Abstract
Cancer cells are characterized by a low antigenic immunogenicity, a rapid growth and an immunosuppressive effect on the extracellular matrix. These properties induce a weak immune response in colorectal cancer (CRC) carcinogenesis. It is therefore crucial to determine the composition of the inflammatory mass, including neutrophils, macrophages and eosinophils in the tumor tissue of patients with CRC, and to analyze other clinicopathological parameters. The present study included 144 patients diagnosed with CRC. Tissue samples obtained from routine histopathological diagnosis were stained with hematoxylin and eosin. Inflammatory cells were assessed in the invasive front and in the center of the tumor by light microscopy under a high-power magnification. The percentage of neutrophils in the invasive front was significantly higher compared with that in the center of the tumor mass (P<0.01). Macrophages and eosinophils were present in the invasive front and in the center of tumor mass in most cases. The presence of neutrophils, macrophages and eosinophils was correlated with various clinicopathological features. Patients with macrophages present in the center of tumor mass had longer disease-free survival time (P=0.041). In conclusion, the present study demonstrated that the inflammatory cell infiltrate served a significant role in the immune response of patients with CRC. It should be noted that the presence of macrophages localized in the stroma of the central part of the primary tumor mass was associated with the survival time of patients with CRC.
Keywords: neutrophils, macrophages, eosinophils, colorectal cancer, inflammatory cells, deposits, prognostic significance
Introduction
Defense mechanisms are effective only in the early stages of cancer development. Throughout tumor growth, cancer cells acquire various properties that allow them to reduce the body defense mechanisms (1). Cancer cells display low antigenic immunogenicity and rapid growth (2). Furthermore, cancer cells exhibit immunosuppressive activity on the extracellular matrix. These properties weaken the immune response during colorectal carcinogenesis (2). In the field of tumor microenvironment research, the role of macrophages, also known as tumor-associated macrophages (TAM), is of particular interest. It has been hypothesized that the macrophages population in the tumor may not be homogeneous, and that certain macrophages may undergo abnormal stimulation. These macrophages may transform from antigen-presenting cells that stimulate the immune system to fight cancer, into cells that secrete factors that increase cancer cell proliferation and stimulate tumor angiogenesis (3). Subsequently, neutrophils serve a fundamental role in the antimicrobial defense system of the body. Neutrophils comprise granules that contain large amounts of free radicals and serine proteases, including peroxidase and lysozyme (4). It has been reported that minor inflammatory activity is maintained in patients with colorectal adenomas, which confirms the influence of inflammation on intestinal cell proliferation (5). In such cases, neutrophils undergoing apoptosis permanently accumulate in the mucous membrane; they are therefore not cleared by macrophages and release their intracellular granules, which can cause tissue damage and loss of control over their regeneration (6). Neutrophil granulocytes and macrophages secrete tumor growth factors, including vascular endothelial growth factor, hepatocyte growth factor, interleukin (IL)-6, IL-8, matrix metalloproteinases and elastase, which can contribute to the stimulation of the tumor microenvironment (7,8). In addition, it has been demonstrated that the presence of eosinophils in the tumor mass may be a prognostic factor in patients with colorectal cancer (CRC) (9). It is therefore crucial to determine the cellular composition of the inflammatory mass, including neutrophils, macrophages and eosinophils in the tissues of patients with CRC, and to evaluate their association with other clinicopathological parameters and disease-free survival (DFS).
Materials and methods
Patients
The present study included 144 patients diagnosed with CRC (88 men and 56 women) who underwent surgery in the Department of Oncological Surgery, Comprehensive Cancer Center (Białystok, Poland) between April 2014 and December 2016. The mean age of the patients was 67.5 years, including 34 patients <60 years-old and 110 patients >60 years-old. The majority of patients presented similar symptoms, including abdominal pain, anemia, rectal bleeding, constipation, diarrhea, vomiting and anorexia. In the majority of cases, patients additionally received treatment for hypertension, type II diabetes, osteoarthritis and coronary heart disease. However, none of the patients had received inflammatory therapy.
All patients underwent routine diagnostic tests, including basic diagnostic laboratory tests (morphological tests and lipid profiles), electrocardiography, spirometry, arterial blood gasometric test, X-ray and computerized chest tomography. The clinical stage of CRC was evaluated according to the Tumor-Node-Metastasis (TNM) classification (10) and the Dukes staging systems (11). Prior to surgery, patients with tumors identified in other sites received no inflammatory or immunosuppressive therapy. The response to preoperative therapy was estimated according to the Response Evaluation Criteria in Solid Tumors (12).
The present study was performed in accordance with the Declaration of Helsinki for Human Experimentation, and the protocol was approved by The Bioethics Committee of the Medical University of Bialystok (approval no. R-I-002/351/2016). Written informed consent was obtained from all participants.
