.534; P=0.425, respectively. Symbols represent censored observations. NSCLC, non-small cell lung cancer; PFS, progression free survival; OS, overall survival; ERβ1, estrogen receptor β1; EGFR, epidermal growth factor receptor.
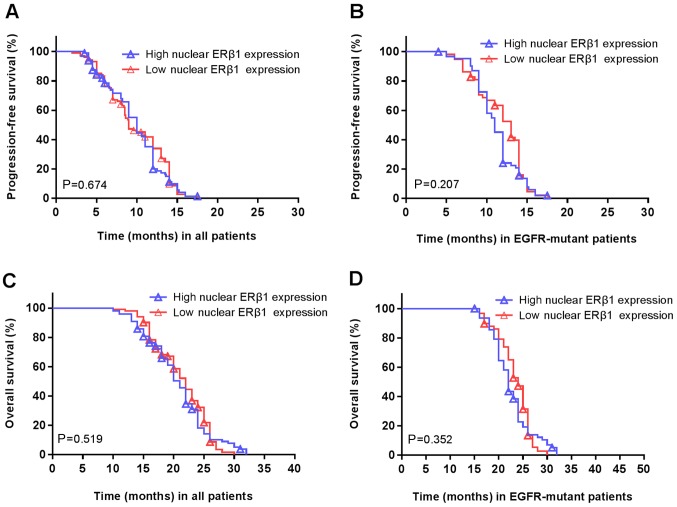
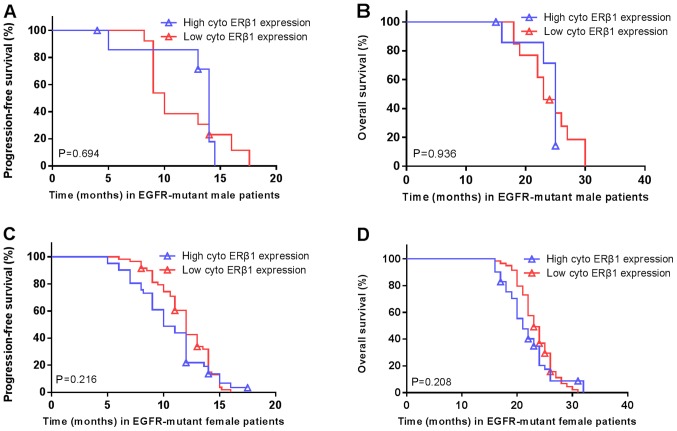
Furthermore, there was no association between high/low nuclear ERβ1 and the PFS (P=0.674; Fig. 6A) or OS (P=0.207; Fig. 6B). Similarly, there was no association between nuclear ERβ1 expression and PFS (P=0.519; Fig. 6C) or OS (P=0.352; Fig. 6D) in patients with EGFR mutations. There was no significant difference in the PFS (14 vs. 10 months; P=0.694; Fig. 7A) or OS (25 vs. 23 months; P=0.936; Fig. 7B) in patients with high/low cytoplasmic ERβ1 expression in the male patients carrying EGFR mutations. Similarly, no significant differences were in PFS (10 vs. 12 months; P=0.216; Fig. 7C) or OS (21 vs. 23 months; P=0.208; Fig. 7D) were observed in the female EGFR-mutant patients with high/low expression. There were no associations between EGFR mutations and nuclear ERβ1 expression or between patients carrying EGFR mutations and total ERβ1 levels in males and females (Table II).
Figure 6.
Nuclear ERβ1 expression is not associated with the survival of patients with. (A) PFS and (B) OS were not significantly altered in patients with high ERβ1 expression compared with low expression. P=0.674; P=0.207, respectively. (C) PFS and (D) OS in patients with EGFR-mutant non-small cell lung cancer were not significantly altered in patients with high ERβ1 expression compared with low expression. P=0.519; P=0.342, respectively. Symbols represent censored observations. NSCLC, non-small cell lung cancer; PFS, progression free survival; OS, overall survival; ERβ1 estrogen receptor β1; EGFR, epidermal growth factor receptor.
Figure 7.
Cytoplasmic ERβ1 expression is not associated with the survival of patients. There was no significant difference in the (A) PFS and (B) OS in male patients with high vs. low cytoplasmic ERβ1 expression. P=0.694; P=0.936, respectively. (C) PFS and (D) OS in female patients with high vs. low cytoplasmic ERβ1 expression. P=0.216; P=0.208, respectively. Symbols represent censored observations. PFS, progression free survival; OS, overall survival; ERβ1, estrogen receptor β1; EGFR, epidermal growth factor receptor; cyto, cytoplasmic.
