Abstract
microRNAs are involved in the tumor progression of various cancer types. The present study aimed to determine the prognostic significance of microRNA-505 (miR-505) in patients with breast cancer and investigate the functional role of miR-505 in BCa progression. The expression of miR-505 was estimated using reverse transcription-quantitative polymerase chain reaction. Kaplan-Meier survival curves and Cox regression analysis were used to evaluate the prognostic value of miR-505 in patients with BCa. Cell experiments were performed to assess the biological function of miR-505 during BCa progression. A significant downregulated expression level of miR-505 was observed in BCa tissues and cells compared with the corresponding controls (P<0.001). The expression of miR-505 was significantly associated with distant metastasis status (P=0.013) and Tumor-Node-Metastasis staging (P=0.002). Furthermore, the overall survival time was significantly shorter for patients with low miR-505 expression compared with those with high miR-505 expression (P<0.001). In addition, miR-505 was identified as an independent prognostic factor for BCa. The results of cell experiments revealed that an overexpression of miR-505 could significantly inhibit BCa cell proliferation, migration and invasion, whereas a downregulation of miR-505 significantly enhanced BCa cell proliferation, migration and invasion (P<0.05). In summary, all data indicated that a low miR-505 expression level is associated with a poor prognosis for patients with BCa and promotes tumor cell proliferation, migration and invasion. Therefore, the aberrant expression of miR-505 may serve as a therapeutic target for BCa.
Keywords: microRNA-505, prognosis, proliferation, migration, invasion, breast cancer
Introduction
Breast cancer (BCa) is the most common type of malignancy in females worldwide and is responsible for cancer-associated mortality (1). BCa is a highly heterogeneous and malignant disease that can be classified into a number of subtypes based on the status of molecular markers, including the expression of estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) (2). BCa is typically considered a complex malignancy, which is predominantly due to its varied etiology, including certain environmental factors and genetic changes (3). Therefore, the majority of BC cases exhibit a range of different pathological entities and clinical manifestations, which can lead to misdiagnosis or delayed diagnosis (4). Despite advancements in diverse therapeutic strategies, including surgical resection, chemotherapy and radiotherapy, the overall survival rate of patients with BCa remains poor (5). Additionally, the current therapeutic strategies have been reported to possess certain adverse side effects, including poor specificity or a limited response to chemotherapy (6). Therefore, the identification of functional molecules that serve crucial roles during BCa initiation and development is urgently required to improve understanding of the underlying mechanisms of BCa and promote the development of novel treatment strategies.
MicroRNAs (miRNAs) are a group of short, non-coding RNA molecules that consist of ~23 nucleotides and serve important regulatory roles in gene expression at the post-transcriptional level (7). miRNAs can bind to the 3′-untranslated region of a target mRNA, which leads to suppression of the target mRNA or promotion of mRNA degradation (8). miRNAs have been reported to be involved in various biological processes, including cell proliferation, differentiation, invasion, migration and apoptosis, in normal and tumor cells (9–12). In recent decades, the functional roles of miRNAs in tumor progression have been identified in different types of human cancer, which has increased attention regarding the aberrant expression of miRNAs in tumor samples (13–15). miRNAs may potentially be used in cancer-targeted therapy and typically exhibit a high diagnostic and prognostic significance in patients with cancer (16,17). Therefore, there is a requirement to identify additional functional miRNAs in BCa progression, as they may be beneficial in BCa treatment. miRNA-505 (miR-505) has been reported to act as a tumor suppressor and inhibit tumor progression in certain types of human cancer, including osteosarcoma (18), cervical cancer (19), hepatoma (20) and endometrial cancer (21). Matamala et al (22) revealed that miR-505 is downregulated in breast cancer tissues. However, the clinical and functional role of miR-505 in breast cancer remains elusive.
The present study investigated the expression levels of miR-505 in BCa tissues and cells. In addition, the prognostic significance of miR-505 was evaluated for patients with BCa. Finally, the effect of miR-505 on the behaviour of BCa cells was evaluated.
Materials and methods
Patients and tissue collection
A total of 128 patients with a mean age of 58.3±12.9 years (range, 35–80 years), who were pathologically diagnosed with BCa and underwent surgical resection between July 2008 and June 2012 at Yidu Central Hospital of Weifang (Weifang, China), were included in the present study. None of the patients had previously received any antitumor therapy and the electronic medical records of all patients were complete. The BCa tissue samples and adjacent normal tissue samples (at least 5 cm from the edge of the tumor tissue) were collected from the patients during surgery and immediately frozen with liquid nitrogen for future use. The demographic and clinicopathologic data are summarized in Table I. The Tumor-Node-Metastasis (TNM) stage of the patients was determined according to the criteria published by the American Joint Committee on Cancer classification (23). Distant metastasis indicated that the tumor had spread to the whole tissue or other organs, including the lungs, brain, bone and liver. Each patient provided written informed consent and their personal information was anonymized. The experimental procedures of the present study were approved by the Ethics Committee of Yidu Central Hospital of Weifang (Weifang, China). The patients were enrolled in a 5-year follow-up survey following surgery and the survival information was obatined by telephone communication.