Histopathological examination of CRC tumor tissue
Tissues obtained from surgery were fixed in 4% buffered formalin for 24 to 72 h at room temperature. Small section of tissue were embedded in paraffin. Sections (4 µm-thick) were cut from paraffin blocks and stained with hematoxylin and eosin (H&E) at room temperature for 4 min (cat. no. 468802128; POCH S.A.; Avantor Performance Materials Poland, Gliwice, Poland) according to the manufacturer's protocol. The slides were deparaffinized in an oven at 60°C for 5 min. Subsequently, slides were rehydrated in xylene (three washes, 10 min each) and graded ethanol (100, 95, 85 and 75%, 1 min at each concentration). Routine histopathological assessment was of the sections was performed by two pathologists blinded to the clinical information. The type of tumor growth, tumor size, histological type, percentage of mucinous components, grade of malignancy, TNM and Dukes stages was determined by the pathologists. Venous, lymphatic and perineural invasion of cancer cells were also analyzed. Characteristic features of lymph node invasion were examined, including the number of resected and invaded lymph nodes, the presence of micro- and macro-metastases, invasion of the pouch lymph node, presence of distant metastases and the size of metastases. The presence, number and size of the deposits of cancer cells were also assessed (13).
The tumor stroma ratio was then determined, as previously described by Huijbers et al (14). The analysis was performed in the most invasive tumor area of each slide by using ×25 or ×50 magnification. The tumor and stromal tissues were identified and subsequently viewed at ×100 magnification. According to above criteria, tumor cells were present at all edges of the image field whereas necrotic areas and mucinous components were excluded. Tumor budding was analyzed as previously described by Morodomi et al (15). The extent of the inflammatory cell reaction in the invasive front of the tumor and the center of the tumor mass was also observed and classified according to the Klintrup-Makinen criteria (16). Inflammatory reaction in the invasive margin and center of the tumor were scored on a 4-point scale: 0, No increase in inflammatory cell infiltrate; 1, a mild or patchy increase; 2, a prominent inflammatory reaction with some evidence of cancer cell destruction; and 3, florid ‘cup-like’ inflammatory infiltrate. The extent of necrosis and fibrosis in the central tumor was evaluated according to Richards et al (17) and graded as follows: Absent, none; focal, <10% of tumor area; moderate, 10–30%; or extensive, >30%. Crohn's-like aggregates of lymphocytes (CLR) were evaluated based on the Väyrynen criteria (18). CLR was defined as lymphoid structures surrounding the primary tumors, excluding mucosa-associated lymphoid tissue or pre-existing lymph nodes. Histological categorization of fibrotic cancer stroma was performed based on the criteria from Ueno et al (19). Fibrotic cancer stroma was classified as follows: Mature, consist of mature collagen fibers and elongated fibers with fibrocytes stratified into multiple layers; intermediate, broad bands of collagen with brightly eosinophilic hyalinization and keloid-like structures; and immature, consist of randomly orientated keloid-like collagen bundles surrounded by myxoid stroma.
Morphological examination of inflammatory cells at the invasive front and in the center of CRC tumor tissue
Tissue samples obtained from routine histopathological diagnosis were stained with H&E, and used to assess inflammatory cells in the invasive front and center of the tumor with light microscopy under a high-power magnification (×400; Leica DM6 B, KAWA.SKA, Sp. z o.o., Piaseczno, Poland). Analysis was performed by two independent pathologists blinded to patients' clinical information. Morphologically, neutrophils are polymorphonuclear cells with segmented nuclei that present clumped chromatin, eosinophilic cytoplasm and pink granules. Cells were counted and quantified as a percentage of all examined cells. Neutrophils were divided into three groups: 1, 2 and 3, whether they corresponded to 0, <20 or >21% of all cells examined. Macrophages are mononuclear cells that contain numerous lysosomal acid phosphatases in their cytoplasm. Eosinophils are double-segmented nuclear cells with acidophilic cytoplasm that contains large Babès-Ernst granules (20). In the present study, macrophages and eosinophils were evaluated as absent (lack of cells) or present (>5 cells observed in the examined field).
Statistical analysis
Statistical analysis was performed using the STATISTICA 10.0 program (Statsoft, Cracow, Poland). A Mann-Whitney U-test was used to compare the groups. Correlations between the parameters were calculated using the Spearman's correlation coefficient test. P<0.05 was considered to indicate a statistically significant difference. DFS time was calculated as the duration between the date of the diagnosis and the date of disease progression, including local or distant relapse. The DFS rate was estimated using the Kaplan Meier estimator method and the survival curves were compared using log-rank tests.
Results
Neutrophils, macrophages and eosinophils in CRC tissue
Neutrophils in the invasive front of the CRC tumor were absent in 48 cases (group 1), present in 77 cases (group 2) and a further 19 cases (group 3; Table I). In addition, neutrophils in the center of the tumor mass were observed in 16 cases (group 2) and 40 cases (group 3; Table I). The percentage of neutrophils in the invasive front were significantly higher compared with that in the center of the tumor mass (P<0.01). Macrophages and eosinophils were present in the invasive front and in the center of the tumor mass in 65–72% cases (Table II).
Table I.
