Abstract
Background
Previous studies have shown the relationship between higher skin thickness score and the existence of organ involvements in systemic sclerosis (SSc). Here, we firstly investigated the correlation between skin thickness score and quantitative measurements of each organ involvement in Japanese patients with SSc.
Methods
All Japanese SSc patients hospitalized to our clinic for initial evaluation of SSc were selected. Skin thickness was evaluated by modified Rodnan total skin thickness score (mRSS). Relationship between mRSS and prevalence or incidence of organ involvements was examined by logistic analyses. Correlation between mRSS and quantitative measurements of organ involvements was examined by correlation analyses and regression analyses.
Results
We recruited 198 patients into our study. The mean disease duration was 7.3 years with the mean follow-up duration of 3.2 years. Multivariate logistic regression analyses revealed that higher mRSS is related to higher prevalence of interstitial lung disease (P < 0.05), restrictive impairment (P < 0.01), and diffusion impairment (P < 0.05) of the lung. Correlation analyses revealed mRSS negatively correlates with forced vital capacity (P < 0.001) and diffusing capacity (P < 0.001) of the lung. Correlation between longitudinal change of mRSS and that of forced vital capacity (P < 0.05) or diffusing capacity (P < 0.001) of the lung was also demonstrated.
Conclusions
Skin thickness score significantly correlates with quantitative measurements of lung involvement in Japanese patients with SSc.
Keywords: Systemic sclerosis, Modified Rodnan total skin thickness score, Interstitial lung disease, Regression analysis
Background
Systemic sclerosis (SSc) is a connective tissue disease characterized by fibrosis, vascular injury, and immunological abnormality across multiple organs [1]. Variable symptoms of SSc are the reflection of each organ involvement by different aspects of the pathology, which makes it difficult to grasp the whole picture of the disease. For example, fibrosis of the dermis causes skin sclerosis [2], while alveolar fibrosis causes systemic sclerosis-related interstitial lung disease (SSc-ILD) [3]. In addition, vascular injury in the extremities causes digital ulcer [4], renal involvement triggers SSc renal crisis (SRC) [5], and impairment of the pulmonary arteries causes pulmonary hypertension [6]. The reflection of immunological abnormality is a variety of autoantibodies detected from the sera of SSc patients [7, 8].
Effective treatment differs by each organ involvement, which requires clinicians to combine multiple therapeutic modalities to treat SSc patients. Some medications have pivotal effect on each organ involvement, making the treatment further difficult. For instance, systemic corticosteroids are used in treating skin fibrosis and SSc-ILD [9, 10], while high-dose corticosteroids are known as a risk factor of SRC development [11, 12]. Calcium channel blockers are sometimes used for treating Raynaud’s phenomenon in SSc patients [13], but they worsen the symptoms of gastroesophageal reflux disease (GERD) [14]. To optimize the combination of such multiple therapeutic modalities, evaluation of severity and disease activity for SSc patients should be multidimensional. Skin thickness is one of such measurements that have long time been used and evaluated as a barometer that mainly reflects the aspect of fibrosis. It has been revealed that tighter skin is a predictive factor of death [15], heart involvement [16], muscle involvement [17], and development of SRC [18, 19]. In addition, higher skin thickness score is related to severer function disability of SSc patients measured by health assessment questionnaire disability index [20, 21]. It has also been reported that rapid increase in skin thickness score is a predictor of higher incidence of early death and SRC [22].
Modified Rodnan total skin thickness score (mRSS) is one of the established methods to examine the skin thickness of SSc patients. It is a semiquantitative, noninvasive, and rapid method to measure the skin thickness with high reproducibility [18], which makes it widely used both in clinical trials and real clinical practice [23]. Previous studies have investigated the relationship between incidence of disease-related events or presence of organ involvement and skin thickness that was quantitatively evaluated [15–19]. However, almost all of them targeted Caucasians, Hispanics, or African American patients.
Herein, we investigated the correlation between mRSS and quantitative measurements of organ involvement by retrospective observation of Japanese SSc cohort. Our goal is to evaluate the utility of mRSS as a quantitative barometer of other organ involvement in SSc patients.
Methods
Subject patients
We included all the patients with SSc hospitalized to our clinic since May 2011 until April 2018. All the new patients arrived at our clinic receives initial evaluation for SSc, including physical examinations, laboratory tests, high-resolution computed tomography (HRCT) scanning of the lung, pulmonary function tests, transthoracic echocardiography, and gastrointestinal endoscopy. Only Japanese patients hospitalized for initial evaluation of SSc were recruited into the present study. We excluded patients who did not meet the classification criteria for SSc established by American College of Rheumatology and European League Against Rheumatism in 2013 (ACR/EULAR criteria 2013) [24]. This study was approved by the ethics committee at The University of Tokyo Hospital.
Data collection
We retrospectively reviewed the electronic medical records. The patients’ demographic information, laboratory results, and examination findings were obtained at the time closest to the first admission to our clinic. The collected demographic information included age, sex, disease duration, follow-up duration, and medication regimen. The laboratory results included autoantibody profiles, blood cell counts, C-reactive protein (CRP) levels, and erythrocyte sedimentation rates (ESRs).
Longitudinal data of mRSS and the result of pulmonary function test were collected from the most recent time when these examinations were performed at the same time.
Qualitative evaluation of organ involvements
Systolic dysfunction of the heart was defined as left ventricle ejection fraction (LVEF) < 50%. Diastolic dysfunction of the heart was defined as ratio between early mitral inflow velocity and mitral annular early diastolic velocity (E/e’) > 15. Pulmonary hypertension was defined as mean pulmonary artery pressure > 25 mmHg on right heart catheterization. Presence of SSc-ILD was determined upon HRCT readings. Restrictive impairment of the lung was defined as the percentage of predicted forced vital capacity (%FVC) < 80%. Patients with the ratio of forced expiratory volume in 1 s to forced vital capacity (FEV1%) < 70% were excluded to rule out the obstructive components of the lung. Diffusion impairment of the lung was defined as the percentage of diffusing capacity for carbon monoxide (%DLco) < 70%. Classification of GERD findings from upper gastrointestinal endoscopy was performed by gastroenterologists, and reflux esophagitis was defined as Grade M or more on Los Angeles classification of esophagus [25]. Myositis was defined as elevation of serum CK twofold higher than the upper normal limit.
