Abstract
Background:
Epilepsy is one of the most common chronic neurological conditions and its treatment during pregnancy is challenging. Levetiracetam (LEV) is an antiepileptic medication frequently used during pregnancy. Only a few small studies have been published on LEV monitoring during pregnancy, demonstrating decreased serum LEV levels during the first and second trimester; however, the most significant decrease was observed during the third trimester of pregnancy. In this study we aimed to evaluate LEV pharmacokinetics during different stages of pregnancy.
Methods:
We followed up and monitored serum levels of pregnant women treated with LEV for epilepsy.
Results:
Fifty-nine women with 66 pregnancies during the study period were included. The lowest raw LEV serum concentrations were observed during the first trimester. Compared with the pre-pregnancy period, raw serum concentration was lower by 5.76 mg/L [95% confidence interval (CI) (2.78, 8.75), p = 0.039] during the first trimester. Comparing the decrease in the first trimester with either the second or the third, no significant changes were observed (p = 0.945, p = 0.866). Compared with pre-pregnancy measurements, apparent clearance was increased by 71.08 L/day [95%CI (16.34, 125.83), p = 0.011] during the first trimester. About 30% of LEV serum levels during pregnancy were below the laboratory quoted reference range.
Conclusions:
Raw LEV serum levels tend to decrease during pregnancy, mainly during the first trimester contrary to previous reports. Monitoring of LEV serum levels is essential upon planning pregnancy and thereafter if pre-pregnancy LEV levels are to be maintained. However, more studies are needed to assess the correlation with clinical outcome.
Keywords: antiepileptic drugs (AEDs), levetiracetam, pharmacokinetics, pregnancy, therapeutic drug monitoring (TDM)
Introduction
Epilepsy is one of the most common chronic neurological conditions.1 The pharmacologic treatment of epilepsy during pregnancy is challenging. The goal is to obtain the best possible control of seizures with minimum adverse effects for the mother and the unborn child.2
Levetiracetam (LEV) is a second-generation antiepileptic drug (AED), indicated for the treatment of generalized and focal seizures.3 Current evidence suggests that LEV overall risk of teratogenicity is within the population baseline risk.4,5
LEV is commonly used in pregnant women, due to its safety profile, with low teratogenic risk and favorable pharmacokinetic characteristics.6–8
The pharmacokinetic properties of LEV include high oral bioavailability (>95%), low plasma protein binding (<10%), low risk of drug–drug interactions and it is not extensively metabolized. LEV has linear kinetics and first-order elimination mechanism.9 The main metabolic pathway (24% of the dose) is an enzymatic hydrolysis of the acetamide group. The major route of LEV excretion is through the urine: 66% of an administered dose is eliminated unchanged, and 24% is excreted in urine as inactive metabolites.10,11 Due to these properties, LEV is a frequently used medication also during pregnancy.7 Physiological changes during different stages of gestation may affect the pharmacokinetic characteristics of LEV as is common with other drugs.11,12
Since the introduction of LEV in the late 1990s, only a few studies on LEV pharmacokinetics during pregnancy, involving a small number of participants, have been published.13–15 These studies have reported some decrease of LEV serum levels during the first and second trimester; however, the most significant decrease in LEV level was observed during the third trimester. López-Fraile et al. prospectively investigated variations in serum concentration of LEV while taking the same dose during pregnancy and post-partum. The study results show a reduction of 20.3% in the serum level of LEV in the first trimester.13 The highest observed reduction (47.8%), compared with the pre-pregnancy period, was during the third trimester.13 Westin et al. reported similar results in a cohort of 20 pregnant women, after dose adjustments.14 Considering the fact that LEV is not metabolized by the liver, and its plasma protein binding is low, these serum concentration changes have been attributed to the increase in volume of distribution, renal clearance and renal blood flow during pregnancy, as renal plasma flow increases by 25–50% and glomerular filtration rate (GFR) by 50–80% above nonpregnant values.16–18 Other possible mechanisms such as an enhancement of hydrolytic processes or an accelerated drug glucuronidation during pregnancy, have not been investigated.12 Clinical guidelines recommend to consider monitoring LEV levels during pregnancy, though, data are insufficient to determine the pharmacokinetic alterations of LEV during this period.18,19 As a result levels are not routinely measured.20
The aim of this study was to measure the potential pharmacokinetic changes of LEV before, during and after pregnancy.
Methods
This is a pharmacokinetic, single site, cohort study conducted at the Assaf Harofeh Medical Center, a 900 bed teaching hospital in central Israel.
