Abstract
Mushroom body (MB) is a prominent structure essential for olfactory learning and memory in the Drosophila brain. The development of the MB involves the appropriate guidance of axon lobes and sister axon branches. Appropriate guidance that accurately shapes MB development requires the integration of various guidance cues provided by a series of cell types, which guide axons to reach their final positions within the MB neuropils. Netrins are axonal guidance molecules that are conserved regulators of embryonic nerve cord patterning. However, whether they contribute to MB morphogenesis has not yet been evaluated. Here, we find that Netrin-B (NetB) is highly expressed in the MB lobes, regulating lobe length through genetic interactions with the receptors Frazzled and Uncoordinated-5 from 24 h after pupal formation onwards. We observe that overexpression of NetB causes severe β lobe fusion in the MB, which is similar to the MB defects seen in the Drosophila model of fragile X syndrome (FXS). Our results further show that fragile-X mental retardation protein FMRP inhibits the translational activity of human ortholog Netrin-1 (NTN1). Knock-down of NetB significantly rescues the MB defects and ameliorates deficits in the learning and memory in FXS model Drosophila. These results indicate a critical role for NetB in MB lobe extension and identify NetB as a novel target of FMRP which contributes to learning and memory.
Electronic supplementary material
The online version of this article (10.1186/s13041-019-0472-1) contains supplementary material, which is available to authorized users.
Keywords: Mushroom body (MB), Netrin-B (NetB), Axon extension, Fragile X syndrome (FXS)
Introduction
To form a complete and functional nervous system, neurons need to extend axons to reach specific targets. Proper axon guidance and extension are controlled by neuronal cell surface receptors and their extracellular ligands, known as axon guidance molecules [1–3]. The mushroom body (MB) neuropils in Drosophila melanogaster are a powerful model system for investigating axonal guidance and extension because of the unique structure of the MB [4–7]. During development, MB neurons, called Kenyon cells, experience a sequential differentiation process into three neuronal sub-types: α/β neurons, α′/β′ neurons and γ neurons. The cell bodies of these MB neurons form a pair of quadruple clusters in the dorsal posterior cortex and project their axons through an axon tract called the peduncle to the anterior region. The axons bifurcate into two branches at the anterior end of the peduncle and segregate into medial (β, β′ and γ) and dorsal (α, α′) lobes. Many axonal guidance cues are known to regulate development of the MB lobes or even sister branch-specific development. For example, Robo2/3 signaling mainly regulates dorsal and medial lobe extension, while Sema-1a signaling directs lobe outgrowth and orientation in a lobe- and axon branch-specific manner. Likewise, Eph signaling guides specific axon branches of MB neurons [3, 4, 6, 8]. As essential chemotropic cues for axon guidance during neural development, netrins are also expressed in the MBs [3], but their function in MB development is not yet clear.
Netrins are a family of laminin-related proteins that function as chemotropic guidance cues for migrating cells and axons during neural development [9, 10]. In Drosophila, two Netrin homologs have been identified, known as NetA and NetB. As key axonal guidance molecules, detailed studies have been conducted to investigate their roles in ventral nerve cord (VNC) development. Both NetA and NetB are highly expressed by the cells of the VNC midline, mainly guiding commissural axons either toward or away from the midline. In Drosophila, Frazzled (Fra), Uncoordinated-5 (Unc-5) and Down syndrome cell adhesion molecule (Dscam) have been identified as the three main receptors of netrins. During VNC development, Fra is involved in the attraction of commissural axon towards the midline, while Unc-5 acts to prevent commissural axons from crossing the midline. Dscam serves as an attractive receptor which acts in parallel with Fra during VNC development, but it can also respond to multiple other as-yet-unidentified ligands [11–14]. During MB development, Dscam is required for MB axon sister branch segregation [15–17], but it is less clear whether Fra and Unc-5 regulate MB development.
As a prominent structure for higher-order functions in the Drosophila protocerebrum, the MB is essential for learning and memory, similar to the hippocampus in mammals [3, 18]. Drosophila with MB defects exhibit impaired memory formation and deficits in sleep homeostasis. Fly models of cognitive disorders, such as fragile X syndrome (FXS) or Alzheimer’s disease, or of inherited cognitive deficits, for example caused by mutation of ZC3H14, show subtle MB defects [18–27]. FXS, the most common form of inherited monogenic disorder caused by mutation of the fragile-X mental retardation 1 (Fmr1) gene, leads to transcriptional silencing of its encoded fragile-X mental retardation protein FMRP [28–30]. In dfmr1 mutant flies, the MB is characterized by the failure of the β lobes to stop at the brain midline. Research by both McBride et al. and Chang et al. has demonstrated that MB defects can be rescued and courtship activity and associated memory can be restored by treatment with metabotropic glutamate receptor (mGluR) antagonists or GABAergic inhibitors [23–25]. These findings highlight the importance of structural integrity of the MB to learning and memory.
In our study, we focused on the roles of netrins in Drosophila MB development. By loss- and gain-of-function experiments we demonstrate that MB axons display lobe-specific NetB signaling, regulating lobe axon outgrowth. Fra and Unc5 were found to participate in lobe extension via genetic interaction with NetB signaling. Overexpression of NetB results in severe β lobe fusion similar to the MB abnormality observed in dfmr1 mutant models, with increased NetB protein levels observed in the brains of dfmr1 mutants. We further report that NetB human ortholog NTN1 mRNA physically interacts with FMRP, and that NTN1 mRNAs exhibit abnormal polyribosome profiles. Importantly, MB and memory defects are ameliorated in FXS Drosophila by knock-down of NetB.
Materials and methods
Drosophila stocks
Flies were cultured on standard Drosophila yeast-cornmeal molasses food at 25 °C. The following RNAi lines were obtained from the Vienna Drosophila Resource Center for use as stocks: UAS-NetB-RNAi (VDRC330183), UAS-NetA-RNAi (VDRC108577), and UAS-Dscam1-RNAi (VDRC108835). The following lines were obtained from the Bloomington Drosophila Stock Center: NetAΔ (BDSC66878), NetBΔ (BDSC66879), NetBtm (BDSC66880), Dscam11 (BDSC5934), Fra3 (BDSC8813), Unc-5MI04273 (BDSC37426), UAS-Fra-RNAi (BDSC40826), UAS- Unc-5-RNAi (BDSC33756), UAS-mCD8-GFP (BDSC5130), dfmr1Δ3, dfmr150M along with Elav-Gal4, OK107-Gal4, repo-Gal4 and other balancer flies. UAS-NetA and UAS-NetB lines were constructed and the expression level of NetA and NetB were verified.
Antibodies and immunodetection
For indirect immunofluorescent staining, larval and adults brains were dissected, fixed and stained as previous described [31]. The following antibody dilutions were used: monoclonal 1D4 antibody (anti-FasII), 1:20 (Developmental Studies Hybridoma Bank, Iowa city, IA, USA); c-myc (9E10, anti-c-myc), 1:20 (Santa Cruz, TX, USA); Cy3-labeled anti-mouse, 1:200 (Jackson Immunoresearch, PA, USA). Confocal imaging was performed using a confocal microscope (TCS SP5; Leica Microsystems, Wetzlar, Germany). Defects in lobe length and width were defined by visually inspecting all brains. Only obvious and unambiguous differences in length or width were considered [3, 5].
For western blotting (WB), the protein quantity of each sample was estimated by the Bradford assay (Thermo Fisher Scientific, MA, USA), before being subjected to SDS-PAGE. The primary antibodies were diluted as follows: c-myc (9E10), 1:50 (Santa Cruz, TX, USA); β-actin, 1 μg/ml (Abcam, UK), anti-FMRP (1C3): 1:500 (Millipore, MA, USA).