The reviewed disease-related events contained death, SRC, ileus, and heart failure that were observed since the primary evaluation until the most recent arrival to our clinic. Occurrence of SRC, ileus, and heart failure was determined upon clinical diagnosis.
Quantitative or categorical evaluation of organ involvements
The degree of skin sclerosis was scored on mRSS by 6 dermatologists at The University of Tokyo Scleroderma Center, all of whom had been trained by repeated teaching method previously described [26]. The evaluation value of lung involvement included serum levels of Krebs von den Lungen-6 (KL-6) and surfactant protein-D (SP-D) levels, and pulmonary function parameters including %FVC and %DLco. Quantitative evaluation of heart involvement encompassed serum level of brain natriuretic peptide (BNP) and the results of echocardiography including LVEF, E/e’, and right ventricle systolic pressure (RVSP). Renal involvement was described by estimated glomerular filtration rate (eGFR). Severity of GERD on upper gastrointestinal endoscopy was categorically evaluated by Los Angeles classification of esophagus. Quantitative measurement of musculoskeletal system included the serum level of creatinine kinase (CK).
Statistical analyses
Univariate analysis was performed by single logistic analysis for categorical variables and single regression analysis for continuous variables. Correlation analysis was also performed between some continuous variables to estimate Pearson’s correlation coefficient. Multivariate analysis was performed by multiple logistic analysis for categorical variables and multiple regression analysis for continuous variables. Explanatory variables in multivariate analysis were age, sex, disease duration, and mRSS. In sensitivity analyses, the following explanatory variables were added: baseline presence of pulmonary hypertension, the use of corticosteroids or immunosuppressants, the use of vasoactive agents (endothelin receptor antagonists, phosphodiesterase 5 inhibitors, beraprost, sarpogrelate hydrochloride, limaprost alfadex, and angiotensin-converting enzyme inhibitors), and the history of smoking. All the analyses were performed using Stata/IC 15 (StataCorp LLC, TX, USA).
Results
Demographics of the subject patients
In total, there were 1101 patients with SSc hospitalized to our clinic during the study period. We selected 228 patients who were hospitalized for initial evaluation of SSc and excluded 28 patients because they did not meet ACR/EULAR criteria 2013. Two patients were excluded because they were from Taiwan and the Philippines. As a result, 198 Japanese patients were recruited into our study (Fig. 1). The background features of the participants are summarized in Table 1. Their mean age was 55.4 years old (standard deviation [SD] = 15.5) with the number of female patients of 177 (89.4%). The mean disease duration was 7.3 years (SD = 8.8) with the mean follow-up duration of 3.2 years (SD = 2.3). The proportion of patients with history of smoking was 21.9%. Almost half of the patients (46.8%) had diffuse cutaneous SSc. The frequency of specific autoantibody positivity in the sera of the patients was as follows: anti-topoisomerase (topo) I antibody (Ab) in 78 patients (39.4%), anti-centromere Ab in 64 patients (32.3%), anti-RNA polymerase III Ab in 21 patients (10.6%), and anti-U1RNP Ab in 22 patients (11.1%). Corticosteroids or immunosuppressants had already been introduced in 50 patients (25.3%), and vasoactive agents had been administered to 74 patients (37.4%).
Fig. 1.
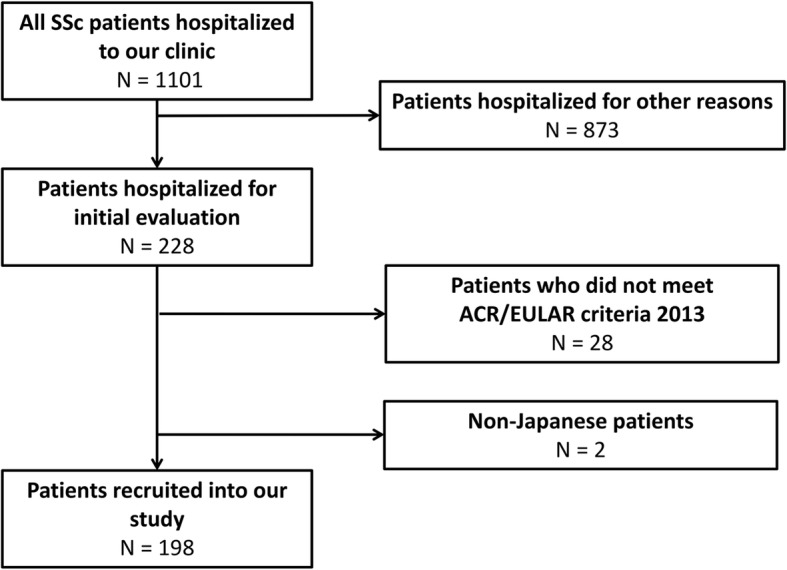
The flow chart of patient recruitment. All Japanese patients with systemic sclerosis (SSc) hospitalized to our clinic for initial evaluation were collected. Patients who did not meet the classification criteria for SSc established by American College of Rheumatology and European League Against Rheumatism in 2013 (ACR/EULAR criteria 2013) were excluded. In total, 198 patients were recruited into our study
Table 1.