Table I.
Association between miR-505 expression and the clinicopathological features of patients with breast cancer.
| miR-505 expression | ||||
|---|---|---|---|---|
| Feature | Total no. (n=128) | Low (n=66) | High (n=62) | P-value |
| Age, years | 0.798 | |||
| ≤50 | 44 | 22 | 22 | |
| >50 | 84 | 44 | 40 | |
| Tumor size, cm | 0.160 | |||
| ≤2 | 62 | 28 | 34 | |
| >2 | 66 | 38 | 28 | |
| ER status | 0.927 | |||
| Negative | 80 | 41 | 39 | |
| Positive | 48 | 25 | 23 | |
| PR status | 0.412 | |||
| Negative | 81 | 44 | 37 | |
| Positive | 47 | 22 | 25 | |
| HER2 status | 0.665 | |||
| Negative | 87 | 46 | 41 | |
| Positive | 41 | 20 | 21 | |
| Distant metastasis | 0.013 | |||
| Negative | 90 | 40 | 50 | |
| Positive | 38 | 26 | 12 | |
| TNM stage | 0.002 | |||
| I–II | 60 | 22 | 38 | |
| III–IV | 68 | 44 | 24 | |
ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; miR-505, microRNA-505; TNM, Tumor-Node-Metastasis.
Cell culture and transfection
The four BCa cell lines MCF-7, BT474, T47D and MDA-MB-231, and the normal mammary epithelial cell line MCF-10A were obtained from American Type Culture Collection (Manassas, MA, USA). The cells were cultured in Dulbecco's modified Eagles medium (DMEM; Gibco; Thermo Fisher Scientific, Inc., Waltham, MA, USA) supplemented with 10% fetal bovine serum (FBS; Gibco; Thermo Fisher Scientific, Inc.) and maintained in a 5% CO2. atmosphere at 37°C.
Two BCa cell lines MCF-7 and MDA-MB-231, which had low expression of miR-505, were seeded in 24-well plates and cultured for 24 h at 37°C. Following incubation, the cells were transfected with 20 nM miR-505 mimic, miR-505 inhibitor, mimic negative control (NC) or inhibitor NC using Lipofectamine® 2000 (Invitrogen; Thermo Fisher Scientific, Inc.), according to the manufacturer's protocol. The vectors used in transfection were obtained from Shanghai GenePharma Co., Ltd. (Shanghai, China). The MiR sequences were as follows: miR-505 mimic, 5′-GGGAGCCAGGAAGUAUUGAUGU-3′; miR-505 inhibitor, 5′-ACAUCAAUACUUCCUGGCUCCC-3′; mimic NC, 5′-UUCUCCGAACGUGUCACGUTT-3′; inhibitor NC, 5′-CAGUACUUUUGUGUAGUACAA-3′. Cells were used for subsequent experimentation 48 h following transfection.
RNA extraction and reverse transcription-quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted from the tissue samples and the five cell lines using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.). A NanoDrop 2000 (Thermo Fisher Scientific, Inc.) was used to evaluate the concentration and quality of the RNA. Single-stranded complementary DNA was synthesized from 1 µg RNA using a PrimeScript RT reagent kit (Takara Bio, Inc., Otsu, Japan) and stored at −20°C.
The expression levels of miR-505 were examined using qPCR, which was performed with a SYBR-Green I Master mix kit (Invitrogen; Thermo Fisher Scientific, Inc.) using a 7300 Real-Time PCR system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The following thermocycling conditions were used for the qPCR: initial denaturation at 95°C for 10 min; 40 cycles of 95°C for 30 sec, 58°C for 30 sec, and 72°C for 20 sec. U6 was used as an internal control. The primers for qPCR were as follows: miR-505 forward, 5′-CTACGTGGGTCACCCCCTC-3′ and reverse, 5′-CCAAAGGAGACCTCGTAG-3′; and U6 forward, 3′-GCTTCGGCAGCACATATACTAAAAT-5′ and reverse, 3′-CGCTTCACGAATTTGCGTGTCAT-5′. The expression data were calculated using the 2−ΔΔCq method (24) and normalized to the U6 expression level.
Cell proliferation analysis
The MCF-7 and MDA-MB-231 cells were transfected with miR-505 mimic, miR-505 inhibitor or NCs as aforementioned. Following transfection, the stably transfected cells were seeded in 96-well plates at a cell concentration of 4×105 cells/well. Following incubation for 0, 24, 48 or 72 h, 20 µl MTT (5 g/l; Ameresco) was added to each well and the cells were then further cultured for 4 h at 37°C. The medium was then removed and 150 µl dimethyl sulfoxide was added to the wells to dissolve the formazan. Cell proliferation was evaluated by examining the absorbance at 490 nm using a microplate reader.