The participating women who were planning pregnancy or pregnant were referred to the Clinical Pharmacology and Toxicology Unit by neurologists from several medical centers. The study was approved by the Assaf Harofeh ethics committee (the number of the IRB protocol is: 127-12-ASF) before the research was started, and a written informed consent for inclusion in the present study was obtained from each patient. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Inclusion criteria were age 18 years and above, in a pre-pregnancy, during pregnancy or post-partum, with LEV level reaching steady state (at least 5 days after the last dose adjustment), and with at least two samples of trough levels. The patients were interviewed by trained staff to verify the inclusion criteria. The women were required to attend the study unit where age, epilepsy type and duration, LEV total daily dose (mg), co-medications and pregnancy week data were recorded.
The frequency of patients’ visits to our unit, and of serum samples collection for LEV analysis, was according to the referring neurologist. Blood samples were drawn immediately before the next dose. Pregnancy stages were classified as: planning pregnancy, first trimester, second trimester, third trimester and post-partum. The post-partum period was defined as 2–12 weeks after delivery.21 Apparent clearance (L/day) was calculated as the ratio between total daily dose (mg/day) and serum level concentration (mg/L). Apparent clearance was calculated for each woman individually, and then the mean value of apparent clearance was calculated for each stage of pregnancy.
Some patients were pregnant more than once during the study period and each pregnancy was considered as a separate case.
Blood was drawn through venipuncture and collected into Vacuette® tube - Z Serum Clot Acti-vator (Greiner Bio-One GmbH, Kremsmünster, Austria). The sample underwent centrifugation for 7 min at 3200 RPM at room temperature and the serum was separated to the secondary tube PS (SARSTEDT AG& Co, Nümbrecht, Germany). Immediately after centrifugation, the sample was frozen at −20°C.
LEV serum concentrations were determined using a commercial kit (Chromsystems Instruments & Chemicals GmbH, Munich, Germany) in high-performance liquid chromatography (HPLC) with photodiode array ultraviolet detection (Varian ProStar). In this method of analysis, the lower limit of quantification of serum LEV is 0.5 mg/l. LEV laboratory quoted reference range for serum concentration is 10–37 mg/l, while toxic level is above 400 mg/l. All samples were tested using the same kit.
Continuous variables were described as mean and range. Univariate and multivariate generalized estimating equations (GEE) models were used to study the changes in apparent clearance, total daily dose, serum concentrations and serum concentrations adjusted for dose during the pregnancy periods. The coefficient (b) and 95% confidence intervals (CIs) were reported. All statistical tests were two-sided and p < 0.05 was considered as statistically significant. SPSS software (IBMS SPSS Statistics for Windows, Version 25, IBM Corp, Armonk, New York, USA) was used for all statistical analysis.
Results
Overall, 76 pregnant women were enrolled in the study, with 90 pregnancies. Out of them, 59 women with 66 pregnancies met the inclusion criteria.
Mean age was 29.1 (range: 20–39) years and mean period since the first diagnosis of epilepsy was 13.6 years (range: 4–27). Polytherapy for epilepsy was recorded in 34 women.
Mean values of the total daily dose, apparent clearance and raw serum LEV concentrations during various study stages are presented in Table 1.
Table 1.
Measurements of total daily dose, raw serum concentration and apparent clearance of Levetiracetam (LEV).
| Trimester | Number of samples |
Total daily dose (mg) | Serum concentration (mg/l) | Apparent clearance (l/day) |
|---|---|---|---|---|
| Pre-pregnancy | 39 | 2051.28 (250–4000) |
18.22 (0.25–45.4) |
166.48 (56.6–1000) |
| 1 | 51 | 2149.02 (250–4000) |
12.45 (0.25–32.5) |
237.56 (97.4–1000) |
| 2 | 88 | 2538.64 (250–5000) |
14.83 (0.25–65.6) |
236.02 (45.7–1000) |
| 3 | 59 | 2770.34 (500–5000) |
13.81 (3.2–39.4) |
242.56 (62.11–784.31) |
| Post-partum | 16 | 2703.13 (1250–5000) |
28.4 (11.1–76.5) |
107.8 (52.96–171.42) |
Values are presented as mean (range min–max).
Comparison between the pre-pregnancy stage, the pregnancy trimesters and post-partum stage
A comparison of all the parameters (apparent clearance, total daily dose, serum concentration) was made between pre-pregnancy stage to all the pregnancy trimesters and post-partum stage (Table 2).
Table 2.
