Abstract
Background
Migraine is a common, disabling condition and a burden for the individual, health services, and society. Many sufferers choose not to, or are unable to, seek professional help and rely on over‐the‐counter analgesics. Naproxen is a non‐steroidal anti‐inflammatory drug (NSAID); its efficacy in acute migraine has not been established by systematic reviews. Co‐therapy with an antiemetic should help to reduce the nausea and vomiting commonly associated with migraine headaches.
Objectives
To determine the efficacy and tolerability of naproxen, alone or in combination with an antiemetic, compared with placebo and other active interventions in the treatment of acute migraine headaches in adults.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) on The Cochrane Library, MEDLINE, EMBASE, and the Oxford Pain Relief Database, together with two online databases (www.gsk‐clinicalstudyregister.com and www.clinicaltrials.gov) and reference lists, for studies to 22 May 2013.
Selection criteria
We included randomised, double‐blind, placebo‐ or active‐controlled studies, with at least 10 participants per treatment arm, using naproxen alone or with an antiemetic to treat a migraine headache episode.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data. We used numbers of participants achieving each outcome to calculate risk ratios and numbers needed to treat (NNT) or harm (NNH) compared with placebo or a different active treatment.
Main results
We included six studies using naproxen 275 mg, 500 mg, or 825 mg to treat attacks of moderate or severe pain intensity. Overall, 1241 participants took naproxen (275 mg to 825 mg), 229 took sumatriptan 50 mg, 173 took naratriptan 2.5 mg, and 1092 took placebo. No studies combined naproxen with an antiemetic. Studies using naproxen 275 mg provided no useable data for analysis.
Naproxen (500 mg and 825 mg) was better than placebo for pain‐free response and headache relief. At two hours, the NNT for pain‐free response was 11 (95% CI 8.7 to 17) (17% response with naproxen, 8% with placebo; risk ratio 2.0 (1.6 to 2.6), moderate quality) and for headache relief was 6.0 (4.8 to 7.9) (45% response with naproxen, 29% with placebo; risk ratio 1.6 (1.4 to 1.8), moderate quality). The NNT for sustained pain‐free response during the 24 hours post dose was 19 (13 to 34) (12% response with naproxen, 6.7% with placebo), and for sustained headache relief during the 24 hours post dose was 8.3 (6.4 to 12) (30% response with naproxen, 18% with placebo). Analysing only the lower dose of 500 mg of naproxen did not significantly change the results. Adverse events, which were mostly mild or moderate in severity and rarely led to withdrawal, were more common with naproxen than with placebo when the 500 mg and 825 mg doses were considered together, but not when the 500 mg dose was analysed alone.
There were insufficient data for analysis of naproxen compared with sumatriptan, and no data suitable for analysis of naproxen compared with naratriptan.
Authors' conclusions
Naproxen is statistically superior to placebo in the treatment of acute migraine, but the NNT of 11 for pain‐free response at two hours suggests that it is not a clinically useful treatment. Cochrane reviews examining other commonly used analgesics for acute migraine have reported better (lower) NNT results for the same outcome. Naproxen is not clinically useful as a stand‐alone analgesic in acute migraine, as it is effective in fewer than 2 people in 10.
Plain language summary
Naproxen for acute migraine in adults
Migraine is a complex condition with a wide variety of symptoms. For many people the main feature is a painful headache. Other symptoms include feeling sick, vomiting, disturbed vision, and sensitivity to light, sound, and smells.
Non‐steroidal anti‐inflammatory drugs (NSAIDs) are used to treat migraine headaches. One NSAID is naproxen. On 22 May 2013, we looked for clinical trials where naproxen was used to treat migraine headache. We found six good quality studies with about 2700 people.
Naproxen was more effective than placebo for relieving migraine headache in adults, but only weakly so. From having headache pain described as moderate or severe, about 2 in 10 people (17%) were pain‐free at two hours when treated with naproxen. However, about 1 in 10 (8%) were pain‐free at two hours when treated with placebo. Almost 5 in 10 had some headache relief with naproxen, and 3 in 10 with placebo. Naproxen is not as good as some other medicines such as ibuprofen or sumatriptan. More dizziness, tingling sensations (paraesthesia), sleepiness (somnolence), nausea, indigestion (dyspepsia), dry mouth, and abdominal discomfort were reported with the 825 mg dose. These effects were generally of mild to moderate severity and rarely led to withdrawal from the studies.
Naproxen is not a good drug for treating migraine at the doses of 500 mg or 825 mg used in the studies we found.
Summary of findings
Summary of findings 1. Naproxen 500 mg or 825 mg compared with placebo for migraine headache.
| Naproxen 500 mg or 825 mg compared with placebo for migraine headache | ||||||
|
Patient or population: migraine headache ‐ moderate or severe pain Settings: community Intervention: naproxen 500 mg or 825 mg Comparison: placebo | ||||||
| Outcomes | Probable outcome with comparator | Probable outcome with intervention | NNT or NNH and/or relative effect (95% CI) | No of studies, attacks, events | Quality of the evidence (GRADE) | Comments |
| Pain‐free response at 2 h | 80 in 1000 | 170 in 1000 | NNT 11 (8.7 to 17) | 4 studies, 2149 attacks, 275 events | Moderate1 | Lower NNTs are better than higher NNTs |
| Headache relief at 2 h | 290 in 1000 | 450 in 1000 | NNT 6.0 (4.8 to 7.9) | 4 studies, 2149 attacks, 793 events | Moderate1 | Lower NNTs are better than higher NNTs |
| Sustained pain‐free during the 24 h post dose | 70 in 1000 | 120 in 1000 | NNT 19 (13 to 34) | 4 studies, 2149 attacks, 202 events | Moderate1 | Lower NNTs are better than higher NNTs |
| Sustained headache relief during the 24 h post dose | 180 in 1000 | 300 in 1000 | NNT 8.3 (6.4 to 12) | 4 studies, 2149 attacks, 505 events | Moderate1 | Lower NNTs are better than higher NNTs |
| At least one AE | 120 in 1000 | 150 in 1000 | NNH 28 (15 to 130) | 4 studies, 2174 attacks, 293 events | Low2 | Higher NNHs are better than lower NNTs |
| Serious AE | Insufficient data | ‐ | ||||
|
AE: adverse event; CI: confidence interval; NNT: number needed to treat; NNH: number needed to harm. Note: NNT or NNH is reported when an outcome is statistically different from placebo or comparator. Where the result is not statistically different, a risk ratio or similar outcome is reported. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 ‐ Quality of evidence downgraded from high because of threat from potential publication bias with modest effect size and numbers of events.
2 ‐ Quality of evidence downgraded from high because of threat from potential publication bias with modest effect size and numbers of events, combined with inconsistent reporting of the outcome.
Background
Description of the condition
Migraine is a common, disabling headache disorder, ranked seventh highest among specific causes of disability globally (Steiner 2013), and with considerable social and economic impact (Hazard 2009). Rcent reviews found a one‐year prevalence of 15% globally (Vos 2012) and for adults in European countries (Stovner 2010), 13% for all ages in the USA (Victor 2010), 21% in Russia (Ayzenberg 2012), and 9% for adults in China (Yu 2012). Migraine is more prevalent in women than in men (by a factor of two to three), and in the age range 30 to 50 years.
The International Headache Society (IHS) classifies two major subtypes (IHS 2013). Migraine without aura is the most common subtype. It is characterised by attacks lasting 4 to 72 hours that are typically of moderate to severe pain intensity (PI), unilateral, pulsating, aggravated by normal physical activity, and associated with nausea with or without photophobia and phonophobia. Migraine with aura is characterised by reversible focal neurological symptoms that develop over a period of at least 5 minutes and last for less than 60 minutes, followed by headache with the features of migraine without aura. In some cases, the headache may lack migrainous features or be absent altogether (IHS 2013).
A large prevalence study in the USA found that over half of migraineurs had severe impairment or required bed rest during attacks. Despite this high level of disability and a strong desire for successful treatment, only a proportion of migraine sufferers seek professional advice for the treatment of attacks. The majority were not taking any preventive medication, although one‐third met guideline criteria for offering or considering it. Nearly all migraineurs (98%) used acute treatments for attacks, with 49% using over‐the‐counter (OTC) medication only, 20% using prescription medication, and 29% using both. OTC medication included aspirin, other non‐steroidal anti‐inflammatory drugs (NSAIDs), paracetamol (acetaminophen), and paracetamol with caffeine (Bigal 2008; Diamond 2007; Lipton 2007). Similar findings have been reported from other large studies in France and Germany (Lucas 2006; Radtke 2009).
The significant impact of migraine with regard to pain, functional health, and well‐being is well documented (Buse 2011; Leonardi 2005); it is ranked in the top 10 disorders for global years lived with disability (Vos 2012). A cross‐sectional survey of eight EU countries (representing 55% of the adult population) has estimated an annual direct and indirect cost of migraine per person of EUR 1222, and a total annual cost for the EU of EUR 111 billion for adults aged 18 to 65 years (Linde 2012). Costs vary between countries, probably due to differences in available therapies and the way they are delivered, and structural differences in healthcare systems (Bloudek 2012). In the USA, the mean annual direct cost per person has been estimated at USD 1757 for episodic migraine and USD 7750 for chronic migraine (Munakata 2009). Whatever the exact direct and indirect costs are for each country, it is clear that migraine presents a significant economic burden. Successful treatment of acute migraine attacks not only benefits patients by reducing their disability and improving health‐related quality of life, but also has the potential to reduce the need for healthcare resources and increase economic productivity.
Description of the intervention
Naproxen is an NSAID first marketed in the mid‐1970s, with confirmed efficacy in acute (Derry 2009), and chronic (Moore 2010a; Moore 2010b) pain. It is a propionic acid derivative (of the same family as ibuprofen), with analgesic, anti‐inflammatory, and antipyretic properties. It has been widely used in treating arthritis, menstrual cramps, gout, sprains and strains, and a variety of acute pain conditions. Naproxen and its soluble sodium salt are commonly available as 250 mg and 500 mg tablets (275 mg and 550 mg of sodium salt). In many parts of the world it remains a prescription‐only drug, but in others such as the USA, UK, and most parts of Canada, it is available OTC in restricted doses.
OTC medications are less expensive, more accessible, and tend to have favourable safety profiles relative to many prescription treatments, although naproxen causes more serious gastrointestinal adverse events than ibuprofen, for example (Hernandez‐Diaz 2000). Naproxen may be a useful alternative OTC treatment of migraine headache in individuals who do not tolerate or respond to commonly used aspirin and ibuprofen.
In order to establish whether naproxen is an effective analgesic at a specified dose in acute migraine attacks, it is necessary to study its effects in circumstances that permit detection of pain relief (PR). Such studies are carried out in individuals with established pain of moderate to severe intensity, using single doses of the interventions. Participants who experience an inadequate response with either placebo or active treatment are permitted to use rescue medication, and the intervention is considered to have failed in those individuals. In clinical practice, however, individuals would not normally wait until pain is of at least moderate severity, and may take a second dose of medication if the first dose does not provide adequate relief. Once analgesic efficacy is established in studies using single doses in established pain, further studies may investigate different treatment strategies and patient preferences. These are likely to include treating the migraine headache early while pain is mild, and using a low dose initially, with a second dose if response is inadequate.
How the intervention might work
NSAIDs act by inhibiting the activity of cyclooxygenase (COX), now recognised to consist of two isoforms (COX‐1 and COX‐2), which catalyses the production of prostaglandins, which are responsible for pain and inflammation. Naproxen inhibits both COX isoforms. Prostaglandins mediate a variety of physiological functions, including maintenance of the gastric mucosal barrier, regulation of renal blood flow, and regulation of endothelial tone, and they also play an important role in inflammatory and nociceptive processes. Naproxen is thought to inhibit central sensitisation by attenuating meningeal inflammation and preventing central sensitisation arising from glial cells in the brain stem. In one study, naproxen suppressed central trigeminal neurons in an animal model of intracranial pain (Jakubowski 2007).