Clinical demographics of the subject patients
| N | n (%) or mean (SD) | |
|---|---|---|
| Male | 198 | 21 (10.6%) |
| Female | 198 | 177 (89.4%) |
| Age (years) | 198 | 55.4 (15.5) |
| Disease duration (years) | 196 | 7.3 (8.8) |
| Follow-up duration (years) | 198 | 3.2 (2.3) |
| History of smoking | 196 | 43 (21.9%) |
| Death | 198 | 4 (2.0%) |
| Raynaud’s phenomenon | 196 | 170 (86.7%) |
| Puffy finger | 144 | 97 (67.4%) |
| Nail fold bleeding | 190 | 139 (73.2%) |
| Telangiectasia | 162 | 65 (40.1%) |
| Autoantibody | ||
| Anti-topo I Ab | 198 | 78 (39.4%) |
| Anti-centromere Ab | 198 | 64 (32.3%) |
| Anti-RNA polymerase III Ab | 198 | 21 (10.6%) |
| Anti-U1RNP Ab | 198 | 22 (11.1%) |
| Medications | ||
| Corticosteroids or immunosuppressants | 198 | 50 (25.3%) |
| Corticosteroids | 198 | 43 (21.7%) |
| Immunosuppressants | 198 | 19 (9.6%) |
| Vasoactive agents | 198 | 74 (37.4%) |
| Endothelin receptor antagonists | 198 | 14 (7.1%) |
| Phosphodiesterase 5 inhibitors | 198 | 5 (2.5%) |
| Beraprost | 198 | 52 (26.3%) |
| Sarpogrelate hydrochloride | 198 | 26 (13.1%) |
| Limaprost alfadex | 198 | 15 (7.6%) |
| Angiotensin-converting enzyme inhibitors | 198 | 3 (1.5%) |
| Others | ||
| Non-steroidal anti-inflammatory drugs | 198 | 27 (13.6%) |
| Tocopherol nicotinate | 198 | 64 (32.3%) |
| Proton pump inhibitors | 198 | 87 (43.9%) |
| Laboratory tests | ||
| White blood cells (/mm3) | 198 | 6700 (2300) |
| Hemoglobin (g/dL) | 198 | 12.3 (1.9) |
| Hematocrit (%) | 198 | 38.5 (5.1) |
| Platelets (× 104/mm3) | 198 | 26.7 (7.8) |
| CRP (mg/dL) | 198 | 0.43 (1.19) |
| ESR (mm/h) | 195 | 25.8 (19.7) |
| Skin involvement | ||
| Qualitative measurement | ||
| Diffuse cutaneous systemic sclerosis | 190 | 89 (46.8%) |
| Quantitative measurement | ||
| mRSS | 177 | 9.9 (8.9) |
| Lung involvement | ||
| Qualitative evaluation | ||
| SSc-ILD | 196 | 87 (44.4%) |
| Restrictive impairment | 197 | 36 (18.3%) |
| Diffusion impairment | 191 | 33 (17.3%) |
| Quantitative evaluation | ||
| KL-6 (U/mL) | 198 | 519 (499) |
| SP-D (ng/mL) | 190 | 107 (98) |
| %FVC (%) | 197 | 95.9 (20.8) |
| %DLco (%) | 191 | 88.3 (20.0) |
| FEV1% (%) | 197 | 82.1 (8.7) |
| Heart involvement | ||
| Qualitative evaluation | ||
| Systolic dysfunction | 184 | 0 (0%) |
| Diastolic dysfunction | 150 | 10 (6.7%) |
| Pulmonary hypertension | 198 | 5 (2.5%) |
| Heart failure | 198 | 3 (1.5%) |
| Quantitative evaluation | ||
| BNP (pg/mL) | 191 | 43.0 (50.0) |
| LVEF (%) | 184 | 70.1 (6.3) |
| E/e’ | 150 | 9.6 (3.5) |
| RVSP (mmHg) | 181 | 27.3 (7.5) |
| Renal involvement | ||
| Qualitative evaluation | ||
| SRC | 198 | 6 (3.0%) |
| Quantitative evaluation | ||
| eGFR (mL/min/1.73 m2) | 198 | 87.8 (25.9) |
| Gastrointestinal involvement | ||
| Qualitative evaluation | ||
| Reflux esophagitis | 179 | 78 (43.6%) |
| Ileus | 198 | 6 (3.0%) |
| Categorical evaluation | ||
| Los Angeles classification | ||
| Grade N | 179 | 101 (56.4%) |
| Grade M | 179 | 30 (16.8%) |
| Grade A | 179 | 32 (17.9%) |
| Grade B | 179 | 11 (6.2%) |
| Grade C | 179 | 4 (2.2%) |
| Grade D | 179 | 1 (0.6%) |
| Musculoskeletal involvement | ||
| Qualitative evaluation | ||
| Myositis | 197 | 7 (3.6%) |
| Quantitative evaluation | ||
| CK (U/L) | 197 | 110 (119) |
N number of the observation, n number of the patients applicable, SD standard deviation
Incidence and prevalence of organ involvements
The number of patients with each organ involvement was as follows: interstitial lung diseases in 87 patients (44.4%), restrictive impairment of the lung in 36 patients (18.3%), diffusion impairment of the lung in 33 patients (17.3%), diastolic dysfunction of the heart in 10 patients (6.7%), pulmonary hypertension in 5 patients (2.5%), heart failure in 3 patients (1.5%), SRC in 6 patients (3.0%), reflux esophagitis in 78 patients (43.6%), ileus in 6 patients (3.0%), and myositis in 7 patients (3.6%). There were no patients with systolic dysfunction of the heart.
Single and multiple logistic analyses revealed that mRSS is associated with death, SRC, and lung involvement
Single logistic analyses revealed that higher mRSS is related to higher incidence of death (P < 0.05) and SRC (P < 0.05). Higher mRSS was also related to baseline presence of SSc-ILD (P < 0.05), restrictive impairment (P < 0.01), and diffusion impairment (P < 0.01) of the lung (Table 2). Multiple logistic analyses showed that relation between mRSS and these organ involvements is statistically significant even after compensating with the patients’ basic characteristics (sex, age, and disease duration). Meanwhile, no relationship was found between mRSS and incidence of ileus and heart failure. Furthermore, there was no relationship between mRSS and presence of reflux esophagitis and myositis. Collectively, higher mRSS was significantly and independently associated with higher incidence of death and SRC and higher prevalence of SSc-ILD, restrictive impairment, and diffusion impairment of the lung.
Table 2.