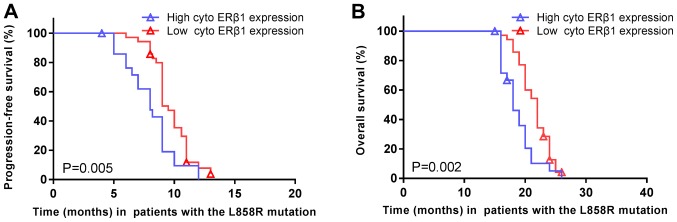
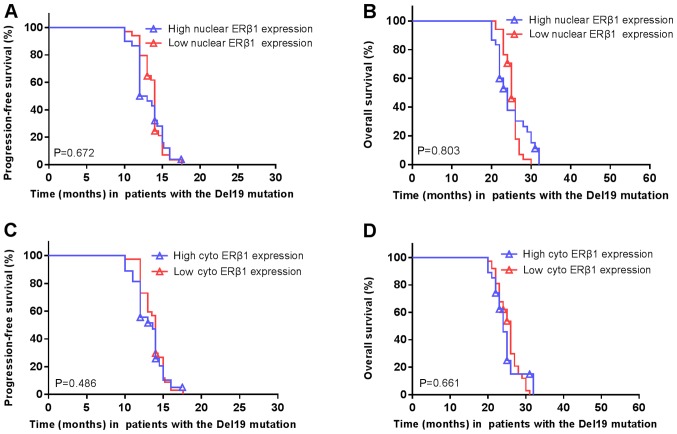
The median PFS of patients carrying EGFR exon 21 L858R mutations was significantly shorter in high cytoplasmic ERβ1 tumors (8.0 months; 95% CI, 6.2–9.8 months) compared with low cytoplasmic ERβ1-expressing tumors (9.5 months; 95% CI, 8.9–10.1 months; P=0.005; HR=1.977; 95% CI, 1.126–3.469; Fig. 8A). Similarly, the median OS was significantly shorter in high cytoplasmic ERβ1-expressing tumors (18.0 months; 95% CI, 16.3–19.7 months) compared with low cytoplasmic ERβ1-expressing tumors (22.0 months; 95% CI, 20.8–23.2 months; P=0.002; HR=2.217; 95% CI, 1.246–3.945; Fig. 8B). A significant difference between the numbers of patients with high cytoplasmic ERβ1 expression compared with low cytoplasmic expression in patients with EGFR mutations may have skewed the data. A total of 122 (60.7%) had EGFR mutations, of which, 64 (31.8%) were EGFR Del19 mutations and 58 (28.9%) were EGFR exon 21 L858R mutation. In patients with EGFR mutations, two patients had the Del19 and an additional T790M mutation, one patient had a S768I/L858R mutation and one patient had a 19Del/G719X mutation (Table III). There were no significant differences in ERβ1 expression between patients with a EGFR Del19 or exon 21 L858R mutation, irrespective of the localization of the protein, although it was observed that the frequency of high cytoplasmic ERβ1 expression was lower (23 patients, 39.6%) compared with that of low expression (35 patients, 60.1%) in patients with EGFR L858R mutation, which was similar to patients carrying EGFR Del19 mutations, this difference was not significant (Table III). In patients with the Del19 EGFR mutation, the survival rate did not differ significantly between patients with high or low nuclear ERβ1 expression (Fig. 9A and B, respectively) or cytoplasmic expression (Fig. 9C and D).
Figure 8.
Cytoplasmic expression of ERβ1 in patients with EGFR exon 21 L858R mutation is associated with survival. (A) Progression-free survival and (B) overall survival were significantly increased in patients with EGFR exon 21 L858R mutated non-small cell lung cancer and low cytoplasmic ERβ1 expression compared with high expression. P=0.005; P=0.002, respectively. Symbols represent censored observations. EGFR, epidermal growth factor receptor; ERβ1, estrogen receptor β1; cyto, cytoplasmic.
Table III.