Cell migration and invasion analysis
The BCa cell migration and invasion abilities were assessed using a Transwell system (Corning, Inc., Corning, NY, USA) with 8-µm pore size membranes. Membranes pre-coated with Matrigel (Corning, Inc.) were used for the invasion analysis. The MCF-7 and MDA-MB-231 cells were harvested at 48 h post-transfection and seeded in the upper chambers of the Transwell system at a concentration of 4×105 cells/well. Serum-free DMEM was added into the upper chambers and the bottom chambers were filled with DMEM supplemented with 10% FBS. After 48 h, the cells in the bottom chambers were stained using 0.1% crystal violet (Sigma-Aldrich; Merck KGaA, Darmstadt, Germany) for 10 min at room temperature. Cells were subsequently counted using an inverted light microscope at a magnification of ×200. The experiments were repeated a minimum of three times.
Statistical analysis
All data are presented as the mean ± standard deviation. All statistical analysis was performed using SPSS 18.0 software (SPSS, Inc., Chicago, IL, USA) or GraphPad Prism 5.0 software (GraphPad Software, Inc., La Jolla, CA, USA). The data from two groups or multiple groups were compared using a Student's t-test or one-way analysis of variance followed by Tukey's post hoc test. The associations between miR-505 expression and clinicopathological parameters were analyzed using χ2 test. Survival analysis was performed using the Kaplan-Meier method and a log-rank test. The prognostic value of miR-505 was evaluated by Cox regression analysis. All the experiments were repeated at least three times. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression of miR-505 in BCa tissues and cells
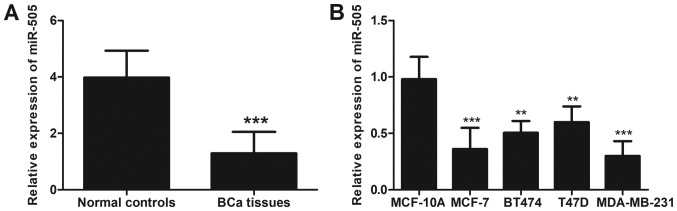
As presented in Fig. 1, RT-qPCR revealed that the expression of miR-505 was significantly downregulated in BCa tissues compared with adjacent normal tissues (P<0.001). Similarly, a significantly lower expression level of miR-505 was identfied in the four BCa cell lines examined compared with the normal mammary cell line (all P<0.01).
Figure 1.
Expression patterns of miR-505 in BCa tissues and cell lines. (A) The expression of miR-505 was downregulated in BCa tissues (n=128) compared with the normal controls (n=128). ***P<0.001 vs. normal controls. (B) The expression level of miR-505 in four BCa cell lines was lower compared with that in normal cells. **P<0.01, ***P<0.001 vs. MCF-10A. BCa, breast cancer.
Associations between miR-505 expression and the clinicopathological features of patients with BCa
Due to the dysreguled expression of miR-505 identified in BCa, we hypothesized that miR-505 may be involved in BCa development. Therefore, the associations between miR-505 expression level and the clinicopathological features of patients with BCa were assessed. The patients with BCa were divided into two groups based on the median expression value of miR-505 (1.124), which generated a low miR-505 expression group and a high miR-505 expression group. Notably, the expression of miR-505 was identifed to be associated with distant metastasis status (P=0.013) and TNM staging (P=0.002). However, no significant associations were revealed between miR-505 expression and other clinical parameters, including age, tumor size, ER status, PR status and HER2 status (all P>0.05; Table I).
Prognostic value of miR-505 in patients with BCa
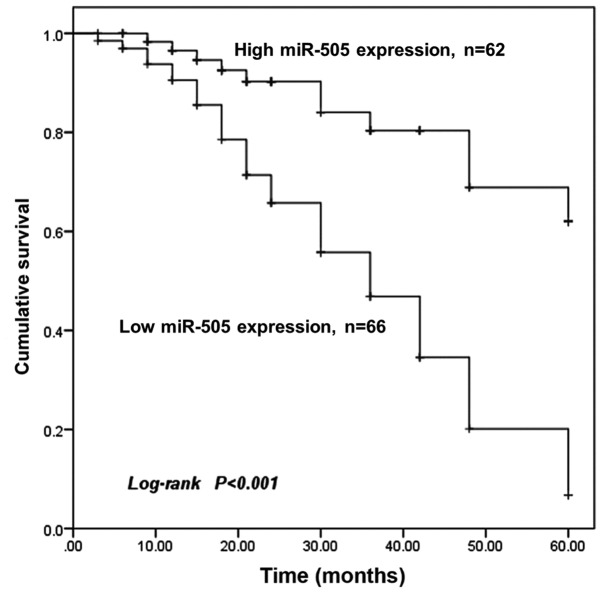
Survival analysis was performed for the patients with different miR-505 expression levels. As presented in Fig. 2, Kaplan-Meier survival curves revealed that the overall survival time was significantly shorter for patients with low miR-505 expression compared with those with high miR-505 expression (P<0.001). Furthermore, miR-505 expression and other clinicopathological parameters were included in Cox regression analysis to identify prognostic factors for the overall survival of patients with BCa. Univariate Cox analysis revealed that miR-505 expression [hazard ratio (HR), 3.972; 95% confidence interval (CI), 2.044–7.720; P=0.012], distant metastasis (HR, 1.974; 95% CI, 0.993–3.927; P=0.022) and TNM stage (HR, 1.678; 95% CI, 0.976–2.886; P=0.031) were significantly associated with the overall survival rate of patients with BCa. Furthermore, multivariate analysis identified miR-505 expression (HR, 5.707; 95% CI, 2.798–11.638; P=0.001) and TNM stage (HR, 2.602; 95% CI, 1.461–4.632; P=0.041) as independent prognostic factors for the overall survival rate of patients with BCa (Table II).