RNA immunoprecipitation (RNA-IP)
Immunoprecipitation was performed according to the modified protocol of Gross et al. [32]. Lysates from HEK293 cells for RIP were prepared in lysis buffer (20 mM Tris-HCl, pH 7.5; 150 mM NaCl; 5 mM MgCl2; 1 mM DTT; and 1% Triton X-100, supplemented with proteinase and RNase inhibitors) on ice. The solution was then precleared with 180 μl Dynabeads protein G (Thermo Fisher Scientific, MA, USA) for 2.5 h. Next, one third of the supernatant was saved as the input fraction for WB. The rest of the supernatant was then incubated with antibody-bound Dynabeads Protein G, blocked beforehand with anti-FMRP antibody (1C3) or normal mouse IgGs, overnight at 4 °C. Then, one sixth of the beads were boiled prior to WB. Total RNA from the remaining beads was extracted with an equal amount of C. elegans RNA added into the reaction mix. The RNA extract underwent reverse transcription, followed by quantitative real-time PCR (RT-qPCR). Relative values were calculated using the ddCt method with 18S rRNA from C. elegans as an external control gene.
Liner sucrose gradient fraction
Cultured normal and fmr1 mutant lymphoblastoid cells were incubated with cycloheximide (100 μg/ml) or 30 mM EDTA at 37 °C for 30 min to arrest polyribosome migration. Cells were lysed using lysis buffer (10 mM HEPES-KOH, pH 7.5; 150 mM KCl; 10 mM MgCl2; 1 mM DTT; 100 μg/mL cycloheximide; and 1% Triton X-100, supplemented with proteinase and RNase inhibitors). Cytoplasmic extracts were loaded on a 15–60% (wt/vol) sucrose gradient and centrifuged at 45,000 rpm in a Beckman SW-55 rotor (Beckman Coulter, CA, USA) for 60 min at 4 °C to separate them into 10 fractions. Each fraction was isolated and analyzed by RT-qPCR [33], with an equal amount of C. elegans RNA used as an external control.
Behavioral training and testing
The behavioral training and testing were performed as described previously [24, 25]. Virgin male flies were collected within 3 h of eclosion. Each male fly was collected in an individual food tube. Virgin W1118 females were collected and kept in food tubes in groups of 10. Flies were aged for 5 d in a 12/12 h light/dark cycle at 25 °C before behavioral training and testing. All testing was performed between 11 am and 3 pm during the relative light phase with comfortable humidity. Mated females were 5 d old and were observed to mate with a male the night before training. All male flies were transferred to fresh food tubes the day before testing. Male flies were assigned randomly to groups for behavioral training and testing in a polystyrene chamber (8 × 10 × 12 mm), with the behavioral training and testing performed blind to group [24, 34, 35]. The total amount of time that a male spent on courtship activity when paired with an unanesthetized female target was scored either during a test period of 10 min or until successful copulation. The courtship index (CI) was measured as the percentage of total time spent courting during the observation [34].
Pavlovian olfactory learning and memory
Flies were trained with the classical conditioning procedure from Tully and Quinn [36]. After one training cycle, the olfactory learning (3 min-memory) was tested. The experiments were carried out at 25 °C and 70% humidity. Flies were collected after eclosion and incubated for 5 days before testing learning.
Statistical methods
To assess lobe length, samples were obtained, with experimenters blind to genotype. Each α/β lobe of a MB was categorized into “normal”, “short” and “overextended” classes. For “normal”, the MBs show a paired neuropil structure in which the α lobes project toward the dorsal surface, while the β and γ lobes project toward the midline of the brain. For “short”, the MBs exhibit obviously reduced or absent lobes. “Overextended α lobes” is defined as overextension of the tip of the dorsal lobes toward the interhemispheric region, “overextended β lobes” as the β lobes cross the MB midline and fused, and “overextended γ lobes” as γ lobes project dorsally outside the normal axis due to overextension [6, 8]. The phenotypic severity in each group was quantified as the percentage of MBs that belong to the defective class, statistical significance was assessed by Fisher’s exact test. ANOVAs were performed for comparison between multiple groups. The other data were analyzed using Student’s two-tailed t-test.
Results
Drosophila NetB regulates MB lobe extension
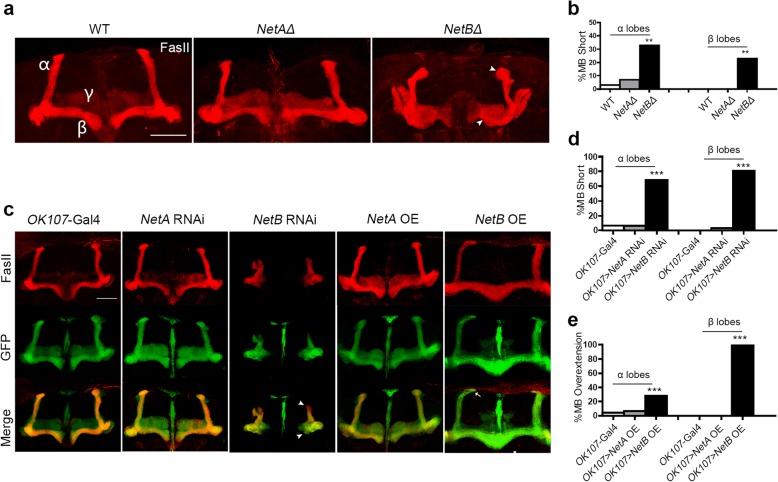
To investigate the roles of Drosophila netrins in MB development, we assessed the overall structure of the adult MB (dissected at 3 d after eclosion) in wild type (WT), NetA deletion mutant NetAΔ, and NetB deletion mutant NetBΔ flies. Anti-Fasciclin (FasII) antibody labels α/β lobes strongly and γ lobes weakly in wild type (WT) strain W1118 adult brains (Fig. 1a). The WT MB showed a paired neuropil structure in which the α lobes project toward the dorsal surface, while the β and γ lobes project toward the midline of the brain. No obvious defects were observed in NetAΔ flies compared with WT flies. However, the stereotyped morphology of MB lobes was disrupted, leading to a relatively smaller MB structure with short lobes in NetBΔ flies (Fig. 1a). We statistically analyzed the MB defects, focusing on α/β lobes, which are easy to visualize. The α/β lobes were significantly shorter in NetBΔ flies compared to those in WT flies, with the percentage of short α and β lobes being 33 and 23% in the two groups, respectively (Fig. 1b, p < 0.01). We also documented the effects of NetB on α’, β’ and γ lobe length (Additional file 1: Table S1).
Fig. 1.
NetB regulates MB lobe extension. a Images of WT, NetAΔ and NetBΔ MBs in adult brains. FasII labeling showed similar lenghth of α/β lobes in WT and NetAΔ, whrease NetBΔ MBs displayed short lobes (arrowhead). b Results from (a) were quantified for the percentage of brain hemispheres with short α/β lobes. (WT, n = 24; NetAΔ, n = 28; netBΔ, n = 33; n indicates fly numbers. **p < 0.01). c MBs from control, NetA RNAi, NetB RNAi, NetA over-expression (OE) and NetB OE were visulized by mCD8-GFP driven by Ok107-Gal4 and immunostaining for FasII. Altered NetA expression caused no neuroanatomical MB defects, wherease NetB knocked-down MBs showed servere short lobes (arrowhead) and NetB OE MBs resulted in overextended α/β lobes (arrow).d The percentage of brain hemispheres with short α/β lobes in NetA/NetB RNAi flies. (OK107-Gal4, n = 30; OK107 > UAS-NetA RNAi, n = 32; OK107 > UAS-NetB RNAi, n = 26; ***p < 0.001). e The percentage of brain hemispheres with overextension of α/β lobes in flies over-expressing Netrins. (OK107-Gal4, n = 30; OK107 > UAS-NetA, n = 30; OK107 > UAS-NetB, n = 28, ***p < 0.001). Significance was determined by Fisher’s exact test. Scale bars: 50 μm
To further analyze how netrins regulate MB morphogenesis, we investigated the effects of knock-down and overexpression of NetA and NetB using the pan-MB neuroblast driver OK107-Gal4. Reductions or increases of NetA activity resulted in no obvious neuroanatomical MB defects compared with control. Knock-down of NetB in MB neurons resulted in significantly greater numbers of short lobes, reminiscent of the phenotype observed in NetBΔ mutants. Overexpression of NetB resulted in slight overextension of the tip of the α lobe toward the interhemispheric region and severe fusion in the β lobe (Fig. 1c, d). Similar phenotypes were also observed using the pan-neuronal elav-Gal4 driver (Additional file 2: Figure S1). These results suggest that NetB can regulate the precise extension of MB lobes.