Association of EGFR mutations with ERβ1 expression in 201 lung adenocarcinoma tissue samples.
| EGFR mutation, n (%) | ||||
|---|---|---|---|---|
| ERβ1 expression | n | Del19 | Exon 21 L858R | P-value |
| Cytoplasmic | 0.63 | |||
| High | 50 | 27 (42.2) | 23 (39.6) | |
| Low | 72 | 37 (57.8) | 35 (60.4) | |
| Nuclear | 0.26 | |||
| High | 63 | 30 (47.6) | 33 (52.4) | |
| Low | 59 | 34 (57.6) | 25 (42.4) | |
| Both | 0.64 | |||
| High | 71 | 36 (51.9) | 35 (49.1) | |
| Low | 51 | 28 (46.8) | 23 (53.2) | |
EGFR, epidermal growth factor receptor; ERβ1, estrogen receptor β1.
Figure 9.
Nuclear or cytoplasmic ERβ1 expression is not associated with survival of patients with the EGFR Del19 mutation. (A) PFS and (B) OS of patients carrying Del19 EGFR mutated NSCLC with high vs. low nuclear ERβ1 expression. P=0.672; P=0.803, respectively. (C) PFS and (D) OS in patients carrying Del19 EGFR mutated NSCLC with high vs. low cytoplasmic ERβ1 expression. P=0.486; P=0.661, respectively. Symbols represent censored observations. EGFR, epidermal growth factor receptor; NSCLC, non-small cell lung cancer; PFS, progression free survival; OS, overall survival; ERβ1, estrogen receptor β1; cyto, cytoplasmic.
Multivariate analysis
The multivariate analysis of ERβ1 expression, age, sex, tobacco smoking and tumor brain metastasis demonstrated that only ERβ1 expression was an independent predictor of PFS (HR=2.847; 95% CI, 1.456 to 5.565; P=0.002) and OS (HR=2.639; 95% CI, 1.283 to 5.036; P=0.003) in patients carrying EGFR 21 L858R mutation (Tables IV and V).
Table IV.
Multivariate analysis of progression free survival in patients with the epidermal growth factor receptor 21 L858R mutation.
| Variable | Hazard ratio | 95% confidence interval | P-value |
|---|---|---|---|
| Sex | 1.901 | 0.807–4.477 | 0.142 |
| Age (years) | 1.021 | 0.575–1.814 | 0.942 |
| Tumor brain metastasis | 1.698 | 0.889–3.240 | 0.109 |
| Smoking status | 0.854 | 0.333–2.187 | 0.742 |
| Performance status | 1.201 | 0.626–2.303 | 0.582 |
| Cytoplasmic estrogen receptor-β1 expression | 2.847 | 1.456–5.565 | 0.002a |
P<0.01.
Table V.
Multivariate analysis of overall survival in patients with the epidermal growth factor receptor 21 L858R mutation.
| Variable | Hazard ratio | 95% confidence interval | P-value |
|---|---|---|---|
| Sex | 1.598 | 0.668–3.824 | 0.293 |
| Age (years) | 1.098 | 0.613–1.969 | 0.753 |
| Tumor brain metastasis | 1.038 | 0.567–1.901 | 0.903 |
| Smoking status | 1.124 | 0.429–2.944 | 0.812 |
| Performance status | 1.396 | 0.716–2.720 | 0.327 |
| Cytoplasmic estrogen receptor-β1 expression | 2.639 | 1.383–5.036 | 0.003a |
P<0.01.
Discussion
Lung cancer is a major cause of cancer-associated mortality worldwide (30). Tobacco smoking is the single largest contributing risk factor in lung cancer development, although certain patients with lung cancer are non-smokers, suggesting that other factors are also important in the pathogenesis of lung cancer. These risk factors may induce mutations of EGFR or alter expression of other genes (6–16). For example, aberrant ERβ expression is associated with lung cancer development and progression (9,11,31), and the survival of patients with NSCLC (32–36). Therefore, the present study further assessed ERβ1 expression in lung adenocarcinoma tissue specimens and determined the outcomes of patients with or without EGFR mutations following treatment with EGFR-TKI. The present study demonstrated that 60.7% of patients carried an EGFR mutation and the majority of these mutations were the EGFR Del19 and exon 21 L858R mutations. The presence of EGFR mutations was significantly higher in females than male patients, and in non-smokers compared with smokers. The median PFS of the cohort of patients was 10 months, whereas the median OS was 21 months. Patients with EGFR mutations had a significantly improved median PFS compared with patients without EGFR mutations following treatment with EGFR-TKIs, and the median PFS was also longer in patients with EGFR Del19 compared with the EGFR exon 21 L858R mutation. Additionally, the median OS was significantly improved in patients with EGFR mutations compared with patients without EGFR mutations. In addition, ERβ1 expression was increased in patients with EGFR mutations compared with patients without EGFR mutations. The median PFS and OS were significantly shorter in patients with the EGFR exon 21 L858R mutation, and in patients with cytoplasmic ERβ1-expressed tumor. Multivariate analysis demonstrated that only ERβ1 expression was an independent predictor of PFS and OS in patients carrying EGFR 21 L858R mutation.