Evaluation of the neutrophils in the stroma at the front and tumor center of colorectal cancer.
| Invasive front (%) | Centre of main tumor mass (%) | ||||||
|---|---|---|---|---|---|---|---|
| Type of cell | P-value | ||||||
| Group (percentage of neutrophils) | 1 (0) | 2 (<20) | 3 (>21) | 1 (0) | 2 (<20) | 3 (>21) | |
| Number of cases | 48 (33.3) | 77 (53.4) | 19 (13.3) | 88 (61.1) | 16 (11.1) | 40 (27.8) | <0.010 |
Table II.
Evaluation of the macrophages and eosinophils in the stroma at the front and tumor center of colorectal cancer.
| Invasive front | Center of main tumor mass | ||||
|---|---|---|---|---|---|
| Type of cell | Absent | Present | Absent | Present | P-value |
| Macrophages | 48 (33.3) | 96 (66.7) | 51 (35.4) | 93 (64.6) | 0.711 |
| Eosinophils | 40 (27.8) | 104 (72.2) | 45 (31.2) | 99 (68.8) | 0.998 |
Correlation analysis between each type of inflammatory cell
According to the observations, neutrophils in the invasive front of the CRC tumor positively correlated with macrophages in the center of the tumor mass (R=0.239, P=0.004). Furthermore, macrophages present in the invasive front were negatively correlated with neutrophils, and positively correlated with eosinophils in the deeper layer of the tumor mass (R=−0.170, P=0.041; and R=0.318, P<0.010, respectively). Positive correlations between eosinophils from the invasive front, and macrophages and eosinophils in the center of the tumor mass were observed (R=0.315, P<0.010; and R=0.353, P<0.010, respectively). However, the presence of eosinophils was negatively correlated with neutrophils in the main mass of CRC tumor (R=−0.207, P=0.013). The results are presented in Table III.
Table III.
Correlations of the cellular composition of inflammatory infiltration in the stroma at the front and tumor center of colorectal cancer.
| Neutrophils | Macrophages | Eosinophils | ||||
|---|---|---|---|---|---|---|
| Centre of main mass Invasive front | R | P-value | R | P-value | R | P-value |
| Neutrophils | −0.094 | 0.262 | 0.239 | 0.004 | 0.206 | 0.013 |
| Macrophages | −0.170 | 0.041 | 0.030 | 0.308 | 0.318 | <0.010 |
| Eosinophils | −0.207 | 0.013 | 0.315 | <0.010 | 0.353 | <0.010 |
Correlation between presence of inflammatory cells and clinicopathological features of patients with CRC
Macrophages localized in the center of the tumor mass negatively correlated with histological type of adenocarcinoma and the percentage of mucinous component (R=−0.171, P=0.040; R=−0.221, P<0.010, respectively; Table IV). Macrophages were also associated with tumor stroma percentage (R=0.220, P=0.011; Table IV). Patients with macrophages in the center of the tumor mass were associated with venous invasion, presence of deposits, and larger deposit size (R=−0.432; P<0.010; R=−0.158, P<0.010; R=−0.203, P=0.015, respectively; Table V).
Table IV.
Correlation between a particular population of inflammatory infiltrate located in the center of the tumor and anatomic parameters in patients with colorectal cancer.
| Variables | N | Neutrophils | Macrophages | Eosinophils |
|---|---|---|---|---|
| Age (years) | ||||
| <60 | 30 | NS | NS | NS |
| ≥60 | 114 | |||
| Sex | ||||
| Female | 56 | NS | NS | NS |
| Male | 88 | |||
| Localization | ||||
| Right-side | 18 | NS | NS | −0.0186 |
| Transverse | 13 | 0.031 | ||
| Left-side | 12 | |||
| Sigmoid | 25 | |||
| Rectum | 76 | |||
| Tumor size (cm) | ||||
| <2.5 | 23 | NS | NS | NS |
| 2.5–5.0 | 99 | |||
| >5.0 | 22 | |||
| TNM stage | ||||
| 1 | 36 | NS | NS | NS |
| 2 | 27 | |||
| 3 | 69 | |||
| 4 | 12 | |||
| Duke stage | ||||
| A | 31 | NS | NS | NS |
| B | 33 | |||
| C | 69 | |||
| D | 11 | |||
| Adenocarcinoma type | ||||
| Partim mucinous | 19 | NS | −0.221 | NS |
| Adenocarcinoma | 125 | <0.010 | ||
| Malignancy grade | ||||
| 2 | 135 | NS | NS | NS |
| 3 | 9 | |||
| pT stage | ||||
| 1 | 3 | NS | NS | NS |
| 2 | 52 | |||
| 3 | 86 | |||
| 4 | 3 | |||
| TSP (%) | ||||
| <50 | 73 | NS | 0.220 | NS |
| >50 | 71 | 0.011 |
NS, non significant.
Table V.