NetB is highly expressed in MB and controls MB lobe morphogenesis from 24 h after pupal formation
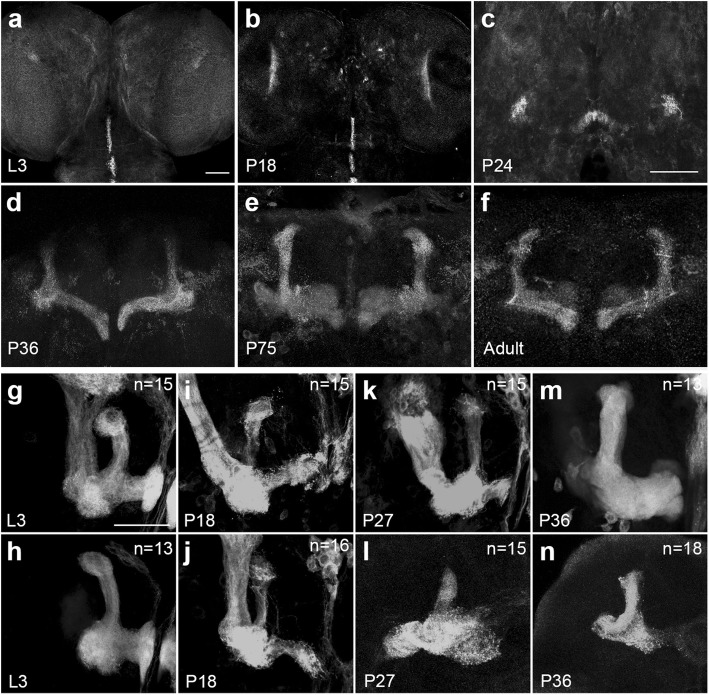
Next, we examined the expression pattern of NetB during Drosophila brain development. We labeled a myc-tagged membrane-tethered version of NetB (NetBtm), expressed under the control of the endogenous Netrin promoter [37, 38]. We observed that during the larval period and early pupal stage, NetB was mainly expressed in the ventral midline (Fig. 2a, b). From 24 h after pupal formation (APF), NetB was expressed in the ellipsoid body (EB) and around the end of peduncles (Fig. 2c). NetB was highly expressed in the MB lobes from 36 h APF into adulthood (Fig. 2d-f).
Fig. 2.
NetB expression pattern and MB phenotypes in NetB RNAi flies during brain development. a-f NetB expression pattern in NetBtm flies, as revealed by anti-myc staining (gray). a-b Strong expression of NetB was observed in the VNC cells at third Larva stage (L3) and 18 h APF. c At stage of the 24 h APF, NetB was enriched around the end of peduncles of MB and in the ellipsoid body (EB). d-f NetB expression from 36 h APF until adult stage. MB lobes were strongly stained with anti-myc. g-n The MB morphology of the control (OK107 > UAS-mCD8-GFP) and NetB RNAi (UAS-NetB RNAi/+; UAS-mCD8-GFP/+;OK107-Gal4/+)flies at L3,18h APF,27 h APF,36 h APF. Upper line shows the control group MBs and lower line shows the NetB RNAi MBs. Numbers in the upper right corners indicate the numbers of observed MBs in corresponding genotypes
During MB development, both γ and α′/β′ neurons are generated and acquire similar projection patterns during the instar stage. After pupal formation, γ neurons undergo a dramatic reorganization and become restricted to the end of peduncle at around 24 h APF, while α′/β′ neurons remain relatively unchanged. Meanwhile, all MB neurons which are born after pupal formation become α/β neurons, and their axons extend to form the adult-specific lobes [39]. To determine the stage at which axonal defects start to be observed, we analyzed the morphology of MB lobes in NetB RNAi flies during metamorphosis. NetB RNAi lobes were indistinguishable from control at larval stage and at 18 h APF (Fig. 2g–j; n = 15 and n = 13 for control and NetB RNAi larval MBs, respectively; n = 15 and n = 16 for control and NetB RNAi 18 h APF MBs, respectively). Notably, at 27 h APF and 36 h APF, NetB RNAi MBs showed a much greater proportion of short lobes (Fig. 2k–n; n = 15 and 15 for control and NetB RNAi 27 h APF MBs, respectively; n = 13 and 18 for control and NetB RNAi 36 h APF MBs, respectively). These results imply that NetB is required for the MB lobe projection to mature after the completion of degeneration.
Receptors Fra and Unc-5 are involved in MB development through genetic interaction with NetB
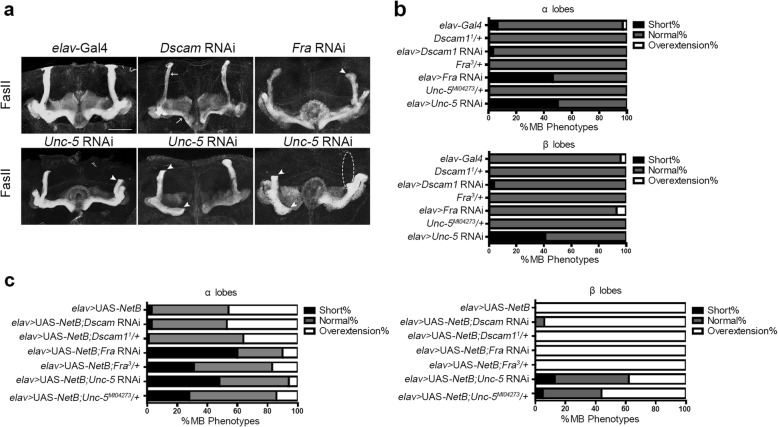
Dscam, Fra, and Unc-5 have been shown to function as netrin receptors during VNC development in Drosophila [11, 13, 40]. We investigated the possible roles of these receptors in MB formation. We made use of RNAi constructs targeting each receptor which were driven by elav-Gal4. Knock-down of Dscam1 resulted in very thin and faint α/β lobes, but had no effect on lobe length (n = 30). Knock-down of Fra led to a significantly greater proportion of short α lobes, but no β lobe defects (n = 28). After knock-down of Unc-5, the dorsal or medial lobes were partially or completely lost compared with control (n = 34) (Fig. 3a, b). Furthermore, we also investigated the MB phenotypes of the receptor heterozygous mutant alleles (the mutants are often recessive lethal). Removing one copy of these receptors was not sufficient to cause any neuroanatomical defects in the MB on its own. Our data broadly indicate roles for Dscam in regulating divergent segregation of axons in the MB, Fra in α lobe outgrowth, and Unc-5 in both α and β lobe extension.
Fig. 3.