Logistic analysis of relationship between mRSS and qualitative evaluation of organ involvement
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| N | OR (95% CI) | N | OR (95% CI) | |
| Death | 177 | 1.15* (1.03 to 1.29) | 176 | 1.23* (1.03 to 1.46) |
| Lung involvement | ||||
| Interstitial lung disease | 175 | 1.04* (1.00 to 1.08) | 174 | 1.05* (1.01 to 1.09) |
| Restrictive impairment | 176 | 1.07** (1.03 to 1.12) | 175 | 1.07** (1.02 to 1.12) |
| Diffusion impairment | 171 | 1.07** (1.02 to 1.11) | 170 | 1.06* (1.01 to 1.11) |
| Heart involvement | ||||
| Diastolic dysfunction | 136 | 1.03 (0.96 to 1.10) | ||
| Pulmonary hypertension | 177 | 0.97 (0.86 to 1.09) | ||
| Heart failure | 177 | 1.11 (0.99 to 1.23) | ||
| Renal involvement | ||||
| SRC | 177 | 1.11* (1.02 to 1.20) | 176 | 1.11* (1.01 to 1.23) |
| Gastrointestinal involvement | ||||
| Reflux esophagitis | 161 | 1.03 (0.99 to 1.07) | ||
| Ileus | 177 | 1.04 (0.95 to 1.13) | ||
| Musculoskeletal involvement | ||||
| Myositis | 177 | 1.03 (0.95 to 1.11) | ||
N number of the observation, OR odds ratio, CI confidence interval. Asterisk (*) indicates statistical significance in logistic analysis.*P < 0.05; **P < 0.01
Single and multiple regression analyses showed the correlation between mRSS and lung involvement
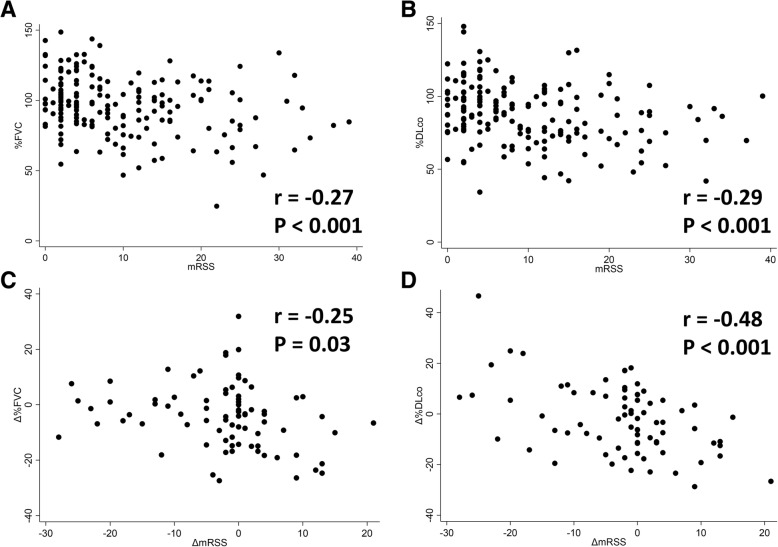
Single regression analyses revealed that mRSS negatively correlates with %FVC (P < 0.001) and %DLco (P < 0.001) and positively correlates with eGFR (P < 0.05) and the serum level of SP-D (P < 0.05; Table 3). Correlation analyses also showed negative correlation between mRSS and %FVC (P < 0.001; Fig. 2a) or %DLco (P < 0.001; Fig. 2b). There was no correlation between mRSS and the serum levels of KL-6 and CK, the results of echocardiography, or endoscopic findings in the esophagus.
Table 3.
Regression analysis of correlation between mRSS and quantitative or categorical evaluation of organ involvement
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| N | β (95% CI) | N | β (95% CI) | |
| Lung involvement | ||||
| KL-6 | 177 | 5.14 (−3.38 to 13.7) | ||
| SP-D | 170 | 1.87* (0.27 to 3.47) | 169 | 1.87* (0.09 to 3.65) |
| %FVC | 176 | − 0.64*** (− 0.98 to − 0.30) | 175 | − 0.61** (− 0.98 to − 0.23) |
| %DLco | 171 | − 0.67*** (− 1.00 to − 0.33) | 170 | − 0.53** (− 0.89 to − 0.16) |
| Heart involvement | ||||
| BNP | 171 | 0.82 (− 0.02 to 1.66) | ||
| LVEF | 166 | 0.06 (− 0.05 to 0.17) | ||
| E/e’ | 136 | 0.02 (− 0.04 to 0.09) | ||
| RVSP | 164 | 0.11 (− 0.02 to 0.25) | ||
| Renal involvement | ||||
| eGFR | 177 | 0.44* (0.03 to 0.85) | 176 | 0.11 (− 0.25 to 0.46) |
| Gastrointestinal involvement | ||||
| Los Angeles classification | 161 | 0.02 (− 0.01 to 0.04) | ||
| Musculoskeletal involvement | ||||
| CK | 177 | 1.99 (− 0.09 to 4.07) | ||
N number of the observation, β regression coefficient, CI confidence interval. Asterisk (*) indicates statistical significance in regression analysis.*P < 0.05; **P < 0.01; ***P < 0.001
Fig. 2.
The scatter plot of mRSS and the result of pulmonary function test. On the baseline, a %FVC and b %DLco negatively correlate with mRSS (correlation coefficient [r] = − 0.27, P < 0.001 and r = − 0.29, P < 0.001, respectively). In addition, the longitudinal analysis showed negative correlation of ΔmRSS with c Δ%FVC and d Δ%DLco (r = − 0.25, P = 0.03 and r = − 0.48, P < 0.001, respectively)
In addition, multiple regression analyses showed that negative correlation between mRSS and the results of pulmonary function test or serum levels of SP-D is statistically significant even after compensation with the patients’ basic characteristics. In contrast, correlation between mRSS and eGFR was not statistically significant in multiple regression analyses.
Sensitivity analyses clarified that correlation between mRSS and pulmonary function is independent from other explanatory variables
Sensitivity analysis of the multivariate regression model was performed by adding other explanatory variables. The significance of mRSS as an explanatory variable of %FVC and %DLco was robust to adding baseline presence of pulmonary hypertension, the use of corticosteroids or immunosuppressants, the use of vasoactive agents, or the history of smoking (Table 4). Thus, mRSS significantly and independently correlated with %FVC and %DLco.
Table 4.