The efficacy of oral medications is reduced in many migraineurs because of impaired gastrointestinal motility, which is associated with nausea, and because of non‐absorption of the drug due to vomiting (Volans 1974). The addition of an antiemetic may improve outcomes by alleviating the often incapacitating symptoms of nausea and vomiting, and (at least potentially) by enhancing the bioavailability of the co‐administered analgesic. In particular, prokinetic antiemetics such as metoclopramide, which stimulate gastric emptying, may improve outcomes by increasing absorption of the analgesic. This has been investigated for metoclopramide and aspirin (Ross‐Lee 1983; Volans 1975). It has been claimed that treatment with intravenous metoclopramide alone can reduce pain in severe migraine attacks (Friedman 2005; Salazar‐Tortolero 2008), but this claim requires further investigation, since metoclopramide has not been shown to be an analgesic in classical pain studies. The present review seeks to determine whether treatment of acute migraine with naproxen plus an antiemetic is in any way superior to treatment with naproxen alone. In a recent review of aspirin with or without an antiemetic for acute migraine (Kirthi 2013), aspirin plus metoclopramide was significantly better than aspirin alone for headache relief and relief of nausea at two hours, but not for pain‐free at two hours or sustained pain‐free during the 24 hours post dose.
Why it is important to do this review
Naproxen has confirmed efficacy in a variety of acute pain situations, is widely available and relatively inexpensive, and it is important to know where it fits in the range of therapeutic options for migraine therapy. For many migraineurs, non‐prescription therapies offer convenience and may be the only therapies available or affordable. This review is one of a series examining the efficacy of OTC treatments for migraine, including aspirin (Kirthi 2013), paracetamol (acetaminophen) (Derry 2013), and ibuprofen (Rabbie 2013), as well as oral sumatriptan (Derry 2012), which is available without prescription in some countries. Naproxen in combination with sumatriptan is the subject of a separate review (Law 2013).
Objectives
To determine the efficacy and tolerability of naproxen, alone or in combination with an antiemetic, compared with placebo and other active interventions in the treatment of acute migraine headaches in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised, double‐blind, placebo‐ or active‐controlled studies using naproxen to treat a migraine headache episode. Studies had to have a minimum of 10 participants per treatment arm and measure at least one of the outcomes specified below. We accepted studies reporting treatment of consecutive headache episodes if outcomes for the first, or each, episode were reported separately; first‐attack data were used preferentially. We accepted cross‐over studies if there was adequate washout (≥ 24 hours) between treatments.
Types of participants
Studies enrolled adults (at least 18 years of age) with episodic migraine. We used the definition of migraine specified by the IHS (IHS 1988; IHS 2004; IHS 2013), although we accepted diagnostic criteria equivalent to IHS 1988 where a specific reference was not provided. We excluded studies evaluating treatments for chronic migraine. There were no other restrictions on migraine frequency, duration, or type (with or without aura). We accepted studies that included participants taking stable prophylactic therapy to reduce the frequency of migraine attacks. If reported, details on any prophylactic therapy prescribed or allowed are provided in the Characteristics of included studies table.
Types of interventions
We included studies that used a single dose of naproxen to treat a migraine headache episode when pain was of moderate to severe intensity, or investigated different dosing strategies or timing of the first dose in relation to headache intensity, or both. There were no restrictions on dose or route of administration.
Included studies could use either naproxen alone or naproxen plus an antiemetic. The antiemetic had to be taken either combined with naproxen in a single formulation or separately not more than 30 minutes before naproxen, and be self administered or possible to self administer (ie given by a nurse in clinic, but could be taken by the participant at home).
A placebo comparator is essential to demonstrate that naproxen is effective in this condition. We considered active‐controlled trials without a placebo as secondary evidence. We excluded studies designed to demonstrate prophylactic efficacy in reducing the number or frequency of migraine headaches.
Types of outcome measures
In selecting the main outcome measures for this review, we considered scientific rigour, availability of data, and patient preferences. Patients with acute migraine headaches have rated complete PR, no headache recurrence, rapid onset of PR, and no side effects as the four most important outcomes (Lipton 1999).
In view of these patient preferences, and in line with the guidelines for controlled trials of drugs in migraine issued by the IHS (IHS 2000), we considered the following main outcomes.
Primary outcomes
Pain‐free at two hours, without the use of rescue medication.
Reduction in headache pain ('headache relief') at two hours (pain reduced from moderate or severe to none or mild without the use of rescue medication).
We would have collected data for pain‐free and headache relief outcomes at one hour if reported and relevant, for example, if a fast‐acting formulation of the intervention was tested.
Secondary outcomes
Sustained pain‐free during the 24 hours post dose (pain‐free within two hours, with no use of rescue medication or recurrence of pain of any intensity within 24 hours).
Sustained headache relief during the 24 hours post dose (headache relief at two hours, with no use of rescue medication or a second dose of study medication, or recurrence of moderate or severe pain within 24 hours).
Adverse events: participants with any adverse event during the 24 hours post dose; serious adverse events; adverse events leading to withdrawal.
Other outcomes
We also collected data for a number of other outcomes, including:
use of rescue medication;
relief of headache‐associated symptoms;
relief of functional disability.
PI or PR had to be measured by the participant (not the investigator or care giver). Pain measures accepted for the main efficacy outcomes were:
PI: 4‐point categorical scale, with wording equivalent to none, mild, moderate, and severe; or 100 mm visual analogue scale (VAS)), where less than 30 mm was considered equivalent to mild or no pain and 30 mm or greater equivalent to moderate or severe pain (Collins 1997);
PR: 5‐point categorical scale, with wording equivalent to none, a little, some, a lot, complete; or 100 mm VAS, where less than 30 mm was considered equivalent to none or a little, and 30 mm or greater equivalent to some, a lot, or complete.
We considered only data obtained directly from the participant.
Definitions of important terms, including all measured outcomes, are provided in Appendix 1.
Search methods for identification of studies
Electronic searches
We searched the following electronic databases.
The Cochrane Central Register of Controlled Trials (CENTRAL),(The Cochrane Library), Issue 4 of 12, 2013.
MEDLINE (via Ovid),1947 to 22 May 2013.
EMBASE (via Ovid), 1974 to 22 May 2013.
Oxford Pain Relief Database, searched on 22 May 2013 (Jadad 1996a).
See Appendix 2, Appendix 3, and Appendix 4 for the search strategies for CENTRAL, MEDLINE (via Ovid), and EMBASE (via Ovid), respectively.
We applied no language restrictions.
Searching other resources
We searched for additional studies in reference lists of retrieved studies and review articles, and in two clinical trials databases (www.clinicaltrials.gov and www.gsk-clinicalstudyregister.com). We did not search grey literature and short abstracts.
Data collection and analysis
Selection of studies
Two review authors independently carried out the searches and selected studies for inclusion. We viewed the titles and abstracts of all studies identified by electronic searches on screen and excluded any that clearly did not satisfy inclusion criteria. We read full copies of the remaining studies to identify those suitable for inclusion. We settled disagreements by discussion with a third review author.
Data extraction and management
Two review authors independently extracted data from included studies using a standard data extraction form. We settled disagreements by discussion with a third review author. One review author entered data into Review Manager 5 (RevMan 2012).
Assessment of risk of bias in included studies
We used the Oxford Quality Score (Jadad 1996b) as the basis for inclusion, limiting inclusion to studies that were randomised and double‐blind as a minimum. The scores for each study are reported in the Characteristics of included studies table.
Two review authors independently assessed risk of bias for each study, using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and adapted from those used by the Cochrane Pregnancy and Childbirth Group, with any disagreements resolved by discussion. We assessed the following for each study.
Random sequence generation (checking for possible selection bias). We assessed the method used to generate the allocation sequence as: low risk of bias (any truly random process, eg random number table; computer random number generator); unclear risk of bias (method used to generate sequence not clearly stated). We excluded studies using a non‐random process (eg odd or even date of birth; hospital or clinic record number).
Allocation concealment (checking for possible selection bias). The method used to conceal allocation to interventions prior to assignment determines whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment. We assessed the methods as: low risk of bias (eg telephone or central randomisation; consecutively numbered sealed opaque envelopes); unclear risk of bias (method not clearly stated). We excluded studies that did not conceal allocation (eg open list).
Blinding of outcome assessment (checking for possible detection bias). We assessed the methods used to blind study participants and outcome assessors from knowledge of which intervention a participant received. We assessed the methods as: low risk of bias (study states that it was blinded and describes the method used to achieve blinding, eg identical tablets; matched in appearance and smell); unclear risk of bias (study states that it was blinded but does not provide an adequate description of how it was achieved). We excluded studies that were not double‐blind.
Incomplete outcome data (checking for possible attrition bias due to the amount, nature, and handling of incomplete outcome data). We assessed the methods used to deal with incomplete data as: low risk (< 10% of participants provided no data without acceptable reason, eg they were randomised but did not have a qualifying headache). We excluded studies with high data loss.
Size of study (checking for possible biases confounded by small size). We assessed studies as being at low risk of bias (≥ 200 participants per treatment arm); unclear risk of bias (50 to 199 participants per treatment arm); high risk of bias (< 50 participants per treatment arm).
Measures of treatment effect
We used risk ratio (RR) to establish statistical difference. We used numbers needed to treat (NNT) and pooled percentages as absolute measures of benefit or harm.
We used the following terms to describe adverse outcomes in terms of harm or prevention of harm:
when significantly fewer adverse outcomes occurred with naproxen than with control (placebo or active) we use the term the number needed to treat to prevent one event (NNTp).
when significantly more adverse outcomes occurred with naproxen compared with control (placebo or active) we use the term the number needed to harm or cause one event (NNH).
Unit of analysis issues
We accepted randomisation to individual patient only.
Dealing with missing data
The most likely source of missing data was in cross‐over studies; we planned to use only first‐period data where possible, but no included studies used a cross‐over design. Where there were substantial missing data in any study, we commented on this and performed sensitivity analyses to investigate their effect.
For all outcomes, we carried out analyses, as far as possible, on a modified intention‐to‐treat basis, that is we included all participants who were randomised and received an intervention. Where sufficient information was reported, we re‐included missing data in the analyses we undertook. We would exclude data from outcomes where results from 10% or greater of participants were missing with no acceptable reason provided or apparent.
Assessment of heterogeneity
We assessed heterogeneity of response rates using L'Abbé plots, a visual method for assessing differences in results of individual studies (L'Abbé 1987). Where data could be pooled, we reported the I2 statistic.
Assessment of reporting biases
We assessed publication bias by examining the number of participants in trials with zero effect (RR of 1.0) needed for the point estimate of the NNT to increase beyond a clinically useful level (Moore 2008). In this case, we specified a clinically useful level as an NNT of 8 or greater for pain‐free at two hours, and NNT 6 or greater for headache relief at two hours.
Data synthesis
We analysed studies using a single dose of naproxen in established pain of at least moderate intensity separately from studies in which medication was taken before pain was well established or in which a second dose of medication was permitted.
We calculated effect sizes and combined data for analysis only for comparisons and outcomes where there were at least two studies and 200 participants (Moore 1998). Relative risk of benefit ('relative benefit') or harm ('relative risk') was calculated with 95% confidence intervals (CIs) using a fixed‐effect model (Morris 1995). We calculated NNT, NNTp, and NNH with 95% CIs using the pooled number of events by the method of Cook and Sackett (Cook 1995). We assumed a statistically significant difference from control when the 95% CI of the RR of benefit or harm did not include the number one.
We used the z test (Tramer 1997) to determine significant differences between NNT, NNTp, and NNH for different groups in subgroup and sensitivity analyses.
We described data from comparisons and outcomes with only one study or fewer than 200 participants in the summary tables and text where appropriate for information and comparison, but we did not analyse these data quantitatively.
Subgroup analysis and investigation of heterogeneity
Issues for potential subgroup analysis were dose, monotherapy versus combination with an antiemetic, route of administration, and formulation. For combined treatment with an antiemetic, the intent was to compare different antiemetics if there were sufficient data.
Sensitivity analysis
We planned sensitivity analysis for study quality (Oxford Quality Score of 2 versus 3 or more) and for migraine type (with aura versus without aura). A minimum of two studies and 200 participants had to be available for any sensitivity analysis.