Receptors Fra and Unc-5 show genetic interaction with NetB in MB lobe extension. a Receptors Dscam, Fra and Unc-5 are involved in MB development. Dscam RNAi MBs displayed thin α/β lobes with normal length compared with control MBs; Fra RNAi MBs displayed short α lobes (arrowhead); Knock-down of Unc-5 caused both short (arrowhead) or even missing (dashed lines) α/β lobes. b The percentage of brain hemispheres with short α/β lobes (elav-Gal4, n = 30; elav > Dscam RNAi, n = 30; elav > Fra RNAi, n = 28; elav > Unc-5 RNAi, n = 34; ***p < 0.001; Dscam11/+, n = 25; Fra3/+, n = 30; Unc-5MI04273/+, n = 30). c Receptors Fra and Unc-5 show genetic interaction with NetB. The lobe phenotypes were classified as short, normal or overextension, and the quantification was shown as the percentages of brain hemispheres in each category. The percentage of overextended α lobes was significantly reduced in elav > UAS-NetB; Unc-5 RNAi (or Unc-5MI04273/+) and in elav > UAS-NetB; Fra RNAi (or Fra3/+) compared with NetB OE (elav > UAS-NetB); The percentage of overextended β lobes was significantly reduced only in elav > UAS-NetB; Unc-5 RNAi (or Unc-5MI04273/+) lines. (elav-Gal4/Y; UAS-NetB/+, n = 30; elav-Gal4/Y; UAS-NetB/+; Unc-5 RNAi/+, n = 48; elav-Gal4/Y; UAS-NetB/ Unc-5MI04273, n = 36; elav-Gal4/Y; UAS-NetB/+; FraRNAi/+, n = 24; elav-Gal4/Y; UAS-NetB/Fra3, n = 36; elav-Gal4/Y; UAS-NetB/+; /Dscam RNAi, n = 30; elav-Gal4/Y; UAS-NetB/Dscam11, n = 34; p < 0.0001). Significance was determined by Fisher’s exact test. Scale bars: 50 μm
We then examined whether NetB signaling affecting MB morphogenesis is dependent on these receptors. As overexpression of NetB results in overextended lobes, disruption of the NetB pathway may reduce these defects. We investigated the genetic interaction between netrin receptors and NetB by combining either a Dscam11, Fra3 or Unc-5MI04273 allele (or an RNAi construct against one of these receptors) with elav-Gal4-driven NetB overexpression. Potential genetic interaction was quantified as the percentage of MB lobes categorized as short, normal, or overextended. We did not observe any significant alterations to the NetB overexpression phenotype when NetB overexpression was combined with mutations (or RNAi knock-down) in Dscam. Loss of one copy (or RNAi-mediated knock-down) of Fra reduced the percentage of α lobe overextension with no alteration to fused β lobe morphology. Introduction of Unc-5MI04273 (or RNAi-mediated knock-down of Unc-5) led to a robust decrease in both α and β lobe extension (Fig. 3c). Furthermore, we examined MB phenotypes in NetBΔ homozygotes in combination with heterozygous mutations of receptor Fra and Unc-5. Introduction of these heterozygous alleles did not aggravate the phenotypes of NetB mutants, implying that NetB is the primary ligand responsible for regulating MB extension via Fra and Unc-5 (Table 1).
Table 1.
No significant alteration of the NetBΔ phenotypes in combination with mutations in Fra and Unc-5 (Fisher’s exact test)
| Genotypes | #brains | Lobe | % Short |
|---|---|---|---|
| NetBΔ/Y | 32 | α | 33 |
| β | 18 | ||
| NetBΔ/Y; Fra3/+ | 25 | α | 32 |
| β | 14 | ||
| NetBΔ/Y; Unc-5MI04273/+ | 26 | α | 33 |
| β | 15 |
Genetic reduction of NetB rescues MB defects and ameliorates the learning and memory in dfmr1 mutants
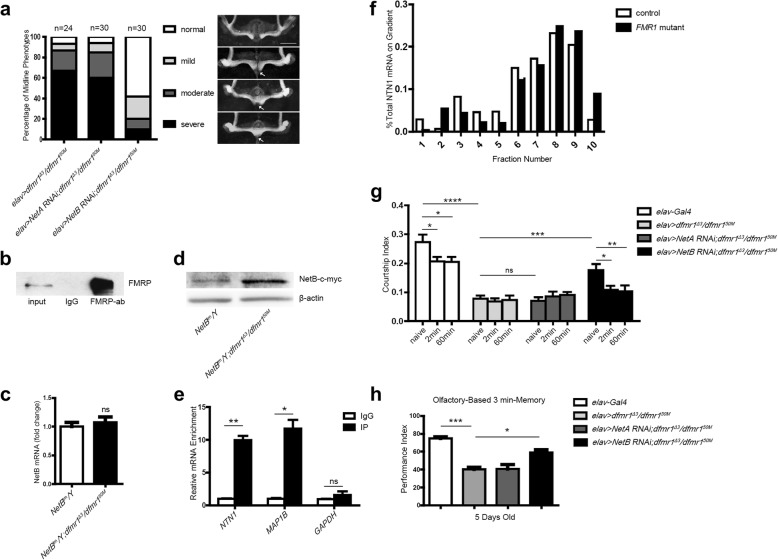
As a result of the deletion of dFMRP, dfmr1 mutant flies exhibit deficits in cognitive function associated with a relatively high frequency of β lobes which cross the midline [24, 25, 41]. In our study, NetB-overexpressing flies exhibited similar defects as dfmr1 mutants. We wondered whether MB defects are influenced by the NetB signaling pathway in dfmr1 mutants. The severity and variability of β lobe midline crossing was classified in a Drosophila FXS model of dfmr1Δ3/dfmr150M trans-heterozygotes as either normal, mild, moderate or severe. The β lobes in dfmr1 mutants cross over the midline and fuse at a fairly high frequency (93% total, with 67% severe, 19% moderate, 7% mild midline crossing; n = 24). The fusion percentage of β lobes after knock-down of NetA in dfmr1 mutant flies did not differ significantly compared with dfmr1 mutants (94% total, with 60% severe, 25% moderate, 9% mild midline crossing; n = 30). However, the fusion percentage was only 42% after knock-down of NetB in dfmr1 mutants (with 10% severe, 10% moderate, 22% mild midline crossing; n = 30), indicating that reduction of NetB can significantly rescue MB defects in dfmr1 mutants (Fig. 4a). Interestingly, a significant increase in NetB protein level was detected in the brains of dfmr1 mutants with an unchanged total mRNA compared with control flies NetBtm (Fig. 4b, c).
Fig. 4.
Knock-down of NetB rescues the MB defects and ameliorates the memory in dfmr1 mutants. a Knock-down of NetB rescued the β lobe fusion in dfmr1 mutants. b, c Western blot and RT-qPCR showed that NetB protein level was obviously elevated in the brains of dfmr1 mutants while mRNA level was not significantly altered compared with control. d, e Interaction of FMRP with NTN1 mRNA in HEK293 cells. d Western blot analysis showed FMRP was precipitated by the anti-FMRP antibody. IgG IPs were used as a negative controls. e RT-qPCR analysis indicated that NTN1 and MAP1B (positive control) mRNAs were significantly enriched in anti-FMRP IPs. *p < 0.05, **p < 0.01. f Polyribosome profile of NTN1 mRNA indicated a clear increase of the NTN1 mRNA in the most actively translating ployribosomes in the Fmr1 mutant lymphoblastoid cells compared with normal control (N = 3, **p < 0.01). g Knock-down of NetB in dfmr1 mutants ameliorated the courtship activity, immediate recall memory and short-term memory of dfmr1 mutant flies (*p = 0.0328, **p = 0.0085, ****p < 0.0001, Two-way ANOVA). h Knock-down of NetB in dfmr1 mutant flies by elav-Gal4 ameliorated the olfactory conditioning short-term memory of dfmr1 mutants (*p < 0.05, ***p < 0.001, One-way ANOVA). Error bars = SEM
The canonical role of FMRP is translation suppression via direct mRNA-binding [42]. We performed RNA coimmunoprecipitation in cultured HEK293 cell extracts to investigate whether FMRP directly interacts with NetB human ortholog NTN1 mRNA. As shown in Fig. 4d, FMRP was precipitated by the 1C3 anti-FMRP antibody from the immunoprecipitates (IPs), Next, we performed RT-qPCR on input, IgG and anti-FMRP Ab immunoprecipitated fractions to assess levels of NTN1 mRNA and control mRNAs. Notably, in comparison to the negative control IgG IPs group, NTN1 mRNA was significantly enriched in the immunoprecipitated complexes. MAP1B, a known target of FMRP, was also significantly enriched in the anti-FMRP IPs. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA levels did not differ between groups, further demonstrating the specificity of the NTN1-mRNA-FMRP interaction (Fig. 4e). Next, a polyribosome profile was performed to test whether FMRP regulates NTN1 mRNA translation. We used a lymphoblastoid cell line derived from an FXS patient who harbored a 237-kb deletion encompassing the entire FMR1 and FMR1NB genes [42]. In normal lymphoblastoid cells, the samples treated by cyclohexamide resulted in the accumulation of stalled polysomes, and FMRP was distributed in every fraction. The samples treated by EDTA completely disrupted polysomes into ribosomal subunits, and FMRP was only distributed in messenger ribonucleoprotein and monosomal fractions (Additional file 3: Figure S2). We analyzed the polyribosome profile of NTN1 mRNA in normal and Fmr1 mutant lymphoblastoid cells treated by cyclohexamide. As shown in Fig. 4f, the majority of NTN1 mRNA was associated with translating polyribosomes (fraction 6–10) in both normal control and Fmr1 mutant cell extracts. Notably, there was a clear increase of NTN1 mRNA in the most actively translating polyribosomes of Fmr1 mutant cells (fractions 8–10) compared with normal cells (**p < 0.01). And the NTN1 mRNA level was not altered in the mRNP/monosomes (fractions 1–5, p > 0.05) but showed a decrease in the fraction 6 and 7 (*p < 0.05) in the Fmr1 mutant cells (Two-way ANOVA). These results indicate that NTN1 translation is negatively regulated by FMRP.