Figure 2.
Kaplan-Meier survival curves for patients with BCa. The overall survival time was significantly shorter for patients with low miR-505 expression (n=66) compared with patients with high miR-505 expression (n=62). BCa, breast cancer; miR-505, microRNA-505.
Table II.
Cox regression analysis for miR-505 expression in patients with breast cancer.
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Variable | HR | 95% CI | P-value | HR | 95% CI | P-value |
| miR-505 expression | 3.972 | 2.044–7.720 | 0.012 | 5.707 | 2.798–11.638 | 0.001 |
| Age | 1.328 | 0.721–2.446 | 0.364 | 1.032 | 0.544–1.958 | 0.923 |
| Tumor size | 1.398 | 0.807–2.420 | 0.232 | 1.269 | 0.715–2.253 | 0.416 |
| ER status | 1.069 | 0.618–1.849 | 0.811 | 1.023 | 0.566–1.895 | 0.939 |
| PR status | 1.036 | 0.587–1.829 | 0.903 | 1.182 | 0.631–2.215 | 0.601 |
| HER2 status | 1.231 | 0.702–2.159 | 0.469 | 1.232 | 0.669–2.268 | 0.503 |
| Distant metastasis | 1.974 | 0.993–3.927 | 0.022 | 1.985 | 0.965–4.086 | 0.062 |
| TNM stage | 1.678 | 0.976–2.886 | 0.031 | 2.602 | 1.461–4.632 | 0.041 |
ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; miR-505, microRNA-505; TNM, Tumor-Node-Metastasis; HR, hazard ratio; CI, confidence interval.
Effects of miR-505 on the proliferation of BCa cells
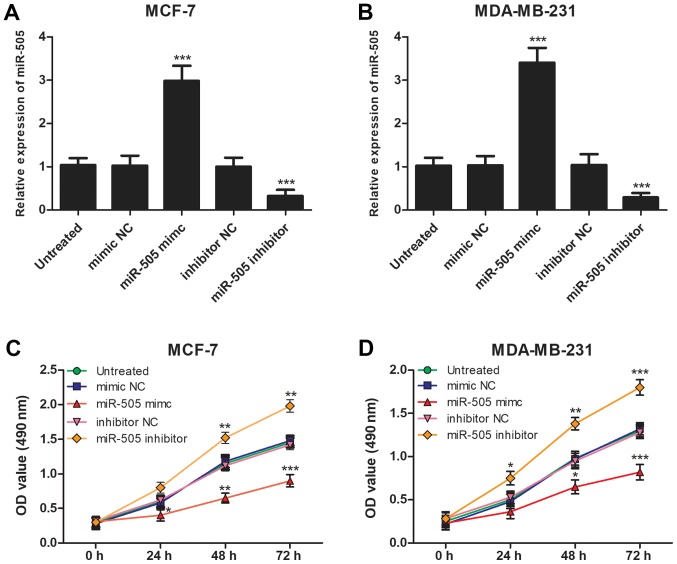
In addition to assessing the expression of miR-505 in patients with BCa patients, the functional role of miR-505 in the progression of BCa was investigated using miR-505 mimic or miR-505 inhibitor to regulate miR-505 expression in BCa cells. MCF-7 and MDA-MB-231 cells were selected for transfection experiments as they exhibited low miR-505 expression levels. RT-qPCR confirmed that the expression of miR-505 was significantly higher in cells transfected with miR-505 mimic (P<0.001) and significantly lower in cells transfected with miR-505 inhibitor compared with the untreated cells (P<0.001; Fig. 3A and B). Subsequently, MTT assay demonstrated that an overexpression of miR-505 significantly inhibited the proliferation and knockdown of miR-505 significantly increased the proliferation of MCF-7 and MDA-MB-231 cells compared with the untreated cells (all P<0.05; Fig. 3C and D).
Figure 3.
Effects of miR-505 on cell proliferation in the MCF-7 and MDA-MB-232 cell lines. The expression of miR-505 in (A) MCF-7 and (B) MDA-MB-232 cells was significantly higher following transfection with miR-505 mimic and significantly lower following transfection with miR-505 inhibitor compared with untreated cells ***P<0.001. Proliferation of (C) MCF-7 and (D) MDA-MB-231 cells was significantly inhibited following transfection with miR-505 mimic and significantly enhanced following overexpression of miR-505. All experiments were repeated three times. *P<0.05, **P<0.01, ***P<0.001 vs. untreated. miR-505, microRNA-505; NC, negative control; OD, optical density.