Summary of findings and assessment of the certainty of the evidence
Results
Description of studies
Results of the search
After screening titles and abstracts of all records retrieved in the searches, we identified 20 potentially relevant completed studies. We found one further study that may be completed but for which there were no data (NCT01390324); details are in Characteristics of studies awaiting classification (Figure 1).
1.
Study flow diagram.
Included studies
Six studies (with data reported in three primary publications and two clinical trial summaries) satisfied all inclusion criteria, and are included in this review (Brandes 2007 Study 1Brandes 2007 Study 2; S2WA4003; S2WA4004; Smith 2005; Wentz 2008). A further publication (Landy 2007) provided data on functional disability for the studies in Brandes 2007 Study 1 and Brandes 2007 Study 2 that were not reported in the primary publication. All studies used a parallel‐group design, and medication was to be taken orally when the PI was of at least moderate intensity. All placebo‐controlled studies treated a single attack with a single dose of study medication, while the active‐controlled studies treated multiple attacks over a 12‐week period, each with a single dose of study medication. No studies employed multiple dosing strategies for individual attacks.
All studies were multicentre and diagnosed migraine (with or without aura) according to IHS criteria. Individuals with frequent migraine headaches (> six or eight attacks per month) were excluded. Most studies required that participants had previously tolerated treatment with an NSAID or had no contraindications, or both. In all studies, participants self treated their headaches at home. The mean age of participants ranged from 40 to 42 years, and between 81% and 91% were female. Participants were eligible for inclusion if they were using stable prophylactic medication in three studies (Brandes 2007 Study 1Brandes 2007 Study 2; Smith 2005), but Wentz 2008 excluded participants if they had been using prophylactic medication within one month of the trial period. S2WA4003 and S2WA4004 did not mention prophylaxis, but it seems likely that it was not allowed. All studies had an appropriate washout period between any analgesic or other prohibited medication that might interfere with the results and study medication.
Three studies gave naproxen 500 mg (Brandes 2007 Study 1Brandes 2007 Study 2; Smith 2005), two gave only 275 mg (S2WA4003; S2WA4004), while Wentz 2008 gave naproxen 825 mg as this is the recommended maximum dose in Europe for acute migraine treatment. Four studies compared naproxen with placebo (Brandes 2007 Study 1Brandes 2007 Study 2; Smith 2005; Wentz 2008); three also included treatment arms using sumatriptan and sumatriptan plus naproxen (Brandes 2007 Study 1Brandes 2007 Study 2; Smith 2005). One study compared placebo and naproxen with a novel COX‐2 NSAID (GW406381) (Wentz 2008); this drug is not marketed, so results are not reported here. Two studies were head‐to‐head comparisons of a low dose (275 mg) of naproxen with naratriptan (S2WA4003; S2WA4004). No studies combined naproxen with an antiemetic.
In total, 1241 participants took naproxen (275 mg to 825 mg), 229 took sumatriptan 50 mg, 173 took naratriptan 2.5 mg, and 1092 took placebo. The number included in efficacy analyses was slightly lower because some participants were excluded from these analyses due to major protocol violations or because they reported no useful results. A further 735 participants took sumatriptan 85 mg, and 737 took a combination of sumatriptan 85 mg and naproxen 500 mg (Brandes 2007 Study 1; Brandes 2007 Study 2). Sumatriptan 85 mg is a dose used only in the context of this particular combination and is not otherwise available; a comparison of naproxen alone with sumatriptan plus naproxen is included in Law 2013.
The outcomes reported by individual studies are listed in the Characteristics of included studies table. All studies measured headache PI using a standard 4‐point scale. The placebo‐controlled studies evaluated pain‐free response at two hours and sustained pain‐free during the 24 hours post dose as the primary outcome measures. Wentz 2008 was concerned with the primary outcomes for the novel NSAID but the relevant primary outcome data for naproxen were available. Dichotomous data could also be extracted from all placebo‐controlled studies for the secondary outcomes of headache relief at two hours and sustained headache relief during the 24 hours post dose. Wentz 2008 and Smith 2005 also provided usable data for headache relief at the earlier time of one hour post dose. Dichotomous data on the use of rescue medication were reported, as were numerical data on adverse events. Data on relief of associated symptoms were reported but not in a consistent way; only one study reported data for calculation of relief of vomiting (Wentz 2008), while specific relief of nausea, photophobia, and phonophobia were available from three out of the four placebo‐controlled studies (Brandes 2007 Study 1; Brandes 2007 Study 2; Wentz 2008).
The two studies with only an active control (no placebo) reported PR at four hours only and did not provide any usable efficacy data (S2WA4003; S2WA4004).
Details of individual studies are in the Characteristics of included studies table.
Excluded studies
We excluded 14 studies. Details are in the Characteristics of excluded studies table.
Risk of bias in included studies
Methodological quality, assessed using the Oxford Quality Scale, was good in all studies. One study scored 5/5 (Wentz 2008), three scored 4/5 (S2WA4003; S2WA4004; Smith 2005), and two scored 3/5 (Brandes 2007 Study 1; Brandes 2007 Study 2). Points were lost due to failure to report adequately the method of randomisation or blinding, or both, although it is likely that these were satisfactory as the trials are recent and would have been carried out in dedicated clinical trial facilities. Full details are in the Characteristics of included studies table.
In addition, we created a Risk of bias table, which considered sequence generation, allocation concealment, blinding, incomplete outcome data, and study size (Figure 2). Only one study adequately reported the method of allocation concealment (Wentz 2008). No study had substantial amounts of missing data, and no study was considered to be at high risk of bias.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Effects of interventions
See: Table 1
The two active‐controlled studies comparing naproxen 275 mg with naratriptan 2.5 mg did not report any of our prespecified efficacy outcomes (S2WA4003; S2WA4004); they did report numbers of participants experiencing our prespecified adverse event and withdrawal outcomes, but combined data for all attacks over a 12‐week period without any explanation of how it was done, so we were unable to use them in analyses.
For analysis of the placebo‐controlled studies, we chose to combine results from the three using naproxen 500 mg with the one using naproxen 825 mg. We carried out a sensitivity analysis to determine the effect of dose.
All included studies used one or more standard scales to measure PI or PR, and reported outcomes as defined in the Types of outcome measures section. Details of efficacy outcomes in individual studies are in Appendix 5 and of adverse events and withdrawals in Appendix 6.
Pain‐free at two hours
Naproxen versus placebo
Four studies (2149 participants) provided data for use of naproxen, compared with placebo, when pain was moderate or severe (Brandes 2007 Study 1; Brandes 2007 Study 2; Smith 2005; Wentz 2008).
The proportion of participants pain‐free at two hours with naproxen 500/825 mg was 17% (183/1064; range 15% to 29%).
The proportion of participants pain‐free at two hours with placebo was 8.5% (92/1085; range 5.8% to 10%).
The relative benefit of treatment compared with placebo was 2.0 (95% CI 1.6 to 2.6) (Figure 3); the NNT was 11 (8.7 to 17).
For naproxen 500 mg alone, the NNT was 13 (9.7 to 22), which was not significantly different from the NNT for the combined dosage.
3.

Forest plot of comparison: 1 Pain, outcome: 1.1 Pain‐free response at two hours.
The L'Abbé plot showed a considerable degree of similarity of response between different studies (Figure 4).
4.
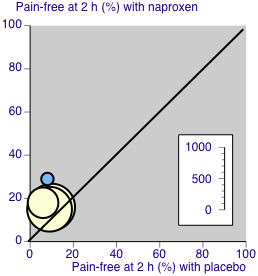
L'Abbé plot for pain‐free response at two hours. Size of circle proportional to size of study (see inset scale). Cream = naproxen 500 mg, blue = naproxen 825 mg.
Naproxen versus sumatriptan 50 mg
One study compared naproxen 500 mg with sumatriptan 50 mg (474 participants) (Smith 2005); 45/248 (18%) were pain‐free at two hours with naproxen 500 mg compared with 45/226 (20%) with sumatriptan 50 mg. There were insufficient data for analysis.
Headache relief at two hours
Naproxen versus placebo
Four studies (2149 participants) provided data for use of naproxen compared with placebo when pain was moderate or severe (Brandes 2007 Study 1; Brandes 2007 Study 2; Smith 2005; Wentz 2008).
The proportion of participants experiencing headache relief at two hours with naproxen 500/825 mg was 45% (482/1064; range 44% to 55%).
The proportion of participants experiencing headache relief at two hours with placebo was 29% (311/1085; range 27% to 34%).
The relative benefit of treatment compared with placebo was 1.6 (1.4 to 1.8) (Figure 5); the NNT was 6.0 (4.8 to 7.9).
For naproxen 500 mg alone, the NNT was 6.2 (4.9 to 8.3), which was not significantly different from the NNT for the combined dosage.
5.

Forest plot of comparison: 1 Pain, outcome: 1.4 Headache relief at two hours.
The L'Abbé plot showed a considerable degree of similarity of response between different studies (Figure 6).
6.
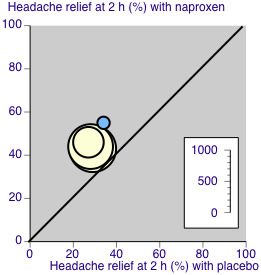
L'Abbé plot for headache relief at two hours. Size of circle proportional to size of study (see inset scale). Cream = naproxen 500 mg, blue = naproxen 825 mg.
Naproxen versus sumatriptan 50 mg
One study compared naproxen 500 mg with sumatriptan 50 mg (474 participants) (Smith 2005); 114/248 (46%) had PR at two hours with naproxen 500 mg compared with 111/226 (49%) with sumatriptan 50 mg. There were insufficient data for analysis.
Sustained pain‐free during the 24 hours post dose
Naproxen versus placebo
Four studies (2149 participants) provided data for use of naproxen, compared with placebo, when pain was moderate or severe (Brandes 2007 Study 1; Brandes 2007 Study 2; Smith 2005; Wentz 2008).
The proportion of participants with a 24‐hour sustained pain‐free response with naproxen 500/825 mg was 12% (129/1064; range 10% to 26%).
The proportion of participants with a 24‐hour sustained pain‐free response with placebo was 6.7% (73/1085; range 5.0% to 8.3%).
The relative benefit of treatment compared with placebo was 1.8 (1.4 to 2.4) (Analysis 1.3); the NNT was 19 (13 to 34).
For naproxen 500 mg alone, the NNT was 25 (16 to 70), which was not significantly different from the NNT for the combined dosage.
1.3. Analysis.

Comparison 1: Naproxen versus placebo, Outcome 3: 24‐h sustained pain‐free
Naproxen versus sumatriptan 50 mg
One study compared naproxen 500 mg with sumatriptan 50 mg (474 participants) (Smith 2005); 30/248 (12%) had a sustained pain‐free response during the 24 hours post dose with naproxen 500 mg compared with 25/226 (11%) with sumatriptan 50 mg. There were insufficient data for analysis.
Sustained headache relief during the 24 hours post dose
Naproxen versus placebo
Four studies (2149 participants) provided data for use of naproxen compared with placebo, when pain was moderate or severe (Brandes 2007 Study 1; Brandes 2007 Study 2; Smith 2005; Wentz 2008).
The proportion of participants with 24‐hour sustained headache relief with naproxen 500/825 mg was 30% (315/1064; range 25% to 46%);
The proportion of participants with 24‐hour sustained headache relief with placebo was 18% (190/1085; range 17% to 21%).
The relative benefit of treatment compared with placebo was 1.7 (1.5 to 2.0) (Analysis 1.4); the NNT was 8.3 (6.4 to 12).
For naproxen 500 mg alone, the NNT was 9.3 (6.9 to 14), which was not significantly different from the NNT for the combined dosage.