Furthermore, we tested courtship-associated learning and memory ability in dfmr1 mutant flies when knock–down of NetB. Learning and memory in Drosophila can be investigated experimentally by taking advantage of conditioned courtship behavior, as described previously. In conditioned courtship, courting Drosophila males perform characteristic behaviors: orienting toward the female; wing extension and vibration; licking; and attempting to copulate. Virgin females generally respond by mating, but recently mated females are unreceptive and will reject to copulate. A naïve male paired with a mated female will initially court her, but his courtship activity soon decreases. After 1 h of training with the mated female, his courtship activity remains depressed for 2 to 3 h when subsequently paired with a virgin female [24, 25]. As the results showed in Fig. 4g, dfmr1 mutants showed low courtship interest in virgin females (naïve), impaired immediate recall memory at 0–2 min post-training, and a short-term memory deficit for up to 1 h, as previously reported [24, 25, 43]. A similar phenotype was also found during knock-down of NetA in dfmr1 mutants. However, knock-down of NetB significantly ameliorated deficits in courtship activity, immediate recall memory and short-term memory.
Considering the olfactory conditioning short-term memory was impaired in dfmr1 mutants [44], we asked whether knock-down of NetB in dfmr1 mutants can also ameliorate the olfactory short-term memory. dfmr1 mutants displayed significant defects in olfactory based 3 min-memory compared with normal control group (elav-Gal4), so did knock-down of NetA in dfmr1 mutants. We found that knock-down of NetB can partially rescue the short-term memory of dfmr1 mutant flies (Fig. 4h). The tests of locomotor and olfactory abilities in dfmr1 mutant flies were replicated, showing no defects as previously reported [44, 45]. We also noticed that knock-down of NetA or NetB in dfmr1 mutants showed no detectable changes in the locomotor and olfactory abilities (Additional file 4: Figure S3). Overall, we conclude that NetB regulates the learning and memory in the FXS Drosophila model.
Discussion
This study has revealed a novel function for the conserved axonal guidance cue NetB which is responsible for regulating MB axon morphology during the development of higher-order brain centers in Drosophila. Although two Drosophila homologs of Netrin have been identified and share high homology, we demonstrate that NetB, but not NetA, regulates axon lobe extension of MB neurons. The amino acid sequence difference in the first EGF repeat of domain V might alter the receptor binding specificities of NetA and NetB [37, 46, 47], yet functional differences between NetA and NetB are still under debate. During embryonic nervous system development, NetA and NetB play similar roles in guiding commissural axon formation. When growing segmental nerve a (SNa) motor axons are repelled outwards from the central nervous system, only NetB is required to respond to the Unc-5 receptor. While ectopic expression of NetA appears to have no effect [37, 48]. These findings imply that they may play distinct roles in mediating axon guidance. The MB is a major source of NetB in the developing protocerebrum, with the precise regulation of MB lobe extension depending on the accurate distribution of NetB expression in space and time. In the current study, NetB exhibited a specific pattern of expression in the MB structure just after 24 h APF, when γ neuron degeneration was complete and axons had started to extend to form the mature lobes. Knock-down of NetB significantly impeded axon extensions at 27 h APF. These observations indicate that NetB is largely responsible for axon projection after degeneration which forms the adult-specific axon lobes.
In our study, we found that the Fra and Unc-5 receptors were involved in NetB signaling which regulates MB lobe extension, while Dscam showed no obvious genetic interaction with NetB. Dscam was involved in the mutual repulsion of sister branches, with the segregation accuracy of the sister branches depending on the repulsive force resulting from expression of the same set of Dscam isoforms. Loss of Dscam results in the failure of sister branch segregation, with flies exhibiting thin MB lobes [49]. Fra and Unc-5 affect lobe length in distinct ways, as Fra is preferential for α lobes, whereas Unc-5 affects the length of both α and β lobes. We wonder whether NetB from the MB cells interacts with the Frazzled and Unc5 of the same cells. NetB overexpression in the MB cells showed severe α and β lobe overextension, however, knock-down of Unc-5 and Fra displayed no obvious MB defects and failed to ameliorate the defects of overextended α or β lobes when overexpressing NetB (Additional file 5: Figure S4a). Knock-down of Fra in glia didn’t alter MB morphology, whereas knock-down of Unc-5 showed significant short MB lobes (Additional file 5: Figure S4b, c and Additional file 6: Table S2). These data imply that NetB from the MB cells interacts with the Unc-5 from neurons and glia surrounding the lobe fibers, and with the Fra from neurons surrounding the lobe fibers. This can be interpreted as possibly indicating a complex expression distribution of these receptors during MB development. The slit/robo2 and slit/robo3 signaling pathways provide a well-understood example of the complexity of regulation of MB lobe extension based on an intricate distribution of receptors. As Robo2 and Robo3 proteins are expressed in many domains in the developing central complex and in fibers which cross the central neuropil, they show elaborate and distinct expression distributions in these domains across space and time. Loss-of-function mutations to each specific receptor results in different MB lobe defects [8]. Thus, further research is needed to fully understand the different processes contributing to lobe formation which depend on the NetB/Fra or NetB/Unc-5 signaling pathways.
Overexpression of NetB in the MB results in severe β lobe fusion, resembling the defect seen in dfmr1 mutants. As a translation suppressor binding to specific mRNAs, lack of FMRP results in dysregulated translation of many mRNAs, including those critical to neuronal development, plasticity, and dendritic spine architecture [30]. In this study, we identified NTN1 mRNA as a specific target of FMRP. NetB protein levels were significantly enhanced in the brains of dfmr1 mutants. Knock-down of NetB significantly rescued dfmr1-related MB defects and ameliorated deficits in short-term learning and memory in FXS model Drosophila. As a conserved axon guidance cue, NTN1 directs synaptic formation and synaptic plasticity during development [50]. Restricted sources of NTN1 can regulate the rapid local recruitment of synaptic proteins during neural development and spatially mediate the local translation of the synaptic proteome in Aplysia neurons for synaptic consolidation [50–53]. In the mature mammalian brain, NTN1 and its receptor DCC (an ortholog of Fra) are enriched at synapses, disruption of NTN1/DCC signaling reduces dendritic spine volume, severely attenuates LTP at hippocampal Schaffer collateral synapses, and impairs hippocampal-dependent learning and memory [54]. These findings suggest that overexpression of NTN1 leads to synaptic structural defects, which may contribute to cognitive impairment in FXS.