Correlation between a particular population of inflammatory infiltrates located in the center of the tumor and disease progression parameters in patients with colorectal cancer.
| Variables | N | Neutrophils | Macrophages | Eosinophils |
|---|---|---|---|---|
| Venous invasion | ||||
| Absent | 123 | −0.432 | ||
| Present | 21 | NS | <0.010 | NS |
| Lymphatic invasion | ||||
| Absent | 118 | NS | NS | NS |
| Present | 26 | |||
| Perineural invasion | ||||
| Absent | 134 | NS | NS | NS |
| Present | 10 | |||
| Lymph node metastasis | ||||
| Absent | 71 | NS | NS | NS |
| Present | 73 | |||
| Distant metastasis | ||||
| Absent | 129 | NS | NS | NS |
| Present | 15 | |||
| Tumor deposits | ||||
| Absent | 122 | 0.354 | −0.220 | −0.158 |
| Present | 22 | 0.000 | 0.008 | 0.000 |
| Size of tumor deposits (mm) | ||||
| <2.5 | 12 | 0.170 | −0.203 | NS |
| ≥2.5 | 10 | 0.041 | 0.015 | |
| K-M grade | ||||
| Low | 76 | NS | NS | NS |
| High | 68 | |||
| Tumor budding | ||||
| Absence | 86 | NS | NS | NS |
| Presence | 58 | |||
| Crohn's-like aggregates of lymphocyte | ||||
| Absence | 112 | NS | NS | 0.236 |
| Presence | 32 | <0.010 | ||
| Necrosis | ||||
| Absent | 42 | NS | NS | NS |
| Focal | 51 | |||
| Moderate | 34 | |||
| Extensive | 17 | |||
| Fibrosis | ||||
| Absent | 9 | NS | NS | NS |
| Focal | 67 | |||
| Moderate | 38 | |||
| Extensive | 30 | |||
| Maturation of fibrotic stroma | ||||
| Immature | 10 | NS | NS | NS |
| Intermediate | 84 | |||
| Mature | 50 |
NS, not significant.
Neutrophils were positively correlated with the presence of deposits of cancer cells deposits (R=0.354, P<0.010; Table V). In addition, neutrophils were positively correlated with deposits of >2.5 mm in size (R=0.170, P=0.041, Table V).
The presence of eosinophils in the center of tumor mass was negatively correlated with tumor localization and deposits presence (R=−0.186, P=0.031, Table V; and R=−0.220, P=0.008, Table IV, respectively), but positively with Crohn's-like aggregates of lymphocytes (R=0.236, P<0.010).
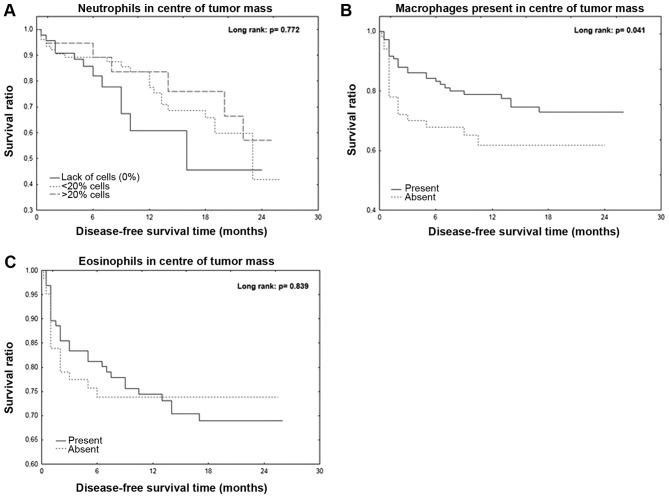
Correlation between DFS time and inflammatory cells in the center of the tumor mass
The mean DFS time was 12.1 months for all patients. Patients with macrophages present in the center of the tumor mass exhibited a significantly longer DFS time (P=0.041; Fig. 1A). However, the Kaplan-Meier curve analyses of neutrophils and eosinophils in the center of tumor mass were not statistically significant (Fig. 1B and C).
Figure 1.
Disease-free survival time is negatively associated with the presence of inflammatory cells in the tumor. (A) Disease-free survival in relation to the percentage of neutrophils present in the tumor mass. Disease-free survival in relation to the presence of (B) macrophages or (C) eosinophils in the tumor mass.
Discussion
Antigens located on cancer cells determine the reactivity and quality of the immune response. Immune system cells migrate to the location of a foreign entity and initiate the inflammatory response. Neutrophil granulocytes represent the first line of immunological defense, and activate or inhibit subsequent populations of immunocompetent cells (21). These mechanisms confirm the interdependencies between certain types of inflammatory cells, including macrophages, neutrophils and eosinophils. The present study demonstrated that neutrophils were mainly present in the invasive front of the primary tumor (60% of cases) compared with in the center of the tumor (30% of patients). Rao et al (22) reported that intratumoral neutrophil infiltration was observed in 45.4% of patients diagnosed with CRC. However, Arelaki et al (23) observed that neutrophils gradually move from the tumor mass to the invasive front. The present study also reported that neutrophils were located in the center of the primary tumor of patients with CRC was positively correlated with the presence of tumor deposits and the size of these deposits. Furthermore, the results from this study were consistent with the observations from Rao et al (22) who reported that the increase in the number of intratumoral neutrophils is associated with determinants of disease progression, including TNM. However, Galdiero et al (24) indicated that the density of tumor-associated neutrophils dramatically decreased in patients with stage IV CRC compared with patients with stages I–III CRC. Neutrophils are the first inflammatory infiltration cells that can activate further inflammatory reaction pathways, such as lymphocyte activation. However, a long-lasting infiltration of numerous neutrophils, particularly in the center of the tumor, causes the degradation of the extracellular matrix, leading to the secretion of metalloproteinases into the extracellular matrix, resulting in its degradation and enabling the progression of cancer development (25). The presence and number of neutrophils in the primary tumor in patients with colorectal cancer may depend on the ability of tumor cells to evade the immune response (26).