Effects of miR-505 on BCa cell migration and invasion
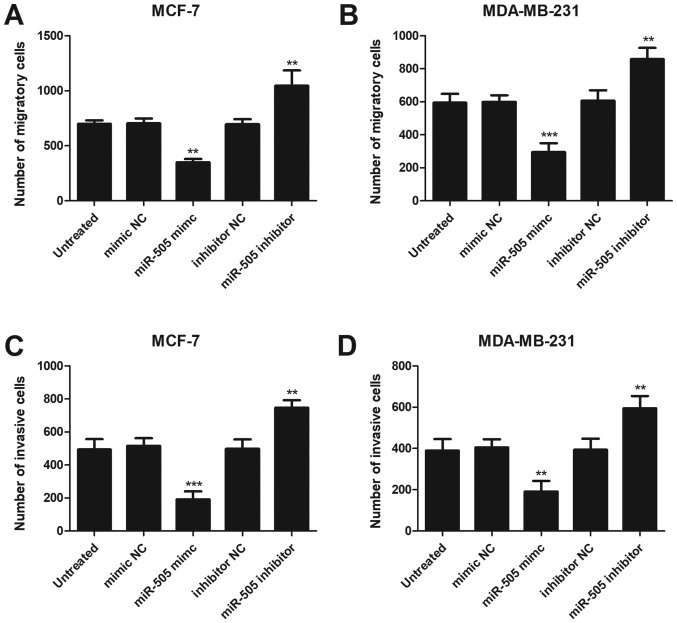
Subsequently, Transwell and Matrigel assays were performed to assess the influence of miR-505 on BCa cell migration and invasion, respectively. As presented in Fig. 4A and B, compared with the untreated cells, the migration abilities of MCF-7 and MDA-MB-231 cells were significaly suppressed following an upregulation of miR-505 (P<0.01 for MCF-7, P<0.001 for MDA-MB-231) and significantly increased following downregulation of miR-505 expression (P<0.01 for both). As expected, an overexpression of miR-505 significantly reduced the number of invasive cells (P<0.001 for MCF-7, P<0.01 for MDA-MB-231) and a downregulation of miR-505 significantly increased the number of invasive MCF-7 and MDA-MB-231 cells compared with the untreated cells (all P<0.01 for both; Fig. 4C and D).
Figure 4.
Effects of miR-505 on the migration and invasion of MCF-7 and MDA-MB-232 cells. An upregulation of miR-505 suppressed, whereas a downregulation of miR-505 promoted the migration of (A) MCF-7 and (B) MDA-MB-232 cells. Cell invasion examined by Transwell assay was inhibited by miR-505 overexpression and enhanced by miR-505 downregulation in (C) MCF-7 and (D) MDA-MB-231 cell lines. All experiments were repeated three times. **P<0.01 and ***P<0.001 vs. untreated. miR-505, microRNA-505; NC, negative control.
Discussion
BCa is one the most frequent types of malignancy among females worldwide (25). Despite the development of various therapeutic strategies, including resection operation, chemotherapy and radiotherapy, the prognosis of patients diagnosed with BCa remains poor, which is predominantly due to the limited sensitivity and specificity of these treatment methods (26,27). Therefore, this is an urgent requirement for the development of efficient treatment strategies for patients with BCa.
It has been reported that the initiation and development of BCa are complex processes that involve a wide range of genetic changes (28). Dysregulation of genes typically serves a pivotal role in the progression of BCa and exhibits significant potential for the improvement of BCa-targeted therapy (29). For example, increased expression of prolyl-4-hydroxylase α subunit 2 (P4HA2) in BCa can promote tumor cell proliferation and aggressive phenotypes, which indicates an oncogenic role of P4HA2 in tumor progression (30). Zhu et al (31) demonstrated that Sulfatase 2 (SULF2) could enhance cell proliferation, invasion, mobility and adhesion, and suppress apoptosis of BCa cells, which suggests that SULF2 may be a therapeutic target for BCa treatment. Over the past decades, a number of studies have reported important roles of miRNAs in numerous types of human cancer (32–34). In addition, miRNAs have been described as functional molecules during the progression of various types of malignancy, including BCa (35). Chai et al (36) indicated that BCa cell proliferation and cell cycle progression were promoted by miR-498, which was demonstrated to serve an oncogenic role in BCa progression by downregulating phosphatatse and tensin homolog expression. Furthermore, a downregulation of miR-202 expression has been observed in BCa tissues, and miR-202 was revealed to exert an inhibitory effect on cell proliferation, migration and invasion in BCa cells (37). These previous studies indicate that aberrant expression levels of miRNAs serve important roles in the tumor progression of BCa.