Changes in apparent clearance, total daily dose and serum concentration from pre-pregnancy status.
| Trimester | Number of samples | Apparent clearance (l/day) | Total daily dose (mg) | Serum concentration (mg/l) | Serum concentration adjusted for dose (mg/l) |
|---|---|---|---|---|---|
| 1 | 51 | ↑71.08 (16.33,125.83) p = 0.011 |
↑97.73 (176.99,372.46) p = 0.486 |
↓5.76 (2.78,8.75) p < 0.001 |
↓6.37 (3.60.9.13) p < 0.001 |
| 2 | 88 | ↑69.54 (15.3,123.79) p = 0.012 |
↑487.35 (171.16,803.54) p = 0.003 |
↓3.39 (0.17,6.60) p = 0.039 |
↓6.39 (3.13,9.66) p < 0.001 |
| 3 | 59 | ↑76.07 (12.74,139.41) p = 0.019 |
↑719.05 (326.95,1111.16) p < 0.001 |
↓4.41 (1.11,7.71) p = 0.009 |
↓8.84 (5.80,11.88) p < 0.001 |
| Post-partum | 16 | ↓58.68 (2.88,114.48) p = 0.039 |
↑651.84 (116.62,1187.06) p = 0.017 |
↑10.17 (1.61,18.73) p = 0.02 |
↑6.15 (−0.055,12.36) p = 0.052 |
Values are presented as mean change (95% CI), p value; ↑ increased; ↓ decreased.
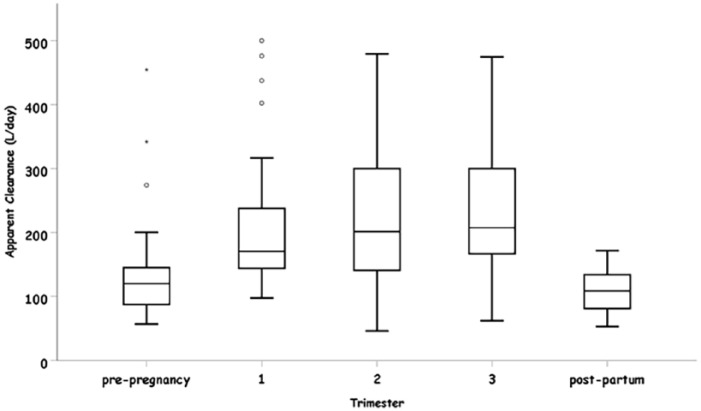
Mean of apparent clearance in all the three trimesters of pregnancy was significantly increased as compared with the pre-pregnancy stage (Table 2). The apparent clearance was increased by 71.08 L/day [95%CI (16.34, 125.83), p = 0.011], 69.55 L/day [95%CI (15.3, 123.79), p = 0.012] and 76.08 L/day [95%CI (12.74, 139.4), p = 0.019] during the first, second and third trimesters, respectively. Mean apparent clearance values demonstrated no significant changes between the three trimesters. The most significant change was observed in the first trimester as compared with pre-pregnancy. When a comparison between pre-pregnancy stage and post-partum stage was made, the mean apparent clearance was decreased significantly by 58.68 L/day [95%CI (2.88–114.48), p = 0.039] (Table 2).
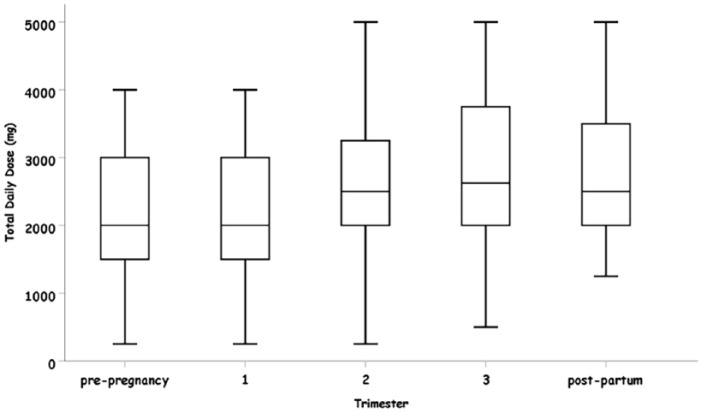
The total daily dose of administered LEV was increased during all stages of pregnancy. During the first trimester the change in the total daily dose was not statistically significant (Table 2). The most significant change was observed in the third trimester. When compared with pre-pregnancy stage, during the post-partum stage the total daily dose was significantly increased by 651.84 mg [95%CI (116.62, 1187.06), p = 0.017)].