The present data on EGFR mutations and association with improved PFS and OS of patients with stage IV lung adenocarcinoma following treatment with EGFR-TKI are consistent with earlier studies (2,3). A significantly longer PFS and OS in patients with EGFR Del19 compared with patients with the EGFR exon 21 L858R mutations was observed, which is also consistent with previous studies (23,24,37). The present data highlighted the importance of the EGFR mutation status in association with survival of patients with lung adenocarcinoma following treatment with EGFR-TKI. Previous studies demonstrated that a high frequency of EGFR mutations occurred in Asian patients with advanced non-tobacco smoking lung adenocarcinoma (38) and treatment of these patients with EGFR-TKI may effectively control disease progression and prolong PFS (20).
The incidence of lung adenocarcinoma is increasing in a number of countries; for example, ~40% of all lung cancer cases are adenocarcinomas in the US (39). Lung adenocarcinoma can occur in tobacco smokers and non-smokers (39,40), and can carry a number of gene mutations, including KRAS, EGFR (20%), HER2 (2%), ALK receptor tyrosine kinase, BRAF, PIK3CA, MET or p53 (41). EGFR mutations in lung adenocarcinoma were first identified in 2004 and more frequent in East Asia compared with Western countries (42,43). EGFR mutations were identified in exon 18 G719S, exon 19 G719C, and exon 21 L858R or L861Q, each of which results in constitutive activation of the EGFR as an oncogene (44), and are associated with treatment responses to gefitinib and erlotinib (41).
The present data demonstrated that ERβ1 expression was increased in lung adenocarcinoma tissues, and additionally ERβ1 expression was significantly increased in patients with EGFR mutations compared with patients without EGFR mutations, which is consistent with previous publications in patients with lung adenocarcinoma (13,14,45). Estrogen can downregulate levels of EGFR expression, whereas EGF can downregulate the level of ERβ expression in NSCLC cell lines (46). Tamoxifen, a selective estrogen-receptor modulator, upregulates EGFR expression, whereas gefitinib, an EGFR-TKI, upregulates ERβ expression (47), suggesting that ERβ signaling interacts with EGFR signaling in patients with lung adenocarcinoma. Furthermore, the present study assessed the association between ERβ1 expression and outcome of lung adenocarcinoma patients with EGFR mutations following treatment with EGFR-TKI; however, no significant differences in survival were observed among patients with high or low total, nuclear or cytoplasmic ERβ1 expression. However, multivariate analysis demonstrated that high cytoplasmic ERβ1 expression was associated with worse PFS and OS in patients with the EGFR exon 21 L858R mutation following treatment with EGFR-TKI. In contrast to the present study, a previous study demonstrated that increases in ERβ1 expression in the cytoplasm or nucleus (independently; however, not simultaneously) was associated with poorer prognosis in patients with EGFR-mutated lung adenocarcinoma (16). Another previous study demonstrated that strong ERβ1 expression was associated with improved prognosis in patients with lung adenocarcinoma treated with EGFR-TKI (15). A possible explanation for the discrepancy between these two studies and the present data may stem from the previous studies not differentiating between patients with EGFR Del19 or exon 21 L858R mutations. However, it is unclear why there was no association of cytoplasmic ERβ1 expression with prognosis of patients with the Del19 EGFR. Estrogen activates ER and EGFR signaling in cells (48) and ERβ1 expression in NSCLC may be involved in EGFR-TKI resistance in the treatment of patients with NSCLC. Fu et al (49) recently demonstrated that ERβ1 induced Erk1/2 and Akt activation, which may have resulted in EGFR-TKI resistance. Ma et al (50) demonstrated that ER directly binds to EGFR to confer tumor cell resistance to EGFR-TKIs, and that a combination of an EGFR-TKI with anti-estrogen therapy may induce tumor cell sensitivity to EGFR-TKI. However, EGFR Del19 or exon 21 L858R mutants are translated into different EGFR protein structures (51). Specifically, in the wild-type EGFR NSCLC, the C-helix is outward rotated and the N-terminal portion of the activation loop forms a helical turn that locks the C-helix in the inactive position. However, the mutant EGFR could destabilize the inactive conformation, e.g., the EGFR L858R mutation has a much larger charged side chain, which will not be able to be contained in the inactive conformation, but can be subsumed within the active and reorganized form of the enzyme (52). Therefore, it is hypothesized that cytoplasmic ERβ1 binds more tightly to the EGFR 21 L858R mutant protein compared with the Del19 mutant EGFR to confer resistance to TKIs. However, further studies are required to confirm this.