Eosinophil granulocytes are involved in the following step of the inflammatory reaction cascade, originally in the allergic reaction, but also in the neoplastic changes (9). The present study demonstrated that eosinophils were present in the invasive tumor front and in the center of the tumor in ~70% of patients with CRC. A previous study by Kiziltaş et al (27) confirmed the presence of eosinophil infiltration in ~75% of patients with CRC. In addition, Cho et al (28) observed a decrease in the number of eosinophils in the tumor tissue of patients with CRC. Furthermore, a previous study reported that a high percentage of eosinophils is present in CRC tissue (29). The present study also demonstrated that the percentage of eosinophils in the center of the tumor mass was negatively correlated with the presence of cancer cell deposits. Prizment et al (9) reported that a high eosinophil score in the tumor is negatively correlated with age and tumor stage. In addition, Harbaum et al (30) demonstrated that increased peri- and intratumoral eosinophil counts are significantly associated with T and N classification, tumor differentiation and vascular invasion. The presence of eosinophils in the tumor center in patients with CRC may therefore influence the activation of the immune system due to their association with other determinants of disease progression.
TAMs are macrophages originating from the circulating monocytes subpopulation, which settle into the tumor tissue stroma (31). TAMs divide into one of two subpopulations, M1 and M2. M2 TAMs possess the ability to secrete various types of growth factors that stimulate tumor growth and neoangiogenesis (32). In addition, TAMs produce numerous proteolytic enzymes, including metalloproteinases and cathepsins, which are involved in extracellular matrix degradation that subsequently conditions the tumor cell aggressiveness and viability (33,34). However, some anti-cancer properties of M1 TAMs have been demonstrated in previous studies (35,36). The presence of both CD68-positive TAMs and VEGF were associated with better prognosis in colon carcinoma (36). Due to their pro- and anti-tumor, the present study investigated the occurrence of TAMs in the tumor tissue of patients with CRC. The results demonstrated that such macrophages were present in 65–72% of all cases, in the invasive front and in center of the tumor. In addition, the present study revealed that presence of macrophages was accompanied by tumors primarily containing nonmucinous component. Colon tumors containing mucus-producing cells typically have poor connective tissue stroma. Compared with tumors with glandular cancer spurs, Nonmucinous tumors are widely distributed in a rich stroma. In this case, inflammatory cells have the possibility to accumulate and freely migrate (37). The results from the present study confirmed that the presence of macrophages in the tumor center was associated with the tumor stroma percentage. In addition, the results revealed that macrophages were present in tumors that contained relatively more stroma compared with cancerous tissue. A previous study reported that TAMs present increased expression of tumor growth factor β and IL-6, which may activate tumor angiogenesis (38). However, Funada et al (39) reported that low levels of macrophage infiltration at the invasive margin were associated with a higher rate of vascular invasion and lymph node metastasis. In addition, Zhou et al (40) demonstrated that a low TAMs density at the invasive front is associated with a higher occurrence of hepatic metastasis. According to Koelzer et al (41), strong infiltration of TAM type M2 predicts a lower tumor grade and reduces lymph node metastasis. Ammendola et al (42) confirmed that macrophages presence is correlated with angiogenesis in patients with locally advanced CRC. Gulubova et al (43) reported that a low number of CD68-positive cells, including macrophages, is associated with disease progression parameters, including the presence of metastases in local lymph nodes, distant metastases, advanced tumor stage, tumor cell invasion of blood and lymph vessels or perineural invasion. This was consistent with the results from the present study, which demonstrated that the presence of macrophages was negatively correlated with the tumor disease progression factors, including blood vessel involvement and the presence and size of cancer cell deposits. The present study confirmed the crucial role of macrophages in the immune response from the tumors of patients with CRC. However, the exact subtype of macrophages involved requires further immunophenotypic assessment.
The cellular composition of inflammatory infiltrate may represent a potential prognostic marker for patients with CRC. The present study demonstrated that the presence of macrophages in the center of the primary tumor was associated with a significantly longer DFS time. The beneficial effect of the presence of macrophage infiltration on the DFS of patients with CRC was also confirmed by Funada et al (39), Zhou et al (40), Gulubova et al (43) and Kinouchi et al (44). The present study investigated the association between neutrophils and eosinophils located in the tumor center with DFS. No significant differences were observed, conversely with previous studies that indicated a beneficial effect of neutrophils and eosinophils in the tumor center on CRC patients DFS (9,44–46).
In conclusion, the results from the present study confirmed the importance of assessing the cellular composition of the inflammatory mass in the primary tumor of patients with CRC. Notably, macrophages located in the stroma of the tumor center were associated with a longer DFS in patients.