In the present study, the expression level of miR-505 was identified to be significantly lower in BCa tissues compared with adjacent normal tissues. Similarly, the expression level of miR-505 was significantly lower in BCa cell lines compared with normal cells. Furthermore, the majority of patients with low miR-505 expression exhibited distant metastasis and presented with an advanced TNM stage. Therefore, it can be suggested that miR-505 may be involved in the development of BCa. The present results were consistent with a previous study, which also identified a decreased expression of miR-505 in BCa tissues (22). Additionally, aberrant expression patterns of miR-505 have been detected in other types of human cancer. In hepatoma cells, the expression of miR-505 has been demonstrated to be downregulated and miR-505 was identified to promote cell proliferation and invasion by regulating high-mobility group box 1 (20). Similarly, downregulated miR-505 expression has been identified in endometrial cancer tissues and was involved in tumor progression, with a tumor suppressor role in this disease (21). Therefore, we hypothesize that miR-505 may be a tumor suppressor in BCa.
Given the dysregulated miR-505 expression in BCa tissues, the current study evaluated the prognostic value of miR-505 in BCa. The clinical significance of miRNAs has received increasing attention due to their high diagnostic and prognostic potential in different types of human cancer (38,39). Certain miRNAs have been identified as diagnostic or prognostic biomarkers in BCa, including miR-204 (40) and miR-301a (41). Ma et al (19) reported that downregulated miR-505 expression predicts poor prognosis in cervical cancer. However, to the best of our knowledge, the clinical significance of miR-505 has infrequently been reported in BCa. In the present study, the Kaplan-Meier method was used to plot survival curves for patients with BCa, which demonstrated that patients with low miR-505 expression exhibited a shorter overall survival time compared with those with high miR-505 expression. Additionally, univariate Cox analysis demonstrated that miR-505 expression, distant metastasis and TNM stage were associated with overall survival of patients with BCa. Multivariate Cox analysis further validated that miR-505 expression and TNM stage were independent prognostic factors for patients with BCa. Although distant metastasis is considered an aggressive behavior in BCa and may predict a poor prognosis, an independent association between distant metastasis and overall survival was not identified during multivariate analysis. This result may be due to the effects of other parameters in the equations of multivariate analysis or due to the cohort size used in the present study, which may be a limitation.
To further investigate the biological function of miR-505 in BCa progression, the effects of miR-505 on BCa cell proliferation, migration and invasion were assessed. The expression of miR-505 was regulated by cell transfection with miR-505 mimic or miR-505 inhibitor. According to MTT and Transwell assays, it was identified that upregulation of miR-505 could inhibit the proliferation, migration and invasion of BCa cells, whereas a downregulation of miR-505 could promote the proliferation, migration and invasion of BCa cells. Therefore, it can be suggested that miR-505 serves an inhibitory role in BCa progression. In endometrial cancer, miR-505 also acts as a tumor suppressor and suppresses tumor cell biological behaviors by targeting tumor growth factor-α (21). In addition, antitumor effects of miR-505 in cervical carcinoma can be achieved by downregulating the expression of Frizzled-4 (19). However, to the best of our knowledge, the molecular mechanisms underlying the role of miR-505 in BCa remain unknown and require further investigation.
In conclusion, the present results demonstrated that miR-505 expression is decreased in BCa and serves as an independent prognostic biomarker. Overexpression of miR-505 was demonstrated to suppress BCa cell proliferation, migration and invasion, which indicates miR-505 may potentially be used to improve targeted therapy for patients with BCa.
Acknowledgements
Not applicable.
Funding
No funding was received.
Availability of data and materials
The analyzed datasets generated during the study are available from the corresponding author on reasonable request.
Authors' contributions
JW and HL designed the study, performed the clinical research, analyzed the data and wrote the manuscript. ML performed the cell experiments. All authors read and approved the final manuscript.
Ethics approval and patient consent
Each patient provided written informed consent and their personal information was anonymized. The experimental procedures of the present study were approved by the Ethics Committee of Yidu Central Hospital of Weifang (Weifang, China).