The present study has certain limitations. For example, only immunohistochemistry was performed to analyze ERβ1 expression in lung cancer tissues; however, RT-qPCR does not easily allow for the determination of the subcellular localization (nuclear or cytoplasmic) of expression in tumor cells and tissue specimens and the presence of a mix of stromal and tumor cells may further complicate the analysis. Furthermore, the All red scoring system of immunohistochemical ERβ1 expression in tissue specimens was performed according to previous studies (14,26) and the median Allred score was defined as the cutoff value of high vs. low expression, which is different from previously published studies that used other scoring systems and cut-off values. Thus, a standardized scoring system is required for ERβ expression in NSCLC tissue specimens. Furthermore, the present study had a relatively small sample size and a future study with a larger sample size with multi-institutional participation is preferable to confirm the findings.
The present study demonstrated that cytoplasmic ERβ1 expression was associated with poor prognosis of patients with stage IV lung adenocarcinoma carrying EGFR exon 21 L858R mutation subsequent to treatment with EGFR-TKI.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of Anhui Provincial Cancer Hospital (Hefei, China; approval no. 201705).
Authors' contributions
YH and CH conceived and designed the experiments. Data collection and experiments were performed by MZ, JW and HL. XH, YF, JZ and WC analyzed the data and all authors contributed to the writing of the manuscript.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252–271. doi: 10.3322/caac.21235. [DOI] [PubMed] [Google Scholar]
- 2.Rosell R, Carcereny E, Gervais R, Vergnenegre A, Massuti B, Felip E, Palmero R, Garcia-Gomez R, Pallares C, Sanchez JM, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): A multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13:239–246. doi: 10.1016/S1470-2045(11)70393-X. [DOI] [PubMed] [Google Scholar]
- 3.Zhou C, Wu YL, Chen G, Feng J, Liu XQ, Wang C, Zhang S, Wang J, Zhou S, Ren S, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12:735–742. doi: 10.1016/S1470-2045(11)70184-X. [DOI] [PubMed] [Google Scholar]
- 4.Ettinger DS, Wood DE, Aisner DL, Akerley W, Bauman J, Chirieac LR, D'Amico TA, DeCamp MM, Dilling TJ, Dobelbower M, et al. Non-small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15:504–535. doi: 10.6004/jnccn.2017.0050. [DOI] [PubMed] [Google Scholar]
- 5.Novello S, Barlesi F, Califano R, Cufer T, Ekman S, Levra MG, Kerr K, Popat S, Reck M, Senan S, et al. Metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27(Suppl 5):v1–v27. doi: 10.1093/annonc/mdw326. [DOI] [PubMed] [Google Scholar]
- 6.Kobayashi S, Boggon TJ, Dayaram T, Jänne PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG, Halmos B. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. N Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- 7.Morgillo F, Della Corte CM, Fasano M, Ciardiello F. Mechanisms of resistance to EGFR-targeted drugs: Lung cancer. ESMO Open. 2016;1:e000060. doi: 10.1136/esmoopen-2016-000060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thomas C, Gustafsson JA. The different roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer. 2011;11:597–608. doi: 10.1038/nrc3093. [DOI] [PubMed] [Google Scholar]
- 9.Siegfried JM, Stabile LP. Estrongenic steroid hormones in lung cancer. Semin Oncol. 2014;41:5–16. doi: 10.1053/j.seminoncol.2013.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marquez-Garban DC, Chen HW, Fishbein MC, Goodglick L, Pietras RJ. Estrogen receptor signaling pathways in human non-small cell lung cancer. Steroids. 2007;72:135–143. doi: 10.1016/j.steroids.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siegfried JM. Smoking out reproductive hormone actions in lung cancer. Mol Cancer Res. 2014;12:24–31. doi: 10.1158/1541-7786.MCR-13-0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stabile LP, Siegfried JM. Estrogen receptor pathways in lung cancer. Curr Oncol Rep. 2004;6:259–267. doi: 10.1007/s11912-004-0033-2. [DOI] [PubMed] [Google Scholar]