1.4. Analysis.

Comparison 1: Naproxen versus placebo, Outcome 4: 24‐h sustained headache relief
Naproxen versus sumatriptan 50 mg
One study (Smith 2005) compared naproxen 500 mg with sumatriptan 50 mg (474 participants); 62/248 (25%) had sustained headache relief during the 24 hours post dose with naproxen 500 mg compared with 66/226 (29%) with sumatriptan 50 mg. There were insufficient data for analysis.
| Summary of results: Pain‐free and headache relief | |||||||
| Baseline pain | Studies |
Attacks treated |
Treatment (%) |
Placebo or comparator (%) |
Relative benefit (95% CI) |
NNT (95% CI) |
|
| Pain‐free at 2 h | |||||||
| Naproxen 500/825 mg vs placebo | ≥ mod | 4 | 2149 | 17 | 8 | 2.0 (1.6 to 2.6) | 11 (8.7 to 17) |
| Naproxen 500 mg vs placebo | ≥ mod | 3 | 1951 | 16 | 9 | 1.9 (1.5 to 2.4) | 13 (9.7 to 22) |
| Naproxen 500 mg vs sumatriptan 50 mg | ≥ mod | 1 | 474 | 18 | 20 | Not calculated | Not calculated |
| Headache relief at 2 h | |||||||
| Naproxen 500/825 mg vs placebo | ≥ mod | 4 | 2149 | 45 | 29 | 1.6 (1.4 to 1.8) | 6.0 (4.8 to 7.9) |
| Naproxen 500 mg vs placebo | ≥ mod | 3 | 1951 | 44 | 28 | 1.6 (1.4 to 1.8) | 6.2 (4.9 to 8.3) |
| Naproxen 500 mg vs sumatriptan 50 mg | ≥ mod | 1 | 474 | 46 | 49 | Not calculated | Not calculated |
| Sustained pain‐free during the 24 h post dose | |||||||
| Naproxen 500/825 mg vs placebo | ≥ mod | 4 | 2149 | 12 | 7 | 1.8 (1.4 to 2.4) | 19 (13 to 34) |
| Naproxen 500 mg vs placebo | ≥ mod | 3 | 1951 | 11 | 7 | 1.6 (1.2 to 2.1) | 26 (16 to 70) |
| Naproxen 500 mg vs sumatriptan 50 mg | ≥ mod | 1 | 474 | 12 | 11 | Not calculated | Not calculated |
| Sustained headache relief during the 24 h post dose | |||||||
| Naproxen 500/825 mg vs placebo | ≥ mod | 4 | 2149 | 30 | 18 | 1.7 (1.5 to 2.0) | 8.3 (6.4 to 12) |
| Naproxen 500 mg vs placebo | ≥ mod | 3 | 1951 | 28 | 17 | 1.7 (1.4 to 2.0) | 9.3 (6.9 to 14) |
| Naproxen 500 mg vs sumatriptan 50 mg | ≥ mod | 1 | 474 | 25 | 29 | Not calculated | Not calculated |
Subgroup analysis of primary outcomes
Subgroup analysis according to dose (500 mg versus 825 mg tablets) has been considered in the main analysis above. Inclusion of the higher dose did not significantly change the results. All studies used the oral route of administration, and none used multiple dosing strategies.
Sensitivity analysis of primary outcomes
All studies scored at least 3/5 for methodological quality on the Oxford Quality Scale, and no studies provided separate data for participants with or without aura, so no sensitivity analysis could be carried out for these criteria.
Adverse events
Any adverse event
All placebo‐controlled studies reported some information about participants who experienced one or more adverse events, but the reporting was inconsistent. Brandes 2007 Study 1, Brandes 2007 Study 2 and Smith 2005 reported the number of participants in each treatment arm with at least one adverse event occurring within 24 hours of taking study medication, while Wentz 2008 reported numbers with adverse events until study discharge. Since there was no obvious relationship between numbers of participants with adverse events and the time over which the data were collected, we have combined data from the different time periods for analysis of the placebo‐controlled studies.
Naproxen 500 mg to 825 mg versus placebo
Four studies (2174 participants) provided data for analysis (Brandes 2007 Study 1; Brandes 2007 Study 2; Smith 2005; Wentz 2008).
The proportion of participants experiencing one or more adverse events with naproxen 500/825 mg was 15% (165/1078; range 13% to 17%).
The proportion of participants experiencing one or more adverse events with placebo was 12% (128/1096; range 10% to 14%).
The relative risk of treatment compared with placebo was 1.3 (1.1 to 1.6) (Analysis 1.5); the NNH was 28 (15 to 132).
The relative risk of naproxen 500 mg compared with placebo was 1.2 (0.96 to 1.5); the NNH was not calculated.
1.5. Analysis.

Comparison 1: Naproxen versus placebo, Outcome 5: Any adverse event
There was no significant difference between naproxen 500 mg and placebo for participants with at least one adverse event.
Two studies comparing naproxen 275 mg with naratriptan 2.5 mg (S2WA4003; S2WA4004) provided data on the number of participants reporting any adverse event, but combined data for all attacks over 12 weeks of treatment without any explanation of how this was done, so we were unable to use them.
Specific adverse events
The most common specific adverse events reported in studies were dizziness, paraesthesia, somnolence, nausea, dyspepsia, dry mouth, and abdominal discomfort. The incidence of any specific event in individual studies was small (5% or less) and considered too low for useful analysis.
Serious adverse events
There were no reported serious adverse events related to study medication.
Withdrawals due to adverse events
Three placebo‐controlled studies reported that there were no adverse event withdrawals due to naproxen (Brandes 2007 Study 1; Brandes 2007 Study 2; Wentz 2008), and another did not mention any adverse event withdrawals (Smith 2005). The active‐controlled studies reported a total of 4/166 adverse event withdrawals with naproxen 275 mg and 3/173 with naratriptan 2.5 mg over the whole 12‐week treatment period (S2WA4003; S2WA4004).
Exclusions of participants from analyses after randomisation were mostly due to protocol violations or failing to take the medication (no qualifying headache), or failure to document results in diary cards; they were generally well reported. Numbers of participants lost to follow‐up, or withdrawing due for unspecified reasons were small and judged unlikely to influence results.
We classified participants who took rescue medication as withdrawals due to lack of efficacy, and details are reported under 'Use of rescue medication' (Appendix 7).
Other outcomes
Results for use of rescue medication, relief of headache‐associated symptoms, and relief of functional disability are in Appendix 7.
Discussion
Summary of main results
This review included four randomised, double‐blind, placebo‐controlled studies using naproxen to treat a migraine attack when the pain was at least of moderate severity. For the primary outcome of pain‐free at two hours, data were available for 1054 headaches treated with naproxen 500 mg or 825 mg and 1085 treated with placebo. Of these, 198 headaches were treated with naproxen 825 mg, and we analysed by both doses combined and by 500 mg alone.
For the IHS‐preferred outcome of pain‐free at two hours, naproxen was better than placebo when taken for moderate or severe PI; the NNT was 11 (8.7 to 17), with 17% and 8% of people being pain‐free with naproxen and placebo, respectively. For headache relief at two hours, naproxen was better than placebo; the NNT was 6.0 (4.8 to 7.9), with 45% of participants responding compared with 29% with placebo. These results were obtained with 500 mg and 825 mg doses combined, but results were very similar with 500 mg alone.
Comparisons with other analgesics reviewed using identical methods show that naproxen was less effective than most other medicines available without prescription. For comparison, the NNT for two‐hour pain‐free response was 8.1 with aspirin 1000 mg (Kirthi 2013), 7.2 with ibuprofen 400 mg (Rabbie 2013), 12 with paracetamol 1000 mg (Derry 2013), and 6.1 with oral sumatriptan 50 mg (Derry 2012). The NNT for two‐hour headache relief was 4.9 with aspirin 1000 mg (Kirthi 2013), 3.2 with ibuprofen 400 mg (Rabbie 2013), 5.0 with paracetamol 1000 mg (Derry 2013), and 4.0 with oral sumatriptan 50 mg (Derry 2012).
Results for sustained (24‐hour) outcomes were modest, with NNTs of about 8 for 24‐hour sustained headache relief, and 19 for 24‐hour sustained pain‐free.
Additional analyses show that naproxen was superior to placebo for use of rescue medication, and relief of migraine‐associated symptoms (nausea, photophobia, and phonophobia) and functional disability, with NNTs of 6 to 10 (Appendix 7).
There was no significant difference in the number of participants experiencing one or more adverse events between naproxen 500 mg and placebo (RR 0.96 to 1.5). However, when the higher dose was included in the analysis there were slightly more adverse events than placebo (RR 1.1 to 1.6), giving an NNH of 28 (15 to 132). Most adverse events were described as mild or moderate, and transient. The incidence of any specific adverse event was low (5% or less), and no serious adverse events or adverse event withdrawals were reported.
Two active‐controlled studies comparing a low dose (275 mg) of naproxen with a standard dose (2.5 mg) of naratriptan did not report any usable data (S2WA4003; S2WA4004).
Overall completeness and applicability of evidence
Included participants all had a diagnosis of migraine according to IHS criteria, and the information for comparators was sufficiently large to allow for comparisons with placebo in order to generate conclusions about relative efficacy and harm. Most participants were recruited from a pool of known headache sufferers, for example from neurology outpatient departments, and so may be more refractory to treatment than the general public as a whole, and were carefully screened and excluded if there was any contraindication to a study medication. These factors could lead to an underestimate of treatment effect, and overestimate of safety. All studies included participants with or without aura, but none reported results for the two types separately. Individual studies often are underpowered to determine differences between treatments for adverse events. Even pooling studies may not provide adequate numbers of events to demonstrate differences or allow confidence in the size of the effect. However, it is likely that adverse event data continued to be recorded after taking rescue medication, which may confound the results due to adverse events associated with the rescue medication itself. Single‐dose studies are certainly unlikely to reveal rare, but potentially serious, adverse events; however, naproxen is a widely used NSAID, and rare, serious adverse events in occasional users for migraine are unlikely to differ in nature from, or be more frequent than, those in patients with conditions such as osteoarthritis who use naproxen regularly. Overall, in these studies, the number of participants experiencing any adverse event with active treatment was greater than with placebo.
We excluded six studies because they did not use IHS criteria to diagnose migraine (Andersson 1989; Johnson 1985; Nestvold 1985; Pradalier 1985; Sargent 1988; Treves 1992). A total of 462 participants were included in these studies, although not all provided evaluable data and not all received naproxen or placebo. None of the studies provided single‐dose data, and none reported any of our primary outcomes.
We identified a small number of studies of a combination formulation of naproxen and metoclopramide (MT100: naproxen sodium 500 mg plus metoclopramide 16 mg) manufactured by Pozen. We were unable to obtain any reports of the individual studies (despite contacting the manufacturer for full details of the trials); the drug did not receive a license from the US Food and Drug Administration (FDA) due to concerns about tardive dyskinesia associated with metoclopramide. For completeness we have included the results, such as they are available to us, in Appendix 8. We are also aware that there may be a combination formulation of naproxen and domperidone available in some parts of Asia (Martindale 2012), but have been unable to identify any studies.
Quality of the evidence
Included studies were of good methodological quality and validity. Some did not adequately describe the method of randomisation or allocation concealment, but this may reflect the limitation of space in published articles rather than any flaw in methodology. Migraine was diagnosed using standard, validated criteria, and outcomes measured were generally those recommended by the IHS as being of clinical relevance, although not all studies reported all the outcomes we sought (eg vomiting).
Potential biases in the review process
The main area of concern is the small numbers of events used to calculate some results, and particularly for specific adverse events, where no analysis was possible. We identified a large amount of data in comparisons with placebo. The NNTs for two‐hour pain‐free and two‐hour headache relief, at 8 and 6, respectively, were already at the preset limits for determining clinical utility, and so the calculation of how much additional data with null effect would be required to reach that point could not be done (Moore 2008). However, a simple calculation showed that even the addition of three times the available data in unpublished trials with null effect would still produce a statistically significant benefit, even if clinically unimportant. It is unlikely that such a large amount of unidentified data exists, so publication bias overturning the direction of the result (that naproxen is statistically better than placebo) is not a concern.
Agreements and disagreements with other studies or reviews
A systematic review of naproxen alone for acute migraine was carried out in Bandolier, identifying five studies up to June 2000 (Bandolier 2000). Only 225 participants were treated with naproxen and 190 with placebo, and most studies were small, predating IHS criteria for diagnosis and IHS‐preferred outcomes; none of those studies is included in this review because of the absence of IHS diagnosis of migraine. Only one study reported the outcome of headache relief at two hours (Andersson 1989). Naproxen at doses ranging from 750 mg/day to 1250 mg/day provided significantly better PR than placebo in the four placebo‐controlled studies, while the two studies comparing it with ergotamine 2 mg to 3 mg showed no significant difference between the two drugs.