Acknowledgements
Not applicable.
Funding
The author(s) received funding support from Medical University of Bialystok for this work.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
KJ is responsible for the conception and design the study, data collection, statistical analysis and writing the paper. MK and LKK performed the histological examination. WK and WF collected the data and reviewed the article. All authors read and approved the final manuscript.
Ethics approval and consent to participate
The present study was performed in accordance with the Declaration of Helsinki for Human Experimentation, and the protocol was approved by The Bioethics Committee of the Medical University of Bialystok (approval no. R-I-002/351/2016). Written informed consent was obtained from all participants.
Patients consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Testa U, Pelosi E, Castelli G. Colorectal cancer: Genetic abnormalities, tumor progression, tumor heterogeneity, clonal evolution and tumor-initiating cells. Med Sci (Basel) 2018;6:E31. doi: 10.3390/medsci6020031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merlano MC, Granetto C, Fea E, Ricci V, Garrone O. Heterogeneity of colon cancer: From bench to bedside. ESMO Open. 2017;2:e000218. doi: 10.1136/esmoopen-2017-000218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eljaszewicz A, Gackowska L, Kubiszewska I, Jankowski M, Urbańska M, Wiese M, Helmin-Basa A, Michałkiewicz J, Zegarski W. Aktywność makrofagów w rozwoju choroby nowotworowej. Współczesna Onkologia. 2010;1:1–6. doi: 10.5114/wo.2010.971. (In Poland) [DOI] [Google Scholar]
- 4.Hong-Fang T, Yan-Hui P, Lei B, Wan-Xing Z. Effects of granulocyte colony-stimulating factor on opsonin receptor expression and neutrophil antibacterial activity in a mouse model of severe acute pancreatitis. Postepy Hig Med Dosw (Online) 2017;71:352–358. doi: 10.5604/01.3001.0010.3819. [DOI] [PubMed] [Google Scholar]
- 5.Dubois RN. Role of inflammation and inflammatory mediators in colorectal cancer. Trans Am Clin Climatol Assoc. 2014;125:358–372. discussion 372–373. [PMC free article] [PubMed] [Google Scholar]
- 6.Dunn GP, Old LJ, Schreiber RD. The immunobiology of cancer immunosurveillance and immunoediting. Immunity. 2004;21:137–148. doi: 10.1016/j.immuni.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 7.McCourt M, Wang JH, Sookhai S, Redmond HP. Activated human neutrophils release hepatocyte growth factor/scatter factor. Eur J Surg Oncol. 2001;27:396–403. doi: 10.1053/ejso.2001.1133. [DOI] [PubMed] [Google Scholar]
- 8.Shamamian P, Schwartz JD, Pocock BJ, Monea S, Whiting D, Marcus SG, Mignatti P. Activation of progelatinase A (MMP-2) by neutrophil elastase, cathepsin G, and proteinase-3: A role for inflammatory cells in tumor invasion and angiogenesis. J Cell Physiol. 2001;189:197–206. doi: 10.1002/jcp.10014. [DOI] [PubMed] [Google Scholar]
- 9.Prizment AE, Vierkant RA, Smyrk TC, Tillmans LS, Lee JJ, Sriramarao P, Nelson HH, Lynch CF, Thibodeau SN, Church TR, et al. Tumor eosinophil infiltration and improved survival of colorectal cancer patients: Iowa women's health study. Mod Pathol. 2016;29:516–527. doi: 10.1038/modpathol.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NCCN Clinical Practice Guidelines in Oncology, corp-author. www.nccn.org.asp. Colon Cancer. doi: 10.6004/jnccn.2009.0056. [DOI] [PubMed] [Google Scholar]
- 11.Kyriakos M. The President cancer, the Dukes classification, and confusion. Arch Pathol Lab Med. 1985;109:1063–1066. [PubMed] [Google Scholar]
- 12.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European organization for research and treatment of cancer, national cancer institute of the United States, national cancer institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 13.Lin Q, Wei Y, Ren L, Zhong Y, Qin C, Zheng P, Xu P, Zhu D, Ji M, Xu J. Tumor deposit is a poor prognostic indicator in patients who underwent simultaneous resection for synchronous colorectal liver metastases. Onco Targets Ther. 2015;8:233–240. doi: 10.2147/OTT.S71414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huijbers A, Tollenaar RA, v Pelt GW, Zeestraten EC, Dutton S, McConkey CC, Domingo E, Smit VT, Midgley R, Warren BF, et al. The proportion of tumor-stroma as a strong prognosticator for stage II and III colon cancer patients: Validation in the VICTOR trial. Ann Oncol. 2013;24:179–185. doi: 10.1093/annonc/mds246. [DOI] [PubMed] [Google Scholar]