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Globalcancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Brenton JD, Carey LA, Ahmed AA, Caldas C. Molecular classification and molecular forecasting of breast cancer: Ready for clinical application? J Clin Oncol. 2005;23:7350–7360. doi: 10.1200/JCO.2005.03.3845. [DOI] [PubMed] [Google Scholar]
- 3.Bombonati A, Sgroi DC. The molecular pathology of breast cancer progression. J Pathol. 2011;223:307–317. doi: 10.1002/path.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reis-Filho JS, Lakhani SR. The diagnosis and management of pre-invasive breast disease: Genetic alterations in pre-invasive lesions. Breast Cancer Res. 2003;5:313–319. doi: 10.1186/bcr650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai K, Qin F, Zhang H, Liu X, Guo C, Zhang M, Gu F, Fu L, Ma Y. Low expression of BMPRIB indicates poor prognosis of breast cancer and is insensitive to taxane-anthracycline chemotherapy. Oncotarget. 2016;7:4770–4784. doi: 10.18632/oncotarget.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schmitz KH, DiSipio T, Gordon LG, Hayes SC. Adverse breast cancer treatment effects: The economic case for making rehabilitative programs standard of care. Support Care Cancer. 2015;23:1807–1817. doi: 10.1007/s00520-014-2539-y. [DOI] [PubMed] [Google Scholar]
- 7.Turchinovich A, Weiz L, Burwinkel B. Extracellular miRNAs: The mystery of their origin and function. Trends Biochem Sci. 2012;37:460–465. doi: 10.1016/j.tibs.2012.08.003. [DOI] [PubMed] [Google Scholar]
- 8.Pillai RS, Bhattacharyya SN, Artus CG, Zoller T, Cougot N, Basyuk E, Bertrand E, Filipowicz W. Inhibition of translational initiation by Let-7 MicroRNA in human cells. Science. 2005;309:1573–1576. doi: 10.1126/science.1115079. [DOI] [PubMed] [Google Scholar]
- 9.Xiang J, Wu Y, Li DS, Wang ZY, Shen Q, Sun TQ, Guan Q, Wang YJ. MiR-584 suppresses invasion and cell migration of thyroid carcinoma by regulating the target oncogene ROCK1. Oncol Res Treat. 2015;38:436–440. doi: 10.1159/000438967. [DOI] [PubMed] [Google Scholar]
- 10.Lu Y, Hu J, Sun W, Li S, Deng S, Li M. MiR-29c inhibits cell growth, invasion, and migration of pancreatic cancer by targeting ITGB1. Onco Targets Ther. 2016;9:99–109. doi: 10.2147/OTT.S92758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bai J, Zhang Z, Li X, Liu H. MicroRNA-365 inhibits growth, invasion and metastasis of malignant melanoma by targeting NRP1 expression. Cancer Biomark. 2015;15:599–608. doi: 10.3233/CBM-150500. [DOI] [PubMed] [Google Scholar]
- 12.Zhou C, Lu Y, Li X. MiR-339-3p inhibits proliferation and metastasis of colorectal cancer. Oncol Lett. 2015;10:2842–2848. doi: 10.3892/ol.2015.3661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dan B, Luo J, Li K, Chen S. Prognostic value of miR-375 for survival outcomes in various cancers: A systematic review and meta-analysis. Oncol Res Treat. 2018;41:47–50. doi: 10.1159/000481708. [DOI] [PubMed] [Google Scholar]
- 14.Lin F, Yao L, Xiao J, Liu D, Ni Z. MiR-206 functions as a tumor suppressor and directly targets K-Ras in human oral squamous cell carcinoma. Onco Targets Ther. 2014;7:1583–1591. doi: 10.2147/OTT.S67624. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Zheng K, Liu W, Liu Y, Jiang C, Qian Q. MicroRNA-133a suppresses colorectal cancer cell invasion by targeting Fascin1. Oncol Lett. 2015;9:869–874. doi: 10.3892/ol.2014.2753. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 16.Yang H, Yu J, Wang L, Ding D, Zhang L, Chu C, Chen Q, Xu Z, Zou Q, Liu X. miR-320a is an independent prognostic biomarker for invasive breast cancer. Oncol Lett. 2014;8:1043–1050. doi: 10.3892/ol.2014.2298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Deng Y, Chen Y. Increased expression of miR-29a and its prognostic significance in patients with cholangiocarcinoma. Oncol Res Treat. 2017;40:128–132. doi: 10.1159/000455869. [DOI] [PubMed] [Google Scholar]
- 18.Liu YJ, Li W, Chang F, Liu JN, Lin JX, Chen DX. MicroRNA-505 is downregulated in human osteosarcoma and regulates cell proliferation, migration and invasion. Oncol Rep. 2018;39:491–500. doi: 10.3892/or.2017.6142. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 19.Ma C, Xu B, Husaiyin S, Wang L, Wusainahong K, Ma J, Zhu K, Niyazi M. MicroRNA-505 predicts prognosis and acts as tumor inhibitor in cervical carcinoma with inverse association with FZD4. Biomed Pharmacother. 2017;92:586–594. doi: 10.1016/j.biopha.2017.04.028. [DOI] [PubMed] [Google Scholar]
- 20.Lu L, Qiu C, Li D, Bai G, Liang J, Yang Q. MicroRNA-505 suppresses proliferation and invasion in hepatoma cells by directly targeting high-mobility group box 1. Life Sci. 2016;157:12–18. doi: 10.1016/j.lfs.2016.05.039. [DOI] [PubMed] [Google Scholar]