Suthisisang 2010 is a meta‐analysis of the four included studies in this review that contributed to analyses (Brandes 2007 Study 1; Brandes 2007 Study 2; Smith 2005; Wentz 2008). The meta‐analysis quotes NNT values for naproxen versus placebo of 10 (7 to 25) for pain‐free at two hours, 15 (9 to 100) for sustained pain‐free during the 24 hours post dose, 7 (5 to 9) for headache relief at two hours, and 9 (7 to 13) for sustained headache relief during the 24 hours post dose. These results are consistent with the results reported in this review for the same comparison with the same studies; minor differences appear to arise from the use of slightly different denominators in the analyses, and do not substantially affect the results. The authors concur that naproxen is unlikely to be considered as a first‐line therapy, given the superiority of other simple analgesics (aspirin and ibuprofen) for important outcomes such as pain‐free at two hours.
Another review of NSAID treatments for migraine was neither systematic nor did it provide any analysis (Pardutz 2010).
Authors' conclusions
Implications for practice.
This review suggests that naproxen is effective in migraine headache for a small proportion of sufferers. Other Cochrane reviews examining alternative monotherapies, such as aspirin, ibuprofen, paracetamol, or sumatriptan have reported better (lower) NNT results for the same outcome, so are effective in more people.
Implications for research.
Analgesics effective in acute and chronic painful conditions typically have a much lesser effect in migraine. This is especially the case when using the IHS criterion of pain‐free at two hours as the outcome. Naproxen is no exception when used alone, and the combination with sumatriptan has been evaluated in clinical trials and a Cochrane review (Law 2013). In these circumstances, no further research with naproxen for migraine is warranted.
What's new
| Date | Event | Description |
|---|---|---|
| 8 July 2020 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 11, 2011 Review first published: Issue 10, 2013
| Date | Event | Description |
|---|---|---|
| 29 May 2019 | Amended | Contact details updated. |
| 20 May 2015 | Review declared as stable | This review will be re‐assessed for updating in 2020. See Published notes. |
| 20 December 2013 | Amended | Minor changes made to definitions of 24‐hour outcomes and Use of rescue medication in Appendix 1, and 95% confidence intervals added to NNTs in Abstract |
Notes
Assessed for updating in 2015
In April 2015, we did not identify any potentially relevant new studies after a restricted search (electronic search strategy run in selected databases). The conclusions of this Cochrane Review, which last had a full search in May 2013, are therefore still considered up‐to‐date. The review will be assessed for further updating in 2020.
Assessed for updating in 2020
At July 2020 we are not aware of any potentially relevant studies likely to change the conclusions. This is not an active area of research and so this review has now been stabilised following discussion with the authors and editors. If appropriate we will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitates major revisions.
Acknowledgements
Institutional support for this review was from the Oxford Pain Research Trust. Lifting The Burden: the Global Campaign against Headache and the International Headache Society provided financial support for the editorial process. See Sources of support for details.
Appendices
Appendix 1. Definitions
All terms relating to primary efficacy outcomes are defined according to the effect of the treatment on headache pain, measured using a 4‐point pain intensity (PI) scale (ranging from 0 to 3 or none, mild, moderate, and severe).
Baseline PI ‐ level of pain participant must be experiencing in order to receive study medication, either 1 (mild pain) or 2/3 (moderate or severe pain).
Pain‐free at two hours ‐ number of participants with a PI of 0 (none) at two hours after administration of study medication, expressed as a fraction of the treated participants with the appropriate baseline pain.
Pain‐free at one hour ‐ number of participants with a PI of 0 (none) at one hour after administration of study medication, expressed as a fraction of the treated participants with the appropriate baseline pain.
Headache relief at two hours ‐ number of participants with a reduction in PI from 2/3 (moderate/severe) to 0/1 (none/mild) at two hours after administration of study medication, expressed as a fraction of the treated participants with grade 2/3 baseline pain.
Headache relief at one hour ‐ number of participants with a reduction in PI from 2/3 (moderate/severe) to 0/1 (none/mild) at one hour after administration of study medication, expressed as a fraction of the treated participants with grade 2/3 baseline pain.
24‐hour sustained headache relief ‐ number of participants with a reduction in PI from 2/3 (moderate/severe) to 0/1 (none/mild) at two hours after administration of study medication which is then sustained between 2 and 24 hours without recurrence of headache or use of additional medication, expressed as a fraction of the treated participants with grade 2/3 baseline pain.
24‐hour sustained pain‐free ‐ number of participants with a PI of 0 (none) at two hours after administration of study medication which is then sustained between 2 and 24 hours without recurrence of headache or use of additional medication expressed as a fraction of the treated participants with the appropriate baseline pain.
Use of rescue medication ‐ number of participants requiring the use of additional medication to treat an inadequate response to study medication, provided that the additional medication is not, or does not include, the study drug.
Relief of associated symptoms ‐ number of participants with an absence of a headache‐associated symptom (nausea, vomiting, photophobia, or phonophobia) at two hours after administration of study medication, expressed as a fraction of the treated participants for whom the symptom was present at baseline.
Complete relief of functional disability ‐ reduction in the level of functional disability, measured using a 4‐point scale, from any degree of disability (grade 1/2/3) at baseline to grade 0 (able to work/function normally) at two hours after administration of study medication, expressed as a fraction of the treated participants with any functional disability at baseline.
Appendix 2. Search strategy for CENTRAL
MeSH descriptor Naproxen
(naproxen or Aleve or Anaprox or Antalgin or Feminax or Flanax or Inza or Midol or Miranax or Nalgesin or Naposin or Naprelan or Naprogesic or Naprosyn or Narocin or Proxen or Synflex or Xenobid):ti,ab,kw.
1 OR 2
MeSH descriptor Headache/ OR MeSH descriptor Headache Disorders explode all trees
MeSH descriptor Migraine Disorders explode all trees
(headach* OR migrain* OR cephalgi* OR cephalalgi*):ti,ab,kw.
4 OR 5 OR 6
Randomized controlled trial:pt.
MESH descriptor Double‐blind Method
random*:ti,ab,kw.
OR/8‐10
3 AND 7 AND 11
Limit 12 to Clinical Trials (CENTRAL)
Appendix 3. Search strategy for MEDLINE (via Ovid)
Naproxen/
(naproxen or Aleve or Anaprox or Antalgin or Feminax or Flanax or Inza or Midol or Miranax or Nalgesin or Naposin or Naprelan or Naprogesic or Naprosyn or Narocin or Proxen or Synflex or Xenobid).mp.
1 OR 2
Headache/ OR exp Headache Disorders/
exp Migraine Disorders/
(headach* OR migrain* OR cephalgi* OR cephalalgi*).mp.
4 OR 5 OR 6
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
OR/8‐15
3 AND 7 AND 16
Appendix 4. Search strategy for EMBASE (via Ovid)
Naproxen/
(naproxen or Aleve or Anaprox or Antalgin or Feminax or Flanax or Inza or Midol or Miranax or Nalgesin or Naposin or Naprelan or Naprogesic or Naprosyn or Narocin or Proxen or Synflex or Xenobid).mp
1 OR 2
Headache/ OR exp Headache and facial pain/
exp Migraine/
(headach* OR migrain* OR cephalgi* OR cephalalgi*).mp.
4 OR 5 OR 6
clinical trials.sh.
controlled clinical trials.sh.
randomized controlled trial.sh.
double‐blind procedure.sh.
(clin* adj25 trial*).ab.
((doubl* or trebl* or tripl*) adj25 (blind* or mask*)).ab.
placebo*.ab.
random*.ab.
OR/8‐15
3 AND 7 AND 16
Appendix 5. Results for individual studies: efficacy in placebo‐controlled studies
| Study ID | Treatment | HR 1 h | HR 2 h | PF 2 h | SHR 24 h | SPF 24 h | Use of rescue medication |
| Brandes 2007 Study 1 | (1) naprox 500 mg, n = 361 (2) placebo, n = 365 | No data | (1) 157/356 (2) 102/360 | (1) 53/356 (2) 33/360 | (1) 107/365 (2) 64/360 | (1) 37/356 (2) 30/360 | From 2 to 24 h (1) 135/356 (2) 192/360 |
| Brandes 2007 Study 2 | (1) naprox 500 mg, n = 371 (2) placebo, n = 387 | No data | (1) 158/364 (2) 109/382 | (1) 57/364 (2) 37/382 | (1) 102/364 (2) 64/382 | (1) 37/364 (2) 25/382 | From 2 to 24 h (1) 143/364 (2) 223/382 |
| Smith 2005 | (1) naprox 500 mg, n = 250 (2) placebo, n = 241 | (1) 67/248 (2) 29/241 | (1) 114/248 (2) 65/241 | (1) 45/248 (2) 14/241 | (1) 62/248 (2) 41/241 | (1) 30/248 (2) 12/241 | From 2 to 24 h (1) 129/248 (2) 154/241 |
| Wentz 2008 | (1) naprox 825 mg, n = 96 (2) placebo, n = 102 |
(1) 32/96 (2) 12/102 | (1) 53/96 (2) 35/102 | (1) 28/96 (2) 8/102 | (1) 44/96 (2) 21/102 | (1) 25/96 (2) 6/102 | (1) 33/96 (2) 61/102 |
| HR: headache relief; naprox: naproxen; PF: pain‐free; SHR: sustained headache relief; SPF: sustained pain free. | |||||||
Appendix 6. Results for individual studies: adverse events and withdrawals in placebo‐controlled studies
| Study ID | Treatment | Any AE | SAE | AE withdrawal | Other withdrawal |
| Brandes 2007 Study 1 | (1) naprox 500 mg, n = 361 (2) placebo, n = 365 | ≤ 24 h: (1) 48/361 (2) 45/365 | No SAE reported | None reported | Exclusions ‐ took medication but no evaluable data:
(1) 5 (2) 5 |
| Brandes 2007 Study 2 | (1) naprox 500 mg, n = 371 (2) placebo, n = 387 | ≤ 24 h: (1) 52/371 (2) 39/387 | No SAE reported | None reported | Exclusions ‐ took medication but no evaluable data: (1) 7 (2) 5 |
| Smith 2005 | (1) naprox 500 mg, n = 250 (2) placebo, n = 241 | AE reported for safety population at 72 h (1) 55/250 (2) 36/242 | None | None | None reported |
| Wentz 2008 | (1) naprox 825 mg, n = 96 (2) placebo, n = 102 |
AE reported until 'study discharge' (1) 22/96 (2) 8/102 |
None | None | 16 excluded (10 had no qualifying headache, 3 lost to follow‐up, 1 protocol violation, 2 'other') |
| AE: adverse event; naprox: naproxen; SAE: serious adverse event. | |||||
Appendix 7. Other outcomes
Use of rescue medication
All studies asked participants whose symptoms were not adequately controlled to wait for two hours before taking any additional medication in order to give the test medication enough time to have an effect. Use of rescue or 'escape' medication (usually a different analgesic) after that time was reported in all studies and is a measure of treatment failure (lack of efficacy). The time over which use of rescue medication was measured was 24 hours.
All four studies (2149 participants) provided data for naproxen compared with placebo, when pain was moderate or severe; 41% of participants needed rescue medication with naproxen 500/825 mg, and 58% with placebo. The relative benefit was 0.71 (0.65 to 0.78) (Analysis 1.6) and the NNTp was 6.0 (4.8 to 8.0). For 500 mg alone, the NNTp was 6.0 (5.0 to 8.7), which was not significantly different.
1.6. Analysis.

Comparison 1: Naproxen versus placebo, Outcome 6: Use of rescue medication
Relief of headache‐associated symptoms
Naproxen versus placebo
Three studies provided data on relief of nausea, photophobia, and phonophobia in comparison with placebo (Brandes 2007 Study 1; Brandes 2007 Study 2; Wentz 2008; Analysis 1.7). Too few participants experienced vomiting to allow any analysis of this symptom.