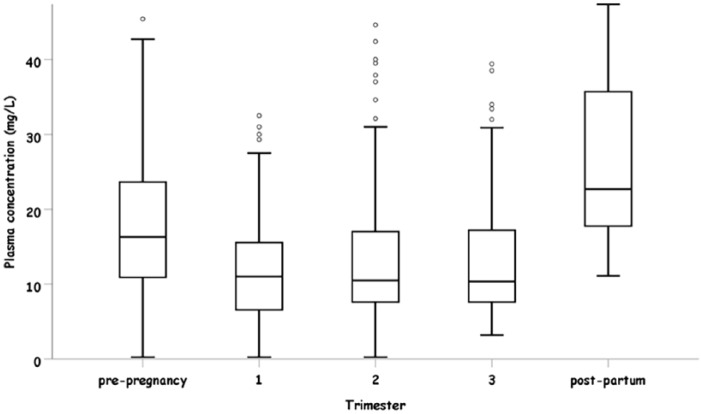
Raw serum concentration of LEV was decreased during all stages of pregnancy. Compared with the pre-pregnancy stage, serum concentration was significantly lower by 5.76 mg/l [95%CI (2.78, 8.75), p = 0.039] 3.39 mg/l [95%CI (0.17, 6.60), p = 0.009] and 4.41 mg/l [95%CI (1.11, 7.71), p = 0.02] during the first, second and third trimesters, respectively. The most significant change was observed in the first trimester. No significant changes between the second and the third trimesters compared with the first trimester were observed (p = 0.945, p = 0.866). During the post-partum period, LEV serum concentrations were significantly higher than in the pre-pregnancy stage by 10.17 mg [95%CI (1.61, 18.73), p = 0.02].
Comparison between the pre-pregnancy stage and pregnancy
A comparison of all parameters was performed for 34 women, with 38 pregnancies. The mean apparent clearance (adjusted to maternal age, trimester and total daily dose) in all three trimesters of pregnancy was significantly increased compared with the pre-pregnancy stage. Compared with the pre-pregnancy stage, apparent clearance was increased by 98.85 l/day [95%CI (34.4, 163.3), p = 0.003], 76.54 l/day [95%CI (14.5, 138.6), p = 0.016] and 100.4 l/day [95%CI (25.95, 174.87), p = 0.008] during the first, second and third trimester respectively. The total daily dose of LEV was increased during all the stages of pregnancy.
Mean raw serum concentration of LEV decreased during the first trimester of pregnancy by 6.32 mg/l [95%CI (3.84, 8.79), p < 0.001] as compared with the pre-pregnancy stage. Additional decrease during the second and the third trimester of pregnancy was statistically insignificant: 1.98 mg/l [95%CI (1.41, 5.37), p = 0.25] and 1.43 mg/l [95%CI (2.79, 5.66), p = 0.44], respectively. When adjusted to the trimester, age and total daily dose, the decrease in the serum concentrations of LEV was statistically significant during all the three trimesters of pregnancy: 6.99 mg/l [95%CI (4.08, 9.9), p < 0.001], 5.83 mg/l [95%CI (2.12, 9.55), p = 0.002] and 7.89 mg/l [95%CI (4.02, 11.76), p < 0.001], respectively.
An additional subanalysis was performed for this group, including all women with pre-pregnancy measurements above the minimal laboratory quoted threshold (10 mg/l) of LEV serum concentration. A total of 30 women exhibited raw LEV serum concentrations above the laboratory quoted threshold while planning pregnancy. During the first trimester, 21 women were evaluated, and, in 7 pregnancies, LEV serum concentration decreased below the lower laboratory quoted reference range; 26 women were tested during the second trimester, out of whom 6 had LEV serum concentration below the laboratory quoted reference range. During the third trimester, 19 pregnancies were evaluated; 6 of them were below the laboratory quoted reference range of serum concentration of LEV. Figure 1 describes number of samples below, above and within the laboratory quoted reference range of raw serum concentration of LEV.
Figure 1.
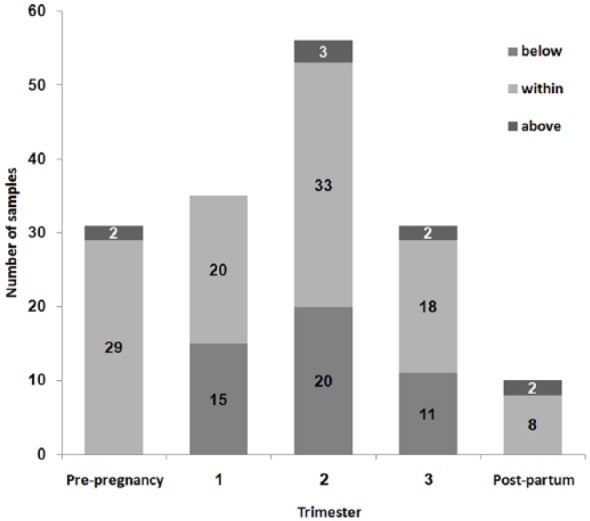
Number of Levetiracetam (LEV) raw serum levels within, below and above the laboratory quoted reference range in each pregnancy stage.