- 15.Morodomi T, Isomoto H, Shirouzu K, Kakegawa K, Irie K, Morimatsu M. An index for estimating the probability of lymph node metastasis in rectal cancers. Lymph node metastasis and the histopathology of actively invasive regions of cancer. Cancer. 1989;63:539–543. doi: 10.1002/1097-0142(19890201)63:3<539::AID-CNCR2820630323>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 16.Klintrup K, Mäkinen JM, Kauppila S, Väre PO, Melkko J, Tuominen H, Tuppurainen K, Mäkelä J, Karttunen TJ, Mäkinen MJ. Inflammation and prognosis in colorectal cancer. Eur J Cancer. 2005;41:2645–2654. doi: 10.1016/j.ejca.2005.07.017. [DOI] [PubMed] [Google Scholar]
- 17.Richards CH, Flegg KM, Roxburgh CS, Going JJ, Mohammed Z, Horgan PG, McMillan DC. The relationships between cellular components of the peritumoural inflammatory response, clinicopathological characteristics and survival in patients with primary operable colorectal cancer. Br J Cancer. 2012;106:2010–2015. doi: 10.1038/bjc.2012.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Väyrynen JP, Sajanti SA, Klintrup K, Mäkelä J, Herzig KH, Karttunen TJ, Tuomisto A, Mäkinen MJ. Characteristics and significance of colorectal cancer associated lymphoid reaction. Int J Cancer. 2014;134:2126–2135. doi: 10.1002/ijc.28533. [DOI] [PubMed] [Google Scholar]
- 19.Ueno H, Jones AM, Wilkinson KH, Jass JR, Talbot IC. Histological categorization of fibrotic cancer stroma in advanced rectal cancer. Gut. 2004;53:581–586. doi: 10.1136/gut.2003.028365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lichtman MA, Kipps TJ, Seligsohn U, Kaushansky K, Prchal JT. 8th. McGraw-Hill Companies; 2010. Williams Hematology; pp. 875–999. [Google Scholar]
- 21.Nowak B. Patomechanizm rozwoju reakcji zapalnej. Przegl Reumatol. 2005 R.1 nr 4; s.5. [Google Scholar]
- 22.Rao HL, Chen JW, Li M, Xiao YB, Fu J, Zeng YX, Cai MY, Xie D. Increased intratumoral neutrophil in colorectal carcinomas correlates closely with malignant phenotype and predicts patients' adverse prognosis. PLoS One. 2012;7:e30806. doi: 10.1371/journal.pone.0030806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arelaki S, Arampatzioglou A, Kambas K, Papagoras C, Miltiades P, Angelidou I, Mitsios A, Kotsianidis I, Skendros P, Sivridis E, et al. Gradient infiltration of neutrophil extracellular traps in colon cancer and evidence for their involvement in tumour growth. PLoS One. 2016;11:e0154484. doi: 10.1371/journal.pone.0154484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galdiero MR, Bianchi P, Grizzi F, Di Caro G, Basso G, Ponzetta A, Bonavita E, Barbagallo M, Tartari S, Polentarutti N, et al. Occurrence and significance of tumor-associated neutrophils in patients with colorectal cancer. Int J Cancer. 2016;139:446–456. doi: 10.1002/ijc.30076. [DOI] [PubMed] [Google Scholar]
- 25.Mizuno R, Kawada K, Itatani Y, Ogawa R, Kiyasu Y, Sakai Y. The role of tumor-associated neutrophils in colorectal cancer. Int J Mol Sci. 2019;20:E529. doi: 10.3390/ijms20030529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pagès F, Galon J. Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol. 2011;29:610–618. doi: 10.1200/JCO.2010.30.5425. [DOI] [PubMed] [Google Scholar]
- 27.Kiziltaş S, Sezgin Ramadan S, Topuzoğlu A, Küllü S. Does the severity of tissue eosinophilia of colonic neoplasms reflect their malignancy potential? Turk J Gastroenterol. 2008;19:239–244. [PubMed] [Google Scholar]
- 28.Cho H, Lim SJ, Won KY, Bae GE, Kim GY, Min JW, Noh BJ. Eosinophils in colorectal neoplasms associated with expression of CCL11 and CCL24. J Pathol Transl Med. 2016;50:45–51. doi: 10.4132/jptm.2015.10.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moezzi J, Gopalswamy N, Haas RJ, Jr, Markert RJ, Suryaprasad S, Bhutani MS. Stromal eosinophilia in colonic epithelial neoplasms. Am J Gastroenterol. 2000;95:520–523. doi: 10.1111/j.1572-0241.2000.01778.x. [DOI] [PubMed] [Google Scholar]
- 30.Harbaum L, Pollheimer MJ, Kornprat P, Lindtner RA, Bokemeyer C, Langner C. Peritumoral eosinophils predict recurrence in colorectal cancer. Mod Pathol. 2015;28:403–413. doi: 10.1038/modpathol.2014.104. [DOI] [PubMed] [Google Scholar]
- 31.Mantovani A, Bottazzi B, Colotta F, Sozzani S, Ruco L. The origin and function of tumor-associated macrophages. Immunol Today. 1992;13:265–270. doi: 10.1016/0167-5699(92)90008-U. [DOI] [PubMed] [Google Scholar]