1.7. Analysis.

Comparison 1: Naproxen versus placebo, Outcome 7: Relief of associated symptoms
For all symptoms, naproxen was better than placebo; lower (better) NNTs were obtained for all three symptoms in comparisons with placebo. The higher dose of naproxen did not significantly affect the result.
| Summary of results: relief of headache‐associated symptoms at two hours | ||||||
| Intervention | Studies | Attacks with symptom present | Treatment (%) | Placebo (%) | Relative benefit (95% CI) | NNT (95% CI) |
| Nausea | ||||||
| Naproxen 500/825 mg vs placebo | 3 | 782 | 39 | 23 | 1.7 (1.4 to 2.1) | 6.1 (4.4 to 11) |
| Naproxen 500 mg vs placebo | 2 | 686 | 36 | 21 | 1.7 (1.3 to 2.2) | 6.8 (4.7 to 12) |
| Photophobia | ||||||
| Naproxen 500/825 mg vs placebo | 3 | 1342 | 32 | 19 | 1.7 (1.4 to 2.1) | 7.3 (5.5 to 11) |
| Naproxen 500 mg vs placebo | 2 | 1185 | 31 | 18 | 1.7 (1.4 to 2.1) | 7.7 (5.6 to 12) |
| Phonophobia | ||||||
| Naproxen 500/825 mg vs placebo | 3 | 1303 | 35 | 23 | 1.7 (1.4 to 2.0) | 7.2 (5.3 to 11) |
| Naproxen 500 mg vs placebo | 2 | 1146 | 32 | 20 | 1.6 (1.3 to 2.0) | 8.1 (5.7 to 14) |
| CI: confidence interval; NNT: number needed to treat. | ||||||
Relief of functional disability
Two studies treating when pain was at least moderate (1348 participants, Brandes 2007 Study 1 and Brandes 2007 Study 2, reported in Landy 2007) reported on participants with functional disability at baseline and at two hours; 20% experienced complete relief of disability with naproxen 500 mg, and 9% with placebo. The relative benefit was 2.1 (1.6 to 2.8) (Analysis 1.8) and the NNT was 9.5 (7.0 to 15). There were no useful data on the higher naproxen dose.
1.8. Analysis.

Comparison 1: Naproxen versus placebo, Outcome 8: Relief of functional disability
Treating headaches with naproxen was significantly better than placebo for relief of functional disability.
Appendix 8. MT100 (naproxen sodium 500 mg + metoclopramide 16 mg) versus placebo
| Outcome | Baseline pain | Studies |
Attacks treated |
Treatment (%) |
Placebo or comparator (%) |
Relative benefit (95% CI) |
NNT (95% CI) |
| Headache relief at 2 h | ≥ mod | 4 | 1622 | 46 | 31 | 1.5 (1.3 to 1.7) | 6.8 (5.1 to 9.9) |
| Sustained headache relief during the 24 h post dose | ≥ mod | 4 | 1622 | 33 | 20 | 1.6 (1.4 to 2.0) | 7.5 (5.7 to 11) |
| CI: confidence interval; mod: moderate; NNT: number needed to treat. | |||||||
Data from Pozen 2005a; Pozen 2005b
Data and analyses
Comparison 1. Naproxen versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Pain‐free response at 2 h | 4 | 2149 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.61, 2.58] |
| 1.2 Headache relief at 2 h | 4 | 2149 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.58 [1.41, 1.77] |
| 1.3 24‐h sustained pain‐free | 4 | 2149 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.81 [1.37, 2.38] |
| 1.4 24‐h sustained headache relief | 4 | 2149 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.45, 1.98] |
| 1.5 Any adverse event | 4 | 2174 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [1.05, 1.62] |
| 1.6 Use of rescue medication | 4 | 2149 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.65, 0.78] |
| 1.7 Relief of associated symptoms | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.7.1 Nausea | 3 | 782 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [1.38, 2.16] |
| 1.7.2 Photophobia | 3 | 1342 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [1.43, 2.10] |
| 1.7.3 Phonophobia | 3 | 1313 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.68 [1.40, 2.01] |
| 1.8 Relief of functional disability | 2 | 1346 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.14 [1.62, 2.84] |
1.1. Analysis.

Comparison 1: Naproxen versus placebo, Outcome 1: Pain‐free response at 2 h
1.2. Analysis.

Comparison 1: Naproxen versus placebo, Outcome 2: Headache relief at 2 h
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brandes 2007 Study 1.
| Study characteristics | ||
| Methods | Multicentre, R, DB, PC, parallel‐group. Single dose to treat a single attack Medication taken when PI ≥ moderate Assessments at 0, 0.5, 1, 1.5, 2, then hourly to 24 h | |
| Participants | Migraine ± aura (IHS 2004), aged 18‐65 years. History: > 6 months with frequency of 2‐6 per month and untreated severity ≥ moderate Excluded: uncontrolled hypertension, cardio‐ or cerebrovascular disease, using MAOI, ergot, SJW, or NSAID N = 1461 F = 86% Mean age 40 years 72% without aura |
|
| Interventions | Sumatriptan 85 mg/naproxen 500 mg, n = 370 (364 analysed for efficacy) Sumatriptan 85 mg, n = 365 (361 for efficacy) Naproxen 500 mg, n = 361 (365 for efficacy) Placebo, n = 365 (360 for efficacy) Rescue medication allowed after 2 h if necessary (as prescribed by physician but not ergot‐containing, serotonin agonist, or NSAID‐containing medications) | |
| Outcomes | Headache relief at 2 h Pain‐free at 2 h 24‐h sustained headache relief 24‐h sustained pain‐free Presence and relief of associated symptoms at 2 h Presence and relief of functional disability at 2 h (from Landy 2007) Use of rescue medication Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs described |
| Size | Low risk | > 200 participants in each treatment arm |
Brandes 2007 Study 2.
| Study characteristics | ||
| Methods | Multicentre, R, DB, PC, parallel‐group. Single dose to treat a single attack Medication taken when PI ≥ moderate Assessments at 0, 0.5, 1, 1.5, 2, then hourly to 24 h | |
| Participants | Migraine ± aura (IHS 2004), aged 18‐65 years. History: > 6 months with frequency of 2‐6 per month and untreated severity ≥ moderate Excluded: uncontrolled hypertension, cardio‐ or cerebrovascular disease, using MAOI, ergot, SJW, or NSAID N = 1495 F = 88% Mean age 40 years 76% without aura |
|
| Interventions | Sumatriptan 85 mg/naproxen 500 mg, n = 367 (362 for efficacy) Sumatriptan 85 mg, n = 370 (362 for efficacy) Naproxen 500 mg, n = 371 (364 for efficacy) Placebo, n = 387 (382 for efficacy) Rescue medication allowed after 2 h if necessary (as prescribed by physician but not ergot‐containing, serotonin agonist, or NSAID‐containing medications) | |
| Outcomes | Headache relief at 2 h Pain‐free at 2 h 24‐h sustained headache relief 24‐h sustained pain‐free Presence and relief of associated symptoms at 2 h Presence and relief of functional disability at 2 h (from Landy 2007) Use of rescue medication Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs described |
| Size | Low risk | > 200 participants in each treatment arm |
S2WA4003.
| Study characteristics | ||
| Methods | Multicentre, R, DB, PC, parallel‐group. Single dose to treat single attack; several attacks treated over 12 weeks
Medication taken when PI ≥ moderate Assessments at 0, 4 h, for efficacy and adverse events, MSQ at baseline and after 12 weeks of treatment |
|
| Participants | Migraine ± aura (IHS 1988), aged 18‐65 years. History: > 12 months with frequency of 1‐6 per month and untreated severity ≥ moderate Excluded: previous use of triptan on > 3 occasions or had prescription for sumatriptan, ischaemic disease or symptoms, cardio‐ or cerebrovascular pathology, uncontrolled hypertension, epilepsy N = 168 F = 85% Mean age 39 years |
|
| Interventions | Naproxen sodium 275 mg, n = 81 Naratriptan 2.5 mg, n = 87 |
|
| Outcomes | Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double dummy" method |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Double dummy" method |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Size | Unclear risk | 50‐200 participants in each treatment arm |
S2WA4004.
| Study characteristics | ||
| Methods | Multicentre, R, DB, PC, parallel‐group. Single dose to treat single attack; several attacks treated over 12 weeks
Medication taken when PI ≥ moderate Assessments at 0, 4 h, for efficacy and adverse events, MSQ at baseline and after 12 weeks of treatment |
|
| Participants | Migraine ± aura (IHS 1988), aged 18‐65 years. History: > 12 months with frequency of 1‐6 per month and untreated severity ≥ moderate Excluded: previous use of triptan on > 3 occasions or had prescription for sumatriptan, ischaemic disease or symptoms, cardio‐ or cerebrovascular pathology, uncontrolled hypertension, epilepsy N = 171 F = 85% Mean age 37 years |
|
| Interventions | Naproxen sodium 275 mg, n = 85 Naratriptan 2.5 mg, n = 86 |
|
| Outcomes | Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double dummy" method |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Double dummy" method |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for |
| Size | Unclear risk | 50‐200 participants in each treatment arm |
Smith 2005.
| Study characteristics | ||
| Methods | Multicentre, R, DB, DD, parallel‐group. Single dose to treat a single attack Medication taken when pain ≥ moderate Assessments at 0, 15 min intervals to 2 h, 30 min to 4 h, hourly to 24 h | |
| Participants | Migraine ± aura (IHS 2004), aged ≥ 18 years. History ≥ 1 year with 2‐6 attacks per month, and able to tolerate oral triptan or ergot derivative N = 972 F = 91% Mean age 42 years Without aura: > 70% | |
| Interventions | Sumatriptan 50 mg + naproxen 500 mg, n = 251 Sumatriptan 50 mg, n = 229 Naproxen 500 mg, n = 250 Placebo, n = 242 Rescue medication allowed after 2 h if necessary (not specified) | |
| Outcomes | Headache relief at 1 and 2 h Pain‐free at 2 h 24‐h sustained headache relief 24‐h sustained pain‐free Presence and relief of functional disability at 2 h Presence and relief of associated symptoms at 2 h Use of rescue medication Adverse events Withdrawals | |
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy method |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐dummy method |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs described |
| Size | Low risk | > 200 participants in each treatment arm |
Wentz 2008.
| Study characteristics | ||
| Methods | Multicentre, multinational, R, DB, PC, parallel‐group, double‐dummy study. Single dose to treat a single attack Medication taken when PI ≥ moderate Assessments at 0, 0.5, 1, 1.5, 2, 3, 4 h |
|
| Participants | Migraine ± aura (IHS 2004), aged 18‐65 years. History > 6 months
Frequency 6/month with untreated severity ≥ moderate
Excluded if > 15 headache days/month, associated disease, or if on acute or prophylactic medication N = 284 (safety population, 283 for efficacy) F = 81% Mean age 41 years Without aura: > 80% |
|
| Interventions | Naproxen 825 mg, n = 109 Placebo, n = 117 Rescue medication allowed after 2 h (patient's usual medication) 111 participants were also treated with an experimental COX‐2 inhibitor (GW406381), which is not marketed |
|
| Outcomes | Headache relief at 1, 2, and 4 h Pain‐free response at 1 and 2 h 24‐h sustained headache relief 24‐h sustained pain‐free Adverse events Withdrawals Presence and relief of headache associated symptoms at 2 hours Use of rescue medication |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by computer‐generated sequence |
| Allocation concealment (selection bias) | Low risk | No concealment of allocations prior to assignments |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐dummy blinding method used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐dummy blinding method used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐outs accounted for |
| Size | Unclear risk | 50‐200 participants in each treatment arm |
COX: cyclooxygenase; DB: double‐blind; IHS: International Headache Society; MAOI: monoamine oxidase inhibitor; MSQ: Migraine‐Specific Quality of Life Questionnaire; N: number of participants in study; n: number of participants in treatment arm; NSAID: non‐steroidal anti‐inflammatory drug; PC: placebo‐controlled; PI: pain intensity; R: randomised; SJW: St John's Wort; W: withdrawals.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Adis 2006 | Not RCT ‐ general review of naproxen sodium/metoclopramide formulation from Pozen Inc. Data from the Pozen studies are included in Appendix 8 |
| Andersson 1989 | Did not use IHS diagnostic criteria (or equivalent) for migraine ‐ diagnostic criteria judged not equivalent to IHS criteria |
| Johnson 1985 | Did not use IHS diagnostic criteria (or equivalent) for migraine ‐ no diagnostic criteria reported |
| Krymchantowski 2005 | Not randomised. Quasi‐randomised only |
| Misra 2010 | Fewer than 10 participants/treatment arm |
| NCT01726920 | Not double blind |
| Nestvold 1985 | Did not use IHS diagnostic criteria (or equivalent) for migraine ‐ diagnostic criteria judged not equivalent to IHS criteria |
| Nestvold 1986 | Supplement article, not original study report (same study as Nestvold 1985), no additional data |
| Pradalier 1985 | Did not use IHS diagnostic criteria (or equivalent) for migraine ‐ diagnostic criteria judged not equivalent to IHS criteria |
| Sargent 1988 | Did not use IHS diagnostic criteria (or equivalent) for migraine ‐ diagnostic criteria judged not equivalent to IHS criteria |
| Smith 2007 | Same study as Smith 2005, but reporting only quality‐of‐life and satisfaction outcomes |
| Stronks 2003 | Low patient numbers (12 only). No data on individual attacks. A comparative study versus naratriptan without placebo |
| Treves 1992 | Did not use IHS diagnostic criteria (or equivalent) for migraine ‐ diagnostic criteria judged not equivalent to IHS criteria |
| Welch 1986 | Supplement article, not original study report |
IHS: International Headache Society; RCT: randomised controlled trial.