About 50% of the patients were treated with additional antiepileptic drugs. These drugs included: clobazam, lacosamide, carbamazepine, oxcarbazepine, topiramate, valproate, clonazepam, lamotrigine and ethosuximide. Only five women in our study were taking enzyme-inducing antiepileptic drugs throughout pregnancy; four women were treated with carbamazepine, and one with oxcarbazepine.
Multiple pregnancies and individual variation
During the study period several women (n = 11) were pregnant more than once. We compared different pregnancies of the same patient at the same trimesters.
In a group of eight women, blood samples were drawn during the first trimester in two consecutives pregnancies. Comparing the first trimester between the two pregnancies of each woman, apparent clearance, serum concentration and total daily dose were not significantly changed.
Six women were evaluated during the second trimester from different pregnancies. There was no difference between the pregnancies with regard to total daily dose. Raw serum concentration levels were lower by 7.26 mg/l [95%CI (13.04, 1.47), p = 0.014] and the apparent clearance was higher by 83.26 l/day [95% CI (8.3, 158.2), p = 0.029] during the consecutive pregnancies.
Three women were tested during the third trimester of both pregnancies. There was no difference between the pregnancies with regard to LEV serum concentration and apparent clearance. Total daily dose was higher during the later pregnancy by 625 mg/day [95%CI (192.64, 247.44), p = 0.001].
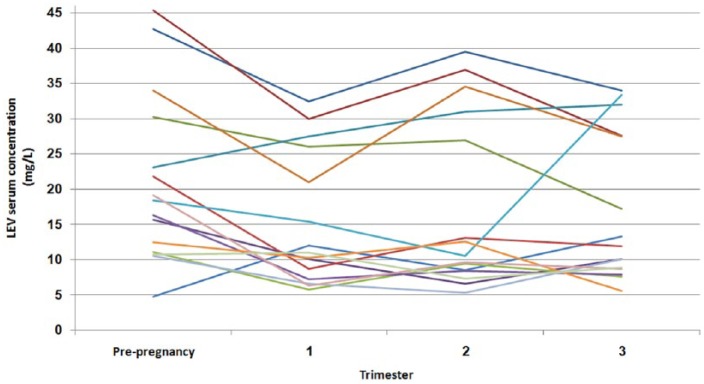
Individual variation in raw LEV serum levels during different stages of pregnancy is presented in Figure 2.
Discussion
To the best of the authors’ knowledge, this is the largest cohort of patients ever analyzed for LEV serum levels before, during and after pregnancy.
Previous studies have shown a reduction of LEV serum concentration during pregnancy, with a maximal reduction during the third trimester when compared with pre-pregnancy state.12–14
Consistent with these studies, our analysis demonstrates an increased apparent clearance rate of LEV during all stages of pregnancy, as compared with the pre-pregnancy state. However, after analyzing all three trimesters of pregnancy, surprisingly, the most significant increase was observed during the first trimester and not during the third trimester (Figure 3).
Figure 3.
Apparent clearance (l/day) in each pregnancy stage.
There was no significant change between either the second or the third trimesters compared with the first trimester. Raw serum LEV concentration remained significantly higher during the post-partum stage, as compared with the pre-pregnancy stage (p = 0.020) (Figure 4). In Table 2, we present data on LEV serum concentration as measured and with the appropriate adjustment for dose administered. The adjusted levels present the decrease in serum level that would have been occurred if the dose was not increased, reflecting the effect of pregnancy on LEV serum concentration.
Figure 4.
Raw serum concentration (mg/l) in each pregnancy stage.
Similarly, in a subanalysis of 34 women, the most significant decrease in raw serum LEV concentrations was observed during the first trimester. When adjusted to trimester, age and total daily dose, the decrease in LEV serum concentration was statistically significant during all three trimesters of pregnancy. This observation may suggest that the dose of LEV should be increased as soon as pregnancy is confirmed.