- 13.He Q, Zhang M, Zhang J, Chen Y, He J, Shen J, Liu Y, Zhong S, Jiang L, Yang C, et al. Correlation between epidermal growth factor receptor mutations and nuclear expression of female hormone receptors in non-small cell lung cancer: A meta-analysis. J Thorac Dis. 2015;7:1588–1594. doi: 10.3978/j.issn.2072-1439.2015.09.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nose N, Sugio K, Oyama T, Nozoe T, Uramoto H, Iwata T, Onitsuka T, Yasumoto K. Association between estrogen receptor-beta expression and epidermal growth factor receptor mutation in the postoperative prognosis of adenocarcinoma of the lung. J Clin Oncol. 2009;27:411–417. doi: 10.1200/JCO.2008.18.3251. [DOI] [PubMed] [Google Scholar]
- 15.Nose N, Uramoto H, Iwata T, Hanagiri T, Yasumoto K. Expression of estrogen receptor beta predicts a clinical response and longer progression-free survival after treatment with EGFR-TKI for adenocarcinoma of the lung. Lung Cancer. 2011;71:350–355. doi: 10.1016/j.lungcan.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Wang Z, Li Z, Ding X, Shen Z, Liu Z, An T, Duan J, Zhong J, Wu M, Zhao J, et al. ERβ localization influenced outcomes of EGFR-TKI treatment in NSCLC patients with EGFR mutations. Sci Rep. 2015;5:11392. doi: 10.1038/srep11392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lim VW, Lim WY, Zhang Z, Li J, Gong Y, Seow A, Yong EL. Serum estrogen receptor beta mediated bioactivity correlates with poor outcome in lung cancer patients. Lung Cancer. 2014;85:293–298. doi: 10.1016/j.lungcan.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 18.Leung YK, Mak P, Hassan S, Ho SM. Estrogen receptor (ER)-beta isoforms: A key to understanding ER-beta signaling. Proc Natl Acad Sci USA. 2006;103:13162–13167. doi: 10.1073/pnas.0605676103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tu HY, Ke EE, Yang JJ, Sun YL, Yan HH, Zheng MY, Bai XY, Wang Z, Su J, Chen ZH, et al. A comprehensive review of uncommon EGFR mutations in patients with non-small cell lung cancer. Lung Cancer. 2017;114:96–102. doi: 10.1016/j.lungcan.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 20.Dahabreh IJ, Linardou H, Siannis F, Kosmidis P, Bafaloukos D, Murray S. Somatic EGFR mutation and gene copy gain as predictive biomarkers for response to tyrosine kinase inhibitors in non-small cell lung cancer. Clin Cancer Res. 2010;16:291–303. doi: 10.1158/1078-0432.CCR-09-1660. [DOI] [PubMed] [Google Scholar]
- 21.Castellanos E, Feld E, Horn L. Driven by mutations: The predictive value of mutation subtype in EGFR-mutated non-small cell lung cancer. J Thorac Oncol. 2017;12:612–623. doi: 10.1016/j.jtho.2016.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Yu HA, Arcila ME, Hellmann MD, Kris MG, Ladanyi M, Riely GJ. Poor response to erlotinib in patients with tumors containing baseline EGFR T790M mutations found by routine clinical molecular testing. Ann Oncol. 2014;25:423–428. doi: 10.1093/annonc/mdt573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choi YW, Jeon SY, Jeong GS, Lee HW, Jeong SH, Kang SY, Park JS, Choi JH, Koh YW, Han JH, Sheen SS. EGFR Exon 19 deletion is associated with favorable overall survival after first-line gefitinib therapy in advanced non-small cell lung cancer patients. Am J Clin Oncol. 2018;41:385–390. doi: 10.1097/COC.0000000000000282. [DOI] [PubMed] [Google Scholar]
- 24.Yang JC, Wu YL, Schuler M, Sebastian M, Popat S, Yamamoto N, Zhou C, Hu CP, O'Byrne K, Feng J, et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): Analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015;16:141–151. doi: 10.1016/S1470-2045(14)71173-8. [DOI] [PubMed] [Google Scholar]