Sensitivity analysis of the multivariate regression model
| Additional variables | %FVC vs mRSS | %DLco vs mRSS | ||
|---|---|---|---|---|
| N | β (95% CI) | N | β (95% CI) | |
| Pulmonary hypertension | 175 | − 0.59** (− 0.97 to − 0.22) | 170 | − 0.52** (− 0.88 to − 0.16) |
| Use of corticosteroids or immunosuppressants | 175 | − 0.52** (− 0.89 to − 0.15) | 170 | − 0.45* (− 0.81 to − 0.10) |
| Use of vasoactive agents | 175 | − 0.59** (− 0.97 to − 0.22) | 170 | − 0.50** (− 0.85 to − 0.14) |
| History of smoking | 174 | − 0.62** (− 0.98 to − 0.27) | 169 | − 0.52** (− 0.88 to − 0.16) |
β regression coefficient, CI confidence interval. Asterisk (*) indicates statistical significance in regression analysis.*P < 0.05; **P < 0.01
Subgroup analyses revealed correlation between mRSS and lung function is significant in patients with anti-topo I Ab and in patients with disease duration shorter than 5 years
We performed subgroup analyses of correlation between mRSS and baseline %FVC or %DLco by autoantibody profile and disease duration (Table 5). In patients with anti-topo I Ab, the correlation to %FVC and %DLco was statistically significant in both single regression analyses (%FVC: P < 0.05; %DLco: P < 0.01) and multiple regression analyses (%FVC: P < 0.01; %DLco: P < 0.05). By contrast, these correlations were not statistically significant among patients with anti-centromere, anti-RNA polymerase III, or anti-U1 RNP Abs. In addition, multivariate regression analysis showed significant correlation between mRSS and both %FVC (P < 0.05) and %DLco (P < 0.05) in SSc patients with disease duration shorter than 5 years, while mRSS significantly correlated with %FVC (P < 0.01) but not with %DLco among patients with disease duration of 5 years or more. Taken together, correlation between mRSS and %FVC or %DLco was significant especially among patients with anti-topo I Ab and patients with disease duration shorter than 5 years.
Table 5.
Subgroup analysis of correlation between mRSS and pulmonary function by autoantibody profile or disease duration
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| N | β (95% CI) | N | β (95% CI) | |
| %FVC | ||||
| Autoantibody profile | ||||
| Anti-topo I Ab | 65 | − 0.68* (− 1.25 to − 0.11) | 65 | − 0.80** (− 1.39 to − 0.20) |
| Anti-centromere Ab | 60 | − 1.36 (− 1.18 to 0.91) | ||
| Anti-RNA polymerase III Ab | 17 | 0.66 (− 0.29 to 1.61) | ||
| Anti-U1RNP Ab | 19 | − 0.09 (− 1.07 to 0.89) | ||
| Disease duration | ||||
| < 5 years | 106 | − 0.41* (− 0.77 to − 0.05) | 106 | − 0.43* (− 0.84 to − 0.02) |
| ≥ 5 years | 69 | − 1.53*** (− 2.36 to − 0.70) | 69 | − 1.48** (− 2.33 to − 0.63) |
| %DLco | ||||
| Autoantibody profile | ||||
| Anti-topo I Ab | 62 | − 0.80** (− 1.36 to − 0.24) | 53 | − 0.68* (− 1.27 to − 0.08) |
| Anti-centromere Ab | 58 | − 0.02 (− 1.02 to 1.07) | ||
| Anti-RNA polymerase III Ab | 17 | − 0.07 (− 1.03 to 0.89) | ||
| Anti-U1RNP Ab | 19 | − 0.63 (− 1.59 to 0.32) | ||
| Disease duration | ||||
| < 5 years | 104 | − 0.70** (− 1.10 to − 0.30) | 104 | − 0.52* (− 0.97 to − 0.07) |
| ≥ 5 years | 66 | − 0.86* (− 1.61 to − 0.11) | 66 | − 0.68 (− 1.43 to 0.06) |
N number of the observation, β regression coefficient, CI confidence interval. Asterisk (*) indicates statistical significance in regression analysis.*P < 0.05; **P < 0.01; ***P < 0.001
Longitudinal analyses showed negative correlation between the change in mRSS and that in %FVC and %DLco
Longitudinal data was available for 84 patients (42.4%). The mean follow-up duration among those patients was 2.5 years (SD = 1.9). We examined the correlation between mRSS change (ΔmRSS) and pulmonary function change (Δ%FVC and Δ%DLco). Correlation analyses showed that ΔmRSS negatively correlated with both Δ%FVC (P = 0.03; Fig. 2c) and Δ%DLco (P < 0.001; Fig. 2d). Thus, the longitudinal change in mRSS negatively correlated with the longitudinal change in %FVC and %DLco.
Discussion
Our retrospective observation of SSc patients revealed that mRSS significantly correlates with quantitative measurements of the lung involvement such as %FVC and %DLco on the baseline. The correlation in multivariate regression analysis was robust to adding baseline presence of pulmonary hypertension, the use of corticosteroids or immunosuppressants, the use of vasoactive agents, and the history of smoking as explanatory variables. Moreover, the longitudinal change in mRSS significantly correlated with that in %FVC and %DLco. Although previous studies have shown that higher skin thickness score is related to the existence of organ involvements [15–19], correlation between skin thickness score and quantitative barometers of each organ involvement has not yet been documented in Japan. This is the first study that revealed correlation between skin thickness score and quantitative measurements of organ involvements in Japanese SSc patients.
Close relationship between skin sclerosis and lung fibrosis in SSc patients is suggested by several aspects of clinical experience. First, skin sclerosis and SSc-ILD share their chronology; they both develop in the first few years in the natural time course of SSc [27]. This corresponds to our result that correlation between skin score and pulmonary function was prominent in patients with shorter disease duration. Second, pathohistological feature of skin involvement and lung involvement in SSc patients is quite similar; invasion of inflammatory cells is seen in their early stage, and proliferation and degeneration of collagen fibers is observed in their late stage [2, 3]. Third, SSc patients with anti-topo I Ab experience combination of severe skin sclerosis and SSc-ILD [7, 8]. Indeed, correlation between mRSS and pulmonary function was prominent in patients with anti-topo I Ab in our study. It suggests that skin and lung fibrosis in SSc has similar abnormality of immune system as its background. Forth, recent clinical experiences have indicated that both skin and lung fibrosis responds well to B cell-targeting therapy, including rituximab and tocilizumab. Previously, our group has revealed that B cells play a key role in the pathogenesis of SSc [28]. Abnormality of B cell function including production of autoantibodies and inflammatory cytokines, such as interleukin-6 (IL-6), contributes to the progression of fibrosis in SSc mouse models [29]. Rituximab, a chimeric monoclonal Ab binding to CD20, ablates B cells from blood circulation via targeting CD20 expressed on the surface of B cells. Some open-label clinical studies [30–33] and a retrospective case-control study [34] revealed that SSc patients on rituximab showed significant improvement of mRSS and %FVC, which is now being verified by an ongoing double-blind randomized placebo-controlled trial (UMIN000030139). Tocilizumab, a humanized monoclonal Ab binding to IL-6 receptors, inhibits the signaling pathway via IL-6 mainly secreted by B cells that modulate inflammation and tissue fibrosis [35, 36]. A double-blind randomized placebo-controlled trial (NCT01532869) indicated that weekly subcutaneous injection of tocilizumab reduces mean mRSS and prevents FVC from decline [37, 38]. These facts highlight the crucial role of B cells in the pathogenesis of both skin and lung involvement of SSc.