- 21.Chen S, Sun KX, Liu BL, Zong ZH, Zhao Y. MicroRNA-505 functions as a tumor suppressor in endometrial cancer by targeting TGF-alpha. Mol Cancer. 2016;15:11. doi: 10.1186/s12943-016-0496-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matamala N, Vargas MT, Gonzalez-Campora R, Minambres R, Arias JI, Menendez P, Andres-Leon E, Gomez-Lopez G, Yanowsky K, Calvete-Candenas J, et al. Tumor microRNA expression profiling identifies circulating microRNAs for early breast cancer detection. Clin Chem. 2015;61:1098–1106. doi: 10.1373/clinchem.2015.238691. [DOI] [PubMed] [Google Scholar]
- 23.Singletary SE, Allred C, Ashley P, Bassett LW, Berry D, Bland KI, Borgen PI, Clark GM, Edge SB, Hayes DF, et al. Staging system for breast cancer: Revisions for the 6th edition of the AJCC Cancer Staging Manual. Surg Clin North Am. 2003;83:803–819. doi: 10.1016/S0039-6109(03)00034-3. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Akram M, Iqbal M, Daniyal M, Khan AU. Awareness and current knowledge of breast cancer. Biol Res. 2017;50:33. doi: 10.1186/s40659-017-0140-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi M, Guo N. MicroRNA expression and its implications for the diagnosis and therapeutic strategies of breast cancer. Cancer Treat Rev. 2009;35:328–334. doi: 10.1016/j.ctrv.2008.12.002. [DOI] [PubMed] [Google Scholar]
- 27.Blank PR, Schwenkglenks M, Moch H, Szucs TD. Human epidermal growth factor receptor 2 expression in early breast cancer patients: A swiss cost-effectiveness analysis of different predictive assay strategies. Breast Cancer Res Treat. 2010;124:497–507. doi: 10.1007/s10549-010-0862-7. [DOI] [PubMed] [Google Scholar]
- 28.Byler S, Goldgar S, Heerboth S, Leary M, Housman G, Moulton K, Sarkar S. Genetic and epigenetic aspects of breast cancer progression and therapy. Anticancer Res. 2014;34:1071–1077. [PubMed] [Google Scholar]
- 29.Lei F, Zhang L, Li X, Lin X, Wu S, Li F, Liu J. Overexpression of prostate tumor overexpressed 1 correlates with tumor progression and predicts poor prognosis in breast cancer. BMC Cancer. 2014;14:457. doi: 10.1186/1471-2407-14-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xiong G, Deng L, Zhu J, Rychahou PG, Xu R. Prolyl-4-hydroxylase alpha subunit 2 promotes breast cancer progression and metastasis by regulating collagen deposition. BMC Cancer. 2014;14:1. doi: 10.1186/1471-2407-14-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhu C, He L, Zhou X, Nie X, Gu Y. Sulfatase 2 promotes breast cancer progression through regulating some tumor-related factors. Oncol Rep. 2016;35:1318–1328. doi: 10.3892/or.2015.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dragomir M, Mafra ACP, Dias SMG, Vasilescu C, Calin GA. Using microRNA networks to understand cancer. Int J Mol Sci. 2018;19:E1871. doi: 10.3390/ijms19071871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang LL, Huang LW, Wang L, Tong BD, Wei Q, Ding XS. Potential role of miR-139-5p in cancer diagnosis, prognosis and therapy. Oncol Lett. 2017;14:1215–1222. doi: 10.3892/ol.2017.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Long JP, Dong LF, Chen FF, Fan YF. miR-146a-5p targets interleukin-1 receptor-associated kinase 1 to inhibit the growth, migration, and invasion of breast cancer cells. Oncol Lett. 2019;17:1573–1580. doi: 10.3892/ol.2018.9769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sun EH, Zhou Q, Liu KS, Wei W, Wang CM, Liu XF, Lu C, Ma DY. Screening miRNAs related to different subtypes of breast cancer with miRNAs microarray. Eur Rev Med Pharmacol Sci. 2014;18:2783–2788. [PubMed] [Google Scholar]
- 36.Chai C, Wu H, Wang B, Eisenstat DD, Leng RP. MicroRNA-498 promotes proliferation and migration by targeting the tumor suppressor PTEN in breast cancer cells. Carcinogenesis. 2018;39:1185–1196. doi: 10.1093/carcin/bgy092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao S, Cao C, Dai Q, Chen J, Tu J. miR-202 acts as a potential tumor suppressor in breast cancer. Oncol Lett. 2018;16:1155–1162. doi: 10.3892/ol.2018.8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu X, Lu J. The significance of detection of serum miR-423-5p and miR-484 for diagnosis of colorectal cancer. Clin Lab. 2015;61:187–190. doi: 10.7754/Clin.Lab.2014.140625. [DOI] [PubMed] [Google Scholar]
- 39.Cong J, Liu R, Wang X, Wang J, Wang H, Hou J. Low miR-498 expression levels are associated with poor prognosis in ovarian cancer. Eur Rev Med Pharmacol Sci. 2015;19:4762–4765. [PubMed] [Google Scholar]
- 40.Li W, Jin X, Zhang Q, Zhang G, Deng X, Ma L. Decreased expression of miR-204 is associated with poor prognosis in patients with breast cancer. Int J Clin Exp Pathol. 2014;7:3287–3292. [PMC free article] [PubMed] [Google Scholar]
- 41.Yu H, Li H, Qian H, Jiao X, Zhu X, Jiang X, Dai G, Huang J. Upregulation of miR-301a correlates with poor prognosis in triple-negative breast cancer. Med Oncol. 2014;31:283. doi: 10.1007/s12032-014-0283-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The analyzed datasets generated during the study are available from the corresponding author on reasonable request.