These results are different from those reported in previous studies, where apparent clearance was mostly increased during the third trimester.13–15 It has been assumed previously that increased renal blood flow and glomerular filtration rate during pregnancy play a role in decreasing AED serum levels.16–18 However, our results could not be explained by an increase in GFR alone.
Hormonal changes and increased estrogen levels may lead to accelerated drug glucuronidation during pregnancy.22 This effect has been studied in pregnant women treated with lamotrigine as a possible explanation for decreased lamotrigine serum levels.22 Another physiological change during pregnancy which may explain our findings could be the increased peripheral hydrolysis of LEV. So far, neither of these metabolic changes has been studied with LEV.12 The results of our study may be explained by additional physiological changes which alter the pharmacokinetics of LEV during pregnancy, such as altered gastrointestinal absorption of LEV due to gastric pH changes, decreased gastrointestinal motility or vomiting during pregnancy.23,24
The total daily dose of LEV was significantly increased during the second and the third trimesters but not during the first trimester (Figure 5). This delay in the LEV dose increase may be explained by the fact that during the second follow-up visit at the neurology clinic, the patients have already entered the second trimester of pregnancy.
Figure 5.
Levetiracetam (LEV) total daily dose (mg) in each pregnancy stage.
About 30% of raw LEV serum concentrations during pregnancy were below the laboratory quoted reference range (Figure 1). Since the most considerable decrease in serum concentration appears to occur during the first trimester, the patient might be exposed to higher risk of seizures during pregnancy. Some data suggest that women with epilepsy are 10 times more likely to die while pregnant than nonepileptic women.25,26 This fact is critical in implementing a routine LEV serum monitoring and dose adjustment in women planning and commencing pregnancy.
The apparent clearance of LEV was significantly higher in the nonpregnant state before pregnancy than in the nonpregnant state after pregnancy. This result may be explained mostly by the fact that the number of measurements post-partum was low compared with other stages and these two groups of women consisted from different subjects. Probably, post-pregnancy weight did not return to its pre-pregnancy value, hence affecting the volume of distribution.
A recent study suggested that enzyme-inducing antiepileptic drugs such as carbamazepine and oxcarbazepine might increase the apparent clearance of LEV by more than 40%.27 In our cohort, only five women were treated with enzyme-inducing antiepileptic drugs. However, these women were already on stable polytherapy before pregnancy and no dose change was made during the pregnancy.
Eleven women were pregnant more than once during the study period. When comparing consecutive pregnancies, the same trend of decreased LEV serum level was observed during the first trimester and thereafter.
Individual variation in raw LEV serum levels is presented in Figure 2. Most of the patients present similar pharmacokinetic profile; however, a few patients demonstrate intrapatient variability (Figure 2).
Figure 2.
Individual variation in raw Levetiracetam (LEV) serum concentration assessed before and during pregnancy (mg/l).
Our study has several limitations. Data regarding the clinical outcome, such as frequency of seizures, were not available in many cases. The frequency of the blood analysis was determined by the neurologist, and, therefore, not all women were regularly tested during each stage of the pregnancy during the study. There were no records of body weight each time women were followed.
The mean apparent clearance values between before, during and after pregnancy have been calculated on the basis of different numbers of subjects at the different stages studied. In order to overcome this limitation we used GEE models. However, this may limit the result of the study and may have confounded the interpretation of the stages of pregnancy when changes occur.
In conclusion, contrary to previous, smaller studies, we found that the most significant increase in apparent clearance of LEV is during the first trimester of pregnancy. About 40–50% of all pregnancies are unplanned.28 Recent data suggest that in women with epilepsy the rates of unplanned pregnancies are even higher – up to 65%.29
The need for monitoring LEV serum levels should be emphasized to physicians caring for women with epilepsy upon planning pregnancy and thereafter, if pre-pregnancy LEV levels are to be maintained. However, more studies are needed to assess correlation with clinical outcome.
Footnotes
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Maya Berlin, Clinical Pharmacology and Toxicology Unit, Assaf Harofeh Medical Center, Zerifin, Affiliated to Sackler Faculty of Medicine, Tel-Aviv University, Israel.
Dana Barchel, Clinical Pharmacology and Toxicology Unit, Assaf Harofeh Medical Center, Zerifin, Affiliated to Sackler Faculty of Medicine, Tel-Aviv University, Israel.
Revital Gandelman-Marton, Epilepsy and EEG Unit, Assaf Harofeh Medical Center, Zerifin, Affiliated to Sackler Faculty of Medicine, Tel-Aviv University, Israel.
Nurit Brandriss, Biochemistry Department, Assaf Harofeh Medical Center, Zerifin, Affiliated to Sackler Faculty of Medicine, Tel-Aviv University, Israel.