- 32.Leek RD, Lewis CE, Whitehouse R, Greenall M, Clarke J, Harris AL. Association of macrophage infiltration with angiogenesis and prognosis in invasive breast carcinoma. Cancer Res. 1996;56:4625–4629. [PubMed] [Google Scholar]
- 33.Mantovani A. Tumor-associated macrophages in neoplastic progression: A paradigm for the in vivo function of chemokines. Lab Invest. 1994;71:5–16. [PubMed] [Google Scholar]
- 34.Afik R, Zigmond E, Vugman M, Klepfish M, Shimshoni E, Pasmanik-Chor M, Shenoy A, Bassat E, Halpern Z, Geiger T, et al. Tumor macrophages are pivotal constructors of tumor collagenous matrix. J Exp Med. 2016;213:2315–2331. doi: 10.1084/jem.20151193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ohtani H, Naito Y, Saito K, Nagura H. Expression of costimulatory molecules B7-1 and B7-2 by macrophages along invasive margin of colon cancer: A possible antitumor immunity? Lab Invest. 1997;77:231–241. [PubMed] [Google Scholar]
- 36.Khorana AA, Ryan CK, Cox C, Eberly S, Sahasrabudhe DM. Vascular endothelial growth factor, CD68, and epidermal growth factor receptor expression and survival in patients with Stage II and Stage III colon carcinoma: A role for the host response in prognosis. Cancer. 2003;97:960–968. doi: 10.1002/cncr.11152. [DOI] [PubMed] [Google Scholar]
- 37.Hamilton SR, Bosman FT, Boffetta P, et al. Carcinoma of the colon and rectum In: WHO classification of tumours of the digestive system. In: Bosman FT, Carneiro F, Hruban RH, Theise ND, editors. 4th. IARC; Lyon: 2010. pp. 134–146. [Google Scholar]
- 38.Popovic ZV, Sandhoff R, Sijmonsma TP, Kaden S, Jennemann R, Kiss E, Tone E, Autschbach F, Platt N, Malle E, Gröne HJ. Sulfated glycosphingolipid as mediator of phagocytosis: SM4s enhances apoptotic cell clearance and modulates macrophage activity. J Immunol. 2007;179:6770–6782. doi: 10.4049/jimmunol.179.10.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Funada Y, Noguchi T, Kikuchi R, Takeno S, Uchida Y, Gabbert HE. Prognostic significance of CD8+ T cell and macrophage peritumoral infiltration in colorectal cancer. Oncol Rep. 2003;10:309–313. [PubMed] [Google Scholar]
- 40.Zhou Q, Peng RQ, Wu XJ, Xia Q, Hou JH, Ding Y, Zhou QM, Zhang X, Pang ZZ, Wan DS, et al. The density of macrophages in the invasive front is inversely correlated to liver metastasis in colon cancer. J Transl Med. 2010;8:13. doi: 10.1186/1479-5876-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koelzer VH, Canonica K, Dawson H, Sokol L, Karamitopoulou-Diamantis E, Lugli A, Zlobec I. Phenotyping of tumor-associated macrophages in colorectal cancer: Impact on single cell invasion (tumor budding) and clinicopathological outcome. Oncoimmunology. 2015;5:e1106677. doi: 10.1080/2162402X.2015.1106677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ammendola M, Patruno R, Sacco R, Marech I, Sammarco G, Zuccalà V, Luposella M, Zizzo N, Gadaleta C, Porcelli M, et al. Mast cells positive to tryptase and tumour-associated macrophages correlate with angiogenesis in locally advanced colorectal cancer patients undergone to surgery. Expert Opin Ther Targets. 2016;20:533–540. doi: 10.1517/14728222.2016.1158811. [DOI] [PubMed] [Google Scholar]
- 43.Gulubova M, Ananiev J, Yovchev Y, Julianov A, Karashmalakov A, Vlaykova T. The density of macrophages in colorectal cancer is inversely correlated to TGF-β1 expression and patients' survival. J Mol Histol. 2013;44:679–692. doi: 10.1007/s10735-013-9520-9. [DOI] [PubMed] [Google Scholar]
- 44.Kinouchi M, Miura K, Mizoi T, Ishida K, Fujibuchi W, Sasaki H, Ohnuma S, Saito K, Katayose Y, Naitoh T, et al. Infiltration of CD40-positive tumor-associated macrophages indicates a favorable prognosis in colorectal cancer patients. Hepatogastroenterology. 2013;60:83–88. doi: 10.5754/hge12372. [DOI] [PubMed] [Google Scholar]
- 45.Fernández-Aceñero MJ, Galindo-Gallego M, Sanz J, Aljama A. Prognostic influence of tumor-associated eosinophilic infiltrate in colorectal carcinoma. Cancer. 2000;88:1544–1548. doi: 10.1002/(SICI)1097-0142(20000401)88:7<1544::AID-CNCR7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 46.Wikberg ML, Ling A, Li X, Öberg Å, Edin S, Palmqvist R. Neutrophil infiltration is a favorable prognostic factor in early stages of colon cancer. Hum Pathol. 2017;68:193–202. doi: 10.1016/j.humpath.2017.08.028. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.