Characteristics of studies awaiting classification [ordered by study ID]
NCT01390324.
| Methods | Multicentre, randomised, double‐blind, parallel‐group |
| Participants | Migraine ± aura (IHS 2004 criteria), male or female, 3‐month history of 2‐6 moderate/severe attacks per month, aged 18‐65 years |
| Interventions | Naratriptan 2.5 mg + naproxen 500 mg Naratriptan 2.5 mg Naproxen 500 mg |
| Outcomes | Headache relief at 2 and 4 h Pain‐free at 2 and 4 h 24‐h sustained headache relief 24‐h sustained pain‐free Associated symptoms Use of rescue medication Adverse events |
| Notes | Scheduled primary completion date October 2012 Contact person asked for update January 2013 |
IHS: International Headache Society.
Differences between protocol and review
After discussion with headache specialists and editorial staff, and in line with Cochrane recommendations, we decided to limit our outcomes for acute migraine headache reviews in order to focus attention on the most important outcomes and to make them more readable for both clinicians and patients. For the majority of interventions we will now include two‐hour pain‐free and headache relief as primary outcomes, and 24‐hour sustained pain‐free, sustained headache relief, and adverse events as secondary outcomes. Pain‐free headache relief outcomes at earlier time points will be included in special circumstances, if reported and relevant (eg if a 'fast acting' formulation is investigated). We have moved results for use of rescue medication and relief of headache‐associated symptoms and functional disability to Appendix 7.
We have expanded the Risk of bias table; this review uses the new criteria for analysis. We have also included an assessment of publication bias, which was not included in the protocol. This assessment is now being added routinely to all our reviews as a measure of reliability/robustness of the results.
Contributions of authors
SD and RAM wrote the protocol.
For the full review, SD and SL searched for studies, identified those for inclusion, and carried out data extraction. RAM checked data extraction. SL entered data into RevMan, and SD checked it. All authors were involved in analysis and writing the final review.
Sources of support
Internal sources
-
Oxford Pain Research Trust, UK
General institutional support
External sources
-
Lifting the Burden: the Global Campaign against Headache, UK
Funding for administrative costs associated with editorial review of the protocol
-
International Headache Society, UK
Funding for administrative costs associated with editorial and peer review of the full review
Declarations of interest
RAM has consulted for various pharmaceutical companies and has received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. RAM and SD have received research support from charities, government, and industry sources at various times. SL has no interests to declare.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Brandes 2007 Study 1 {published data only}
- Brandes JL, Kudrow D, Stark SR, O'Carroll CP, Adelman JU, O'Donnell FJ, et al. Sumatriptan-naproxen for acute treatment of migraine: a randomized trial. JAMA 2007;297(13):1443-54. [DOI: 10.1001/jama.297.13.1443 ] [DOI] [PubMed] [Google Scholar]
- Landy S, DeRossett SE, Rapoport A, Rothrock J, Ames MH, McDonald SA, et al. Two double-blind, multicenter, randomized, placebo-controlled, single-dose studies of sumatriptan/naproxen sodium in the acute treatment of migraine: function, productivity, and satisfaction outcomes. Medscape General Medicine 2007;9(2):53. [PMC free article] [PubMed] [Google Scholar]
Brandes 2007 Study 2 {published data only}
- Brandes JL, Kudrow D, Stark SR, O'Carroll CP, Adelman JU, O'Donnell FJ, et al. Sumatriptan-naproxen for acute treatment of migraine: a randomized trial. JAMA 2007;297(13):1443-54. [DOI: 10.1001/jama.297.13.1443 ] [DOI] [PubMed] [Google Scholar]
- Landy S, DeRossett SE, Rapoport A, Rothrock J, Ames MH, McDonald SA, et al. Two double-blind, multicenter, randomized, placebo-controlled, single-dose studies of sumatriptan/naproxen sodium in the acute treatment of migraine: function, productivity, and satisfaction outcomes. Medscape General Medicine 2007;9(2):53. [PMC free article] [PubMed] [Google Scholar]
S2WA4003 {published data only}
- Anonymous. A randomized, double-blind, double-dummy, active-placebo controlled, parallel group evaluation of oral naratriptan (2.5mg) compared to oral naproxen sodium (275mg) on migraine-related quality of life. Result summary for S2WA4003. download.gsk-clinicalstudyregister.com/files/2014.pdf (accessed 19 September 2013).
S2WA4004 {published data only}
- Anonymous. A randomized, double-blind, double-dummy, active-placebo controlled, parallel group evaluation of oral naratriptan (2.5mg) compared to oral naproxen sodium (275mg) on migraine-related quality of life. Result summary for S2WA4004. download.gsk-clinicalstudyregister.com/files/2041.pdf (accessed 19 September 2013).
Smith 2005 {published data only}
- Smith TR, Sunshine A, Stark SR, Littlefield DE, Spruill SE, Alexander WJ. Sumatriptan and naproxen sodium for the acute treatment of migraine. Headache 2005;45(8):983-91. [DOI: 10.1111/j.1526-4610.2005.05178.x] [DOI] [PubMed] [Google Scholar]
Wentz 2008 {published data only}
- Wentz AL, Jimenez TB, Dixon RM, Aurora SK, Gold M, CXA20008 Study Investigators. A double-blind, randomized, placebo-controlled, single-dose study of thecyclooxygenase-2 inhibitor, GW406381, as a treatment for acute migraine. European Journal of Neurology 2008;15(4):420-7. [DOI: 10.1111/j.1468-1331.2008.02093.x] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Adis 2006 {published data only}
- Adis International Limited. Naproxen sodium/metoclopramide: metoclopramide/naproxen-sodium, MT 100, naproxen-sodium/metoclopramide. Drugs in R and D 2006;7(4):259-61. [Google Scholar]
Andersson 1989 {published data only}
- Andersson PG, Hinge HH, Johansen O, Andersen CU, Lademann A, Gøtzsche PC. Double-blind study of naproxen vs placebo in the treatment of acute migraine attacks. Cephalalgia 1989;9(1):29-32. [DOI] [PubMed] [Google Scholar]
Johnson 1985 {published data only}
- Johnson E, Ratcliffe D, Wilkinson M. Naproxen sodium in the treatment of migraine. Cephalalgia 1985;5(1):5-10. [DOI] [PubMed] [Google Scholar]
Krymchantowski 2005 {published data only}
- Krymchantowski AV, Peixoto P, Higashi R, Silva A Jr, Schutz V. Lysine clonixinate vs naproxen sodium for the acute treatment of migraine: a double-blind, randomized, crossover study. Medscape General Medicine 2005;7(4):69. [PMC free article] [PubMed] [Google Scholar]
Misra 2010 {published data only}
- Misra M, Sharma T, Kalra J, Goel D, Dhasmana DC. Comparative efficacy and tolerability of sumatriptan, ergotamine, naproxen and rizatriptan in moderate to severe acute attack of migraine. JK Science 2010;12(4):175-9. [Google Scholar]
NCT01726920 {published data only}
- Peron C. Efficacy and safety of a fixed-dose combination of naratriptan and naproxen in acute treatment of migraine (Copérnico). clinicaltrials.gov/show/NCT01726920 (accessed 19 September 2013). [ACH-NRP-03(04/12)]
Nestvold 1985 {published data only}
- Nestvold K, Kloster R, Partinen M, Sulkava R. Treatment of acute migraine attack: naproxen and placebo compared. Cephalalgia 1985;5(2):115-9. [DOI] [PubMed] [Google Scholar]
Nestvold 1986 {published data only}
- Nestvold K. Naproxen and naproxen sodium in acute migraine attacks. Cephalalgia 1986;6 Suppl 4:81-4. [DOI] [PubMed] [Google Scholar]
Pradalier 1985 {published data only}
- Pradalier A, Rancurel G, Dordain G, Verdure L, Rascol A, Dry J. Acute migraine attack therapy: comparison of naproxen sodium and an ergotamine tartrate compound. Cephalalgia 1985;5(2):107-13. [DOI] [PubMed] [Google Scholar]
Sargent 1988 {published data only}
- Sargent JD, Baumel B, Peters K, Diamond S, Saper JR, Eisner LS, et al. Aborting a migraine attack: naproxen sodium v ergotamine plus caffeine. Headache 1988;28(4):263-6. [DOI] [PubMed] [Google Scholar]
Smith 2007 {published data only}
- Smith T, Blumenthal H, Diamond M, Mauskop A, Ames M, McDonald S, et al. Sumatriptan/naproxen sodium for migraine: efficacy, health related quality of life, and satisfaction outcomes. Headache 2007;47(5):683-92. [DOI: 10.1111/j.1526-4610.2007.00790.x] [DOI] [PubMed] [Google Scholar]
Stronks 2003 {published data only}
- Stronks DL, Tulen JH, Bussmann HB, Mulder LJ, Passchier J. Effects of naratriptan versus naproxen on daily functioning on the acute treatment of migraine. Headache 2003;43(8):845-52. [DOI: 10.1046/j.1526-4610.2003.03162.x] [DOI] [PubMed] [Google Scholar]
Treves 1992 {published data only}
- Treves T, Streiffler M, Korczyn A. Naproxen sodium versus ergotamine tartrate in the treatment of acute migraine attacks. Headache 1992;32(6):280-2. [DOI] [PubMed] [Google Scholar]
Welch 1986 {published data only}
- Welch K. Naproxen sodium in the treatment of migraine. Cephalalgia 1986;6 Suppl 4:85-92. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT01390324 {published data only}
- Peron C. Efficacy and safety of a fixed-dose combination of naratriptan and naproxen in acute treatment of migraine (Atenéia). clinicaltrials.gov/show/NCT01390324 (accessed 19 September 2013). [ACH-NRP-03(05/11)]
Additional references
Ayzenberg 2012
- Ayzenberg I, Katsarava Z, Sborowski A, Chernysh M, Osipova V, Tabeeva G, et al. The prevalence of primary headache disorders in Russia: a countrywide survey. Cephalalgia 2012;32(5):373-81. [DOI: 10.1177/0333102412438977 ] [DOI] [PubMed] [Google Scholar]
Bandolier 2000
- Anon. Naproxen for acute migraine, 2000. www.medicine.ox.ac.uk/bandolier/booth/Migraine/NapORacu.html (19 September 2013).