Ilan Blatt, Epilepsy Clinic and EEG Laboratory, Department of Neurology, Sheba Medical Center, Tel Hashomer, Israel.
Tomer Ziv-Baran, Department of Epidemiology and Preventive Medicine, School of Public Health, Sackler Faculty of Medicine, Tel-Aviv University, Israel.
Miri Y. Neufeld, EEG and Epilepsy Unit, Department of Neurology, Tel Aviv Medical Center, Sackler Faculty of Medicine, Tel Aviv University, Israel
Natalie Dinavitser, Clinical Pharmacology and Toxicology Unit, Assaf Harofeh Medical Center, Zerifin, Affiliated to Sackler Faculty of Medicine, Tel-Aviv University, Tel Aviv, Israel and Department of Clinical Biochemistry and Pharmacology, Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer Sheva, Israel.
Elkana Kohn, Clinical Pharmacology and Toxicology Unit, Assaf Harofeh Medical Center, Zerifin, Affiliated to Sackler Faculty of Medicine, Tel-Aviv University, Israel.
Dotan Shaniv, Clinical Pharmacology and Toxicology Unit, Assaf Harofeh Medical Center, Zerifin, Affiliated to Sackler Faculty of Medicine, Tel-Aviv University, Israel.
Tal De-Haan, Clinical Pharmacology and Toxicology Unit, Assaf Harofeh Medical Center, Zerifin, Affiliated to Sackler Faculty of Medicine, Tel-Aviv University, Israel.
Fanny Ofek, Pharmacy Department, Assaf Harofeh Medical Center, Zerifin, Affiliated to Sackler Faculty of Medicine, Tel-Aviv University, Israel.
Gideon Koren, Clinical Pharmacology and Toxicology Unit, Assaf Harofeh Medical Center, Zerifin, Affiliated to Sackler Faculty of Medicine, Tel-Aviv University, Israel and Maccabi-Kahn Institute of Research and Innovation, Tel Aviv, Israel.
David Stepensky, Department of Clinical Biochemistry and Pharmacology, Faculty of Health Sciences, Ben-Gurion University of the Negev, Beer Sheva, Israel.
Matitiahu Berkovitch, Clinical Pharmacology and Toxicology Unit, Assaf Harofeh Medical Center, Affiliated to Sackler Faculty of Medicine, Tel-Aviv University, Zerifin, 7033001, Israel.
Reference
- 1. Wiebe S, Bellhouse DR, Fallahay C, et al. Burden of epilepsy: the Ontario Health Survey. Can J Neurol Sci 1999; 26: 263–270. [DOI] [PubMed] [Google Scholar]
- 2. Morrell MJ. Epilepsy in women. Am Fam Physician 2002; 66: 1489–1494. [PubMed] [Google Scholar]
- 3. Arroyo S, Crawford P. Safety profile of levetiracetam. Epileptic Disord. 2003; 5(Suppl. 1): S57–S63. [PubMed] [Google Scholar]
- 4. Chaudhry SA, Jong G, Koren G. The fetal safety of Levetiracetam: a systematic review. Reprod Toxicol 2014; 46: 40–45. [DOI] [PubMed] [Google Scholar]
- 5. Weston J, Bromley R, Jackson CF, et al. Monotherapy treatment of epilepsy in pregnancy: congenital malformation outcomes in the child. Cochrane Database Syst Rev 2016; 11: CD010224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Martinez Ferri M, Peña Mayor P, Perez López-Fraile I, et al. Comparative study of antiepileptic drug use during pregnancy over a period of 12 years in Spain. Efficacy of the newer antiepileptic drugs lamotrigine, levetiracetam, and oxcarbazepine. Neurologia 2018; 33: 78–84. [DOI] [PubMed] [Google Scholar]
- 7. Garrity LC, Turner M, Standridge SM. Increased levetiracetam clearance associated with a breakthrough seizure in a pregnant patient receiving once/day extended-release levetiracetam. Pharmacotherapy 2014; 34: e128–e132. [DOI] [PubMed] [Google Scholar]
- 8. Veroniki AA, Cogo E, Rios P, et al. Comparative safety of anti-epileptic drugs during pregnancy: a systematic review and network meta-analysis of congenital malformations and prenatal outcomes. BMC Med 2017; 15: 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Patsalos PN. Pharmacokinetic profile of levetiracetam: toward ideal characteristics. Pharmacol Ther 2000; 85: 77–85. [DOI] [PubMed] [Google Scholar]
- 10. Radtke RA. Pharmacokinetics of levetiracetam. Epilepsia 2001; 42(Suppl. 4): 24–27. [DOI] [PubMed] [Google Scholar]