Furthermore, our study showed that higher mRSS is predictive for higher mortality and higher incidence of SRC. These results from Japanese SSc cohort are consistent with those of previous studies in other ethnic populations [18, 19], which indicates that skin thickness may be an indicator of not only organ fibrosis represented by skin fibrosis and SSc-ILD, but also other aspects of SSc such as vasculopathy.
From a viewpoint of clinical application, mRSS is promised as a good surrogate marker of lung involvement in SSc patients. Our study showed that mRSS significantly correlates with %FVC and %DLco in SSc patients both on the baseline and along the time course. Other evaluation methods for SSc-ILD, including serologic marker measurements, HRCT, or 6-min walk test, are more invasive or time-consuming than measuring mRSS. Moreover, it has also been reported that mRSS is a measurement tool with less inter- and intra-observer variation [39]. Skin thickness score might be a reasonable way of monitoring the efficacy of treatments for SSc-ILD in both clinical trials and real clinical settings. Furthermore, mRSS possibly reflects the global disease activity and severity of SSc. Indeed, Composite Response Index for Systemic Sclerosis includes mRSS in its scoring [40]. Taken together, although further studies are warranted, evaluation of mRSS is useful as a surrogate marker of organ involvements, global disease activity, and severity in SSc.
The major limitation of the present study is its retrospective design. There is some missing in the data especially in longitudinal data, which may bias the result of the analyses. Moreover, the small sample size and short observation term leads to the low power of the analysis, especially for rare disease-related events or subgroup analyses. Prospective observational studies with bigger sample size and longer follow-up are desirable. In addition, the present study includes only Japanese patients. The prevalence of SSc-ILD, decreased %DLco [41], pulmonary hypertension [42], and esophagitis [43] was lower than those reported in previous studies in other populations. Validation of our study should be conducted targeting other ethnicities in the future.
Conclusions
Retrospective observation of Japanese SSc patients revealed significant correlation between skin thickness and pulmonary function. Skin thickness score is a promising surrogate marker of lung involvement in systemic sclerosis.
Acknowledgements
None.
Abbreviations
- %DLco
Percentage of predicted diffusing capacity of the lungs for carbon monoxide
- %FVC
Percentage of predicted forced vital capacity
- 95% CI
95% confidence interval
- Ab
Antibody
- ACR
American College of Rheumatology
- BNP
Brain natriuretic peptide
- CK
Creatinine kinase
- CRP
C-reactive protein
- E/e’
Ratio between early mitral inflow velocity and mitral annular early diastolic velocity
- eGFR
Estimated glomerular filtration rate
- ESR
Erythrocyte sedimentation rate
- EULAR
European League Against Rheumatism
- FEV1%
The ratio of forced expiratory volume in 1 s to forced vital capacity
- GERD
Gastroesophageal reflux disease
- HRCT
High-resolution computed tomography
- IL-6
Interleukin-6
- ILD
Interstitial lung disease
- KL-6
Krebs von den Lungen-6
- LVEF
Left ventricle ejection fraction
- mRSS
Modified Rodnan total skin thickness score
- N
Number of the observation
- n
Number of the patients applicable
- OR
Odds ratio
- r
Correlation coefficient
- RVSP
Right ventricle systolic pressure
- SD
Standard deviation
- SP-D
Surfactant protein-D
- SRC
Systemic sclerosis renal crisis
- SSc
Systemic sclerosis
- β
Regression coefficient
Authors’ contributions
KMM analyzed and interpreted the patient data and was a major contributor in writing the manuscript. AY launched this study and was involved in revising the manuscript. The others participated in collecting the patient data. All authors read and approved the final manuscript.
Funding
There is no funding to declare.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics approval and consent to participate
This study was approved by the ethics committee at The University of Tokyo Hospital. Informed consent was obtained on document from the subject patients.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kazuki M. Matsuda, Email: mkazuki.kom@gmail.com
Ayumi Yoshizaki, Phone: +81-3-3815-5411, Email: yoshizakiay-der@h.u-tokyo.ac.jp.
Ai Kuzumi, Email: kuzumia-der@h.u-tokyo.ac.jp.
Takemichi Fukasawa, Email: fukasawat-der@h.u-tokyo.ac.jp.
Satoshi Ebata, Email: ebatas-der@h.u-tokyo.ac.jp.
Shunsuke Miura, Email: miuras-der@h.u-tokyo.ac.jp.
Tetsuo Toyama, Email: toyamat-der@h.u-tokyo.ac.jp.
Asako Yoshizaki, Email: yoshizakia-der@h.u-tokyo.ac.jp.
Hayakazu Sumida, Email: sumidah-der@h.u-tokyo.ac.jp.
Yoshihide Asano, Email: asanoy-der@h.u-tokyo.ac.jp.
Koji Oba, Email: oba@epistat.m.u-tokyo.ac.jp.
Shinichi Sato, Email: satos-der@h.u-tokyo.ac.jp.