In summary, we provide evidence for a significant regulation of NetB signaling in the MB lobe extension. We describe a novel FMRP target and find that knock-down of NetB can partially restore learning and memory in dfmr1 mutants.
Additional files
Table S1. NetB affects α’/β’and γ lobe length (Fisher exact test). (DOCX 15 kb)
Figure S1. a-c. Knock-down of Netrins with pan-neuronal driver elav-Gal4 showed similar phenotypes as driven by OK107-Gal4. a. MBs from control (elav-Gal4 > mCD8-GFP), NetA RNAi, NetB RNAi, NetA OE and NetB OE were visulized by mCD8-GFP driven by elav-Gal4 and immunostaining for FasII. The Control, NetA RNAi, NetA OE MBs showed normal structures, but knock-down of NetB showed short lobes (arrowhead). NetB OE MBs showed overextended lobes (arrow). b. The percentage of brain hemispheres with short α/β lobes in NetA/NetB RNAi flies. (elav-Gal4, n = 35; elav > NetA RNAi, n = 40; elav > NetB RNAi, n = 30; ***p < 0.001). c. The percentage of brain hemispheres with overextension of α/β lobes in flies over-expressing Netrins. (elav-Gal4, n = 35; elav > UAS-NetA, n = 30; elav > UAS-NetB, n = 30, ***p < 0.001). Significance was determined by Fisher exact test. Scale bars: 50 μm. (TIF 6225 kb)
Figure S2. The distribution of FMRP in the normal lymphoblastoid cell extracts treated with cycloheximide and EDTA. After cycloheximide treatment, FMRP was distributed across all the fractions. However, in the EDTA treated samples, most of the FMRP was in fractions 1–5, which correspond to the free messenger ribonucleoprotein (mRNP) and monosomal fraction. (TIF 685 kb)
Figure S3. Analysis of locomotor and olfactory abilities. a. Locomotor activity was measured by a line crossing assay [34]. All genotypes had similar locomotor activity profiles (elav-Gal4/Y, elav-Gal4/Y;dfmr1Δ3/dfmr150M, elav-Gal4/Y; UAS-NetA RNAi/+;dfmr1Δ3/dfmr150M, elav-Gal4/Y; UAS-NetB RNAi/+; dfmr1Δ3/dfmr150M, for each genotype, we tested 20 flies). b. Olfactory capabilities were measured by the olfactory trap assay [35]. No differences were found between any of the genotypes tested with this assay at the 60 h time point. (TIF 6938 kb)
Figure S4. NetB from the MB cells doesn’t interact with the Fra and Unc5 of the same cells. a. Knock-down of Fra and Unc-5 with NetB overexpression in the MB cells failed to ameliorate the defects of overextended α and β lobes (OK107 > UAS-NetB, n = 28; UAS-NetB/+; UAS-Fra RNAi/+; OK107-Gal4/+, n = 30; UAS-NetB/+; UAS-Unc-5 RNAi/+; OK107-Gal4/+, n = 28). b. Knock-down of Fra and Unc-5 with OK107-Gal4 displayed normal α/β lobes with normal length. Knock-down of Fra by a glia specific driver repo-Gal4, the MBs showed normal structure. However, knock-down of Unc-5 with repo-Gal4 caused short α/β lobes (arrowhead). c. The percentage of brain hemispheres with short α/β lobes (OK107-Gal4, n = 30; repo-Gal4, n = 28; OK107 > Fra RNAi, n = 25, OK107 > Unc-5 RNAi, n = 20; repo > Fra RNAi, n = 20; repo > Unc-5 RNAi, n = 22, ***p < 0.001). Significance was determined by Fisher’s exact test. Scale bars: 50 μm. (TIF 5078 kb)
Table S2. The summary of the α/β lobe defects in knocking-down Fra or Unc-5 by pan-neuron, MB and glia drivers respectively. (DOCX 15 kb)
Acknowledgements
The authors thank H, Qin (College of Biology, Hunan University) for his help in the test of olfactory conditioning paradigm.
Funding
This study was supported by the National Natural Science Foundation of China (grant number 81571253, 81771385) and the Hunan Provincial Natural Science Foundation of China (grant number 2016JJ3135).
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Abbreviations
- APF
After pupal formation
- CI
Courtship index
- Dscam
Down syndrome cell adhesion molecule
- EB
Ellipsoid body
- Fmr1
Fragile-X mental retardation 1
- FMRP
Fragile-X mental retardation protein
- Fra
Frazzled
- FXS
Fragile X syndrome
- GAPDH
Glyceraldehyde-3-phosphate dehydrogenase
- IPs
Immunoprecipitates
- MAP1B
Micro-tubule associated protein
- MB
Mushroom body
- NetA
Netrin-A
- NetB
Netrin-B
- NTN1
Netrin-1
- RNA- IP
RNA immunoprecipitation
- SNa
Segmental nerve a
- Unc-5
Uncoordinated-5
- Unc-5
Uncoordinated-5
- VNC
ventral nerve cord
Authors’ contributions
RD and HC designed research; HK, JZ and RD wrote the article; HK, JZ, XJ, GL, WH performed research; HK analyzed data. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Written informed consent was obtained from parents and patients older than age 18 years for participation in this study, which was approved by the ethics committee of the state key laboratory of medical genetics, Central South University (approval number: 2013051201).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Huaixing Kang, Email: kanghuaixin@163.com.
Juan Zhao, Email: zhaojuan@sklmg.edu.cn.
Xuan Jiang, Email: jiangxuan@sklmg.edu.cn.
Guangxu Li, Email: liguangxu@sklmg.edu.cn.
Wen Huang, Email: huangwen@sklmg.edu.cn.
Huili Cheng, Email: 2556266023@qq.com.
Ranhui Duan, Email: duanranhui@sklmg.edu.cn.