- 11. KEPPRA® (Levetiracetam), film-coated tablets, summary of product characteristics. Belgium: UCB Pharma, Inc, 2016, pp.1–12. [Google Scholar]
- 12. Reimers A, Brodtkorb E. Second-generation antiepileptic drugs and pregnancy: a guide for clinicians. Expert Rev Neurother 2012; 12: 707–717. [DOI] [PubMed] [Google Scholar]
- 13. López-Fraile IP, Cid AO, Juste AO, et al. Levetiracetam plasma level monitoring during pregnancy, delivery, and postpartum: clinical and outcome implications. Epilepsy Behav 2009; 15: 372–375. [DOI] [PubMed] [Google Scholar]
- 14. Westin AA, Reimers A, Helde G, et al. Serum concentration/dose ratio of levetiracetam before, during and after pregnancy. Seizure 2008; 17: 192–198. [DOI] [PubMed] [Google Scholar]
- 15. Tomson T, Palm R, Källén K, et al. Pharmacokinetics of levetiracetam during pregnancy, delivery, in the neonatal period, and lactation. Epilepsia 2007; 48: 1111–1116. [DOI] [PubMed] [Google Scholar]
- 16. De Sousa Mendes M, Hirt D, Urien S, et al. Physiologically-based pharmacokinetic modeling of renally excreted antiretroviral drugs in pregnant women. Br J Clin Pharmacol 2015; 80: 1031–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sturgiss SN, Dunlop W, Davison JM. Renal haemodynamics and tubular function in human pregnancy. Baillieres Clin Obstet Gynaecol 1994; 8: 209–234. [DOI] [PubMed] [Google Scholar]
- 18. Reisinger TL, Newman M, Loring DW, et al. Antiepileptic drug clearance and seizure frequency during pregnancy in women with epilepsy. Epilepsy Behav 2013; 29: 13–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harden CL, Hopp J, Ting TY, et al. Management issues for women with epilepsy-focus on pregnancy (an evidence-based review): I. Obstetrical complications and change in seizure frequency: Report of the Quality Standards Subcommittee and Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsia 2009; 50: 1229–1236. [DOI] [PubMed] [Google Scholar]
- 20. Richards N, Reith D, Stitely M, et al. Are doses of lamotrigine or levetiracetam adjusted during pregnancy? Epilepsia Open 2018; 3: 86–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Andrew MA, Easterling TR, Carr DB, et al. Amoxicillin pharmacokinetics in pregnant women: modeling and simulations of dosage strategies. Clin Pharmacol Ther 2007; 81: 547–556. [DOI] [PubMed] [Google Scholar]
- 22. Öhman I, Beck O, Vitols S, et al. Plasma concentrations of lamotrigine and its 2-N-glucuronide metabolite during pregnancy in women with epilepsy. Epilepsia 2008; 49: 1075–1080. [DOI] [PubMed] [Google Scholar]
- 23. Pennell PB. Antiepileptic drug pharmacokinetics during pregnancy and lactation. Neurology 2003; 61: S35–S42. [DOI] [PubMed] [Google Scholar]
- 24. Tomson T, Landmark CJ, Battino D. Antiepileptic drug treatment in pregnancy: changes in drug disposition and their clinical implications. Epilepsia 2013; 54: 405–414. [DOI] [PubMed] [Google Scholar]
- 25. Kapoor D, Wallace S. Trends in maternal deaths from epilepsy in the United Kingdom: a 30-year retrospective review. Obstet Med 2014; 7: 160–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. MacDonald SC, Bateman BT, McElrath TF, et al. Mortality and morbidity during delivery hospitalization among pregnant women with epilepsy in the United States. JAMA Neurol 2015; 72: 981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Aldaz A, Alzueta N, Viteri C. Influence of comedication on levetiracetam pharmacokinetics. Ther Drug Monit 2017; 1. [DOI] [PubMed] [Google Scholar]
- 28. Bearak J, Popinchalk A, Alkema L, et al. Global, regional, and subregional trends in unintended pregnancy and its outcomes from 1990 to 2014: estimates from a Bayesian hierarchical model. Lancet Glob Heal. 2018; 6: e380–e389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Herzog AG, Mandle HB, Cahill KE, et al. Predictors of unintended pregnancy in women with epilepsy. Neurology 2017; 88: 728–733. [DOI] [PubMed] [Google Scholar]