- 25.Mirsadraee S, Oswal D, Alizadeh Y, Caulo A, van Beek E., Jr The 7th lung cancer TNM classification and staging system: Review of the changes and implications. World J Radiol. 2012;4:128–134. doi: 10.4329/wjr.v4.i4.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malalasekera A, Tan CSY, Phan V, Yip PY, Vardy J, Clarke SJ, Kao S. Eastern cooperative oncology group score: Agreement between non-small-cell lung cancer patients and their oncologists and clinical implications. Cancer Treat Commun. 2016;5:17–21. doi: 10.1016/j.ctrc.2015.11.009. [DOI] [Google Scholar]
- 27.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 28.Lopez-Rios F, Angulo B, Gomez B, Mair D, Martinez R, Conde E, Shieh F, Tsai J, Vaks J, Current R, et al. Comparison of molecular testing methods for the detection of EGFR mutations in formalin-fixed paraffin-embedded tissue specimens of non-small cell lung cancer. J Clin Pathol. 2013;66:381–385. doi: 10.1136/jclinpath-2012-201240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abe K, Miki Y, Ono K, Mori M, Kakinuma H, Kou Y, Kudo N, Koguchi M, Niikawa H, Suzuki S, et al. Highly concordant coexpression of aromatase and estrogen receptor beta in non-small cell lung cancer. Hum Pathol. 2010;41:190–198. doi: 10.1016/j.humpath.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 30.Chen W, Zheng R, Zhang S, Zeng H, Fan Y, Qiao Y, Zhou Q. Esophageal cancer incidence and mortality in China, 2010. Thorac Cancer. 2014;5:343–348. doi: 10.1111/1759-7714.12100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burns TF, Stabile LP. Targeting the estrogen pathway for the treatment and prevention of lung cancer. Lung Cancer Manag. 2014;3:43–52. doi: 10.2217/lmt.13.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hsu LH, Liu KJ, Tsai MF, Wu CR, Feng AC, Chu NM, Kao SH. Estrogen adversely affects the prognosis of patients with lung adenocarcinoma. Cancer Sci. 2015;106:51–59. doi: 10.1111/cas.12558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ma L, Zhan P, Liu Y, Zhou Z, Zhu Q, Miu Y, Wang X, Jin J, Li Q, Lv T, Song Y. Prognostic value of the expression of estrogen receptor β in patients with non-small cell lung cancer: A meta-analysis. Transl Lung Cancer Res. 2016;5:202–207. doi: 10.21037/tlcr.2016.04.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwartz AG, Prysak GM, Murphy V, Lonardo F, Pass H, Schwartz J, Brooks S. Nuclear estrogen receptor beta in lung cancer: Expression and survival differences by sex. Clin Cancer Res. 2005;11:7280–7287. doi: 10.1158/1078-0432.CCR-05-0498. [DOI] [PubMed] [Google Scholar]
- 35.Skov BG, Fischer BM, Pappot H. Oestrogen receptor beta over expression in males with non-small cell lung cancer is associated with better survival. Lung Cancer. 2008;59:88–94. doi: 10.1016/j.lungcan.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 36.Skjefstad K, Grindstad T, Khanehkenari MR, Richardsen E, Donnem T, Kilvaer T, Andersen S, Bremnes RM, Busund LT, Al-Saad S. Prognostic relevance of estrogen receptor α, β and aromatase expression in non-small cell lung cancer. Steroids. 2016;113:5–13. doi: 10.1016/j.steroids.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 37.Sutiman N, Tan SW, Tan EH, Lim WT, Kanesvaran R, Ng QS, Jain A, Ang MK, Tan WL, Toh CK, Chowbay B. EGFR mutation subtypes influence survival outcomes following first-line gefitinib therapy in advanced asian NSCLC patients. J Thorac Oncol. 2017;12:529–538. doi: 10.1016/j.jtho.2016.11.2225. [DOI] [PubMed] [Google Scholar]