References
- 1.Asano Y. Systemic sclerosis. J Dermatol. 2018;45:128–138. doi: 10.1111/1346-8138.14153. [DOI] [PubMed] [Google Scholar]
- 2.Jinnin M. Mechanisms of skin fibrosis in systemic sclerosis. J Dermatol. 2010;37(1):11–25. doi: 10.1111/j.1346-8138.2009.00738.x. [DOI] [PubMed] [Google Scholar]
- 3.Arroliga AC, Podell RAM DN. Pulmonary manifestations of scleroderma. J Thorac Imaging. 1992;7(2):30–45. doi: 10.1097/00005382-199203000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Giuggioli D, Manfredi A, Lumetti F, Colaci M, Ferri C. Scleroderma skin ulcers definition, classification and treatment strategies our experience and review of the literature. Autoimmun Rev. 2018;17:155–164. doi: 10.1016/j.autrev.2017.11.020. [DOI] [PubMed] [Google Scholar]
- 5.Denton CP, Lapadula G, Mouthon L, Müller-Ladner U. Renal complications and scleroderma renal crisis. Rheumatology. 2006;48:iii32–iii35. doi: 10.1093/rheumatology/ken483. [DOI] [PubMed] [Google Scholar]
- 6.Proudman SM, Stevens WM, Sahhar J, Celermajer D. Pulmonary arterial hypertension in systemic sclerosis: the need for early detection and treatment. Intern Med J. 2007;37:485–494. doi: 10.1111/j.1445-5994.2007.01370.x. [DOI] [PubMed] [Google Scholar]
- 7.Hamaguchi Y. Autoantibody profiles in systemic sclerosis: predictive value for clinical evaluation and prognosis. J Dermatol. 2010;37(1):42–53. doi: 10.1111/j.1346-8138.2009.00762.x. [DOI] [PubMed] [Google Scholar]
- 8.Nihtyanova SI, Denton CP. Autoantibodies as predictive tools in systemic sclerosis. Nat Rev Rheumatol. 2010;6(2):112–116. doi: 10.1038/nrrheum.2009.238. [DOI] [PubMed] [Google Scholar]
- 9.Sharada B, Kumar A, Kakker R, Adya CM, Pande I, Uppal SS, et al. Intravenous dexamethasone pulse therapy in diffuse systemic sclerosis - a randomized placebo-controlled study. Rheumatol Int. 1994;14:91–94. doi: 10.1007/BF00300808. [DOI] [PubMed] [Google Scholar]
- 10.Ando K, Motojima S, Doi T, Nagaoka T, Kaneko N, Aoshima M, et al. Effect of glucocorticoid monotherapy on pulmonary function and survival in Japanese patients with scleroderma-related interstitial lung disease. Respir Investig. 2013;51:69–75. doi: 10.1016/j.resinv.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 11.Steen VD, Medsger TA, Osial TA, Ziegler GL, Alvin Shapiro BP, Rodnan GP. Factors predicting development of renal involvement progressive systemic sclerosis. Am J Med. 1984;76:779–786. doi: 10.1016/0002-9343(84)90986-0. [DOI] [PubMed] [Google Scholar]
- 12.Helfrich DJ, Banner B, Steen VD, Medsger TA. Normotensive renal failure in systemic sclerosis. Arthritis Rheum. 1989;32(9):1128–1134. doi: 10.1002/anr.1780320911. [DOI] [PubMed] [Google Scholar]
- 13.Thompson AE, Shea B, Welch V, Fenlon D, Pope JE. Calcium-channel blockers for Raynaud’s phenomenon in systemic sclerosis. Arthritis Rheum. 2001;44(8):1841–1847. doi: 10.1002/1529-0131(200108)44:8<1841::AID-ART322>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 14.Konrad-Dalhoff I, Baunack AR, Rämsch KD, Ahr G, Kraft H, Schmitz H, et al. Effect of the calcium antagonists nifedipine, nitrendipine, nimodipine and nisoldipine on oesophageal motility in man. Eur J Clin Pharmacol. 1991;41:313–316. doi: 10.1007/BF00314958. [DOI] [PubMed] [Google Scholar]
- 15.Clements PJ, Lachenbruch PA, Ng SC, Simmons M, Sterz M, Furst DE. Skin score: a semiquantitative measure of cutaneous involvement that improves prediction of prognosis in systemic sclerosis. Arthritis Rheum. 1990;33(8):1256–1263. doi: 10.1002/art.1780330828. [DOI] [PubMed] [Google Scholar]
- 16.Clements PJ, Hurwitz EL, Wong WK, Seibold JR, Mayes M, White B, et al. Skin thickness score as a predictor and correlate of outcome in systemic sclerosis: high-dose versus low-dose penicillamine trial. Arthritis Rheum. 2000;45(11):2445–2454. doi: 10.1002/1529-0131(200011)43:11<2445::AID-ANR11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 17.Wiese AB, Berrocal VJ, Furst DE, Seibold JR, Merkel PA, Mayes MD, et al. Correlates and responsiveness to change of measures of skin and musculoskeletal disease in early diffuse systemic sclerosis. Arthritis Care Res. 2014;66(11):1731–1739. doi: 10.1002/acr.22339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amjadi S, Maranian P, Furst DE, Clements PJ, Weng KW, Postlethwaite AE, et al. Course of the modified Rodnan skin thickness score in systemic sclerosis clinical trials: analysis of three large multicenter, double-blind, randomized controlled trials. Arthritis Rheum. 2009;60(8):2490–2498. doi: 10.1002/art.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shand L, Lunt M, Nihtyanova S, Hoseini M, Silman A, Black CM, et al. Relationship between change in skin score and disease outcome in diffuse cutaneous systemic sclerosis: application of a latent linear trajectory model. Arthritis Rheum. 2007;56(7):2422–2431. doi: 10.1002/art.22721. [DOI] [PubMed] [Google Scholar]
- 20.Clements PJ, Wong WK, Hurwitz EL, Furst DE, Mayes M, White B, et al. The disability index of the health assessment questionnaire is a predictor and correlate of outcome in the high-dose versus low-dose penicillamine in systemic sclerosis trial. Arthritis Rheum. 2001;44(3):653–661. doi: 10.1002/1529-0131(200103)44:3<653::AID-ANR114>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 21.Clements PJ, Wong K, Hurwitz EL, Furst DE, Mayes M, White B, et al. Correlates of the disability index of the health assessment questionnaire: a measure of functional impairment in systemic sclerosis. Arthritis Rheum. 1999;42(11):2372–2380. doi: 10.1002/1529-0131(199911)42:11<2372::AID-ANR16>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 22.Domsic RT, Rodriguez-Reyna T, Lucas M, Fertig N, Medsger TA. Skin thickness progression rate: a predictor of mortality and early internal organ involvement in diffuse scleroderma. Ann Rheum Dis. 2011;70:104–109. doi: 10.1136/ard.2009.127621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khanna D, Furst DE, Clements PJ, Allanore Y, Baron M, Czirjak L, et al. Standardization of the modified Rodnan skin score for use in clinical trials of systemic sclerosis. J Scleroderma Relat Disord. 2017;2(1):11–18. doi: 10.5301/jsrd.5000231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against rheumatism collaborative initiative. Arthritis Rheum. 2013;65(11):2737–2747. doi: 10.1002/art.38098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Armstrong D, Bennett JR, Blum AL, Dent J, De Dombal FT, Galmiche P, et al. The endoscopic assessment of esophagitis: a progress report on observer agreement. Gastroenterology. 1996;111:85–92. doi: 10.1053/gast.1996.v111.pm8698230. [DOI] [PubMed] [Google Scholar]