References
- 1.Dickson BJ. Molecular mechanisms of axon guidance. Science. 2002;298(5600):1959–1964. doi: 10.1126/science.1072165. [DOI] [PubMed] [Google Scholar]
- 2.Lowery LA, Van Vactor D. The trip of the tip: understanding the growth cone machinery. Nat Rev Mol Cell Biol. 2009;10(5):332. doi: 10.1038/nrm2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliva C, Soldano A, Mora N, De Geest N, Claeys A, Erfurth ML, et al. Regulation of Drosophila brain wiring by neuropil interactions via a slit-Robo-RPTP signaling complex. Dev Cell. 2016;39(2):267–278. doi: 10.1016/j.devcel.2016.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyle M, Nighorn A, Thomas JB. Drosophila Eph receptor guides specific axon branches of mushroom body neurons. Development. 2006;133(9):1845–1854. doi: 10.1242/dev.02353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shin J. E., DiAntonio A. Highwire Regulates Guidance of Sister Axons in the Drosophila Mushroom Body. Journal of Neuroscience. 2011;31(48):17689–17700. doi: 10.1523/JNEUROSCI.3902-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zwarts L, Goossens T, Clements J, Kang YY, Callaerts P. Axon branch-specific Semaphorin-1a signaling in DrosophilaMushroom body development. Front Cell Neurosci. 2016;10(29):1–11. [DOI] [PMC free article] [PubMed]
- 7.Marchetti G, Reichardt I, Knoblich JA, Besse F. The TRIM-NHL protein brat promotes axon maintenance by repressing src64B expression. J Neurosci. 2014;34(41):13855–13864. doi: 10.1523/JNEUROSCI.3285-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicolas E, Preat T. Drosophila central brain formation requires Robo proteins. Dev Genes Evol. 2005;215(10):530–536. doi: 10.1007/s00427-005-0009-8. [DOI] [PubMed] [Google Scholar]
- 9.Serafini T, Colamarino SA, Leonardo ED, Wang H, Beddington R, Skarnes WC, et al. Netrin-1 is required for commissural axon guidance in the developing vertebrate nervous system. Cell. 1996;87(6):1001. doi: 10.1016/S0092-8674(00)81795-X. [DOI] [PubMed] [Google Scholar]
- 10.Lai Winq Sun K, Correia JP, Kennedy TE. Netrins: versatile extracellular cues with diverse functions. Development. 2011;138(11):2153. doi: 10.1242/dev.044529. [DOI] [PubMed] [Google Scholar]
- 11.Kolodziej PA, Timpe LC, Mitchell KJ, Fried SR, Goodman CS, Jan LY, et al. Frazzled encodes a Drosophila member of the DCC immunoglobulin subfamily and is required for CNS and motor axon guidance. Cell. 1996;87(2):197–204. doi: 10.1016/S0092-8674(00)81338-0. [DOI] [PubMed] [Google Scholar]
- 12.Hiramoto M, Hiromi Y, Giniger E, Hotta Y. The Drosophila netrin receptor frazzled guides axons by controlling netrin distribution. Nature. 2000;406(6798):886–889. doi: 10.1038/35022571. [DOI] [PubMed] [Google Scholar]
- 13.Keleman K, Dickson BJ. Short- and long-range repulsion by the Drosophila Unc5 netrin receptor. Neuron. 2001;32(4):605. doi: 10.1016/S0896-6273(01)00505-0. [DOI] [PubMed] [Google Scholar]
- 14.Lewis TL, Jr, Courchet J, Polleux F. Cellular and molecular mechanisms underlying axon formation, growth, and branching. J Cell Biol. 2013;202(6):837–848. doi: 10.1083/jcb.201305098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hattori D, Demir E, Kim HW, Viragh E, Zipursky SL, Dickson BJ. Dscam diversity is essential for neuronal wiring and self-recognition. Nature. 2007;449(7159):223. doi: 10.1038/nature06099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goyal G, Zheng J, Adam E, Steffes G, Jain M, Klavins K, et al. Sphingolipid-dependent Dscam sorting regulates axon segregation. Nat Commun. 2019;10(813):1–17. [DOI] [PMC free article] [PubMed]
- 17.Zhan XL, Clemens JC, Neves G, Hattori D, Flanagan JJ, Hummel T, et al. Analysis of Dscam diversity in regulating axon guidance in Drosophila mushroom bodies. Neuron. 2004;43(5):673–686. doi: 10.1016/j.neuron.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 18.Fiala A. Olfaction and olfactory learning in Drosophila: recent progress. Curr Opin Neurobiol. 2007;17(6):720–726. doi: 10.1016/j.conb.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 19.Davis RL. Traces of Drosophila memory. Neuron. 2011;70(1):8–19. doi: 10.1016/j.neuron.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kahsai L, Zars T. Learning and memory in Drosophila: behavior, genetics, and neural systems. Int Rev Neurobiol. 2011;99:139. doi: 10.1016/B978-0-12-387003-2.00006-9. [DOI] [PubMed] [Google Scholar]
- 21.Waddell S. Reinforcement signalling in Drosophila ; dopamine does it all after all. Curr Opin Neurobiol. 2013;23(3):324–329. doi: 10.1016/j.conb.2013.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bushey D, Cirelli C. From genetics to structure to function: exploring sleep in Drosophila. Int Rev Neurobiol. 2011;99(24):213. doi: 10.1016/B978-0-12-387003-2.00009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drozd M, Bardoni B, Capovilla M. Modeling Fragile X Syndrome in Drosophila. Front Mol Neurosci. 2018;11(124):1–15. [DOI] [PMC free article] [PubMed]
- 24.Mcbride SM, Choi CH, Wang Y, Liebelt D, Braunstein E, Ferreiro D, et al. Pharmacological Rescue of Synaptic Plasticity, courtship behavior, and mushroom body defects in a Drosophila model of fragile X syndrome. Neuron. 2005;45(5):753–764. doi: 10.1016/j.neuron.2005.01.038. [DOI] [PubMed] [Google Scholar]
- 25.Chang S, Bray SM, Li Z, Zarnescu DC, He C, Jin P, et al. Identification of small molecules rescuing fragile X syndrome phenotypes in Drosophila. Nat Chem Biol. 2008;4(4):256. doi: 10.1038/nchembio.78. [DOI] [PubMed] [Google Scholar]
- 26.Chakraborty R, Vepuri V, Mhatre SD, Paddock BE, Miller S, Michelson SJ, et al. Characterization of a Drosophila Alzheimer's disease model: pharmacological Rescue of Cognitive Defects. PLoS One. 2011;6(6):e20799. doi: 10.1371/journal.pone.0020799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kelly SM, Bienkowski R, Banerjee A, Melicharek DJ, Brewer ZA, Marenda DR, et al. The Drosophila ortholog of the ZC3H14 RNA binding protein acts within neurons to pattern axon projection in the developing brain. Dev Neurobiol. 2016;76(1):93–106. doi: 10.1002/dneu.22301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Santoro MR, Bray SM, Warren ST. Molecular mechanisms of fragile X syndrome: a twenty-year perspective. Annu Rev Pathol. 2011;7(1):219–245. doi: 10.1146/annurev-pathol-011811-132457. [DOI] [PubMed] [Google Scholar]
- 29.Jacquemont S, Hagerman RJ, Hagerman PJ, Leehey MA. Fragile-X syndrome and fragile X-associated tremor/ataxia syndrome: two faces of FMR1. Lancet Neurol. 2007;6(1):45–55. doi: 10.1016/S1474-4422(06)70676-7. [DOI] [PubMed] [Google Scholar]
- 30.Davis JK, Broadie K. Multifarious functions of the fragile X mental retardation protein. Trends Genet. 2017;33(10):703. doi: 10.1016/j.tig.2017.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rollmann SM, Zwarts L, Edwards AC, Yamamoto A, Callaerts P, Norga K, et al. Pleiotropic effects of Drosophila neuralized on complex behaviors and brain structure. Genetics. 2008;179(3):1327–1336. doi: 10.1534/genetics.108.088435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gross C, Nakamoto M, Yao X, Chan CB, Yim SY, Ye K, et al. Excess phosphoinositide 3-kinase subunit synthesis and activity as a novel therapeutic target in fragile X syndrome. J Neurosci. 2010;30(32):10624. doi: 10.1523/JNEUROSCI.0402-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lu R, Wang H, Liang Z, Ku L, O'Donnell WT, Li W, et al. The fragile X protein controls microtubule-associated protein 1B translation and microtubule stability in brain neuron development. Proc Natl Acad Sci U S A. 2004;101(42):15201–15206. doi: 10.1073/pnas.0404995101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Siegel RW, Hall JC. Conditioned responses in courtship behavior of normal and mutant Drosophila. Proc Natl Acad Sci U S A. 1979;76(7):3430–3434. doi: 10.1073/pnas.76.7.3430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mcbride SM, Giuliani G, Choi C, Krause P, Correale D, Watson K, et al. Mushroom body ablation impairs short-term memory and long-term memory of courtship conditioning in Drosophila melanogaster. Neuron. 1999;24(4):967–977. doi: 10.1016/S0896-6273(00)81043-0. [DOI] [PubMed] [Google Scholar]