- 38.Shi Y, Au JS, Thongprasert S, Srinivasan S, Tsai CM, Khoa MT, Heeroma K, Itoh Y, Cornelio G, Yang PC. A prospective, molecular epidemiology study of EGFR mutations in asian patients with advanced non-small-cell lung cancer of adenocarcinoma histology (PIONEER) J Thorac Oncol. 2014;9:154–162. doi: 10.1097/JTO.0000000000000033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stewart BW, Wild CP, editors. World Health Organization, IARC Publications; Lyon, France: 2014. World Cancer Report 2014; pp. 489–508. [Google Scholar]
- 40.Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I, Singh B, Heelan R, Rusch V, Fulton L, et al. EGF receptor gene mutations are common in lung cancers from ‘never smokers’ and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA. 2004;101:13306–13311. doi: 10.1073/pnas.0405220101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Greulich H. The genomics of lung adenocarcinoma: Opportunities for targeted therapies. Genes Cancer. 2010;1:1200–1210. doi: 10.1177/1947601911407324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Paez JG, Jänne PA, Lee JC, Tracy S, Greulich H, Gabriel S, Herman P, Kaye FJ, Lindeman N, Boggon TJ, et al. Mutations in lung cancer: Correlation with clinical response to gefitinib therapy. Science. 2004;304:1497–1500. doi: 10.1126/science.1099314. [DOI] [PubMed] [Google Scholar]
- 43.Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW, Harris PL, Haserlat SM, Supko JG, Haluska FG, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 44.Greulich H, Chen TH, Feng W, Jänne PA, Alvarez JV, Zappaterra M, Bulmer SE, Frank DA, Hahn WC, Sellers WR, Meyerson M. Oncogenic transformation by inhibitor-sensitive and -resistant EGFR mutants. PLoS Med. 2005;2:e313. doi: 10.1371/journal.pmed.0020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hsu LH, Chu NM, Kao SH. Estrogen, estrogen receptor and lung cancer. Int J Mol Sci. 2017;18:E1713. doi: 10.3390/ijms18081713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Stabile LP, Lyker JS, Gubish CT, Zhang W, Grandis JR, Siegfried JM. Combined targeting of the estrogen receptor and the epidermal growth factor receptor in non-small cell lung cancer shows enhanced antiproliferative effects. Cancer Res. 2005;65:1459–1470. doi: 10.1158/0008-5472.CAN-04-1872. [DOI] [PubMed] [Google Scholar]
- 47.Shen H, Yuan Y, Sun J, Gao W, Shu YQ. Combined tamoxifen and gefitinib in non-small cell lung cancer shows antiproliferative effects. Biomed Pharmacother. 2010;64:88–92. doi: 10.1016/j.biopha.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 48.Zhao XZ, Liu Y, Zhou LJ, Wang ZQ, Wu ZH, Yang XY. Role of estrogen in lung cancer based on the estrogen receptor-epithelial mesenchymal transduction signaling pathways. OncoTargets Ther. 2015;8:2849–2863. doi: 10.2147/OTT.S90085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Fu S, Liu C, Huang Q, Fan S, Tang H, Fu X, Ai B, Liao Y, Chu Q. Estrogen receptor β1 activation accelerates resistance to epidermal growth factor receptor-tyrosine kinase inhibitors in non-small cell lung cancer. Oncol Rep. 2018;39:1313–1321. doi: 10.3892/or.2018.6186. [DOI] [PubMed] [Google Scholar]
- 50.Ma S, Yin N, Qi X, Pfister SL, Zhang MJ, Ma R, Chen G. Tyrosine dephosphorylation enhances the therapeutic target activity of epidermal growth factor receptor (EGFR) by disrupting its interaction with estrogen receptor (ER) Oncotarget. 2015;6:13320–13333. doi: 10.18632/oncotarget.3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rosell R, Taron M, Reguart N, Isla D, Moran T. Epidermal growth factor receptor activation: How exon 19 and 21 mutations changed our understanding of the pathway. Clin Cancer Res. 2006;12:7222–7231. doi: 10.1158/1078-0432.CCR-06-0627. [DOI] [PubMed] [Google Scholar]
- 52.Yun CH, Boggon TJ, Li Y, Woo MS, Greulich H, Meyerson M, Eck MJ. Structures of lung cancer-derived EGFR mutants and inhibitor complexes: Mechanism of activation and insights into differential inhibitor sensitivity. Cancer Cell. 2007;11:217–227. doi: 10.1016/j.ccr.2006.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.