- 26.Czirjak L, Nagy Z, Aringer M, Riemekasten G, Matucci-cerinic M, Furst DE. The EUSTAR model for teaching and implementing the modified Rodnan skin score in systemic sclerosis. Ann Rhem Dis. 2007;66:966–969. doi: 10.1136/ard.2006.066530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Steen VD, Medsger TA. Severe organ involvement in systemic sclerosis with diffuse scleroderma. Arthritis Rheum. 2000;43(11):2437–2444. doi: 10.1002/1529-0131(200011)43:11<2437::AID-ANR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 28.Yoshizaki A, Sato S. Abnormal B lymphocyte activation and function in systemic sclerosis. Ann Dermatol. 2015;27(1):1–9. doi: 10.5021/ad.2015.27.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasegawa M, Hamaguchi Y, Yanaba K, Bouaziz JD, Uchida J, Fujimoto M, et al. B-lymphocyte depletion reduces skin fibrosis and autoimmunity in the tight-skin mouse model for systemic sclerosis. Am J Pathol. 2006;169(3):954–966. doi: 10.2353/ajpath.2006.060205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith V, Van Praet JT, Vandooren B, Van Der Cruyssen B, Naeyaert JM, Decuman S, et al. Rituximab in diffuse cutaneous systemic sclerosis: an open-label clinical and histopathological study. Ann Rheum Dis. 2010;69(1):193–197. doi: 10.1136/ard.2008.095463. [DOI] [PubMed] [Google Scholar]
- 31.Daoussis D, Liossis S-NC, Tsamandas AC, Kalogeropoulou C, Kazantzi A, Sirinian C, et al. Experience with rituximab in scleroderma: results from a 1-year, proof-of-principle study. Rhematology (Oxford) 2010;49:271–280. doi: 10.1093/rheumatology/kep093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Daoussis D, Liossis S-NC, Tsamandas Christina Kalogeropoulou AC, Paliogianni F, Sirinian C, Yiannopoulos G, et al. Effect of long-term treatment with rituximab on pulmonary function and skin fibrosis in patients with diffuse systemic sclerosis. Clin Exp Rheumatol. 2012;30:17–22. [PubMed] [Google Scholar]
- 33.Daoussis D, Melissaropoulos K, Sakellaropoulos G, Antonopoulos I, Markatseli TE, Simopoulou T, et al. A multicenter, open-label, comparative study of B-cell depletion therapy with rituximab for systemic sclerosis-associated interstitial lung disease. Semin Arthritis Rheum. 2017;46(5):625–631. doi: 10.1016/j.semarthrit.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Jordan S, Distler JHW, Maurer B, Huscher D, Van Laar JM, Allanore Y, et al. Effects and safety of rituximab in systemic sclerosis: an analysis from the European Scleroderma Trial and Research (EUSTAR) group. Ann Rheum Dis. 2015;74(6):1188–1194. doi: 10.1136/annrheumdis-2013-204522. [DOI] [PubMed] [Google Scholar]
- 35.Snir A, Kessel A, Haj T, Rosner I, Slobodin G, Toubi E, et al. Anti-IL-6 receptor antibody (tocilizumab): a B cell targeting therapy. Ecp Rheumatol. 2011;29:697–700. [PubMed] [Google Scholar]
- 36.Roll P, Muhammad K, Schumann M, Kleinert S, Einsele H, Dörner T, et al. In vivo effects of the anti-interleukin-6 receptor inhibitor tocilizumab on the B cell compartment. Arthritis Rheum. 2011;2011(63):1255–1264. doi: 10.1002/art.30242. [DOI] [PubMed] [Google Scholar]
- 37.Khanna D, Denton CP, Jahreis A, van Laar JM, Frech TM, Anderson ME, et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet. 2016;387:2630–2640. doi: 10.1016/S0140-6736(16)00232-4. [DOI] [PubMed] [Google Scholar]
- 38.Khanna D, Denton CP, Lin CJF, Van Laar JM, Frech TM, Anderson ME, et al. Safety and efficacy of subcutaneous tocilizumab in systemic sclerosis: results from the open-label period of a phase II randomised controlled trial (faSScinate) Ann Rheum Dis. 2018;77(2):212–220. doi: 10.1136/annrheumdis-2017-211682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clements P, Lachenbruch P, Siebold J, White B, Weiner S, Martin R, Weinstein A, Weisman M, Mayes MCD. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol. 1995;22:1281–1285. [PubMed] [Google Scholar]
- 40.Khanna D, Berrocal VJ, Giannini EH, Seibold JR, Merkel PA, Mayes MD, et al. The American College of Rheumatology provisional composite response index for clinical trials in early diffuse cutaneous systemic sclerosis. Arthritis Care Res. 2016;68(2):167–178. doi: 10.1002/acr.22804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Suliman YA, Dobrota R, Huscher D, Nguyen-Kim TDL, Maurer B, Jordan S, et al. Pulmonary function tests: high rate of false-negative results in the early detection and screening of scleroderma-related interstitial lung disease. Arthritis Rheumatol. 2015;67(12):3256–3261. doi: 10.1002/art.39405. [DOI] [PubMed] [Google Scholar]
- 42.Hachulla E, Gressin V, Guillevin L, Carpentier P, Diot E, Sibilia J, et al. Early detection of pulmonary arterial hypertension in systemic sclerosis: a French nationwide prospective multicenter study. Arthritis Rheum. 2005;52(12):3792–3800. doi: 10.1002/art.21433. [DOI] [PubMed] [Google Scholar]
- 43.Akesson BYA, Wollheim FA. Organ manifestations in 100 patients with progressive systemic sclerosis: a comparison between the CREST syndrome and diffuse scleroderma. Br J Rheumatol. 1989;28(4):281–286. doi: 10.1093/rheumatology/28.4.281. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.