- 36.Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79(1):35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 37.Brankatschk M, Dickson BJ. Netrins guide Drosophila commissural axons at short range. Nat Neurosci. 2006;9(2):188. doi: 10.1038/nn1625. [DOI] [PubMed] [Google Scholar]
- 38.Orr BO, Borgen MA, Caruccio PM, Murphey RK. Netrin and frazzled regulate presynaptic gap junctions at a Drosophila giant synapse. J Neurosci. 2014;34(16):5416–5430. doi: 10.1523/JNEUROSCI.3183-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee T, Lee A, Luo L. Development of the Drosophila mushroom bodies: sequential generation of three distinct types of neurons from a neuroblast. Development. 1999;126(18):4065–4076. doi: 10.1242/dev.126.18.4065. [DOI] [PubMed] [Google Scholar]
- 40.Andrews GL, Tanglao S, Farmer WT, Morin S, Brotman S, Berberoglu MA, et al. Dscam guides embryonic axons by netrin-dependent and -independent functions. Development. 2008;135(23):3839–3848. doi: 10.1242/dev.023739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Michel CI, Kraft R, Restifo LL. Defective neuronal development in the mushroom bodies of Drosophila fragile X mental retardation 1 mutants. J Neurosci. 2004;24(25):5798–5809. doi: 10.1523/JNEUROSCI.1102-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo S, Huang W, Xia Q, Du Q, Wu L, Duan R. Mutational analyses of the FMR1 Gene in Chinese pediatric population of fragile X suspects: low tolerance for point mutation. J Child Neurol. 2015;30(6):803–806. doi: 10.1177/0883073814538508. [DOI] [PubMed] [Google Scholar]
- 43.Monyak RE, Emerson D, Schoenfeld BP, Zheng X, Chambers DB, Rosenfelt C, et al. Insulin signaling misregulation underlies circadian and cognitive deficits in a Drosophila fragile X model. Mol Psychiatry. 2016;22(8):1140–1148. doi: 10.1038/mp.2016.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bolduc FV, Bell K, Cox H, Broadie KS, Tully T. Excess protein synthesis in Drosophila fragile X mutants impairs long-term memory. Nat Neurosci. 2008;11(10):1143–1145. doi: 10.1038/nn.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dockendorff TC, Su HS, Mcbride SM, Yang Z, Choi CH, Siwicki KK, et al. Drosophila lacking dfmr1 activity show defects in circadian output and fail to maintain courtship interest. Neuron. 2002;34(6):973–984. doi: 10.1016/S0896-6273(02)00724-9. [DOI] [PubMed] [Google Scholar]
- 46.Mitchell KJ, Doyle JL, Serafini T, Kennedy TE, Tessier-Lavigne M, Goodman CS, et al. Genetic analysis of netrin genes in Drosophila: netrins guide CNS commissural axons and peripheral motor axons. Neuron. 1996;17(2):203–215. doi: 10.1016/S0896-6273(00)80153-1. [DOI] [PubMed] [Google Scholar]
- 47.Harris R, Sabatelli LM, Seeger MA. Guidance cues at the Drosophila CNS midline: identification and characterization of two Drosophila netrin/UNC-6 homologs. Neuron. 1996;17(2):217. doi: 10.1016/S0896-6273(00)80154-3. [DOI] [PubMed] [Google Scholar]
- 48.Newquist G, Drennan JM, Lamanuzzi M, Walker K, Clemens JC, Kidd T. Blocking apoptotic signaling rescues axon guidance in netrin mutants. Cell Rep. 2013;3(3):595–606. doi: 10.1016/j.celrep.2013.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang J, Zugates CT, Liang IH, Lee CH, Lee T. Drosophila Dscam is required for divergent segregation of sister branches and suppresses ectopic bifurcation of axons. Neuron. 2002;33(4):559–571. doi: 10.1016/S0896-6273(02)00570-6. [DOI] [PubMed] [Google Scholar]
- 50.Goldman JS, Ashour MA, Magdesian MH, Tristsch NX, Harris SN, Christofi N, et al. Netrin-1 promotes excitatory synaptogenesis between cortical neurons by initiating synapse assembly. J Neurosci. 2013;33(44):17278–17289. doi: 10.1523/JNEUROSCI.1085-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Colón-Ramos DA, Margeta MA, Shen K. Glia promote local synaptogenesis through UNC-6 (netrin) signaling in C. elegans. Science. 2007;318(5847):103–106. doi: 10.1126/science.1143762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Poon VY, Klassen MP, Shen K. UNC-6/netrin and its receptor UNC-5 locally exclude presynaptic components from dendrites. Nature. 2008;455(7213):669–673. doi: 10.1038/nature07291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kim S, Martin KC. Neuron-wide RNA transport combines with netrin-mediated local translation to spatially regulate the synaptic proteome. eLife. 2015;4(2015-01-08):1–24. [DOI] [PMC free article] [PubMed]
- 54.Horn KE, Glasgow SD, Gobert D, Bull SJ, Luk T, Girgis J, et al. DCC expression by neurons regulates synaptic plasticity in the adult brain. Cell Rep. 2013;3(1):173–185. doi: 10.1016/j.celrep.2012.12.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. NetB affects α’/β’and γ lobe length (Fisher exact test). (DOCX 15 kb)
Figure S1. a-c. Knock-down of Netrins with pan-neuronal driver elav-Gal4 showed similar phenotypes as driven by OK107-Gal4. a. MBs from control (elav-Gal4 > mCD8-GFP), NetA RNAi, NetB RNAi, NetA OE and NetB OE were visulized by mCD8-GFP driven by elav-Gal4 and immunostaining for FasII. The Control, NetA RNAi, NetA OE MBs showed normal structures, but knock-down of NetB showed short lobes (arrowhead). NetB OE MBs showed overextended lobes (arrow). b. The percentage of brain hemispheres with short α/β lobes in NetA/NetB RNAi flies. (elav-Gal4, n = 35; elav > NetA RNAi, n = 40; elav > NetB RNAi, n = 30; ***p < 0.001). c. The percentage of brain hemispheres with overextension of α/β lobes in flies over-expressing Netrins. (elav-Gal4, n = 35; elav > UAS-NetA, n = 30; elav > UAS-NetB, n = 30, ***p < 0.001). Significance was determined by Fisher exact test. Scale bars: 50 μm. (TIF 6225 kb)
Figure S2. The distribution of FMRP in the normal lymphoblastoid cell extracts treated with cycloheximide and EDTA. After cycloheximide treatment, FMRP was distributed across all the fractions. However, in the EDTA treated samples, most of the FMRP was in fractions 1–5, which correspond to the free messenger ribonucleoprotein (mRNP) and monosomal fraction. (TIF 685 kb)
Figure S3. Analysis of locomotor and olfactory abilities. a. Locomotor activity was measured by a line crossing assay [34]. All genotypes had similar locomotor activity profiles (elav-Gal4/Y, elav-Gal4/Y;dfmr1Δ3/dfmr150M, elav-Gal4/Y; UAS-NetA RNAi/+;dfmr1Δ3/dfmr150M, elav-Gal4/Y; UAS-NetB RNAi/+; dfmr1Δ3/dfmr150M, for each genotype, we tested 20 flies). b. Olfactory capabilities were measured by the olfactory trap assay [35]. No differences were found between any of the genotypes tested with this assay at the 60 h time point. (TIF 6938 kb)
Figure S4. NetB from the MB cells doesn’t interact with the Fra and Unc5 of the same cells. a. Knock-down of Fra and Unc-5 with NetB overexpression in the MB cells failed to ameliorate the defects of overextended α and β lobes (OK107 > UAS-NetB, n = 28; UAS-NetB/+; UAS-Fra RNAi/+; OK107-Gal4/+, n = 30; UAS-NetB/+; UAS-Unc-5 RNAi/+; OK107-Gal4/+, n = 28). b. Knock-down of Fra and Unc-5 with OK107-Gal4 displayed normal α/β lobes with normal length. Knock-down of Fra by a glia specific driver repo-Gal4, the MBs showed normal structure. However, knock-down of Unc-5 with repo-Gal4 caused short α/β lobes (arrowhead). c. The percentage of brain hemispheres with short α/β lobes (OK107-Gal4, n = 30; repo-Gal4, n = 28; OK107 > Fra RNAi, n = 25, OK107 > Unc-5 RNAi, n = 20; repo > Fra RNAi, n = 20; repo > Unc-5 RNAi, n = 22, ***p < 0.001). Significance was determined by Fisher’s exact test. Scale bars: 50 μm. (TIF 5078 kb)
Table S2. The summary of the α/β lobe defects in knocking-down Fra or Unc-5 by pan-neuron, MB and glia drivers respectively. (DOCX 15 kb)
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.