Bigal 2008
- Bigal ME, Serrano D, Reed M, Lipton RB. Chronic migraine in the population: burden, diagnosis, and satisfaction with treatment. Neurology 2008;71(8):559-66. [DOI: 10.1212/01.wnl.0000323925.29520.e7] [DOI] [PubMed] [Google Scholar]
Bloudek 2012
- Bloudek LM, Stokes M, Buse DC, Wilcox TK, Lipton RB, Goadsby PJ, et al. Cost of healthcare for patients with migraine in five European countries: results from the International Burden of Migraine Study (IBMS). Journal of Headache and Pain 2012;13(5):361-78. [DOI: 10.1007/s10194-012-0460-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Buse 2011
- Buse D, Manack A, Serrano D, Reed M, Varon S, Turkel C, et al. Headache impact of chronic and episodic migraine: results from the American Migraine Prevalence and Prevention study. Headache 2012;52(1):3-17. [DOI: 10.1111/j.1526-4610.2011.02046.x] [DOI] [PubMed] [Google Scholar]
Collins 1997
- Collins SL, Moore RA, McQuay HJ. The visual analogue pain intensity scale: what is moderate pain in millimetres? Pain 1997;72(1-2):95-7. [DOI] [PubMed] [Google Scholar]
Cook 1995
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995;310(6977):452-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2009
- Derry C, Derry S, Moore RA, McQuay HJ. Single dose oral naproxen and naproxen sodium for acute postoperative pain in adults. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD004234.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2012
- Derry C, Derry S, Moore A. Sumatriptan (oral route of administration) for acute migraine attacks in adults. Cochrane Database of Systematic Reviews 2012;2:CD008615. [DOI: 10.1002/14651858.CD008615.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2013
- Derry S, Moore RA, McQuay HJ. Paracetamol (acetaminophen) with or without an antiemetic for acute migraine headaches in adults. Cochrane Database of Systematic Reviews 2013;4:CD008040. [DOI: 10.1002/14651858.CD008040.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Diamond 2007
- Diamond S, Bigal ME, Silberstein S, Loder E, Reed M, Lipton RB. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention study. Headache 2007;47(3):355-63. [DOI: 10.1111/j.1526-4610.2006.00631.x] [DOI] [PubMed] [Google Scholar]
Friedman 2005
- Friedman BW, Corbo J, Lipton RB, Bijur PE, Esses D, Solorzano C, et al. A trial of metoclopramide vs sumatriptan for the emergency department treatment of migraines. Neurology 2005;64(3):463-8. [DOI: 10.1212/01.WNL.0000150904.28131.DD] [DOI] [PubMed] [Google Scholar]
Hazard 2009
- Hazard E, Munakata J, Bigal ME, Rupnow MF, Lipton RB. The burden of migraine in the United States: current and emerging perspectives on disease management and economic analysis. Value in Health 2009;12(1):55-64. [DOI: 10.1111/j.1524-4733.2008.00404.x] [DOI] [PubMed] [Google Scholar]
Hernandez‐Diaz 2000
- Hernández-Diaz S, García Rodriguez LA. Association between nonsteroidal anti-inflammatory drugs and upper gastrointestinal tract bleeding and perforation: an overview of epidemiological studies published in the 1990s. Archives of Internal Medicine 2000;160(14):2093-9. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Chapter 8: Assessing risk of bias in included studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011 Available from www.cochrane-handbook.org.
IHS 1988
- Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia 1988;8 Suppl 7:1-96. [PubMed] [Google Scholar]
IHS 2000
- International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of drugs in migraine: second edition. Cephalalgia 2000;20(9):765-86. [DOI] [PubMed] [Google Scholar]
IHS 2004
- Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004;24 Suppl 1:1-160. [DOI] [PubMed] [Google Scholar]
IHS 2013
- Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia 2013;33(9):629-808. [DOI: 10.1177/0333102413485658] [DOI] [PubMed] [Google Scholar]
Jadad 1996a
- Jadad AR, Carroll D, Moore A, McQuay H. Developing a database of published reports of randomised clinical trials in pain research. Pain 1996;66(2-3):239-46. [DOI] [PubMed] [Google Scholar]
Jadad 1996b
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Controlled Clinical Trials 1996;17(1):1-12. [DOI] [PubMed] [Google Scholar]
Jakubowski 2007
- Jakubowski M, Levy D, Kainz V, Zhang XC, Kosaras B, Burstein R. Sensitization of central trigeminovascular neurons: blockade by intravenous naproxen infusion. Neuroscience 2007;148(2):573-83. [DOI: 10.1016/j.neuroscience.2007.04.064] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirthi 2013
- Kirthi V, Derry S, Moore RA, McQuay HJ. Aspirin with or without an antiemetic for acute migraine headaches in adults. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD008041.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
L'Abbé 1987
- L'Abbé KA, Detsky AS, O'Rourke K. Meta-analysis in clinical research. Annals of Internal Medicine 1987;107(2):224-33. [DOI] [PubMed] [Google Scholar]
Law 2013
- Law S, Derry S, Moore A. Sumatriptan plus naproxen for acute migraine attacks in adults. Cochrane Database of Systematic Reviews 2013, Issue 10. [DOI: 10.1002/14651858.CD008541] [DOI] [PubMed] [Google Scholar]
Leonardi 2005
- Leonardi M, Steiner TJ, Scher AT, Lipton RB. The global burden of migraine: measuring disability in headache disorders with WHO's Classification of Functioning, Disability and Health (ICF). Journal of Headache and Pain 2005;6(6):429-40. [DOI: 10.1007/s10194-005-0252-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Linde 2012
- Linde M, Gustavsson A, Stovner LJ, Steiner TJ, Barré J, Katsarava Z, et al. The cost of headache disorders in Europe: the Eurolight project. European Journal of Neurology 2012;19(5):703-11. [DOI: 10.1111/j.1468-1331.2011.03612.x] [DOI] [PubMed] [Google Scholar]
Lipton 1999
- Lipton RB, Stewart WF. Acute migraine therapy: do doctors understand what patients with migraine want from therapy? Headache 1999;39 Suppl 2:S20-S26. [Google Scholar]
Lipton 2007
- Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, AMPP Advisory Group, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007;68(5):343-9. [DOI: 10.1111/j.1468-2982.2006.01275.x] [DOI] [PubMed] [Google Scholar]
Lucas 2006
- Lucas C, Géraud G, Valade D, Chautard MH, Lantéri-Minet M. Recognition and therapeutic management of migraine in 2004, in France: results of FRAMIG 3, a French nationwide population-based survey. Headache 2006;46(5):715-25. [DOI: 10.1111/j.1526-4610.2006.00430.x] [DOI] [PubMed] [Google Scholar]
Martindale 2012
- Brayfield A. Martindale: the complete drug reference. www.medicinescomplete.com/mc/martindale/current/ (19 September 2013).
Moore 1998
- Moore RA, Gavaghan D, Tramer MR, Collins SL, McQuay HJ. Size is everything - large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209-16. [DOI: 10.1016/S0304-3959(98)00140-7] [DOI] [PubMed] [Google Scholar]
Moore 2008
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA, editors(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15-24. [ISBN: 978-0-931092-69-5] [Google Scholar]
Moore 2010a
- Moore RA, Moore OA, Derry S, Peloso PM, Gammaitoni AR, Wang H. Responder analysis for pain relief and numbers needed to treat in a meta-analysis of etoricoxib osteoarthritis trials: bridging a gap between clinical trials and clinical practice. Annals of the Rheumatic Diseases 2010;69(2):374-9. [DOI: 10.1136/ard.2009.107805] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2010b
- Moore RA, Smugar SS, Wang H, Peloso PM, Gammaitoni A. Numbers-needed-to-treat analyses - do timing, dropouts, and outcome matter? Pooled analysis of two randomized, placebo-controlled chronic low back pain trials. Pain 2010;151(3):592-7. [DOI: 10.1016/j.pain.2010.07.013] [DOI] [PubMed] [Google Scholar]
Morris 1995
- Morris JA, Gardner MJ. Calculating confidence intervals for relative risk, odds ratios and standardised ratios and rates. In: Gardner MJ, Altman DG, editors(s). Statistics with Confidence - Confidence Intervals and Statistical Guidelines. London: British Medical Journal, 1995:50-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Munakata 2009
- Munakata J, Hazard E, Serrano D, Klingman D, Rupnow MF, Tierce J, et al. Economic burden of transformed migraine: results from the American Migraine Prevalence and Prevention (AMPP) Study. Headache 2009;49(4):498-508. [DOI: 10.1111/j.1526-4610.2009.01369.x] [DOI] [PubMed] [Google Scholar]
Pardutz 2010
- Pardutz A, Schoen J. NSAIDs in the acute treatment of migraine: a review of clinical and experimental data. Pharmaceuticals 2010;3:1966-87. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pozen 2005a
- Pozen Inc. MT 100 (naproxen sodium and metoclopramide hydrochloride) Tablets. Briefing document for peripheral and central nervous system drugs advisory committee meeting, 4 August 2005, 2005. www.fda.gov/ohrms/dockets/ac/05/briefing/2005-4167B1_01_01-Pozen-Backgrounder.pdf (accessed 19 September 2013).
Pozen 2005b
- Pozen Inc. MT100 for the treatment of migraine, 2005. www.fda.gov/ohrms/dockets/ac/05/briefing/ 2005-4167S1_01_POZEN-Presentation.ppt (accessed 17 April 2012).
Rabbie 2013
- Rabbie R, Derry S, Moore RA, McQuay HJ. Ibuprofen with or without an antiemetic for acute migraine headaches in adults. Cochrane Database of Systematic Reviews 2013;4:CD008039. [DOI: 10.1002/14651858.CD008039.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Radtke 2009
- Radtke A, Neuhauser H. Prevalence and burden of headache and migraine in Germany. Headache 2009;49(1):79-89. [DOI: 10.1111/j.1526-4610.2008.01263.x] [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Ross‐Lee 1983
- Ross-Lee LM, Eadie MJ, Heazlewood V, Bochner F, Tyrer JH. Aspirin pharmacokinetics in migraine. The effect of metoclopramide. European Journal of Clinical Pharmacology 1983;24(6):777-85. [DOI] [PubMed] [Google Scholar]
Salazar‐Tortolero 2008
- Salazar-Tortolero G, Huertas-Campistol A, Vergez-Pinto L, Ramos-Brunet A, Lluch-López J. Metoclopramide as a painkiller for intense migraine headache in emergency departments [Metoclopramida como analgésicoen la cefalea migrañosa intensa en urgencias]. Revista de Neurologia 2008;47(10):506-8. [PMID: ] [PubMed] [Google Scholar]
Steiner 2013
- Steiner TS, Stovner LJ, Birbeck GL. Migraine: the seventh disabler. Journal of Headache and Pain 2013;14:1. [DOI: 10.1186/1129-2377-14-1] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stovner 2010
- Stovner LJ, Andree C. Prevalence of headache in Europe: a review for the Eurolight project. Journal of Headache and Pain 2010;11(4):289-99. [DOI: 10.1007/s10194-010-0217-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Suthisisang 2010
- Suthisisang CC, Poolsup N, Suksomboon N, Lertpipopmetha V, Tepwitukgid B. Meta-analysis of the efficacy and safety of naproxen sodium in the acute treatment of migraine. Headache 2010;50(5):808-18. [DOI: 10.1111/j.1526-4610.2010.01635.x] [DOI] [PubMed] [Google Scholar]
Tramer 1997
- Tramèr MR, Reynolds DJM, Moore RA, McQuay HJ. Impact of covert duplicate results on meta-analysis: a case study. BMJ 1997;315(7109):635-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Victor 2010
- Victor TW, Hu X, Campbell JC, Buse DC, Lipton RB. Migraine prevalence by age and sex in the United States: a life-span study. Cephalalgia 2010;30(9):1065-72. [DOI: 10.1177/0333102409355601 ] [DOI] [PubMed] [Google Scholar]
Volans 1974
- Volans GN. Absorption of effervescent aspirin during migraine. British Medical Journal 1974;4(5939):265-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Volans 1975
- Volans GN. The effect of metoclopramide on the absorption of effervescent aspirin in migraine. British Journal of Clinical Pharmacology 1975;2(1):57-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vos 2012
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012;380(9859):2163-96. [DOI: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yu 2012
- Yu S, Liu R, Zhao G, Yang X, Qiao X, Feng J, et al. The prevalence and burden of primary headaches in China: a population-based door-to-door survey. Headache 2012;52(4):582-91. [DOI: 10.1111/j.1526-4610.2011.02061.x] [DOI] [PubMed] [Google Scholar]


